Abstract
Background:
Identifying a suitable volunteer unrelated donor (UD) in South Africa is challenging due to the highly diverse ethnic groups and mixed-race populations in this region. Haplo-identical Haematopoietic Cell Transplantation (HaploHCT) is thus an attractive procedure for patients with high risk haematological malignancies.
Objectives:
To assess the safety and feasibility of haploHCT in South Africa.
Study Design:
We retrospectively analyzed the outcome of 134 patients with haematological malignancies who received unmanipulated haploHCT with Post-Transplant Cyclophosphamide at two high volume HCT centers between 2014 and 2019. We assessed overall survival (OS), disease free survival (DFS), non-relapse mortality (NRM), relapse incidence (RI), and incidence of acute GVHD.
Results:
Median recipient and donor age was 44 years (range, 15–73) and 36 years (range, 9–68) respectively. Acute myeloid leukaemia or myelodysplastic syndrome (AML/MDS) and acute lymphoblastic leukaemia (ALL) were the most common indications for haploHCT (61.2%). The EBMT risk score was ≥ 5 in 44 patients (32.8%). Seventy-seven patients (57.4%) received myeloablative conditioning regimens. The majority of patients (57.4%) received gender matched transplants and peripheral blood stem cells (PBSC) (70.9%) as cell source. Sixteen patients (11.9%) had an incongruent CMV serostatus at transplant. Median follow up was 10.8 months (range 0.36–70.8).
Overall Survival (OS) at 1 and 3 years was 56% (95% CI 47–64) and 37% (95% CI 28–47), respectively. Disease Free Survival (DFS) at 1 and 3-years was 47% (95% CI 38–55) and 32% (95% CI 24–41), respectively. The 100-day and 3-year cumulative incidence of NRM was 18% (95% CI 11–25) and 41% (95% CI 32–50) respectively, whereas the 1 and 3-year Cumulative Relapse incidence (RI) was 16% (95% CI 11–24%) and 21% (95% CI 14–29) respectively. The 1-year OS for AML/MDS was 55% (95% CI 40–67) vs. 41% (95% CI 21–60) for ALL. Forty-five patients (41.7%) developed aGVHD by day 100; Of these, 80% had grades I & II disease.
In multivariable analysis, older donor age was an independent risk factor for lower DFS. RI was higher for diagnoses other than acute leukaemia/MDS (RR=2.62; 95% CI 1.12–6.15; p=0.027), decreased for PBSC vs BM (RR=0.43; 95% CI 0.19–0.95; p=0.038) and decreased for offspring donors (RR=0.25; 95% CI 0.09–0.67; p=0.006).
Conclusion:
These data support the feasibility of haploHCT and suggest that unmanipulated haploHCT utilizing a younger parent or offspring donor are viable options for adults with acute leukaemia and MDS who lack a suitable RD or UD in sub-Saharan Africa.
Keywords: Haploidentical stem cell transplant, Post-transplant cyclophosphamide, Sub-Saharan Africa, South-Africa, Haematological malignancies
INTRODUCTION
Allogeneic haematopoietic stem cell transplantation (HCT) from an HLA matched related donor (RD) or matched unrelated donor (UD) is a curative option for many high-risk haematological malignancies1. Finding a fully HLA matched family donor occurs in about one-third of patients. Other donor sources available are matched unrelated donors, mismatched unrelated donors, haploidentical donors and single or double umbilical cord blood units. Haploidential HCT (HaploHCT) has historically been associated with high rates of graft-versus-host-disease (GVHD) and graft failure leading to high non-relapse mortality2,3. However, using the post-transplant cyclophosphamide (PTCY) approach4, haploHCT has become more effective, limiting GVHD and treatment-related mortality and leading to its increasing adoption5. In addition, a recent meta-analysis confirms its equivalent all-cause mortality and relapse incidence for acute leukaemia in comparison to unrelated donor transplants6. HaploHCT is therefore an attractive approach in patients that require an urgent allograft.
The South African Bone Marrow Registry (SABMR) for recruitment of local volunteer unrelated stem cell (SC) donors, had 73 027 locally registered donors in 2017, of whom the majority were of Caucasian origin. However, the majority of the South African population consists of other ethnic groups and mixed-race backgrounds7. Additionally, the potential donor pool is negatively impacted by the high seroprevalence of human immunodeficiency virus (13.1%) in South Africa8. The HLA alleles in people of African descent are genetically highly diverse9. Therefore, finding a matched related, local unrelated and even international unrelated donor for the South African patient of African or mixed-race descent, is exceptionally challenging. If an international matched UD can be identified, it is often prohibitively expensive, requiring a high tier private medical insurance to fund the transplant, a benefit that is affordable to only 17% of South-Africans10. Identification and recruitment of a suitable unrelated donor can also lead to a delay in receiving a time sensitive transplant. HaploHCT is therefore a potentially meaningful therapeutic intervention for patients in South Africa with high risk haematological malignancies.
HaploHCT was implemented in 2014 at the two largest public (Groote Schuur Hospital) and private (Pretoria East Netcare Hospital) transplant centers in South Africa. We describe our first five years of experience, focusing on the efficacy and outcome of haploHCT. Our objective was to review all patients with high risk haematological malignancies that underwent T-cell replete haploHCT with PTCY. In order to determine the feasibility of this procedure we assessed the overall survival (OS), disease free survival (DFS), non-relapse mortality (NRM), relapse incidence (RI), incidence of acute GVHD and the risk factors that influenced these outcomes.
MATERIALS AND METHODS
Patients
Consecutive patients undergoing haploHCT at Pretoria East Netcare Hospital, and the public-academic center at Groote Schuur Hospital/University of Cape Town from January 2014 to December 2019 were included in this retrospective cohort study. Patients were ≥ 15 years of age, with high-risk haematological malignancies undergoing first haploHCT (previous autologous and allogeneic transplants permitted), family donor with ≥ 2 HLA mismatches and no matched unrelated donor. Peripheral blood (PB) or bone marrow (BM) or both were used as SC source and GVHD prophylaxis was performed with PTCY28 without ex-vivo T cell depletion. The University of Cape Town research ethics committee approved this study (HREC REF: 592/2018) and patient data were captured in a REDCap® database.
