Author's summary
This study compared a novel telemonitoring system using a single-lead electrocardiogram (ECG) patch with a conventional telemonitoring system in a real-time inpatient setting. The novel telemonitoring system offers performance comparable to a conventional system while significantly reducing signal loss owing to the seamless wireless network through the dual-connection configuration between an ECG patch and neighboring gateways, efficiently communicated by the Bluetooth Low Energy protocol. The signal noise was also significantly reduced by preventing motion artifacts or poor contact between the electrodes and skin using a lightweight single-lead ECG patch.
Keywords: Electrocardiogram, Telemedicine, Wearable electronic devices
Graphical Abstract

Abstract
Background and Objectives
Although a single-lead electrocardiogram (ECG) patch may provide advantages for detecting arrhythmias in outpatient settings owing to user convenience, its comparative effectiveness for real-time telemonitoring in inpatient settings remains unclear. We aimed to compare a novel telemonitoring system using a single-lead ECG patch with a conventional telemonitoring system in an inpatient setting.
Methods
This was a single-center, prospective cohort study. Patients admitted to the cardiology unit for arrhythmia treatment who required a wireless ECG telemonitoring system were enrolled. A single-lead ECG patch and conventional telemetry were applied simultaneously in hospitalized patients for over 24 hours for real-time telemonitoring. The basic ECG parameters, arrhythmia episodes, and signal loss or noise were compared between the 2 systems.
Results
Eighty participants (mean age 62±10 years, 76.3% male) were enrolled. The three most common indications for ECG telemonitoring were atrial fibrillation (66.3%), sick sinus syndrome (12.5%), and atrioventricular block (10.0%). The intra-class correlation coefficients for detecting the number of total beats, atrial and ventricular premature complexes, maximal, average, and minimal heart rates, and pauses were all over 0.9 with p values for reliability <0.001. Compared to a conventional system, a novel system demonstrated significantly lower signal noise (median 0.3% [0.1–1.6%] vs. 2.4% [1.4–3.7%], p<0.001) and fewer episodes of signal loss (median 22 [2–53] vs. 64 [22–112] episodes, p=0.002).
Conclusions
The novel telemonitoring system using a single-lead ECG patch offers performance comparable to that of a conventional system while significantly reducing signal loss and noise.
Trial Registration
Clinical Research Information Service Identifier: KCT0008176
INTRODUCTION
Electrocardiogram (ECG) plays a pivotal role in managing cardiovascular patients. Continuous ECG monitoring, as recommended by the American Heart Association (AHA), can improve the early detection and treatment of cardiac events.1),2) With the incidence of in-hospital cardiac arrests reported to be as high as 5 events per 1,000 hospital admissions,3) the compelling need for continuous ECG telemonitoring in selected hospitalized patients is underscored.
Recent technological advancements have led to developing single-lead ECG patches for continuous monitoring to detect and manage arrhythmias.4),5) Despite their convenience, these devices have raised concerns regarding diagnostic accuracy owing to their single-lead design.6) Nonetheless, they offer considerable advantages over traditional methods such as Holter recording, particularly in diagnosing arrhythmias, owing to their long-term duration of monitoring.7),8),9),10)
Most evaluations of single-lead ECG patches have been conducted in outpatient settings, leaving the efficacy of these patches for real-time telemonitoring in inpatient settings,11) unlike conventional telemetry systems that require clarification. The complexities of hospital environments and the diversity of patient profiles necessitate a comprehensive evaluation.
Unlike outpatient settings, inpatient settings require continuous real-time monitoring of ECG signals to detect cardiac events without missing data. A wired connection between the electrodes and monitoring devices may be sufficient for bedridden patients. However, for ambulatory patients, seamless wireless networks between ECG sensors and signal receivers, such as gateways or access points (APs), are required for real-time continuous telemonitoring. This highlights the need for a reliable system that uses convenient wearable devices capable of telemonitoring for extended periods. However, the efficacy of telemonitoring using a single-lead ECG patch in inpatient settings compared with a conventional telemetry system remains uncertain. Therefore, we compared the efficacy of a novel telemonitoring system using a single-lead ECG patch with that of a conventional telemetry system in an inpatient setting.
METHODS
Ethical statement
The study adhered to the guidelines of the Declaration of Helsinki (revised in 2013) and was approved by the Clinical Research Information Service of the Republic of Korea (CRIS No. KCT0008176).
