Abstract
Cancer remains a leading global cause of mortality, demanding early diagnosis and effective treatment. Traditional therapeutic methods often fall short due to their need for more specificity and systemic toxicity. In this challenging landscape, nanodiamonds (ND) emerge as a potential solution, mitigating the limitations of conventional approaches. ND are tiny carbon particles that mimic traditional diamonds chemical stability and hardness and harness nanomaterials’ advantages. ND stands out for the unique properties that make them promising nanotheranostics candidates, combining therapeutic and imaging capabilities in one platform. Many of these applications depend on the design of the particle’s surface, as the surface’s role is crucial in transporting bioactive molecules, preventing aggregation, and building composite materials. This review delves into ND’s distinctive features, structural and optical characteristics, and their profound relevance in advancing cancer diagnosis and treatment methods. The report delves into how these exceptional ND properties drive the development of state-of-the-art techniques for precise tumor targeting, boosting the effectiveness of chemotherapy as a chemosensitizer, harnessing immunotherapy strategies, facilitating precision medicine, and creating localized microfilm devices for targeted therapies.
Keywords: Bioimaging, drug-delivery, nanotheranostic, nanodiamonds, nano-immunotherapy, microfilm devices
1. Introduction
Despite numerous advancements in oncology over the centuries, conventional chemotherapy remains the primary treatment approach. However, its nonselective biodistribution poses challenges, as it often results in low bioavailability at the tumor site, inducing detrimental effects on healthy tissues [1]. Consequently, patients experience dose-limiting adverse effects such as myelosuppression, leukopenia, and thrombocytopenia, which may necessitate treatment discontinuation [2–4]. Uncontrolled rapid cell division and growth are the hallmarks of cancer cells, during which they reprogram themselves to overcome a stressed environment [5, 6]. This helps them to adapt various strategies to bypass the effect of chemotherapy. Overexpression of drug efflux transporters is a strategy that rapidly effluxes drugs from the cancer cell and decreases the drug retention time within the tumor, eventually limiting the drug’s effect [7]. As a result, higher dosing regimens become necessary, affecting treatment compliance and patient morbidity and ultimately leading to treatment failure [8].
Moreover, chemotherapeutic drugs are low molecular weight, have short half-lives in the blood, and are rapidly distributed in healthy tissues, limiting their therapeutic efficacy [6]. Other than chemotherapy, treatments like radiation, surgery, and immunotherapy have been used for several decades. While they can be effective, they also come with toxic side effects, trauma from surgery, post-treatment complications, and the risk of disease recurrence [1]. These challenges result in suboptimal treatment outcomes, making achieving the necessary target doses for effective results difficult. Consequently, alternative techniques are imperative to overcome these obstacles and improve treatment efficacy. The rapid expansion of nanomedicine unfolds a juncture of innovative cancer therapy approaches, including early diagnosis with nanomaterials, targeted drug carriers [8], localized therapeutic elution using embedded micro-devices [9], and precision medicine [10]. Manipulating materials and devices at the nanoscale offers exciting potential in overcoming cancer therapy challenges. Nanoparticles, ranging from 1 to 1000 nanometers, possess distinct properties that improve anticancer drug efficacy and reduce side effects. Diverse nanoparticles, like liposomes, polymeric, gold, magnetic, and carbon-based nanoparticles, offer specific advantages, finding applications in cancer therapy [11–15]. Currently under exploration for biological applications, carbon-based nanomaterials such as fullerene, carbon nanotubes, graphene, carbon dots, and nanodiamonds are gaining significant attention owing to their exceptional properties. The versatility of each carbon-based nanoparticle arises from its distinct characteristics, making them adaptable materials chosen based on specific needs. Notably, nanodiamonds, characterized by diamond nanoparticles at the nanoscale, are particularly distinguished for their hardness and biocompatibility. This unique profile positions nanodiamonds as valuable materials with drug delivery and imaging applications. ND are carbon-based crystalline structures resembling diamonds and possess diamond-like properties, such as chemical stability, extreme hardness, and stiffness, while offering nanomaterial benefits like a large surface area, high drug-carrying capacity, precise surface modifications, and optical properties [16].
ND shows promise in cancer therapy due to its biocompatibility and potential for therapeutic and imaging applications [16]. Figure 1 shows the advantages of ND as a theranostic agent, implicating its application in diagnostics and cancer treatment. Therapy includes application in targeted drug delivery, gene delivery, ND embedded in drug-loaded microfilms enabling localized chemotherapeutic elution, photothermal and photodynamic therapy, i.e., laser irradiation based heat generated elimination of tumor cells. Apart from this, ND is also used in marker-based cancer diagnosis. It provides specificity and serves as a carrier for immunotherapeutics, such as immune checkpoint inhibitors or cytokines, tumor-specific antigens, or peptides. It effectively presents them to immune cells followed by their activation. This activation boosts the potency of immunotherapy approaches [17]. While there is promising progress in using ND for cancer drug delivery, the ongoing research is focused on optimizing various aspects of their application. This review article explores nanodiamonds’ distinctive features and properties of ND and their significance in advancing cancer diagnosis and treatment methods. The report delves into how these unique properties of ND contribute to developing cutting-edge approaches for precise tumor targeting, enhancing the efficacy of chemotherapy (chemosensitizer), utilizing immunotherapy strategies, enabling precision medicine, and localized microfilm devices for targeted therapies.
Figure 1:

Illustrates schematic of the advantages of nanodiamond-based theranostics in cancer treatment.
2. Nanodiamonds: Unique properties and their applicability for cancer theranostics
Nanodiamonds were initially synthesized in 1963 through the detonation of carbon-based explosives [18, 19]. However, due to limited industrial interest in nanotechnology during that period, the applications of these nanodiamonds remained largely unknown and underutilized until approximately two decades ago. It was in 2005 when Yu et al. published the first report on utilizing nanodiamonds for fluorescence imaging and showcased their low toxicity in 293T human kidney cells [20]. Since then, numerous studies have emerged, highlighting the diverse potential of nanodiamonds in various cellular systems and applications. ND exhibits variations in size, shape, and surface potential. These distinctive properties stem primarily from different preparation methods, significantly impacting the nanodiamonds’ characteristics and optimal applications. The two main approaches for obtaining nanodiamonds suitable for biomedical purposes are detonation (DND) and growth under high-pressure high-temperature (HPHT) conditions (as shown in Figure 2a) [21]. Detonation nanodiamonds (DND) typically have smaller sizes ranging from 1 to 10 nm, a positive surface charge, and a higher content of sp2 carbon. On the other hand, HPHT particles are larger, ranging from 35 to 100 nm, and can carry a negative or positive charge with a lower content of sp2 carbon [22]. A size comparative transmission electron microscopy (TEM) image of DND and HPHT ND is shown in Figure 2d [23]. Additionally, HPHT nanodiamonds undergo a milling procedure that imparts them with sharp edges, resulting in a non-spherical shape [23]. The size of ND significantly affects their behavior in the bloodstream and their accumulation at specific sites. Tiny ND (around 5 nm) are quickly cleared by the kidneys, limiting their collection in the reticuloendothelial system (RES) and tumor sites [24]. In contrast, larger nanodiamonds (approximately 50 nm) remain in circulation for extended periods, allowing increased accumulation in tumors and RES [25, 26]. Nanodiamonds have shown no substantial harm at the organ or organism levels despite RES accumulation [27, 28]. Notably, the larger size of HPHT particles allows them to possess luminescent centers, known as nitrogen-vacancy (NV) centers, that would enable real-time imaging of drug transport via fluorescence microscopy, magnetic resonance imaging (MRI), and photoacoustic imaging [7].
