Abstract
Purpose:
The purpose of this study series, which involves a questionnaire survey and qualitative interviews, was to (a) evaluate patient-reported usefulness of continuous glucose monitor (CGM) hypoglycemia-informing features and (b) identify challenges in using these features (ie, CGM glucose numbers, trend arrows, trend graphs, and hypoglycemia alarms) during hypoglycemia in adults with type 1 diabetes (T1DM).
Methods:
A cross-sectional questionnaire survey study was conducted with adults who have T1DM and were using CGMs to assess the perceived usefulness of hypoglycemia-informing features. A semistructured interview study with T1DM CGM-using adults and inductive thematic analysis were subsequently performed to identify challenges in using CGM hypoglycemia-informing features to manage hypoglycemia.
Results:
In the survey study (N = 252), the CGM glucose numbers, trend arrows, trend graphs, and hypoglycemia alarms were found to be very useful by 79%, 70%, 43%, and 64% of participants, respectively. Several challenges in using these features to manage hypoglycemia were identified in the qualitative study (N = 23): (1) hypoglycemia information not fully reliable,; (2) unpredictability of future blood glucose levels, (3) lack of awareness about how information can be used, and (4) disruptions associated with information.
Conclusions:
Although the majority of T1DM adults found their CGMs’ hypoglycemia-informing features helpful, challenges in optimally using these features persisted. Targeted knowledge and behavioral interventions could improve CGM use to reduce hypoglycemia.
Real-time continuous glucose monitors (CGMs) are devices that assess and provide glucose level information in real time to help people living with diabetes improve their diabetes self-management, including reducing hypoglycemia.1 CGMs have multiple features that can inform users about hypoglycemia, including displaying current glucose numbers, trend arrows, and trend graphs. CGMs can also generate alarms to warn users about potential impending and ongoing hypoglycemia based on built-in mathematical algorithms that integrate glucose numbers, trends in and rates of changes, and prespecified glucose thresholds to predict upcoming possibly dangerously low blood glucose levels.2 Clinical trials have demonstrated CGMs’ efficacy in reducing hypoglycemia in people with type 1 diabetes (T1DM).3–7 However, growing evidence suggests that clinically significant hypoglycemia (ie, severe hypoglycemia or spending ≥1% of time with glucose levels <54 mg/dL) continues to affect about 15% to 35% of people with T1DM despite using CGMs.8-11 This pattern underscores that health care gaps remain in eliminating factors that contribute to hypoglycemia development and mismanagement.
Surveys and qualitative studies have evaluated patients’ perceived strengths and limitations of CGMs, including CGM use for blood glucose management,12,13 life experiences with CGMs,14 perceived CGM accuracy,15 psychosocial factors around CGM use,13,16-18 and barriers to using CGMs.13 Hypoglycemia-informing features are integral to CGM-facilitated diabetes management. However, sparse research has reported patient–CGM interactions during hypoglycemia, namely, patient-perceived usefulness and challenges in adopting these features for effective hypoglycemia self-management. Such information could inform diabetes care and education specialists (DCESs), health care providers, and researchers for improving CGM user education or developing behavioral interventions to lessen hypoglycemia risks.
The purpose of this study series, which involves a questionnaire survey and qualitative interviews, was to evaluate patient-reported usefulness of CGM hypoglycemia-informing features and to identify challenges in using these features during hypoglycemia in adults with T1DM.
Research Methods
Study Overviews and Settings
A cross-sectional questionnaire survey study and subsequent qualitative interview study were conducted at the University of Michigan. The survey evaluated patient-reported usefulness of CGM hypoglycemia-informing features; semistructured interviews explored challenges in using these features, including explaining why some participants did not find certain features helpful. The interview study was part of a larger project qualitatively assessing people’s experiences with hypoglycemia while using CGMs.
Survey data were collected between January and April 2021, and interviews were held from October 2021 to April 2022. Both studies were approved by the University of Michigan’s Institutional Review Board (HUM00189672; HUM00197194). All participants provided consent prior to completing study-related activities. Two checklists, STrengthening the Reporting of OBservational studies in Epidemiology (STROBE)19 and Consolidated criteria for Reporting Qualitative research (COREQ),20 were used to ensure the integrity of the study design and data reporting.
Questionnaire Survey Study
Eligibility and recruitment.