Statistical analysis and definitions
The primary aim was to determine patient characteristics, DFS and OS of the cohort. Secondary aims were to determine relapse incidence (RI), non-relapse mortality (NRM) and the incidence of acute GVHD by day 100 after transplant. Pre-transplantation disease status was evaluated before HCT which included a bone marrow aspiration and biopsy with flow cytometry and cytogenetics for patients with leukaemia, or computed tomography and/or a positron emission tomography scan for patients with lymphoma. Morphological complete remission (CR) of leukaemia was defined as <5% blasts on BM biopsy and absence of active extramedullary disease. Patients not in morphologic CR were considered to have active disease. Untreated myelodysplastic syndrome (MDS) patients without response to therapy prior to transplant were considered to have active disease regardless of blast count. DFS was defined as time to death or relapse/progression, whichever came first. OS was defined as time to death from all causes. NRM was defined as death without evidence of relapse or progression. Neutrophil engraftment was defined as the first day of 3 consecutive days when absolute neutrophil count was >= 0.5 × 109/L while platelet engraftment was defined as the first day of 3 consecutive days when platelet count was > 20 × 109/L without a platelet transfusion in the past 7 days. Acute GVHD was graded according to the modified Seattle Glucksberg criteria29. DFS and OS were estimated by the Kaplan-Meier method30. Cumulative incidence (CI) functions were used to estimate engraftment, RI and NRM31. The competing risks were specified as follows: death for engraftment, death for RI and relapse for NRM. The incidence of acute GVHD was estimated for those who had not died. Univariate analyses were performed for OS and DFS using the Cox Proportional Hazards model, while risk factors for RI, NRM and engraftment were analysed using competing-risks Cox Proportional Hazards regression analysis with Gray’s test32,33,34. The following variables were assessed in the univariate analysis: disease type, disease status at transplant (CR1/2 vs > CR2), SC source (BM vs PB vs PB+BM), intensity of conditioning regimen (myeloablative [MAC] vs reduced intensity [RIC]), recipient age at transplant (categorised into quartiles), donor age (categorised into quartiles), recipient/donor gender mismatch, recipient/donor cytomegalovirus (CMV) status, donor family relationship, number of previous autologous, matched related or matched unrelated transplantations, and transplant centre. Categories with less than 15 patients overall were not included in analyses. Study variables identified in the univariate analysis that were significant at p<0.20 were combined into the multivariable model, after examining each pair of variables for possible confounding using the chi-squared test (or Fisher’s exact test for 2×2 tables). A value of Cramer’s V (or the phi coefficient for Fisher’s exact test) > 0.50 was regarded as too strong an association to include both variables in the multivariable model. Non-significant variables were sequentially removed from the multivariable model. The association between cell source and acute GVHD was determined by Fisher’s exact test. Statistical analysis was carried out using SAS version 9.4 for Windows. A 5% significance level was used.
RESULTS
Patient characteristics
Patient, donor and transplant characteristics are summarised in Table 1. The median recipient age was 44 years (range, 15–73) and the median donor age was 36 years (range, 9–68). AML, MDS and ALL were the most common indications for transplant, comprising 61.2% of the cohort. The EBMT risk score35 was used to assign disease risk which included 27 patients (20.1%) with a score of less than 2, 63 patients (47%) with a score between 2–4, and 44 patients (32.8%) with a score >= 5. Therefore, 79.8% of patients had high risk disease. Seventy seven patients (57.4%) received myeloablative conditioning regimens. The majority of patients (57.4%) received a gender matched donor transplant and peripheral blood stem cells (70.9%) as cell source. Sixteen patients (11.9%) had an incongruent CMV serostatus with their donor at transplant. Median follow up of surviving patients was 10.9 months (range 0.36–70.8).
Table 1.
Patient, donor and transplant characteristics (N=134)
| Characteristic | Value |
|---|---|
| Age at transplant, median (range), years | 44 (15–73) |
| Patient gender, male, n (%) | 83 (62) |
| Disease, n (%) | |
| AML and MDS | 60 (44.8) |
| - CR1/CR2 | 42 |
| - > CR2 | 18 |
| B and T-ALL | 22 (16.4) |
| - CR1/CR2 | 18 |
| - > CR2 | 4 |
| “Other” diagnoses included | |
| HL | 9 (6.7) |
| - CR1/CR2 | 3 |
| - > CR2 | 6 |
| MM | 8 (6.0) |
| - CR | 3 |
| - PR/VGPR | 5 |
| CML | 6 (4.5) |
| - 2nd CP | 2 |
| - AP | 3 |
| - BP | 1 |
| NHLa | 17 (12.7) |
| - CR1/CR2 | 10 |
| - > CR2 | 7 |
| CLL | 4 (3.0) |
| - CR1/CR2 | 1 |
| - > CR2 | 3 |
| Therapy related myeloid neoplasm | 3 (2.2) |
| - PR | 2 |
| - Active disease | 1 |
| CMML | 3 (2.3) |
| - Active disease | 3 |
| MPN (myelofibrosis) | 1 (0.8) |
| - Active disease | 1 |
| Blastic plasmacytoid dendritic cell tumor | 1 (0.8) |
| - CR1 | 1 |
| EBMT risk score | |
| 1–2 | 27 (20.1) |
| 2–4 | 63 (47.0) |
| >= 5 | 44 (32.8) |
| Conditioning | |
| - MAC-busulphan based | 46 (34.3) |
| - MAC-TBI based | 31 (23.1) |
| - RIC-TBI based | 57 (42.6) |
| Donor age, median (range), y | 36 (9–68) |
| Donor gender, male, n (%) | 78 (58) |
| Donor to recipient gender mismatch | |
| - Female to male | 31 (23.1) |
| - Male to female | 26 (19.4) |
| - Matched | 77 (57.5) |
| Stem cell source | |
| - PBSC | 95 (70.9) |
| - BM | 36 (26.9) |
| - PBSC and BM | 3 (2.2) |
| Recipient/donor CMV serostatus | |
| - Neg/neg | 7 (5.2) |
| - Neg/pos | 9 (6.7) |
| - Pos/neg | 7 (5.2) |
| - Pos/pos | 77 (57.5) |
| - Missing | 34 (25.4) |
| Donor relationship | |
| - Son | 46 (34.3) |
| - Mother | 25 (18.7) |
| - Brother | 24 (17.9) |
| - Sister | 17 (12.7) |
| - Daughter | 14 (10.5) |
| - Father | 5 (3.7) |
| - Other family member male/female | 3 (2.3) |
| Number of previous transplants | |
| - 0 | 110 (82.1) |
| - 1 | 19 (14.2) |
| - 2 | 5 (3.7) |
| Centre | |
| - Groote Schuur hospital | 24 (17.9) |
| - Pretoria East Netcare | 110 (82.1) |
| Acute GVHD | |
| - Yes | 45 (41.7) |
| - No | 63 (58.3) |
| Acute GVHD grade | |
| - I | 17 (37.8) |
| - II | 19 (42.2) |
| - III | 6 (13.3) |
| - IV | 3 (6.7) |
Abbreviations: AML, acute myeloid leukaemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukaemia; HL, Hodgkin lymphoma; MM, multiple myeloma; CML, chronic myeloid leukaemia; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukaemia; CMML, chronic myelomonocytic leukaemia; MPN, myeloproliferative neoplasm; PBSC, peripheral blood stem cells; BM, bone marrow; CMV, cytomegalovirus; CR, complete remission; PR, partial remission; VGPR, very good partial response; CP, chronic phase; AP, accelerated phase; BP, blastic phase; MAC, myeloablative chemotherapy; RIC, reduced intensity conditioning; TBI, total body irradiation
NHL includes high grade B cell, low grade B cell and T cell not otherwise specified.
