Abstract
Background
Obesity increases risk of pre-eclampsia, but the association with haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome is understudied.
Objective
To examine the association between prepregnancy body mass index (BMI) and HELLP syndrome, including early-onset versus late-onset disease.
Study design
A retrospective cohort study using population-based data.
Setting
British Columbia, Canada, 2008/2009–2019/2020.
Population
All pregnancies resulting in live births or stillbirths at ≥20 weeks’ gestation.
Methods
BMI categories (kg/m2) included underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9) and obese (≥30.0). Rates of early-onset and late-onset HELLP syndrome (<34 vs ≥34 weeks, respectively) were calculated per 1000 ongoing pregnancies at 20 and 34 weeks’ gestation, respectively. Cox regression was used to assess the associations between risk factors (eg, BMI, maternal age and parity) and early-onset versus late-onset HELLP syndrome.
Main outcome measures
Early-onset and late-onset HELLP syndrome.
Results
The rates of HELLP syndrome per 1000 women were 2.8 overall (1116 cases among 391 941 women), and 1.9, 2.5, 3.2 and 4.0 in underweight, normal BMI, overweight and obese categories, respectively. Overall, gestational age-specific rates of HELLP syndrome increased with prepregnancy BMI. Obesity (compared with normal BMI) was more strongly associated with early-onset HELLP syndrome (adjusted HR (AHR) 2.24 (95% CI 1.65 to 3.04) than with late-onset HELLP syndrome (AHR 1.48, 95% CI 1.23 to 1.80) (p value for interaction 0.025). Chronic hypertension, multiple gestation, bleeding (<20 weeks’ gestation and antepartum) also showed differing AHRs between early-onset versus late-onset HELLP syndrome.
Conclusions
Prepregnancy BMI is positively associated with HELLP syndrome and the association is stronger with early-onset HELLP syndrome. Associations with early-onset and late-onset HELLP syndrome differed for some risk factors, suggesting possible differences in aetiological mechanisms.
Keywords: Obesity, EPIDEMIOLOGY, OBSTETRICS
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We were able to describe gestational age-specific incidence of haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, based on population data on all pregnancies.
The population-based design coupled with detailed information about demographic, behavioural and clinical factors allowed robust adjustment for possible confounding.
We did not have detailed information on laboratory values used for the diagnosis of HELLP syndrome, and therefore, we were not able to estimate the severity of HELLP syndrome.
We did not have information about race/ethnicity, socioeconomic status and prior history of pregnancy with pre-eclampsia/eclampsia or HELLP syndrome.
Approximately 25% of women had missing information about body mass index, and we used multiple imputation methods to address this limitation.
Introduction
Hypertensive disorders of pregnancy, such as pre-eclampsia (PE), are among the leading causes of maternal morbidity and mortality, affecting 3%–5% of pregnancies worldwide1 2 and accounting for up to 14% of maternal deaths.3 Early-onset PE at <34 weeks’ gestation is often associated with placental insufficiency whereas late-onset PE is often associated with pre-existing maternal health conditions such as metabolic syndrome and obesity.4 Early-onset versus late-onset PE differ with regard to some risk factors, clinical management and rates of adverse perinatal outcomes.5 6 A related condition, namely, haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome occurs in 0.2%–0.8% of pregnancies7–9 and 10%–20% of cases of severe PE.10 Although HELLP syndrome has been distinguished from PE as a separate disease,11 it is still commonly viewed as a form of severe PE.9 While the distinction between early-onset and late-onset PE and the difference in the associations between prepregnancy obesity and these conditions has been established, such differences have not been studied with regard to HELLP syndrome.
Prepregnancy obesity is a known modifiable risk factor for PE.12–15 To date, the world prevalence of obesity has nearly tripled since 197516 and the proportion of pregnant women with obesity ranges from 1.8% to 25.3% globally.17 The prevalence of prepregnancy obesity was 17.8% in 2012–2016 in Ontario, Canada18 and 29.0% in 2019 in the USA.19 Despite the large increases in obesity in high-income countries, the association between maternal prepregnancy body mass index (BMI) and HELLP syndrome has not been adequately assessed in a large population-based study to date.
We carried out a population-based, retrospective cohort study to examine the association between maternal prepregnancy BMI and HELLP syndrome and to assess differences in this association in early-onset versus late-onset HELLP syndrome. We hypothesised that maternal obesity is a risk factor for HELLP syndrome, and this relationship may be different in early-onset compared with late-onset disease. In additional analyses, we examined other risk factors for HELLP syndrome in terms of their association with early-onset versus late-onset HELLP syndrome.
