Abstract
Neurodegenerative pathologies such as Alzheimer disease neuropathologic change (ADNC), Lewy body disease (LBD), limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), and cerebrovascular disease (CVD) frequently coexist, but little is known about the exact contribution of each pathology to cognitive decline and dementia in subjects with mixed pathologies. We explored the relative cognitive impact of concurrent common and rare neurodegenerative pathologies employing multivariate logistic regression analysis adjusted for age, gender, and level of education. We analyzed a cohort of 6,262 subjects from the National Alzheimer’s Coordinating Center database, ranging from 0 to 6 comorbid neuropathologic findings per individual, where 95.7% of individuals had at least 1 neurodegenerative finding at autopsy and 75.5% had at least 2 neurodegenerative findings. We identified which neuropathologic entities correlate most frequently with one another and demonstrated that the total number of pathologies per individual was directly correlated with cognitive performance as assessed by Clinical Dementia Rating (CDR®) and Mini-Mental State Examination (MMSE). We show that ADNC, LBD, LATE-NC, CVD, hippocampal sclerosis, Pick disease, and FTLD-TDP significantly impact overall cognition as independent variables. More specifically, ADNC significantly affected all assessed cognitive domains, LBD affected attention, processing speed, and language, LATE-NC primarily affected tests related to logical memory and language, while CVD and other less common pathologies (including Pick disease, progressive supranuclear palsy, and corticobasal degeneration) had more variable neurocognitive effects. Additionally, ADNC, LBD, and higher numbers of comorbid neuropathologies were associated with the presence of at least one APOE ε4 allele, and ADNC and higher numbers of neuropathologies were inversely correlated with APOE ε2 alleles. Understanding the mechanisms by which individual and concomitant neuropathologies affect cognition and the degree to which each contributes is an imperative step in the development of biomarkers and disease-modifying therapeutics, particularly as these medical interventions become more targeted and personalized.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-024-02716-y.
Keywords: Alzheimer disease neuropathologic change, Limbic-predominant age-related TDP-43 encephalopathy, Lewy body dementia, Age-related tauopathy, Pick disease, Frontotemporal lobar dementia, Progressive supranuclear palsy, Corticobasal degeneration, Cerebrovascular disease, MMSE, CDR
Introduction
Globally, the number of individuals living with dementia or some form of cognitive impairment is approximately 55–60 million individuals, but this is expected to increase approximately threefold by 2050 with an exponential rise in the yearly cost to patients, their families, and society at large [15, 31, 58, 68, 100]. Alzheimer disease (AD) neuropathologic change (ADNC) remains the most common underlying pathological finding in individuals with cognitive impairment, however it has become clear over the past decade that other neurodegenerative pathologies, including cerebrovascular disease (CVD), Lewy body disease (LBD), and limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), among others, are frequent comorbid findings [38, 56, 59, 63, 70, 75]. Recently, a number of studies have examined the cognitive effects of concomitant neuropathologies, and have suggested that a large percentage of cognitive impairment and dementia may be due to the additive or synergistic effects of comorbid disease states; however, it is unclear exactly how much each neurodegenerative disease contributes to overall cognitive decline and more specific cognitive and neuropsychological symptoms in individual patients or at the population level [3, 10, 11, 20, 22, 27, 29, 30, 34, 37, 38, 40, 45, 46, 53, 55, 56, 59–63, 70, 76, 84, 91, 95, 99]. There is evidence to suggest there are “normal levels” of common neurodegenerative pathologies at any given age, and the relatively recent concepts of “resistance” to developing neurodegenerative pathology with aging and cognitive “resilience” against the effects of pathology that is present have also been established [1, 51, 78, 93, 95]. There is also increasing evidence that “resilience” against a particular pathology may involve “resistance” to developing others; for example, a cognitively intact individual who is considered resilient against ADNC may have significantly less comorbid LATE-NC or CVD pathology compared to a cognitively impaired individual with similar levels of ADNC [1, 45, 50, 85].
A number of previous studies have focused on isolating individual disease processes in large cohorts to determine the specific cognitive contributions and other symptoms of a given (or combined) pathology [6–9, 13, 23, 25, 42, 91, 95]. This strategy is limited given the frequency with which many of these diseases co-occur and the relative scarcity of some isolated pathologies. This is particularly challenging in non-AD studies given the near ubiquity of some degree of ADNC findings in the aged population. Herein, we attempt to circumvent this issue using multivariate statistical models to disentangle and quantify the relative contributions of a number of common and rare neurodegenerative pathologies in 6,262 subjects with mixed pathologies from the National Alzheimer’s Coordinating Center (NACC) database. We evaluate the correlation between pathologies including ADNC, primary age-related tauopathy (PART), LBD, LATE-NC, hippocampal sclerosis, frontotemporal lobar degeneration with TDP-43 (FTLD-TDP), amyotrophic lateral sclerosis(ALS)/motor neuron disease (MND), Pick disease, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and various forms of CVD (as well as additional covariates, including cerebral amyloid angiopathy (CAA), multiple system atrophy (MSA), chronic traumatic encephalopathy (CTE), and prion disease). In addition, we assess the cognitive impact of cumulative neurodegenerative pathologies, determine the amount of variation in cognition between subjects that can be directly attributed to each disease process, and determine the relative likelihood of impairment of global cognition and specific cognitive/neuropsychological domains (memory, attention, executive function, processing speed, and language) for each neurodegenerative pathology in an effort to determine the relative contribution of each individual pathology to cognitive impairment, irrespective of comorbid findings.
Methods
Case selection and exclusion criteria
Data for this study were downloaded with permission from the NACC (sourced from 37 ADRC collection centers located across the United States), which is a widely utilized cohort with available neuropathologic and neurocognitive data [7–9, 13, 23–28, 30, 39, 54, 67, 71, 72, 79–81, 91, 93, 95], established with funding from the National Institute on Aging (U01 AG016976) (https://naccdata.org/). We utilized standardized Uniform Data Set (UDS), version 3 variable definitions (https://naccdata.org/data-collection/forms-documentation/uds-3), Neuropathology (NP) Data Set, version 11 variable definitions (https://naccdata.org/data-collection/forms-documentation/np-11), and Genetic Data Set (Gen) variable definitions (https://files.alz.washington.edu/documentation/rdd-genetic-data.pdf) from NACC, as previously described [4, 5, 93]. A total of 6,262 unique NACC cases with global Clinical Dementia Rating (CDR®) Dementia Staging Instrument at the final clinical visit and recorded neuropathological autopsy data were identified for analysis.
Neuropathologic, genetic, and demographic variables
Each neurodegenerative pathology was assessed from NACC variables. Where available, ADNC level was determined from the NACC NP dataset variable NPADNC. In instances where NPADNC was not available, ADNC levels were derived from a combination of Braak stage (NACCBRAA), Thal phase (NPTHAL), and CERAD neuritic plaque (NP) score (NACCNEUR) [12, 32, 52, 83]. A total of 4,137 cases had discernable ADNC levels (66.1% of all cases). Definite PART was assessed from a combination of NACCBRAA, NPTHAL, and NACCNEUR, and was defined here as Braak stage III-IV in the absence of diffuse or neuritic plaques in the neocortex (Thal phase 0 and CERAD NP score “none”) [17, 91, 96]. A total of 245 cases met these criteria for definite PART. LBD stage [47] was assessed using the NACC NP dataset variable NACCLEWY, which was available for 5,980 cases (95.5%).
FTLD-TDP, ALS/MND, and LATE-NC were assessed using NACC NP dataset variables NPFTDTDP, NPALSMND, NPTDPA (TDP-43 immunoreactive inclusions in the spinal cord), NPTDPB (TDP-43 immunoreactive inclusions in amygdala), NPTDPC (TDP-43 immunoreactive inclusions in the hippocampus), and NPTDPE (TDP-43 immunoreactive inclusions in neocortex). Cases with a neuropathologic diagnosis of FTLD-TDP and TDP-43 immunoreactive inclusions in the neocortex were included as FTLD-TDP. Cases were assigned LATE-NC stage 0 in the absence of TDP-43 immunoreactivity in any region, LATE-NC stage 1 with TDP-43 immunoreactive inclusions in the amygdala only, LATE-NC stage 2 with TDP-43 immunoreactive inclusions in the amygdala and hippocampus but not neocortex, and LATE-NC stage 3 with TDP-43 inclusions in the amygdala, hippocampus, and neocortex and an absence of a diagnosis of FTLD-TDP or ALS/MND [18, 30, 39, 43, 53, 55]. A total of 2,483 cases had sufficient data to determine FTLD-TDP status (39.7%), 2,960 cases had sufficient data to determine ALS/MND status (47.3%), and 1,916 cases had sufficient data to determine LATE-NC status (30.6%).
Hippocampal sclerosis was determined with the NACC NP dataset variable NPHIPSCL (n = 3,011; 48.1%). Pick disease was determined with the NACC NP dataset variable NACCPICK (n = 6,182; 98.7%). PSP was determined with the NACC NP dataset variable NACCPROG (n = 6,141; 98.1%). CBD was determined with the NACC NP dataset variable NACCCBD (n = 6,141; 98.1%). MSA was determined with the NACC NP dataset variable NPPDXB (n = 3,098; 49.5%). CTE was determined with the NACC NP dataset variable NPFTDT7 (n = 3,054; 48.8%). Prion disease was determined with the NACC NP dataset variable NACCPRIO (n = 6,067; 96.9%). CVD was determined using a combination of infarcts/lacunes (NACCINF; n = 6,217; 99.3%), hemorrhages/microbleeds (NACCHEM; n = 6,110; 97.6%), arteriolosclerosis (NACCARTE; n = 5,608; 89.6%), and white matter rarefaction (NPWMR; n = 2,757; 44.0%). CAA was determined with the NACC NP dataset variable NACCAMY (n = 6,116; 97.7%). Of note, CTE, MSA, and prion disease were used as covariates for multivariate logistic regression analysis, but are not displayed in figures.
