Abstract
Background
The male condom, which consists of a thin sheath placed over the glans and shaft of the penis, is designed to prevent pregnancy by providing a physical barrier against the deposition of semen into the vagina during intercourse. Beginning in the 1990s, nonlatex male condoms made of polyurethane film or synthetic elastomers were developed as alternative male barrier methods for individuals with allergies, sensitivities or preferences that prevented the consistent use of condoms made of latex.
Objectives
The review sought to evaluate nonlatex male condoms in comparison with latex condoms in terms of contraceptive efficacy, breakage and slippage, safety, and user preferences.
Search methods
In December 2012, we searched computerized databases for randomized controlled trials (RCTS) of nonlatex condoms (MEDLINE, CENTRAL, POPLINE, LILACS, ClinicalTrials.gov, ICTRP). Previous searches also included EMBASE. For the initial review, we wrote to the manufacturers of nonlatex condoms and known investigators to locate other trials not identified in our search.
Selection criteria
The review included RCTs that evaluated a male nonlatex condom made of polyurethane film or synthetic elastomers in comparison with a latex condom.
Data collection and analysis
We evaluated all titles and abstracts located in the literature searches for inclusion. Two authors independently extracted data from the identified studies. We analyzed data with RevMan. The Peto odds ratio (Peto OR) with 95% confidence interval (CI) was calculated for each outcome of contraceptive efficacy, condom breakage and slippage, discontinuation of use, safety, and user preference. Contraceptive efficacy, early discontinuation, and safety outcomes were also measured with survival analysis techniques.
Main results
While the eZ·on condom did not protect against pregnancy as well as its latex comparison condom, no differences were found in the typical‐use efficacy between the Avanti and the Standard Tactylon and their latex counterparts. The nonlatex condoms had higher rates of clinical breakage than their latex comparison condoms: the Peto OR for clinical breakage ranged from 2.64 (95% CI 1.63 to 4.28) to 4.95 (95% CI 3.63 to 6.75). Few adverse events were reported. Substantial proportions of participants preferred the nonlatex condom or reported that they would recommend its use to others.
Authors' conclusions
Although the nonlatex condoms were associated with higher rates of clinical breakage than their latex comparison condoms, the new condoms still provide an acceptable alternative for those with allergies, sensitivities, or preferences that might prevent the consistent use of latex condoms. The contraceptive efficacy of the nonlatex condoms requires more research.
Keywords: Humans, Male, Latex, Polyurethanes, Condoms, Condoms/standards, Contraception, Contraception/instrumentation, Polystyrenes, Randomized Controlled Trials as Topic
Plain language summary
Nonlatex compared to latex male condoms for birth control
The male condom can prevent pregnancy by keeping sperm out of the birth canal. Nonlatex condoms can be used by people who are allergic or sensitive to latex. Some people may not have used latex condoms because they did not like them. This review compared nonlatex condoms with latex condoms. The main issues were effect on birth control, whether the condom broke or slipped, and which condom people liked.
In December 2012, we used a computer to find randomized trials of nonlatex condoms. For the initial review, we also wrote to researchers and makers of nonlatex condoms to find other trials. We included all studies that compared a male nonlatex condom with a latex condom.
The eZ·on condom did not prevent pregnancy as well as latex condoms. The Avanti and the Standard Tactylon condoms were similar to latex condoms for birth control. The nonlatex condoms broke more often than the latex condoms. However, many people liked the nonlatex condoms better. They may be useful for people who are allergic or sensitive to latex.
Background
The male condom, the only reversible male contraceptive method, consists of a thin sheath placed over the glans and shaft of the penis to provide a physical barrier against the deposition of semen into the vagina during intercourse. Most commercially‐produced condoms are made of latex. A small proportion (about 5% in the U.S.) is made of the intestinal cecum of lamb (Murphy 1990). These 'natural membrane' or 'lambskin' condoms are considered inferior to latex condoms in that they do not provide adequate protection against sexually transmitted infections (Lytle 1990; Minuk 1989). Latex male condoms have been mass‐produced since the mid‐1800s (Murphy 1990) and, currently, are widely used in many nations for contraception. In the United States, for example, an estimated 13% of women of reproductive age reported using male condoms for contraception in 1995 (Abma 1997). The latex condom offers a safe, effective, user‐controlled contraceptive method that is easy to use and relatively inexpensive.
A basic measure of contraceptive effectiveness is the first‐year failure rate, that is, the probability of pregnancy in the initial year of use. Although male condoms have an estimated method‐specific failure rate of 2% for the first year of use, the typical‐use failure rate is estimated to be 15% (Trussell 2007). The difference between the method‐specific failure rate and the user failure rate can be attributed to improper and inconsistent condom use. For example, fewer than half of condom users in the U.S. reported using condoms consistently at every act of intercourse (Mosher 1993). Due to the difficulties in conducting efficacy studies, comparative studies of condoms have often evaluated surrogate endpoints, such as condom breakage and slippage. Prospective studies of condoms used during vaginal intercourse have shown 2% breakage and complete slippage 2% of the time (Warner 2007).
Several factors could deter couples from using condoms or could contribute to their inconsistent use. Condom users have reported decreased sensitivity and sexual enjoyment. In a U.S. national survey, for example, almost 75% of men stated that condoms decreased sensation (Grady 1993). Difficulties in donning and removing condoms could also reduce their efficacy and acceptability; latex condoms are tight for retention during coitus and must be unrolled in only one direction onto the penis. Furthermore, latex allergies could preclude the use of condoms. An estimated 1% to 6% of the general U.S. population is allergic to latex, and the proportion may be much higher among populations with greater exposure to latex, such as health care workers (Warner 2007). Poor heat conductivity and relatively low strength at maximum stretch are demonstrated disadvantages of condoms made of latex. Latex condoms can also deteriorate during storage due to the susceptibility of latex to oxidation (Free 1996). In addition, the use of oil‐based lubricants, including hand oils and body lotions, can deteriorate latex.
Beginning in the 1990s, male condoms composed of polyurethane film or synthetic elastomers were developed to address these limitations. These nonlatex condoms provide an option for those with allergies or sensitivities to latex. Nonlatex condoms can also be safely used with oil‐based lubricants and have the potential for an increased shelf life due to their ability to withstand a broader range of storage conditions. In addition, nonlatex condoms were suggested to have a less noticeable odor, less constricting fit, and an improved ability to conduct body heat. Nonlatex condoms that are more effective and acceptable to the user than traditional latex condoms could be an important factor in increasing the consistent use of condoms as a method of contraception.
Objectives
To compare the contraceptive efficacy, breakage, slippage, safety, and user preference of nonlatex male condoms versus latex male condoms.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials in any language comparing a nonlatex male condom not made of natural membrane with a latex condom were eligible for inclusion. Although protection against sexually transmitted infections was not an outcome of the review, trials of natural membrane condoms were excluded since their use is not generally recommended due to their recognized inadequacy in protecting against the transmission of viruses (Lytle 1990; Minuk 1989).
Types of participants
Eligible participants were sexually active couples engaging in heterosexual, vaginal intercourse and without contraindications to latex or nonlatex condoms.
Types of interventions
Any nonlatex condom not made out of natural membrane was eligible to be included. Currently, five types of nonlatex condoms are manufactured: eZ·on, Avanti, Tactylon, the Protex Original, and the Trojan Supra. Randomized controlled trials evaluating the first four types of condoms were found.
The nonlatex eZ·on condom (Family Health International, Research Triangle Park, NC and Mayer Laboratories, Inc., Oakland, CA) is a baggy polyurethane condom that can be donned in either direction. The eZ·on condom is 28 mm in diameter in the opening, 171 mm in length and 70 mm in width. The condom is packaged with a silicone‐based lubricant.
The nonlatex Avanti condom (SSL International plc, Knutsford, UK) is a nipple‐tipped polyurethane condom that is 33 mm in diameter in the opening, 180 mm in length, 0.035 to 0.040 mm in thickness and 64 mm in width. The condom is packaged with a silicone‐based lubricant. The Avanti Super Thin condom (SSL International plc, Knutsford, UK) has the same dimensions but slightly more lubricant. Both styles of Avanti condoms were treated as the same product by the U.S. regulatory agency, the Food and Drug Administration (FDA).
The nonlatex Tactylon condoms (Sensicon Corporation, Vista, CA) are made of styrene ethylene butylene styrene (SEBS), a synthetic polymer used in Tactylon surgical and examination gloves (SmartPractice). The condoms come in three styles: Standard Tactylon, Baggy Tactylon, and Low‐Modulus Tactylon. The Standard Tactylon has a standard cylindrical shape with a reservoir tip. The Baggy Tactylon has a diameter at the opening that is similar to traditional condoms, but the diameter is larger immediately below the open end. The Low‐Modulus Tactylon has a standard cylindrical shape with a low modulus (i.e., low resistance to stretch) with a high elongation. The three condoms are packaged with a silicone‐based lubricant and have similar dimensions (180 mm in length, 0.07 mm in thickness, and 52 mm in width) except that the width of the Baggy Tactylon ranges from 49 mm to 81 mm.
The nonlatex condom Sagami Protex Original (Sagami Rubber Industries Co, Ltd., Tokyo, Japan) is made of polyurethane. The Protex Original is 193 mm in length, 0.03 mm in thickness, and 58 mm in width. The condom is packaged with a silicone‐based lubricant.
Any latex male condom could be the comparison method.
Types of outcome measures
Outcome measures included contraceptive efficacy, condom breakage and slippage, discontinuation of use, safety, and user preference. We used the condom breakage and slippage measures proposed by Steiner and colleagues (Steiner 1994): (1) Nonclinical breakage The number of condoms that break before intercourse while package is being opened or while condom is being put on divided by the number of condoms attempted to be used. (2) Clinical breakage The number of condoms that break during intercourse or withdrawal divided by the number of condoms used during intercourse. (3) Total breakage Both clinical and nonclinical breakage divided by the number of condoms attempted to be used. (4) Complete slippage The number of condoms that completely slip off the penis during intercourse or withdrawal divided by the number of condoms used during intercourse. (5) Partial slippage The number of condoms that partially slip off the penis during intercourse or withdrawal divided by the number of condoms used during intercourse. (6) Total clinical failure The number of condoms that break or slip completely off during intercourse or withdrawal divided by the number of condoms used during intercourse. (7) Total failure The number of condoms that break (both nonclinical and clinical breakage) or completely slip off divided by the number of condoms attempted to be used.
Search methods for identification of studies
Electronic searches
In December 2012, we searched the computerized databases of MEDLINE using PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), POPLINE, and LILACS for studies of nonlatex condoms. We also searched for recent trials via ClinicalTrials.gov and the search portal of the International Clinical Trials Registry Platform (ICTRP). The 2012 strategies can be found in Appendix 1. The 2010 strategies can be found in Appendix 2. We also searched EMBASE for the initial review and the 2006 and 2008 updates (Appendix 3).
Searching other resources
The references of identified publications were assessed for inclusion. For the initial review, we also wrote to the manufacturers of nonlatex condoms and known investigators to request information about any other published or unpublished trials not discovered in our search.
Data collection and analysis
One author evaluated all titles and abstracts located in the literature searches to determine whether they met the inclusion criteria. Two authors independently extracted data from the studies identified for inclusion. Data were entered and analyzed with RevMan. The Peto odds ratio (Peto OR) with 95% confidence interval (CI) was calculated for each outcome with the number of condoms, men, women, or couples used as the denominator. Contraceptive efficacy and early discontinuation were also measured using survival analysis techniques and entered into 'Additional tables.'
We could not use paired analyses for the crossover trials since the data were not presented in this manner. Elbourne 2002 suggests using only the data from the first treatment period when paired data are not available; however, data by treatment period were not provided. Therefore, we treated the data from the crossover trials as if they had come from parallel trials. That is, even though the same participants were in each condom group, we analyzed the data defined by the latex and nonlatex condom groups. This approach does not take advantage of the within‐participant correlations present in crossover trials. Also, the assumption of independence required by most statistical methods is violated since the same participants were included in both groups.
Although couples in each of the eligible trials were assigned to use multiple condoms during both the latex and nonlatex periods, the present review ignores the cluster design of the trials. Four trials (Bounds 2002; Callahan 2000; Cook 2001; Steiner 2003) accounted for potential cluster effects in at least some outcomes by modeling using generalized estimating equation methods. Since data from cluster analyses were not available for most outcomes and since RevMan does not support cluster data, we treated the data as if they came from independent observations. This method, though, is less than ideal given that the probability of condom function outcomes could vary substantially between couples.
Due to the differences in the dimensions and materials of condom types, study results were combined for meta‐analysis only when identical comparisons of nonlatex and latex condom types were made. The homogeneity of the meta‐analyses was assessed by examining the results from both a fixed‐effects model and a random‐effects model. Since the chi‐squared test for heterogeneity used in RevMan is a low‐power test, the alpha level was set at 0.10. Sensitivity analyses were conducted to test the robustness of the results that appeared to be based on heterogeneous combinations. The effect of deleting each study in turn was assessed. All trials were critically appraised by examining factors that can potentially contribute to biases: the study design, blinding, randomization method, group allocation concealment, and loss to follow up and early discontinuation.
Results
Description of studies
Eight randomized crossover trials (Bounds 2002; Callahan 2000; Cook 2001; Frezieres 1998; Frezieres 2000; Potter 2003; Steiner 1993; Trussell 1992) and three randomized parallel trials (Frezieres 1999; Nelson 2001; Steiner 2003) satisfied the criteria for inclusion. The 11 eligible trials recruited sexually active, adult couples in a monogamous, heterosexual relationship and who were not at risk for sexually transmitted diseases. Nine trials were conducted in the U.S., one trial (Bounds 2002) was located in the U.K., and one was done in France (Potter 2003). Two trials differed from the others by restricting participation to couples using condoms for contraception prior to study entry (Frezieres 2000; Potter 2003). Couples in the crossover trials were to use two to six condoms of each type. The study periods were 5 to 12 weeks, except for two trials (Bounds 2002; Potter 2003) that did not report the duration for each condom period. The three randomized parallel trials (Frezieres 1999; Nelson 2001; Steiner 2003) were the only studies that were designed to measure contraceptive efficacy. Although the two earlier trials (Frezieres 1999; Nelson 2001) had a longer (six‐month) duration than the crossover studies, the condom breakage, slippage and acceptability data were based on a nested study of the condoms used for the first five acts of intercourse, and therefore are comparable with data from the crossover trials in terms of the number of condoms used and the duration of the study period. In contrast, the breakage and slippage data from the most recent efficacy study (Steiner 2003) were collected from the entire seven‐month study.
Four types of nonlatex condoms and eight latex condoms were evaluated in 14 nonlatex and latex condom combinations. The baggy polyurethane eZ·on condom was compared to the latex Kimono Select condom in two studies (Cook 2001; Steiner 2003) and to the latex Durex Gossamer condom in a third study (Bounds 2002). Both the Kimono Select (180 mm in length, 54 mm in diameter and 0.06 mm in thickness) and Durex Gossamer (178 mm in length, 52 mm in diameter and 0.065 mm in thickness) condoms are standard‐shaped devices that are packaged in a silicone‐based lubricant.
The second nonlatex condom, the polyurethane Avanti condom, was evaluated in four trials (Bounds 2002; Frezieres 1998; Frezieres 1999; Frezieres 2000). The Bounds 2002 study compared the Avanti condom to the latex Durex Gossamer condom. Frezieres 1998 and Frezieres 1999 compared the Avanti with the latex Ramses Sensitol condom. The Ramses Sensitol condom is identical to the Avanti condom in length and open‐end circumference. Also, both are reservoir‐tipped and packaged in a silicone‐based lubricant. However, the Avanti condom is thinner (0.035 to 0.040 mm versus 0.070 to 0.080 mm) and wider when laid flat (64 mm versus 52 mm) than the Ramses Sensitol condom. Frezieres 2000 compared the Avanti with the latex Trojan‐Enz condom. Both condoms are cylindrical with a reservoir tip and are similar in length (180 mm), but the Trojan‐Enz condom is thicker (0.075 mm versus 0.04 to 0.05 mm) and is narrower when laid flat (52 mm versus 57 mm). Also, the Trojan‐Enz is packaged in an aqueous‐based lubricant while the Avanti condom comes in a silicone‐based lubricant. Both condoms were distributed to the study participants with the lubricant Astroglide (Biofilm, Inc.).