Engraftment
Neutrophils and platelets recovered at a median of 20 (95% CI, 19–21 days) and 29 days (95% CI, 27–33 days), respectively. The cumulative incidence of neutrophil and platelet recovery at day 28 was 87% (95% CI, 80–92%) and 42% (95% CI, 32–51%) respectively and overall were 90% (95% CI, 83–94%) and 82% (95% CI, 74–88%). Thirteen patients had primary graft failure (9.7%), of whom only one survived. For both neutrophil and platelet engraftment, there was a significantly faster neutrophil (HR=1.74; 95% CI 1.18–2.59; p=0.006) and platelet recovery (HR=1.56; 95% CI 1.03–2.37; p=0.038) when PBSC were used as cell source in comparison with bone marrow (Figure 1A & 1B).
Figure 1A.
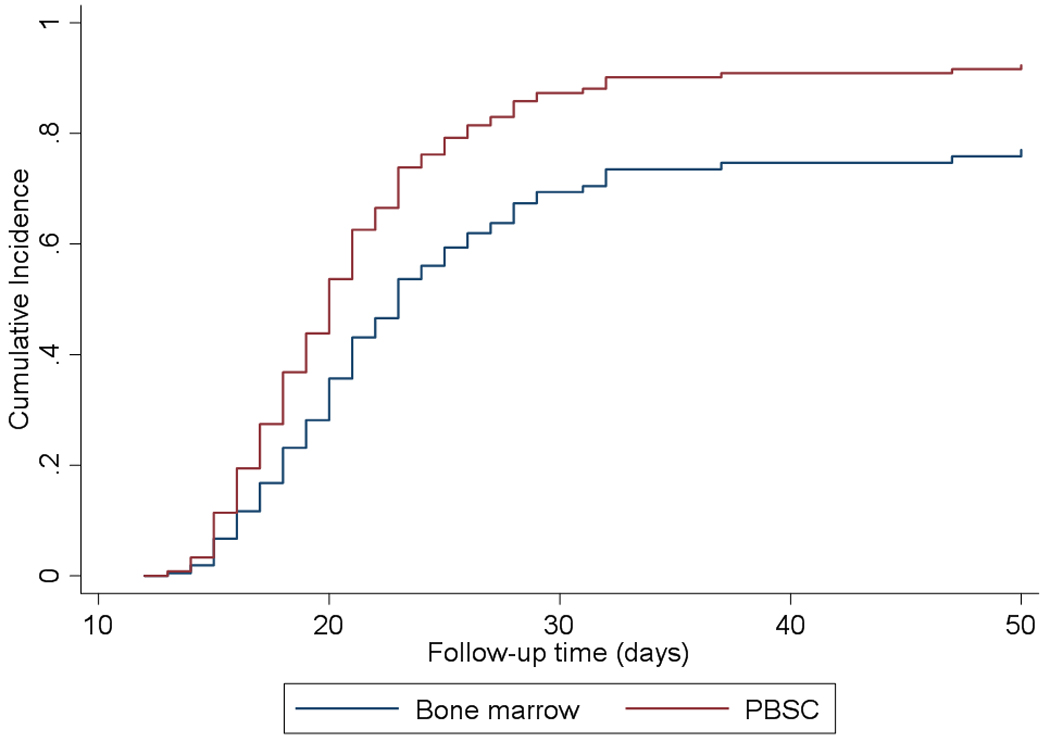
Neutrophil engraftment significantly faster for PBSC vs BM (p=0.006)
Figure 1B.
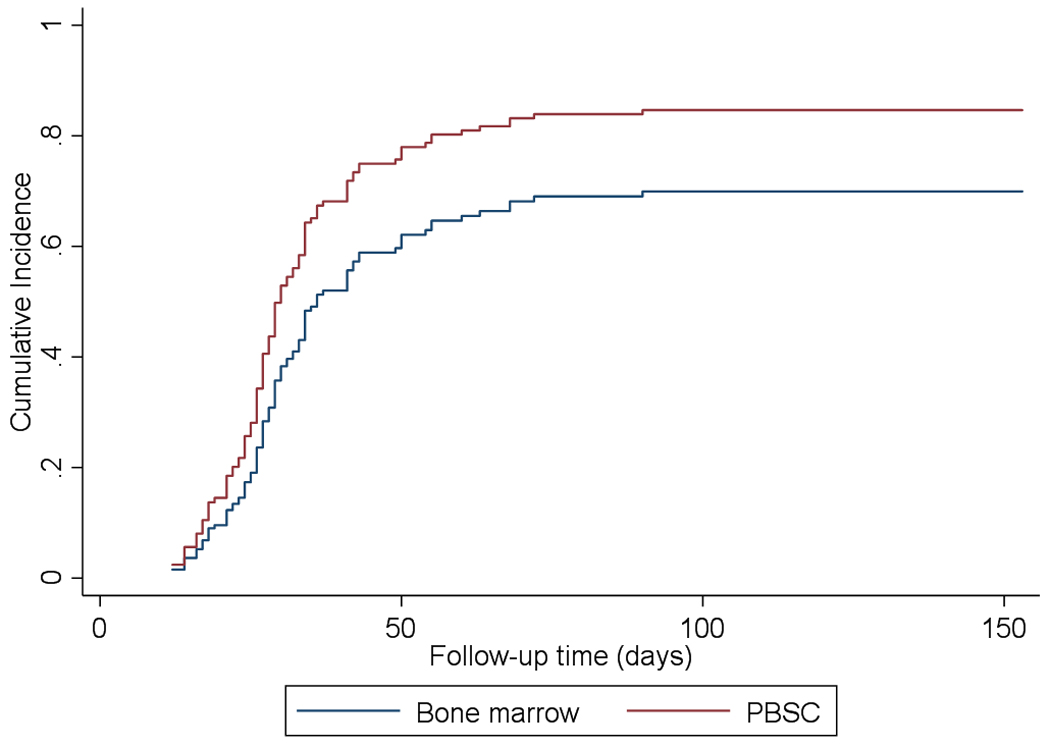