Figure 2:
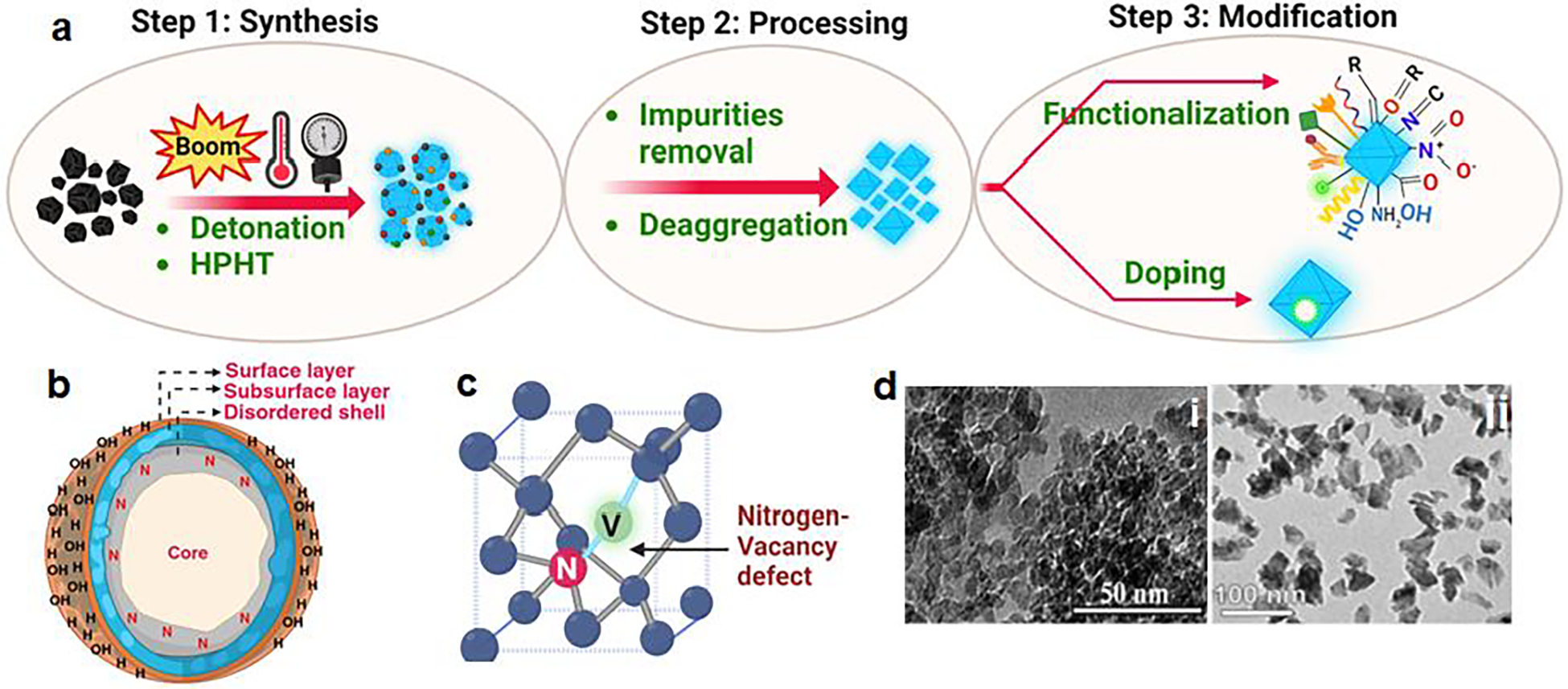
(a) Schematic for synthesis and modification of nanodiamonds (b) outer and inner core structure of nanodiamond (c) structure of nitrogen–vacancy (N–V) defect as color centers in nanodiamond (d) TEM image of DND and HPHT ND (Reproduced with permission [23]).
2.1. Biocompatibility:
Biocompatibility is the quality that characterizes a material’s ability to peacefully coexist with living tissues without inducing any detrimental effects [29]. Extensive research has been undertaken to analyze the potential toxicity of nanodiamonds (ND) by employing both in vitro and in vivo approaches. These studies have examined various factors, including ND origin, size, morphology, and surface characteristics [30]. The biocompatibility of raw DND, including carboxylated DND and base functionalized DND (DND-COONa & DND-SO3Na), with sizes ranging from 2 to 10 nm, has been demonstrated by their low cytotoxicity. Cell viability was retained when neuroblastoma cells, macrophages, keratinocytes, and PC-12 cells were incubated with these DND (100 μg/mL), showing no significant reduction in cell viability compared to controls. Additionally, incubation with these DNDs did not induce reactive oxygen species (ROS) generation or cell morphological changes [31]. Similarly, carboxylated-HPHT ND with different diameters (20 and 100 nm) showed no significant cytotoxicity or genotoxicity at an exposure dose of 250 μg/mL in six human cell lines, including liver (HepG2 and Hep3B), kidney (Caki-1 and Hek-293), intestine (HT29), and lung (A549) cells [32]. These findings indicate carboxylated-HPHT nanodiamonds’ safety and low toxicity when exposed to various cell types [33]. Compared with the in vitro cellular experiment, the results from the in vivo biocompatibility evaluation using animal models can further reflect the potential impact of ND on human health. One notable study by Chow et al. focused on using NDs as drug delivery platforms for treating liver tumors and mammary carcinomas in mice. It showed that even a higher dosage of 500 μg of ND did not increase the bloodstream’s interleukin-6 (IL-6) concentrations. This suggests the absence of systemic inflammatory responses to ND. Furthermore, the ND treatment did not lead to elevated serum alanine transferase (ALT) levels, indicating no adverse effects on liver function. Histological analysis of multiple tissues after ND treatment also revealed no significant changes, further supporting the non-toxic nature of ND [34]. Yuan et al. conducted a study to examine the effects of ND of different sizes on pulmonary toxicity and translocation in the lungs of mice after intratracheal instillation. The mice were assessed at various time points, 1 to 28 days after exposure, followed by biochemical, ultrastructural, and histopathological evaluations. They didn’t find any noticeable adverse effects on the lungs [35]. These nanodiamonds were well tolerated by multiple cell types and animal models and had minimal impact on their metabolic activity, suggesting their suitability for biomedical applications [30]. This highlights nanodiamonds’ potential for use in diverse cellular systems without significant adverse effects.
2.2. Surface chemistry:
Diamonds are known for their exceptional hardness and transparency, qualities that stem from the specific arrangement of carbon atoms within a crystalline lattice structure, with each carbon atom forming strong covalent bonds with four neighbouring carbon atoms in tetrahedral sp3 hybridization [21]. ND possesses an inner core made of diamond with sp3 carbon atoms and an outer shell composed of graphitic carbon with a mixture of sp3 and sp2 hybridization, as depicted in Figure 2b. The outer shell is decorated without passivation by ‘dangling bonds’[36]. These bonds are energetically unfavorable and highly reactive, which compel ND surfaces to undergo various interactions and transformations with their surrounding environment, which can occur naturally or be induced in a controlled environment [37]. The precise nature of the outer shell is still being investigated; however, two general models have been proposed. One model suggests an amorphous shell with a substantial amount of sp2 carbon. In contrast, the other model offers a structure resembling a sp2 graphene sheet or a buckyball, resulting in a “bucky-diamond” design [18]. The sp2 and sp3 bonds in ND allow for interconversion, making ND flexible templates, particularly on curved surfaces where electron stability is a concern. ND exhibits a distinct truncated octahedral architecture, facilitating strong binding to drugs and enabling their dispersibility in water [38]. The surface of ND primarily consists of carbon, phenols, pyrones, sulfonic acid, and carboxylic acid functional groups. ND may also contain anhydrides, hydroxyl groups, and epoxide groups in smaller quantities. The existence of carboxylic groups serves as a promising initial stage for subsequent functionalization and conversion[39] This characteristic enhances the water stability of ND suspensions and facilitates the formation of complexes with water-insoluble drugs like 4-hydroxytamoxifen (employed in breast cancer treatment [40, 41]) and purvalanol A (utilized for liver cancer treatment [42, 43]). This interaction contributes to elevated overall solubility and prolonged drug release [21]. Moreover, ND may naturally contain nitrogen-vacancy (N-V) centers or nitrogen impurities, as shown in Figure 2c. ND with N-V centers exhibit fluorescence properties, making them useful in fluorescent bio-labeling agents [44]. Similarly, these impurities in the lattice can also be created by doping with the desired atoms like tritium (3H); doping introduces radioactive isotopes, enabling radiolabelling. Boron doping enhances the electro-conductivity and is used in electrochemical sensors to diagnose cancer [45, 46].
2.3. Surface functionalization:
Since ND are typically of small size and the presence of surface functional groups prone to agglomerate and limit their dispersion in aqueous solutions [47, 48]. These aggregates pose challenges in their circulation in the body, as they can block blood vessels and reduce their effectiveness as drug delivery carriers [46]. Various forces influence ND aggregation, including hydrogen bonding, capillary pressure, and Van der Waals forces [49]. These forces tend to bring the particles together, leading to the formation of larger aggregates. However, it’s worth noting that in some instances, controlled aggregation of smaller particles (around 80 nm) can be favorable as this arrangement can improve efficacy and prolong half-life, particularly in drug-resistant tumors [38]. To address aggregation challenges and achieve a more desirable particle size distribution, researchers have developed methods to modify the surface of ND mechanically or chemically. Mechanical approaches such as ultrasonic dispersion and ball milling can disperse ND [50]. The chemical technique involves a three-step process starting with the surface homogenization of ND. The homogenization of nanodiamonds (ND) can be achieved through either oxidative or reductive conditions, depending on the desired functionalities [51]. Under oxidative conditions, treatments such as sulfuric acid, nitric acid, or hydrogen peroxide, coupled with sonication, can increase the amount of carboxylic acids on the surface of ND. Alternatively, ND can undergo a reduction process using wet chemistry that involves reductants like hydrides or plasma treatment that utilizes hydrogen (H-plasma). Oxidation of ND enhances the formation of carboxylic acid (−COOH) group terminated ND, while the reduction process improves the appearance of hydroxyl (−OH) groups terminated ND [52]. The presence of carboxylic acid groups on ND serves as an excellent starting point as it improves solubility in polar organic solvents, stability by preventing agglomeration, and diverse functionalization, enabling the attachment of various organic molecules and polymers, facilitating targeted applications in drug delivery, biosensing and the development of novel materials. After homogenization, the NDs are subjected to further functionalization, which can be achieved by physisorption or covalent attachment [52]. The surface functional group of ND can influence their interactions with biological systems by modulating the interaction with the vascular barrier and induce leakiness [2]. Overall, the surface functionalization of ND enables their tailored use in biomedicine by improving stability, dispersion, and the attachment of biomarkers, thereby expanding their applications in drug delivery, diagnosis, and bio-labeling.
