Abstract
The safety and efficacy of both cell and gene therapies have been demonstrated in numerous preclinical and clinical trials. Chimeric antigen receptor T (CAR-T) cell therapy, which leverages the technologies of both cell and gene therapies, has also shown great promise for treating various cancers. Advancements in pertinent fields have also highlighted challenges faced while manufacturing cell and gene therapy products. Potential problems and obstacles must be addressed to ease the clinical translation of individual therapies. Literature reviews of representative cell-based, gene-based, and cell-based gene therapies with regard to their general manufacturing processes, the challenges faced during manufacturing, and QC specifications are limited. We review the general manufacturing processes of cell and gene therapies, including those involving mesenchymal stem cells, viral vectors, and CAR-T cells. The complexities associated with the manufacturing processes and subsequent QC/validation processes may present challenges that could impede the clinical progression of the products. This article addresses these potential challenges. Further, we discuss the use of the manufacturing model and its impact on cell and gene therapy.
Keywords: Cell therapy, Chimeric antigen receptor T, Clinical translation, Gene therapy, Manufacturing, Mesenchymal stem cells, Viral vectors
INTRODUCTION
In recent years, the demand for cell and gene therapy (CGT) has surged as the number of United States Food and Drug Administration (U.S. FDA)-approved CGT products has increased annually [1]. This increasing demand underscores the pressing need for manufacturers to improve and advance their production processes to keep pace with the rapidly evolving CGT field. The entire CGT manufacturing process must occur in a facility that complies with good manufacturing practice (GMP) regulations and has received full authorization to manufacture CGTs or advanced-therapy medicinal products [2], as they are referred to in Europe, for human use. Numerous clinical trials have been conducted to assess the safety and efficacy of CGTs for a wide range of clinical indications, including cancer [3-5]. CGT includes the injection of cells, genes, or gene-modified cells into a patient with the purpose of preventing or treating a disease. Cell therapy involves the injection of cells to replace or aid the repair of damaged cells, whereas gene therapy involves the injection of genes into cells to alter the genetic makeup of the recipient cells [1, 6, 7]. Cell-based gene therapies, such as chimeric antigen receptor T (CAR-T) therapy, represent combinations of both approaches.
According to a recent survey of CGT investors conducted by Bloomberg Intelligence and the Commercialization Committee of the International Society for Cell & Gene Therapy, clinically significant data are the number one consideration for investors in the CGT field [8]. Maintaining the efficacy of a cell and/or gene therapy product during the manufacturing process is a key factor that can influence overall clinical outcomes. Challenges faced when manufacturing therapeutic products must be addressed to ensure their efficacious clinical translation. In this review, we provide a general overview of representative CGTs, namely mesenchymal stem cells (MSCs) for cell therapy, lentiviral vectors (LVs) and adeno-associated viral vectors (AAVs) for gene therapy, and CAR-T cells for cell-based gene therapy. We also summarize the general CGT manufacturing processes and highlight challenges encountered during manufacturing that may potentially hinder the clinical use of the respective CGTs.
MSC-BASED CELL THERAPIES
Various cell types, including T cells, stem cells, dendritic cells, and natural killer (NK) cells, have been investigated in clinical trials involving MSC-based cell therapies [9]. Cancer is the main indication for leukocyte (T cells, dendritic cells, and NK cells) therapy. Following leukocytes, the second most commonly used type of cell therapy is stem cell-based therapy, which involves hematopoietic stem cells (HSCs) or MSCs [10]. Although numerous autologous stem cell transplantations have been performed for MSC therapy, allogeneic cells have been predominantly used for that purpose [11, 12]. MSCs are readily available and can be acquired from various sources, including the bone marrow, adipose tissue, and umbilical cord tissue [13, 14]. The mechanism of action of MSCs has been attributed to paracrine activity rather than the direct replacement of damaged cells [15]. Paracrine activity refers to the ability of MSCs to secrete paracrine factors into the microenvironment [16].
