Correction to: Adv Ther (2019) 36:1786–1811 10.1007/s12325-019-00985-8
In this article, the drug name in figure 2 is given as Atorvastatin 20 mg incorrectly. The correct Fig. 2 is given below.
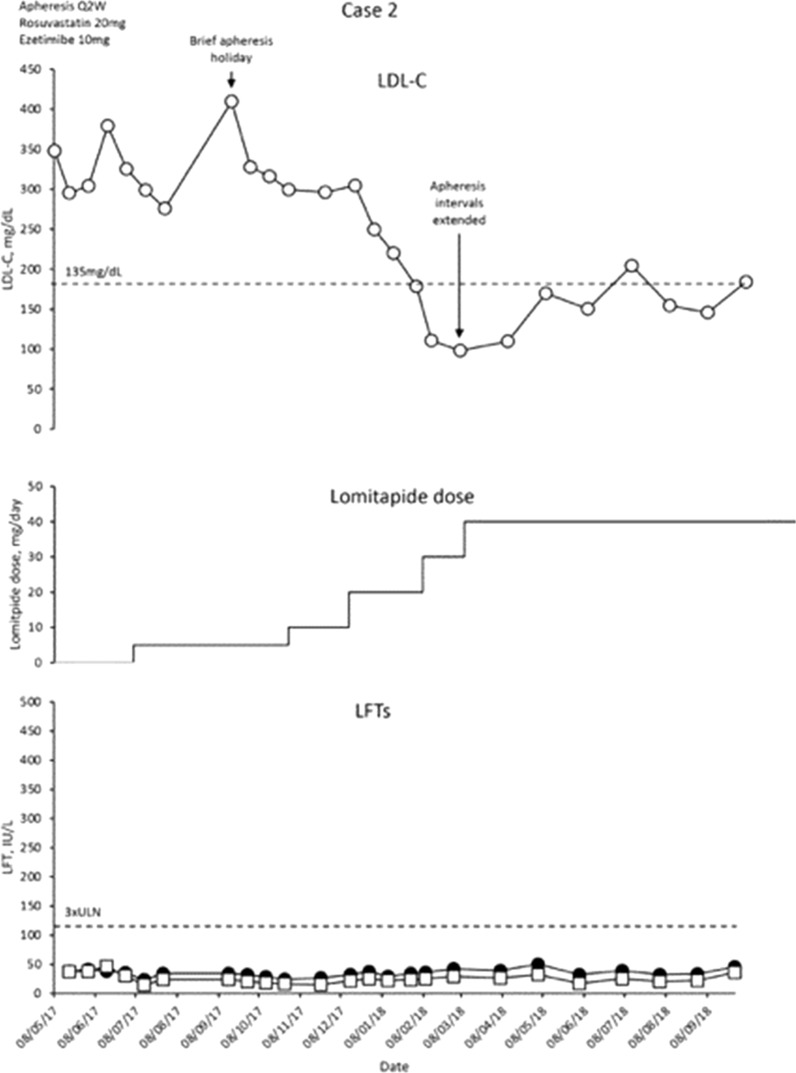
Fig. 2.
Evolution of LDL-C values in case 2 with lomitapide therapy. Upper panel shows mean interval LDL-C levels for patient 2. Middle panel shows lomitapide dose changes over time. Lower panel shows corresponding ALT (closed circles) and AST (open squares) levels over the same period. Dotted line on upper panel shows EAS targets for LDL-C levels in children with HoFH. Dotted line on lower panel indicates 3× upper limit of normal for LFTs; ALT alanine aminotransferase, AST aspartate aminotransferase, EAS European Atherosclerosis Society, HoFH homozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, LFTs liver function tests, Q2W every 2 weeks, ULN upper limit of normal
The Tables 1 and 2 consists few errors in published article. The correct Tables 1 and 2 are given below.
Table 1.
Individual data for the 11 patients
| Parameter | Patient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Sex | Female | Male | Male | Male | Female | Male | Female | Male | Male | Female | Male |
| Age, years | 13 | 12 | 16 | 7 | 11 | 16 | 4 | 14 | 15 | 11 | 9 |
| Genetic variant |
LDLR c.313+5 G>A |
LDLR c.682G>T | LDLR c.119_1207del | LDLR c.666C>A, c.1646C>A | c.-187-? 940+? Dup | LDLR c.131G>A, c.2043C>A | LDLR c.2043C>A | LDLR c.1846-? 2311+?del, c.1895A>T |
LDLR c.313+1 G>A, del exon 1–6 |
LDLR c.1731G>T | LDLR c.1731G>T |
| LDL-C at diagnosis, mg/dL | 799 | 672 | 981 | 1008 | 1009 | 901 | 739 | 474 | 982 | 1002 | 824 |
| LLT prior to lomitapide | Statins, ezetimibe, LA | Statins, ezetimibe, LA | Statins, ezetimibe, LA, EV | Statins, ezetimibe, bile acid sequestrant | Statins, ezetimibe | Statins, ezetimibe, LA, EV | Statins, ezetimibe | Statins, ezetimibe | Statins, ezetimibe, LA, EV | Statins, ezetimibe, bile acid sequestrant | Statins, ezetimibe, bile acid sequestrant |
| Duration of therapy prior to lomitapide, years | 11 | 2 | 14 | 3 | 8 | 6 | < 1 | 6 | 11 | 8 | 8 |
| LDL-C prior to lomitapide, mg/dL | 299 | 326 | 187 | 833 | 443 | 274 | 649 | 223 | 81 | 630 | 705 |
| LDL-C at nadir, mg/dL | 56 | 98 | 73 | 360 | 231 | 23 | 236 | 75 | 62 | 441 | 460 |
| Concomitant LLT |
Atv 40mg Ez 10mg LA Q2W |
Ro 20mg Ez 10mg LA Q15D |
Ro 20mg Ez 10mg Ev 420mg QW Co 3250mg LA Q1W |
Ro 20mg Ez 10mg Co 625mg |
Atv 10mg Ez 10mg |
Ro 20mg Ez 10mg LA Q2W |
Atv 10mg Ez 5mg |
Ro 30mg Ez 10mg |
Atv 40mg Ez 10mg LA 2xW |
Atv 40mg Ez 10mg Cholestyramine 4g |
Atv 40mg Ez 10mg Cholestyramine 4g |
| Maximal reduction with lomitapide, % | 81 | 70 | 61 | 57 | 48 | 92 | 64 | 66 | 24 | 27 | 34 |
| Maximum dose of lomitapide, mg/day | 20 | 40 | 60 | 30 | 20** | 30 | 15 | 15*** | 15 | 20 | 20 |
| Length of lomitapide exposure, months | 17 | 15 | 20 | 15 | 48 | 15 | 12 | 22 | 18 | 19 | 19 |
| Change in concomitant LLT |
Ev stopped§ LA stopped |
Ev stopped§ LA reduced to Q4W |
Ev stopped§ Ro stopped LA reduced to Q2W |
None |
Atv 40mg† Atv 60mg† |
Ev stopped§ Ro 30mg Ro 40mg**** LA stopped |
None |
Ez stopped§ LA stopped |
LA reduced 75% Ev stopped§ |
None | None |
