Abstract
Background
Oesophagectomy followed by oesophagogastrostomy is the preferred treatment for early‐stage oesophageal cancer. It carries the risk of anastomotic leakage after oesophagogastric anastomosis, which causes considerable morbidity and mortality and is one of the most dangerous complications. Omentoplasty has been recommended by some researchers to prevent anastomotic leaks associated with oesophagogastrostomy. However, the value of omentoplasty for oesophagogastrostomy after oesophagectomy has not been systematically reviewed.
Objectives
To assess the effects of omentoplasty for oesophagogastrostomy after oesophagectomy in patients with oesophageal cancer.
Search methods
A comprehensive search to identify eligible studies for inclusion was conducted using the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PubMed and other reliable resources.
Selection criteria
Randomised controlled trials comparing omentoplasty versus no omentoplasty for oesophagogastrostomy after oesophagectomy in patients with oesophageal cancer were eligible for inclusion.
Data collection and analysis
Two review authors (Yong Yuan and Xiaoxi Zeng) independently assessed the quality of included studies and extracted data; disagreements were resolved through arbitration by another review author. Results of dichotomous outcomes were expressed as risk ratios (RRs) with 95% confidence intervals (CIs), and continuous outcomes were expressed as mean differences (MDs) with 95% CIs. Meta‐analysis was performed when available data were sufficiently similar. Subgroup analysis was carried out on the basis of different approaches to surgery.
Main results
Three randomised controlled trials (633 participants) were included in this updated review. No significant differences in hospital mortality were noted between the study group (with omentoplasty) and the control group (without omentoplasty) (RR 1.28, 95% CI 0.49 to 3.39). None of the included studies reported differences in long‐term survival between the two groups. The incidence of postoperative anastomotic leakage was significantly less among study participants treated with omentoplasty than among those treated without (RR 0.25, 95% CI 0.11 to 0.55), but the additional benefit was seen in the subgroup analysis only for participants undergoing a transhiatal oesophagogastrectomy (THE) procedure (RR 0.23, 95% CI 0.07 to 0.79); transthoracic oesophagogastrectomy (TTE) (RR 0.19, 95% CI 0.03 to 1.03); or three‐field oesophagectomy (RR 0.33, 95% CI 0.09 to 1.19 ). Omentoplasty did not significantly improve other surgery‐related complications, such as anastomotic stricture (RR 0.91, 95% CI 0.33 to 2.57). However, participants treated with omentoplasty could reduce the duration of hospitalisation compared with that seen in the control group (MD ‐2.13, 95% CI ‐3.57 to ‐0.69).
Authors' conclusions
Omentoplasty may provide additional benefit in decreasing the incidence of anastomotic leakage after oesophagectomy and oesophagogastrostomy for patients with oesophageal cancer without increasing or decreasing other complications, especially among those treated with THE. It also has the potential to reduce the duration of hospital stay after operation. Further randomised controlled trials are needed to investigate the influences of omentoplasty on the incidence of anastomotic leakage and anastomotic stricture, long‐term survival, duration of hospital stay and quality of life after oesophagectomy and oesophagogastrostomy when different surgical approaches are used.
Keywords: Humans; Esophagectomy; Anastomosis, Surgical; Anastomosis, Surgical/adverse effects; Anastomosis, Surgical/methods; Anastomotic Leak; Anastomotic Leak/etiology; Anastomotic Leak/prevention & control; Esophageal Neoplasms; Esophageal Neoplasms/surgery; Esophagostomy; Esophagostomy/methods; Esophagus; Esophagus/surgery; Gastrostomy; Gastrostomy/methods; Length of Stay; Omentum; Omentum/surgery; Randomized Controlled Trials as Topic; Stomach; Stomach/surgery
Plain language summary
Omentoplasty for oesophagogastrostomy after oesophagectomy
Oesophagectomy followed by oesophagogastrostomy, in which an anastomosis between the residual oesophagus and the stomach substitute is made, remains the standard surgery for patients with oesophageal cancer. Whichever surgical procedure is chosen, that is, transthoracic oesophagectomy (TTE) with direct visualisation of the thoracic oesophagus or transhiatal oesophagectomy (THE) with avoidance of a thoracic incision, postoperative anastomotic leakage causes considerable morbidity and mortality. Omentoplasty, in which the omentum is used to wrap the anastomosis, has been recommended by some researchers to prevent postoperative anastomotic leakage—one of the most serious complications of oesophagectomy followed by oesophagogastrostomy for patients with oesophageal cancer. This updated systematic review, including 633 participants in three randomised controlled trials, suggests that omentoplasty could reduce the incidence of anastomotic leakage and the duration of hospital stay after operation. Although the difference in anastomotic leakage was significant only among patients undergoing THE, the risk ratios of omentoplasty for THE and TTE were similar. In addition, omentoplasty does not appear to increase or decrease hospital mortality nor the incidence of postoperative complications, such as anastomotic stricture, pulmonary and cardiac complications, infection, vocal cord palsy and perijejunostomy leakage. Additional clinical trials are needed to investigate the influences of omentoplasty on the incidence of anastomotic leakage and anastomotic stricture, long‐term survival, duration of hospital stay and quality of life after oesophagectomy and oesophagogastrostomy when different surgical approaches are used.
Summary of findings
Summary of findings for the main comparison. Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy after oesophagectomy.
| Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy after oesophagectomy | ||||||
| Patient or population: patients with oesophagogastrostomy after oesophagectomy Settings: inpatient Intervention: omentoplasty with oesophagogastrostomy versus oesophagogastrostomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy | |||||
| Mortality Clinical manifestation Follow‐up: 30 daysa | Study population | RR 1.28 (0.49 to 3.39) | 633 (3 studies) | ⊕⊕⊕⊕ high | None | |
| 22 per 1000 | 28 per 1000 (11 to 75) | |||||
| Moderate | ||||||
| 31 per 1000 | 40 per 1000 (15 to 105) | |||||
| Anastomotic leakage Radiographic contrast study and/or clinical manifestation Follow‐up: 6 to 14 daysb | Study population | RR 0.25 (0.11 to 0.55) | 633 (3 studies) | ⊕⊕⊕⊝ moderatec | None | |
| 95 per 1000 | 24 per 1000 (10 to 52) | |||||
| Moderate | ||||||
| 98 per 1000 | 25 per 1000 (11 to 54) | |||||
| Anastomotic leakage—TTE Radiographic contrast study Follow‐up: 6 to 12 daysb | Study population | RR 0.19 (0.03 to 1.03) | 272 (2 studies) | ⊕⊕⊕⊝ moderatec | None | |
| 60 per 1000 | 11 per 1000 (2 to 61) | |||||
| Moderate | ||||||
| 73 per 1000 | 14 per 1000 (2 to 75) | |||||
| Anastomotic leakage—THE Radiographic contrast study Follow‐up: 6 to 12 daysb | Study population | RR 0.23 (0.07 to 0.79) | 177 (2 studies) | ⊕⊕⊕⊝ moderatec | None | |
| 144 per 1000 | 33 per 1000 (10 to 114) | |||||
| Moderate | ||||||
| 143 per 1000 | 33 per 1000 (10 to 113) | |||||
| Anastomotic leakage—3‐field oesophagectomy Radiographic contrast study and/or clinical manifestation Follow‐up: 7 to 14 days | Study population | RR 0.33 (0.09 to 1.19) | 184 (1 study) | ⊕⊕⊕⊝ moderatec | None | |
| 98 per 1000 | 32 per 1000 (9 to 116) | |||||
| Moderate | ||||||
| 98 per 1000 | 32 per 1000 (9 to 117) | |||||
| Anastomotic strictures Barium swallow and/ or endoscope examinationd Follow‐up: 3 yearse | Study population | RR 0.91 (0.33 to 2.57) | 631 (3 studies) | ⊕⊕⊕⊝ moderatef | None | |
| 92 per 1000 | 84 per 1000 (30 to 237) | |||||
| Moderate | ||||||
| 72 per 1000 | 66 per 1000 (24 to 185) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aMortality was assessed within a 30‐day postoperative period. bPostoperative leakage was assessed on postoperative days 6 to 14. cSome minor leaks might be undetectable by contrast study. dAssessment for stricture was unclear in Bhat's study (Bhat 2006). In Dai's study (Dai 2010), barium swallow and endoscopic study were used for stricture measurement, and diagnosis was made by endoscopy for Zheng's trial (Zheng 2013). eIn Bhat's study (Dai 2010), length of follow‐up for anastomotic stricture was not specified. For Dai's study (Dai 2010), 1‐year follow‐up was conducted. In Zheng's study (Zheng 2013), assessment of stricture was performed during the 3‐year follow‐up period. fThe detailed method and criteria used for anastomotic stricture assessment were unclear in Bhat's study (Bhat 2006).
Background
Description of the condition
Oesophageal cancer, characterised by rapid development and fatal prognosis in most patients, is the sixth most frequent tumour disease worldwide (Kollarova 2007). In 2002, 462,000 new cases of oesophageal cancer and 386,000 related deaths were reported worldwide (Parkin 2005). The incidence of oesophageal carcinoma varies considerably among different geographic locations and ethnic groups. The highest rates were reported in northern China and northern Iran, where the incidence was over 100 in 100,000 individuals (Koshy 2004). In contrast, the incidence in the United States was fewer than five per 100,000, even though the rate was about quadruple for African Americans (Fisher 1998). The predominant histological type of oesophageal cancer is squamous cell carcinoma, although the incidence of oesophageal adenocarcinoma has dramatically increased in some areas (Blot 1999).
