Abstract
Introduction
A new, citrate-free ixekizumab formulation, which is bioequivalent to the original formulation, was associated with significant reduction in injection site pain. This study evaluates patient satisfaction with the first injection experience of citrate-free ixekizumab in a real-world setting.
Methods
A non-interventional, observational, web-based survey of adults (≥ 18 years) with psoriasis, psoriatic arthritis, or axial spondyloarthritis was conducted between August 2022 and March 2023. Patients enrolled in the Taltz US Customer Support Program were identified as receiving either the original ixekizumab or initiating citrate-free ixekizumab. Patients receiving original ixekizumab completed one survey at baseline to assess satisfaction with the formulation and one survey after switching to assess satisfaction, willingness to continue using and recommending citrate-free ixekizumab, and formulation preference. Participants previously exposed to ixekizumab completed one survey to assess their satisfaction and willingness to continue using and recommending citrate-free ixekizumab. Descriptive and comparative statistics are reported for patients that switched from original to citrate-free ixekizumab (n = 361); and descriptive statistics are reported for patients not previously exposed to ixekizumab (n = 90).
Results
A total of 451 patients were included in the analysis. Significantly more patients were satisfied with their first injection with citrate-free ixekizumab compared to original ixekizumab (83.9% vs. 71.7% respectively; p = 0.0001). Almost all patients who switched from original ixekizumab were definitely or mostly willing to continue using and recommending citrate-free ixekizumab (93.9% and 93.4%, respectively). Additionally, 94.2% of patients who switched from original to citrate-free ixekizumab preferred citrate-free ixekizumab or had no preference. Three-fourths of patients not previously exposed to ixekizumab were satisfied with their first injection with citrate-free ixekizumab and 94.5% were definitely or mostly willing to continue using citrate-free ixekizumab.
Conclusion
The citrate-free ixekizumab formulation was preferred and well accepted by most patients who switched from the original ixekizumab formulation. Similar findings were seen for those newly initiating citrate-free ixekizumab.
Keywords: Ankylosing spondylitis, Axial spondyloarthritis, Citrate-free buffer, Health-related quality of life, Injection site pain, Patient satisfaction, Psoriasis, Psoriatic arthritis, Subcutaneous injection
Key Summary Points
| Why carry out this study? |
| The citrate-free ixekizumab formulation has been commercially available in the USA since August 2022 and has replaced the original formulation of ixekizumab. |
| However, patients’ experience with the new formulation has not been evaluated in a real-world setting. |
| Patient satisfaction with the first injection experience with citrate-free ixekizumab was evaluated in patients who had switched from the original ixekizumab formulation and in patients not previously exposed to ixekizumab. |
| What was learned from the study? |
| Significantly more patients were satisfied with citrate-free ixekizumab compared to original ixekizumab; and almost all patients who switched from original ixekizumab were definitely or mostly willing to continue using citrate-free ixekizumab. |
| Citrate-free ixekizumab was preferred and well accepted by most patients who switched from original ixekizumab. |
| Similar findings were seen across indications and regardless of prior biologic use. |
Introduction
Psoriasis (PsO), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA) including ankylosing spondylitis (AS) and non-radiographic axSpA (nr-axSpA) are chronic, immune-mediated, inflammatory disorders [1–4]. PsO affects 2–4% of the global population, and its symptoms include disfigurement, scaling, skin pain, and itching [5]. Among patients with PsO, up to 30% develop PsA; symptoms of PsA include joint pain and stiffness, joint damage, and disability [5, 6]. AxSpA, which affects 0.1–1.4% of the population, has two subtypes: AS and nr-axSpA [4]. Symptoms of axSpA include spinal pain, reduced mobility, and fatigue [4]. Symptoms related to these inflammatory disorders can profoundly impair patients’ health-related quality of life [1, 2, 7, 8]. Treatment goals for these patients include alleviating symptoms, improving functioning, and decreasing disease complications [2, 3, 9].
