Abstract
Introduction
Patients with a first venous thromboembolism (VTE) are at risk of recurrence. Recurrent VTE (rVTE) can be prevented by extended anticoagulant therapy, but this comes at the cost of an increased risk of bleeding. It is still uncertain whether patients with an intermediate recurrence risk or with a high recurrence and high bleeding risk will benefit from extended anticoagulant treatment, and whether a strategy where anticoagulant duration is tailored on the predicted risks of rVTE and bleeding can improve outcomes. The aim of the Leiden Thrombosis Recurrence Risk Prevention (L-TRRiP) study is to evaluate the outcomes of tailored duration of long-term anticoagulant treatment based on individualised assessment of rVTE and major bleeding risks.
Methods and analysis
The L-TRRiP study is a multicentre, open-label, cohort-based, randomised controlled trial, including patients with a first VTE. We classify the risk of rVTE and major bleeding using the L-TRRiP and VTE-BLEED scores, respectively. After 3 months of anticoagulant therapy, patients with a low rVTE risk will discontinue anticoagulant treatment, patients with a high rVTE and low bleeding risk will continue anticoagulant treatment, whereas all other patients will be randomised to continue or discontinue anticoagulant treatment. All patients will be followed up for at least 2 years. Inclusion will continue until the randomised group consists of 608 patients; we estimate to include 1600 patients in total. The primary outcome is the combined incidence of rVTE and major bleeding in the randomised group after 2 years of follow-up. Secondary outcomes include the incidence of rVTE and major bleeding, functional outcomes, quality of life and cost-effectiveness in all patients.
Ethics and dissemination
The protocol was approved by the Medical Research Ethics Committee Leiden-Den Haag-Delft. Results are expected in 2028 and will be disseminated through peer-reviewed journals and during (inter)national conferences.
Trial registration number
Keywords: Thromboembolism, Clinical trials, EPIDEMIOLOGIC STUDIES, Anticoagulation
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The models can be applied to all patients with a first venous thromboembolism (VTE) without cancer, irrespective of whether this event was provoked or unprovoked.
The study is designed to follow usual clinical procedures as much as possible to increase the generalisability of the results.
Primary outcomes will be adjudicated by a committee blinded for treatment.
The open-label design might increase cross-over between treatment groups and might influence assessment and reporting of study outcomes by the patient or treating physician.
Questionnaires are used for follow-up which might result in missing outcome data, despite procedures to limit this, such as regular phone contact and collecting information from treating physicians.
Introduction
Patients with a first venous thromboembolism (VTE) are at risk of a recurrent event, especially when the first event was unprovoked. The estimated risk of recurrence in patients with a first unprovoked VTE was 10% in the first year and 36% after 10 years,1 whereas patients with a first VTE provoked by a transient risk factor have an estimated risk of 1%–6% in the first year and 3%–15% after 5 years, depending on whether the provoking factor was a minor or major transient risk factor.2 3 A recurrent VTE has serious consequences with estimated case fatality rates of 4%.1 4 In addition, compared with the initial event, recurrent VTE is associated with a higher risk of long-term complications such as post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension.5 6 Recurrent VTE can be prevented by prolonged oral anticoagulant therapy, but this comes at the cost of an increased risk of major bleeding compared with ceasing treatment.7 8 A recent meta-analysis reported an overall major bleeding incidence of 1.7 per 100 person-years during extended use of vitamin K antagonists (VKAs) and 1.1 per 100 person-years during extended use of direct oral anticoagulants (DOACs), with a case fatality rate of 8.4%.9 Importantly, the same meta-analysis reported limited safety information on long-term anticoagulation in patients with VTE, in particular for DOAC recipients where information beyond 1 year of treatment was sparse. Indeed, indefinite use of anticoagulant therapy may result in a significant lifetime risk of major bleeding, a risk that is still to be quantified.
Consequently, the optimal duration of anticoagulant treatment is still under debate. Previously, patients received oral anticoagulant treatment for a fixed period (ie, 3–6 months) after a first VTE, whereas current guidelines recommend to base treatment duration (ie, either a limited period or indefinite duration), on the balance between the risk of recurrent VTE and major bleeding.10–15 Indefinite treatment should be considered for patients with a first unprovoked VTE given its higher associated recurrence risk, and it is recommended to discontinue anticoagulant treatment after 3 months for patients with a provoked VTE. However, the definition of provoked VTE varies between guidelines, between centres and over time, highlighting the clinical ambiguity surrounding this decision.16 In addition, basing the decision on treatment duration solely on the classification of the first event into provoked or unprovoked may be too crude: a study from our group showed that the c-statistic of the (un)provoked status was only 0.61, indicating that the ability to distinguish patients at low and high risk of recurrence is limited. In fact, 15% of patients with a first provoked VTE had a predicted 2-year recurrence risk of more than 10%, whereas this risk was below 10% in 45% of the patients with a first unprovoked VTE.17 This finding indicates that these patient groups would have been undertreated or overtreated if the current guidelines were strictly followed (without accounting for bleeding risk or patient preferences).11–15 17 Furthermore, guidelines advise to take the risk of major bleeding into account, but guidance on how to best assess the risk of major bleeding and balance this against the risk of VTE is not available.11–15 18 Moreover, studies investigating the optimal duration of anticoagulation in relation to patient-relevant outcomes such as quality of life are lacking.19 Therefore, in current clinical practice the decision to stop or continue treatment indefinitely is based on insufficient information. For these reasons, more elaborate individualised risk stratification in combination with knowledge on the optimal treatment duration, linked to these risks, is expected to reduce both types of serious complications.