2.4. Optical properties:
ND possesses unique optical characteristics as they have in-built fluorescence properties, enabling them to be used for real-time imaging during drug delivery; researchers can monitor drug distribution within the body, track treatment response, and evaluate the effectiveness of drug delivery systems [19]. The fluorescence exhibited by ND can be attributed to the presence of optical defects within the diamond structure. Notably, these defects, known as color centers, facilitate electron transitions within the forbidden band, resulting in the absorption and emission of light. This unique property of color centers allows them to selectively absorb visible light energy and emit fluorescence (as shown in Figure 2c). Among 700 defects, nitrogen-vacancy (N–V) centers play a significant role and exhibit desirable fluorescence quality. The formation of N–V centers involves irradiating ND with high-energy particles such as electrons, protons, or helium ions, followed by vacuum annealing at temperatures ranging from 600 to 800 °C [23, 53]. Irradiation introduces vacancies in the diamond structure, and during annealing, these vacancies migrate and are trapped by nitrogen atoms that are naturally present in the diamond. Two types of N–V centers are formed: neutral (NV0) and negatively charged (NV–). These two types have different emission spectra. When excited, the N–V center emits far-red fluorescence with a near-unity quantum yield, characterized by a zero-phonon line (ZPL) at 637 nm. In contrast, the NV0 center emits characteristic orange luminescence with a ZPL at around 575 nm [54, 55]. The NV− center is exciting due to its S = 1 spin ground state, which can be manipulated through optical pumping and electron paramagnetic resonance and exhibits a long spin coherence time. Notably, the fluorescence emitted by N–V centers remains stable without experiencing blinking or bleaching, even under continuous high-power laser excitation at room temperature. N–V centers in ND are also being investigated for their potential in high-resolution magnetic sensing Fields[54, 56, 57], super-resolution microscopy, fluorescence resonance energy transfer [58], and biomedical imaging [59].
Moreover, ND exhibits broad emission spectra ranging from ultraviolet to near-infrared wavelengths. This wide spectral range allows ND to absorb light across different wavelengths, including the near-infrared (NIR) region. NIR has excellent tissue penetration capabilities, which means it can pass through biological tissues more effectively than shorter wavelengths. This property is crucial for biomedical applications as it enables deeper light penetration into the body, providing an added advantage of obtaining high-resolution imaging. Moreover, when ND are exposed to NIR light, they can efficiently convert the light energy into heat through photothermal conversion. This occurrence results from imperfections within the diamond lattice, capable of absorbing light and transforming it into localized heat, thereby increasing the local temperature. This process facilitates the thermal ablation of cancer cells while minimizing harm to the surrounding healthy tissue. Zhang et al. reported a novel oral drug delivery system for colon cancer therapy using polydopamine-coated nanodiamonds (PND) as the photothermal carrier. They coupled sulfhydryl-polyethylene glycol-folate (SH-PEG-FA) on the PND surface to achieve targeted delivery to colon tumors, and curcumin was loaded as the model drug and coated with chitosan (CS) for prolonged gastrointestinal retention and localization in the colon. The ND showed high photothermal efficiency by converting NIR light into heat, NIR laser-responsive drug release, and improved cellular uptake by cancer cells [60]. Thus showing the promising ability to develop effective and targeted cancer treatment strategies.
3. Applications of nanodiamonds in cancer therapeutics
3.1. Drug delivery:
Selective targeting of cancer cells is crucial in developing next-generation nanocarriers for drug delivery in cancer therapy [16, 61]. Drugs can be delivered and accumulated in the tumor via direct or indirect routes mediated or affected by nanoparticles. ND as carriers offer unique advantages, allowing for the precise delivery of therapeutic agents to cancer cells while minimizing systemic toxicity [7]. It’s a complex process that can be influenced by various factors, including the size, surface properties, charge, and functionalization of the nanodiamonds, as well as the type of cells and target approaches [62].
Uptake pathways, tumor targeting, and drug delivery using nanodiamonds:
To comprehend the interaction and crosstalk between ND drug carrier platforms and tumor biology, it is crucial to explore the targeting mechanisms, which can be categorized as passive and active targeting (mechanism shown in Figure 3). In passive targeting, ND is designed to take advantage of the tumor’s unique features, such as leaky blood vessels and poor drainage. The rapidly proliferating cancer cells lead to the development of new blood vessels (called neovascularization) with larger pores resulting in poor perm selectivity compared to normal vessels. The variance in vascular structure leads to heightened permeability of blood vessels in tumors, enabling nanodiamonds (ND) to exit from the bloodstream and gather in the tumor tissue [63]. Additionally, the flawed angiogenesis and impaired lymphatic drainage contribute to the increased retention of ND within the tumor, aiding in the discharge of drug payloads to the cancer cells. This event is known as the enhanced permeability and retention (EPR) effect, a critical element in the spontaneous buildup of drug-laden ND within the tumor site, obviating the need for specialized guidance [64, 65]. The size of the ND also influences the EPR effect. Research has shown smaller particles have better penetration capabilities, specifically targeting tumor tissues without significant leakage into normal vessels. Conversely, larger particles are more likely to be cleared by the immune system, limiting their effectiveness as drug carriers [66]. Therefore, striking a balance between particle size and EPR effect is essential to optimize passive targeting strategies for drug delivery in cancer treatment [62]. Additionally, the surrounding environment in and around tumor cells, i.e., tumor microenvironment (TME), also plays a critical role in passive targeting [61]. For instance, the rapidly proliferating tumor cells rely on glycolysis, a process in which glucose is converted into energy without oxygen (anaerobic metabolism). This phenomenon is known as the Warburg effect and is a key feature and chief energy source of many cancer cells [67]. As a result of glycolysis, tumor cells generate substantial amounts of lactic acid, causing a reduction in the pH of the TME. The TME becomes more acidic in contrast to the adjacent healthy tissues. Consequently, pH-sensitive ND can be engineered to respond to the lower pH conditions and release their cargo, such as drugs, into the TME. In a study, Li et al. demonstrated that doxorubicin (DOX)-loaded-polyethylene glycol-conjugated ND exhibited a pH-sensitive sustained release of the drug in a low-pH environment and selective accumulation in MCF-7 cells compared to non-malignant cells, indicating selective targeting TME and cytotoxicity of the drug delivery system [68]. Passive targeting, despite its advantages, suffers several drawbacks. One significant obstacle is suboptimal bio-distribution, where particles are trapped in the liver and spleen due to reticuloendothelial function. Moreover, the extent of the EPR effect varies not only between different tumors but even within the same tumor due to heterogeneity and differences in vascular permeability [67, 69]. To overcome these limitations and enhance the effectiveness of targeted drug delivery, researchers are working on synergizing passive targeting with more dynamic methods, which involve actively guiding ND to specific disease sites. This approach is known as active targeting and aims to improve spatial localization while reducing off-target effects in normal tissues. Active targeting involves modifying nanoparticles with ligands or functional moieties that recognize and bind to receptors or markers expressed explicitly on the tumor cells. When ligands interact with their target receptors in the cancer cells, it leads to the inward folding of the cell membrane and the internalization of ligand-loaded ND through receptor-mediated endocytosis, enhancing spatial localization and intracellular drug penetration [70, 71]. Various ligands have been investigated as targeting moieties to improve the specificity of ND for active targeting. These ligands, including monoclonal antibodies, peptides, amino acids, vitamins, and carbohydrates, are designed to bind specifically to receptors overexpressed on the surface of targeted cells. Notable receptors that have been extensively studied include the transferrin receptor, folate receptor, glycoproteins, and the epidermal growth factor receptor (EGFR). These ligands allow ND to be directed toward specific cells or tissues, improving their selectivity and potential for therapeutic applications [72, 73]. Zhang et al. developed a versatile ND-based drug delivery system with receptor-mediated targeting, imaging, and enhanced therapy capabilities. They covalently grafted fluorescently labeled Paclitaxel-DNA conjugates and anti-epidermal growth factor receptor (EGFR) monoclonal antibody onto cross-linker sulfosuccinimidyl 6-(3′-[2-pyridyldithio] propionamide) hexanoate (sulfo-LC-SPDP) modified ND surface, creating a targeted drug delivery platform for MCF-7 and MDA-MB-231 breast cancer cell lines (Figure 4a) [74]. One challenge in drug delivery using nano-carriers is difficulty escaping from the endosome and reaching the cytoplasm after being taken up by a cancer cell. However, HPHT-ND carriers have demonstrated efficient drug delivery into the cell cytoplasm. According to Chu et al., NDs succeed in escaping endosomes due to their sharp edges and potentially positive surface charge [75]. Once the drug is released within the endosome, the ND’s sharp edges and positive charge destabilize the endosomal membrane, allowing the ND and their drug to escape into the cytoplasm [76]. Consequently, ND has a tendency to gather in the cytoplasm, typically in proximity to the nuclear envelope, but they seldom penetrate the nuclei [77–79]. In a recent study by Pall et al., a novel approach was introduced, combining Raman imaging with the precise localization of fluorescent nanodiamonds (ND). This innovative method enables the detection of NV luminescence integrated with confocal Raman microscopy within a single scan, enhancing the understanding of the cellular environment. The study involved the use of mammalian breast cancer (MCF7) cell lines, mammalian breast cell lines (184A1), and human dental pulp stem cells (DPSC), all incubated with luminescent fluorescent ND. The methodology leveraged the C-H Raman peak for nucleus visualization, shifting the spectral detection window to longer wavelengths. This adjustment facilitated the collection of the majority of NV photons, enabling sensitive chemical localization of even small (5–50 nm) fluorescent ND. Notably, the research demonstrated the colocalization of luminescent probes with cell organelles, highlighting the identification of fluorescent ND in the vicinity of the cell nucleus [76]. On the contrary, Gismondi et al. employed confocal microscopy to illustrate that nanodiamonds could penetrate the cell cytoplasm but remained embedded in the nuclear membrane, exposing only small portions to the nuclear area. They utilized plant secondary metabolites, ciproten, and quercetin, for nanodiamond conjugation and treated human (HeLa) and murine (B16F10) tumor cells [77]. Currently, no clinical trials utilize ND as a delivery system; however, they show promise in cancer treatment, with patents exploring their potential as drug delivery systems. A patent by Petit et al. showed an intriguing study that ND can act as a drugs after uptake, generating free radicals when irradiated for cancer treatment [80]. Future advancements using NDs could lead to innovative approaches in cancer therapy.