Patient age at death was derived from the UDS variable NACCDAGE, patient sex was assessed with the UDS variable SEX, race was determined from the UDS variable RACE, and education was assessed with the UDS variable EDUC. Clinical assessment of normal cognition, mild cognitive impairment (MCI), or dementia was assessed with the UDS variable NACCUDSD. APOE genotype (ε2/2, ε2/3, ε2/4, ε3/3, ε3/4, ε4/4) were assessed with the variable NACCAPOE. Demographic, genetic, and pathologic data on all individuals included in this study can be found in Table 1.
Table 1.
Demographic, genetic, and pathologic features
| All CDR | CDR = 0 | CDR = 0.5 | CDR = 1 | CDR = 2 | CDR = 3 | p-value | |
|---|---|---|---|---|---|---|---|
| n | 6262 | 668 | 961 | 1068 | 1376 | 2189 | − |
| Age (years) | 80.0 ± 0.1 | 85.8 ± 0.4 | 84.3 ± 0.3 | 79.5 ± 0.4 | 79.1 ± 0.3 | 77.3 ± 0.3 | < 0.0001 |
| Gender (M:F) | 3385:2877 | 271:397 | 537:424 | 643:425 | 791:585 | 1143:1046 | < 0.0001 |
| Education (years) | 15.3 ± 0.1 | 15.7 ± 0.1 | 15.6 ± 0.1 | 15.1 ± 0.1 | 15.1 ± 0.1 | 15.2 ± 0.1 | 0.0046 |
| APOE Status | |||||||
| ≥ 1 APOE ε2 allele | 11.1% (607) | 17.4% (110) | 14.6% (127) | 12.9% (119) | 9.5% (116) | 7.3% (135) | < 0.0001 |
| ≥ 1 APOE ε4 allele | 44.0% (2416) | 19.7% (124) | 31.7% (276) | 44.2% (408) | 49.4% (605) | 54.6% (1003) | < 0.0001 |
| Total number of pathologies | 1.93 ± 0.01 | 0.87 ± 0.03 | 1.43 ± 0.03 | 1.86 ± 0.03 | 2.31 ± 0.03 | 2.19 ± 0.02 | < 0.0001 |
| ADNC | |||||||
| Not | 9.9% (408) | 23.3% (79) | 15.8% (84) | 10.5% (70) | 5.1% (50) | 7.7% (125) | < 0.0001 |
| Low | 13.1% (544) | 40.7% (138) | 22.2% (118) | 12.0% (80) | 10.1% (99) | 6.7% (109) | |
| Intermediate | 14.1% (582) | 29.8% (101) | 25.4% (135) | 14.9% (99) | 10.3% (10)1 | 9.0% (146) | |
| High | 62.9% (2603) | 6.2% (21) | 36.5% (194) | 62.6% (416) | 74.4% (726) | 76.6% (1246) | |
| Braak stage | |||||||
| 0 | 6.9% (426) | 5.4% (36) | 5.5% (52) | 8.7% (91) | 8.1% (74) | 8.1% (173) | < 0.0001 |
| I | 8.2% (505) | 19.9% (132) | 10.9% (103) | 7.0% (73) | 7.2% (82) | 6.2% (115) | |
| II | 10.5% (646) | 27.0% (179) | 16.4% (155) | 10.3% (108) | 6.7% (87) | 5.5% (117) | |
| III | 10.1% (620) | 24.1% (160) | 15.8% (149) | 10.6% (111) | 7.9% (98) | 4.8% (102) | |
| IV | 12.9% (795) | 17.1% (113) | 22.4% (212) | 15.9% (166) | 11.5% (155) | 6.9% (149) | |
| V | 17.8% (1091) | 5.1% (34) | 15.3% (145) | 19.6% (205) | 23.0% (310) | 18.5% (397) | |
| VI | 33.5% (2061) | 1.2% (8) | 13.6% (129) | 27.8% (291) | 40.1% (540) | 50.9% (1093) | |
| Thal phase | |||||||
| 0 | 13.7% (418) | 23.7% (79) | 18.9% (89) | 13.8% (71) | 8.1% (53) | 11.7% (126) | < 0.0001 |
| 1 | 8.8% (268) | 20.7% (69) | 11.1% (52) | 6.6% (34) | 7.2% (47) | 6.2% (66) | |
| 2 | 5.8% (178) | 10.5% (35) | 8.9% (42) | 5.% (29) | 6.7% (44) | 2.6% (28) | |
| 3 | 11.6% (353) | 20.1% (67) | 14.5% (68) | 13.0% (67) | 7.9% (52) | 9.2% (99) | |
| 4 | 18.4% (559) | 18.0% (60) | 20.0% (94) | 20.6% (106) | 19.2% (126) | 16.1% (173) | |
| 5 | 41.7% (1268) | 6.9% (23) | 26.6% (125) | 43.2% (207) | 50.8% (333) | 54.1% (580) | |
| CERAD NP score | |||||||
| None | 23.5% (1462) | 47.4% (316) | 31.7% (303) | 25.7% (274) | 17.1% (234) | 15.4% (335) | < 0.0001 |
| Sparse | 23.7% (791) | 23.4% (156) | 19.6% (188) | 10.9% (116) | 10.2% (139) | 8.8% (192) | |
| Moderate | 18.6% (1161) | 18.3% (122) | 22.9% (219) | 20.2% (215) | 17.1% (234) | 17.1% (371) | |
| Frequent | 45.2% (2818) | 10.8% (72) | 25.8% (247) | 43.2% (460) | 55.7% (762) | 58.7% (1277) | |
| Definite PART (Braak III-IV) | 4.0% (245) | 10.4% (69) | 6.3% (60) | 4.0% (42) | 2.0% (27) | 2.2% (47) | < 0.0001 |
| Lewy body stage | |||||||
| None | 68.8% (4114) | 83.9% (547) | 74.4% (694) | 67.4% (700) | 64.4% (841) | 64.8% (1332) | < 0.0001 |
| Brainstem | 3.9% (231) | 5.4% (35) | 5.9% (55) | 3.9% (41) | 3.1% (40) | 2.9% (60) | |
| Limbic | 14.3% (857) | 7.7% (50) | 10.4% (97) | 14.8% (154) | 16.3% (213) | 16.7% (343) | |
| Neocortical | 13.0% (779) | 3.1% (20) | 9.3% (87) | 13.8% (143) | 16.1% (210) | 15.5% (319) | |
| LATE-NC stage | |||||||
| 0 | 70.3% (1347) | 88.6% (156) | 77.4% (229) | 72.4% (249) | 61.0% (280) | 67.6% (433) | < 0.0001 |
| 1 | 7.2% (137) | 2.8% (5) | 6.1% (18) | 8.1% (28) | 9.2% (42) | 6.9% (44) | |
| 2 | 17.8% (341) | 6.8% (12) | 13.9% (41) | 14.8% (51) | 23.5% (108) | 20.1% (129) | |
| 3 | 4.7% (91) | 1.7% (3) | 2.7% (8) | 4.7% (16) | 6.3% (29) | 5.5% (35) | |
| Hippocampal sclerosis | 13.9% (420) | 2.7% (9) | 8.2% (37) | 15.0% (76) | 17.8% (114) | 17.1% (184) | < 0.0001 |
| FTLD-TDP | 6.4% (159) | 1.5% (4) | 2.5% (10) | 6.9% (31) | 5.5% (32) | 10.5% (82) | < 0.0001 |
| ALS/MND | 1.7% (49) | 3.1% (10) | 1.7% (8) | 2.2% (11) | 2.4% (15) | 0.5% (5) | 0.0036 |
| Pick’s disease | 1.6% (101) | 0.3% (2) | 0.4% (4) | 1.4% (15) | 1.5% (21) | 2.8% (59) | < 0.0001 |
| PSP | 4.1% (221) | 1.1% (7) | 5.8% (55) | 4.7% (50) | 2.7% (37) | 3.4% (72) | < 0.0001 |
| CBD | 1.9% (121) | 0.5% (3) | 2.0% (19) | 1.8% (19) | 2.1% (28) | 2.4% (52) | 0.0343 |
| CTE | 0.6% (17) | 0.3% (1) | 0.4% (2) | 0.8% (4) | 1.0% (7) | 0.3% (3) | 0.2617 |
| Prion disease | 1.5% (90) | 0.2% (1) | 0.8% (7) | 2.7% (28) | 1.2% (16) | 1.8% (38) | 0.0001 |
| Gross infarcts | 18.6% (158) | 18.1% (121) | 24.3% (231) | 20.7% (218) | 16.4% (223) | 16.8% (365) | < 0.0001 |
| Gross hemorrhage | 6.6% (407) | 6.1% (42) | 8.3% (77) | 6.8% (70) | 6.4% (85) | 6.2% (133) | 0.2434 |
| Arteriolosclerosis | |||||||
| None | 20.2% (1134) | 22.0% (128) | 19.8% (172) | 20.5% (204) | 19.8% (248) | 19.9% (382) | 0.0002 |
| Mild | 35.8% (2007) | 43.2% (251) | 37.1% (322) | 36.5% (362) | 34.1% (426) | 33.7% (646) | |
| Moderate | 30.8% (1730) | 26.7% (155) | 29.7% (258) | 31.5% (313) | 31.1% (389) | 32.1% (615) | |
| Severe | 13.1% (737) | 8.1% (47) | 13.4% (116) | 11.5% (114) | 15.0% (188) | 14.2% (272) | |
| White matter rarefaction | |||||||
| None | 41.7% (1149) | 45.3% (146) | 45.6% (183) | 48.5% (217) | 40.2% (231) | 36.7% (372) | < 0.0001 |
| Mild | 28.4% (784) | 33.9% (109) | 29.2% (117) | 27.5% (123) | 32.6% (187) | 24.5% (248) | |
| Moderate | 19.5% (537) | 12.7% (41) | 17.5% (70) | 17.2% (77) | 18.3% (105) | 24.1% (244) | |
| Severe | 10.4% (287) | 8.1% (26) | 7.7% (31) | 6.7% (30) | 8.9% (51) | 14.7% (149) | |
| CAA | |||||||
| None | 40.9% (2499) | 59.0% (386) | 49.9% (469) | 41.4% (432) | 34.1% (463) | 35.3% (749) | < 0.0001 |
| Mild | 28.1% (1717) | 27.2% (178) | 26.7% (251) | 29.4% (307) | 30.8% (417) | 26.6% (564) | |
| Moderate | 19.9% (1219) | 10.2% (67) | 14.1% (132) | 19.6% (205) | 23.2% (315) | 23.5% (500) | |
| Severe | 11.1% (682) | 3.5% (23) | 9.3% (87) | 9.6% (100) | 11.9% (161) | 14.6% (311) | |
Cognitive and neuropsychological variables
Representative cognitive and neuropsychological variables encompassing overall cognition and specific neuropsychological domains were assessed using the UDS variables. These included global CDR (CDRGLOB; n = 6,262; 100%), CDR Sum of Boxes (CDRSUM; n = 6,262; 100%), Mini-Mental State Examination (MMSE; NACCMMSE; n = 3,548; 56.7%), logical memory immediate recall (LMI) (LOGIMEM; n = 2,778; 44.4%), logical memory delayed recall (LMD) (MEMUNITS; n = 2,735; 43.7%), digit span forward (DSF) (DIGIF; n = 2,867; 45.8%), digit span backward (DSB) (DIGIB; n = 2,828; 45.2%), Trail Making Test Part A (TMT-A) (TRAILA; n = 2,604; 41.6%), Trail Making Test Part B (TMT-B) (TRAILB; n = 1,870; 29.9%), Wechsler Adult Intelligence Scale Digit Symbol Substitution Test (WAIS DS) (WAIS; n = 1,970; 31.5%), animal fluency (ANIMALS; n = 3,310; 52.9%), vegetable fluency (VEG; n = 3,190; 50.9%), and Boston Naming Test, 30 odd items (BNT) (BOSTON; n = 2,725; 43.5%), as previously described [7, 27, 28, 30, 36, 80, 91]. The total number of subjects with available data for each neuropathologic feature and cognitive test combination is available in Supplemental Table 1.