Study population
This single-center prospective cohort study was conducted at the Department of Cardiology, Hallym University Sacred Heart Hospital. The inclusion criteria were as follows:1) patients aged 19 years or older, 2) patients admitted for cardiac arrhythmia treatment requiring continuous ECG telemonitoring, and 3) patients hospitalized in a cardiology unit equipped with a wireless real-time ECG telemonitoring system. Patients were excluded from the study if they could not understand or comply with the study protocol, did not express their opinions, or had skin reactions or allergies that prevented the application of the ECG patch. Data were collected from August 3, 2022, to December 31, 2022, with a final enrollment of 80 participants.
Wireless electrocardiogram telemonitoring in inpatient settings
The participants were subjected to simultaneous wireless ECG telemonitoring using a single-lead ECG patch (ThynC™; Seers Technology Co., Seongnam, Korea) and a conventional telemetry device (IntelliVue MX40 Patient Wearable Monitor; Philips, New York, NY, USA) for over 24 hours. The patient was required to carry a conventional device with electrodes on the chest to facilitate ECG sensing along leads I, V1, and V6. The single-lead ECG patch was also affixed to the precordium and oriented 45° from the inter-nipple line to record lead II (Supplementary Figure 1). The ECG was continuously monitored and recorded on a central monitoring platform in the cardiology unit via wireless networks between each sensing device and its corresponding gateway.
Figure 1 illustrates the novel telemonitoring platform using a single-lead ECG patch in an inpatient setting. Wireless communication between the ECG sensing device and the signal receiver was facilitated through Bluetooth Low Energy (BLE), which operates over a 2.4 GHz frequency band. To ensure reliable signal reception and transmission to the central monitoring platform, the number and spacing of the gateways were tested and adjusted when the telemonitoring system was installed. The gateways installed in the unit received ECG data from the patches applied to the patients, which were then wirelessly transmitted to ward monitors, dashboards, workstations, and mobile devices for real-time telemonitoring.
Figure 1. Schematic illustration of an inpatient setting for a novel telemonitoring system using a single-lead electrocardiogram patch.
Patients in a cardiology unit wear a single-lead ECG patch. ECG data from the patch can be wirelessly transmitted to the central monitoring platform and real-time telemonitored at ward monitors, a dashboard in the nurse station, and a workstation. The gateways as ECG signal receivers are strategically installed to facilitate seamless reception of ECG data.
ECG = electrocardiogram.
The wireless communication between a single-lead ECG patch and its corresponding gateway utilizes a dual-connection method to mitigate signal loss as patients move around in a cardiology unit (Figure 2). In the single-connection setup, only one nearby gateway was connected to the ECG patch to receive signals. Therefore, a single-connection configuration may lead to transient signal losses when the patient moves out of the effective range of a previously connected gateway. However, the dual-connection approach allows the ECG patch to communicate with 2 nearby gateways simultaneously. It prevents the risk of signal loss because the patient is within the effective range of another gateway, even if it is outside the effective range of one gateway. This process may minimize signal loss, thereby enhancing the reliability of continuous wireless ECG telemonitoring.
Figure 2. Schematic illustration of the dual connection configuration to prevent signal loss in ambulatory patient settings.
The BLE protocol efficiently communicated the dual connection configuration between the ECG patch and the neighboring gateways. It allows the ECG patch to connect with 2 nearby gateways simultaneously. It prevents the risk of signal loss because the patient is within the effective range of another gateway, even if it is outside the effective range of one gateway.
BLE = Bluetooth Low Energy; ECG = electrocardiogram.
Measurements and parameters
The baseline characteristics of the study population were analyzed. These included demographic information (age, sex, height, body weight, and body mass index), comorbidities (heart failure, hypertension, diabetes mellitus, stroke, and vascular disease), echocardiographic parameters (left ventricular ejection fraction, left atrial diameter, and left atrial volume index), blood test parameters (serum creatinine and creatinine clearance), and indications for wireless ECG telemonitoring (atrial fibrillation [AF], sick sinus syndrome, atrioventricular block, and ventricular tachycardia).
Furthermore, a comparative assessment was performed between the ECG parameters recorded by a single-lead ECG patch and those recorded by a conventional telemetry device. These parameters included the total monitoring time, total QRS complex, total atrial premature complex (APC), total ventricular premature complex (VPC), AF burden, minimum, average, and maximum heart rates (HRs), and pauses. The effectiveness of continuous telemonitoring was also evaluated by measuring the proportion of noise data, episodes of signal loss, and the minimum and maximum durations of signal loss in both devices.
Noise is defined as uninterpretable signals mainly attributable to motion artifacts or poor contact between the electrodes and the skin, excluding signal loss. Most signal losses occurred because of the loss of wireless connections when patients moved outside the effective range of the gateways. Supplementary Figure 2 shows examples of signal noise and signal loss. The ECG signals from both devices were reviewed and validated by 2 cardiologists.
