Abstract
Introduction
Respiratory syncytial virus (RSV) represents a considerable burden on the healthcare system and hospital resources. This study explored the impact of universal immunoprophylaxis with long-acting monoclonal antibody (nirsevimab) during infants’ first RSV season on RSV-induced health events and related costs in the Kingdom of Saudi Arabia (KSA).
Methods
The burden of RSV-induced health events and related costs under the current standard of practice (SoP) and the impact of universal immunoprophylaxis with nirsevimab was estimated using a static decision-analytic model in a cohort of infants experiencing their first RSV season in the KSA. The model estimated hospital admissions (including pediatric intensive care unit [PICU] admissions and mechanical ventilation [MV]), emergency room (ER) visits, primary care (PC) visits, long-term sequelae, and RSV mortality.
Results
The model estimated that under the current SoP, RSV results in 17,179–19,607 hospitalizations (including 2932–3625 PICU and 172–525 MV admissions), 57,654–191,115 ER visits, 219,053–219,970 PC visits, 14 deaths, 12,884–14,705 cases of recurrent wheezing, and a total cost of SAR 480–619 million. Universal nirsevimab immunoprophylaxis was estimated to avert 58% of hospitalizations (58% PICU admissions, 58% MV episodes), 53% of ER visits, 53% of PC visits, 58% of episodes of recurrent wheezing, 8 deaths, and result in savings of SAR 274–343 million in total healthcare cost.
Conclusion
Compared with current SoP, an nirsevimab immunoprophylaxis strategy in the KSA for all infants during their first RSV season was estimated to dramatically decrease healthcare resource use, and economic burden associated with RSV.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02798-w.
Keywords: Hospitalization, Immunization, Infant, Nirsevimab, Public health, Respiratory syncytial viruses, Respiratory tract infections, Kingdom of Saudi Arabia, Model, RSV
Key Summary Points
| Why carry out the study? |
| Respiratory syncytial virus (RSV) is associated with substantial use of inpatient and outpatient resources in the Kingdom of Saudi Arabia (KSA). |
| Nirsevimab, a long-acting monoclonal antibody indicated for the prevention of RSV lower respiratory tract disease in neonates and infants during their first RSV season, was compared with the current standard of practice (SoP) in the entire birth cohort of the KSA over its first RSV season. |
| What was learned from the study? |
| Nirsevimab use in all infants at the beginning and during the RSV season was estimated to substantially reduce the RSV-related clinical and economic burden, compared with the current SoP. |
| Implementation of a universal nirsevimab immunoprophylaxis strategy in the KSA could prevent thousands of RSV-related health events and save considerable costs. |
Introduction
Respiratory syncytial virus (RSV) is responsible for various respiratory illnesses, particularly lower track respiratory disease (LRTD), and is the primary cause of bronchiolitis and pneumonia. While individuals of all ages can be infected, RSV infection in infants aged < 12 months is more likely to be severe and fatal. In 2015, an estimated 33.1 million cases of RSV infection occurred worldwide in children aged < 5 years; of these, approximately 3.2 million required hospitalization, and approximately 59,600 cases resulted in death. Children aged < 6 months accounted for approximately 45% of the severe outcomes of RSV-induced LRTD (i.e., hospitalization and death) [1].
RSV is a seasonal virus, and its transmission and seasonality are driven by geographical location and climate. In the Kingdom of Saudi Arabia (KSA), the circulation of the RSV virus is mostly confined to the winter season (from September to March) with a peak in December and January [2]. From 2001 to 2015, approximately 23% of acute respiratory illnesses in the pediatric population in the KSA were attributable to RSV infection, with children aged < 1 year representing the majority of cases [3]. In a recent study of children aged < 6 years of age (median 5 months) in the KSA hospitalized with bronchiolitis, 53% and 64% of general ward admissions and pediatric intensive care unit (PICU) admissions, respectively, were attributable to RSV infection, as determined by the multiplex polymerase chain reaction test. These estimates are in line with those seen in other countries such as Spain [4]. In addition to high clinical burden, RSV infections are associated with a significant economic burden. Globally, it has been estimated that the total direct medical cost associated with acute medically attended lower respiratory tract disease (MA-LRTD) amount to almost €5 billion, with just over half of the total cost being spent on hospitalizations [5].
Although the risk of severe illness is the highest among infants born prematurely and in those with underlying conditions [6], most infants hospitalized for RSV infection were born full-term and/or are otherwise healthy [2]. Therefore, the implementation of an immunoprophylaxis strategy aiming to protect all infants during their first RSV season is being considered in the KSA.
Currently, in the KSA there are no prevention options available for children with an RSV infection who are otherwise healthy [1]. Palivizumab, a monoclonal antibody targeting RSV F glycoprotein, is the only available prophylaxis product; however, it has only been studied in and its use is restricted to high-risk children with underlying conditions. According to the guidelines from Saudi Initiative of Bronchiolitis Diagnosis, Management, and Prevention (SIBRO), palivizumab should be used between the beginning of October and the end of March in the following groups: (a) children born prematurely at 29–35 weeks gestational age (wGA) or < 29 wGA; aged 6 months or 12 months, respectively, at the start of the RSV season who are at a high risk of respiratory sequelae, (b) high-risk children aged < 2 years born prematurely at ≤ 35 wGA who required treatment for bronchopulmonary dysplasia within the prior 6 months; and (c) children aged < 2 years with congenital heart defects (CHD), or with cystic fibrosis with manifestations of severe lung disease, or requiring treatments that adversely affect respiratory function, or who are immunocompromised [1].
