Abstract
Background
Coronavirus disease (COVID-19) currently named SARS-CoV-2 is a contagious disease caused by a coronavirus. The virus may infect the hair follicles directly or indirectly through systemic changes in the immune or hormonal systems.
Aims
In the current study we aimed to determine the prevalence of hair disorders in females infected with COVID-19.
Methods
Data was collected using a questionnaire covering four main domains: personal data, past medical history, COVID-19 history and treatment, and existence of any hair problems and their management. No identifier or sensitive data were collected. Those complaining of hair loss were subjected to complete general and local hair examination using trichoscopy to confirm hair loss.
Results
Hair problems were reported in 307 (61.4%) of COVID-19-infected female subjects. A total of 68.1% patients reported that hair loss existed and increased after COVID-19; 29.6% reported their hair problems only post-COVID-19 while 2.3% had hair shedding issues during infection only. The main reported hair problems were telogen effluvium (60.8%), increased gray hair (13.8%), seborrheic dermatitis (5.6%) trichotillomania (3.6%), and alopecia areata (2.2%).
Conclusion
In conclusion, we reported prevalence of post-COVID hair fall that was confirmed by trichoscopy and which affected approximately 61.4% of infected females.
Keywords: Alopecia, Telogen effluvium, Trichoscopy
Background
The coronavirus disease 2019 (COVID-19) has become the most emergent health issue globally. SARS-CoV-2, the pathogen of COVID-19, directly infects multiple tissues and organs, possibly involving skin and hair follicles, and profoundly affects the immune system [1, 2].
Telogen effluvium (TE) and hair loss following bacterial, viral, or protozoal infection had been earlier reported and was of major concern during the 1918 influenza epidemic [3]. Literature review in terms of the impact of COVID-19 infection on the hair follicle reveals hair loss caused during and post-recovery, majorly manifesting as TE [1–3].
The virus may infect the hair follicles directly or indirectly through systemic changes in the immune or hormonal systems [4]. Furthermore, it is plausible to believe that alopecia may influence or predict the severity and course of COVID-19. A growing number of reports have focused on the clinical manifestations and pathophysiology of COVID-19-associated hair diseases [4, 5].
In the current study we aimed to determine the prevalence of hair disorders in females infected with COVID-19. For that we enrolled 500 female patients post-COVID-19 and they were asked questions about pre- and post-pandemic hair diseases. All patients were subjected to online questionnaire containing 25 questions which have been divided into four domains (personal data, medical past history, diagnoses and treatment of COVID-19 infections, hair problems and their management).
Methods
This was a multi-center, cross-sectional study conducted from January 2022 to January of 2023. A total of 500 female participants attending dermatology clinics at university as well as ministry of health hospitals were included in the study after their consenting. The study followed the Helsinki declaration principals. Ethical approval was obtained from the institutional review board of Damietta Faculty of Medicine (Al-Azhar University). Written informed consent was obtained from every patient at recruitment. Inclusions included females aged 18–55 years of age whom were diagnosed with COVID-19 infection confirmed by using the reverse transcription polymerase chain reaction (RT-PCR) test of pharyngeal and nasal swabs (only those who finished the management protocol and had been discharged at least 6 months prior to joining the study). Those who were aged less than 18 years or above 55 years were excluded.
Data was collected using a questionnaire covering four main domains: personal data, past medical history, COVID-19 history and treatment, and existence of any hair problems and their management. No identifier or sensitive data were collected. Those complaining of hair loss were subjected to complete general and local hair examination using trichoscopy to confirm hair loss. Dermoscopic evaluation (DermLite DL4) and imaging were reproduced and saved.
The study was conducted in accordance with the Declaration of Helsinki and upon approval by the Local Ethical Committee—Al Azhar University IRB (00012367–21-06–005). Statistical analysis using continuous variables were presented as mean ± SD (standard deviation) for parametric data and median (min–max) for non-parametric data. Qualitative data were described using number and percent. Association between categorical variables was tested using Chi-square test. For all statistical tests p-value > 0.05 was considered not-significant and p-value < 0.05 was considered significant. All data were analyzed using IBM SPSS Statistics for Windows, Version 26, and Microsoft Excel 365.
