Abstract
Immune checkpoint inhibitors (ICIs) lead to durable responses in a subset of patients with cancer, but most patients do not respond to ICI, prompting interest in combining immunotherapy with other therapeutic regimens. Preclinical evidence supports the potential for therapeutic synergy between immunotherapy and radiation therapy through modulation of the tumor microenvironment and antitumor immune responses. Local therapy also has the potential to overcome localized sites of relative immune suppression and resistance. Prospective clinical trials have been initiated to test these hypotheses in the clinic as well as to investigate the toxicities and adverse events associated with combination immunotherapy and radiation therapy. In this review, we discuss the emerging results from prospective clinical trials of combination immunotherapy and radiation therapy, the safety and efficacy of their combination, concordance with preclinical and retrospective data, and some of the remaining open questions to be addressed by future clinical trials.
Introduction
Over the past decade the approval and clinical implementation of immunotherapy and specifically immune checkpoint inhibitors (ICIs) have transformed our ability to treat cancer, as some patients demonstrate durable responses and cures. There are a wide range of response rates to ICI across solid tumor types, but other than higher response rates in specific malignancies such as melanoma, renal cell carcinoma, cutaneous squamous cell carcinoma, Merkel cell carcinoma, and mismatch repair deficient carcinomas, in most other solid tumor types less than 20% of patients respond to treatment, with fewer still demonstrating durable benefit.1 Although overall response rates are low, the ability of ICIs to affect benefit in tumors often refractory to other treatment modalities and the so-called “tail” of extended survival in immunotherapy trials have led to mounting interest in increasing response rates by combining immunotherapy with other treatment regimens such as cytotoxic agents, molecular targeted treatments, or radiation therapy.
Preclinical studies have investigated a potential interactive relationship between immunotherapies and radiation therapy. The results of these studies suggest that radiation therapy may have both immunostimulatory and immunosuppressive effects.2 The ability of radiation therapy to induce immunogenic cell death resulting in an inflammatory tumor microenvironment has led to the hypothesis that immunotherapy and radiation therapy may in many cases be synergistic.3,4 Abscopal responses in which out-of-field tumors dramatically respond to combination ICIs and radiation therapy have been reported in case reports but are infrequent,5,6 supporting the hypothesis that immunotherapy is likely needed to propagate local immunologic effects of radiation to achieve systemic benefit in nonradiated tumor environments. Retrospective analyses have also suggested potential clinical benefit and safety of combinations of ICIs with radiotherapy.7–11 These preclinical and retrospective clinical data suggesting potential benefit of radiation therapy in priming response to ICIs have led to an increasing number of clinical trials investigating this hypothesis over the past several years. Conversely, most patients with cancer receive radiation over the course of their care, and immunotherapy offers the possibility of augmenting radiation responses. This offers the potential for a less toxic alternative to chemotherapy in targeting occult micrometastases in locoregionally advanced cancers or in patients with limited burden of metastatic disease or oligometastases.
Prospective clinical trials investigating the safety and efficacy of combination immunotherapy and radiation therapy have been initiated in multiple cancer types and span phases I, II, and III trials. In this review we summarize the results of these initial clinical trials to identify trends, find areas for further investigation, and provide evidence for or against hypotheses formed based on pre-clinical data. We identified clinical trials by querying clinicaltrials.gov and PubMed, as well as soliciting advice from the the National Cancer Institute Immuno-Oncology Translational Network and the National Cancer Institute Radiation and Immunotherapy Working Group (date: March 21, 2021; database: clinicaltrials.gov, pubmed.gov; search terms: “immunotherapy” AND “radiotherapy”; condition: “cancer”). Notably, clinical trials with negative results that were not reported may be under-represented in the published literature. Here we summarize the results of 20 phase I trials, 17 phase II trials, and 4 phase III trials (Table 1 and Table E1). A search of clinicaltrials.gov identified 97 phase I trials, 214 phase II trials, and 31 phase III trials that are ongoing and actively recruiting patients. Initial retrospective and prospective clinical studies suggest that combination immunotherapy and palliative radiation therapy is generally safe without site-specific increases in toxicity, such as pneumonitis or rates of immune-related adverse events (irAEs).11,12 Previous reviews have mainly focused on preclinical and translational data to make speculative hypotheses. Only recently have larger phase I and phase II/III studies with efficacy endpoints reported initial results. Here, we summarize the results of recently published prospective clinical trials to address the knowledge gap regarding the validity of initial preclinical hypotheses. These initial data can provide insights regarding the populations of patients who might benefit from combined radiation-immunotherapy approaches as well as provide guidance for clinical practice and the design of future trials, such as the sequencing of therapy and specifics of radiation targeting, dosing, and fractionation. This review summarizes the results from prospective clinical trials of combination immunotherapy and radiation therapy, focusing predominantly on efficacy endpoints and potential determinants of response.
Table 1.
Phase II and III clinical trials investigating the efficacy of combination radiotherapy with immune checkpoint inhibition.