Children born full-term and those born prematurely at > 35 wGA are not eligible to receive palivizumab; the current standard of practice (SoP) for these children only involves supportive care, including respiratory support and hydration maintenance.
Nirsevimab, a neutralizing monoclonal antibody targeting the RSV F protein, was developed as a novel prophylactic option for the protection of infants against RSV-induced MA-LRTD. Nirsevimab has an extended half-life, and a rapid onset of protection, allowing for at least 5 months of protection after a single dose.
The safety and efficacy of nirsevimab have been evaluated in studies involving large samples of infants, including preterm and term infants as well as infants who are at a high risk of severe disease and otherwise healthy children. Given the results of these trials, the European Medicines Agency (EMA) [7] and Medical and Healthcare Regulations Agency authorized the use of nirsevimab in newborns and infants in their first RSV season for the prevention of RSV-induced LRTD. In July 2023 the US Food and Drug Administration (FDA) approved nirsevimab in an extended indication, including children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season [8], and the Centers for Disease Control and Prevention (CDC) recommended its use in all infants according to the US FDA label. At the time of the study, nirsevimab was not yet approved by the Saudi Food and Drug Authority (SFDA).
This study aimed to estimate the public health and economic impact of a universal immunoprophylaxis strategy with nirsevimab to protect all infants in their first RSV season versus SoP in the KSA.
Methods
Model Overview
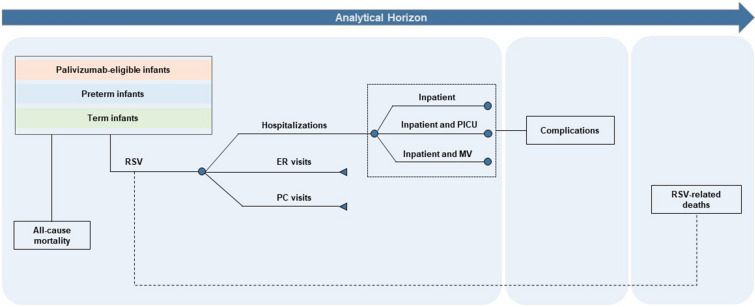
A decision analytic model (Fig. 1) was used to track infants in the KSA during their first RSV season. The model stratified infants by their month of birth and estimated health and cost outcomes for the first RSV season. Demography, seasonality, epidemiology, health event risk, prevention effectiveness, and coverage rate data derived from published literature, publicly available sources, and expert clinical opinion were used to inform the model and assess the impact of the prophylaxis measures evaluated.
Fig. 1.
Model structure. ER emergency room, LRTD lower respiratory tract disease, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit, RSV respiratory syncytial virus
Seasonality was based on Osman et al. [2], a retrospective cohort study conducted between 2016 and 2021 in a tertiary hospital in the KSA, that defined RSV seasonality in the KSA as the period from October to February. The model also considered low levels of RSV circulation beyond the RSV specific season (between March and September). All infants enter the model as susceptible to an RSV infection, with the risk of MA-LRTD varied by the age at infection, subpopulation, and the RSV circulation density.
Target Population and Immunoprophylaxis Strategies
Following clinical trials investigating nirsevimab, the infant population in the model was stratified into the following three mutually exclusive subpopulations because the risk of RSV-induced MA-LRTDs varies across these subpopulations: (a) palivizumab-eligible infants (i.e., infants born at < 35 wGA and infants with chronic lung disease (CLD) of prematurity or CHD); (b) infants born preterm between 35 wGA and 36 weeks and 6 days gestational age who are not eligible for palivizumab; and (c) infants born full-term or ≥ 37 wGA who are not eligible for palivizumab.
The model performed a pairwise comparison of two immunoprophylaxis strategies to estimate the impact of nirsevimab. The first strategy included the current SoP for each subpopulation, where infants not eligible for palivizumab did not receive prophylaxis, while palivizumab-eligible infants received one dose of palivizumab monthly throughout the RSV season for a maximum of five doses. In the second strategy, all infants were immunized with a single dose of nirsevimab either at birth or during the start of the RSV season. Infants born out of the RSV season (OoS; from March to September) received nirsevimab at the beginning of the RSV season, while those born within the season (WiS; from October to February) received nirsevimab at birth. The economic impact of these scenarios was estimated on the basis of the number of RSV-induced MA-LRTD cases and the associated healthcare costs.
The analysis was based on a payer perspective and estimated the following outcomes: inpatient hospital admissions, PICU admissions, mechanical ventilation (MV), emergency room (ER) visits, primary care (PC) visits, RSV-related deaths, complications, and the associated costs. A time horizon capturing the infants’ first RSV season was used in the analysis, except for complications (recurrent wheezing) and mortality, which were analyzed over a 3-year and lifetime horizon, respectively. This time horizon was selected to capture the typical RSV infection-associated costs and resource use [9].
Model Inputs
The model was parameterized using two approaches, based on published studies or expert clinical opinion. As a result of limited local data, a panel of eight experts in RSV disease, epidemiology, and health economics were consulted through one-to-one interviews with each expert followed by a consensus meeting with the whole panel to confirm the validity of the input data related to population, seasonality, coverage, risk of health events, and costs. The panel members are members of the KSA Pediatric Bronchiolitis Association, and they represented the majority of KSA regions and healthcare systems. At least one expert from each type of healthcare system in the KSA was interviewed (public institutions, university hospitals, private hospitals). Model parameters and values applied in the analysis are summarized in Table 1.
