Abstract
A kinetic model of inhibition by the potassium-containing compound potassium bicarbonate is suggested. The model is based on the previous work concerning kinetic studies of suppression of secondary flashes, inhibition by alkali metals and the emission of sulfates and chlorides during biomass combustion. The kinetic model includes reactions with the following gas-phase potassium-containing species: K, KO, KO2, KO3, KH, KOH, K2O, K2O2, (KOH)2, K2CO3, KHCO3 and KCO3. Flame equilibrium calculations demonstrate that the main potassiumcontaining species in the combustion products are K and KOH. The main inhibition reactions, which comprise the radical termination inhibition cycle are KOH+H=K+H2O and K+OH+M=KOH+M with the overall termination effect: H+OH=H2O. Numerically predicted burning velocities for stoichiometric methane/air flames with added KHCO3 demonstrate reasonable agreement with available experimental data. A strong saturation effect is observed for potassium compounds: approximately 0.1% volume fraction of KHCO3 is required to decrease burning velocity by a factor of 2, however an additional 0.6% volume fraction is required to reach a burning velocity of 5 cm/s. Analysis of the calculation results indicates that addition of the potassium compound quickly reduces the radical super-equilibrium down to equilibrium levels, so that further addition of the potassium compound has little effect on the flame radicals.
Keywords: potassium-containing fire suppressant, potassium bicarbonate, flame inhibition, kinetic model, saturation effect
1. Introduction
Experimental data have shown that for addition at low volume fraction, compounds containing alkali metals (K, Na) are highly effective flame inhibitors (Birchall, 1970; Friedman and Levy, 1963; Rosser et al., 1963; Hastie, 1973; Iya et al., 1974; Baratov et al., 1991; Hoffmann, 1971; Tapscott et al., 2001; Chatrathi and Going, 2000; Linteris et al., 2008; Hoorelbeke, 2011), about an order of magnitude more effective than CF3Br on a molar basis. This performance is approaching that of Fe-, Pb- and Cr-containing compounds (Babushok and Tsang, 2000). While potassium-containing compounds have been found to be very effective at reducing burning velocity (Rosser et al., 1963; Hoffmann, 1971; Hoorelbeke, 2011), much of the research has aimed to determine the concentration of agent required to extinguish a flame, using a variety of configurations (e.g., opposed-flow diffusion, cup-burner, liquid pan flame) and for different fuels (methane, propane, heptane, hydrogen) (Ewing et al., 1989; Baratov and Korol’chenko, 1990; Chattaway et al., 1995; Reed et al., 1997a; Chatrathi and Going, 2000; Hoorelbeke, 2011). Because of their proven effectiveness, potassium compounds are the primary constituents of several commercial dry chemical fire suppression compositions, e.g. Monnex, Purple K, Super K.
The mechanism of flame inhibition by potassium was analyzed in several works (Birchall, 1970; Hastie, 1973; McHale, 1975; Jensen et al., 1979; Hastie et al., 1986; Heimerl, 1984; Slack et al., 1989; Baratov et al., 1991). It was suggested that the main radical scavenging reactions (chain carrier termination reactions) are the termolecular recombination reaction OH+K+M=KOH+M and the radical scavenging reactions of hydrogen atom and hydroxyl radical with potassium hydroxide, which regenerates the potassium atom: H+KOH=H2O+K, and OH+KOH=KO+H2O with further reactions of KO with radicals KO + (H,O,OH)=K+ (OH,O2,HO2). Kinetic models of the behavior of potassium containing species in combustion processes were suggested for the analysis of secondary flash suppression (Heimerl, 1984; Slack et al., 1989) and for the analysis of sulfur and chlorine partitioning with potassium salts in the products of biomass combustion (Hindlyarti et al., 2006; Li et al., 2013).
The purpose of the present work is to develop a detailed gas-phase kinetic model of inhibition of hydrocarbon-air flames by potassium compounds. To this end we present the thermochemistry of K-containing species relevant to the combustion environment, using data in literature when available. When data are absent the thermochemistry is estimated. The kinetic model was compiled from the data available in literature and additional reactions were considered. Using the suggested model, we analyzed the inhibition mechanism of potassium bicarbonate in methane-air flames.
2. Kinetic model. Modeling procedure
Table 1 lists the potassium-containing species considered in the kinetic model, as well as their enthalpy of formation H, standard entropy S, and specific heat at constant pressure Cp (all at 298.15 K). Reactions for most of these species were discussed in previous studies of combustion processes with added potassium compounds (Heimerl, 1984; Slack et al., 1989; Baratov et al., 1991; Glarborg and Marshall, 2005; Hindlyarti et al., 2006). Data have been added for several species in a condensed phase and for the potassium dimer (Table 1). The Supplemental Materials include the data in the format of the Chemkin suite of programs (Kee et al., 1986, 1989, 1991), which were used for the present simulations.
Table 1.
Thermodynamic data for potassium-containing species (kJ, J, mole, K)
| Species | H(298 K) | S(298 K) | Cp (298 K) | Reference * |
|---|---|---|---|---|
| K | 89.0 | 160.3 | 20.8 | Goos et al., 2012 |
| K(s) | 0.0 | 64.9 | 29.6 | Goos et al., 2012; Gurvich et al., 1993 |
| K2 | 127.1 | 249.7 | 37.9 | Kee et al., 1990 |
| KH | 123.0 | 197.9 | 31.0 | Kee et al., 1990 |
| KH(s) | −57.7 | 50.2 | 37.9 | Kee et al., 1990 |
| KO | 64.9 | 241.1 | 35.4 | Gurvich et al., 1993 |
| KO2 | −86.6 | 268.6 | 48.1 | Glarborg and Marshall, 2005; Sander et al., 2011 |
| KO2(s) | −284.5 | 122.6 | 77.4 | Kee et al., 1990 |
| K2O | −74.1 | 286.6 | 54.2 | Goos et al., 2012 |
| K2O(s) | −363.2 | 94.1 | 72.0 | est; Kee et al., 1990; Gurvich et al., 1993 |
| K2O2 | −191.6 | 306.5 | 71.0 | Goos et al., 2012 |
| K2O2(s) | −495.8 | 113.4 | 100.0 | Kee et al., 1990 |
| KOH | −231.8 | 275.7 | 49.2 | Glarborg and Marshall, 2005 |
| KOH(s) | −424.7 | 79.1 | 64.9 | Gurvich et al., 1993 |
| (KOH)2 | −638.9 | 342.7 | 105.9 | Glarborg and Marshall, 2005; Gurvich et al., 1993 |
| K2CO3 | −811.7 | 345.5 | 90.0 | Gurvich et al., 1993 |
| K2CO3(s) | −1150.2 | 155.6 | 114.2 | Kee et al., 1990; Gurvich et al., 1993 |
| KO3 | −77.0 | 285.8 | 64.4 | est; Sander et al., 2011; Vasiliu et al., 2010 |
| KHCO3 | −739.7 | 315.5 | 74.5 | est; Plane et al., 2014 |
| KHCO3(s) | −963.2 | 115.5 | 89.1 | est; Meng et al., 1995; Wagman et al., 1982 |
| KCO3 | −523.0 | 311.3 | 68.6 | est; Plane et al., 2014 |
est – estimate in this work
The kinetic model consists of two sets of reactions. The first is for hydrocarbon combustion, for which Grimech-3.0 is adopted (Smith et al., 2000), and the second is for the reactions of potassium-containing species with the species of the hydrocarbon system. Table 2 shows the reactions of potassium-containing species, consisting of 85 reactions of 12 species (K, KH, KO, KO2, KO3, K2O, K2O2, KOH, (KOH)2, K2CO3, KCO3 and KHCO3). The set of reactions with K-containing species includes reactions considered in the literature along with their rate parameters. Additional reactions were also added from the analysis of plausible reactions based on thermochemical considerations, with their rates estimated by analogy or using empirical correlations. Note that the kinetic mechanism for the behavior of potassium-containing species was analyzed in several works for different environments (Hastie, 1973; Hastie et al.; 1986, Jensen et al., 1979; Slack et al., 1989; Baratov et al., 1991; Benilov et al., 1994; Glarborg and Marshall, 2005; Hindlyarti et al., 2006; Plane et al., 2014).
Table 2.
