Fullerenes: The extensive development of nanoscience that has marked this century continues to evolve, producing new materials, structures, and devices for the treatments of diverse pathologies. Fullerenes are a family of nanoparticles with great applicative promise due to their small size (approximately 1 nm in diameter), structure, and capacity to cross biological barriers. Fullerene is the third pure crystal carbon form, with carbon atoms forming fused rings containing five to seven atoms that have been accidentally synthesized in 1985. This discovery led to the authors' Nobel Prize in Chemistry award in 1996, and afterward, fullerenes were detected in nature and outer space. The Fullerene family is named after buckminsterfullerene (C60). This most famous member, in turn, is named as an homage to Buckminster Fuller, an American architect, systems theorist, author, designer, inventor, and futurist (who used similar structural principles for architectural objects he designed).
In addition to their application in technology and electronics, fullerenes display a broad range of biological activities. One of the major obstacles to the biomedical application of fullerenes was their insolubility in aqueous media and their tendency to form aggregates. Thus, the important advancement in developing fullerene's treatment potential is the creation of water-soluble fullerenols. One such hydrophilic compound is the harmonized-hydroxylated fullerene-water complex - 3HFWC (C60(OH)24–45) (Koruga, 2011, 2021). The important characteristic of 3HFWC is that it is made by the functionalization of C60 molecule with OH groups (C60(OH)x) and through the addition of OH groups by water layers C60(OH)36 ± 12@ (H2O)144–2528 (Koruga, 2011). These water layers-water liquid phase (H2O)144–2528, surround and protect the solid phase-hydrogen bonded C60(OH)36 ± 12 nanostructure. Most importantly, at the same time, they protect the surrounding biomolecules from the potentially toxic effects of the C60. Besides their ability to inhibit the activity of HIV protease and bacterial binding to the host cells, fullerenes can interact with the immune system and cancer cells, inhibiting cancer growth in experimental conditions. Importantly, fullerenes have strong anti-oxidative properties due to their ability to absorb free radicals (reviewed in Castro et al., 2017).
Anti-amyloid properties of fullerenes: The application of nanotechnology has been exploited in the treatment of Alzheimer's disease (AD), a devastating disease without a cure, marked by gradual cognitive decline, depression, anxiety, and increased frailty that slowly unables patients to live independently. Advanced age is the single most major risk for AD, with the prevalence doubling every five years between the ages of 65 and 95 years and increasing from 2% at 65 years to 40% at over 85 years of age. Paradoxically, the life expectancy in 2023 is 73.16 years, with a steady increase of 0.23% yearly. Regrettably, AD patients' choice of treatments after diagnosis is limited and not very prospective, as these treatments decelerate but do not cure the disease. AD currently affects about 50 million people worldwide, and this number is estimated to increase by 62% by 2030, leading to complex health and socio-economic problems. Thus, the potential use of nanotechnology in AD treatment is urgently tested in various aspects, trying, if not to cure, then to curtail this disease. Nanoparticles are tested to improve drug delivery, facilitate beta-amyloid removal, improve imaging and diagnosis, induce neuroprotection and repair, and for early detection.
Remarkably, fullerene and its derivatives were shown to have anti-amyloid properties (Podolsky et al., 2007), suggesting their usage as promising candidates in AD treatment. The prevalent “amyloid hypothesis” that attempts to explain the etiology of AD deems the aggregation of amyloid-β (Aβ) peptide and the formation of senile plaques responsible for the development and progression of neurodegeneration and cognitive impairment in AD. Several findings showed that fullerenes and their derivatives can bind Aβ in vitro and in silico (Liu et al., 2019; Pandya et al., 2022). A modified form of fullerene (dimethoxylmethanofullerene) is able to specifically bind to the central hydrophobic motif KLVFF, interfering with intra- and inter-peptide interactions and inhibiting Aβ1–42 dimerization, therefore, suppressing Aβ fibrillation. In addition, the hydrated fullerene (C60: (H2O)n) can inhibit the fibril formation of Aβ (25–35) peptides (Podolski et al., 2007). In another study, Xie et al. (2014) have shown that the strong interactions between the fullerene nanoparticles and Aβ16–22 peptides are responsible for the significant weakening of the peptide-peptide interaction that is important for β-sheet formation, thus retarding Aβ16–22 fibrillation.