Ixekizumab (Taltz®) is a highly selective interleukin-17A monoclonal antibody approved for the treatment of moderate-to-severe plaque PsO and active PsA, active AS, and active nr-axSpA with objective signs of inflammation [10, 11]. After initial dosing, which varies by indication, ixekizumab is administered every 4 weeks by subcutaneous injection [12]. Although ixekizumab significantly reduces symptoms and improves the health-related quality of life for patients, injection site pain after ixekizumab injection has been reported [13–16]. Although the etiology of injection site reactions can be multifactorial (i.e., due to immunologic or non-immunologic factors such as volume, pH, temperature, and speed of injection, and size of the injection needle), citric acid, a commonly used ingredient in pharmaceuticals including ixekizumab, has been known to cause injection site pain by activating the acid-sensing ion channel 1, and solutions containing citrate buffers may cause more pain after subcutaneous injection than citrate-free solutions [17–19]. Lilly recently developed a citrate-free formulation of ixekizumab that is intended to reduce injection site pain [18]. Two phase 1 studies have shown that citrate-free ixekizumab was bioequivalent to the original formulation of ixekizumab and was associated with less injection site pain [20].
The citrate-free ixekizumab formulation has been commercially available in the USA since August 2022 replacing the original formulation of ixekizumab. However, patients’ experience with the new formulation has not been evaluated in a real-world setting. In this study, we evaluated patient satisfaction with the first injection experience with citrate-free ixekizumab in patients who had switched from the original ixekizumab formulation and in patients not previously exposed to ixekizumab.
Methods
Study Design
This was a non-interventional, observational, web-based survey of commercially insured patients receiving either the original or citrate-free ixekizumab formulation. The study was conducted between August 2022 and March 2023 in the USA. Eligible patients were adults (≥ 18 years) diagnosed with PsO, PsA, AS, or nr-axSpA who were enrolled in the Taltz US Customer Support Program and had agreed to be contacted for research studies. Patients were also required to have access to a smartphone or computer with internet access and were receiving either original ixekizumab for ≤ 1 year or citrate-free ixekizumab for ≤ 1 month.
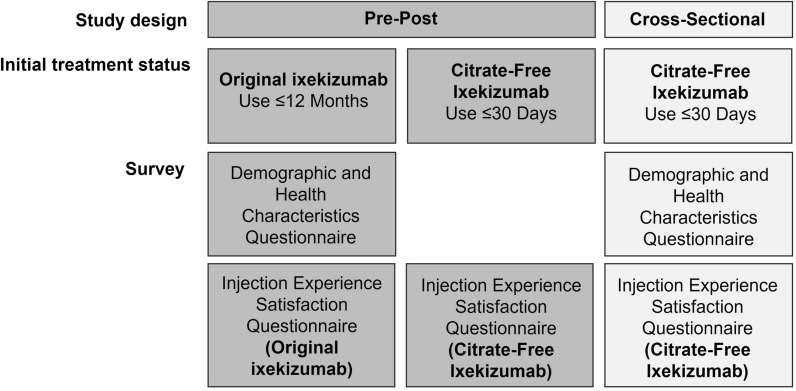
Patients enrolled in the Taltz US Customer Support Program were invited to participate by a third-party recruitment and web-survey hosting vendor, Global Perspectives, which specializes in health outcomes market research. Potential participants were invited by email to participate in the study; interested patients opened the survey link and completed the screening questions and the informed consent form. Participants receiving the original ixekizumab completed two surveys: one survey at baseline to assess their experience with original ixekizumab and one survey within 30 days after switching to assess their experience with citrate-free ixekizumab (Fig. 1). Participants initiating citrate-free ixekizumab who had not been previously exposed to ixekizumab completed one survey to assess their experience with the citrate-free formulation (Fig. 1). All participants who completed the survey(s) were remunerated for their time.
Fig. 1.
Study design
Survey
Participants completed the web-based survey (ca. 10 min) after providing electronic informed consent. The Taltz Injection Experience Satisfaction Questionnaire, a bespoke and non-validated questionnaire, was used to assess patient satisfaction with the ixekizumab injection experience. The language for item stems and response options was adapted from patient-reported measures validated for other disorders [21, 22].
Patients receiving original ixekizumab completed a three-item survey to assess overall satisfaction with their first injection at the time they initiated ixekizumab for the first time, their overall satisfaction with their most recent injection, and the importance of key features of the injection experience (e.g., anxiety regarding self-injection). Patients receiving citrate-free ixekizumab after switching from original ixekizumab completed a five-item survey to assess overall satisfaction with their first injection experience with citrate-free ixekizumab, the importance of key features of the injection experience, their willingness to continue using citrate-free ixekizumab and recommending it to others, and their preferred ixekizumab formulation based on their injection experience. Patients with no prior ixekizumab use and who had received citrate-free ixekizumab completed a four-item survey to assess overall satisfaction with their first injection, the importance of key features of the injection experience (i.e., anxiety regarding self-injection), and their willingness to continue using citrate-free ixekizumab and recommending it to others.