Figure 3:
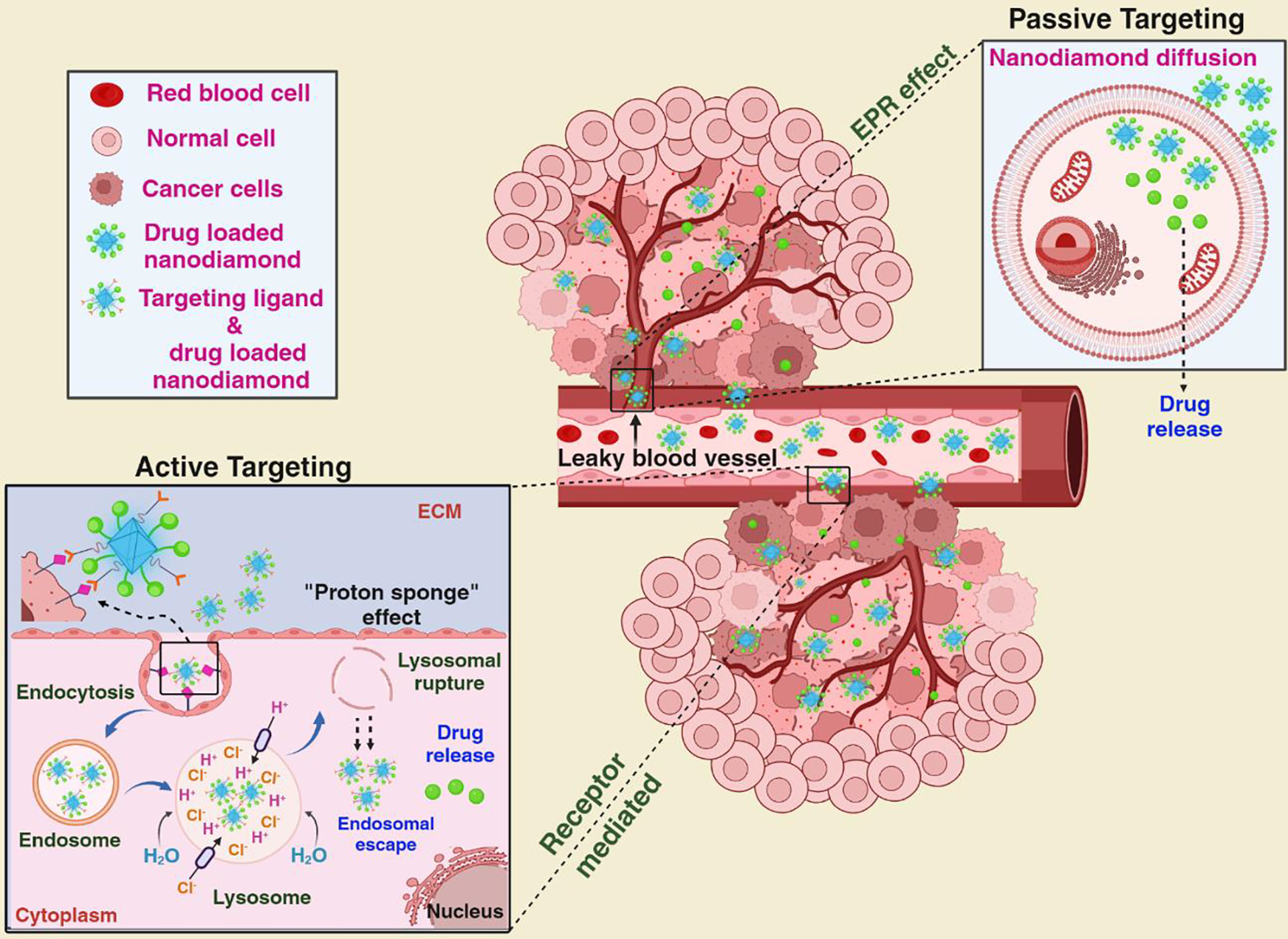
Contrasting passive and active drug targeting: Passive targeting banks on the EPR effect to facilitate the accumulation of ND in tumor tissue, enabling the drug they encapsulate to be released in the cell. While, active targeting entails the attachment of cargo with the ND, which bind to receptors that are overexpressed on the surface of tumor cells. The ND enter the tumor cells through receptor-mediated endocytosis, facilitating drug release. (Adapted from reference with modification [64])
Figure 4:

(a) Synthetis scheme of fluorescently labelled paclitaxel-DNA and anti-epidermal growth factor receptor (EGFR) monoclonal antibody onto cross-linker sulfosuccinimidyl 6-(3′-[2-pyridyldithio] propionamido) hexanoate modified NDs surface (b) A schematic representation of ND-drug conjugates in efflux-transporter–expressing cells: (i) ABC transporter proteins efflux drug out of the cell (ii) Endocytosis of ND-drug conjugates (iii) Diffusion of the free drug across the cell membrane. The ND-drug conjugate is more difficult to remove from the cell than free drugs, which is rapidly eliminated (c) Schematic model showing (i) nanodiamond-Epirubicin (Ep) synthesis and aggregation of EPND (ii) Administering ND-epirubicin (EPND) to mice afflicted with hepatic tumors effectively eliminated hepatic cancer stem cells (CSCs) and successfully thwarted the emergence of secondary tumors, a response not observed in those treated with unaltered epirubicin (Ep), (black arrows indicate treatment condition and number). (Adapted from reference with modifications: [71] (a), [85] (b), [87] (c))
3.2. Bioimaging and tracking:
The nitrogen defects present in ND serve as light-emitting sources resembling individual atoms. These centers facilitate electron transitions within the band gap, making it possible for them to absorb visible light energy and emit fluorescence. Notably, a substantial portion of their emitted light falls within the near-infrared (NIR) spectrum. This characteristic enhances the effectiveness of high-resolution imaging and diagnostic processes. Additionally, in contrast to organic dyes, which often experience a rapid loss of fluorescence, ND fluorescence remains stable and enables extended, long-term tracking [51]. ND is engineered to incorporate particular biomarkers or molecules, directing them to gather at the site of a tumor. Simultaneously, the fluorescence emitted by ND serves as a fluorescent marker, allowing for the accurate pinpointing of tumor locations. In their study, Chang and colleagues introduced fluorescently stable ND, which were employed to monitor individual particles within cells three-dimensionally using either single-photon or two-photon excitation fluorescence microscopy [81]. Su et al. successfully employed fluorescence lifetime imaging microscopy and time-gated fluorescence imaging to perform bioimaging and track individual human mesenchymal stem cells in non-rodent animal models. They accomplished this by utilizing human serum albumin (HSA) to encapsulate 100-nanometer fluorescent nanodiamond (FND) that contained NV-center defects through physical adsorption, resulting in HSA-FND. Subsequently, they used HSA-FND to tag human placenta choriodecidual membrane-derived mesenchymal stem cells (pcMSCs), which were then introduced into a pig via its left internal jugular vein. This allowed for in vivo imaging and precise quantitative tracking of these cells. It is evident that employing time gating in fluorescence imaging results in higher resolution and greatly enhances the ease of localizing and tracking pcMSCs in porcine lung tissue sections [82]. Faklaris et al. employed sub-50 nanometer-sized fluorescent ND to label the interior of cells and delved into how living cells take them up [83]. Hsiao and colleagues accomplished uniform labeling and super-resolution imaging of cells using albumin-conjugated fluorescent ND [79]. Tian and colleagues reported successful use of diazoxide-modified FND (FND-Dia) for efficient delivery to HeLa cells, ensuring sustained drug release. In addition, they could monitor and directly measure drug activity at the specific particle location and the release site, capitalizing on the advantages of FND. They also analyzed the concentration-dependent free radical response within the FND-Dia group, employing FND quantum sensing capabilities for detection. Importantly, since FND do not experience fading, it became possible to continuously track individual particles within single cells throughout the release experiment [84]. ND is also used as a contrast agent for multimodal imaging by combining ND with other imaging agents or techniques like positron emission tomography (PET) and magnetic resonance imaging (MRI). While this technology is still in the research and development stage, it is promising to enhance the accuracy and effectiveness of cancer diagnosis and treatment. It’s essential to continue research and clinical trials to validate its safety and efficacy for widespread clinical use.