Oesophagectomy, with an anastomosis between the residual oesophagus and the stomach substitute made in the chest or in the neck, remains the standard surgical treatment and is effective for early‐stage tumours confined to the oesophagus and the para‐oesophageal region (Barreto 2010; Bhat 2006; Donohoe 2012). Unfortunately, many complications may occur after radical oesophagectomy and reconstruction of the oesophagus, such as anastomotic leakage, anastomotic stenosis, blood loss, recurrent laryngeal nerve injury, thoracic duct injury and tracheal injury. Among these complications, anastomotic leaks cause considerable morbidity and mortality after oesophagectomy. Cervical anastomoses are associated with a higher rate of leakage than is seen with intrathoracic anastomoses, but leaks from intrathoracic anastomoses cause greater morbidity (Urschel 1995).
As the main curative option for early‐stage oesophageal cancer is currently surgery, improving the outcome of surgery is an effective way to reduce mortality (Mody 2002; Verhoef 2007). Prevention of anastomotic leaks would therefore improve patient outcomes following oesophagectomy.
Description of the intervention
The omentum has been widely used in the management of various thoracic and abdominal problems. Omentoplasty, which means omental wrapping of the anastomosis, has been strongly recommended by some researchers for preventing anastomotic leaks associated with oesophagogastrostomy after oesophagectomy (Ohwada 2000; Ohwada 2002; Thakur 2004). Usually a tongue of omentum along the greater curvature of the stomach is taken, with its vessels preserved, and is sutured to the circumference of the anastomosis after completion. This procedure is feasible only when the stomach is used as a substitute in oesophagectomy, and it can be applied in both intrathoracic and neck anastomoses (Wilkins 2002).
How the intervention might work
Many risk factors are considered to be related to anastomotic leaks, among which gastric conduit vascularity has been established as a major determinant of anastomotic wound healing (Blewett 2001; Urschel 1995). It has been shown that the omentum could produce vascular endothelial growth factor (VEGF) protein, which is an important angiogenic factor possibly contributing to omentum‐induced angiogenesis. Furthermore, enhanced expression of VEGF by omental cells under a condition of hypoxia may be responsible for improving the angiogenic activity of the omentum (Zhang 1997). However, the pedicled omentum, which provides an extra protective layer for anastomosis, can supply the nutrition and oxygen necessary for anastomotic wound healing. It may therefore work to minimise the incidence of anastomotic leaks and to improve outcomes for patients with oesophageal cancer.
Why it is important to do this review
Some studies have reported that omentoplasty is effective for oesophagogastrostomy after oesophagectomy (Ohwada 2000; Ohwada 2002; Thakur 2004); however unsuccessful cases of omentoplasty have also been reported (Kurahashi 2004). An animal experiment indicated that omentoplasty reinforcement of oesophagogastric anastomoses had no beneficial impact on anastomotic healing (Cui 2000). However, the evidence on omentoplasty for oesophagogastrostomy after oesophagectomy is not strong enough to permit a sound recommendation; therefore this review update is important.
Objectives
To assess the effects of omentoplasty for oesophagogastrostomy after oesophagectomy in patients with oesophageal cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing omentoplasty versus no omentoplasty for oesophagogastrostomy after oesophagectomy in patients with oesophageal cancer.
Types of participants
Patients with a diagnosis of oesophageal cancer, according to pathology, who received omentoplasty or no omentoplasty for oesophagogastrostomy after oesophagectomy, regardless of age, sex, race, stage and histological type of cancer.
Types of interventions
Omentoplasty for oesophagogastrostomy after oesophagectomy in patients with oesophageal cancer, regardless of anastomotic site (neck or chest anastomosis), anastomotic method (manual or mechanical anastomosis) or surgical approach (transhiatal or transthoracic).
The control group received no omentoplasty.
Types of outcome measures
Primary outcomes
Mortality (death from any cause in the early postoperative period: within one month after surgery).
Survival (during the follow‐up period): at one, three and five years.
Anastomotic leakage rate after surgery.
Secondary outcomes
Surgery‐related complications, such as major vascular injury, tracheal injury, recurrent laryngeal nerve injury or thoracic duct injury.
Omentoplasty‐related complications, such as peritonitis, intestinal obstruction or infection.
Anastomotic stenosis after surgery.
Duration of hospital stay.
Quality of life.
Search methods for identification of studies
Electronic searches
We searched the following.
Cochrane Central Register of Controlled Trials (CENTRAL) (December 2013).
Ovid MEDLINE(R) Daily Update (5 February 2014); Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) (1946 to present).
EMBASE (1980 to week 5, 2014).
The search strategy for MEDLINE, using a combination of controlled vocabulary and text‐word terms, is shown in Appendix 1 and was modified to suit other databases, such as CENTRAL (Appendix 2) and EMBASE (Appendix 3).
Searching other resources
We also searched the following clinical trial registers.
National Institute of Health clinical trials database (www.clinicaltrials.gov).
Trials Central (www.trialscentral.org).
Current Controlled Trials (www.controlled‐trials.com).
Center Watch (www.centerwatch.com).
Chinese Cochrane Centre Controlled Trials Register (www.chictr.org).
PubMed and reference lists were searched for related information. Trials published in English and in other languages were included.
Data collection and analysis
Selection of studies
Two review authors (Yong Yuan and Xiaoxi Zeng) independently reviewed the titles, abstracts and full texts of studies. Only studies that met the inclusion criteria were included. We used methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) to identify multiple reports from the same study, and we corresponded with the original study authors to clarify eligibility of studies when necessary. Disagreements on selection of studies for inclusion were resolved by consensus discussion or by discussion with a third party.
Data extraction and management
Two review authors (Yong Yuan and Xiaoxi Zeng) independently extracted data concerning details of study participant characteristics, methods, interventions and outcomes using a data extraction form. Disagreements were resolved by discussion or were arbitrated by another review author (Yang Hu). We used Microsoft Excel and Access in managing the data, if necessary.
Assessment of risk of bias in included studies
Two review authors (Yong Yuan and Xiaoxi Zeng) independently assessed the risk of bias of included studies using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Other potential threats to validity.
This was achieved by answering a prespecified question about the adequacy of the study in relation to the above specific domains, such that a judgement of 'low risk' indicated low risk of bias, 'high risk' indicated high risk of bias and 'unclear risk' indicated unclear or unknown risk of bias. Disagreements between review authors arising at any stage were resolved by discussion or with the assistance of a third party when necessary (Figure 1).
1.
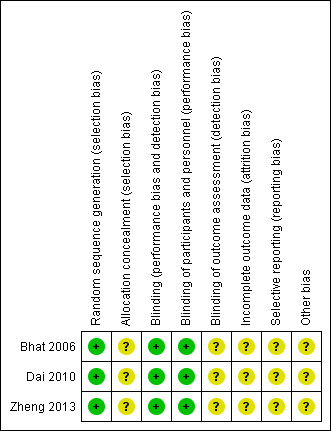
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We expressed treatment effects as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and as weighted mean differences (WMDs) or standardised mean differences (SMDs) (with 95% CIs) for continuous outcomes.
Unit of analysis issues
We found only three individually randomised controlled trials (RCTs) for this specific topic; no cluster‐randomised trials or cross‐over trials were included in this systematic review.
Dealing with missing data
We tried to obtain missing data from the original study authors by email when possible. Sensitivity analyses were not performed in this review because of the limited available data. We addressed the potential impact of missing data on the findings of the review in the Discussion section, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Assessment of heterogeneity
We assessed heterogeneity using a standard Chi2 test with significance set at P value < 0.1 or an I2 statistic > 75%. If evidence of heterogeneity was found, we explored which factor caused it and performed a subgroup analysis based on the possible reasons.
Assessment of reporting biases
We aimed to identify and minimise reporting biases (publication bias, time lag bias, duplicate publication bias, location bias, citation bias, language bias or outcome reporting bias) through a comprehensive search for studies, inclusion of unpublished studies and use of trial registries. Funnel plot asymmetry testing and sensitivity analysis were not performed because only three studies were included in this review.
Data synthesis
We performed a meta‐analysis for the outcomes listed above when available data were sufficiently similar. The primary analysis looked at effects on the three primary outcome measures. We planned to consider the three primary outcomes for data synthesis, including the RRs of postoperative death, anastomotic leakage rate and long‐term survival after operation, but no data were available regarding long‐term survival. We also included anastomotic strictures in data synthesis, as this is an important and common complication of oesophagogastrostomy. A fixed‐effect model was used unless heterogeneity was significant, in which case we applied a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We intended to explore the following potential sources of heterogeneity using subgroup analysis or meta‐regression.
Differences in the follow‐up period.
Differences in the surgical procedure: anastomotic site (neck or chest anastomosis), anastomotic method (manual or mechanical anastomosis), surgical approach (transhiatal or transthoracic).
Finally, meta‐regression was not conducted, as only three studies are included in this review.
Sensitivity analysis
The following sensitivity analyses were performed.
Different effect methods for meta‐analysis were performed to confirm that results were robust regardless of whether a random‐effects or a fixed‐effect model was used.
For anastomotic stricture, repeat analysis was performed by including participants who were excluded owing to short‐term in‐hospital mortality.
