Abstract
Human studies of fear neurobiology have established neural circuits that are activated to threatening stimuli, whether it be during Pavlovian fear conditioning or in response to naturally occurring threats. This circuitry involves the central and basolateral amygdala, as well as the bed nucleus of the stria terminalis, insula, hippocampus, and regulatory regions such as the anterior cingulate cortex and ventromedial prefrontal cortex. While research has found that fear-based disorders, such as anxiety and post-traumatic stress disorder, as associated with dysfunction in these circuits, there is substantial individual heterogeneity in the clinical presentation of symptoms. Recent work has used data-driven methods to derive brain biotypes that capitalize on the activity of the fear circuit and its interaction with other regions of the brain. These biotypes have great utility in both describing individual variation in psychopathology and in identifying individuals at greater risk for fear-based disorders after an environmental stressor, such as a traumatic event. The review discusses recent examples of how fear neurobiology studies can be leveraged to derive biotypes that may ultimately lead to improved treatment.
Keywords: Fear conditioning, Biotypes, Trauma, Neuroimaging
Trauma, defined as exposure to actual or threatened death, serious injury, or sexual violence, is highly prevalent: global data indicate that 70% of individuals around the world have experienced at least one major traumatic event in their lives (Kessler et al., 2017). Those who have experienced trauma are at risk for multiple mental health disorders (Green et al., 2010), including fear-based disorders characterized by pathological fear or worry, and heightened physiological arousal triggered by stimuli or situations in the absence of actual danger (Singewald et al., 2015). Pathological fear or worry is present in a range of psychiatric conditions, including anxiety disorders (e.g. generalized anxiety disorder, specific phobias, and panic disorder) and trauma-related disorders (e.g. posttraumatic stress disorder, PTSD). Despite the prevalent link between trauma exposure and fear-based disorders, there is clear evidence for heterogeneity in the type and severity of outcomes following trauma, and within clinical presentations of fear-based disorders. In fact, there are 3,150 different symptom combinations which would satisfy diagnostic criteria for PTSD (Galatzer-Levy and Bryant, 2013). The heterogeneity in clinically observable symptoms suggests that there are multiple homogeneous biologically-based clusters within and between diagnostic categories (Insel and Cuthbert, 2015). However, the neurobiological basis of these clusters remains poorly understood. Identifying unique profiles of brain activation underlying heterogeneity in fear-based disorders may help improve diagnostic specificity, treatment response, and aid in predicting divergent outcomes following trauma.
In response, the National Institutes of Mental Health advanced the Research Domain Criteria (RDoC) framework with the primary goal to restructure mental health classification given that these disorders emerge from dysfunction in neural circuits (Insel et al., 2010). Aligned with this approach, current research has empirically distinguished unique clinical symptom profiles, or subtypes, of PTSD which showed differential associations with functional impairment (Breslau et al., 2005; Naifeh et al., 2010). In trauma-exposed youth, clinical subtypes were differentially associated with longitudinal risk for worsening symptoms and functional impairment (Ayer et al., 2011). As such, identifying associations between these clinical subtypes and their biological substrates may provide more precise biomarkers in vulnerable populations like trauma-exposed youth. Neuroimaging studies play a key role in filling this gap by identifying neural correlates of fear that are predictive of subsequent mental health outcomes following trauma.