Platelet engraftment significantly faster for PBSC vs BM (p=0.038)
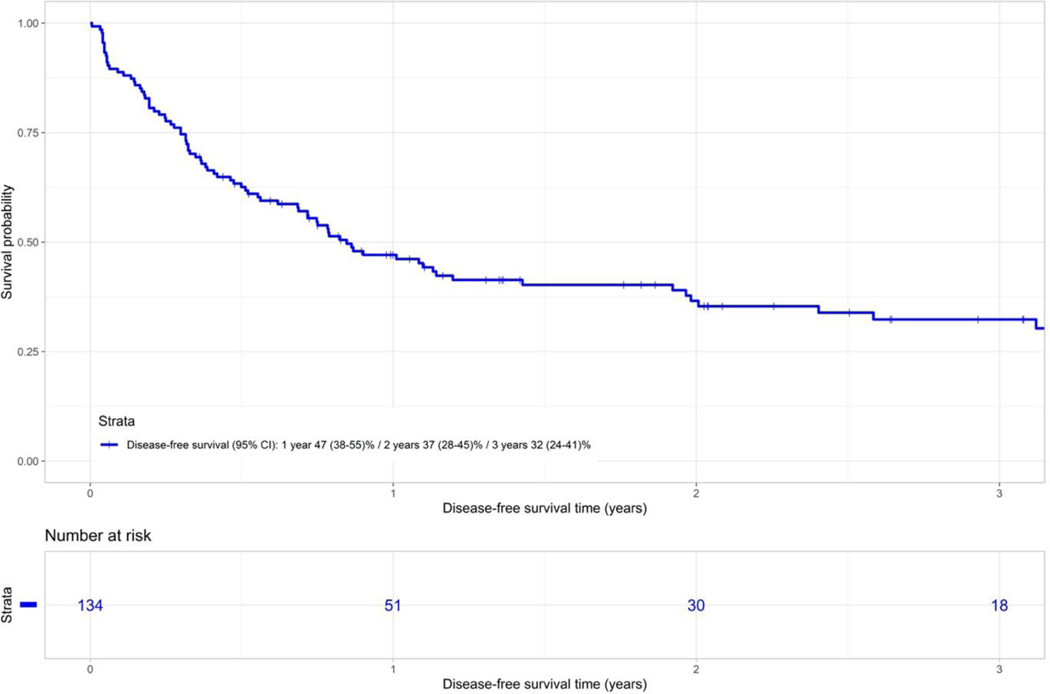
Disease free survival (DFS) and overall survival (OS)
The probability of DFS at 1 and 3-years was 47% (95% CI 38–55) and 32% (95% CI 24–41) respectively for the entire cohort (Figure 2A). In univariate analysis, lower DFS was associated with older donor age (46–68y vs. 9–25y) (HR 1.90; 95% CI 1.02–3.5; p=0.43) and remained an independent risk factor in multivariable analysis.
Figure 2A.
Disease free survival of whole cohort of patients.
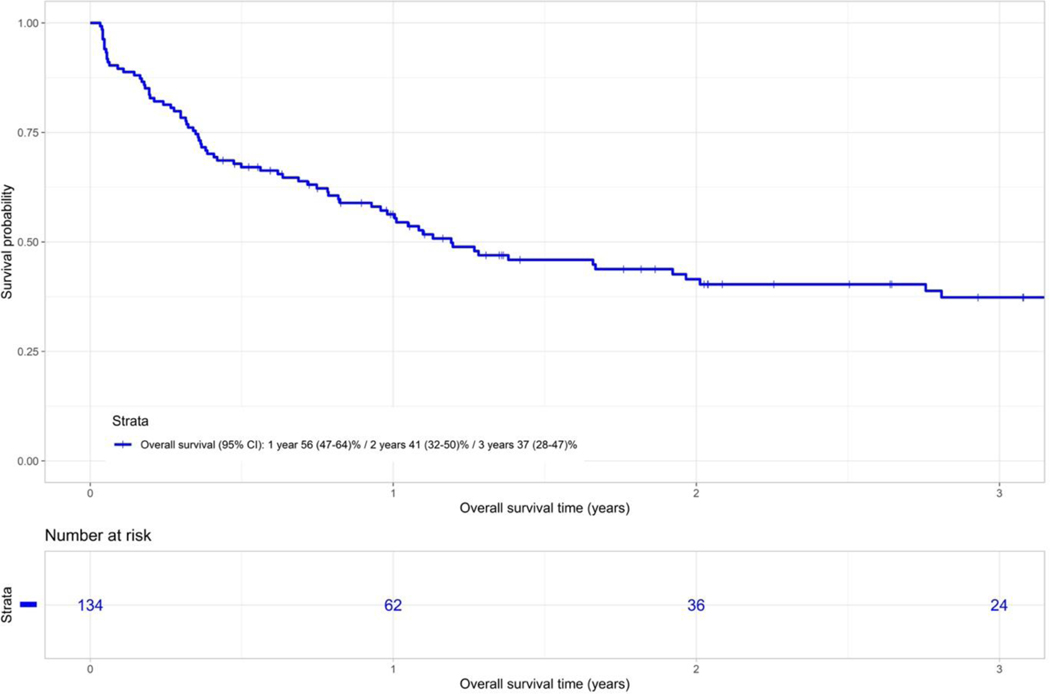
The probability of OS at 1 and 3 years was 56% (95% CI 47–64) and 37% (95% CI 28–47) respectively for the entire cohort (Figure 2B). In univariate analysis no significant risk factors could be identified for OS.
Figure 2B.
Overall survival of whole cohort of patients.