The third nonlatex condom comes in three styles: Standard Tactylon, Baggy Tactylon, and Low‐Modulus Tactylon. One study (Steiner 1993) compared the three lubricated Tactylon styles to the standard, lubricated latex condom distributed by the U.S. Agency for International Development (USAID). A second study (Callahan 2000) compared the three Tactylon condoms to the latex Aladan condom. The lubricated Aladan condom has a standard cylindrical, reservoir‐tipped shape 183 mm in length, 52 mm in width and 0.07 mm in thickness. Three trials (Frezieres 2000; Nelson 2001; Trussell 1992) evaluated the Standard Tactylon condom versus the latex Trojan‐Enz condom. While the Standard Tactylon and the Trojan‐Enz condoms were lubricated in two trials (Frezieres 2000; Nelson 2001), the condoms were distributed without lubrication in the third (Trussell 1992). Nelson 2001 also compared the Standard Tactylon to the LifeStyles condom (52 mm in width, 180 mm in length and 0.06 mm thick) with the lubricant Astroglide distributed to the study participants.
The fourth nonlatex condom was made of polyurethane and known as Protex Original in Europe and Sagami Original in Japan. Potter 2003 compared the Protex Original with a control latex condom supplied by the same manufacturer, Sagami Rubber Industries Co., Ltd. The polyurethane condom was thinner than the latex condom (0.03 mm versus 0.06 mm). The Protex Original was also slightly wider (58 mm versus 52 mm) and slightly longer (193 mm versus 189 mm). Both types of condoms were packaged with the same quantity of a silicone‐based lubricant.
Risk of bias in included studies
The 'Characteristics of included studies' table includes details related to the methodological quality of each of the studies. Despite the provision of condoms in similar packages (Callahan 2000; Frezieres 1999), the participants could not be blinded to the group assignment in any of the trials due to differences in condom attributes. Study investigators and staff were blinded in three studies (Frezieres 1998; Frezieres 1999; Nelson 2001) and outcome assessors were blinded in five studies (Cook 2001; Frezieres 1998; Frezieres 1999; Nelson 2001; Steiner 2003). Randomization was conducted using random sampling numbers (Bounds 2002); computer‐generated numbers (Frezieres 1998; Frezieres 1999; Nelson 2001); a computer‐generated, permuted block scheme stratified by site only (Cook 2001) or site and prior condom experience (Steiner 2003); or an undescribed method (Callahan 2000; Frezieres 2000; Potter 2003; Steiner 1993; Trussell 1992). Group allocation was concealed using sealed, sequentially‐numbered containers (Frezieres 1998); sealed, sequentially‐numbered opaque containers (Cook 2001; Frezieres 1999; Nelson 2001); or a centralized telephone allocation process (Steiner 2003). The remaining six trials did not report the method of allocation concealment.
The proportion of eligible couples who were recruited but subsequently declined participation ranged from 11% to 64% for the six trials that reported this information (Frezieres 1998; Frezieres 1999; Nelson 2001; Potter 2003; Steiner 1993; Trussell 1992). The combined loss to follow up and early discontinuation rates ranged from 2% to 47% in the 11 trials. About 8% of couples in the Nelson 2001 trial were disqualified after randomization and excluded from the analyses. Pregnancy at enrollment was the most common reason given for disqualification (13 women in the Tactylon, 3 in the LifeStyles, and 9 in the Trojan‐Enz group). Two couples were excluded from the analyses in Potter 2003, due to ambiguous responses. The exclusion of randomized participants from the analysis is inappropriate since it can bias the results (Schulz 2002b).
Callahan 2000 deviated from the proposed condom breakage and slippage standard definitions (Steiner 1994) by classifying breaks that occurred after withdrawal as nonclinical breakage. The remaining trials either followed the standard breakage and slippage definitions or presented the data in a manner that allowed their extraction. The randomized study design ideally prevents bias due to a learning effect. Almost a quarter (23.9%) of the couples in the crossover trial by Steiner 1993 did not follow perfectly the randomized order for the use of the four study condoms and 8.8% did not use the two assigned condoms of the same type consecutively. Steiner 1993 argued that the lack of compliance with the designated order was unlikely to have more than a minimal effect on the measures of condom functionality since most couples were experienced condom users and would have gained an inconsequential amount of condom experience during the study. While departure from the randomized condom order potentially is an issue in a second crossover trial (Trussell 1992), the authors did not describe any violations in condom use order. The remaining five crossover studies used a study design that was unlikely to lead to changes in the assigned order of condom use.
Effects of interventions
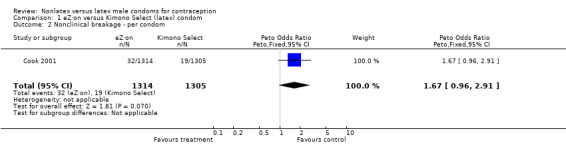
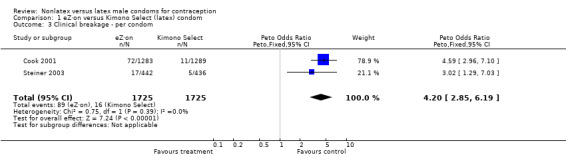
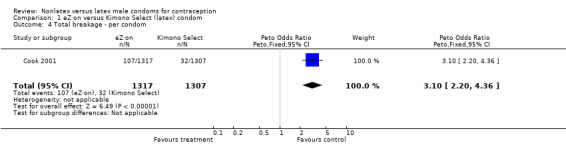
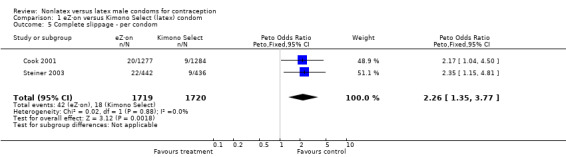
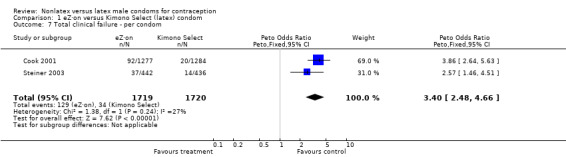
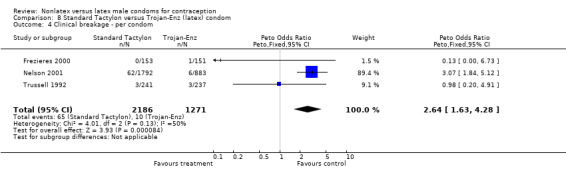
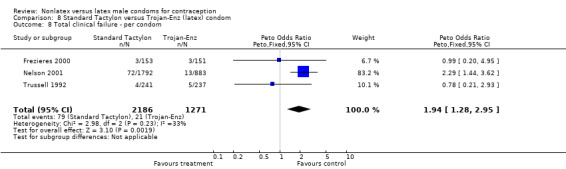
The nonlatex condoms did not fare as well as the latex condoms in terms of total failure and total clinical failure. For five comparisons, the Peto OR of total failure for the nonlatex condoms versus their latex comparisons varied between 1.92 (95% CI 1.08 to 3.40) and 3.47 (95% CI 2.82 to 4.27). Six comparisons did not have statistically significantly different Peto ORs and three comparisons did not report data for total failure. For eight comparisons, the Peto OR of total clinical failure for the nonlatex versus latex condoms ranged from 1.94 (95% CI 1.28 to 2.95) to 4.41 (95% CI 3.51 to 5.54), but it was not statistically significantly different for six comparisons. Clinical breakage, rather than nonclinical breakage or slippage, was responsible for the higher rates of condom failures with the nonlatex condoms. The Peto OR of clinical breakage for the nonlatex condoms versus their latex comparison condoms ranged from 2.64 (95% CI 1.63 to 4.28) to 4.95 (95% CI 3.63 to 6.75), except for five comparisons that did not show statistically significant differences. Most comparisons for nonclinical breakage, complete slippage, or partial slippage did not find differences between the nonlatex and latex condoms. The Avanti versus the Ramses Sensitol condom (Peto OR 0.23; 95% CI 0.06 to 0.90) and the Baggy Tactylon versus the USAID condom (Peto OR 3.73; 95% CI 1.43 to 9.72) were the only comparisons with statistically significant findings for nonclinical breakage. The only comparisons with statistically significant Peto ORs of complete slippage were for the eZ·on versus the latex Kimono Select (Peto OR 2.26; 95% CI 1.35 to 3.77) and for the Avanti versus the latex Ramses Sensitol condom (Peto OR 3.57; 95% CI 2.58 to 4.95).
Only one trial found an important difference in contraceptive efficacy. Since the a priori null hypothesis of the inferiority of the nonlatex condom for typical‐use efficacy was not rejected, Steiner 2003 concluded that the eZ·on condom did not protect against pregnancy as well as the nonlatex Kimono Select condom (Table 1). However, no statistically significant differences in typical‐use efficacy were found for the Avanti versus the latex Ramses Sensitol or the Standard Tactylon versus the combined LifeStyles and Trojan‐Enz latex condoms (Table 2). The Peto OR of pregnancy, calculated with the number of condoms (Frezieres 1999) or the number of women (Nelson 2001) as the denominator, also did not show any statistically significant advantages of either condom group in preventing pregnancies.
1. Nonlatex versus latex: hazard ratio of pregnancy.
| Trial | Comparison | Nonlatex probability | Latex probability |
| Steiner 2003 | eZ·on versus Kimono Select | 9.0 (95% CI 5.9 to 12.2)* | 5.4 (95% CI 2.9 to 7.8)* |
*stratified by site and prior condom experience
2. Nonlatex versus latex: 6‐month cumulative lifetable pregnancy rate per 100 women.
| Trial | Comparison | Nonlatex rate | Latex rate |
| Frezieres 1999 | Avanti versus Ramses Sensitol | 4.1 (95% CI 1.9 to 6.3) | 6.2 (95% CI 3.6 to 8.8) |
| Nelson 2001 | Standard Tactylon versus LifeStyles/Trojan‐Enz | 10.8 | 7.9 |
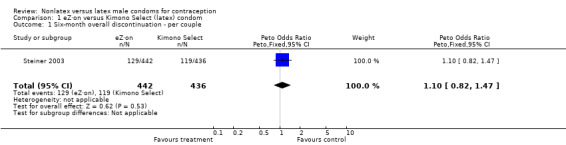
Discontinuation rates varied widely. Two parallel trials (Frezieres 1999; Nelson 2001) reported six‐month cumulative life‐table rates of early discontinuation per 100 women. Women in the Avanti group were significantly more likely to discontinue the trial early (P value 0.002 from article; Table 3) than those in the latex Ramses Sensitol group. The Avanti condom users also were significantly more likely to discontinue for condom‐related reasons than the latex condom users (P value 0.01 from article). The life‐table overall discontinuation rates for the Standard Tactylon users compared to the combined group of latex Lifestyles and Trojan‐Enz users were not significantly different (Table 3). In the third parallel trial (Steiner 2003), the Peto OR showed that the eZ·on and the latex Kimono Select groups were similar for overall discontinuation.
3. Nonlatex versus latex: 6‐month cumulative lifetable discontinuation rate per 100 women.
| Outcome | Trial | Comparison | Nonlatex rate | Latex rate |
| Discontinuation ‐ overall | Frezieres 1999 | Avanti versus Ramses Sensitol | 37.6 | 27.6 |
| Nelson 2001 | Standard Tactylon versus LifeStyles/Trojan‐Enz | 30.9 | 26.0 | |
| Discontinuation ‐ condom‐related | Frezieres 1999 | Avanti versus Ramses Sensitol | 17.5 (13.5 to 21.5) | 11.0 (7.8 to 14.3) |
| Nelson 2001 | Standard Tactylon versus LifeStyles/Trojan‐Enz | 17.7 | 12.7 | |
| Discontinuation ‐ not condom‐related | Frezieres 1999 | Avanti versus Ramses Sensitol | 20.6 (16.2 to 25.0) | 15.9 (12.1 to 19.7) |
| Nelson 2001 | Standard Tactylon versus LifeStyles/Trojan‐Enz | 24.9 | 21.6 |
Several important differences were found in the frequency of adverse events. Two trials reported data on "medical events," which the authors defined as any genital problem that remained for less than 24 hours (Callahan 2000; Cook 2001). These transient symptoms included genital burning, irritation, itching, rash, and bruising. No differences in medical events were detected in Cook 2001. In Callahan 2000, the Standard Tactylon was associated with fewer medical events when compared with the latex Aladan condom; the Peto OR of medical events was 0.35 (95% CI 0.19 to 0.63) for males and 0.39 (95% CI 0.27 to 0.57) for females. The Baggy Tactylon (Peto OR 0.49; 95% CI 0.35 to 0.70) and the Low‐Modulus Tactylon (Peto OR 0.61; 95% CI 0.43 to 0.85) resulted in fewer medical events for females when compared with the Aladan condom (Callahan 2000). Irritation, burning, itching and genital pain were the most commonly reported medical events in the trial comparing the three Tactylon styles to the Aladan condom (Callahan 2000). The Frezieres 1999 efficacy study reported on transitory discomfort to males, including painful constriction, irritation, itching and burning. The Avanti users were less likely than users of the Ramses Sensitol condom to report these events (Peto OR 0.63; 95% CI 0.58 to 0.68). The Steiner 2003 efficacy trial reported on "genital adverse experiences," which they defined as possibly or probably product‐related adverse events. Males did not report differences in genital adverse experiences by condom type (Table 4). However, female eZ·on condom users were less likely to report genital problems than their latex Kimono Select counterparts, with a hazard ratio of 0.6 (95% CI 0.5 to 0.8) stratified by center and prior condom experience (Table 5). In Potter 2003, males reported more itching, burning, and prickling when using the Protex Original than when using the latex condom (Peto OR 1.65; 95% CI 1.21 to 2.24).
4. Nonlatex versus latex: hazard ratio of genital irritation ‐ males.
| Study | Comparison | Hazard Ratio* | 95% CI |
| Steiner 2003 | eZ·on versus Kimono Select (latex) condom | 1.0 | 0.7 to 1.5 |
*stratified by site and prior condom experience
5. Nonlatex versus latex: hazard ratio of genital irritation ‐ females.
| Study | Comparison | Hazard Ratio* | 95% CI |
| Steiner 2003 | eZ·on versus Kimono Select (latex) condom | 0.6 | 0.5 to 0.8 |
*stratified by site and prior condom experience
Most studies either did not report adverse events or did not have enough power to adequately detect differences between groups. In Cook 2001, adverse events included two men who reported genital irritation or mild genital rash and four women who reported mild or moderate genital irritation, genital rash, severe genital edema, or labial edema. The participants with adverse events were evenly divided between the eZ·on and the latex Kimono Select condom groups. Callahan 2000 reported one adverse event, which was a case of vaginitis by a woman using the Baggy Tactylon condom. In the Nelson 2001 trial, none of the male participants reported adverse events that they believed to be probably or possibly related to the study condoms. Women reported 15 adverse events, including yeast infection, urinary tract infection, allergy to latex condom, and undiagnosed events.
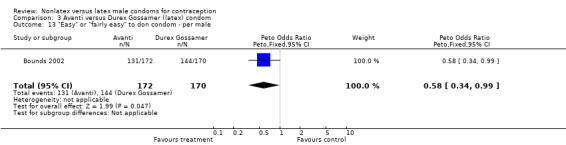
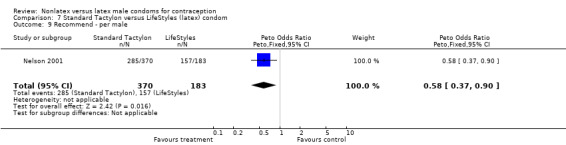
Few of the trials found statistically significant differences in the acceptability of the condom types. Males in the Bounds 2002 trial reported less often that the eZ·on was easy to don than the latex Durex Gossamer (Peto OR 0.49; 95% CI 0.29 to 0.82). In the Frezieres 1999 efficacy study, the male users of the Avanti condom reported less often that they would recommend their assigned condom than the latex Ramses Sensitol condom users (Peto OR 0.34; 95% CI 0.25 to 0.47). In a second efficacy trial (Nelson 2001), male Standard Tactylon users reported less often than male Lifestyles users that they would recommend their study condom (Peto OR 0.58; 95% CI 0.37 to 0.90). Female Standard Tactylon users reported less often than female Trojan‐Enz users that they would recommend their study condom (Peto OR 0.55; 95% CI 0.34 to 0.88) (Nelson 2001). Finally, the Low‐Modulus Tactylon condom was chosen as the overall preferred condom more often than the latex comparison condom among the participants in the two crossover trials who used the three types of Tactylon condoms and either the latex Aladan condom (Callahan 2000) or the standard USAID latex condom (Steiner 1993). The Peto OR of preferred condom for the Low‐Modulus Tactylon versus the Aladan condom was 1.63 (95% CI 1.20 to 2.23) for males and 1.48 (95% CI 1.08 to 2.01) for females. The Peto OR for the Low‐Modulus Tactylon versus the standard USAID latex condom was 1.83 (95% CI 1.24 to 2.69) for males and 1.52 (95% CI 1.04 to 2.23) for females. In comparing the Protex Original with a standard latex condom, Potter 2003 used eight condom properties rather than an overall preference item. The Protex Original was preferred for thinness and odor, while the latex condom was preferred for its sound, touch and feel, and ease of unrolling.