Survey eligibility criteria were as follows: diagnosis of T1DM, ≥18 years old, and CGM usage time ≥70%.21 Recruitment emails were sent to 1024 T1DM CGM users identified via electronic medical records (EMRs) of the University of Michigan health care complexes, which provides care to a population of greater than 1 million people living in southeast Michigan. Telephone calls or physical letters were used to recruit individuals without valid email addresses in EMRs. Surveys were administered through REDCap.22 The study team provided telephone-based surveys for participants without immediate internet access.
Data collection and statistical analysis.
Survey questions assessed participants’ diabetes duration, CGM use history, and reported usefulness of CGM hypoglycemia-informing features (Table 1). Medical records were reviewed to collect demographic, A1C, and insulin pump use information. CGM reports were obtained to record each participant’s average glucose level and time with glucose <70 mg/dL. Descriptive analysis was conducted to summarize survey results, with data presented in percentage or median (interquartile range).
Table 1.
Question Assessing Patient-Reported Usefulness of CGM Hypoglycemia-Informing Features
| For making a decision on treating hypoglycemia, how useful do you find the various features of your CGM device? | |
| a. | CGM glucose numbers |
| 0 (not at all useful) | |
| 1 (somewhat not useful) | |
| 2 (somewhat useful) | |
| 3 (very useful) | |
| b. | CGM glucose arrows (up or down) |
| 0 (not at all useful) | |
| 1 (somewhat not useful) | |
| 2 (somewhat useful) | |
| 3 (very useful) | |
| c. | CGM glucose graph |
| 0 (not at all useful) | |
| 1 (somewhat not useful) | |
| 2 (somewhat useful) | |
| 3 (very useful) | |
| d. | CGM glucose alarms |
| 0 (not at all useful) | |
| 1 (somewhat not useful) | |
| 2 (somewhat useful) | |
| 3 (very useful) | |
Abbreviation: CGM, continuous glucose monitor.
Qualitative Interview Study
Eligibility and recruitment.
Interview eligibility criteria were as follows: diagnosis of T1DM, ≥18 years old, using a CGM for >6 months, and CGM usage time ≥70%.21 Individuals with uncontrolled psychological conditions or chronic cognitive impairment were excluded. Study candidates were identified through University of Michigan EMRs. Purposive sampling based on the time spent in hypoglycemia, age, sex, race/ethnicity, and socioeconomic status were considered during recruitment to ensure sample diversity. Candidates were invited through emails and telephone calls. Demographic information, CGM usage time, and the time spent with glucose <70 mg/dL were collected at screening. After recruitment, A1C and insulin pump information was extracted from EMRs; CGM average glucose levels were collected from participants’ CGM reports.
Semistructured interviews and analysis.
A semistructured interview guide was developed to explore participants’ experiences using CGM hypoglycemia-informing features for hypoglycemia management. The guide also covered how certain features (ie, CGM glucose numbers, trend arrows, trend graphs, and hypoglycemia alarms) impeded participants’ self-management (sample question and probe: “What CGM information gets in the way rather than helps? Tell me more about how [a CGM feature] works for or against your low management”). To refine the interview questions, pilot interviews were held with 2 eligible volunteers who had T1DM and who were using CGMs; these data were not included in the final analysis. Authors MD (PhD, mixed methodologist, woman) and YKL (MD, clinical diabetes researcher/endocrinologist, man) conducted one-on-one interviews. Due to the COVID-19 pandemic, interviews were completed through HIPAA-compliant Zoom video/telephone calls. All recorded audio (range = 37:25-96:40 minutes) was professionally transcribed. One participant had a second interview to answer additional questions that emerged during data analysis. No participant had an established relationship with the interviewer prior to the study.
Inductive thematic analysis23 was conducted by 4 members of the research team, including YKL, MD, AA (undergraduate research assistant, woman), and SC (research program manager, woman), all of whom are trained in qualitative analysis. This process was performed with analysis software MAXQDA. Six transcripts were initially coded together to develop and ensure a shared understanding of the early coding scheme. Each transcript was subsequently assigned to at least 2 team members, who individually applied existing codes to segments of text in the interview transcripts. Additional codes were generated and applied as needed based on new information in subsequent transcripts. After individual coding, the team members met to review which codes applied to which segments, address disagreements, and jointly determine a final set of codes. Participant checking of findings was not conducted on the findings. In team meetings, data saturation was discussed, and potential themes and supporting quotes were reviewed. Themes were established by linking related codes and synthesizing participants’ experiences within the combined codes. The wording of themes was subsequently finalized.
