Abstract
Background
JYNNEOS™ vaccine has been used as post-exposure prophylaxis (PEP) during a mpox outbreak in New York City (NYC). Data on effectiveness are limited.
Methods
Effectiveness of a single dose of JYNNEOS™ vaccine administered subcutaneously ≤14 days as PEP for preventing mpox disease was assessed among individuals exposed to case-patients from May 22, 2022–August 24, 2022. Individuals were evaluated for mpox through 21 days post-exposure. An observational study was conducted emulating a sequence of nested “target” randomized trials starting each day after exposure. Results were adjusted for exposure risk and race/ethnicity. Analyses were conducted separately based on last (PEPL) and first (PEPF) exposure date. We evaluated the potential to overestimate PEP effectiveness when using conventional analytic methods due to exposed individuals developing illness before they can obtain PEP (immortal time bias) compared to the target trial.
Results
Median time from last exposure to symptom onset (incubation period) among cases that did not receive PEPL was 7 days (range 1—16). Time to PEPL receipt was 7 days (range 0—14). Among 549 individuals, adjusted PEPL and PEPF effectiveness was 19% (95% Confidence Interval [CI], −54% to 57%) and −7% (95% CI, −144% to 53%) using the target trial emulation, respectively, and 78% (95% CI, 50% to 91%) and 73% (95% CI, 31% to 91%) using conventional analysis.
Conclusions
Determining PEP effectiveness using real-world data during an outbreak is challenging. Time to PEP in NYC coupled with the observed incubation period resulted in overestimated PEP effectiveness using a conventional method. The target trial emulation, while yielding wide confidence intervals due to small sample size, avoided immortal time bias. While results from these evaluations cannot be used as reliable estimates of PEP effectiveness, we present important methodologic considerations for future evaluations.
Background
Ongoing transmission of mpox disease is occurring in the United States (U.S.), with the initial case detected on May 17, 2022. As of July 5, 2023, there have been >30,000 cases of mpox disease in the U.S., with >3,800 case-patients in New York City (NYC).1,2 JYNNEOS™ (Modified Vaccinia Ankara-Bavarian Nordic vaccine (MVA-BN)), a live, non-replicating Vaccinia virus vaccine, was licensed by the U.S. Food and Drug Administration (FDA) in 2019 as a 2-dose series (4 weeks apart) for the prevention of mpox and smallpox disease and has been used in the U.S. for the mpox outbreak response since 2022.3 The standard route of administration of JYNNEOS™ approved by the FDA was via the subcutaneous route (0.5mL dosage). On August 9, 2022, intradermal administration (0.1mL dosage) of JYNNEOS™ received Emergency Use Authorization as an alternate route.3 The U.S. Centers for Disease Control and Prevention (CDC) recommends that unvaccinated people exposed to mpox be vaccinated against mpox within 4 days after exposure for the greatest likelihood of preventing disease, though there may still be benefit to vaccination ≤14 days of exposure.4,5 Licensure of JYNNEOS™ was supported by animal studies and immunogenicity studies.6–10 Data on real-world vaccine effectiveness of post-exposure prophylaxis (PEP) against mpox disease are limited.11–13 We aimed to evaluate the effectiveness of JYNNEOS™ vaccine administered subcutaneously as PEP among a cohort of individuals with known exposures to mpox case-patients using surveillance data collected as part of case investigations conducted during the mpox outbreak in NYC. However, given an unanticipatedly short incubation period and challenges in administering PEP quickly during an outbreak, we were concerned about the potential for immortal time bias, which would occur if exposed individuals develop illness before they can obtain PEP.14 Motivated by recent work on emulating trials of PEP from observational data, we evaluated PEP effectiveness using a target trial approach, which avoids immortal time bias. To demonstrate the potential for overestimation of PEP effectiveness using conventional approaches, we compare results from a conventional method to this alternative analytic method.
Methods
Routine surveillance
The New York City (NYC) Department of Health and Mental Hygiene (DOHMH) receives Orthopoxvirus and mpox virus Polymerase Chain Reaction (PCR) diagnostic test results reported through the New York State (NYS) Electronic Clinical Laboratory Reporting System (ECLRS) and reports of suspected cases of mpox disease from medical providers. Individuals with confirmed and probable mpox had DNA detected by PCR for mpox virus or Orthopoxvirus, respectively.15 As per routine DOHMH protocols, persons with confirmed and probable mpox disease16 were interviewed by DOHMH staff and asked about contacts who were exposed during their infectious period. Because the infectious period for mpox disease can last for many days (i.e., until all lesions have scabbed over and have fallen off, and a fresh layer of healthy tissue is visible), exposures to other people can occur over multiple days. For individuals exposed to an individual with mpox disease, dates of last and first exposure were documented in DOHMH’s surveillance database. All individuals named by a case-patient who met criteria for having had a high-risk exposure, and most with an intermediate-risk exposure, defined according to the CDC Interim Community Exposure Risk Assessment and Recommendations16* were recommended to receive a 2-dose JYNNEOS™ subcutaneous vaccine series as PEP if first vaccine dose could be administered ≤14 days after their last exposure. Vaccination providers in NYC are required to report all immunization doses administered to children ages ≤18 years to the NYC Citywide Immunization Registry (CIR); prior to July 29, 2022, reporting of JYNNEOS™ doses to individuals aged ≥19 years required consent of the vaccine recipient.17 From July 29, 2022 to October 27, 2022, a NYS Executive Order was in place, which suspended the requirement for obtaining consent and mandated reporting of mpox immunizations administered to individuals of all ages to the CIR. During the period of this evaluation, individuals were not charged for the cost of vaccination. DOHMH performed daily monitoring by phone or text message of exposed individuals with high or intermediate risk exposures to ask if they developed symptoms of mpox disease during the 21 days following their last exposure.