Results
A total of 500 patients were enrolled in the study using a data collection sheet. Patients’ ages ranged from 18 to 55 years with a mean age of 37.5 ± 8.4 years. A total of 360 (72%) patients were married and 114 (22.8%) patients were single. Smoking was reported among 16 (3.2%) patients. A total of 232 (46.4%) patients were health care workers (HCWs). Regarding chronic diseases, 15 (3%) patients were diabetic, 55 (11%) patients had hypertension, 20 (4%) had a hypercoagulable condition, while 384 (76.8%) patients had no chronic health problem. Only 9 (1.8%) of patients had a psychiatric health condition and 17 (3.4%) complained of hypothyroidism (Table 1).
Table 1.
Sociodemographic characteristics of the studied cases
| Sociodemographic characteristics | n = 500 | % |
|---|---|---|
|
Age/years 19–30 31–40 41–55 |
138 222 140 |
27.6 44.4 28.0 |
|
Occupation Not working Retired Health care worker Governmental employee Special business Others |
92 9 232 132 19 16 |
18.4 1.8 46.4 26.4 3.8 3.2 |
|
Marital status Single Married Divorced Widow |
114 360 10 16 |
22.8 72.0 2.0 3.2 |
|
Educational level Middle or lower Secondary Higher education |
26 32 442 |
5.2 6.4 88.4 |
|
Income Low Middle High |
6 448 46 |
1.2 89.6 9.2 |
|
Special habits Smoking |
16 | 3.2 |
The COVID-19 infection data among study patients demonstrated that among the 500 patients studied, 78 (15.6%) were hospitalized, and 10 (2%) needed ICU admission. Medications used during COVID-19 infection included vitamins (89%), antipyretics (85.8%), antibiotics (76.6%), expectorants (43.2%), and anticoagulants (37.4%).
All participants (100%) were diagnosed mainly by PCR, 34% diagnosed further clinically, and 23% needed CT scan for diagnosis confirmation. The five main symptoms found among participants were bone and muscle aches (85.4%), fever (74.2%), loss of smell and taste (714%), cough (62.0%), and shortness of breath (51.8%) (Table 2).
Table 2.
COVID-19 diagnosis and symptoms among studied cases
| Method of diagnosis | n = 500 | % |
|---|---|---|
|
Clinically PCR CT scan Others |
170 500 119 16 |
34.0 100.0 23.8 3.2 |
| Symptoms during COVID-19 infection | ||
|
Fever Bone and muscle aches Cough Shortness of breath Eye inflammation Stomach aches Diarrhea Vomiting Severe respiratory symptoms Loss of smell and taste Others |
371 427 310 259 71 142 155 64 73 357 68 |
74.2 85.4 62.0 51.8 14.2 28.4 31.0 12.8 14.6 71.4 13.6 |
| Symptoms needed hospitalization | 78 | 15.6 |
| Symptoms needed ICU admission | 10 | 2.0 |
COVID-19 coronavirus disease, PCR polymerase chain reaction, CT computerized tomography
Hair problems were reported in 307 (61.4%) of COVID-19-infected female subjects. A total of 68.1% patients reported that hair loss existed and increased after COVID-19; 29.6% reported their hair problems only post-COVID-19, while 2.3% had hair shedding issues during infection only. The main reported hair problems were telogen effluvium (60.8%), increased gray hair (13.8%), seborrheic dermatitis (5.6%), trichotillomania (3.6%), and alopecia areata (2.2%). Hair loss occurred after 2–3 months of COVID-19 infection among 93 (18.6%) cases, while 213 (42.6%) patients noticed hair loss after 6 months of the infection. Of the participants with hair problems, 65.2% did not use any treatment; 8.2% of them used treatment based on past experiences; 7.6% received prescription medications through physician telemedicine calls; and only 10.4% visited a dermatologist (Figs. 1, 2, 3 and Table 3).
Fig. 1.
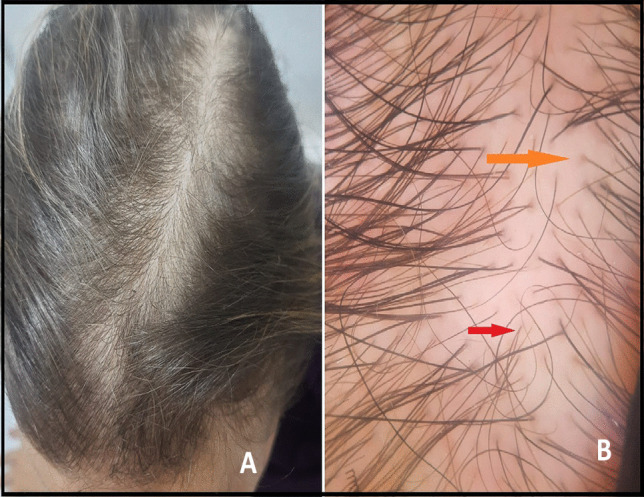
A Clinical image of a 32-year-old female showing reduced hair density with widened hair over the vertex and mid-frontal scalp. B Dermoscopy image showing great variability in the thickness of hair shaft or hair shaft diversity, vellus hair (red arrow), and peripilar sign (orange arrow) suggestive of androgenetic alopecia
Fig. 2.