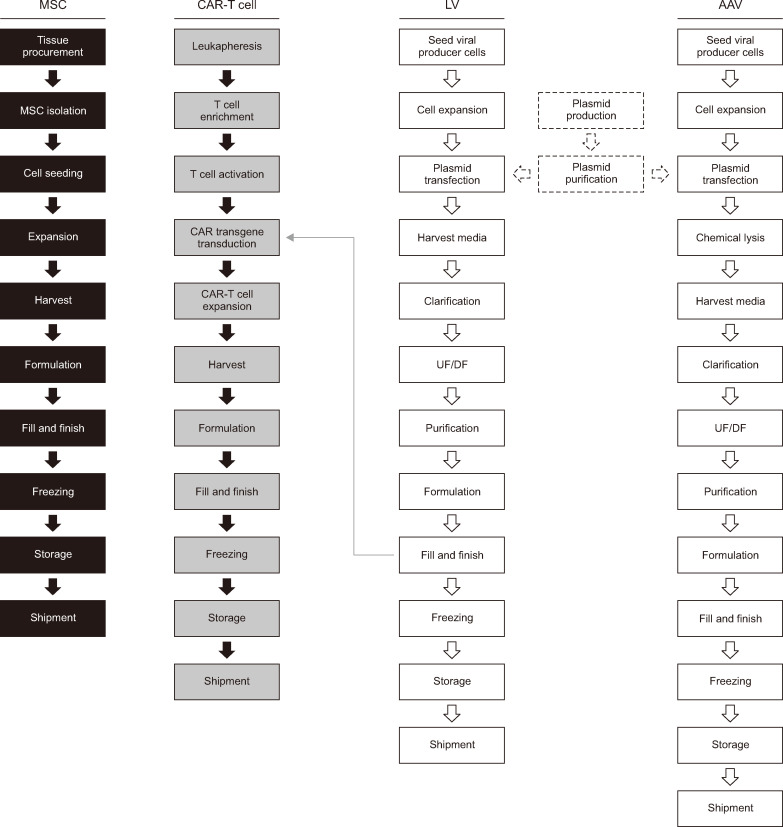
The upstream MSC manufacturing process (Fig. 1) starts with tissue procurement, followed by MSC isolation from the tissue source. Different techniques are employed to isolate MSCs from tissues, depending on the tissue source [14]. Cells are cultured in a conventional culture medium that usually contains supplements, such as fetal bovine serum (FBS). The MSCs are then expanded on different scales, ranging from multilayer flasks to bioreactors [17, 18]. The duration of cultivation and number of passages differ among manufacturers and institutions. The identity, potency, and safety of the cells are confirmed via QC analysis. The minimum criteria for characterizing MSCs include (but are not limited to) their identity, sterility, viability, purity, potency, and efficacy [19]. To prepare and distribute cells for injection, they are harvested after expansion and subjected to the following steps: formulation, fill and finish, freezing, storage, and shipment.
Fig. 1.
Cell and gene therapy manufacturing processes. Black boxes represent the flow diagram for MSC-based cell therapy. Gray boxes denote the flow diagram for CAR-T cell-based gene therapy. The process flows for LV- and AAV-based gene therapies are indicated in white boxes with solid lines. Plasmid production and purification are indicated in white boxes with dashed lines.
Abbreviations: MSC, mesenchymal stem cell; CAR-T, chimeric antigen receptor T; LV, lentiviral vector; AAV, adeno-associated virus; DF, diafiltration; UF, ultrafiltration.
Common challenges faced in MSC manufacturing are presented in Table 1. To achieve clinical doses, MSCs must be expanded extensively. However, during expansion, variabilities may arise depending on the donor, the condition/sterility of the tissue source or starting material, the isolation technique, and the cultivation method. Such factors influence MSC heterogeneity and potency. Sequential passaging and long-term culture of MSCs may also affect their potency, specifically their proliferation capabilities [20]. Prolonging the MSC culture duration implies the use of more culture mediums and supplements, such as FBS, raising the overall manufacturing cost. Moreover, increased exposure to FBS can amplify pre-existing safety concerns, including adverse immunological reactions elicited in recipients and the high batch-to-batch variability of FBS [17, 18]. To ensure the advancement of MSC-based cell therapies in clinical settings, it is critical to employ methods that enable rapid, large-scale MSC expansion, balancing the therapeutic potency of the cells with acceptable manufacturing expenses.
Table 1.
Challenges faced during CGT product manufacturing that may affect their clinical use
| Type of therapy | Type of cell or vector | Points to consider during the manufacturing process |
|---|---|---|
| Cell-based therapy | MSCs |
Quality of the tissue source (starting material) Duration of cell cultivation Presence of residual xenogeneic serum |
| Gene-based therapy | AAV | Separation of empty from full AAV capsids |
| LV |
Presence of residual impurities (including xenogeneic serum) Optimization of transfection conditions Development of (a) stable producer cell line(s) |
|
| Cell-based gene therapy | CAR-T cells |
Quality of leukapheresis material Impurities Complex, multistep procedures |
Abbreviations: CGT, cell and gene therapy; MSCs, mesenchymal stem cells; AAV, adeno-associated virus; LV, lentiviral vector; CAR-T, chimeric antigen receptor T.
AAV AND LV-BASED GENE THERAPIES
AAVs and LVs are important viral vectors used for gene therapies [21]. Currently, FDA-approved, commercially available AAV-based therapies target diseases such as retinal dystrophy and spinal atrophy [22], whereas LV-based gene therapies target diseases such as B-cell lymphoma, β-thalassemia, and cerebral adrenoleukodystrophy [23]. Both vectors package genes and enable direct administration of the gene of interest to patients [24]. A major difference between the two types of vectors is that a single DNA strand is packaged in AAVs, whereas an RNA genome is packaged in LVs. The advantages of AAVs include low immunogenicity, high delivery and efficiency, and long-term expression. The advantages of LVs include long-term transgene expression and the ability to transduce nondividing cells [25]. LVs permanently integrate into the host genome. Although the overall frequency is low, genomic integration is also possible for AAVs [26, 27].