Results
Description of studies
Results of the search
The initial search revealed 230 studies (MEDLINE 81, EMBASE 128, CENTRAL 21). After obviously irrelevant records and duplicates were removed, 27 studies remained. Of the remaining 27 studies, 24 were excluded when abstracts and full texts were read. No other studies were identified by a search of other resources. The reference lists of relevant studies were also checked for additional studies (Figure 2). In addition to the two studies included in our last version of this systematic review (Bhat 2006; Dai 2010), one study (Zheng 2013) was deemed eligible for inclusion in this updated review. Detailed information on included and excluded studies is presented in the Characteristics of included studies and Characteristics of excluded studies sections.
2.
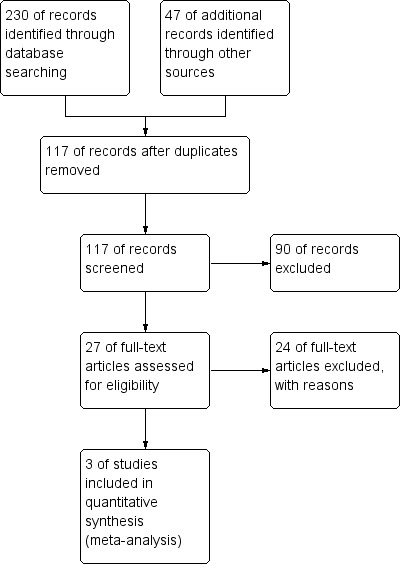
Study flow diagram.
Included studies
Participants
A total of 633 patients with previously untreated oesophageal cancer were included in the three RCTs. The sex ratio in all studies showed a male preponderance (3:1.8 (Bhat 2006), 4:1 (Dai 2010), 1.5:1 (Zheng 2013)). The mean age of participants was 52.5 (Bhat 2006), 63.5 (Dai 2010) and 66.2 (Zheng 2013) years in these studies, respectively. In the studies of Bhat and Dai, only patients with stage I, II or III oesophageal cancer (according to tumour‐node‐metastasis (TNM) classification of oesophagus and oesophagogastric junctions in the Seventh Edition of the AJCC [American Joint Committee on Cancer] Cancer Staging Manual; AJCC 2009) were eligible for inclusion (Bhat 2006; Dai 2010), of whom 417 patients were diagnosed with squamous cell carcinoma and the others with adenocarcinoma. In Zheng's study, patients with stage I through IV disease (based on Union for International Cancer Control (UICC)/TNM classification, Seventh Edition, 2009) who underwent radical oesophagectomy with three‐field lymphadenectomy were included; no detailed information about the histological types of cancer was provided (Zheng 2013).
Interventions
All studies included two groups: One was treated with oesophagogastrostomy following oesophagectomy and reinforcement of the anastomosis with the pedicled omentum, and the other with only oesophagogastrostomy following oesophagectomy (Bhat 2006; Dai 2010; Zheng 2013). All study participants underwent standard surgical procedures for radical surgery, and participants in Zheng's study underwent three‐field lymphadenectomy. Two surgical approaches consisting of transthoracic oesophagogastrectomy with intrathoracic anastomosis (TTE) and transhiatal oesophagogastrectomy with left‐sided neck anastomosis (THE) were performed in the studies of Bhat (Bhat 2006) and Dai (Dai 2010), and Zheng's study adopted the three‐incision oesophagectomy with three‐field lymphadenectomy (Zheng 2013). In terms of methods of anastomosis, mechanically stapled oesophagogastric anastomosis was used in the study of Dai (Dai 2010), and manual anastomosis was performed in the studies of Baht (Bhat 2006) and Zheng (Zheng 2013). Surgical procedures for omentoplasty were similar among studies, involving omental mobilisation (omental flap near prospective gastric resection line nourished by right gastroepiploic artery) and omental wrapping around the anastomosis with interrupted sutures. In addition, participants in two studies (Bhat 2006; Dai 2010) underwent postoperative chemoradiotherapy, and the remaining study provided no information on postoperative chemotherapy (Zheng 2013).
Outcomes
Hospital mortality (Dai et al reported only mortality due to pulmonary insufficiency (Dai 2010)), anastomotic leakage and anastomotic strictures were reported in all three included studies. In addition, other postoperative complications (pulmonary complications, infection), cardiac complications, vocal cord palsy and perijejunostomy leakage were reported in Bhat's study (Bhat 2006). Dai et al reported other postoperative complications, duration of hospital stay and median duration from operation to the development of benign strictures (without standard deviation (SD) value) (Dai 2010), and Zheng et al provided data on duration of hospitalisation and tumour recurrence (Zheng 2013).
Excluded studies
After irrelevant records were removed, 27 studies remained. Another 24 studies were excluded after abstracts and full texts were read because they did not employ a randomised controlled design (Characteristics of excluded studies).
Risk of bias in included studies
Allocation
Restricted randomisation with the use of permuted blocks to generate allocation sequences was applied in two studies (Bhat 2006; Dai 2010); both studies stated that, to reduce the bias caused by prediction of length of the block, length of the blocks was varied randomly. In Zheng's study, individual randomisation was performed using the sealed envelope technique (Zheng 2013), and the allocation procedure was blinded to participants and surgeons despite uncertainty among personnel about who actually generated the allocation sequence. No detailed information on allocation concealment was provided for the other two studies (Bhat 2006; Dai 2010).
Blinding
The allocation procedure was blinded to participants and surgeons in Zheng's study (Zheng 2013). Although it was not clear whether surgeons or outcome assessors knew about allocation during outcome measurements and data analysis, it was very likely that blinding was carried out at least among participants. No information on blinding was clearly stated in the two remaining studies; however, study authors for both stated that only surgeons knew the allocation of study participants (Bhat 2006; Dai 2010). Thus, it was likely that single or double (if the outcome assessors were not the surgeons) blinding was performed.
Incomplete outcome data
All studies reported hospital mortality and causes of death (Bhat 2006; Dai 2010; Zheng 2013). Two participants were lost to follow‐up in Zheng's study (Zheng 2013), and Dai et al did not provide data on deceased participants in their analysis of anastomotic strictures (Dai 2010). However, the studies of Dai and Bhat did not list prespecified periods of follow‐up, percentages of participants who completed prespecified follow‐up or causes of loss to follow‐up. None of these studies provided data on long‐term survival.
Selective reporting
None of the included studies provided sufficient information to permit judgement on selective reporting because none provided study protocols (Bhat 2006; Dai 2010; Zheng 2013).
Other potential sources of bias
None of the included studies reported funding sources (Bhat 2006; Dai 2010; Zheng 2013). In addition, Dai et al did not compare differences in the numbers of participants receiving TTE and THE (Dai 2010).
Effects of interventions
See: Table 1
Survival, omentoplasty‐related complications and quality of life were not addressed in the included studies; other primary and secondary outcomes were compared and analysed.
Primary outcomes
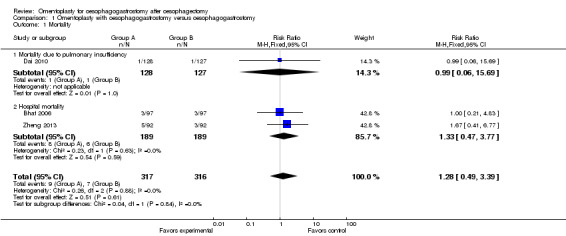
Mortality (death from any cause in the early postoperative period: within one month after surgery)
All studies reported hospital mortality, and no significant difference was observed between the study group (oesophagogastrostomy plus omentoplasty) and the control group (oesophagogastrostomy alone) (RR 1.28, 95% CI 0.49 to 3.39, I2 = 0%) (Bhat 2006; Dai 2010; Zheng 2013) (Figure 3).
3.
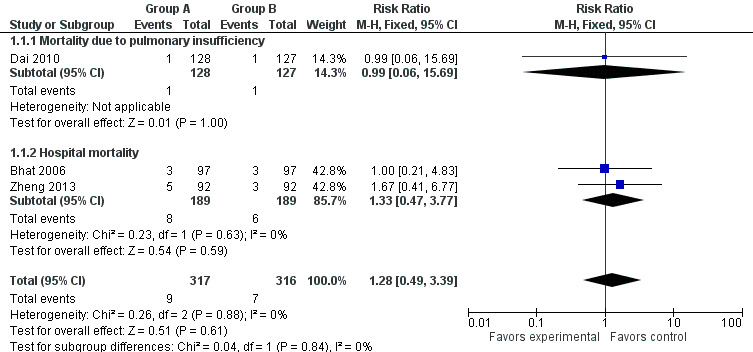
Forest plot of comparison: 1 Omentoplasty + oesophagogastrostomy versus oesophagogastrostomy, outcome: 1.1 Mortality.
Survival (during the follow‐up period): at one, three and five years
None of the included studies reported differences in long‐term survival between the two groups. We tried to contact the original study authors to obtain detailed information on survival, but we received no response, or we learned that no data were available.
Anastomotic leakage rate after surgery
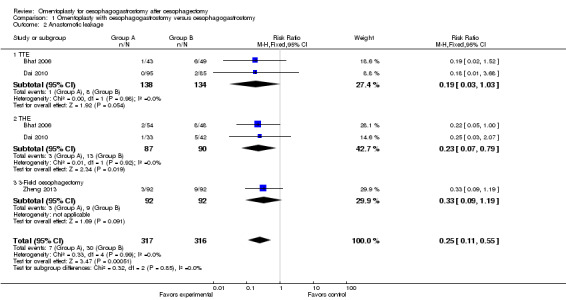
Data from the included studies show that the incidence of anastomotic leakage was significantly less in the study group than in the control group (RR 0.25, 95% CI 0.11 to 0.55, I2 = 0%) (Bhat 2006; Dai 2010; Zheng 2013).