Table 1.
Model Inputs
| Input | Palivizumab-eligible infants | Preterm infants | Term infants |
|---|---|---|---|
| Demographic and epidemiologic inputs | |||
| Annual births | World Bank data—Saudi Arabia [10] | ||
| Proportion of births by month, % | 8.33 | ||
| Population size, n (%) | 10,183 (2.0) | 20,366 (4.0) | 478,590 (94.0) |
| Immunoprophylaxis period | October to February | ||
| Inpatient hospitalizationsa, % | 8.9 [12, 14, 15]–14.5* | 7.6 [18]–14.5* | 3.0*–3.8 [17] |
| PICU (conditional to inpatient hospitalization), % | 16.1 [2] | 16.1 [2] | 16.1 [2] |
| MV (conditional to PICU), % | 1.0*–2.7 [18] | 1.0*–2.7 [18] | 1.0*–2.7 [18] |
| ER visitsa, % | 12.2 [19]–43.4* | 12.2 [19]–43.4* | 12.2 [19]–43.4* |
| PC visits, % | 45.9 [19]–50.0* | 45.9 [19]–50.0* | 45.9 [19]–50.0* |
| Recurrent wheezing (year 1), % | 31.0 [28] | 31.0 [28] | 31.0 [28] |
| Recurrent wheezing (year 2), % | 27.0 [28] | 27.0 [28] | 27.0 [28] |
| Recurrent wheezing (year 3), % | 17.0 [28] | 17.0 [28] | 17.0 [28] |
| RSV-related mortality |
0.000042 (0–5 months) [29] 0.000021 (6–11 months) [29] |
||
| Nirsevimab profile | |||
| Efficacy—inpatient, % | 51.0 [27] | 83.2 [20] | 83.2 [20] |
| Efficacy—outpatient, % | 51.0 [27] | 86.2 [21] | 74.5 [22] |
| Duration of protection | 5 months | 5 months | 5 months |
| Coverage rate, % | 90.0 | 90.0 | 90.0 |
| Palivizumab profile | |||
| Efficacy—inpatient, % | 51.0 [27] | – | – |
| Efficacy—outpatient, % | 51.0 [27] | – | – |
| Duration of protection | 1 month | – | – |
| Coverage rate, % | 55.0 | – | – |
| Costs of health events | |||
| Inpatient hospitalizationsb | SAR 6649 | SAR 6649 | SAR 6649 |
| PICUc | SAR 63,065 | SAR 63,065 | SAR 63,065 |
| MVd | SAR 67,890 | SAR 67,890 | SAR 67,890 |
| ER visitse | SAR 1500 | SAR 1500 | SAR 1500 |
| PC visitse | SAR 180 | SAR 180 | SAR 180 |
| Recurrent wheezing year 1f | SAR 990 | SAR 990 | SAR 990 |
| Recurrent wheezing year 2g | SAR 961 | SAR 961 | SAR 961 |
| Recurrent wheezing year 3g | SAR 933 | SAR 933 | SAR 933 |
ER emergency room, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit
*Denotes inputs based on local expert opinion
aValues described in the table are an average of the risk of health events by age at infection in months that are provided in Supplementary Material
bWeighted average of MOH and NGHA cost estimates (70% MOH, 30% NGHA Business Center). To estimate the cost of hospitalization per patient, unit cost of hospitalization per day was multiplied by 5. NGHA costs were used as a proxy for all other health institutions in the KSA
cWeighted average of Medical Cities and NGHA cost estimates (70% Medical Cities, 30% NGHA Business Center). To estimate the cost of PICU per patient, unit cost of PICU per day was multiplied by 10. NGHA costs were used as a proxy for all other health institutions in the KSA
dWeighted average of MOH and NGHA cost estimates (70% MOH, 30% NGHA Business Center). To estimate the cost of MV per patient, unit cost of MV per day was multiplied by 5. NGHA costs were used as a proxy for all other health institutions in the KSA
eWeighted average of MOH and NGHA cost estimates (70% MOH, 30% NGHA Business Center). NGHA costs were used as a proxy for all other health institutions in the KSA
fEstimated as 5.5 × unit price of PC visit
gCost of recurrent wheezing in years 2 and 3 was discounted, using 3% discount rate
The total number of annual births was informed by the World Bank database and distributed equally by month across the year [10].
As a result of limited published literature on the guidelines for palivizumab use in the KSA, the size of the three model subpopulations was defined on the basis of expert opinion and the published data. To estimate the proportion of infants eligible to receive palivizumab, palivizumab sales data in 2021 and estimated coverage rate were used. The proportion of infants born preterm was determined on the basis of KSA Ministry of Health [11] data and calculated as a proportion of all preterm infants born in the KSA minus the estimated proportion of palivizumab-eligible infants. Infants not belonging to either of the aforementioned subpopulations were included in the term population, which was calculated as the remaining distribution of infants (not preterm or eligible for palivizumab).
As younger infants are typically at a higher risk of severe RSV requiring hospitalization, the inputs related to the disease burden were differentiated by age (in months). As a result of limited availability of published local data, two sources of disease burden inputs were used in the model. The first source was a consensus of local experts (described above). The experts provided an overall rate of events during the first year of infants’ lives, and the estimates for inpatient hospitalization, ER visits, and PC visits were additionally distributed by month of age based on a trend derived from Hall 2013 [12] (inpatient hospitalization) and Lively 2019 [13] (ER and PC visits).