Each cognitive/neuropsychological test was adjusted for age, sex, and education level as previously described [73, 91, 98]. The neurocognitive tests (LMI, LMD, DSF, DSB, TMT-A, TMT-B, WAIS DS, animals, vegetables, and BNT) were converted into z-scores and the percentile was determined for each with corrections for age, sex, and education, where “mild impairment” was defined as the 2–8.99 percentile, “moderate impairment” was defined as the 1–1.99 percentile, and severe impairment was defined as < 1 percentile [73]. We defined impairment for the purposes of multivariate logistic regression analysis as < 9th percentile, including mild-severe impairment. Global CDR was defined as impaired using both 0.5 and 1 as thresholds. CDR sum of boxes (SOB) was defined as impaired using both 3 and 4.5 as thresholds. MMSE was defined as impaired using scores of 21 and 24 as thresholds. All figures and tables presented here display data using global CDR ≥ 1, CDR SOB ≥ 4.5, and MMSE ≤ 24 [2, 21, 57, 64, 88].
Data analysis
Multivariate logistic regression analysis was performed with MedCalc (MedCalc Software Ltd, Ostend, Belgium). All other statistical analyses were performed with GraphPad Prism version 9.5.1 (GraphPad Software, Inc., La Jolla, CA, USA). All graphs were created using GraphPad Prism; graphs of linear regression analysis between cognitive status (global CDR, CDR SOB, and MMSE) and the total number of neurodegenerative pathologies were created as composites, combining multiple variable bubble plots and linear regression analysis, where the size of each data point represents the number of subjects. Differences in the proportion of gender, APOE status, and neuropathologic variables among cohorts were calculated using Fisher’s exact test. Differences between age, education, and total number of pathologies between groups were evaluated using multiple t-tests. Correlations with total number of pathologies were modeled using linear regression and Pearson correlation coefficient. Percent contributions of each pathology to cognitive impairment was determined using multiple regression analysis where the calculated β coefficient for each pathology was divided by the sum of all β values, as previously described in detail [10, 66]. Statistical significance was set at α = 0.05.
Results
Demographic features of the cohort as a whole
There were a total of 6,262 individuals with available CDR, with score groups ranging from 0 to 3 (Table 1). CDR score of 0 represents an individual with no cognitive impairment. CDR scores of 0.5, 1, 2, and 3 represent individuals with questionable, mild, moderate, and severe cognitive impairment, respectively. Clinically, 13.7% of the total cohort were not impaired at the last clinical visit, while 10.4% had MCI, 74.1% had dementia, and 1.8% were impaired but did not meet criteria for either MCI or dementia. The average age for the general cohort was 80 years old (80.0 ± 0.1 for all CDR). There was a statistically significant difference in age among CDR score groups that highlights the relationship between cognitive impairment severity and mortality, where age was inversely correlated to overall CDR, suggesting that subjects with the most severe disease may die earlier, or perhaps there is a degree of selective attrition. The average age for individuals with no cognitive impairment (CDR = 0) was 85.8 ± 0.4 years old, while the average age for individuals with severe cognitive impairment (CDR = 3) was 77.3 ± 0.3 years old (p < 0.0001). Gender was predominantly male in groups with questionable to severe cognitive impairment (55.7% male in CDR 0.5–3), but cognitively intact individuals were significantly more likely to be female (59.4% female in CDR = 0 group) (p < 0.0001). The average level of education was 15.3 ± 0.1 years, and individuals with no cognitive impairment display a modest but statistically significant higher level of education when compared to cognitively impaired individuals (15.7 ± 0.1 years for CDR = 0 compared to 15.2 ± 0.1 years for CDR = 3; p = 0.0046). For the entire cohort (all CDR), 607 individuals (11.1%) have ≥ 1 APOE ε2 allele and 2,416 individuals (44.0%) have ≥ 1 APOE ε4 allele. The frequency of APOE ε4 alleles was positively correlated with CDR; 19.7% of subjects with CDR = 0 had at least one APOE ε4 allele, while 54.6% of subjects with CDR = 3 had at least one APOE ε4 allele (p < 0.0001). Conversely, the frequency of APOE ε2 alleles was inversely correlated with CDR; 17.4% of subjects with CDR = 0 had at least one APOE ε2 allele, while only 7.3% of subjects with CDR = 3 had at least one APOE ε2 allele (p < 0.0001) (Table 1).
Pathologic features of the cohort as a whole
In subjects with at least ADNC, LATE-NC, LBD, and cerebrovascular pathology data available (n = 1,847), the total number of neuropathologic findings was close to 2 per individual (1.93 ± 0.01 for all CDR). 95.7% of individuals had at least one identified neuropathologic finding at autopsy, and 75.5% had at least two neuropathologic findings. The number of pathologies per individual was directly correlated with global CDR; there were 0.87 ± 0.03 neuropathologic diagnoses in cognitively intact subjects (CDR = 0) compared to 2.19 ± 0.02 neuropathologic diagnoses in subjects with severe cognitive impairment (CDR = 3; p < 0.0001) (Table 1).
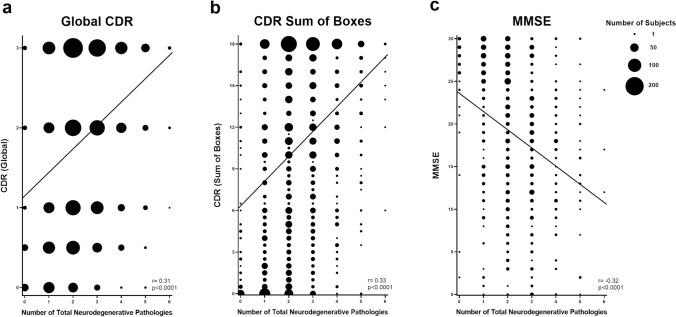
The number of neurodegenerative pathologies identified at autopsy was proportional to measures of global cognition by linear regression analysis. There was a positive correlation between the number of total neurodegenerative pathologies and the global CDR (r = 0.31, p < 0.0001) (Fig. 1a), which assesses memory, orientation, judgement, and problem solving, as well as functioning across community affairs, home and hobbies, and personal care domains [57, 64]. Similarly, there was a linear relationship between the number of total neurodegenerative pathologies and CDR sum of boxes (r = 0.33, p < 0.0001) (Fig. 1b). MMSE includes tests assessing orientation, memory, attention/concentration, naming, verbal repetition and comprehension, reading and writing, and visuospatial abilities. A perfect score is 30 points, while a score of less than 25 is consistent with cognitive impairment [2, 21]. There was an inverse relationship between number of total neurodegenerative pathologies and MMSE (r = -0.32, p < 0.0001) (Fig. 1c). These same trends were present in patients with intermediate or high level ADNC; there was a direct correlation between the number of additional pathologies co-occurring in patients with intermediate or high level ADNC and global CDR (r = 0.20, p < 0.0001) and CDR sum of boxes (r = 0.21, p < 0.0001), and an indirect correlation with MMSE (r = −0.15, p = 0.0012). Taken together, these data demonstrate that individuals with higher numbers of concurrent pathologies generally have more severe levels of cognitive impairment as measured by CDR and MMSE.
Fig. 1.