In addition, subgroup analysis was performed on the basis of the surgical approach used (TTE, THE or three‐field oesophagectomy). For participants treated with THE, the incidence of anastomotic leakage was obviously different between the study group and the control group (RR 0.23, 95% CI 0.07 to 0.79, I2 = 0%). Nonetheless, among those treated with TTE, no significant difference was identified (RR 0.19, 95% CI 0.03 to 1.03, I2 = 0%). Among participants treated with three‐field oesophagectomy, no significant difference in the rate of anastomotic leakage was noted between the two groups (RR 0.33, 95% CI 0.09 to 1.19) (Figure 4).
4.
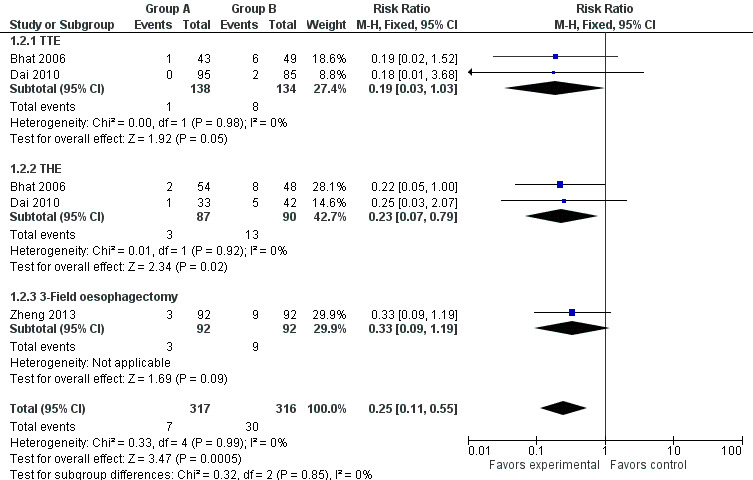
Forest plot of comparison: 1 Omentoplasty + oesophagogastrostomy versus oesophagogastrostomy, outcome: 1.2 Anastomotic leakage.
Secondary outcomes
Surgery‐related complications, such as major vascular injury, tracheal injury, recurrent laryngeal nerve injury or thoracic duct injury
Pulmonary complications
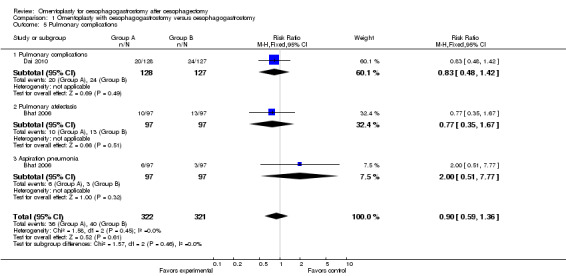
Bhat et al and Dai et al reported pulmonary complications (Bhat 2006; Dai 2010), and Bhat et al provided detailed information on the incidences of pulmonary atelectasis and aspiration pneumonia (Bhat 2006). Omentoplasty did not significantly increase the incidence of pulmonary complications (RR 0.90, 95% CI 0.59 to 1.36, I2 = 0%).
Wound infections
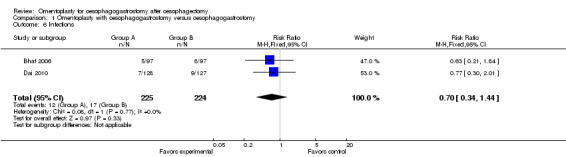
Bhat et al reported the incidence of wound sepsis (Bhat 2006), and Dai et al provided information on abdominal/thoracic infections (Dai 2010). Pooled analysis showed no significant differences between the study group and the control group (RR 0.70, 95% CI 0.34 to 1.44, I2 = 0%).
Cardiac complications
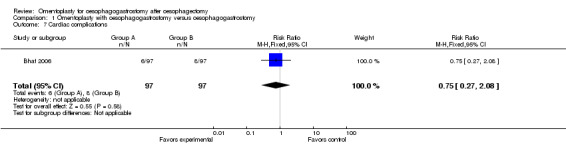
According to Bhat's study, the incidence of cardiac complications did not differ significantly between groups (RR 0.75, 95% CI 0.27 to 2.08) (Bhat 2006).
Vocal cord palsy
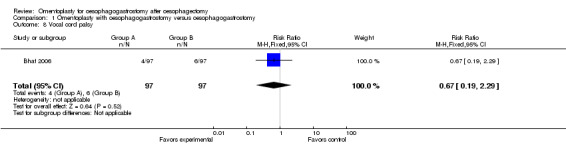
Bhat's study revealed no obvious differences in vocal cord palsy between the two groups (RR 0.67, 95% CI 0.19 to 2.29) (Bhat 2006).
Perijejunostomy leakage
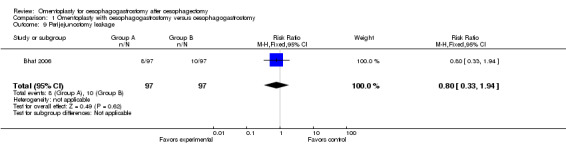
According to Bhat's study, omentoplasty did not reduce significantly the incidence of perijejunostomy leakage (RR 0.80, 95% CI 0.33 to 1.94) (Bhat 2006).
Other surgery‐related complications
No other surgery‐related complications were identified in the included studies (Bhat 2006; Dai 2010).
Omentoplasty‐related complications, such as peritonitis, intestinal obstruction or infection
No omentoplasty‐related complications were reported in any of the studies.
Anastomotic stenosis after surgery
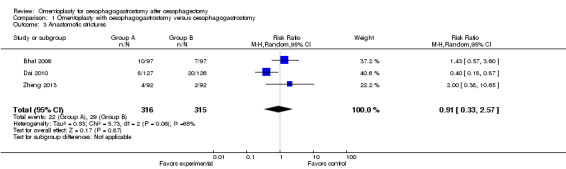
Information on anastomotic strictures was obtained for 631 participants (two were excluded owing to hospital mortality); results showed no significant differences between the study group and the control group (RR 0.91, 95% CI 0.33 to 2.57, I2 = 65%) (Bhat 2006; Dai 2010; Zheng 2013) (Figure 5). Heterogeneity was identified; thus, a random‐effects model was adopted for data synthesis. We intended to perform a subgroup analysis based on different follow‐up periods and surgical procedures, but available data from the included studies were insufficient.
5.
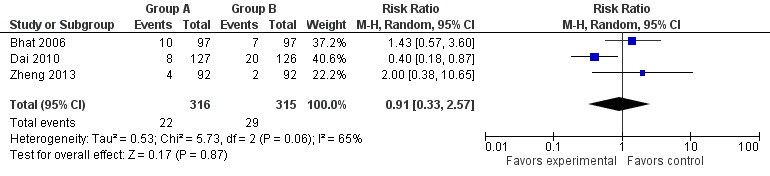
Forest plot of comparison: 1 Omentoplasty + oesophagogastrostomy versus oesophagogastrostomy, outcome: 1.3 Anastomotic strictures.
Duration of hospital stay
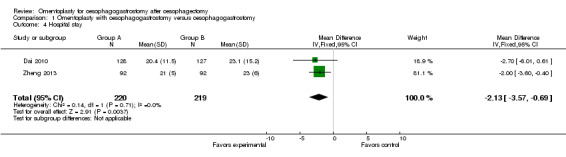
Two studies addressed this issue (Dai 2010; Zheng 2013). The duration of hospitalisation was shorter in the study group than in the control group (MD ‐2.13, 95% CI ‐3.57 to ‐0.69, I2 = 0%) (Figure 6).
6.
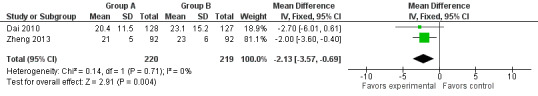
Forest plot of comparison: 1 Omentoplasty + oesophagogastrostomy versus oesophagogastrostomy, outcome: 1.4 Hospital stay.
Quality of life
Quality of life was not addressed in the included studies.
Sensitivity analysis
We also performed meta‐analyses on mortality (RR 1.28, 95% CI 0.48 to 3.41), anastomotic leakage (RR 0.25, 95% CI 0.12 to 0.56), pulmonary complications (RR 0.88, 95% CI 0.58 to 1.34) and infections (RR 0.70, 95% CI 0.34 to 1.44) using the random‐effects method, and on anastomotic stricture (RR 0.76, 95% CI 0.44 to 1.28) using the fixed‐effect method: The robustness of study results did not differ significantly.
Whether the deceased participant in the study group was assigned as having postoperative anastomotic stricture and the participant in the control group as not having the complication (RR 1.21, 95% CI 0.37 to 3.92), or vice versa (RR 0.64, 95% CI 0.33 to 1.23), the two groups did not differ significantly.
Discussion
Summary of main results
This review confirms that application of omentoplasty with oesophagogastrostomy after oesophagectomy for oesophageal cancer can reduce the incidence of anastomotic leakage—one of the most severe and fatal complications of this surgical intervention. In addition, the duration of hospital stay was shorter for patients treated with omentoplasty. However, omentoplasty did not significantly increase or decrease hospital mortality nor the incidence of postoperative complications (such as anastomotic strictures, pulmonary complications, infections, cardiac complications, vocal cord palsy and perijejunostomy leakage).