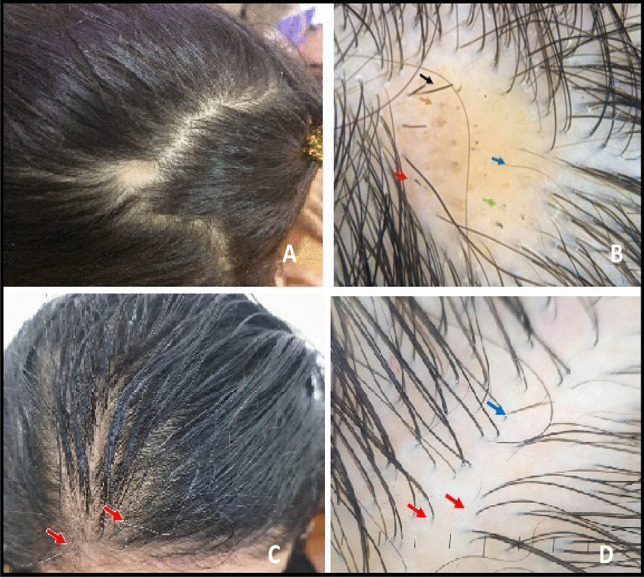
A Clinical image showing localized shedding of hair in the frontal area of the scalp surrounded by preserved hairs in a 22-year-old female. B Dermoscopy image showing yellow dots (orange arrow), black dots (green arrow), broken hair (red arrow), and tapering hair (black arrow) suggestive of alopecia areata. C Clinical image of a 21-year-old female showing reduced hair density all over the scalp with gray hair appear recently (red arrows). D Dermoscopy image showing upright re-growing hair (blue arrow), single hair emerging from the hair follicles (red arrows), and no hair shaft diversity suggestive of telogen effluvium
Fig. 3.
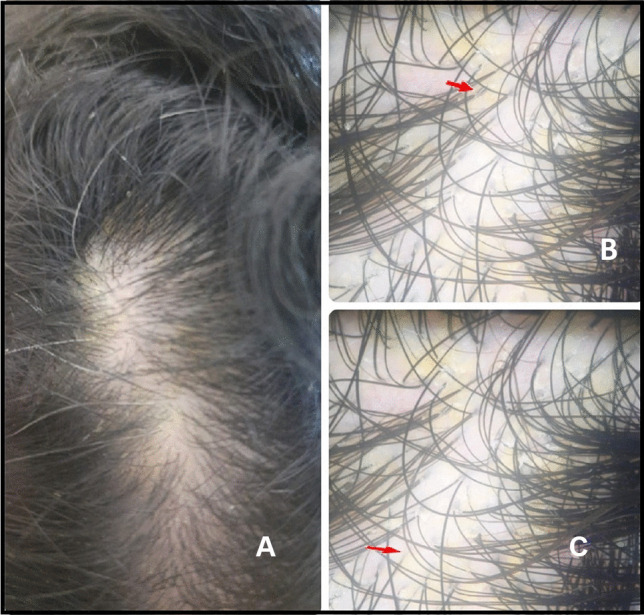
A A 21-year-old female patient complaining of clinical image showing diffuse scales of frontal and middle areas of the scalp with preservation of hair structure and density. B and C Dermoscopy images showing yellowish scales among follicular units (red arrow) suggestive of seborrheic dermatitis
Table 3.