Multiple prediction models have been developed to assess the risk of VTE recurrence and major bleeding in patients with VTE.20 21 At the time we started to design the present study (2018), models for the prediction of VTE recurrence included the Men and HERDOO2 rule, Vienna prediction model, DASH score, DAMOVES score, pre and post D-dimer strategy, Worcester VTE score and L-TRRiP (Leiden Thrombosis Recurrence Risk Prevention) model.17 22–27 Of these, the L-TRRiP model is the only externally validated model that predicts long-term recurrence risk after a provoked as well as an unprovoked first VTE, which allows for easier use given the problems related to the distinction between provoked and unprovoked VTE as described above. In addition, it allows for more precise risk stratification by providing an absolute recurrence risk, rather than dichotomising high and low recurrence risk. Another advantage of the L-TRRiP model is that all parameters can be determined during anticoagulant treatment, so interruption or discontinuation of the treatment is not required, in contrast to some other models that include D-dimer, a biomarker predictor that needs to be measured after a short interruption of anticoagulation. Besides being unpractical, such interruption—although relatively rare—may lead to early recurrent VTE events shortly after discontinuation.28
Models to predict major bleeding during anticoagulant therapy have mainly been developed for patients with atrial fibrillation (AF). Examples of such models are the HAS-BLED score and HEMORR2HAGES score.29 30 Nevertheless, in current clinical practice these models are sometimes also used to predict major bleeding among patients with VTE.12 18 However, patient characteristics differ between patients with AF and VTE, and the predictive performance of these models in patients with VTE is limited.20 Therefore, dedicated models for patients with VTE have been developed, which include the score developed by Kuijer et al, the ACCP risk table, the RIETE score and VTE-BLEED score.11 31–34 Of these, the VTE-BLEED score is among the most externally validated models, has been validated during extended anticoagulant therapy and has shown a good predictive performance in patients using VKAs, as well as in those using DOACs.18 35–38
Previous attempts have been made to optimise the length of treatment of patients after a first VTE based on individualised assessment of recurrent VTE risk.28 39 One study showed a clear benefit of prolonged anticoagulant treatment compared with discontinuation on recurrent VTE in patients with an unprovoked VTE and elevated d-dimer levels 1 month after ceasing anticoagulant treatment (2.9% vs 15% during 9–18 months of follow-up, respectively).39 However, the incidence of recurrent VTE in patients with normal d-dimer levels (in whom anticoagulation was therefore stopped) was still high (6%–7% per patient-year),39 40 indicating d-dimer alone cannot be used to guide anticoagulant treatment duration. Another study showed that prolonging anticoagulant treatment based on the Vienna score versus routine clinical care did not improve the clinical outcome in the randomised groups, although that the risk of actual recurrent VTE was indeed low in those with a low predicted risk based on the Vienna score.28 Likewise, a management study implementing the HERDOO2 rule showed that women with a low predicted recurrence risk had indeed a low risk of VTE recurrence after anticoagulant discontinuation.41 However, the benefit of extended anticoagulation in the patients with a high risk of VTE recurrence remains uncertain. Furthermore, none of these studies included patients with a first provoked VTE or applied a bleeding risk model next to the prediction of recurrence risk. Currently, none of these strategies is recommended by the guidelines.
In summary, the current strategy to decide on (dis)continuation of anticoagulant treatment after a first VTE is not optimal since (1) the definition of provoked VTE is subject to debate, (2) the insufficient discriminative power of a distinction between provoked and unprovoked VTE is disregarded, (3) the risk of major bleeding is not properly taken into account and (4) patient relevant outcomes such as quality of life are not taken into account. This results in both overtreatment and undertreatment with anticoagulants in a proportion of patients with a first VTE, leading to unnecessary high lifetime risks of major bleeding or recurrent VTE, respectively. Although some novel strategies have been studied, this has not resulted in a more tailored strategy to determine optimal treatment duration. Therefore, in the L-TRRiP study, we aim to evaluate outcomes of tailored duration of anticoagulant treatment based on individualised risk assessment of a patient’s recurrent VTE and major bleeding risk, using both the L-TRRiP and VTE-BLEED models.
Methods and analysis
Study design
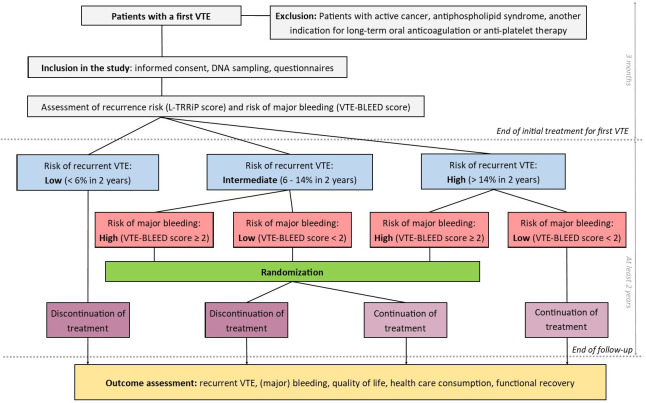
The L-TRRiP study is a multicentre, open-label, cohort-based randomised controlled trial. The L-TRRiP and VTE-BLEED prediction models are used to individually classify patients according to their risk of recurrent VTE (as low, intermediate or high) and major bleeding (as low or high), respectively. After the initial 3 months, anticoagulant treatment is stopped in patients with a low recurrent VTE risk, while patients with a high recurrent VTE risk and low major bleeding risk continue treatment. Patients in the other risk groups (ie, patients with an intermediate recurrent VTE risk or a high recurrent VTE risk and high bleeding risk) are randomised to continue or discontinue anticoagulant treatment (figure 1). All patients, both in the non-randomised and randomised arms, are followed up for at least 2 years, following the same procedures. Academic hospitals, teaching hospitals and general hospitals from the Netherlands participate in this trial. At this time, the trial has started enrolment in 17 hospitals (see online supplemental appendix I). Study enrolment started in 2021, the first patient was enrolled in June 2021. The planned end date of the study is 2027, 2 years after enrolment of the last patient, which is expected to be in 2025. The L-TRRiP study is registered at the Dutch Trial Registry: NL9003 and ClinicalTrials.gov: NCT06087952. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines were followed when drafting the study protocol.
Figure 1.