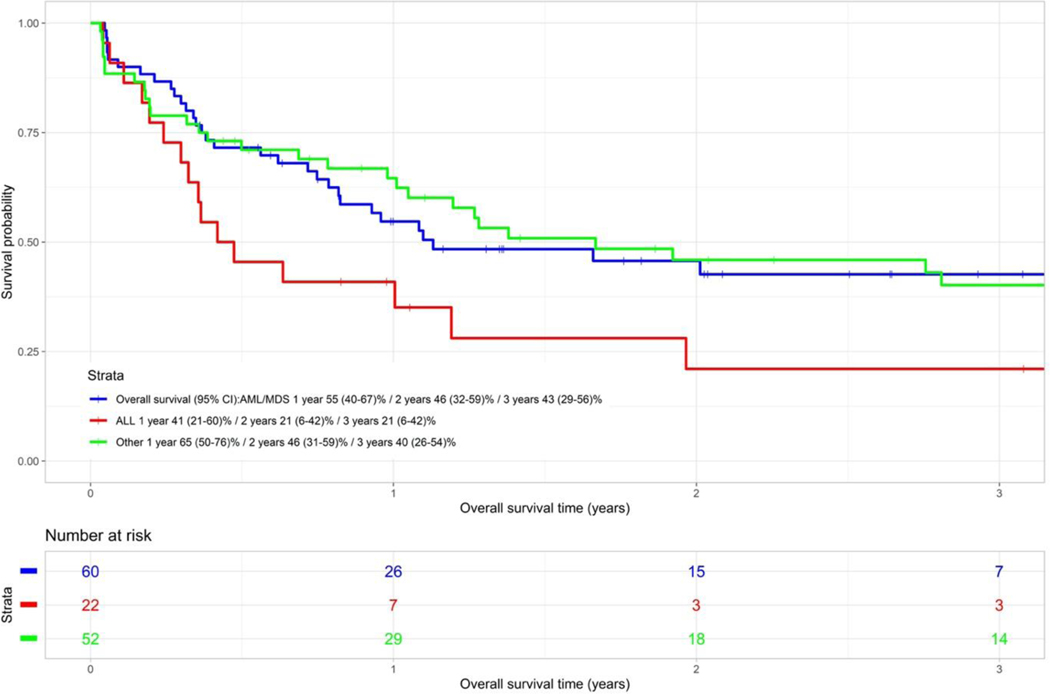
The 1 and 3-year OS of the ALL group was 41% (95% CI 21–60) and 21% (95% CI 6–42) respectively. In comparison, the 1 and 3-year OS of the AML/MDS group was 55% (95% CI 41–67) and 43% (95% CI 29–56) respectively, and the 1 and 3-year OS of the “other” diagnostic group (predominantly lymphoma) was 65% (95% CI 50–76) and 40% (95% CI 26–54) respectively (Figure 2C). There was a trend towards better OS for the AML/MDS group versus the ALL group (p=0.067).
Figure 2C.
Overall survival categorised by disease type (P=0.067)
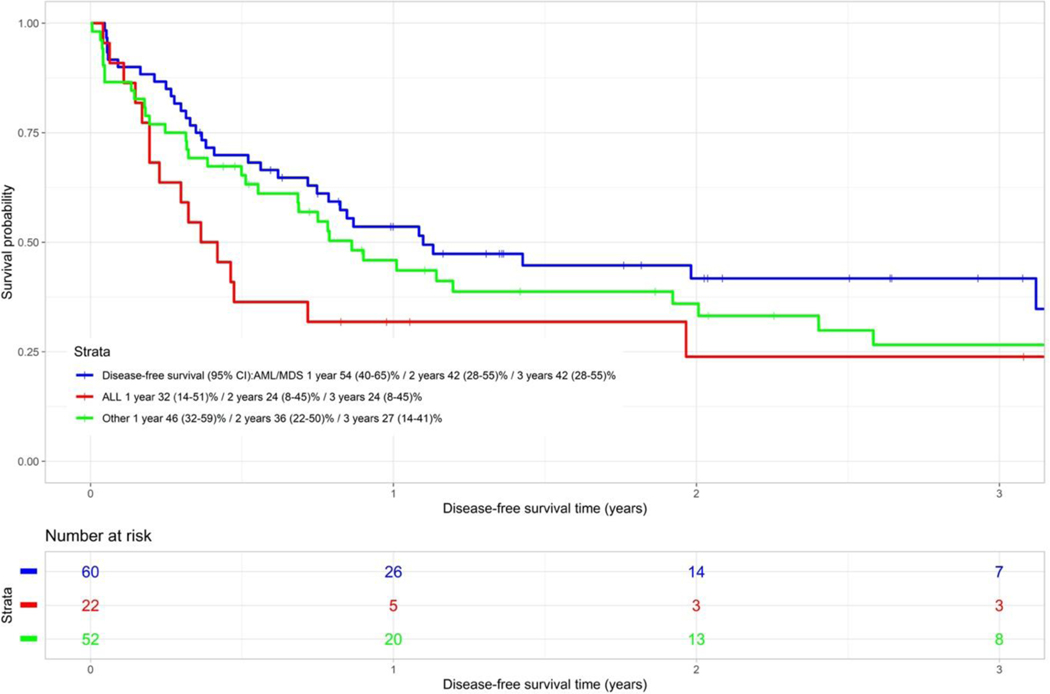
The 1 and 3-year DFS of the ALL group was 32% (95% CI 14–51) and 24% (95% CI 8–45) respectively. The 1 and 3-year DFS of the AML/MDS group was 54% (95% CI 40–65) and 42% (95% CI 28–55) respectively and the 1 and 3-year DFS of the other diagnostic group were 46% (95% CI 32–59) and 27% (95% CI 14–41) respectively (Figure 2D). The DFS of the AML/MDS group was marginally significantly better versus the ALL group (p=0.053).
Figure 2D.