| Study | Ref | Disease | Arms | Outcomes (experimental vs control) | |
|---|---|---|---|---|---|
| Phase II (single arm) | Formenti et al, 2018 | 30 | Metastatic NSCLC | RT with concurrent ICI | OR, 18%; OS, 7.36 mo; PFS, 3.81 mo |
| Bauml et al, 2019 | 33 | Oligometastatic NSCLC with prior LAT (surgery, chemo RT, SBRT, interventional ablation) | RT with adjuvant ICI | PFS, 18.7 mo; OS, 77.5% at 24 mo | |
| Lin et al, 2019 | 34 | Locally advanced NSCLC | ChemoRT with concurrent ICI | PFS, 13.2 mo; OS, not reached | |
| Ho et al, 2020 | 36 | Metastatic TNBC | RT with adjuvant ICI | ORR, 17.6% | |
| Voorwerk et al, 2019 | 38 | Metastatic TNBC | RT with adjuvant ICI | ORR, 8% | |
| Barroso-Sousa et al, 2020 | 35 | HR + metastatic breast cancer | RT with concurrent ICI | ORR, 0%; PFS, 1.4 mo; OS, 2.9 mo | |
| Chintakuntlawar et al, 2019 | 39 | Anaplastic thyroid cancer | ChemoRT with concurrent ICI | OS, 2.76 mo | |
| Peters et al, 2021 | 32 | Stage III NSCLC | ChemoRT with concurrent ICI | PFS, 12.7 mo; OS, 38.8 mo | |
| Phase II (randomized) | McBride et al, 2020 | 44 | Metastatic HNSCC | RT with concurrent ICI vs ICI alone | ORR, 34.5% vs 29.0% (P = .86); OS (P = .75); PFS (P = .79) |
| Theelen et al, 2019 | 42 | Metastatic NSCLC | RT with adjuvant ICI vs ICI alone | ORR, 36% vs 18% (P = .07); PFS, 6.6 vs 1.9 mo (P = .19); OS, 15.9 vs 7.6 mo (P = .16) | |
| Welsh et al, 2020 | 41 | Metastatic NSCLC | RT with concurrent ICI vs ICI alone | ORR, 22% vs 25% (P = .99); PFS, 9.1 vs 5.1 mo (P = .52) | |
| Monjazeb et al, 2021 | 45 | Metastatic microsatellite stable CRC | HFRT with concurrent ICI vs LDFRT with concurrent ICI | OS, 3.8 mo | |
| Mahmood et al, 2020 | 43 | Metastatic adenoid cystic carcinoma | RT with concurrent ICI vs ICI alone | SD, 50% vs 70% (P = .65); PFS, 4.5 vs 6.6 mo (P = .63) | |
| Rahma et al, 2021 | 46 | Locally advanced rectal cancer | Neoadjuvant chemoRT and concurrent ICI vs chemoRT | NAR, 11.53 vs 14.08 (P = .26) | |
| Phase III | Antonia et al, 2018 | 51 | Stage III NSCLC | ChemoRT with adjuvant ICI versus chemoRT with placebo | OSR, 66.3% vs 55.6% (P = .005); OS (HR, 0.68; P = .0025); PFS, 17.2 vs 5.6 mo (HR, 0.51) |
| Kwon et al, 2014 | 47 | Metastatic castration-resistant prostate cancer | RT with adjuvant ICI vs RT with placebo | OS, 11.2 vs 10 mo (HR, 0.85; P = .053); PFS, 4.0 vs 3.1 mo (HR, 0.70; P < .0001) | |
| Lee et al, 2021 | 53 | Locally advanced HNSCC | ChemoRT with concurrent and adjuvant ICI vs chemoRT with placebo | PFS, not reached (HR, 1.21; P = .92) | |
| Kelly et al, 2021 | 52 | Esophageal or GEJ cancer | Neoadjuvant chemoRT and surgery with adjuvant ICI vs neoadjuvant chemoRT and surgery with placebo | DFS, 22.4 vs 11.0 mo (HR, 0.69; P < .001) |
Abbreviations: ChemoRT = chemoradiotherapy; CRC = colorectal cancer; DFS = disease-free survival; GEJ = gastroesophageal junction; HFRT = hypofractionated radiation therapy; HNSCC = head and neck squamous cell carcinoma; HR = hazard ratio; ICI = immune checkpoint inhibitor; LAT = locally ablative therapy; LDFRT = low dose fraction radiation therapy; NAR = neoadjuvant rectal score; NSCLC = non-small cell lung cancer; OR = odds ratio; ORR = objective response rate; OS = overall survival; OSR = overall survival rate; PFS = progression free survival; RT = radiation therapy; SBRT = stereotactic body radiation therapy; SD = stable disease; TNBC = triple negative breast cancer.
Phase I Trials
The vast majority of phase I trials have confirmed that combination immunotherapy and radiation therapy is well-tolerated. Radiation therapy with concurrent atezolizumab13 or pembrolizumab14 is well tolerated in patients with metastatic non-small cell lung cancer (NSCLC). Similarly, radiation therapy with pembrolizumab,15 ipilimumab,16–18 or nivolumab15 is well tolerated in patients with metastatic melanoma. Chemoradiotherapy with pembrolizumab19 or avelumab20 is well tolerated in patients with advanced head and neck squamous cell carcinoma (HNSCC). The safety of combination immunotherapy and radiation therapy has also been demonstrated in metastatic breast cancer,21 metastatic urothelial carcinoma,22 metastatic solid tumors,23 and extensive stage small cell lung cancer.24 One phase I trial was temporarily stopped due to dose-limiting toxicities of pembrolizumab with adjuvant hypofractionated radiation therapy in metastatic bladder cancer,25 and the trial was amended to reduce the radiation therapy dose. Overall, these phase I trials provide encouraging evidence that combination immunotherapy and radiation therapy is safe without significant site-specific toxicities or serious irAEs.
Early clinical trials have also contributed to our understanding and hypotheses of the biology underlying the use of combination immunotherapy and radiation therapy. A relatively large phase I study evaluating the combination of stereotactic body radiation therapy (SBRT) delivered to 30 to 50 Gy over 5 fractions up to 7 days before pembrolizumab in patients with metastatic solid tumors resulted in progression-free survival (PFS) of 3.1 months and overall survival (OS) of 9.6 months and dose-limiting toxicities in 6 of 73 patients.26 Responsiveness of unirradiated lesions to combination therapy correlated with interferon-γ associated gene expression, while responsiveness of irradiated lesions correlated with DNASE1 expression.27 In-field radiation responses were observed even when large lesions were partially irradiated in some cases. These findings suggest that different mechanisms might underly in-field and out-of-field responses, and that radiation-related immune activation might contribute to responsiveness to combination therapy in a subset of patients.
In another phase I study, hypofractionated radiation therapy combined with adjuvant ipilimumab in patients with stage IV melanoma resulted in a PFS of 3.8 months and OS of 10.7 months.28 Partial responses in unirradiated lesions were observed in 18% of patients, and unresponsiveness in unirradiated lesions was associated with increased and not decreased programmed death-ligand 1 (PD-L1) expression. Limited systemic responses rates were also observed in another single-arm prospective study of concurrent ipilimumab with radiation therapy in patients with metastatic solid cancers of multiple types, resulting in partial responses in unirradiated lesions in 10% of patients.29 The observations of out-of-field responses suggest that abscopal responses with combination ICIs and radiation therapy might be rare, and their significance needs to be evaluated in the context of clinical benefit and biology. Ongoing preclinical and early phase clinical trials are now testing additional immunotherapy combinations and alternative approaches to radiation therapy delivery to determine whether the immune effects of radiation may be harnessed to achieve clinical benefit.
