Abstract
The glucagon-like peptide 1 is a pleiotropic hormone that has potent insulinotropic effects and is key in treating metabolic diseases such as diabetes and obesity. Glucagon-like peptide 1 exerts its effects by activating a membrane receptor identified in many tissues, including different brain regions. Glucagon-like peptide 1 activates several signaling pathways related to neuroprotection, like the support of cell growth/survival, enhancement promotion of synapse formation, autophagy, and inhibition of the secretion of proinflammatory cytokines, microglial activation, and apoptosis during neural morphogenesis. The glial cells, including astrocytes and microglia, maintain metabolic homeostasis and defense against pathogens in the central nervous system. After brain insult, microglia are the first cells to respond, followed by reactive astrocytosis. These activated cells produce proinflammatory mediators like cytokines or chemokines to react to the insult. Furthermore, under these circumstances, microglia can become chronically inflammatory by losing their homeostatic molecular signature and, consequently, their functions during many diseases. Several processes promote the development of neurological disorders and influence their pathological evolution: like the formation of protein aggregates, the accumulation of abnormally modified cellular constituents, the formation and release by injured neurons or synapses of molecules that can dampen neural function, and, of critical importance, the dysregulation of inflammatory control mechanisms. The glucagon-like peptide 1 receptor agonist emerges as a critical tool in treating brain-related inflammatory pathologies, restoring brain cell homeostasis under inflammatory conditions, modulating microglia activity, and decreasing the inflammatory response. This review summarizes recent advances linked to the anti-inflammatory properties of glucagon-like peptide 1 receptor activation in the brain related to multiple sclerosis, Alzheimer's disease, Parkinson's disease, vascular dementia, or chronic migraine.
Keywords: astrocytes, brain, glucagon-like peptide 1 receptor, inflammation, microglia
Introduction
Glucagon-like peptide 1 (GLP-1) can have broad pharmacological and therapeutic uses (Müller et al., 2019). Among its hormonal effects, it should be included its capability to decrease gastric emptying, inhibit food intake, or control metabolism (Decara et al., 2016; Müller et al., 2019; Brierley et al., 2021; Imbernon et al., 2022; Singh et al., 2022). Preclinical studies have demonstrated the capacity of the activation of the GLP-1 receptor to induce protective effects, modulating the hypothalamic-pituitary-adrenal axis, heart, and lungs (Gil-Lozano et al., 2014; Romaní-Pérez et al., 2015; Drucker, 2018; Müller et al., 2019; Fandiño et al., 2020; Diz-Chaves et al., 2022b; Li et al., 2022; Reich and Hölscher, 2022). Moreover, GLP-1 modulates learning and memory, rewards behavior, and reduces hunger-driven feeding, food's hedonic value, and motivation (Skibicka, 2013; Müller et al., 2019), in addition to its well-known incretin effect in hyperglycaemic conditions (Müller et al., 2019).
Moreover, in the inflammatory response, GLP-1 acts as a target and mediator (Drucker, 2018). In rodents, the secretion of the peptide increases after inflammatory stimuli (Kahles et al., 2014; Nguyen et al., 2014). GLP-1 modulates inflammation in multiple areas, demonstrating anti-inflammatory effects like pancreatic islets and adipose tissue (Langlois et al., 2016; Lee and Jun, 2016). Also, the liver, gut, kidney, lung, testis or skin, and the vascular system, which includes the aorta and vein endothelial cells, GLP-1 can reduce the production of inflammatory cytokines, chemokines, and the infiltration of immune cells in the tissues (Drucker, 2016; Lee and Jun 2016; Fandiño et al., 2020; Yang et al., 2022) and mediate the inflammatory response (Drucker, 2018). Moreover, GLP-1 and the GLP-1 receptor analogs display a wide range of neuroprotective and anti-inflammatory effects in the brain in several animal models of neuroinflammatory diseases (Diz-Chaves et al., 2018; Diz-Chaves, et al., 2022b). These molecules emerge essential in treating brain inflammatory-related pathologies by restoring brain cell homeostasis under inflammatory conditions. This review summarizes recent advances in the anti-inflammatory properties of GLP-1 receptor activation in the brain.
Retrieval Strategy
The studies cited in this review were retrieved from the PubMed database. The appropriate literature published from 2018 and 2023 was screened. Even though, for the writing of the manuscript, relevant studies before these year periods were included when necessary. The following words combined with Boolean operators (AND and OR) were used to maximize search specificity and sensitivity: microglia, astrocytes, GLP-1 receptor, GLP-1 receptor analogs, brain inflammation, neuroinflammation, GLP-1 effects, brain inflammatory diseases, multiple sclerosis, Alzheimer's disease, vascular dementia, chronic migraine. The results were further screened by title and abstract. Furthermore, the role of microglia and its contribution to neuroinflammation events was included. No language or study type restrictions were applied.
Glucagon-Like Peptide 1 and Its Derivatives
Cell-specific posttranslational processing of the proglucagon gene leads to the synthesis of GLP-1 (Müller et al., 2019). In pancreatic α-cells, in enteroendocrine L-cells throughout the gut, and in the caudal portion of the nucleus tractus solitary of the brainstem, the preproglucagon (PPG) is processed by the prohormone convertase 1/3 into GLP-1 and other peptides (Larsen et al., 1997; Müller et al., 2019). Moreover, in the brain, PPG cell bodies were also described in a population of glutamatergic olfactory bulb interneurons in the adjacent medullary reticular formation, the lumbar-sacral spinal cord and the piriform cortex (Holt et al., 2019; Thiebaud et al., 2019; Diz-Chaves, et al., 2022b).
The processing of proglucagon results in the gut and, to a smaller extent, in the pancreatic α-cells, in three GLP-1 peptides of 37, 31, and 30 residues (Diz-Chaves, et al., 2022a). The pancreatic receptor recognizes a glycine-extended peptide GLP-1(7–37) and GLP-1(7–36) amide, two N-terminally truncated products (Drucker et al., 1986; Campos et al., 1994). However, in vitro and in vivo studies have demonstrated that its metabolite, GLP-1(9–36), reduces glucose production alone or induced by positive allosteric modulators (Elahi et al., 2008; Willard et al., 2021) and has protective effects in neuronal and microglial cell lines (Li et al., 2021).
The GLP-1(7–36)amide is rapidly degraded (1–2 minutes in humans) by the ubiquitous enzyme dipeptidyl peptidase-4 and forms two N-terminally truncated peptides, the GLP-1 (9–37) and the GLP-1 (9–36)amide (Holst et al., 2019). The short half-life of GLP-1 limits its therapeutic use. For this reason, different GLP-1 derivatives with extended half-lives and improved bioavailability have been developed in the last years to treat metabolic diseases like type 2 diabetes and obesity (Müller et al., 2018, 2019; Knudsen and Lau, 2019; Arslanian et al., 2022). These molecules bind to the GLP-1 receptor (GLP-1R) and can activate downstream signaling, modulating cell growth and repair similarly to GLP-1 (Müller et al., 2018). There are currently seven approved GLP-1 receptor agonists (GLP-1 RAs) (Trujillo et al., 2021). Short-acting: exenatide twice daily (Byetta®), lixisenatide once daily (Adlyxin®, Lyxumia®); and long-acting: liraglutide once daily (Victoza®), exenatide once weekly (Bydureon®), dulaglutide (Trulicity®), semaglutide (Ozempic®) and oral semaglutide (Rybelsus®) (Trujillo et al., 2021).
Classifying the GLP-1 RAs into several groups concerning the structural modification of the GLP-1(7–37) molecule is possible: The Exendin-based therapies, based on the exendin-4, protect from dipeptidyl peptidase-4 cleavage. This molecule bears a 53% homology to human GLP-1 (Müller et al., 2018; Knudsen and Lau, 2019; Diz-Chaves, et al., 2022b). Modifying several amino acid sequences results in molecules that are dipeptidyl peptidase-4 resistant and present increased half-lives, such as Lixisenatide (Sanofi, Paris, France), a 44-amino-acid derivative of Exenatide (Müller et al., 2018; Knudsen and Lau, 2019; Diz-Chaves et al., 2022b). There are GLP-1 RAs with a slow-down renal excretion by conjugating the peptide to substances such as fatty acids and ovalbumin (among others). Like liraglutide (Novo Nordisk, Copenhagen, Denmark), based upon the native GLP-1 sequence, is palmitoylated (C16:0) at the side chain of lysine 20 through a γ-glutamic acid spacer-molecule (Knudsen and Lau, 2019; Diz-Chaves, et al., 2022b) or Semaglutide (Novo Nordisk), a chemically optimized analog of liraglutide with enhanced pharmacological properties (Müller et al., 2019; Diz-Chaves, et al., 2022b). Nowadays, it has developed various classes of peptide-based multi-agonists, and peptide-small molecule conjugates with probed superior preclinical efficacy and is currently undergoing clinical evaluation (Clemmensen et al., 2019). A single molecule with complementary signaling characterizes the multi-agonists through more than one receptor, each offering beneficial effects on systems (Müller et al., 2018, 2019) like the dual or triple agonist of the GLP-1 receptor, including glucagon or GIP (glucose-dependent insulinotropic polypeptide) or single-molecule hybrids of GLP1 and GLP2 still under study (Müller et al., 2018; Clemmensen et al., 2019).
