Abstract
Background
Following surgery, incisions are usually closed by fixing the edges together with sutures (stitches), staples, adhesive glue or clips. This process helps the cut edges heal together and is called 'healing by primary intention'. However, not all incised wounds are closed in this way: where there is high risk of infection, or when there has been significant tissue loss, wounds may be left open to heal from the 'bottom up'. This delayed healing is known as 'healing by secondary intention'. Negative pressure wound therapy (NPWT) is one treatment option for surgical wounds that are healing by secondary intention.
Objectives
To assess the effects of negative pressure wound therapy (NPWT) on the healing of surgical wounds healing by secondary intention (SWHSI) in any care setting.
Search methods
For this review, in May 2015 we searched the following databases: the Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials; Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations; Ovid EMBASE; and EBSCO CINAHL. There were no restrictions based on language or date of publication.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) comparing the effects of NPWT with alternative treatments or different types of NPWT in the treatment of SWHSI. We excluded open abdominal wounds from this review as they are the subject of a separate Cochrane review that is in draft.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction.
Main results
We located two studies (69 participants) for inclusion in this review. One study compared NPWT with an alginate dressing in the treatment of open, infected groin wounds. and one study compared NPWT with a silicone dressing in the treatment of excised pilonidal sinus. The trials reported limited outcome data on healing, adverse events and resource use.
Authors' conclusions
There is currently no rigorous RCT evidence available regarding the clinical effectiveness of NPWT in the treatment of surgical wounds healing by secondary intention as defined in this review. The potential benefits and harms of using this treatment for this wound type remain largely uncertain.
Keywords: Humans; Surgical Procedures, Operative; Wound Healing; Bandages; Negative‐Pressure Wound Therapy; Negative‐Pressure Wound Therapy/methods; Pilonidal Sinus; Pilonidal Sinus/surgery; Randomized Controlled Trials as Topic; Surgical Wound Infection; Surgical Wound Infection/therapy
Plain language summary
Negative pressure wound therapy for treating surgical wounds healing by secondary intention (open surgical wounds)
Background
Following surgery, incisions are usually closed by fixing the edges together with sutures (stitches), staples, adhesive glue or clips. This process helps the cut edges heal together and is called 'healing by primary intention'. However, not all incised wounds are closed in this way: where there is high risk of infection, or when there has been significant tissue loss, wounds may be left open to heal from the 'bottom up'.
Treating open surgical wounds can be challenging ‐ the wounds can be large, deep, at risk of infection and can produce a lot of liquid (called exudate) which is difficult to manage. Treatment options include wound dressings and the use of negative pressure wound therapy (NPWT), which is becoming a common treatment for a variety of wound types. NPWT involves the application of a wound dressing to the wound, followed by the attachment of a machine that applies a carefully controlled negative pressure (or vacuum) to the dressing. This sucks any wound and tissue fluid away from the treated area into a canister. NPWT may have a more positive effect on wound healing than alternative treatments. We investigated the evidence for the effectiveness of NPWT as a treatment for surgical wounds healing by secondary intention.
What we found
Despite extensive searching for all relevant medical studies that might provide evidence about the effectiveness of NPWT for treating surgical wounds healing by secondary intention, we found only two eligible studies. One study compared NPWT with use of an alginate dressing in surgical wounds healing by secondary intention. The study was small, with only 20 participants, and reported very limited information (data) for wound healing, which was the outcome in which we were most interested. Time to healing was shorter for participants in the NPWT group than participants in the alginate dressing group (median of 57 days to healing for NPWT group compared with 104 days for alginate dressing group). Although some participants in this very small study needed an amputation or died, there was no difference between treatments for the number of amputations or number of deaths. A second study compared NPWT with a silicone dressing in participants who had undergone surgical removal of a pilonidal sinus: median time to healing in the NPWT group was 84 days compared to 93 days in the dressing group.
There is currently a lack of evidence for both the benefits and potential harms of NPWT. More, better quality research is needed to determine the effectiveness of using NPWT on surgical wounds that are healing by secondary intention.
This research was assessed as being up to date in May 2015.
Background
Description of the condition
Following surgery, incisions are usually closed by fixing the edges together with sutures (stitches), staples, tissue adhesive (glue) or clips. This process helps the cut edges to heal together and is called 'healing by primary intention'. However, not all incised wounds are closed in this way: where there is high risk of infection, or when there has been significant tissue loss, wounds may be left open to heal from the 'bottom up'. This delayed healing is known as 'healing by secondary intention'. As well as being planned ‐ for example following excision of a pilonidal sinus, or incision and drainage of a peri‐anal abscess ‐ healing by secondary intention also occurs when wound closure fails and wounds dehisce (wound edges separate), for example due to inflammation and oedema (fluid collection), and cannot be re‐closed. These wounds may be left to heal completely by secondary intention, or be surgically closed after partial healing has occurred (sometimes known as delayed healing by primary intention).
Currently, there are very few data available on the incidence and prevalence of surgical wounds healing by secondary intention (SWHSI). Two published audit studies from the north of England (Bradford (Vowden 2009), and Hull (Srinivasaiah 2007)) estimated that SWHSI constitute approximately 28% of all prevalent acute (mainly surgical/traumatic) wounds that receive wound care provision. A more recent survey, also undertaken in the north of England (Hull), collected data on the prevalence of SWHSI over a two‐week period across community, primary care and acute care settings (Ashby 2015‐ personal communication ‐ paper in preparation). The study identified a total of 187 surgical wounds deemed to be healing by secondary intention during this period from a population of 590,585. We have not been able to locate non‐United Kingdom (UK) data regarding the epidemiology of SWHSI.
As with epidemiological data, there is also a lack of data on the impact of SWHSI on people's health‐related quality of life. A recent qualitative study explored the impact of these wounds on 20 patients and carers (McCaughan 2015‐ personal communication ‐ paper in preparation); its findings highlighted the shock and anguish that these wounds can cause people, as well as the feeling of frustration and powerlessness felt when living with them. We have not been able to locate any other data on the impact of living with SWHSI.
Furthermore, without good epidemiological data it is difficult to estimate how much the management of SWHSI costs healthcare providers. SWHSI are managed in both acute and community settings and can be difficult and time consuming to treat, as they present specific management problems ‐ such as very high levels of exudate ‐ and must be protected from infection and trauma. Given that these wounds are healing from the bottom up and some may be large, they may also take a reasonable amount of time to heal, which also increases healthcare costs.
The traditional approach to treating SWHSI involves daily or more frequent dressing changes, sometimes involving packing of a wound cavity. There are a number of different dressing options, from simple dressings ‐ such as non‐adherent dressings ‐ to more modern options such as foam, hydrocolloid and alginate dressings. A Cochrane systematic review found 13 randomised controlled trials comparing dressings and topical treatments for SWHSI (Vermeulen 2004). All of the trials were over ten years old, small and of poor quality. There was no evidence that the choice of dressing or topical treatment had any impact on healing rates. Given the potential complexity, size and longevity of SWHSI, interest is increasing in alternative treatment options.
Description of the intervention
Negative pressure wound therapy (NPWT) is a technology that is used in wound care on complex wounds (e.g. Guy 2012). NPWT involves the application of a wound dressing through which a negative pressure (or vacuum) is applied, often with any wound and tissue fluid that is drawn away from the area being collected in a canister. The intervention was developed in the 1990s, and the uptake of NPWT in the healthcare systems of developed countries has been dramatic. In the USA a US Department of Health report estimated that between 2001 and 2007, Medicare payments for NPWT pumps and associated equipment increased from USD 24 million to USD 164 million (an increase of almost 600%; Department of Health and Human Services 2009). Initially only one NPWT manufacturer supplied NPWT machines (the V.A.C system: KCI, San Antonio Texas), however, as the NPWT market has grown, a number of different commercial NPWT systems have been developed, with machines becoming smaller and more portable. Indeed, the most recent introduction to the market is a single use, or 'disposable', negative pressure product. Ad hoc, non‐commercial negative pressure devices are also used, especially in resource‐poor settings. These devices tend to use simple wound dressings, such as gauze, or transparent occlusive (non‐permeable) dressings, with negative pressure generated in hospital by vacuum suction pumps.
A number of different healthcare professionals prescribe and apply NPWT, and it is now used both in secondary and primary (community) care, particularly following the introduction of ambulatory systems. Whilst the NPWT systems outlined above differ in a number of respects ‐ such as type of pressure (constant or cyclical) applied to the wound, the material in contact with the surface of the wound and also the type of dressing used ‐ the principle of applying a negative pressure to the wound in a closed environment is the same for all products.
