Abstract
Background
Acute otitis media (AOM) is a common problem in children, for which amoxicillin, with or without clavulanate, is frequently prescribed as a treatment of choice. The conventional recommendation is either three or four daily doses. However, nowadays it is frequently prescribed as once or twice daily doses. If once or twice daily amoxicillin, with or without clavulanate, is as effective for acute otitis media as three or four times a day, it may be more convenient to give the medication once or twice a day to children and hence improve compliance.
Objectives
To compare the effectiveness of one or two daily doses with three or four daily doses of amoxicillin, with or without clavulanate, for the treatment of AOM in children; and to compare complication rates and adverse reactions.
Search methods
We searched CENTRAL 2013, Issue 2, MEDLINE (January 1950 to March week 1, 2013), EMBASE (1974 to March 2013) and the Science Citation Index (2001 to March 2013).
Selection criteria
We included randomised controlled trials (RCTs) of children aged 12 years or younger with AOM, diagnosed by acute ear pain (otalgia) and inflamed ear drum (confirmed by positive tympanocentesis or tympanogram of type B or C).
Data collection and analysis
Two review authors independently extracted data on treatment outcomes from individual trials and assessed trial quality based on selection bias, performance bias and detection bias, attrition bias, reporting bias and other biases. We defined the quality grading as low risk of bias, high risk of bias or unclear risk of bias. We summarised the results as risk ratio (RR) with 95% confidence intervals (CI).
Main results
We included five studies with 1601 children in the review. Pooled analysis demonstrated that the following outcomes were comparable between the two groups: clinical cure at the end of therapy (RR 1.03, 95% CI 0.99 to 1.07); during therapy (RR 1.06, 95% CI 0.85 to 1.33) and at follow‐up (RR 1.02, 95% CI 0.95 to 1.09); recurrent AOM (RR 1.21, 95% CI 0.52 to 2.81); compliance rate (RR 1.04, 95% CI 0.98 to 1.10) and overall adverse events (RR 0.92, 95% CI 0.52 to 1.63). When we performed subgroup analysis separately for trials with amoxicillin only and amoxicillin/clavulanate only, it showed that all important outcomes were comparable between once or twice daily groups and the three times daily group. The risk of bias amongst the five included studies was as follows: for random sequence generation we graded two studies as low and three unclear risk of bias; for allocation concealment all studies were at unclear risk of bias; for blinding (performance and detection bias) we graded four as high and one as unclear risk of bias; for incomplete outcome data (attrition bias) we graded two low, two high and one as unclear risk of bias; for reporting bias four were at low and one at high risk; and for ‘other’ bias four were at low and one at unclear risk of bias.
Authors' conclusions
This review showed that the results of using once or twice daily doses of amoxicillin, with or without clavulanate, were comparable with three doses for the treatment of AOM.
Keywords: Child; Humans; Acute Disease; Amoxicillin; Amoxicillin/administration & dosage; Anti‐Bacterial Agents; Anti‐Bacterial Agents/administration & dosage; Clavulanic Acid; Clavulanic Acid/administration & dosage; Drug Administration Schedule; Drug Therapy, Combination; Drug Therapy, Combination/methods; Otitis Media; Otitis Media/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Dosage intervals of amoxicillin for the treatment of acute middle ear infection
Acute middle ear infection (acute otitis media) is a very common disease in children and may cause pain and hearing loss. Delayed or ineffective treatment may lead to serious complications such as ear drum perforation, sensorineural hearing loss or the disease becoming chronic. Amoxicillin, with or without clavulanate, is the most commonly used antibiotic for treating acute otitis media. Currently, a reduction in the dosing interval to one or two daily doses is being used, in preference to the conventional three or four daily doses, to aid compliance.
We identified five randomised clinical studies with 1601 children comparing two dosing schedules. Participants were aged 12 years or younger with AOM. The primary outcome was clinical cure rate in terms of resolution of otalgia and fever at the end of antibiotic therapy (days seven to 15). The secondary outcomes were clinical cure rate in terms of middle ear effusion during therapy, clinical cure rate post‐treatment (one to three months) in terms of resolution of middle ear infection, AOM complications and adverse events to medication. The results showed that treating acute middle ear infection with either once/twice daily or three times daily amoxicillin, with or without clavulanate, has the same results using our outcome measures, including adverse events such as diarrhoea and skin reactions.
The evidence is current to March 2013.
Background
Description of the condition
Acute otitis media (AOM) is one of the most common diseases in children. During the first six months of life about 48% of infants have one episode of AOM or otitis media with effusion (OME) and about 20% have two or more episodes (Daly 1999). The peak incidence of AOM occurs between 6 and 12 months of age. It has been found that by the end of the first year of life 62.4% of infants have had one or more episodes of AOM and 17.3% have had three or more episodes (Teele 1989). The risk of developing another episode within one month after the onset of the primary infection is estimated at 35% (Carlin 1987).
The common bacterial pathogens of AOM are Streptococcal pneumoniae (S. pneumoniae), Haemophilus influenzae (H. influenzae) and Moraxella catarrhalis (M. catarrhalis) (Jacobs 1998). These bacteria are becoming increasingly resistant to antibiotics (Barnett 1995; Faden 1994; Henderson 1988; Johnson 1996; Kaplan 1995). Systematic reviews have demonstrated that in uncomplicated AOM, about 20 children must be treated with antibiotics to prevent one child having some pain after two days (Venekamp 2013) and about eight children must receive antibiotics to avoid one clinical failure (Rosenfeld 2001). A study by Rovers 2006 demonstrated that antibiotics seemed to have most benefit in children younger than two years of age with bilateral AOM, and in children with both AOM and otorrhoea. However, the emergence of multiple‐drug resistant strains, particularly S. pneumoniae, complicates the management of AOM and increases the risk of treatment failure. Antibiotics are frequently used for AOM in the US (Froom 1997; Venekamp 2013), where it is the most frequent disease that antibiotics are used to treat in outpatient departments (McCaig 1995). In contrast, the national Dutch guidelines recommend that children be treated for symptoms but do not receive antibiotics unless fever or pain persists (Froom 1997).
Description of the intervention
Due to growing bacterial resistance, the Center for Disease Control and Prevention and the American Academy of Pediatrics promotes the judicious use of antibiotics in the treatment of AOM. Antibiotic therapy remains an appropriate treatment option for most children with AOM because spontaneous cure rates are lower in complicated AOM and AOM secondary to S. pneumoniae infection. When amoxicillin, the treatment of choice in AOM, is not effective or not tolerated in children, an alternative antibiotic such as amoxicillin/clavulanate, or second‐ and third‐generation cephalosporins, which can cover beta‐lactamase producing bacteria, should be considered (Pichichero 2003).
The effectiveness of antibiotics does not depend solely on their antimicrobial activity against the suspected pathogens, but also on characteristics such as dosage, appropriate dosing intervals, tolerability and palatability, which promote compliance and adherence. A convenient once‐ or twice‐daily dosing schedule increases the likelihood of compliance with a full course of therapy (Leibovitz 2003). The traditional dosing interval for prescribing amoxicillin, with or without clavulanate, is every six to eight hours. These dosing intervals may result in poor compliance especially for children at school or daycare centres, which necessitates the involvement and co‐operation of a third person.
How the intervention might work
The length of time that antibiotic serum levels are above the Minimal Inhibitory Concentration (MIC), or time above the MIC, has been demonstrated to be a major determinant in predicting successful clinical outcomes for beta‐lactam antimicrobial agents (Cars 1997; Drusano 1997). This finding denotes that the dosing frequency of beta‐lactam antimicrobial agents could be reduced by increasing the dose, in order to maximise time above the MIC. An increased dose, instead of three times daily dosing, will enhance compliance (Grob 1992; Urquhart 1992). However, these studies relate to conditions other than otitis media.
Why it is important to do this review
In recent years, an increased dosing interval of amoxicillin, with or without clavulanate, has been more frequently used to treat AOM (AAPS 2004). It is therefore reasonable to assess clinical trials comparing the effectiveness of reduced dosing intervals (one or two daily doses) with traditional dosing intervals (three or four daily doses) of amoxicillin, with or without clavulanate, for the treatment of AOM in children.
Objectives
To compare the effectiveness of one or two daily doses with three or four daily doses of amoxicillin, with or without clavulanate, for the treatment of AOM in children; and to compare complication rates and adverse reactions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing two different dosing intervals of the same intervention, amoxicillin, with or without clavulanate.
Types of participants
Participants aged 12 years or younger, with AOM diagnosed by acute ear pain (otalgia) and an inflamed ear drum (confirmed by positive tympanocentesis or tympanogram of type B or C). A tympanogram is the print‐out of an impedance bridge showing the stiffness or the compliance of the middle ear structures as it varies with changes in pressure within the external ear canal. A type B tympanogram suggests fluid in the middle ear; type C suggests that the pressure within the middle ear is below atmospheric pressure.
Types of interventions
Once or twice daily doses compared with three or four daily doses of amoxicillin, with or without clavulanate.
Types of outcome measures
Primary outcomes
Clinical cure rate at the end of antibiotic therapy (days 7 to 15), in terms of resolution of otalgia, resolution of fever and bacteriological cure rate, if data are provided.
Secondary outcomes
Clinical cure rate during therapy in terms of resolution of otalgia and resolution of fever.