Of late, small molecules' positive allosteric modulators of the GLP-1 receptor have been characterized (Nakane et al., 2015; Willard et al., 2021) even though they still not have been approved for clinical use yet (Clemmensen et al., 2019). These compounds can enhance the affinity of GLP-1R for GLP-1 and provide effective direct agonism. Some of these positive allosteric modulators can potentiate the activity of weaker metabolite GLP-1 (9–36) by interacting with both the receptor and the peptide (Nakane et al., 2015; Willard et al., 2021).
Glucagon-Like Peptide 1 Receptor in the Brain: Signaling Pathways
GLP-1 promotes biological actions by binding to a class B family's G protein-coupled receptor (Müller et al., 2019). Seven transmembrane domains form the GLP-1R. It contains an amino-terminal hydrophobic region followed by a hydrophilic domain with three N-linked glycosylation sites essential for the receptor function as standard folding and trafficking to the cell surface (Chen et al., 2010; Yang et al., 2016).
It is possible to find receptor mRNA transcripts widely distributed in peripheral tissues or the central nervous system (CNS; Müller et al., 2019; McLean et al., 2021). PPG neurons display axonal inputs with the most GLP-1R-expressing brain regions and can be activated by the GLP-1 originating from those cells (McLean et al., 2021). However, brainstem PPG neurons do not project to the hippocampus (Llewellyn-Smith et al., 2011) or the olfactory bulb, where GLP-1R has been described (McLean et al., 2021). Moreover, GLP-1Rs are highly abundant in circumventricular organs (Müller et al., 2019; McLean et al., 2021; Nowell et al., 2022), as well as nuclei involved in the regulation of glucose metabolism and energy balance (McLean et al., 2021), as was demonstrated recently, using far-red fluorescent labels for the real-time detection of endogenous GLP-1R in live cells (Ast et al., 2020). The passage of GLP-1 agonists across the blood-brain barrier (BBB) is incompletely understood. However, a mechanism that acts as the conduit between the peripheral circulation and the brain may exist for GLP-1 receptor-dependent ligand uptake and transcytosis across tanycytes, the choroid plexus, or endothelial cells (Jones, 2022).
The GLP-1 receptor is present in neurons and glial cells in several brain regions (Diz-Chaves et al., 2022b). However, species differences related to brain regions may exist about the expression of the receptor. In fact, in the spinal cord in mice, the receptor appears in neurons and microglia but not in astrocytes (Qian et al., 2022). However, in the hypothalamus, GLP-1R is not expressed in microglia, but it was described in neurons, vascular cells, and astrocytes expressing aquaporin associated with vascular end feet (Heiss et al. 2021; Smith et al. 2022). It is essential to point out that the lack in the expression of the receptor in the microglia in several brain regions may be related to the necessity of the existence of a brain inflammatory insult to increase its density, related to its role in controlling inflammation (Diz-Chaves et al., 2022b).
In the brain, the activation of GLP-1R coupled Gα subunit pathway activates the adenylate cyclase system, which stimulates cAMP formation through the conversion of adenosine triphosphate, continued with protein kinase A (PKA) activation (McLean et al., 2021; Nowell et al., 2022; Figure 1). Then, PKA can promote cAMP-response element binding protein phosphorylation. The activity of PKA can augment the release of neurotransmitters into the synapse, thus facilitating long-term potentiation and giving support to cell growth and survival as well as synaptic plasticity (Diz-Chaves et al., 2022b; Nowell et al., 2022; Reich and Hölscher, 2022). Furthermore, the metabolic ligand adenosine diphosphate, derived from adenylate cyclase, acts on K+ channels, facilitating depolarisation of the cell membrane after the closure of the channel, and reducing repolarisation, leading to the opening of voltage-dependent L-type Ca2+ channels, leading to the increase of the intracellular concentration of Ca2+ and facilitating that way the neurotransmitter release (Calsolaro and Edison, 2015; Nowell et al., 2022). Moreover, an alternative cAMP signaling mechanism has been described by activating cAMP-binding proteins designated as cAMP-regulated guanine nucleotide exchange factors (cAMP/GEFs, also known as Epac) (Holz, 2004). EPAC leads to the activation of mitogen-activated protein kinase (MAPK), starting a slower process of gene activation (Hölscher and Li, 2010).
Figure 1.
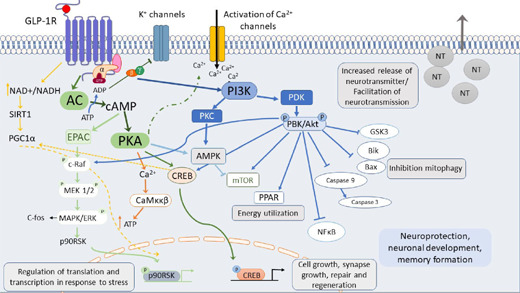
Downstream GLP-1 receptor activation signaling.
Downstream GLP-1 receptor activation of the GLP-1 receptor promotes several cell-signaling cascades involved in GLP-1RAS effects: memory formation, synapse repair, neuroprotection, activation of Ca2+, and regeneration of neurotransmitter release. Reprinted with permission from Diz-Chaves et al. (2022b). AC: Adenylate cyclase; AMPK: AMP-activated protein kinase; Bax, Bik: Bcl2-interacting killer; Ca2+: calcium ions; c-Raf: cellular Raf gene (rapidly accelerated fibrosarcoma); CREB: cyclic AMP response element-binding protein; EPAC: exchange proteins directly activated by cAMP; ERK: extracellular signal-regulated kinase; MAPK: mitogen-activated protein kinase; MEK1/2: MAPK or Erk kinases; mTOR: mammalian target of rapamycin; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; P90RSK: ribosomal S6 kinase; PGC1α: peroxisome proliferator-activated receptor γ co-activator; PI3K: phospho- inositide 3 kinase; PKB: protein kinase B; PKC: protein kinase C; PKD: protein kinase D; SIRT1: sirtuin 1.
When the GLP-1 receptor coupled Gβγ subunits activate PI3K, it consequently initiates MAPK cascade. It promotes gene expression controlling different cellular repair mechanisms, cell growth, and differentiation (Nowell et al., 2022). Moreover, the pathway PI3K/AKT can be activated. The AKT pathway can phosphorylate many substrate proteins and modulate different processes, such as autophagy, long-term potentiation, the synapse formation, as well as inhibiting the secretion of proinflammatory cytokines, apoptosis, microglial activation, tau phosphorylation, and the accumulation of α-synuclein and amyloid-β (Aβ; Grieco et al., 2019; Cao et al., 2022; Guan et al., 2022; Nowell et al., 2022).
Microglia and Astrocytes in the Inflammatory Response
In the brain, microglia are the principal resident immune cells involved in homeostasis and host defense against pathogens and CNS disorders (Hickman et al., 2018). These cells are highly dynamic and undergo intense remodeling processes throughout their lifespan (El Ali and Rivest, 2016). Their physiological functions could include the control of synaptic remodeling, the maintenance of myelin homeostasis, or the migration to sites of neuronal death to phagocytose dead or dying cells or debris (Hickman et al., 2018). Microglia mediate host defense against infectious pathogens, harmful self-proteins such as Aβ, aggregated α-synuclein, mutant or oxidized superoxide dismutase, mutant huntingtin or prions, as well as primary or metastatic CNS tumors (Hickman et al., 2018).
Microglia express several receptors involved in the control of innate immune functions, mainly the pattern recognition receptors that regroup three principal receptor families: nucleotide-binding oligomerization domain (nod)-like receptors (NLRs), toll-like receptors (TLRs), and the retinoic acid-inducible gene-1-like receptors (El Ali and Rivest, 2016). These receptors recognize specific ligands called pathogen-associated molecular patterns and danger-associated molecular patterns (DAMPs; Rivest, 2009; El Ali and Rivest, 2016). The activation of these receptors induces specific signaling pathways involved in modulating microglial functions (El Ali and Rivest, 2016). After an injury or disease, microglia respond by undergoing a process of cellular activation characterized by phenotypical morphological changes, increased cell proliferation, and modifying their function by leading to the production of proinflammatory molecules (Rivest, 2009; Astiz et al., 2016; Plastini et al., 2020; Diz-Chaves et al., 2022b). After CNS insult, microglia respond to the subsequent activation of the astrocytes in a process called astrocytosis (DiSabato et al., 2016). Moreover, related to these effects, chronic microglia activation is associated with the etiology and progression of different neurodegenerative diseases (DiSabato et al., 2016; Hickman et al., 2018).