Study population
Individuals aged ≥18 years who resided in NYC, and met criteria for having had a high-risk or intermediate-risk exposure to a person with confirmed or probable mpox disease*16 from May 22, 2022–August 24, 2022, were included in the evaluation if they had no mpox disease and no JYNNEOS™ vaccination prior to the first documented exposure (Figure 1). Immunization status was ascertained through immunization records received from vaccination referral sites and through a match to the CIR. Vaccinations were not assessed through self-report. Age group, sex at birth, gender identity, race, and ethnicity were obtained from routinely collected surveillance data, including interviews with exposed individuals, ECLRS laboratory reports, and CIR. If race, ethnicity, or gender identity differed by reporting source, data from interviews were used; otherwise, data from ECLRS and CIR were used.
Figure 1.
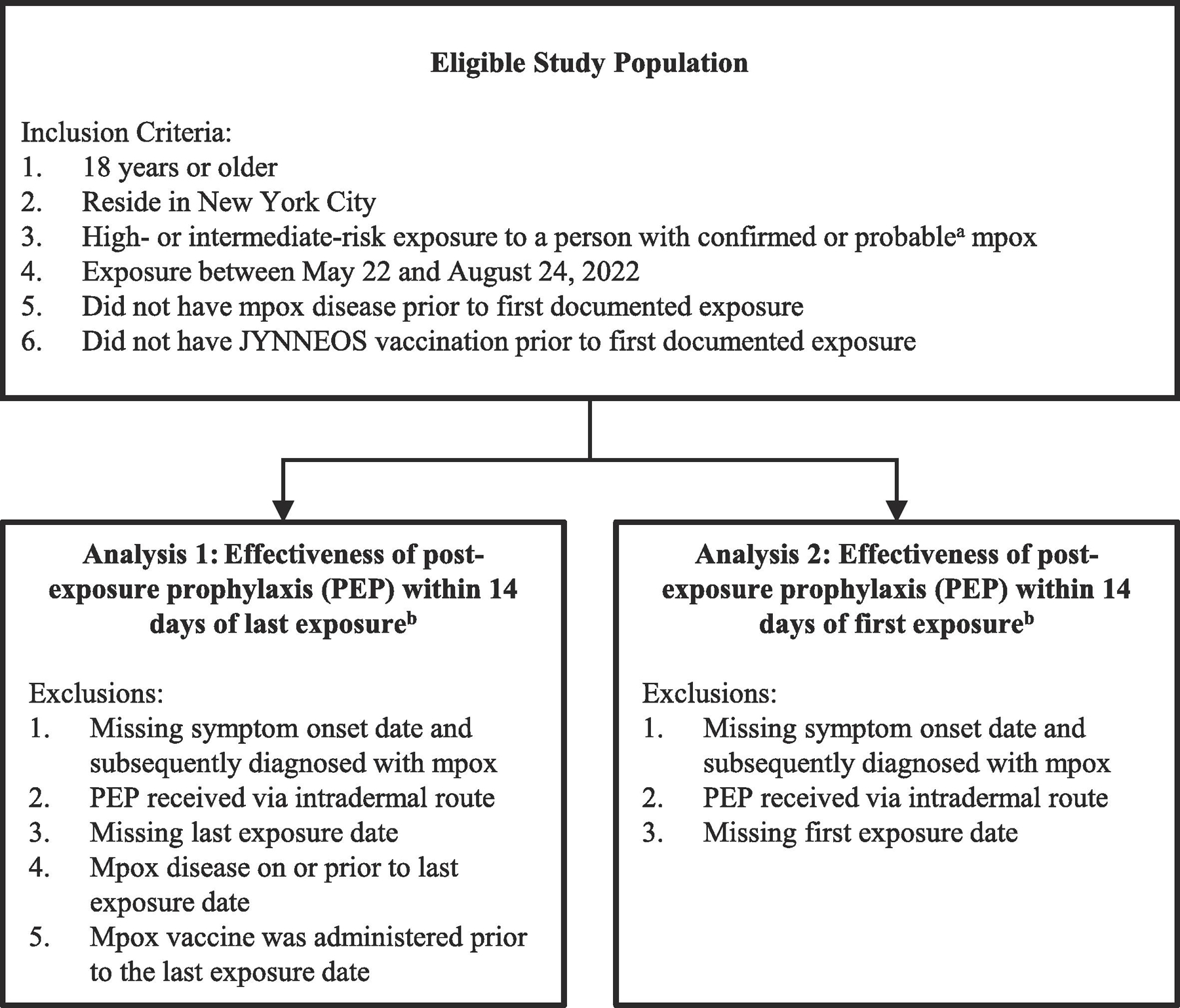
Eligibility criteria and exclusion criteria for evaluation of effectiveness of one dose of JYNNEOS™ vaccine as post-exposure prophylaxis (PEP) for mpox
a Centers for Disease Control and Prevention. Case Definitions for Use in the 2022 Mpox Response. 2022. (Accessed October 15, 2022, at www.cdc.gov/poxvirus/mpox/clinicians/case-definition.html#:~:text=and%20specimen%20collection.-,Confirmed%20Case,culture%20from%20a%20clinical%20specimen.)
b Analyses 1 and 2 were conducted independently and are not mutually exclusive.