Single-Arm Phase II Trials
Single-arm phase II trials have documented responses to combination immunotherapy and radiation therapy and have furthered our understanding of the biology underlying these responses. In NSCLC, concurrent ipilimumab with radiation therapy targeting a single tumor site in patients with metastatic disease resulted in an 18% objective response rate (ORR), median PFS of 3.8 months, and median OS of 7.4 months,30 which is difficult to interpret in the absence of a ipilimumab monotherapy comparator arm. Neoantigen-specific T cell expansion and increased neoantigen expression were observed after treatment. A limited number of patients had prolonged survival compared with what would have been expected with ipilimumab monotherapy. It is possible that such a benefit of radiation in augmenting response to ICIs might be amplified in clinical settings where radiation therapy was delivered to all tumor sites.31
In nonmetastatic or limited metastatic NSCLC, such approaches have been possible with the use of external beam radiation in combination with ICIs. A study of concurrent nivolumab with chemoradiotherapy in patients with stage III NSCLC resulted in a median PFS of 12.7 months and median OS of 38.8 months.32 In another study in patients with oligometastatic NSCLC with less than or equal to 4 metastases, local ablative therapy (surgery, chemoradiation, ablation, or SBRT) to all visible lesions with adjuvant pembrolizumab started 4 to 12 months later resulted in a median PFS of 18.7 months, compared with a historical control median PFS of 6.6 months.33 Surprisingly, in contrast to the response to anti-PD-1/anti-PD-L1 alone in metastatic NSCLC, PFS in this study using combined ICI and radiation therapy was not associated with PD-L1 expression or CD8 T cell infiltration of tumor. Concurrent PD-L1 inhibition with atezolizumab with definitive chemoradiotherapy in locally advanced NSCLC resulted in a PFS of 13.2 months,34 and baseline tumor biopsy PD-L1 status was similarly not associated with recurrence. This suggests that radiation may play a role in priming an effective response, particularly for patients with tumors expressing little or no PD-L1, which do not typically respond to ICIs targeting this pathway.
Combination of ICIs with radiation therapy has also demonstrated mixed results in single-arm phase II trials in other cancer types, with limited overall response rates. Palliative radiation therapy to a dose of 20 Gy in 4 fractions started 2 to 7 days after pembrolizumab in hormone receptor positive metastatic breast cancer resulted in a PFS of 1.4 months and OS of 2.9 months, with no increase in unexpected adverse events.35 Pembrolizumab started within 3 days after the first of 5 fractions of 6 Gy in patients with metastatic triple-negative breast cancer resulted in a PFS of 2.6 months and an ORR of 17.6%,36 compared with a historic response rate of 5.3% for pembrolizumab monotherapy in a similar cohort.37 Notably, PD-L1 expression was again not associated with response rate or PFS in this study. In another study conducted in patients with metastatic triple-negative breast cancer, adjuvant nivolumab after 3 fractions of 8 Gy resulted in an ORR of 8%.38
As has been suggested in the NSCLC studies noted previously, more promising clinical outcomes may be achievable in settings where a limited overall burden of disease is irradiated, such as oligometastatic disease as suggested Bauml et al,33 and this is currently being investigated in phase II trials (NCT0482176, NCT03808337). As was observed in NSCLC, PD-L1 expression was not demonstrated to be predictive of response in these single-arm phase II studies.36 In particularly aggressive disease with high burdens of occult micrometastatic disease, such approaches may not be effective with external beam targeting only grossly visible tumor sites. For example, a small prospective study of chemoradiotherapy with concurrent pembrolizumab in locally advanced anaplastic thyroid cancer resulted in study closure after all 3 patients died within 6 months of treatment initiation.39
It is difficult to draw conclusions regarding the efficacy of combination radiation ICI approaches from these singlearm phase II trials. Correlative studies have demonstrated changes in local and systemic immunity and in some cases appear to corroborate preclinical data that suggest radiation can modulate local and systemic immunity. However, irrespective of the immunologic changes observed, systemic response rates have generally been limited. This underscores the importance of integrating detailed scientific analysis of clinical trial specimens with parallel studies in preclinical models to identify and target mechanisms of treatment resistance.
Randomized Phase II trials
Randomized phase II studies have, to date, been among the most effective in exploring the ability of radiation to improve systemic response rates to ICIs (Fig. 1). The pembrolizumab after SBRT (PEMBRO-RT) trial examined the efficacy of pembrolizumab started within 7 days after 24 Gy in 3 fractions of SBRT delivered to a single site of metastatic disease compared with pembrolizumab alone in patients with metastatic NSCLC.40 Prior radiation therapy did not exacerbate toxicity, and there was an improved overall response rate that did not achieve statistical significance (ORR, P = .07; PFS, P = .19; and OS, P = .16). Interestingly, patients with PD-L1 negative tumors had a significant improvement in PFS (P = .03) and OS (P = .046) from radiation therapy that was not observed in the PD-L1 positive group.
Fig. 1.