Given that fear-based disorders are often characterized by exaggerated threat reactivity, experimental models of these disorders include direct associative learning of threat and safety, as well as social threat processing (Shackman and Fox, 2021). Extensive work describing the neural correlates of Pavlovian fear conditioning has been conducted in rodent models (Ganella and Kim, 2013) and humans (Jovanovic et al., 2013) and has identified an evolutionarily conserved neurocircuit underlying the acquisition, expression, and modulation of learned threat responses. Work with rodent models has implicated the amygdala and its connections to the brain stem in the execution of efferent behavioral responses to threatening or unconditioned aversive stimuli (Davis, 1992; Gafford and Ressler, 2016). Microcircuits within the central and basolateral amygdala are involved in consolidating threat during fear conditioning, by associating an unconditioned stimulus (US) with a conditioned stimulus (CS+) (Fanselow and LeDoux, 1999). Translational work in monkeys and humans has shown that, in addition to the central amygdala, the bed nucleus of the stria terminalis supports increased physiological arousal to both acute and potential threat (Shackman and Fox, 2016). Neuroimaging research in humans has extended the fear conditioning circuitry beyond amygdala microcircuits to include the dorsal anterior cingulate cortex (dACC), hippocampus, insula, and the dorsolateral prefrontal cortex (dlPFC) during the acquisition of fear (Alvarez et al., 2008; Fullana et al., 2016; Milad et al., 2007a), and the ventromedial prefrontal cortex (vmPFC) during the extinction of fear (Milad et al., 2007b). This same neurocircuitry may also mediate responses to stimuli that are not inherently threatening, but may convey information about potential threats nearby. For example, the socio-cultural environment can provide such information through observation of fearful emotional states of others (Bandura and Walters, 1977; Olsson and Phelps, 2007). The recognition of these social threat cues is an important survival mechanism to appropriately respond to environmental threats. In humans, patterns of brain activation similar to those evoked by fear conditioning paradigms, can be observed when fear is acquired via social observation (Olsson et al., 2007) and passive viewing of negative emotions (Shin and Liberzon, 2010).
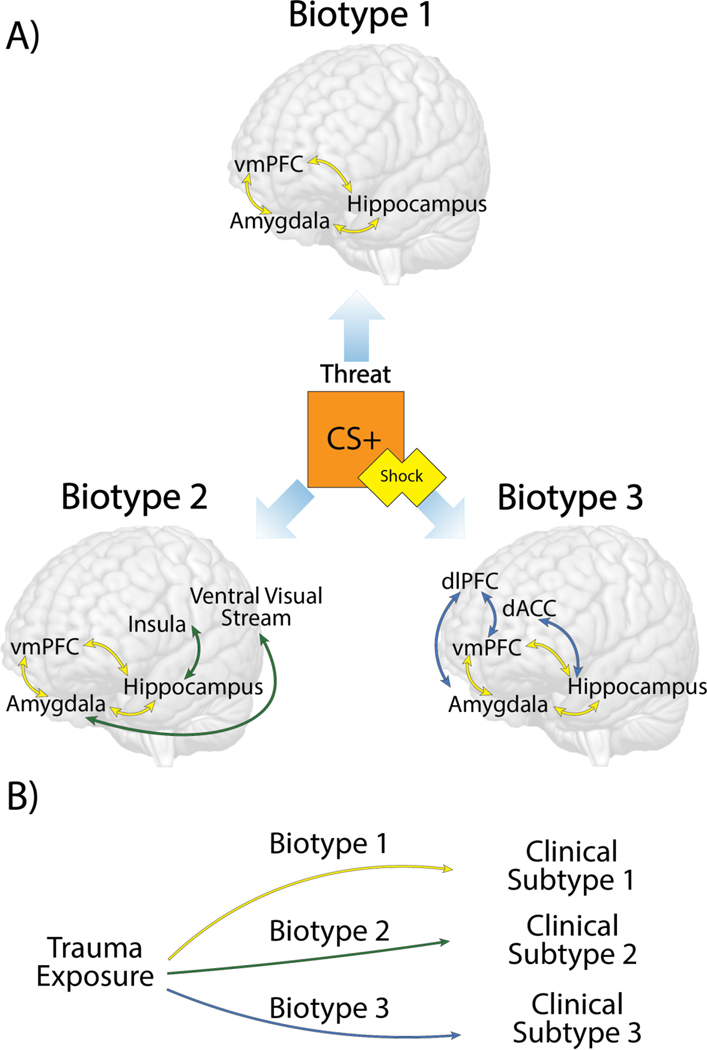
While neural correlates of threat may underlie hallmark symptoms of fear-based disorders (Etkin and Wager, 2007; Wen et al., 2021), focusing exclusively on these brain regions may obscure meaningful differences in neural engagement giving rise to individual variation in fear-based symptomology (LeDoux and Pine, 2016). Heterogeneity in fear-based symptoms may be explained in part by interactions between fear conditioning circuitry with other brain regions that are not typically considered part of the this circuit (Insel and Cuthbert, 2015; Pessoa and Adolphs, 2010). For example, the ventral visual stream was recently implicated in reexperiencing symptoms in PTSD patients and predicted increasing symptom severity over time (Harnett et al., 2022). To broaden the scope of fear conditioning circuit interactions, a data-driven approach can be applied to identify patterns of activation or connectivity across multiple networks to describe unique functional brain profiles, or biotypes, in the context of threat (Figure 1a). This approach may explain heterogeneity within clinical presentations of fear-based disorders (Maron-Katz et al., 2020) and different mental health outcomes following trauma exposure (Stevens et al., 2021)(Figure 1b). This review will provide a focused discussion of the recent studies which derived threat-related biotypes to test their associations with fear-based symptoms, and their predictive value in identifying differing outcomes following trauma exposure.