Hair loss data among studied cases
| Hair loss data | n = 500 | % |
|---|---|---|
| Complaining of hair problems before and or/after | 307 | 61.4 |
|
Yes before and become worse after infection Yes post-COVID-19 only Yes, only during infection only |
209 91 7 |
68.1 29.6 2.3 |
|
Hair problems Telogen effluvium Alopecia areata Seborrheic dermatitis Increased gray hair Trichitollamania |
304 11 28 69 18 164 |
60.8 2.2 5.6 13.8 3.6 32.8 |
|
No treatment used Used treatment based on past experience Recommended treatment by others Based on physician telephone call Visit to non-specialist doctor Visit to a dermatologist |
326 41 7 38 36 52 |
65.2 8.2 1.4 7.6 7.2 10.4 |
COVID-19 coronavirus disease
The prevalence of hair loss was significantly higher among those in the 31–40-year age group when compared to other age groups (p < 0.001). In addition, hair loss was significantly higher among HCWs, divorced, and widow females (Table 4). The prevalence of post-infection hair loss was significantly higher among females complaining of a hypercoagulable state while a significantly less prevalence of hair loss was found among females with hypertension and those complaining of a psychiatric condition (p < 0.01). Other factors were insignificantly associated with post-infection hair loss. More prevalent hair loss was reported among those who needed hospital and ICU admission as well as among those complaining of severe respiratory symptoms and shortness of breath (p = 0.001, p = 0.002, respectively). Of notice hair loss was significantly higher among those who used antibiotics for COVID treatment (p = 0.027) (Tables 5, 6 and 7) .
Table 4.
Comparison of sociodemographic characteristics among studied cases
| Sociodemographic characteristics |
No hair problems n = 193 (%) |
Hair problems n = 307 (%) |
Test of significance |
|---|---|---|---|
|
Age/years 19–30 31–40 41–55 |
67 (34.7) 52 (26.9) 74 (38.3) |
71 (23.1) 170 (55.4) 66 (21.5) |
χ2 = 39.35 p < 0.001* |
|
Occupation Not working Retired Health care worker Governmental employee Special business |
56 (29.0) 9 (4.7) 46 (23.8) 63 (32.6) 9 (4.7) 10 (5.2) |
36 (11.7) 0 186 (60.6) 69 (22.5) 10 (3.3) 6 (2.0) |
χ2MC = 77.18 p < 0.001* |
|
Marital status Single Married Divorced Widow |
54 (28.0) 139 (72.0) 0 0 |
60 (19.5) 221 (72.0) 10 (3.3) 16 (5.2) |
χ2MC = 77.18 p < 0.001* |
|
Educational level Middle or lower Secondary Higher education |
13 (6.7) 32 (16.6) 148 (76.7) |
13 (4.2) 0 294 (95.8) |
χ2 = 57.21 p < 0.001* |
|
Income Low Middle High |
6 (3.1) 187 (96.9) 0 |
0 261 (85.0) 46 (15.0) |
χ2MC = 40.33 p < 0.001* |
|
Special habits Smoking |
16 (8.3) | 0 |
χ2 = 26.29 p < 0.001* |
χ2: Chi-square test
*Statistically significant
Table 5.
Comparison of associated comorbidities among studied cases
| Associated comorbidities |
No hair problems n = 193 (%) |
Hair problems n = 307 (%) |
Test of significance |
|---|---|---|---|
|
Hypertension Diabetes mellitus Hypercoagulation Psychiatric conditions Hypothyroidism |
37 (19.2) 9 (4.7) 0 9 (4.7) 7 (3.6) |
18 (5.9) 6 (2.0) 20 (6.5) 0 10 (3.3) |
χ2 = 21.44, p < 0.001* χ2 = 2.98, p = 0.084 χ2 = 13.09, p < 0.001* χ2 = 14.58, p < 0.001* χ2 = 0.049, p = 0.806 |
χ2: Chi-square test
*Statistically significant
Table 6.
Comparison of COVID-19 symptoms among studied cases
| Symptoms during COVID-19 infection |
No hair problems n = 193 (%) |
Hair problems n = 307 (%) |
Test of significance |
|---|---|---|---|
|
Fever Bone and muscle aches Cough Shortness of breath Eye inflammation Stomach aches Diarrhea Vomiting Severe respiratory symptoms Loss of smell and taste |
148 (76.7) 175 (90.7) 124 (64.2) 117 (60.6) 26 (13.5) 61 (31.6) 78 (40.4) 44 (22.8) 25 (8.1) 123 (63.7) |
223 (72.6) 252 (82.1) 186 (60.6) 142 (46.3) 45 (14.7) 81 (26.4) 77 (25.1) 20 (6.5) 48 (24.9) 234 (76.2) |
χ2 = 1.013, p = 0.314 χ2 = 7.01, p = 0.008* χ2 = 0.675, p = 0.411 χ2 = 9.79, p = 0.002* χ2 = 0.137, p = 0.711 χ2 = 1.58, p = 0.207 χ2 = 13.03, p < 0.001* χ2 = 28.15, p < 0.001* χ2 = 26.59, p < 0.001* χ2 = 9.05, p = 0.003* |
| Symptoms needed hospitalization | 18 (9.3) | 60 (19.5) | χ2 = 10.71, p = 0.001* |
| Symptoms needed ICU admission | 0 (0.0) | 10 (3.3) | χ2 = 6.42, p = 0.001* |
χ2: Chi-square test, ICU intensive care unit
*Statistically significant
Table 7.