Only three comparisons included identical latex and nonlatex condom types and, thus, were eligible to be combined in meta‐analyses: 1) the eZ·on versus the Kimono Select (Cook 2001; Steiner 2003); 2) the Avanti versus the latex Ramses Sensitol condom (Frezieres 1998; Frezieres 1999); and 3) the Standard Tactylon versus the latex Trojan‐Enz condom (Frezieres 2000; Nelson 2001; Trussell 1992). The trial results included in the first two comparisons appeared to be homogenous using either a fixed‐effects or a random‐effects model. The Standard Tactylon versus the Trojan‐Enz condom comparisons also appeared to be homogeneous with two exceptions: the results for clinical breakage and total breakage appeared to differ between the studies. The three trials differed in that the condoms were lubricated in two trials (Frezieres 2000; Nelson 2001) but unlubricated in the third (Trussell 1992). However, the estimates for clinical breakage and total breakage from Trussell 1992 appeared to be homogenous with the estimates from the two trials that used lubricated condoms. Sensitivity analysis revealed that the statistically significant results for the two outcomes were both dependent on the inclusion of Nelson 2001.
Discussion
Although the nonlatex condoms had breakage and slippage rates similar to those found in the literature (Warner 2007), they did not perform as well as the latex condoms. In general, the nonlatex condoms were more likely to break during intercourse or withdrawal than were the latex condoms. While the eZ·on condom did not protect against pregnancy as well as its latex comparison condom, no differences were found in the typical‐use efficacy in the comparisons between the Avanti and the Standard Tactylon and their latex counterparts. Substantial proportions of study participants reported preferences for the nonlatex condoms. Therefore, the nonlatex condoms appear to be an acceptable alternative for those with sensitivities, aversion, or reluctance to the use of latex condoms.
An interpretation of the findings of the trials should include a consideration of several limitations. Breakage and slippage outcomes appear to be useful for comparative studies of condom types, and the eligible trials collected data on these measures of functionality; however, breakage and slippage have not been established to be valid surrogate endpoints for contraceptive efficacy. The two randomized parallel trials with a breakage and slippage component nested within a longer efficacy study (Frezieres 1999; Nelson 2001) provided an opportunity to evaluate the ability of breakage and slippage rates to predict contraceptive efficacy. Neither trial found evidence to suggest that measures of condom functionality predicted typical‐use pregnancy rates. Since slippage and breakage do not appear to be valid surrogate endpoints for pregnancy, these outcomes should not be used in future studies (Grimes 2005).
Second, differences in experience with latex and nonlatex condoms may have resulted in unbalanced measures of condom functionality. The proportion of participants in the 10 trials who were experienced condom users (with the definition of "experienced" varying between trials) ranged from 73% to 100%. Because nonlatex condoms were new products that were not widely available at the time of the trials, prior condom experience probably was limited to the latex devices. The participants were unlikely to have become as proficient during the trials in donning, fitting, and using the condoms made from the new materials as they were with nonlatex condoms, since most trials assigned few condoms of each type to be used in a short period of time.
Third, despite the crossover design in eight of the included trials, the results were analyzed as if they were from parallel trials. This analytic method is not a preferred approach since it fails to account for the within‐participant correlation present in crossover trials and also violates the assumption of independence required for most statistical methods (Elbourne 2002).
Fourth, since the trials assigned multiple condoms of each type, couples who may have been predisposed to condom failures could have contributed a disproportionate number of failures. For example, Frezieres 1998 found that only 4% of the couples broke more than one of the polyurethane condoms, but those couples accounted for 39% of the clinical breaks with the polyurethane condoms. The low number of condoms assigned to be used by the couples in each of the trials reduced the potential impact of couples who might have been prone to condom failure. However, the review would have been strengthened with the use of cluster analyses to account for inter‐couple differences.
Fifth, the studies relied on self‐reported outcomes, which might not have provided adequate assessments of slippage and breakage. The validity of self‐reported condom use has been examined, and may depend on factors related to the population or intervention (Chen 2007; Gallo 2007).
Sixth, couples at a greater risk of experiencing condom failure might have discontinued the trial at a higher rate than couples at lower risk of condom failure. If a disproportionate rate of study discontinuation occurred between these two groups, then the reported rates for condom slippage and breakage would underestimate the actual rates. Also, if study discontinuation occurred in a different pattern between the study condom groups, then the comparative measures of slippage and breakage could be biased. For example, potential for bias exists in the Frezieres 1999 efficacy study since 33% of the polyurethane users who discontinued before completing two months reported a condom breakage versus 3% of the latex condom users who discontinued during this period.
Seventh, the participants could not be blinded as to their assigned device, which introduces the potential for bias due to media exposure or personal experiences with the study condom type. This might bias acceptability outcomes, but probably would have less effect on efficacy comparisons. Also, six of the trials did not describe any attempt to conceal the allocation process; lack of adequate allocation concealment could have introduced bias (Schulz 2002a).
Furthermore, the generalizability of the findings might be limited. The high proportion of experienced condom users could limit the ability to extrapolate the results of the trials to populations with less condom experience. Also, since the couples in the trials self‐selected for participation and were required to meet eligibility criteria, the results from these participants may not apply to those not in a monogamous relationship, younger than 18 years of age, or with a known risk for sexually transmitted infections (STIs). For example, since the study couples were not at a high risk for STIs and the majority of the trials required the use of another effective contraceptive method, the compliance and diligence in condom use in these trials may not be applicable to other settings. Finally, the short duration of the trials may not be adequate for predicting experience with the condoms during longer, real‐life use.
Authors' conclusions
Implications for practice.
Only three trials examined contraceptive efficacy. While the eZ·on condom did not perform as well as its comparison latex condom in terms of preventing pregnancy, the Avanti and the Standard Tactylon had pregnancy rates similar to their latex comparisons. Despite the higher rate of clinical breakages with the nonlatex condoms, condoms made of the new materials could provide an acceptable alternative for individuals with allergies, sensitivities, or personal preferences that might prevent the consistent use of latex condoms.
Implications for research.
Since nonlatex condoms could be appropriate for certain subgroups, efficacy studies of the condom types are warranted. Breakage and slippage have not been found to be valid surrogate endpoints, so future studies should focus on pregnancy rates.
We only examined trials on the use of nonlatex condoms during vaginal intercourse to prevent pregnancies. The ability of the nonlatex condoms to protect against the transmission of HIV or other sexually transmitted infections (STIs) has not been established. While the new condoms are thought to provide protection comparable to that of latex condoms (Trussell 2007), the consequences of infection justify research on STI transmission.
What's new
| Date | Event | Description |
|---|---|---|
| 16 March 2015 | Amended | Added further detail in Declarations of interest |
| 15 January 2014 | Review declared as stable | No longer being updated |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 18 December 2012 | New search has been performed | Updated searches; no new studies found. |
| 4 November 2010 | New search has been performed | Searches were updated for MEDLINE, CENTRAL, POPLINE, LILACS, ClinicalTrials.gov, and ICTRP. No new studies were found. |
| 27 May 2008 | New search has been performed | Searches were updated; no new trials were found. |
| 21 September 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of FHI 360 assisted with the literature searches.
Appendices
Appendix 1. Search 2012
MEDLINE via PubMed (01 Mar 2010 to 17 Dec 2012)
condom*[tiab] AND latex[tiab] AND Clinical Trial[ptyp]
CENTRAL (2010 to 17 Dec 2012)
latex AND condom* in title, abstract, or keywords
POPLINE (2010 to 2012)
Global: condom* AND latex
LILACS (through 17 Dec 2012)
latex and (condom or condoms or condon or condones or preservativo or preservativos) [Words]
ClinicalTrials.gov (01 Jun 2010 to 17 Dec 2012)
1) Search terms: latex AND condom* AND contracept* Study type: Interventional
2) Intervention: latex AND condom* Study type: Interventional
ICTRP (01 Jun 2010 to 17 Dec 2012)
Intervention: latex AND condom*
Appendix 2. Search 2010
MEDLINE via PubMed (2008 to 02 Nov 2010)
This was based on the recommended Cochrane search strategy revised for PubMed searches (Robinson 2002): (("condom"[title/abstract word]) AND ("latex*"[title/abstract word])) AND ((randomized controlled trials [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ("latin square" [tw]) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh]))
CENTRAL (through 02 Nov 2010)
latex AND condom* in title, abstract, or keywords
POPLINE (2008 to 03 Nov 2010)
(condom & latex) & (compar* / clinical trials / comparative studies / random / double‐blind studies)
LILACS (through 03 Nov 2010)
latex and (condom or condoms or condon or condones or preservativo or preservativos) [Words]
ClinicalTrials.gov (through 02 Nov 2010)
Search terms: latex AND condom* AND contraception
ICTRP (through 02 Nov 2010)
Condition: contraceptive OR contraception Intervention: latex AND condom
Appendix 3. Previous searches
Previous searches used the strategies listed for 2010 and also included the following:
EMBASE (initial review and updates in 2006 and 2008)
latex(w)condom?
Data and analyses
Comparison 1. eZ·on versus Kimono Select (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐month overall discontinuation ‐ per couple | 1 | 878 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.82, 1.47] |
| 2 Nonclinical breakage ‐ per condom | 1 | 2619 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.96, 2.91] |
| 3 Clinical breakage ‐ per condom | 2 | 3450 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.20 [2.85, 6.19] |
| 4 Total breakage ‐ per condom | 1 | 2624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.10 [2.20, 4.36] |
| 5 Complete slippage ‐ per condom | 2 | 3439 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.26 [1.35, 3.77] |
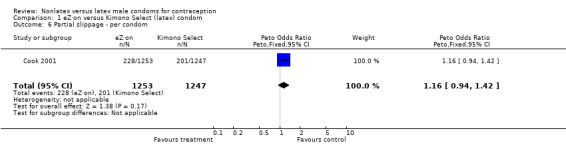
| 6 Partial slippage ‐ per condom | 1 | 2500 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.94, 1.42] |
| 7 Total clinical failure ‐ per condom | 2 | 3439 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.40 [2.48, 4.66] |
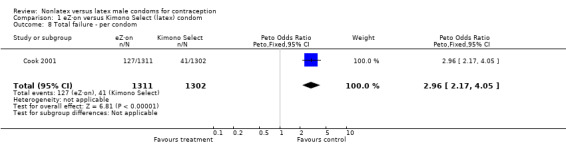
| 8 Total failure ‐ per condom | 1 | 2613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.96 [2.17, 4.05] |
| 9 Medical event ‐ per male | 1 | 2807 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [0.97, 4.56] |
| 10 Medical event ‐ per female | 1 | 2807 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.98, 3.00] |
| 11 Adverse genital experience ‐ per male | 1 | 878 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.59, 1.42] |
| 12 Adverse genital experience ‐ per female | 1 | 878 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.46, 0.90] |
| 13 Preferred choice ‐ per male | 1 | 674 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| 14 Preferred choice ‐ per female | 1 | 674 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.67, 1.30] |
| 15 Would recommend to friend ‐ per male | 1 | 579 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.56, 1.11] |
| 16 Would recommend to friend ‐ per female | 1 | 579 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.75, 1.52] |
1.1. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 1 Six‐month overall discontinuation ‐ per couple.
1.2. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 2 Nonclinical breakage ‐ per condom.
1.3. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 3 Clinical breakage ‐ per condom.
1.4. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 4 Total breakage ‐ per condom.
1.5. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 5 Complete slippage ‐ per condom.
1.6. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 6 Partial slippage ‐ per condom.
1.7. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 7 Total clinical failure ‐ per condom.
1.8. Analysis.
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 8 Total failure ‐ per condom.
1.9. Analysis.
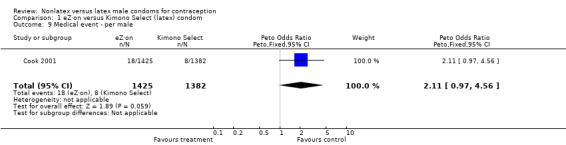
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 9 Medical event ‐ per male.
1.10. Analysis.
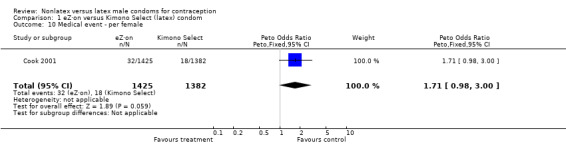
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 10 Medical event ‐ per female.
1.11. Analysis.
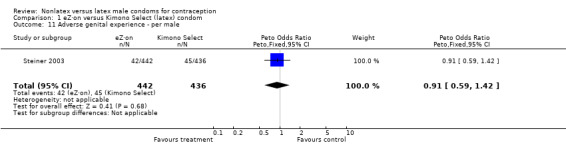
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 11 Adverse genital experience ‐ per male.
1.12. Analysis.
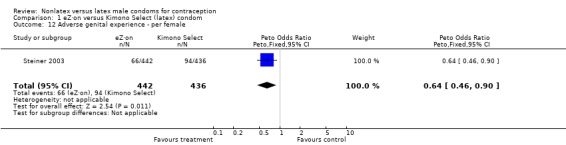
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 12 Adverse genital experience ‐ per female.
1.13. Analysis.
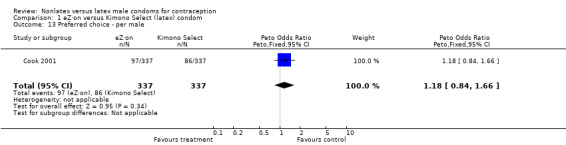
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 13 Preferred choice ‐ per male.
1.14. Analysis.
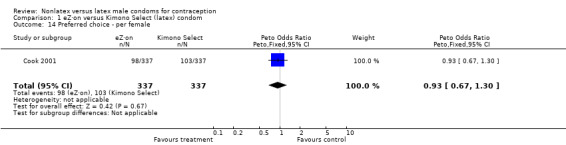
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 14 Preferred choice ‐ per female.
1.15. Analysis.
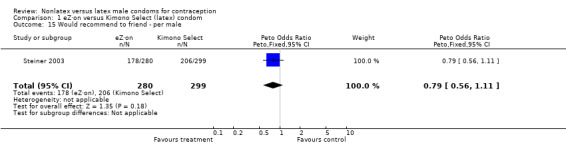
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 15 Would recommend to friend ‐ per male.
1.16. Analysis.
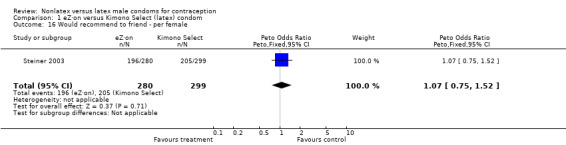
Comparison 1 eZ·on versus Kimono Select (latex) condom, Outcome 16 Would recommend to friend ‐ per female.