Kinetic model for potassium-containing species (AT)
| A | b | E | Reference* | |
|---|---|---|---|---|
| K atom reactions | ||||
| 1. K+O2(+M)=KO2(+M) | 3.60E+14 | 0.0 | 0.0 | (Glarborg and Marshall, 2005, Sorvajarvi et al., 2015) |
| LOW /2.0e22 −1.55 0./ | ||||
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 2. K+OH+M=KOH+M | 2.18E+21 | −1.0 | 0.0 | (Jensen and Jones, 1982),a |
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 3. K+H+M=KH+M | 3.00E+17 | −1.0 | 0.0 | (Hindlyarti et al., 2006) |
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 4. k+o+m=ko+m | 1.50E+21 | −1.5 | 0.0 | (Glarborg and Marshall, 2005) |
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 5. K+HCO=KH+CO | 3.00E+13 | 0.0 | 14.64 | (Williams and Fleming, 1999),b |
| 6. K+HO2=KH+O2 | 2.00E+13 | 0.0 | 58.58 | b |
| 7. K+HO2=KOH+O | 7.00E+12 | 0.0 | 31.38 | (Baratov et al., 1991),b |
| 8. K+H2O2=KOH+OH | 2.50E+13 | 0.0 | 0.0 | (silver et al., 1984),Na |
| 9. K+H2O2=KO+H2O | 1.60E+13 | 0.0 | 0.0 | (silver et al., 1984),Na |
| KO reactions | ||||
| 10. KO+H=K+OH | 2.00E+14 | 0.0 | 6.28 | (Williams and Fleming, 1999),b |
| 11. KO+O=K+O2 | 1.20E+14 | 0.0 | 1.00 | (plane et al., 2014) |
| 12. KO+OH=KOH+O | 2.00E+13 | 0.0 | 0.0 | (Glarborg and Marshall, 2005, Williams and Fleming, 1999) |
| 13. KO+OH=K+HO2 | 3.00E+13 | 0.0 | 41.84 | b |
| 14. KO+HO2=KOH+O2 | 5.00E+13 | 0.0 | 0.0 | (Glarborg and Marshall, 2005, Williams and Fleming, 1999) |
| 15. KO+HO2=KO2+OH | 3.00E+12 | 0.0 | 16.74 | (Heimerl and Keller, 1987),b |
| 16. KO+H2=KOH+H | 1.60E+13 | 0.0 | 0.0 | (williams and Fleming, 1999),Na |
| 17. KO+H2=K+H2O | 3.10E+12 | 0.0 | 0.0 | (Glarborg and Marshall, 2005, Williams and Fleming, 1999),Na |
| 18. KO+H2O=KOH+OH | 6.00E+11 | 0.5 | 0. 0 | (Slack et al., 1989) |
| 19. KO+CO=K+CO2 | 1.00E+14 | 0.0 | 0.0 | (Heimerl and Keller, 1987) |
| 20. KO+HCO=KOH+CO | 3.00E+13 | 0.0 | 0.0 | (Williams and Fleming, 1999),Na |
| 21. KO+CH4=KOH+CH3 | 1.20E+12 | 0.0 | 14.64 | (Baratov et al., 1991) |
| 22. KO+CH2O=KOH+HCO | 1.20E+13 | 0.0 | 16.74 | b |
| 23. KO+KO=KO2+K | 1.00E+13 | 0.0 | 0.0 | (Singh and Weaver, 1991) |
| 24. ko+h+m=koh+m | 3.60E+22 | −2.0 | 0.0 | (Baratov et al., 1991) |
| 25. KO+O+M=KO2+M | 1.45E+19 | −1.0 | 0.0 | (Baratov et al., 1991) |
| 26. KO+CH3=K+CH3O | 2.30E+13 | 0.0 | 2 5.10 | b |
| 27. KO+C2H6=KOH+C2H5 | 1.20E+13 | 0.0 | 2 5.10 | b |
| 28. KO+C2H4=KOH+C2H3 | 1.50E+13 | 0.0 | 33.47 | b |
| 29. KO+C2H2=KOH+C2H | 2.00E+13 | 0.0 | 37.66 | b |
| 30. KO+C3H8=KOH+C3H7 | 1.50E+13 | 0.0 | 20.92 | b |
| KO2 reactions | ||||
| 31. KO2+H=K+HO2 | 1.90E+12 | 0.0 | 0.0 | (Singh and Weaver, 1991) |
| 32. KO2+H=KOH+O | 1.00E+13 | 0.0 | 0.0 | (singh and Weaver, 1991) |
| 33. KO2+H=KO+OH | 5.00E+13 | 0.0 | 29.29 | (Babushok et al., 2003),Na |
| 34. KO2+O=KO+O2 | 1.30E+13 | 0.0 | 0.0 | (DeMore et al., 1997, Glarborg and Marshall, 2005),Na |
| 35. KO2+CO=KO+CO2 | 6.00E+13 | 0.0 | 96.23 | (Baratov et al., 1991) |
| 36. KO2+OH=KOH+O2 | 1.20E+13 | 0.0 | 0.0 | (Baratov et al., 1991) |
| 37. KO2+H2=KOH+OH | 1.80E+12 | 0.0 | 83.14 | (Baratov et al., 1991) |
| 38. KO2+HCO=KOH+CO2 | 6.00E+12 | 0.0 | 0.0 | (Baratov et al., 1991) |
| 39. KO2+CH3=KOH+CH2O | 6.00E+12 | 0.0 | 0.0 | (Baratov et al., 1991) |
| KOH reactions | ||||
| 40. KOH+H=K+H2O | 2.20E+12 | 0.5 | 0.0 | (Slack et al., 1989) |
| 41. KOH+CH3=K+CH3OH | 3.50E+12 | 0.0 | 41.84 | b |
| 42. KOH+HO2=KO2+H2O | 6.00E+12 | 0.0 | 22.18 | (Baratov et al., 1991) |
| 43. KOH+KOH=(K0H)2 | 8.00E+13 | 0.0 | 0.0 | (Glarborg and Marshall, 2005) |
| KH reactions | ||||
| 44. KH+H=K+H2 | 1.00E+14 | 0.0 | 0.0 | (Hindlyarti et al., 2006) |
| 45. KH+O=K+OH | 5.00E+13 | 0.0 | 0.0 | (Hindlyarti et al., 2006) |
| 46. KH+O=KO+H | 6.00E+12 | 0.0 | 29.29 | b |
| 47. KH+OH=K+H2O | 1.00E+14 | 0.0 | 0.0 | (Heimerl and Keller, 1987) |
| 48. KH+OH=KOH+H | 1.00E+13 | 0.0 | 0.0 | (Heimerl and Keller, 1987) |
| 49. KH+HCO=K+CH2O | 2.00E+13 | 0.0 | 0.0 | b |
| 50. KH+CH3=CH4+K | 1.00E+14 | 0.0 | 0.0 | (Babushok et al., 2003),Na |
| 51. KH+CH3O=K+CH3OH | 2.00E+13 | 0.0 | 0.0 | b |
| 52. KH+KO=K+KOH | 1.00E+14 | 0.0 | 0.0 | (Heimerl and Keller, 1987) |
| K2O reactions | ||||
| 53. KO+K+M=K2O+M | 1.00E+16 | 0.0 | 0.0 | b |
| 54. K2O+H=K+KOH | 5.00E+12 | 0.0 | 12.55 | (Babushok et al., 2003),Na |
| 55. K2O+O=K2O2 | 1.00E+14 | 0.0 | 0.0 | b |
| 56. K2O+O=KO+KO | 1.00E+13 | 0.0 | 41.84 | b |
| 57. K2O+OH=KO+KOH | 1.00E+13 | 0.0 | 20.92 | b |
| K2O2 reactions | ||||
| 58. KO+KO=K2O2 | 1.00E+14 | 0.0 | 0.0 | b |
| 59. K202+H=K0H+K0 | 1.50E+13 | 0.0 | 4.18 | (Babushok et al., 2003), Na |
| 60. K202+0=K02+K0 | 1.00E+13 | 0.0 | 33.47 | b |
| 61. K202+0H=K0H+K02 | 1.00E+13 | 0.0 | 12.55 | b |
| KO3 reacti ons | ||||
| 62. KO2+O+M=KO3+M | 1.45E+19 | −1.0 | 0.0 | (Baratov et al., 1991. ) |
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 63. H+KO3=KO2+OH | 2.00E+13 | 0.0 | 0.0 | b |
| 64. O+KO3=KO2+O2 | 2.00E+13 | 0.0 | 0.0 | b |