| Liver status | Liver enzymes normal | Liver enzymes normal | Elevated liver enzymes resolved after Ro stopped | Liver enzymes normal | Liver enzymes normal | Minimal ALT increase resolved without intervention | Liver enzymes normal | ALT increases managed with lomitapide dose reduction | Liver enzymes and liver imaging normal | Liver enzymes normal | Liver enzymes normal |
| Adverse events¶ | Nausea, vomiting, Diarrhoea, frequent bowel move-ments | Diarrhoea, vomiting | Flatulence, hypertransaminasaemia | None | Diarrhoea | Gastrointestinal pain, Hypertransaminasaemia | Diarrhoea | Hypertransaminasaemia | None | None | None |
AE adverse events, ALT alanine aminotransferase, At atorvastatin, Co colesevalem, Ev evolocumab (all Ev stopped prior to lomitapide), Ez ezetimibe, GI gastrointestinal, LA lipoprotein apheresis, LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapies
All oral drug doses are daily
*Q2W
**patient briefly received 30mg/day before back-titration to 20mg/day
***patient briefly received 20mg/day before back-titration to 15mg/day
****subsequent, post-hoc reduction to Ro 35mg
†atorvastatin dose changes – Atv dose increased to 60mg near end of observation period
§Patient had also received evolocumab (no response), which had been stopped before commencement on lomitapide
¶MedDRA preferred term
Table 2.
Summary data for the 11 patients
| Parameter | Age | Baseline LDL-C, mg/dL | Nadir LDL-C, mg/dL | Percentage reduction in LDL-C from baseline to nadir, % | Lomitapide dose, mg/day | Lomitapide exposure, months |
|---|---|---|---|---|---|---|
| Mean | 11.6 | 422.7 | 192.2 | 56.7 | 25.0 | 20.0 |
| Median | 12.0 | 325.5 | 98.0 | 61.2 | 20.0 | 18.2 |
| SD | 3.8 | 245.4 | 163.2 | 21.7 | 13.8 | 9.5 |
LDL-C low-density lipoprotein cholesterol, SD standard deviation
In the result section of abstract, text has been updated. The incorrect text is “In the 11 cases, mean baseline LDL-C was 419 ± 74.6 mg/dL and was markedly reduced by lomitapide to a nadir of 176.7 ± 46.3 mg/dL (58.4 ± 6.8% decrease). Six patients achieved recommended target levels for children below 135 mg/dL, five of whom had LA frequency reduced.”
The correct text is “In the 11 cases, mean baseline LDL-C was 422 ± 245.4 mg/dL and was markedly reduced by lomitapide to a nadir of 192.2 ± 163.2 mg/dL (56.7 ± 21.7% decrease). Six patients achieved recommended target levels for children below 135 mg/dL, three of whom had LA frequency reduced and a further three stopped LA.”
In the section of Summary of the Case Series, text has been updated. The incorrect text is “Table 2 provides summary descriptive statistics for all 11 patients. Baseline LDL-C was 419.9 ± 74.6 mg/dL. The mean at nadir was 176.7 ± 46.3 mg/dL, representing a 58.4 ± 6.8% reduction in LDL-C. Note that patients 9–11 had modest decreases in LDL-C levels (patient 9 was treated to reduce LA frequency, and patients 10 and 11 had compliance issues). These LDL-C reductions were achieved with a mean dose of lomitapide 24.5 ± 4.3 mg/day over a mean period of 20.0 ± 2.9 months.”
The correct text is “Table 2 provides summary descriptive statistics for all 11 patients. Baseline LDL-C was 422.7 ± 245.4 mg/dL. The mean at nadir was 192.2 ± 163.2 mg/dL, representing a 56.7 ± 21.7% reduction in LDL-C. Note that patients 9–11 had modest decreases in LDL-C levels (patient 9 was treated to reduce LA frequency, and patients 10 and 11 had compliance issues). These LDL-C reductions were achieved with a mean dose of lomitapide 25.0 ± 13.8 mg/day over a mean period of 20.0 ± 2.9 months.”