Comparison of laboratory findings and medications used during COVID-19 among studied cases
| Laboratory findings during COVID-19 infection |
No hair problems n = 193 (%) |
Hair problems n = 307 (%) |
Test of significance |
|---|---|---|---|
|
CBC CRP ESR LDH D-dimer Serum ferritin |
84 (43.5) 35 (18.1) 25 (13.0) 0 27 (14.0) 17 (8.8) |
174 (56.7) 90 (29.3) 15 (4.9) 10 (3.3) 56 (18.2) 18 (5.9) |
χ2 = 8.21, p = 0.004* χ2 = 7.90, p = 0.005* χ2 = 10.48, p = 0.001* χ2 = 6.42, p = 0.01* χ2 = 1.55, p = 0.214 χ2 = 1.58, p = 0.209 |
| Medications during COVID-19 infection |
No hair problems n = 193 (%) |
Hair problems n = 307 (%) |
Test of significance |
|
Antibiotics Antipyretics Vitamins Expectorant Antitussive Corticosteroids Anticoagulant |
158 (81.9) 159 (82.4) 161 (83.4) 60 (31.1) 34 (17.6) 66 (34.2) 65 (33.7) |
225 (73.3) 270 (87.9) 284 (92.5) 156 (50.8) 143 (46.6) 97 (31.6) 122 (39.7) |
χ2 = 4.86, p = 0.027* χ2 = 3.01, p = 0.083 χ2 = 9.99, p = 0.002* χ2 = 18.79, p < 0.001* χ2 = 43.47, p < 0.001* χ2 = 0.365, p = 0.546 χ2 = 1.86, p = 0.173 |
χ2: Chi-square test, CBC complete blood picture, CRP C reactive protein, ESR erythrocyte sedimentation rate, LDH lactate dehydrogenases
*Statistically significant
Discussion
The current study was conducted to assess the prevalence of hair loss after COVID-19 infection and the factors associated with post-COVID-19 hair loss among 500 female study patients. In the present study, only confirmed COVID-19 cases were analyzed. Hair problems were reported in more than half of the participants (307; 61.4%) whom 68.1% of them reported worsening of their hair loss following contracting COVID; 29.6% of them reported noticing hair problem post-COVID only while 2.3% developed transient hair loss only during the infection.
Results in the current study were in accordance with a major Saudi study that assessed the prevalence of hair loss following COVID-19 infection as well as the factors associated with post-COVID-19 which revealed that nearly half of their participants (48.5%) noticed an increase in hair loss of more than 120 hairs per day after COVID-19 infection, and half of the participants (52.6%) reported hair accumulation on a pillow [6]. Another study recruiting a total of 806 participants reported that 52.7% experienced hair loss after COVID-19 infection. Age, gender, high temperature during, and the presence of hair loss prior to infection were significantly associated with the incidence of telogen effluvium (TE) [7]. According to a study conducted in Brazil via remote SMS messages and electronic form fill ups by patients of COVID-19 saved on the national registry, hair loss was the most frequently reported post-COVID-19 manifestation in 2800 subjects that comprised 48% of the studied cohort [8].
A meta-analysis performed on 15 published studies included 47,910 patients (age 17–87 years). The included studies defined long-COVID as ranging from 14 to 110 days post-viral infection. It was estimated that 80% of the infected patients with SARS-CoV-2 developed one or more long-term symptoms. The five most common symptoms were fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnea (24%) [9].