| 65. OH+KO3=KO2+HO2 | 1.00E+13 | 0.0 | 37.66 | b |
| 66. CH3+K03=CH30+K02 | 1.00E+13 | 0.0 | 20.92 | b |
| 67. KO+O2(+M)=KO3(+M) | 3.40E+14 | 0.0 | 0.0 | (DeMore et al., 1997), Na |
| LOW /1.1e23 −2.0 0.0/ | ||||
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| KHCO3,KCO3,K2CO3 reactions | ||||
| 68. KOH+CO2(+M)=KHCO3(+M) | 4.09E+14 | 0.0 | 0.0 | (DeMore et al., 1997),Na |
| LOW /1.25e30 −4.21 0. / | (Plane et al., 2014) | |||
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 69. KO+CO2(+M)=KCO3(+M) | 3.90E+14 | 0.0 | 0.0 | (DeMore et al., 1997),Na |
| LOW /3.8e2 5 −2.0 0.0/ | ||||
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 70. KCO3+H+M=KHCO3+M | 2.00E+16 | 0.0 | 0.0 | b |
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 71. KO2+CO+M=KCO3+M | 3.00E+16 | 0.0 | 0.0 | (DeMore et al., 1997),Na |
| 72. K+KCO3+M=K2CO3+M | 2.00E+18 | 0.0 | 0.0 | b |
| H2/2.0/ H2O /6.0/ CH4/2.0/ CO/1.5/ CO2/2.0/ C2H6 /3.0/ | ||||
| 73. KHCO3+H=K+CO2+H2O | 2.70E+13 | 0.0 | 29.83 | (Plane et al., 2014) |
| 74. KHCO3+H=KCO3+H2 | 1.50E+13 | 0.0 | 41.84 | b |
| 75. KHCO3+O=KCO3+OH | 1.00E+13 | 0.0 | 52.30 | b |
| 76. KHCO3+OH=KCO3+H2O | 2.00E+13 | 0.0 | 31.38 | b |
| 77. KCO3+H=KOH+CO2 | 3.00E+12 | 0.0 | 16.74 | b |
| 78. KCO3+O=KO2+CO2 | 5.00E+12 | 0.0 | 12.55 | b |
| 79. KHCO3+KO=K2CO3+OH | 6.00E+12 | 0.0 | 29.29 | b |
| 80. KHCO3+KOH=K2CO3+H2O | 3.00E+12 | 0.0 | 37.66 | b |
| 81. KCO3+KO=K2CO3+O | 7.00E+12 | 0.0 | 20.92 | b |
| 82. KCO3+KO2=K2CO3+O2 | 1.00E+13 | 0.0 | 12.55 | b |
| 83. K2CO3+M=K2O+CO2+M | 5.00E+16 | 0.0 | 338.90 | b |
| 84. K2CO3+OH=KCO3+KOH | 3.00E+14 | 0.0 | 46.02 | b |
| 85. K2CO3+O=K2O2+CO2 | 3.00E+14 | 0.0 | 29.29 | b |
a-Na, increased 20%; b-estimation by analogy or using empirical correlations
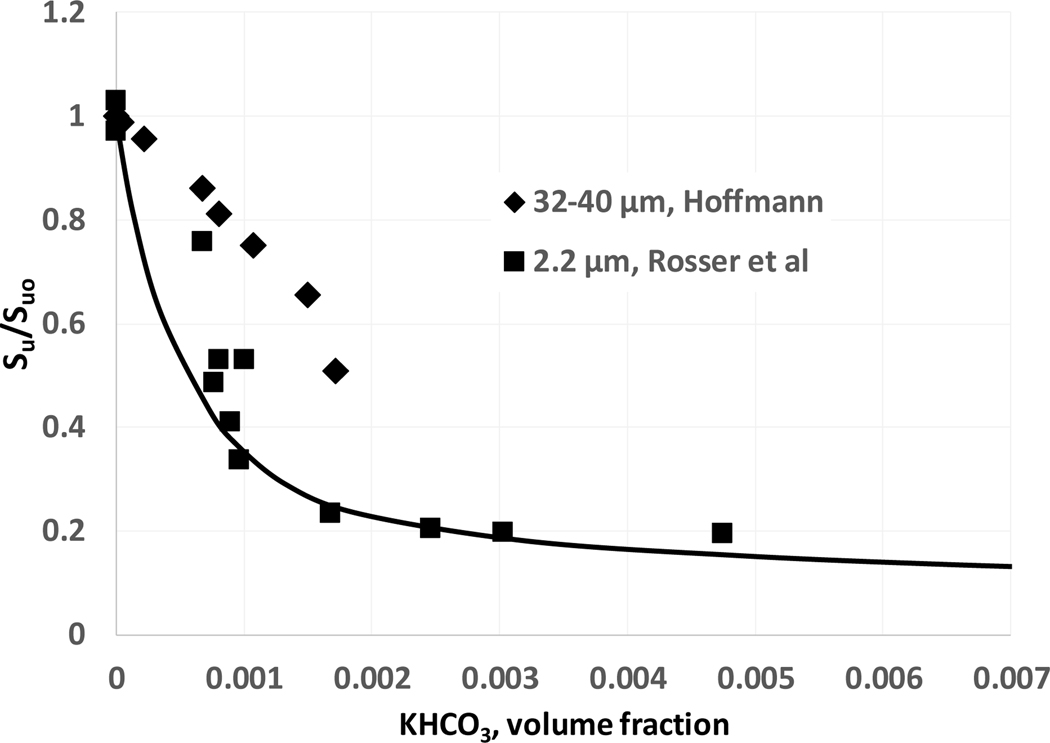
There are very few experimental data on the influence of potassium compounds on the burning velocity of hydrocarbon systems. For comparison with model predictions we have used experimental data of Rosser et al. (1963) and Hoffmann (1971) on the influence of KHCO3 on burning velocity of a stoichiometric methane-air flame. Note that KHCO3 is added to the flames as solid particles, for two ranges of diameter: 2.2 μm (Rosser et al., 1963) and 32–40 μm (Hoffmann, 1971). The experimental data demonstrate that small particles (i.e., those with diameter less than roughly 5–25 μm) provide the maximum inhibition effect (Rosser et al., 1963; Ewing et al., 1989; Ewing et al., 1992); whereas larger particles have lower inhibition effectiveness. This is likely due to the slower evaporation of the larger particles, leading to lower gas-phase potassium species volume fractions in the reactions zone. Experimental studies on the effect of large size KHCO3 particles on burning velocity of propane were conducted by Hoorelbeke and van Wingerden (2009) and Hoorelbeke (2011). The present comparison of modeling results with experimental data assumes complete evaporation of KHCO3 particles in the flame reaction zone. Note that in the simulations, although the species KHCO3 is added as a solid, all references to its concentration in the flames are listed as the volume fraction of the gasphase species that it evaporates to. This facilitates the comparisons with other agents, for which the volume basis (i.e., molar) is the most tractable. Figure 1 shows the measured and numerically predicted normalized burning velocity of premixed stoichiometric methane-air flames as a function of the KHCO3 volume fraction (added as solid particles); the agreement is reasonable.
Figure 1.
Dependence of burning velocity Su normalized by the uninhibited burning velocity Suo on KHCO3 volume fraction for stoichiometric methane-air flame (line: calculations; symbols: experimental data; initial conditions: 298 K, 101.33 kPa).
3. Results and discussion
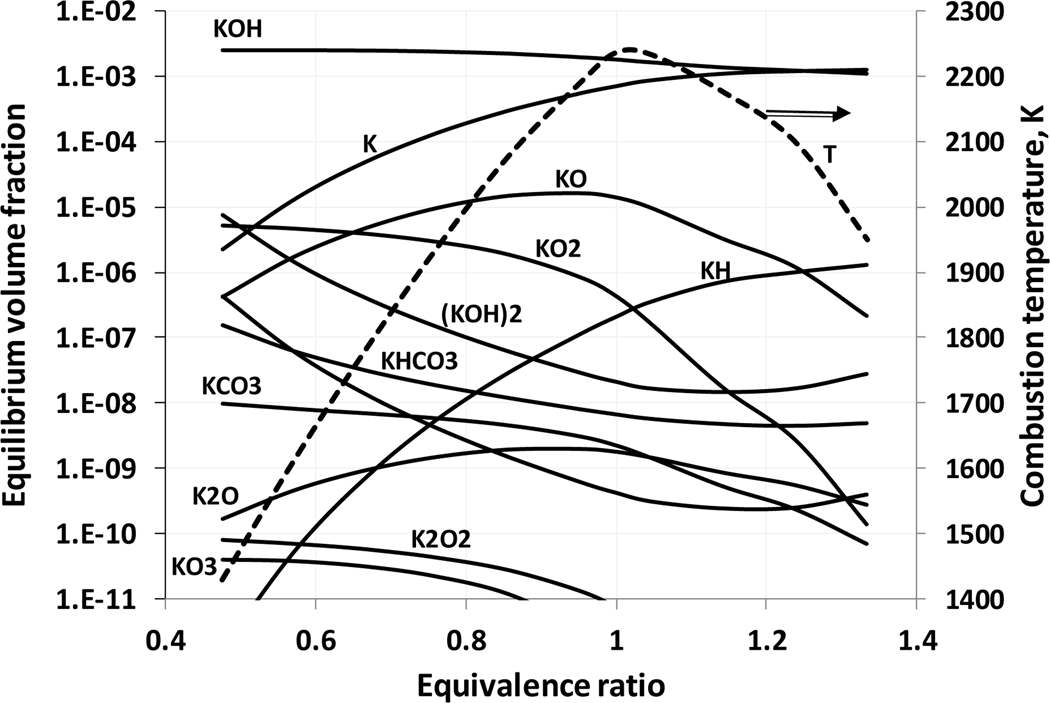
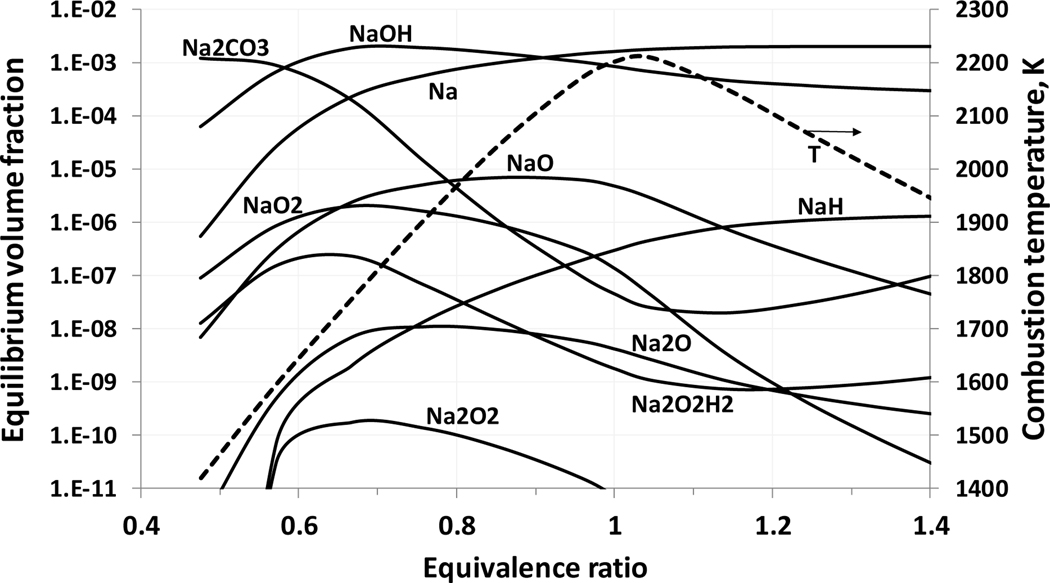
Figure 2 shows equilibrium volume fractions of potassium containing species in methane-air flames with added KHCO3(s) (volume fraction 0.25%) as a function of the fuel-air equivalence ratio. For comparison purposes, Figure 3 provides similar results for sodium bicarbonate (solid). Despite the differences in the absolute level of the equilibrium concentrations, the alkali metal atom and the corresponding hydroxide (KOH and NaOH) are the major species in the combustion products. For very lean mixtures, KO2 and KO become significant species. For sodium, low temperature combustion products contain rather large equilibrium concentrations of Na2CO3. These calculations indicate that that the main alkali metal-containing species in a flame reaction zone are potassium and sodium atoms and their hydroxides. Note that compounds of potassium in a condensed phase were not considered in flame equilibrium calculations except of initial agent KHCO3.