Figure 1. Schematic of different theoretical biotypes identified during fear learning.
A) The threat stimuli represented here is a reinforced conditioned stimulus (CS+) paired with an aversive shock. In the context of this threat, different patterns of neural interactions with the fear conditioning circuit (yellow) may emerge within groups. This example demonstrates three hypothetical functional profiles of brain activation or connectivity, or biotypes, defined by differing interactions with the ventral visual stream (green) or prefrontal regions (blue). Bidirectional arrows denote connectivity between regions. Brain-based biotypes may aid in characterizing heterogeneity in fear-based symptomology. B) Following trauma exposure, these brain-based biotypes can provide predictive value in determining mental health outcomes. This example demonstrates how hypothetical biotypes 1, 2, and 3 from panel A may aid in predicting different clinical symptom clusters, or subtypes, following trauma.
Fear conditioning (i.e. acquisition) and extinction paradigms serve as an experimental model of fear-based disorders and elucidate pathological processes underlying differences in learned fear and safety (Graham and Milad, 2011; Jovanovic and Ressler, 2010). Although much progress had been made in identifying the neurocircuitry involved in fear acquisition and extinction, relatively few studies have used these paradigms to define biotypes. To address this gap in the literature, Ahrenholtz and co-authors applied a data-driven approach to functional magnetic resonance imaging (fMRI) data collected during a fear acquisition and extinction task in adult women with PTSD (Ahrenholtz et al., 2021). A K-means clustering analysis identified 3 distinct biotypes of functional brain activation when contrasting CS+>CS- during acquisition and extinction separately. The first biotype was characterized by greater engagement of the salience network, which may indicate greater attentional awareness of potentially threatening stimuli. The second biotype was represented by greater activation in the ventral visual network during both acquisition and extinction, and may be associated with processes related to object recognition. The last biotype was characterized by increased engagement of the default mode network (DMN) during acquisition. These biotypes demonstrated distinct associations with clinical, behavioral, and trauma-related measures, such that biotype 1 was uniquely associated with higher working memory, better emotion regulation, and less distress; in addition, biotypes 1 and 3 were associated with less early life sexual abuse, as well as lower skin conductance response (SCR) and reexperiencing symptoms. Moreover, individuals whose functional profiles most closely resembled the second biotype were more likely to demonstrate greater SCR and threat expectancy ratings specific to the CS+ during acquisition compared to extinction. These results show that, although all women in the sample were diagnosed with PTSD, there are apparent distinct functional profiles of brain activation during fear acquisition and extinction with differing clinical characteristics.
To further understand the neural bases of heterogeneity in fear-based disorders, Wen and colleagues used a whole brain approach to evaluate altered dynamic functional connectivity (FC) in healthy controls, anxiety patients, and PTSD patients (Wen et al., 2022). Participants underwent fear acquisition and extinction learning, and extinction recall the following day. During fear extinction learning and recall, the authors used dynamic FC to capture temporal changes in the functional coupling of brain regions between large-scale brain networks related to fear-based disorders. Specifically, during extinction learning, healthy controls demonstrated increasing FC to the CS+ from early to late extinction, whereas FC decreased in both anxiety and PTSD groups. These FC reductions predominately occurred between the DMN, frontoparietal control network (CON), somatomotor network (SMN), and ventral and dorsal attentional networks (VAN/DAN). FC reductions were associated with subjective measures of fear across all subjects and with clinical measures of anxiety and PTSD symptoms, in the anxiety and PTSD group, respectfully. These results suggest functional coupling of wide-spread brain networks beyond fear conditioning circuitry can explain symptoms of fear-based disorders. FC profiles observed during extinction learning were then compared between the anxiety and PTSD patient groups. Partial overlap in FC profiles was demonstrated involving the CON, DMN, and SMN during extinction learning, and may explain common symptomology between anxiety disorders and PTSD. However, distinct connections were also observed between the anxiety and PTSD groups. Specifically, connections between the dlPFC and other regions across the fear conditioning circuitry (including the dACC, vmPFC, and insula), were markedly different across patient groups. One interesting distinction was that the dlPFC-hippocampus FC was impaired in the PTSD group only, whereas the dlPFC-insula was impaired in the anxiety group. As such, heterogeneity in fear-based symptoms may be explained by differing interactions between the fear conditioning circuitry and other brain regions. In addition to describing heterogeneity among disorders, differences in such interactions in the context of threat could be used to prospectively predict varying clinical outcomes following trauma exposure.