Clinical cure rate post‐treatment (one to three months), in terms of resolution of middle ear effusion, as determined by tympanometry, assessed only in those who do not have recurrences of AOM after completion of therapy.
AOM complications: recurrent AOM (after completion of therapy), acute mastoiditis.
Adverse reactions to medication.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 2, part of The Cochrane Library, www.thecochranelibrary.com (accessed 15 March 2013), which contains the Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (January 1950 to March week 1, 2013), EMBASE (July 2010 to August 2012) and the Science Citation Index (2001 to March 2013). See Appendix 1 for details of previous search.
We used the following terms to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision): Ovid format (Lefebvre 2011). We adapted the search strategy to search other databases. See Appendix 2 for the EMBASE search strategy and Appendix 3 for the Science Citation Index search strategy.
MEDLINE (OVID)
1 exp Otitis Media/ 2 otitis media.tw. 3 (AOM or OME).tw. 4 ((infect* or inflam*) adj2 middle ear*).tw. 5 or/1‐4 6 exp Amoxicillin/ 7 (amoxicillin* or amoxycillin*).tw,nm. 8 6 or 7 9 5 and 8
Searching other resources
We checked the reference lists of identified clinical trials and any relevant reviews or meta‐analyses. We contacted major pharmaceutical companies that manufacture antibiotics and relevant experts in the field for additional trial information. We contacted the first author of relevant trials if any questions arose.
Data collection and analysis
Selection of studies
Two review authors (ST, PV) retrieved titles and abstracts from the literature search for checking against the inclusion/exclusion criteria and recorded data on a pre‐designed screening form. The same review authors independently screened results for each title/abstract. A third review author (ML) resolved any disagreements. We requested the full‐text articles if the title or the abstract were unclear.
Data extraction and management
Two review authors (ST, PV) independently reviewed each article and completed data extraction forms. We resolved disagreements through group discussion.
Assessment of risk of bias in included studies
We assessed the methodological quality of the selected trials using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The methods used for the generation of the randomisation sequence were described for each trial.
1. Random sequence generation
We assessed the possibility of selection bias for random sequence generation, using the following criteria:
'Low' risk of bias: description of a random component in the sequence generation process was shown.
'High' risk of bias: the investigators describe a non‐random component in the sequence generation process such as randomisation based on date of birth, case record number or date of presentation.
'Unclear' risk of bias: insufficient information about the sequence generation process to permit a judgement of 'Low' risk or 'High' risk.
2. Allocation sequence concealment
'Low' risk of bias: participants and investigators enrolling participants could not foresee assignment due to central allocation, sequentially numbered drug containers of identical appearance, sequentially numbered, opaque, sealed envelopes.
'High' risk of bias: participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias.
'Unclear' risk of bias: insufficient information about the allocation concealment to permit a judgement of 'Low' risk or 'High' risk.
3. Blinding (performance bias and detection bias)
-
'Low' risk of bias: any one of the following:
No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
'High' risk of bias: any one of the following:
No blinding or incomplete blinding, but the review authors judge that the outcome is likely to be influenced by lack of blinding.
Blinding of participants and key study personnel attempted, and likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
-
'Unclear' risk of bias: any of the following:
Insufficient information to permit a judgement of 'Low' risk or 'High' risk.
The study did not address this outcome.
4. Incomplete outcome data
-
'Low' risk of bias: any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome.
Missing outcome data balanced in number across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
-
'High' risk of bias: any one of the following:
Reasons for missing outcome data likely to be related to true outcome with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size among missing outcomes enough to induce clinically relevant bias in observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
-
'Unclear' risk of bias: any one of the following:
Insufficient reporting of attrition/exclusions to permit a judgement of 'Low' risk or 'High' risk (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Selective outcome reporting
-
'Low' risk of bias: any of the following:
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
-
'High' risk of bias: any of the following:
Not all of the study's pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
'Unclear' risk of bias: insufficient information to permit a judgement of 'Low' risk or 'High' risk. It is likely that the majority of studies will fall into this category.
6. Other bias
'Low' risk of bias: the study appears to be free of other sources of bias.
-
'High' risk of bias: there is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem
-
'Unclear' risk of bias: there may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Measures of treatment effect
For categorical data (for example, cure or not cure), we used risk ratios (RR) and 95% confidence intervals (CI). We showed continuous differences between groups in the meta‐analysis (for example, pain relief on a visual analogue scale) as a mean difference (MD) and 95% CI.
Unit of analysis issues
We did not find any cluster‐randomised trials.
Dealing with missing data
We performed data analysis using the intention‐to‐treat (ITT) principle. Regarding drop‐outs, we applied the worst‐case scenario to the once or twice daily groups.
Assessment of heterogeneity
When important heterogeneity was suspected from the Chi2 test for heterogeneity (at 10%), or from visual inspection of the results, we investigated this by looking for differences in clinical and methodological factors between the trials. When concern about heterogeneity persisted, we considered using a random‐effects model. We calculated the I2 statistic to estimate the degree of heterogeneity. If the I2 statistic was more than 50% or the P value less than 0.10, we used a random‐effects model.
Assessment of reporting biases
We planned to examine publication bias by using funnel plots (Light 1984) and when asymmetry was observed, we would use the trim and fill method to assess the effect of this asymmetry on the conclusions (Duval 2000). However, we did not produce the funnel plots because there were only six studies. When meta‐analysis was inappropriate, we drew conclusions from the trials' descriptive elements, methodological quality, the number of trials with consistent findings, the plausibility of the results and the strength of the associations in the primary trials, as well as consensus among the authors.
Data synthesis
We performed meta‐analyses of the five studies (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Murph 1993; Principi 1986) using Review Manager (RevMan 2012) software.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses and investigated heterogeneity on the basis of types of interventions (i.e. amoxicillin alone (Murph 1993; Principi 1986), amoxicillin with clavulanate (Behre 1997; Damrikarnlert 2000; Hoberman 1997)).
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality on the review's conclusions. Analysis of trial quality was based on allocation concealment. We excluded trials with clearly inadequate allocation concealment to assess differences in the overall result.
Results
Description of studies
Results of the search
The MEDLINE search retrieved 171 studies. However, there were only six studies relevant to the objectives of this review (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Jacobsson 1993; Murph 1993; Principi 1986). Among the 229 results retrieved by the EMBASE search, six relevant studies were found (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Jacobsson 1993; Murph 1993; Principi 1986). No randomised controlled trial (RCT) was retrieved by searching the Science Citation Index. We found six studies in health services research meeting abstracts but only one was relevant to the review objectives (Damrikarnlert 2000). We retrieved six out of 98 studies from the CENTRAL search (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Jacobsson 1993; Murph 1993; Principi 1986).
Included studies
We included five studies involving 1601 children (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Murph 1993; Principi 1986).
Four studies were supported by pharmaceutical funding (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Principi 1986). Two trials compared amoxicillin without clavulanate (Murph 1993; Principi 1986) and used once and two daily versus three times daily dosing, while the other four studies compared the effectiveness of amoxicillin/clavulanate two times versus three times daily (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Jacobsson 1993). Treatment duration was 10 days in four trials (Behre 1997; Hoberman 1997; Murph 1993; Principi 1986) and 7 to 10 days in one trial (Damrikarnlert 2000).
Excluded studies
We excluded the study by Jacobsson (Jacobsson 1993) because it included only recurrent AOM cases or cases that were not responsive to amoxicillin/penicillin or cefaclor, which differed from the other studies. In addition, the total daily dosage for amoxicillin was quite low (20 to 33.2 mg/kg/day).
Risk of bias in included studies
The included studies are summarised in the Characteristics of included studies tables and the quality assessment of each study is summarised in 'Risk of bias' tables. The detailed risks of bias and quality of each study is shown in Table 1. We found that all trials had unclear risk of bias for allocation concealment. A graphical representation of risk of bias among the included studies is shown in Figure 1. A funnel plot is shown in Figure 2 which shows that there was no publication bias.
1. Quality assessment for individual trials.
| Trial | Randomisation process | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias | Drop‐outs |
| Principi 1986 | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | 0% |
| Murph 1993 | Low risk | Unclear risk | Low risk | Unclear risk | High risk | Low risk | 7.7% |
| Behre 1997 | Unclear risk | Unclear risk | Low risk | High risk | Low risk | Low risk | 4.8% to 8.2% |
| Hoberman 1997 | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | 5.2% to 5.6% |
| Damrikarnlert 2000 | Low risk | Unclear risk | Low risk | High risk | Low risk | Low risk | 10.7% to 11% |
1.
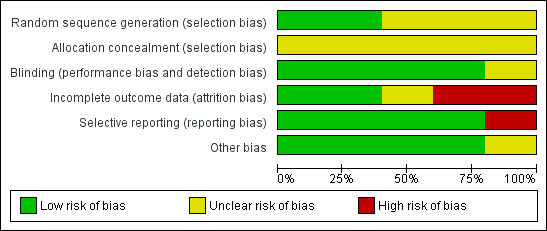
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
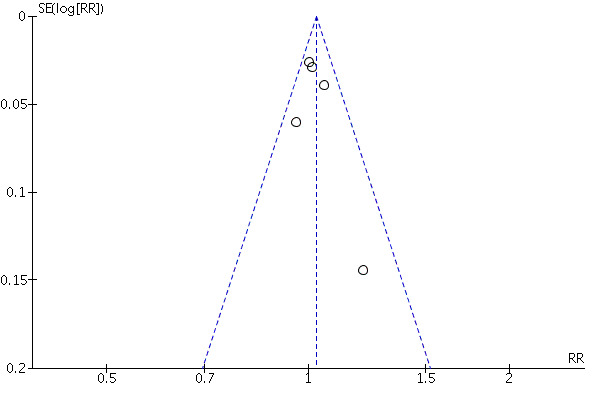
Funnel plot of the five trials included.