Figure 2.
Equilibrium volume fraction of potassium-containing species in the combustion products of a methane-air flame as a function of equivalence ratio (initial conditions: 298 K, 101.33 kPa, KHCO3(s) mole fraction of 0.25%).
Figure 3.
Equilibrium volume fraction of sodium-containing species in the combustion products of a methane-air flame as a function of equivalence ratio (initial conditions: 298 K, 101.33 kPa, NaHCO3(s) mole fraction of 0.25%).
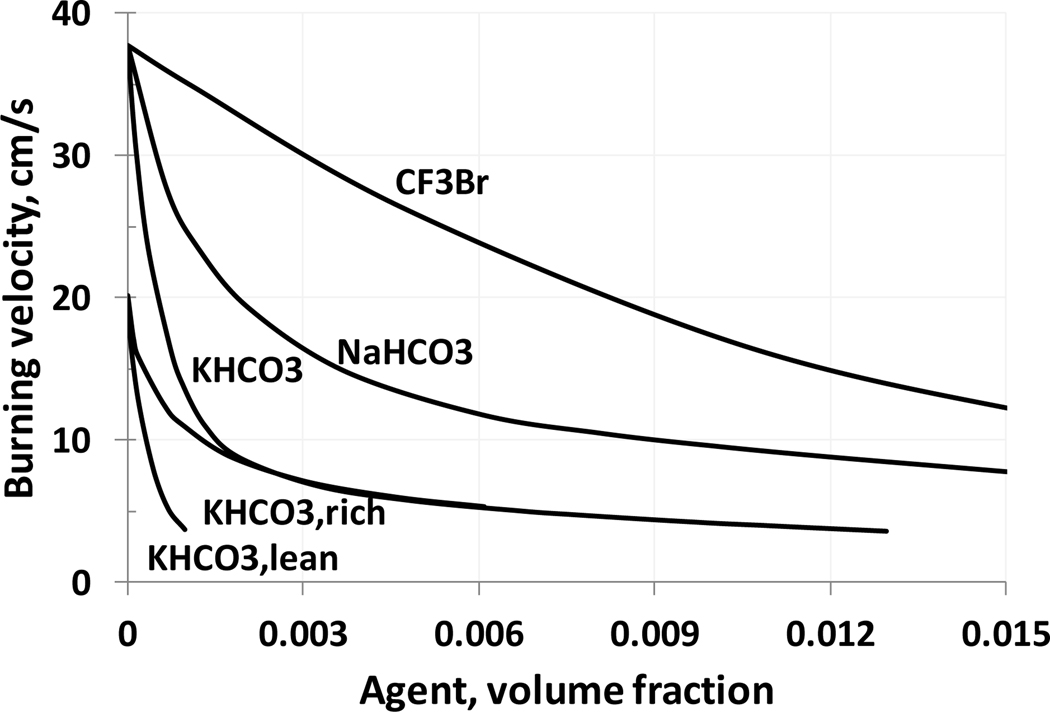
Using the above mechanism, simulations for a stoichiometric methane-air flame with added inhibitor (KHCO3, NaHCO3, and CF3Br) are shown in Figure 4. For comparison purposes, calculations are also performed for NaHCO3, using the model of Babushok et al. (2003), and for CF3Br, using the model from Babushok et al. (2015). For KHCO3 or NaHCO3 addition, it is assumed that the particles are small and that they rapidly evaporate and decompose. The simulations indicate that KHCO3 is a more effective inhibitor than sodium bicarbonate, and is a significantly more effective inhibitor than CF3Br (by a factor of 21, for achieving a 10% reduction in burning velocity), which is consistent with previous experimental results (Babushok and Tsang, 2000).
Figure 4.
Dependence of burning velocity on inhibitor mole fraction for added CF3Br, NaHCO3, and KHCO3 (initial conditions: methane-air, 298 K, 101.33 kPa; stoichiometric, lean and rich mixtures, ϕ=0.7, 1.0, and 1.34).
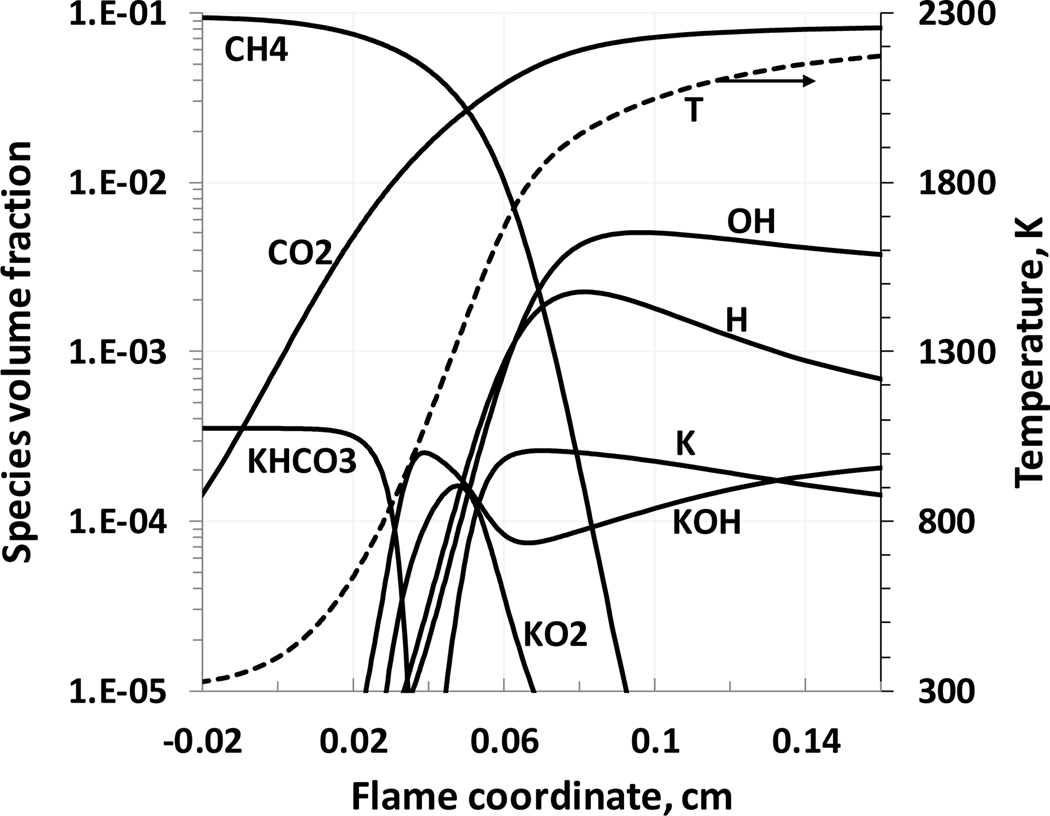
Figure 5 illustrates the flame structure of a stoichiometric methane-air flame inhibited by KHCO3. The main potassium-containing species in the flame zone are potassium atom and potassium hydroxide. A relatively large concentration of KO2 is observed in a low temperature range as a result of relatively fast formation through the termolecular reaction K+O2+M=KO2+M. The KO2 concentration decreases, and its contribution to the flame inhibition decreases, nearer to the high temperature region of the flame. A relatively large concentration of (KOH)2 is observed in the low temperature region of the flame, as a result of association reaction KOH+KOH=(KOH)2. Nonetheless, in the higher temperature regions of the flame, the concentration of (KOH)2 is low and its reactions are not important for flame inhibition (hence it is not shown in Figure 5).
Figure 5.
Flame structure of KHCO3-inhibited stoichiometric methane flame (initial conditions: 298 K, 101.33 kPa, KHCO3(s) mole fraction of 0.036%).