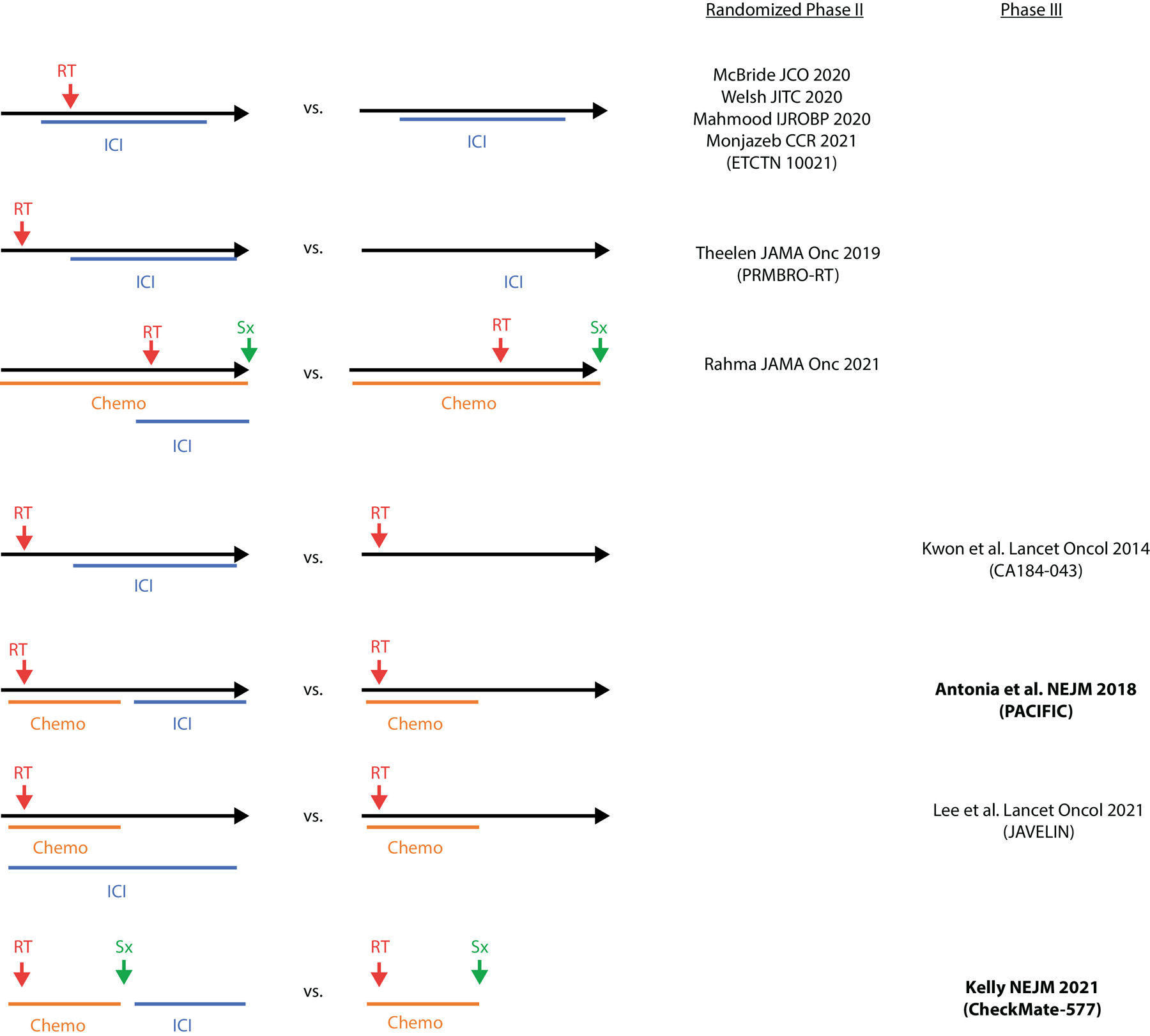
Prospective clinical trial design. Schematic illustrating different treatment arms of randomized clinical trials used to assess the safety and efficacy of combining radiation therapy (RT) with immune checkpoint inhibition (ICI) and/or surgery (Sx) and chemotherapy (Chemo). Clinical trials that adhere to a given design are referenced on the right. Bold studies indicate studies that achieved their primary endpoint.
A single institution phase II trial also failed to demonstrate a significant improvement in ORR or PFS with the addition of radiation therapy to a single site delivered in either 50 Gy in 4 fractions or 45 Gy in 15 fractions to pembrolizumab in patients with metastatic NSCLC.41 This study observed an improvement in PFS in patients with low PD-L1 expression assessed by biopsy. A pooled analysis of this study and the PEMBRO-RT trial suggested a significant improvement in PFS (hazard ratio [HR], 0.67; P = .045) and OS (HR, 0.67; P = .0004) with combination pembrolizumab and radiation therapy in patients with metastatic NSCLC42; however, this unplanned analysis of secondary endpoints from 2 distinct trials should be interpreted with caution.
Additional randomized phase II trials have also not demonstrated improved systemic response rates with combination radiation therapy and ICI in other cancer types besides NSCLC. In patients with metastatic adenoid cystic carcinoma, the addition of 30 Gy in 6 fractions of radiation therapy to pembrolizumab was well tolerated but did not result in objective responses outside of the radiation field.43 Notably, significant local responses were observed in the radiation treatment field, which is encouraging given the limited radiation dose used. Similarly, patients with metastatic HNSCC randomized to receive SBRT at 27 Gy in 3 fractions between the first 2 nivolumab doses (n = 32) versus nivolumab alone (n = 30) did not experience increased toxicity, but also did not experience improved ORR, PFS, or OS with addition of radiotherapy.44 NCI Experimental Therapeutics Clinical Trials Network 10021 was a multi-center phase II trial that evaluated the addition of different radiation therapy regimens (hypofractionated radiation [HFRT] to 24 Gy in 3 fractions or low-dose fractionated radiation of 0.5 Gy twice daily for 2 days repeated for up to 4 cycles) or no radiation in combination with the PD-L1 inhibitor durvalumab and the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab in patients with metastatic microsatellite stable colorectal cancer.45 Although no significant radiation therapy-related toxicities were observed, addition of either HFRT or low-dose fractionated radiation did not improve PFS or OS compared with no radiation.45 However, cyclic GMP−AMP and the cyclic GMP−AMP receptor stimulator of interferon genes activation, micronuclei formation, and primary nuclear rupture were observed even after low-dose radiation. This supports the ability of radiation therapy to activate the tumor immune microenvironment, particularly in the case of HFRT where Ki67 + PD-1 + (activated) CD8+ T-cells were observed more frequently.45 Here, the combination of PDL1 and CTLA-4 blockade and radiation was employed based on preclinical data suggesting potential mechanisms of benefits related to immune escape mechanisms present in patients treated with PD(L)-1 inhibitor and radiation28 as well as the potential for modulation of T-regulatory cells and initial antigen specific T-cell responses via the addition of a CTLA-4 inhibitor. In a recently published study, the addition of concurrent pembrolizumab to neoadjuvant chemoradiotherapy with capecitabine and 50.4 Gy for locally advanced rectal cancer after folinic acid, fluorouracil, and oxaliplatin chemotherapy was safe but did not improve the neoadjuvant rectal score nor pathologic and clinical complete response rates.46
In summary, phase II trial results to date have highlighted the challenges in reliably improving ICI response rates with radiation therapy. The radiation employed in these phase II trials is generally hypofractionated, not the more protracted fractionation schedules used in curative standard-of-care regimens generally employed in the phase III trials mentioned in the following sections. As in the phase III trials described in the following sections, promising results were observed in the PEMBRO-RT trial, suggesting that sequential radiation therapy followed by ICI might be effective. Greater treatment effect observed in PD-L1 low or negative patients through stratified analyses in these phase II trials suggests that PD-L1 status might be inversely correlated with responsiveness to combination ICI and radiation therapy, suggesting this as a reasonable treatment to test in patients refractory to ICI monotherapy.