Linear regression analysis demonstrating strong correlation between the total number of neuropathologic variables identified at autopsy (including ADNC, PART, LBD, LATE-NC, hippocampal sclerosis, FTLD-TDP, ALS/MND, Pick disease, PSP, CBD, CVD, CTE, prion disease, AGD, and MSA) and (a) global CDR, (b) CDR sum of boxes, and (c) MMSE (size of each data point corresponds to the number of subjects)
Compared to cognitively intact subjects, individuals with moderate and severe cognitive impairment were found to have higher levels of certain pathologies like ADNC (74.4% of CDR 2 and 76.6% of CDR 3 subjects had high level ADNC compared to only 6.2% of CDR 0 subjects; p < 0.0001), limbic and neocortical stage LBD (p < 0.0001), hippocampal and neocortical stage LATE-NC (p < 0.0001), hippocampal sclerosis (p < 0.0001), FTLD-TDP (p < 0.0001), infarcts (p < 0.0001), arteriolosclerosis (p = 0.0002), white matter rarefaction (p < 0.0001), and CAA (p < 0.0001), while the frequency of definite PART decreased (p < 0.0001) (Table 1). The frequency of ALS/MND was also inversely proportional to cognitive decline (p = 0.0036), which may be due to these patients succumbing to the non-cognitive components of their illness before developing TDP-43-associated cognitive impairment.
Features of cognitively intact individuals
Interestingly, many cognitively normal individuals (CDR scores of 0) displayed some degree of pathology, and in a minority of cases very severe levels of individual pathologies and co-morbid neuropathologies. Individuals with no cognitive impairment averaged less than one neurodegenerative finding at autopsy (Table 1); however, a small percentage of individuals exhibited significant neuropathologic changes. Among those with no cognitive impairment (n = 668), 101 and 21 individuals had intermediate and high level ADNC respectively, 50 and 20 individuals had limbic and neocortical stage LBD respectively, 12 and 3 had LATE-NC stage 2 and 3 respectively, 9 had hippocampal sclerosis, 4 had FTLD-TDP, 2 had Pick disease, 10 had ALS/MND, 121 had gross infarcts, 42 had gross hemorrhage, 47 had severe arteriolosclerosis, 26 had severe white matter rarefaction, and 23 had severe CAA [82, 87, 97]. Only 17.3% of subjects with a CDR of 0 had no identifiable pathology, while 82.7% had at least one significant neuropathologic finding and 42.8% had at least 2 concurrent neurodegenerative pathologies, with up to 5 of the assessed neurodegenerative findings identified in one individual with a CDR of 0 and MMSE of 30, suggesting a significant level of resilience in the face of neurodegenerative pathology in a minority of subjects.
Correlation between neurodegenerative pathologies
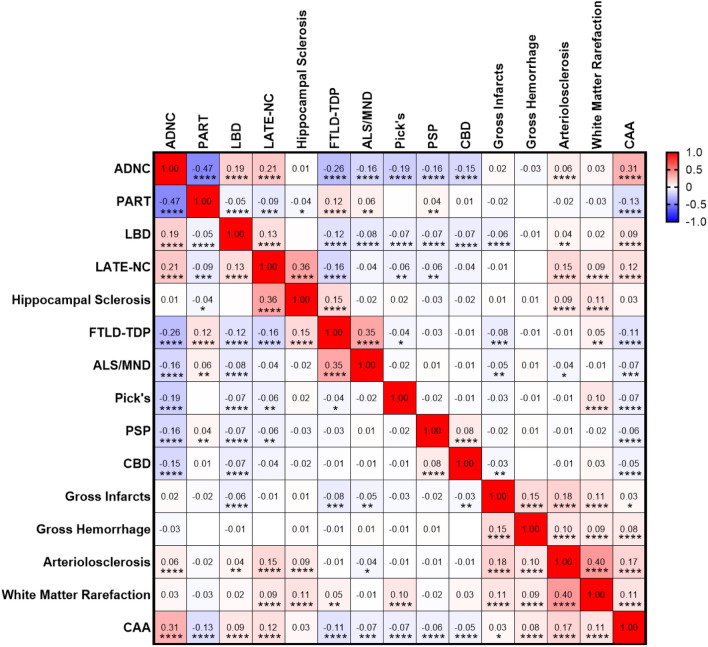
In this cohort, some neurodegenerative pathologies correlated more frequently with others. For example, ADNC showed a high correlation with CAA (Pearson r = 0.31; p < 0.0001), LATE-NC (r = 0.21; p < 0.0001), LBD (r = 0.19; p < 0.0001), and arteriolosclerosis (r = 0.06; p < 0.0001). In contrast, ADNC was negatively correlated with FTLD-TDP (r = −0.26; p < 0.0001), Pick disease (r = -0.19; p < 0.0001), ALS/MND (r = −0.16; p < 0.0001), PSP (r = -0.16; p < 0.0001), and CBD (r = -0.15; p < 0.0001). By definition, ADNC was inversely correlated with definite PART (r = −0.47; p < 0.0001) since the diagnosis of definite PART requires the absence of β-amyloid [17, 52, 96]. Hippocampal sclerosis was significantly correlated with TDP-43 pathologies (both LATE-NC and FTLD-TDP), as well as arteriolosclerosis and white matter rarefaction, but not other neurodegenerative pathologies, including ADNC [19, 26, 30, 39]. Individual cerebrovascular pathologies tended to correlate with one another, and in particular arteriolosclerosis was highly correlated with white matter rarefaction (r = 0.40; p < 0.0001) (Fig. 2). Braak stage, Thal phase, CERAD neuritic plaque density, diffuse plaque density, CAA, and arteriolosclerosis were all significantly correlated, while hippocampal sclerosis was again most correlated to TDP-43 and, to a lesser extent, vascular variables (Supplemental Fig. 1).
Fig. 2.
Correlation matrix assessing different autopsy-proven neuropathologic findings across 6,262 subjects in the NACC dataset. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
Univariate and multivariate analysis of cognitive impact of comorbid neuropathologies
Due to the frequency with which subjects (particularly those with greater levels of cognitive impairment) had more than one neurodegenerative pathology (Table 1 and Fig. 1), and the relative frequency with which some neuropathologic findings (ADNC, LATE-NC, LBD, CVD) tended to co-occur (Fig. 2), we explored which neuropathologies contributed to global and specific aspects of cognitive impairment [61]. To unravel which of these pathologies were associated with varying severities of cognitive impairment, we examined all variables by performing multivariate logistic regression analysis after adjusting each individual test for age, gender, and years of education [63, 73].
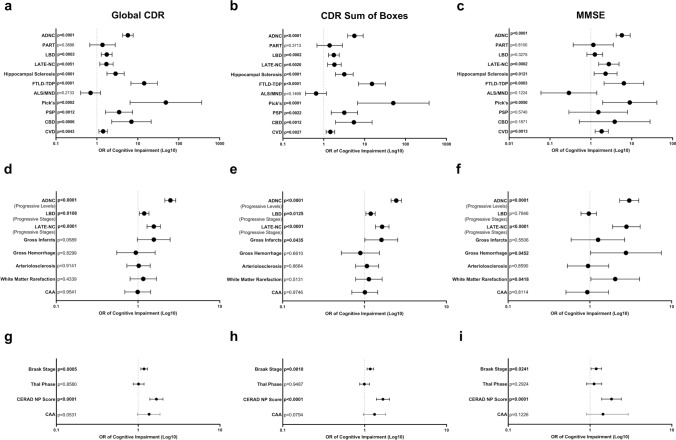
Neuropathologic, cognitive, and neuropsychological variables were studied to analyze the contribution of each pathology to impairment of overall cognition, as well as impairment of specific neuropsychological domains. In terms of global CDR (using a threshold of CDR = 1), the presence of ADNC (combined intermediate and high level), LBD (combined stage 2–3), LATE-NC (combined stage 2–3), hippocampal sclerosis, FTLD-TDP, Pick disease, PSP, CBD, and CVD all demonstrated a significant and independent risk of cognitive impairment (Fig. 3a). The multivariate odds ratio for cognitive impairment of individuals with level 2 or 3 ADNC was 5.72 (4.25–7.72 95% CI; p < 0.0001) (Supplemental Table 2), indicating that individuals with level 2 or 3 ADNC were 5.72 times more likely to experience cognitive impairment when compared to individuals without this pathology, due to the contribution of ADNC alone (i.e., regardless of comorbid pathologies). The multivariate odds ratio for cognitive impairment for individuals with Pick disease was 48.45 (6.47–362.62 95% CI; p = 0.0002), with FTLD-TDP was 14.30 (6.79–30.12 95% CI; p < 0.0001), with CBD was 6.98 (2.30–21.21 95% CI; p = 0.0006), with PSP was 3.49 (1.63–7.44 95% CI; p = 0.0012), with hippocampal sclerosis was 2.86 (1.76–4.65 95% CI; p < 0.0001), with stage 2 or 3 LBD was 1.74 (1.28–2.35 95% CI; p = 0.0003), with stage 2 or 3 LATE-NC was 1.71 (1.18–2.49 95% CI; p = 0.0051), and with CVD was 1.42 (1.12–1.80 95% CI; p = 0.0043). Similar results were seen for CDR sum of boxes using 4.5 as a threshold for cognitive impairment (Fig. 3b). These same interactions were also seen using global CDR = 0.5 and CDR sum of boxes = 3.0 as thresholds.
Fig. 3.