These cells can lose their homeostatic molecular signature and functions during many diseases, such as synaptic plasticity roles, becoming chronically inflammatory (Butovsky and Weiner, 2018). Remarkably, microglia have a typical neurodegenerative signature for conditions such as Alzheimer's disease (AD), multiple sclerosis (MS), or Parkinson's disease (PD) (Butovsky and Weiner, 2018), reacting to injury through morphological changes by increasing microglial proliferation, migration to the target, phagocytosis, activating the inflammasome, and releasing proinflammatory molecules (Diz-Chaves et al., 2022b).
Astrocytes are involved in almost every aspect of CNS function. These cells modulate synapse formation and maturation, homeostasis of ions and neurotransmitters, and BBB maintenance. They also are critical for maintaining neuronal metabolic support (Bonvento and Bolaños, 2021; Torres-Ceja and Olsen, 2022). Astrocyte activation has two faces, a beneficial one, promoting tissue repair and homeostasis, or a detrimental, exacerbating inflammatory response and tissue damage, depending on the stimuli' nature (Jha et al., 2019). Microglia activation stimulates the secretion of several proinflammatory molecules like tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, nitric oxide, vascular endothelial growth factor-B, or complement factor Cq1 that can induce a modification of the astrocyte phenotype towards A1 reactive (Liddelow et al., 2017). Under these circumstances, astrocytes lose the capacity to promote neuronal survival, outgrowth, synaptogenesis, and phagocytosis, inducing the death of close neurons and oligodendrocytes (Liddelow et al., 2017). Also, A1 reactive astrocytes have decreased metabolic and homeostatic roles, impairing glutamate uptake (resulting in excitotoxicity) and decreasing lactate release from astrocytes (Liddelow et al., 2017; Jha et al., 2019).
Glucagon-Like Peptide-1 Receptor Activation in the Brain Concerning Inflammation
Neuroinflammation is related to the chronic activation of microglia and astrocytes accompanied by significant cytokine and chemokine production, the infiltration of peripheral immune cells and edema formation, and increased BBB permeability or even breakdown (DiSabato et al., 2016; Diz-Chaves et al., 2022b). However, the context, duration, and course of the primary stimulus or insult may determine the degree of neuroinflammation (DiSabato et al., 2016).
Most neurological and neurodegenerative disorders are influenced by inflammatory processes (Gilhus and Deuschl, 2019), as well as many neuropsychiatric disorders, retina, and optic nerve degenerative diseases, epilepsy, traumatic brain injury or stroke (DW et al., 2017; Rossi et al., 2021; Diz-Chaves et al., 2022b; Reich and Hölscher, 2022). This review focused on multiple sclerosis, PD and AD, vascular dementia, and migraine.
Multiple sclerosis
Multiple sclerosis (MS) is the most prevailing chronic inflammatory disease of CNS (Dendrou et al., 2015). It is characterized by immune cell infiltration across the BBB and the apparition of lesion sites, a usual hallmark of MS. Lesioned sites are related to inflammation, demyelination, activation of glia, and neuroaxonal degeneration, conducting to disruption of neuronal signaling (Diz-Chaves et al., 2022b). These lesions are considerably recognized in the white matter but may appear throughout the CNS; they are related to focal areas of glial reaction, inflammation, and demyelination (Reich et al., 2018).
In this context, the therapeutic efficacy of the activation of the GLP-1R in “in vivo” and in “in vitro” MS models has been described (DellaValle et al., 2016; Lee et al., 2018; Chiou et al., 2019; Gharagozloo et al., 2021; Park et al., 2021; Ammar et al., 2022). The therapy with GLP-1 RAs as Liraglutide, exendin-4, or dulaglutide can ameliorate the disease score both in experimental autoimmune encephalomyelitis (EAE) and Cup-mice (DellaValle et al., 2016; Lee et al., 2018; Chiou et al., 2019; Gharagozloo et al., 2021; Song et al., 2022) independently of the dose, time, and via administration (subcutaneously or intraperitoneally).
Related to the anti-inflammatory effects of GLP-1R activation, NLY01 (a pegylated long-acting exendin 4 GLP-1R agonist with an extended half-life) suppresses the activation and expansion of innate immune cells in the myeloid lineage at the early stage of EAE (Yun et al., 2018; Gharagozloo et al., 2021). In addition, dulaglutide treatment reduces lymphocyte infiltration into the CNS at PID 14 in EAE mice (Chiou et al., 2019). It suppresses the development of highly encephalitogenic Th1/Th17 cells in the CNS, suggesting a critical role in the modulation of T cell pathogenicity in the CNS (Chiou et al., 2019). Moreover, NLY01 lowers the percentage of infiltrating leukocytes (CD45high cells) and effector/memory T cells (CD4+CD44+) in the CNS (Gharagozloo et al., 2021).
In MS, disease progression induces a well-known pathogenic event characterized by an excessive immune response and the production of diverse proinflammatory cytokines, such as IL-1, IL-6, IL-12, IL-18, and IL-23 (Diz-Chaves et al., 2022b). These molecules expand Th1 and Th17 cells (Govindarajan et al., 2020). Following the breakdown of BBB, the activation of Th1, Th17, and γδ T cells occurs, facilitating the entry of these cells into the brain and spinal cord (Govindarajan et al., 2020). Microglia, the infiltrating monocytes, and neutrophils begin to secrete IL-1β and IL-23 to activate further and facilitate these cells' expansion (Govindarajan et al., 2020). In this regard, Exendin-4 decreases the mRNA expression level of proinflammatory cytokines (IL-17, IL-1β, IL-6, and TNF-α) in the spinal cords of EAE mice (Lee et al., 2018).
In the EAE model, activated macrophages, monocytes, dendritic cells, as well as apoptotic oligodendrocytes release pathogen-associated molecular pattern or DAMP, like the high mobility group box protein-1 to the extracellular environment, and initiate an inflammatory response characterized by the activation of the inflammasome (Barclay and Shinohara, 2017; Song et al., 2017; Govindarajan et al., 2020). These molecules activate TLRs that recruit the adaptor protein myeloid differentiation primary response 88 into the receptor complex, leading to phosphorylation of the inhibitor of nuclear factor-kB (NF-κB) IκB through interaction with the p50 and p65 transcription factors. Activation of the NF-κB pathway causes the synthesis of pro-IL-1β or pro-IL-18 (Govindarajan et al., 2020; Diz-Chaves et al., 2022b). About the GLP-1 RAs effects, it has been described that Exendin-4 attenuates NF-κB p65 expression in the EAE spinal cord and blocks IκBα degradation in LPS-stimulated primary microglia, additionally demonstrating that exendin-4 can stop microglial polarization (Lee et al., 2018; Figure 2). Moreover, inflammasome activation is a critical step in autoimmune and proinflammatory responses in MS (Govindarajan et al., 2020). Its assembly outcomes are the caspase-1 activation and processing and release of IL-1β and IL-18 (Song et al., 2017; Zhang et al., 2018a; Govindarajan et al., 2020). In addition, caspase-1 cleaves gasdermin D (GSDMD), which initiates pyroptosis leading to the upregulation of IL-1β (Govindarajan et al., 2020). Liraglutide downregulates the mRNA expression levels of NLRP3, ASC, caspase 1, and GSDMD induced by EAE (Song et al., 2017, 2022). Liraglutide also prevents Cup-induced indirect and direct overexpression of HMGB1 and TLR-4, respectively (Ammar et al., 2022), and attenuates the activation and release of inflammatory modulators such as caspase-1 and IL-1β mitigating NLRP3 overexpression (Ammar et al., 2022).
Figure 2.
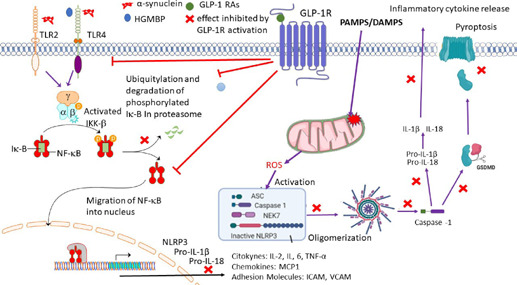
The activation of the GLP-1 receptor induces anti-inflammatory effects.