The main reported hair problems in the current study were telogen effluvium (TE) (60.8%), increased gray hair (13%), seborrheic dermatitis (SE) (5.6%), trichotillomania (3.6%), and alopecia areata (AA) (2.2%). There is a wide discrepancy between studies regarding the prevalence of different related hair problems in post-COVID-19. An online-based self-administered cross-sectional study on 404 medical students in Bangladesh found prevalence of TE, AA, and SD to be 61, 25, and 58%, respectively [10]. In Turkey, one study reported the prevalence of TE, AA, and SD to be 28, 2.8, and 20%, respectively [11], while another conducted on 2171 post-COVID-19 patients found that TE (85%) was the most common type of hair loss followed by worsening of androgenetic alopecia (AGA) (7%) [12]. A total of 321 (29.13%) patients developed graying/whitening of hair after getting infected with COVID-19.
TE is a type of reactive non-scarring hair loss that causes hair shedding and is more common in women. TE is a harmless disorder that has been linked to hormonal changes, extreme dieting, infections, and the use of anticoagulant medications, prescribed for treating COVID-19 patients [13].
In the current study, there was higher significance of hair loss among users of vitamins, expectorants, and antitussive medications (p < 0.05) while a non-significant prevalence of hair loss was encountered among users of anticoagulants (p = 0.173). Of notice hair loss was significantly higher among those who used antibiotics for COVID treatment (p = 0.027). Some medications used to treat COVID infection were reported to trigger telogen effluvium (enoxaparin, hydroxychloroquine, azithromycin, etc.). On the other hand, there is currently conflicting evidence regarding their mechanisms. Regarding the prophylactic anticoagulant therapy used in patients affected by SARS-CoV-2, most studies reported important associations of hair loss in these patients, possibly due to collagen degeneration of the follicular sheath [14].
The COVID-19 pandemic has increased the levels of anxiety and stress-related disorders among individuals which contributed to hair shedding and TE. Rates of acute hair shedding during the pandemic increased for which patients requested treatments to break such cycle. Interestingly, we found that only 25.2% of patients sought medical advice through phone calls and visit to general physician or dermatologists, similar to the rate of 18.9% reported by Turkmen et al. [11] and 22.9% reported by Alsalhi et al. [15].
In the current report, more prevalent hair loss was reported among those who needed hospital and ICU admission as well as among those complaining of severe respiratory symptoms and shortness of breath (p = 0.001, p = 0.002, respectively). Regarding the severity of the infectious episode, different studies revealed a positive correlation of hair loss and severity of infection requiring hospital or ICU admission and related this to increase in proinflammatory cytokines [14].
In specialized studies, smoking was frequently associated with the appearance of TE, due to influencing follicular apoptosis due to altering the sensitivity of acetyl choline receptor [10]. In our case smoking did not appear to affect hair loss. The relationship between smoking and COVID-19 seems to be very controversial while smokers are susceptible to COVID-19; some reports suggested that it may also have a protective effect on the respiratory tract [16, 17].
A number of mechanisms had been proposed for post-COVID hair loss involving direct endothelial cell damage; in addition, aggravation of existing hair loss was related to SARS-CoV-2 virus acting on the transmembrane serine protease 2 genes (TMPRSS2)—which has a role in the regulation of androgen pathways [4]. Another cause of hair loss related the severity of the viral inflammation and cytokine storm to hair matrix cell destruction [5]. Moreover, perifollicular inflammation manifested by the accumulation of activated macrophages and mast cell degranulation in the context of psychological stress as well as the ischemia and vascular decompensation caused by the hypercoagulability state and microthrombus formation were described as major culprits in inducing hair loss [18]. Moreover, a recent study reported an increased incidence of hair shedding following COVID-19 vaccinations [19].
The following study is limited by its cross-sectional nature and by its gender bias on a female population in a localized community. Objective measures such as inflammatory biomarkers and oxygen saturation which are detrimental for the severity of infection have not been measured. Moreover, a lack of scalp biopsy remains to be a limitation.
Conclusion
In conclusion, we reported prevalence of post-COVID hair fall that was confirmed by trichoscopy and which affected approximately 61.4% of infected females. Other factors, such as stress and infection, cannot be excluded and remain to be further investigated by larger multicenter studies.
Acknowledgements
Authors are grateful to all participants in the study.