Materials and methods
Data sources and study population
The study included all live births and stillbirths at ≥20 weeks’ gestation in British Columbia (BC), Canada, between 1 April 2008 and 31 March 2020, with data obtained from the British Columbia Perinatal Database Registry (BCPDR).20 The BCPDR includes information on >99% of births in BC, with detailed data on maternal demographic characteristics, prenatal care, pregnancy complications, labour and delivery characteristics and neonatal outcomes. Each record, abstracted from medical charts (or midwives’ notes), includes up to 25 International Classification of Diseases, 10th Edition, Canadian version (ICD-10) codes for diagnoses related to the delivery hospitalisation. Chart abstraction is standardised and conducted by trained personnel, and data quality is routinely assessed. Prior validation studies showed high accuracy of collected information on labour and delivery.21
Prepregnancy BMI and HELLP syndrome
Prepregnancy weight and height were based on maternal self-report or healthcare provider assessment at ≤11 weeks’ gestation.22 BMI was classified as follows (in kg/m2): underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9) and obese (≥30.0).23 The primary outcome of this study was a physician diagnosis of HELLP syndrome documented in the medical chart, and abstracted and recorded in the BCPDR. In Canada, HELLP syndrome is typically diagnosed using the Tennessee classification criteria, namely lactate dehydrogenase ≥600 IU/L, liver transaminases (aspartate aminotransferase and alanine aminotransferase) elevated more than twice the upper limit of normal and a platelet count <100 000/μL (×109/L).24 Early-onset and late-onset HELLP syndrome were defined as HELLP syndrome with delivery at <34 weeks and ≥34 weeks’ gestation, respectively. Early pregnancy ultrasound was used to ascertain gestational age, and the last menstrual period estimate of gestational age was used for those without early pregnancy ultrasound information.
Covariates
In addition to BMI, we examined the association between maternal age, nulliparity, pre-existing diabetes, chronic hypertension, in vitro fertilisation (IVF) conception, multiple gestation, bleeding before 20 weeks, antepartum bleeding or haemorrhage, substance use and smoking during pregnancy and early-onset versus late-onset HELLP syndrome. Alcohol use and prior adverse birth outcomes (prior stillbirth or neonatal death) were included as potential confounders; all these factors are known to be associated with HELLP syndrome.25 Maternal age was categorised as <25, 25–34 and ≥35 years. All chronic conditions and pregnancy complications were identified using ICD-10 codes or data fields abstracted from medical charts to the BCPDR (online supplemental table A.1).
bmjopen-2023-079131supp001.pdf (103.8KB, pdf)
Statistical analyses
The rates of HELLP syndrome per 1000 deliveries were compared between women in each BMI category. Complete-case analyses were performed for individuals with known BMI. The association between prepregnancy BMI and HELLP syndrome was first expressed using crude HRs and 95% CIs, obtained from a Cox model without adjustment for other risk factors.
Gestational age-specific rates of HELLP syndrome were compared between women in the various BMI categories, using undelivered pregnancies at each gestational week as the denominator. These rates were plotted, and splines with 95% CIs were fitted by the generalised additive model (‘gam’) smoothing method. Cox models with interaction terms between prepregnancy BMI categories and gestational age at HELLP onset (<34 vs ≥34 weeks’ gestation) were used to obtain crude HRs and 95% CIs. This analysis was carried out to assess whether gestational age at onset modified the association between BMI and HELLP syndrome.
In multivariable analyses, Cox models were also used to adjust for covariates (listed above) and to also examine their associations with early onset versus late onset of HELLP syndrome using interaction terms. We did not assess early onset versus late onset of HELLP syndrome interactions with risk factors including alcohol use and prior adverse birth outcomes due to a low number of women with HELLP syndrome in these categories, but adjusted for them in the model as potential confounders.
Sensitivity analyses included multiple imputations for missing BMI values based on a multiple imputation procedure using SAS statistical software (PROC MI).26 Variables included in the imputation were those also included in the regression analyses. 10 imputed datasets were created, with the final results obtained using Rubin’s rule.27 All analyses were repeated with the imputed dataset and results were compared with the primary analyses.
All analyses were carried out using SAS V.9.4 (SAS Institute) and RV.4.0.3.28
Patient and public involvement
Neither patients nor the public were involved in the design, or conduct, or reporting, or dissemination plans of our research. We used only deidentified information and the need for patient’s consent was waived.
Results
Study population
Overall, 538 683 women had a live birth or stillbirth in BC between 1 April 2008 and 31 March 2020 (online supplemental figure 1). Records with missing gestational age or those with <20 weeks’ gestational duration were excluded (n=14 206, 2.6%). The study population for the primary analyses included 391 941 pregnancies, after exclusion of women with missing BMI (n=1 32 536; 24.6%). The overall incidence of HELLP syndrome was 2.85 (95% CI 2.68 to 3.01) per 1000 pregnancies (n=1116).
bmjopen-2023-079131supp002.pdf (1,006.7KB, pdf)
The proportion of women who were in underweight, normal BMI, overweight and obese categories prior to pregnancy was 5.7%, 59.1%, 21.4% and 13.8%, respectively. Pre-existing diabetes, chronic hypertension, prior adverse pregnancy outcomes (stillbirth or neonatal death), multiple gestation, gestational hypertension, gestational diabetes, proteinuria and alcohol use during pregnancy were more frequent in women with overweight and obesity compared with women with normal BMI (table 1). Nulliparity and ultrasound-diagnosed fetal growth restriction were observed more frequently in the underweight group. Substance use and smoking during pregnancy were more frequent in underweight, overweight, and obese groups compared with women with normal BMI.
Table 1.