Design of the Leiden Thrombosis Recurrence Risk Prevention (L-TRRiP) study. VTE, venous thromboembolism.
bmjopen-2023-078676supp001.pdf (171KB, pdf)
Study population
Patients with a first confirmed symptomatic distal or proximal deep venous thrombosis (DVT) of the lower extremity or pulmonary embolism (PE) with an indication for anticoagulant treatment for at least 3 months, aged 18 years or above, who provide informed consent prior to any study specific procedure, are eligible to participate in this trial. Patients with active cancer, known antiphospholipid syndrome, those who have an indication other than VTE for prolonged anticoagulant treatment (eg, AF), who have an indication for long-term antiplatelet therapy despite the use of oral anticoagulation (eg, recent myocardial infarction) or who have an extremely high bleeding risk necessitating discontinuation of anticoagulant treatment will be excluded. Diagnostic testing for malignancy or antiphospholipid syndrome after the index VTE diagnosis is performed at the discretion of the treating physician. Patients with VTE related to severe COVID-19 (ie, requiring hospital admission in 3 months before the index event) as well as patients with vaccine-induced immune thrombotic thrombocytopenia are not eligible to participate in this trial since the effect of these conditions on recurrence is not known, and such, patients were not included in derivation of the L-TRRiP model.17
Risk prediction models
The L-TRRiP model includes sex, type and location of VTE, risk factors for VTE, history of cardiovascular disease as well as blood group non-O and the factor V Leiden mutation to predict the absolute 2-year risk of recurrent VTE. A predicted 2-year VTE risk below 6% is classified as low, a VTE risk of 6%–14% as intermediate and a VTE risk above 14% as high (see table 1).17 The VTE-BLEED model uses age of 60 years or higher, renal dysfunction, anaemia, history of clinically relevant or major bleeding, active malignancy, and uncontrolled hypertension in male patients to predict major bleeding risk. A score <2 is classified as low bleeding risk and a score ≥2 as high bleeding risk (table 2).33
Table 1.
L-TRRiP model
| Factor | Coefficient |
| Male sex | 0.63 |
| Type of first VTE | |
| PE | −0.61 |
| PE+DVT | 0.32 |
| Location of DVT | |
| Popliteal DVT* | −0.46 |
| Surgery† | −0.51 |
| Pregnancy/puerperium† | −1.49 |
| Hormone use‡ | −0.67 |
| Plaster cast† | −0.79 |
| Immobility in bed, in hospital†§ | −0.31 |
| History of cardiovascular disease¶ | −0.35 |
| Blood group, non-O | 0.24 |
| Factor V Leiden mutation** | 0.40 |
| Calculation of the L-TRRiP score | |
| Prognostic score | Beta1*x1+beta2*x2+beta3*x3 + …. The x1, x2, x3, etc, represent the factors in the model, and beta1, beta2, beta3, etc, represent the corresponding coefficients. |
| Absolute 2-year risk of VTE recurrence | 0.9235595ˆexp(prognostic score) |
| Classification of patients with the L-TRRiP score | |
| Low recurrent VTE risk | 2-year risk <0.06 |
| Intermediate recurrent VTE risk | 2-year risk 0.06–0.14 |
| High recurrent VTE risk | 2-year risk >0.14 |
Table adapted from Timp et al. 17
*DVT at the level of the vena poplitea or below.
†Within 3 months before VTE.
‡Use of hormonal contraceptives or hormone replacement therapy at the time of VTE.
§Confinement to bed ≥3 days.
¶Including a history of heart failure, angina pectoris, peripheral artery vascular disease (claudication), acute myocardial infarction.
**Homozygous or heterozygous.
DVT, deep venous thrombosis; L-TRRiP, Leiden Thrombosis Recurrence Risk Prevention; PE, pulmonary embolism; VTE, venous thromboembolism.
Table 2.
VTE-BLEED model
| Factor | Score |
| Active cancer* | 2 |
| Male with uncontrolled arterial hypertension† | 1 |
| Anaemia‡ | 1.5 |
| History of bleeding§ | 1.5 |
| Age ≥60 years old | 1.5 |
| Renal dysfunction¶ | 1.5 |
| Classification of patients with the VTE-BLEED score | |
| Low bleeding risk | Total score <2 |
| High bleeding risk | Total score ≥2 |
Table adapted from Klok et al. 35
*Cancer diagnosed within 6 months before diagnosis of VTE (excluding basal-cell or squamous-cell carcinoma of the skin), recently recurrent or progressive cancer or any cancer that required anti-cancer treatment within 6 months before the VTE was diagnosed.
†Value of systolic blood pressure ≥140 mm Hg at baseline.
‡Haemoglobin <13 g/dL in men or <12 g/dL in women.
§Including prior major or non-major clinically relevant bleeding events, rectal bleeding (more than spotting on toilet paper), frequent nose bleeding or haematuria.
¶Estimated glomerular filtration rate <60 mL/min at baseline (calculated with Cockcroft-Gault formula).
VTE, venous thromboembolism.
Procedures
After providing informed consent, patients are asked to fill in a questionnaire including demographic variables, clinical circumstances and risk factors for the first VTE and medical history including previous bleeding. Furthermore, a self-administered buccal swab is taken to assess the factor V Leiden mutation and ABO blood group by DNA analysis. Information is obtained from the electronic health records from the hospital including recent haemoglobin level, renal function, blood pressure, comorbidities and details regarding the first VTE event (type and location of VTE).
Based on this information, the L-TRRiP and VTE-BLEED scores and corresponding risk categories are calculated in the coordinating centre (Leiden University Medical Center). Depending on the risk category of the patient, a decision on duration of treatment is either made immediately, or the duration of treatment is randomised (figure 1).
When applicable, randomisation is performed shortly before the routine 3-month visit in the coordinating centre using the randomisation function in CastorEDC to ensure concealment of treatment allocation.42 Randomisation is performed in a 1:1 ratio, using variable block randomisation with a block size of two, four or six stratified by study centre, risk group for recurrent VTE and bleeding to ensure equal distribution of the patients. The treating physician receives the risk classification of recurrent VTE and major bleeding risk, and the corresponding treatment duration or outcome of randomisation shortly before the routine 3-month visit and discusses this with the patient.