Overall completeness and applicability of evidence
In this systematic review, included samples consisted of patients with oesophageal cancer. At least two studies (Bhat 2006; Dai 2010) enrolled patients with two histological types: squamous cell carcinoma and adenocarcinoma; the former was the predominant type. However, no conclusions can be drawn regarding omentoplasty for different types of cancer, as no separate comparisons were provided. In addition, all of the included RCTs were carried out in China and India, and no data on ethnic or racial groups of study participants were available. It is well established that the frequency of different histological types of oesophageal cancer varies considerably in different geographic locations and ethnic groups (Chalasani 1998; DeMeester 1997; Li 1997). Thus, applicability of the evidence might be limited for ethnic groups in which the incidence of adenocarcinoma is increasing. Nevertheless, given that this is a surgical technique, it is reasonable to say that omentoplasty may have similar effects on people from different ethnic groups.
With regards to different surgical procedures, first, the RCTs included in this review represent two methods of anastomosis for esophagogastrostomy, namely, manual (Bhat 2006; Zheng 2013) and mechanical (Dai 2010) anastomosis. With both methods, omentoplasty was reported to significantly reduce the incidence of leakage of anastomosis. Nonetheless, data were insufficient for assessment of the influences of manual and mechanical methods on participant outcomes. The latest systematic review on the influence of different anastomotic methods on postoperative anastomotic leakage revealed that manual and mechanical stapler anastomoses yielded similar results (Honda 2013).
Second, in the three included studies, 177 study participants underwent THE with cervical anastomosis, 272 received TTE with intrathoracic anastomosis and 184 were treated with three‐field oesophagectomy (right thoracotomy with cervical anastomosis and three‐field lymphadenectomy). In practice, the three different approaches have their own pros and cons. TTE, which provides direct exposure of the thoracic oesophagus, might be beneficial for individuals with positive lymph node metastasis or with tumour at the distal oesophagus (Barreto 2010; Omloo 2007); however, it can lead to higher risks of cardiorespiratory dysfunction and other catastrophic consequences, such as mediastinitis. Treatment with THE might be preferred for patients with respiratory co‐morbidity or advanced age who suffer from early‐stage oesophageal cancer without lymph node involvement (Boshier 2011; Donohoe 2012). As a thoracotomy incision is not required with THE, risks of postoperative pulmonary complications and mediastinitis are reduced; however, THE is associated with poorer visualisation of the upper and middle oesophagus and greater risk of subsequent stricture and recurrent laryngeal nerve injury (Barreto 2010; Boshier 2011; Donohoe 2012). Three‐field oesophagectomy provides the advantage of radical lymphadenectomy, which has the potential to improve patient survival. However, it causes significant trauma and may increase surgical risks and postoperative complications as well. In the current systematic review, omentoplasty was associated with a decreased incidence of anastomotic leakage in THE procedures. Meanwhile, for TTE and three‐field oesophagectomy, although statistical significance was not identified, leakage was less common in the omentoplasty group, indicating that patients treated with oesophagogastrostomy, regardless of the anastomotic approach selected, might benefit from omentoplasty.
Furthermore, two studies reported long‐term outcomes. Zheng's study provided data on tumour recurrence (Zheng 2013) and suggested that omentoplasty did not significantly influence this outcome. Bhat's study reported overall two‐year and five‐year survival rates (Bhat 2006)—a major concern for patients with cancer. However, the study did not perform separate analyses for the study group and the control group; therefore conclusions on long‐term survival require further investigation.
Finally, none of the included studies evaluated quality of life after oesophagectomy. The volume of residual stomach is decreased significantly after oesophagectomy, and postoperative gastro‐oesophageal reflux persists in most patients. There is no doubt that oesophagectomy followed by oesophagogastrostomy would have a negative impact on quality of life after the operation (Biere 2011; Teoh 2011). Given this information and the fact that overall five‐year survival for oesophageal cancer remains very low, it is important to address this issue when a new surgical technique is introduced, especially when it may have an effect on subsequent quality of life.
Quality of the evidence
In this review, we identified three RCTs including 633 study participants with oesophageal cancer. All included studies provided sufficient details to confirm the randomisation procedure. The main limitations of design and implementation of the included studies consisted of the following.
Allocation concealment, which might lead to bias in sequence generation procedures (Higgins 2008), was not mentioned or was not clear in the included studies.
Authors of all included studies pointed out that surgeons were the only people who knew the allocation, but whether surgeons interacted with outcome assessors was not stated. Thus, we have reason to doubt whether outcome assessors were blinded (surgeons can also be the assessors), and the quality of the evidence provided by these studies might be weakened, as it has been proposed that blind assessment of outcomes may be more important than blind administration of treatment (Day 2000).
No studies provided information on clinical trial registration or on study protocols, making it difficult to judge the predetermined duration of follow‐up and the corresponding rate of loss to follow‐up, as well as the bias caused by selective reporting.
Potential biases in the review process
For a comprehensive search of related studies, the literature search was conducted independently by two different parties (review authors and Cochrane Trials Search Co‐ordinator) according to specific search strategies. To minimise bias in the review process, two review authors independently carried out assessment of eligibility and risk of bias for all studies, as well as data extraction.
Anastomotic leaks may present a broad spectrum of manifestations, ranging from asymptomatic and minor anastomotic defects that are observed only on contrast studies to fulminant leaks with systemic sepsis and multi‐organ failure (Alanezi 2004; Urschel 1995). Some scholars have pointed out that lack of an accurate definition of an anastomotic leak might be a main reason for reported variation in the incidence of anastomotic leakage (Lerut 2002). However, one study provided a detailed definition (Zheng 2013) and the other two (Bhat 2006; Dai 2010) did not provide a clear definition of anastomotic leak—one of the most important outcome measurements. Also, available data were insufficient to confirm whether the same definitions or standards were adopted for outcomes reported in both studies, such as anastomotic stricture.
Funnel plot asymmetry testing was not performed to test publication bias as only three studies were included.
Agreements and disagreements with other studies or reviews
Anastomotic leakage of oesophagogastrostomy causes considerable morbidity and mortality after oesophagectomy (Ancona 2006; Urschel 1995). A postoperative mortality rate as high as 40% could be related to this complication (Turkyilamz 2009). In general, our study provided results similar to those of former studies, including RCTs included in this review (Bhat 2006; Dai 2010; Zheng 2013) and other case‐control studies (Ohwada 2002; Thakur 2004), showing that omentoplasty decreases the incidence of anastomotic leaks. One of the included studies showed that the THE procedure had a higher risk of anastomotic leakage than TTE (Dai 2010), which is consistent with other findings (Chang 2008; Hulscher 2001; Rindani 1999). However, another included study identified no difference in anastomotic leakage between THE and TTE (Bhat 2006). Future RCTs are needed to investigate this discrepancy.
Pooled analysis of anastomotic strictures in the three studies revealed heterogeneity. In Dai's study, the incidence of this complication in the study group was much lower than in the control group (Dai 2010); in the other two studies, although statistical significance was not identified, strictures were more common in the study group (Bhat 2006; Zheng 2013). Bhat et al did not provide the definition of anastomotic stricture applied (Bhat 2006), and follow‐up periods for assessment of stricture were different among the three studies. Thus, we assume that inconsistency might be attributed to the different diagnostic standards of anastomotic stricture applied and to different follow‐up periods. The two different methods of anastomosis involving manual (Bhat 2006; Zheng 2013) or mechanical anastomosis (Dai 2010) can also contribute to the different incidences of postoperative stricture reported (Honda 2013). Heterogeneity might be explained by the different ratios of TTE to THE in the two studies (92:102 in Bhat's study, 180:75 in Dai's study), for these two surgical procedures could lead to differences in the incidence of stricture of anastomosis and the need for oesophageal dilatation (Chang 2008; Raz 2008). However, a subgroup analysis based on different surgical procedures was not performed because no relevant data were available.
Authors' conclusions
Implications for practice.
This systematic review suggests that omentoplasty may provide additional benefit in decreasing the incidence of anastomotic leakage and the duration of hospital stay after oesophagectomy and oesophagogastrostomy for oesophageal cancer, although omentoplasty does not increase the rate of other postoperative complications, including pulmonary complications, infection, cardiac complications, vocal cord palsy and perijejunostomy leakage. Additional RCTs are needed to confirm the effects of omentoplasty in oesophagogastrostomy.
Implications for research.
A series of studies, including RCTs, have been carried out to investigate the effects and safety of omentoplasty for oesophagogastrostomy in patients with oesophageal cancer. Nonetheless, additional RCTs are needed to address the issues for which no conclusions have been reached, such as:
benefits of omentoplasty in different subgroups of patients (e.g. different cancer stages, ages, genders, races);
benefits of omentoplasty in different surgical procedures of oesophagectomy and oesophagogastrostomy (i.e. TTE vs THE vs three‐field oesophagectomy; manual vs mechanical anastomosis; traditional vs minimal invasive oesophagectomy);
influence of omentoplasty on anastomotic stricture after oesophagogastrostomy and oesophagectomy, as well as its effects on different surgical procedures;
effects of omentoplasty on long‐term survival; and
effects of omentoplasty on quality of life.
Additional attention should be paid to:
registering trials before implementation;
performing and reporting on randomisation, allocation concealment and blinding (when appropriate); and
defining the target period for follow‐up and reporting cases of loss to follow‐up in the study protocol.
In addition, large multi‐centre trials that can include more participants are welcome.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2014 | New search has been performed | The search was rerun and the review updated |
| 9 June 2014 | New citation required and conclusions have changed | One new study was identified, and the conclusion has changed |
Acknowledgements
We would like to thank C Xie, R Malthaner and G Leontiadis for their comments on protocol development; and R Malthaner, M Walsh and S Rhodes for their comments on development of the full review. We also thank the Co‐ordinator of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, Karin Dearness, and the Cochrane Trials Search Co‐ordinator, Racquel Simpson, for their work on this review.