Study design
Analyses of vaccine effectiveness were conducted separately for JYNNEOS™ vaccine administered as PEP ≤14 days after the last exposure (PEPL) and for PEP administered ≤14 days after the first exposure (PEPF). Exposed individuals in the study population were evaluated for mpox disease (“case-patients”), the outcome of interest, through 21 days after exposure (defined as the 21-day reference period, corresponding to the incubation period); for analyses of PEPL, the reference period was day 0 through 21 after last exposure and for analyses of PEPF, the reference period was day 0 through day 21 after first exposure date. Mpox disease was based on a PCR result positive for mpox virus (confirmed case) or Orthopoxvirus (probable case) with symptom onset date within the 21-day reference period. For the purposes of PEP, second doses administered ≥28 days after first doses would not prevent disease from exposures that occurred prior to first doses; because our analysis was restricted to case-patients occurring within the 21-day incubation period following exposure, second doses were not included in this analysis. Individuals were excluded from both PEPL and PEPF analyses if they were missing symptom onset date (for individuals who were subsequently diagnosed with mpox disease) or if PEP was given via the intradermal route; see Figure 1. Individuals were excluded from the PEPL analysis if (1) they were missing last exposure date; (2) mpox disease was on or prior to last exposure date; or (3) JYNNEOS™ mpox vaccine was administered prior to the last exposure date. Individuals were excluded from the PEPF analysis if (1) they were missing first exposure date. Because the 21-day reference period differs for PEPL and PEPF analyses (for individuals exposed on >1 date) and because individuals may only have had a last or first exposure date documented, some individuals were included in the analysis of either PEPL or PEPF but not both, and their vaccination or disease classification may have differed for first versus last exposure analyses.
Time from exposure to receipt of PEP among individuals who received PEP was determined separately for individuals included in the PEPL and PEPF analyses. Time from exposure to symptom onset (incubation period) among individuals who developed mpox and had not received PEP was determined separately for individuals included in the PEPL and PEPF analyses.
Analysis
To illustrate the challenges of analyzing real world data from postexposure studies, two different methods were used to estimate of vaccine effectiveness. Method 1, a target trial emulation, was conducted to account for the possibility of immortal time bias by emulating a sequence of daily trials postexposure in which there is clear alignment of eligibility, assignment to a vaccination strategy, and time zero (Figure 2). By contrast, Method 2 was a more conventional approach, in which the potential for immortal time bias is not accounted for. It is included for illustrative purposes and to highlight how immortal time bias can impact results of vaccine effectiveness.
Figure 2*.
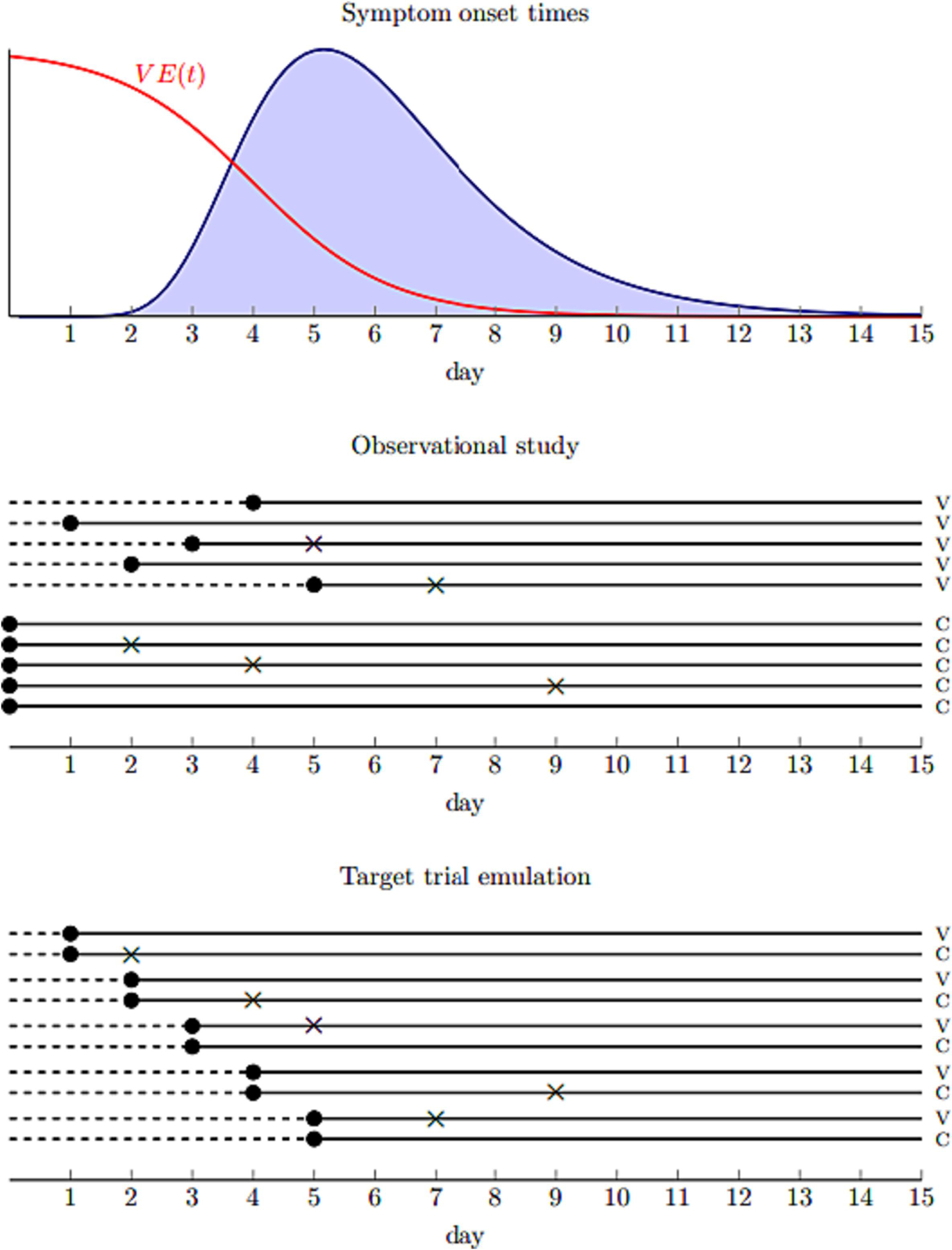
Illustration of the challenges of evaluating postexposure vaccination using observational data. The top panel shows the distribution of symptom onset times among cases as well as vaccine efficacy as a function of postexposure day of administration for a hypothetical pathogen. The middle panel shows an observational study with 5 vaccinated (V) and 5 unvaccinated (C) individuals in which there are delays in receiving vaccines. Dots show the time exposure status is first defined and Xs show symptom onset. The dashed line represents possible immortal time among vaccinated who have to survive symptom free long enough to be vaccinated. The bottom panel shows a nested sequence of daily trials among the same individuals in which there is no immortal time bias because the timing of enrollment and exposure assignment coincides in each trial.