Comparison 2. eZ·on versus Durex Gossamer (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 337 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.11] |
| 2 Clinical breakage ‐ per condom | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.33, 3.28] |
| 3 Total breakage ‐ per condom | 1 | 337 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.29, 2.69] |
| 4 Total breakage ‐ per couple | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.23, 4.34] |
| 5 Complete slippage ‐ per condom | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.41, 10.24] |
| 6 Complete slippage ‐ per couple | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.85 [0.38, 21.23] |
| 7 Partial slippage ‐ per condom | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.65, 2.24] |
| 8 Partial slippage ‐ per couple | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.73, 6.52] |
| 9 Total clinical failure ‐ per condom | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.51, 3.39] |
| 10 Total failure ‐ per condom | 1 | 337 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.46, 2.94] |
| 11 Total failure ‐ per couple | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.43, 5.17] |
| 12 Felt "identical" or "almost identical" to coitus without condom ‐ per male | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.72, 1.70] |
| 13 "Easy" or "fairly easy" to don condom ‐ per male | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.29, 0.82] |
| 14 Coitus with condom was "comfortable" ‐ per male | 1 | 334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.52, 1.79] |
| 15 "Excellent" or "good" acceptability ‐ per male | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.27, 1.82] |
| 16 "Excellent" or "good" acceptability ‐ per female | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.48, 3.36] |
| 17 Preferred choice ‐ per male | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.45, 4.23] |
| 18 Preferred choice ‐ per female | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.53, 4.77] |
2.1. Analysis.
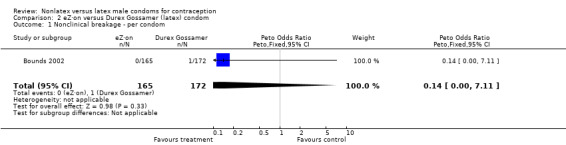
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 1 Nonclinical breakage ‐ per condom.
2.2. Analysis.
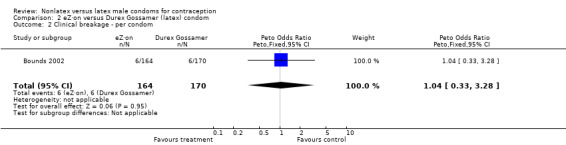
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 2 Clinical breakage ‐ per condom.
2.3. Analysis.
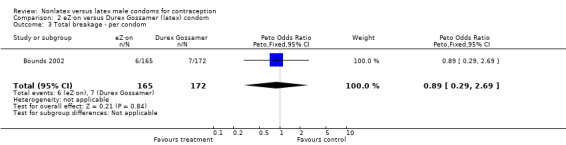
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 3 Total breakage ‐ per condom.
2.4. Analysis.
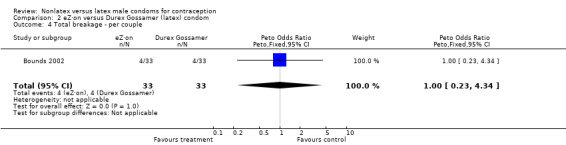
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 4 Total breakage ‐ per couple.
2.5. Analysis.
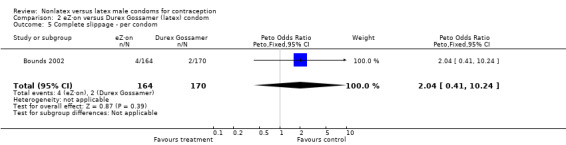
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 5 Complete slippage ‐ per condom.
2.6. Analysis.
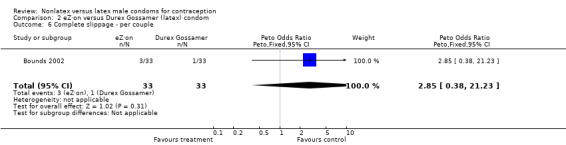
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 6 Complete slippage ‐ per couple.
2.7. Analysis.
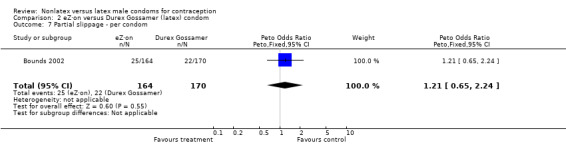
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 7 Partial slippage ‐ per condom.
2.8. Analysis.
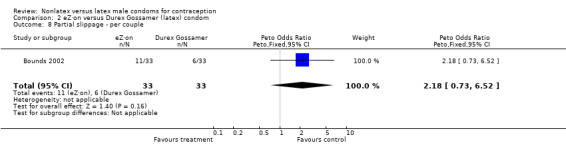
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 8 Partial slippage ‐ per couple.
2.9. Analysis.
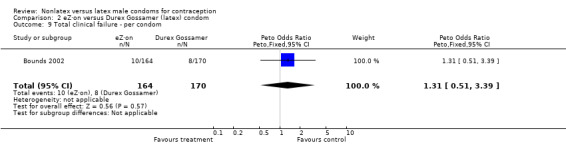
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 9 Total clinical failure ‐ per condom.
2.10. Analysis.
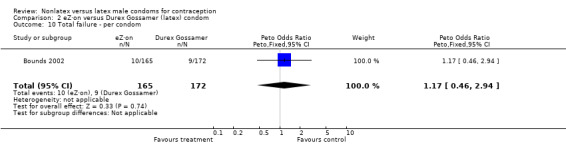
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 10 Total failure ‐ per condom.
2.11. Analysis.
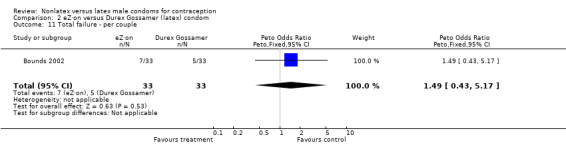
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 11 Total failure ‐ per couple.
2.12. Analysis.
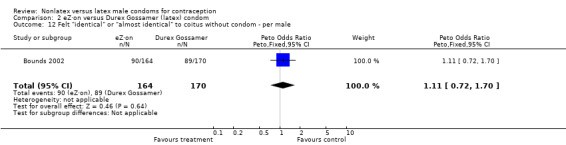
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 12 Felt "identical" or "almost identical" to coitus without condom ‐ per male.
2.13. Analysis.
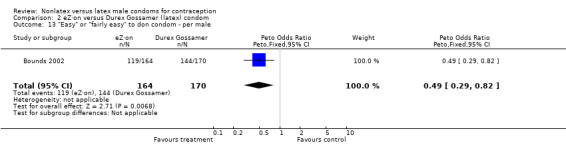
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 13 "Easy" or "fairly easy" to don condom ‐ per male.
2.14. Analysis.
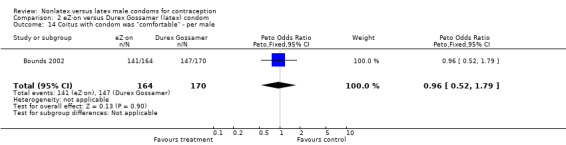
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 14 Coitus with condom was "comfortable" ‐ per male.
2.15. Analysis.
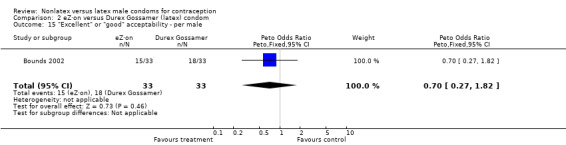
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 15 "Excellent" or "good" acceptability ‐ per male.
2.16. Analysis.
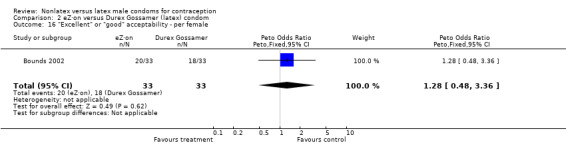
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 16 "Excellent" or "good" acceptability ‐ per female.
2.17. Analysis.
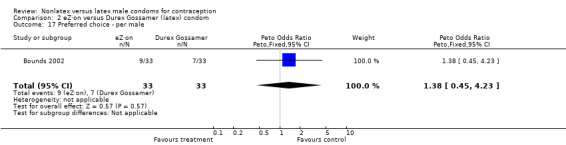
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 17 Preferred choice ‐ per male.
2.18. Analysis.
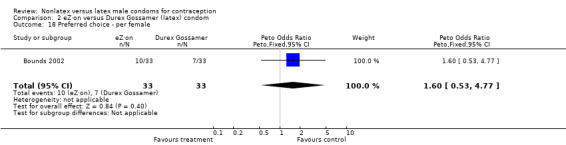
Comparison 2 eZ·on versus Durex Gossamer (latex) condom, Outcome 18 Preferred choice ‐ per female.
Comparison 3. Avanti versus Durex Gossamer (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [0.20, 18.60] |
| 2 Clinical breakage ‐ per condom | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.25, 2.72] |
| 3 Total breakage ‐ per condom | 1 | 347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.34, 2.86] |
| 4 Total breakage ‐ per couple | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.45, 6.55] |
| 5 Complete slippage ‐ per condom | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.53, 10.54] |
| 6 Complete slippage ‐ per couple | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.86 [0.63, 23.61] |
| 7 Partial slippage ‐ per condom | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.75, 2.46] |
| 8 Partial slippage ‐ per couple | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [0.92, 8.02] |
| 9 Total clinical failure ‐ per condom | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.48, 3.22] |
| 10 Total failure ‐ per condom | 1 | 347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.55, 3.21] |
| 11 Total failure ‐ per couple | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.56 [0.81, 8.06] |
| 12 Felt "identical" or "almost identical" to coitus without condom ‐ per male | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.50, 1.15] |
| 13 "Easy" or "fairly easy" to don condom ‐ per male | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.34, 0.99] |
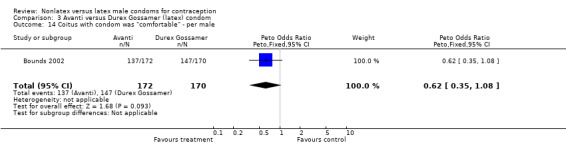
| 14 Coitus with condom was "comfortable" ‐ per male | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.35, 1.08] |
| 15 "Excellent" or "good" acceptability ‐ per male | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 16 "Excellent" or "good" acceptability ‐ per female | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.38, 2.69] |
| 17 Preferred choice ‐ per male | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.58, 5.25] |
| 18 Preferred choice ‐ per female | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.41, 4.06] |
3.1. Analysis.
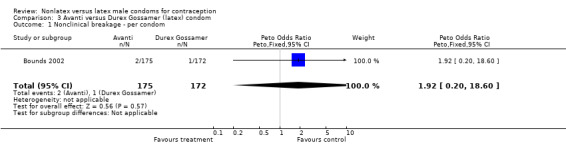
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 1 Nonclinical breakage ‐ per condom.
3.2. Analysis.
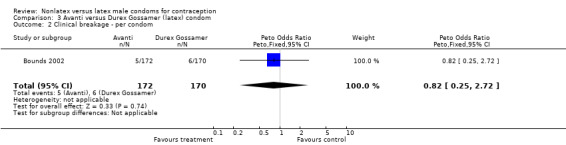
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 2 Clinical breakage ‐ per condom.
3.3. Analysis.
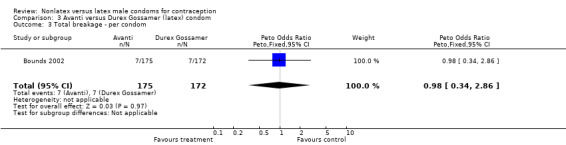
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 3 Total breakage ‐ per condom.
3.4. Analysis.
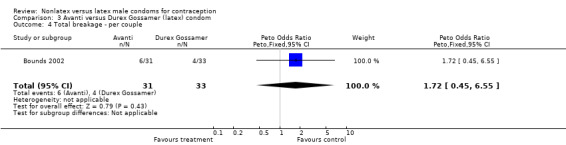
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 4 Total breakage ‐ per couple.
3.5. Analysis.
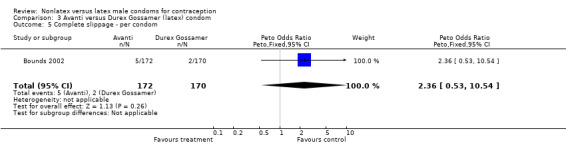
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 5 Complete slippage ‐ per condom.
3.6. Analysis.
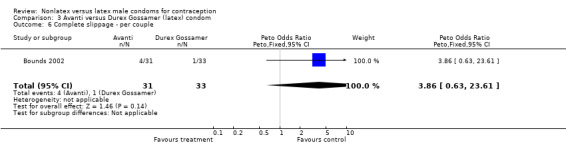
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 6 Complete slippage ‐ per couple.
3.7. Analysis.
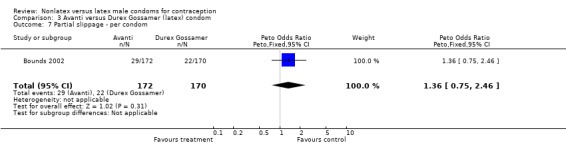
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 7 Partial slippage ‐ per condom.
3.8. Analysis.
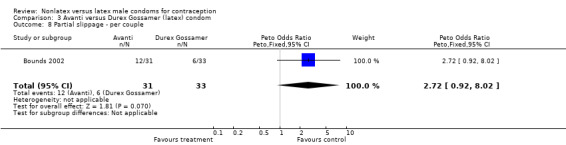
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 8 Partial slippage ‐ per couple.
3.9. Analysis.
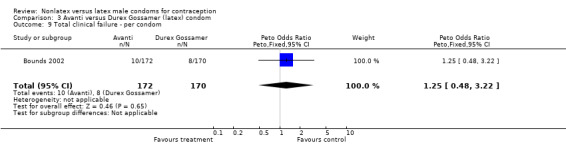
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 9 Total clinical failure ‐ per condom.
3.10. Analysis.
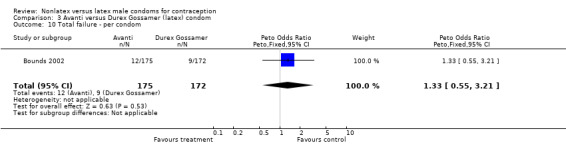
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 10 Total failure ‐ per condom.
3.11. Analysis.
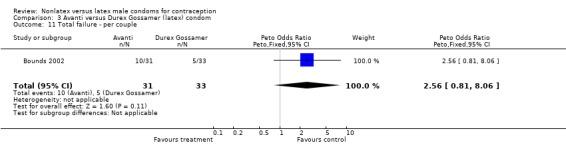
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 11 Total failure ‐ per couple.
3.12. Analysis.
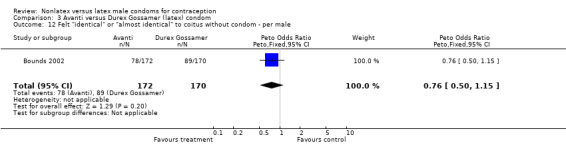
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 12 Felt "identical" or "almost identical" to coitus without condom ‐ per male.
3.13. Analysis.
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 13 "Easy" or "fairly easy" to don condom ‐ per male.
3.14. Analysis.
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 14 Coitus with condom was "comfortable" ‐ per male.
3.15. Analysis.
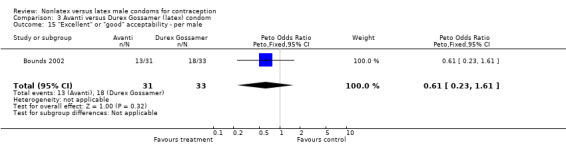
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 15 "Excellent" or "good" acceptability ‐ per male.
3.16. Analysis.
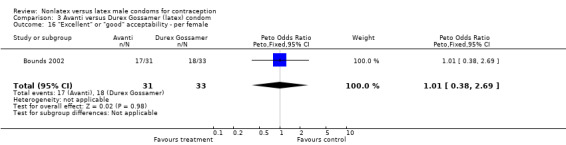
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 16 "Excellent" or "good" acceptability ‐ per female.
3.17. Analysis.
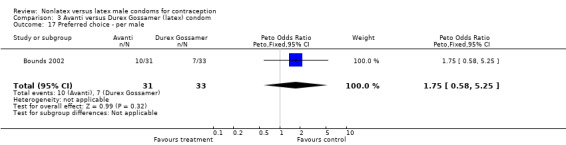
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 17 Preferred choice ‐ per male.
3.18. Analysis.
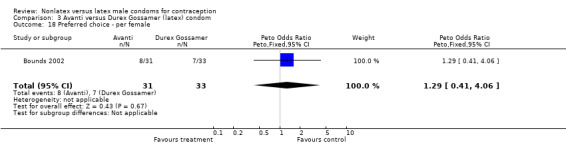
Comparison 3 Avanti versus Durex Gossamer (latex) condom, Outcome 18 Preferred choice ‐ per female.