A plasmid-based approach is commonly used to manufacture AAVs. The upstream AAV manufacturing process (Fig. 1) [28] starts with large-scale plasmid production and purification. The following plasmids must be prepared for transfection: (1) a cis-acting plasmid that carries the therapeutic gene of interest, (2) a trans-acting plasmid containing protein-coding genes necessary for AAV replication and capsid formation, and (3) a trans-acting plasmid that enables AAV replication in the host cells [29]. All three plasmids are transfected into an AAV-producing cell line, such as human embryonic kidney 293 (HEK293) cells, to produce viral vectors. Cell stocks stored in a cell bank are thawed and cultured before large-scale expansion (growth in single-use bioreactors). Following chemical lysis, which involves the disruption of cells to release AAVs into the culture medium, nuclease treatment is performed to purify the vectors by selectively degrading DNA impurities. Subsequently, the harvested viral supernatant undergoes a purification process comprising centrifugation, chromatography, and ultrafiltration (UF) and diafiltration (DF) to remove cellular debris and impurities. The impurities consist of plasmid DNA, proteins and DNA from AAV-producing cells, and empty capsids.
QC assays must be performed to assess the identity, potency, and safety of the viral vectors. Examples of QC tests include the detection of viral contamination, residual DNA, and residual proteins, as well as the measurement of the vector genome concentration [30]. The final manufacturing steps include formulation followed by fill and finish, freezing, storage, and shipment. Processing, filtration, and/or centrifugation methods are implemented to remove impurities from the viral supernatant. The final formulation of LVs is achieved during the final fill-and-finish step. As for AAVs, QC requirements have been established for LVs and include tests such as assessing their physical and functional titers and detecting and removing impurities [31].
The upstream LV manufacturing process (Fig. 1) [32] starts with the culture and large-scale expansion (i.e., in a bioreactor) of an LV-producer cell line, such as HEK293T, a derivative of the HEK293 cell line that expresses the SV40 T-antigen. SV40 T-antigen expression prevents innate immune response activation. HEK293T cells can also be used to produce LVs at high titers [33]. However, the presence of the SV40 T-antigen raises safety concerns regarding clinical manufacturing [34]. Therefore, LV-producing cells that do not express the SV40 T-antigen are being developed for clinical purposes. Furthermore, because of concerns regarding the use of xenogeneic serum (FBS) and the removal of residual serum during purification, well-established methods have been implemented to culture LVs in serum-free medium that yields titers comparable to those obtained with serum-supplemented culture medium [33]. Plasmids encoding the transgene of interest and the vesicular stomatitis virus G protein, envelope, gag, and rev genes can be transiently expressed in HEK293T cells using a transfection reagent. Usually, the cells are co-transfected with three to four plasmids. Unlike AAVs, most LV vectors are released from the cells, and the culture supernatant is used for the downstream process [32].
Although commonly used, transient expression may not be ideally suited for large-scale manufacturing of AAVs and LVs because of scalability issues. Large amounts of transfection reagents and genetic material (DNA) are required to scale up transient expression systems, which leads to increased production costs and, potentially, batch-to-batch variability [35]. An alternative option is to establish and use stable producer cell lines. Stable producer cell line production requires the use of helper viruses, such as an adenovirus [36]. However, it does not require plasmid DNA because the genes required for viral vector production are already integrated into the cell lines. Using stable cell lines results in fewer empty capsids and better vector quality than transient expression [37]. Using stable producer cell lines can also reduce manufacturing costs and maximize the titers of both AAVs and LVs [36, 38]. Therefore, employing stable producer cell lines can help overcome challenges currently faced in gene therapy, such as a low product yield.
Challenges faced in AAV and LV manufacturing are presented in Table 1. Purification is a major hurdle for both AAVs and LVs. The purity of AAVs and LVs varies depending on the vector-biomanufacturing process, and residual impurities may affect the final product yield. Developing an effective, scalable purification method is crucial to reduce the overall cost of GMP-compliant production and increase vector production [39]. Empty-full separation (i.e., the separation of empty and partial capsids from full AAV capsids) is another challenge that may affect the clinical use of AAV-based therapies. Contamination with empty AAV capsids may generate immunological reactions in the host, such as enhanced T-cell proliferation [40]. Transfection conditions must be optimized to maximize AAV and LV production. Although the use of stable producer cell lines may serve clinically beneficial purposes, several points must be considered during manufacturing to facilitate clinical translation, such as contamination with helper viruses or cytotoxicity induced by vector components required for AAV production [41]. The purification process is another critical step in LV manufacturing because impurities (such as residual DNA, HEK293T cell proteins, medium components, and transfection reagents) can generate unwanted inflammatory responses. The inherently unstable nature of LVs [42] is a major challenge during production, in which environmental factors can influence and abrogate LV functionality [43].
CAR-T CELL-BASED GENE THERAPIES
Both cell and gene manufacturing are required for the production of CAR-T cells (Fig. 1). Predominantly used in cancer immunotherapy as a treatment option for B-cell lymphoma and leukemia, CAR-T cell therapy involves genetically modifying T cells ex vivo, which enables cancer-cell targeting and elimination after CAR-T cells are infused into the patient. T cells can be genetically engineered to recognize B-cell maturation antigen (BCMA) [44] or B-cell surface antigens, such as CD19 and CD20 [45, 46]. To evade on-target toxicity in normal cells, CAR-T cell targets should be highly selective [47].