*Figure and accompanying text copied with permission from authors from: Boyer C, Lipsitch M. Defining and emulating target trials of the effects of postexposure vaccination using observational data. medrxiv 2023.
Target trial emulation method
In Table S3 of the Supplement, we specify the protocol for the target trial that we sought to emulate using the observational data. We emulated a sequence of 15 nested trials starting each day after exposure from day 0 through day 14. Individuals were eligible for a given trial if (1) they had not received JYNNEOS™ vaccine prior to the start day of the trial and (2) they had not developed mpox on or prior to the start day of the trial. Eligible individuals were assigned to the vaccinated group if they received vaccine on the start day of each trial and assigned to the unvaccinated group if they had not received a vaccine on or prior to that date. In each trial, individuals were then followed until they developed mpox disease or reached day 21 post-exposure, whichever occurred first. A per-protocol analysis was used in which we censored (removed from contributing further person-time in each trial) individuals in the unvaccinated group who later received the vaccine on the day of vaccination and adjusted for possible selection bias due to censoring using inverse probability of censoring weights. JYNNEOS™ PEP effectiveness in each trial was estimated using pooled logistic regression across all trials for post-exposure time. Vaccine effectiveness was calculated as [(1 – Odds Ratio) × 100%] where the odds ratio from a discrete-time pooled logistic regression approximates the hazard ratio. Models included covariates for exposure risk (high or intermediate) and race/ethnicity. To account for the fact that individuals contribute person-time in multiple trials in the pooled dataset, we used cluster-robust variance estimator to calculate 95% CIs.
Conventional method
Individuals in the retrospective cohort were considered to have received PEP if they received a first dose of JYNNEOS™ vaccine anytime ≤14 days of exposure and prior to date of symptom onset, if applicable. Individuals who were vaccinated on days 15–21 after exposure (i.e., beyond the window for PEP) were considered to have not received PEP. Individuals were considered “case-patients” if they met the case criteria defined above with onset anytime ≤21 days of exposure. Multivariable logistic regression was performed adjusting for exposure risk and race/ethnicity. JYNNEOS™ PEP effectiveness was calculated as [(1 – Odds Ratio) × 100%], where the odds ratio represents the odds of mpox disease among exposed individuals who received PEP compared with those who did not receive PEP, controlling for exposure risk and race/ethnicity; effectiveness therefore represents the proportionate reduction in mpox disease among individuals who received PEP relative to those who did not. The 95% confidence intervals (CIs) were constructed around the odds ratio and converted using the effectiveness formula; exact intervals were calculated to account for small cell sizes.15
Sensitivity analyses
Because some people with symptoms of mpox disease may not have sought diagnostic testing, a sensitivity analysis was conducted to also count individuals with suspected mpox15 as “case-patients” (subject to the same inclusion and exclusion criteria as listed above). Suspected cases were counted as case-patients if they had reported symptom onset during the 21-day reference period to DOHMH but either laboratory testing was not performed (based on the absence of a negative or positive test result reported to ECLRS/DOHMH) or results of testing were inconclusive. Symptomatic persons who had negative test results were not counted as case-patients.
For the conventional method, a separate sensitivity analysis was also conducted in which individuals who were vaccinated on days 15–21 after exposure were counted as having received PEP.
The protocol was reviewed by the NYC Department of Health and Mental Hygiene Institutional Review Board and was determined to be public health surveillance, non-research. This activity was reviewed by CDC and was conducted following applicable federal law and CDC policy (45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.)
Analyses were conducted using SAS version 9.4.
Results
Among 594 individuals included in the analysis of PEPL effectiveness, median age was 35 years (range 18 to 87 years) with similar distribution of age groups between those who did and did not receive PEPL (Table 1). Differences in demographic distribution included a greater proportion of people who were non-Hispanic White (43.5%) and a smaller proportion who were non-Hispanic Black (14.7%), among individuals who received PEPL compared with those who did not receive PEPL (26.4% and 28.0%, for non-Hispanic White and Black persons, respectively). Among those receiving PEPL, a greater proportion reported male sex at birth (75.4%) and male gender (80.8%) compared with those who did not receive PEPL (male sex 66.3%, male gender 70.9%, female sex 21.1%, and female gender 19.5%). Race/ethnicity, sex, and gender were more frequently unknown or listed as “other” among individuals who did not receive PEPL. Demographic characteristics for individuals included in the analysis of PEPF are listed in Supplement Table S1.
Table 1.