Statistical Analyses
Descriptive and comparative statistics were used to summarize the demographics, clinical characteristics, and survey items for all enrolled patients who had a baseline value. Continuous variables were summarized using means and standard deviations or medians and ranges, and comparisons between groups were made using t tests. Categorical variables were summarized using frequencies and percentages, and comparisons between groups were made using the chi-square test or Fisher’s exact test when the expected frequency was ≤ 5. Subgroup analyses were conducted by indication (i.e., PsO only, PsA only, PsO + PsA, and AS + nr-axSpA) and prior biologic use status in the past 2 years (i.e., 0 prior biologics, 1 prior biologic, and ≥ 2 prior biologics). Comparison of patient satisfaction before and after switching to the new citrate-free ixekizumab formulation from the original ixekizumab formulation was conducted using McNemar tests based on the observed data. All statistical analyses were conducted using observed data.
Compliance with Ethics Guidelines
This study was conducted in accordance with the ethical principles based on the Declaration of Helsinki and was approved by an institutional review board (Advarra CIRBITM; Pro00065375) on 05 August 2022. All participants provided informed consent electronically.
Results
Patient Population
Four hundred and fifty-one patients completed the baseline survey and were included in the analysis (Table 1). Of these, 361 patients completed two surveys, one survey while on original ixekizumab and another after switching to citrate-free ixekizumab; and 90 patients completed a survey after initiating citrate-free ixekizumab (i.e., had no prior ixekizumab use). Most patients were White (85.4%), had PsO and/or PsA (91.8%), and were 45.3 years of age on average. Over half the patients had no prior biologic use in the past 2 years (53.2%), while some had used one prior biologic (28.6%) or two or more biologics (18.2%).
Table 1.
Baseline demographics and clinical characteristics
| Overall N = 451 | |
|---|---|
| Age (years) | |
| Mean (SD) | 45.3 (12.59) |
| Median (range) | 46.0 (18–77) |
| Gender, n (%) | |
| Female | 265 (58.8%) |
| Male | 185 (41.0%) |
| Other | 1 (0.2%) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 400 (88.7) |
| Hispanic or Latino | 51 (11.3) |
| Race, n (%) | |
| White | 385 (85.4) |
| Black or African American | 24 (5.3) |
| Asian | 18 (4.0) |
| Multiracial (no primary race) | 14 (3.1) |
| American Indian or Alaska Native | 7 (1.6) |
| Native Hawaiian or Other Pacific Islander | 4 (0.9) |
| Other | 12 (2.7) |
| Overall health, n (%) | |
| Excellent | 48 (10.6) |
| Very good | 163 (36.1) |
| Good | 180 (39.9) |
| Fair | 54 (12.0) |
| Poor | 6 (1.3) |
| Indication, n (%) | |
| Psoriasis only | 197 (43.7) |
| Psoriasis + psoriatic arthritis | 154 (34.1) |
| Psoriatic arthritis only | 63 (14.0) |
| Axial spondyloarthritis | 37 (8.2) |
| Duration of indication (years), mean (SD) | |
| Psoriasis | 15.3 (12.76) |
| Psoriatic arthritis | 9.0 (9.47) |