Patients who are allocated to continue anticoagulant treatment can remain on the same anticoagulant or switch anticoagulants at the discretion of their treating physician. In the Netherlands, DOACs (apixaban, dabigatran, edoxaban and rivaroxaban) as well as VKAs (acenocoumarol and phenprocoumon) and low-molecular-weight heparins are registered for the treatment of VTE. Dose reduction of apixaban or rivaroxaban according to current guidelines after the initial 6 months is allowed, at the discretion of the treating physician. In case the treating physician and/or patient decides to deviate from the treatment duration, the reasons for deviation are registered, and patients will complete follow-up as usual.
Follow-up
All patients (both the randomised and the non-randomised groups) are followed up for at least 2 years. The follow-up starts at the routine 3-month visit after the first VTE, shortly after randomisation, if applicable. During the first 2 years, they will fill in a standardised questionnaire every 3 months, which is sent and processed by the coordinating centre. After the first 2 years of follow-up, patients will fill in a questionnaire once every year for the remaining study duration (ie, as expected until 2027), implying that the total duration of follow-up is expected to vary between two (patients enrolled in 2025) and 6 years (patients enrolled in 2021). Since the follow-up beyond 2 years was not originally planned, but added to the protocol in an amendment which was approved in October 2023, patients enrolled before this time will be asked separately for informed consent for the additional follow-up period.
The follow-up questionnaires are set up to screen for recurrent VTE, (major) bleeding events and other (severe) adverse events. To prevent missing outcome information, we will contact patients by telephone when they do not return the questionnaire. In addition, at the time of inclusion patients provide consent to request information on recurrent VTE and bleeding from their treating physician and general practitioner, which allows us to collect information from them and detect the primary outcomes even if a patient does not respond to the questionnaires.
In case of a reported recurrent VTE or bleeding event, additional information is retrieved from the medical records of the hospital or general practitioner for adjudication. Adverse events related to the study intervention are registered. All severe adverse events, including death and non-elective hospitalisation, are reported to the institutional review board. The questionnaire is also used to evaluate anticoagulant treatment use and remaining symptoms of VTE. Furthermore, we evaluate quality of life by means of the EuroQol 5-dimensional 5-level (EQ-5D-5L) questionnaire.43 Also, functional recovery is assessed using the Post-VTE Functional Scale (PVFS).44 45 In order to perform a cost-effectiveness analysis, we measure healthcare consumption and productivity losses during the first 2 years of follow-up by using Medical Consumption Questionnaire (iMTA MCQ) and Productivity Costs Questionnaire (iMTA PCQ) from the institute for Medical Technology Assessment. All questionnaires are offered digitally (via CastorEDC) or by regular mail as preferred by the participant.
Overall, the study is designed to follow general clinical practice as closely as possible, to optimise generalisability of the results and to lower the burden for the patients.
Outcomes
For the randomised group, the primary outcome is a composite endpoint of recurrent VTE and major bleeding at 2 years. Recurrent VTE is diagnosed after clinical suspicion is objectively confirmed by diagnostic imaging, according to current guidelines.46 47 Bleeding events will be classified as major, clinically relevant non-major (CRNMB) or minor according to the current guidelines of the International Society of Thrombosis and Haemostasis (ISTH): major bleeding is defined as fatal bleeding, symptomatic bleeding in a critical area or organ or bleeding causing a decrease in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells; CRNMB is defined as any bleeding that does not fit the criteria for major bleeding, but does require medical intervention, lead to hospitalisation or increased care level or prompt face to face evaluation.48 49
All clinical outcomes will be evaluated and classified by an independent committee blinded for treatment allocation using discharge letters, radiology reports and other relevant information retrieved from the medical records. In case of a recurrent VTE or (major) bleeding event, patients will be treated according to the local clinical practice, meaning that (dis)continuing anticoagulant treatment at that point is at the discretion of the treating physician.
Secondary outcomes are (1) the combined incidence of recurrent VTE and major bleeding events (primary outcome) weighted by the associated loss of quality-adjusted life years (QALYs) and functional limitations (PFVS) in the randomised group; (2) cost-effectiveness of prolonged anticoagulant treatment compared with discontinuation in the randomised groups; (3) the incidence of recurrent VTE and major bleeding and CRNMB at 2 years and during entire follow-up in in all groups; (4) the predictive performance (discrimination and calibration) of the L-TRRiP and VTE-BLEED model in the arms that discontinue and continue, respectively; and (5) the natural course of recovery from a first acute VTE with regard to long-term functional limitations using the PVFS.
Data collection
Data are collected and stored pseudonymised using the web-based data management platform, CastorEDC.42 Personal information of included participants is securely shared with the coordinating centre for them to send the questionnaires and buccal swab and contact the participants if needed. To optimise data quality, the digital data collection forms include checks for important study variables, such as range checks for continuous variables, check of the assigned risk categories and verification of relevant medical history included in the prediction models by both the study team and the patient (via the baseline questionnaire).
Sample size calculation
The sample size of this study is based on the randomised part of the study. Based on the estimated risks of recurrent VTE and major bleeding as observed in the derivation studies of both prediction models,17 33 we assume an overall 2-year recurrent VTE risk of 10% in the discontinuation arm of the randomised groups and a major bleeding risk of 0.6%. Assuming a reduction of the recurrent VTE risk of 85% by anticoagulant treatment, the recurrent VTE risk of the group that continues anticoagulant treatment will be 1.5%. Furthermore, we estimate this will lead to an increase in the overall risk of major bleeding to 2.1%. To demonstrate a 7% absolute difference in the combined endpoint (ie, 10.6% vs 3.6%) with an alpha of 0.05 and a power of 90%, we need a sample size of 552 subjects for the randomised part of the study. Taking into account a drop-out rate of 10%, we aim to include 608 patients in the randomised part of the study. Based on the derivation studies, we expect the randomised group to form about 38% of the total included population, in which case we expect to include approximately 1600 patients in total; 848 (53%) in the low VTE recurrence risk group and 144 (9%) in the high recurrence and low bleeding risk group.17 33 Of note, these numbers may change depending on the final proportion of the randomised group.