Appendices
Appendix 1. MEDLINE search strategy
randomised controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
exp esophagus/
esophag$.mp.
oesophag$.mp.
paraesophageal region.mp.
or/12‐15
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or cyst$ or growth$ or adenocarcin$ or malig$).mp.
Precancerous Conditions/ or Carcinoma, Squamous Cell/ or precursor lesion$.mp. or Esophageal Neoplasms/ or Ulcer/ or Peptic Ulcer/
or/17‐18
16 and 19
exp Esophagostomy/ or exp Anastomosis, Surgical/ or $sophagogastr$.mp.
exp Esophagoplasty/
exp Reconstructive Surgical Procedures/
anastomo$.mp.
or/21‐24
exp Omentum/
omentum.mp.
omentoplasty.mp.
omental wrapping.mp.
or/26‐29
exp Peritoneum/
peritoneum.mp.
31 or 32
exp Surgical Flaps/
Surgical Flap$.mp.
34 or 35
33 and 36
Peritoneum/tr [Transplantation]
37 or 38
(20 or 25) and (30 or 39)
11 and 40
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
exp esophagus/
esophag$.mp.
oesophag$.mp.
paraesophageal region.mp.
or/1‐4
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or cyst$ or growth$ or adenocarcin$ or malig$).mp.
Precancerous Conditions/ or Carcinoma, Squamous Cell/ or precursor lesion$.mp. or Esophageal Neoplasms/ or Ulcer/ or Peptic Ulcer/
or/6‐7
5 and 8
exp Esophagostomy/ or exp Anastomosis, Surgical/ or $sophagogastr$.mp.
exp Esophagoplasty/
exp Reconstructive Surgical Procedures/
anastomo$.mp.
or/10‐13
exp Omentum/
omentum.mp.
omentoplasty.mp.
omental wrapping.mp.
or/15‐18
exp Peritoneum/
peritoneum.mp.
20 or 21
exp Surgical Flaps/
Surgical Flap$.mp.
23 or 24
22 and 25
Peritoneum/tr [Transplantation]
26 or 27
(9 or 14) and (19 or 28)
Appendix 3. EMBASE search strategy
Clinical trial/
Randomized controlled trial/
Randomization/
Single‐Blind Method/
Double‐Blind Method/
Cross‐Over Studies/
Random Allocation/
Placebo/
Randomi?ed controlled trial$.tw.
Rct.tw.
Random allocation.tw.
Randomly allocated.tw.
Allocated randomly.tw.
(allocated adj2 random).tw.
Single blind$.tw.
Double blind$.tw.
((treble or triple) adj blind$).tw.
Placebo$.tw.
Prospective study/
or/1‐19
Case study/
Case report.tw.
Abstract report/ or letter/
or/21‐23
20 not 24
exp esophagus/
esophag$.mp.
oesophag$.mp.
paraesophageal region.mp.
or/26‐29
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or cyst$ or growth$ or adenocarcin$ or malig$).mp.
Precancerous Conditions/ or Carcinoma, Squamous Cell/ or precursor lesion$.mp. or Esophageal Neoplasms/ or Ulcer/ or Peptic Ulcer/
31 or 32
30 and 33
exp Esophagostomy/ or exp Anastomosis, Surgical/ or $sophagogastr$.mp.
exp Esophagoplasty/
exp Reconstructive Surgical Procedures/
anastomo$.mp.
or/35‐38
exp Omentum/
omentum.mp.
omentoplasty.mp.
omental wrapping.mp.
or/40‐43
exp Peritoneum/
peritoneum.mp.
45 or 46
exp Surgical Flaps/
Surgical Flap$.mp.
48 or 49
47 and 50
Transplantation.mp. or exp Transplantation/
47 and 52
51 or 53
(34 or 39) and (44 or 54)
25 and 55
Data and analyses
Comparison 1. Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.49, 3.39] |
| 1.1 Mortality due to pulmonary insufficiency | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.69] |
| 1.2 Hospital mortality | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.47, 3.77] |
| 2 Anastomotic leakage | 3 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.11, 0.55] |
| 2.1 TTE | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.03, 1.03] |
| 2.2 THE | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.79] |
| 2.3 3‐Field oesophagectomy | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.19] |
| 3 Anastomotic strictures | 3 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.33, 2.57] |
| 4 Hospital stay | 2 | 439 | Mean Difference (IV, Fixed, 95% CI) | ‐2.13 [‐3.57, ‐0.69] |
| 5 Pulmonary complications | 2 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.36] |
| 5.1 Pulmonary complications | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.42] |
| 5.2 Pulmonary atelectasis | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.67] |
| 5.3 Aspiration pneumonia | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.51, 7.77] |
| 6 Infections | 2 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.44] |
| 7 Cardiac complications | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.27, 2.08] |
| 8 Vocal cord palsy | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.29] |
| 9 Perijejunostomy leakage | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.33, 1.94] |
1.1. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 2 Anastomotic leakage.
1.3. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 3 Anastomotic strictures.
1.4. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 4 Hospital stay.
1.5. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 5 Pulmonary complications.
1.6. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 6 Infections.
1.7. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 7 Cardiac complications.
1.8. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 8 Vocal cord palsy.
1.9. Analysis.
Comparison 1 Omentoplasty with oesophagogastrostomy versus oesophagogastrostomy, Outcome 9 Perijejunostomy leakage.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhat 2006.
| Methods | Design: prospective randomised trial Time for follow‐up: median follow‐up time for surviving participants: 22 months (range 3 to 52 months) |
|
| Participants | Total number of participants: 194 Setting: single medical centre (Sher‐i‐Kashmir Institute of Medical Sciences) Inclusion criteria: oesophageal carcinoma Exclusion criteria: (1) previous or co‐existing cancer; (2) previous gastric or oesophageal surgery, neoadjuvant chemotherapy or radiation therapy; (3) recurrent laryngeal nerve palsy; (4) tumour invading the peri‐oesophageal tissues Age: mean age of included participants: 52.5 Sex: male/female ratio of included participants: 3:1.8 |
|
| Interventions | Group A: oesophagogastrectomy along with reinforcement of the anastomosis with the pedicled omentum (manual anastomosis) + postoperative chemoradiation therapy Group B: oesophagogastrectomy without using the omentum around the anastomosis (manual anastomosis) + postoperative chemoradiation therapy |
|
| Outcomes | Outcomes reported in both groups: hospital mortality, anastomotic leakage, anastomotic strictures, pulmonary atelectasis, aspiration pneumonia, wound sepsis, cardiac complications, vocal cord palsy, perijejunostomy leakage, anastomotic strictures Time points for follow‐up: every 3 months for the first 3 years and every 4 to 6 months thereafter |
|
| Notes | Operation procedure (TTE/THE): group A: 43:54; group B: 49:48 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A restricted randomisation plan was used. Patients were assigned randomly to permuted blocks of 4 to 6 patients" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The surgeons only knew inside the operating theatre whether a patient belonged to the study group or the control group" However, the authors did not specify whether single blinding or double blinding was performed (they did not indicate whether surgeons were the assessors) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding could not be applied to surgeons. However participants could have been blinded to surgical treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Hospital mortality was reported and causes were listed; however, study authors did not list the percentages of participants who completed prespecified follow‐up or causes of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol of this study is not available |
| Other bias | Unclear risk | Funding source was not stated |
Dai 2010.
| Methods | Design: prospective randomised trial Time for follow‐up: median follow‐up time for surviving patients: 22 months (range 3 to 52 months) |
|
| Participants | Total number of participants: 255 Setting: single medical centre (Xinqiao Hospital of the Third Military Medical University) Inclusion criteria: (1) oesophageal cancer (stage I, II and III) according to TNM classification of oesophagus and oesophagogastric junctions in the Seventh Edition of the AJCC Cancer Staging Manual (AJCC 2009); (2) previously untreated patients Exclusion criteria: (1) other previous or concomitant malignant diseases; (2) previous gastric or oesophageal surgery, neoadjuvant chemotherapy or radiation therapy; (3) advanced tumour stage (T4 disease), advanced lymph node involvement or distant metastasis (M1 lymph or M1 disease); (4) poor pulmonary reserve (forced expiratory volume < 50% of normal) Age: group A: 62 ± 9 years; group B: 64 ± 8 years Sex (male/female): group A: 98:30; group B: 105:22 |
|
| Interventions | Group A: oesophagogastrectomy along with the pedicle omental flap (stapled anastomosis) + postoperative chemoradiation therapy Group B: oesophagogastrectomy without the pedicle omental flap around the anastomosis (stapled anastomosis) + postoperative chemoradiation therapy |
|
| Outcomes | Outcomes reported in both groups: mortality due to pulmonary insufficiency; anastomotic leakage; anastomotic strictures; pulmonary complications; abdominal or thoracic infections; hospital stay (days); medium duration from operation to development of benign strictures (standard deviation not provided) Time points for follow‐up: every 3 months for the first 3 years and every 4 to 6 months thereafter |
|
| Notes | Operation procedure (TTE/THE): group A: 95:33; Group B: 85:42 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A restricted randomization plan was used. Patients were assigned randomly to permuted blocks of 4 to 6 patients" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The surgeon inside the operating theatre was the only one to know whether a patient belonged to the study group or the control group" However, study authors did not specify whether single blinding or double blinding was performed (they did not indicate whether surgeons were the assessors) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding could not be applied to the surgeons. However, participants could be well blinded to the surgical treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Hospital mortality was reported, and causes were listed; however, study authors did not list percentages of participants who completed prespecified follow‐up or causes of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available; survival rate and all‐cause mortality (only mortality due to pulmonary complications was provided) were not reported |
| Other bias | Unclear risk | Funding source was not stated Comparison of surgical procedure (TTE and THE) between 2 groups was not provided |
Zheng 2013.