| Ankylosing spondylitis | 11.0 (10.92) |
| Non-radiographic axial spondyloarthritis | 1.7 (1.13) |
| Description of indicationa, n (%) | |
| Excellent | 132 (29.3) |
| Very good | 140 (31.0) |
| Good | 102 (22.6) |
| Fair | 57 (12.6) |
| Poor | 20 (4.4) |
| Number of biologics used in past 2 years, n (%) | |
| No prior biologics | 240 (53.2) |
| 1 prior biologics | 129 (28.6) |
| ≥ 2 prior biologics | 82 (18.2) |
SD standard deviation
aPsoriasis and/or psoriatic arthritis or axial spondyloarthritis
Satisfaction with Injection Experience for Patients Who Switched from Original to Citrate-Free Ixekizumab
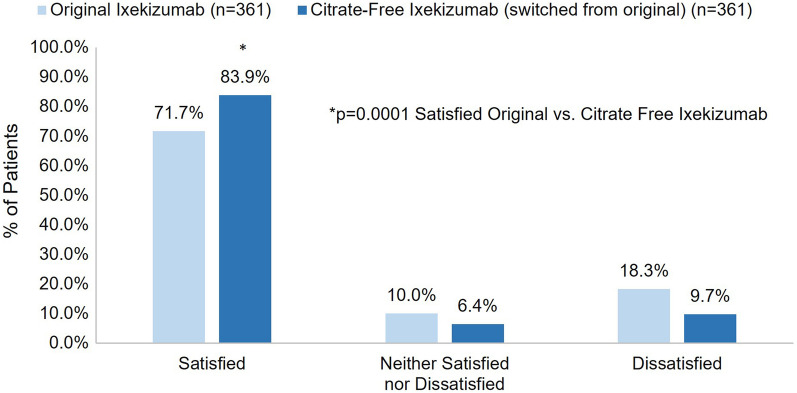
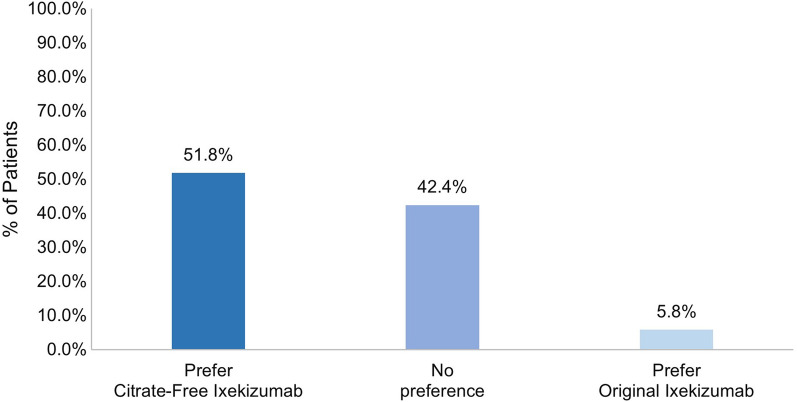
Overall, significantly more patients reported feeling very or somewhat satisfied with their first injection experience with citrate-free ixekizumab compared to original ixekizumab (83.9% vs. 71.7% respectively; p = 0.0001) (Fig. 2). The percentage of patients receiving original ixekizumab who were very or somewhat satisfied with their first injection was similar for different treatment durations: ≤ 1 month (n = 40, 70.0%), 2–5 months (n = 151, 70.2%), and 6–12 months (n = 170, 73.5%) (p = 0.7768). Most patients who switched from original ixekizumab were definitely or mostly willing to continue using citrate-free ixekizumab (93.9%) as well as to recommend it to others (93.4%) (Fig. 3 and Fig. 4). Additionally, most patients (94.2%) who switched from original to citrate-free ixekizumab preferred citrate-free ixekizumab or had no preference (Fig. 5).
Fig. 2.
Overall satisfaction with first injection of citrate-free ixekizumab and first injection of the original ixekizumab
Fig. 3.
Willingness to continue using citrate-free ixekizumab among participants who had switched from the original ixekizumab
Fig. 4.
Willingness to recommend citrate-free ixekizumab to a friend or family member among participants who had switched from the original ixekizumab
Fig. 5.
Preference for ixekizumab by formulation among participants who had switched from the original ixekizumab
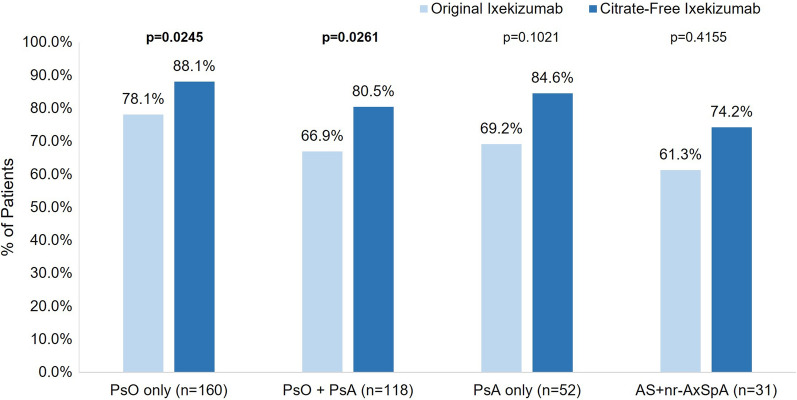
Subgroup Analysis by Indication for Patients Who Switched from Original to Citrate-Free Ixekizumab
For those with PsO and PsO + PsA, significantly more patients were very or somewhat satisfied with their first injection experience with citrate-free ixekizumab compared to their first injection experience with original ixekizumab (PsO n = 141/160, 88.1% vs. n = 125/160, 78.1%, respectively, p = 0.0245; PsO + PsA n = 95/118, 80.5% vs. n = 79/118, 66.9%, respectively, p = 0.0261) (Fig. 6). In general, the percentage of patients that were very or somewhat satisfied with their first injection experience with citrate-free and original ixekizumab was similar for patients with PsA as well as those with AS + nr-axSpA; however, these findings were not statistically significant (PsA n = 44/52, 84.6% vs. n = 36/52, 69.2%, respectively, p = 0.1021; AS + nr-axSpA n = 23/31, 74.2% vs. n = 19/31, 61.3%, respectively, p = 0.4155).