CAR-T cell manufacturing [48, 49] starts with leukapheresis, i.e., the collection of leukocytes from the blood of the patient (autologous) or a healthy donor (allogeneic). The leukapheresis material is then processed to enrich and isolate T cells. Viral vectors, usually LVs or retroviral vectors, must be prepared along with the T cells to transduce the latter with the CAR transgene. With respect to clinical applications, LVs are attractive candidates because they can transduce slowly proliferating or non-proliferating cells more efficiently than other vectors [50]. Antigen-presenting cells (such as dendritic cells) are used to activate T cells in vivo, facilitating their stimulation for proliferation and differentiation [48]. For the purposes of clinical manufacturing, the most common approach to activate T cells involves the use of a monoclonal antibody (such as an anti-CD3 antibody) and an interleukin (such as interleukin-2) [48]. Activated T cells are transduced with the CAR transgene via LVs and expanded in culture vessels. The use of serum in the culture medium must be contemplated. Previous reports have shown that serum can affect T-cell functionality [51] and that using serum-free medium enhances the killing potential of both T cells and CAR-T cells in vitro and in vivo [52]. After formulation and filling, the final steps of CAR-T cell manufacturing include freezing, storage, and shipment.
The quality of the starting leukapheresis material must be scrutinized to increase the efficacy and potency of CAR-T cell therapy during the clinical stage. For CAR-T cell therapy, the purity and potency of both the isolated T cells and the LVs must be considered. The technique used to select and isolate T cells must be optimized to ensure that a pure T cell population is used as the starting material. Contaminating monocytes remaining in the starting material disrupt T-cell activation and transduction, potentially resulting in poor T-cell quality and CAR-T cell manufacturing failure [53]. In addition to confirming the purity of the T cells, QC requirements for CAR-T cells include examining cell-surface CAR expression and sterility testing (i.e., potency assays), which must be conducted quickly as CAR-T cells are generally infused shortly after production.
The time between T-cell collection and CAR-T cell infusion (i.e., the vein-to-vein time) must be considered during manufacturing. Vein-to-vein time optimization is crucial for patients in need of urgent treatment. The vein-to-vein time, including the time required for manufacturing and quality assessment, reportedly is three to five weeks [54]. Concerns of deterioration of the patient’s condition during the interim and adverse disease progression are always present. CAR-T cell manufacturing is shifting toward the use of a closed, automated, single-use GMP-compliant system that can yield a CAR-T cell product within two weeks [55, 56]. This advancement will provide a standardized method for producing CAR-T cells and eliminate the influence of manual handling or manipulations on the overall quality of the manufactured CAR-T cell product.
QC TESTING
As mentioned earlier, the overall manufacturing process for CGTs is arduous and complicated. Performing proper in-process QC and validation tests is of utmost importance for translating the respective therapeutic products into clinical use. Several attributes, such as identity, purity, potency, and safety (sterility and the presence of adventitious agents, endotoxins, or mycoplasma), are commonly examined for different CGTs. Table 2 lists various tests and measurement methods used to assess quality attributes [19,30,31,57,58]. Considering the importance of time for CAR-T cell therapies, efficient and rapid QC tests should be implemented in manufacturing. Nucleic acid amplification tests that can detect mycoplasma infections more rapidly than conventional methods are currently available. Demonstrating that a viral vector is not contaminated with replication-competent vectors (RCVs) is essential for related therapies because RCVs may affect the behavior of the viral vector [59].
Table 2.
Measurement of QC specifications
| Quality attribute | Measurement methods for CGT therapies | ||
|---|---|---|---|
| Cell-based (i.e., MSCs) [19] | Gene-based (i.e., AAV, LV) [30, 31] | Cell-based gene (i.e., CAR-T cell) [57, 58] | |
| Identity |
Morphology (microscopy) Immunophenotyping (FACS) Differentiation potential (respective staining method) |
Viral protein (ELISA) Vector genome copies (PCR) Specific viral proteins (western blot) or sequences (sequencing) |
% of T cells % of CAR-expressing T cells (FACS) |
| Viability | Cell counting (i.e., acridine orange, propidium iodide, or trypan blue) | NA | Cell counting (i.e., acridine orange, propidium iodide, or trypan blue) |
| Purity | Residual contaminants, such as FBS (ELISA) | Residual contaminants, such as host cell proteins or DNA (ELISA, qPCR) |
% of other immune cells (FACS) Residual contaminants, such as beads and feeder cells (FACS) |
| Potency | Secretion of paracrine factors (ELISA) |
Titer (qPCR) In vitro functional assay |
In vitro assay (i.e., cytotoxic T lymphocyte assay) |
| Endotoxin | Bacterial endotoxin test (i.e., LAL) | LAL | LAL |
| Adventitious agents | Adventitious assay (in vitro and in vivo) | Adventitious assay (in vitro and in vivo) | Adventitious assay (in vitro and in vivo) |
| Mycoplasma | Test for mycoplasma (NAT) | Test for mycoplasma | Test for mycoplasma (NAT) |
| Sterility | Sterility testing (bacteria and fungi) | Sterility testing (bacteria and fungi) | Sterility testing (bacteria and fungi) |
| Replication competence | NA | RCV assay | RCV assay |
Each manufacturer has specifications that must be met to release a finished product. Publicly disclosed lot-release specifications for four representative FDA-approved CAR-T cell products, namely Kymriah [60], Yescarta [61], Tecartus [62], and Carvykti [63], are presented in Table 3. Kymriah, Yescarta, and Tecartus target CD19, whereas Carvykti targets BCMA. Although Kymriah targets CD19 and Carvykti targets BCMA, the presence of CARs is verified for both products to confirm their identity. Potency testing for Kymriah focuses on interferon-gamma release, whereas Carvykti testing focuses on CAR expression in viable T cells, along with a non-disclosed component. Although Kymriah, Yescarta, and Tecartus all target CD19, different methods are used to evaluate their quality and safety before the final product release. Major distinctions exist regarding purity assessments. Purity specifications for Tecartus are unavailable, preventing a direct comparison with the other CD19-targeting CAR-T cell therapies. However, the evaluation methods used for Kymriah and Yescarta differ markedly. Gentamicin, endotoxin, and a non-disclosed reagent are assessed when testing the purity of Yescarta, whereas the percentage of viable T cells or CD19-positive B cells is quantified for Kymriah.