Phase III Trials
In contrast to the phase II studies that largely test the ability of radiation to improve systemic response rates to ICI, phase III trials have generally evaluated the addition of immunotherapy to standard-of-care radiation approaches in attempts to address micrometastatic disease and/or improve the effectiveness of radiation and chemoradiation (Fig. 2). These studies have evaluated OS and PFS endpoints in prostate cancer, NSCLC, and esophagogastric cancer.
Fig. 2.
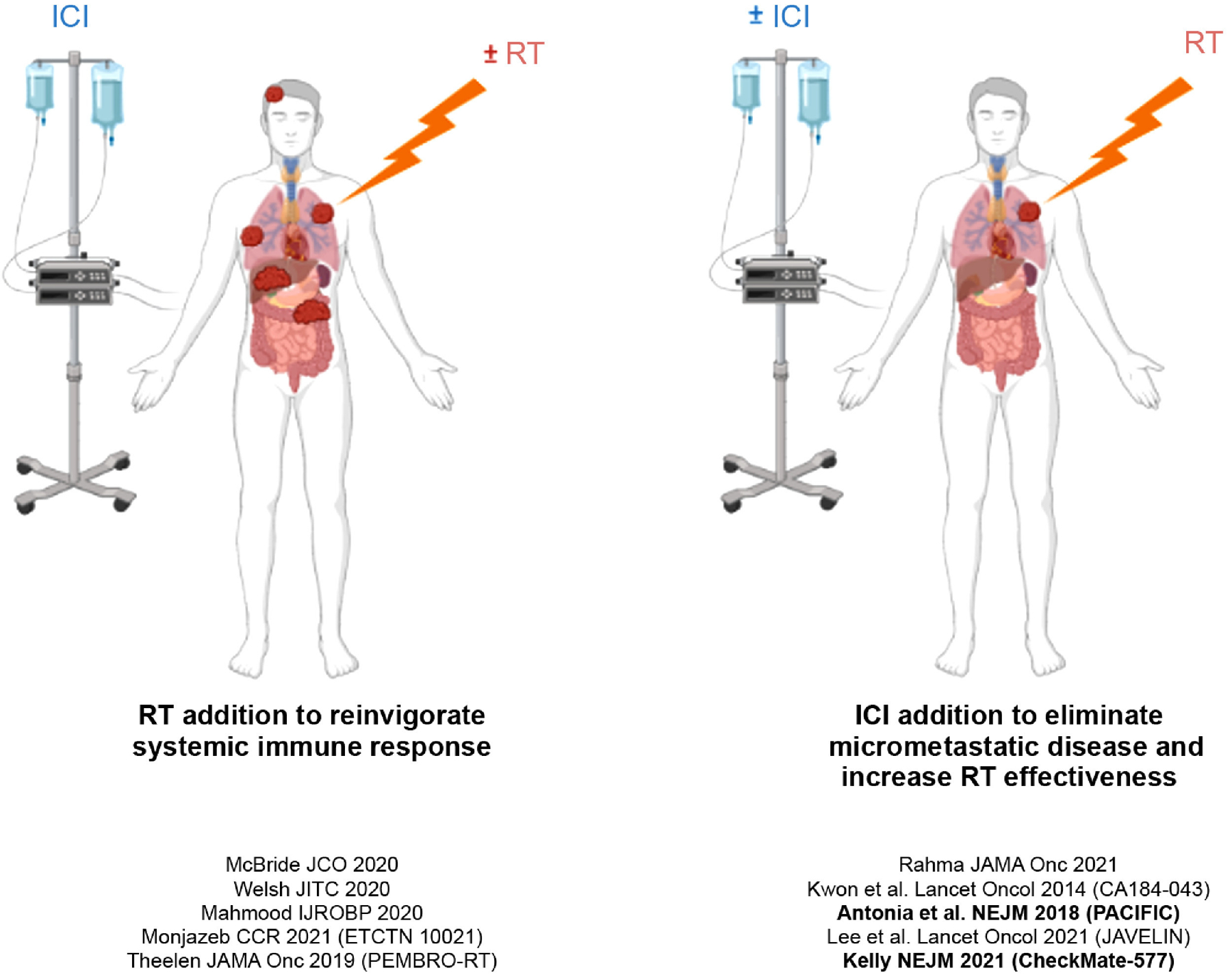
Radiation therapy (RT) and immune checkpoint inhibition (ICI) synergism. Schematic illustrating 2 potential conceptual frameworks of RT and ICI synergism. Clinical trials that adhere to a given approach are referenced. Bold studies indicate studies that achieved their primary endpoint. Created using BioRender.com.
The CA184–043 trial was the earliest phase III study that investigated combination immunotherapy and radiation therapy and enrolled patients with castrate-resistant metastatic prostate cancer and at least 1 bone metastasis.47 In this study, 799 patients were randomized to receive CTLA-4 inhibition with ipilimumab (10 mg/kg, n = 399) or placebo (n = 400) administered within 2 days after radiation therapy (8 Gy in 1 fraction) to 1 to 5 bone metastases, with a primary endpoint of OS assessed in the intention-to-treat population. The improved survival observed in the ipilimumab arm did not achieve statistical significance (HR, 0.85; P = .053) although PFS was improved (HR, 0.7; 95% confidence interval, 0.61–0.82; P < .0001). Adjuvant ipilimumab increased incidence of grade 3 to 4 irAEs (26% with ipilimumab compared with 3% for placebo), most commonly including diarrhea, fatigue, and anemia. Notably, the dose of ipilimumab used in this study (10 mg/kg) is higher than doses currently used in clinical practice. Encouragingly, long-term prespecified survival analyses demonstrated a benefit in patients receiving ipilimumab and radiation therapy, with a 2- to 3-fold increased OS at 3 years and beyond.48 In contrast, another randomized study testing treatment of patients with castrate-resistant prostate cancer with ipilimumab versus placebo without the addition of preceding radiation failed to demonstrate an improvement in OS.49
The placebo-controlled phase III PACIFIC trial investigated combination immunotherapy and radiation therapy in patients with unresectable stage III NSCLC.50,51 The trial randomized 713 patients, not selected based on PD-L1 expression, to receive placebo (n = 236) or the PD-L1 inhibitor durvalumab (10 mg/kg, n = 473) administered as consolidation therapy 1 to 42 days after definitive radiation (54–66 Gy) with at least 2 cycles of concurrent platinum-based chemotherapy (containing etoposide, vinblastine, vinorelbine, a taxane [paclitaxel or docetaxel], or pemetrexed), with primary endpoints of OS and PFS assessed according to Response Evaluation Criteria in Solid Tumors, version 1.1. Durvalumab improved both PFS and OS (HR, 0.51 and HR, 0.68, respectively), leading to U.S. Food and Drug Administration approval for durvalumab in this setting. Importantly, incidence of grade 3 or 4 pneumonitis was similar between patients treated with combination immunotherapy and radiation therapy compared with radiation therapy alone (3.6% vs 2.6%). Interestingly, in an unplanned subset analysis, benefit of durvalumab was greatest when patients were randomized <14 days after completing radiation therapy. This practice-changing study highlighted the potential of sequential immunotherapy after chemoradiation targeting all gross disease in patients with locally advanced NSCLC.