The activation of the GLP-1 receptor by GLP-1 Ras promotes several anti-inflammatory mechanisms. Reprinted with permission from Diz-Chaves et al. (2022b). AMPK: Adenosine monophosphate-activated protein kinase; ASC: apoptosis-associated speck-like protein containing a CARD; GSMD: gasdermin D; ICAM: intracellular adhesion molecule-1; IKKK: IκB kinase kinase; IKKβ: IκB kinase β; IL-1β: interleukin-1β; IL-2: interleukin-2; IL-6: interleukin-6; MCP1: monocyte chemoattractant protein 1; NAD/NADH: nicotinamide adenine dinucleotide; NEK7: NIMA related kinase 7; NFκB: nuclear factor kappa B; pro-IL-18: pro-interleukin-18; pro-IL-1β: pro-interleukin-1β; SIRT1 sirtuin 1; VCAM: vascular cell adhesion molecule-1.
The AMPK system activation induces the neuroprotective anti-inflammatory downstream signaling cascade (Peixoto et al., 2017). The GLP-1 R activation can phosphorylate AMPK, activating multiple downstream pathways through molecules such as p53, SIRT1, and peroxisome proliferator-activated receptor γ coactivator-1 (PGC1α; Peixoto et al., 2017; Diz-Chaves et al., 2022b). Inducing the inhibition of the NF-κB translocation and indirectly suppressing proinflammatory gene transcriptions (Muraleedharan and Dasgupta, 2022). It is known that EAE and cup MS models show in the lumbar spinal cord and brain a reduced phosphorylated AMPK expression. Furthermore, liraglutide administration partly restores the effect (Chiou et al., 2019; Song et al., 2022). Moreover, semaglutide decreases NF-κB and TNF-α levels by activating the PI3K/AKT signal pathway and hampering GSK3β (Sadek et al., 2022).
About microglia, the EAE increases the number of amoeboid Iba1-positive cells in the spinal cord, an effect reversed by exendin-4 that changes their morphological transformation into ramified cells (Lee et al., 2018).
Parkinson's disease
Parkinson's disease (PD) is the second most expected neurodegenerative disorder. The degeneration of dopaminergic neurons in the substantia nigra pars compacta is responsible for the motor impairments of the disease (Diz-Chaves et al., 2022b), as well as the intraneuronal inclusions of α-synuclein protein commonly referred to as Lewy bodies or Lewy neurites (Kam et al., 2020), a critical hallmarks of the disease. Moreover, iron accumulation, elevated oxidative stress, and lipid peroxidation damage are other conspicuous features of PD (Mahoney-Sánchez et al., 2021). A crucial aspect of PD is chronic inflammation by activating microglia and astrocytes, leading to the chronic production of cytokines, NF-κB pathway activation, and oxidative damage to proteins (Diz-Chaves et al., 2022b).
α-Synuclein can be secreted from neurons and transmitted to neighboring cells, including neurons and glia, which underlie the spreading of Lewy body pathology (Choi et al., 2020). Extracellular α-synuclein oligomers function as DAMPs, activating innate immune receptors on the surface of microglia, including TLR2 (Kam et al., 2020). It also binds to TLR4, which interacts with α-synuclein to induce the transcriptional upregulation of Sqstm1/p62 through the NFKB/NF-κB pathway; SQSTM1 then binds and recruits the α-synuclein to the phagophore for degradation through a lysosome (Choi et al., 2020). α-Synuclein likewise binds Fc gamma receptor IIB on microglial surfaces reducing microglial phagocytosis, which could impair the clearance of aggregated species or other parenchymal debris (Choi et al., 2015). Moreover, Fyn kinase, in conjunction with the class B scavenger receptor CD36, regulates the microglial uptake of aggregated human α-synuclein (Panicker et al., 2019). In the microglia, fibrillar α-synuclein activates the NF-κB pathway (Kam et al., 2020), central to the inflammatory microglial response. But also, Fyn kinase mediates PKCδ-dependent NF-κB–p65 nuclear translocation, leading to inflammasome priming, facilitating α-synuclein import into microglia, contributing to the generation of mitochondrial reactive oxygen species and consequently to NLRP3 inflammasome activation, thereby driving IL-1β release by microglia (Panicker et al., 2019).
GLP-1R activation is neuroprotective in cellular and animal models of dopaminergic degeneration (Jones-Tabah, 2022). While the signaling pathways activated downstream of GLP-1R have not been extensively characterized directly in DA neurons, evidence from other brain areas indicates that GLP-1R activates PKA and MAPK, and PI3/AKT/mTOR signaling pathways (Jones-Tabah, 2022). In 1-methyl-4-phenyl-1,2,3,6-tetrahydropypridine (MPTP) mice, the anti-inflammatory effect of liraglutide is due to the activation of the AMPK/PGC1α/NF-κB signaling pathway by increasing p-AMPK expression and reducing NF-κB protein levels (Cao et al., 2022). A complementary mechanism that may contribute to GLP-1R-mediated neuroprotection is the regulation of insulin receptors and the reversal of insulin insensitivity in DA neurons (Jones-Tabah, 2022).
GLP-1 RAs are neuroprotective in PD models improving motor and non-motor deficits (Hölscher, 2018; Labandeira et al., 2022). It has been described that semaglutide, liraglutide, exendin-4, NLY01, and the GLP-1/GIP dual receptors agonist (DA-JC1, DA-JC4, DA5-CH, and DA3-CH), enclose neuroprotective properties in the MPTP mouse and the 6-hydroxydopamine (6-OHDA)-lesion induced rat models (Cao et al., 2016; Yuan et al., 2017; Feng et al., 2018; Zhang et al., 2018b, 2019; Lv et al., 2021; Cao et al., 2022; Zhang et al., 2022). In this respect, the GLP-1 RAs: semaglutide, DA-JC1DA3-CH, and DA5-CH have anti-inflammatory effects reducing the activation of microglia and astrocytes in the MPTP mouse model (Cao et al., 2016, 2022; Yuan et al., 2017; Lv et al., 2021) and in a 6-OHDA-lesion rat model of PD (Zhang et al., 2022). Moreover, the dual GLP-1/GIP receptor agonist, DA5-CH, decreases proinflammatory mediators like IL-1β and TNF-α in MPTP-mice and has shown to be superior to semaglutide, reducing these inflammatory cytokines in 6-OHDA-lesion rats (Lv et al., 2021; Zhang et al., 2022). Furthermore, DA5-CH also lowers the number of TL4-and NF-κB- positive cells and increases the growth factor β1 (TGF-β1)-positive cells in the striatum (Lv et al., 2021). They are reducing IL-6 and increasing IL-10 expression levels in the striatum (Lv et al., 2021).
An MPTP-treated Parkinson's disease model in mice was generated with the constructed commensal MG1363-pMG36e-GLP-1 able to express GLP-1 continuously to treat PD (Fang et al., 2019). MG1363-pMG36e-GLP-1 relieves inflammation by reducing TLR-4 expression, down-regulating p-NF-κB in the NF-κB signaling pathway, and reducing the gene and protein levels expression of proinflammatory mediators like IL-1β, IL-6, and TNF-α. However, it is possible that some of the beneficial effects observed by the action of this constructed commensal MG1363-pMG36e-GLP-1 could be independent of the GLP-1 production and be mediated by the commensal itself through another mechanism (Fang et al., 2019). It is known that oxidative stress plays a role in dopaminergic nerve cell death in PD, which is related to ferroptosis (Mahoney-Sánchez et al., 2021). MPTP increases the levels of ROS, whereas L. lactis MG1363-pMG36e-GLP-1 can effectively decrease the ROS levels via activating the Keap1/Nrf2/GPX4 signaling pathway down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis (Yue et al., 2022).
Using a single-blind trial design, a proof of concept has evaluated the progress of 45 patients with moderate PD, randomly assigned to receive subcutaneous exenatide injections for 12 months or as controls (Aviles-Olmos et al., 2013). In preliminary clinical trials, exenatide-treated patients have shown to improve at 12 months on the MDS-UPDRS, a mean of 2.7 points, compared with a mean decline of 2.2 points in control patients (Aviles-Olmos et al., 2013). Nowadays, three GLP-1 RAs are being tested in four clinical trials in PD: exenatide (NCT04305002, NCT03456687), semaglutide (NCT03659682), or liraglutide (NCT02953665) (Diz-Chaves al., 2022b).