Results
Survey Participant Characteristics
A total of 252 participants were included in the analysis: 65% women, median (interquartile range) age of 43 (32-59), diabetes duration of 23 (14–32) years, A1C of 7.2% (6.4%–7.8%), 55.2 mmol/mol (46.4–61.7 mmol/mol), time with glucose levels <70 mg/dL of 1.4 (0.6%–3.0%; Table 2). Based on EMRs, when the CGM was initiated, all participants had at least 1 visit with a DCES at the University of Michigan, which has an Association of Diabetes Care and Education Specialists-accredited Diabetes Self-Management Education and Support program.24
Table 2.
Patient Demographic and Glycemic Characteristics (N = 23)
| Characteristics | Outcomes, percentage or median (interquartile range) |
|
|---|---|---|
| Questionnaire survey participants (n = 252) |
Qualitative interview participants (n = 23) |
|
| Age, y | 43 (32-59) | 47 (33-60) |
| Sex | ||
| FemaLe | 65 | 48 |
| MaLe | 35 | 52 |
| Race | ||
| Caucasian | 94 | 87 |
| African American | 2 | 13 |
| Asian American | 1 | 0 |
| Other | 3 | 0 |
| Ethnicity | ||
| Non-Hispanic | 94 | 100 |
| Hispanic | 4 | 0 |
| Refused/unknown | 2 | 0 |
| Duration of diabetes, y | 23 (14–32) | 25 (15–33) |
| A1C level, % | 7.2 (6.4–7.8) | 6.7 (6.2–7.6) |
| A1C level, mmol/mol | 55.2 (46.4–61.7) | 49.7 (44.3–59.6) |
| CGM type | ||
| Dexcom | 92 | 83 |
| Medtronic | 8 | 17 |
| Insulin pump use | 80 | 91 |
| With autosuspension feature | 70 | 61 |
| With hybrid closed-loop feature | 64 | 57 |
| CGM usage time, % | 97 (92–99) | 94 (87–100) |
| Average CGM glucose level, mg/dL | 158 (141–176) | 147 (133–163) |
| Time with glucose levels <70 mg/dL (3.9 mmol/L) on CGM, % | 1.4 (0.6–3.0) | 2.5 (1.0–5.9) |
Abbreviation: CGM, continuous glucose monitor.
Usefulness of CGM Hypoglycemia-Informing Features
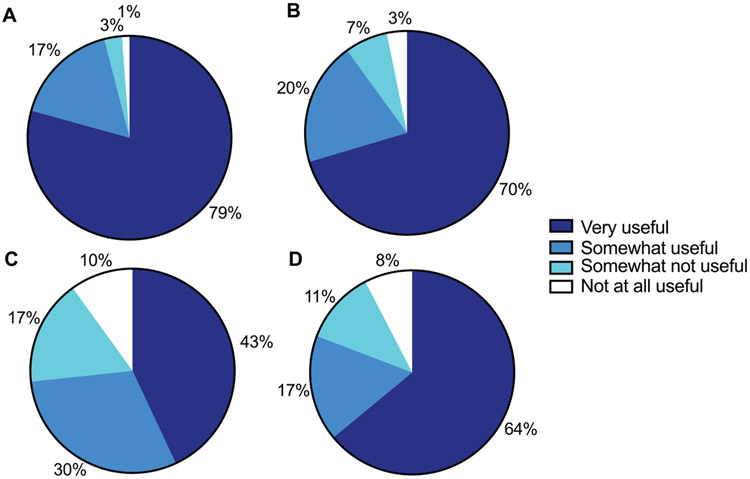
Among survey participants, 79% reported finding the CGM glucose numbers very useful in managing hypoglycemia; only 4% reported this feature was either somewhat not or not at all useful (Figure 1). Similarly, 70% considered the CGM glucose trend arrows very useful in managing hypoglycemia, and 64% found the CGM hypoglycemia alarms very useful in managing hypoglycemia; 10% and 19%, respectively, found these 2 components either somewhat not or not at all useful. Among all CGM features, the lowest proportion of survey participants (43%) found CGM trend graphs very useful, and 27% found this feature either somewhat not or not at all useful.