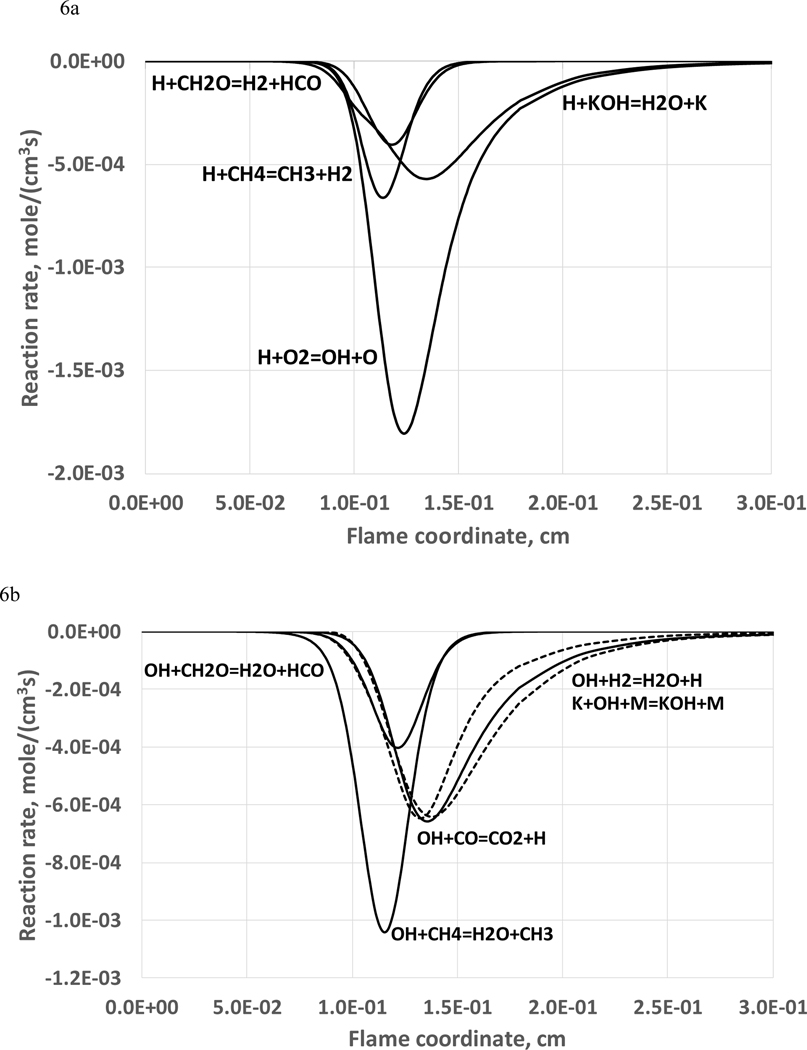
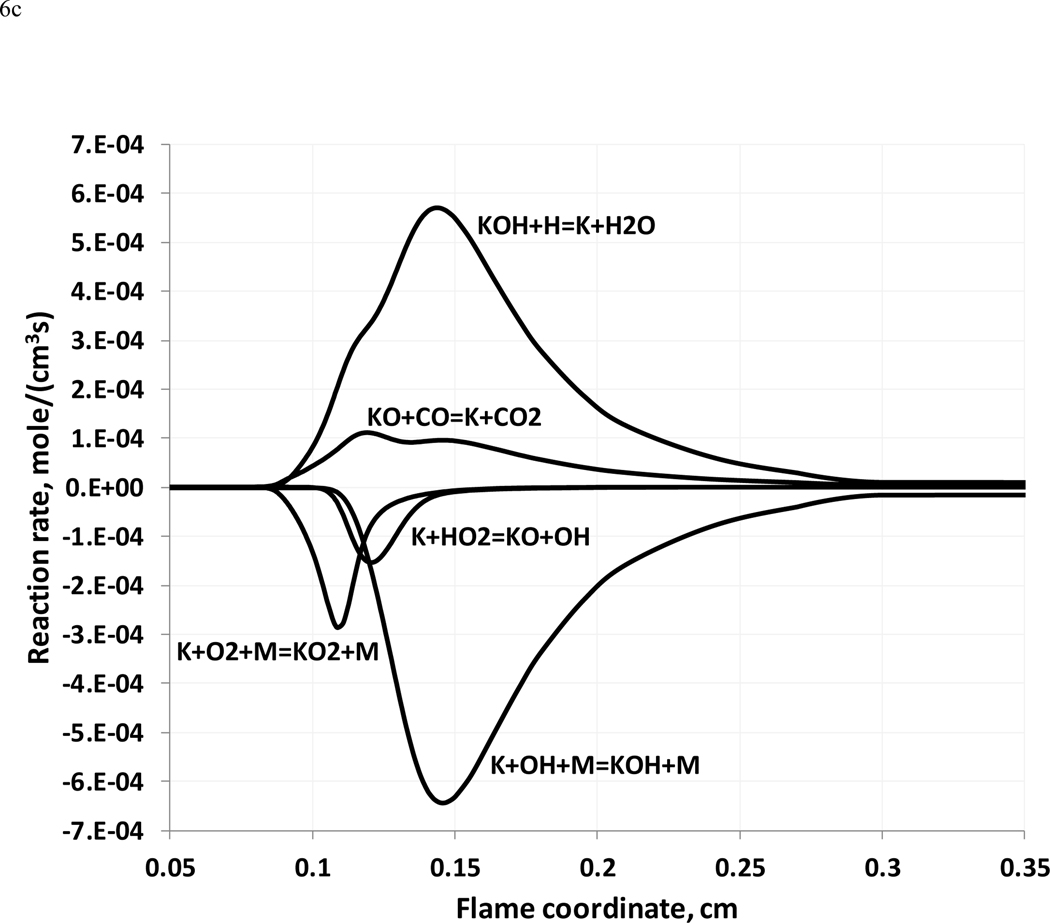
Figures 6 (a,b) show the reaction rate profiles of the main reactions for consumption of H and OH radicals, and Figure 6c shows the main reactions for the formation and consumption of potassium atom in the main reaction zone of the flame. As can be seen, for potassium species reactions, the main H atom scavenging reaction is KOH+H=H2O+K, and the OH radical termination reaction is OH+K+M=KOH+M. For a KHCO3 additive mole fraction of 0.05% (corresponding to a burning velocity decrease about 45 %), the rate of the reaction KOH+H is about 30 % of the rate of the chain-branching reaction H+O2 = OH + H .
Figure 6.
Reaction fluxes as a function of position in the flame, for the main reactions of: (a) H atom consumption atom (rates of H+CH2O and H+KOH – dotted lines); (b) OH radical consumption (rates of OH+H2 and K+OH+M – dotted lines); (c) formation and consumption of potassium atom (stoichiometric methane-air flame, 298 K, 101.33 kPa, KHCO3(s) mole fraction of 0.05%).
Thus, the main radical scavenging reactions of potassium-containing species are K+OH+M=KOH+M and KOH+H=K+H2O. The sequence of these reactions represents a relatively simple radical recombination cycle: potassium atom recombines mostly with hydroxyl radical with formation of potassium hydroxide. The further reaction of potassium hydroxide with hydrogen atom regenerates the potassium atom and completes the inhibition cycle with the overall radical recombination: OH+H=>H2O. This radical recombination cycle was considered in earlier work studying potassium-compound flame inhibition (Birchall, 1970; Hastie, 1973; Jensen et al., 1979) and suppression of secondary combustion (Hastie et al., 1986; Heimerl, 1984). Comparison with the inhibition mechanism of sodium compounds: Na+OH+M=NaOH+M and H+NaOH=Na+H2O, indicates that the potassium radical recombination cycle is similar, in agreement with earlier studies (Hastie, 1973; Jensen and Jones, 1982; Williams and Fleming, 1999; Babushok et al., 2003).
Additional reactions of KO and KO2 may also be important (Hindlyarti et al., 2006; Friedman and Levy, 1963). Hindlyarti et al. (2006) studied the influence of potassium chloride on the CO oxidation by water vapor in nitrogen diluted mixtures in a flow reactor at 773–1373 K. The reaction KOH+OH=KO+H2O was included in the model as the reverse reaction, and they concluded that it is the main reaction for KOH removal for their experimental conditions. The H atom concentration was very low at these conditions (unlike flame conditions, for which H-atom concentration is relatively high, comparable to that of OH). For the estimate of the rate constant for the reverse process (KO+H2O), Hindiyarti et al (2006) used 1.3×1014 (cm3/mole/s) with zero activation energy. We have used the rate constant of Slack et al. (1989), which at flame temperatures is roughly comparable with the data of Hindiyarti et al (2006).