Allocation
All five studies claimed that they were RCTs but only two studies clearly mentioned the method of randomisation (Damrikarnlert 2000; Murph 1993). One used a computer generation method (Damrikarnlert 2000) and the other generated a table of random numbers (Murph 1993). All studies had an unclear risk of bias because there was no information on the method of allocation concealment.
Blinding
There was only one study that had a placebo control and the investigator, participants and assessors were blinded to this (Murph 1993). Two studies blinded only the observers (Behre 1997; Damrikarnlert 2000). There was no information on blinding process in one study (Principi 1986).
Incomplete outcome data
There was one study with no drop‐outs (Principi 1986). Three studies had less than 10% drop‐outs (Behre 1997; Hoberman 1997; Murph 1993) and one study had more than 10% drop‐outs (Damrikarnlert 2000). There were three studies with high risk of incomplete outcome data (Behre 1997; Damrikarnlert 2000). Two studies with low risk of bias (Hoberman 1997; Principi 1986) and one study with unclear risk of bias (Murph 1993).
Selective reporting
Four studies had important clinical outcomes (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Principi 1986), except one study which had high risk of bias for selective reporting (Murph 1993). Four trials assessed the primary outcome at the end of treatment (Behre 1997; Damrikarnlert 2000; Murph 1993; Principi 1986), while Hoberman 1997 assessed the primary outcome at the follow‐up visit, days 32 to 38. Thus we used the secondary outcome (clinical response at days 12 to 14) by Hoberman as the primary outcome in this review and vice versa. The study by Murph 1993 did not reported clinical cure rate at follow‐up (post‐treatment), including AOM complications.
Other potential sources of bias
Four studies reported comparable baseline characteristics and compliance rates for both treatment and control groups (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Murph 1993). However, the study by Principi 1986 did not report compliance rates.
Effects of interventions
We analysed the effectiveness of the interventions based on one or two daily doses of amoxicillin, with or without clavulanate versus three daily doses. Subgroup analyses were performed based on amoxicillin treatment trials only, amoxicillin/clavulanate treatment trials only and exclusion of the studies with many unclear risk of bias characteristics.
1. All included studies
Primary outcome
Clinical cure rate at the end of therapy
(Table 2; Analysis 1.1; Figure 3)
2. Clinical cure rate at the end of therapy.
| Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Principi 1986 | 49/55 | 51/55 | 0.96 | 0.85 to 1.08 |
| Murph 1993 | 27/33 | 23/34 | 1.21 | 0.91 to 1.60 |
| Hoberman 1997 | 241/287 | 229/288 | 1.06 | 0.98 to 1.14 |
| Behre 1997 | 212/231 | 210/232 | 1.01 | 0.96 to 1.07 |
| Damrikarnlert 2000 | 187/199 | 175/187 | 1.00 | 0.95 to 1.06 |
CI: confidence interval
1.1. Analysis.
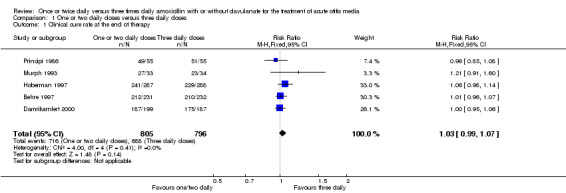
Comparison 1 One or two daily doses versus three daily doses, Outcome 1 Clinical cure rate at the end of therapy.
3.
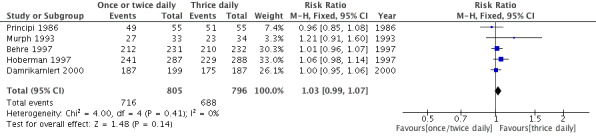
Forest plot of comparison: Clinical cure rate at the end of therapy.
Five trials involving 1601 participants compared one or two daily doses of amoxicillin, with or without clavulanate, with three daily doses. The risk ratio (RR) showed comparable results between groups (RR 1.03, 95% confidence interval (CI) 0.99 to 1.07).
Secondary outcomes
Clinical cure rate during therapy
(Table 3; Analysis 1.2; Figure 4)
3. Clinical cure rate during therapy.
| Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Behre 1997 | 30/30 | 28/33 | 1.17 | 1.01 to 1.37 |
| Damrikarnlert 2000 | 48/199 | 45/186 | 1 | 0.80 to 1.25 |
CI: confidence interval
1.2. Analysis.
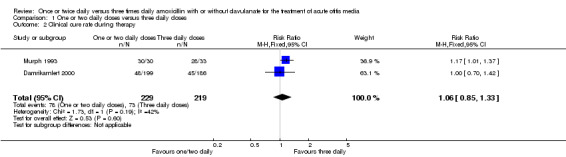
Comparison 1 One or two daily doses versus three daily doses, Outcome 2 Clinical cure rate during therapy.
4.
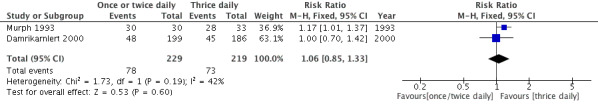
Forest plot of comparison: Clinical cure rate during therapy.
Two studies reported clinical cure during therapy and demonstrated a RR of 1.06 (95% CI 0.85 to 1.33) (Damrikarnlert 2000; Murph 1993).
Clinical cure at post‐treatment (one to three months)
(Table 4; Analysis 1.3; Figure 5)
4. Clinical cure rate at post‐treatment (1 to 3 months).
| Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Principi 1986 | 42/46 | 48/49 | 0.93 | 0.85 to 1.03 |
| Behre 1997 | 185/220 | 180/232 | 1.25 | 0.96 to 1.66 |
| Hoberman 1997 | 172/287 | 176/288 | 0.98 | 0.83 to 1.15 |
| Damrikarnlert 2000 | 168/180 | 153/174 | 1.44 | 0.81 to 2.29 |
CI: confidence interval
1.3. Analysis.
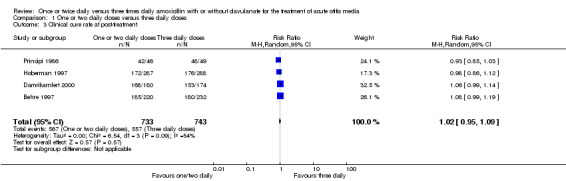
Comparison 1 One or two daily doses versus three daily doses, Outcome 3 Clinical cure rate at post‐treatment.
5.
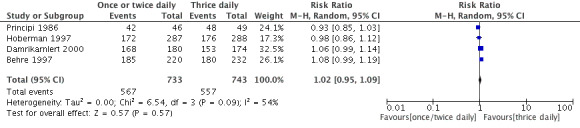
Forest plot of comparison: Clinical cure at post‐treatment (one to three months).
Four trials reported clinical cure rate after therapy which showed no difference in either group (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Principi 1986). The results were comparable (RR 1.02, 95% CI 0.95 to 1.09).
AOM complications: Recurrent AOM after completion of therapy
(Table 5; Analysis 1.4; Figure 6)
5. AOM complications: Recurrent AOM after completion of therapy.
| Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Principi 1986 | 4/49 | 1/51 | 4.16 | 0.48 to 35.95 |
| Hoberman 1997 | 51/287 | 54/288 | 0.97 | 0.78 to 1.2 |
| Damrikarnlert 2000 | 7/80 | 12/174 | 1.19 | 0.64 to 2.2 |
CI: confidence interval
1.4. Analysis.
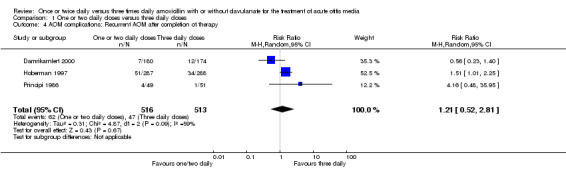
Comparison 1 One or two daily doses versus three daily doses, Outcome 4 AOM complications: Recurrent AOM after completion of therapy.
6.
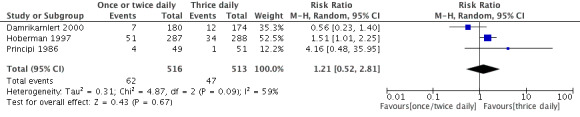
Forest plot of comparison: AOM complications: Recurrent AOM after completion of therapy.
The pooled data from three studies showed a RR of 1.21 (95% CI 0.52 to 2.81) (Damrikarnlert 2000; Hoberman 1997; Principi 1986).
Adverse reactions to medication
(Table 6; Table 7; Analysis 1.5; Analysis 1.6; Analysis 1.7).