Maternal demographic and clinical characteristics by prepregnancy body mass index (BMI); British Columbia, 2008/2009–2019/2020*
| Underweight | Normal BMI | Overweight | Obese | |
| n=22 392 | n=231 517 | n=83 864 | n=54 168 | |
| Maternal age (years) | ||||
| <25 | 4018 (17.9) | 26 332 (11.4) | 9733 (11.6) | 6947 (12.8) |
| 25–34 | 14 392 (64.3) | 146 790 (63.4) | 52 138 (62.2) | 33 948 (62.7) |
| ≥35 | 3982 (17.8) | 58 395 (25.2) | 21 993 (26.2) | 13 273 (24.5) |
| Nullipara | 12 551 (56.1) | 117 740 (50.9) | 37 202 (44.4) | 22 020 (40.7) |
| Pre-existing diabetes | 26 (0.1) | 693 (0.3) | 672 (0.8) | 1006 (1.9) |
| Chronic hypertension | 23 (0.1) | 717 (0.3) | 727 (0.8) | 1380 (2.6) |
| Prior stillbirth/neonatal death | 130 (0.6) | 1894 (0.8) | 979 (1.2) | 791 (1.5) |
| IVF conception | 496 (2.2) | 6835 (3.0) | 2579 (3.1) | 1639 (3.0) |
| Multiple gestation | ||||
| Twins | 253 (1.1) | 3318 (1.4) | 1340 (1.6) | 895 (1.7) |
| Triplets/quadruplets† | <5 (0) | 34 (0) | 26 (0) | 19 (0) |
| Bleeding <20 weeks | 483 (2.2) | 4116 (1.8) | 1572 (1.9) | 1166 (2.2) |
| Antepartum bleeding/haemorrhage (≥20 weeks) | 374 (1.7) | 3352 (1.5) | 1227 (1.5) | 706 (1.3) |
| Intrauterine growth restriction‡ | 987 (4.4) | 5445 (2.4) | 1472 (1.8) | 953 (1.8) |
| Gestational hypertension | 547 (2.4) | 8551 (3.7) | 5694 (6.8) | 6332 (11.7) |
| Gestational diabetes | 1680 (7.5) | 19 492 (8.4) | 11 548 (13.8) | 11 452 (21.1) |
| Proteinuria | 161 (0.7) | 2236 (1.0) | 1267 (1.5) | 1337 (2.5) |
| Alcohol use | 192 (0.9) | 2240 (1.0) | 944 (1.1) | 786 (1.5) |
| Substance use | 1118 (5.0) | 8099 (3.5) | 3514 (4.2) | 2970 (5.5) |
| Smoking | 1727 (7.7) | 12 943 (5.6) | 6155 (7.3) | 5576 (10.3) |
| Gestational age at delivery (weeks) | ||||
| 20–27 | 102 (0.5) | 881 (0.4) | 393 (0.5) | 358 (0.7) |
| 28–33 | 387 (1.7) | 3423 (1.5) | 1473 (1.8) | 1158 (2.1) |
| 34–36 | 1620 (7.2) | 15 119 (6.5) | 6142 (7.3) | 4680 (8.6) |
| 37–41 | 20 076 (89.7) | 209 831 (90.6) | 75 017 (89.5) | 47 448 (87.6) |
| ≥42 | 207 (0.9) | 2263 (1.0) | 839 (1.0) | 524 (1.0) |
*Data are shown as n (%).
†Information on cell numbers <5 was suppressed due to confidentiality reasons.
‡Ultrasound-diagnosed intrauterine growth restriction.
IVF, in vitro fertilisation.
Unadjusted analyses for prepregnancy BMI
The rates of HELLP syndrome in women in underweight, normal, overweight and obese categories were 1.9, 2.5, 3.2 and 4.0 per 1000 pregnancies, respectively (table 2). Overall, crude HRs for HELLP syndrome in women who were in the overweight and obese categories were 1.29 (95% CI 1.12 to 1.49) and 1.62 (95% CI 1.39 to 1.90), respectively, compared with women who had normal BMI (online supplemental table A.2).
Table 2.
Rates of early-onset and late-onset HELLP syndrome per 1000 ongoing pregnancies by maternal demographic and clinical characteristics; British Columbia, 2008/2009–2019/2020
| Early-onset HELLP syndrome |
Late-onset HELLP syndrome |
Overall | |
| Prepregnancy BMI category | |||
| Underweight | 8 (0.4) | 35 (1.6) | 43 (1.9) |
| Normal weight | 125 (0.5) | 462 (2.0) | 587 (2.5) |
| Overweight | 73 (0.9) | 199 (2.4) | 272 (3.2) |
| Obese | 69 (1.3) | 145 (2.8) | 214 (4.0) |
| Maternal age (years) | |||
| <25 | 30 (0.6) | 97 (2.1) | 127 (2.7) |
| 25–34 | 158 (0.6) | 512 (2.1) | 670 (2.7) |
| ≥35 | 87 (0.9) | 232 (2.4) | 319 (3.3) |
| Nullipara | 188 (1.0) | 629 (3.4) | 817 (4.3) |
| Pre-existing diabetes | 6 (2.5) | 14 (6.3) | 20 (8.3) |
| Chronic hypertension | 19 (6.7) | 20 (7.7) | 39 (13.7) |
| Prior stillbirth/neonatal death* | <5 (<1.0) | <5 (<1.0) | 5 (1.3) |
| IVF conception | 19 (1.6) | 73 (6.7) | 92 (8.0) |
| Multiple gestation | 33 (5.6) | 91 (19.3) | 124 (21.1) |
| Bleeding (<20 weeks) | 12 (1.6) | 10 (1.5) | 22 (3.0) |
| Antepartum bleeding/haemorrhage (≥20 weeks) | 15 (2.7) | 12 (2.5) | 27 (4.8) |
| Alcohol use* | <5 (<1.0) | <11 (<2.7) | 12 (2.9) |
| Substance use | 14 (0.9) | 25 (1.6) | 39 (2.5) |
| Smoking | 14 (0.5) | 36 (1.4) | 50 (1.9) |
*Information on cell numbers <5 was suppressed due to confidentiality reasons. Other numbers were suppressed if needed to avoid back-calculation from the total.