Table 3.
Lot-release specifications of four FDA-approved CAR-T therapies
| Quality attribute | Measurement methods | |||
|---|---|---|---|---|
| Kymriah [60] | Yescarta [61] | Tecartus [62] | Carvykti [63] | |
| Identity | Presence of CAR signal (qPCR) | Linker and CD28 sequences (scFv heavy-chain variable region) | Linker and CD28 sequences (scFv heavy-chain variable region) | Presence of CAR signal |
| Potency | Detection of CAR expression (FACS) | Detection of CAR expression | Detection of CAR expression | Detection of CAR expression |
| Detection of IFN-γ release | Detection of cell viability | Detection of cell viability | ||
| Not disclosed | Not disclosed | Not disclosed | ||
| Purity | % of viable T cells | Endotoxin | Not available | Not disclosed |
| Residual beads | Gentamicin | % of NK cells | ||
| % of viable CD19+ B cells | Not disclosed | Detection of viability | ||
| Safety | Mycoplasma | Mycoplasma | Mycoplasma | Mycoplasma |
| Sterility | Sterility | Sterility | Sterility | |
| Bacterial endotoxin | RCR | Endotoxin | Endotoxin | |
| VSV-G DNA (qPCR) | Not disclosed | RCL | ||
| Not disclosed | ||||
Abbreviations: FDA, Food and Drug Administration; CAR-T, chimeric antigen receptor T; CAR, chimeric antigen receptor; qPCR, quantitative PCR; scFv, single-chain variable fragment; FACS, fluorescence-activated cell sorting; NK, natural killer; VSV-G, vesicular stomatitis virus glycoprotein G; RCR, replication-competent retrovirus; RCL, replication-competent lentivirus.
The variable methods used to evaluate the quality and safety of different FDA-approved CAR-T cell therapies underscore the necessity for a standardized testing approach. The U.S. FDA’s draft guidance for the industry on developing CAR-T cell therapies [64] offers potentially valuable insights into assay development and evaluation methods for critical quality attributes, including identity and potency. Regarding identity, the recommendations advocate using flow cytometry or PCR to detect the transgene. The document suggests employing cell-surface markers to observe the cellular composition of the final products and emphasizes testing the potency of both vectors and CAR-T cells [64]. It also strongly recommends using a matrix approach involving various assays, such as cell-killing assays, cytokine-secretion assays, and transduction-efficiency measurements to confirm the potency.
FUTURE PERSPECTIVES
Determining whether centralized or decentralized manufacturing is a more appropriate manufacturing model is a subject of debate in the CGT field [65-67]. Centralized manufacturing involves the manufacturing of products at a centralized GMP facility and the distribution of the products to point-of-care locations. With decentralized manufacturing, local production is possible when the GMP facility is close to the point-of-care location, which substantially reduces the time required to deliver the products. Applying the decentralized model to cell-based therapies, such as CAR-T cell therapy, will be advantageous, considering that rapid product delivery to patients with cancer is essential. Facilitating multi-center manufacturing, however, would require standardizing the manufacturing protocol to minimize product variation.
To successfully deliver the manufactured products for clinical use, patient accessibility must be prioritized, regardless of which manufacturing model is implemented. In-house manufacturing in a GMP facility of a hospital [68] represents a centralized approach to manufacturing and secures patient accessibility. In-house manufacturing within the hospital can help overcome many challenges faced when manufacturing CGTs for clinical use. The quality of the tissue source (starting material) can be ensured through rapid delivery from the operation room to the GMP facility, which would reduce the time delay between manufacturing and clinical application and enable parallel production and patient monitoring at a single location.
These features of in-house manufacturing within the hospital setting will benefit allogeneic and autologous therapies involving both stem cell and CAR-T cell therapies. If a hospital has no GMP facility, CAR-T cell production must be outsourced, involving local cell collection, cryopreservation, and shipping to the manufacturing site. Outsourcing of the manufacturing process (including QC), followed by the shipment of the final product back to the point-of-care location, increases the vein-to-vein time. Real-world analysis has indicated that the vein-to-vein time of representative, currently available CAR-T cell products is longer than 28 days [69]. In-house GMP manufacturing within the hospital may serve as a solution to reduce the vein-to-vein time.