The placebo-controlled phase III CheckMate-577 trial investigated the efficacy of adjuvant nivolumab after definitive chemoradiation and surgical resection in patients with locally advanced esophagogastric carcinoma.52 This trial randomized 794 patients to receive placebo (n = 262) or nivolumab (240 mg, n = 532) administered 4 to 16 weeks after surgery with prior neoadjuvant chemoradiation (median 45 Gy and carboplatin/paclitaxel, cisplatin/fluorouracil, or fluorouracil/oxaliplatin), with a primary endpoint of disease-free survival. Adjuvant nivolumab improved disease-free survival from 11.0 months in the placebo arm to 22.4 months in the treatment arm (HR, 0.69; P < .001). Clinical benefit was observed in both PD-L1 low and high expressing tumors. Grade 3 or 4 events related to the treatment occurred in 13% of patients receiving adjuvant nivolumab and 6% of patients receiving placebo. Like the PACIFIC trial, the CheckMate-577 trial investigated the efficacy of adjuvant ICI (rather than concurrent ICI) in locally advanced (rather than metastatic) disease. Together, these 2 phase III trials provide encouraging support for sequential immunotherapy after chemoradiation and/or surgery in locally advanced disease.
In contrast to the PACIFIC and CheckMate-577 trials, other phase III immunotherapy radiation studies have failed to demonstrate benefit adding ICI to standard radiation or chemoradiation approaches. The placebo-controlled phase III JAVELIN trial showed no improvement in PFS with concurrent and maintenance (up to 1 year) PD-L1 inhibition with avelumab and chemoradiotherapy with cisplatin 100 mg/m2 delivered every 3 weeks compared with chemoradiotherapy alone in patients with previously untreated locally advanced squamous cell carcinoma of the oropharynx, hypopharynx, larynx, or oral cavity.53 Notably, this study included comprehensive elective nodal irradiation as is standard in definitive head and neck treatment, which could have detrimental immunomodulatory effects as suggested by preclinical studies54 and clinical trials that demonstrate increased metabolic activity in pathologically negative cervical lymph nodes.55
In summary, recently published phase III studies have demonstrated mixed results regarding the benefit of adding CTLA-4 or PD(L)-1 inhibition to standard-of-care radiation in the palliative or locally advanced setting in patients with metastatic castrate resistant prostate cancer, locally advanced NSCLC, esophagogastric cancer, and squamous cell head and neck cancer. In contrast to the more equivocal or negative phase II studies adding radiation to standard-of-care ICI approaches, the benefit of adding ICI after definitive chemoradiation in NSCLC or after chemoradiation and surgery in esophagogastric cancer is unequivocal. More positive results were observed with sequential administration of immune checkpoint blockade after radiation or chemoradiation in the CA184–043, PACIFIC, and Checkmate 577 studies compared with the JAVELIN HN study that used concurrent radiation and immune checkpoint inhibition. The potential effect of sequencing of therapies is particularly notable in the case of the JAVELIN HN study, as ICIs have demonstrated benefit in the metastatic setting, yet this benefit failed to translate to patients with locally advanced disease when avelumab was added concurrently to definitive chemoradiation. Enhanced benefit seen with sequential administration of radiation followed by PD(L)-1 inhibition is consistent with recently published preclinical data56; however, the use of PD-L1 inhibitor as opposed to a PD-1 inhibitor, administration of elective nodal irradiation in this trial, and patient selection may have also contributed to the failure of JAVELIN. Additional ongoing phase III studies in head and neck cancer will help clarify these questions (NCT03452137, NCT03576417, NCT03040999). Finally, the choice of study endpoint and patient selection is critical to establishing benefit in future trials given the observation that relatively few long term responding patients are responsible for the benefit observed in PACIFIC and other ICI trials.57
Future Directions: Preoperative Immunotherapy and Novel Agents
Preoperative radiation therapy/ICI combinations have demonstrated safety and encouraging efficacy in “window of opportunity” trials. In a phase I/Ib trial, 21 patients with HNSCC received 3 cycles of nivolumab with SBRT 5 weeks before surgery followed by adjuvant nivolumab. Major pathologic responses were observed in 86% of patients and only 1 grade 4 pneumonitis was observed.58 In a randomized phase II trial, 60 patients with resectable NSCLC were randomized to receive durvalumab monotherapy or durvalumab in combination with SBRT at 24 Gy in 3 fractions delivered 1 to 2 weeks before surgery. Preoperative durvalumab with radiation therapy was well tolerated and resulted in major pathologic responses in 53.3% of patients compared with 6.7% in patients who received durvalumab monotherapy, with increased responses also observed in unirradiated lymph nodes in the durvalumab/ radiation arm.59
Several notable prospective clinical studies have investigated the efficacy of radiation therapy and immunotherapy combinations using novel radiation delivery strategies or standard-of-care radiation therapy with other types of immunotherapy besides ICI. In a randomized phase II trial with crossover design, radiation therapy followed by interleukin 2 improved overall response in patients with metastatic melanoma from 35% to 54%, although there were no significant differences in PFS or OS compared with interleukin 2 monotherapy.60 In an additional randomized phase II trial, transforming growth factor beta inhibition with fresolimumab combined with focal radiation in patients with metastatic breast cancer was well tolerated and improved OS (HR, 2.73; P = .039).61 These studies suggest that other immunomodulatory agents, in addition to ICIs, might be appealing agents to combine with radiation therapy. On the other hand, based on preclinical data31,62 a growing number of clinical studies are evaluating novel approaches of delivering radiation therapy to more effectively prime and propagate systemic antitumor immunity in combination with immunotherapies. In a phase I study, combining the targeted radionuclide therapy Lutathera with nivolumab in 9 patients with neuroendocrine tumors was well tolerated and resulted in 1 partial response in a patient with extensive stage small cell lung cancer.63 Additional studies are now being advanced to test this growing class of radiotherapeutics in combination with immunotherapies.