Another source for the MA-LRTD rates was published data. As a result of limited data available specific to the KSA, rates of all MA-LRTD events except for PICU were sourced from international data. The risk of inpatient hospitalization in palivizumab-eligible infants was sourced from global randomized studies comparing palivizumab vs placebo (Feltes 2003 [14] study of infants with CHD, and IMpact [15] study of infants with bronchopulmonary dysplasia and below 29 wGA). The overall risk of inpatient hospitalization was based on the placebo arm of these trials and was distributed by month of age based on a trend derived from Hall 2013 [12] to account for a greater risk in younger infants. Risks of inpatient hospitalization in preterm and term infants were informed by Spanish retrospective studies Muñoz-Quiles 2016 [16] and Heppe Montero 2022 [17], respectively. The risk of PICU admissions was derived from a local retrospective study Osman 2023 [2] that reported PICU admission among children hospitalized with bronchiolitis in a KSA tertiary hospital between 2016 and 2021, while the risk of MV among those hospitalized was informed by a retrospective study of outcomes in children with bronchiolitis in Spain [18]. As more granular data were not available, risks of PICU admission and MV among hospitalized infants were assumed to be constant over the first year of infants’ lives. The risks of outpatient MA-LRTD, ER visits and PC visits, were also informed by retrospective data from Spain (the BARI study [19]). The same probabilities of PICU admission, MV, ER visits, and PC visits were applied to all three subpopulations because of the lack of more granular data.
Detailed inputs based on expert opinion and on international literature stratified by age at infection are summarized in Supplementary Material.
The efficacy of prophylaxis products was defined by the percent reduction in the number of RSV MA-LRTDs in both the inpatient and outpatient settings. The efficacy of nirsevimab against inpatient hospitalization of 83.2% in preterm and term infants was informed by the HARMONIE study [20], a phase 3b large, multi-country European clinical trial that evaluated the efficacy of nirsevimab in a real-world setting during the 2022–2023 RSV season. Nirsevimab efficacy against outpatient events was informed by randomized, placebo-controlled trials: a phase 2b study in preterm infants [21] and a phase 3 MELODY study in term infants [22]. The model assumed an immediate onset of protection following nirsevimab immunoprophylaxis and a duration of protection of 5 months. No residual protection was assumed after 5 months, which is a conservative assumption as preliminary results suggest extended protection beyond 5 months [22, 23]. A 90% nirsevimab coverage rate was applied to all three subpopulations on the basis of clinical expert opinion and local vaccine coverage rate of vaccines included in the National Immunization Schedule [24]. In palivizumab-eligible infants, nirsevimab efficacy against inpatient hospitalization and outpatient events, expected to be similar to that observed in preterm and term infants [25], was assumed non-inferior to that of palivizumab; the results of the phase 2/3 trial (MEDLEY) provided the basis for the non-inferiority assumption [26].
The efficacy of palivizumab in eligible infants was derived from a meta-analysis of three clinical trials exploring the impact of palivizumab on the frequency of RSV-induced hospitalizations [27]. The same palivizumab efficacy estimate was applied regardless of whether treatment was received in the inpatient or outpatient setting. The model assumed that palivizumab-eligible infants received up to five doses of palivizumab with a coverage rate of 55%.
Costs were informed by national databases: Ministry of Health database, Medical Cities database, and National Guard Health Affairs (NGHA) database.
Analysis
The model estimated disease burden under two immunoprophylaxis strategies. First, the disease burden under the current SoP was assessed by estimating the impact of the current SoP on healthcare resource utilization (i.e., inpatient hospitalization, PICU admissions, the requirement for MV, ER visits, and PC visits) and associated costs. Then, the disease burden with universal nirsevimab immunoprophylaxis was assessed by estimating the number of cases averted and costs saved compared to the SoP. The results reflect the entire birth cohort, and detailed health event and cost results are presented by subpopulation (i.e., risk groups) and month of birth (i.e., within season versus outside of the season). To account for the uncertainty in the incidence rates of MA-LRTDs stemming from both approaches used to inform the model (sourced from local expert consensus or international published data), the results are reported as a range of values.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Under the current SoP the model estimated a disease burden of 296,344–428,264 cases of RSV-induced MA-LRTDs during the first RSV season, with an associated cost of SAR 466–607 million (USD 124–162 million, conversion rate 1 SAR = 0.26666667 USD as of 16 October 2023). Among these, 17,179–19,607, 57,654–191,115, and 219,083–219,970 infants required inpatient hospitalizations (including PICU admission and MV), ER visits, and PC visits, respectively. The model also estimated 14 RSV-related deaths (Table 2).
Table 2.