BMI, body mass index; HELLP, haemolysis, elevated liver enzymes and low platelets; IVF, in vitro fertilisation.
The rates of early-onset and late-onset HELLP syndrome were 0.7 (n=275) and 2.2 (n=841) per 1000 ongoing pregnancies at 20 weeks’ and 34 weeks’ gestation, respectively (online supplemental table A.3). Most cases of HELLP syndrome occurred at or after 34 weeks (75.4%; 841 out of total 1116 cases). Frequencies of overweight and obesity, older maternal age (≥35), pre-existing diabetes, chronic hypertension, multiple gestation, bleeding before 20 weeks of gestation, antepartum bleeding/haemorrhage, substance use and smoking were higher among women with early-onset versus late-onset HELLP syndrome. Frequencies of underweight, younger maternal age (<25 years), nulliparity, IVF conception and alcohol use were higher among women with late-onset HELLP syndrome (online supplemental table A.3).
The rates of late-onset HELLP syndrome were higher than early-onset HELLP syndrome regardless of BMI category and maternal age group (table 2). Nulliparous women, those with pre-existing diabetes, chronic hypertension, prior stillbirth/neonatal death, IVF conception, multiple gestation, alcohol use and substance use also had higher rates of late-onset than early-onset HELLP syndrome. Women with multiple gestation had highest rate of HELLP syndrome, followed by those with chronic hypertension.
Differences in gestational age-specific incidence rates of HELLP syndrome by BMI group are shown in figure 1A,B (shows log-transformed gestational-age specific rates).
Figure 1.
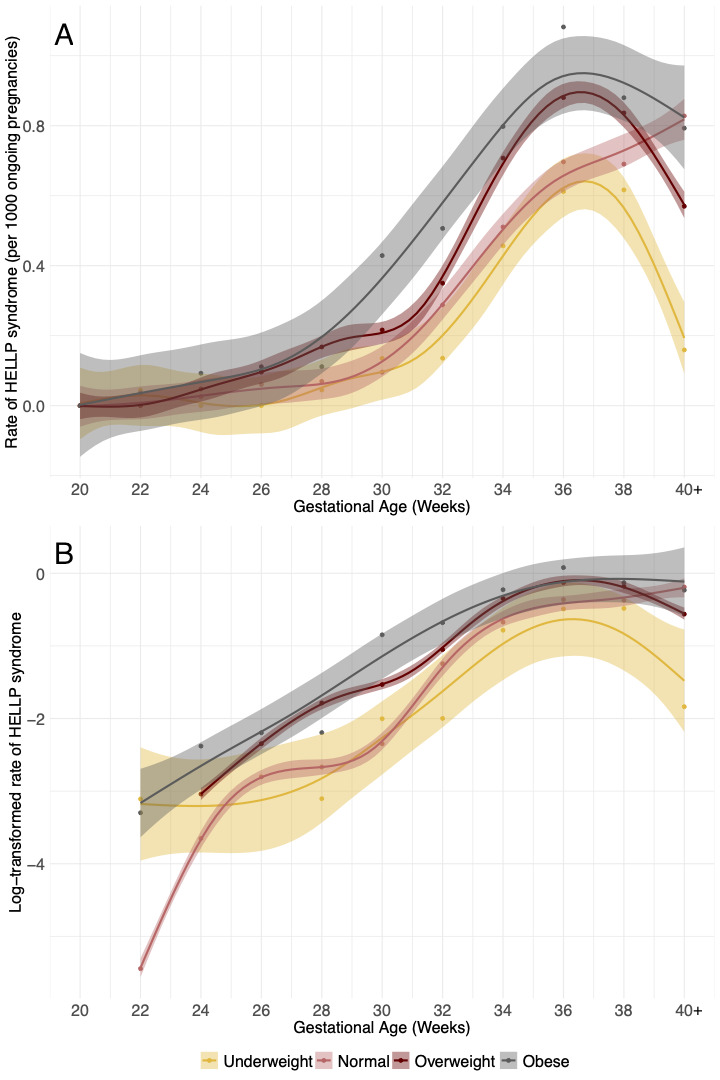
Gestational age-specific rates of HELLP syndrome for each BMI category (A) and log-transformed rates (B). Rates from 40 to 45 weeks were combined. Splines with 95% CIs were fitted by the generalised additive model (‘gam’) smoothing method. BMI, body mass index; HELLP, haemolysis, elevated liver enzymes and low platelets.
Gestational age-specific rates of HELLP syndrome increased over the course of pregnancy, with higher rates at 36–37 weeks and a subsequent decline among women with prepregnancy BMI below or above normal values but not among those with normal BMI (figure 1, online supplemental table A.4). Crude analyses showed that HRs for early-onset HELLP syndrome in women in overweight and obese groups were 1.62 (95% CI 1.21 to 2.16) and 2.37 (95% CI 1.77 to 3.18), respectively, compared with women with normal BMI. These HRs were 1.21 (95% CI 1.02 to 1.42) and 1.42 (95% CI 1.17 to 1.71) for late-onset HELLP syndrome, respectively (online supplemental table A.2).