Fig. 6.
Overall satisfaction with citrate-free ixekizumab injection and the original ixekizumab injection by indication. AS, ankylosing spondylitis; AxSpA, axial spondyloarthritis; PsA, psoriatic arthritis; PsO, psoriasis
At least 93% of patients who switched from the original ixekizumab were definitely or mostly willing to continue using citrate-free ixekizumab, regardless of the indication (range from n = 149, 93.1% PsO only to n = 30, 96.8% AS + nr-axSpA), and at least 90% of patients were definitely or mostly willing to recommend citrate-free ixekizumab to others (range from n = 49, 94.2% PsA only to n = 28, 90.3% AS + nr-axSpA). Most patients preferred citrate-free ixekizumab or had no preference, regardless of the indication (range from n = 47, 90.3% PsA only to n = 31, 100.0% AS + nr-axSpA).
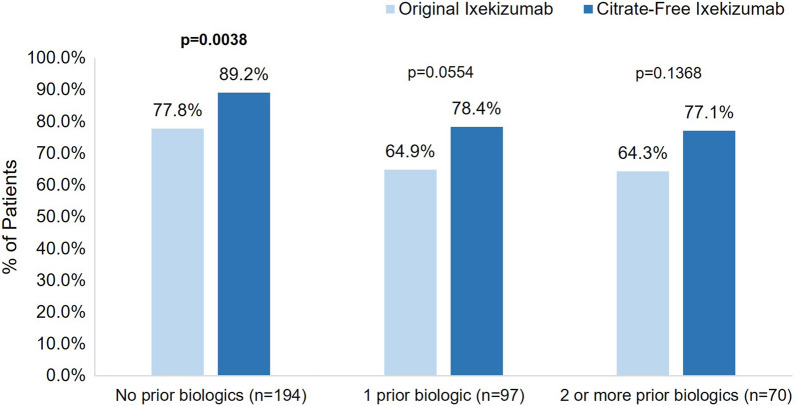
Subgroup Analysis by Prior Biologic Use Status for Patients Who Switched from Original to Citrate-Free Ixekizumab
For those who did not use biologics prior to ixekizumab in the past 2 years, significantly more patients were very or somewhat satisfied with their first injection experience with citrate-free ixekizumab compared to their first injection experience with original ixekizumab (n = 173, 89.2% vs. n = 151, 77.8%, respectively; p = 0.0038) (Fig. 7). A similar difference in the proportion of patients who were very or somewhat satisfied was seen for those who used one or two or more biologics in the previous 2 years; however, these findings were not statistically significant. At least 93% of patients who switched from original ixekizumab were definitely or mostly willing to continue using citrate-free ixekizumab, regardless of biologic use in the previous 2 years (range from n = 181, 93.3% no prior biologics to n = 67, 95.7% ≥ 2 biologics), and at least 91% of patients were definitely or mostly willing to recommend the citrate-free ixekizumab to others (range from n = 64, 91.4% ≥ 2 biologics to n = 183, 94.3% no prior biologics). Nearly all patients preferred citrate-free ixekizumab or had no preference regardless of biologic status (n = 182, 93.8% no prior biologics; n = 91, 93.8% one prior biologic; and n = 67, 95.7% ≥ 2 biologics).
Fig. 7.