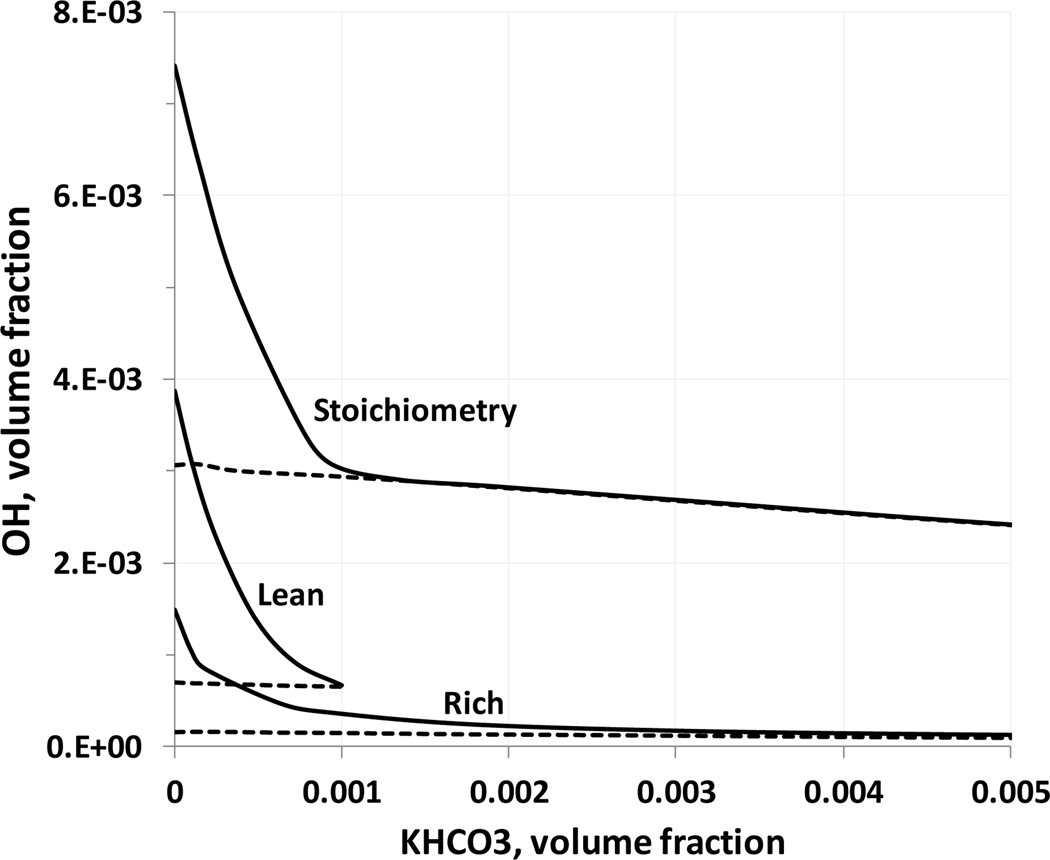
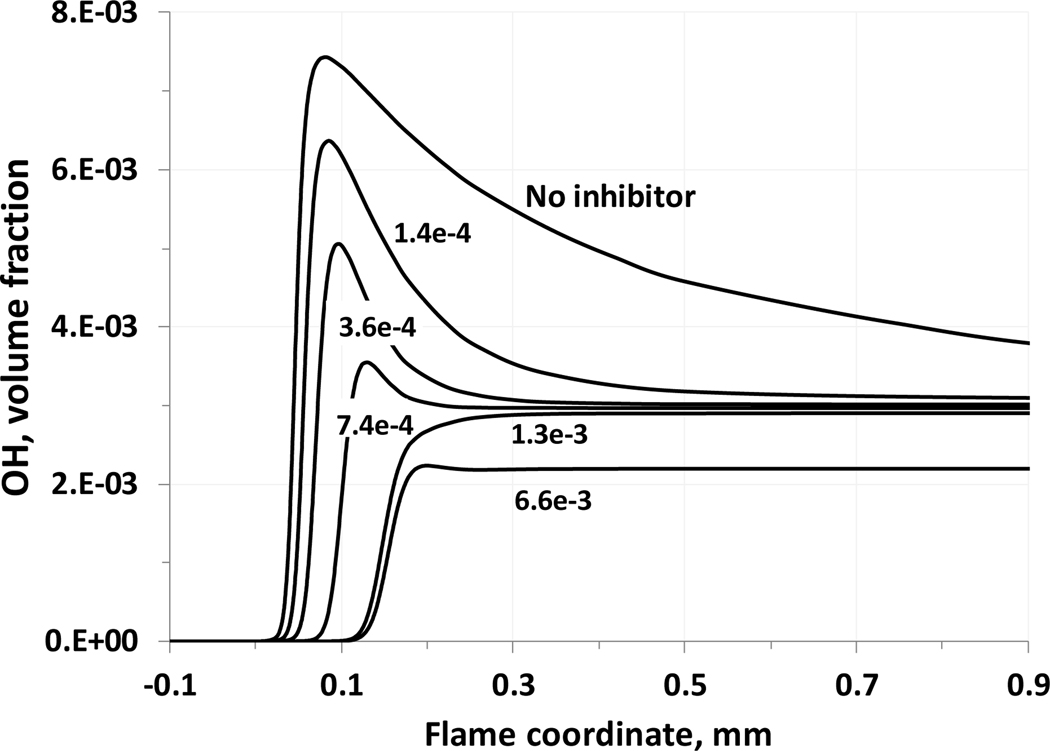
For addition of KHCO3 to premixed methane-air flames (ϕ=0.7, 1.0, and 1.34), the calculations show a strong decrease in the reduction in burning velocity with increasing addition of agent (Figure 4). This saturation in the inhibition effect is pronounced for gas-phase KHCO3 volume fractions in the range 0.001 to 0.002, and appears to occur at lower agent loadings for initially richer flames. The inhibition at low loading is also less for richer premixed CH4-air flames, as was also found in experimental studies (Hoorelbeke, 2011; Hoorelbeke and van Wingerden, 2009), and has been shown for iron (Reinelt and Linteris, 1996; Rumminger et al, 1999), phosphorus (Babushok et al., 2016), HBr (Westbrook, 1980), and Br2 (Rosser et al, 1958). Figure 7 shows the maximum (solid lines) and equilibrium (dotted lines) concentrations of hydroxyl radical as a function of KHCO3 concentration. It can be seen that the radical overshoot (the difference between maximum radical concentration and the equilibrium concentration) is decreasing with inhibitor concentration. At a gas-phase volume fraction of KHCO3 around 0.1 % the OH radical overshoot is disappearing, meaning that the maximum radical concentration does not occur in the main reaction zone of the flame. This can be interpreted as the suppression of chain-branching processes in a flame, as discussed in the work (Noto et al., 1998). Since the burning velocity is generally correlated with radical concentrations (Tanford and Pease, 1947), further reduction in burning velocity would require an inhibitor with some physical effect to lower flame temperature. Figure 8 shows the hydroxyl radical profiles in a methane/air stoichiometric flame at different inhibitor volume fractions. Again, it can be seen that at a KHCO3 volume fraction of about 0.1%, the radical overshoot disappears, and the maximum hydroxyl concentration is the flame equilibrium concentration. The further addition of agent leads to a decrease of equilibrium concentration as a result of decrease in the equilibrium temperature.
Figure 7.
Peak (solid lines) and equilibrium (dotted lines) OH volume fraction as a function of KHCO3 mole fraction (initial conditions: stoichiometric, lean and rich mixtures, ϕ=0.7, 1.0, and 1.34; 298 K, 101.33 kPa).
Figure 8.
Flame concentration profiles of hydroxyl radical at different initial KHCO3(s) loadings (stoichiometric methane-air flame, initial conditions:298 K, 101.33 kPa).
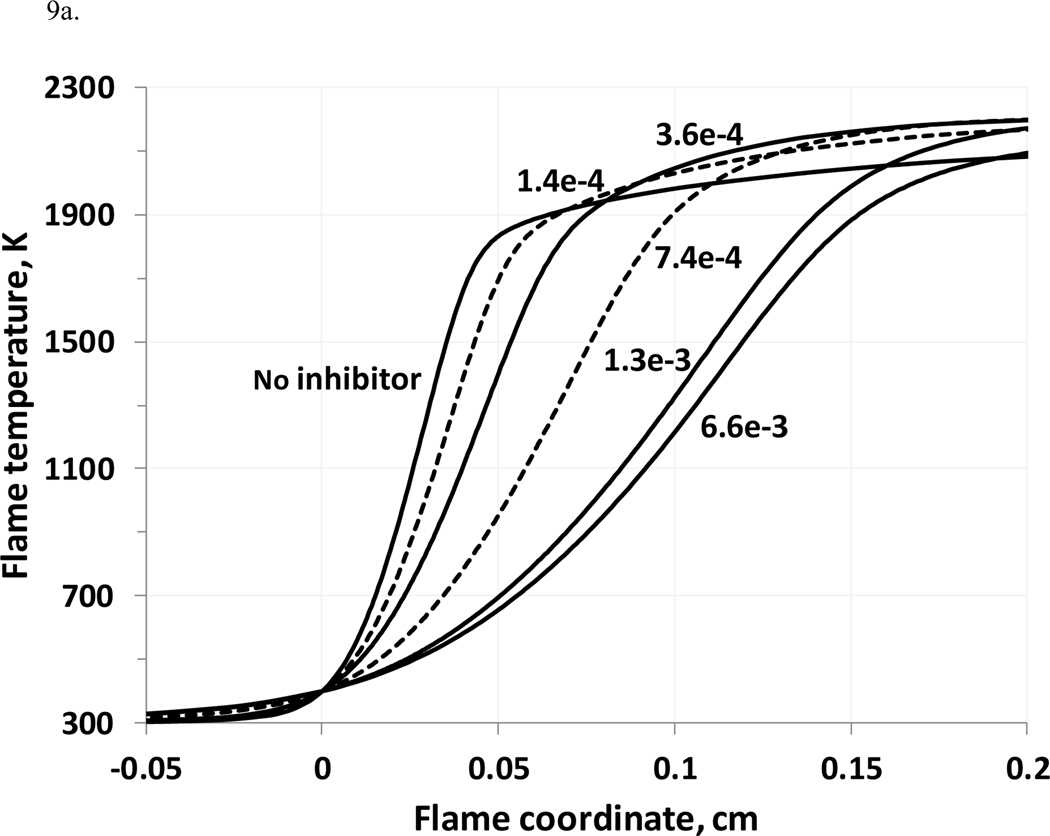
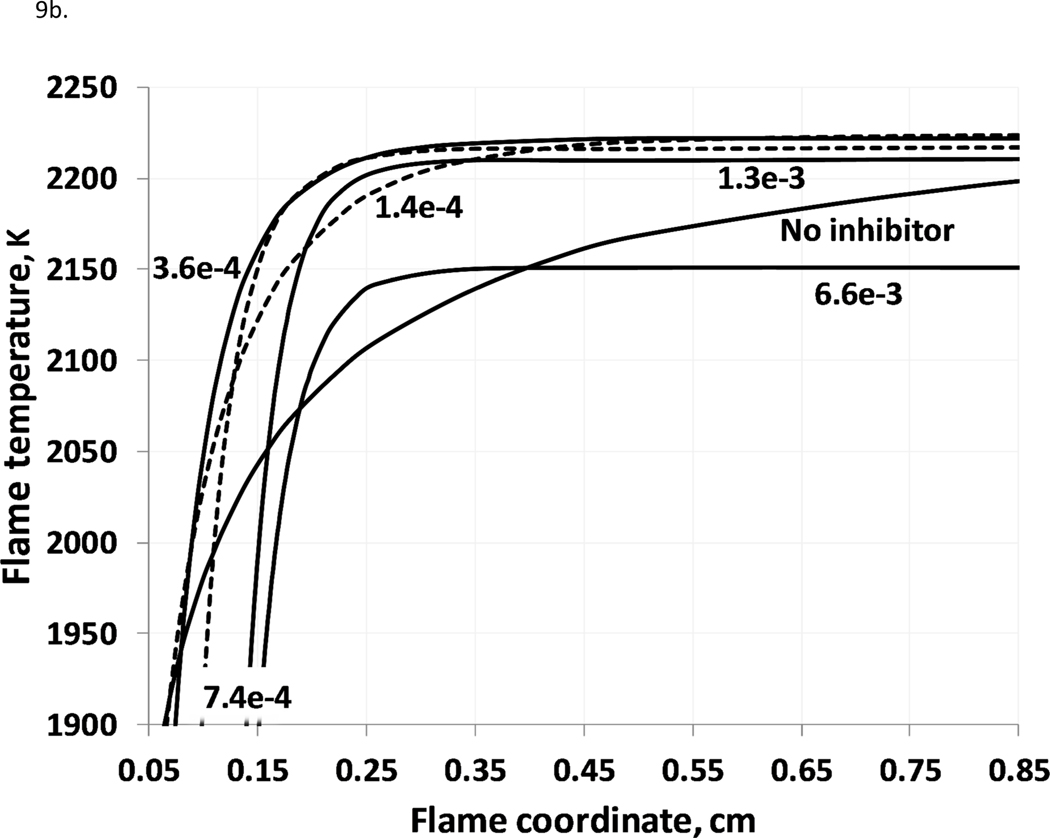
Addition of potassium compounds to a flame accelerates combustion in some regions, and retards it in others. Figure 9a shows the temperature profiles corresponding to the OH profiles of Fig. 8, for the early parts of the flames, while Fig. 9b shows the latter parts. As discussed by Dixon-Lewis and Simpson (1976), addition of the inhibitor decreases the reaction rate in the flame reaction zone (the initial tangent of temperature increase), due to reduced rates of buildup of the chain branching radical pool. Of course, the adiabatic combustion temperature is reduced with addition of potassium bicarbonate due to its endothermic decomposition and release of the relatively inert CO2 which acts as a diluent. Nonetheless, as shown in Figure 9b, downstream of the main reaction zone of the flame, the presence of the potassium compound leads to a higher rate of temperature increase towards the equilibrium value. Examination of the reaction fluxes in the downstream region shows that this is due to CO conversion to CO2 via: KO + CO = K + CO2. , which does not occur in in the flames without KHCO3.