| Methods | Design: prospective randomised trial Time for follow‐up: 3 years after surgery |
|
| Participants | Total number of participants: 184 Setting: single medical centre (no clear information was provided) Inclusion criteria: patients who underwent radical oesophagectomy with 3‐field lymphadenectomy Exclusion criteria: patients who received chemotherapy or radiotherapy before surgery; patients undergoing emergency surgery Age: group A: 67.5 ± 11.2 years; group B: 65.7 ± 9.4 years Sex (male/female): group A: 56:36; group B: 54:38 |
|
| Interventions | Group A: thoracic oesophagectomy + 3‐field lymphadenectomy (hand‐sewn 2‐layered anastomosis) + omentoplasty Group B: thoracic oesophagectomy + 3‐field lymphadenectomy (hand‐sewn 2‐layered anastomosis) |
|
| Outcomes | Outcomes reported in both groups: hospital mortality; anastomotic leakage; anastomotic strictures; hospital stay (days); tumour recurrence Time points for follow‐up: 7th and 14th days after the operation, and within 3 years following surgery |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed in the operating room in a blind manner for both the patient and the surgeon, using the sealed envelope technique" |
| Allocation concealment (selection bias) | Unclear risk | "Randomization was performed in the operating room in a blind manner for both the patient and the surgeon, using the sealed envelope technique." But it's not clear who performed the randomisation procedure |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Randomisation was blinded to participants and surgeons. It's very likely that blinding was performed at least on participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Whether surgeons and outcome assessors knew about participants' procedures, participants could be well blinded to surgical treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Hospital mortality and numbers lost‐to‐follow‐up were reported; however, study authors did not list numbers of participants lost to follow‐up in each group nor causes of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol of this study is not available |
| Other bias | Unclear risk | Funding source was not stated |
AJCC: American Joint Committee on Cancer; TNM: tumour‐node‐metastasis; THE: transhiatal oesophagogastrectomy; TTE: transthoracic oesophagogastrectomy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cui 2000 | Research in animal models |
| Dockendorf 1993 | Research in animal models |
| Fekete 1981 | Retrospective study |
| Freeman 1982 | Not about omentoplasty after oesophagectomy |
| Goldsmith 1968 | Retrospective study |
| Goldsmith 1973 | Not about omentoplasty after oesophagectomy for oesophageal cancer |
| Hayari 2004 | Research in animal models |
| Ignjatovic 1998 | Not about omentoplasty after oesophagectomy for oesophageal cancer |
| Karaoglanoglu 2007 | Commentary |
| Liebermann‐Meffert 1991 | Review |
| Liebermann‐Meffert 2000 | Review about omentum |
| Liu 2006 | Retrospective study |
| Maeda 1999 | Retrospective study |
| Martins 1999 | Investigation on protection of great vessels, not on anastomosis |
| Nikoladze 1993 | Retrospective study |
| Ohwada 2000 | Retrospective study |
| Ohwada 2002 | Retrospective study |
| Thakur 2004 | Retrospective study |
| Thakur 2007 | Retrospective study about oesophagectomy and other thoracic diseases |
| Yasunori 2004 | Retrospective study |
| Yener 2010 | Retrospective study |
| Yuan 2011 | Editorial |
| Yuan 2012 | Prior version of this systematic review |
| Zhang 1987 | Retrospective study |
Differences between protocol and review
None.
Contributions of authors
Yong Yuan and Xiaoxi Zeng contributed equally to development of the review.
Yong Yuan developed the protocol, extracted and analysed data, assessed the quality of included studies and drafted the review.
Xiaoxi Zeng extracted and analysed data and assessed the quality of included studies.
Yang Hu contributed to data analysis and interpretation.
Tianpeng Xie contributed to interpretation of results.
Yongfan Zhao organised the team and made important intelligent contributions.
Sources of support
Internal sources
West China Hospital of Sichuan University, China.
External sources
No sources of support supplied
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bhat 2006 {published data only}
- Bhat MA, Dar MA, Lone GN, Dar AM. Use of pedicled omentum in esophagogastric anastomosis for prevention of anastomotic leak. Annals of Thoracic Surgery 2006;82(2):1857‐62. [DOI] [PubMed] [Google Scholar]
Dai 2010 {published data only}
- Dai JG, Zhang ZY, Min JX, Huang XB, Wang JS. Wrapping of the omental pedicle flap around esophagogastric anastomosis after esophagectomy for esophageal cancer. Surgery 2010;149(3):404‐10. [DOI] [PubMed] [Google Scholar]
Zheng 2013 {published data only}
- Zheng QF, Wang JJ, Ying MG, Liu SY. Omentoplasty in preventing anastomotic leakage of oesophagogastrostomy following radical oesophagectomy with three‐field lymphadenectomy. European Journal of Cardio‐thoracic Surgery 2013;43(2):274‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cui 2000 {published data only}
- Cui Y, Urschel JD. Omentoplasty reinforcement of esophagogastric anastomoses in rats. Annals of Thoracic and Cardiovascular Surgery 2000;6(6):361‐2. [PubMed] [Google Scholar]
Dockendorf 1993 {published data only}
- Dockendorf BL, Frazee RC, Matheny RG. Omental pedicle graft to improve ischemic anastomoses. Southern Medical Journal 1993;86(6):628‐32. [DOI] [PubMed] [Google Scholar]
Fekete 1981 {published data only}
- Fekete F, Breil P, Ronsse H, Tossen JC, Langonnet F. EEA stapler and omental graft in esophagogastrectomy: experience with 30 intrathoracic anastomoses for cancer. Annals of Surgery 1981;193(6):825‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1982 {published data only}
- Freeman JL, Brondbo K, Osborne M, Noyek AM, Shaw HJ, Rubin A, et al. Greater omentum used for carotid cover after pharyngolaryngoesophagectomy and gastric 'pull‐up' or colonic 'swing'. Archives of Otolaryngology 1982;108(11):685‐7. [DOI] [PubMed] [Google Scholar]
Goldsmith 1968 {published data only}
- Goldsmith HS, Kiely AA, Randall HT. Protection of intrathoracic esophageal anastomoses by omentum. Surgery 1968;63(3):464‐6. [PubMed] [Google Scholar]
Goldsmith 1973 {published data only}
- Goldsmith HS, Alday ES, Mikoshiba Y. Esophageal graft protection with intact omentum. Surgery, Gynecology and Obstetrics 1973;86(6):628‐32. [PubMed] [Google Scholar]
Hayari 2004 {published data only}
- Hayari L, Hershko DD, Shoshani H, Maor R, Mordecovich D, Shoshani G. Omentopexy improves vascularization and decreases stricture formation of esophageal anastomoses in a dog model. Journal of Pediatric Surgery 2004;39(4):540‐4. [DOI] [PubMed] [Google Scholar]
Ignjatovic 1998 {published data only}
- Ignjatovic M, Cuk V, Zivotic‐Vanovic M, Minic L. New surgical technique of omental pedicle graft preparation for omentomyelopexy. Vojnosanitetski Pregled 1998;55(3):247‐54. [PubMed] [Google Scholar]
Karaoglanoglu 2007 {published data only}
- Karaoglanoglu N, Turyilmaz A, Eroglu A. Use of pedicled omentum and endostaplers in esophagogastric anastomosis. Annals of Thoracic Surgery 2007;83(6):2259‐60. [DOI] [PubMed] [Google Scholar]
Liebermann‐Meffert 1991 {published data only}
- Liebermann‐Meffert DMI, Siewert JR. The role of greater omentum in intrathoracic transposition. Netherlands Journal of Surgery 1991;43(5):154‐60. [PubMed] [Google Scholar]
Liebermann‐Meffert 2000 {published data only}
- Liebermann‐Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surgical Clinics of North America 2000;80(1):275‐93. [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu K, Zhang GC, Cai ZJ. Avoiding anastomotic leakage following esophagogastrostomy. Journal of Thoracic and Cardiovascular Surgery 1983;86(1):142‐5. [PubMed] [Google Scholar]
Maeda 1999 {published data only}
- Maeda M, Matsuzaki Y, Edagawa M, Shimizu T, Onitsuka T. Usefulness of the pedicled omental graft in thoracic surgery. Annals of Thoracic and Cardiovascular Surgery 1999;5(4):220‐4. [PubMed] [Google Scholar]
Martins 1999 {published data only}
- Martins AS, Lage HT, Lopes LR, Brandalise NA. Use of omentum pedicled graft to protect great vessels in gastric transposition for pharyngoesophageal cancer. Journal of Surgical Oncology 1999;70(3):181‐4. [DOI] [PubMed] [Google Scholar]
Nikoladze 1993 {published data only}
- Nikoladze GD, Songulashvili ZE. Use of great omentum in esophageal surgery. Khirurgiia 1993;2:77‐80. [PubMed] [Google Scholar]
Ohwada 2000 {published data only}