Statistical analyses
Data are presented as number (%), mean ± standard deviation, or median (interquartile range) based on the variable characteristics. A descriptive analysis was conducted for baseline characteristics. The ECG parameters were compared using the intra-class correlation coefficient (ICC) with a 95% confidence interval (CI) to evaluate the similarity between the wireless ECG recordings from the 2 devices. To compute the ICC, we first processed the raw ECG data through the proprietary analysis tools provided by each telemetry system's manufacturer. After extracting a set of ECG parameters for each system and participant, we applied these parameters to calculate the ICC for each corresponding parameter. Reliability p values were also calculated. An ICC exceeding 0.9 indicated excellent reliability.
For inter-observer correlation analysis, 2 electrophysiologists independently reviewed the raw ECG data of the 2 telemonitoring systems. The Bland-Altman plot12) was employed to assess the agreement in measuring ECG parameters between the 2 devices, and 95% limits of agreement were calculated for each parameter. Any measurement difference between the 2 devices was considered a good agreement within the limits of the agreement. If an ECG parameter had outliers beyond the 95% limits of agreement in the Bland-Altman plot, the raw data of both telemonitoring systems were evaluated to identify the reasons. The paired t-test or Wilcoxon signed-rank test, as appropriate, was used to compare the differences in ECG parameters, signal noise, and signal loss between the 2 devices. The distributions of signal noise, signal loss, and the minimum and maximum durations of signal loss were plotted and compared between the 2 devices. A p value less than 0.05 was considered statistically significant for all statistical analyses; thus, the null hypothesis was rejected. Statistical analyses were performed using IBM SPSS Statistics for Windows (version 22.0; IBM Corp., Armonk, NY, USA).
RESULTS
The baseline characteristics of the study population are summarized in Table 1. Eighty participants were evaluated. Their mean age was 61.8±10.1 years, and the proportion of men was 76.3%. Indications for ECG telemonitoring, in descending order, included AF (66.3%), sick sinus syndrome (12.5%), atrioventricular block (10.0%), premature ventricular complex (6.3%), and ventricular tachycardia (5.0%).
Table 1. Baseline characteristics of the study participants (total n=80).
| Characteristics | Value | |
|---|---|---|
| Demographics | ||
| Age (year) | 61.8±10.1 | |
| Male | 61 (76.3) | |
| Height (cm) | 167.8±9.3 | |
| Body weight (kg) | 72.6±14.3 | |
| Body mass index (kg/m2) | 25.7±3.7 | |
| Comorbidities | ||
| Heart failure | 27 (33.8) | |
| Hypertension | 64 (80.0) | |
| Diabetes mellitus | 25 (31.3) | |
| Stroke | 13 (16.3) | |
| Vascular disease | 5 (6.3) | |
| Laboratory results | ||
| Left ventricular ejection fraction (%) | 61.4±6.9 | |
| Left atrial volume index (mL/m2) | 57.6±23.0 | |
| Left atrial diameter (mm) | 44.5±5.8 | |
| Serum creatinine (mg/dL) | 1.00±0.22 | |
| Creatinine clearance (mL/min) | 80.3±26.7 | |
| Indications for wireless ECG monitoring | ||
| Atrial fibrillation | 53 (66.3) | |
| Sick sinus syndrome | 10 (12.5) | |
| Atrioventricular block | 8 (10.0) | |
| Ventricular premature complex | 5 (6.3) | |
| Ventricular tachycardia | 4 (5.0) | |
Data are presented as mean ± standard deviation or number (%).
ECG = electrocardiogram.
Comparison of novel telemonitoring system with a conventional telemonitoring system: electrocardiogram parameters
The median duration of telemonitoring was the same for both the single-lead ECG patch and the conventional telemetry device. All ECG parameters demonstrated excellent reliability for both devices (Table 2). The total QRS complex, atrial premature complex, ventricular premature complex, and pause showed ICCs of 0.999 (95% CI, 0.998–0.999), 0.998 (0.997–0.999), 0.999 (0.998–0.999), and 0.997 (0.996–0.998), respectively (all p values for reliability <0.001). Both ECG telemonitoring systems identified AF episodes in the same 9 patients, and the AF burden had an ICC of 0.995 (0.978–0.999). The ICCs were 0.906 (0.853–0.940), 0.967 (0.949–0.979), and 0.986 (0.978–0.991) for the minimum, average, and maximum HRs, respectively (all p-values for reliability <0.001). Although all ECG parameters showed excellent reliability between the 2 devices, significant differences were observed in the total QRS complex, atrial premature complex, ventricular premature complex, maximum HR, minimum HR, and pause duration (p<0.001, p=0.001, p=0.003, p<0.001, p=0.049, and p<0.001, respectively) (Table 2). Inter-observer correlation analysis showed that in both telemonitoring systems, an excellent degree of agreement was observed regardless of ECG parameters (Supplementary Table 1).