Figure 9.
(a,b). Flame temperature profiles at different KHCO3(s) loadings (stoichiometric methane-air flame, initial conditions:298 K, 101.33 kPa; temperature profiles for 1.4e-4 and 7.4e-4 – dotted lines).
It is of interest to compare available experimental data on suppression concentrations of KHCO3 with modeling predictions based on the suggestion that a burning velocity of 5 cm/s roughly corresponds to the flammability limits, although of course this is a very approximate estimation (Egerton and Thabet, 1952; Westbrook, 1983). The calculations (Figure 4) demonstrate that a KHCO3 volume fraction of 0.7 % is required to reach the burning velocity of 5 cm/s burning velocity for a stoichiometric mixture. For NaHCO3 (Babushok et al., 2003), a volume fraction of about 2 % is required. Experimental data demonstrate the following suppression gas-phase volume fractions for KHCO3: 0.57 % (premixed methane flame, (Ewing et al., 1984)), 0.78% (diffusion flame, heptane, (Ewing et al., 1992)) and 0.61% (pan fire, heptane, Purple-K, (Chattaway et al., 1995)), which reasonably corresponds to the 0.7 % estimate above. For sodium compounds, experimental data generally show about twice as much required (on a mass basis) as compared to potassium, consistent with the above simulations (Birchall, 1970; Fischer and Leonard, 1995; Reed et al., 1997b).
4. Conclusions
A detailed kinetic model of influence of potassium-containing compounds on hydrocarbon-air flames has been developed, and includes 85 reactions of 12 potassium-containing species (K, KO, KO2, KO3, K2O, KH, KOH, KCO3, KHCO3, K2CO3, K2O2, (KOH)2). Simulations employing the mechanism to model premixed methane-air flames inhibited by potassium bicarbonate, have indicated the following:
The calculated burning velocity of premixed CH4-air flames with added KHCO3 agrees reasonably well with available experimental data assuming fast evaporation of inhibitor particles in a flame reaction zone.
Flame equilibrium calculations demonstrate that the main K-containing species in the combustion products are potassium hydroxide and potassium atom. The species KO and KO2 are always more than an order of magnitude lower in volume fraction than K and KOH, over a range of equivalence ratios. For very lean conditions (ϕ less than about 0.6) concentrations of KO, KO2 and (KOH)2 become comparable with K, but are still substantially less than that of KOH.
For CH4/air flames, the main inhibition reactions are K+OH+M=KOH+M and H+KOH=K+H2O.
The marginal effect of added KHCO3 to lower the burning velocity in near-stoichiometric CH4-air flames is reduced greatly above volume fractions from 0.1 % to 0.2 %. At these loadings, the peak OH radical volume fraction is lowered to equilibrium values, and the burning velocity is about 10 cm/s. Further decrease in the burning velocity (down to suppression level) is likely the result of reductions in the radical concentrations due to lower temperatures from thermal effects of agent addition.
The inhibition effectiveness of KHCO3 is higher in lean mixtures than in rich fuel mixtures.
5. Acknowledgements
The work of VB was supported by Total Refining &Chemicals.
Footnotes
Official contribution of NIST, not subject to copyright in the United States. Certain commercial equipment, instruments, and materials are identified in this paper to adequately specify procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology.
References:
- Babushok V. & Tsang W. 2000. Inhibitor rankings for alkane combustion. Combust. Flame, 123, 488–506. [Google Scholar]
- Babushok VI, Linteris GT, Burgess DR & Baker PT 2015. Hydrocarbon flame inhibition by C3H2F3Br (2-BTP). Combust. Flame, 162, 1104–1112. [Google Scholar]
- Babushok VI, McNesby KL, Miziolek AW & Skaggs RR 2003. Modeling of synergistic effects in flame inhibition by 2-H heptafluoropropane blended with sodium bicarbonate. Combust. Flame, 133, 201–205. [Google Scholar]
- Babushok VI, Linteris GT, Katta VR & Takahashi F. 2016. Influence of hydrocarbon moiety of DMMP on flame propagation in lean mixtures. Combust. Flame, 171, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratov AN, Dobrikov VV & Shamonin VG 1991. On the role of homogeneous factors in inhibition of methane-air flames with powders. Soviet J. Chem. Phys, 7, 1395–1403. [Google Scholar]
- Baratov AN & Korol’chenko AY (Eds.) 1990. Fire and explosion safety of substances and materials, and fire suppression means. Reference Handbook (In Russian), Moscow: Publishing House “Khimiya”. [Google Scholar]
- Baratov AN & Vogman LP 1982. Fire Suppresant Dry Chemicals (in Russian), Moscow, Stroiizdat. [Google Scholar]
- Benilov MS, Pozdeev PA, Rogov BV & Sinelschchikov VA 1994. Nonequilibrium boundary-layer of potassium-seeded combustion products. Combust. Flame, 98, 313–325. [Google Scholar]
- Birchall JD 1970. Mechanism of flame inhibition by alkali metal salts. Combust. Flame, 14, 85–95. [Google Scholar]
- Chatrathi K. & Going J. 2000. Dust deflagration extinction. Process Saf. Prog, 19, 146–153. [Google Scholar]
- Chattaway A, Dunster RG, Gall R. & Spring DJ 1995. The evaluation of non-pyrotechnically generated aerosols as fire suppressants. In Proceedings of HOTWC-1995, Albuquerque, NM, 473–483. [Google Scholar]
- DeMore WB, Sander SP, Golden DM, Hampson RF, Kurylo MJ, Howard CJ, Ravishankara AR, Kolb CE & Molina MJ 1997. Chemical kinetics and photochemical data for use in stratospheric modeling. Evaluation number 12. JPL Publication 97–4. [Google Scholar]
- Dixon-Lewis G. & Simpson RJ 1976. Aspects of flame inhibition by halogen compounds. Proc. Combust. Inst, 16, 1111–1119. [Google Scholar]
- Egerton A. & Thabet SK 1952. Flame propagation - the measurement of burning velocities of slow flames and the determination of limits of combustion. Proc. R. Soc. A, 211, 445–471. [Google Scholar]
- Ewing CT, Faith FR, Hughes JT & Carhart HW 1989. Flame extinguishment properties of dry chemicals: extinction concentrations for small diffusion pan fires. Fire Technol., 134–149. [Google Scholar]
- Ewing CT, Faith FR, Romans F, Hughes JT & Carhart HW 1992. Flame extinguishment properties of dry chemicals: extinction weights for small diffusion pan fires and additional evidence for flame extinguishment by thermal mechanism. J. Fire Prot. Eng, 4, 35–52. [Google Scholar]
- Ewing CT, Hughes JT & Carhart HW 1984. The extinction of hydrocarbon flames based on the heat-absorption processes which occur in them. Fire Mater., 8, 148–156. [Google Scholar]
- Fischer G. & Leonard JT 1995. Effectiveness of Fire Extinguishing Powders Based on Small Scale Suppression Tests, Report NRL/MR/6180–95-7778, NRL, Wasington, DC [Google Scholar]
- Friedman R. & Levy JB 1963. Inhibition of opposed-jet methane air diffusion flames - the effects of alkali metal vapours and organic halides. Combust. Flame, 7, 195–201. [Google Scholar]
- Glarborg P. & Marshall P. 2005. Mechanism and modeling of the formation of gaseous alkali sulfates. Combust. Flame, 141, 22–39. [Google Scholar]
- Goos E, Burcat A. & Ruscic B. 2012. Extended Third Millennium Thermodynamic Database for Combustion and Air-Pollution Use with updates from Active Thermochemical Tables. TechnionIIT, Haifa, Israel. ftp://ftp.technion.ac.il/pub/supported/aetdd/thermodynamics/BURCAT.THR (Accessed August 2012). [Google Scholar]
- Gurvich LV, Iorish VS, Chekhovskoi DV, Ivanisov AD, Proskurnev AY, Yungman VS, Medvedev VA, Veits IV & Bergman GA 1993. IVTHANTHERMO. Database on THermodynamic Properties of Individual Substances, Institute of High Temperatures, Moscow: (NIST Special Database 5, IVTANTHERMO-PC, 1998). [Google Scholar]