Disease free survival categorised by disease type (P=0.053)
Non relapse mortality
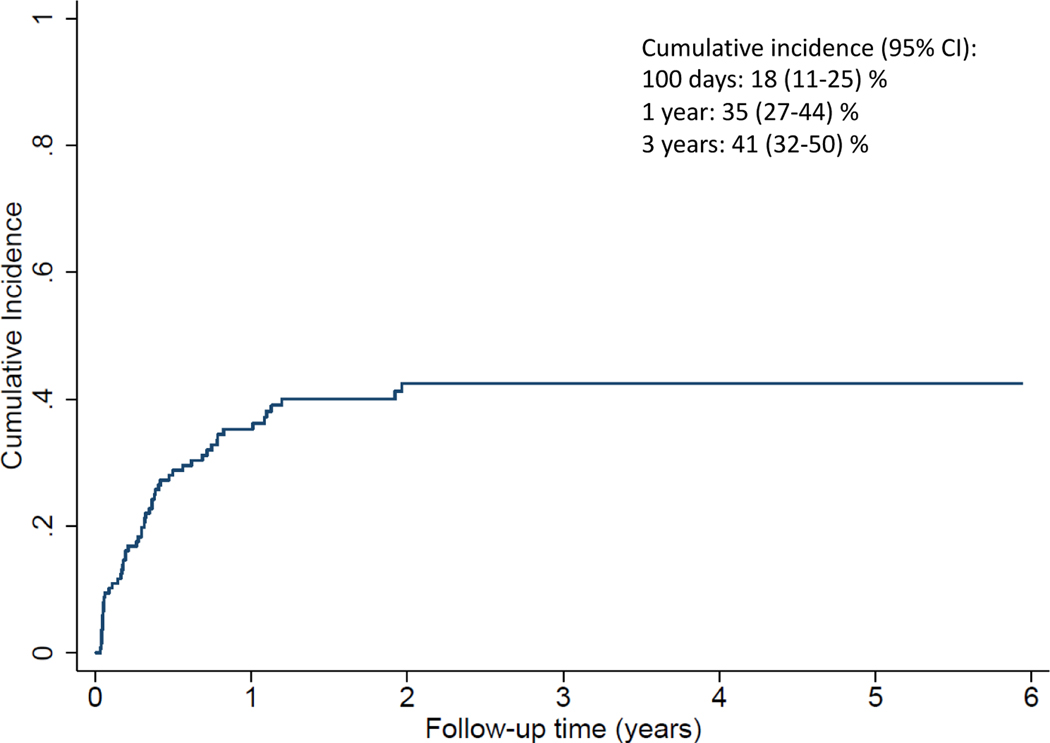
The cumulative incidence (CI) of NRM at day 100 and at 3 years was 18% (95% CI 11–25%) and 41% (95% CI 32–50%) respectively (Figure 3). Table 2 summarizes causes and contributory causes of death.
Figure 3.
Cumulative incidence of non-relapse mortality.
Table 2.
Causes and contributory causes of death.
| Characteristic | Value |
|---|---|
| Cause of death, n (%) | 76 (100) |
| - HCT related cause* | 53 (69.7) |
| - Relapse or progression | 20 (26.3) |
| - Unknown | 3 (3.9) |
| Contributary cause of death, n (%) | 76 |
| - Bacterial infection | 31 (40.8) |
| - Graft failure/poor graft function | 23 (30.3) |
| - Viral infection | 22 (28.9) |
| - Fungal infection | 20 (26.3) |
| - GVHD | 19 (25.0) |
| - Multiple organ failure | 13 (17.1) |
| - Pulmonary toxicity | 5 (6.6) |
| - Renal failure | 5 (6.6) |
| - Cardiac toxicity | 3 (3.9) |
Death due to a complication of transplant.
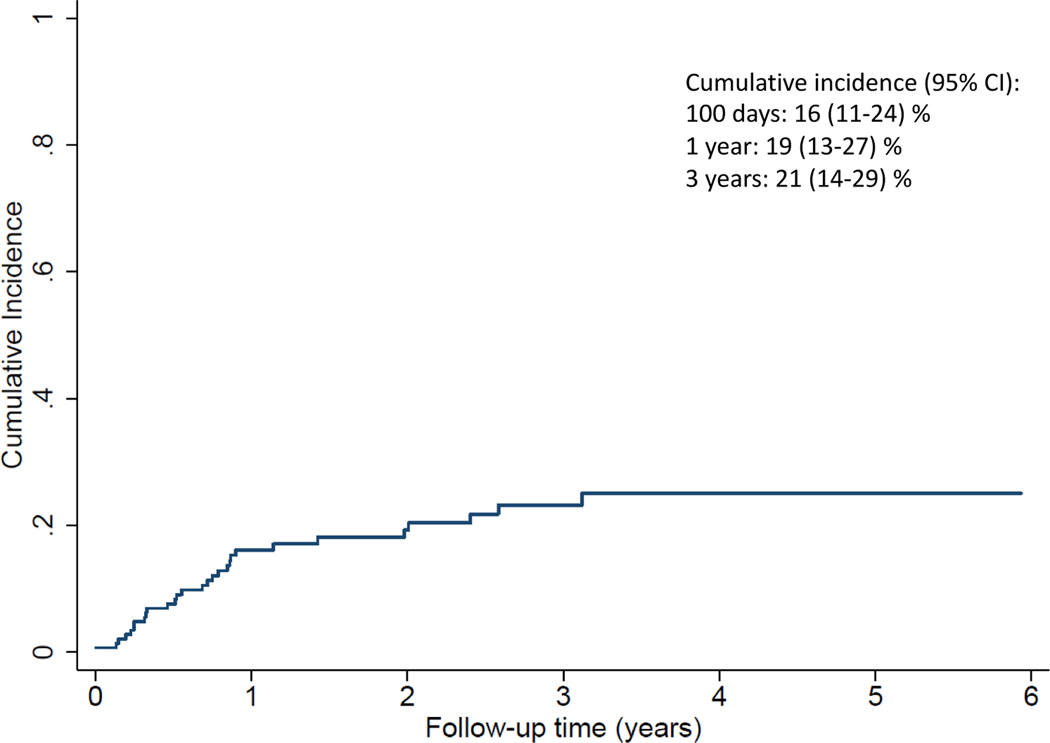
Relapse incidence (RI)
The CI of relapse at 1 and 3-years from transplant was 16% (95% CI 11–24%) and 21% (95% CI 14–29%) respectively (Figure 4). In total, 29 patients relapsed: 8 AML (5 in CR1/CR2 and 3 in CR3+), 5 ALL (all 5 in CR1/CR2) and 16 from other diagnostic groups (predominantly lymphoma) (5 in CR1/CR2 while the remaining 11 were in CR3+).
Figure 4.
Cumulative incidence of relapse.
In multivariable analysis, RI was higher for “other” diagnoses (predominantly lymphoma) vs. AML/MDS (HR=2.62; 95% CI 1.12–6.15), decreased for PBSC vs BM (RR=0.43; 95% CI 0.19–0.95; p=0.038) and decreased for offspring donors (RR=0.25; 95% CI 0.09–0.67; p=0.006). See Table 3.
Table 3.