Alzheimer's disease
Alzheimer's disease (AD) is the most specific cause of dementia (Knopman et al., 2021). Different potential risk factors increment the risk of dementia in midlife, principally metabolic factors such as diabetes mellitus or obesity, but also hearing loss, traumatic brain injury, and alcohol abuse (Knopman et al., 2021; De Felice et al., 2022). AD dementia pathology involves Aβ-containing extracellular neuritic plaques throughout the cerebral cortex (Knopman et al., 2021). Alterations in insulin signaling appear directly linked to this Aβ oligomer formation in the brain (De Felice et al., 2022). Furthermore, proteins necessary for synaptic plasticities, such as N-methyl-D-aspartate-and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid-type glutamate receptors, are drawn from the cell surface after neural oligomer exposition, indicating a vast impact on synapses (De Felice et al., 2022). The progress of the disease is related to the inflammatory process. They are linked to the apparition of neurofibrillary tangles, Aβ deposits, damaged neurons, insulin resistance, and endoplasmatic reticulum stress (De Felice et al., 2022). Microglial activation and neurotoxicity are crucial in AD's pathogenesis (Leng and Edison, 2021). In this regard, glial cell inflammation is critical in synapse damage or elimination and brain dysfunction, contributing to cognitive and non-cognitive symptoms of AD (Ferreira, 2021).
The exposition of amyloid-β oligomers induces the removal of the insulin receptors from the plasma membrane in the neurons, relating a state of neuronal insulin resistance with AD (Ferreira, 2021). Liraglutide decreases by 50% the number of activated microglia in the hippocampus and cortex (Diz-Chaves et al., 2022b). This molecule also reduces cortical microglia burden and the number in the proximity of amyloid plaques in APP/PS1xdb/db and APPswe/PS1dE9 mice (Long-Smith et al., 2013; Carranza-Naval et al., 2021; Diz-Chaves et al., 2022b) as well as, reactive astrocytes in APPswe/PS1dE9 mice (Long-Smith et al., 2013). Correspondingly, in APP/PS1 mice, lasting liraglutide treatment (8 weeks/i.p/once daily) to mice halves microglia in the hippocampus, demonstrating a prophylactic effect (McClean et al., 2015; Diz-Chaves et al., 2022b).
In APP/PS1 mice, the GLP-1/GIP dual receptor agonists DA4-JC or DA-JC1, compared to liraglutide, are superior in most parameters tested, showing anti-inflammatory effects (Maskery et al., 2020; Salles et al., 2020) by decreasing TNF-α and IL-1β production and lowering the amyloid plaque load (Maskery et al., 2020). Moreover, in AD/STZ mice, DA4-JC reduces microglia and astrocyte activation (Shi et al., 2017). The triple receptor agonist (GLP-1/GIP/glucagon) reduces the reactivity of microglia and astrocytes in APP/PS1 (Tai et al., 2018). Several drugs used to treat T2DM and normalize insulin signaling are being tested in clinical trials for AD treatment (Gejl et al., 2016; Femminella et al., 2019).
Vascular dementia
Neuroinflammation due to cerebral perfusion deficit is a key causative factor of vascular dementia (VD) (Duncombe et al., 2017). In the rat animal model, VD is characterized by increased Iba1-positive and GFAP-positive cells in the hippocampal CA1, CA3, and dentate gyrus regions (Guan et al., 2022). This effect is reversed by the treatment with dulaglutide (Guan et al., 2022).
Chronic migraine
Chronic migraine (CM) is a severe neurological disease that manifests as neuronal plastic changes in the trigeminal nucleus caudalis (Iyengar et al., 2019). By using chronic nitroglycerin injection-stimulated mouse model, it was observed that Liraglutide suppressed the nitroglycerin-induced upregulation of microglia in these mice, shortening both total and mean lengths of microglial processes and reduced nitroglycerin-induced upregulation of Iba-1, as well as, IL-1β and TNF-α protein levels (Jing et al., 2021).
In resume, activation of the GLP-1 receptor (Figure 2) may prevent DAMPS, like high mobility group box protein-1, from being released to the extracellular environment from the monocytes, activated macrophages, dendritic cells, or apoptotic oligodendrocytes and bind to the TLR (Chiou et al., 2019). Also, it may directly or indirectly act to inhibit NKκB transcription through the attenuation of NF-κB p65 expression and blocking of IκBα degradation (DellaValle et al., 2016; Zhang et al., 2018b) induced by extracellular α-synuclein oligomers or high mobility group box protein-1. As well, the activation of the receptor can attenuate NKκB translocation and the proinflammatory mediators' production (Chiou et al., 2019). Moreover, blocking NKκB transcription may prevent the display of pro-IL-1β and pro-IL-18 and the oligomerization of NLPR3 inflammasome (Peixoto et al., 2017; Shi et al., 2017; Cai et al., 2021). Therefore, the activation of caspase 1 that produces IL-1β cleaves from GSDMD, inducing pyroptosis and releasing proinflammatory cytokines (Shi et al., 2017; Cai et al., 2021). Moreover, the activation of downstream signaling cascade through the AMPK system may block nuclear NF-κB (Muraleedharan and Dasgupta, 2022) and may suppress proinflammatory gene transcriptions inhibiting the synthesis of proinflammatory mediators (cytokines, chemokines, and adhesion molecules) (Peixoto et al., 2017; Chiou et al., 2019; Lv et al., 2021).
Moreover, GLP-1R activation decreases the number and reactivity of microglia under inflammatory circumstances (Figure 3; DellaValle et al., 2016; Lee and Jun 2016; Shi et al., 2017; Cai et al., 2021; Jing et al., 2021; Lv et al., 2021; Guan et al., 2022). Microglia are the first cells to respond to CNS insults inducing astrogliosis. It is well known that astrocytes act, promoting tissue repair and homeostasis, but also, they can be detrimental by exacerbating inflammatory reactions and tissue damage, depending on the stimuli' nature (Jha et al., 2019). The chronic activation of microglia can convert astrocytes into a neurotoxic A1 phenotype (Yun et al., 2018) by secreting different molecules such as TNF-α, IL-1β, nitric oxide, vascular endothelial growth factor-B, or complement factor Cq1 (Haidet-Phillips et al., 2011; Meoni et al., 2020; Chowen and Garcia-Segura, 2021). The modification in the astrocyte phenotype is directly related to altered homeostatic and diminished metabolic functions, an increase in excitotoxicity related to impaired glutamate uptake, and a decrease in lactate release from astrocytes (Jha et al., 2019; Meoni et al., 2020). GLP-1 RAs activation (Figure 3) prevents the microglial-mediated transformation of astrocytes to an A1 neurotoxic phenotype (Cao et al., 2016; Yun et al., 2018; Zhang et al., 2018b).
Figure 3.
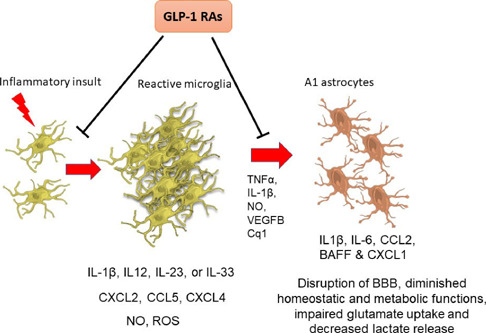
Chronic microglia activation facilitates changes in the astrocyte phenotype to the reactive A1.
Microgliosis and astrocytosis induced by neurodegenerative diseases are blocked by GLP-1 receptor activation. Reprinted with permission from Diz-Chaves et al. (2022b). BAFF: B cell-activating factor of the TNF family; CCL2: C-C motif chemokine ligand 2; CCL5: CC chemokine ligand 5; Cq1: complement component 1q; CXCL-1: chemokine (C-X-C motif) ligand 1; CXCL2: chemokine (C-X-C motif) ligand 2; CXCL4: chemokine (C-X-C motif) ligand 4; IL-12: interleukin-12; IL-1β: interleukin-1β; IL-23: interleukin-23; IL-33: interleukin-33; IL-6: interleukin-6; NO: nitric oxide; ROS: reactive oxygen species; TNF-α: tumor necrosis factor α; VEGFB: vascular endothelial growth factor-B.
Conclusion
GLP-1 receptor analogs play a critical role in neuroprotective and anti-inflammatory effects in the brain in several inflammatory-related brain diseases. Several clinical trials have been performed based on the encouragingly preclinical results. These molecules have demonstrated tremendous positive results in clinical trials, demonstrating that a better mechanistic understanding of the action of these molecules could highlight their pleiotropic effects, strengthening their clinical outcomes.