- Hastie JW 1973. Molecular basis of flame inhibition. J. Res. Natl. Bur. Stand. Sect. A, A 77, 733–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie JW, Bonnell DW & Schenck PK 1986. Molecular Basis for Secondary Flash Suppression. Report ARO 18375-CH/MIPR 102–84, NBS, Washington, DC. [Google Scholar]
- Heimerl JM 1984. Advanced Flash Suppression Network Involving Alkali Salts. Report NTIS:ADA149988, ABRL, Aberdeen Proving Ground, MD. [Google Scholar]
- Heimerl JM & Keller GE 1987. Kinetics networks and MEFF-code predictions. A progress report. In Proceedings of the Workshop on the Chemical Suppression of Rocket Afterburning and of Gun Muzzle Flash, SpeciaL Report BRL-SP-59, Aberdeen Proving Ground, MD, 45–51. [Google Scholar]
- Hindlyarti L, Frandsen F, Livbjerg H. & Glarborg P. 2006. Influence of potassium chloride on moist CO oxidation under reducing conditions: Experimental and kinetic modeling study. Fuel, 85, 978–988. [Google Scholar]
- Hoffmann W. 1971. Influence of alkali-metal salts on laminar flame velocity. Chem. Ing. Tech, 43, 556–560. [Google Scholar]
- Hoorelbeke L. 2011. Numerical and Experimental Study of Vapour Cloud Explosions – Mitigation by Inhibitors. Vrije Universiteit, Brussel. [Google Scholar]
- Hoorelbeke P. & Van WIngerden K. 2009. Experimental study of inhibition of premixed flames. In 22nd International Colloquium on the Dynamics of Explosions and Reactive Systems. Abstracts., Minsk, Belarus. [Google Scholar]
- Iya KS, Wollowitz S & Kaskan WE 1974. Measure of inhibition of quenched premixed flames. Combust. Flame, 22, 415–417. [Google Scholar]
- Jensen DE & Jones GA 1982. Kinetics of flame inhibition by sodium. J. Chem. Soc., Faraday Trans. I, 78, 2843–2850. [Google Scholar]
- Jensen DE, Jones GA & Mace ACH 1979. Flame inhibition by potassium. J. Chem. Soc., Faraday Trans. I, 75, 2377–2385. [Google Scholar]
- Kee RJ, Dixon-Lewis G, Warnatz J, Coltrin RE & Miller JA 1986. A fortran computer package for the evaluation of gas-phase, multicomponent transport properties. Report SAND86–8246, Sandia National Laboratories. [Google Scholar]
- Kee RJ, Rupley FM & Miller JA 1989. CHEMKIN-II: A fortran chemical kinetics package for the analysis of gas phase chemical kinetics. Report SAND89–9009B, Sandia National Laboratories.. [Google Scholar]
- Kee RJ, Rupley FM & Miller JA 1990. The Chemkin Thermodynamic Data Base. Report SAND-87–8215B, Sandia National Laboratories. [Google Scholar]
- Kee RJ, Grcar JF, Smooke MD & Miller JA 1991. PREMIX: A fortran computer program for modeling steady laminar one-dimensional premixed flames. Report SAND85–8240, Sandia National Laboratories. [Google Scholar]
- Li B, Sun ZW, Li ZS, Alden M, Jakobsen JG, Hansen S. & Glarborg P. 2013. Post-flame gas-phase sulfation of potassium chloride. Combust. Flame, 160, 959–969. [Google Scholar]
- Linteris GT, Rumminger MD & Babushok VI 2008. Catalytic inhibition of laminar flames by transition metal compounds. Prog. Energy Combust. Sci, 34, 288–329. [Google Scholar]
- McHale ET 1975. Flame inhibition by potassium compounds. Combust. Flame, 24, 277–279. [Google Scholar]
- Meng ZY, Seinfeld JH, Saxena P. & Kim YP 1995. Atmospheric gas-aerosol equilibrium .4. Thermodynamics of carbonates. Aerosol Sci. Technol, 23, 131–154. [Google Scholar]
- Noto T, Babushok V, Hamins A. & Tsang W. 1998. Inhibition effectiveness of halogenated compounds. Combust. Flame, 112, 147–160. [Google Scholar]
- Plane JMC, Feng W, Dawkins E, Chipperfield MP, Hoffner J, Janches D. & Marsh DR 2014. Resolving the strange behavior of extraterrestrial potassium in the upper atmosphere. Geophys. Res. Lett, 41, 4753–4760. [Google Scholar]
- Reed MD, Fleming JW, Williams BA, Sheinson RS, Chattaway A, Laverty N. & Spring DJ 1997. Laboratory evaluation of bicarbonate powders as fire suppressants. In Proceedings of the International Conference of Ozone Protection Technologies, Baltimore, MD, 333–344. [Google Scholar]
- Reed MD, Williams BA, Sheinson RS, Fleming JW, 1997. Behavior of bicarbonate powders in counterflow diffusion flames. In Chemical and Physical Processes in Combustion. Proceedings of Fall Technical Meeting of Estearn State Section of the Combustion Institute, 83–86. [Google Scholar]
- Reinelt R. & Linteris GT 1996. Experimental study of the inhibition of premixed and diffusion flames by iron pentacarbonyl. Proc. Combust. Inst, 26, 1421–1428. [Google Scholar]
- Rosser WA, Wise H. & Miller J. 1958. Mechanism of combustion inhibition by compounds containing halogen. Symp (Int) Combust, 7, 175–182. [Google Scholar]
- Rosser WA, Inami SH & Wise H. 1963. The effect of metal salts on premixed hydrocarbon air flames. Combust. Flame, 7, 107–119. [Google Scholar]
- Rumminger MD, Reinelt D, Babushok V. & Linteris GT 1999. Numerical study of the inhibition of premixed and diffusion flames by iron pentacarbonyl. Combust. Flame, 116, 207–219. [Google Scholar]
- Sander SP, Abbatt J, Barker JR, Burkholder JB, Friedl RR, Golden DM, Huie RE, Kolb CE, Kurylo MJ, Moortgat GK, Orkin VL & Wine PH 2011. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 17. Jet Propulsion Laboratory, Pasadena. [Google Scholar]
- Silver JA, Stanton AC, Zahniser MS & Kolb CE 1984. Gas-phase reaction-rate of sodium-hydroxide with hydrochloric-acid. J. Phys. Chem, 88, 3123–3129. [Google Scholar]
- Singh T. & Weaver DP 1991. Suppression kinetics of KOH in H2-air flames. In Heat and Mass Transfer in Fires and Combustion Systems, ASME HTD, 176, 31–42. [Google Scholar]
- Slack M, Cox JW, Grillo A, Ryan R. & Smith O. 1989. Potassium kinetics in heavily seeded atmospheric-pressure laminar methane flames. Combust. Flame, 77, 311–320. [Google Scholar]
- Smith GP, Golden DM, Frenklach M, Moriarty NW, Eiteneer B, Goldenberg M, Bowman CT, Hanson RK, Song S, Gardiner JWC, Lissianski VV & Qin Z. 2000. GRI-Mech 3.0, http://www.me.berkeley.edu/gri_mech. [Google Scholar]
- Sorvajarvi T, Viljanen J, Toivonen J, Marshall P. & Glarborg P. 2015. Rate Constant and Thermochemistry for K + O-2 + N-2 = KO2 + N-2. J. Phys. Chem. A, 119, 3329–3336. [DOI] [PubMed] [Google Scholar]
- Tanford C, Pease RN 1947. Equilibrium Atom and Free Radical Concentrations in Carbon Monoxide Flames and Correlation with Burning Velocities. J. Chem. Phys, 15, 413–433. [Google Scholar]
- Tanford C, Pease RN 1947. Equilibrium Atom and Free Radical Concentrations in Carbon Monoxide Flames and Correlation with Burning Velocities. J. Chem. Phys, 15, 413–433. [Google Scholar]
- Tapscott RE, Sheinson RS, Babushok VI, Nyden MR & Gann RG 2001. Alternative Fire Suppressant Chemicals: A Research Review With Recommendations. NIST TN 1443, NIST, Gaithersburg, MD. [Google Scholar]
- Vasiliu M, Li SG, Peterson KA, Feller D, Gole JL & Dixon DA 2010. Structures and Heats of Formation of Simple Alkali Metal Compounds: Hydrides, Chlorides, Fluorides, Hydroxides, and Oxides for Li, Na, and K. J. Phys. Chem. A, 114, 4272–4281. [DOI] [PubMed] [Google Scholar]
- Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM, Churney KL & Nuttall RL 1982. The NBS tables of chemical thermodynamic properties - selected values for inorganic and C-1 and C-2 organic-substances in SI units. J. Phys. Chem. Ref. Data, 11, Supplement No 2. [Google Scholar]
- Westbrook CK 1980. Inhibition of laminar methane-air and methanol-air flames by hydrogen bromide. Combust. Sci. Technol, 23, 191–202. [Google Scholar]
- Westbrook CK 1983. Numerical modeling of flame inhibition by CF3BR. Combust. Sci. Technol, 34, 201–225. [Google Scholar]
- Williams BA & Fleming JW 1999. Suppression mechanisms of alkali metal compounds. In Proceedings of Halon Options Technical Working Conference, Albuquerque, NM. 157–169. [Google Scholar]