Table 2. Comparison of electrocardiographic parameters between novel telemonitoring system using a single-lead ECG patch and the conventional telemonitoring system in inpatient setting.
| Variables | Novel telemonitoring system* (n=80) | Conventional telemonitoring system† (n=80) | 95% limits of agreement | ICC (95% CI) | p value for reliability | p value for difference |
|---|---|---|---|---|---|---|
| Monitoring time (minute) | 1,408 (1,215–1,827) | 1,408 (1,215–1,827) | - | - | - | - |
| Total QRS complex (No.) | 69,635 (54,756–90,223) | 66,601 (53,749–88,667) | −14,573, 10,851 | 0.999 (0.998–0.999) | <0.001 | <0.001 |
| Total atrial premature complex (No.) | 99 (19–375) | 100 (19–377) | −181, 137 | 0.998 (0.997–0.999) | <0.001 | 0.001 |
| Total ventricular premature complex (No.) | 5 (0–97) | 5 (0–88) | −308, 335 | 0.999 (0.998–0.999) | <0.001 | 0.003 |
| AF burden‡ (%) | 26.1 (10.1–100.0) | 27.2 (10.0–100.0) | −3.8, 4.3 | 0.995 (0.978–0.999) | <0.001 | 0.116 |
| Maximum HR (/min) | 114.1±27.6 | 104.8±21.6 | −37.8, 19.3 | 0.906 (0.853–0.940) | <0.001 | <0.001 |
| Minimum HR (/min) | 54.7±10.9 | 55.5±10.1 | −6.6, 8.2 | 0.967 (0.949–0.979) | <0.001 | 0.049 |
| Average HR (/min) | 72.2±12.1 | 71.7±12.0 | −6.1, 5.1 | 0.986 (0.978–0.991) | <0.001 | 0.151 |
| Pause (second) | 1.3 (1.2–1.7) | 1.4 (1.3–1.8) | −0.09, 0.17 | 0.997 (0.996–0.998) | <0.001 | <0.001 |
Data are mean ± standard deviation or median (interquartile range).
AF = atrial fibrillation; ECG = electrocardiogram; HR = heart rate; ICC = intra-class correlation coefficient.
*ThynC™, Seers Technology, Seongnam, Korea.
†IntelliVue MX40 Patient Wearable Monitor, Philips, New York, NY, USA.
‡Among a total of 80 participants, AF was detected in 9 participants. Both the novel telemonitoring system using a single-lead ECG patch and the conventional system detected AF in the same 9 participants. The AF burden data was calculated from the 9 participants only.
Bland-Altman plots demonstrated that measurements of ECG parameters from both devices were within the limits of agreement for most participants, indicating a high degree of agreement between the 2 telemonitoring systems (Figure 3). Figure 4 presents examples of various arrhythmic episodes comparing the findings of the 2 telemonitoring systems. All arrhythmic episodes were simultaneously identifiable via the single-lead ECG patch and conventional telemetry device.
Figure 3. Bland-Altman plots for ECG parameters.
The limits of agreement and mean difference are represented. Most ECG parameters displayed good agreement between the two devices. The annotations (A-G) denote some outliers for each parameter. The analyses for the outliers are presented in the RESULT.
AF = atrial fibrillation; APC = atrial premature complex; ECG = electrocardiogram; ICC = intraclass correlation; HR = heart rate; VPC = ventricular premature complex.
Figure 4. Various arrhythmic episodes recorded simultaneously by a novel telemonitoring system using a single-lead ECG patch and the conventional telemonitoring system.
Arrhythmic episodes were identifiable in both systems simultaneously. The upper panel shows the conventional telemonitoring system with three channels, while the lower panel presents a novel telemonitoring system using a single-lead ECG patch.
ECG = electrocardiogram.