Multivariable regression model: Disease free survival and relapse incidence.
| Characteristic | Disease free survival | Relapse incidence | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| Donor age | ||||||
| 46 – 68 years | 1.9 | 1.02–3.53 | 0.043 | - | - | - |
| 36 – 45 years | 1.4 | 0.7–2.8 | 0.34 | - | - | - |
| 26 – 35 years | 1.16 | 0.6–2.28 | 0.66 | - | - | - |
| 9 – 25 years | 1.0 | reference | - | - | - | - |
| Diagnostic group | ||||||
| Other* | - | - | - | 2.62 | 1.12–6.15 | 0.027 |
| ALL group | - | - | - | 1.51 | 0.38–5.96 | 0.56 |
| AML/MDS group | - | - | - | 1.0 | reference | - |
| Stem cell source | ||||||
| PBSC | - | - | - | 0.43 | 0.19–0.95 | 0.038 |
| BM | - | - | - | 1.0 | reference | - |
| Donor relation | ||||||
| Offspring | - | - | - | 0.25 | 0.09–0.67 | 0.006 |
| Sibling | - | - | - | 0.39 | 1.15–1.01 | 0.053 |
| Parent | - | - | - | 1.0 | reference | - |
predominantly lymphoma
Graft versus host disease (GVHD)
Forty-five patients (41.7%) developed acute graft versus host disease before day 100 – the majority developed grade I (37.8%) and grade II (42.2%) disease, with 6 patients (13.3%) and 3 patients (6.7%) developing grade III and IV disease respectively. We analyzed if there was an association between the cell source and development of acute GVHD, however no significant association could be found (Supplementary Table 2).
DISCUSSION:
This is the first analysis of the South African experience with T-cell replete haploHCT in adults with haematological malignancies. Our study supports the feasibility and safety of haploHCT and suggests that unmanipulated haploHCT is a viable option, especially for patients with acute leukaemia and MDS. Finding a RD or UD in SA is challenging for three reasons: Firstly, this region of the world faces an ongoing HIV epidemic, limiting our local volunteer donor pool. Secondly, the local population is genetically highly diverse and finally, the costs associated with finding and recruiting an international UD is prohibitive for most patients. The applicability of HaploHCT, as compared to unrelated donor transplantation, is consistent with the imperative to provide affordable and equitable access to stem cell transplantation for all patients in South Africa irrespective of socio-economic status. HaploHCT is therefore an attractive option for some patients with high risk haematological malignancies.
We observed a 3-year probability of OS and DFS of 37% and 32% respectively for the entire cohort. The outcome of the AML/MDS sub-group approximates that of other international transplant centres12,13 with a 3-year OS and DFS of 43% and 42% respectively. However, the results of the ALL group appear disappointing compared to other publications6,14 with a 3-year OS and DFS of only 21% and 24%, respectively. The heterogeneity of minimal residual disease (MRD) assessment, the lack of MRD standardization, unavailability of novel ALL drugs and distinct disease biology in this population can be possible explanations for this observation.
In multivariable analysis, we were unable to identify any risk factors associated with OS, whereas increased donor age was associated with inferior DFS. Similarly, Canaani et al16 observed the importance of donor age > 40 years leading to inferior leukaemia free survival and OS when recipient age is > 40 years in patients with acute leukaemia. In contrast, McCurdy25 and Solomon26 et al have reported that donor age was not associated with inferior patient outcomes. These conflicting results notwithstanding, our data suggest that donor age should be taken into account when deciding on a potential donor for a patient if more than one donor is available. Other advantages of using younger donors is the lower probability of clonal haematopoiesis in younger individuals and better CD34 stem cell yields36.
The 3-year probability of relapse was 21% for the entire cohort, 12% for AML/MDS, 23% for ALL and 31% for the ‘other’ group, mostly comprising of advanced lymphomas. For this last group a higher relapse rate was not unexpected, and was in keeping with other studies6,12.
The use of PBSC as cell source led to significantly faster rates of neutrophil and platelet engraftment17, but had no significant impact on rates of acute GVHD (supplementary table 2). However, this must be interpreted with caution as comparative groups are small, with insufficient statistical power. This is in contrast to Ruggeri18 and the meta-analyses of Durer19 and Yu et al20 which have shown a higher likelihood of acute GVHD with no impact on OS and RI with the use of PBSC. A limitation of our study is the missing data on the specific date of diagnosis of acute and chronic GVHD, preventing us from calculating the cumulative incidence of acute and chronic GVHD.
The favorable association of offspring donors with a lower RI in our study has also been observed by Solomon et al26. In addition, they showed a worse OS with the use of parental donors vs sibling or offspring donors using the PTCY method. McCurdy et al25 has also recently shown that recipients of haploHCT from parental donors was associated with increased risk of graft failure, while no differences were seen with sibling or offspring donors. These observations together with our data, suggest that the use of offspring or sibling donors is preferred above parental donors. Finally, most studies argue in favor for the use of offspring or sibling donors, and our data confirm this observation.
The 100-day and 3-year NRM in our cohort was 18% and 41% respectively. The former rate is similar, but the latter rate higher than the rates recently reported by Piemontese et al12 (3-year NRM 28%), yet lower than historical haploHCT reports2 (55–67%). The only risk factor identified on univariate analysis for NRM, was older recipient age (> 57 years), an expected conventional risk factor for NRM. Half of our cohort was > 44 years of age and the majority (79.8%) had high EBMT risk scores; both these factors can contribute to the relatively high 3-year NRM. As the cohort that we describe represents our early experience of haploHCT with PTCY in Southern Africa, this could contribute to the high NRM and we expect NRM to improve over time as our experience with this procedure matures. Our data can now serve as a comparator for future studies of haploHCT, and to assist clinicians and patients to make evidence-informed clinical decisions. Whether myeloablative conditioning, CMV incongruence, gender-mismatched transplants and older maternal donors contributed to the relatively high NRM in our cohort remains unanswered and needs further investigation.
We also observed a relatively high rate of graft failure and poor graft function (23 patients ; 17.2% of whole cohort) as compared to recent reports12,27. Further analysis is needed to understand the contribution of donor specific antibodies, desensitisation techniques and cell source on engraftment in order to create tailored preventative strategies.
As with any retrospective analysis, our results should be interpreted cautiously owing to inherent biases in data collection, presence of missing data, the variations in clinical practice among the public and private transplant centers and the heterogeneity of this group of haematological malignancies. However, consecutive patients that received a haploHCT at the two centres were included in this analysis, removing potential selection bias.
We conclude that our data confirms the feasibility of unmanipulated haploHCT in South Africa and suggests that utilizing younger parental or offspring donors are valid options for adults with acute leukaemia and MDS lacking a related or unrelated donor.
Supplementary Material
Funding:
This work was supported in part by the National Institutes of Health Fogarty International Center training grant D43-TW010345.
Footnotes
Declaration of competing interests: None.