Results
| Standard of practice | Nirsevimab | Change (%) | |
|---|---|---|---|
| Health events | |||
| Inpatient hospitalizations (incl. PICU and MV) | 17,179*–19,607 | 7330*–8128 | 9849 (57)*–11,479 (59) |
| PICU (incl. MV) | 2932*–3675 | 1251*–1523 | 1681 (57)*–2151 (59) |
| MV | 172*–525 | 73*–217 | 98 (57)*–307 (59) |
| ER visits | 57,654–191,115* | 27,100–88,748* | 30,554 (53)–102,367 (54)* |
| PC visits | 219,083–219,970* | 102,147*–102,719 | 116,364 (53)–117,823 (54)* |
| RSV-related mortality | 14*–14 | 7*–7 | 8 (53)*–8 (53) |
| Recurrent wheezing | 12,884*–14,705 | 5498*–6096 | 7386 (57)*–8609 (59) |
| Costs | |||
| Inpatient hospitalizations | SAR 94,726,973*–105,929,818 | SAR 40,420,233*–43,912,600 | SAR 54,306,740 (57)*–62,017,217 (59) |
| PICU | SAR 174,070,120*–198,670,409 | SAR 74,276,150*–82,357,682 | SAR 99,793,970 (57)*–116,312,726 (59) |
| MV | SAR 11,662,796*–35,614,793 | SAR 4,976,544*–14,763,909 | SAR 6,686,252 (57)*–20,850,884 (59) |
| ER visits | SAR 86,480,896–286,672,815* | SAR 40,650,224–133,122,187* | SAR 45,830,672 (53)–153,550,628 (54)* |
| PC visits | SAR 39,434,984–39,594,586* | SAR 18,386,470*–18,489,444 | SAR 20,945,540 (53)–21,208,116 (54)* |
| Recurrent wheezing | SAR 12,455,582*–14,215,854 | SAR 5,314,827*–5,893,101 | SAR 7,140,754 (57)*–8,322,753 (59) |
| Total hospitalization cost (incl. PICU and MV) | SAR 280,459,889*–340,215,019 | SAR 119,672,927*–141,034,192 | SAR 160,786,962 (57)*–199,180,828 (59) |
| Total MA-LRTD cost (hospitalization, ER and PC visits) | SAR 466,130,899–606,727,290* | SAR 200,173,860–271,181,584* | SAR 265,957,040 (57)–335,545,706 (55)* |
| Total healthcare cost | SAR 480,346,754–619,182,871* | SAR 206,066,961–276,496,411* | SAR 274,279,793 (57)–342,686,460 (55)* |
ER emergency room, MA-LRTD medically attended lower respiratory tract disease, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit, SoP standard of practice
*Denotes results estimated using inputs based on local expert opinion
Among infants with an inpatient admission, 12,884–14,705 long-term sequelae (recurrent wheezing) were estimated to occur over a 3-year time horizon, and the costs associated with wheezing amounted to SAR 12.5–14.2 million (Table 2).
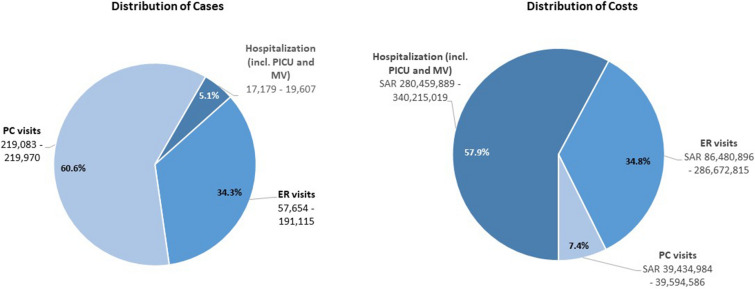
The analysis showed that the majority of the MA-LRTD burden under the current SoP occurs in the outpatient setting, with PC visits representing 61% of the burden, followed by ER visits representing 34% of all cases. While outpatient visits accounted for over 90% of all MA-LRTDs, the associated costs only represented 42% of the overall economic burden. On the other hand, while inpatient hospitalizations (including PICU admissions and MV) only accounted for 5% of the hospital resource use it represented the majority of the annual economic burden (58%), with the total costs estimated at over SAR 280–340 million (Fig. 2). Among those hospitalized, 18% were admitted to a PICU (2932–3675 cases), and 2% required MV (172–525 cases).
Fig. 2.
RSV MA-LRTD burden during the first RSV season under the SoP strategy. Ranges of cases and costs reflect the uncertainty in RSV MA-LRTDs associated with uncertainty in RSV MA-LRTDs rates. Percentage distribution is calculated as an average of two sets of results sourced from local expert consensus and from international published data. ER emergency room, MA-LRTD medically attended lower respiratory tract disease, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit
The model showed that nearly all hospitalizations (97%), ER visits (98%), and PC visits (98%) occurred in preterm and term infants not eligible for palivizumab (i.e., the populations that do not receive prophylaxis under the current SoP). Similarly, most of the economic burden (97% of the total MA-LRTD cost) was attributable to these two subpopulations. A similar trend was observed for recurrent wheezing, with 97% of cases occurring in these two subgroups over 3 years (Supplementary Material).
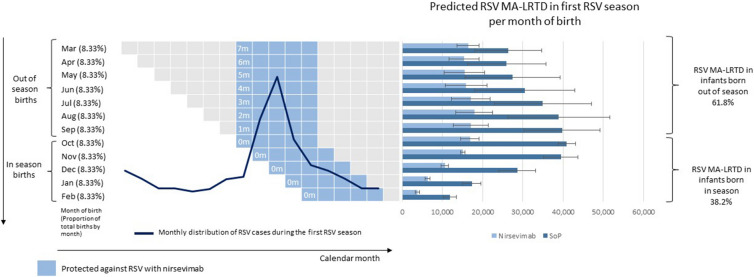
The majority of RSV MA-LRTD burden was estimated to occur in infants born OoS (March to September), compared with those born WiS (October to February). Infants born OoS accounted for 61.8% of all MA-LRTD events and 56.7% of costs associated with MA-LRTD. (Fig. 3, Supplementary Material).
Fig. 3.