Data analysis plan
Baseline characteristics will be summarised using descriptive statistics (mean, SD or medium, IQR; number, percentage). Furthermore, we will present the number of patients who continued anticoagulant treatment while being allocated to discontinuation and vice versa (cross-over), including the reason for switching anticoagulant treatment. In case of missing data, we will perform multiple imputation if indicated (depending on the amount and nature of the missingness) and pool the results according to Rubin’s rules.50
Randomised group
Following an intention-to-treat analysis, the cumulative incidence of the primary outcome in the randomised group at 2 years will be estimated using the cumulative incidence competing risk method, accounting for the competing risk of death from other causes than VTE or major bleeding. Follow-up will start at the time of the 3-month visit. We will censor patients when they withdraw informed consent, are lost to follow-up or reach the end of the study follow-up period. HRs and corresponding 95% CIs will be estimated using a Cox regression model.
As secondary analyses, we will perform a per-protocol analysis, in which patients who did not receive the allocated treatment during the complete follow-up will be censored at the time of the protocol deviation. In case of a different distribution of risk factors between the treatment groups due to chance, adjusted HRs and 95% CIs will be estimated. The primary outcome (ie, recurrent VTE and major bleeding) will be weighted for the impact on quality of life (EQ-5D) and functional limitations (PFVS) (in two separate analyses) using the difference between the measures taken after and the last one before the event as weights. Furthermore, we will estimate the incidence of recurrent VTE and major bleeding during the entire follow-up, estimate the cumulative incidence of CRNMB and assess repeated events (eg, CRNMB followed by major bleeding) using negative binomial regression.
Healthcare costs will be calculated using Dutch standard prices for economic evaluations.51 52 Absence from work will be valued with friction cost method. QALYs will be assessed using the EQ-5D-5L scores (Dutch tariff53) at different timepoints, using the area-under-the-curve approach. The economic evaluation will consist of a cost-effectiveness analysis, comparing costs per event, as well as a cost-utility analysis, comparing costs per QALY. In net-benefit analysis, costs will be related to effectiveness and presented in a cost-effectiveness acceptability curve.
Non-randomised group
The cumulative incidences of recurrent VTE, major bleeding and CRNMB at 2 years and during the entire follow-up in the non-randomised groups will be calculated, using the same approach as in the randomised groups.
All participants
We will assess the difference in recommended treatment duration as allocated in the study to treatment duration according to the guidelines (ie, continuation in unprovoked and discontinuation in provoked VTE). We will determine the predictive performance of the L-TRRiP model in all patients that discontinued anticoagulant treatment (since the L-TRRiP model is developed to predict the risk of VTE recurrence after discontinuation) by creating a calibration plot containing the observed and predicted 2-year risks of recurrent VTE. Likewise, we will determine the predictive performance of the VTE-BLEED model in all patients who continued anticoagulant treatment, although observed risks will be plotted against the total score as absolute predicted risks are not provided by the model. For the analysis of functional recovery, an ordinal logistic regression model will be used.
Patient and public involvement statement
The L-TRRiP study is investigator initiated. An advisory board, consisting of five patients with a history of VTE, is involved in the practical implementation of the trial, such as patient recruitment and dissemination of study results among patients. In order to make the results of the study accessible to patients, we will publish a Dutch summary.
Ethics and dissemination
The L-TRRiP study will be conducted according to the principles of Good Research Practice and in accordance with the applying Dutch laws (the Medical Research Involving Human Subjects Act (WMO) and General Data Protection Regulation (GDPR)). The protocol is approved by the Medical Research Ethics Committee Leiden-Den Haag-Delft, the Netherlands. Monitoring will be executed by monitors working for the coordinating centre who are independent of the study investigators, to ensure compliance with the protocol, Good Research Practice and legal aspects.
Results are expected in 2028. Our aim is to disseminate the results by publication in peer-reviewed journals, professional societies and through presentations on (inter)national conferences according to publication standards. After data collection and data cleaning are finished, deidentified data will be registered in a repository and be made available for further research on reasonable request to the corresponding author.
Supplementary Material
Acknowledgments
The authors especially acknowledge the participating centres (see online supplemental appendix I) and the Dutch Thrombosis Network.