Funding Statement
Funding: This work was supported by the European Union Grant Alehoop (H2020-BBIJTI-2019-887259). And from the Xunta de Galicia (Centro singular de Investigación de Galicia accreditation 2016–2019), ED431G/02 (to FM).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Ammar RA, Mohamed AF, Kamal MM, Safar MM, Abdelkader NF. Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: role of AMPK/SIRT1 signaling and NLRP3 inflammasome. Inflammopharmacology. 2022;30:919–934. doi: 10.1007/s10787-022-00956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Pérez M, Bismuth E, Dib S, Cho JI, Cox D; AWARD-PEDS Investigators Once-weekly dulaglutide for the treatment of youths with type 2 diabetes. N Engl J Med. 2022;387:433–443. doi: 10.1056/NEJMoa2204601. [DOI] [PubMed] [Google Scholar]
- Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, Koszegi Z, Bacon A, Jones BJ, Lucey MA, Sasaki S, Brierley DI, Hastoy B, Tomas A, D'Agostino G, Reimann F, Lynn FC, Reissaus CA, Linnemann AK, D'Este E, et al. Super-resolution microscopy-compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat Commun. 2020;11:467. doi: 10.1038/s41467-020-14309-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiz M, Pernía O, Barrios V, Garcia-Segura LM, Diz-Chaves Y. Short-term high-fat diet feeding provides hypothalamic but not hippocampal protection against acute infection in male mice. Neuroendocrinology. 2016;104:40–50. doi: 10.1159/000444527. [DOI] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay W, Shinohara ML. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Brain Pathol. 2017;27:213–219. doi: 10.1111/bpa.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvento G, Bolaños JP. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021;33:15461564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Brierley DI, Holt MK, Singh A, de Araujo A, McDougle M, Vergara M, Afaghani MH, Lee SJ, Scott K, Maske C, Langhans W, Krause E, de Kloet A, Gribble FM, Reimann F, Rinaman L, de Lartigue G, Trapp S. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021;3:258–273. doi: 10.1038/s42255-021-00344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018;19:622–635. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai HY, Yang D, Qiao J, Yang JT, Wang ZJ, Wu MN, Qi JS, Holscher C. A GLP-1/GIP dual receptor agonist DA4-JC effectively attenuates cognitive impairment and pathology in the APP/PS1/Tau model of Alzheimer's disease. J Alzheimers Dis. 2021;83:799–818. doi: 10.3233/JAD-210256. [DOI] [PubMed] [Google Scholar]
- Calsolaro V, Edison P. Novel GLP-1 (glucagon-like peptide-1) analogues and insulin in the treatment for Alzheimer's disease and other neurodegenerative diseases. CNS Drugs. 2015;29:1023–1039. doi: 10.1007/s40263-015-0301-8. [DOI] [PubMed] [Google Scholar]
- Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- Cao B, Zhang Y, Chen J, Wu P, Dong Y, Wang Y. Neuroprotective effects of liraglutide against inflammation through the AMPK/NF-κB pathway in a mouse model of Parkinson's disease. Metab Brain Dis. 2022;37:451–462. doi: 10.1007/s11011-021-00879-1. [DOI] [PubMed] [Google Scholar]
- Cao L, Li D, Feng P, Li L, Xue GF, Li G, Hölscher C. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson's disease by reducing chronic inflammation in the brain. Neuroreport. 2016;27:384–391. doi: 10.1097/WNR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- Carranza-Naval MJ, Del Marco A, Hierro-Bujalance C, Alves-Martinez P, Infante-Garcia C, Vargas-Soria M, Herrera M, Barba-Cordoba B, Atienza-Navarro I, Lubian-Lopez S, Garcia-Alloza M. Liraglutide reduces vascular damage, neuronal loss, and cognitive impairment in a mixed murine model of Alzheimer's disease and type 2 diabetes. Front Aging Neurosci. 2021;13:741923. doi: 10.3389/fnagi.2021.741923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Miller LJ, Dong M. Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor. Am J Physiol Endocrinol Metab. 2010;299:E62–8. doi: 10.1152/ajpendo.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou HC, Lin MW, Hsiao PJ, Chen CL, Chiao S, Lin TY, Chen YC, Wu DC, Lin MH. Dulaglutide modulates the development of tissue-infiltrating Th1/Th17 cells and the pathogenicity of encephalitogenic Th1 cells in the central nervous system. Int J Mol Sci. 2019;20:1584. doi: 10.3390/ijms20071584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Seegobin SP, Liang D, Yue Z. Synucleinphagy: a microglial “community cleanup program” for neuroprotection. Autophagy. 2020;16:1718–1720. doi: 10.1080/15548627.2020.1774149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YR, Kang SJ, Kim JM, Lee SJ, Jou I, Joe EH, Park SM. FcγRIIB mediates the inhibitory effect of aggregated α-synuclein on microglial phagocytosis. Neurobiol Dis. 2015;83:90–99. doi: 10.1016/j.nbd.2015.08.025. [DOI] [PubMed] [Google Scholar]
- Chowen JA, Garcia-Segura LM. Role of glial cells in the generation of sex differences in neurodegenerative diseases and brain aging. Mech Ageing Dev. 2021;196:111467. doi: 10.1016/j.mad.2021.111473. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Finan B, Müller TD, DiMarchi RD, Tschöp MH, Hofmann SM. Emerging hormonal-based combination pharmacotherapies for the treatment of metabolic diseases. Nat Rev Endocrinol. 2019;15:90–104. doi: 10.1038/s41574-018-0118-x. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Gonçalves RA, Ferreira ST. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci. 2022;23:215–230. doi: 10.1038/s41583-022-00558-9. [DOI] [PubMed] [Google Scholar]
- Decara J, Arrabal S, Beiroa D, Rivera P, Vargas A, Serrano A, Pavón FJ, Ballesteros J, Dieguez C, Nogueiras R, Rodríguez de Fonseca F, Suárez J. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. Biofactors. 2016;42:600–611. doi: 10.1002/biof.1295. [DOI] [PubMed] [Google Scholar]
- DellaValle B, Brix GS, Brock B, Gejl M, Landau AM, Møller A, Rungby J, Larsen A. Glucagon-like peptide-1 analog, liraglutide, delays onset of experimental autoimmune encephalitis in lewis rats. Front Pharmacol. 2016;7:433. doi: 10.3389/fphar.2016.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem 139 Suppl. 2016;2:136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y, Toba L, Fandiño J, González-Matías LC, Garcia-Segura LM, Mallo F. The GLP-1 analog, liraglutide prevents the increase of proinflammatory mediators in the hippocampus of male rat pups submitted to maternal perinatal food restriction. J Neuroinflammation. 2018;15:337. doi: 10.1186/s12974-018-1370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y, Herrera-Pérez S, González-Matías LC, Mallo F. Effects of glucagon-like peptide 1 (GLP-1) analogs in the hippocampus. Vitam Horm. 2022a;118:457–478. doi: 10.1016/bs.vh.2021.12.005. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Mastoor Z, Spuch C, González-Matías LC, Mallo F. Anti-inflammatory effects of GLP-1 receptor activation in the brain in neurodegenerative diseases. Int J Mol Sci. 2022b;23:9583. doi: 10.3390/ijms23179583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Mojsov S, Habener JF. Cell-specific posttranslational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. J Biol Chem. 1986;261:9637–9643. [PubMed] [Google Scholar]
- Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci. 2017;131:2451–2468. doi: 10.1042/CS20160727. [DOI] [PubMed] [Google Scholar]
- Elahi D, Egan JM, Shannon RP, Meneilly GS, Khatri A, Habener JF, Andersen DK. GLP-1 (9-36) amide, cleavage product of GLP-1 (7-36) amide, is a glucoregulatory peptide. Obesity (Silver Spring) 2008;16:1501–1509. doi: 10.1038/oby.2008.229. [DOI] [PubMed] [Google Scholar]
- ElAli A, Rivest S. Microglia ontology and signaling. Front Cell Dev Biol. 2016;4:72. doi: 10.3389/fcell.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandiño J, Toba L, González-Matías LC, Diz-Chaves Y, Mallo F. GLP-1 receptor agonist ameliorates experimental lung fibrosis. Sci Rep. 2020;10:18091. doi: 10.1038/s41598-020-74912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Tian P, Zhao X, Jiang C, Chen T. Neuroprotective effects of an engineered commensal bacterium in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine Parkinson disease mouse model via producing glucagon-like peptide-1. J Neurochem. 2019;150:441–452. doi: 10.1111/jnc.14694. [DOI] [PubMed] [Google Scholar]