3.3. ND as chemosensitizer: Overcoming chemoresistance and metastasis prevention:
Chemotherapy is commonly used for tumor treatment; however, its effectiveness is limited due to adverse reactions and chemoresistance, a significant factor leading to treatment failure in cancer, particularly in tumor-initiating cancer stem cells. Finding ways to overcome chemoresistance can significantly improve cancer therapy outcomes, preventing cancer recurrence and metastasis [85]. Various mechanisms can lead to drug resistance, such as alterations in cellular targets, increased repair of drug-induced DNA damage, rapid drug degradation, avoidance of apoptosis, and drug sequestration within cells [86]. The most common resistance mechanism involves ATP-binding cassette (ABC) transporters that pump drugs out of cancer cells, reducing their effectiveness by keeping drug concentrations low [87]. These transporters play essential roles in healthy cells but contribute to chemoresistance in cancer cells (Figure 4b)[86]. To overcome these drawbacks, chemosensitizers can enhance tumor cells’ sensitivity to drugs and combat multidrug resistance. Chemosensitizers are substances that sensitize cancer cells to the effects of chemotherapy, making the treatment more effective. Nanodiamond has emerged as a promising chemosensitizing material to address drug resistance using various mechanisms such as targeted drug delivery, improved cellular uptake, and enhanced bioavailability for drugs with challenging physicochemical properties. ND-based delivery of anthracyclines can overcome ABC transporter-mediated drug resistance in cancer cells, which makes ND an effective drug delivery platform for treating cancer stem cells (CSCs). In hepatic cancers with chemoresistant and metastatic CSCs expressing ABC transporter proteins, ND-delivering epirubicin showed better efficacy in killing CSCs than epirubicin alone (Figure 4c) [88]. Toh et al. investigated ND to improve drug retention in lentivirally transduced mitoxantrone (MTX) resistant triple negative breast cancer cells (MDA-MB-231). They engineered nanodiamonds to generate ND-MTX complex and compared drug retention in complex and bare MTX forms over a period of 24 hours. ND–MTX showed a higher retention efficiency of 39 ± 5.4% compared to MTX 6 ± 1.9% [89]. However, the successful implementation depends on the ND carrier and drug development strategies that can modify drug distribution and pharmacokinetics, thereby reducing side effects.
3.4. NDs in immunotherapy
In recent decades, cancer immunotherapy has witnessed remarkable clinical achievements and emerged as a promising cornerstone in cancer treatment. Cancer immunotherapy is a treatment approach aimed at identifying, destroying, and eliminating tumor cells by activating or enhancing the function of the body’s immune response while minimizing damage to normal cells [90]. These approaches include “immune checkpoint blockade therapy,” “cancer vaccine therapy,” “chimeric antigen receptor (CAR)-T cell therapy,” and “immune system modulator therapy” [90–92]. Despite its success, challenges persist in the form of low tumor antigen responsiveness and multiple escape mechanisms within the immunosuppressive microenvironment. As a result, the clinical application of immunotherapy often faces issues like low response rates, limited treatment durations, and the potential for severe immunotoxicity [62]. By utilizing ND in immunotherapy, researchers aim to enhance the efficacy of cancer treatment by promoting immune activation and enabling real-time monitoring of the immune response. Immunotherapy using nanodiamonds (ND) involves diverse approaches, including nano-vaccines, artificial antigen-presenting cells (aAPCs), and interventions targeting the immunosuppressed tumor microenvironment (TME) [90]. The successful development of nano-vaccine formulations relies on the efficient and targeted delivery of immunomodulatory and immunostimulatory molecules to specific cells [17]. Unlike conventional methods, nanoparticles offer advantages such as shielding the payload (antigen/adjuvant) from the biological environment, prolonging its half-life, reducing systemic toxicity, facilitating delivery to antigen-presenting cells (APCs), and potentially directly activating TAA-specific T-cells. A study by Yuan et al. caught attention for its focus on triple-negative breast cancer, a subtype known for its poor prognosis and resistance to chemotherapy. They utilized nanodiamonds coated with polyglycerol and doxorubicin for their study (as illustrated in Figure 5) [45]. Since immunosuppression is a significant factor in this type of cancer, they investigated changes in the tumor environment and adjacent immunological factors. An important discovery of the study was that ND-doxorubicin did not induce the upregulation of P-glycoprotein or interleukin-6, both of which are associated with doxorubicin resistance. Moreover, applying these ND-based complexes reduced secretion of the granulocyte-colony stimulating factor produced by the tumor and decreased myeloid-derived suppressor cells (MDSCs). MDSCs are myeloid cells that the cancer manipulates to suppress anti-tumor immune responses. These favorable outcomes created an environment conducive to activating macrophages, dendritic cells, and lymphocytes, effectively initiating an anti-tumor response in triple-negative breast cancer [45]. In another research, Zhang et al. introduced a novel ND-based agent designed to efficiently deliver immunostimulatory cytosine–phosphate–guanine (CpG) oligonucleotides (ODN). The combination of CpG ODN with ND showed a remarkable increase in cellular uptake, surpassing three orders of magnitude. Inside the cells, CpG-ND localizes in lysosomes and interacts with Toll-like receptor 9 (TLR9), resulting in notable cytokine secretion. The immunostimulatory CpG-ND has a long-lasting immunoregulatory effect, persisting for at least three days at the cellular level and two days in mice. This enduring impact was attributed to NDs’ unique “spongelike” porous structure, which ensures the protection and gradual release of CpG ODN. The potential of CpG-ND was validated by administering through tail-vein injections every three days in murine tumor models (B16 melanoma and 4T1 breast carcinoma xenografts) that showed highly promising results, with significant suppression of tumor growth (Figure 6a) [93]. However, it’s important to note that the application of nanodiamonds in immunotherapy is still an area of active research, and further studies are required to fully understand their potential and optimize their use in cancer treatment.
Figure 5:

Illustrating doxorubicin-polyglycerol-nanodiamond (ND-DOX) conjugate (top), weight of 4T1 tumor xenografts in mice at the end of 3-week treatment of ND-DOX/DOX (below left) and Spleen size of 4T1-tumor bearing mice at the end of 3-week treatment of ND-DOX/DOX (below right). (Adapted from reference with modifications: [42])
Figure 6:

(a) Schematic for preparing ND and their immunomodulatory activity for cancer therapy (b) Hybrid films of varied size and shapes and schematic of hybrid film patch synthesis. ND and DOX molecules bound through physical interactions are deposited on top of a base layer of parylene. A final layer of parylene film is then deposited for additional elution control. (Adapted from reference with modification [92] (a), [9] (b).
3.5. Precision medicine
Personalized medicine, often called precision medicine, presents an innovative approach aiming to customize therapeutic approaches based on the distinctive attributes of each patient. The primary goal of precision medicine is to develop individualized treatment regimens by considering specific patient data, including genetic profiles, environmental exposures, and other health factors. This methodology considers the diversity among patients, resulting in enhanced patient categorization, increased drug precision, and the optimization of dosage or combined treatments [10]. In precision medicine, gene therapy holds significant potential for tailoring cancer treatments. In a study carried out by Alhaddad et al. [94], ND was utilized as a delivery system for siRNA targeting Ewing sarcoma cells. In vitro, results showed that complexes consisting of nanodiamonds coated with a polymer Polyethylenimine, combined with EWS-Fli1-specific siRNA, effectively suppressed the expression of the EWS-Fli1 gene and its associated protein. Furthermore, the intrinsic luminescence of nanodiamonds enabled real-time monitoring of the cargo. These designs are customized based on individual patient data and are engineered to address specific barriers in diverse patient populations, holding great promise. Nevertheless, like others, precision therapy challenges related to biological barriers limit their full clinical potential regarding delivery.
3.6. Microfilm devices for localized elimination of cancer using nanodiamond
Nanodiamonds have versatile applications beyond targeted drug delivery. They can be integrated into nanostructured devices, where they are embedded in drug-loaded microfilms [95]. Microfilm devices refer to a specific drug delivery system that uses thin films or patches containing medication for target and controlled release at a particular site in the body. The devices are designed to adhere to the skin or mucosal surfaces or are transplanted at the tumor site directly following surgery or radiation, allowing the drug to be released slowly and steadily over an extended period and preventing cancer recurrence [31, 96]. These ND-microfilms can be utilized as anti-tumor patches, facilitating localized and controlled release of chemotherapy drugs. Lam et al. developed a scalable and flexible microfilm patch device that uses nanodiamonds to achieve significantly extended drug release capabilities in a localized format (Figure 6b). The developed microfilm was composed of a chemotherapeutic Doxorubicin and nanodiamond (DOX-ND) conjugate sandwiched between a base and thin permeable layer of parylene C (a biocompatible and FDA-approved flexible and robust framework). The drug was gradually and consistently released from the embedded nanodiamond clusters over one month, with additional Doxorubicin reserved, suggesting the potential for an extended release lasting several months or more (Figure 6b) [9]. Notably, the device circumvented the common issue of a sudden and sizeable initial drug release, often seen as a drawback in conventional treatments. Thus, the microfilm devices have several advantages that potentially improve therapeutic outcomes: 1) are implanted directly into the tumor, allowing the controlled release of chemotherapy drugs into the cancerous tissue. This targeted approach helps to increase the drug concentration at the tumor site, enhancing the drug’s effectiveness against cancer cells; 2) The microfilm devices are designed to provide sustained drug release over an extended period. This sustained release ensures the drug remains in the tumor environment longer, increasing its exposure to cancer cells. 3) By delivering drugs directly to the tumor, the risk of systemic toxicity is reduced. This localized drug delivery approach may allow for higher drug concentrations within the tumor while sparing healthy tissues from unnecessary exposure to the medication. 4) Some microfilm devices are made from biodegradable materials, which gradually break down over time. As the film degrades, it releases the drug and eliminates the need for device removal after treatment [95]. It is important to note that while microfilm devices in localized drug delivery hold promise, they are still under investigation.