Current RSV burden over the first RSV season. RSV MA-LRTDs include hospitalizations (including PICU and MV cases), ER visits, and PC visits. Error bars reflect the uncertainty in RSV MA-LRTDs associated with uncertainty in RSV MA-LRTDs rates. Percentage distribution is calculated as an average of two sets of results sourced from local expert consensus and from international published data. ER emergency room, MA-LRTD medically attended lower respiratory tract disease, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit, RSV respiratory syncytial virus
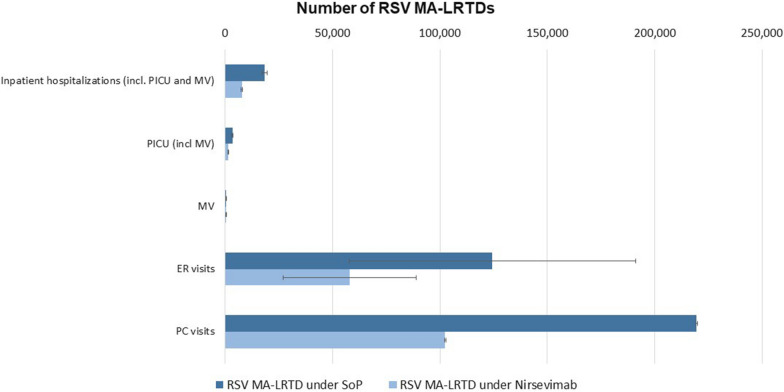
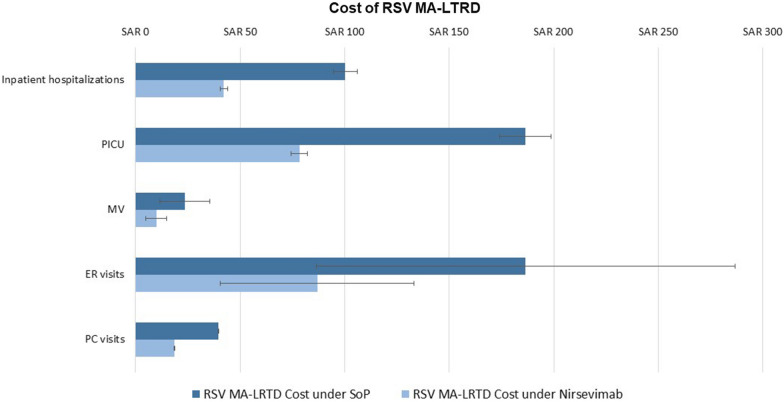
The introduction of universal immunoprophylaxis with nirsevimab was predicted to prevent 9849–11,479 inpatient hospitalizations (including PICU and MV), 116,634–117,823 PC visits, and 30,554–102,267 ER visits, representing average MA-LRTD burden reductions of 58%, 53%, and 53%, respectively. Compared to the SoP, universal immunoprophylaxis with nirsevimab would result in a reduction in spending on MA-LRTD events of SAR 266–336 million (excluding the cost of prophylaxis and complications) (Table 2, Figs. 4, 5).
Fig. 4.
Current RSV MA-LRTD burden over the first RSV season and impact of nirsevimab. Error bars reflect the uncertainty in RSV MA-LRTDs associated with uncertainty in RSV MA-LRTDs rates. ER emergency room, MA-LRTDs medically attended lower respiratory tract disease, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit, SoP standard of practice
Fig. 5.
Current RSV MA-LRTD cost burden over the first RSV season and impact of nirsevimab. Error bars reflect the uncertainty in RSV MA-LRTDs associated with uncertainty in RSV rates. ER emergency room, MA-LRTD medically attended lower respiratory tract disease, MV mechanical ventilation, PC primary care, PICU pediatric intensive care unit, SoP standard of practice
Nirsevimab was also estimated to prevent 8 deaths (35% reduction) and 7386–8609 long-term sequelae (recurrent wheezing, 58% reduction) cases over 3 years. Expected savings associated with the reduction of wheezing cases amounted to SAR 7.1–8.3 million (Table 2).
The greatest impact of nirsevimab was seen in term infants, averting 7984–10,493 inpatient hospitalizations (including PICU and MV), 28,944–96,968 ER visits, and 110,230–111,608 PC visits, representing an average reduction of 60%, 53%, and 54% in inpatient hospitalizations, ER visits, and PC visits, respectively (Supplementary Material).
Among infants born OoS, introduction of universal nirsevimab immunoprophylaxis would avert 4110–4466 inpatient hospitalizations (including PICU and MV admissions), 12,248–70,101 ER visits, and 45,819–80,685 PC visits, representing average MA-LRTD burden reductions of 46%, 47%, and 47%, respectively, and cost savings of SAR 22.7–24.1 million (46%), 18.4–105.2 million (47%), and 8.2–14.5 million (47%), respectively. Among infants born WiS, universal nirsevimab immunoprophylaxis was predicted to prevent 5738–7013 inpatient hospitalizations (including PICU and MV admissions, average 70% reduction), 18,306–32,266 ER visits (61%), and 37,138–70,545 PC visits (61%). The associated cost savings were SAR 31.6–37.9 million (70%), SAR 27.5–48.4 million (61%), and SAR 6.7–12.7 million (61%), respectively (Supplementary Material).