Multivariate logistic regression analysis demonstrating the odds ratios (OR) and 95% confidence intervals of cognitive impairment in the presence of ADNC (level 2–3), definite PART (Braak III-IV), LBD (stage 2–3), LATE-NC (stage 2–3), hippocampal sclerosis, FTLD-TDP, ALS/MND, Pick disease, PSP, CBD, and CVD in terms of (a) global CDR, (b) CDR sum of boxes, and (c) MMSE. CTE, MSA, and prion disease were included as covariates in multivariate analysis model but are not shown here for simplicity. Multivariate logistic regression analysis performed on progressive levels/stages of ADNC, LBD, LATE-NC, infarcts, hemorrhage, arteriolosclerosis, white matter rarefaction, and cerebral amyloid angiopathy in terms of (d) global CDR, (e) CDR sum of boxes, and (f) MMSE. Multivariate logistic regression analysis was also performed on progressive Braak stage, Thal phase, CERAD NP score, and CAA (with LBD, LATE-NC, and CVD used as covariates) in terms of (g) global CDR, (h) CDR sum of boxes, and (i) MMSE
For MMSE, the multivariate odds ratio for cognitive impairment was significant for ADNC, LATE-NC, hippocampal sclerosis, FTLD-TDP, Pick disease, and CVD (Fig. 3c). The multivariate odds ratio for cognitive impairment for individuals with intermediate or high level ADNC was 5.72 (3.58–9.15 95% CI; p < 0.0001), for individuals with stage 2 or 3 LATE-NC was 2.75 (1.55–4.88 95% CI; p = 0.0002), with hippocampal sclerosis was 2.29 (1.20–4.36 95% CI; p = 0.0121), with FTLD-TDP was 6.41 (2.10–19.64 95% CI; p = 0.0011), with Pick disease was 8.93 (1.94–41.23 95% CI; p = 0.0050), and with CVD was 1.83 (1.27–2.66 95% CI; p = 0.0013). Taken together, these results demonstrate that ADNC, LATE-NC, CVD, hippocampal sclerosis, Pick disease, and FTLD-TDP significantly impact overall cognition as independent variables, as evidenced by a poor cognitive performance in terms of global CDR, CDR sum of boxes, and MMSE (Fig. 3a-c). LBD, PSP, and CBD were found to independently impact cognitive performance as measured by global CDR and CDR sum of boxes but this association was not found with MMSE. These findings highlight the relative contribution of each neuropathology to overall cognition and specific neuropsychological domains (Supplemental Table 2).
Multivariate logistic regression analysis was also performed to evaluate the effects of progressive levels of the most commonly encountered neurodegenerative findings, including ADNC, LATE-NC, LBD, and cerebrovascular disease to increase the granularity of these findings. Progressive levels of ADNC had an odds ratio of cognitive impairment of 2.49 (2.13–2.89 95% CI; p < 0.0001) for global CDR (Fig. 3d and Supplemental Table 3), each progressive stage of LBD had an odds ratio of 1.19 (1.04–1.36 95% CI; p = 0.0108, and each progressive stage of LATE-NC had an odds ratio of 1.56 (1.29–1.88 95% CI; p < 0.0001). In contrast, specific cerebrovascular pathologies (infarcts, hemorrhage, arteriolosclerosis, white matter rarefaction) and CAA did not have a significant contribution to cognitive impairment as examined by global CDR. In general, similar results are seen for CDR sum of boxes (Fig. 3e), apart from the fact that gross infarcts displayed a significant contribution to cognitive impairment with an odds ratio of 1.61 (1.01–2.55 95% CI; p = 0.0435). For MMSE, the multivariate odds ratio for cognitive impairment was significant for progressive levels of ADNC, progressive stages of LATE-NC, the presence of hemorrhage and white matter rarefaction (Fig. 3f). The multivariate odds ratio for cognitive impairment for progressive levels of ADNC was 3.03 (2.31–3.96 95% CI; p < 0.0001), for progressive stages of LATE-NC was 2.80 (1.90–4.12 95% CI; p < 0.0001), for gross hemorrhage was 2.76 (1.02–7.47 95% CI; p = 0.0452), and for white matter rarefaction was 2.04 (1.03–4.06 95% CI; p = 0.0418). We also performed an analysis of the impact of individual components of ADNC [52]. Using multivariate logistic regression analysis (with CVD, LATE-NC, and LBD as covariates) we identified progressive Braak stage and CERAD NP score as significantly affecting the global CDR, CDR sum of boxes, and MMSE, while progressive Thal phase and presence of moderate-severe CAA were not significantly associated with cognitive impairment (Fig. 3g–i).
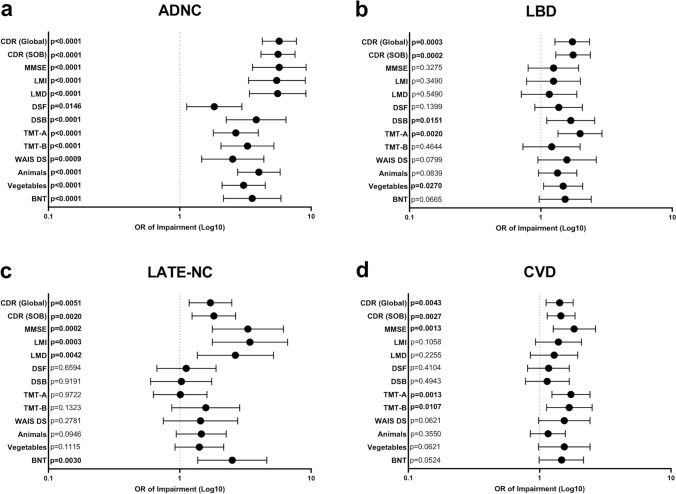
Using multiple regression analysis, we determined that the four most common neuropathologic features (ADNC, LATE-NC, LBD, and CVD) explained 42.2–58.4% of the variation in global cognitive impairment between subjects as measured by CDR and MMSE, with ADNC explaining the majority of this variation (21.5–31.5%). LBD explained an additional 3.2–6.1% of variation in cognitive impairment, LATE-NC explained 7.1–14.5%, and CVD as a pooled group explained 6.9–10.2%; all other neuropathologic entities each explained ≤ 3% of variation in cognitive function when included in the model (Supplemental Fig. 2). Additionally, we performed multivariate logistic regression analysis on more specific cognitive/neuropsychological domains [30, 73, 98]. ADNC significantly affected all assessed cognitive domains (memory, attention, executive function, processing speed, and language), while LBD affected some domains related to attention, processing speed, and language (DSB, TMT-A, and vegetable naming), LATE-NC primarily affected tests related to logical memory and language domains (LMI, LMD, and BNT), while CVD only independently affected TMT-A and TMT-B tests (Fig. 4a-d and Supplemental Table 2). Furthermore, FTLD-TDP significantly impacted all tests related to logical memory, attention, and language domains (with additional effects in some measurements of processing speed), and Pick disease significantly affected a subset of tests associated with these same domains (Supplemental Table 2). Isolating cases with only the most common pathologies (ADNC, LBD, LATE-NC, and CVD) demonstrated significant effects across all domains for each progressive level of ADNC and impairment of logical memory and language domains for progressive stages of LATE-NC with less consistent associations with LBD and cerebrovascular disease (Supplemental Table 3).
Fig. 4.
Multivariate logistic regression analysis demonstrating the odds ratios (OR) and 95% confidence intervals of impairment for global CDR, CDR sum of boxes, MMSE, logical memory immediate recall (LMI), logical memory delayed memory (LMD), digit span forward (DSF), digit span backward (DSB), trail making test A (TMT-A), trail making test B (TMT-B), Wechsler adult intelligence scale digit symbol substitution (WAIS DS), animal fluency (Animals), vegetable fluency (Vegetables), and Boston naming test (BNT) in the four most commonly identified neuropathologies, (a) ADNC, (b) LBD, (c) LATE-NC, and (d) CVD (including infarcts/lacunes, hemorrhages/microhemorrhages, moderate-severe arteriolosclerosis, and moderate-severe white matter rarefaction). Significance and odds ratios for each cognitive/neuropsychological test was determined in the context of ADNC, PART, LBD, LATE-NC, hippocampal sclerosis, FTLD-TDP, ALS/MND, Pick disease, PSP, CBD, CVD, CTE, prion disease, AGD, and MSA
Impact of APOE status on each disease process
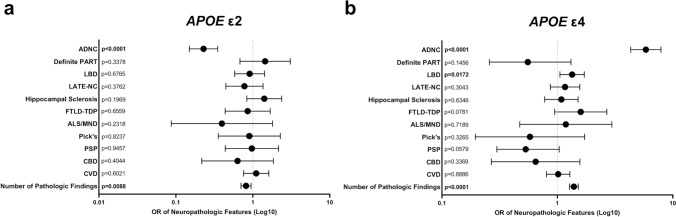
Using multivariate logistic regression analysis, we assessed the relationship between the presence of APOE ε2 and APOE ε4 alleles and each individual neurodegenerative process, as well as with the total number of neuropathologic features identified at autopsy. The presence of at least one APOE ε2 allele was inversely correlated with intermediate-high level ADNC (0.23 OR; 0.15–0.35 95% CI; p < 0.0001) and increasing numbers of total neuropathologies (0.82 OR; 0.70–0.95 95% CI; p = 0.0088) (Fig. 5a). The presence of at least one APOE ε4 allele was significantly associated with the presence of intermediate-high level ADNC (5.85 OR; 4.33–7.91 95% CI; p < 0.0001), limbic and neocortical stage LBD (1.34 OR; 1.05–1.71 95% CI; p = 0.0172), and increasing numbers of total neuropathologies (1.39 OR; 1.27–1.52 95% CI; p < 0.0001) (Fig. 5b). No significant interaction was noted between any other neurodegenerative disease process and the presence of either APOE allele.
Fig. 5.