4. Limitation of nanodiamond
Using nanodiamonds in cancer therapy encounters numerous challenges, impeding its broader application and progression to clinical trials. The existing barriers underscore concerns related to ND’s distribution, toxicity, and biodegradability. A pivotal limitation is the absence of efficient methods to determine the post-administration distribution of ND within the body. Studies examining the biodistribution of nanodiamonds in animal bodies, particularly in organs like the lungs and liver, reveal potential entrapment without lethal impacts. However, uncertainties persist regarding the long-term effects, emphasizing the imperative for ongoing research to grasp the implications of nanodiamonds in vivo fully. While nanodiamonds exhibit significant biocompatibility, there is still toxicity toward specific tissues and organs, contingent on size, shape, and functionalization. The lack of research on the biodegradability of ND poses a limitation to its precise scalability in cargo delivery studies, further hindering its widespread use. Achieving successful drug delivery with nanodiamonds requires well-dispersed and disaggregated particles with a known surface structure conducive to bioconjugation. Despite the notable solubility of ND, surface functionalization demands special attention, involving homogenization, linker attachment, and cargo loading before its application in cancer treatment. Researchers have made significant advancements in response to these challenges, developing mature characterization and conjugation methodologies. However, ongoing exploration is essential to address the remaining uncertainties and propel nanodiamonds toward effective and safe applications in cancer therapy.
5. Conclusion
Nanodiamonds are emerging as a highly promising tool within the realm of nanomedicine, with a particular focus on their applications in cancer therapy and diagnostics. They possess unique characteristics that render them well-suited for employment in theranostics, which encompasses both therapeutic and diagnostic functions. The potential of ND-based theranostics is indeed significant, yet several challenges must be addressed. These challenges encompass the need to meticulously refine ND’s design, size, shape, and surface properties for specific applications to ensure their long-term safety and stability and optimize their clinical implementation scalability. The characteristics of ND are propelling the advancement of cutting-edge methods for accurate tumor targeting, enhancing the efficacy of chemotherapy as a chemosensitizer, enabling the utilization of immunotherapy approaches, facilitating precision medicine, and enabling the creation of localized microfluidic devices for precise targeted therapies. As of now, there are no ongoing clinical trials employing ND as a drug delivery platform. Nevertheless, ND shows considerable promise in cancer therapy, actively exploring their potential as drug carriers. Ongoing research in this field strongly indicates that nanodiamonds hold the potential to revolutionize cancer therapy by providing more precise and effective treatment modalities, all the while minimizing adverse side effects.
Highlights.
Cancer is a global cause of death demanding early diagnosis and improved treatments
Combination therapy based on nanodiamond
Gene delivery based on nanodiamond
NDs unite treatment and imaging and address limitations of traditional therapies
NDs’ properties drive techniques for precise tumor targeting and chemosensitizer
ND-based immunotherapy, precision medicine and localized microfilm patches
Acknowledgment
This work was partially supported by the National Science Foundation [grant number 1912322]; American Cancer Society [grant number DICRIDG-22–1037199-01]; and National Institutes of Health grants [R25AG070244 and UO1GM132769] to Manoj Mishra.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Wang X, Yang L, Chen ZG, Shin DM, Application of nanotechnology in cancer therapy and imaging, CA Cancer J Clin, 58 (2008) 97–110. [DOI] [PubMed] [Google Scholar]
- [2].Setyawati MI, Mochalin VN, Leong DT, Tuning Endothelial Permeability with Functionalized Nanodiamonds, ACS Nano, 10 (2016) 1170–1181. [DOI] [PubMed] [Google Scholar]
- [3].Sharma A, Goyal AK, Rath G, Recent advances in metal nanoparticles in cancer therapy, Journal of Drug Targeting, 26 (2018) 617–632. [DOI] [PubMed] [Google Scholar]
- [4].Tjo K, Varamini P, Nanodiamonds and their potential applications in breast cancer therapy: a narrative review, Drug Delivery and Translational Research, 12 (2022) 1017–1028. [DOI] [PubMed] [Google Scholar]
- [5].Gupta C, Prakash D, Gupta S, Cancer treatment with nano-diamonds, FBS, 9 (2017) 62–70. [DOI] [PubMed] [Google Scholar]
- [6].Wong XY, Sena-Torralba A, Álvarez-Diduk R, Muthoosamy K, Merkoçi A, Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs, ACS Nano, 14 (2020) 2585–2627. [DOI] [PubMed] [Google Scholar]
- [7].Benson V, Amini A, Why nanodiamond carriers manage to overcome drug resistance in cancer, Cancer Drug Resist, 3 (2020) 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat Biotechnol, 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lam R, Chen M, Pierstorff E, Huang H, Osawa E, Ho D, Nanodiamond-Embedded Microfilm Devices for Localized Chemotherapeutic Elution, ACS Nano, 2 (2008) 2095–2102. [DOI] [PubMed] [Google Scholar]
- [10].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, Engineering precision nanoparticles for drug delivery, Nat Rev Drug Discov, 20 (2021) 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alavi M, Hamidi M, Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles, Drug Metabolism and Personalized Therapy, 34 (2019). [DOI] [PubMed] [Google Scholar]
- [12].Marchesan S, Kostarelos K, Bianco A, Prato M, The winding road for carbon nanotubes in nanomedicine, Materials Today, 18 (2015) 12–19. [Google Scholar]
- [13].Vlamidis Y, Voliani V, Bringing Again Noble Metal Nanoparticles to the Forefront of Cancer Therapy, Frontiers in Bioengineering and Biotechnology, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tsai L-W, Lin Y-C, Perevedentseva E, Lugovtsov A, Priezzhev A, Cheng C-L, Nanodiamonds for Medical Applications: Interaction with Blood in Vitro and in Vivo, Int J Mol Sci, 17 (2016) 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Priyadarshni N, Singh P, Mahato K, Magnetic Nanoparticle-Based Sensing Strategies for Clinical Analysis and Environmental Safety Assessment, in: Purohit B, Chandra P(Eds.) Surface Engineering and Functional Nanomaterials for Point-of-Care Analytical Devices, Springer Nature Singapore, Singapore, 2023, pp. 67–102. [Google Scholar]
- [16].Chauhan S, Jain N, Nagaich U, Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents, Journal of Pharmaceutical Analysis, 10 (2020) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Leleux J, Roy K, Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective, Adv Healthc Mater, 2 (2013) 72–94. [DOI] [PubMed] [Google Scholar]
- [18].Zhu Y, Li J, Li W, Zhang Y, Yang X, Chen N, Sun Y, Zhao Y, Fan C, Huang Q, The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems, Theranostics, 2 (2012) 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zupančič D, Veranič P, Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis, Int J Mol Sci, 23 (2022) 8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yu S-J, Kang M-W, Chang H-C, Chen K-M, Yu Y-C, Bright Fluorescent Nanodiamonds: No Photobleaching and Low Cytotoxicity, Journal of the American Chemical Society, 127 (2005) 17604–17605. [DOI] [PubMed] [Google Scholar]
- [21].Kaur R, Badea I, Nanodiamonds as novel nanomaterials for biomedical applications: drug delivery and imaging systems, Int J Nanomedicine, 8 (2013) 203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Turcheniuk K, Mochalin VN, Biomedical applications of nanodiamond (Review), Nanotechnology, 28 (2017) 252001. [DOI] [PubMed] [Google Scholar]