Adjusted analyses
The associations did not change substantially after adjusting for other risk factors (table 3). Gestational age at onset of HELLP syndrome modified the effect of maternal BMI on HELLP syndrome, but only in the obese group. Specifically, obesity was more strongly associated with early-onset HELLP syndrome (adjusted HR, AHR 2.24) than with late-onset HELLP syndrome (AHR 1.48, p value for interaction=0.025; table 3).
Table 3.
Adjusted HRs for early-onset and late-onset HELLP syndrome with 95% CIs; British Columbia, 2008/2009–2019/2020
| Overall AHR (95% CI)* |
Early-onset HELLP AHR (95% CI) |
Late-onset HELLP AHR (95% CI) |
P value† | |
| Prepregnancy BMI category | ||||
| Underweight | 0.79 (0.58 to 1.07) | 0.67 (0.33 to 1.38) | 0.82 (0.58 to 1.16) | 0.628 |
| Normal weight | Ref | Ref | Ref | Ref |
| Overweight | 1.34 (1.16 to 1.55) | 1.63 (1.22 to 2.18) | 1.26 (1.07 to 1.49) | 0.129 |
| Obese | 1.65 (1.41 to 1.94) | 2.24 (1.65 to 3.04) | 1.48 (1.23 to 1.80) | 0.025 |
| Maternal age (years) | ||||
| <25 | 0.92 (0.76 to 1.12) | 0.92 (0.62 to 1.38) | 0.92 (0.74 to 1.15) | 0.998 |
| 25–34 | Ref | Ref | Ref | Ref |
| ≥35 | 1.27 (1.11 to 1.47) | 1.39 (1.06 to 1.83) | 1.23 (1.05 to 1.45) | 0.445 |
| Nullipara | 2.93 (2.56 to 3.36) | 2.56 (1.97 to 3.33) | 3.09 (2.63 to 3.63) | 0.229 |
| Pre-existing diabetes | 2.40 (1.51 to 3.80) | 1.64 (0.71 to 3.81) | 2.88 (1.66 to 5.00) | 0.273 |
| Chronic hypertension | 3.93 (2.80 to 5.51) | 5.95 (3.62 to 9.79) | 2.92 (1.83 to 4.66) | 0.041 |
| Prior stillbirth/neonatal death‡ | 0.88 (0.36 to 2.13) | N/A | N/A | N/A |
| IVF conception | 1.21 (0.95 to 1.55) | 0.83 (0.50 to 1.41) | 1.37 (1.04 to 1.80) | 0.101 |
| Multiple gestation | 13.66 (11.06 to 16.87) | 8.31 (5.59 to 12.35) | 17.81 (13.89 to 22.83) | 0.001 |
| Bleeding at <20 weeks | 0.95 (0.62 to 1.45) | 1.89 (1.05 to 3.39) | 0.60 (0.32 to 1.12) | 0.008 |
| Antepartum bleeding or haemorrhage (≥20 weeks) | 2.10 (1.43 to 3.08) | 3.75 (2.22 to 6.35) | 1.37 (0.77 to 2.43) | 0.011 |
| Alcohol use‡ | 1.07 (0.60 to 1.90) | N/A | N/A | N/A |
| Substance use | 0.98 (0.70 to 1.36) | 1.38 (0.78 to 2.42) | 0.84 (0.56 to 1.27) | 0.166 |
| Smoking | 0.71 (0.53 to 0.96) | 0.70 (0.40 to 1.23) | 0.71 (0.50 to 1.01) | 0.963 |
*AHR, with 95% CI in parentheses, was obtained from the Cox model that included all variables in the table.
†p value for interaction with early-onset versus late-onset HELLP syndrome.
‡N/A, We did not examine differences by early onset versus late onset for prior stillbirth/neonatal death or alcohol use due to small sample size.
AHR, adjusted HR; BMI, body mass index; HELLP, haemolysis, elevated liver enzymes and low platelets; IVF, in vitro fertilisation; N/A, not applicable.
AHRs for each risk factor calculated separately for early-onset versus late-onset HELLP syndrome are shown in table 3. Risk factors significantly associated with HELLP syndrome included overweight, obesity, advanced maternal age (≥35 years), nulliparity, pre-existing diabetes, chronic hypertension, multiple gestation and antepartum bleeding/haemorrhage. Smoking during pregnancy had an inverse association with HELLP syndrome. IVF conception was a risk factor for late-onset but not early-onset HELLP syndrome. Bleeding before 20 weeks and antepartum bleeding/haemorrhage were risk factors for early-onset but not late-onset HELLP syndrome. Obesity (p=0.025), chronic hypertension (p=0.041), multiple gestation (p=0.001), bleeding before 20 weeks (p=0.008) and antepartum bleeding/haemorrhage (p=0.011) differed significantly in their associations with early-onset versus late-onset HELLP syndrome (p values for interaction).
Sensitivity analyses
Women with missing BMI were not substantially different from women with known BMI (online supplemental table A.5); and the results were not appreciably changed after the analyses were repeated using imputed BMI values (online supplemental table A.6).