Footnotes
Twitter: @gjgeersing
Collaborators: L-TRRiP investigators – Participating centres: Yavuz Bilgin; Marleen Goddrie; Pieter Jobse; Suzanne Jong; Saskia Kuipers; Brianne Murphy; Carolien van Netten (Adrz, Goes). Carla Boekholt; Coen van Guldener; Danick Werner (Amphia Ziekenhuis, Breda). Michiel Coppens; Nick van Es (Amsterdam University Medical Centers, Amsterdam). Laura Kratz; Marjolein Kremers; Monique Schilders (Catharina Ziekenhuis, Eindhoven). Gideon Hajer; Bas Langeveld; Saskia Teunisse-de Recht (Deventer Ziekenhuis). Annemiek Bogerd; Ymke Broers; Stan Kolman; Marcel A van de Ree; Sanjay Sankatsing (Diakonessenhuis, Utrecht). Marissa Cloos-van Balen; Ted Koster; Lenneke van Tol (Groene Hart Ziekenhuis, Gouda). Edith Beishuizen; Yvonne Ende-Verhaar; Milou Stals (Haaglanden Medisch Centrum, the Hague). Shantie Bharatsingh; Edith Boersma; Annemarie van der Kraan-Donker; Albert T A Mairuhu; Rick Roos (Haga Teaching Hospital, the Hague). Sabine van Arnhem; Fransien Croon-de Boer; Ad Dees; Matthijs Eefting; J P (Hanneke) van Embden; Roxane Heller; Merel Hoogendorp; Roel Jonkhoff; Roel J J M van de Laar; Corry Leunis-de Ruiter; Patricia Scherpenisse – Klopstra (Ikazia ziekenhuis, Rotterdam). Jan-Willem K van den Berg; Tom L H Stellema; Kim Warink (Isala, Zwolle). M Elske van den Akker - van Marle; Lizanne E van den Akker; J Louise I Burggraaf; Eleonora C Camilleri; Suzanne C Cannegieter; Tess R C Huibregtse; Menno V Huisman; Ingeborg de Jonge; Frederikus A Klok; Ruben Y Kok; Inger N Kunnekes; Saskia le Cessie; Dieuwke Luijten; Lejla Mahic; Hinke C Nagtegaal; Petra J Noordijk; Hülya Öztürk; Alexia M van der Ploeg; Nienke van Rein; Vibeke Schmidt; Anne-Marie Schuitemaker; Vera C Slootweg; Mark J R Smeets; Milou Thibaudier (Leiden University Medical Centre, Leiden). Coty Y Bruggeman; Annette W G van der Velden (Martini Ziekenhuis, Groningen). Marco Dam; Swopkje de Jong; Cees Kroon; Hanneke van der Velde (Nij Smellinghe Ziekenhuis, Drachten). Evertine Abbink; Jenneke Leentjens; Saskia Middeldorp (Radboud University Medical Center, Nijmegen). Carlinda Bresser; Laura M Faber; Fleur Kleijwegt; Tjerk de Nijs; Simone Sissing (Rode Kruis Ziekenhuis, Beverwijk). Soerajja Bhoelan; Tessa Elling; Èmese Heijkoop; Francien Huisman; Mark Lenssen; Anja B U Makelburg; Karina Meijer; Karen H Thedinga; Marja A J Voskuilen; Femke Yspeerd (University Medical Center Groningen, Groningen). Sandra Brookman; Titia Lamberts; Inge Paas; Janneke Swart-Heikens (Wilhelmina Ziekenhuis, Assen). Remy H H Bemelmans; Janneke van den Brink; Wouter K de Jong; Aline van de Vendel (Ziekenhuis Gelderse Vallei, Ede). Independent physician: Marieke J.H. Wermer (Department of neurology, Leiden university Medical Center); Ellis S. van Etten (Department of neurology, Leiden university Medical Center).
Contributors: SCC, MVH, FAK, G-JG and SM designed the study. MEvdA-vM (healthcare economics) and SlC (statistics) contributed to the parts in the protocol on their specific disciplines. JLIB-vD wrote the first manuscript draft, supervised by NvR and SCC. RHHB, JWKvdB, CYB, MC-vB, MC, ME, YE-V, NvE, CvG, WKdJ, FK, TK, CK, SK, JL, DL, ATAM, KM, MAvdR, RR, IS, JSH and AWGvdV are involved in the trial conduct in their affiliations and revised the manuscript. All authors gave final approval of the version to be published.
Funding: The L-TRRiP study is supported by ZonMw (program Goed Gebruik Geneesmiddelen), the Netherlands (grant number: 848017007). The funder did not participate in the design of the study and will have no role in the study conduct, data analysis, interpretation and publication of the data.
Competing interests: MC has received financial support for research from Bayer, CSL Behring, Roche, Novo Nordisk and UniQure and lees for lecturing or consultancy from Alexion, Bayer, CSL Behring, Daiichi Sankyo, Sobi and Viatris, all unrelated to the present work and paid to his institution. NvE has received a lecture fee from Bristol Myers Squibb, which was unrelated to this work and paid to his institution. JL reports grants or contracts from BMS-Pfizer, Viatris, AstraZeneca and Synapse, all unrelated to this work and paid to her institution. KM reports speaker fees from Alexion, Bayer and CSL Behring, participation in trial steering committees for Bayer and AstraZeneca, consulting fees from Uniqure, participation in data monitoring and endpoint adjudication committee for Octapharma. All payments are made to her institution. SM reports grants and personal fees from Daiichi-Sankyo, Bayer, Pfizer and Boehringer-Ingelheim, personal fees from Portola/Alexion, AbbVie, Pfizer/Bristol-Meyers Squibb, Norgine, Viatris and Sanofi, all paid to her institution and outside the submitted work. MVH reports grants from Dutch Heart Foundation, Netherlands Organisation for Health Research and Development, Bayer Health Care, Pfizer-BMS Leo Pharma Boehringer-Ingelheim, all outside this work. FAK reports grants or contracts from Bayer, BMS, BSCI, MSD, Leo Pharma, Actelion, Farm-X, The Netherlands Organisation for Health Research and Development, the Dutch Thrombosis Association, The Dutch Heart Foundation and the Horizon Europe Program, all unrelated to this work and paid to his institution. All others report no conflicts of interest related to this project.