Figure 1.
Patient-reported usefulness of CGM hypoglycemia-informing features: (A) CGM glucose number, (B) CGM glucose trend arrow, (C) CGM glucose trend graph, and (D) CGM hypoglycemia alarm.
Abbreviation: CGM, continuous glucose monitor.
Interview Participant Characteristics
Twenty-three participants were enrolled, and all completed the interview study: 48% women, median (interquartile range) age of 47 (33–60), diabetes duration of 25 (15–33) years, A1C of 6.7% (6.2%–7.6), 49.7 mmol/mol (44.3–59.6 mmol/mol), and time with glucose levels <70 mg/dL of 2.5% (1.0%–5.9%; Table 2). Participants’ household income ranged from $50 000 to >$200 000/year. Their education level ranged from high school graduate to doctorate holder. All participants had at least 1 EMR-documented visit with a DCES at the University of Michigan when the CGM was being initiated.
Themes Related to Challenges in Using CGM Hypoglycemia-Informing Features
Four themes were identified regarding challenges in using CGM hypoglycemia-informing features.
Theme 1. Hypoglycemia information not fully reliable.
Some participants described the technological challenge of CGM glucose numbers being inaccurate compared with their self-monitoring blood glucose (SMBG) devices. This circumstance limited these participants’ use of CGM glucose numbers. Given this perceived lack of accuracy, one participant described using her CGM only as a screening tool for hypoglycemia:
My CGM is probably about 50% accurate. I don’t rely on it for 100%. I rely on it more as a warning alert to say, “Start paying attention.”
(55-year-old Caucasian woman)
Some participants related CGM glucose number inaccuracy to either the start (Table 3, Quote 1) or near the end (Table 3, Quote 2) of sensor sessions. Some also reported that sensor compressions could lead to false CGM hypoglycemia readings (Table 3, Quote 3). In response to these accuracy concerns, several participants described always checking their glucose levels via SMBG to avoid treating falsely reported hypoglycemia (Table 3, Quote 4).
Table 3.
Themes, Relevant CGM Features, Sample Quotes, and Participant Profiles for Main Challenges in Using CGM Hypoglycemia Information
| Quote | CGM feature | Quote | Participant profile |
|---|---|---|---|
| Theme 1 – Hypoglycemia information not fully reliable | |||
| 1 | Glucose number | “The only times that I've ever kind of noticed that the CGM might be off a little bit is right after insertion. So some of those times I’ll test a couple of times and then recalibrate for about an hour or so.” | 34-year-old Caucasian woman |
| 2 | Glucose number | “I just rely a lot on that information, so I’m just hoping it’s accurate. And I know sometimes it's not always—sometimes at the beginning of a session or at the end when it's starting to not work properly anymore, I definitely would rather have good information.” | 20-year-old African American woman |
| 3 | Glucose number | “If I look at my measurements and it’s been very steady, and then all of a sudden there’s a dramatic drop, I’ve learned that that seems to be an artifact of the sensor, like if I’ve been laying on it or something. Then it might give me what seems to be a false reading, because I’ve learned that if I treat then, sometimes then I will spike really high.” | 33-year-old Caucasian woman |
| 4 | Glucose number | “[When I see lows on my CGM] I’ll finger prick myself just to make sure. Because I’ve had some—I mean even last night, my CGM told me my sugar was 40 and I tested it [with a finger prick], and it was 180.” | 24-year-old Caucasian man |
| Theme 2 – Unpredictability of future blood glucose levels | |||
| 5 | Trend arrow | “Sometimes I’ll have a double arrow going down, and then 5 minutes later I’ll have an arrow going kind of at an angle down. So I’m trying to figure out if it went from double arrow 5 minutes before the angle arrow, then what happened in those 5 minutes? Why did it change from two arrows to one arrow going at an angle?” | 47-year-old Caucasian woman |
| Theme 3 – Lack of awareness about how information can be used | |||
| 6 | Trend graph | “[The trend graph is] not so much for—well, not so much [helpful] for managing the lows because it’s helpful to see where it was, but really, I’m looking at kind of the future. Where it’s going. So it’s really that trend arrow that gives me more—it’s just more important, I guess, for where I need to go.” | 56-year-old Caucasian man |
| Theme 4 – Disruptions associated with information | |||
| 7 | Alarm | “I hate the alarms on the CGM for being low. I would turn [them] off if I could. Sometimes I do turn the Bluetooth off because it’s just annoying to me. I’m like, ‘I know that I’m low. I don’t need it to tell me that I’m low ever … ’I know that it’s important for other people to have the—I understand why there’s an alarm. I just don’t like that I can’t turn it off.” | 36-year-old Caucasian woman |
| 8 | Alarm | “I guess I like it when it’s telling me when it’s initially, but when it keeps beeping continuously after I’m already attempting to fix the problem, that’s when it gets annoying.” | 21-year-old multiracial woman |
| 9 | Alarm | “I wish I could silence [the alarms]. Acknowledge yes, I’m low. Yes, I’m treating this low blood sugar…. If it could have a screen pop up, ‘Are you treating this low?’ ‘Yes.’ Just to say that I’ve acknowledged this. Yes, I’m treating it. Alert me again in 10 minutes or something like that.” | 33-year-old Caucasian woman |
Abbreviation: CGM, continuous glucose monitor.