REFERENCES:
- 1.Yanada M, Matsuo K, Emi N et al. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a meta-analysis. Cancer 2005. Apr 15;103(8):1652–8. [DOI] [PubMed] [Google Scholar]
- 2.Szydlo R, Goldman JM, Klein JP et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. Journal of clinical Oncology. 1997. May;15(5):1767–77. [DOI] [PubMed] [Google Scholar]
- 3.Beatty PG, Clift RA, Mickelson EM et al. Marrow transplantation from related donors other than HLA-identical siblings. NEJM 1985. Sep 26;313(13):765–71. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell PV, Luznik L, Jones RJ et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biology of Blood and Bone Marrow Transplant. 2002;8(7):377–86. [DOI] [PubMed] [Google Scholar]
- 5.Passweg JR, Baldomero H, Bader P et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 2017. Jun;52(6):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagelmann N, Bacigalupo A, Rambaldi A et al. Haploidentical Stem Cell Transplantation With Posttransplant Cyclophosphamide Therapy vs Other Donor Transplantations in Adults With Hematologic Cancers: A Systematic Review and Meta-analysis. Jama Oncology 2019. Oct 17;5(12):1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.South African Bone Marrow Registry Fact Sheet as of 31 December 2017. Website: www.sabmr.co.za.
- 8.Statistics South Africa. Midyear Population Estimates 2018. https://www.statssa.gov.za/publications/P0302/P03022018.pdf (accessed 7 February 2021).
- 9.Mellet J, Gray C, Pepper M et al. HLA typing: Conventional techniques v. next-generation sequencing. South African Medical Journal 2016;106(1):88–91. DOI: 10.7196/SAMJ.2016.v106i1.9571. [DOI] [PubMed] [Google Scholar]
- 10.Statistics South Africa. General Household Survey 2017. http://www.statssa.gov.za/publications/P0318/P03182017.pdf (accessed 7 February 2021).
- 11.World Bank. Data: South Africa, 2021. http://data.worldbank.org/country/south-africa (accessed 7 February 2021).
- 12.Piemontese S, Ciceri F, Labopin M et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia (2015) 29, 1069–1075; doi: 10.1038/leu.2014.336. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Huang X, Fei Q et al. Comparison analysis between haplo identical stem cell transplantation and matched sibling donor stem cell transplantation for high-risk acute myeloid leukemia in first complete remission. Science China Life Sciences 2019. May;62(5):691–697. [DOI] [PubMed] [Google Scholar]
- 14.Al Malki M, Yang D, Labopin M et al. Comparing transplant outcomes in ALL patients after haploidentical with PTCy or matched unrelated donor transplantation. Blood Adv (2020) 4 (9): 2073–2083. 10.1182/bloodadvances.2020001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciurea S, Shah MV, Saliba R et al. Haploidentical Transplantation for Older Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biology of blood and marrow transplantation 2018. Jun;24(6):1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canaani J, Savani BN, Labopin M et al. Donor age determines outcome in acute leukemia patients over 40 undergoing haploidentical hematopoietic cell transplantation. American Journal of Hematology 2018. Feb;93(2):246–253. [DOI] [PubMed] [Google Scholar]
- 17.Champlin RE, Schmitz N, Horowitz MM et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood 2000. Jun 15;95(12):3702–9. [PubMed] [Google Scholar]
- 18.Ruggeri A, Labopin M, Bacigalupo A et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer 2018. Apr 1;124(7):1428–1437. [DOI] [PubMed] [Google Scholar]
- 19.Durer S, Durer C, Jamil F et al. The Comparison of Unmanipulated Bone Marrow Versus Peripheral Blood Haploidentical Stem Cell Transplantation in Adult Acute Leukemia: A Systematic Review and Meta-Analysis. Blood (2018) 132 (Supplement 1): 5768. 10.1182/blood-2018-99-109924. [DOI] [Google Scholar]
- 20.Yu X, Liu L, Xie Z et al. Bone marrow versus peripheral blood as a graft source for haploidentical donor transplantation in adults using post-transplant cyclophosphamide—A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology. Volume 133, January 2019, Pages 120–128. 10.1016/j.critrevonc.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Mariotti J, Devillier R, Bramanti S et al. Peripheral Blood Stem Cells versus Bone Marrow for T Cell-Replete Haploidentical Transplantation with Post-Transplant Cyclophosphamide in Hodgkin Lymphoma. Biology of blood and marrow transplantation 2019. Sep;25(9):1810–1817. [DOI] [PubMed] [Google Scholar]
- 22.Nagler A, Dholaria B, Labopin M. et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia 34, 2766–2775 (2020). 10.1038/s41375-020-0850-9. [DOI] [PubMed] [Google Scholar]
- 23.Servais S, Porcher R, Xhaard A et al. Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica 2014. Mar;99(3):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollman C, Spellmann S, Zhang MJ et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 2016. Jan 14;127(2):260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCurdy S, Zhang MJ, St Martin A et al. Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood advances 2018. Feb 13;2(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon S, Aubrey M, Zhang X et al. Selecting the Best Donor for Haploidentical Transplant: Impact of HLA, Killer Cell Immunoglobulin-Like Receptor Genotyping, and Other Clinical Variables. Biology of blood and marrow transplantation. 2018. Apr;24(4):789–798. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs EJ HLA-haploidentical blood or marrow transplantation with high-dose, post-transplantation cyclophosphamide. Bone marrow transplantation. 2015. Jun;50 Suppl 2(0 2):S31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luznik L, O’Donnell P et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008. Jun; 14(6): 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on AGvHD grading. Bone Marrow Transplant 1995; 15: 825–828. [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 31.Gooley TA, Leisenring W et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706. [DOI] [PubMed] [Google Scholar]
- 32.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy Rep 1966. Mar;50(3):163–70. [PubMed] [Google Scholar]
- 33.Scrucca L, Santucci A et al. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010. Sep;45(9):1388–95. [DOI] [PubMed] [Google Scholar]
- 34.Cox DR. Regression models and life tables. J R Stat Soc 1972; 34: 187–202. [Google Scholar]
- 35.Gratwohl A. The EBMT risk score. Bone Marrow Transplant. 2012. Jun;47(6):749–56. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Chen XH, Zhang X, Gao L, Gao L, Kong PY, et al. Stem cell collection in unmanipulated HLA-haploidentical/mismatched related transplantation with combined granulocytecolony stimulating factor-mobilised blood and bone marrow for patients with haematologic malignancies: the impact of donor characteristics and procedural settings. Transfusion medicine (Oxford, England). 2010;20(3):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.