Detailed analysis for the outliers
The detailed analysis for some outliers in Figure 3 was performed by comparing raw data from the 2 systems. For the outlier ‘A,’ the total count of QRS complexes was 361,392 for the conventional system and 398,539 for the novel system. The conventional system exhibited a higher noise proportion (4.6% vs. 1.3%) and a greater number of signal loss episodes (90 vs. 73). Consequently, it is concluded that the conventional system reported fewer QRS complexes due to reduced monitoring time when excluding noise. For the outlier ‘B,’ the AF burden was 57.6% and 39.3% for the conventional and novel systems. However, the conventional telemetry system erroneously includes the signal loss proportion as part of the AF episode if it directly precedes or follows an AF episode, leading to overestimating AF duration (Supplementary Figure 3). This results in a higher calculated AF burden when compared to the novel system. On the other hand, the novel system did not include the signal loss portion to calculate the AF burden. As a result, the conventional system calculated a total AF duration of 2,679 minutes (AF burden of 57.9%), while the novel system calculated 1,678 minutes (AF burden of 40.4%). If we manually calculate the AF burden excluding the signal loss proportion in the conventional system, the same AF burden of 40.4% was observed as in the novel system. For the outlier ‘C,’ the lower APC count in the conventional system (5,240 vs. 5,826) was mainly due to the higher noise proportion (3.0% vs. 0.2%) that led to a decrease in analyzable monitoring time. For the outlier ‘D,’ The novel system reported a lower count of VPCs (21,769 vs. 23,222) due to the misclassification of coupled VPCs with fusion beats as normal beats (Supplementary Figure 4). This misclassification occurred because the fusion beats presented as a narrower QRS complex on the single-lead ECG strip in the novel system. For the outlier ‘E,’ the novel system recorded a lower minimum HR (21 vs. 39 per min). This discrepancy can be attributed to the higher noise proportion in the conventional system (4.9% vs. 0.3%). The conventional system disregarded the episode of minimal HR detected by the novel system due to signal noise. For the outlier ‘F,’ the maximum HR was higher in the novel system (86 vs. 160 per min). This discrepancy was because the novel system included tachyarrhythmic episodes for calculating maximum HR while the conventional system included only sinus rhythm episodes. Similarly, for the outlier ‘G,’ the difference in average HR was due to the systems’ different algorithms for calculating HR.
Comparison of novel telemonitoring system with a conventional telemonitoring system: signal noise and signal loss
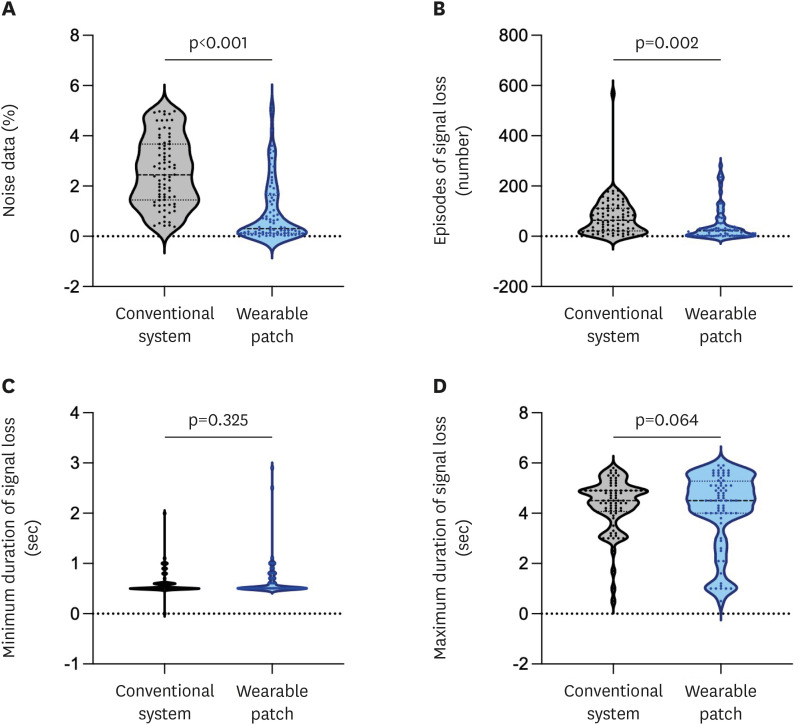
Table 3 and Figure 5 summarize the signal noise and loss comparison between the 2 telemonitoring systems. Compared to the conventional system, the novel system using a single-lead ECG patch showed a significantly reduced proportion of noise data: 2.4% (1.4–3.7%) vs. 0.3% (0.1–1.6%); p<0.001. In addition, the total number of episodes of signal loss was significantly lower with the novel telemonitoring system; 64 (22–112) vs. 22 (2–53); p =0.002. However, the minimum and maximum durations of signal loss were not significantly different between the 2 telemonitoring systems (p=0.325 and p=0.064, respectively).
Table 3. Comparison of signal loss and noise between novel telemonitoring system using a single-lead ECG patch and the conventional telemonitoring system.
| Variables | Novel telemonitoring system* | Conventional telemonitoring system† | p value for difference |
|---|---|---|---|
| Proportion of noise data‡ (%) | 0.3 (0.1–1.6) | 2.4 (1.4–3.7) | <0.001 |
| Episodes of signal loss§ (N) | 22 (2–53) | 64 (22–112) | 0.002 |
| Minimum duration of signal loss (sec) | 0.5 (0.5–0.6) | 0.5 (0.5–0.6) | 0.325 |
| Maximum duration of signal loss (sec) | 4.5 (2.5–5.1) | 4.5 (4.1–4.9) | 0.064 |
Data are presented as median (interquartile range).