6. Overall adverse events.
| Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Behre 1997 | 111/231 | 94/232 | 1.19 | 0.97 to 1.46 |
| Damrikarnlert 2000 | 25/209 | 37/206 | 0.67 | 0.42 to 1.07 |
CI: confidence interval
7. Specific adverse events to medication.
| Adverse events | Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Diarrhoea | Principi 1986 | 1/55 | 1/55 | 1.00 | 0.06 to 16.40 |
| Hoberman 1997 | 16/287 | 20/288 | 0.80 | 0.42 to 1.52 | |
| Behre 1997 | 15/231 | 24/232 | 0.63 | 0.34 to 1.17 | |
| Damrikarnlert 2000 | 15/209 | 22/206 | 0.65 | 0.3 to 1.28 | |
| Skin adverse events | Principi 1986 | 3/55 | 3/55 | 1.00 | 0.19 to 5.19 |
| Hoberman 1997 | 23/287 | 30/288 | 0.77 | 0.46 to 1.29 | |
| Damrikarnlert 2000 | 2/209 | 5/206 | 0.39 | 0.07 to 2.02 |
CI: confidence interval
1.5. Analysis.
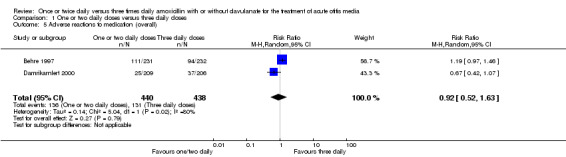
Comparison 1 One or two daily doses versus three daily doses, Outcome 5 Adverse reactions to medication (overall).
1.6. Analysis.
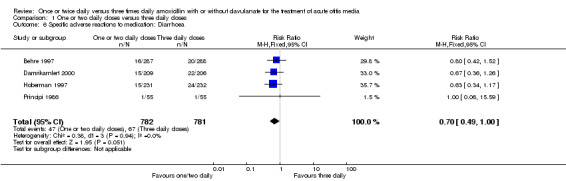
Comparison 1 One or two daily doses versus three daily doses, Outcome 6 Specific adverse reactions to medication: Diarrhoea.
1.7. Analysis.
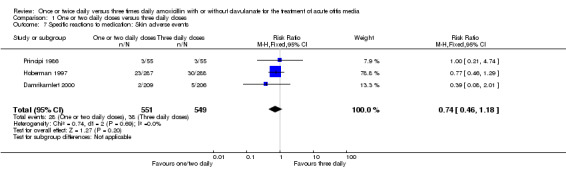
Comparison 1 One or two daily doses versus three daily doses, Outcome 7 Specific reactions to medication: Skin adverse events.
The RR of overall adverse events between groups did not show any statistically significant difference (RR 0.92, 95% CI 0.52 to 1.63) (Behre 1997; Damrikarnlert 2000).
Diarrhoea and skin adverse events were comparable (diarrhoea RR 0.70, 95% CI 0.49 to 1.00; skin RR 0.74; 95% CI 0.46 to 1.18) (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Principi 1986).
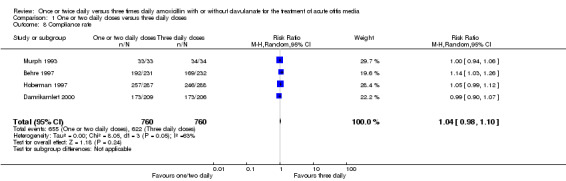
Compliance rates were also reported in four trials (Behre 1997; Damrikarnlert 2000; Hoberman 1997; Murph 1993). The pooled data showed no difference in compliance rate (RR 1.04, 95% CI 0.98 to 1.10) (Table 8; Analysis 1.8).
8. Compliance rate.
| Study | One or two daily doses (n/N) | Three daily doses (n/N) | Risk ratio | 95% CI |
| Murph 1993 | 33/33 | 34/34 | 1 | 0.94 to 1.06 |
| Hoberman 1997 | 257/287 | 246/288 | 1.05 | 0.99 to 1.12 |
| Behre 1997 | 192/231 | 169/232 | 1.14 | 1.03 to 1.26 |
| Damrikarnlert 2000 | 173/209 | 173/206 | 0.99 | 0.90 to 1.07 |
CI: confidence interval
1.8. Analysis.
Comparison 1 One or two daily doses versus three daily doses, Outcome 8 Compliance rate.
2. Analysis of amoxicillin treatment trials only
The results are shown in Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.7; Analysis 2.5 and Analysis 2.6, which are comparable for all outcomes.
2.1. Analysis.
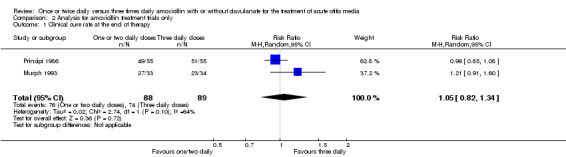
Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 1 Clinical cure rate at the end of therapy.
2.2. Analysis.

Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 2 Clinical cure rate during therapy.
2.3. Analysis.
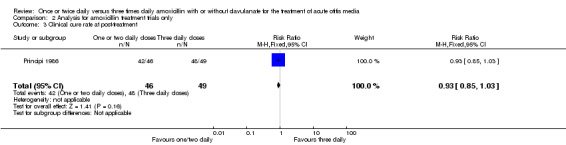
Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 3 Clinical cure rate at post‐treatment.
2.4. Analysis.
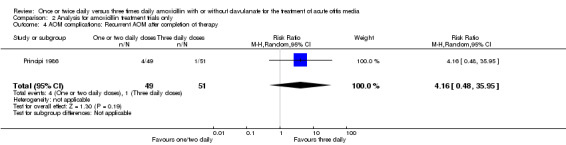
Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 4 AOM complications: Recurrent AOM after completion of therapy.
2.7. Analysis.
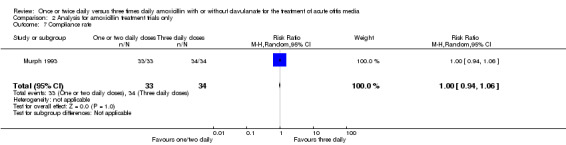
Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 7 Compliance rate.
2.5. Analysis.
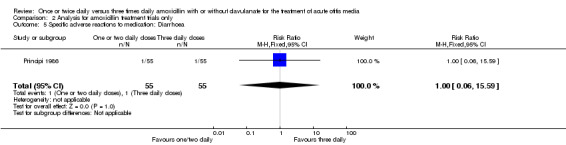
Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 5 Specific adverse reactions to medication: Diarrhoea.
2.6. Analysis.
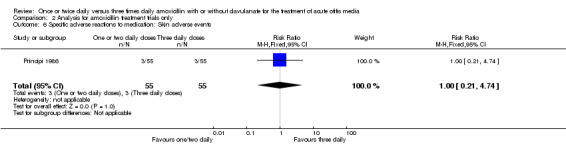
Comparison 2 Analysis for amoxicillin treatment trials only, Outcome 6 Specific adverse reactions to medication: Skin adverse events.
3. Analysis of amoxicillin/clavulanate treatment trials only
The results are shown in Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.8; Analysis 3.5; Analysis 3.6 and Analysis 3.7, which are comparable for all outcomes.
3.1. Analysis.
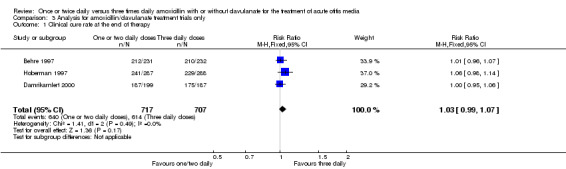
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 1 Clinical cure rate at the end of therapy.
3.2. Analysis.
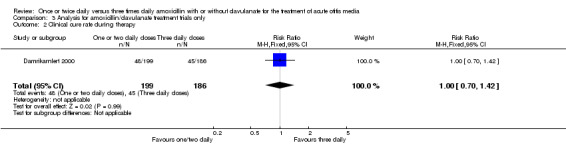
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 2 Clinical cure rate during therapy.
3.3. Analysis.
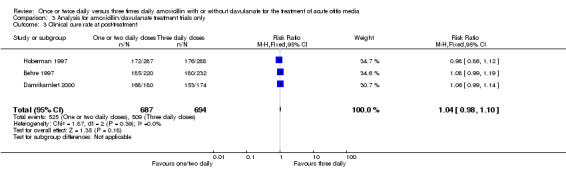
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 3 Clinical cure rate at post‐treatment.
3.4. Analysis.
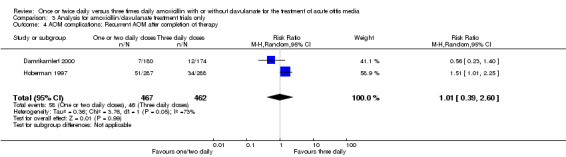
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 4 AOM complications: Recurrent AOM after completion of therapy.
3.8. Analysis.
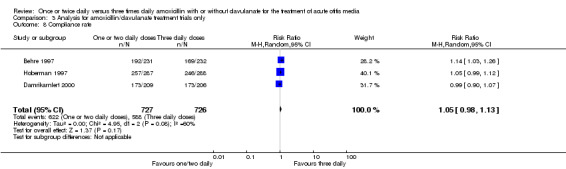
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 8 Compliance rate.