How the intervention might work
NPWT ostensibly assists in wound management by collecting high volumes of wound exudate, reducing the frequency of dressing changes by keeping anatomically‐challenging wounds clean, and reducing odour. Manufacturers, however, also suggest that the application of mechanical force to the wound provides biologically‐plausible processes by which wound healing is promoted, i.e. the drawing together of wound edges, increased perfusion, and the removal of infectious material and exudate (KCI 2012). NPWT might have a beneficial effect by preventing unnecessary dressing changes and reducing exposure to the environment.
There are some potentially negative aspects associated with NPWT; these include wound maceration (softening of tissue due to exposure to liquid), retention of dressings, and wound infection, as well as other injuries (FDA 2011). NPWT devices are usually worn continually by patients during treatment; they can interfere with mobility, and, anecdotally, are often noisy, which prevents some patients from sleeping.
Why it is important to do this review
It is important to assess the current evidence about the effects of NPWT, given its widespread use. No current systematic reviews on NWPT have focused on types of SWHSI or clearly summarise information on this wound type. A recent National Institute for Health and Care Excellence (NICE) interventional procedure guidance document, 'Negative Pressure Wound Therapy for the Open Abdomen' states that "Current evidence on the safety and efficacy of negative pressure wound therapy (NPWT) for the open abdomen is adequate to support the use of this procedure provided that normal arrangements are in place for consent, audit and clinical governance" (NICE 2013). Conclusions for this type of open surgical wound were based on a range of data sources, including randomised controlled trials, case series and specialist advisors. An on‐going Cochrane review is underway that is investigating NPWT for the treatment of abdominal wounds. There is also a current review investigating NPWT for the treatment of foot ulcers in people with diabetes (Dumville 2013). A bespoke systematic review of randomised controlled trials that investigates the clinical and cost effectiveness of NPWT for other types of SWHSI is required.
Objectives
To assess the effects of negative pressure wound therapy (NPWT) on the healing of surgical wounds healing by secondary intention (SWHSI) in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), including cluster RCTs, irrespective of language of report. We included cross‐over trials only if they reported outcome data at the end of the first treatment period and prior to cross‐over. We excluded studies that used quasi‐randomisation.
Types of participants
Men and women aged 18 years and over with surgical wounds healing by secondary intention (SWHSI). We excluded skin graft donor sites, as they form a specific group of superficial wounds, which require specific care. We also excluded wounds of non‐surgical origin, such as trauma wounds, as well as wounds of non‐surgical origin that had undergone surgical or sharp debridement or other surgical treatments. Finally, we also excluded people with open abdominal wounds (wound extending into the abdominal cavity with open fascia) as these are a very specific type of surgical wound that heal by secondary intention and are the subject of a separate protocol that has been drafted. Studies that recruited participants with a SWHSI alongside people with other types of chronic or acute wounds were included if the results for people with relevant wounds were presented separately (or were available from the study authors).
Types of interventions
The primary intervention of interest was negative pressure wound therapy (NPWT), both commercial and non‐commercial treatments. We included any RCT in which use of a specific NPWT machine during the treatment period was the only systematic difference between treatment groups. We anticipated that likely comparisons would include the use of NPWT during the care pathway compared with no use of NPWT, or comparison of different types/brands of NPWT used during the care pathway.
Types of outcome measures
We list primary and secondary outcomes below. If a study was eligible (i.e. correct study design, population and intervention/comparator), but did not report a listed outcome, we contacted the study authors where possible to establish whether an outcome of interest to this review was measured but not reported.
It is important to take time into account in the reporting of outcome measures. We categorised outcome measure data for complete wound healing from:
less than one week to eight weeks as short‐term;
over eight weeks to 26 weeks as medium‐term; and
over 26 weeks as long term.
We report all outcome measures at the latest time point available (assumed to be length of follow‐up if not specified) and the time point specified in the methods as being of primary interest (if this is different from latest time point available).
Primary outcomes
The primary outcomes for this review were complete wound healing and adverse events.
Complete wound healing
For this review we regarded the following as providing the most relevant and rigorous measures of outcome:
time to complete wound healing: for information we note whether this outcome has been correctly analysed using censored data and with adjustment for prognostic covariates such as baseline size;
the proportion of wounds healed (frequency of complete healing).
Where both these outcomes were reported we present all data in a summary outcome table for reference, but focus on reporting time to healing. We accepted authors’ definitions of what constituted a healed wound.
Adverse events (generic)
We extracted data reported on adverse events, and classified them as 'serious adverse events' and 'non‐serious adverse events' where trials provided a clear methodology for the collection of adverse event data. The methodology needed to make it clear whether events were reported at the participant level, or, where multiple events per person were reported, that an appropriate adjustment had been made for data clustering. We did not extract individual types of adverse events, such as pain or infection that require specific assessment, under this outcome ‐ instead this assessment included any event classed as adverse by the patient and or health professional during the trial.
Secondary outcomes
Participant health‐related quality of life/health status (measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12 or SF‐6). We did not include ad hoc measures of quality of life that were not likely to be validated and would not be common to multiple trials.
Wound infection (as defined by trial author).
Mean pain scores (including pain at dressing change) are included only where pain is reported as a continuous outcome using a validated scale such as a visual analogue scale (VAS).
Resource use: including measurements of resource use such as number of dressing changes, nurse visits, length of hospital stay and re‐operation/intervention.
Costs: any costs applied to resource use.
Complete fascia closure: reported as median time to closure or number of wounds closed
Proportion of wounds closed or time to wound closure: complete wound closure (including skin) that was the result of surgical closure rather than healing where the role of treatments were to prepare the wound for closure.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
The Cochrane Wounds Group Specialised Register (searched 19 May 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2015, Issue 4);
Ovid MEDLINE (1946 to 18 May 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 16 July 2014);
Ovid EMBASE (1974 to 18 May 2015);
EBSCO CINAHL (1982 to 18 May 2015).
We used the following search strategy in The Cochrane Central Register of Controlled Trials (CENTRAL). Other search strategies are listed in (Appendix 1):
#1 MeSH descriptor: [Negative‐Pressure Wound Therapy] explode all trees #2 MeSH descriptor: [Suction] explode all trees #3 MeSH descriptor: [Vacuum] explode all trees #4 ("negative pressure" or negative‐pressure or TNP or NPWT):ti,ab,kw #5 (sub‐atmospheric or subatmospheric):ti,ab,kw #6 ((seal* next surface*) or (seal* next aspirat*)):ti,ab,kw #7 (wound near/3 suction*):ti,ab,kw #8 (wound near/3 drainage):ti,ab,kw #9 (foam next suction) or (suction next dressing*):ti,ab,kw #10 (vacuum assisted closure or VAC):ti,ab,kw #11 (vacuum next therapy) or (vacuum next dressing*) or (vacuum next seal*) or (vacuum next assist*) or (vacuum near closure) or (vacuum next compression) or (vacuum next pack*) or (vacuum next drainage) or (suction* adj drainage):ti,ab,kw #12 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11) #13 MeSH descriptor: [Surgical Wound Infection] explode all trees #14 MeSH descriptor: [Surgical Wound Dehiscence] explode all trees #15 (surg* near/5 infect*):ti,ab,kw #16 (surg* near/5 wound*):ti,ab,kw #17 (surg* near/5 site*):ti,ab,kw #18 (surg* near/5 incision*):ti,ab,kw #19 (surg* near/5 dehisc*):ti,ab,kw #20 (wound* near/5 dehisc*):ti,ab,kw #21 (wound* near/5 infect*):ti,ab,kw #22 (wound near/5 disruption*):ti,ab,kw #23 (wound next complication*):ti,ab,kw #24 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25 (intent* or second* or heal* or complic*):ti,ab,kw #26 ((open* or clos*) near/5 wound*):ti,ab,kw #27 #25 or #26 #28 #24 and #27 #29 #12 and #28
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision; Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
Clinical Trials.gov (www.clinicaltrials.gov);
WHO International Clinical Trials Registry Platform (ICTR) (apps.who.int/trialsearch/Default.aspx);
The EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
We contacted corresponding authors and the manufacturers and distributors of NPWT devices. We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials as well as relevant systematic reviews, meta‐analyses, and health‐technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of the citations retrieved by the searches for relevance. After this initial assessment, we obtained full text copies of all studies felt to be potentially relevant. Two review authors independently checked the full papers for eligibility; disagreements were resolved by discussion and, where required, the input of a third review author. We recorded all reasons for the exclusion of studies for which we had obtained full copies. We have completed a PRISMA flowchart to summarise this process (Liberati 2011).
Where studies were reported multiple times, we obtained all publications. Whilst the study was included only once in the review, we extracted data from all reports to ensure that we obtained maximal relevant data.