Conclusions
Preclinical evidence from animal models and retrospective studies has led to interest in combining immunotherapy and radiation therapy for the treatment of cancer, resulting in a series of prospective clinical trials, many of which are ongoing or in development. Data from these prospective trials have demonstrated that combination immunotherapy and radiation therapy is well-tolerated across a variety of tumor types, radiation techniques, and sites irradiated. However, efficacy data have been mixed. The addition of immune checkpoint blockade with durvalumab in patients with NSCLC after treatment with chemoradiation has suggested practice-changing benefit in PFS and OS. Interestingly, clinical trials completed to date that have sequenced immunotherapy after the completion of radiation and chemoradiation have also demonstrated more favorable outcomes compared with concurrent treatment strategies. Reasons for this potential difference are unknown, but there has long been concern that larger field, fractionated radiation therapy can result in negative immunologic effects that could affect the success of combined treatment. Several phase II studies have suggested a potential benefit for radiation-ICI combination approaches in PD-L1 low or negative tumors and in patients with oligometastatic disease or undergoing preoperative treatment delivering radiation to all known tumor sites before surgery (Fig. 2). Notably, most associations between radiation-ICI combination benefit and tumor PD-L1 negativity have been observed in tumor types that have demonstrated responsiveness to ICI, such as lung cancer, but not in other tumors that are generally refractory to ICI treatment and are often PD-L1 negative, such as colorectal cancer, suggesting that the prognostic utility of PD-L1 status in the setting of radiation/immunotherapy combinations is likely tumor-type dependent. Additionally, tumor mutational burden is associated with survival and response to ICI treatment64,65 and should be evaluated as a potential predictor of response to combination radiation-ICI. The potential for enhanced local effects within the radiation treatment field are also notable in studies that used a moderate hypofractionated treatment dose or partially irradiated larger tumors. Finally, although correlative biomarker studies confirm that radiation can have local and systemic immune effects in patients, these have unfortunately so far proven insufficient or too rare to reliably generate an enhanced systemic response rate in relatively small studies of patients treated with ICIs. These data should guide current clinical practice and be used in combination with data from preclinical studies to design future trials in novel settings and with unique combinations aimed at maximizing antitumor immune responses and providing patient benefit.
Supplementary Material
Acknowledgments—
This manuscript evolved from discussions among members of the NCI’s Immunooncology Translational Network (IOTN) and the NCI Radiation and Immunotherapy Working Group.
Sources of support:
E.A.G. was supported by NIH grant T32GM007753. A.G.S. was supported by NIH grant U01DE028233-04. Z. S.M was supported by NIH grant U01CA233102.
J.D.S. reports: Research support paid to the institution: Merck, BMS, Regeneron, Debiopharm. Consulting/scientific advisory board/travel fees: Genentech, Immunitas, Debiopharm, BMS, Nanobiotix, Tilos, AstraZeneca, LEK, Catenion, ACI Clinical, Astellas, Stimit. Expert witness fees. Stock options: Immunitas. Stock: Doximity. R.W. reports stock and other ownership interests with Boost Therapeutics, Immvira LLC, Reflexion Pharmaceuticals, Coordination Pharmaceuticals Inc, Magi Therapeutics, Oncosenescence. He has served in a consulting or advisory role for Aettis Inc, Astrazeneca, Coordination Pharmaceuticals, Genus, Merck Serono S.A., Nano proteagen, NKMax America Inc, Shuttle Pharmaceuticals, Highlight Therapeutics, S.L. He has research grants with Varian and Regeneron. He has received compensation including travel, accommodations, or expense reimbursement from Astrazeneca, Boehringer Ingelheim LTD, and Merck Serono S.A. Z.S.M. reports being a member of the scientific advisory board for Archeus Technologies and Seneca Therapeutics and has received equity options for these companies. Z.S.M. is an inventor on patents or filed patents managed by the Wisconsin Alumni Research Foundation relating to the interaction of targeted radionuclide therapies and immunotherapies, nanoparticles designed to augment the antitumor immune response following radiation therapy, and the development of a brachytherapy catheter capable of delivering intratumor injectables.
Footnotes
Disclosures: A.G.S. has served in a consulting or advisory role for Roche/Genentech.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijrobp.2021.08.009.