Demographic characteristics of individuals exposed to persons with mpox included in the target trial evaluation of post-exposure prophylaxis (PEP) within 14 days of last exposure — New York City, May 22, 2022–August 24, 2022
| Characteristic | All individuals (N=594) |
Received PEP (N=333) |
Did not receive PEP (N=261) |
|||
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Median | 35 | 35 | 35 | |||
| Range | 18–87 | 19–76 | 18–87 | |||
| Age group (years) — no. (%) | ||||||
| 18–44 | 451 | 75.9% | 248 | 74.5% | 203 | 77.8% |
| 45–64 | 117 | 19.7% | 73 | 21.9% | 44 | 16.9% |
| 65 and older | 26 | 4.4% | 12 | 3.6% | 14 | 5.4% |
| Race/Ethnicity — no. (%)* | ||||||
| White, non-Hispanic | 214 | 36.0% | 145 | 43.5% | 69 | 26.4% |
| Black, non-Hispanic | 122 | 20.5% | 49 | 14.7% | 73 | 28.0% |
| Asian, non-Hispanic | 38 | 6.4% | 26 | 7.8% | 12 | 4.6% |
| Multiple races, non-Hispanic | 5 | 0.8% | 3 | 0.9% | 2 | 0.8% |
| Hispanic | 178 | 30.0% | 101 | 30.3% | 77 | 29.5% |
| Other or Unknown | 37 | 6.2% | 9 | 2.7% | 28 | 10.7% |
| Sex — no. (%) | ||||||
| Male | 424 | 71.4% | 251 | 75.4% | 173 | 66.3% |
| Female | 103 | 17.3% | 48 | 14.4% | 55 | 21.1% |
| Other or Unknown | 67 | 11.3% | 34 | 10.2% | 33 | 12.6% |
| Gender — no. (%) | ||||||
| Men | 454 | 76.4% | 269 | 80.8% | 185 | 70.9% |
| Women | 101 | 17.0% | 50 | 15.0% | 51 | 19.5% |
| Transgender/ Gender Non-Conforming/ Non-Binary | 23 | 3.9% | 14 | 4.2% | 9 | 3.4% |
| Unknown | 16 | 2.7% | 0 | 0.0% | 16 | 6.1% |
No individuals reported their race/ethnicity as non-Hispanic Native Hawaiian/Pacific Islander, Native American, or Alaska Native
A total of 47 individuals were excluded from the analysis of PEPL (Table 2). Most exclusions were due to receipt of a first dose of JYNNEOS™ vaccine prior to their last exposure date (n=31). For the analysis of PEPF, 170 individuals were excluded, with almost all (168) due to missing date of first exposure (Supplement Table S2).
Table 2.
Reasons for exclusion from evaluation of mpox post-exposure prophylaxis (PEP) within 14 days of last exposure — New York City, May 22, 2022–August 24, 2022
| Reason for exclusion* | No. |
|---|---|
| Developed mpox disease and missing symptom onset date | 2 |
| Developed mpox disease with onset date on or prior to last exposure date** | 14 |
| Vaccinated prior to last exposure date | 31 |
| Route of administration was intradermal | 2 |
A total of 641 individuals met inclusion criteria; of those, 47 individuals were excluded with 49 reasons for exclusion (exclusion categories are not mutually exclusive).
Onset occurred prior to last exposure date but after first exposure date so included in the analysis of first exposure date
Of the 471 individuals included in the analysis of PEPF effectiveness, 183 received PEPF and 288 did not (Table 3).
Table 3.
Results of effectiveness of one dose of JYNNEOS™ vaccine as post-exposure prophylaxis (PEP) for mpox within 14 days after last and first exposure by level of exposure risk— New York City, May 22, 2022–August 24, 2022 using Method 1 (multivariable regression) and Method 2 (target trial)
| PEP timeframe | All individuals | Received PEP | Did Not Receive PEP | PEP effectiveness* (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|---|
| Developed mpox | Did not develop mpox | Developed mpox | Did not develop mpox | Method 1 (Multivariable regression) |
Method 2 (Target trial) |
||
| 0 to 14 days after last exposure (PEPL) | 594 | 10 | 323 | 29 | 232 | 78% (50%, 91%) |
19% (−54%, 57%) |
| 0 to 14 days after first exposure (PEPF) | 471 | 6 | 177 | 29 | 259 | 73% (31%, 91%) |
−7% (−144%, 53%) |
Results adjusted for exposure risk category (high vs. intermediate) and race/ethnicity.
Centers for Disease Control and Prevention. Monitoring Persons Exposed. 2022. www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
Target trial emulation
Based on results adjusted for exposure risk and race/ethnicity, estimated PEPL effectiveness was 19% (95% CI, −54% to 57%) and estimated PEPF effectiveness was −7% (95% CI, −144% to 53%) (Table 3). Unadjusted analyses yielded similar results for both PEPL and PEPF.
In the sensitivity analysis in which individuals with suspected mpox disease who had no laboratory testing or inconclusive test results for mpox were counted as “case-patients” based on symptom onset within the 21-day reference (incubation) period, the resulting estimated PEPL effectiveness was similar to that in the primary PEPL analysis, at 13% (95% CI, −47% to 49%).
Time to PEP and symptom onset
Of the 594 individuals included in analysis of PEPL effectiveness, 333 received PEPL and 261 did not. The median time from last exposure to symptom onset (incubation period) among exposed individuals who did not receive PEP and became subsequent case-patients (n=29) was 7 days (range 1 to 16) (Figure 3). The median time to receipt of PEP among PEPL recipients (n=333) was 7 days (range 0 to 14) (Figure 3). Results for these analyses based on PEPF are presented in Figure S1. Because the median time to symptom onset and the time to receipt of PEP overlap, the risk of immortal time bias with a conventional method was high.
Figure 3.