Overall satisfaction with first injection of citrate-free ixekizumab and first injection of the original ixekizumab formulation by prior biologic use
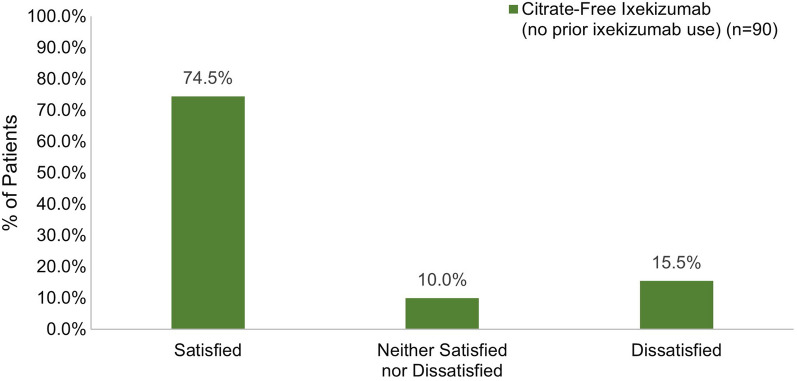
Satisfaction with Injection Experience for Patients Initiating Citrate-Free Ixekizumab Without Prior Ixekizumab Use
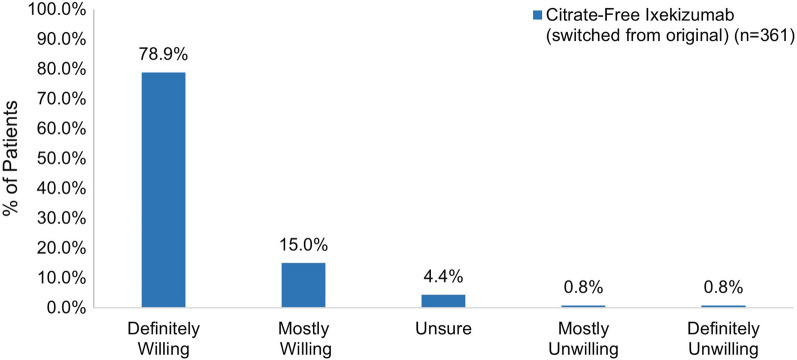
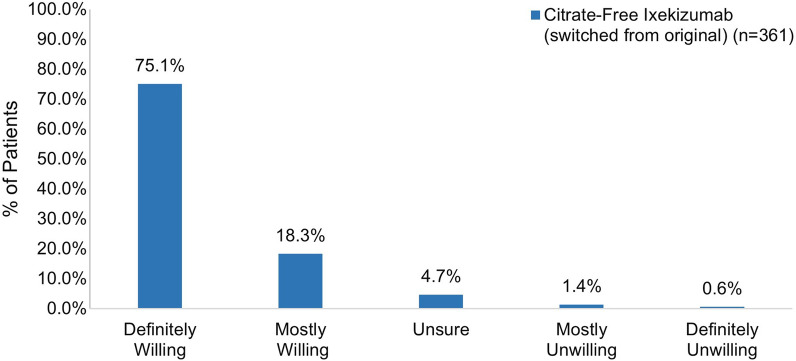
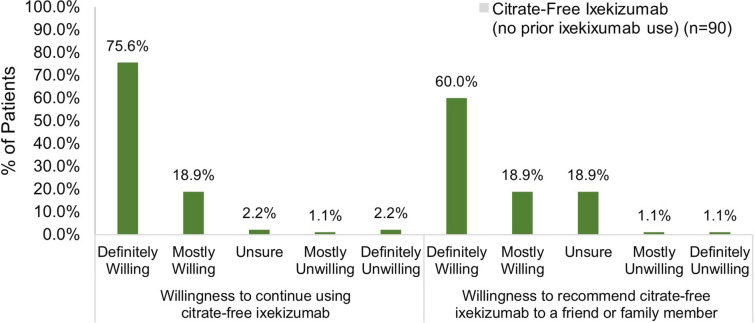
Among patients who had not been previously exposed to ixekizumab, 74.5% reported feeling very or somewhat satisfied with their first injection experience with citrate-free ixekizumab (Fig. 8). Most patients were definitely or mostly willing to continue using citrate-free ixekizumab (94.5%) and were definitely or mostly willing to recommend it to others (78.9%) (Fig. 9).
Fig. 8.
Overall satisfaction with the first injection of citrate-free ixekizumab in patients initiating ixekizumab
Fig. 9.
Willingness to use and recommend ixekizumab in patients initiating ixekizumab
Discussion
The main objective of this real-world study was to evaluate whether citrate-free ixekizumab, a new formulation developed to reduce injection-site pain, was associated with greater satisfaction with their treatment experience compared with the original, citrate-containing formulation. To the best of our knowledge, this is the first study comparing satisfaction with the injection experience between those receiving citrate-free versus original ixekizumab. We showed that patients who switched from original ixekizumab were significantly more satisfied with the first injection of citrate-free ixekizumab compared to original ixekizumab, with 16.6% more patients being very satisfied. Almost all of these patients (approximately 93%) were willing to continue using or to recommend citrate-free ixekizumab, suggesting they were satisfied with the injection experience. In addition, the majority of patients preferred citrate-free ixekizumab, while less than 6% of patients preferred original ixekizumab.