3.5. Analysis.
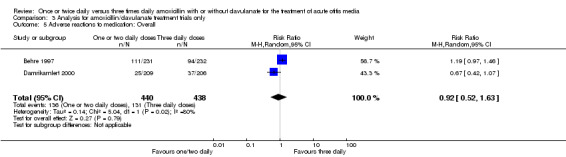
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 5 Adverse reactions to medication: Overall.
3.6. Analysis.
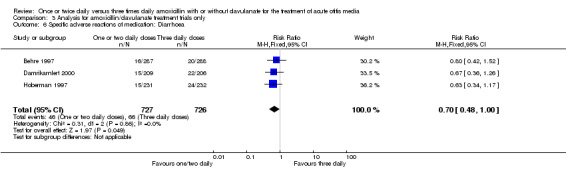
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 6 Specific adverse reactions of medication: Diarrhoea.
3.7. Analysis.
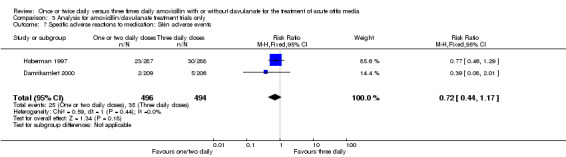
Comparison 3 Analysis for amoxicillin/clavulanate treatment trials only, Outcome 7 Specific adverse reactions to medication: Skin adverse events.
4. Exclusion of the studies with many unclear risk of bias characteristics
The study by Principi 1986 had unclear risk of bias for random sequence generation, allocation concealment, blinding and 'other' bias. When we excluded this study from the analysis, both treatment groups still had comparable results (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6).
4.1. Analysis.
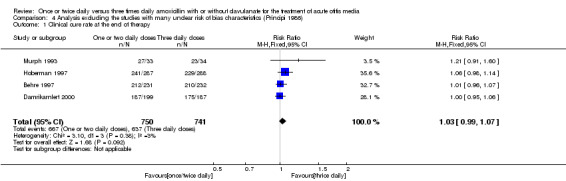
Comparison 4 Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986), Outcome 1 Clinical cure rate at the end of therapy.
4.2. Analysis.
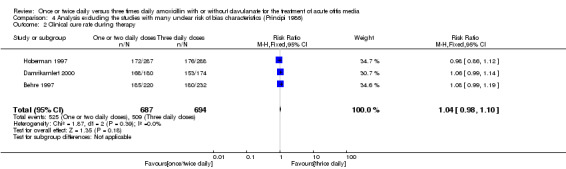
Comparison 4 Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986), Outcome 2 Clinical cure rate during therapy.
4.3. Analysis.
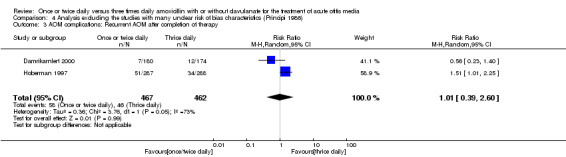
Comparison 4 Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986), Outcome 3 AOM complications: Recurrent AOM after completion of therapy.
4.4. Analysis.
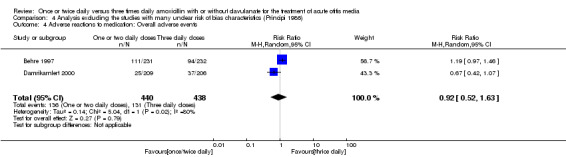
Comparison 4 Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986), Outcome 4 Adverse reactions to medication: Overall adverse events.
4.5. Analysis.
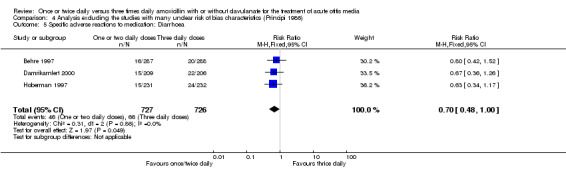
Comparison 4 Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986), Outcome 5 Specific adverse reactions to medication: Diarrhoea.
4.6. Analysis.
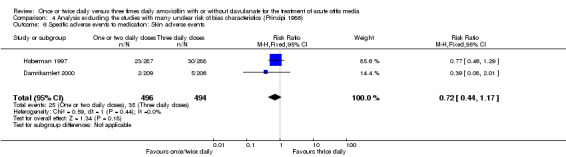
Comparison 4 Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986), Outcome 6 Specific adverse events to medication: Skin adverse events.
Discussion
Acute otitis media (AOM) is one of the most common infections for which antibacterial agents are prescribed for children in the United States (Rovers 2004). The diagnosis and management of AOM has a significant impact on the health of children, the cost of providing health care and the use of antibiotics. Although there has been much discussion surrounding the treatment of uncomplicated AOM using antibiotics or observation, antibiotics are still widely used. The first‐line antibiotic of choice for AOM is amoxicillin, with or without clavulanate (AAPS 2004). The effectiveness of antibiotics does not depend solely on their antimicrobial activity against the suspected pathogens, but also on factors such as dosage, appropriate dosing intervals, tolerability and palatability. One or two daily doses of oral antibiotics have a higher compliance rate than doses taken three times daily (Kardas 2007; Pechere 2007).
This systematic review demonstrated that when pooling all data from published trials of treatment with amoxicillin or amoxicillin/clavulanate for uncomplicated AOM in children, the results are comparable for all outcomes when comparing a once or twice daily dose with thrice daily doses. These results may show that both dosages have the same effectiveness or that uncomplicated AOM can self resolve.
Summary of main results
This review identified five RCTs reporting clinical outcomes in children treated with one or two daily doses of amoxicillin, with or without clavulanate, compared to three daily doses. No trial used four daily doses. There were no significant statistical differences in the effectiveness of the two different dosages of amoxicillin, with or without clavulanate.
Overall completeness and applicability of evidence
This review demonstrates that both one or two daily doses of amoxicillin or amoxicillin/clavulanate have comparable effectiveness for the treatment of uncomplicated AOM in children. One or two daily doses might increase compliance in daily practice.
Quality of the evidence
All of the included studies had an unclear risk of bias for allocation concealment and three studies had an unclear risk of bias for randomisation as they did not describe the details of randomisation sequence generation or methods to conceal allocation.
Potential biases in the review process
We have not identified any potential biases in the review process.
Agreements and disagreements with other studies or reviews
There have been no other published studies or reviews comparing the two dosages of amoxicillin, with or without clavulanate, for the treatment of AOM.
Authors' conclusions
Implications for practice.
In routine practice amoxicillin with/without clavulanate has been used as a twice daily dose for the treatment of AOM and other infectious diseases of the upper respiratory tract. Our findings confirm that using twice daily doses has the same effectiveness as thrice daily doses for uncomplicated AOM.
Implications for research.
More than 90% of AOM cases occur in children less than two years of age. However, in all of the included studies the age ranges were between two months and 12 years, with few cases less than two years of age. A good quality equivalence or non‐inferiority trial should be performed to see whether the treatments are either equally effective or whether a lower dosage scheme is as effective as a higher dosage scheme. Children less than two years of age should also be included in the study and a microbiological study of middle ear fluid should be performed.
Feedback
Once or twice daily versus three times daily amoxicillin with or without clavulanate for the treatment of acute otitis media, 8 May 2014
Summary
The new version of the systematic review “Once or twice daily versus three times daily amoxicillin with or without clavulanate for the treatment of acute otitis media” (last assessed as up‐to‐date: 15 March 2013) includes the same 5 randomized controlled trials (1601 children) that were included in the previous one (last assessed as up‐to‐date: 10 July 2010). In the Publication History it is reported “New search for studies and content updated (no change to conclusions)”.
We believe this is incorrect; in the 2010 version in fact there was not pooling of the data, as it was judged to be not appropriate, and the authors’ conclusions were: “This review showed insufficient evidence to judge whether once or twice daily doses of amoxicillin, with or without clavulanate, were comparable with three or four daily doses for the treatment of AOM. The evidence appears to be biased and therefore no firm conclusions can be drawn”. On the contrary, in the last updated version of the systematic review data were pooled and conclusions reported: “This review showed that the results of using once or twice daily doses of amoxicillin, with or without clavulanate, were comparable with three doses for the treatment of AOM”.
This change is very relevant for clinicians, policy makers and the public; thus, it should be supported by a thorough discussion, explaining the rationale behind the change. Moreover, we suggest to state clearly in the Publication History: “New search for studies and content updated, no new trials identified, conclusions changed”, highlighting the change accordingly using the [CC] dark blue flag (There has been an important change to the conclusions of the review published in the most recent issue).
Best regards,
Simona Di Mario1, Carlo Gagliotti2, Maria Luisa Moro2
1 SaPeRiDoc, Servizio assistenza distrettuale, medicina generale, pianificazione e sviluppo dei servizi sanitari. Direzione generale sanità e politiche sociali. Regione Emilia‐Romagna, Bologna, Italy
2 Area Rischio Infettivo. Agenzia Sanitaria e Sociale Regionale. Regione Emilia‐Romagna, Bologna, Italy
I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2014 | Feedback has been incorporated | Feedback comment added. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 15 March 2013 | New search has been performed | We updated the electronic searches but did not identify any new studies for inclusion or exclusion in our review. |
| 15 March 2013 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 10 July 2010 | New search has been performed | We conducted searches but identified no new studies as suitable for inclusion or exclusion in our update. The conclusions remain unchanged. We assessed all risk of bias items. |
| 18 March 2008 | New search has been performed | Searches conducted. |
| 12 March 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to thank Elizabeth Dooley and Professor Pisake Lumbiganon for their support and advice, Sarah Thorning for undertaking the literature searches, and Prof. Bruce Arroll, Maroeska Rovers, Eugene Leibovitz, Rick Shoemaker and Dilip Raghavan for commenting on the draft review.