Multivariate logistic regression analysis demonstrating the odds ratios (OR) and 95% confidence intervals of an individual having (a) at least one APOE ε2 allele or (b) at least one APOE ε4 allele with the presence of each pathology: ADNC (level 2–3), definite PART (Braak III-IV), LBD (stage 2–3), LATE-NC (stage 2–3), hippocampal sclerosis, FTLD-TDP, ALS/MND, Pick disease, PSP, CBD, and CVD, as well as an increasing number of total pathologic findings
Discussion
Dementia is one of the leading causes of morbidity and mortality in the elderly population worldwide, with a significant anticipated rise in prevalence in coming decades [15, 58]. Alzheimer disease remains the most common underlying pathology associated with dementia, however it has recently become clear that many cases of dementia that are attributed to clinical Alzheimer disease have a variety of other comorbid neurodegenerative pathologies at the time of autopsy which may be responsible for some of the cognitive symptoms [3, 22, 25, 34, 37, 38, 40, 45, 56, 59–61, 63, 70, 76, 84, 85, 95, 99]. Given the degree of overlap among neurodegenerative diseases, particularly ADNC, LBD, LATE-NC, and various forms of CVD, it has been difficult to determine the exact contribution of each pathologic finding to an individual patient’s cognitive status, or particular cognitive/neuropsychological test scores. Moreover, established concepts such as resilience against Alzheimer disease pathology must be considered in the context of a wider array of neurodegenerative diseases, as this resilience may be related in part to a relative lack of comorbidities (i.e., resilience against Alzheimer disease pathology may be related to an individual’s resistance to developing comorbid TDP-43 or vascular pathology) [1, 45, 61, 63, 93, 95]. It is important to understand the biology underlying these individual and concomitant neuropathologies, as well as their additive and synergistic clinical effects, as the development of biomarkers, preventative measures, and both symptomatic and disease-modifying therapeutics will depend on an accurate and complete assessment of all factors contributing to cognitive impairment, particularly as these medical interventions become more personalized and targeted toward specific neuronal populations and protein accumulations [59, 85].
To this end, we analyzed a cohort of 6,262 subjects from the NACC database, ranging from 0–6 comorbid neuropathologic entities in individual patients, using multivariate logistic regression analysis to help unravel the relative contributions of ADNC, CAA, PART, LBD, LATE-NC, hippocampal sclerosis, FTLD-TDP, ALS, Pick disease, PSP, CBD, and CVD. As expected, the average number of neurodegenerative findings increases from less than one per cognitively intact subject to more than 2 per subject with moderate-severe cognitive impairment, and there is a direct correlation between cognitive impairment and the progressive level of common pathologies such as ADNC, LATE-NC, LBD, CVD, and CAA and frequency of rarer pathologies such as FTLD-TDP, Pick disease, PSP, CBD, while the frequency of definite PART decreases with increasing global CDR (Table 1). We found significant correlations between many of these pathologies (Fig. 2) and a direct correlation between the number of pathologies and cognitive impairment (Fig. 1). ADNC was the only underlying neurodegenerative pathology that significantly impaired all neuropsychological and cognitive domains as an independent variable (Fig. 4a), although notably many of the individual cognitive domains were more affected by other pathologies, in particular FTLD-TDP and Pick disease, which had greater effects than ADNC in many measures. A number of other neurodegenerative pathologies were significantly associated with more selective deficits (Supplemental Table 2). These results also demonstrate that Braak stage and CERAD NP score are the important determinants of cognitive impairment in ADNC, while Thal phase is not correlated with cognitive status (Fig. 3g-i) [72]. The presence of ADNC and increased numbers of neurodegenerative pathologies were inversely correlated with APOE ε2, while ADNC, LBD, and increased neurodegenerative pathologies were positively correlated with APOE ε4 (Fig. 5), suggesting that APOE status has minimal impact on non-ADNC neurodegenerative processes in isolation, but may play a role in the development of multiple concurrent proteinopathies [62, 95].
We did not find any significant cognitive impairment associated with definite PART (Supplemental Table 2) and definite PART was found more frequently in patients with lower global CDR scores (Table 1). This was similar to our previous findings in pure PART [91], although those demonstrated some isolated effects on processing speed, executive function, and visuospatial function, which were not found in the present study. This is consistent with previous observations that cognitive impairment in PART is correlated more with the overall hippocampal p-tau burden (as opposed to Braak stage), the presence of white matter pathology, and other comorbidities, including LATE-NC and CVD [7, 33, 44, 48, 74, 90, 91, 96]. This also supports the idea that definite PART is a separate process from ADNC, and may represent more of a normal aging pattern [16, 17, 35, 90, 94, 96]. The frequency of ALS/MND was also inversely correlated with global CDR (Table 1), which may be explained by subjects dying of ALS-related complications earlier than subjects without ALS, before more severe cognitive impairment from associated FTLD-TDP could develop. Another interesting finding is that hippocampal sclerosis is significantly associated with cognitive impairment, apparently independent of TDP-43 pathology (Fig. 3a-c). 81 cases of hippocampal sclerosis did not have a concurrent diagnosis of FTLD-TDP or LATE-NC (19.3% of total cases with hippocampal sclerosis) and in 9 cases (2.1%) hippocampal sclerosis was the only pathology identified, and these 9 cases had significant cognitive impairment (global CDR of 1.7 and MMSE of 19.6). This may be due to a wider range of underlying causes of CA1 neuron loss in the hippocampus, including epilepsy and severe global hypoxic-ischemic injury, two etiologies excluded from our earlier studies [30].
There are also a number of cases in which there was an apparent mismatch between the severity of pathology identified and the cognitive status (Fig. 1 and Table 1). 122 cognitively intact patients had intermediate or high level ADNC (36% of CDR = 0 patients with ADNC data available), 70 had limbic or neocortical LBD (10.8%), 15 had stage 2 or 3 LATE-NC (8.5%), 4 had FTLD-TDP (1.5%), and 2 had Pick disease (0.2%), among other pathologies. Perhaps most interestingly, only 17.3% had no significant neuropathologic findings, while 42.8% had 2 or more, and 1 subject had 5 pathologies (high level ADNC, LBD stage 2, LATE-NC stage 2, hippocampal sclerosis, and CVD). These data suggest that a subset of these cases are individuals who are resilient against one or more pathologies, a population which warrants additional study as there may be underlying biological differences that are unassessed with routine neuropathologic diagnosis [92]. There are also rare cases with a CDR score of 2–3 that lack any significant identified neuropathologic diagnoses. These cases may represent subjects with underlying pathologies that were unassessed due to previous versions of the NACC NP dataset, subjects with very low levels of multiple different pathologies adding up to produce a cognitive effect (i.e., low level ADNC in combination with LATE-NC stage 1, LBD stage 1, and/or relatively mild cerebrovascular changes), pathologic findings that do not fit into one or more of the designated NACC categories, or subjects with unspecified/undocumented genetic alterations [22]. Similar to previous studies [10, 66], between 42.2% and 58.4% of the variance in cognitive impairment was accounted for by the most common neuropathological findings (ADNC, LATE-NC, LBD, and CVD) (Supplemental Fig. 2). This suggests that the development of successful therapies with the capacity to remove or prevent any of these pathologies would remove a significant portion of the dementia burden from a given population. For example, successful treatment of CVD could eliminate up to 10% of cognitive impairment from the population as a whole, while a successful treatment of ADNC could potentially eliminate 30% of cognitive impairment [14].
There are a number of limitations associated with this study. While the study is based on a large patient population (total n = 6,262 subjects), all subjects are drawn from the NACC dataset, which is not necessarily representative of the population at large [69]. The NACC dataset is enriched for subjects with Caucasian ancestry, high levels of education, rare diseases/pathologies, more frequent APOE ε4 alleles, more severe dementia, and more severe neuropathologic findings, which may be related to population-specific selection and recruitment biases, including enrolling a higher number of patients with existing dementia compared to cognitively normal individuals [24, 86]. The variables included in both the clinical and neuropathological datasets have also undergone numerous revisions, and autopsy data on TDP-43 and Thal phase were not included until relatively recently with the NACC NP dataset version 10 [13]. The provided data for many variables include only the presence of regional pathology and in some cases the general distribution without severity/density/burden of pathology, making distinction between FTLD-TDP and LATE-NC difficult in some instances [55]. Most variables do not take into account bilateral pathologic features, which may be important as pathologic asymmetry may provide a source of cognitive reserve or resilience against certain pathologies, and may result in deficits to specific cognitive/neuropsychological domains [41, 49, 65, 77, 89]. While this study may not be fully representative of the relationship between mixed pathologies and cognition in the population at large as a result of these limitations, the methods employed here may serve as a framework which can be applied in additional clinic- and community-based cohorts to better elucidate the relative effects of each of these neurodegenerative processes individually and in combination.
In the context of the existing literature, our findings are consistent with the hypothesis that the additive effects of multiple pathologies may be responsible for a large portion of cognitive impairment experienced by elderly subjects. These results suggest that ADNC is the most common and most consistent factor affecting all cognitive domains, while others (including LATE-NC, LBD, and CVD) are more selective in their cognitive effects, and some frontotemporal dementias (FTLD-TDP, Pick disease, PSP, CBD) may have greater effects in some specific cognitive domains than ADNC. Given current trend toward developing personalized therapies and treatments designed to target specific protein aggregates and neuronal subtypes and populations, there is a critical need for the development of in vivo biomarkers that can accurately distinguish between neuropathologic processes (and progression/severity within processes), as well as distinguish which processes underlie specific cognitive symptoms, and which are modifiable [85]. The data presented in this report offer a step toward determining the relative effects of many of these disease processes and how they may interact, which is critical for accurate clinical diagnosis, as well as biomarker and drug development.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD).
Authors contribution
Conception of the work: J.M.W. and T.E.R.; Design of the work: C.M.-D., S.H., J.M.W., and T.E.R.; Acquisition/analysis/interpretation of the data: C.M.-D., S.H., R.T.Y., K.F., G.A.M., J.K., E.V.D., M.M.G., A.S.P., L.C., L.S.K.M., T.E.R.; Drafted the work or substantially revised it: C.M.-D., S.H., E.V.D., C.L.W., J.F.C., J.M.W., T.E.R.; All authors have reviewed and approved of the final draft.
Funding
J.M.W. and T.E.R. are supported in part by National Institute on Aging (NIA) R21 AG078505 and Texas Alzheimer’s Research and Care Consortium (TARCC) grants 957581 and 957607. E.V.D. is also supported in part by TARCC grant 957607. M.M.G. is supported in part by NIA R01 AG077472. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data availability
The data presented in this manuscript are derived from the National Alzheimer’s Coordinating Center (NACC) dataset, and are available upon request from https://naccdata.org/.
Declarations
Conflict of interest
The authors have no conflict of interest to report.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carolina Maldonado-Díaz and Satomi Hiya have contributed equally to this work and are co-first authors.