The satisfaction, willingness to continue using or recommending, and preference results for patients who switched from original ixekizumab were similar and consistent across indications (i.e., PsO, PsA, PsO + PsA, and AS + nr-axSpA) and the number of prior biologics used (i.e., none, one, or two or more). More patients were satisfied with their first injection of citrate-free ixekizumab compared to original ixekizumab (ranges in satisfaction across indication and prior biologic use subgroups were 74–89% for citrate-free and 61–78% for original); and the magnitude of differences in satisfaction between the first injection of citrate-free and original ixekizumab also was similar across indications (range 10–15%) and prior biologic use groups (range 11–14%) favoring citrate-free ixekizumab.
At least 90% of patients who switched from original to citrate-free ixekizumab were willing to continue using and recommending citrate-free ixekizumab—regardless of indication or biologic use status; and at least 90% of patients in each of the indication and biologic use status groups preferred citrate-free ixekizumab or had no preference.
In general, the results for the citrate-free ixekizumab also were similar between patients who switched from the original formulation and those who never used ixekizumab. At least three-fourths of patients on citrate-free ixekizumab were satisfied with their overall injection experience; however, approximately 10% more patients who switched from original ixekizumab were satisfied compared to those who never used ixekizumab and approximately 6% more of those who never used ixekizumab were dissatisfied. These findings suggest that experience with the original formulation may have positively impacted perceptions of satisfaction with the new formulation. Almost all patients were willing to continue using and recommending citrate-free ixekizumab to friends or family members regardless of whether they switched from the original formulation or never used ixekizumab.
The satisfaction and preference findings in this study highlight the acceptability of citrate-free ixekizumab and build on prior research demonstrating less injection site pain with the new formulation [19]. These findings are relevant in the context of other prior research showing a significant, positive association between treatment satisfaction and better medication adherence and persistence [5, 6]; and in research showing a significant association between adherence and use of a citrate-free buffer formulation [23]. In particular, adherence rates were significantly greater for patients with rheumatoid arthritis receiving a citrate-free biologic formulation compared to those receiving a citrate-containing formulation [23]; and these findings were consistent across indications. As non-adherence to biologics can result in poorer clinical outcomes [5, 6], finding ways to improve satisfaction and adherence is of paramount importance.
This study has some limitations, which should be considered when reviewing the findings described here. First, since the study included only patients enrolled in a Customer Support Program, the study population may not be representative of the general PsO, PsA, and axSpA populations. Second, patients who were insured by public payers (e.g., Medicaid and Medicare) were not included in the study, which may limit generalizability to minority and elderly populations. Third, ratings for the first injection of the original ixekizumab formulation may have been affected by recall bias in patients who had been treated with ixekizumab for 6–12 months. Last, patients who had discontinued the original ixekizumab formulation in the past and were not using ixekizumab at the time of the study were excluded, and the reasons for discontinuing treatment (e.g., side effects such as injection site pain due to treatment with the original ixekizumab formulation) were not known; therefore, their experience and satisfaction with citrate-free ixekizumab could not be compared.
Conclusion
Citrate-free ixekizumab was preferred and well accepted by most patients who switched from original ixekizumab. Most patients who switched from original ixekizumab were satisfied with their first injection experience, were willing to continue using and recommend to others, and preferred the new formulation. Similar findings were seen for those newly initiating citrate-free ixekizumab.
Acknowledgements
The authors thank the patients who participated in this survey study.
Medical Writing and Editorial Assistance.
Data analysis services were provided by Ray Hsieh (Evidera Inc.) and funded by Eli Lilly and Company. Medical writing was provided by Surayya Taranum, PhD, CMPP (Evidera Inc.) in accordance with Good Publication Practice (GPP 2022) guidelines and funded by Eli Lilly and Company.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of its research and content, and have given their approval for this version to be published.
Author Contributions
Julie Birt, Rebecca Bolce, Jeffrey Lisse, William N. Malatestinic, Baojin Zhu, Miriam Kimel, Julie McCormack, and Marissa Stefan contributed to the study conception and design. Miriam Kimel, Julie McCormack, and Marissa Stefan performed material preparation, data collection, and/analysis. Sanjay Chabra and W. Chad Cragun provided clinical expert review. All authors interpreted the data. All authors contributed to the drafting of the manuscript and approved the final manuscript.