Data extraction and management
We extracted and summarised details of the eligible studies. Two review authors extracted data independently and resolved disagreements by discussion, drawing on a third review author where required. Where data were missing from reports, we attempted to contact the study authors to obtain this information. If a study with more than two intervention arms were included, we would extract data only from those intervention and control groups that met the eligibility criteria.
Where possible we extracted the following data, according to treatment group, for the pre‐specified interventions and outcomes in this review. We collected outcome data for relevant time points as described in Types of outcome measures.
Country of origin.
Type of wound and surgery.
Unit of randomisation (per patient) ‐ single wound or multiple wounds on the same patient.
Unit of analysis.
Trial design e.g. parallel, cluster.
Care setting.
Number of participants randomised to each trial arm.
Eligibility criteria and key baseline participant data.
Details of treatment regimen received by each group.
Duration of treatment.
Details of any co‐interventions.
Primary and secondary outcome(s) (with definitions).
Outcome data for primary and secondary outcomes (by group).
Duration of follow‐up.
Number of withdrawals (by group).
Publication status of study.
Source of funding for trial.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting and other issues – in this review we will record issues with unit of analysis, for example where a cluster trial has been undertaken but analysed at the individual level in the study report. We assessed blinding and completeness of outcome data for each of the review outcomes separately. We note that, since wound healing is a subjective outcome, it can be at a high risk of measurement bias when outcome assessment is not blinded. We have presented our assessment of risk of bias using two 'risk of bias' summary figures; one is a summary of bias for each item across all studies, and the second shows a cross‐tabulation of each trial by all of the risk of bias items. We classed studies with an assessment of high risk of bias for any one of three key risk domains, namely the randomisation sequence, allocation concealment and blinding of outcome assessment domains (the latter for a specified outcome), as being at overall high risk of bias (for a specified outcome).
Measures of treatment effect
For dichotomous outcomes we calculated the risk ratio (RR) with 95% confidence intervals (CI). For continuously distributed outcome data from trials that used the same assessment scale we used the mean difference (MD) with 95% CIs. When trials used different assessment scales, we used the standardised mean difference (SMD) with 95% CIs. We considered mean or median time to healing without survival analysis as a valid outcome only if reports specified that all wounds healed (i.e. if the trial authors regarded time to healing as a continuous measure as there is no censoring). We reported time‐to‐event data (e.g. time‐to‐complete wound healing) as hazard ratios (HR) where possible in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio, then, where feasible, we planned to estimate this using other reported outcomes, such as the numbers of events, through the application of available statistical methods (Parmar 1998).
Dealing with missing data
It is common to have data missing from trial reports. Excluding participants post‐randomisation from analysis, or ignoring those participants who are lost to follow‐up compromises the randomisation, and potentially introduces bias into trials. Where there were missing data that we thought should be included in the analyses, we contacted the relevant study authors to enquire whether these data were available.
Where 'proportion of wounds healed' data remained missing, for analysis we assumed that if randomised participants were not included in an analysis, their wound did not heal (i.e. they would be considered in the denominator but not the numerator).
In a time‐to‐healing analysis using survival analysis methods, drop‐outs should be accounted for as censored data, so we took no action regarding missing data.
For continuous variables, for example length of hospital stay, and for all secondary outcomes, we presented the data available from the study reports/study authors and did not anticipate imputing missing data. Where measures of variance were missing, we calculated these where possible. If calculation was not possible, we contacted the study authors. Where these measures of variation were not available, we excluded the study from any relevant meta‐analyses that were conducted.
Assessment of heterogeneity
Assessment of heterogeneity can be a complex, multi‐faceted process. Firstly, we considered clinical and methodological heterogeneity, that is the degree to which the included studies varied in terms of participants, interventions, outcomes and characteristics such as length of follow‐up. This assessment of clinical and methodological heterogeneity was supplemented by information regarding statistical heterogeneity ‐ assessed using the Chi² test (a significance level of P < 0.10 was considered to indicate statistically significant heterogeneity) in conjunction with the I² measure (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). Very broadly, it is considered that I² values of 25% or less may mean a low level of heterogeneity (Higgins 2003), and values of 75% or more indicate very high heterogeneity (Deeks 2011). Should there be evidence of high heterogeneity, we plan to explore this further where possible: see Data synthesis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of 'small study effects', that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial’s size or precision (Sterne 2011). We planned to present funnel plots for meta‐analyses comprising 10 RCTs or more using RevMan 5.3 (RevMan 2014).
Data synthesis
We combined details of included studies in a narrative review according to type of comparator, possibly by location of or type of wound, and then by outcomes and time period. Where appropriate and required, we considered clinical and methodological heterogeneity, and anticipated pooling data when studies appeared appropriately similar in terms of wound type, intervention type, duration of follow‐up and outcome type, in order to ensure any synthesis was valid.
Our standard approach for meta‐analytical analyses was to employ the more conservative random‐effects model, because statistical assessments can miss potentially important between‐study heterogeneity in small samples, (Kontopantelis 2012).
A fixed‐effect analyses was only planned when, in the judgement of the review authors, there was minimal clinical heterogeneity that was supported by a statistically non‐significant Chi² value and an I² of 0% (Kontopantelis 2013). In all other circumstances we would adopt a random‐effects model. If relevant, where clinical heterogeneity was thought to be acceptable or of interest, we planned to meta‐analyse even when statistical heterogeneity was high, and to consider the use of meta‐regression to attempt to interpret the causes behind this heterogeneity (Thompson 1999; Thompson 2002).
Data were presented using forest plots where possible. For dichotomous outcomes we presented the summary estimate as a risk ratio (RR) with 95% CI. Where continuous outcomes were measured in the same way across studies, we planned to present a pooled mean difference (MD) with 95% CI; we planned to pool mean difference (MD) estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of hazard ratios and 95% CIs as presented in the study reports using the generic inverse variance method in RevMan 5.3. Where time to healing was analysed as a continuous measure, but it was not clear if all wounds healed, the use of the outcome in the study would be documented but we would not summarise the data or use them in any meta‐analysis.
We would obtain pooled estimates of treatment effect by using Cochrane RevMan software (version 5.3; RevMan 2014).
'Summary of findings' tables
We planned to present the main results of the review in 'summary of findings' tables. These tables are used to present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision (or imprecision) of effect estimates and risk of publication bias (Schünemann 2011b).
time to complete wound healing where analysed using appropriate survival analysis methods;
proportion of wounds completely healing during the trial period;
adverse events.
Where data were not pooled it was decided to conduct the GRADE assessment for each comparison and present this narratively within the results section without the presentation of separate summary of finding tables.
Subgroup analysis and investigation of heterogeneity
We planned to assess potential heterogeneity across the following areas, specifically, where there was evidence of between‐trial heterogeneity we envisaged a subgroup analysis being conducted for:
locations of wound types i.e. abdominal wounds and other wound types;
type of negative pressure system being used.
Sensitivity analysis
Where possible we planned to perform sensitivity analyses to explore the effect of the following criteria:
concealment of allocation (allocation adequately concealed versus not reported or inadequate);
type of randomisation (truly randomised with adequate method of generating the randomisation sequence versus not reported).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
The search generated 586 records: we obtained 32 records pertaining to 26 studies. We included only two of these 26 studies in this review and excluded the remaining 24 studies (Figure 1). We are aware of one potentially relevant on‐going study (Seidel 2013; registers checked 18 May 2015).
1.

Study flow diagram
Included studies
This review includes two studies. The first contained 20 participants (Monson 2014) and had an unclear follow‐up time. The study had two‐arms and was conducted in Sweden. Monson 2014 compared NPWT with an alginate dressing for the treatment of infected open groin wounds that followed from arterial surgery. The NPWT system used was the V.A.C. system (KCI‐Medical, TX, USA).
The second included study (Ulas Biter 2014) randomised 49 participants who had undergone surgical excision of a pilonidal sinus. The study, conducted in the Netherlands, had 6 months follow‐up (although it seems that healing data were collected for a longer period). Ulas Biter 2014 evaluated the use of NPWT (type not given) compared to open wound care.
Excluded studies
In total 24 studies for which full text information was obtained were excluded from the review for the following reasons (see Characteristics of excluded studies).
It was not clear what type of wounds were included in the study (one study; Albert 2012).
Participants did not have open surgical wounds (seven studies; Bee 2008; Liao 2012; Oh 2013; Pachowsky 2012; Quantz 2010; Stannard 2009; Tuncal 2013).
The study was not an RCT (three studies; Mees 2012; Pan 2013; Pellino 2014).
It was not clear whether the study was an RCT or whether participants had relevant wounds (one study; Huang 2006).