- Femminella GD, Frangou E, Love SB, Busza G, Holmes C, Ritchie C, Lawrence R, McFarlane B, Tadros G, Ridha BH, Bannister C, Walker Z, Archer H, Coulthard E, Underwood BR, Prasanna A, Koranteng P, Karim S, Junaid K, McGuinness B, et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer's disease: study protocol for a randomised controlled trial (ELAD study) Trials. 2019;20:191. doi: 10.1186/s13063-019-3259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Zhang X, Li D, Ji C, Yuan Z, Wang R, Xue G, Li G, Hölscher C. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson's disease. Neuropharmacology. 2018;133:385–394. doi: 10.1016/j.neuropharm.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Ferreira ST. Brain insulin, insulin-like growth factor 1 and glucagon-like peptide 1 signalling in Alzheimer's disease. J Neuroendocrinol. 2021;33:e12959. doi: 10.1111/jne.12959. [DOI] [PubMed] [Google Scholar]
- Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht A, Møller N, Brock B, Rungby J. In Alzheimer's disease, 6-month treatment with GLP-1 analog prevents the decline of brain glucose metabolism: Randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozloo M, Smith MD, Sotirchos ES, Jin J, Meyers K, Taylor M, Garton T, Bannon R, Lord HN, Dawson TM, Dawson VL, Lee S, Calabresi PA. Therapeutic potential of a novel glucagon-like peptide-1 receptor agonist, NLY01, in experimental autoimmune encephalomyelitis. Neurotherapeutics. 2021;18:1834–1848. doi: 10.1007/s13311-021-01088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, Vigo E, González-Matías LC, Brubaker PL, Mallo F. Corticotropin-releasing hormone and the sympathoadrenal system are major mediators in the effects of peripherally administered exendin-4 on the hypothalamic-pituitary-adrenal axis of male rats. Endocrinology. 2014;155:2511–2523. doi: 10.1210/en.2013-1718. [DOI] [PubMed] [Google Scholar]
- Gilhus NE, Deuschl G. Neuroinflammation — a common thread in neurological disorders. Nat Rev Neurol. 2019;15:429–430. doi: 10.1038/s41582-019-0227-8. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, de Rivero Vaccari JP, Keane RW. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J Neuroinflammation. 2020;17:260. doi: 10.1186/s12974-020-01944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M, Giorgi A, Gentile MC, d'Erme M, Morano S, Maras B, Filardi T. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci. 2019;13:1112. doi: 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T, Xiao Y, Xie X, Meng N, Qi Q, Xu J, Jiang X, Zhang Z, Teng Z, Lv P. Dulaglutide improves gliosis and suppresses apoptosis/autophagy through the PI3K/Akt/mTOR signaling pathway in vascular dementia rats. Neurochem Res. 2023;48:1561–1579. doi: 10.1007/s11064-022-03853-0. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss CN, Mannerås-Holm L, Lee YS, Serrano-Lobo J, Håkansson Gladh A, Seeley RJ, Drucker DJ, Bäckhed F, Olofsson LE. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021;35:109163. doi: 10.1016/j.celrep.2021.109163. [DOI] [PubMed] [Google Scholar]
- Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer's and Parkinson's disease models. Neuropharmacology. 2018;136:251–259. doi: 10.1016/j.neuropharm.2018.01.040. [DOI] [PubMed] [Google Scholar]
- Hölscher C, Li L. New roles for insulin-like hormones in neuronal signalling and protection: new hopes for novel treatments of Alzheimer's disease? Neurobiol Aging. 2010;31:1495–1502. doi: 10.1016/j.neurobiolaging.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Albrechtsen NJW, Rosenkilde MM, Deacon CF. Physiology of the incretin hormones, GIP and GLP-1-regulation of release and posttranslational modifications. Compr Physiol. 2019;9:1339–1381. doi: 10.1002/cphy.c180013. [DOI] [PubMed] [Google Scholar]
- Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68:21–33. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbernon M, Saponaro C, Helms HCC, Duquenne M, Fernandois D, Deligia E, Denis RGP, Chao DHM, Rasika S, Staels B, Pattou F, Pfrieger FW, Brodin B, Luquet S, Bonner C, Prevot V. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 2022;34:1054–1063. doi: 10.1016/j.cmet.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59:659–681. doi: 10.1111/head.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Jo M, Kim JH, Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25:227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- Jing F, Zou Q, Wang Y, Cai Z, Tang Y. Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J Headache Pain. 2021;22:86. doi: 10.1186/s10194-021-01302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. The therapeutic potential of GLP-1 receptor biased agonism. Br J Pharmacol. 2022;179:492–510. doi: 10.1111/bph.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Tabah J. Targeting G protein-coupled receptors in the treatment of Parkinson's disease. J Mol Biol. 2022;435:167927. doi: 10.1016/j.jmb.2022.167927. [DOI] [PubMed] [Google Scholar]
- Kahles F, Meyer C, Möllmann J, Diebold S, Findeisen HM, Lebherz C, Trautwein C, Koch A, Tacke F, Marx N, Lehrke M. GLP-1 secretion is increased by inflammatory stimuli in an IL-6–dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. 2014;63:3221–3229. doi: 10.2337/db14-0100. [DOI] [PubMed] [Google Scholar]
- Kam TI, Hinkle JT, Dawson TM, Dawson VL. Microglia and astrocyte dysfunction in Parkinson's disease. Neurobiol Dis. 2020;144:105028. doi: 10.1016/j.nbd.2020.105028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, Nixon RA, Jones DT. Alzheimer disease. Nat Rev Dis Primers. 2021;7:33. doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne) 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira CM, Fraga-Bau A, Arias Ron D, Alvarez-Rodriguez E, Vicente-Alba P, Lago-Garma J, Rodriguez-Perez AI. Parkinson's disease and diabetes mellitus: common mechanisms and treatment repurposing. Neural Regen Res. 2022;17:1652–1658. doi: 10.4103/1673-5374.332122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A, Dal S, Vivot K, Mura C, Seyfritz E, Bietiger W, Dollinger C, Peronet C, Maillard E, Pinget M, Jeandidier N, Sigrist S. Improvement of islet graft function using liraglutide is correlated with its anti-inflammatory properties. Br J Pharmacol. 2016;173:3443–3453. doi: 10.1111/bph.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Jeon SJ, Cho KS, Moon E, Sapkota A, Jun HS, Ryu JH, Choi JW. Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol Neurobiol. 2018;55:3007–3020. doi: 10.1007/s12035-017-0550-2. [DOI] [PubMed] [Google Scholar]
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer's disease: where do we go from here? Nat Rev Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- Li JY, Cui LY, Sun XH, Shen DC, Yang XZ, Liu Q, Liu MS. Alterations in metabolic biomarkers and their potential role in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2022;9:1027–1038. doi: 10.1002/acn3.51580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen X, Vong JSL, Zhao L, Huang J, Yan LYC, Ip B, Wing YK, Lai HM, Mok VCT, Ko H. Systemic GLP-1R agonist treatment reverses mouse glial and neurovascular cell transcriptomic aging signatures in a genome-wide manner. Commun Biol. 2021;4:656. doi: 10.1038/s42003-021-02208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith CM, Manning S, McClean PL, Coakley MF, O'Halloran DJ, Holscher C, O'Neill C. The diabetes drug liraglutide ameliorates aberrant insulin receptor localization and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer's disease. Neuromolecular Med. 2013;15:102–114. doi: 10.1007/s12017-012-8199-5. [DOI] [PubMed] [Google Scholar]
- Lv M, Xue G, Cheng H, Meng P, Lian X, Hölscher C, Li D. The GLP-1/GIP dual-receptor agonist DA5-CH inhibits the NF-κB inflammatory pathway in the MPTP mouse model of Parkinson's disease more effectively than the GLP-1 single-receptor agonist NLY01. Brain Behav. 2021;11:e2231. doi: 10.1002/brb3.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney-Sánchez L, Bouchaoui H, Ayton S, Devos D, Duce JA, Devedjian JC. Ferroptosis and its potential role in the physiopathology of Parkinson's disease. Prog Neurobiol. 2021;196:101890. doi: 10.1016/j.pneurobio.2020.101890. [DOI] [PubMed] [Google Scholar]
- Maskery M, Goulding EM, Gengler S, Melchiorsen JU, Rosenkilde MM, Hölscher C. The dual GLP-1/GIP receptor agonist DA4-JC shows superior protective properties compared to the GLP-1 analogue liraglutide in the APP/PS1 mouse model of Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2020;35:1533317520953041. doi: 10.1177/1533317520953041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean PL, Jalewa J, Hölscher C. Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation, and memory impairment in APP/PS1 mice. Behav Brain Res. 2015;293:96–106. doi: 10.1016/j.bbr.2015.07.024. [DOI] [PubMed] [Google Scholar]
- McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr Rev. 2021;42:101–132. doi: 10.1210/endrev/bnaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meoni S, Macerollo A, Moro E. Sex differences in movement disorders. Meoni S, Macerollo A, Moro E 2020. 2020;16:84–96. doi: 10.1038/s41582-019-0294-x. [DOI] [PubMed] [Google Scholar]
- Müller TD, Clemmensen C, Finan B, DiMarchi RD, Tschöp MH. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol Rev. 2018;70:712–746. doi: 10.1124/pr.117.014803. [DOI] [PubMed] [Google Scholar]
- Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraleedharan R, Dasgupta B. AMPK in the brain: its roles in glucose and neural metabolism. FEBS J. 2022;289:2247–2262. doi: 10.1111/febs.16151. [DOI] [PubMed] [Google Scholar]
- Nakane A, Gotoh Y, Ichihara J, Nagata H. New screening strategy and analysis for identification of allosteric modulators for glucagon-like peptide-1 receptor using GLP-1 (9-36) amide. Anal Biochem. 2015;491:23–30. doi: 10.1016/j.ab.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Mandard S, Dray C, Deckert V, Valet P, Besnard P, Drucker DJ, Lagrost L, Grober J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes. 2014;63:471–482. doi: 10.2337/db13-0903. [DOI] [PubMed] [Google Scholar]
- Nowell J, Blunt E, Edison P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer's and Parkinson's disease. Mol Psychiatry. 2023;28:217–229. doi: 10.1038/s41380-022-01792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker N, Sarkar S, Harischandra DS, Neal M, Kam TI, Jin H, Saminathan H, Langley M, Charli A, Samidurai M, Rokad D, Ghaisas S, Pletnikova O, Dawson VL, Dawson TM, Anantharam V, Kanthasamy AG, Kanthasamy A. Fyn kinase regulates misfolded α-synuclein uptake and NLRP3 inflammasome activation in microglia. J Exp Med. 2019;216:1411–1430. doi: 10.1084/jem.20182191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kam TI, Lee S, Park H, Oh Y, Kwon SH, Song JJ, Kim D, Kim H, Jhaldiyal A, Na DH, Lee KC, Park EJ, Pomper MG, Pletnikova O, Troncoso JC, Ko HS, Dawson VL, Dawson TM, Lee S. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer's disease. Acta Neuropathol Commun. 2021;9:78. doi: 10.1186/s40478-021-01180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto CA, Oliveira WH, Araújo SMDR, Nunes AKS. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 2017;298(Pt A):31–41. doi: 10.1016/j.expneurol.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Plastini MJ, Desu HL, Brambilla R. Dynamic responses of microglia in animal models of multiple sclerosis. Front Cell Neurosci. 2020;14:269. doi: 10.3389/fncel.2020.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Chen H, Xia M, Chang J, Li X, Ye S, Wu S, Jiang S, Bao J, Wang B, Kong R, Zhang S, Zheng S, Cao X, Hong X. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury. Int J Biol Sci. 2022;18:1328–1346. doi: 10.7150/ijbs.68974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N, Hölscher C. The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: an in-depth review. Front Neurosci. 2022;16:970925. doi: 10.3389/fnins.2022.970925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, Santisteban P, González-Matías LC, Vigo E, Mallo F. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- Rossi GCM, Gandini Wheeler-Kingshott CAM, Toosy A. Editorial: neuroinflammation and the visual system. Front Neurol. 2021;12:1412. doi: 10.3389/fneur.2021.724447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek MA, Kandil EA, El Sayed NS, Sayed HM, Rabie MA. Semaglutide, a novel glucagon-like peptide-1 agonist, amends experimental autoimmune encephalomyelitis-induced multiple sclerosis in mice: involvement of the PI3K/Akt/GSK-3β pathway. Int Immunopharmacol. 2022;115:109647. doi: 10.1016/j.intimp.2022.109647. [DOI] [PubMed] [Google Scholar]
- Salles GN, Calió ML, Hölscher C, Pacheco-Soares C, Porcionatto M, Lobo AO. Neuroprotective and restorative properties of the GLP-1/GIP dual agonist DA-JC1 compared with a GLP-1 single agonist in Alzheimer's disease. Neuropharmacology. 2020;162:107813. doi: 10.1016/j.neuropharm.2019.107813. [DOI] [PubMed] [Google Scholar]
- Shi L, Zhang Z, Li L, Hölscher C. A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer's icv. STZ rat model. Behav Brain Res. 2017;327:65–74. doi: 10.1016/j.bbr.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Wang L, Xia B, Liu J, Tahiri A, El Ouaamari A, Wheeler MB, Pang ZP. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 2022;12:178. doi: 10.1186/s13578-022-00914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Patterson-Cross R, Woodward O, Lewis J, Chiarugi D, Merkle F, Gribble F, Reimann F, Adriaenssens A. A comparative transcriptomic analysis of glucagon-like peptide-1 receptor- and glucose-dependent insulinotropic polypeptide-expressing cells in the hypothalamus. Appetite. 2022;174:106022. doi: 10.1016/j.appet.2022.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Pei L, Yao S, Wu Y, Shang Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. doi: 10.3389/fncel.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Guo R, Mehmood A, Zhang L, Yin B, Yuan C, Zhang H, Guo L, Li B. Liraglutide attenuates central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway. CNS Neurosci Ther. 2022;28:422–434. doi: 10.1111/cns.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai J, Liu W, Li Y, Li L, Hölscher C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer's disease. Brain Res. 2018;1678:64–74. doi: 10.1016/j.brainres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Thiebaud N, Gribble F, Reimann F, Trapp S, Fadool DA. A unique olfactory bulb microcircuit driven by neurons expressing the precursor to glucagon-like peptide 1. Sci Rep. 2019;9:15542. doi: 10.1038/s41598-019-51880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ceja B, Olsen ML. A closer look at astrocyte morphology: Development, heterogeneity, and plasticity at astrocyte leaflets. Curr Opin Neurobiol. 2022;74:102550. doi: 10.1016/j.conb.2022.102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. doi: 10.1177/2042018821997320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Wainscott DB, Showalter AD, Stutsman C, Ma W, Cardona GR, Zink RW, Corkins CM, Chen Q, Yumibe N, Agejas J, Cumming GR, Minguez JM, Jiménez A, Mateo AI, Castaño AM, Briere DA, Sloop KW, Bueno AB. Discovery of an orally efficacious positive allosteric modulator of the glucagon-like peptide-1 receptor. J Med Chem. 2021;64:3439–3448. doi: 10.1021/acs.jmedchem.1c00029. [DOI] [PubMed] [Google Scholar]
- Yang D, de Graaf C, Yang L, Song G, Dai A, Cai X, Feng Y, Reedtz-Runge S, Hanson MA, Yang H, Jiang H, Stevens RC, Wang MW. Structural determinants of binding the seventransmembrane domain of the glucagon-like peptide-1 receptor (GLP-1R) J Biol Chem. 2016;291:12991–13004. doi: 10.1074/jbc.M116.721977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Luo X, Li J, Lei Y, Zeng F, Huang X, Lan Y, Liu R. Application of glucagon-like peptide-1 receptor antagonists in fibrotic diseases. Biomed Pharmacother. 2022;52:113236. doi: 10.1016/j.biopha.2022.113236. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Li D, Feng P, Xue G, Ji C, Li G, Hölscher C. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson's disease. Eur J Pharmacol. 2017;812:82–90. doi: 10.1016/j.ejphar.2017.06.029. [DOI] [PubMed] [Google Scholar]
- Yue M, Wei J, Chen W, Hong D, Chen T, Fang X. Neurotrophic role of the next-generation probiotic strain L. lactis MG1363-pMG36e-GLP-1 on Parkinson's disease via inhibiting ferroptosis. Nutrients. 2022;14:4886. doi: 10.3390/nu14224886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CJ, Jiang M, Zhou H, Liu W, Wang C, Kang Z, Han B, Zhang Q, Chen X, Xiao J, Fisher A, Kaiser WJ, Murayama MA, Iwakura Y, Gao J, Carman J, Dongre A, Dubyak G, Abbott DW, Shi FD, et al. TLR-stimulated IRAKM activates caspase-8 inflammasome in microglia and promotes neuroinflammation. J Clin Invest. 2018a;128:5399–5412. doi: 10.1172/JCI121901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang L, Li L, Hölscher C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson's disease mouse model. Neuropeptides. 2018b;71:70–80. doi: 10.1016/j.npep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang L, Li L, Hölscher C. Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of Parkinson's disease. J Parkinsons Dis. 2019;9:157–171. doi: 10.3233/JPD-181503. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li C, Zhang Z, Zhang Z, Jin QQ, Li L, Hölscher C. DA5-CH and semaglutide protect against neurodegeneration and reduce α-synuclein levels in the 6-OHDA Parkinson's disease rat model. Parkinsons Dis. 2022;2022:1428817. doi: 10.1155/2022/1428817. [DOI] [PMC free article] [PubMed] [Google Scholar]