Funding
This study, including the journal’s Rapid Service and Open Access fees, was funded by Eli Lilly and Company.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the language in the patient informed consent, which does not permit the dataset to be made public.
Declarations
Conflict of Interest
Julie Birt, Rebecca Bolce, Jeffrey Lisse, William N. Malatestinic, and Baojin Zhu are employees of Eli Lilly and Company and hold stock in the company. Sanjay Chabra has served as speaker for Eli Lilly and Company. W. Chad Cragun has served as advisor and speaker for Eli Lilly and Company. Miriam Kimel, Julie McCormack, and Marissa Stefan are employees of Evidera Inc.
Ethical Approval
This study was conducted in accordance with the ethical principles based on the Declaration of Helsinki and was approved by an institutional review board (Advarra CIRBITM; Pro00065375) on 05 August 2022. All participants provided informed consent electronically.
References
- 1.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Guyatt G, Ogdie A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019;71(1):5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–1613. doi: 10.1002/art.41042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Medina C, Moltó A. Update on the epidemiology, risk factors, and disease outcomes of axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2018;32(2):241–253. doi: 10.1016/j.berh.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–65.e19. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(5):405–417. doi: 10.1080/1744666X.2018.1468252. [DOI] [PubMed] [Google Scholar]
- 8.Mayo Clinic. Ankylosing spondylitis. 2021. https://www.mayoclinic.org/diseases-conditions/ankylosing-spondylitis/symptoms-causes/syc-20354808. Accessed 22 Aug 2022.
- 9.Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298. doi: 10.1016/j.jaad.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50. doi: 10.2147/JIR.S100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US FDA. FDA approves new psoriasis drug Taltz 2016. https://cacmap.fda.gov/news-events/press-announcements/fda-approves-new-psoriasis-drug-taltz. Accessed 22 Aug 2023.
- 12.TALTZ (ixekizumab) injection, for subcutaneous use. Summary of Product Characteristics 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125521s014lbl.pdf. Accessed 22 Aug 2023.
- 13.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 14.Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158(5):533–541. doi: 10.1001/jamadermatol.2022.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang YK, Shin JU, Lee HJ, Yoon MS, Kim DH. Injection site reactions due to the use of biologics in patients with psoriasis: a retrospective study. JAAD Int. 2023;10:36–38. doi: 10.1016/j.jdin.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loft ND, Vaengebjerg S, Halling AS, Skov L, Egeberg A. Adverse events with IL-17 and IL-23 inhibitors for psoriasis and psoriatic arthritis: a systematic review and meta-analysis of phase III studies. J Eur Acad Dermatol Venereol. 2020;34(6):1151–1160. doi: 10.1111/jdv.16073. [DOI] [PubMed] [Google Scholar]
- 17.Yang YL, Lai TW. Citric acid in drug formulations causes pain by potentiating acid-sensing ion channel 1. J Neurosci. 2021;41(21):4596–4606. doi: 10.1523/JNEUROSCI.2087-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laursen T, Hansen B, Fisker S. Pain perception after subcutaneous injections of media containing different buffers. Basic Clin Pharmacol Toxicol. 2006;98(2):218–221. doi: 10.1111/j.1742-7843.2006.pto_271.x. [DOI] [PubMed] [Google Scholar]
- 19.Shear NH, Paul C, Blauvelt A, et al. Safety and tolerability of ixekizumab: integrated analysis of injection-site reactions from 11 clinical trials. J Drugs Dermatol. 2018;17(2):200–206. [PubMed] [Google Scholar]
- 20.Chabra S, Gill BJ, Gallo G, et al. Ixekizumab citrate-free formulation: results from two clinical trials. Adv Ther. 2022;39(6):2862–2872. doi: 10.1007/s12325-022-02126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boye KS, Jordan JB, Malik RE, Currie BM, Matza LS. Patient perceptions of and preferences between characteristics of injectable diabetes treatments. Diabetes Ther. 2021;12(9):2387–2403. doi: 10.1007/s13300-021-01097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollema ED, Snoek FJ, Pouwer F, Heine RJ, van der Ploeg HM. Diabetes fear of injecting and self-testing questionnaire: a psychometric evaluation. Diabetes Care. 2000;23(6):765–769. doi: 10.2337/diacare.23.6.765. [DOI] [PubMed] [Google Scholar]
- 23.Bluett J, Morgan C, Thurston L, et al. Impact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohort. Rheumatology (Oxford) 2015;54(3):494–499. doi: 10.1093/rheumatology/keu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the language in the patient informed consent, which does not permit the dataset to be made public.