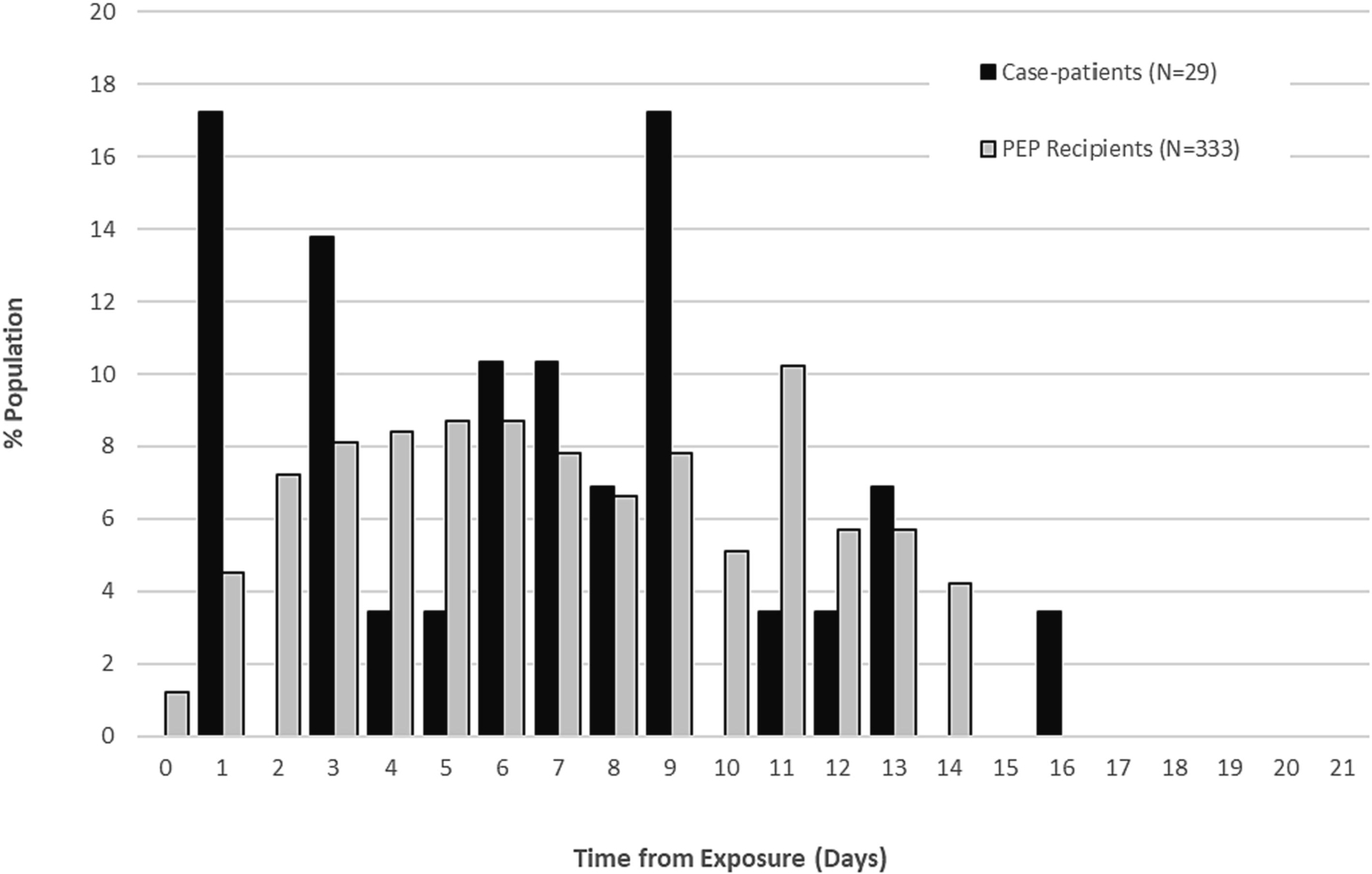
Distribution of time from date of last exposure to receipt of post-exposure prophylaxis (PEP) among individuals who received PEP and to date of symptom onset among individuals who developed mpox disease but did not receive PEP*
*Includes individuals included in the analysis of PEP effectiveness following date of last exposure (PEPL)
Cases excludes individuals that received PEPL.
The denominator for “% Population” is 29 for case-patients and 333 for PEP recipients.
Conventional method
Based on multivariable regression adjusted for exposure risk and race/ethnicity (Table 3), PEPL effectiveness was 78% (95% CI, 50% to 91%) and PEPF effectiveness was 73% (95% CI, 31% to 91%) (Table 3). Unadjusted analyses yielded similar results for both PEPL and PEPF.
In the sensitivity analysis in which individuals with suspected mpox disease who had no laboratory testing or inconclusive test results for mpox were counted as “case-patients” based on symptom onset within the 21-day reference (incubation) period, the resulting PEPL effectiveness was 81% (95% CI, 62% to 91%). In the sensitivity analysis considering individuals who were vaccinated on days 15–21 after exposure as having received PEP, the resulting PEPL effectiveness was 85% (95% CI, 65% to 94%).
Discussion
Despite the recommendation for use of JYNNEOS™ as PEP during the mpox outbreak, data on the effectiveness of PEP against mpox are lacking. Unfortunately, results from these analyses cannot be used as reliable estimates of PEP effectiveness. Several challenges exist to the analysis of PEP effectiveness using real-world data during an outbreak. PEP in NYC was often administered many days after last exposure (7-day median); contributing factors include potential delays in seeking care, laboratory confirmation of case-patients, identification and notification of exposed individuals, and time to presentation of exposed individuals for vaccination. We show that with an incubation period similar to the time to receipt of PEP, the risk of immortal time bias is high and PEP effectiveness is overestimated using conventional methods. This form of reverse causality, whereby the outcome (disease) precludes the exposure (vaccination), would result in overestimation of effectiveness, as the validity of the effectiveness estimate depends on the assumption that vaccination reduces the risk of disease, rather than development of the disease reducing the chance of the individual being eligible for vaccination. To appropriately account for this issue, a target trial approach was conducted in which a randomized controlled trial was emulated using observational data. This approach allowed us to account for time that individuals spent being unvaccinated (i.e., prior to any vaccination) equally for individuals who developed mpox disease and those who did not, to avoid inflated estimates due to immortal time bias. However, because infections often occurred before PEP was administered, there was inadequate power even to detect a high estimate of vaccine effectiveness, which explains the extremely wide confidence intervals and the inability to draw conclusions about vaccine effectiveness from this data.