Comparison 4. Avanti versus Ramses Sensitol (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancies ‐ per condom | 1 | 3686 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.41, 1.44] |
| 2 Six‐month condom‐related discontinuation ‐ per couple | 1 | 767 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.11, 2.57] |
| 3 Six‐month condom‐unrelated discontinuation ‐ per couple | 1 | 767 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.86, 1.86] |
| 4 Nonclinical breakage ‐ per condom | 2 | 5776 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.06, 0.90] |
| 5 Clinical breakage ‐ per condom | 2 | 5712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.95 [3.63, 6.75] |
| 6 Total breakage ‐ per condom | 2 | 5776 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.30 [3.18, 5.83] |
| 7 Complete slippage ‐ per condom | 2 | 5712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.57 [2.58, 4.95] |
| 8 Total clinical failure ‐ per condom | 2 | 5712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.41 [3.51, 5.54] |
| 9 Total failure ‐ per condom | 2 | 5776 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.47 [2.82, 4.27] |
| 10 Transitory discomfort ‐ per condom by male | 1 | 37743 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.58, 0.68] |
| 11 Acceptability ‐ per male | 1 | 687 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.84, 1.53] |
| 12 Acceptability ‐ per female | 1 | 687 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.81, 1.48] |
| 13 Recommend ‐ per male | 1 | 723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.25, 0.47] |
4.1. Analysis.
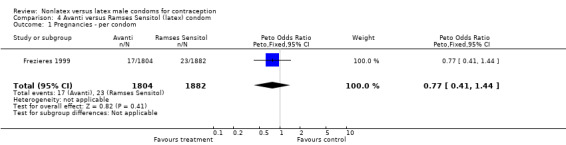
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 1 Pregnancies ‐ per condom.
4.2. Analysis.
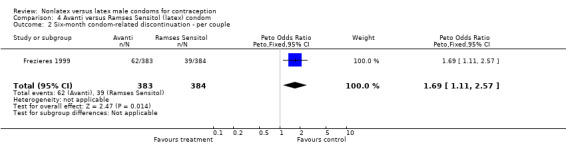
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 2 Six‐month condom‐related discontinuation ‐ per couple.
4.3. Analysis.
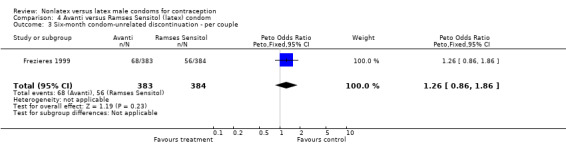
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 3 Six‐month condom‐unrelated discontinuation ‐ per couple.
4.4. Analysis.
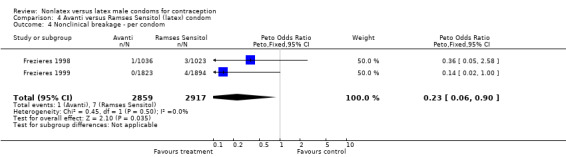
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 4 Nonclinical breakage ‐ per condom.
4.5. Analysis.
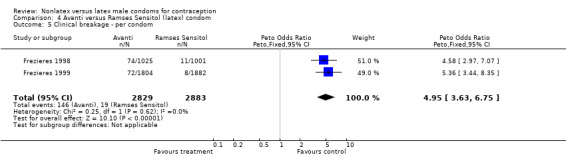
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 5 Clinical breakage ‐ per condom.
4.6. Analysis.
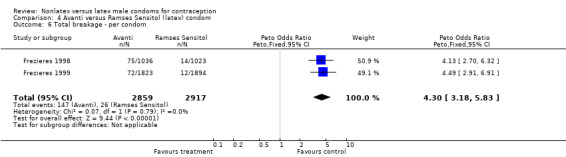
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 6 Total breakage ‐ per condom.
4.7. Analysis.
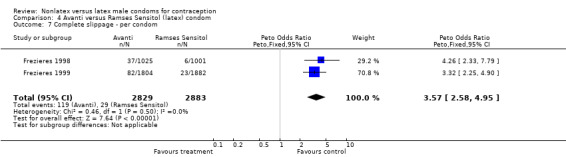
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 7 Complete slippage ‐ per condom.
4.8. Analysis.
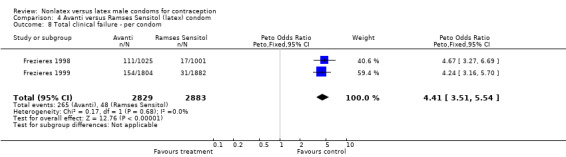
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 8 Total clinical failure ‐ per condom.
4.9. Analysis.
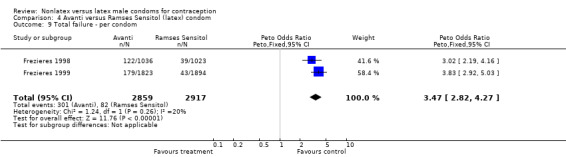
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 9 Total failure ‐ per condom.
4.10. Analysis.
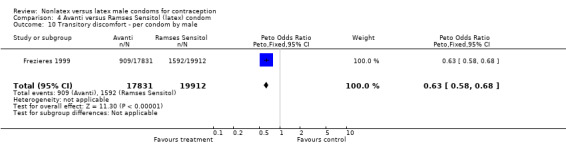
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 10 Transitory discomfort ‐ per condom by male.
4.11. Analysis.
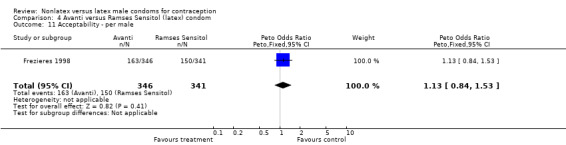
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 11 Acceptability ‐ per male.
4.12. Analysis.
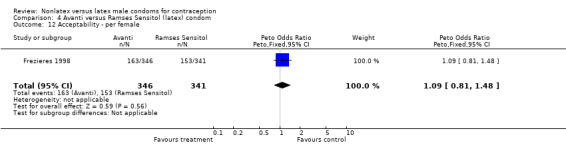
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 12 Acceptability ‐ per female.
4.13. Analysis.
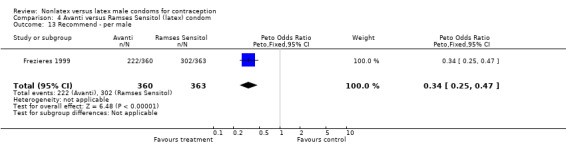
Comparison 4 Avanti versus Ramses Sensitol (latex) condom, Outcome 13 Recommend ‐ per male.
Comparison 5. Avanti versus Trojan‐Enz (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 306 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 2 Clinical breakage ‐ per condom | 1 | 301 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.76 [0.39, 19.82] |
| 3 Total breakage ‐ per condom | 1 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.26, 8.82] |
| 4 Complete slippage ‐ per condom | 1 | 301 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.41 [0.54, 10.78] |
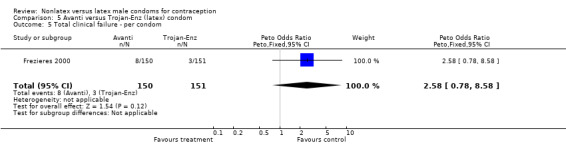
| 5 Total clinical failure ‐ per condom | 1 | 301 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.58 [0.78, 8.58] |
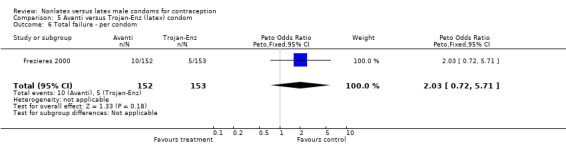
| 6 Total failure ‐ per condom | 1 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [0.72, 5.71] |
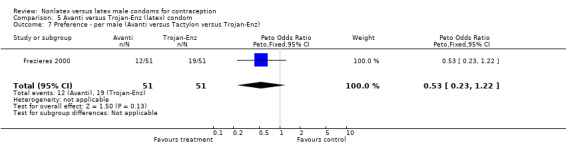
| 7 Preference ‐ per male (Avanti versus Tactylon versus Trojan‐Enz) | 1 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.23, 1.22] |
| 8 Preference ‐ per female (Avanti versus Tactylon versus Trojan‐Enz) | 1 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.33, 1.76] |
5.1. Analysis.
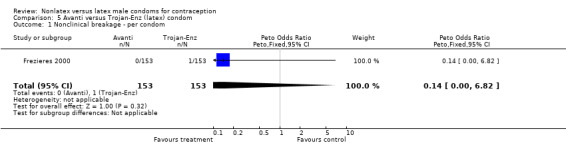
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 1 Nonclinical breakage ‐ per condom.
5.2. Analysis.
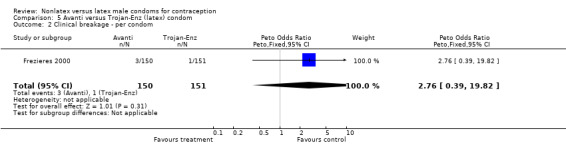
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 2 Clinical breakage ‐ per condom.
5.3. Analysis.
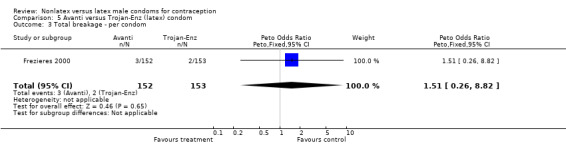
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 3 Total breakage ‐ per condom.
5.4. Analysis.
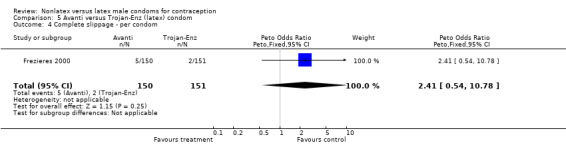
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 4 Complete slippage ‐ per condom.
5.5. Analysis.
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 5 Total clinical failure ‐ per condom.
5.6. Analysis.
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 6 Total failure ‐ per condom.
5.7. Analysis.
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 7 Preference ‐ per male (Avanti versus Tactylon versus Trojan‐Enz).
5.8. Analysis.
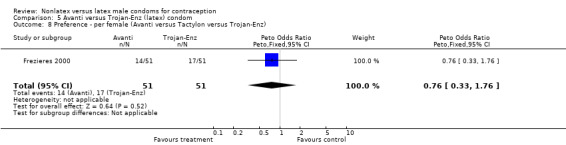
Comparison 5 Avanti versus Trojan‐Enz (latex) condom, Outcome 8 Preference ‐ per female (Avanti versus Tactylon versus Trojan‐Enz).
Comparison 6. Standard Tactylon versus Aladan (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical breakage ‐ per condom | 1 | 2309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.48 [1.99, 6.09] |
| 2 Complete slippage ‐ per condom | 1 | 2309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.27, 1.49] |
| 3 Total clinical failure ‐ per condom | 1 | 2309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.32, 3.38] |
| 4 Medical event ‐ per condom by male | 1 | 2378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.19, 0.63] |
| 5 Medical event ‐ per condom by female | 1 | 2378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.27, 0.57] |
6.1. Analysis.
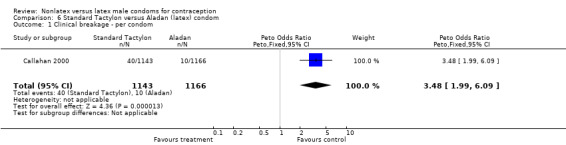
Comparison 6 Standard Tactylon versus Aladan (latex) condom, Outcome 1 Clinical breakage ‐ per condom.
6.2. Analysis.
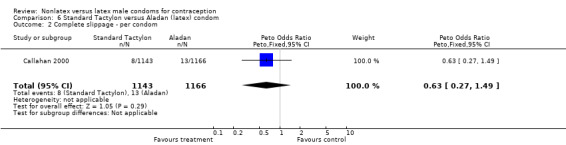
Comparison 6 Standard Tactylon versus Aladan (latex) condom, Outcome 2 Complete slippage ‐ per condom.
6.3. Analysis.
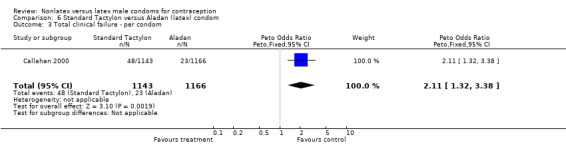
Comparison 6 Standard Tactylon versus Aladan (latex) condom, Outcome 3 Total clinical failure ‐ per condom.
6.4. Analysis.
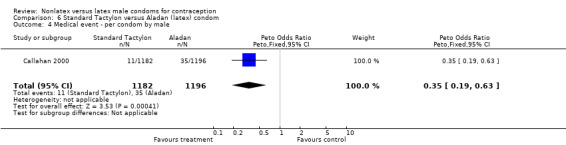
Comparison 6 Standard Tactylon versus Aladan (latex) condom, Outcome 4 Medical event ‐ per condom by male.
6.5. Analysis.
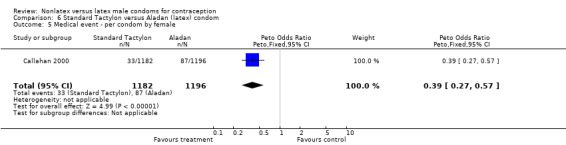
Comparison 6 Standard Tactylon versus Aladan (latex) condom, Outcome 5 Medical event ‐ per condom by female.
Comparison 7. Standard Tactylon versus LifeStyles (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
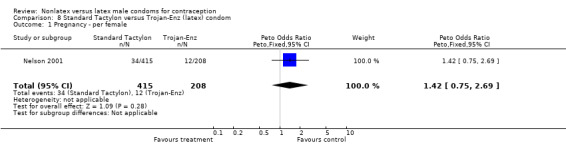
| 1 Pregnancy ‐ per female | 1 | 622 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.75, 2.68] |
| 2 Six‐month discontinuation ‐ per couple | 1 | 622 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.81, 1.58] |
| 3 Nonclinical breakage ‐ per condom | 1 | 2758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.13, 1.30] |
| 4 Clinical breakage ‐ per condom | 1 | 2714 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.09 [2.42, 6.90] |
| 5 Total breakage ‐ per condom | 1 | 2758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.78 [1.72, 4.49] |
| 6 Complete slippage ‐ per condom | 1 | 2714 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.21, 1.42] |
| 7 Total clinical failure ‐ per condom | 1 | 2714 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.59 [1.63, 4.11] |
| 8 Total failure ‐ per condom | 1 | 2758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [1.32, 3.11] |
| 9 Recommend ‐ per male | 1 | 553 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.37, 0.90] |
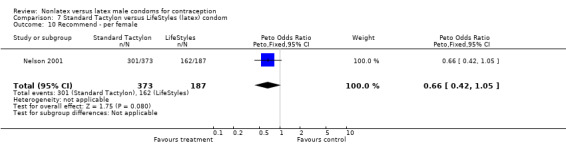
| 10 Recommend ‐ per female | 1 | 560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.42, 1.05] |
7.1. Analysis.
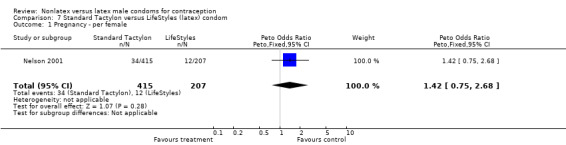
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 1 Pregnancy ‐ per female.
7.2. Analysis.
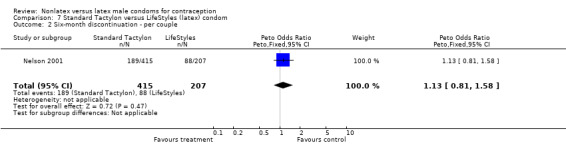
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 2 Six‐month discontinuation ‐ per couple.
7.3. Analysis.
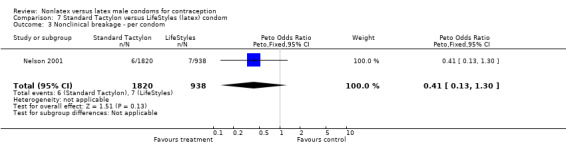
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 3 Nonclinical breakage ‐ per condom.