Appendices
Appendix 1. Previous search
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 2) which contains the Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (January 1950 to July 2010), EMBASE (1974 to July 2010), the Science Citation Index (2001 to July 2010) and NLM Gateway (HSRProj) (July 2010).
We used the following terms to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision): Ovid format (Lefebvre 2011). We adapted the search strategy to search other databases. See Appendix 2 for the EMBASE search strategy; Appendix 3 for the Science Citation Index search strategy; and Appendix 4 for the NLM Gateway search strategy. There were no language or publication restrictions.
MEDLINE (OVID)
1 otitis media.mp. 2 acute.tw 3 1 or 2 4 amoxicillin.mp. 5 amoxycillin.mp. 6 4 or 5 7 (singl* or one or once or two or twice).tw. 8 ("q.d." or "qd" or "o.d." or "od" or "b.d." or "bd").tw. 9 (three or thrice or four).tw. 10 ("t.d.s." or "tds" or "q.i.d." or "qid").tw. 11 7 or 8 12 9 or 10 13 11 and 12 14 3 and 6 and 13 15 randomized controlled trial.pt. 16 controlled clinical trial.pt. 17 randomized.ab. 18 placebo.ab. 19 randomly.ab. 20 trial.ab. 21 groups.ab. 22 15 or 16 or 17 or 18 or 19 or 20 or 21 23 humans.sh. 24 22 and 23 25 14 and 24
EMBASE search strategy
#1. 'otitis media'/exp AND [embase]/lim #2. 'acute otitis media'/exp AND [embase]/lim #3. 'acute suppurative otitis media'/exp AND [embase]/lim #4. 'otitis media':ti,ab AND [embase]/lim #5. 'aom':ti,ab AND [embase]/lim #6. 'asom':ab,ti AND [embase]/lim #7. #1 OR #2 OR #3 OR #4 OR #5 OR #6 #8. 'amoxicillin'/exp AND [embase]/lim #9. 'amoxicillin plus clavulanic acid'/exp AND [embase]/lim #10. amoxicillin:ti,ab AND [embase]/lim #11. amoxycillin:ab,ti AND [embase]/lim #12. #8 OR #9 OR #10 OR #11 #13. (singl*:ti,ab OR one:ti,ab OR once:ti,ab OR two:ti,ab OR twice:ti,ab) AND [embase]/lim #14. ('q.d.':ti,ab OR 'qd':ti,ab OR 'o.d.':ti,ab OR 'od.':ti,ab OR 'b.d.':ti,ab OR 'bd':ti,ab) AND [embase]/lim #15. (three:ti,ab OR thrice:ti,ab OR four:ti,ab) AND [embase]/lim #16. ('t.d.s.':ti,ab OR 'tds':ti,ab OR 'q.i.d.':ti,ab OR 'qid':ti,ab) AND [embase]/lim #17. #13 OR #14 #18. #15 OR #16 #19. #17 AND #18 #20. #7 AND #12 AND #19 #21. 'randomized controlled trial'/exp AND [embase]/lim #22. 'controlled study'/exp AND [embase]/lim #23. 'single blind procedure'/exp AND [embase]/lim #24. 'double blind procedure'/exp AND [embase]/lim #25. 'phase 3 clinical trial'/exp AND [embase]/lim #26. random*:ab,ti AND [embase]/lim #27. placebo*:ti,ab AND [embase]/lim #28. 'clinical trial':it AND [embase]/lim #29. 'randomized controlled trial':it AND [embase]/lim #30. (singl*:ti,ab OR doubl*:ti,ab OR tripl*:ti,ab OR trebl*:ti,ab) AND (blind*:ti,ab OR mask*:ti,ab) AND [embase]/lim #31. 'controlled clinical trial':ti,ab AND [embase]/lim #32. 'controlled clinical trials':ti,ab AND [embase]/lim #33. #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR # 31 OR #32 #34. ('nonhuman'/exp OR 'animals'/exp) NOT 'human'/exp AND [embase]/lim #35. #33 NOT #34 #36. #20 AND #35
Science Citation Index
Science Citation Index searched by ISI Web of Knowledge (http://apps.isiknowledge.com) using advanced search
#1 TI = (otitis media) #2 TI = amoxicillin #3 TI=amoxycillin #4 #2 OR #3 #5 TI = once #6 TI = one #7 TI = single #8 TI = two #9 TI = twice #10 #5 OR #6 OR #7 OR #8 OR #9 #11 TI = three #12 TI=thrice #13 TI=four #14 #11 OR #12 OR #13 #15 #10 AND #14 #16 #1 AND #4 AND #15
NLM Gateway
We used the terms ((otitis media) AND (amoxycillin OR amoxicillin) AND (one OR once OR two OR twice OR single) AND (thrice OR three OR four)) to search NLM Gateway.
Appendix 2. EMBASE search strategy
#16 #7 AND #15 #15 #10 NOT #14 #14 #11 NOT #13 #13 #11 AND #12 #12 'human'/de 2 #11 'animal'/de OR 'animal experiment'/de OR 'nonhuman'/de 8 #10 #8 OR #9 #9 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti AND trial:ti AND (doubl* NEXT/1 blind*):ab,ti #8 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #7 #3 AND #6 #6 #4 OR #5 #5 amoxycillin*:ab,ti OR amoxicillin*:ab,ti #4 'amoxicillin'/de #3 #1 OR #22 #2 'otitis media':ab,ti OR ome:ab,ti OR aom:ab,ti OR ((infect* OR inflam*) NEAR/2 'middle ear'):ab,ti OR ((infect* OR inflam*) NEAR/2 'middle ears'):ab,ti #1 'acute otitis media'/exp OR 'suppurative otitis media'/exp OR 'otitis media'/de
Appendix 3. Science Citation Index search strategy
| # 4 | 25 | #2 AND #1 Refined by: Publication Years=( 2011 OR 2012 OR 2010 ) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All Years Lemmatization=On |
|
| # 3 | 335 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All Years Lemmatization=On |
|
| # 2 | 1,129,266 | Title=(trial) OR Topic=(random* or placebo* OR ((singl* OR doubl*) NEAR/1 blind*)) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All Years Lemmatization=On |
|
| # 1 | 939 | Topic=("otitis media" OR ((infect* OR inflam*) NEAR/2 "middle ear")) AND Topic=(amoxicillin* OR amoxycillin*) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All Years Lemmatization=On |
Data and analyses
Comparison 1. One or two daily doses versus three daily doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure rate at the end of therapy | 5 | 1601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 2 Clinical cure rate during therapy | 2 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.85, 1.33] |
| 3 Clinical cure rate at post‐treatment | 4 | 1476 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.09] |
| 4 AOM complications: Recurrent AOM after completion of therapy | 3 | 1029 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.52, 2.81] |
| 5 Adverse reactions to medication (overall) | 2 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.52, 1.63] |
| 6 Specific adverse reactions to medication: Diarrhoea | 4 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 1.00] |
| 7 Specific reactions to medication: Skin adverse events | 3 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.18] |
| 8 Compliance rate | 4 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.10] |
Comparison 2. Analysis for amoxicillin treatment trials only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure rate at the end of therapy | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.82, 1.34] |
| 2 Clinical cure rate during therapy | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.01, 1.37] |
| 3 Clinical cure rate at post‐treatment | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.03] |
| 4 AOM complications: Recurrent AOM after completion of therapy | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 4.16 [0.48, 35.95] |
| 5 Specific adverse reactions to medication: Diarrhoea | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.59] |
| 6 Specific adverse reactions to medication: Skin adverse events | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.74] |
| 7 Compliance rate | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.94, 1.06] |
Comparison 3. Analysis for amoxicillin/clavulanate treatment trials only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure rate at the end of therapy | 3 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 2 Clinical cure rate during therapy | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.42] |
| 3 Clinical cure rate at post‐treatment | 3 | 1381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 4 AOM complications: Recurrent AOM after completion of therapy | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.39, 2.60] |
| 5 Adverse reactions to medication: Overall | 2 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.52, 1.63] |
| 6 Specific adverse reactions of medication: Diarrhoea | 3 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.00] |
| 7 Specific adverse reactions to medication: Skin adverse events | 2 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Compliance rate | 3 | 1453 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.13] |
Comparison 4. Analysis excluding the studies with many unclear risk of bias characteristics (Principi 1986).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure rate at the end of therapy | 4 | 1491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 2 Clinical cure rate during therapy | 3 | 1381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 3 AOM complications: Recurrent AOM after completion of therapy | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.39, 2.60] |
| 4 Adverse reactions to medication: Overall adverse events | 2 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.52, 1.63] |
| 5 Specific adverse reactions to medication: Diarrhoea | 3 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.00] |
| 6 Specific adverse events to medication: Skin adverse events | 2 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.44, 1.17] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Principi 1986.