Another published manuscript aimed to evaluate PEP effectiveness among individuals with known contact to individuals with mpox disease who received PEP ≤14 days compared with those who did not receive PEP.13 In this evaluation by Morales et. al., PEP effectiveness was 88.8% (95% CI, 76.0% to 94.7%), similar to the results we obtained using conventional methodology. As acknowledged by the authors, there is the potential for bias if contacts with early onset of mpox disease did not receive PEP, although it was not clear if immortal time bias was adequately controlled for.18 In an evaluation by C.E. van Ewijk et. al., high-risk individuals exposed to mpox were monitored for infection and PEP receipt; authors reported similar challenges with measuring PEP vaccine effectiveness as half of the high-risk contacts included developed symptoms prior to the opportunity to have received PEP.19
The CDC recommends that vaccination be administered within 4 days of exposure, and that subsequent vaccination through 14 days after exposure might be less effective.20 While PEP administered as soon as possible after the earliest exposure is ideal, there is no established guidance on whether PEP should be restricted to the interval after the first or last exposure. Given potential delays in seeking care, laboratory confirmation of case-patients, identification and notifications of exposed individuals, and presentation of exposed individuals for vaccination, PEP in NYC was most often administered >4 days after last exposure; as a result, we were unable to determine effectiveness of PEP within 4 days after exposure with any degree of precision. Because NYC DOHMH PEP referrals were based on last exposure date, DOHMH staff documented the last exposure date more completely than the first exposure date in the DOHMH surveillance database, regardless of whether there was one or more than one date of exposure; as a result, many people included in analyses of effectiveness of PEPL were not included in analyses of PEPF. However, among individuals with both a first and last exposure date documented who were included in both PEPL and PEPF analyses, 44% had a single date of exposure and the first and last exposures occurred a median of 1 day apart (range 0 to 32 days).
Target trial emulation approaches would be important methods for consideration of future PEP effectiveness studies to address immortal time bias from conventional methods. Pooling of data across multiple jurisdictions to have sufficient sample size might be helpful for overcoming the realities of delayed PEP. Standardized protocols and data collection should be developed in advance of a future surge of mpox to consider using pooled data for these analyses and should be a consideration as part of planning before the next increase in mpox and for future vaccine-preventable disease outbreaks. The analyses presented may be useful for planning future randomized evaluations of PEP or observational emulations of them for mpox or other infections. The 7-day median incubation period which was observed may also have implications for future PEP administration guidelines.
This evaluation was subject to other limitations. Attempts to evaluate effectiveness of PEP via the intradermal route were not conducted because the subcutaneous route was the approved method for most of the timeframe in this evaluation and continues to be an option for use. Information on exposure dates reported by case-patients and exposed individuals may not have been exact due to limited recall, particularly if exposures occurred over a prolonged period. Further, NYC DOHMH staff could not confirm the validity of self-reported information regarding exposed individuals and the nature of their exposure. Not all exposed individuals were reached for follow-up to ascertain symptoms after exposure; while DOHMH received all positive test results, it is possible that symptomatic individuals may not have sought testing, so they would have been misclassified as non-case-patients. It is possible that individuals who did not receive PEP were less likely to have access to or to have pursued diagnostic testing compared with those who received PEP, which could result in an underestimate of the vaccine effectiveness. However, in the sensitivity analysis accounting for individuals who did not have testing performed but reported symptoms to NYC DOHMH, PEP estimates were similar to those of the primary analysis. There were differences in the distribution of race/ethnicity between exposed individuals who received PEP and those who did not receive PEP, which was similar to what has been seen among individuals who received JYNNEOS™ as expanded post-exposure prophylaxis (PEP++) in NYC and nationally.1,21 Another limitation was a lack of data on underlying medical conditions for individuals included in the evaluation, including immunocompromising conditions or treatments that have the potential to reduce immunogenicity for vaccinations.22 Further, although JYNNEOS™ vaccine is recommended for mpox PEP regardless of receipt of previous smallpox vaccine (which had been routinely recommended until the 1970s), it is possible that previous smallpox vaccine could have conferred some protection against mpox23; however, in a univariate analysis, age (<50 years and ≥50 years) was not significantly associated with mpox disease. In addition, we did not evaluate mpox disease severity, which might differ among people with mpox disease who did and did not receive PEP.
At the start of the outbreak, given limited national supply of JYNNEOS™, vaccination was reserved for individuals with known high- and intermediate-risk exposures to mpox case-patients. As both vaccine supply and mpox disease incidence increased, vaccination efforts expanded to a broader group of individuals with presumed or potential exposure in the prior 14 days (PEP++). This has since expanded to include pre-exposure prophylaxis (PrEP) for individuals who are at increased risk for potential future exposure and vaccination prior should continue to be offered to persons at risk of mpox to prevent infection.20
JYNNEOS™ continues to be licensed and recommended as a 2-dose series. While the national strategy shifts to a PrEP model, PEP following a known exposure continues to be recommended. Additional studies are needed to determine JYNNEOS™ vaccine effectiveness as PEP, including among different populations at risk of mpox disease including persons living with HIV infection and the optimal timing of PEP.
Supplementary Material
Acknowledgements
The authors thank Judy Chen and Tingting Gu-Templin for assuring the quality of data, and Naama Kipperman, Kimberly Johnson, and Pierre Amiel for their assistance with developing and reviewing code. We thank all case and contact investigators in the Incident Command System Surveillance and Epidemiology Emergency Response Group for the collection of interview data that made this analysis possible. We also thank the Integrated Data Team and the Citywide Immunization Registry team for their collection and management of immunization data. Marc Lipsitch and Christopher Boyer were supported by the Morris-Singer Fund and National Institutes of Health (1R01GM139926).
Marc Lipsitch reports financial support was provided by National Institutes of Health (1R01GM139926) and the Morris-Singer Fund.
He is seconded part-time to the US Centers for Disease Control and Prevention (CDC) on an Intergovernmental Personnel Agreement. This work is in his academic capacity and not his CDC role and does not represent the views of CDC.
Christopher Boyer reports financial support was provided by National Institutes of Health (1R01GM139926) and the Morris-Singer Fund.