7.4. Analysis.
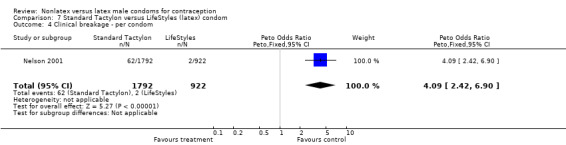
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 4 Clinical breakage ‐ per condom.
7.5. Analysis.
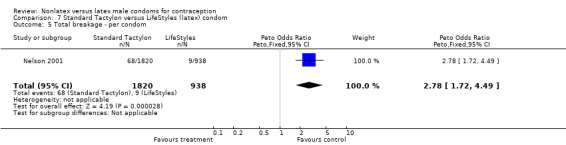
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 5 Total breakage ‐ per condom.
7.6. Analysis.
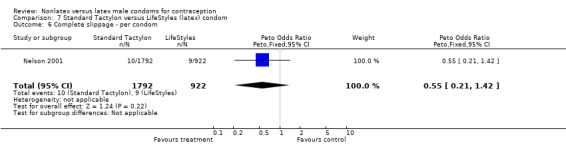
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 6 Complete slippage ‐ per condom.
7.7. Analysis.
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 7 Total clinical failure ‐ per condom.
7.8. Analysis.
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 8 Total failure ‐ per condom.
7.9. Analysis.
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 9 Recommend ‐ per male.
7.10. Analysis.
Comparison 7 Standard Tactylon versus LifeStyles (latex) condom, Outcome 10 Recommend ‐ per female.
Comparison 8. Standard Tactylon versus Trojan‐Enz (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy ‐ per female | 1 | 623 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.75, 2.69] |
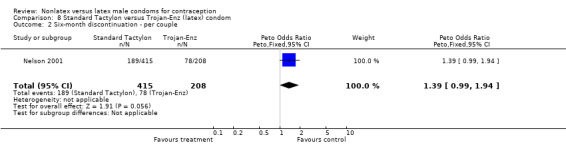
| 2 Six‐month discontinuation ‐ per couple | 1 | 623 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.99, 1.94] |
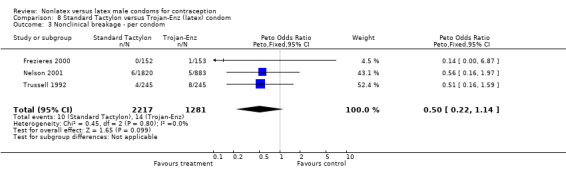
| 3 Nonclinical breakage ‐ per condom | 3 | 3498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.22, 1.14] |
| 4 Clinical breakage ‐ per condom | 3 | 3457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [1.63, 4.28] |
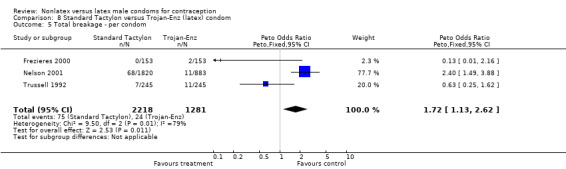
| 5 Total breakage ‐ per condom | 3 | 3499 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.13, 2.62] |
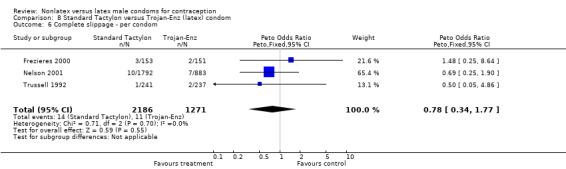
| 6 Complete slippage ‐ per condom | 3 | 3457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.34, 1.77] |
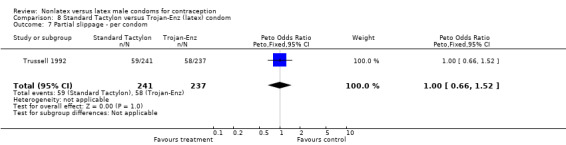
| 7 Partial slippage ‐ per condom | 1 | 478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.66, 1.52] |
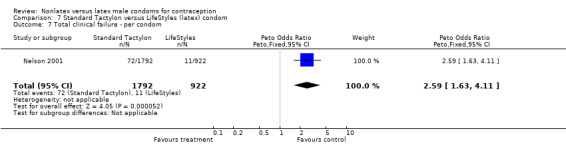
| 8 Total clinical failure ‐ per condom | 3 | 3457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [1.28, 2.95] |
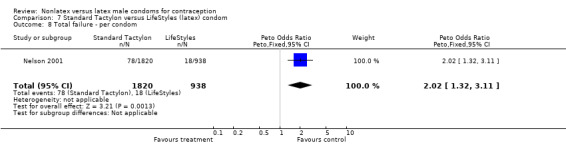
| 9 Total failure ‐ per condom | 3 | 3499 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.99, 2.09] |
| 10 Preference ‐ per male (Avanti versus Tactylon versus Trojan‐Enz) | 1 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.45, 2.22] |
| 11 Preference ‐ per male (Tactylon versus Trojan‐Enz) | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.91 [0.87, 4.20] |
| 12 Preference ‐ per female (Avanti versus Tactylon versus Trojan‐Enz) | 1 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.53, 2.66] |
| 13 Preference ‐ per female (Tactylon versus Trojan‐Enz) | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [0.74, 3.57] |
| 14 Recommend ‐ per male | 1 | 552 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.44, 1.04] |
| 15 Recommend ‐ per female | 1 | 556 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.34, 0.88] |
8.1. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 1 Pregnancy ‐ per female.
8.2. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 2 Six‐month discontinuation ‐ per couple.
8.3. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 3 Nonclinical breakage ‐ per condom.
8.4. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 4 Clinical breakage ‐ per condom.
8.5. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 5 Total breakage ‐ per condom.
8.6. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 6 Complete slippage ‐ per condom.
8.7. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 7 Partial slippage ‐ per condom.
8.8. Analysis.
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 8 Total clinical failure ‐ per condom.
8.9. Analysis.
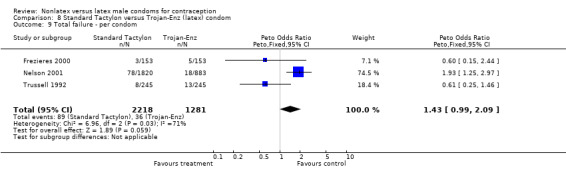
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 9 Total failure ‐ per condom.
8.10. Analysis.
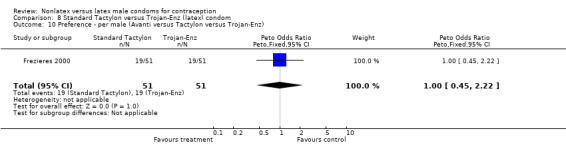
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 10 Preference ‐ per male (Avanti versus Tactylon versus Trojan‐Enz).
8.11. Analysis.
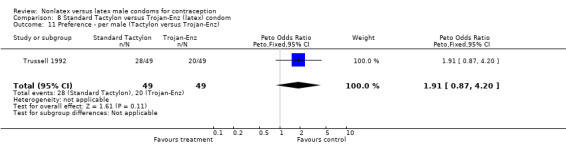
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 11 Preference ‐ per male (Tactylon versus Trojan‐Enz).
8.12. Analysis.
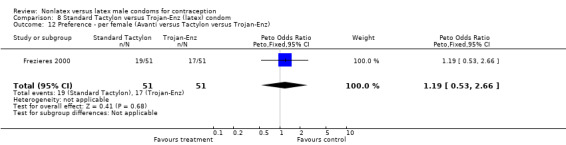
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 12 Preference ‐ per female (Avanti versus Tactylon versus Trojan‐Enz).
8.13. Analysis.
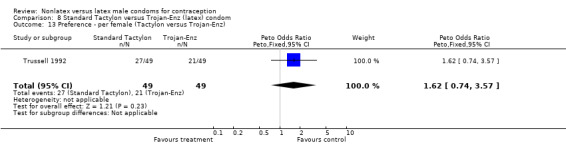
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 13 Preference ‐ per female (Tactylon versus Trojan‐Enz).
8.14. Analysis.
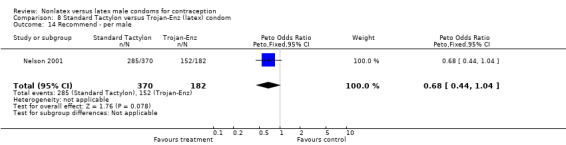
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 14 Recommend ‐ per male.
8.15. Analysis.
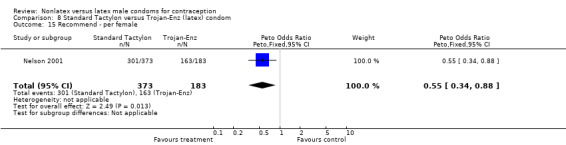
Comparison 8 Standard Tactylon versus Trojan‐Enz (latex) condom, Outcome 15 Recommend ‐ per female.
Comparison 9. Standard Tactylon versus standard USAID (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 1128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.77 [0.89, 8.65] |
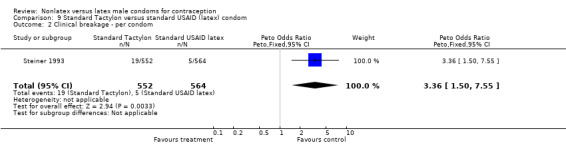
| 2 Clinical breakage ‐ per condom | 1 | 1116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.36 [1.50, 7.55] |
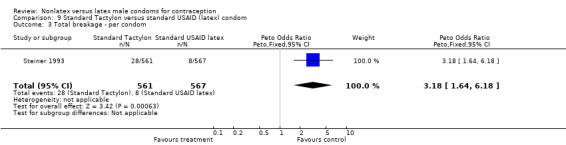
| 3 Total breakage ‐ per condom | 1 | 1128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.18 [1.64, 6.18] |
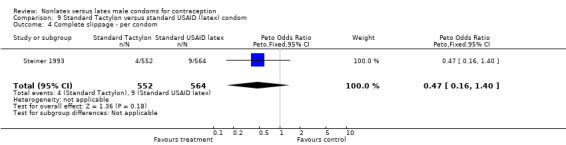
| 4 Complete slippage ‐ per condom | 1 | 1116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.16, 1.40] |
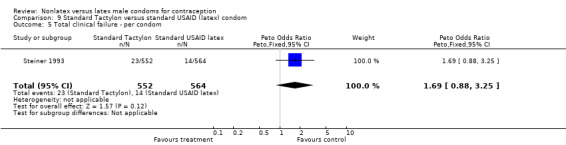
| 5 Total clinical failure ‐ per condom | 1 | 1116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [0.88, 3.25] |
| 6 Total failure ‐ per condom | 1 | 1128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [1.08, 3.40] |
| 7 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex) | 1 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.97, 2.17] |
| 8 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex) | 1 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.86, 1.89] |
9.1. Analysis.
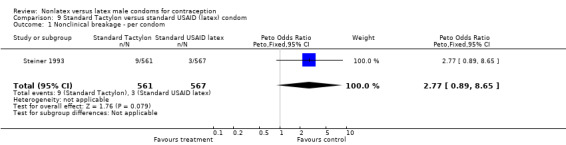
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 1 Nonclinical breakage ‐ per condom.
9.2. Analysis.
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 2 Clinical breakage ‐ per condom.
9.3. Analysis.
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 3 Total breakage ‐ per condom.
9.4. Analysis.
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 4 Complete slippage ‐ per condom.
9.5. Analysis.
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 5 Total clinical failure ‐ per condom.
9.6. Analysis.
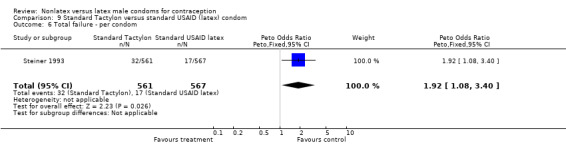
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 6 Total failure ‐ per condom.
9.7. Analysis.
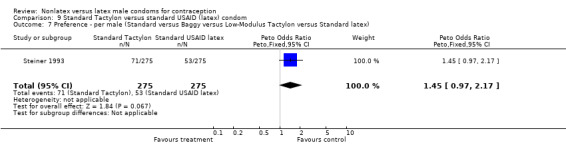
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 7 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex).
9.8. Analysis.
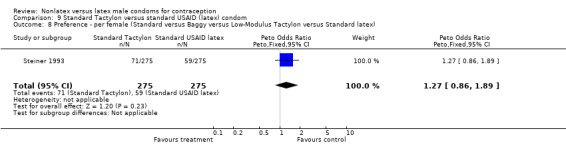
Comparison 9 Standard Tactylon versus standard USAID (latex) condom, Outcome 8 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex).
Comparison 10. Baggy Tactylon versus Aladan (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical breakage ‐ per condom | 1 | 2314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.52 [2.02, 6.13] |
| 2 Complete slippage ‐ per condom | 1 | 2314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.56, 2.47] |
| 3 Total clinical failure ‐ per condom | 1 | 2314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.41 [1.54, 3.78] |
| 4 Medical event ‐ per condom by male | 1 | 2384 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.57, 1.49] |
| 5 Medical event ‐ per condom by female | 1 | 2384 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.35, 0.70] |
10.1. Analysis.
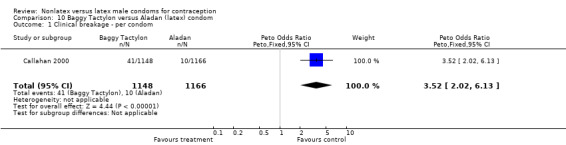
Comparison 10 Baggy Tactylon versus Aladan (latex) condom, Outcome 1 Clinical breakage ‐ per condom.
10.2. Analysis.
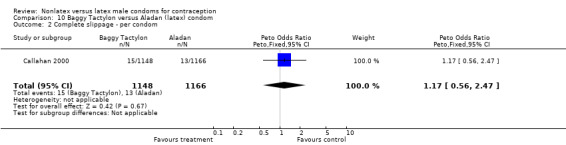
Comparison 10 Baggy Tactylon versus Aladan (latex) condom, Outcome 2 Complete slippage ‐ per condom.
10.3. Analysis.
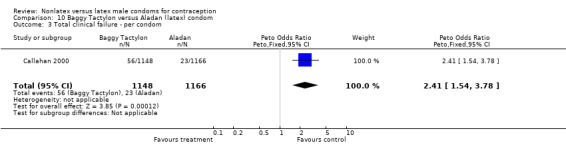
Comparison 10 Baggy Tactylon versus Aladan (latex) condom, Outcome 3 Total clinical failure ‐ per condom.
10.4. Analysis.
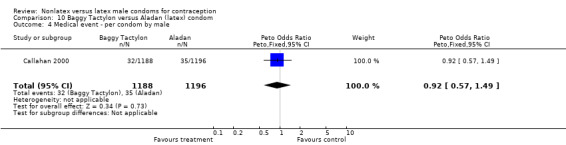
Comparison 10 Baggy Tactylon versus Aladan (latex) condom, Outcome 4 Medical event ‐ per condom by male.
10.5. Analysis.
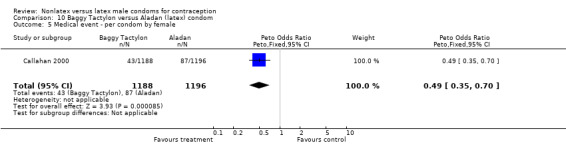
Comparison 10 Baggy Tactylon versus Aladan (latex) condom, Outcome 5 Medical event ‐ per condom by female.
Comparison 11. Baggy Tactylon versus standard USAID (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.73 [1.43, 9.72] |
| 2 Clinical breakage ‐ per condom | 1 | 1115 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.91, 6.15] |
| 3 Total breakage ‐ per condom | 1 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [1.51, 5.91] |
| 4 Complete slippage ‐ per condom | 1 | 1115 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.76, 3.83] |
| 5 Total clinical failure ‐ per condom | 1 | 1115 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.98 [1.06, 3.69] |
| 6 Total failure ‐ per condom | 1 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.61 [1.56, 4.35] |
| 7 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex) | 1 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.90, 2.02] |
| 8 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex) | 1 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.74, 1.66] |
11.1. Analysis.
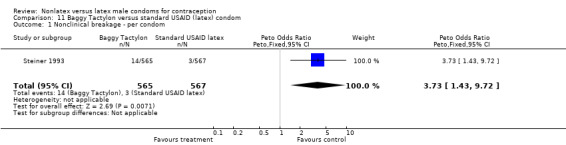
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 1 Nonclinical breakage ‐ per condom.