Study population had a range of wounds, and we were unable to obtain any separate data relevant to this review (six studies; Braakenburg 2006; Hu 2009; Lei 2011; Mody 2008; Moues 2005; Orgill 2004; Perez 2010).
No relevant outcome data were available (three studies; de Laat 2011; Dorafshar 2011; Jeschke 2004).
NPWT was not the only systematic difference between study groups (one study; Pliakos 2010).
No study information was available (one study; Walker 2005).
Risk of bias in included studies
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
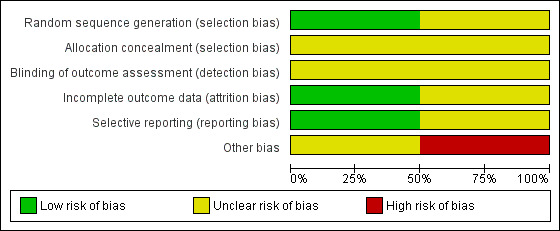
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
We classed Monson 2014 as being at unclear risk of bias, as the trialists reported no information about methods used to generate the randomisation sequence or allocation procedure. Ulas Biter 2014 was classed at low risk of bias.
Blinding
Neither study mentioned, or allude to, any kind of blinding.
Incomplete outcome data
We judged Monson 2014 to be at unclear risk of bias and Ulas Biter 2014 to be at low risk of bias for the domain of incomplete outcome data.
Selective reporting
We judged Monson 2014 to be at low risk of bias for the domain of selective reporting, as the trial report suggested that all outcome data collected were reported. Ulas Biter 2014 was classed at unclear risk of bias.
Other potential sources of bias
We classed Monson 2014 as being at high risk of bias as it seems that the time to event data presented in the trial report had been calculated using the incorrect statistical methods. Ulas Biter 2014 was classed at unclear risk of bias as it was not clear what methods had been used to calculate time to event data ‐ the use of non‐parametric methods was noted but with no further detail. Given the way data are presented it seems unlikely that time‐to‐event analysis was conducted.
Effects of interventions
Comparison 1: NPWT compared with alginate dressing (one study; 20 participants)
One study was included in this comparison (Monson 2014). It allocated people with an open and infected groin wound to either an alginate dressing or NPWT. The duration of the study follow‐up was unclear. We classed the study as being at unclear risk of selection bias and at low risk of attrition and reporting biases. We considered the time‐to‐healing data to be at high risk of bias, as it was not clear that the data had been analysed using the correct methods.
Primary outcome: time to wound healing
The reported median time to healing in the NPWT group was 57 days compared with a median time to healing in the alginate group of 104 days. The number of healing events were not presented, neither were variation data (only ranges around the medians were supplied, see Table 1). We considered these data to be of limited value as they did not seem to have been calculated in an appropriate way for time‐to‐event data. As a result of this we have not analysed these data further.
1. Study outcomes.
| Study ID | Length of follow‐up | Intervention Group A | Control Group B | Complete wound healing | Adverse events | Wound infection | Mean pain scores | Participant health‐related quality of life/health status | Resource use | Costs | Fascia closure | Wound closure |
| Monson 2014 | Unclear | NPWT (n = 10) |
Alginate dressing (n = 10) |
Time to healing* Median (range) Group A: 57 days (25‐115) Group B: 104 days (57‐175) *Not thought to be calculated using standard time‐to‐event statistical methods |
Amputation (per participant) Group A: 3/10 Group B: 2/10 Mortality Group A: 0/10 Group B: 1/10 |
Not reported | Not reported | Not reported | Not reported | Not reported | ||
| Ulas Biter 2014 | 6 months | NPWT | Open wound care (n=25) |
Time to healing Median (range) Group A: 84 days (34‐349) Group B: 93 days (43‐264) * The statistical methods used for this time‐to‐event analysis were not specified methods note that "statistical analysis appropriate for nonparametric data was used" it is not clear if censoring was accounted for. |
not reported |
Infection (and abscesses) Group A: 2 Group B: 2 |
Not reported | Not reported |
Time to resume work or school, (days) Median (range) Group A: 27 (7 to 126) Group B: 29 (6 to 63) |
Not reported | Not reported | Not reported |
Abbreviation
NPWT: negative pressure wound therapy
Primary outcome: adverse events
It was not clear how adverse event data had been collected. The study summarised data on death and amputation. There was no evidence of a difference in the number of participants with amputation between the NPWT group (0%: 0/10) and the alginate group (10%: 1/10): RR 1.50 (95% CI 0.32 to 7.14; Analysis 1.1). The study was very small and was underpowered for this comparison, which is imprecise and uncertain. There was no evidence of a difference in the number of participants who died between the NPWT group (30%: 3/10) and the alginate group (20%: 2/10): RR 0.33 (95% CIs 0.02 to 7.32; Analysis 1.2). Again the limited sample size and lack of outcome events mean this comparison is also underpowered and uncertain.We considered these data to be of very low quality due to imprecision.
1.1. Analysis.
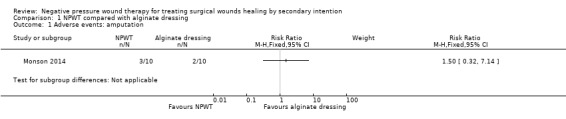
Comparison 1 NPWT compared with alginate dressing, Outcome 1 Adverse events: amputation.
1.2. Analysis.
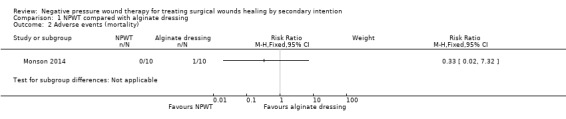
Comparison 1 NPWT compared with alginate dressing, Outcome 2 Adverse events (mortality).
No data on other review outcomes were presented
Comparison 2: NPWT compared with silicone wound dressing (one study; 49 participants)
One study was included in this comparison (Ulas Biter 2014) it allocated people undergoing surgical excision of a pilonidal sinus to treatment with NPWT for14 days following surgery and then standard care or standard wound care (use of a silicone wound dressing). We classed the study as being at unclear risk of selection and detection bias and low risk of attrition bias. We considered the time‐to‐healing data to be at unclear risk of bias, as it was not clear that the data had been analysed using the correct methods.
Primary outcome: time to wound healing
The reported median time to healing in the NPWT group was 84 days. This was compared with a median time to healing in the silicone group of 93 days. The number of healing events were not presented, neither were variation data (only ranges around the medians were supplied, see Table 1). We considered these data to be of limited value as it was not clear how they had been calculated. As a result of this we have not presented them further here.
Secondary outcomes: wound infection
Wound infection (defined as redness or pain in the wound) data was only presented combined with data on abscesses (defined as retention of pus in the wound and bleeding). We present these data although we can't be clear about how many of the events were infections per se. In total, 2 participants in the NPWT group (2/24 = 8%) and 2 participants in the silicone dressing group (2/25 = 8%) had an infection/abscess: RR: 1.04 95% CI 0.16 to 6.81 (Analysis 2.1). The limited sample size and lack of outcome events mean this comparison is also underpowered and uncertain. We considered these data to be of very low quality due to imprecision and potential indirectness of outcome.
2.1. Analysis.
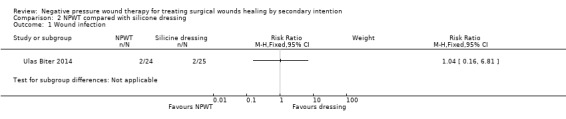
Comparison 2 NPWT compared with silicone dressing, Outcome 1 Wound infection.
Secondary outcomes: Resource use
The median number of days to resume school or work was 27 days in the NPWT group and 29 days in the silicone dressing group: only ranges around the medians were supplied, see Table 1). Data have not been analysed further.
No data on other review outcomes were presented
Discussion
Summary of main results
Two studies were included in this review (Monson 2014; Ulas Biter 2014). The studies included 20 participants (Monson 2014) and 49 participants (Ulas Biter 2014). We deemed the studies to be at unclear risk of selection and detection bias and at low risk of attrition bias. The studies were small and, as such, was underpowered. The data reported were limited and it is difficult to draw conclusions about the potential effects (positive or negative, or both) of NPWT from the studies.
Overall there is low quality and inconclusive evidence regarding the clinical effectiveness of NPWT as a treatment for surgical wounds healing by secondary intention.
Quality of the evidence
RCTs need to be adequately powered so that they are able to detect treatment effects of a specified size, if they exist. This means that sample size calculations should be used to help estimate the number of people that should be recruited to a trial. Additionally trials should have an adequate follow‐up period so that there is enough time in which important outcome events, such as complete wound healing, can occur.The trials included in this review were small, and the follow‐up period of the study was uncertain. This results in a limited evidence base, with further problems in quality caused by the reporting of limited outcomes. Wound healing, and preparation for closure surgery, as well as adverse events, constitute potentially important outcomes. Such outcomes should be collected rigorously with a clear methodology. Ultimately, there were few studies that could be considered for inclusion in the review, and the two studies included presented limited outcome data.