Theme 2. Unpredictability of future blood glucose levels.
Although many participants found CGM trend arrow information useful for managing hypoglycemia, some were worried about the biologically unpredictable nature of blood glucose dynamics (Table 3, Quote 5) and wondered how to interpret the trend arrow details:
Although I know [the CGM] is pretty accurate, [the trend arrow] just makes you wonder … where it’s going to go next, regardless of what the arrows say…. Especially when you see the double arrows rising or falling. Like, “Oh, this sucks. What am I going to spike to? What am I going to drop to? How do I correct for this?”
(33-year-old Caucasian woman)
Theme 3. Lack of awareness about how information can be used.
Several participants described only using CGM glucose numbers and trend arrows to manage their hypoglycemia without acknowledging how trend graphs could provide additional information (Table 3, Quote 6). One participant explained:
I don’t use [the CGM trend graph] as much as I use the numbers and the arrows….I mean I look at it when I turn my phone on, but I already know that it’s going to be going down when I have low numbers. I know that the trend is going down. I don’t know why I don’t pay attention to the graph, to be honest with you.
(49-year-old Caucasian woman)
Theme 4. Disruptions associated with information.
Some participants described feeling sufficiently informed about hypoglycemia with their own hypoglycemia symptoms and other CGM features. They therefore found CGM hypoglycemia alarms redundant and sometimes even annoying (Table 3, Quote 7). Several participants did not believe that turning off CGM alarms would be problematic or dangerous:
I left the helpful alarms on there, but the ones that are saying, “Oh, you’re going to be low in 30 minutes,” or whatever they are, I shut all those off. I look at [the CGM] so often that I don’t need that alarm. It doesn’t really harm me any.
(33-year-old Caucasian woman)
Several participants also reported frustration related to a continuous alarm after treatment (Table 3, Quote 8). They wished there were an option to temporarily silence this type of alarm (Table 3, Quote 9).
Conclusions
In this questionnaire survey and qualitative interview study series evaluating patient-reported usefulness of and challenges in using CGM hypoglycemia-informing features, the majority of the T1DM adult CGM users reported CGM glucose numbers, trend arrows, and hypoglycemia alarms to be helpful in managing hypoglycemia. Conversely, more than a quarter of participants found trend graphs either less or not useful. Challenges in using these features included perceived CGM inaccuracy, which affected the trustworthiness of hypoglycemia information. The unpredictability of blood glucose also influenced participants’ use of trend arrows. Finally, CGM hypoglycemia information, particularly hypoglycemia alarms, could be disruptive to patients and lead them to stop using this feature.