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: on behalf of the L-TRRiP investigators, Yavuz Bilgin, Marleen Goddrie, Pieter Jobse, Suzanne Jong, Saskia Kuipers, Brianne Murphy, Carolien van Netten, Carla Boekholt, Coen van Guldener, Danick Werner, Michiel Coppens, Nick van Es, Laura Kratz, Marjolein Kremers, Monique Schilders, Gideon Hajer, Bas Langeveld, Saskia Teunisse-de Recht, Annemiek Bogerd, Ymke Broers, Stan Kolman, Marcel A van de Ree, Sanjay Sankatsing, Marissa Cloos-van Balen, Ted Koster, Lenneke van Tol, Edith Beishuizen, Yvonne Ende-Verhaar, Milou A M Stals, Shantie Bharatsingh, Edith Boersma, Annemarie van der Kraan-Donker, Albert T A Mairuhu, Rick Roos, Sabine van Arnhem, Fransien Croon-de Boer, Ad Dees, Matthijs Eefting, J P (Hanneke) van Embden, Roxane Heller, Merel Hoogendorp, Roel Jonkhoff, Roel J J M van de Laar, Corry Leunis-de Ruiter, Patricia Scherpenisse-Klopstra, Jan-Willem K van den Berg, Tom L H Stellema, Kim Warink, M Elske van den Akker-van Marle, Lizanne E van den Akker, J Louise I Burggraaf-van Delft, Eleonora C Camilleri, Suzanne C Cannegieter, Tess R C Huibregtse, Menno V Huisman, Ingeborg de Jonge, Frederikus A Klok, Ruben Y Kok, Inger N Kunnekes, Saskia le Cessie, Dieuwke Luijten, Lejla Mahic, Hinke C Nagtegaal, Petra J Noordijk, Hülya Oztürk, Alexia M van der Ploeg, Nienke van Rein, Vibeke Schmidt, Anne-Marie Schuitemaker, Vera C Slootweg, Mark J R Smeets, Milou Thibaudier, Coty Y Bruggeman, Annette W G van der Velden, Marco Dam, Swopkje de Jong, Cees Kroon, Hanneke van der Velde, Evertine Abbink, Jenneke Leentjens, Saskia Middeldorp, Carlinda Bresser, Laura M Faber, Fleur Kleijwegt, Tjerk de Nijs, Simone Sissing, Soerajja Bhoelan, Tessa Elling, Èmese Heijkoop, Francien Huisman, Mark Lenssen, Anja B U Makelburg, Karina Meijer, Karen H Thedinga, Marja A J Voskuilen, Femke Yspeerd, Sandra Brookman, Titia Lamberts, Inge Paas, Janneke Swart Heikens, Remy H H Bemelmans, Janneke van den Brink, Wouter K de Jong, Aline van de Vendel, Marieke J.H. Wermer, and Ellis S. van Etten
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Khan F, Rahman A, Carrier M, et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ 2019;366:l4363. 10.1136/bmj.l4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010;170:1710–6. 10.1001/archinternmed.2010.367 [DOI] [PubMed] [Google Scholar]
- 3. Weitz JI, Prandoni P, Verhamme P. Anticoagulation for patients with venous thromboembolism: when is extended treatment required TH Open 2020;4:e446–56. 10.1055/s-0040-1721735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrier M, Le Gal G, Wells PS, et al. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 2010;152:578–89. 10.7326/0003-4819-152-9-201005040-00008 [DOI] [PubMed] [Google Scholar]
- 5. Kahn SR. Determinants and time course of the Postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698. 10.7326/0003-4819-149-10-200811180-00004 [DOI] [PubMed] [Google Scholar]
- 6. Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017;49:1601792. 10.1183/13993003.01792-2016 [DOI] [PubMed] [Google Scholar]
- 7. Middeldorp S, Prins MH, Hutten BA. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev 2014;2014:CD001367. 10.1002/14651858.CD001367.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan F, Tritschler T, Kimpton M, et al. Long-term risk of recurrent venous thromboembolism among patients receiving extended oral anticoagulant therapy for first unprovoked venous thromboembolism.. J Thromb Haemost 2021;19:2801–13. 10.1111/jth.15491 [DOI] [PubMed] [Google Scholar]
- 9. Khan F, Tritschler T, Kimpton M, et al. Long-term risk for major bleeding during extended oral anticoagulant therapy for first unprovoked venous thromboembolism: A systematic review and meta-analysis. Ann Intern Med 2021;174:1420–9. 10.7326/M21-1094 [DOI] [PubMed] [Google Scholar]
- 10. Baglin T, Bauer K, Douketis J, et al. Duration of anticoagulant therapy after a first episode of an unprovoked pulmonary Embolus or deep vein thrombosis: guidance from the SSC of the ISTH. J Thromb Haemost 2012;10:698–702. 10.1111/j.1538-7836.2012.04662.x [DOI] [PubMed] [Google Scholar]
- 11. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 12. NICE . Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. London: National Institue for Health and Care Excellence, 2020. [Google Scholar]
- 13. Konstantinides SV, Meyer G, Becattini C, et al. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J 2020;41:543–603. 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 14. Ortel TL, Neumann I, Ageno W, et al. American society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020;4:4693–738. 10.1182/bloodadvances.2020001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest 2021;160:e545–608. 10.1016/j.chest.2021.07.055 [DOI] [PubMed] [Google Scholar]
- 16. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 2016;14:1480–3. 10.1111/jth.13336 [DOI] [PubMed] [Google Scholar]
- 17. Timp JF, Braekkan SK, Lijfering WM, et al. Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: the Leiden thrombosis recurrence risk prediction model (L-Trrip). PLoS Med 2019;16:e1002883. 10.1371/journal.pmed.1002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. den Exter PL, Woller SC, Robert‐Ebadi H, et al. Management of bleeding risk in patients who receive anticoagulant therapy for venous thromboembolism: communication from the ISTH SSC subcommittee on predictive and diagnostic variables in thrombotic disease. J Thromb Haemost 2022;20:1910–9. 10.1111/jth.15776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Jong CMM, Rosovsky RP, Klok FA. Outcomes of venous thromboembolism care: future directions. J Thromb Haemost 2023;21:1082–9.:S1538-7836(23)00163-0. 10.1016/j.jtha.2023.02.015 [DOI] [PubMed] [Google Scholar]
- 20. de Winter MA, van Es N, Büller HR, et al. Prediction models for recurrence and bleeding in patients with venous thromboembolism: A systematic review and critical appraisal. Thromb Res 2021;199:85–96.:S0049-3848(21)00008-6. 10.1016/j.thromres.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 21. Burggraaf JLI, van Rein N, Klok FA, et al. How to predict recurrent venous thromboembolism and bleeding? A review of recent advances and their implications. Pol Arch Intern Med 2023;133:16492. 10.20452/pamw.16492 [DOI] [PubMed] [Google Scholar]
- 22. Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. Canadian Medical Association Journal 2008;179:417–26. 10.1503/cmaj.080493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010;121:1630–6. 10.1161/CIRCULATIONAHA.109.925214 [DOI] [PubMed] [Google Scholar]
- 24. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25. 10.1111/j.1538-7836.2012.04735.x [DOI] [PubMed] [Google Scholar]