3HFWC nanoparticles decreased the plaque load in the 5×FAD mouse AD model: The creation of water-soluble fullerene compound – 3HFWC opened the possibility of per os treatments of experimental animals, and we tested the effects of 3HFWC on the amyloid plaque load in an AD transgenic mouse model, the 5×FAD mice (Perovic et al., 2023). 5×FAD mice express human amyloid precursor protein (APP) and presenilin 1 (PSEN1) transgenes with a total of five AD-linked mutations (three in APP and two in PSEN1) under transcriptional control of neuron-specific murine Thy-1 promoter (Oakley et al., 2006). The 5×FAD mice were drinking 3HFWC dissolved in water ad libitum, instead of the regular drinking water, for three months in the presymptomatic, prodromal phase of the disease progression (Figure 1). The prodromal phase of AD progression represents a sizable window of opportunity for testing various treatments. The main finding of this study is that 3HFWC treatment was able to induce a 14–30% decrease in amyloid plaque load in the brains of 5×FAD mice. Considering that the 5×FAD transgenic AD model is characterized by the aggressive production of Aβ and an extremely fast development of AD pathology, the achieved effect of 3HFWC treatment is even more significant. More humanized AD mouse models that recapitulate this disease in ways comparable to its progression in human patients are more suitable for assessing of the treatment's anti-AD effects.
Figure 1.
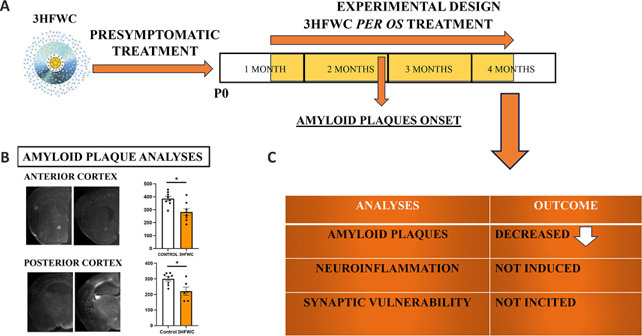
Effect of per os presymptomatic treatment with 3HFWC nanosubstance on amyloid plaque burden, neuroinflammation, and synaptic plasticity in 5×FAD animal Alzheimer's disease model.
(A) Experimental design: 5×FAD animals were treated with 3HFWC for three months starting at the postnatal day 21. (B) Amyloid plaque burden was analyzed in the anterior and posterior cortex, and a decrease of 14% and 20%, respectively, was observed after the 3HFWC treatment. (C) The analyses conducted after three months of 3HFWC treatment showed that amyloid plaque burden was reduced, neuroinflammation was not induced, and synaptic vulnerability was not incited. *P < 0.05. Unpublished data. Created with Microsoft PowerPoint. 3HFWC: Harmonized-hydroxylated fullerene-water complex; P0: postnatal day zero.
It remains to be explored if 3HFWC effects will be different in the animal models with the slower progression of the disease. The observed plaque-load decrease in the brains of 5×FAD mice differed depending on the region examined. These specific changes may result from the regional differences in Aβ production, diverse regional susceptibility to the 3HFWC treatments, or the different infiltration of nanoparticles. Methods that will enable us to track the distribution of 3HFWC particles should be devised, and a thorough investigation of 3HFWC distribution, using different doses and duration of treatments, should be conducted. Particularly interesting is the lack of a 3HFWC effect on the plaque load in the hippocampus. Considering the importance of this brain region in memory and cognition, its unresponsiveness to the treatment should be further investigated, potentially revealing mechanisms that were able to inhibit 3HFWC effects and penetrability. Based on previous research, the decrease in the plaque load is assumed to result from 3HFWC binding to Aβ and preventing its fibrillation. The question that presents itself is, what happens with the putative 3HFWC-Aβ complex? Is it cleared from the brain? If so, what is the mechanism of its clearance? If not, are these complexes deposited in the brain parenchyma? Most importantly, what is the half-life of such a complex? These questions must be answered as these findings will define future therapeutic regimes.