The high usefulness of CGM hypoglycemia-informing features reported by this cohort is consistent with users’ predominantly positive CGM experiences described in prior studies.13,25,26 Challenges in using these features to manage hypoglycemia, which can be technological (Theme 1), biological (Theme 2), informational (Theme 3), and emotional (Theme 4), could help explain the unfavorable reflections to the CGM features. Knowledge-based interventions can be implemented to address some of these obstacles. Under Theme 1, participants reported comparing CGM glucose numbers with SMBG information to determine CGM accuracy. Although CGMs’ glucose accuracy is comparable to most commercially available glucometers,27 the CGM sampling rate during daily use is much higher than SMBG (eg, 288 vs 6 times per 24 hours, respectively). CGMs will therefore likely produce more false glucose numbers than SMBG.28 In addition, participants are often instructed to check SMBG if they suspect inaccurate CGM glucose readings. This behavior may lead to sampling bias and perpetuate the impression that CGM glucose numbers are inaccurate or even simply not useful.15 Setting expectations by explaining the nature of CGMs and how these devices differ from SMBG could calibrate users’ accuracy perceptions and thus improve the utility of CGM glucose information.
Knowledge about how to use trend graphs could also help improve the utility of this feature for hypoglycemia self-management. A trend graph uniquely presents longitudinal glucose information to assess glucose dynamics related to insulin, food consumption, exercise, and other factors contributing to glucose changes, including hypoglycemia.29 Research has demonstrated favorable outcomes in hypoglycemia reduction among people who favor pattern analysis over minute-by-minute data.16 By contrast, trend graph interpretation can be complex and may require substantial numeracy skills,30 limiting the use of this feature. Knowledge gaps exist in how to use trend graph information and need to be better assessed and addressed by DCESs and adapted for varied literacy and numeracy skills. Studies on the feasibility of increasing trend graph utility may also inform interventions for targeted populations to improve hypoglycemia self-management.
The unpredictability of blood glucose continues to affect hypoglycemia self-management despite the availability of continuous glucose information. Current CGM trend arrows are determined based on historical CGM glucose data31 and can often forecast hypoglycemia development.20 However, the findings of this research indicate that CGMs cannot predict hypoglycemia recovery after patients’ treatment responses to hypoglycemia. Such a function would inform patients’ decisions about whether additional treatment is needed; users could then prevent ongoing hypoglycemia due to undertreatment and rebound hyperglycemia due to overtreatment. Automated insulin delivery systems are capable of providing additional protection for hypoglycemia reduction by reactively reducing insulin doses or suspending insulin,32,33 yet active support for hypoglycemia recovery (eg, instructions on whether food treatment is needed based on glucose trends) is limited. The early-phase clinical trial with the dual hormone system has returned promising early data.34 Furthermore, initial data on mini-dose glucagon have shown the possibility of reducing hypoglycemia and rebound hyperglycemia.35
Scholars have identified the roles of psychological factors in hypoglycemia self-management11,36,37 and CGM use.13,16-18 Hypoglycemia alarms are a powerful feature that can provide additional support to reduce hypoglycemia, even with the availability of other continuous glucose information38,39; however, this feature can also generate patient discomfort20,40 and thus discontinued use. Both hypoglycemia and currently available sharp, audible hypoglycemia alarms can produce poor patient experiences. Real-time educational, behavioral, and psychosocial interventions could enable better user experiences with hypoglycemia alarms and enhance this feature’s adoption. Opportunities exist where DCESs, diabetes researchers, and patient experts can collaboratively develop effective educational patient-centered messages and programs to ensure that CGM and other technologies are used safely and for maximum benefit.
Strengths and Limitations
This study, to our knowledge, is one of the first to focus on patient-reported usefulness and challenges of using CGM hypoglycemia-informing features to manage hypoglycemia. This work combined a survey to demonstrate the prevalence of perceived feature usefulness followed by a qualitative exploration of what and how challenges compromise these features’ utility. The distribution of survey participants across racial/ethnic groups and the proportion who reported using an insulin pump were similar to the 2016 to 2018 T1D Exchange national report.41 The interview cohort also included a wide-spectrum population based on time in hypoglycemia, age, sex, race/ethnicity, and socioeconomic characteristics. Because participants were exclusively recruited from a tertiary academic hospital and had received structured diabetes education, findings’ generalizability may be limited. However, this study highlights that challenges persist in using CGM hypoglycemia-informing features despite the current standard of care. In addition, the qualitative study was planned after the survey study. The opportunity to design a sequential mixed-methods study using the survey responses to guide interviews was hence missed. Even so, the qualitative study reached content saturation, and the results were accordingly valid.
In summary, this quantitative–qualitative study series demonstrates that although most T1DM adults found CGM hypoglycemia-informing features helpful, challenges in optimally using these features remain. Additional knowledge about these features together with ongoing advances in diabetes technologies and behavioral science could further improve CGM use for reducing hypoglycemia.