| Methods | Randomised controlled study conducted in the outpatient ward of the Pediatric Department IV of the University of Milan between October 1984 and 1985 AOM was defined on clinical data (fever or otalgia or both), otoscopic finding of ear drum, and presence of ear effusion shown by tympanograms. Children with spontaneously perforated ear drums were also included only if the ear had been draining for no longer than 12 hours |
|
| Participants | 55 AOM children in each treatment group (total 110), aged 6 months to 12 years | |
| Interventions | 10 days of amoxicillin 60 mg/kg/day 2 or 3 times daily | |
| Outcomes | Primary outcome
1. Clinical cure rate at the end of therapy ‐ Clinical cure (normalisation of clinical or relief of acute signs and symptoms of AOM) assessed at day 15 Secondary outcome 1. Clinical cure rate during therapy (days 2 to 3), in terms of resolution of otalgia and resolution of fever: not reported 2. Clinical cure rate at post‐treatment (1 to 3 months) ‐ Clinical cure at days 30, 60 and 90 3. AOM complications after completion of therapy: recurrent AOM ‐ Recurrent at days 30, 60 and 90 4. Adverse reactions to medication ‐ Diarrhoea which necessitated discontinuation of treatment ‐ Generalised rash ‐ Urticaria 5.Other outcomes ‐ Compliance: mentioned to be assessed but not reported |
|
| Notes | Supported in part by a Farmitalia‐Carlo Erba SpA Milano grant Intention‐to‐treat principle analysis was not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment methods were not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low rate of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported |
| Other bias | Unclear risk | Comment: no report of compliance rate |
Murph 1993.
| Methods | Double‐blind, placebo‐controlled study. A table of random numbers was used to generate a randomised sequence AOM was defined as evidence on otoscopic assessment of an inflamed tympanic membrane and presence of middle ear effusion |
|
| Participants | 77 AOM children, aged 7 months to 12 years old, recruited from the Pediatric Child Health Clinic of the University of Iowa Hospitals and Clinics 10/77 (7.7%) children could not be evaluated because of failure to return for follow‐up or because they withdrew from the study; 33 and 34 children in the once and 3 times daily groups, respectively, were left for the analysis |
|
| Interventions | 10 days of amoxicillin 40 mg/kg/day 1 versus 3 times daily | |
| Outcomes | Primary outcome 1. Clinical cure rate at the end of therapy (days 7 to 14) ‐ Resolved AOM with or without MEE at days 10 to 14 Secondary outcome 1. Clinical cure rate during therapy (days 2 to 3), in terms of resolution of otalgia and resolution of fever 2. Clinical cure rate at post‐treatment (1 to 3 months) ‐ not reported 3. AOM complications after completion of therapy: Recurrent AOM ‐ not reported 4. Adverse reactions to medication ‐ There were no differences in the 2 groups for the total number of complaints of diarrhoea (P = 0.30), vomiting (P = 0.66) or stomach pain (P = 0.23). No details were described Other outcome 1. Compliance rate |
|
| Notes | No pharmaceutical industry support Intention‐to‐treat principle analysis was not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A table of random numbers was used to assign children to one of two treatment groups" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | The authors did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled study" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "10 children (14.9%) could not be evaluated (failed to return for follow‐up or withdrew from the study)" Comment: no information on whether those lost to follow‐up or who withdrew were in the once or thrice daily dose group |
| Selective reporting (reporting bias) | High risk | Comments: clinical cure rate at follow‐up (1 to 3 months) and AOM complications were not reported |
| Other bias | Low risk | |
Hoberman 1997.
| Methods | Multicentre, randomised, non‐placebo‐controlled trial conducted at 4 university‐affiliated hospitals and 20 private practices in the US and Canada between January and July 1994 AOM was defined as either presence of purulent otorrhoea for less than 24 hours or evidence of middle ear effusion in addition to at least 1 indicator of acute middle ear inflammation Investigators were blinded to treatment assignments and participants, parents and guardians were asked not to discuss medications or duration of treatment with investigators |
|
| Participants | 575 children aged 2 months to 12 years were included (287 and 288 in the 2 and 3 times daily groups, respectively) | |
| Interventions | 10 days of amoxicillin/clavulanate 40/10 mg/kg/day 2 times daily versus 45/6.4 mg/kg/day 3 times daily | |
| Outcomes | Primary outcome 1. Clinical cure rate at the end of therapy (days 7 to 15) ‐ Clinical cure (completely/improved symptoms and signs of AOM at days 12 to 14 Secondary outcomes 1. Clinical cure rate during therapy (days 2 to 3), in terms of resolution of otalgia and resolution of fever: not accounted 2. Clinical cure rate at post‐treatment (1 to 3 months): clinical cure at days 31 to 38 3. AOM complications after completion of therapy: recurrent AOM 4. Adverse reactions to medication ‐ Adverse events: diaper rash: vomiting, diarrhoea Other outcome ‐ Compliance rate |
|
| Notes | Supported by SmithKline Beecham Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned randomly" Comment: method of randomisation was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Investigators were blinded to treatment assignments" Comment: no information on the allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "..parents and guardians were asked not to discuss medications or duration of treatment with investigators" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All important outcomes were accounted for |
| Other bias | Low risk | Quote: "Comparable demographic data and compliance rate" |
Behre 1997.
| Methods | Multicentre, randomised, single‐blinded (observer‐blinded) controlled trial conducted in 26 centres in Germany, Belgium, Switzerland, the UK and Eire AOM was defined by the visual appearance of the ear drum or presence of middle ear effusion on otoscopy, and the presence of 2 of the following symptoms: ear pain, ear discharge, hearing loss, fever, lethargy, irritability, anorexia, vomiting or diarrhoea Analysis was performed based on the intention‐to‐treat and per‐protocol principle, however only the intention‐to‐treat analysis was shown |
|
| Participants | 463 AOM children aged 2 to 12 years (231 and 232 children in the 2 and 3 times daily groups, respectively) | |
| Interventions | 10 days with amoxicillin/clavulanate (70/10 mg/kg/day and 60/15 mg/kg/day for the 2 and 3 times daily groups, respectively) | |
| Outcomes | Primary outcome
1. Clinical cure at the end of therapy (days 10 to 17) ‐ defined as complete or partial resolution Secondary outcomes 1. Clinical cure rate during therapy (days 2 to 3), in terms of resolution of otalgia and resolution of fever, was not described 2. Clinical cure rate at post‐treatment: ‐ Clinical cure at day 28 3. AOM complications after completion of therapy: recurrent AOM ‐ not described 4. Adverse reactions to medication: other outcome ‐ Compliance rate (at least 80%) |
|
| Notes | ‐ Supported by SmithKline Beecham Pharmaceuticals ‐ 34 and 44 children were noted to withdraw from the study, the total number of children at the end of therapy and follow‐up visit was the same as at the entry ‐ For clinical cure rate post‐treatment, indeterminate (10 versus 11) and 'lost' patients (11 versus 19) were counted as failures in both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised to treatment" Comment: the authors did not describe the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors did not mention anything about allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The study was observer‐blind" Comment: probably done to prevent detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "This fall in the success rate is partly accounted for by increased numbers of patients lost to follow‐up and those with an indeterminate outcome at follow‐up who were categorised as failures" Comment: for robustness, 'loss to follow‐up' and 'indeterminate outcome' should be counted as failure in the 2 times daily and success in the 3 times daily groups. If it was recalculated, success rate should be 185/231(80.1%) and 210/232 (90.5%) for the 2 times daily and 3 times daily groups, respectively |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes were accounted for |
| Other bias | Low risk | |
Damrikarnlert 2000.
| Methods | Multicentre, randomised, single‐blinded (observer‐blinded) trial conducted at 18 centres in Argentina, Brazil, Costa Rica, India, Kenya, Mexico, Morocco, Nigeria, Thailand and Turkey between August 1996 and March 1998 Block of 6 randomisation was performed using SAS software. Although analyses using the intention‐to‐treat and per‐protocol principle were planned, only the intention‐to‐treat principle analysis was shown |
|
| Participants | 415 AOM children aged 2 months to 12 years (209 and 206 children in the 2 and 3 times daily groups, respectively). However, 199 and 187 participants were analysed for primary outcome | |
| Interventions | 7 to 10 days (depending on national prescribing practice) with amoxicillin/clavulanate 45/6.4 mg/kg/day and 40/10 mg/kg/day (2 versus 3 times daily groups, respectively) | |
| Outcomes | Primary outcome
1. Clinical cure rate at the end of therapy ‐ Clinical success at days 7 to 12 Secondary outcome 1. Clinical cure rate during therapy 2. Clinical cure rate post‐treatment (1 to 3 months): ‐ Successful clinical response at days 38 to 42 3. AOM complications after completion of therapy: recurrent AOM 4. Adverse events: other outcomes ‐ Bacteriological response rate at the end of therapy ‐ Compliance rate (at least 80%) |
|
| Notes | Supported by Smithkline Beecham Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in a 1:1 ratio for the two treatments in blocks of 6 using a randomisation schedule generated by the sponsor using SAS software" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: did not mention the method of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "This was a single‐blind, randomised, comparative, parallel‐group study". "These data were recorded in a dispensing register rather than the case report form to maintain observer blinding" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The primary efficacy variable was the clinical response (success or failure) at the end of therapy (Day 7‐12). Secondary efficacy variables were clinical response at follow‐up (Day 38‐42) and bacteriological response (success or failure) at the end of therapy. A tertiary efficacy variable was the clinical response at the on‐therapy visit (Day 3‐5)" |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
AOM: acute otitis media MEE: middle ear effusion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jacobsson 1993 | 1. Included only recurrence or cases that were not responsive to amoxicillin/penicillin or cefaclor, which was different from other studies 2. The total daily dosage for amoxicillin was quite low (20 to 33.2 mg/kg/day) |
Contributions of authors
Sanguansak Thanaviratananich (ST) was responsible for conceiving, designing and co‐ordinating the protocol, developing the search strategy, assessing the quality of studies, entering text into RevMan and writing the review. Patravoot Vatanasapt (PV) was responsible for designing and co‐ordinating the review, assessing the quality of the studies and providing general advice on the review. Malinee Laopaiboon (ML) was responsible for entering text into RevMan and providing general advice on the statistical analysis and the review generally.