Rigorous RCTs in wound care are feasible ‐ they should follow good practice conduct and reporting guidelines e.g. CONSORT (Schulz 2010). Key areas of good practice are: the robust generation of a randomisation sequence, for example, computer generated; robust allocation concealment, for example the use of a telephone randomisation service; and blinded outcome assessment, where possible. All this information should be clearly stated in the study report as, these days, all trial authors should anticipate the inclusion of their trials in systematic reviews. Additionally, studies should clearly report how the collection of adverse event data was planned and how this process was standardised for both treatment arms. In terms of analysis, where possible, data from all participants should be included, that is an intention‐to‐treat analysis should be conducted, and measures of variation such as the standard deviation or standard error should be presented around measures where appropriate. Steps should be taken during the trial to prevent missing data as far as possible.
Potential biases in the review process
In this review we considered as much evidence as it was possible to obtain, including studies that were not published in English language journals. It is possible that there may be unpublished data that we have not been able to access, and there is a potential for publication bias, however, this is likely to be a limited issue.
Agreements and disagreements with other studies or reviews
This is the only review we are aware of that focuses on the effectiveness of NPWT on surgical wounds healing by secondary intention as we have defined them (that is excluding open abdomen wounds which are the subject of another Cochrane review that is in draft). Our results do agree with the general findings of a recently updated systematic review that looked at evidence for the effectiveness of NPWT in treating chronic and acute wounds (Peinemann 2011). This previous review concluded that "Although there may be a positive effect of NPWT, we did not find clear evidence that wounds heal any better or worse with NPWT than with conventional treatment. Good RCTs are still needed to evaluate NPWT."
Authors' conclusions
Implications for practice.
This comprehensive review of current randomised controlled trial (RCT) evidence highlights the current uncertainty regarding the effectiveness of NPWT as a treatment for surgical wounds healing by secondary intention. Given these uncertainties, practitioners' choice of treatment for open surgical wounds may be informed by the costs and symptom management properties of the options available.
Implications for research.
Where it is a priority for patients, carers and health professionals, further research to evaluate the clinical and cost effectiveness of NPWT is warranted. Large and robust RCTs would likely be the most appropriate study design, but other epidemiological research may be as important. Undertaking such studies represents an investment in terms of research costs as well as the opportunity cost of health professionals and patient time. Thus, any future research must follow good practice guidelines for design, implementation and reporting so that the data produced provide maximum use to patients, health professionals and policy makers.
Acknowledgements
The authors are grateful to the following peer reviewers for their time and comments: Duncan Chambers, Andrea Nelson, Elmer Villanueva, Janet Wale and Carolina Weller. The authors would like to acknowledge the contribution of copy‐editors Megan Prictor and Elizabeth Royle. We would also like to thank Evan Kontopantelis for his valuable advice generously provided. This report describes independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grant 13/89/08‐High Priority Cochrane Reviews in Wound Prevention and Treatment).
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) <1946 to July Week 2 2014> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Negative‐Pressure Wound Therapy/ 2 exp Suction/ 3 exp Vacuum/ 4 (negative pressure or negative‐pressure or TNP or NPWT).tw. 5 (sub‐atmospheric or subatmospheric).tw. 6 ((seal$ adj surface$) or (seal$ adj aspirat$)).tw. 7 (wound adj2 suction$).tw. 8 (wound adj5 drainage).tw. 9 ((foam adj suction) or (suction adj dressing$)).tw. 10 (vacuum assisted closure technique or VAC).tw. 11 ((vacuum adj therapy) or (vacuum adj dressing$) or (vacuum adj seal$) or (vacuum adj closure) or (vacuum adj compression) or (vacuum adj pack$) or (vacuum adj drainage) or (suction$ adj drainage)).tw. 12 or/1‐11 13 exp Surgical Wound Infection/ 14 exp Surgical Wound Dehiscence/ 15 (surg* adj5 infect*).tw. 16 (surg* adj5 wound*).tw. 17 (surg* adj5 site*).tw. 18 (surg* adj5 incision*).tw. 19 (surg* adj5 dehisc*).tw. 20 (wound* adj5 dehisc*).tw. 21 (wound* adj5 infect*).tw. 22 (wound adj5 disrupt*).tw. 23 wound complication*.tw. 24 or/13‐23 25 (intent* or second* or heal* or complic*).tw. 26 ((open* or clos*) adj5 wound*).tw. 27 25 or 26 28 24 and 27 29 12 and 28 30 randomised controlled trial.pt. 31 controlled clinical trial.pt. 32 randomi?ed.ab. 33 placebo.ab. 34 clinical trials as topic.sh.
35 randomly.ab.
36 trial.ti. 37 exp animals/ not humans.sh. 38 29 and 37
Database: Embase <1974 to 2014 July 16> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Suction drainage/ 2 exp Vacuum assisted closure/ 3 (negative pressure or negative‐pressure or TNP or NPWT).tw. 4 (sub‐atmospheric or subatmospheric).tw. 5 ((seal$ adj surface$) or (seal$ adj aspirat$)).tw. 6 (wound adj2 suction$).tw. 7 (wound adj5 drainage).tw. 8 ((foam adj suction) or (suction adj dressing$)).tw. 9 (vacuum assisted closure technique or VAC).tw. 10 ((vacuum adj therapy) or (vacuum adj dressing$) or (vacuum adj seal$) or (vacuum adj closure) or (vacuum adj compression) or (vacuum adj pack$) or (vacuum adj drainage) or (suction$ adj drainage)).tw. 11 or/1‐10 12 exp Surgical Wound Infection/ 13 exp Surgical Wound Dehiscence/ 14 (surg* adj5 infect*).tw. 15 (surg* adj5 wound*).tw. 16 (surg* adj5 site*).tw. 17 (surg* adj5 incision*).tw. 18 (surg* adj5 dehisc*).tw. 19 (wound* adj5 dehisc*).tw. 20 (wound* adj5 infect*).tw. 21 (wound adj5 disrupt*).tw. 22 wound complication*.tw. 23 or/12‐22 24 (intent* or second* or heal* or complic*).tw. 25 ((open* or clos*) adj5 wound*).tw. 26 24 or 25 27 23 and 26 28 11 and 27 29 Randomized controlled trials/ 30 Single‐Blind Method/ 31 Double‐Blind Method/ 32 Crossover Procedure/ 33 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 34 (doubl$ adj blind$).ti,ab. 35 (singl$ adj blind$).ti,ab. 36 or/29‐35 37 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 38 human/ or human cell/ 39 and/37‐38 40 37 not 39 41 36 not 40 42 28 and 41
CINAHL SS July 17, 2014 S40 S27 AND S39 S39 S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 S38 MH "Quantitative Studies" S37 TI placebo* or AB placebo* S36 MH "Placebos" S35 TI random* allocat* or AB random* allocat* S34 MH "Random Assignment" S33 TI randomi?ed control* trial* or AB randomi?ed control* trial* S32 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S31 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S30 TI clinic* N1 trial* or AB clinic* N1 trial* S29 PT Clinical trial S28 MH "Clinical Trials+" S27 S12 AND S26 S26 S22 AND S25 S25 S23 OR S24 S24 TI ( ((open* or clos*) n5 wound*) ) OR AB ( ((open* or clos*) n5 wound*) ) S23 TI ( intent* or second* or heal* or complic* ) OR AB ( intent* or second* or heal* or complic* ) S22 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 S21 TI wound complication* or AB wound complication* S20 TI wound* N5 dehisc* or AB wound* N5 dehisc* S19 TI surg* N5 dehisc* or AB surg* N5 dehisc* S18 TI surg* N5 incision* or AB surg* N5 incision* S17 TI surg* N5 site* or AB surg* N5 site* S16 TI surg* N5 wound* or AB surg* N5 wound* S15 TI surg* N5 infection* or AB surg* N5 infection* S14 (MH "Surgical Wound Dehiscence") S13 (MH "Surgical Wound Infection") S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 TI foam suction or suction dressing* or suction drainage or AB foam suction or suction dressing* or suction drainage S10 AB vacuum therapy or vacuum dressing* or vacuum seal* or vacuum closure or vacuum compression or vacuum pack or vacuum drainage S9 TI vacuum therapy or vacuum dressing* or vacuum seal* or vacuum closure or vacuum compression or vacuum pack or vacuum drainage S8 TI wound N5 drainage or AB wound N5 drainage S7 TI wound N5 suction* or AB wound N5 suction* S6 TI seal* N1 surface* or seal* N1 aspirat* or AB seal* N1 surface* or seal* N1 aspirat* S5 TI sub‐atmospheric or subatmospheric or AB sub‐atmospheric or subatmospheric S4 TI negative pressure or negative‐pressure or TNP or AB negative pressure or negative‐pressure or TNP S3 (MH "Negative Pressure Wound Therapy") S2 (MH "Vacuum") S1 (MH "Suction+")
Appendix 2. Risk of bias assessment (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process available to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information available to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding (participants and personnel, outcome assessment) ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement were not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following.