Discussion
RSV is associated with a considerable public health and economic burden, particularly in infants during the winter, due to its high incidence and limited prophylaxis options. This study investigated the impact of universal immunoprophylaxis with nirsevimab in all infants experiencing their first RSV season versus the current SoP. Under the current SoP, RSV was estimated to result in 17,179–19,607 infant hospitalizations, including 2932–3675 PICU admissions and 172–525 MV, and was associated with a total hospitalization cost of SAR 280–340 million (USD 75–91 million), representing a significant part of the economic burden (58%). Additionally, under the current SoP, RSV resulted in an estimated 57,654–191,115 ER visits and 219,083–219,970 PC visits, and their associated costs constitute 35% the total economic burden of RSV MA-LRTD. The model estimated that 12,884–14,705 infants suffered from recurrent wheezing over 3 years with an associated cost of SAR 12.5–14.2 million (USD 3.3–3.8 million). In this study, the preterm and term subpopulations (i.e., infants not eligible for palivizumab) accounted for most hospitalizations (95%), ER visits (98%), and PC visits (98%). Notably, universal immunoprophylaxis with nirsevimab was predicted to avert 56% of all MA-LRTDs compared with the current SoP. According to the analysis, nirsevimab would avert 158,397–230,039 MA-LRTD events, including 30,554–102,367 ER visits, 116,364–117,823 PC visits, and 9849–11,479 inpatient hospitalizations, significantly reducing the burden on the healthcare system and reducing total healthcare costs by SAR 274–343 million (USD 73–91 million), representing a 56% decrease in the total MA-LRTD costs.
Although infants with CHD or CLD and those born prematurely are at the highest risk of severe disease outcomes, the greatest public health and economic impact (i.e., events averted, and costs saved) of nirsevimab immunoprophylaxis was estimated to occur in term infants (constituting 94% of the entire birth cohort).
RSV displays a seasonal circulation pattern in the KSA; the RSV season typically occurs from September to March, with peaks in RSV infections in December and January. To provide protection during the peak of the RSV season, the 5-month period from October to February was defined as the immunoprophylaxis period, which is consistent with the duration of protection by nirsevimab conservatively assumed by the model.
Our findings align with those from alternative models evaluating the impact of nirsevimab on RSV MA-LRTDs in other countries, showing similar relative reduction in RSV MA-LRTDs compared to SoC. Similarly to the findings from the US model [30], most RSV MA-LRTDs and associated costs were for infants born outside of the RSV season.
As a result of the lack of local published data, our analysis was based on two sets of inputs related to the disease burden—one based on a consensus of local clinical experts, and the other based on published data, predominantly from Spanish studies. To reflect the uncertainty of estimates, the results were published as ranges. The largest discrepancy between the two sets of inputs was seen for rates of ER visits (average 43% estimated by the experts vs 12% sourced from a study conducted in Spain [19]). This was reflected in the computed results, with ranges of ER estimates being the widest among all MA-LRTDs. This variation is likely a result of differences between KSA and the EU countries influencing caregivers’ willingness and decision to seek treatment at the ER.
Several limitations of this study should be noted. Some inputs did not differ by model subpopulation, such as the probability of PICU admission and ER visits. The waning of immunity after 5 months was not considered because of a lack of data. Therefore, a 5-month duration of protection was conservatively applied, and no residual protection was considered. This study only considered costs related to RSV-induced disease, while the costs associated with the acquisition and administration of the current SoP and nirsevimab were not considered. Additionally, secondary impact of RSV (schedule overload and disruption, PICU management) and other costs associated with medical care were not considered, including doctor calls, home visits, medication, etc. Finally, the model did not consider the societal perspective; the impact of RSV on quality of life of infants, their caregivers, and other family members was not considered and should be explored in the future to fully ascertain the benefits of a universal nirsevimab immunoprophylaxis program. It should be noted, however, that estimating quality of life using EQ-5D or similar scores would not be possible for the population modeled in our study.
Conclusion
Our findings suggest that the RSV-induced disease burden in the KSA is driven by the term and preterm infant subpopulation as these infants accounted for most MA-LRTD events. Notably, no prophylaxis or antiviral treatments are available for this pediatric subpopulation, representing an urgent unmet need. Our model estimates that compared to the current SoP, nirsevimab can confer consistent protection across their first RSV season in all infants regardless of their health status or birth month. Its extended half-life is expected to make the immunization campaign more feasible and acceptable for the public. Therefore, the implementation of a universal nirsevimab immunoprophylaxis strategy in the KSA could alleviate the burden associated with RSV among infants in their first RSV season.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the input of Aditya Sardesai (Evidera, San Francisco, USA) and Solene de Boisvilliers (Evidera, London, UK) to this study.
Medical Writing/Editorial Assistance
Editorial assistance was provided by Hannah Floyd (Evidera, Bethesda, USA) and funded by Sanofi and AstraZeneca.
Author Contributions
Paulina Kazmierska, Matthieu Beuvelet contributed to the design of the study. All authors (Adel Alharbi, Abdullah Yousef, Amal Zubani, Mohammad Alzahrani, Mohammad Alhindi, Saleh Alharbi, Turki Alahmadi and Hana Alabdulkarim, Paulina Kazmierska, Matthieu Beuvelet) were responsible for the data interpretation. Paulina Kazmierska, Matthieu Beuvelet were responsible for the data analysis. All authors (Adel Alharbi, Abdullah Yousef, Amal Zubani, Mohammad Alzahrani, Mohammad Alhindi, Saleh Alharbi, Turki Alahmadi and Hana Alabdulkarim, Paulina Kazmierska, Matthieu Beuvelet) contributed to revising the manuscript, and all reviewed and approved the final version.