*ThynC™, Seers Technology, Seongnam, Korea.
†IntelliVue MX40 Patient Wearable Monitor, Philips, New York, NY, USA.
‡Most noise data were due to signal noise from motion artifacts or poor contact between electrodes and skin. The noise data excluded the episodes with signal loss.
§Most episodes of signal loss were due to transient loss of wireless connection due to participants wandering outside the effective range of the AP.
AP = access point; ECG = electrocardiogram.
Figure 5. Distributions of signal noise and signal loss between novel telemonitoring system using a single-lead ECG patch and the conventional telemonitoring system.
A novel telemonitoring system using a single-lead ECG patch demonstrated a significant reduction in (A) proportions of noise data and (B) the total number of episodes of signal loss, compared to the conventional telemonitoring system. However, (C) the minimum or (D) maximum durations of signal loss were not significantly different between the two telemonitoring systems.
ECG = electrocardiogram.
DISCUSSION
In this study, we compared a novel telemonitoring system that uses a single-lead ECG patch with a conventional telemonitoring system in an inpatient setting. The findings revealed that the novel telemonitoring system exhibited excellent reliability and agreement in assessing ECG parameters compared with the conventional telemonitoring system. The novelty of the present study is that it significantly reduces signal loss owing to the seamless wireless network through the dual-connection configuration between an ECG patch and neighboring gateways, which was efficiently communicated by the BLE protocol. In addition, the signal noise was significantly reduced by preventing motion artifacts or poor contact between the electrodes and skin using a lightweight single-lead ECG patch.
Various portable and wearable devices have been developed with technological advancements, facilitating comprehensive bio-signal monitoring and recording for a long time.13),14),15) Single-lead ECG patches can record ECG continuously for up to a few weeks, either by transmitting these data to a remote server via mobile devices or storing them internally.16),17) Although single-lead ECG recording might be perceived as inferior to multi-channel ECG recording, these devices often receive patients’ preferences because of their convenience and minimal intrusion into daily life if long-term recordings are necessary. Previous studies have demonstrated the ability of single-lead ECG patches to detect arrhythmic episodes accurately.18),19) These studies were primarily conducted in outpatient settings where continuous but not necessarily real-time telemonitoring and recording suffices. However, some hospitalized patients require real-time telemonitoring to detect urgent cardiac events,1) and therefore, reliable and seamless transmission of ECG data from wearable devices to a central monitoring system, such as ward monitors, dashboards in nurse stations, or workstations, is required. In this regard, the usefulness of single-lead ECG patches has not yet been validated.
This study focused on hospitalized ambulatory patients requiring wireless real-time ECG telemonitoring and demonstrated excellent reliability and agreement between the novel telemonitoring system using a single-lead ECG patch and the conventional telemonitoring system using a device with 3-channel ECGs. Although our results are in accordance with the findings of a previous study,19) ECG data in the previous study were recorded in outpatient settings and not in real-time telemonitoring through wireless communication in inpatient settings. Furthermore, a significant difference between the 2 telemonitoring systems was observed in some ECG parameters, including the total QRS complex, atrial premature complex, ventricular premature complex, maximum HR, minimum HR, and pause duration. These discrepancies could potentially be attributed to the lower signal noise and signal loss exhibited by the novel telemonitoring system, which resulted in a 4.6% increase in QRS complexes compared to conventional telemetry. Indeed, the analysis for the outliers suggested that most discrepancies between the 2 systems originated from the neglected recordings from the conventional telemonitoring system due to noise or signal loss. In addition, the maximum and minimum HRs, which were calculated using the algorithms from each system, may have contributed to these disparities. Therefore, although there were some discrepancies in the ECG parameters between the 2 systems, we concluded that the new telemonitoring system had excellent agreement with the conventional system.
The detection of most arrhythmic episodes relies primarily on analyzing the RR intervals between 2 consecutive QRS complexes. Therefore, precise detection of QRS complexes is crucial in patients with arrhythmia and is possible with single-lead ECG patches. However, the accuracy of single-lead ECG monitoring may be compromised in detecting and diagnosing arrhythmias in patients with less pronounced P-waves, especially in lead II.19) In these circumstances, multi-lead ECG monitoring can provide a clearer P-wave for a more accurate diagnosis, but wearable devices with multi-channel ECGs are limited for long-term monitoring owing to their heavy weight, which may cause signal losses and/or noise. Nevertheless, our results revealed that both telemonitoring systems identified AF episodes in the same 9 patients, and the AF burden had an ICC of 0.995 (0.978–0.999). Furthermore, all arrhythmic episodes were simultaneously identifiable via the single-lead ECG patch and the conventional telemetry device (Figure 4).