Insufficient information available to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information available to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Risk of bias (cluster randomised controlled trials)
In cluster‐randomised trials, particular biases to consider include:
recruitment bias;
baseline imbalance;
loss of clusters;
incorrect analysis; and
comparability with individually randomised trials.
Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Baseline imbalance: cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Loss of clusters: occasionally complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
Incorrect analysis: many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a 'unit of analysis error' and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
Comparability with individually randomised trials: in a meta‐analysis that includes both cluster and individually randomised trials, or cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by a Cochrane review of hip protectors discussed by Hahn 2005, where cluster trials showed a large positive effect whereas individually randomised trials did not show any clear benefit. One possible explanation for this is that there was a 'herd effect' in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such 'contamination' would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated, despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and 'herd effects' may be different for different types of cluster.
Data and analyses
Comparison 1. NPWT compared with alginate dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events: amputation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Adverse events (mortality) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. NPWT compared with silicone dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Monson 2014.
| Methods | 2‐arm RCT Undertaken in 1 centre in Sweden Follow‐up time was unclear, it seems that wounds might have been followed for 4 months, but this is not certain |
|
| Participants | 20 participants who had a groin infection in a surgical wound following arterial surgery. The wounds were revised and then left to heal by secondary intention Inclusion criteria: deep perivascular groin infections (Szilagyi grade III) that occurred after arterial surgery Exclusion criteria: none listed |
|
| Interventions | Group A: NPWT (VAC system: KCL‐Medical, San Antonio Texas, USA; n = 10). VAC therapy was started the day after the surgical revision, and a polyurethane sponge was applied with a continuous topical negative pressure of 125 mmHg. Changes of VAC dressings were performed 3 times/week Group B: Alginate dressing (Sorbalgon HARTMANN ScandiCare AB, Anderstorp, Sweden or Melgisorb (Mölnlycke Health Care AB, Götenburg, Sweden); n = 10). Reported that after adequate haemostasis Aquacel (ConvaTec, Bromma, Sweden) was applied to fill the cavity and an outer wound dressing Sterilet Ontex (Selefa Trade AB, Spånga, Sweden), was placed over this. Dressings were changed as often as indicated clinically |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Reported as an interim analyses. Planned sample size was 42 participants Mean size of wound was larger in the dressing group (20.5 cm²) than the NPWT group (13 cm²) The paper presented median time to healing data, but the statistical section suggests that this was calculated as a median (and a range) with the group difference analysed with the Mann‐Whitney U test. It was not clear from the paper whether time‐to‐event methods were used to take time into account in the analysis of healing events Source of funding: not reported, but authors noted no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed by C. M. after surgical debridement, where each patient withdrew a marked note, “VAC” or “alginate,” from an envelope." Comment: method of sequence generation unclear. No other detail reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by C. M. after surgical debridement, where each patient withdrew a marked. note, “VAC” or “alginate,” from an envelope." Comment: method of allocation unclear. No other detail reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods seem to be reported. No protocol obtained |
| Other bias | High risk | Quote: "Continuous variables were expressed in median and range, and group difference analyzed with the Mann‐Whitney U test." Comment: no mention of time‐to‐event methods to produce median time to healing data. It is not clear how the median values were calculated. The number of healed events was not reported |
Ulas Biter 2014.
| Methods | 2‐arm RCT Undertaken in one centre in The Netherlands Follow‐up period of 6 months. Participants were assessed post‐operatively at days 3, 7, 10 and 14. After 2 weeks, all patients visited the outpatient clinic on a weekly basis for inspection and wound measurement until the wound was closed completely. Six months after wound closure, there was 1 final check. |
|
| Participants | 49 participants who underwent surgery to excise a pilonidal sinus. Inclusion criteria: a symptomatic pilonidal sinus with or without a previous abscess of the sinus. Exclusion criteria: less than 16 years of age, a previous attempt at surgical excision of pilonidal disease; the inability to undergo frequent follow‐up; pilonidal sinus situated less than 3 cm from the anus (as the NPWT device could not be placed into position locally). |
|
| Interventions | Group A: NPWT (N=24) (no details of type) applied directly after surgery for 14 days. First, a sponge was positioned in the wound on top of the surrounding skin. A topical skin adhesive (Dermabond) was applied to the borders to prevent skin damage. The NPWT device (consisting of an open‐pore foam covered by an adhesive semipermeable dressing) was applied. A negative pressure of 125mm Hg was adjusted to the wound 24 hours a day. During follow‐up, the sponge was replaced at postoperative days 3, 7, and 10 at the outpatient clinic. At day 14, NPWT was finished, and regular wound care was started: patients were advised to rinse the wound 3 times daily until a superficial wound was achieved; hereafter, the rinsing frequency was reduced. Group B: (N=25) Standard open wound care. A silicone wound dressing was applied with an absorbent bandage on top. Patients were advised to rinse the wound 3 times daily during the first 2 weeks after excision. No special dressings were applied, unless the wound appeared sloughy and/or retention of pus was noted. The same surgical technique was applied in all patients. Paracetamol used advised for pain relief with use of non‐steroidal anti‐inflammatory drugs if required. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Source of funding: not reported, but authors noted no financial disclosures Some events that could be considered adverse events were reported for the NPWT group including number of participants reporting bad odour, pain, noise and air leakage. The number of patients reporting wound leakage was reported in the dressing group. As comparable data has not clearly been reported these data have not been extracted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization took place 1 day before the scheduled operation to facilitate the presence of a NPWT device in the operating room when required. The randomization process was conducted by the principal investigator of the study by using a computer‐generated randomization list without any restrictions such as block size." Comment: Adequate generation of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No information report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "After surgical excision, the installation of the NPWT device was not possible in 2 patients who were randomly assigned to NPWT because of the anatomical site of the wound (close to the anus)... ....Early termination of NPWT (at a median of 7 days postoperatively) was seen in 4 of 24 patients (17%) owing to pain (n = 2), bad odour (n = 1), or practical considerations (n = 1). Two patients were lost to follow‐up after having completed the 2‐week examination. All 49 patients were included in the current data analysis according to the intention‐to‐treat principle." Comment: An intention to treat approach appears to have been followed |
| Selective reporting (reporting bias) | Unclear risk | Reports that pain was collected using visual analogue scale ‐ these data are not reported. |
| Other bias | Unclear risk | Quote: States that "Data analysis was performed in SPSS version 17.0 (SPSS, Chicago, IL). Statistical analysis appropriate for nonparametric data was used." Comment: Not clear if time to event data were correctly analysed. |
Abbreviation
NPWT: negative pressure wound therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albert 2012 | Wound type unclear ‐ we contacted the authors |
| Bee 2008 | Participants did not have open surgical wounds |
| Braakenburg 2006 | Study population had range of wounds ‐ unable to contact authors to assess whether any wounds were relevant to this review |
| de Laat 2011 | No relevant outcome data |
| Dorafshar 2011 | No relevant outcome data ‐ we were unable to contact authors |
| Hu 2009 | Study population had range of wounds ‐ data unavailable for population of interest: based on translation |
| Huang 2006 | We were unclear whether this was an RCT and whether the wounds were all open surgical wounds. We contacted the authors |
| Jeschke 2004 | No relevant outcome data ‐ we contacted the authors who confirmed that the study wounds were open surgical wounds; no healing data currently available |
| Lei 2011 | Study population had range of wounds ‐ data unavailable for population of interest: based on translation |
| Liao 2012 | Participants did not have open surgical wounds |
| Mees 2012 | Not an RCT |
| Mody 2008 | Study population had range of wounds ‐ we contacted the authors to assess whether any data were relevant to this review |
| Moues 2005 | Mixed group ‐ we contacted the author; data unavailable for population of interest |
| Oh 2013 | Participants did not have open surgical wounds |
| Orgill 2004 | Study population had range of wounds ‐ we contacted the authors to assess whether any data were relevant to this review |
| Pachowsky 2012 | Participants did not have open surgical wounds |
| Pan 2013 | Not an RCT |
| Pellino 2014 | Not an RCT |
| Perez 2010 | Study population had range of wounds ‐ we contacted the authors; open surgical wound data not available |
| Pliakos 2010 | Delivery of NPWT was not the only systematic difference between study groups |
| Quantz 2010 | Participants did not have open surgical wounds |
| Stannard 2009 | Participants did not have open surgical wounds |
| Tuncal 2013 | Participants did not have open surgical wounds |
| Walker 2005 | No information available regarding the participants in this study. We could not located a full publication. The study data were also unclear, but 2005 was listed as the date when information was last accessed by authors of other systematic reviews from which this citation was located |
Abbreviations
NPWT: negative pressure wound therapy RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Bayer 2004.