The high cost and GMP regulatory compliance are just a few of the multiple challenges hospitals face in building a GMP facility. Hospitals must be able to pay for the costs of building and maintaining a manufacturing facility, and the facility must abide by GMP laws and regulations [68]. If hospitals cannot overcome obstacles associated with building an in-house GMP facility, an alternative would be for them to start a spin-off company. These spin-off firms could maintain a strong network with local hospitals and specialize in meeting their manufacturing needs. Ideally, the spin-off companies would provide one-stop shopping services where the production and manufacturing of cell, gene, and cell-based gene therapies occur at a single location near the point-of-care location and, most importantly, the patient.
CONCLUSION
CGT is a pertinent topic because it represents a promising strategy for treating a broad spectrum of diseases. The increasing attention to CGTs highlights the complexities of the manufacturing process, which can impede the clinical application of the manufactured products. To progress from demonstrating potential to showcasing robust clinical efficacy, addressing and overcoming the manufacturing challenges associated with CGTs is imperative.
ACKNOWLEDGEMENTS
We thank Dr. Sun Jae Kwon for constructive feedback.
Funding Statement
RESEARCH FUNDING This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant No.: HR22C1363).
Footnotes
AUTHOR CONTRIBUTIONS
Lee NK and Chang JW contributed manuscript writing, editing, and reviewing. Both authors have read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
None declared.
References
- 1.Arabi F, Mansouri V, Ahmadbeigi N. Gene therapy clinical trials, where do we go? An overview. Biomed Pharmacother. 2022;153:113324. doi: 10.1016/j.biopha.2022.113324. [DOI] [PubMed] [Google Scholar]
- 2.Sanz-Nogués C, O'Brien T. Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomater Biosyst. 2021;2:100018. doi: 10.1016/j.bbiosy.2021.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaioannou I, Owen JS, Yáñez-Muñoz RJ. Clinical applications of gene therapy for rare diseases: a review. Int J Exp Pathol. 2023;104:154–76. doi: 10.1111/iep.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043.f017bcafff6042bc80af1d5d1aae7c5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heathman TR, Nienow AW, McCall MJ, Coopman K, Kara B, Hewitt CJ. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med. 2015;10:49–64. doi: 10.2217/rme.14.73. [DOI] [PubMed] [Google Scholar]
- 6.Kim I. A brief overview of cell therapy and its product. J Korean Assoc Oral Maxillofac Surg. 2013;39:201–2. doi: 10.5125/jkaoms.2013.39.5.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Kadiry AE, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med (Lausanne) 2021;8:756029. doi: 10.3389/fmed.2021.756029.4b199b82483b4804886346411fff7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moody J, Milligan WD, St Onge M, Goonewardene A, Rivers P. Cell and gene therapy: a snapshot of investor perspectives. Cytotherapy. 2021;23:256–60. doi: 10.1016/j.jcyt.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang LL, Janes ME, Kumbhojkar N, Kapate N, Clegg JR, Prakash S, et al. Cell therapies in the clinic. Bioeng Transl Med. 2021;6:e10214. doi: 10.1002/btm2.10214.579dc17147a447c4878ce11b58f3e9cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumann GG, Fuchs NV, Tristán-Ramos P, Sebe A, Ivics Z, Heras SR. The impact of transposable element activity on therapeutically relevant human stem cells. Mob DNA. 2019;10:9. doi: 10.1186/s13100-019-0151-x.754709407bfe48578aa76e7b6d842beb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Hao J, Hu Z, Yang YG, Zhou Q, Sun L, et al. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review. Stem Cell Res Ther. 2022;13:93. doi: 10.1186/s13287-022-02751-0.a55056317223417d8d35a4a7773ee2ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Santos ME, Garcia-Arranz M, Andreu EJ, García-Hernández AM, López-Parra M, Villarón E, et al. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front Immunol. 2022;13:918565. doi: 10.3389/fimmu.2022.918565.859113e2a67f49de8a632985ba59dbc7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costela-Ruiz VJ, Melguizo-Rodríguez L, Bellotti C, Illescas-Montes R, Stanco D, Arciola CR, et al. Different sources of mesenchymal stem cells for tissue regeneration: a guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci. 2022;23:6356. doi: 10.3390/ijms23116356.778d4cd136e743f0ab982af16e2e914c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–31. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 17.Jossen V, van den Bos C, Eibl R, Eibl D. Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol. 2018;102:3981–94. doi: 10.1007/s00253-018-8912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan MNFB, Yazid MD, Yunus MHM, Chowdhury SR, Lokanathan Y, Idrus RBH, et al. Large-scale expansion of human mesenchymal stem cells. Stem Cells Int. 2020;2020:9529465. doi: 10.1155/2020/9529465.76b3f189901d4e48bcc7597e660650ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guadix JA, López-Beas J, Clares B, Soriano-Ruiz JL, Zugaza JL, Gálvez-Martín P. Principal criteria for evaluating the quality, safety and efficacy of hMSC-based products in clinical practice: current approaches and challenges. Pharmaceutics. 2019;11:552. doi: 10.3390/pharmaceutics11110552.42c29149602241f084188c2e66b1a87b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x.84bd903655854a26891e4357d80ddf37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z, Anselmo AC, Mitragotri S. Viral vector-based gene therapies in the clinic. Bioeng Transl Med. 2022;7:e10258. doi: 10.1002/btm2.10258.862afd99f3c44e21b81c77ad10af25b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang L, Jin S, Wang J, Lv Z, Xin C, Tan C, et al. AAV vectors applied to the treatment of CNS disorders: clinical status and challenges. J Control Release. 2023;355:458–73. doi: 10.1016/j.jconrel.2023.01.067. [DOI] [PubMed] [Google Scholar]