While acknowledging some limitations of single-lead monitoring, the novel single-lead telemetry system may present significant benefits over conventional multi-lead systems, particularly regarding reduced signal loss and noise. Minimizing signal loss is vital in real-time monitoring to ensure documentation of critical episodes. For instance, in the case of the outlier ‘E,’ the novel system detected a minimum HR of 21 per min, which the conventional system missed due to signal loss. Additionally, we propose that the novel system offers improved patient compliance. The ECG patch utilizes only 2 adhesive electrodes, while the conventional system requires 5. Moreover, the conventional system requires the carrying of a recorder (Supplementary Figure 1). Hence, the single-lead ECG patch telemetry system could be a good alternative to traditional multi-lead systems, offering distinct advantages regarding signal integrity and patient comfort.
The present study has some limitations. First, although our study conducted a head-to-head comparison between the 2 telemonitoring systems, it focused on basic ECG parameters and cardiac arrhythmic episodes, not on myocardial ischemic events. Although early detection of ST changes may be helpful in inpatient settings, a single-lead ECG exhibits substantially low sensitivity in discerning ST segment changes typically associated with non-arrhythmic cardiac events such as myocardial ischemia or infarction. Further investigations using artificial intelligence analysis to detect subtle ST changes are warranted to assess the efficacy of wireless ECG telemonitoring in inpatient settings. Secondly, we cannot conclude whether the novel telemonitoring system using a single-lead ECG patch is superior to conventional telemonitoring systems in signal loss and noise. Although the number and spacing of signal receivers were tested and adjusted when installing the 2 telemonitoring systems to ensure reliable signal reception and transmission to the central monitoring platform, the duration of use of signal receivers or sensors and the number and spacing of signal receivers were significantly different between the 2 telemonitoring systems, which might have affected the results. Thirdly, the study’s confinement to a single center and the lack of testing in different wards with variable settings are acknowledged as limitations, and future research is necessary to extend the validation of the system to multiple institutions, which will provide a more robust evaluation of its performance in diverse situations. Fourthly, as no adverse events leading to study withdrawal were reported, long-term compliance with the ECG patch could not be assessed within this study’s scope. However, given that the patch uses fewer adhesive electrodes of the same type as the conventional system, it is reasonable to expect comparable or improved compliance rates. It is worth noting that out of 80 participants, only one experienced a minor skin allergy related to the ECG patch, which resolved without medical intervention after the patch was removed.
In conclusion, the present study revealed that the novel system using a single-lead ECG patch could provide seamless ECG telemonitoring with excellent reliability and agreement compared with conventional telemonitoring systems in inpatient settings. This innovative system could enhance the convenience and effectiveness of the early detection of cardiac arrhythmias in hospitalized patients.
Footnotes
Funding: This work was supported by the Hallym University Medical Center (HALLYM 2021-08-010-006). This research was supported by a grant from the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (project number: HI20C1662, 1711138358, KMDF_PR_20200901_0173).
Conflict of Interest: Kwon S, Lee SR, Oh S, Han SJ, and Lim HE have none to declare. Choi EK received grants from Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daewoong Pharmaceutical Co, Daiichi-Sankyo, DeepQure, Dreamtech Co., EIL Pharmaceutical Co., Medtronic, Sanofi-Aventis, Samjinpharm, Seers Technology Co, Ltd., and Skylabs outside the submitted work, and Stock options from Seers Technology Co, Ltd. Song HS and Lee YS are stockholders of Seers Technology Co, Ltd.
Data Sharing Statement: The data for the present study is not publicly available but may be available to qualified researchers on reasonable requests from the corresponding author (Lim HE).
- Conceptualization: Kwon S, Choi EK, Lee SR, Oh S, Lee YS, Han SJ, Lim HE.
- Data curation: Kwon S, Choi EK, Song HS, Lee YS, Han SJ, Lim HE.
- Formal analysis: Kwon S, Choi EK, Lee SR, Oh S, Han SJ, Lim HE.
- Funding acquisition: Choi EK, Lim HE.
- Investigation: Kwon S, Choi EK, Lee SR, Oh S, Song HS, Lee YS, Han SJ, Lim HE.
- Methodology: Kwon S, Choi EK, Lee SR, Oh S, Lee YS, Han SJ, Lim HE.
- Project administration: Choi EK, Song HS, Lim HE.
- Resources: Kwon S, Choi EK, Song HS, Lee YS, Han SJ, Lim HE.
- Software: Kwon S, Lee SR, Song HS, Lim HE.