| Methods | No further details |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | As yet unable to obtain paper/abstract |
Herrle 2010.
| Methods | Title suggests a RCT |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | As yet unable to obtain paper |
Kutovoi 2011.
| Methods | Unclear from abstract |
| Participants | Complex wounds ‐ no further detail |
| Interventions | Described in abstract as vacuum‐therapy |
| Outcomes | Possibly healing ‐ unclear from available abstract |
| Notes | As yet unable to obtain paper |
Characteristics of ongoing studies [ordered by study ID]
Seidel 2013.
| Trial name or title | SAWHI‐V.A.C.®‐study |
| Methods | "The trial compares the treatment outcome of the application of a technical medical device which is based on the principle of negative pressure wound therapy (intervention group) and standard conventional wound therapy (control group) in the treatment of subcutaneous abdominal wounds after surgery." |
| Participants | People with subcutaneous abdominal wound healing impairment after surgery |
| Interventions | NPWT versus standard care |
| Outcomes | Time to healing. Economic evaluation also planned |
| Starting date | To be confirmed |
| Contact information | Doerthe.Seidel@uni‐wh.de |
| Notes | Seidel D, Lefering R, Neugebauer EA Treatment of subcutaneous abdominal wound healing impairment after surgery without fascial dehiscence by vacuum assisted closureTM (SAWHI‐V.A.C.‐study) versus standard conventional wound therapy: study protocol for a randomised controlled trial Trials 2013;14;394 |
Abbreviation
NPWT: negative pressure wound therapy
Differences between protocol and review
We removed wound recurrence as an outcome as it was not deemed to be a key measure for this review question. We added two secondary outcomes on wound closure and closure of the fascia. It is increasingly apparent that some clinical aims of using of NPWT are to reduce time to closure surgery or to achieve closure of fascia. Whilst healing is still the primary outcome for these wounds, we felt it was important to include these intermediate outcomes here. It was also decided to exclude open abdominal wounds from this review, as they are the subject of another Cochrane review.
Two studies were included in this review ‐ thus planned data pooling and investigation of reporting biases, heterogeneity and sub‐group analysis that were planned where possible were not conducted.
Contributions of authors
Jo Dumville: conceived, designed and co‐ordinated the review; extracted data, checked quality of data extraction, and analysed and interpreted data; performed statistical analysis; completed the first draft of the review and approved final version before submission; secured funding and is the guarantor of the review. Gemma Owens: designed the review; analysed and interpreted data; completed the first draft of the review, and approved final version before submission. Emma Crosbie: conceived and designed the review; analysed and interpreted data; completed the first draft of the review, and approved final version before submission. Frank Peinemann: designed the review; checked quality of data extraction; analysed and interpreted data; completed the first draft of the review, and approved final version before submission. Zhenmi Liu: extracted data, checked quality of data extraction and undertook quality assessment; completed the first draft of the review, and approved final version before submission.
Contributions of editorial base
Joan Webster, Editor: commented on the review and approved the review for submission. Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; edited the protocol and the review. Amanda Briant: designed the search strategy.
Sources of support
Internal sources
School of Nursing, Midwifery and Social Work. University of Manchester, UK.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 – High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Jo Dumville: none known. Gemma Owens: none known. Emma Crosbie: I am a Scientific Editor for BJOG. I have received funding from an NIHR Clinician Scientist Award, the HTA, Wellbeing of Women/the Wellcome Trust and Central Manchester University Hospitals NHS Foundation Trust. I am an employee of the University of Manchester. I received funding from GSK in 2012 to attend EUROGIN in Portugal and the International HPV Conference in Germany. Frank Peinemann: none known. Zhenmi Lui: none known.
New
References
References to studies included in this review
Monson 2014 {published data only}
- Acosta S, Monsen C, Dencker M. Clinical outcome and microvascular blood flow in VAC‐ and Sorbalgon‐treated peri‐vascular infected wounds in the groin after vascular surgery ‐ an early interim analysis. International Wound Journal 2013;10:377‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsen C, Wann‐Hansson C, Wictorsson C, Acosta S. Vacuum‐assisted wound closure versus alginate for the treatment of deep perivascular wound infections in the groin after vascular surgery. Journal of Vascular Surgery 2014;59:145‐51. [DOI] [PubMed] [Google Scholar]
Ulas Biter 2014 {published data only}
- Biter LU, Beck GMN, Mannaerts GHH, Stok MM, Ham AC, Grotenhuis BA. The use of negative‐pressure wound therapy in pilonidal sinus disease: a randomized controlled trial comparing negative‐pressure wound therapy versus standard open wound care after surgical excision. Diseases of the colon and rectum 2014;57:1406‐1411. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albert 2012 {published data only}
- Albert NM, Rock R, Sammon MA, Bena JF, Morrison SL, Whitman A, et al. Do patient and nurse outcome differences exist between two negative pressure wound therapy systems?. Journal of Wound Ostomy and Continence Nursing 2012;39:259‐66. [DOI] [PubMed] [Google Scholar]
Bee 2008 {published data only}
- Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO, Minard G, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum‐assisted closure. Journal of Trauma 2008;65:337‐42. [DOI] [PubMed] [Google Scholar]
Braakenburg 2006 {published data only}
- Braakenburg A, Obdeijn MC, Feitz R, Rooij IA, Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plastic and Reconstructive Surgery 2006;118:390‐7. [DOI] [PubMed] [Google Scholar]
de Laat 2011 {published data only}
- Laat EHEW, Boogaard MHWA, Spauwen PHM, Kuppevelt DHJM, Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult‐to‐heal wound. A prospective randomized controlled trial. Annals of Plastic Surgery 2011;67:626–31. [DOI] [PubMed] [Google Scholar]
Dorafshar 2011 {published data only}
- Dorafshar AH, Franczyk M, Gottlieb LJ, Wroblewski KE, Lohman RF. A prospective randomized trial comparing subatmospheric wound therapy with a sealed gauze dressing and the standard vacuum‐assisted closure device. Annals of Plastic Surgery 2011;27:79‐84. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu KX, Zhang HW, Zhou F, Yao G, Shi JP, Cheng Z, et al. Observation on the therapeutic effects of negative‐pressure wound therapy on the treatment of complicated and refractory wounds. Chinese Journal of Burns 2009;25:249‐52. [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang WS, Hsieh SC, Hsieh CS, Schoung JY, Huang T. Use of vacuum‐assisted wound closure to manage limb wounds in patients suffering from acute necrotizing fasciitis. Asian Journal of Surgery 2006;29:135‐9. [DOI] [PubMed] [Google Scholar]
Jeschke 2004 {published data only}
- Jeschke MG, Rose C, Angele P, Faffchtmeier B, Nerlich MN, Bolder U. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative‐pressure therapy for reconstruction of acute and chronic wounds. Plastic and Reconstructive Surgery 2004;113:525‐30. [DOI] [PubMed] [Google Scholar]
Lei 2011 {published data only}
- Lei J, Li HS, Hao ZM, Duan P, Hao WJ. Mode of debridement, negative‐pressure therapy combined with tissue transplantation for treatment of complicated and refractory wounds. Chinese Journal of Burns 2011;27:456‐60. [PubMed] [Google Scholar]
Liao 2012 {published data only}
- Liao Q, Xu J, Weng X, Zhong D, Liu Z, Wang C. Effectiveness of vacuum sealing drainage combined with anti‐taken skin graft for primary closing of open amputation wound. Chinese Journal of Reparative and Reconstructive Surgery 2012;26:558‐62. [PubMed] [Google Scholar]
Mees 2012 {published data only}
- Mees ST, Palmes D, Mennigen R, Senninger N, Haier J, Bruewer M. Endo‐vacuum assisted closure treatment for rectal anastomotic insufficiency. Diseases of the Colon and Rectum 2008;51:404‐10. [DOI] [PubMed] [Google Scholar]
Mody 2008 {published data only}
- Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy/Wound Management 2008;54:36‐46. [PubMed] [Google Scholar]
Moues 2005 {published data only}
- Moues CM, Bemd GJCM, Heule F, Hovius SER. A prospective randomized trial comparing vacuum therapy to conventional moist gauze therapy. 2nd World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris. 2004:Abstract no A001.