- 23.Kingwell K. Lentiviral vector gene therapies come of age with two FDA approvals. Nat Rev Drug Discov. 2022;21:790–1. doi: 10.1038/d41573-022-00176-1. [DOI] [PubMed] [Google Scholar]
- 24.van der Walle CF, Dufès C, Desai AS, Kerby J, Broadhead J, Tam A, et al. Report on webinar series cell and gene therapy: from concept to clinical use. Pharmaceutics. 2022;14:168. doi: 10.3390/pharmaceutics14010168.9bd39ba2c93c48cab4aae13fdbdd5c9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munis AM. Gene therapy applications of non-human lentiviral vectors. Viruses. 2020;12:1106. doi: 10.3390/v12101106.7d8f816a82164ee08495b330db61dba9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabatino DE, Bushman FD, Chandler RJ, Crystal RG, Davidson BL, Dolmetsch R, et al. Evaluating the state of the science for adeno-associated virus integration: an integrated perspective. Mol Ther. 2022;30:2646–63. doi: 10.1016/j.ymthe.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisserier M, Sun XQ, Fazal S, Turnbull IC, Bonnet S, Hadri L. Novel insights into the therapeutic potential of lung-targeted gene transfer in the most common respiratory diseases. Cells. 2022;11:984. doi: 10.3390/cells11060984.d8fda3586b1a42f8b1182e6fa9c274bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava A, Mallela KMG, Deorkar N, Brophy G. Manufacturing challenges and rational formulation development for AAV viral vectors. J Pharm Sci. 2021;110:2609–24. doi: 10.1016/j.xphs.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6.63666028eb554ff786d8586456529231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright JF. Quality control testing, characterization and critical quality attributes of adeno-associated virus vectors used for human gene therapy. Biotechnol J. 2021;16:e2000022. doi: 10.1002/biot.202000022. [DOI] [PubMed] [Google Scholar]
- 31.Shi R, Jia S, Liu H, Nie H. Clinical grade lentiviral vector purification and quality control requirements. J Sep Sci. 2022;45:2093–101. doi: 10.1002/jssc.202100937. [DOI] [PubMed] [Google Scholar]
- 32.Labbé RP, Vessillier S, Rafiq QA. Lentiviral vectors for T cell engineering: clinical applications, bioprocessing and future perspectives. Viruses. 2021;13:1528. doi: 10.3390/v13081528.2d62769bd9554d35a58221ab3996c089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry C, Rayat ACME. Lentiviral vector bioprocessing. Viruses. 2021;13:268. doi: 10.3390/v13020268.db12b1bd63064381b315ae5a169c013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croissant C, Armitano J, Lazuech B, Švec D, Pugin C, Guesdon A, et al. A new T-antigen negative HEK293 cell line with improved AAV productivity. Biotechnol Bioeng. 2023;120:1953–60. doi: 10.1002/bit.28414. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira MV, Cabral ET, Coroadinha AS. Progress and perspectives in the development of lentiviral vector producer cells. Biotechnol J. 2021;16:e2000017. doi: 10.1002/biot.202000017. [DOI] [PubMed] [Google Scholar]
- 36.Yuan Z, Qiao C, Hu P, Li J, Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum Gene Ther. 2011;22:613–24. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvaraj N, Wang CK, Bowser B, Broadt T, Shaban S, Burns J, et al. Detailed protocol for the novel and scalable viral vector upstream process for AAV gene therapy manufacturing. Hum Gene Ther. 2021;32:850–61. doi: 10.1089/hum.2020.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YH, Pallant C, Sampson CJ, Boiti A, Johnson S, Brazauskas P, et al. Rapid lentiviral vector producer cell line generation using a single DNA construct. Mol Ther Methods Clin Dev. 2020;19:47–57. doi: 10.1016/j.omtm.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada M, Uchida N, Posadas-Herrera G, Hayashita-Kinoh H, Tsunekawa Y, Hirai Y, et al. Large-scale purification of functional AAV particles packaging the full genome using short-term ultracentrifugation with a zonal rotor. Gene Ther. 2023;30:641–8. doi: 10.1038/s41434-023-00398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pei X, Earley LF, He Y, Chen X, Hall NE, Samulski RJ, et al. Efficient capsid antigen presentation from adeno-associated virus empty virions in vivo. Front Immunol. 2018;9:844. doi: 10.3389/fimmu.2018.00844.de5b08b5f607400c82643773f48e5af6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Q, Polanco A, Lee YS, Yoon S. Critical challenges and advances in recombinant adeno-associated virus (rAAV) biomanufacturing. Biotechnol Bioeng. 2023;120:2601–21. doi: 10.1002/bit.28412. [DOI] [PubMed] [Google Scholar]