Sources of support
Internal sources
Faculty of Medicine, Khon Kaen, Thailand.
External sources
Thai Cochrane Network, Thailand.
Declarations of interest
No conflict of interest known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Behre 1997 {published data only (unpublished sought but not used)}
- Behre U, Burow HM, Quinn P, Cree F, Harrison HE. Efficacy of twice‐daily dosing of amoxycillin/clavulanate in acute otitis media in children. Infection 1997;25(3):163‐6. [DOI] [PubMed] [Google Scholar]
Damrikarnlert 2000 {published and unpublished data}
- Damrikarnlert L, Jauregui AC, Kzadri M. Efficacy and safety of amoxycillin/clavulanate (Augmentin) twice daily versus three times daily in the treatment of acute otitis media in children. The Augmentin 454 Study Group. Journal of Chemotherapy 2000;12(1):78‐97. [DOI] [PubMed] [Google Scholar]
Hoberman 1997 {published data only}
- Hoberman A, Paradise JL, Burch DJ, Valinski WA, Hedrick JA, Aronovitz GH, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin) for treatment of acute otitis media in children. Pediatric Infectious Diseases Journal 1997;16(5):463‐70. [DOI] [PubMed] [Google Scholar]
Murph 1993 {published data only}
- Murph JR, Dusdieker LB, Booth B, Murph WE. Is treatment of acute otitis media with once‐a‐day amoxicillin feasible? Results of a pilot study. Clinical Pediatrics 1993;32(9):528‐34. [DOI] [PubMed] [Google Scholar]
Principi 1986 {published data only}
- Principi N, Marchisio P, Bigalli L, Massironi E. Amoxicillin twice daily in the treatment of acute otitis media in infants and children. European Journal of Pediatrics 1986;145(6):522‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Jacobsson 1993 {published data only}
- Jacobsson S, Fogh A, Larsson A, Lomborg S. Evaluation of amoxicillin clavulanate twice daily versus thrice daily in the treatment of otitis media in children. Danish‐Swedish Study Group. European Journal of Clinical Microbiology and Infectious Diseases 1993;12(5):319‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
AAPS 2004
- American Academy of Pediatrics Subcommittee. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451‐65. [PUBMED: 15121972] [DOI] [PubMed] [Google Scholar]
Barnett 1995
- Barnett ED, Klein JO. The problem of resistant bacteria for the management of acute otitis media. Pediatric Clinics of North America 1995;42(3):509‐17. [PUBMED: 7761138] [DOI] [PubMed] [Google Scholar]
Carlin 1987
- Carlin SA, Marchant CD, Shurin PA, Johnson CE, Murdell‐Panek D, Barenkamp SJ. Early recurrences of otitis media: reinfection or relapse?. Journal of Pediatrics 1987;110(1):20‐5. [PUBMED: 3540247] [DOI] [PubMed] [Google Scholar]
Cars 1997
- Cars O. Efficacy of beta‐lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagnostic Microbiology and Infectious Disease 1997;27:29‐33. [PUBMED: 9127103] [DOI] [PubMed] [Google Scholar]
Daly 1999
- Daly KA, Brown JE, Lindgren BR, Meland MH, Le CT, Giebink GS. Epidemiology of otitis media onset by six months of age. Pediatrics 1999;103(6 pt 1):1158‐66. [PUBMED: 10353923] [DOI] [PubMed] [Google Scholar]
Drusano 1997
- Drusano GL, Craig WA. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. Journal of Chemotherapy 1997;9(Suppl 3):38‐44. [PUBMED: 9248979] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [PUBMED: 10877304] [DOI] [PubMed] [Google Scholar]
Faden 1994
- Faden H, Doern G, Wolf J, Blocker M. Antimicrobial susceptibility of nasopharyngeal isolates of potential pathogens recovered from infants before antibiotic therapy: implications for the management of otitis media. Pediatric Infectious Diseases Journal 1994;13(7):609‐12. [PUBMED: 7970948] [DOI] [PubMed] [Google Scholar]
Froom 1997
- Froom J, Culpepper L, Jacobs M, DeMelker RA, Green LA, Buchem L, et al. Antimicrobials for acute otitis media? A review from the International Primary Care Network. BMJ 1997;315(7100):98‐102. [PUBMED: 9240050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grob 1992
- Grob PR. Antibiotic prescribing practices and patient compliance in the community. Scandinavian Journal of Infectious Diseases ‐ Supplementum 1992;83:7‐14. [PUBMED: 1488629] [PubMed] [Google Scholar]
Henderson 1988
- Henderson FW, Gilligan PH, Wait K, Goff DA. Nasopharyngeal carriage of antibiotic‐resistant pneumococci by children in group day care. Journal of Infectious Diseases 1988;157:256‐63. [PUBMED: 3257247] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jacobs 1998
- Jacobs MR, Dagan R, Appelbaum PC, Burch DJ. Prevalence of antimicrobial‐resistant pathogens in middle ear fluid: multinational study of 917 children with acute otitis media. Antimicrobial Agents and Chemotherapy 1998;42(3):589‐95. [PUBMED: 9517937] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1996
- Johnson AP, Speller DC, George RC, Warner M, Domingue G, Efstratiou A. Prevalence of antibiotic resistance and serotypes in pneumococci in England and Wales: results of observational surveys in 1990 and 1995. BMJ 1996;312:1454‐6. [PUBMED: 8664623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 1995
- Kaplan SL. The emergence of resistant pneumococcus as a pathogen in childhood upper respiratory infections. Seminars in Respiratory Infections 1995;10:31‐6. [PUBMED: 7761712 ] [PubMed] [Google Scholar]
Kardas 2007
- Kardas P. Comparison of patient compliance with once‐daily and twice‐daily antibiotic regimens in respiratory tract infections: results of a randomized trial. Journal of Antimicrobial Chemotherapy 2007;59:531‐6. [PUBMED: 17289766] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville G. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Leibovitz 2003
- Leibovitz E. Acute otitis media in pediatric medicine: current issues in epidemiology, diagnosis, and management. Paediatric Drugs 2003;5(Suppl 1):1‐12. [PUBMED: 14632101] [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing Up: The Science of Reviewing Research. Cambridge, Massachusetts: Harvard University Press, 1984. [Google Scholar]
McCaig 1995
- McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among of office‐based physicians the United States. JAMA 1995;273:214‐9. [PUBMED: 7807660] [PubMed] [Google Scholar]
Pechere 2007
- Pechere JC, Hughes D, Kardas P, Cornaglia G. Non‐compliance with antibiotic therapy for acute community infections: a global survey. International Journal of Antimicrobial Agents 2007;29:245‐53. [PUBMED: 17229552] [DOI] [PubMed] [Google Scholar]
Pichichero 2003
- Pichichero ME, Casey JR. Acute otitis media disease management. Minerva Pediatrica 2003;55(5):415‐38. [PUBMED: 14608265] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rosenfeld 2001
- Rosenfeld RM, Casselbrant ML, Hannley MT. Implications of the AHRQ evidence report on acute otitis media. Otolaryngology ‐ Head and Neck Surgery 2001;125(5):440‐8. [PUBMED: 14608265] [DOI] [PubMed] [Google Scholar]
Rovers 2004
- Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet 2004;363:465‐73. [PUBMED: 14962529] [DOI] [PubMed] [Google Scholar]
Rovers 2006
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta‐analysis with individual patient data. Lancet 2006;368(9545):1429‐35. [PUBMED: 17055944] [DOI] [PubMed] [Google Scholar]
Teele 1989
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. Journal of Infectious Diseases 1989;160(1):83‐94. [PUBMED: 2732519] [DOI] [PubMed] [Google Scholar]
Urquhart 1992
- Urquhart J. Ascertaining how much compliance is enough with outpatient antibiotic regimens. Postgraduate Medical Journal 1992;68(Suppl 3):49‐58. [PUBMED: 1287619] [PubMed] [Google Scholar]
Venekamp 2013
- Venekamp RP, Sanders S, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000219.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Thanaviratananich 2008
- Thanaviratananich S, Laopaiboon M, Vatanasapt P. Once or twice daily versus three times daily amoxicillin with or without clavulanate for the treatment of acute otitis media. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004975.pub2] [DOI] [PubMed] [Google Scholar]
Thanaviratananich 2011
- Thanaviratananich S, Laopaiboon M, Vatanasapt P. Once or twice daily versus three times daily amoxicillin with or without clavulanate for the treatment of acute otitis media. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD004975.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