- Ohwada S, Ogawa T, Kawate S, Kawashima Y, Takeyoshi I, Koyama T, et al. Omentoplasty for cervical esophagogastrostomy following radical esophagectomy with three‐field dissection. Hepato‐Gastroenterology 2000;47(35):1305‐9. [PubMed] [Google Scholar]
Ohwada 2002 {published data only}
- Ohwada S, Ogawa T, Kawate S, Koyama T, Yamada T, Yoshimura S, et al. Omentoplasty versus no omentoplasty for cervical esophagogastrostomy following radical esophagectomy. Hepato‐Gastroenterology 2002;49(43):181‐4. [PubMed] [Google Scholar]
Thakur 2004 {published data only}
- Thakur B, Zhang CS, Tan ZB. Omentoplasty versus no omentoplasty for esophagogastrostomy after surgery for cancer of cardia and esophagus. Indian Journal of Cancer 2004;41(4):167‐9. [PubMed] [Google Scholar]
Thakur 2007 {published data only}
- Thakur B, Zhang CS, Cao FM. Use of greater omentum in thoracic onco‐surgery. Kathmandu University Medical Journal 2007;5(17):129‐32. [PubMed] [Google Scholar]
Yasunori 2004 {published data only}
- Yasunori K, Kenichi O, Hiroyuki C, Toshihiko S, Jun I, Yoichiro U. Omentoplasty for thoracic problems—usefulness of pedicled omentum and review of unsuccessful cases. Journal of the Japanese Association for Chest Surgery 2004;18(4):532‐7. [Google Scholar]
Yener 2010 {published data only}
- Yener A, Atila T, Atilla E, Hacı AA, Nurettin K. The use of pedicled omentum flap in the prevention of esophagogastric anastomotic leak in esophageal cancer. Turkish Journal of Thoracic and Cardiovascular Surgery 2010;18(4):300‐4. [Google Scholar]
Yuan 2011 {published data only}
- Yuan Y, Hu Y, Xie TP, Zhao YF. Omentoplasty for preventing anastomotic leaks after esophagogastrostomy. Surgery 2011;149(6):853‐4. [DOI] [PubMed] [Google Scholar]
Yuan 2012 {published data only}
- Yuan Y, Zeng X, Hu Y, Xie TP, Zhao Y. Omentoplasty for esophagogastrostomy after esophagectomy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008446.pub2] [DOI] [PubMed] [Google Scholar]
Zhang 1987 {published data only}
- Zhang K, Yang Y. Use of pedicled omentum in oesophagogastric anastomosis: analysis of 100 cases. Annals of the Royal College of Surgeons of England 1987;69:209‐11. [PMC free article] [PubMed] [Google Scholar]
Additional references
AJCC 2009
- Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al (editors). AJCC Cancer Staging Manual. 7th Edition. New York: Springer, 2009. [Google Scholar]
Alanezi 2004
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Annals of Thoracic and Cardiovascular Surgery 2004;10(2):71‐5. [PubMed] [Google Scholar]
Ancona 2006
- Ancona E, Cagol M, Epifani M, Cavallin F, Zaninotto G, Castoro C, et al. Surgical complications do not affect long term survival after esophagectomy for carcinoma of the thoracic esophagus and cardia. Journal of the American College of Surgeons 2006;203(5):661‐9. [DOI] [PubMed] [Google Scholar]
Barreto 2010
- Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World Journal of Gastroenterology 2010;14(16):3804‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biere 2011
- Biere SS, Maas KW, Bonavina L, Roig Garcia J, Berge Henegouwen MI, Rosman C, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi‐centered, randomized trial (TIME‐trial). BMC Surgery 2011;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blewett 2001
- Blewett CJ, Miller JD, Yong JEM, Bennett WF, Urschel JD. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Annals of Thoracic and Cardiovascular Surgery 2001;7(2):75‐8. [PubMed] [Google Scholar]
Blot 1999
- Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Seminars in Oncology 1999;26(5 Suppl 15):2‐8. [PubMed] [Google Scholar]
Boshier 2011
- Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: a meta‐analysis. Annals of Surgery 2011;254(6):894‐906. [DOI] [PubMed] [Google Scholar]
Chalasani 1998
- Chalasani N, Wo JM, Waring JP. Racial differences in the histology, location, and risk factors of esophageal cancer. Journal of Clinical Gastroenterology 1998;26(1):11‐3. [DOI] [PubMed] [Google Scholar]
Chang 2008
- Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Annals of Thoracic Surgery 2008;85(2):424‐9. [DOI] [PubMed] [Google Scholar]
Day 2000
- Day SJ, Altman DG. Blinding in clinical trials and other studies. British Medical Journal 2000;321(7259):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMeester 1997
- DeMeester TR. Esophageal carcinoma: current controversies. Seminars in Surgical Oncology 1997;13(4):217‐33. [DOI] [PubMed] [Google Scholar]
Donohoe 2012
- Donohoe CL, O'Farrell NJ, Ravi N, Reynolds JV. Evidence‐based selective application of transhiatal esophagectomy in a high‐volume esophageal center. World Journal of Surgery 2012;36(1):98‐103. [DOI] [PubMed] [Google Scholar]
Fisher 1998
- Fisher S, Brady L. Esophagus. In: Perez CA, Brady LJ editor(s). Principles and Practice of Radiation Oncology. Third Edition. Philadelphia: Lippincott‐Raven, 1998:1241‐56. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. www.cochrane‐handbook.org.
Honda 2013
- Honda M, Kuriyama A, Noma H, Nunobe S, Furukawa TA. Hand‐sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta‐analysis. Annals of Surgery 2013;257(2):238‐48. [DOI] [PubMed] [Google Scholar]
Hulscher 2001
- Hulscher JB, Tijssen JG, Obertop H, Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta‐analysis. Annals of Thoracic Surgery 2001;72(1):306‐13. [DOI] [PubMed] [Google Scholar]
Kollarova 2007
- Kollarova H, Machova L, Horakova D, Janoutova G, Janout V. Epidemiology of esophageal cancer. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 2007;151(1):17‐20. [DOI] [PubMed] [Google Scholar]
Koshy 2004
- Koshy M, Esiashvilli N, Landry JC, Thomas CR, Matthews RH Jr. Multiple management modalities in esophageal cancer: epidemiology, presentation and progression, work‐up, and surgical approaches. The Oncologist 2004;9(2):137‐46. [DOI] [PubMed] [Google Scholar]
Kurahashi 2004
- Kurahashi Y, Okubo K, Cho H, Sato T, Isobe J, Ueno Y. Omentoplasty for thoracic problems—usefulness of pedicled omentum and review of unsuccessful cases. Journal of the Japanese Association for Chest Surgery 2004;18(4):532‐7. [Google Scholar]
Lerut 2002
- Lerut T, Coosemans W, Decker G, Leyn P, Nafteux P, Raemdonck D. Anastomotic complications after esophagectomy. Digestive Surgery 2002;19:92‐8. [DOI] [PubMed] [Google Scholar]
Li 1997
- Li H, Yao SC. Surgical treatment for carcinoma of the oesophagus in Chinese language publications. British Journal of Surgery 1997;84(6):855‐7. [PubMed] [Google Scholar]
Mody 2002
- Mody RP. Carcinoma oesophagus—overview, update of literature. Journal of the Indian Medical Association 2002;100(9):569‐72. [PubMed] [Google Scholar]
Omloo 2007
- Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, Dekken H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five‐year survival of a randomized clinical trial. Annals of Surgery 2007;246(6):992‐1000. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Raz 2008
- Raz DJ, Tedesco P, Herbella FAM, Nipomnick I, May LW, Patti MG. Side‐to‐side stapled intra‐thoracic esophagogastric anastomosis reduces the incidence of leaks and stenosis. Diseases of the Esophagus 2008;21(1):69‐72. [DOI] [PubMed] [Google Scholar]
Rindani 1999
- Rindani R, Martin CJ, Cox MR. Transhiatal versus Ivor‐Lewis oesophagectomy: is there a difference?. The Australian and New Zealand Journal of Surgery 1999;69:187‐94. [DOI] [PubMed] [Google Scholar]
Teoh 2011
- Teoh AY, Yan Chiu PW, Wong TC, Liu SY, Hung Wong SK, Ng EK. Functional performance and quality of life in patients with squamous esophageal carcinoma receiving surgery or chemoradiation: results from a randomized trial. Annals of Surgery 2011;253(1):1‐5. [DOI] [PubMed] [Google Scholar]
Turkyilamz 2009
- Turkyilamz A, Eroglu A, Aydin Y, Tekinbas C, Erol MM, Karaoglanoglu N. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Diseases of the Esophagus 2009;22(2):119‐26. [DOI] [PubMed] [Google Scholar]
Urschel 1995
- Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. American Journal of Surgery 1995;169(6):634‐40. [DOI] [PubMed] [Google Scholar]
Verhoef 2007
- Verhoef C, Weyer R, Schaapveld M, Bastiaannet E, Plukker JTM. Better survival in patients with esophageal cancer after surgical treatment in university hospitals: a plea for performance by surgical oncologists. Annals of Surgical Oncology 2007;14(5):1678‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkins 2002
- Wilkins EW. Left thoracoabdominal approaches. In: Pearson FG, Cooper JD, Deslauriers J editor(s). Esophageal Surgery. Second Edition. Philadelphia: Churchill Livingstone, 2002:802‐9. [Google Scholar]
Zhang 1997
- Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of omentum‐mediated angiogenesis. Journal of Surgical Research 1997;67(2):147‐54. [DOI] [PubMed] [Google Scholar]