- [23].Chang Y-R, Lee H-Y, Chen K, Chang C-C, Tsai D-S, Fu C-C, Lim T-S, Tzeng Y-K, Fang C-Y, Han C-C, Chang H-C, Fann W, Mass production and dynamic imaging of fluorescent nanodiamonds, Nature Nanotechnology, 3 (2008) 284–288. [DOI] [PubMed] [Google Scholar]
- [24].Longmire M, Choyke PL, Kobayashi H, Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats, Nanomedicine, 3 (2008) 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu M, Zheng J, Clearance Pathways and Tumor Targeting of Imaging Nanoparticles, ACS Nano, 9 (2015) 6655–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fang J, Nakamura H, Maeda H, The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect, Advanced Drug Delivery Reviews, 63 (2011) 136–151. [DOI] [PubMed] [Google Scholar]
- [27].Mohan N, Chen C-S, Hsieh H-H, Wu Y-C, Chang H-C, In Vivo Imaging and Toxicity Assessments of Fluorescent Nanodiamonds in Caenorhabditis elegans, Nano Letters, 10 (2010) 3692–3699. [DOI] [PubMed] [Google Scholar]
- [28].Vaijayanthimala V, Cheng P-Y, Yeh S-H, Liu K-K, Hsiao C-H, Chao J-I, Chang H-C, The long-term stability and biocompatibility of fluorescent nanodiamond as an in vivo contrast agent, Biomaterials, 33 (2012) 7794–7802. [DOI] [PubMed] [Google Scholar]
- [29].Crawford L, Wyatt M, Bryers J, Ratner B, Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration, Adv Healthc Mater, 10 (2021) e2002153–e2002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moore L, Grobárová V, Shen H, Man HB, Míčová J, Ledvina M, Štursa J, Nesladek M, Fišerová A, Ho D, Comprehensive interrogation of the cellular response to fluorescent, detonation and functionalized nanodiamonds, Nanoscale, 6 (2014) 11712–11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schrand AM, Huang H, Carlson C, Schlager JJ, Ōsawa E, Hussain SM, Dai L, Are Diamond Nanoparticles Cytotoxic?, The Journal of Physical Chemistry B, 111 (2007) 2–7. [DOI] [PubMed] [Google Scholar]
- [32].Paget V, Sergent JA, Grall R, Altmeyer-Morel S, Girard HA, Petit T, Gesset C, Mermoux M, Bergonzo P, Arnault JC, Chevillard S, Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines, Nanotoxicology, 8 (2014) 46–56. [DOI] [PubMed] [Google Scholar]
- [33].Lai H, Stenzel MH, Xiao P, Surface engineering and applications of nanodiamonds in cancer treatment and imaging, International Materials Reviews, 65 (2020) 189–225. [Google Scholar]
- [34].Chow EK, Zhang X-Q, Chen M, Lam R, Robinson E, Huang H, Schaffer D, Osawa E, Goga A, Ho D, Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment, Science Translational Medicine, 3 (2011) 73ra21–73ra21. [DOI] [PubMed] [Google Scholar]
- [35].Yuan Y, Wang X, Jia G, Liu J-H, Wang T-C, Gu Y, Yang S-T, Zhen S, Wang H, Liu Y, Pulmonary toxicity and translocation of nanodiamonds in mice, Diamond and Related Materials, 19 (2010) 291–299. [Google Scholar]
- [36].Slocombe D, Porch A, Bustarret E, Williams OA, Microwave properties of nanodiamond particles, Applied Physics Letters, 102 (2013). [Google Scholar]
- [37].Barnard AS, Diamond standard in diagnostics: nanodiamond biolabels make their mark, Analyst, 134 (2009) 1751–1764. [DOI] [PubMed] [Google Scholar]
- [38].Ho D, Wang C-HK, Chow EK-H, Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine, Science Advances, 1 (2015) e1500439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Krueger A, Lang D, Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond, Advanced Functional Materials, 22 (2012) 890–906. [Google Scholar]
- [40].Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N, Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study, J Natl Cancer Inst, 97 (2005) 1652–1662. [DOI] [PubMed] [Google Scholar]
- [41].Nayfield SG, Karp JE, Ford LG, Dorr FA, Kramer BS, Potential role of tamoxifen in prevention of breast cancer, J Natl Cancer Inst, 83 (1991) 1450–1459. [DOI] [PubMed] [Google Scholar]
- [42].Chen M, Pierstorff ED, Lam R, Li SY, Huang H, Osawa E, Ho D, Nanodiamond-mediated delivery of water-insoluble therapeutics, ACS Nano, 3 (2009) 2016–2022. [DOI] [PubMed] [Google Scholar]
- [43].Goga A, Yang D, Tward AD, Morgan DO, Bishop JM, Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC, Nat Med, 13 (2007) 820–827. [DOI] [PubMed] [Google Scholar]
- [44].Hui YY, Cheng C-L, Chang H-C, Nanodiamonds for optical bioimaging, Journal of Physics D: Applied Physics, 43 (2010) 374021. [Google Scholar]
- [45].Yuan S-J, Xu Y-H, Wang C, An H-C, Xu H-Z, Li K, Komatsu N, Zhao L, Chen X, Doxorubicin-polyglycerol-nanodiamond conjugate is a cytostatic agent that evades chemoresistance and reverses cancer-induced immunosuppression in triple-negative breast cancer, J Nanobiotechnology, 17 (2019) 110–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mumtaz M, Hussain N, Salam S, Bilal M, Multifunctional nanodiamonds as emerging platforms for cancer treatment, and targeted delivery of genetic factors and protein medications—a review, Journal of Materials Science, 57 (2022) 8064–8099. [Google Scholar]
- [47].Tinwala H, Wairkar S, Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics, Materials Science and Engineering: C, 97 (2019) 913–931. [DOI] [PubMed] [Google Scholar]
- [48].Qin J-X, Yang X-G, Lv C-F, Li Y-Z, Liu K-K, Zang J-H, Yang X, Dong L, Shan C-X, Nanodiamonds: Synthesis, properties, and applications in nanomedicine, Materials & Design, 210 (2021) 110091. [Google Scholar]
- [49].Zhao J, Lu M, Lai H, Lu H, Lalevée J, Barner-Kowollik C, Stenzel MH, Xiao P, Delivery of Amonafide from Fructose-Coated Nanodiamonds by Oxime Ligation for the Treatment of Human Breast Cancer, Biomacromolecules, 19 (2018) 481–489. [DOI] [PubMed] [Google Scholar]
- [50].Uthappa UT, Arvind OR, Sriram G, Losic D, Ho Young J, Kigga M, Kurkuri MD, Nanodiamonds and their surface modification strategies for drug delivery applications, Journal of Drug Delivery Science and Technology, 60 (2020) 101993. [Google Scholar]
- [51].Chipaux M, van der Laan KJ, Hemelaar SR, Hasani M, Zheng T, Schirhagl R, Nanodiamonds and Their Applications in Cells, Small, 14 (2018) 1704263. [DOI] [PubMed] [Google Scholar]
- [52].Reina G, Zhao L, Bianco A, Komatsu N, Chemical Functionalization of Nanodiamonds: Opportunities and Challenges Ahead, Angewandte Chemie International Edition, 58 (2019) 17918–17929. [DOI] [PubMed] [Google Scholar]
- [53].Rondin L, Dantelle G, Slablab A, Grosshans F, Treussart F, Bergonzo P, Perruchas S, Gacoin T, Chaigneau M, Chang HC, Jacques V, Roch JF, Surface-induced charge state conversion of nitrogen-vacancy defects in nanodiamonds, Physical Review B, 82 (2010) 115449. [Google Scholar]
- [54].Balasubramanian G, Lazariev A, Arumugam SR, D.-w. Duan, Nitrogen-Vacancy color center in diamond—emerging nanoscale applications in bioimaging and biosensing, Current Opinion in Chemical Biology, 20 (2014) 69–77. [DOI] [PubMed] [Google Scholar]
- [55].Lai H, Stenzel M, Xiao P, Surface engineering and applications of nanodiamonds in cancer treatment and imaging, International Materials Reviews, 65 (2019) 1–37. [Google Scholar]
- [56].Maze JR, Stanwix PL, Hodges JS, Hong S, Taylor JM, Cappellaro P, Jiang L, Dutt MVG, Togan E, Zibrov AS, Yacoby A, Walsworth RL, Lukin MD, Nanoscale magnetic sensing with an individual electronic spin in diamond, Nature, 455 (2008) 644–647. [DOI] [PubMed] [Google Scholar]
- [57].Bradac C, Gaebel T, Naidoo N, Sellars MJ, Twamley J, Brown LJ, Barnard AS, Plakhotnik T, Zvyagin AV, Rabeau JR, Observation and control of blinking nitrogen-vacancy centres in discrete nanodiamonds, Nature Nanotechnology, 5 (2010) 345–349. [DOI] [PubMed] [Google Scholar]
- [58].Tisler J, Reuter R, Lämmle A, Jelezko F, Balasubramanian G, Hemmer PR, Reinhard F, Wrachtrup J, Highly Efficient FRET from a Single Nitrogen-Vacancy Center in Nanodiamonds to a Single Organic Molecule, ACS Nano, 5 (2011) 7893–7898. [DOI] [PubMed] [Google Scholar]
- [59].Mochalin VN, Shenderova O, Ho D, Gogotsi Y, The properties and applications of nanodiamonds, Nature Nanotechnology, 7 (2012) 11–23. [DOI] [PubMed] [Google Scholar]