Acknowledgments
We appreciate all assistance from the University of Michigan Adult Diabetes Education Program and Data Office for Clinical and Translational Research as well as from the Michigan Institute for Clinical and Health Research. We also sincerely thank all the research participants, without whom this study would not have been possible.
Funding
This work was supported by Michigan Diabetes Research Center Diabetes Interdisciplinary Study Program Award (P30DK020572, 2021) and by the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK129724, 2021); Michigan Center for Translational Diabetes Research Pilot and Feasibility Award (P30DK092926, 2020); REDCap was supported by the National Center for Advancing Translational Sciences (UL1TR002240).
Footnotes
Declaration of Conflicting Interests
The authors of this manuscript have no conflicts of interest relevant to this study to disclose.
Contributor Information
Yu Kuei Lin, Division of Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan.
Annika Agni, Department of Family Medicine, University of Michigan Medical School, Ann Arbor, Michigan.
Samantha Chuisano, Department of Family Medicine, University of Michigan Medical School, Ann Arbor, Michigan.
Michael D. Fetters, Department of Family Medicine, University of Michigan Medical School, Ann Arbor, Michigan; Mixed Methods Program, University of Michigan Medical School, Ann Arbor, Michigan.
Martha Funnell, Department of Learning Health Sciences, University of Michigan Medical School, Ann Arbor, Michigan.
Rodica Pop-Busui, Division of Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan.
Melissa J. DeJonckheere, Department of Family Medicine, University of Michigan Medical School, Ann Arbor, Michigan; Mixed Methods Program, University of Michigan Medical School, Ann Arbor, Michigan.
References
- 1.American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97–S112. doi: 10.2337/dc22-S007 [DOI] [PubMed] [Google Scholar]
- 2.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178–192. doi: 10.1016/j.diabres.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 3.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–387. doi: 10.1001/jama.2016.19976 [DOI] [PubMed] [Google Scholar]
- 4.Beck RW, Riddlesworth TD, Ruedy K, et al. ; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. doi: 10.7326/m16-2855 [DOI] [PubMed] [Google Scholar]
- 5.Mizokami-Stout KR, Li Z, Foster NC, et al. ; for T1D Exchange Clinic Network. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D exchange. Diabetes Care. 2020:43(4):806–812. doi: 10.2337/dc19-1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–1377. doi: 10.1016/s0140-6736(18)30297-6 [DOI] [PubMed] [Google Scholar]
- 7.van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902. doi: 10.1016/s2213-8587(16)30193-0 [DOI] [PubMed] [Google Scholar]
- 8.Lin YK, Hung M, Sharma A, et al. Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring systems in type 1 diabetes. Endocr Pract. 2019;25(6):517–525. doi: 10.4158/ep-2018-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyed Ahmadi S, Westman K, Pivodic A, et al. The association between HbA1c and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4). Diabetes Care. 2020;43(9):2017–2024. doi: 10.2337/dc19-2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akturk HK, Dowd R, Shankar K, Derdzinski M. Real-world evidence and glycemic improvement using Dexcom G6 features. Diabetes Technol Ther. 2021;23(S1):S21–S26. doi: 10.1089/dia.2020.0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YK, Richardson CR, Dobrin I, et al. Beliefs around hypoglycemia and their impacts on hypoglycemia outcomes in individuals with type 1 diabetes and high risks for hypoglycemia despite using advanced diabetes technologies. Diabetes Care. 2022;45(3):520–528. doi: 10.2337/dc21-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tansey M, Laffel L, Cheng J, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Satisfaction with continuous glucose monitoring in adults and youths with type 1 diabetes. Diabet Med. 2011;28(9):1118–1122. doi: 10.1111/j.1464-5491.2011.03368.x [DOI] [PubMed] [Google Scholar]
- 13.Pickup JC, Ford Holloway M, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative frame-work analysis of patient narratives. Diabetes Care. 2015;38(4):544–550. doi: 10.2337/dc14-1855 [DOI] [PubMed] [Google Scholar]