Marc Lipsitch reports financial support was provided by National Institutes of Health (1R01GM139926) and the Morris-Singer Fund. Marc Lipsitch is seconded part-time to the US Centers for Disease Control and Prevention (CDC) on an Intergovernmental Personnel Agreement; this work is in his academic capacity and not his CDC role and does not represent the views of CDC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
High degree of exposure defined by Centers for Disease Control and Prevention as: Contact between an exposed individual’s broken skin or mucous membranes with the skin lesions or bodily fluids from a person with mpox; OR any sexual or intimate contact involving mucous membranes (e.g., kissing, oral-genital, oral-anal, vaginal, or anal sex (insertive or receptive)) with a person with mpox; OR contact between an exposed individual’s broken skin or mucous membranes with materials (e.g., linens, clothing, objects, sex toys) that have contacted the skin lesions or bodily fluids of a person with mpox (e.g., sharing food, handling or sharing of linens used by a person with mpox without having been disinfected or laundered). Intermediate degree of exposure defined by Centers for Disease Control and Prevention as: Being within 6 feet for a total of 3 hours or more (cumulative) of an unmasked person with mpox without wearing a surgical mask or respirator; OR contact between an exposed individual’s intact skin with the skin lesions or bodily fluids from a person with mpox, OR contact between an exposed individual’s intact skin with materials (e.g., linens, clothing, sex toys) that have contacted the skin lesions or bodily fluids from a person with mpox without having been disinfected or laundered; OR contact between an exposed individual’s clothing with the person with mpox’s skin lesions or bodily fluids, or their soiled linens or dressings (e.g., during turning, bathing, or assisting with transfer).
References
- 1.Centers for Disease Control and Prevention. 2022 U.S. Map & Case Count. 2022. (Accessed October 15, 2022, at www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html.)
- 2.New York City Department of Health and Mental Hygiene. Monkeypox (MPV): Data. 2022. (Accessed October 15, 2022, at https://www1.nyc.gov/site/doh/data/health-tools/monkeypox.page#surveillance.)
- 3.United States Food and Drug Administration. Monkeypox Update: FDA Authorizes Emergency Use of JYNNEOS Vaccine to Increase Vaccine Supply. 2022. (Accessed October 14, 2022, at www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply.)
- 4.Kecmanović M, Suvaković V. Uticaj vaktsinalnog statusa na klinicki oblik i ishod obolenih od velikih boginja [Influence of the vaccinal status on the clinical form and results of patients with smallpox]. Med Pregl 1974;27:13–9. [PubMed] [Google Scholar]
- 5.Sommer A. The 1972 smallpox outbreak in Khulna municipality, Bangladesh: II. Effectiveness of surveillance and containment in urban epidemic control. American Journal of Epidemiology 1974;99:303–13. [DOI] [PubMed] [Google Scholar]
- 6.Earl PL, Americo JL, Wyatt LS, et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci U S A 2008;105:10889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatch GJ, Graham VA, Bewley KR, et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol 2013;87:7805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keckler MS, Salzer JS, Patel N, et al. IMVAMUNE(®) and ACAM2000(®) Provide Different Protection against Disease When Administered Postexposure in an Intranasal Monkeypox Challenge Prairie Dog Model. Vaccines (Basel) 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittman PR, Hahn M, Lee HS, et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N Engl J Med 2019;381:1897–908. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson C, Hausmann J, Lauterbach H, et al. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest 2008;118:1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houhou N, Tarhini H, Bertin C, et al. Breakthrough Infections after Postexposure Vaccination against Mpox. N Engl J Med;387:2477–9. [DOI] [PubMed] [Google Scholar]
- 12.Merad Y, Gaymard A, Cotte L, et al. Outcomes of post-exposure vaccination by modified vaccinia Ankara to prevent mpox (formerly monkeypox): a retrospective observational study in Lyon, France, June to August 2022. Eurosurveillance 2022;27:2200882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales LM, Barbas del Buey JF, García MA, et al. Post-exposure vaccine effectiveness and contact management in the mpox outbreak, Madrid, Spain, May to August 2022. Eurosurveillance 2023;28:2200883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer C, Lipsitch M. Defining and emulating target trials of the effects of postexposure vaccination using observational data. medrxiv 2023. [Google Scholar]
- 15.Centers for Disease Control and Prevention. Case Definitions for Use in the 2022 Monkeypox Response. 2022. (Accessed October 15, 2022, at www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html#:~:text=and%20specimen%20collection.-,Confirmed%20Case,culture%20from%20a%20clinical%20specimen.)
- 16.Centers for Disease Control and Prevention. Monitoring Persons Exposed. 2022. (Accessed October 15, 2022, at www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html.)
- 17.New York City Health Code. §11.07, Article 11, Reportable Diseases and Conditions. Chapter 24, Rules of the City of New York. [Google Scholar]
- 18.Van Ewijk CE, Hahne SJM. Letter to the editor: Bias in the vaccine effectiveness estimates of one-dose post-exposure prophylaxis against mpox. Eurosurveillance 2023;28:2300358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Ewjik CE, Smit C, Bavalia R, et al. Acceptance and timeliness of post-exposure vaccination against mpox in high-risk contacts, Amsterdam, the Netherlands, May–July 2022. Vaccine 2023;41:6952–9. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Monkeypox Vaccination. 2022. (Accessed October 15, 2022, at www.cdc.gov/poxvirus/monkeypox/interim-considerations/overview.html.)
- 21.Mpox 2022 Summary: Cases. 2022. (Accessed December 19, 2023, 2023,
- 22.Kroger A, Bahta L, Hunter P. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). (Accessed October 15, 2022, at www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf.) [Google Scholar]
- 23.van Ewijk CE, Miura F, van Rijckevorsel G, et al. Monkeypox outbreak in the Netherlands in 2022: public health response, epidemiological and clinical characteristics of the first 1000 cases and protection of the first-generation smallpox vaccine medRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