- [60].Li Y, Su Y, Pan H, Deng W, Wang J, Liu D, Pan W, Nanodiamond-based multifunctional platform for oral chemo-photothermal combinational therapy of orthotopic colon cancer, Pharmacological Research, 176 (2022) 106080. [DOI] [PubMed] [Google Scholar]
- [61].Cheng Z, Li M, Dey R, Chen Y, Nanomaterials for cancer therapy: current progress and perspectives, J Hematol Oncol, 14 (2021) 85–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J, Shao A, Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance, Frontiers in Molecular Biosciences, 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Carmeliet P, Jain RK, Angiogenesis in cancer and other diseases, Nature, 407 (2000) 249–257. [DOI] [PubMed] [Google Scholar]
- [64].Maeda H, Nakamura H, Fang J, The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo, Advanced Drug Delivery Reviews, 65 (2013) 71–79. [DOI] [PubMed] [Google Scholar]
- [65].Narum SM, Le T, Le DP, Lee JC, Donahue ND, Yang W, Wilhelm S, Chapter 4 - Passive targeting in nanomedicine: fundamental concepts, body interactions, and clinical potential, in: Chung EJ, Leon L, Rinaldi C(Eds.) Nanoparticles for Biomedical Applications, Elsevier2020, pp. 37–53. [Google Scholar]
- [66].Perevedentseva E, Hong SF, Huang KJ, Chiang IT, Lee CY, Tseng YT, Cheng CL, Nanodiamond internalization in cells and the cell uptake mechanism, Journal of Nanoparticle Research, 15 (2013) 1834. [Google Scholar]
- [67].Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF, An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites, J Pharm Pharmacol, 71 (2019) 1185–1198. [DOI] [PubMed] [Google Scholar]
- [68].Li L, Tian L, Zhao W, Li Y, Yang B, Acetate ions enhance load and stability of doxorubicin onto PEGylated nanodiamond for selective tumor intracellular controlled release and therapy, Integrative Biology, 8 (2016) 956–967. [DOI] [PubMed] [Google Scholar]
- [69].Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O, Cancer nanomedicine: from targeted delivery to combination therapy, Trends Mol Med, 21 (2015) 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ali ES, Sharker SM, Islam MT, Khan IN, Shaw S, Rahman MA, Uddin SJ, Shill MC, Rehman S, Das N, Ahmad S, Shilpi JA, Tripathi S, Mishra SK, Mubarak MS, Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives, Seminars in Cancer Biology, 69 (2021) 52–68. [DOI] [PubMed] [Google Scholar]
- [71].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D, Progress and challenges towards targeted delivery of cancer therapeutics, Nature Communications, 9 (2018) 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Danhier F, Feron O, Préat V, To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, Journal of Controlled Release, 148 (2010) 135–146. [DOI] [PubMed] [Google Scholar]
- [73].Gavas S, Quazi S, Karpiński TM, Nanoparticles for Cancer Therapy: Current Progress and Challenges, Nanoscale Research Letters, 16 (2021) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhang X-Q, Lam R, Xu X, Chow EK, Kim H-J, Ho D, Multimodal Nanodiamond Drug Delivery Carriers for Selective Targeting, Imaging, and Enhanced Chemotherapeutic Efficacy, Advanced Materials, 23 (2011) 4770–4775. [DOI] [PubMed] [Google Scholar]
- [75].Chu Z, Zhang S, Zhang B, Zhang C, Fang CY, Rehor I, Cigler P, Chang HC, Lin G, Liu R, Li Q, Unambiguous observation of shape effects on cellular fate of nanoparticles, Sci Rep, 4 (2014) 4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lukowski S, Neuhoferova E, Kinderman M, Krivohlava R, Mineva A, Petrakova V, Benson V, Fluorescent Nanodiamonds are Efficient, Easy-to-Use Cyto-Compatible Vehicles for Monitored Delivery of Non-Coding Regulatory RNAs, J Biomed Nanotechnol, 14 (2018) 946–958. [DOI] [PubMed] [Google Scholar]
- [77].Faklaris O, Joshi V, Irinopoulou T, Tauc P, Sennour M, Girard H, Gesset C, Arnault J-C, Thorel A, Boudou J-P, Curmi PA, Treussart F, Photoluminescent Diamond Nanoparticles for Cell Labeling: Study of the Uptake Mechanism in Mammalian Cells, ACS Nano, 3 (2009) 3955–3962. [DOI] [PubMed] [Google Scholar]
- [78].McGuinness LP, Yan Y, Stacey A, Simpson DA, Hall LT, Maclaurin D, Prawer S, Mulvaney P, Wrachtrup J, Caruso F, Scholten RE, Hollenberg LCL, Quantum measurement and orientation tracking of fluorescent nanodiamonds inside living cells, Nature Nanotechnology, 6 (2011) 358–363. [DOI] [PubMed] [Google Scholar]
- [79].Hsiao WW-W, Hui YY, Tsai P-C, Chang H-C, Fluorescent Nanodiamond: A Versatile Tool for Long-Term Cell Tracking, Super-Resolution Imaging, and Nanoscale Temperature Sensing, Accounts of Chemical Research, 49 (2016) 400–407. [DOI] [PubMed] [Google Scholar]
- [80].Petit J-CAT, Girard H, Grall R, Chevillard S and Delic J, Use of nanodiamands for generating therapeutic free radicals under irradiation, in: U.S. Patent Application Publication (Ed.)United States, 2015. [Google Scholar]
- [81].Chang YR, Lee HY, Chen K, Chang CC, Tsai DS, Fu CC, Lim TS, Tzeng YK, Fang CY, Han CC, Chang HC, Fann W, Mass production and dynamic imaging of fluorescent nanodiamonds, Nat Nanotechnol, 3 (2008) 284–288. [DOI] [PubMed] [Google Scholar]
- [82].Su LJ, Wu MS, Hui YY, Chang BM, Pan L, Hsu PC, Chen YT, Ho HN, Huang YH, Ling TY, Hsu HH, Chang HC, Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs, Sci Rep, 7 (2017) 45607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Faklaris O, Joshi V, Irinopoulou T, Tauc P, Sennour M, Girard H, Gesset C, Arnault JC, Thorel A, Boudou JP, Curmi PA, Treussart F, Photoluminescent diamond nanoparticles for cell labeling: study of the uptake mechanism in mammalian cells, ACS Nano, 3 (2009) 3955–3962. [DOI] [PubMed] [Google Scholar]
- [84].Tian Y, Nusantara AC, Hamoh T, Mzyk A, Tian X, Perona Martinez F, Li R, Permentier HP, Schirhagl R, Functionalized Fluorescent Nanodiamonds for Simultaneous Drug Delivery and Quantum Sensing in HeLa Cells, ACS Applied Materials & Interfaces, 14 (2022) 39265–39273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Longley DB, Johnston PG, Molecular mechanisms of drug resistance, J Pathol, 205 (2005) 275–292. [DOI] [PubMed] [Google Scholar]
- [86].Merkel TJ, DeSimone JM, Dodging drug-resistant cancer with diamonds, Sci Transl Med, 3 (2011) 73ps78. [DOI] [PubMed] [Google Scholar]
- [87].Eckford PDW, Sharom FJ, ABC Efflux Pump-Based Resistance to Chemotherapy Drugs, Chemical Reviews, 109 (2009) 2989–3011. [DOI] [PubMed] [Google Scholar]
- [88].Wang X, Low XC, Hou W, Abdullah LN, Toh TB, Mohd Abdul Rashid M, Ho D, Chow EK, Epirubicin-adsorbed nanodiamonds kill chemoresistant hepatic cancer stem cells, ACS Nano, 8 (2014) 12151–12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Toh T-B, Lee D-K, Hou W, Abdullah LN, Nguyen J, Ho D, Chow EK-H, Nanodiamond–Mitoxantrone Complexes Enhance Drug Retention in Chemoresistant Breast Cancer Cells, Molecular Pharmaceutics, 11 (2014) 2683–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zang X, Zhao X, Hu H, Qiao M, Deng Y, Chen D, Nanoparticles for tumor immunotherapy, Eur J Pharm Biopharm, 115 (2017) 243–256. [DOI] [PubMed] [Google Scholar]
- [91].Quazi S, An Overview of CAR T Cell Mediated B Cell Maturation Antigen Therapy, Clin Lymphoma Myeloma Leuk, 22 (2022) e392–e404. [DOI] [PubMed] [Google Scholar]
- [92].Quazi S, Elucidation of CRISPR-Cas9 application in novel cellular immunotherapy, Mol Biol Rep, 49 (2022) 7069–7077. [DOI] [PubMed] [Google Scholar]
- [93].Zhang Y, Cui Z, Kong H, Xia K, Pan L, Li J, Sun Y, Shi J, Wang L, Zhu Y, Fan C, One-Shot Immunomodulatory Nanodiamond Agents for Cancer Immunotherapy, Adv Mater, 28 (2016) 2699–2708. [DOI] [PubMed] [Google Scholar]
- [94].Alhaddad A, Durieu C, Dantelle G, Le Cam E, Malvy C, Treussart F, Bertrand J-R, Influence of the Internalization Pathway on the Efficacy of siRNA Delivery by Cationic Fluorescent Nanodiamonds in the Ewing Sarcoma Cell Model, PLOS ONE, 7 (2012) e52207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ho D, Beyond the Sparkle: The Impact of Nanodiamonds as Biolabeling and Therapeutic Agents, ACS Nano, 3 (2009) 3825–3829. [DOI] [PubMed] [Google Scholar]
- [96].Ho D, Nanomaterial-based therapy: a new generation of cancer treatment, Therapy, 6 (2009) 99–104. [Google Scholar]