Discussion
Main findings
To our knowledge, this is the largest contemporary study examining the association between prepregnancy BMI and HELLP syndrome, including early-onset and late-onset disease. We showed that the majority of HELLP syndrome (75.4%) occurred at or after 34 weeks’ gestation, with the rate of early-onset HELLP syndrome being substantially lower than that of late-onset HELLP syndrome. Women in overweight or obese groups were at elevated risk for developing HELLP syndrome. Obesity was more strongly associated with early-onset than late-onset HELLP syndrome. In addition to BMI, our study showed that chronic hypertension, bleeding before 20 weeks’ gestation and antepartum bleeding/haemorrhage were stronger risk factors for early-onset HELLP syndrome, whereas multiple gestation was a stronger risk factor for late-onset HELLP syndrome.
Interpretation in the context of scientific literature
The rate of HELLP syndrome in our study (2.8 per 1000 women) was similar to the previously reported rate of 2.5 per 1000 singleton pregnancies in Canada in 2012–2016.25 Prior studies describing the association between prepregnancy obesity and HELLP syndrome are sparse and results vary. In a retrospective cohort study from a single tertiary hospital in the USA (n=434), Martin et al found that maternal weight was not associated with HELLP syndrome.29 Similarly, a case–control study (n=129 cases and 476 controls) found no association between obesity and HELLP syndrome.30 Furthermore, a retrospective case–control study (including n=687 cases and 601 controls) showed that prepregnancy BMI was associated with PE but not HELLP syndrome and suggested that PE and HELLP may have different pathophysiology.12 In contrast, a population-based cohort study from Norway (n=418 897) found that prepregnancy BMI≥30 kg/m2 was associated with HELLP syndrome in the first but not the second pregnancy.9 However, in that study, only 25% of women with a first pregnancy and 30% of women with a second pregnancy had information on BMI. More recently, a population-based study from Canada (n=1 078 323) showed that obesity documented in medical charts was a risk factor for HELLP syndrome,31 however, obesity rates were underestimated and information on BMI was not available, precluding more detailed analyses.
While PE is typically recognised as early-onset versus late-onset disease (before vs ≥34 weeks gestation, respectively), this distinction is rarely made for HELLP syndrome. A prior population-based cohort study (n=96 861) showed that high prepregnancy BMI is a stronger risk factor for late-onset PE than early-onset PE.15 That study also demonstrated a correlation between increased prevalence of maternal obesity in parallel with late-onset PE during the 18-year period, while the incidence of early-onset PE stayed relatively constant.15 In contrast, our study shows a stronger association between overweight/obesity and early-onset HELLP syndrome compared with late-onset HELLP syndrome. This suggests varying pathophysiological pathways between PE and HELLP syndrome or additional obesity-related pathophysiology associated with PE that leads to liver damage at earlier gestation, for instance, obesity-associated steatosis and non-alcoholic fatty liver disease.32 We chose the same gestational age cut-off of 34 weeks for early-onset versus late-onset HELLP syndrome as in PE. However, our data suggest an increase in gestational age-specific rates after 28 weeks’ gestation in women with obesity and after 30 weeks’ gestation in women without obesity. A previous study showed a high proportion of HELLP syndrome cases occurring between 27 and 37 weeks,33 which indicates potential dissimilarities with early-onset versus late-onset PE. Chronic hypertension, however, was found to be a stronger risk factor for early-onset disease for both PE6 and HELLP syndrome compared with late-onset disease. It is worth mentioning that the known inverse association between smoking and PE6 was also observed in HELLP syndrome in our study, and this warrants further investigation.
Clinical and research implications
Our findings show that increases in gestational age-specific rates of HELLP syndrome vary by maternal prepregnancy BMI. The rates declined after 37 weeks’ gestation in women who were in the underweight, overweight and obese categories, but continued increasing in women with normal BMI. This could be due to higher rates of medically indicated early-term deliveries in groups with low or high BMI, which has been shown to reduce maternal morbidity compared with expectant management.34 It is possible that women whose prepregnancy BMI was below and above normal range were more likely to be considered at-risk (due to the abnormal BMI or associated comorbidity) and therefore delivered at early term (37–38 weeks) gestation to prevent adverse maternal and infant outcomes. However, further research is needed to confirm this hypothesis. In addition to BMI, we also showed that chronic hypertension, bleeding before 20 weeks’ gestation and antepartum bleeding/haemorrhage were more strongly associated with early-onset HELLP syndrome, while multiple gestation was more strongly associated with late-onset HELLP syndrome. The association between bleeding at <20 weeks gestation and early-onset HELLP syndrome is novel. Such bleeding can be caused by abnormal placental conditions (eg, abnormal implantation and associated bleeding), which may play a role in the development of HELLP syndrome. These findings are exploratory and require confirmation by other studies. However, they raise the intriguing possibility that determinants of HELLP syndrome (such as antepartum bleeding) have different associations with early-onset and late-onset HELLP syndrome depending on whether they occur at <20 weeks or at ≥20 weeks’gestation. In our study, the association between antepartum bleeding at ≥20 weeks’gestation and HELLP syndrome (which could have been explained as being a consequence of HELLP syndrome causing placental abruption) was not significant in adjusted models.
Strengths and limitations
The strengths of this study include its population-based design coupled with detailed information about demographic, behavioural and clinical factors that allowed for robust adjustment for possible confounding. We had a large enough sample to provide precise estimates for associations with HELLP syndrome, a rare outcome.