- 14.Lawton J, Blackburn M, Allen J, et al. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. 2018;18(1):12. doi: 10.1186/s12902-018-0239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polonsky WH, Hessler D. Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol. 2015;9(2):339–341. doi: 10.1177/1932296814559302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritholz MD, Atakov-Castillo A, Beste M, et al. Psychosocial factors associated with use of continuous glucose monitoring. Diabet Med. 2010;27(9):1060–1065. doi: 10.1111/j.1464-5491.2010.03061.x [DOI] [PubMed] [Google Scholar]
- 17.Tanenbaum ML, Adams RN, Iturralde E, et al. From Wary Wearers to d-Embracers: personas of readiness to use diabetes devices. J Diabetes Sci Technol. 2018;12(6):1101–1107. doi: 10.1177/1932296818793756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messer LH, Cook PF, Tanenbaum ML, Hanes S, Driscoll KA, Hood KK. CGM benefits and burdens: two brief measures of continuous glucose monitoring. J Diabetes Sci Technol. 2019;13(6):1135–1141. doi: 10.1177/1932296819832909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vloemans AF, van Beers CAJ, de Wit M, et al. Keeping safe. Continuous glucose monitoring (CGM) in persons with type 1 diabetes and impaired awareness of hypoglycaemia: a qualitative study. Diabet Med. 2017;34(10):1470–1476. doi: 10.1111/dme.13429 [DOI] [PubMed] [Google Scholar]
- 21.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a meta-data-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 24.Davis J, Fischl AH, Beck J, et al. 2022 National standards for diabetes self-management education and support. Sci Diabetes Self Manag Care. 2022;48(1):44–59. doi: 10.1177/26350106211072203 [DOI] [PubMed] [Google Scholar]
- 25.Polonsky WH, Hessler D, Ruedy KJ, Beck RW; DIAMOND Study Group. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40(6):736–741. doi: 10.2337/dc17-0133 [DOI] [PubMed] [Google Scholar]
- 26.Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15(4):295–301. doi: 10.1089/dia.2012.0298 [DOI] [PubMed] [Google Scholar]
- 27.Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558–566. doi: 10.1177/1932296816672237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol. 2019;13(3):575–583. doi: 10.1177/1932296818812062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yost O, DeJonckheere M, Stonebraker S, et al. Continuous glucose monitoring with low-carbohydrate diet coaching in adults with prediabetes: mixed methods pilot study. JMIR Diabetes. 2020;5(4):e21551. doi: 10.2196/21551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737–746. doi: 10.7326/0003-4819-148-10-200805200-00006 [DOI] [PubMed] [Google Scholar]
- 31.Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc. 2017;1(12):1445–1460. doi: 10.1210/js.2017-00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown SA, Kovatchev BP, Raghinaru D, et al. ; iDCL Trial Research Group. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown SA, Forlenza GP, Bode BW, et al. ; Omnipod 5 Research Group. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44(7):1630–1640. doi: 10.2337/dc21-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389(10067):369–380. doi: 10.1016/s0140-6736(16)32567-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rickels MR, DuBose SN, Toschi E, et al. ; T1D Exchange Mini-Dose Glucagon Exercise Study Group. Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diabetes Care. 2018;41(9):1909–1916. doi: 10.2337/dc18-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers HA, de Zoysa N, Amiel SA. Patient experience of hypoglycaemia unawareness in type 1 diabetes: are patients appropriately concerned? Diabet Med. 2012;29(3):321–327. doi: 10.1111/j.1464-5491.2011.03444.x [DOI] [PubMed] [Google Scholar]
- 37.Speight J, Barendse SM, Singh H, et al. Cognitive, behavioural and psychological barriers to the prevention of severe hypoglycaemia: a qualitative study of adults with type 1 diabetes. SAGE Open Med. 2014;2:2050312114527443. doi: 10.1177/2050312114527443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2018;35(4):483–490. doi: 10.1111/dme.13561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YK, Groat D, Chan O, et al. Alarm settings of continuous glucose monitoring systems and associations to glucose outcomes in type 1 diabetes. J Endocr Soc. 2020;4(1):bvz005. doi: 10.1210/jendso/bvz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobry EC, Karami AJ, Meltzer LJ. Friend or foe: a narrative review of the impact of diabetes technology on sleep. Curr Diabetes Rep. 2022;22(7):283–290. doi: 10.1007/s11892-022-01468-x [DOI] [PubMed] [Google Scholar]
- 41.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66–72. doi: 10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]