11.2. Analysis.
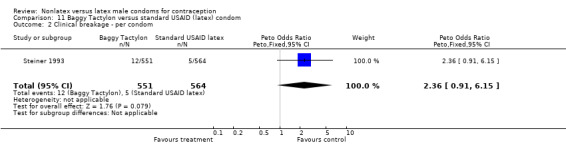
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 2 Clinical breakage ‐ per condom.
11.3. Analysis.
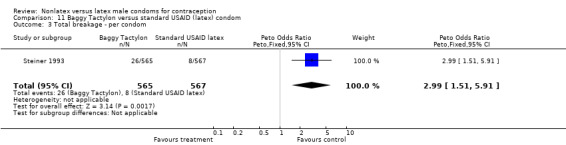
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 3 Total breakage ‐ per condom.
11.4. Analysis.
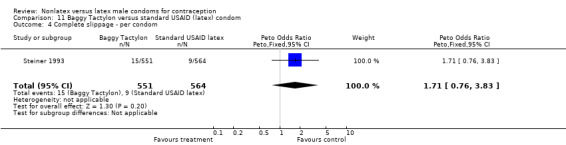
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 4 Complete slippage ‐ per condom.
11.5. Analysis.
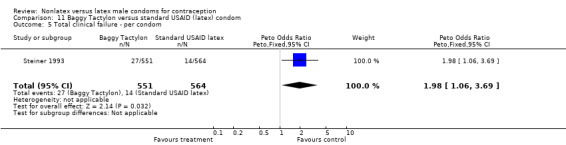
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 5 Total clinical failure ‐ per condom.
11.6. Analysis.
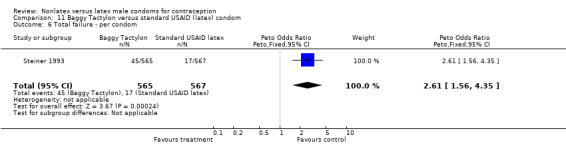
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 6 Total failure ‐ per condom.
11.7. Analysis.
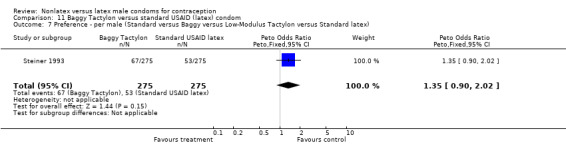
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 7 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex).
11.8. Analysis.
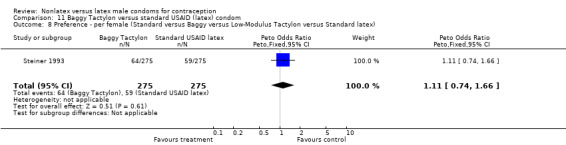
Comparison 11 Baggy Tactylon versus standard USAID (latex) condom, Outcome 8 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex).
Comparison 12. Low‐Modulus Tactylon versus Aladan (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical breakage ‐ per condom | 1 | 2341 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.85 [2.30, 6.45] |
| 2 Complete slippage ‐ per condom | 1 | 2341 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.30, 1.59] |
| 3 Total clinical failure ‐ per condom | 1 | 2341 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.43 [1.56, 3.78] |
| 4 Medical event ‐ per condom by male | 1 | 2393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.37, 1.06] |
| 5 Medical event ‐ per condom by female | 1 | 2393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.43, 0.85] |
| 6 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Aladan) | 1 | 790 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.20, 2.23] |
| 7 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Aladan) | 1 | 790 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [1.08, 2.01] |
12.1. Analysis.
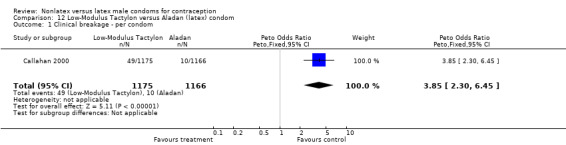
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 1 Clinical breakage ‐ per condom.
12.2. Analysis.
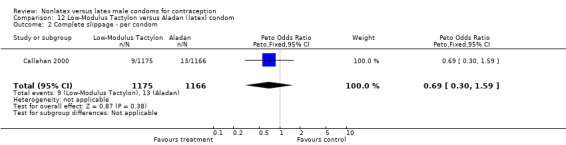
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 2 Complete slippage ‐ per condom.
12.3. Analysis.
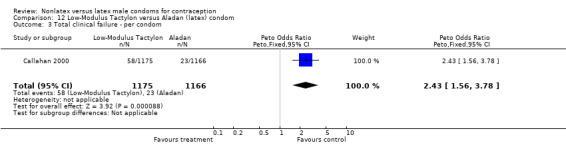
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 3 Total clinical failure ‐ per condom.
12.4. Analysis.
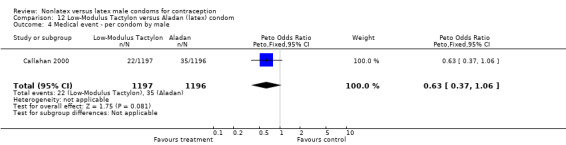
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 4 Medical event ‐ per condom by male.
12.5. Analysis.
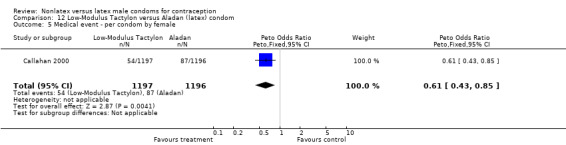
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 5 Medical event ‐ per condom by female.
12.6. Analysis.
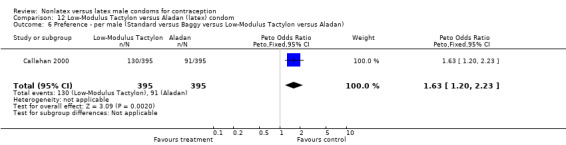
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 6 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Aladan).
12.7. Analysis.
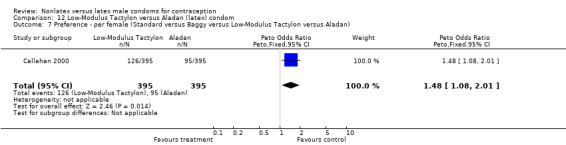
Comparison 12 Low‐Modulus Tactylon versus Aladan (latex) condom, Outcome 7 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Aladan).
Comparison 13. Low‐Modulus Tactylon versus standard USAID (latex) condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.41, 6.67] |
| 2 Clinical breakage ‐ per condom | 1 | 1124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.06 [1.32, 7.12] |
| 3 Total breakage ‐ per condom | 1 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.62 [1.27, 5.40] |
| 4 Complete slippage ‐ per condom | 1 | 1124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.29, 2.10] |
| 5 Total clinical failure ‐ per condom | 1 | 1124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.91, 3.31] |
| 6 Total failure ‐ per condom | 1 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [1.00, 3.20] |
| 7 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex) | 1 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.83 [1.24, 2.69] |
| 8 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex) | 1 | 550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [1.04, 2.23] |
13.1. Analysis.
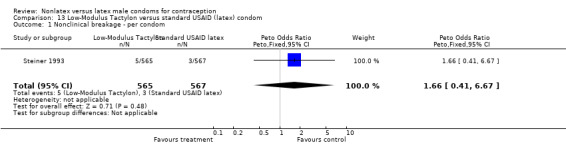
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 1 Nonclinical breakage ‐ per condom.
13.2. Analysis.
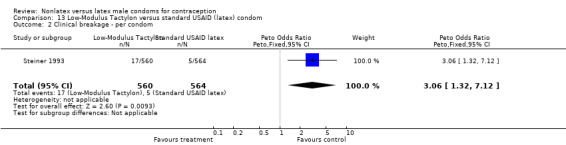
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 2 Clinical breakage ‐ per condom.
13.3. Analysis.
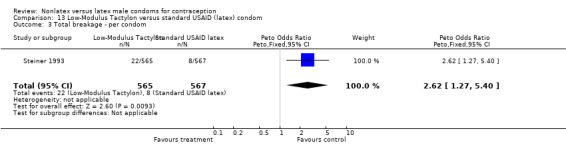
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 3 Total breakage ‐ per condom.
13.4. Analysis.
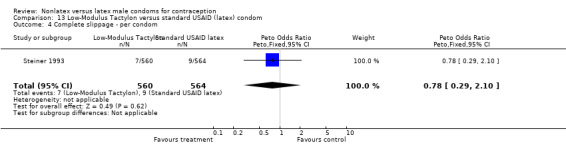
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 4 Complete slippage ‐ per condom.
13.5. Analysis.
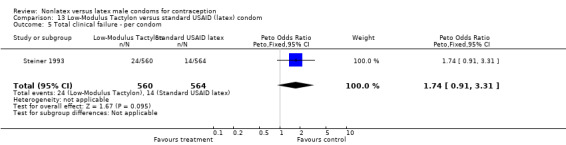
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 5 Total clinical failure ‐ per condom.
13.6. Analysis.
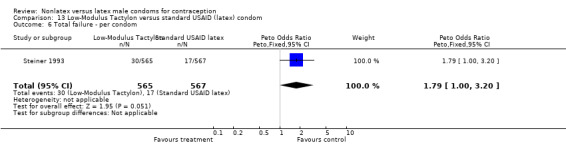
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 6 Total failure ‐ per condom.
13.7. Analysis.
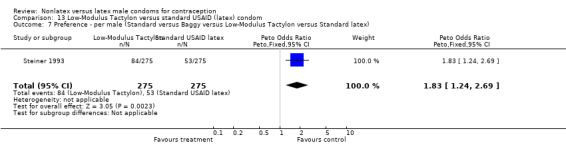
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 7 Preference ‐ per male (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex).
13.8. Analysis.
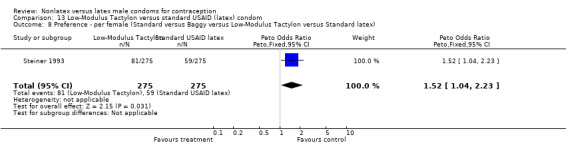
Comparison 13 Low‐Modulus Tactylon versus standard USAID (latex) condom, Outcome 8 Preference ‐ per female (Standard versus Baggy versus Low‐Modulus Tactylon versus Standard latex).
Comparison 14. Protex (Sagami) Original versus Sagami latex condom.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nonclinical breakage ‐ per condom | 1 | 1901 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.21] |
| 2 Clinical breakage ‐ per condom | 1 | 1897 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.21, 1.32] |
| 3 Total breakage ‐ per condom | 1 | 1901 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.19, 1.10] |
| 4 Complete slippage ‐ per condom | 1 | 1897 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.00 [0.72, 5.52] |
| 5 Total clinical failure ‐ per condom | 1 | 1897 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.48, 1.91] |
| 6 Total failure ‐ per condom | 1 | 1901 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.44, 1.67] |
| 7 Adverse event by male ‐ per condom | 1 | 1897 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.21, 2.24] |
| 8 Adverse event by female ‐ per condom | 1 | 1897 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.93, 1.48] |
14.1. Analysis.
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 1 Nonclinical breakage ‐ per condom.
14.2. Analysis.
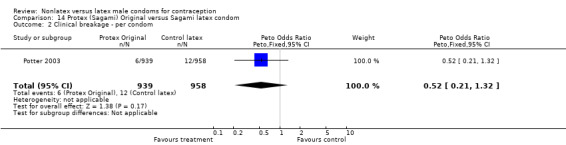
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 2 Clinical breakage ‐ per condom.
14.3. Analysis.
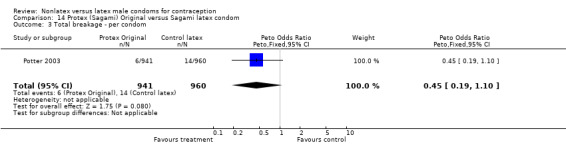
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 3 Total breakage ‐ per condom.
14.4. Analysis.
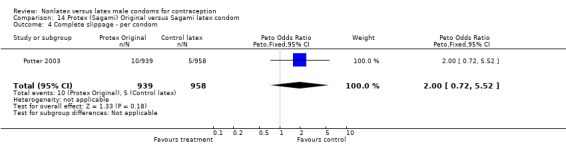
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 4 Complete slippage ‐ per condom.
14.5. Analysis.
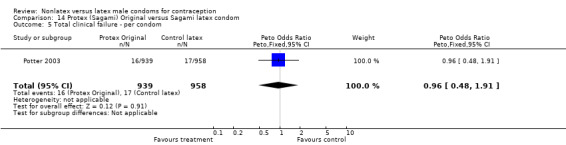
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 5 Total clinical failure ‐ per condom.
14.6. Analysis.
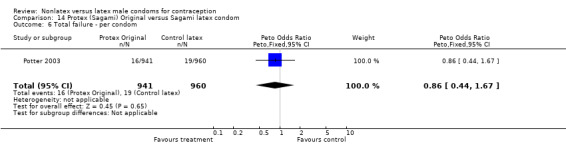
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 6 Total failure ‐ per condom.
14.7. Analysis.
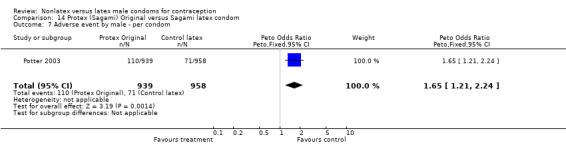
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 7 Adverse event by male ‐ per condom.
14.8. Analysis.
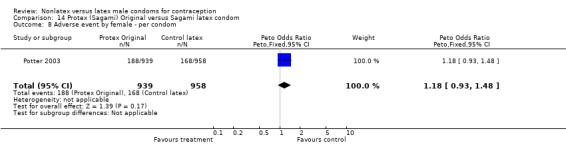
Comparison 14 Protex (Sagami) Original versus Sagami latex condom, Outcome 8 Adverse event by female ‐ per condom.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bounds 2002.
| Methods | Randomized, crossover trial. Postal and telephone contact (no clinic visits) with UK population. Participants were not blinded. Investigator and outcome assessor blinding was not reported. | |
| Participants | 43 healthy, sexually active couples aged 18 to 50 years in a stable monogamous relationship. Exclusions included allergy to latex, current STI; planned genital surgery; require condom use for specific STI protection. | |
| Interventions | Couples were assigned to use 6 condoms of each of the 3 condom types in a randomized sequence. The eZ·on condom (Family Health International, Research Triangle Park, NC and Mayer Laboratories, Inc., Oakland, CA) versus Avanti (SSL International plc, Knutsford, UK) versus the latex Durex Gossamer (SSL International plc, Knutsford, UK). | |
| Outcomes | Ease of use and acceptability; breakage and slippage. Breakage and slippage outcomes were abstracted in method consistent with Steiner 1994 definitions. | |
| Notes | Randomized with random sampling numbers. Allocation concealment methods not reported. 47% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Callahan 2000.
| Methods | Randomized crossover trial. Two centers in United States. Blinding not reported. | |
| Participants | 443 healthy, sexually active couples aged 18 to 45 years in a mutually monogamous, heterosexual relationship using an effective nonbarrier method of contraception. Exclusions included history of sensitivity to latex, Tactylon, silicone oil or water‐based lubricants; at risk for STDs; recent abnormal Papanicolaou smear. | |
| Interventions | Couples were assigned to use 3 condoms of each of the 4 condom types with each type used during a 3‐week period in a randomized sequence. Standard Tactylon condom versus Baggy Tactylon condom versus Low‐Modulus Tactylon condom versus the standard latex condom Aladan (Dothan, AL). All Tactylon condoms were manufactured by Sensicon Corp., Vista, CA (formerly Tactyl Technologies). | |
| Outcomes | Breakage and slippage; medical events; adverse events; acceptability. Breakage and slippage definitions consistent with Steiner 1994, except that breakage while removing condom after withdrawal was classified as nonclinical breakage. | |
| Notes | Randomization and allocation concealment methods not reported. 11% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Cook 2001.