- Supervision: Choi EK, Lee SR, Oh S, Han SJ, Lim HE.
- Validation: Kwon S, Choi EK, Lee SR, Oh S, Han SJ.
- Visualization: Kwon S, Lim HE.
- Writing - original draft: Kwon S.
- Writing - review & editing: Kwon S, Choi EK, Lee SR, Oh S, Han SJ, Lim HE.
SUPPLEMENTARY MATERIALS
Inter-observer correlation analysis
Equipment arrangement for wireless real-time ECG telemonitoring within hospital settings.
Examples of signal noise and signal loss.
Analysis of raw data for the outlier ‘B.’
Analysis of raw data for the outlier ‘D.’
References
- 1.Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110:2721–2746. doi: 10.1161/01.CIR.0000145144.56673.59. [DOI] [PubMed] [Google Scholar]
- 2.Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation. 2017;136:e273–e344. doi: 10.1161/CIR.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 3.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. 2007;33:237–245. doi: 10.1007/s00134-006-0326-z. [DOI] [PubMed] [Google Scholar]
- 4.Svennberg E, Tjong F, Goette A, et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace. 2022;24:979–1005. doi: 10.1093/europace/euac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandberg EL, Halvorsen S, Berge T, et al. Fully digital self-screening for atrial fibrillation with patch electrocardiogram. Europace. 2023;25:euad075. doi: 10.1093/europace/euad075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manninger M, Zweiker D, Svennberg E, et al. Current perspectives on wearable rhythm recordings for clinical decision-making: the wEHRAbles 2 survey. Europace. 2021;23:1106–1113. doi: 10.1093/europace/euab064. [DOI] [PubMed] [Google Scholar]
- 7.Kwon S, Lee SR, Choi EK, et al. Comparison between the 24-hour Holter test and 72-hour single-lead electrocardiogram monitoring with an adhesive patch-type device for atrial fibrillation detection: prospective cohort study. J Med Internet Res. 2022;24:e37970. doi: 10.2196/37970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127:95.e11–95.e17. doi: 10.1016/j.amjmed.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CM, Chang SL, Yeh YH, et al. Enhanced detection of cardiac arrhythmias utilizing 14-day continuous ECG patch monitoring. Int J Cardiol. 2021;332:78–84. doi: 10.1016/j.ijcard.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Oh IY, Lee H, et al. The efficacy of detecting arrhythmia is higher with 7-day continuous electrocardiographic patch monitoring than with 24-h Holter monitoring. J Arrhythm. 2023;39:422–429. doi: 10.1002/joa3.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouzid Z, Al-Zaiti SS, Bond R, Sejdić E. Remote and wearable ECG devices with diagnostic abilities in adults: a state-of-the-science scoping review. Heart Rhythm. 2022;19:1192–1201. doi: 10.1016/j.hrthm.2022.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Bansal A, Joshi R. Portable out-of-hospital electrocardiography: a review of current technologies. J Arrhythm. 2018;34:129–138. doi: 10.1002/joa3.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Alusi MA, Ding E, McManus DD, Lubitz SA. Wearing your heart on your sleeve: the future of cardiac rhythm monitoring. Curr Cardiol Rep. 2019;21:158. doi: 10.1007/s11886-019-1223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leclercq C, Witt H, Hindricks G, et al. Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: proceedings of the European Society of Cardiology Cardiovascular Round Table. Europace. 2022;24:1372–1383. doi: 10.1093/europace/euac052. [DOI] [PubMed] [Google Scholar]
- 16.Pevnick JM, Birkeland K, Zimmer R, Elad Y, Kedan I. Wearable technology for cardiology: an update and framework for the future. Trends Cardiovasc Med. 2018;28:144–150. doi: 10.1016/j.tcm.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermans PA, Solosko TA, Spencer EC, et al. A user-friendly integrated monitor-adhesive patch for long-term ambulatory electrocardiogram monitoring. J Electrocardiol. 2012;45:148–153. doi: 10.1016/j.jelectrocard.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Ramkumar S, Nerlekar N, D’Souza D, Pol DJ, Kalman JM, Marwick TH. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ Open. 2018;8:e024178. doi: 10.1136/bmjopen-2018-024178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon S, Lee SR, Choi EK, et al. Validation of adhesive single-lead ECG device compared with Holter monitoring among non-atrial fibrillation patients. Sensors (Basel) 2021;21:3122. doi: 10.3390/s21093122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inter-observer correlation analysis
Equipment arrangement for wireless real-time ECG telemonitoring within hospital settings.
Examples of signal noise and signal loss.
Analysis of raw data for the outlier ‘B.’
Analysis of raw data for the outlier ‘D.’