As noted above, trauma exposure increases risk for a wide range of fear-based disorders, including PTSD. Stevens and colleagues conducted a longitudinal fMRI study tracking clinical outcomes of individuals exposed to acute traumatic events from the emergency department with the goal of identifying biotypes relevant to stress vulnerability (Stevens et al., 2021). Using a hierarchical clustering approach, they identified 3 replicated biotypes that demonstrated distinct brain activation patterns across threat, reward, and response inhibition fMRI tasks. Individuals in the first biotype were classified as “reactive/disinhibited” as they demonstrated greater activity in amygdala, insula, and dACC during both threat and reward tasks, and less engagement of regulatory regions such as the hippocampus and vmPFC during the inhibition task. Biotype 2 was coined “low reward/high threat” and was characterized by greater engagement of fear circuits specifically during the threat task and less activation to the reward task. Lastly, biotype 3, or “inhibited,” demonstrated deactivation of the fear circuits during the threat task, and higher activation of the hippocampus and vmPFC during the response inhibition task. They then identified associations between these biotypes, psychophysiological measures during a fear-conditionings task, and the later emergence of symptoms. Notably, the “low reward/high threat” biotype also showed higher levels of fear-potentiated startle during a fear conditioning task compared to the other groups. Mental health outcomes were then tracked at multiple time points from 2 weeks to 6 months following trauma exposure across the biotypes. The “reactive/disinhibited” biotype was associated with greater PTSD and anxiety symptoms over the six months post-trauma. In order to control for pre-existing symptoms, a regression analysis controlled for PTSD and anxiety symptom levels prior to the emergency room trauma, since a history of psychopathology was not an exclusion for this study. The “reactive/disinhibited” biotype remained significant in predicting future anxiety, suggesting that biotype membership provided unique predictive value in mental health outcomes above prior psychopathology. These findings are significant as they provide strong foundational support for the theoretical model that distinct biotypes can predict heterogeneity in outcomes following trauma exposure. A notable caveat is that while this study included an internal replication, external replications are still necessary to demonstrate generalizability.
Importantly, this same approach can be applied to prospectively predict risk for fear-based disorders in developmental populations that have experienced trauma. Most anxiety disorders emerge during early adolescence (Wehry et al., 2015), making this an important developmental period in understanding the neurobiological mechanisms contributing to the heterogeneity in outcomes following trauma (Teicher et al., 2016). As an initial step in this direction, Sellnow and colleagues conducted a study characterizing biotypes of emotion processing circuitry in adolescents exposed to early life interpersonal violence (Sellnow et al., 2020). Data were collected during a negative and neutral emotional faces fMRI task, and biotypes were created using a data-driven K-means clustering approach. Three biotypes of emotional processing circuitry were identified, differing in activation of the mPFC, IPFC, hippocampus, insula, posterior cingulate, parietal cortex, and ventral visual cortex. However, these biotypes were derived using the main effect of task (combined fearful and neutral faces), and not specific valence, meaning the biotypes could be related to face processing more generally rather than specific to negative emotion. Nevertheless, the individual differences found in emotion processing circuitry were related to individual differences in trauma exposure and symptomology. A parallel cluster analysis was performed with self-report measures of internalizing symptoms and trauma exposure, yielding three distinct subtypes; those with low levels of trauma exposure and low internalizing symptomology, or the “asymptomatic” group; those with high trauma exposure and moderate symptomology, or the “high trauma moderate symptoms” group; and individuals with high trauma exposure and high symptomology, or the “high trauma high symptoms” group. The authors then tested if individual differences in similarity with these clinical subtypes was related to similarity with emotional processing biotypes. Specifically, classification into biotype 1 was associated with the asymptomatic subtype, classification into biotype 2 associated with the high trauma moderate symptoms subtype, and classification into biotype 3 associated with the high trauma high symptoms subtype. In a subset of the sample, biotypes were derived before and after administration of cognitive behavioral therapy. It was found that PTSD symptom reduction following treatment was associated with changes in biotypes, such that participants who demonstrated the largest symptom reduction also demonstrated increased similarity with biotype 1. These results demonstrate that the derived biotypes have direct clinical relevance in youth populations exposed to trauma and are related to treatment response.