3HFWC treatment did not incite inflammation or synaptic vulnerability in 5×FAD mice: The treatment with 3HFWC (TFT Nano Center, Belgrade) was shown recently to be able to alter the metabolic state of specific brain regions (Lazovic et al., 2021) using functional magnetic resonance imaging analyses indicating the direct central nervous system (CNS) effects. However, one of the main concerns in the use of nanoparticles is their potentially harmful effects. Carbon-based nanoparticles were shown previously to induce inflammation in the brain and affect astrocytes (Park et al., 2010). We, thus, assessed the effects of 3HFWC on inflammation and synaptic plasticity in 5×FAD mice (Perovic et al., 2023). The expression levels of analyzed markers of inflammation glial fibrillary acidic protein and Iba-1 (markers of astrocytes and microglia, respectively) were unaltered after the 3HFWC treatment indicating the absence of induced inflammation. As the application of carbon-based nanoparticles was harmful to synaptic plasticity, we assessed the levels of postsynaptic density‐95, growth‐associated protein‐43, and synaptophysin, markers of pre- and post-synaptic plasticity in the CNS. As no difference in the relative abundance of proteins was observed after the 3HFWC treatment, we concluded that this nanoparticle did not induce synaptic vulnerability even after 3 months of treatment.
The confirmed lack of increased inflammation and gliosis, or incited synaptic vulnerability in the brains of 3HFWC-treated 5×FAD mice, strengthens the suitability of the 3HFWC compound for treatment. The water layers that surround and protect the solid phase—hydrogen bonded C60(OH)36 ± 12 nanostructure are responsible for protecting the surrounding biomolecules from the potentially toxic effects of the C60. Nevertheless, more stringent testing should be conducted, considering the different dosages and duration of the treatment. In addition, changes in inflammation and synaptic vulnerability should be evaluated at different time points post-3HFWC treatment before we can rule out any long-term side effects.
Using machine learning in the 3HFWC treatment evaluation: To estimate the functional effects of 3HFWC on the CNS, we (Perovic et al., 2023) exploited the analytical benefit of NIR spectroscopy for detecting pattern differences between control and 3HFWC-treated brain tissue samples. Using machine learning through the training of artificial neural networks, the highly reliable and accurate area under the curve values ≥ 0.92 obtained by the chosen classifier revealed the high precision of prediction between the control and treated samples, confirming that 3HFWC-treatment induced specific changes in the CNS. It is important to note that AD is characterized by a long prodromal phase that can last several decades before the first clinical symptoms present themselves. Thus, the effects of the chosen therapeutic are most effective when applied during this period. Nevertheless, suitable biomarkers that detect subtle changes in the prodromal phase are lacking. Using sophisticated algorithms operating on large-scale, heterogeneous datasets, machine learning can uncover functional patterns that would be difficult or impossible for even well-trained individuals to identify (Goecks et al., 2020). Many machine learning applications detect altered CNS properties, including AD, indicating the significance of machine learning applications in biomedicine. Research into using NIRS for AD diagnosis is ongoing. While it shows promise, it is often used with other imaging modalities and clinical assessments to provide a more comprehensive picture of the disease. As technology advances and our understanding of AD improve, NIRS might play a valuable role in the early detection and monitoring of AD. Recently, the changes in the AD retina have been emphasized as potential biomarkers to define changes in the CNS. The significant correlation between the retinal and brain changes in AD, together with the fact that the neurosensory retina is the only part of the CNS that is readily available for non-invasive imaging and treatment, brands the retina as a worthy candidate for further focused research to gain the better insight into the changes in the diseased brain.