This study also has several limitations. First, we did not have detailed information on laboratory values important for the diagnosis of HELLP syndrome, and therefore, we were not able to estimate the severity of HELLP. We assumed that the diagnosis of HELLP syndrome would lead to a prompt delivery to prevent worsening of maternal condition. However, in milder cases, expectant management with close observation may have led to a delay between the diagnosis and delivery, especially at very preterm gestation. As a result, incidence of early-onset HELLP syndrome may have been underestimated in our study. However, we do not expect a large inaccuracy in this regard because HELLP syndrome is considered a potentially life-threatening condition and delivery is typically not delayed. Second, we did not have information about race/ethnicity, socioeconomic status and prior history of pregnancy with PE/eclampsia or HELLP syndrome, which could have resulted in residual confounding in the assessments of the relation between BMI and HELLP syndrome. However, we adjusted for several possible confounders and did not observe changes in the association between BMI and HELLP syndrome, suggesting that our results are robust. Third, prepregnancy BMI was largely self-reported, which may have led to some misclassification. Several validation studies have shown relatively good accuracy of self-reported weight and height for epidemiological studies,35–37 suggesting that a large misclassification bias is unlikely. A systematic review of BMI self-report misclassification showed minimal influence on associations between BMI and pregnancy outcomes.38
Approximately 25% of women had missing information about BMI. These women were relatively similar to those with known BMI and sensitivity analyses using imputed BMI values yielded results almost identical to the main analyses. Lastly, the analyses examining differences between early-onset and late-onset HELLP and risk factors other than BMI were exploratory, and further studies are required to confirm our findings.
Conclusions
Consistent with what is known about PE, prepregnancy BMI was found to be a risk factor for HELLP syndrome. However, contrary to the documented association between BMI and PE, with obesity being associated more strongly with late-onset than early-onset PE, our study showed that obesity was more strongly associated with early-onset than with late-onset HELLP syndrome. This suggests potentially different underlying pathophysiology for the various hypertensive disorders of pregnancy. Our findings can help maternity care providers with regard to prepregnancy counselling. Clinicians can better identify women who may benefit from obstetric intervention, as the risk of HELLP increases at late preterm gestation in all women and continues to increase at term and post-term gestation in women with normal prepregnancy BMI. More research on the gestational age-specific effects of prepregnancy BMI is needed to elucidate the underlying causes of HELLP syndrome.
bmjopen-2023-079131supp003.pdf (97.8KB, pdf)
Supplementary Material
Acknowledgments
We thank the Women’s Health Research Institute (WHRI) for providing us with access to the BCPDR database.
Footnotes
Twitter: @Li_Qing_Wang, @sarkalis
Contributors: LQW and SL were involved in the conception, planning, carrying out and analysing data of the project. JNB, GMM, KSJ and NR provided helpful suggestions for the analysis. LQW led the writing of the manuscript and received feedback from JNB, GMM, SL, KSJ and NR. LQW and SL are the guarantors of this study.
Funding: This study was funded by the Canadian Institutes for Health Research (CIHR) and the SickKids Foundation (CIHR SKF–154852). LQW receives support from a CIHR Doctoral Fellowship, KSJ is supported by an investigator award from the BC Children’s Hospital Research Institute, Canada, NR is supported by a grant from the Swedish Research Council for Health, Working Life and Welfare (grant no. 2019-00041).
Disclaimer: All inferences, opinions and conclusions drawn in this publication are those of the authors and do not reflect the opinions or policies of Perinatal Services BC. The funding sources were not involved in study design, data collection, analysis and interpretation, writing of the manuscript and/or decision to submit the article for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The third party is the Women’s Health Research Institute (WHRI) (https://whri.org/).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethics approval was obtained from the University of British Columbia/Children’s and Women’s Hospital and Health Centre of British Columbia Research Ethics Board (#H20-03985).
References
- 1. Burton GJ, Redman CW, Roberts JM, et al. Pre-eclampsia: pathophysiology and clinical implications. BMJ 2019;366:l2381. 10.1136/bmj.l2381 [DOI] [PubMed] [Google Scholar]
- 2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. 10.1016/j.bpobgyn.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 3. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 4. Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia. Hypertension 2008;52:873–80. 10.1161/HYPERTENSIONAHA.108.117358 [DOI] [PubMed] [Google Scholar]
- 5. Wadhwani P, Saha PK, Kalra JK, et al. A study to compare maternal and perinatal outcome in early vs. late onset preeclampsia. Obstet Gynecol Sci 2020;63:270–7. 10.5468/ogs.2020.63.3.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013;209:544. 10.1016/j.ajog.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 7. Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. Eur J Obstet Gynecol Reprod Biol 2013;166:117–23. 10.1016/j.ejogrb.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 8. Martin JN, Rinehart BK, May WL, et al. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol 1999;180:1373–84. 10.1016/s0002-9378(99)70022-0 [DOI] [PubMed] [Google Scholar]