While data-driven clustering approaches may aid in advancing our understanding of mental health disorders, there are several limitations to this approach. For example, clustering analysis relies on expert knowledge to interpret the results, and derive meaning from the groups. Moreover, the number of groups are either determined a priori or derived from the analysis based on a series of user-defined hyperparameters. As such, variability among clustering approaches may dampen the ability to replicate results, and full transparency is essential to derive reproducible biotypes from neuroimaging data (Garcia-Dias 2020). More research is needed to assess the test-retest reliability of biotypes; to test the reliability of brain derived biotypes across different statistical clustering analyses, scan sites, and different MRI acquisition protocols and functional paradigms; and to test their generalizability across different racial/ethnic backgrounds. Nevertheless, clustering approaches have been used across many domains of research and have identified brain-based biotypes distinct from clinical diagnoses (Clementz et al., 2016) and associated with distinct clinical symptom subtypes (Drysdale et al., 2017). Among individuals with PTSD, other investigations have identified biotypes from resting state functional connectivity with unique clinical features (Maron-Katz et al., 2020), although these have not always replicated in the same symptom domains (Esterman et al., 2020). With regard to treatment, some biotypes derived from resting state functional connectively have predicted poor response to psychotherapy (Etkin et al., 2019). Therefore, these data-driven approaches are aligned with the goal of translational research to utilize pattern-recognition techniques and predictive modeling to serve as a clinical tool (Woo et al., 2017). Translating these metrics in the clinic could aid in developing targeted treatments and therapeutics, which parallels new initiatives in other areas of medicine (Rosell et al., 2013).
In summary, these recent data-driven studies demonstrate the high potential for functional neuroimaging in defining threat-related functional brain profiles, or biotypes, to help explain the neurobiological underpinnings of heterogeneity in fear-based disorders. These biotypes may serve as important clinical tools to identify risk and resilience to posttraumatic stress. In a developmental context, these biotypes may serve as important intermediate phenotypes and help predict the emergence of anxiety disorders during adolescence. In the future, this line of research has the potential to improve diagnostic precision and treatment response, and offer effective early interventions in youth exposed to trauma.
Acknowledgements
This work has been supported by funding from the National Institutes of Health (R01 MH111682, MH122867) to T.J. and the Wayne State University New Investigator Grant to J.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ahrenholtz R, Hiser J, Ross MC, Privratsky A, Sartin-Tarm A, James GA, Cisler JM, 2021. Unique neurocircuitry activation profiles during fear conditioning and extinction among women with posttraumatic stress disorder. J Psychiatr Res 141, 257–266. 10.1016/j.jpsychires.2021.07.007 [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C, 2008. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci Official J Soc Neurosci 28, 6211–9. 10.1523/jneurosci.1246-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer L, Danielson CK, Amstadter AB, Ruggiero K, Saunders B, Kilpatrick D, 2011. Latent classes of adolescent posttraumatic stress disorder predict functioning and disorder after 1 year. Compr Psychiat 52, e2. 10.1016/j.comppsych.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, Walters RH, 1977. Social Learning Theory. [Google Scholar]
- Breslau N, Reboussin BA, Anthony JC, Storr CL, 2005. The Structure of Posttraumatic Stress Disorder: Latent Class Analysis in 2 Community Samples. Arch Gen Psychiat 62, 1343–1351. 10.1001/archpsyc.62.12.1343 [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, 2016. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiat 173, 373–384. 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 1992. The Role of the Amygdala in Fear and Anxiety. Annu Rev Neurosci 15, 353–375. 10.1146/annurev.ne.15.030192.002033 [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey B, Dubin MJ, Liston C, 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23, 28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Stumps A, Jagger-Rickels A, Rothlein D, DeGutis J, Fortenbaugh F, Romer A, Milberg W, Marx BP, McGlinchey R, 2020. Evaluating the evidence for a neuroimaging subtype of posttraumatic stress disorder. Sci Transl Med 12. 10.1126/scitranslmed.aaz9343 [DOI] [PubMed] [Google Scholar]