References
- 1.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology. 2018;90:695–703. doi: 10.1212/WNL.0000000000005303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arevalo-Rodriguez I, Smailagic N, Roque-Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI) Cochrane Database Syst Rev. 2021;7:CD010783. doi: 10.1002/14651858.CD010783.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beach TG, Malek-Ahmadi M. Alzheimer's disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis. 2021;79:389–400. doi: 10.3233/JAD-201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimers Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 5.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA. The National Alzheimer’s Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimers Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 6.Bell WR, An Y, Kageyama Y, English C, Rudow GL, Pletnikova O, Thambisetty M, O'Brien R, Moghekar AR, Albert MS, et al. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer’s disease. Alzheimers Dement. 2019;15:8–16. doi: 10.1016/j.jalz.2018.07.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besser LM, Crary JF, Mock C, Kukull WA. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology. 2017;89:1707–1715. doi: 10.1212/WNL.0000000000004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besser LM, Mock C, Teylan MA, Hassenstab J, Kukull WA, Crary JF. Differences in cognitive impairment in primary age-related tauopathy versus Alzheimer disease. J Neuropathol Exp Neurol. 2019;78:219–228. doi: 10.1093/jnen/nly132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besser LM, Teylan MA, Nelson PT. Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological associations. J Neuropathol Exp Neurol. 2020;79:305–313. doi: 10.1093/jnen/nlz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle PA, Wang T, Yu L, Wilson RS, Dawe R, Arfanakis K, Schneider JA, Bennett DA. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144:2166–2175. doi: 10.1093/brain/awab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83:74–83. doi: 10.1002/ana.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Butler Pagnotti RM, Pudumjee SB, Cross CL, Miller JB. Cognitive and clinical characteristics of patients with limbic-predominant age-related TDP-43 encephalopathy. Neurology. 2023;100:e2027–e2035. doi: 10.1212/WNL.0000000000207159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cholerton B, Latimer CS, Crane PK, Corrada MM, Gibbons LE, Larson EB, Kawas CH, Keene CD, Montine TJ. Neuropathologic Burden and Dementia in Nonagenarians and Centenarians. Neurology. 2024;102:e208060. doi: 10.1212/WNL.0000000000208060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crary JF. Primary age-related tauopathy and the amyloid cascade hypothesis: the exception that proves the rule? J Neurol Neuromedicine. 2016;1:53–57. doi: 10.29245/2572.942x/2016/6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cykowski MD, Arumanayagam AS, Powell SZ, Rivera AL, Abner EL, Roman GC, Masdeu JC, Nelson PT. Patterns of amygdala region pathology in LATE-NC: subtypes that differ with regard to TDP-43 histopathology, genetic risk factors, and comorbid pathologies. Acta Neuropathol. 2022;143:531–545. doi: 10.1007/s00401-022-02416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cykowski MD, Takei H, Van Eldik LJ, Schmitt FA, Jicha GA, Powell SZ, Nelson PT. Hippocampal sclerosis but not normal aging or Alzheimer disease is associated with TDP-43 pathology in the Basal forebrain of aged persons. J Neuropathol Exp Neurol. 2016;75:397–407. doi: 10.1093/jnen/nlw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Flores R, Wisse LEM, Das SR, Xie L, McMillan CT, Trojanowski JQ, Robinson JL, Grossman M, Lee E, Irwin DJ, et al. Contribution of mixed pathology to medial temporal lobe atrophy in Alzheimer’s disease. Alzheimers Dement. 2020;16:843–852. doi: 10.1002/alz.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Forrest SL, Kovacs GG. Current concepts of mixed pathologies in neurodegenerative diseases. Can J Neurol Sci. 2023;50:329–345. doi: 10.1017/cjn.2022.34. [DOI] [PubMed] [Google Scholar]
- 23.Gauthreaux K, Bonnett TA, Besser LM, Brenowitz WD, Teylan M, Mock C, Chen YC, Chan KCG, Keene CD, Zhou XH, et al. Concordance of clinical Alzheimer diagnosis and neuropathological features at autopsy. J Neuropathol Exp Neurol. 2020;79:465–473. doi: 10.1093/jnen/nlaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthreaux K, Kukull WA, Nelson KB, Mock C, Chen YC, Chan KCG, Fardo DW, Katsumata Y, Abner EL, Nelson PT. Different cohort, disparate results: Selection bias is a key factor in autopsy cohorts. Alzheimers Dement. 2023 doi: 10.1002/alz.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthreaux K, Mock C, Teylan MA, Culhane JE, Chen YC, Chan KCG, Katsumata Y, Nelson PT, Kukull WA. Symptomatic profile and cognitive performance in autopsy-confirmed limbic-predominant age-related TDP-43 encephalopathy with comorbid Alzheimer disease. J Neuropathol Exp Neurol. 2022;81:975–987. doi: 10.1093/jnen/nlac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthreaux KM, Teylan MA, Katsumata Y, Mock C, Culhane JE, Chen YC, Chan KCG, Fardo DW, Dugan AJ, Cykowski MD, et al. Limbic-predominant age-related TDP-43 encephalopathy: medical and pathologic factors associated with comorbid hippocampal sclerosis. Neurology. 2022;98:e1422–e1433. doi: 10.1212/WNL.0000000000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassenstab J, Monsell SE, Mock C, Roe CM, Cairns NJ, Morris JC, Kukull W. Neuropsychological markers of cognitive decline in persons with Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2015;74:1086–1092. doi: 10.1097/NEN.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayden KM, Jones RN, Zimmer C, Plassman BL, Browndyke JN, Pieper C, Warren LH, Welsh-Bohmer KA. Factor structure of the National Alzheimer’s Coordinating Centers uniform dataset neuropsychological battery: an evaluation of invariance between and within groups over time. Alzheimer Dis Assoc Disord. 2011;25:128–137. doi: 10.1097/WAD.0b013e3181ffa76d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 30.Hiya S, Maldonado-Diaz C, Walker JM, Richardson TE. Cognitive symptoms progress with limbic-predominant age-related TDP-43 encephalopathy stage and co-occurrence with Alzheimer disease. J Neuropathol Exp Neurol. 2024;83:2–10. doi: 10.1093/jnen/nlad098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida MA, Farrell K, Walker JM, Richardson TE, Marx GA, Bryce CH, Purohit D, Ayalon G, Beach TG, Bigio EH, et al. Predictors of cognitive impairment in primary age-related tauopathy: an autopsy study. Acta Neuropathol Commun. 2021;9:134. doi: 10.1186/s40478-021-01233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139:2983–2993. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, Dickson DW, Hof PR, Hyman BT, Jack CR. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol. 2015;129:757–762. doi: 10.1007/s00401-015-1407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM, Weigand SD, Boeve BF, Kantarci K, Petrucelli L, et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART) Acta Neuropathol. 2017;133:705–715. doi: 10.1007/s00401-017-1681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–186. doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karanth S, Nelson PT, Katsumata Y, Kryscio RJ, Schmitt FA, Fardo DW, Cykowski MD, Jicha GA, Van Eldik LJ, Abner EL. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 2020;77:1299–1307. doi: 10.1001/jamaneurol.2020.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsumata Y, Fardo DW, Kukull WA, Nelson PT. Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer’s disease and cerebrovascular disease pathologies. Acta Neuropathol Commun. 2018;6:142. doi: 10.1186/s40478-018-0641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim G, Vahedi S, Gefen T, Weintraub S, Bigio EH, Mesulam MM, Geula C. Asymmetric TDP pathology in primary progressive aphasia with right hemisphere language dominance. Neurology. 2018;90:e396–e403. doi: 10.1212/WNL.0000000000004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiby AC, Scambray KA, Nguyen HL, Basith F, Fakhraee S, Melikyan ZA, Bukhari SA, Montine TJ, Corrada MM, Kawas CH, et al. Characterizing limbic-predominant age-related TDP-43 encephalopathy without Alzheimer’s disease and lewy body dementia in the oldest old: a case series. J Alzheimers Dis. 2023 doi: 10.3233/JAD-230238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marx GA, Koenigsberg DG, McKenzie AT, Kauffman J, Hanson RW, Whitney K, Signaevsky M, Prastawa M, Iida MA, White CL. Artificial intelligence-derived neurofibrillary tangle burden is associated with antemortem cognitive impairment. Acta Neuropathol Commun. 2022;10:157. doi: 10.1186/s40478-022-01457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAleese KE, Colloby SJ, Thomas AJ, Al-Sarraj S, Ansorge O, Neal J, Roncaroli F, Love S, Francis PT, Attems J. Concomitant neurodegenerative pathologies contribute to the transition from mild cognitive impairment to dementia. Alzheimers Dement. 2021 doi: 10.1002/alz.12291. [DOI] [PubMed] [Google Scholar]