- 25. Franco Moreno AI, García Navarro MJ, Ortiz Sánchez J, et al. A risk score for prediction of recurrence in patients with unprovoked venous thromboembolism (DAMOVES). Eur J Intern Med 2016;29:59–64.:S0953-6205(15)00438-0. 10.1016/j.ejim.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 26. Ensor J, Riley RD, Jowett S, et al. Prediction of risk of recurrence of venous thromboembolism following treatment for a first unprovoked venous thromboembolism: systematic review, Prognostic model and clinical decision rule, and economic evaluation. Health Technol Assess 2016;20:i–xxxiii, 10.3310/hta20120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang W, Goldberg RJ, Anderson FA, et al. Occurrence and predictors of recurrence after a first episode of acute venous thromboembolism: population-based Worcester venous thromboembolism study. J Thromb Thrombolysis 2016;41:525–38. 10.1007/s11239-015-1301-8 [DOI] [PubMed] [Google Scholar]
- 28. Geersing G-J, Hendriksen JMT, Zuithoff NPA, et al. Effect of Tailoring anticoagulant treatment duration by applying a recurrence risk prediction model in patients with venous thromboembolism compared to usual care: A randomized controlled trial. PLoS Med 2020;17:e1003142. 10.1371/journal.pmed.1003142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 2010;138:1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 30. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of atrial fibrillation (NRAF). Am Heart J 2006;151:713–9. 10.1016/j.ahj.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 31. Kuijer PM, Hutten BA, Prins MH, et al. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med 1999;159:457–60. 10.1001/archinte.159.5.457 [DOI] [PubMed] [Google Scholar]
- 32. Ruíz-Giménez N, Suárez C, González R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Thromb Haemost 2008;100:26–31. 10.1160/TH08-03-0193 [DOI] [PubMed] [Google Scholar]
- 33. Klok FA, Hösel V, Clemens A, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J 2016;48:1369–76. 10.1183/13993003.00280-2016 [DOI] [PubMed] [Google Scholar]
- 34. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th Ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e419S–e496S. 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klok FA, Barco S, Konstantinides SV. External validation of the VTE-BLEED score for predicting major bleeding in stable Anticoagulated patients with venous thromboembolism. Thromb Haemost 2017;117:1164–70. 10.1160/TH16-10-0810 [DOI] [PubMed] [Google Scholar]
- 36. Klok FA, Barco S, Turpie AGG, et al. Predictive value of venous thromboembolism (VTE)-BLEED to predict major bleeding and other adverse events in a practice-based cohort of patients with VTE: results of the XALIA study. Br J Haematol 2018;183:457–65. 10.1111/bjh.15533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klok FA, Huisman MV. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood 2020;135:724–34. 10.1182/blood.2019001605 [DOI] [PubMed] [Google Scholar]
- 38. Nishimoto Y, Yamashita Y, Morimoto T, et al. Validation of the VTE-BLEED score’s long-term performance for major bleeding in patients with venous Thromboembolisms: from the COMMAND VTE Registry. J Thromb Haemost 2020;18:624–32. 10.1111/jth.14691 [DOI] [PubMed] [Google Scholar]
- 39. Palareti G, Cosmi B, Legnani C, et al. D-Dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9. 10.1056/NEJMoa054444 [DOI] [PubMed] [Google Scholar]
- 40. Kearon C, Spencer FA, O’Keeffe D, et al. D-Dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy: a cohort study. Ann Intern Med 2015;162:27–34. 10.7326/M14-1275 [DOI] [PubMed] [Google Scholar]
- 41. Rodger MA, Le Gal G, Anderson DR, et al. Validating the Herdoo2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017;356:j1065. 10.1136/bmj.j1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castor EDC . Castor electronic data capture. 2019. Available: https://castoredc.com [Accessed 28 Aug 2019].
- 43. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). quality of life research: an international Journal of quality of life aspects of treatment. Care Rehabilitation 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boon GJAM, Barco S, Bertoletti L, et al. Measuring functional limitations after venous thromboembolism: optimization of the post-VTE functional status (PVFS) scale. Thromb Res 2020;190:45–51.:S0049-3848(20)30102-X. 10.1016/j.thromres.2020.03.020 [DOI] [PubMed] [Google Scholar]
- 45. Gwozdz AM, de Jong CMM, Fialho LS, et al. Development of an international standard set of outcome measures for patients with venous thromboembolism: an international consortium for health outcomes measurement consensus recommendation. Lancet Haematol 2022;9:e698–706.:S2352-3026(22)00215-0. 10.1016/S2352-3026(22)00215-0 [DOI] [PubMed] [Google Scholar]
- 46. Diagnostiek, Preventie en Behandeling Van Veneuze Trombo-Embolie en Secundaire Preventie Van Arteriële Trombose: Kwaliteitsinstituut Voor de Gezondheidszorg CBO. 2008.
- 47. Richtlijn Antitrombotisch Beleid - Continueren Antistolling NA acute Veneuze Tromboembolie: Federatie Medisch Specialisten. Available: https://richtlijnendatabase.nl/richtlijn/antitrombotisch_beleid/therapie_vte/continueren_antistolling_na_acute_veneuze_tromboembolie.html [Accessed 2 Nov 2022].
- 48. Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119–26. 10.1111/jth.13140 [DOI] [PubMed] [Google Scholar]
- 49. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of Antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 50. Rubin DB. Multiple imputation for Nonresponse in surveys. New York: Wiley J & Sons, 9 June 1987. 10.1002/9780470316696 [DOI] [Google Scholar]
- 51. Hakkaart-van Roijen L, van der Linden N, Bouwmans CAM, et al. Costing manual: methodology of costing research and reference prices for economic evaluations in Healthcare [in Dutch: Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg]. 2015.
- 52. Kanters TA, Bouwmans CAM, van der Linden N, et al. Update of the Dutch manual for costing studies in health care. PLoS One 2017;12:e0187477. 10.1371/journal.pone.0187477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Versteegh MM, Vermeulen KM, Evers SMAA, et al. Dutch tariff for the five-level version of EQ-5D. Value Health 2016;19:343–52. 10.1016/j.jval.2016.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078676supp001.pdf (171KB, pdf)