- Etkin A, Maron-Katz A, Wu W, Fonzo GA, Huemer J, Vértes PE, Patenaude B, Richiardi J, Goodkind MS, Keller CJ, Ramos-Cejudo J, Zaiko YV, Peng KK, Shpigel E, Longwell P, Toll RT, Thompson A, Zack S, Gonzalez B, Edelstein R, Chen J, Akingbade I, Weiss E, Hart R, Mann S, Durkin K, Baete SH, Boada FE, Genfi A, Autea J, Newman J, Oathes DJ, Lindley SE, Abu-Amara D, Arnow BA, Crossley N, Hallmayer J, Fossati S, Rothbaum BO, Marmar CR, Bullmore ET, O’Hara R, 2019. Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci Transl Med 11. 10.1126/scitranslmed.aal3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiat 164, 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE, 1999. Why We Think Plasticity Underlying Pavlovian Fear Conditioning Occurs in the Basolateral Amygdala. Neuron 23, 229–232. 10.1016/s0896-6273(00)80775-8 [DOI] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, Radua J, 2016. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatr 21, 500–508. 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- Gafford GM, Ressler KJ, 2016. Mouse models of fear-related disorders: Cell-type-specific manipulations in amygdala. Neuroscience 321, 108–120. 10.1016/j.neuroscience.2015.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bryant RA, 2013. 636,120 Ways to Have Posttraumatic Stress Disorder. Perspect Psychol Sci 8, 651–662. 10.1177/1745691613504115 [DOI] [PubMed] [Google Scholar]
- Ganella DE, Kim JH, 2013. Developmental rodent models of fear and anxiety: from neurobiology to pharmacology. Brit J Pharmacol 171, 4556–74. 10.1111/bph.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR, 2011. The Study of Fear Extinction: Implications for Anxiety Disorders. Am J Psychiat 168, 1255–1265. 10.1176/appi.ajp.2011.11040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC, 2010. Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I: Associations With First Onset of DSM-IV Disorders. Arch Gen Psychiat 67, 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Finegold KE, Lebois LAM, Rooij SJH van Ely TD, Murty VP, Jovanovic T, Bruce SE, House SL, Beaudoin FL, An X, Zeng D, Neylan TC, Clifford GD, Linnstaedt SD, Germine LT, Bollen KA, Rauch SL, Haran JP, Storrow AB, Lewandowski C, Musey PI, Hendry PL, Sheikh S, Jones CW, Punches BE, Kurz MC, Swor RA, Hudak LA, Pascual JL, Seamon MJ, Harris E, Chang AM, Pearson C, Peak DA, Domeier RM, Rathlev NK, O’Neil BJ, Sergot P, Sanchez LD, Miller MW, Pietrzak RH, Joormann J, Barch DM, Pizzagalli DA, Sheridan JF, Harte SE, Elliott JM, Kessler RC, Koenen KC, McLean SA, Nickerson LD, Ressler KJ, Stevens JS, 2022. Structural covariance of the ventral visual stream predicts posttraumatic intrusion and nightmare symptoms: a multivariate data fusion analysis. Transl Psychiat 12, 321. 10.1038/s41398-022-02085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiat 167, 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, 2015. Brain disorders? Precisely. Science 348, 499–500. 10.1126/science.aab2358 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, 2013. Translational neuroscience measures of fear conditioning across development: applications to high-risk children and adolescents. Biology Mood Anxiety Disord 3, 17. 10.1186/2045-5380-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ, 2010. How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. Am J Psychiat 167, 648–662. 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, Degenhardt L, Girolamo G. de, Dinolova RV, Ferry F, Florescu S, Gureje O, Haro JM, Huang Y, Karam EG, Kawakami N, Lee S, Lepine J-P, Levinson D, Navarro-Mateu F, Pennell B-E, Piazza M, Posada-Villa J, Scott KM, Stein DJ, Have MT, Torres Y, Viana MC, Petukhova MV, Sampson NA, Zaslavsky AM, Koenen KC, 2017. Trauma and PTSD in the WHO World Mental Health Surveys. Eur J Psychotraumato 8, 1353383. 