References
- 1.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wennerberg E, Lhuillier C, Vanpouille-Box C, et al. Barriers to radiation-induced in situ tumor vaccination. Front Immunol 2017;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498–e509. [DOI] [PubMed] [Google Scholar]
- 4.Weichselbaum RR, Liang H, Deng L, Fu Y-X. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol 2017;14: 365–379. [DOI] [PubMed] [Google Scholar]
- 5.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res 2013;1:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang A, Wilhite TJ, Pike LRG, et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys 2017;98:344–351. [DOI] [PubMed] [Google Scholar]
- 12.Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol 2018;15:477–494. [DOI] [PubMed] [Google Scholar]
- 13.Qin A, Rengan R, Lee S, et al. A pilot study of atezolizumab plus hypofractionated image guided radiation therapy for the treatment of advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2020;108:170–177. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: A nonrandomized controlled trial. JAMA Oncol 2020;6:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratnayake G, Reinwald S, Shackleton M, et al. Stereotactic radiation therapy combined with immunotherapy against metastatic melanoma: Long-term results of a phase 1 clinical trial. Int J Radiat Oncol Biol Phys 2020;108:150–156. [DOI] [PubMed] [Google Scholar]
- 16.Sundahl N, De Wolf K, Kruse V, et al. Phase 1 dose escalation trial of ipilimumab and stereotactic body radiation therapy in metastatic melanoma. Int J Radiat Oncol Biol Phys 2018;100:906–915. [DOI] [PubMed] [Google Scholar]
- 17.Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 2017;99:22–30. [DOI] [PubMed] [Google Scholar]
- 18.Hiniker SM, Reddy SA, Maecker HT, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys 2016;96:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell SF, Gold KA, Gitau MM, et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: A phase IB study. J Clin Oncol 2020;38:2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbers JBW, Al-Mamgani A, Tesseslaar MET, et al. Immuno-radio-therapy with cetuximab and avelumab for advanced stage head and neck squamous cell carcinoma: Results from a phase-I trial. Radiother Oncol 2020;142:79–84. [DOI] [PubMed] [Google Scholar]
- 21.Jiang DM, Fyles A, Nguyen LT, et al. Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget 2019;10:2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundahl N, Vandekerkhove G, Decaestecker K, et al. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur Urol 2019;75:707–711. [DOI] [PubMed] [Google Scholar]
- 23.Maity A, Mick R, Huang AC, et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br J Cancer 2018;119:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma V, Cushman TR, Selek U, Tang C, Welsh JW. Safety of combined immunotherapy and thoracic radiation therapy: Analysis of 3 single-institutional phase I/II trials. Int J Radiat Oncol Biol Phys 2018;101:1141–1148. [DOI] [PubMed] [Google Scholar]
- 25.Tree AC, Jones K, Hafeez S, et al. Dose-limiting urinary toxicity with pembrolizumab combined with weekly hypofractionated radiation therapy in bladder cancer. Int J Radiat Oncol Biol Phys 2018;101:1168–1171. [DOI] [PubMed] [Google Scholar]
- 26.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke JJ, Onderdonk BE, Bhave SR, et al. Improved survival associated with local tumor response following multisite radiotherapy and pembrolizumab: Secondary analysis of a phase I trial. Clin Cancer Res 2020;26:6437–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 2017;23:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formenti SC, Rudqvist N-P, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24: 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jagodinsky JC, Morris ZS. Priming and propagating anti-tumor immunity: Focal hypofractionated radiation for in situ vaccination and systemic targeted radionuclide theranostics for immunomodulation of tumor microenvironments. Semin Radiat Oncol 2020;30:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters S, Felip E, Dafni U, et al. Progression-free and overall survival for concurrent nivolumab with standard concurrent chemoradiotherapy in locally advanced stage IIIA-B NSCLC: Results from the European Thoracic Oncology Platform NICOLAS phase II trial (European Thoracic Oncology Platform 6–14). J Thorac Oncol 2021;16:278–288. [DOI] [PubMed] [Google Scholar]
- 33.Bauml JM, Mick R, Ciunci C, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: A phase 2 trial. JAMA Oncol 2019;5:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin SH, Lin Y, Yao L, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol 2020;15:248–257. [DOI] [PubMed] [Google Scholar]
- 35.Barroso-Sousa R, Krop IE, Trippa L, et al. A phase II study of pembrolizumab in combination with palliative radiotherapy for hormone receptor-positive metastatic breast cancer. Clin Breast Cancer 2020;20:238–245. [DOI] [PubMed] [Google Scholar]
- 36.Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020;126:850–860. [DOI] [PubMed] [Google Scholar]
- 37.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:397–404. [DOI] [PubMed] [Google Scholar]
- 38.Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat Med 2019;25:920–928. [DOI] [PubMed] [Google Scholar]
- 39.Chintakuntlawar AV, Yin J, Foote RL, et al. A phase 2 study of pembrolizumab combined with chemoradiotherapy as initial treatment for anaplastic thyroid cancer. Thyroid 2019;29:1615–1622. [DOI] [PubMed] [Google Scholar]
- 40.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019;5:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J Immunother Cancer 2020;8: e001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467–475. [DOI] [PubMed] [Google Scholar]
- 43.Mahmood U, Bang A, Chen Y-H, et al. A randomized phase 2 study of pembrolizumab with or without radiation in patients with recurrent or metastatic adenoid cystic carcinoma. Int J Radiat Oncol Biol Phys 2021;109:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride S, Sherman E, Tsai CJ, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol 2020;39:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monjazeb AM, Giobbie-Hurder A, Lako A, et al. A randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation for patients with metastatic colorectal cancer. Clin Cancer Res 2021;27:2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahma OE, Yothers G, Hong TS, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: Initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol 2021;7:1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fizazi K, Drake CG, Beer TM, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol 2020;78:822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase iii trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 2016;35: 40–47. [DOI] [PubMed] [Google Scholar]
- 50.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 51.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non−small-cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 52.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021;384:1191–1203. [DOI] [PubMed] [Google Scholar]
- 53.Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 2021;22:450–462. [DOI] [PubMed] [Google Scholar]
- 54.Marciscano AE, Nirschl TR, Francica BJ, et al. Does prophylactic nodal irradiation inhibit potential synergy between radiation therapy and immunotherapy? Int J Radiat Oncol Biol Phys 2016;96: S88. [Google Scholar]
- 55.Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: A phase 2 open-label randomized clinical trial. JAMA Oncol 2020;6:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei J, Montalvo-Ortiz W, Yu L, et al. Sequence of aPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol 2021;6:eabg0117. [DOI] [PubMed] [Google Scholar]
- 57.Bomze D, Asher N, Hasan Ali O, et al. Survival-inferred fragility index of phase 3 clinical trials evaluating immune checkpoint inhibitors. JAMA Netw Open 2020;3:e2017675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leidner R, Crittenden M, Young K, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer 2021;9: e002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol 2021;22:824–835. [DOI] [PubMed] [Google Scholar]
- 60.Curti B, Crittenden M, Seung SK, et al. Randomized phase II study of stereotactic body radiotherapy and interleukin-2 versus interleukin-2 in patients with metastatic melanoma. J Immunother Cancer 2020;8: e000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Formenti SC, Lee P, Adams S, et al. Focal irradiation and systemic TGFb blockade in metastatic breast cancer. Clin Cancer Res 2018;24:2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy 2019;18:240. [DOI] [PubMed] [Google Scholar]
- 63.Kim C, Liu SV, Subramaniam DS, et al. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer 2020;8:e000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