- 9. Malmström O, Morken NH. HELLP syndrome, risk factors in first and second pregnancy: a population-based cohort study. Acta Obstet Gynecol Scand 2018;97:709–16. 10.1111/aogs.13322 [DOI] [PubMed] [Google Scholar]
- 10. Karumanchi SA, Maynard SE, Stillman IE, et al. Preeclampsia: a renal perspective. Kidney Int 2005;67:2101–13. 10.1111/j.1523-1755.2005.00316.x [DOI] [PubMed] [Google Scholar]
- 11. Weinstein L. Classic pages-syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol 2005;193:859. 10.1016/j.ajog.2005.02.113 [DOI] [PubMed] [Google Scholar]
- 12. Leeners B, Rath W, Kuse S, et al. BMI: new aspects of a classical risk factor for hypertensive disorders in pregnancy. Clin Sci 2006;111:81–6. 10.1042/CS20060015 [DOI] [PubMed] [Google Scholar]
- 13. Schummers L, Hutcheon JA, Bodnar LM, et al. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol 2015;125:133–43. 10.1097/AOG.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bodnar LM, Catov JM, Klebanoff MA, et al. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology 2007;18:234–9. 10.1097/01.ede.0000254119.99660.e7 [DOI] [PubMed] [Google Scholar]
- 15. Robillard P-Y, Dekker G, Scioscia M, et al. Increased BMI has a linear association with late-onset preeclampsia: a population-based study. PLoS One 2019;14:e0223888. 10.1371/journal.pone.0223888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization . Home/Newsroom/fact sheets/detail/obesity and overweight. obesity and overweight. n.d. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 17. Guelinckx I, Devlieger R, Beckers K, et al. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 2008;9:140–50. 10.1111/j.1467-789X.2007.00464.x [DOI] [PubMed] [Google Scholar]
- 18. Dzakpasu S, Fahey J, Kirby RS, et al. Contribution of prepregnancy body mass index and gestational weight gain to caesarean birth in Canada. BMC Pregnancy Childbirth 2014;14:106. 10.1186/1471-2393-14-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief 2020:1–8. [PubMed] [Google Scholar]
- 20. Perinatal Services BC . Perinatal data registry. n.d. Available: http://www.perinatalservicesbc.ca/health-professionals/data-surveillance/perinatal-data-registry
- 21. Frosst G, Hutcheon J, Joseph KS, et al. Validating the British Columbia perinatal data Registry: a chart re-abstraction study. BMC Pregnancy Childbirth 2015;15:123. 10.1186/s12884-015-0563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. British Columbia perinatal data registry reference manual version 6.01 – addendum. Perinatal Services BC: Vancouver (BC); 2017. [Google Scholar]
- 23. World Health Organization . A healthy lifestyle - WHO recommendations. 2010. Available: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations
- 24. Khalid F, Mahendraker N, Tonismae T. HELLP syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2023. [PubMed] [Google Scholar]
- 25. Lisonkova S, Bone JN, Muraca GM, et al. Incidence and risk factors for severe preeclampsia, hemolysis, elevated liver enzymes, and low platelet count syndrome, and Eclampsia at preterm and term gestation: a population-based study. Am J Obstet Gynecol 2021;225:538. 10.1016/j.ajog.2021.04.261 [DOI] [PubMed] [Google Scholar]
- 26. SAS Institute Inc . The MI procedure: PROC MI statement. n.d. Available: https://documentation.sas.com/doc/en/statug/15.2/statug_mi_syntax01.htm
- 27. Rubin DB, Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken: WILEY; 2009. Available: https://go.exlibris.link/fnXsLRMW [Google Scholar]
- 28. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2022. Available: https://www.R-project.org/ [Google Scholar]
- 29. Martin JN, May WL, Rinehart BK, et al. Increasing maternal weight: a risk factor for preeclampsia/Eclampsia but apparently not for HELLP syndrome. South Med J 2000;93:686–91. [PubMed] [Google Scholar]
- 30. Fitzpatrick KE, Hinshaw K, Kurinczuk JJ, et al. Risk factors, management, and outcomes of hemolysis, elevated liver enzymes, and low platelets syndrome and elevated liver enzymes, low platelets syndrome. Obstet Gynecol 2014;123:618–27. 10.1097/AOG.0000000000000140 [DOI] [PubMed] [Google Scholar]
- 31. Lisonkova S, Razaz N, Sabr Y, et al. Maternal risk factors and adverse birth outcomes associated with HELLP syndrome: a population-based study. BJOG 2020;127:1189–98. 10.1111/1471-0528.16225 [DOI] [PubMed] [Google Scholar]
- 32. Sarkar M, Grab J, Dodge JL, et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol 2020;73:516–22. 10.1016/j.jhep.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol 1993;169:1000–6. 10.1016/0002-9378(93)90043-i [DOI] [PubMed] [Google Scholar]
- 34. Chappell LC, Brocklehurst P, Green ME, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet 2019;394:1181–90. 10.1016/S0140-6736(19)31963-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davies A, Wellard-Cole L, Rangan A, et al. Validity of self-reported weight and height for BMI classification: a cross-sectional study among young adults. Nutrition 2020;71:110622. 10.1016/j.nut.2019.110622 [DOI] [PubMed] [Google Scholar]
- 36. Fonseca H, Silva AM, Matos MG, et al. Validity of BMI based on self-reported weight and height in adolescents. Acta Paediatr 2010;99:83–8. 10.1111/j.1651-2227.2009.01518.x [DOI] [PubMed] [Google Scholar]
- 37. Hodge JM, Shah R, McCullough ML, et al. Validation of self-reported height and weight in a large, nationwide cohort of U.S. adults. PLOS ONE 2020;15:e0231229. 10.1371/journal.pone.0231229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Headen I, Cohen AK, Mujahid M, et al. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev 2017;18:350–69. 10.1111/obr.12486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-079131supp001.pdf (103.8KB, pdf)
bmjopen-2023-079131supp002.pdf (1,006.7KB, pdf)
bmjopen-2023-079131supp003.pdf (97.8KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The third party is the Women’s Health Research Institute (WHRI) (https://whri.org/).