10.1080/20008198.2017.1353383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Pine DS, 2016. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. Am J Psychiat 173, 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Maron-Katz A, Zhang Y, Narayan M, Wu W, Toll RT, Naparstek S, Angeles CDL, Longwell P, Shpigel E, Newman J, Abu-Amara D, Marmar C, Etkin A, 2020. Individual Patterns of Abnormality in Resting-State Functional Connectivity Reveal Two Data-Driven PTSD Subgroups. Am J Psychiat 177, 244–253. 10.1176/appi.ajp.2019.19010060 [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL, 2007a. A Role for the Human Dorsal Anterior Cingulate Cortex in Fear Expression. Biol Psychiat 62, 1191–1194. 10.1016/j.biopsych.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL, 2007b. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol Psychiat 62, 446–454. 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Naifeh JA, Richardson JD, Ben KSD, Elhai JD, 2010. Heterogeneity in the Latent Structure of PTSD Symptoms Among Canadian Veterans. Psychol Assessment 22, 666–674. 10.1037/a0019783 [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA, 2007. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neur 2, 3–11. 10.1093/scan/nsm005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA, 2007. Social learning of fear. Nat Neurosci 10, 1095–1102. 10.1038/nn1968 [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R, 2010. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci 11, 773–782. 10.1038/nrn2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R, Bivona TG, Karachaliou N, 2013. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 382, 720–731. 10.1016/s0140-6736(13)61715-8 [DOI] [PubMed] [Google Scholar]
- Sellnow K, Sartin-Tarm A, Ross MC, Weaver S, Cisler JM, 2020. Biotypes of functional brain engagement during emotion processing differentiate heterogeneity in internalizing symptoms and interpersonal violence histories among adolescent girls. J Psychiatr Res 121, 197–206. 10.1016/j.jpsychires.2019.12.002 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, 2021. Two Decades of Anxiety Neuroimaging Research: New Insights and a Look to the Future. Am J Psychiat 178, 106–109. 10.1176/appi.ajp.2020.20121733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, 2016. Contributions of the Central Extended Amygdala to Fear and Anxiety. J Neurosci 36, 8050–8063. 10.1523/jneurosci.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I, 2010. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacol 35, 169–191. 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ, 2015. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Therapeut 149, 150–190. 10.1016/j.pharmthera.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Harnett NG, Lebois LAM, Rooij SJH van Ely TD, Roeckner A, Vincent N, Beaudoin FL, An X, Zeng D, Neylan TC, Clifford GD, Linnstaedt SD, Germine LT, Rauch SL, Lewandowski C, Storrow AB, Hendry PL, Sheikh S, Musey PI, Haran JP, Jones CW, Punches BE, Lyons MS, Kurz MC, McGrath ME, Pascual JL, Datner EM, Chang AM, Pearson C, Peak DA, Domeier RM, O’Neil BJ, Rathlev NK, Sanchez LD, Pietrzak RH, Joormann J, Barch DM, Pizzagalli DA, Sheridan JF, Luna B, Harte SE, Elliott JM, Murty VP, Jovanovic T, Bruce SE, House SL, Kessler RC, Koenen KC, McLean SA, Ressler KJ, 2021. Brain-Based Biotypes of Psychiatric Vulnerability in the Acute Aftermath of Trauma. Am J Psychiat 178, 1037–1049. 10.1176/appi.ajp.2021.20101526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17, 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR, 2015. Assessment and Treatment of Anxiety Disorders in Children and Adolescents. Curr Psychiat Rep 17, 52. 10.1007/s11920-015-0591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Chen ZS, Milad MR, 2021. Fear extinction learning modulates large-scale brain connectivity. Neuroimage 238, 118261–118261. 10.1016/j.neuroimage.2021.118261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Seo J, Pace-Schott EF, Milad MR, 2022. Abnormal dynamic functional connectivity during fear extinction learning in PTSD and anxiety disorders. Mol Psychiatr 27, 2216–2224. 10.1038/s41380-022-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Chang LJ, Lindquist MA, Wager TD, 2017. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20, 365–377. 10.1038/nn.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]