- Moues CM, Bemd GJCM, Meerding WJ, Hovius SER. Cost analysis comparing vacuum‐assisted closure wound therapy to conventional moist gauze therapy. 2nd World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris. 2004; Vol. 87:Abstract no A008.
- Moues CM, Vos MC, Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair and Regeneration 2004;12:11‐7. [DOI] [PubMed] [Google Scholar]
- Moues CM, Vos MC, Bemd GJCM, Stijnen T, Hovius SER. Bacterial load in relation to vacuum‐assisted closure wound therapy. 13th conference of the European Wound Management Association; 2003 May 22‐24; Pisa, Italy. 2003:Presentation 42.
- Moues CM, Bemd GCM, Meerding WJ, Hovius SER. An economic evaluation of the use of topical negative pressure on full‐thickness wounds. Journal of Wound Care 2005;14:224‐7. [DOI] [PubMed] [Google Scholar]
- Moues CM, Bemd GJCM, Heule F, Hovius SER. Comparing conventional gauze therapy to vacuum‐assisted closure wound therapy: a prospective randomised trial. Journal of Plastic and Reconstructive Aesthetic Surgery 2007;60:672‐81. [DOI] [PubMed] [Google Scholar]
Oh 2013 {published data only}
- Oh BH, Lee SH, Nam KA, Lee HB, Chung KY. Comparison of negative pressure wound therapy and secondary intention healing after excision of acral lentiginous melanoma on the foot. British Journal of Dermatology 2013;168:333–8. [DOI] [PubMed] [Google Scholar]
Orgill 2004 {published data only}
- Orgill DP, Bayer L. Preliminary results indicate VAC therapy facilitates faster closure of open abdominal wounds. 2nd World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris. 2004:77.
Pachowsky 2012 {published data only}
- Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. International Orthopaedics 2012;36:712‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2013 {published data only}
- Pan A, Angelis G, Nicastri E, Sganga G, Tacconelli E. Topical negative pressure to treat surgical site infections,with a focus on post‐sternotomy infections: a systematic review and meta‐analysis. Infection 2013;41:1129–35. [DOI] [PubMed] [Google Scholar]
Pellino 2014 {published data only}
- Pellino G, Sciaudone G, Candilio G, Campitiello F, Selvaggi F, Canonico S. Effects of a new pocket device for negative pressure wound therapy on surgical wounds of patients affected with Crohn’s disease: a pilot trial. Surgical Innovation 2014;21:204–12. [DOI] [PubMed] [Google Scholar]
Perez 2010 {published data only}
- Perez D, Bramkamp M, Exe C, Ruden C, Ziegler A. Modern wound care for the poor: a randomized clinical trial comparing the vacuum system with conventional saline‐soaked gauze dressings. American Journal of Surgery 2010;199:14‐20. [DOI] [PubMed] [Google Scholar]
Pliakos 2010 {published data only}
- Pliakos I, Papavramidis TS, Mihalopoulos N, Koulouris H, Kesisoglou I, Sapalidis K, et al. Vacuum‐assisted closure in severe abdominal sepsis with or without retention sutured sequential fascial closure: a clinical trial. Surgery 2010;148:947‐53. [DOI] [PubMed] [Google Scholar]
Quantz 2010 {published data only}
- Quantz MA. Does incisional VAC therapy (IVAC) reduce wound complications in high risk patients?. Canadian Cardiovascular Congress 2010 Abstracts; Montreal, QC Canada, October. 2010:145D.
Stannard 2009 {published data only}
- Stannard JP, Volgas DA, Stewart R, McGwin G Jr, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. Journal of Orthopaedic Trauma 2009;23:552‐7. [DOI] [PubMed] [Google Scholar]
Tuncal 2013 {published data only}
- Tuncel U, ErkorkmazU, Turan A. Clinical evaluation of gauze‐based negative pressure wound therapy in challenging wounds. International Wound Journal 2013;10:152‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2005 {published data only}
- Walker PA. Randomised, prospective trial of standard wound drainage medinorm vs constant vacuum drainage to determine whether there is any effect on the amount of wound exudate, haemoglobin & wound bruising. National Research Register, Department of Health: London, United Kingdom 2005.
References to studies awaiting assessment
Bayer 2004 {published data only}
- Bayer L, A‐C P, Orgill DP. Has the Wound Vac become the standard of care for sternal wounds?. 2nd World Union of Wound Healing Societies Meeting; ,8‐13 July; Paris. 2004; Vol. 76.
Herrle 2010 {published data only}
- Herrle F, Jonescheit J‐O, Kirby J, Kienle P, Post S, Niedergethmann M. Open abdomen treatment with vacuum‐therapy randomized pilot‐trial comparing fascial closure and survival with abdominal‐dressing versus vacuum‐pack‐technique‐rationale and design of the ABDOVAC‐trial. Inflammation Research 2010 2010;59:s136. [Google Scholar]
Kutovoi 2011 {published data only}
- Kutovoi AB, Kosul'nikov SO, Tarnopol'skii SA, Karpenko SI, Kravchenko KV. Treatment of purulent wounds using vacuum‐therapy. Klinichna khirurhiia 2011;6:59‐61. [PubMed] [Google Scholar]
References to ongoing studies
Seidel 2013 {published data only}
- Seidel D, Lefering R, Neugebauer EAM. Treatment of subcutaneous abdominal wound healing impairment after surgery without fascial dehiscence by vacuum assisted closure™(SAWHI‐V.A.C.®‐study) versus standard conventional wound therapy: study protocol for a randomized controlled trial. Trials 2013;14:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ashby 2015
- Ashby R, Dumville JC, Oswald AV, Fletcher M, Cullum NA, Chetter IC. A survey of surgical wounds healing by secondary intention: assessment of prevalence, aetiology, duration and management (paper in preparation). personal communication. [DOI] [PMC free article] [PubMed]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The CochraneCollaboration, 2011. Available from www.cochrane‐handbook.org.
Department of Health and Human Services 2009
- Department of Health and Human Services, Office of Inspector General. Comparison of prices for negative pressure wound therapy pumps (Edition: OEI‐02‐07‐00660). oig.hhs.gov/oei/reports/oei‐02‐07‐00660.pdf (accessed Dec 2012) 2009.
Dumville 2013
- Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI] [PubMed] [Google Scholar]
FDA 2011
- US Food, Drug Administration. FDA Safety Communication: update on serious complications associated with negative pressure wound therapy systems. www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm244211.htm (accessed November 2012) 2011.
Guy 2012
- Guy H, Grothier I. Using negative pressure therapy in wound healing. Nursing Times 2012;108:18, 20. [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
KCI 2012
- Kinetic Concepts Inc. Science behind wound therapy. /www.kci1.com/KCI1/sciencebehindwoundtherapy (accessed 11 May 2012).
Kontopantelis 2012
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed. Statistical Methods Methodology Research 2012;21:409‐26. [DOI] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analysis. PLoS One 2013;8:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 (updated March 2011) edition. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2011
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCaughan 2015
- McCaughan D, Sheard L, Dumville JC, Cullum NA, Chetter IC. Patients’ perceptions and experiences of living with a surgical wound healing by secondary intention: an in‐depth qualitative study (paper in preparation). personal communication. [DOI] [PMC free article] [PubMed]
NICE 2013
- National Institute for Health and Care Excellence. Negative pressure wound therapy for the open abdomen. NICE interventional procedure guidance 467. www.nice.org.uk/nicemedia/live/14315/65841/65841.pdf. Accessed 20th Feb 2015 2013.
Parmar 1998
- Parmar MK, Torri V, Stewert L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Peinemann 2011
- Peinemann F, Sauerland S. Negative‐pressure wound therapy systematic review of randomized controlled trials. Deutsches Arzteblatt International 2011;108:381‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2011
- Scottish Intercollegiate Guidelines Network. Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed December 2012) 2011.
Srinivasaiah 2007
- Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north‐east England. Journal of Wound Care 2007;16:413‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18:2693‐708. [DOI] [PubMed] [Google Scholar]
Vermeulen 2004
- Vermeulen H, Ubblink DT, Goossens A, Vos R, Legemate DA, Westerbos SJ. Dressings and topical agents for surgical wounds healing by secondary intention. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003554.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vowden 2009
- Vowden KR, Vowden P. The prevalence, management and outcome for acute wounds identified in a wound care survey within one English health care district. Journal of Tissue Viability 2009;18:7‐12. [DOI] [PubMed] [Google Scholar]