Funding
This study, the Rapid Service Fee and the Open Access Fee were funded by Sanofi and AstraZeneca.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of Interest
Matthieu Beuvelet is an employee of Sanofi and may hold shares/stocks in Sanofi. Paulina Kazmierska is employed by Evidera, a part of Thermo Fisher Scientific, which received funding for research from Sanofi and AstraZeneca. Adel Alharbi, Abdullah Yousef, Amal Zubani, Mohammad Alzahrani, Mohammad Alhindi, Saleh Alharbi, Turki Alahmadi and Hana Alabdulkarim have no relevant financial or non-financial interests to disclose. Nirsevimab is being developed and commercialized in partnership between AstraZeneca and Sanofi.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Alharbi AS, Alqwaiee M, Al-Hindi MY, et al. Bronchiolitis in children: the Saudi initiative of bronchiolitis diagnosis, management, and prevention (SIBRO) Ann Thorac Med. 2018;13(3):127–143. doi: 10.4103/atm.ATM_60_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osman S, Alaa Adeen A, Hetta O, et al. Epidemiology and risk factor analysis of children with bronchiolitis admitted to the intensive care unit at a tertiary care center in Saudi Arabia. Children (Basel) 2023;10(4):646. doi: 10.3390/children10040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Parveen S, Al-Hassinah SM, et al. An overview of respiratory syncytial virus infections in Saudi Arabia. J Infect Dev Ctries. 2018;12(11):929–936. doi: 10.3855/jidc.10736. [DOI] [PubMed] [Google Scholar]
- 4.Viguria N, Martinez-Baz I, Moreno-Galarraga L, et al. Respiratory syncytial virus hospitalization in children in northern Spain. PLoS ONE. 2018;13(11):e0206474. doi: 10.1371/journal.pone.0206474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Akmar LZ, Bailey F, et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis. 2020;222(Suppl 7):S680–S687. doi: 10.1093/infdis/jiz683. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Beyfortus, nirsevimab. EMA/CHMP/647784/2022 2022 [August 2023]. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-beyfortus_en.pdf. Accessed 12 Dec 2023.
- 8.Food and Drug Administration. FDA Approves New Drug to Prevent RSV in Babies and Toddlers 2023 [August 2023]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers. Accessed 12 Dec 2023.
- 9.Bont L, Steijn M, Van Aalderen WM, et al. Seasonality of long term wheezing following respiratory syncytial virus lower respiratory tract infection. Thorax. 2004;59(6):512–516. doi: 10.1136/thx.2003.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The World Bank. Birth rate, crude (per 1000 people)—Saudi Arabia [August 2023]. https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?locations=SA. Accessed 12 Dec 2023.
- 11.Ministry of Health. Premature Babies 2019 [August 2023]. https://www.moh.gov.sa/en/awarenessplateform/ChildsHealth/Pages/PrematureBabies.aspx. Accessed 12 Dec 2023.
- 12.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 13.Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc. 2019;8(3):284–286. doi: 10.1093/jpids/piz011. [DOI] [PubMed] [Google Scholar]
- 14.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–540. doi: 10.1067/S0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 15.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz-Quiles C, Lopez-Lacort M, Ubeda-Sansano I, et al. Population-based analysis of bronchiolitis epidemiology in Valencia. Spain Pediatr Infect Dis J. 2016;35(3):275–280. doi: 10.1097/INF.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 17.Heppe Montero M, Gil-Prieto R, Walter S, et al. Burden of severe bronchiolitis in children up to 2 years of age in Spain from 2012 to 2017. Hum Vaccin Immunother. 2022;18(1):1883379. doi: 10.1080/21645515.2021.1883379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hervas D, Reina J, Yanez A, et al. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis. 2012;31(8):1975–1981. doi: 10.1007/s10096-011-1529-y. [DOI] [PubMed] [Google Scholar]
- 19.Martinon-Torres F, Carmo M, Platero L, et al. Clinical and economic burden of respiratory syncytial virus in Spanish children: the BARI study. BMC Infect Dis. 2022;22(1):759. doi: 10.1186/s12879-022-07745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drysdale S. Efficacy of nirsevimab against RSV lower respiratory tract infection hospitalization in infants: preliminary data from the HARMONIE phase 3b trial. In: 41st Annual Meeting of the European Society for Paediatric Infectious Diseases; Lisbon 2023.
- 21.Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–425. doi: 10.1056/NEJMoa1913556. [DOI] [PubMed] [Google Scholar]
- 22.Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–846. doi: 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins D, Yuan Y, Chang Y, et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat Med. 2023;29(5):1172–1179. doi: 10.1038/s41591-023-02316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Vaccination schedule for Saudi Arabia 2023. https://immunizationdata.who.int/pages/schedule-by-country/sau.html?DISEASECODE=&TARGETPOP_GENERAL. Accessed 12 Dec 2023.
- 25.Simoes EAF, Madhi SA, Muller WJ, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc Health. 2023;7(3):180–189. doi: 10.1016/S2352-4642(22)00321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domachowske J, Madhi SA, Simoes EAF, et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. N Engl J Med. 2022;386(9):892–894. doi: 10.1056/NEJMc2112186. [DOI] [PubMed] [Google Scholar]
- 27.Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;30(4):CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 28.Sigurs N, Bjarnason R, Sigurbergsson F, et al. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–505. doi: 10.1542/peds.95.4.500. [DOI] [PubMed] [Google Scholar]
- 29.Taylor S, Taylor RJ, Lustig RL, et al. Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open. 2016;6(6):e009337. doi: 10.1136/bmjopen-2015-009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieffer A, Beuvelet M, Sardesai A, et al. Expected impact of universal immunization with nirsevimab against RSV-related outcomes and costs among all US infants in their first RSV season: a static model. J Infect Dis. 2022;226(Suppl 2):S282–S292. doi: 10.1093/infdis/jiac216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.