- 46.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol. 2017;27:472–479. doi: 10.1111/bpa.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie AT, Marx GA, Koenigsberg D, Sawyer M, Iida MA, Walker JM, Richardson TE, Campanella G, Attems J, McKee AC, et al. Interpretable deep learning of myelin histopathology in age-related cognitive impairment. Acta Neuropathol Commun. 2022;10:131. doi: 10.1186/s40478-022-01425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montine KS, Bersonm E, Phongpreecha T, Huang Z, Aghaeepour N, Zou JY, MacCoss MJ, Montine TJ. Understanding the molecular basis of resilience to Alzheimer’s disease. Front Neurosci. 2023;17:1311157. doi: 10.3389/fnins.2023.1311157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montine TJ, Cholerton BA, Corrada MM, Edland SD, Flanagan ME, Hemmy LS, Kawas CH, White LR. Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther. 2019;11:22. doi: 10.1186/s13195-019-0479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142:1503–1527. doi: 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, Cooper G, Smith CD, Markesbery WR. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127–1133. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson PT, Lee EB, Cykowski MD, Alafuzoff I, Arfanakis K, Attems J, Brayne C, Corrada MM, Dugger BN, Flanagan ME, et al. LATE-NC staging in routine neuropathologic diagnosis: an update. Acta Neuropathol. 2023;145:159–173. doi: 10.1007/s00401-022-02524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nichols E, Merrick R, Hay SI, Himali D, Himali JJ, Hunter S, Keage HAD, Latimer CS, Scott MR, Steinmetz JD, et al. The prevalence, correlation, and co-occurrence of neuropathology in old age: harmonisation of 12 measures across six community-based autopsy studies of dementia. Lancet Healthy Longev. 2023;4:e115–e125. doi: 10.1016/S2666-7568(23)00019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(63–75):e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Rabinovici GD, Carrillo MC, Forman M, DeSanti S, MIller DS, Kozauer N, Petersen RC, Randolph C, Knopman DS, Smith EE, , et al. Multiple comorbid neuropathologies in the setting of Alzheimer's disease neuropathology and implications for drug development. Alzheimers Dement (N Y) 2016;3:83–91. doi: 10.1016/j.trci.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6:82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX, Lee VM, Kawas CH, Trojanowski JQ. Non-Alzheimer's contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol. 2018;136:377–388. doi: 10.1007/s00401-018-1872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. doi: 10.1093/brain/awy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson JL, Richardson H, Xie SX, Suh E, Van Deerlin VM, Alfaro B, Loh N, Porras-Paniagua M, Nirschl JJ, Wolk D, et al. The development and convergence of co-pathologies in Alzheimer's disease. Brain. 2021;144:953–962. doi: 10.1093/brain/awaa438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rockwood K, Strang D, MacKnight C, Downer R, Morris JC. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J Am Geriatr Soc. 2000;48:558–559. doi: 10.1111/j.1532-5415.2000.tb05004.x. [DOI] [PubMed] [Google Scholar]
- 65.Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, Mesulam MM. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology. 2014;83:1184–1191. doi: 10.1212/WNL.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saltiel N, Tripodis Y, Menzin T, Olaniyan A, Baucom Z, Yhang E, Palmisano JN, Martin B, Uretsky M, Nair E, et al. Relative contributions of mixed pathologies to cognitive and functional symptoms in brain donors exposed to repetitive head impacts. Ann Neurol. 2023 doi: 10.1002/ana.26823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scambray KA, Nguyen HL, Sajjadi SA. Association of vascular and degenerative brain pathologies and past medical history from the National Alzheimer's Coordinating Center Database. J Neuropathol Exp Neurol. 2023;82:390–401. doi: 10.1093/jnen/nlad020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 71.Sennik S, Schweizer TA, Fischer CE, Munoz DG. Risk factors and pathological substrates associated with agitation/aggression in Alzheimer's disease: a preliminary study using NACC data. J Alzheimers Dis. 2017;55:1519–1528. doi: 10.3233/JAD-160780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serrano-Pozo A, Qian J, Muzikansky A, Monsell SE, Montine TJ, Frosch MP, Betensky RA, Hyman BT. Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the alzheimer disease continuum. J Neuropathol Exp Neurol. 2016;75:516–526. doi: 10.1093/jnen/nlw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, Atri A. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther. 2011;3:32. doi: 10.1186/alzrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smirnov DS, Salmon DP, Galasko D, Edland SD, Pizzo DP, Goodwill V, Hiniker A. TDP-43 Pathology exacerbates cognitive decline in primary age-related tauopathy. Ann Neurol. 2022;92:425–438. doi: 10.1002/ana.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spina S, La Joie R, Petersen C, Nolan AL, Cuevas D, Cosme C, Hepker M, Hwang JH, Miller ZA, Huang EJ, et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer's disease. Brain. 2021;144:2186–2198. doi: 10.1093/brain/awab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spires-Jones TL, Attems J, Thal DR. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017;134:187–205. doi: 10.1007/s00401-017-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefanits H, Budka H, Kovacs GG. Asymmetry of neurodegenerative disease-related pathologies: a cautionary note. Acta Neuropathol. 2012;123:449–452. doi: 10.1007/s00401-011-0936-6. [DOI] [PubMed] [Google Scholar]
- 78.Stern Y, Albert M, Barnes CA, Cabeza R, Pascual-Leone A, Rapp PR. A framework for concepts of reserve and resilience in aging. Neurobiol Aging. 2023;124:100–103. doi: 10.1016/j.neurobiolaging.2022.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teylan M, Besser LM, Crary JF, Mock C, Gauthreaux K, Thomas NM, Chen YC, Kukull WA. Clinical diagnoses among individuals with primary age-related tauopathy versus Alzheimer's neuropathology. Lab Invest. 2019;99:1049–1055. doi: 10.1038/s41374-019-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teylan M, Mock C, Gauthreaux K, Chen YC, Chan KCG, Hassenstab J, Besser LM, Kukull WA, Crary JF. Cognitive trajectory in mild cognitive impairment due to primary age-related tauopathy. Brain. 2020;143:611–621. doi: 10.1093/brain/awz403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teylan MA, Mock C, Gauthreaux K, Culhane JE, Jicha G, Chen YC, Chan KCG, Kukull WA, Nelson PT, Katsumata Y. Differences in symptomatic presentation and cognitive performance among participants with LATE-NC compared to FTLD-TDP. J Neuropathol Exp Neurol. 2021;80:1024–1032. doi: 10.1093/jnen/nlab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 83.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/Wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 84.Thomas DX, Bajaj S, McRae-McKee K, Hadjichrysanthou C, Anderson RM, Collinge J. Association of TDP-43 proteinopathy, cerebral amyloid angiopathy, and Lewy bodies with cognitive impairment in individuals with or without Alzheimer's disease neuropathology. Sci Rep. 2020;10:14579. doi: 10.1038/s41598-020-71305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tome SO, Thal DR. Co-pathologies in Alzheimer's disease: just multiple pathologies or partners in crime? Brain. 2021;144:706–708. doi: 10.1093/brain/awab027. [DOI] [PubMed] [Google Scholar]
- 86.Tsuang D, Simpson KL, Li G, Barnhart RL, Edland SD, Bowen J, McCormick W, Teri L, Nochlin D, Larson EB, et al. Evaluation of selection bias in an incident-based dementia autopsy case series. Alzheimer Dis Assoc Disord. 2005;19:67–73. doi: 10.1097/01.wad.0000165507.67993.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 88.Wada-Isoe K, Kikuchi T, Umeda-Kameyama Y, Mori T, Akishita M, Nakamura Y. Global clinical dementia rating score of 0.5 may not be an accurate criterion to identify individuals with mild cognitive impairment. J Alzheimers Dis Rep. 2019;3:233–239. doi: 10.3233/ADR-190126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker JM, Fudym Y, Farrell K, Iida MA, Bieniek KF, Seshadri S, White CL, Crary JF, Richardson TE. Asymmetry of hippocampal tau pathology in primary age-related tauopathy and alzheimer disease. J Neuropathol Exp Neurol. 2021;80:436–445. doi: 10.1093/jnen/nlab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker JM, Goette W, Farrell K, Iida MA, Karlovich E, Part Working G, White CL, III, Crary JF, Richardson TE. The relationship between hippocampal β-amyloid burden and spatial distribution of neurofibrillary degeneration. Alzheimers Dement. 2023 doi: 10.1002/alz.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walker JM, Gonzales MM, Goette W, Farrell K, White Iii CL, Crary JF, Richardson TE. Cognitive and neuropsychological profiles in Alzheimer's disease and primary age-related tauopathy and the influence of comorbid neuropathologies. J Alzheimers Dis. 2023;92:1037–1049. doi: 10.3233/JAD-230022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker JM, Kazempour Dehkordi S, Fracassi A, Vanschoiack A, Pavenko A, Taglialatela G, Woltjer R, Richardson TE, Zare H, Orr ME. Differential protein expression in the hippocampi of resilient individuals identified by digital spatial profiling. Acta Neuropathol Commun. 2022;10:23. doi: 10.1186/s40478-022-01324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker JM, Kazempour Dehkordi S, Schaffert J, Goette W, White CL, Richardson TE, Zare H. The spectrum of Alzheimer-type pathology in cognitively normal individuals. J Alzheimers Dis. 2023;91:683–695. doi: 10.3233/JAD-220898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker JM, Orr ME, Orr TC, Thorn EL, Christie TD, Yokoda RT, Vij M, Ehrenberg AJ, Marx GA, McKenzie AT, et al. Spatial proteomics of hippocampal subfield-specific pathology in Alzheimer's disease and primary age-related tauopathy. Alzheimers Dement. 2023 doi: 10.1002/alz.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker JM, Richardson TE. Cognitive resistance to and resilience against multiple comorbid neurodegenerative pathologies and the impact of APOE status. J Neuropathol Exp Neurol. 2023;82:110–119. doi: 10.1093/jnen/nlac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walker JM, Richardson TE, Farrell K, Iida MA, Foong C, Shang P, Attems J, Ayalon G, Beach TG, Bigio EH, et al. Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol. 2021;80:102–111. doi: 10.1093/jnen/nlaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walker JM, Richardson TE, Farrell K, White Iii CL, Crary JF. The frequency of cerebral amyloid angiopathy in primary age-related tauopathy. J Neuropathol Exp Neurol. 2022;81:246–248. doi: 10.1093/jnen/nlab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, Uyehara-Lock JH, Gelber RP, Ross GW, Petrovitch H, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86:1000–1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wimo A, Seeher K, Cataldi R, Cyhlarova E, Dielemann JL, Frisell O, Guerchet M, Jonsson L, Malaha AK, Nichols E, et al. The worldwide costs of dementia in 2019. Alzheimers Dement. 2023;19:2865–2873. doi: 10.1002/alz.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this manuscript are derived from the National Alzheimer’s Coordinating Center (NACC) dataset, and are available upon request from https://naccdata.org/.