| Methods | Randomized crossover trial. Two centers in United States. Participants were not blinded. Outcome assessors were blinded. | |
| Participants | 360 sexually active couples in a mutually monogamous, heterosexual relationship using an effective nonbarrier method of contraception. Women aged 18 to 45 years and males aged 18 years or older. Exclusions included risk for STI. | |
| Interventions | Couples were assigned to use 4 condoms of each of the 2 condom types with each type used during a 3‐week period in a randomized sequence. The eZ·on condom (Family Health International, Research Triangle Park, NC and Mayer Laboratories, Inc., Oakland, CA) versus a latex condom (Kimono Select, Mayer Laboratories, Inc., Oakland, CA). | |
| Outcomes | Breakage and slippage; medical events; adverse events; acceptability. | |
| Notes | Randomized with computer‐generated permuted block scheme stratified by site. 4% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by sealed, opaque, sequentially‐numbered envelopes opened at admission. |
Frezieres 1998.
| Methods | Randomized crossover trial. One center in United States. Participants were not blinded. Investigators and outcome assessors were blinded. | |
| Participants | 360 couples aged 18 to 45 years in monogamous, heterosexual relationship without known risk of STD. | |
| Interventions | Couples were assigned to use 3 condoms of each of the 2 condom types with each type used during a 2‐week period in a randomized sequence. The polyurethane condom Avanti or Avanti Super Thin (London International Group) versus the latex condom Ramses Sensitol (London International Group). | |
| Outcomes | Breakage and slippage; acceptability. | |
| Notes | Randomized with computer‐generated sequence of binary numbers. 49% of eligible couples declined participation. 6% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed with sealed containers. Packages of 3 condoms were numbered to specify order of use and sealed in envelope that was labeled by couple identification number. |
Frezieres 1999.
| Methods | Randomized parallel trial. One center in United States. Participants were not blinded. Investigators and research staff were blinded. Six‐month trial. Nested breakage, slippage and acceptability study within the efficacy study based on data from the condoms used for the first five acts of intercourse. | |
| Participants | 805 couples aged 18 to 45 years in monogamous heterosexual relationship without known risk of STD or infertility. | |
| Interventions | Polyurethane condom (similar to the Avanti, London International Group condom commercially produced after April 1996) versus the latex condom Ramses Sensitol (London International Group). | |
| Outcomes | Contraceptive efficacy; breakage and slippage; continuation; acceptability. | |
| Notes | Randomized with computer‐generated sequence of binary numbers. 64% of eligible couples declined participation. 31% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Manufacturer provided study condoms packaged in sealed opaque foil wrappers, which were then sealed in opaque containers labeled with the couple identification number. |
Frezieres 2000.
| Methods | Randomized crossover trial. One center in United States. Unblinded. | |
| Participants | 54 couples aged 18 to 45 years in monogamous heterosexual relationship and currently using condoms. | |
| Interventions | Couples were assigned to use 3 condoms of each of the 3 condom types with each type used during a 2‐week period in a randomized sequence. The polyurethane condom Avanti (London International Group) versus the synthetic elastomers condom Tactylon, (Sensicon Corp., Vista, CA) versus the latex condom Trojan‐Enz (Carter Wallace, Inc.). | |
| Outcomes | Condom breakage and slippage; acceptability. | |
| Notes | Randomization and allocation concealment methods not described. 6% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Nelson 2001.
| Methods | Randomized parallel trial. Seven centers in United States. Six‐month trial. Nested breakage, slippage and acceptability study within the efficacy study based on data from the condoms used for the first five acts of intercourse. Participants were unblinded. Investigators and outcome assessors were blinded. | |
| Participants | 830 sexually active, healthy couples (women aged 18 to 40 years and men aged 18 to 50 years) in monogamous heterosexual relationship. Exclusions included irregular menses, known STI, known infertility, and allergies to study products. | |
| Interventions | Tactylon (Sensicon Corp., Vista, CA) versus either latex condom Trojan‐Enz (Carter Wallace) or LifeStyles (Ansell). Study groups given Astroglide water‐based lubricant. | |
| Outcomes | Contraceptive efficacy; breakage and slippage; semen exposure from condom failure; safety; acceptability. | |
| Notes | Randomized with computer‐generated batch scheme. 31% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Condoms were supplied in sealed, opaque containers, which were labeled with subject identification numbers. |
Potter 2003.
| Methods | Randomized crossover trial. Couples in region of Paris (France) were recruited by a market research organization. Couples were asked to complete a brief questionnaire immediately after using each condom. | |
| Participants | 250 couples (aged 18 to 55 years) in monogamous heterosexual relationship and using condoms as current contraceptive method. Exclusions included using other contraceptive methods, known STI, and women with history of serious complications in pregnancy or birth. | |
| Interventions | Couples were assigned to use 5 Protex (or Sagami) Original and 5 latex control condoms supplied by Sagami Rubber Industries Co, Ltd. (Tokyo, Japan). Assignment was random for which condom type to use first. Time period was not specified. | |
| Outcomes | Clinical and nonclinical breakage, clinical and nonclinical slippage. | |
| Notes | No mention of blinding, allocation concealment, or randomization method. 250 couples were recruited, 36 did not return any questionnaires, 6 returned only some of the questionnaires. Of 208 with all questionnaires, 2 were excluded from analysis due to response "errors." Independent audit conducted of analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Steiner 1993.
| Methods | Randomized crossover trial. One center in United States. Unblinded. | |
| Participants | 320 couples aged 21 years or older in monogamous, heterosexual relationship and currently using hormonal method, IUD or sterilization for contraception. Excluded pregnancy, lactation, STI, and allergies or sensitivities to latex. | |
| Interventions | Couples were assigned to use 2 condoms of each of the 4 condom types in a randomized sequence during the 6‐week study period. Standard Tactylon versus Baggy Tactylon versus Low‐Modulus Tactylon versus the standard lubricated USAID latex condom (Ansell, Inc.). All Tactylon condoms were manufactured by Sensicon Corp., Vista, CA. | |
| Outcomes | Condom breakage and slippage; acceptability. | |
| Notes | Randomization and allocation concealment methods not reported. 11% did not complete questionnaires; 24% did not follow perfectly the randomized order for condoms; and 9% did not use the two condoms of the same type consecutively. 11% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Steiner 2003.
| Methods | Randomized crossover trial. Ten centers in United States. Participant and investigator were unblinded. Outcome assessor was blinded. | |
| Participants | Healthy, sexually active females aged 18 to 35 years in a stable, mutually monogamous relationship with regular menses and willing to use condoms only for contraception. Excluded recent, current or contraindications to pregnancy; lactation; certain pap exam results; infertility or conditions associated with infertility; or HIV or STI or high risk for HIV. | |
| Interventions | Women were assigned to one condom type for 30 weeks. The eZ·on condom (Family Health International, Research Triangle Park, NC and Mayer Laboratories, Inc., Oakland, CA) versus a latex condom (Kimono Select, Mayer Laboratories, Inc., Oakland, CA). | |
| Outcomes | Contraceptive efficacy; breakage and slippage; safety; acceptability. Breakage and slippage outcomes were abstracted in method consistent with Steiner 1994 definitions. | |
| Notes | Randomized with computer‐generated, permuted blocked randomization scheme, stratified by site and prior condom experience. 27% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed with centralized telephone system. |
Trussell 1992.
| Methods | Randomized, crossover trial. One center in United States. Blinding not reported. | |
| Participants | 50 couples aged 18 to 55 years in monogamous heterosexual relationship and currently using oral contraceptive, Norplant, IUD or sterilization for contraception if still fertile. Excluded pregnancy, lactation and STI. | |
| Interventions | Couples were assigned to use 5 condoms of each of the 2 condom types in an alternating sequence over a 5‐week period. Half of the couples started with the polyurethane condom and the other half started with the latex condom. The polyurethane condom Tactylon (Tactyl Technologies) versus the latex condom Trojan‐Enz (Carter‐Wallace, Inc.). | |
| Outcomes | Condom breakage and slippage; acceptability. | |
| Notes | Randomization and allocation concealment methods not described. 2% loss to follow up and early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
STD = sexually transmitted disease(s)
STI = sexually transmitted infection(s)
Contributions of authors
Maria Gallo drafted the review. Maria Gallo and David Grimes extracted data for the initial review. Laureen Lopez reviewed the searches for the updates (2006 through 2012), extracted data, and revised the review as needed. David Grimes and Kenneth Schulz edited and approved the review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Child Health and Human Development, USA.
U.S. Agency for International Development, USA.
Declarations of interest
FHI (now FHI 360) developed the eZ·on condom and subsequently licensed the product to Mayer Laboratories, Inc. (Oakland, CA, USA). Two authors are employed at FHI 360 (LM Lopez and KF Schulz), and two were previously employed at FHI 360 (MF Gallo and DA Grimes). However, the authors were not involved in the development of the eZ·on condom, and were not employed at FHI 360 when the licensing agreement was executed with Mayer Laboratories for the eZ·on condom. FHI 360 is a nonprofit human development organization.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bounds 2002 {published data only}
- Bounds W, Molloy S, Guillebaud J. Pilot study of short‐term acceptability and breakage and slippage rates for the loose‐fitting polyurethane male condom eZ·on bi‐directional: a randomized cross‐over trial. European Journal of Contraception & Reproductive Health Care 2002;7:71‐8. [PubMed] [Google Scholar]
Callahan 2000 {published data only}
- Callahan M, Mauck C, Taylor D, Frezieres R, Walsh T, Martens M. Comparative evaluation of three Tactylon(TM) condoms and a latex condom during vaginal intercourse: breakage and slippage. Contraception 2000;61:205‐15. [DOI] [PubMed] [Google Scholar]
Cook 2001 {published and unpublished data}
- Cook L, Nanda K, Farr G, Taylor D. A comparative assessment of the eZ·on™ plastic condom and a latex condom: breakage and slippage. Final clinical study report. Family Health International, Research Triangle Park, NC 1999.
- Cook L, Nanda K, Taylor D. Randomized crossover trial comparing the eZ.on plastic condom and a latex condom. Contraception 2001;63:25‐31. [DOI] [PubMed] [Google Scholar]
Frezieres 1998 {published and unpublished data}
- Frezieres RG, Walsh TL, Nelson AL, Clark VA, Coulson AH. Breakage and acceptability of a polyurethane condom: a randomized, controlled study. Family Planning Perspectives 1998;30:73‐8. [PubMed] [Google Scholar]
- Nelson A, Frezieres R, Walsh T, Clark V, Coulson A. Controlled, randomized evaluation of a commercially available polyurethane and latex condom (Avanti versus Ramses Sensitol). Final Report. N01‐HD‐1‐3109 1996.
Frezieres 1999 {published and unpublished data}
- Frezieres RG, Walsh TL, Nelson AL, Clark VA, Coulson AH. Evaluation of the efficacy of a polyurethane condom: results from a randomized, controlled clinical trial. Family Planning Perspectives 1999;31:81‐7. [PubMed] [Google Scholar]
- Nelson A, Bernstein GS, Frezieres R, Walsh T, Clark V, Coulson A. Study of the efficacy, acceptability and safety of a non‐latex (polyurethane) male condom. N01‐HD‐1‐3109 1997.
Frezieres 2000 {published data only}
- Frezieres RG, Walsh TL. Acceptability evaluation of a natural rubber latex, a polyurethane, and a new non‐latex condom. Contraception 2000;61:369‐77. [DOI] [PubMed] [Google Scholar]
Nelson 2001 {published and unpublished data}
- Nelson A, Frezieres R, Walsh T, Bernstein L, Clark V. Phase II/III contraceptive efficacy trial comparing male latex condoms and a male non‐latex condom. Final report. N01‐HD‐7‐3275 2001.
- Walsh TL, Frezieres RG, Peacock K, Nelson AL, Clark VA, Bernstein L. Evaluation of the efficacy of a nonlatex condom: results from a randomized, controlled clinical trial. Perspectives on Sexual and Reproductive Health 2003;35:79‐83. [PubMed] [Google Scholar]
Potter 2003 {published data only}
- Potter WD, Villemeur M. Clinical breakage, slippage and acceptability of a new commercial polyurethane condom: a randomized, controlled study. Contraception 2003;68:39‐45. [DOI] [PubMed] [Google Scholar]
Steiner 1993 {unpublished data only}
- Steiner M, Joanis C, Taylor B, Glover L. Functionality and acceptability study of three lubricated Tactylon™ condoms and a standard lubricated latex condom. Final Report. Family Health International 1993.
Steiner 2003 {published data only}
- Steiner MJ, Dominik R, Rountree RW, Nanda K, Dorflinger LJ. Contraceptive effectiveness of a polyurethane condom and a latex condom: a randomized controlled trial. Obstetrics and Gynecology 2003;101:539‐47. [DOI] [PubMed] [Google Scholar]
Trussell 1992 {published data only}
- Trussell J, Warner DL, Hatcher R. Condom performance during vaginal intercourse: comparison of Trojan‐Enz and Tactylon condoms. Contraception 1992;45:11‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Abma 1997
- Abma J, Chandra A, Mosher W, Peterson L, Piccinino L. Fertility, family planning, and women's health: New data from the 1995 National Survey of Family Growth. National Center for Health Statistics. Vital Health Statistics 1997;23:1‐114. [PubMed] [Google Scholar]
Chen 2007
- Chen MP, Macaluso M, Blackwell R, Galvao L, Kulczycki A, Diaz J, et al. Self‐reported mechanical problems during condom use and semen exposure. Sexually Transmitted Diseases 2007;34:557‐62. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Free 1996
- Free MJ, Srisamang V, Vail J, Mercer D, Kotz R, Marlowe DE. Latex rubber condoms: predicting and extending shelf life. Contraception 1996;53:221‐9. [DOI] [PubMed] [Google Scholar]
Gallo 2007
- Gallo MF, Behets FM, Steiner MJ, Thomsen SC, Ombidi W, Luchters S, et al. Validity of self‐reported 'safe sex' among female sex workers in Mombasa, Kenya ‐ PSA analysis. International Journal of STD & AIDS 2007 2007;18:33‐8. [DOI] [PubMed] [Google Scholar]
Grady 1993
- Grady WR, Klepinger DH, Billy JOG, Tanfer K. Condom characteristics: the perceptions and preferences of men in the United States. Family Planning Perspectives 1993;25:67‐73. [PubMed] [Google Scholar]
Grimes 2005
- Grimes DA, Schulz KF. Surrogate end points in clinical research: hazardous to your health. Obstetrics and Gynecology 2005;105:1114‐8. [DOI] [PubMed] [Google Scholar]
Lytle 1990
- Lytle CD, Carney PG, Vohra S, Cyr WH, Bockstahler LE. Virus leakage through natural membrane condoms. Sexually Transmitted Diseases 1990;17:58‐62. [DOI] [PubMed] [Google Scholar]
Minuk 1989
- Minuk GY. Condoms and hepatitis B virus infection. Condoms in the prevention of sexually transmitted diseases. Sponsored by the American Social Health Association, the U.S. Centers for Disease Control, and Family Health International; 1987 Feb; Atlanta (GA). Research Triangle Park (NC): American Social Health Association, 1989:4‐5.
Mosher 1993
- Mosher WD, Pratt WF. AIDS‐related behavior among women 15‐44 years of age: United States, 1988 and 1990. Advance Data from Vital and Health Statistics 1993;239:1‐15. [DOI] [PubMed] [Google Scholar]
Murphy 1990
- Murphy JS. The condom industry in the United States. Jefferson NC: McFarland & Company, Inc., 1990. [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [DOI] [PubMed] [Google Scholar]
Schulz 2002a
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐18. [DOI] [PubMed] [Google Scholar]
Schulz 2002b
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781‐5. [DOI] [PubMed] [Google Scholar]
Steiner 1994
- Steiner M, Trussell J, Glover L, Joanis C, Spruyt A, Dorflinger L. Standardized protocols for condom breakage and slippage trials: a proposal. American Journal of Public Health 1994;84:1897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trussell 2007
- Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D, et al. editor(s). Contraceptive Technology. 19th Edition. New York: Ardent Media, Inc., 2007:747‐826. [Google Scholar]
Warner 2007
- Warner L, Steiner MJ. Male condoms. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D, et al. editor(s). Contraceptive Technology. 19th Edition. New York: Ardent Media, Inc., 2007:297‐316. [Google Scholar]
References to other published versions of this review
Gallo 2003
- Gallo M, Grimes D, Schulz K. Nonlatex versus latex male condoms for contraception. Contraception 2003;68:319‐26. [DOI] [PubMed] [Google Scholar]