Abbreviations
- COVID-19
Coronavirus disease (COVID-19)
- TE
Telogen effluvium
- AA
Alopecia areata
- NK cells
Natural killer cells
- IFN
Interferon
- HCW
Health care workers
- TMPRSS2
Transmembrane protease, serine 2
- CK
Creatine kinase
- CRP
C-reactive protein
- DM
Diabetes mellitus
Author contribution
N. A., Z. O., M. Z., and M. E. designed and performed the research. N. A., Z. O., M. Z., and M. E. performed the work. N. A., Z. O., M. Z., and M. E. analyzed and wrote the paper. All authors contributed equally in production of this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by ethics committee on human research by Al Azhar Faculty of Medicine IRB (00012367-21-06-005). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consents were received from participants upon explanation of the study.
Consent for publication
Not applicable. The manuscript does not contain any individual personal data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33(4):e13666. doi: 10.1111/dth.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seyfi S, Alijanpour R, Aryanian Z, Ezoji K, Mahmoudi M. Prevalence of telogen effluvium hair loss in COVID-19 patients and its relationship with disease severity. J Med Life. 2022;15:631–634. doi: 10.25122/jml-2021-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iancu GM, Molnar E, Ungureanu L, Șenilă SC, Hașegan A, Rotaru M. SARS-CoV-2 infection—a trigger factor for telogen effluvium: review of the literature with a case-based guidance for clinical evaluation. Life (Basel) 2023;13(7):1576. doi: 10.3390/life13071576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdulwahab RA, Aldajani BM, Natto NK, Janabi AM, Alhijaili OI, Faqih NT, Alharbi A. Prevalence of hair loss after COVID-19 infection in Makkah Region, Saudi Arabia. Cureus. 2022;14(9):e29285. doi: 10.7759/cureus.29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkeraye S, Alrashidi A, Alotaibi NS, Almajli N, Alkhalifah B, Bajunaid N, Alharthi R, AlKaff T, Alharbi K. The association between hair loss and COVID-19: the impact of hair loss after COVID-19 infection on the quality of life among residents in Saudi Arabia. Cureus. 2022;14(10):e30266. doi: 10.7759/cureus.30266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller-Ramos P, Ianhez M, Silva de Castro CC et al (2022) Post-COVID-19 hair loss: prevalence and associated factors among 5,891 patients. Int J Dermatol 61(5):e162–e164 [DOI] [PubMed]
- 9.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahadi AR, Rafi MA, Shahriar T, Seemanta S, Rabbani MG, Akter M, Majumder MI, Hasan MT. Association between hair diseases and COVID-19 pandemic-related stress: a cross-sectional study analysis. Front Med (Lausanne) 2022;12(9):876561. doi: 10.3389/fmed.2022.876561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turkmen D, Altunisik N, Sener S, Colak C. Evaluation of the effects of COVID-19 pandemic on hair diseases through a web-based questionnaire. Dermatol Ther. 2020;33(6):e13923. doi: 10.1111/dth.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutlu Ö, Demircan YT, Yıldız K, Kalkan G, Demirseren DD, et al. The effect of COVID-19 on development of hair and nail disorders: a Turkish multicenter, controlled study. Int J Dermatol. 2023;62(2):202–211. doi: 10.1111/ijd.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abushukur Y, Mansour M, Rehman R, Rida A, Daveluy S. A systematic review of hair loss as a consequence of COVID-19 infection. Int J Dermatol. 2023;62(8):e436–e439. doi: 10.1111/ijd.16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? A review of the literature. Drugs Real World Outcomes. 2016;3:1–6. doi: 10.1007/s40801-015-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlSahli W, Almulhim Y, Issa NT. Cross-sectional study to determine the prevalence of telogen effluvium among patients experiencing COVID-19 infection or vaccination in Saudi Arabia and Arabic countries. European Journal of Molecular & Clinical Medicine. 2022;9(7):4663–4674. [Google Scholar]
- 16.Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur J Intern Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lippi G, Sanchis-Gomar F, Henry BM. Active smoking and COVID-19: a double-edged sword. Eur J Intern Med. 2020;77:123–124. doi: 10.1016/j.ejim.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tešanović Perković D, Vukojević M, Bukvić MZ. Post-COVID telogen effluvium. Acta Dermatovenerol Croat. 2022;30(4):220–226. [PubMed] [Google Scholar]
- 19.Ammar AM, Ibrahim IS, Mohamed AN, Elsaie ML. Dermoscopy-assisted prevalence of hair loss after COVID-19 vaccination among an Egyptian population: a cross-sectional study. Ir J Med Sci. 2023 doi: 10.1007/s11845-023-03493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