Perspective: The positive effects of 3HFWC treatment in the presymptomatic phase of AD pathology progression are promising. Particularly reassuring are the findings that the 3HFWC treatment did not incite inflammation or synaptic vulnerability, the two permanent features of neurodegenerative diseases. The application of nanoparticles in biomedical treatments is relatively young and unexplored, and caution about developing therapies is necessary. As neuroinflammation is causal or secondary to many neurodegenerative diseases, including AD, Parkinson's disease, Amyotrophic lateral sclerosis, and others, the treatments with 3HFWC and fullerenols derivatives could be extremely beneficial. The main finding, the decreased amyloid plaque load in the cortex of 5×FAD mice, is encouraging because the formation of amyloid aggregates is considered perilous for the progression of neurodegeneration in AD. These amyloid accumulations occur due to exaggerated production and misfolding of toxic proteins and impaired Aβ clearance from the blood because of the compromised blood-brain barrier.
In summary, developing novel treatments or adjuvant therapies with 3HFWC or similar fullerenols derivatives can sequester the abundant free toxic Aβ and prevent its fibrillation and accumulation. It remains to be established if 3HFWC will have similar effects in the case of other proteinopathies such as the Creutzfeldt-Jakob disease and other prion diseases, AD, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders whose etiology involves misfolding of toxic proteins.
This work was supported by Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja, Grant/Award Number: 451-03-9/2021-14/200007 and 451-03-9/2021-14/200017; Zepter International Foundation, Grant/Award Number: 5/2019 (to SI).
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Castro E, Hernandez Garcia A, Zavala G, Echegoyen L. Fullerenes in biology and medicine. J Mater Chem B. 2017;5:6523–6535. doi: 10.1039/C7TB00855D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecks J, Jalili V, Heiser L, Gray JW. How machine learning will transform biomedicine. Cell. 2020;181:92–101. doi: 10.1016/j.cell.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koruga D. Composition of matter containing harmonized hydroxyl modified fullerene substances. US Patent 8058483B2. 2011:1–16. [Google Scholar]
- Koruga D. Compositions comprising hyper harmonized hydroxyl modified fullerene substances. WO2021110234A1. 2021:1–43. [Google Scholar]
- Lazovic J, Zopf LM, Hren J, Gajdos M, Slavkovic M, Jovic Z, Stankovic I, Matovic V, Koruga DJ. Fullerene-filtered light spectrum and fullerenes modulate emotional and pain processing in mice. Symmetry. 2021;13:2004. [Google Scholar]
- Liu Z, Zhou Y, Zhang Q, Chen P, Liu Y, Qian Z. Distinct binding dynamics, sites and interactions of fullerene and fullerenols with amyloid-β peptides revealed by molecular dynamics simulations. Int J Mol Sci. 2019;20:2048. doi: 10.3390/ijms20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in trans- genic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya V, Baweja L, Dhawan A. Preferential binding of fullerene and fullerenol with the N-terminal and middle regions of amyloid beta peptide: an in silico investigation. Int J Nanomed. 2018;13:71–73. doi: 10.2147/IJN.S125011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Roh J, Kim Y, Park K. Induction of inflammatory responses by carbon fullerene (C60) in cultured RAW264.7 cells and in intraperitoneally injected mice. Toxicol Res. 2010;26:267–273. doi: 10.5487/TR.2010.26.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perovic M, Ciric J, Matovic V, Srbovan M, Koruga Dj, Kanazir S, Ivkovic S. The presymptomatic treatment with 3HFWC nanosubstance decreased plaque load in 5XFAD mouse model of Alzheimer's disease. CNS Neurosci Ther. 2023 doi: 10.1111/cns.14188. doi: 10.1111/cns.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolski IY, Podlubnaya ZA, Kosenko EA, Mugantseva EA, Makarova EG, Marsagishvilli LG, Shpagina MD, Kaminsky YG, Andrievsky GV, Klochkov VK. Effects of hydrated forms of C 60 fullerene on amyloid β-peptide fibrillization in vitro and performance of the cognitive task. J Nanosci Nanotechnol. 2007;7:1479–1485. doi: 10.1166/jnn.2007.330. [DOI] [PubMed] [Google Scholar]
- Xie L, Luo Y, Lin D, Xi W, Yang X, Wei G. The molecular mechanism of fullerene-inhibited aggregation of Alzheimer's β-amyloid peptide fragment. Nanoscale. 2014;6:9752–9762. doi: 10.1039/c4nr01005a. [DOI] [PubMed] [Google Scholar]