- 42.Timmins LM, Patel RS, Teryek MS, Parekkadan B. Real-time transfer of lentiviral particles by producer cells using an engineered coculture system. Cytotechnology. 2019;71:1019–31. doi: 10.1007/s10616-019-00343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran MY, Kamen AA. Production of lentiviral vectors using a HEK-293 producer cell line and advanced perfusion processing. Front Bioeng Biotechnol. 2022;10:887716. doi: 10.3389/fbioe.2022.887716.8dff0a1f979149dda7991e6c635cdb6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roex G, Campillo-Davo D, Flumens D, Shaw PAG, Krekelbergh L, De Reu H, et al. Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells. J Transl Med. 2022;20:124. doi: 10.1186/s12967-022-03326-6.05c1c30b2722445782796239a7b077f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei J, Han X, Bo J, Han W. Target selection for CAR-T therapy. J Hematol Oncol. 2019;12:62. doi: 10.1186/s13045-019-0758-x.8f487526a3864704af296aaea874cc21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–9. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]
- 47.Lee HJ, Kim SK, Cho D, Lee JJ. Cellular immunotherapy as a beacon of hope for hematological malignancies. Blood Res. 2015;50:126–8. doi: 10.5045/br.2015.50.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 2018;53:164–81. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 49.Lv Z, Luo F, Chu Y. Strategies for overcoming bottlenecks in allogeneic CAR-T cell therapy. Front Immunol. 2023;14:1199145. doi: 10.3389/fimmu.2023.1199145.3b821db9babb429b95c2839304624d3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milone MC, O'Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–41. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medvec AR, Ecker C, Kong H, Winters EA, Glover J, Varela-Rohena A, et al. Improved expansion and in vivo function of patient T cells by a serum-free medium. Mol Ther Methods Clin Dev. 2018;8:65–74. doi: 10.1016/j.omtm.2017.11.001.4b72adeeb7ef495f864b298b3073f32a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koh SK, Park J, Kim SE, Lim Y, Phan MT, Kim J, et al. Natural killer cell expansion and cytotoxicity differ depending on the culture medium used. Ann Lab Med. 2022;42:638–49. doi: 10.3343/alm.2022.42.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidts A, Marsh LC, Srivastava AA, Bouffard AA, Boroughs AC, Scarfò I, et al. Cell-based artificial APC resistant to lentiviral transduction for efficient generation of CAR-T cells from various cell sources. J Immunother Cancer. 2020;8:e000990. doi: 10.1136/jitc-2020-000990.1383b25b9a8e4f49a0640c17e9556951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18:800–7. doi: 10.1200/OP.22.00315. [DOI] [PubMed] [Google Scholar]
- 55.Jackson Z, Roe A, Sharma AA, Lopes FBTP, Talla A, Kleinsorge-Block S, et al. Automated manufacture of autologous CD19 CAR-T cells for treatment of non-Hodgkin lymphoma. Front Immunol. 2020;11:1941. doi: 10.3389/fimmu.2020.01941.8d0bad17627d4c10b8f6a1d433064624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gajra A, Zalenski A, Sannareddy A, Jeune-Smith Y, Kapinos K, Kansagra A. Barriers to chimeric antigen receptor T-cell (CAR-T) therapies in clinical practice. Pharmaceut Med. 2022;36:163–71. doi: 10.1007/s40290-022-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15.5311327946444c02a2cb7b3e9317382a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Huo Y, Yu L, Wang J. Quality control and nonclinical research on CAR-T cell products: general principles and key issues. Engineering. 2019;5:122–31. doi: 10.1016/j.eng.2018.12.003. [DOI] [Google Scholar]
- 59.Allen JM, Debelak DJ, Reynolds TC, Miller AD. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J Virol. 1997;71:6816–22. doi: 10.1128/jvi.71.9.6816-6822.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Food and Drug Administration, author. Summary basis for regulatory action. [Updated on Aug 2017]. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/August-30--2017-Summary-Basis-for-Regulatory-Action---KYMRIAH.pdf.
- 61.Food and Drug Administration, author. Summary basis for regulatory action. [Updated on Oct 2017]. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/October-18--2017-Summary-Basis-for-Regulatory-Action---YESCARTA.pdf.
- 62.Food and Drug Administration, author. Summary basis for regulatory action. [Updated on July 2020]. https://www.fda.gov/media/141093/download#:~:text=TECARTUS%27%20mechanism%20of%20action%20is,tumor%20toxicity%20from%20CD19%20targeting.
- 63.Food and Drug Administration, author. Summary basis for regulatory action. [Updated on Feb 2022]. https://www.fda.gov/media/156999/download.
- 64.U.S. Food and Drug Administration, author. Considerations for the development of chimeric antigen receptor (CAR) T cell products: draft guidance for industry. [Updated on Jan 2024]. https://www.fda.gov/media/156896/download.
- 65.Harrison RP, Rafiq QA, Medcalf N. Centralised versus decentralised manufacturing and the delivery of healthcare products: a United Kingdom exemplar. Cytotherapy. 2018;20:873–90. doi: 10.1016/j.jcyt.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Harrison RP, Ruck S, Medcalf N, Rafiq QA. Decentralized manufacturing of cell and gene therapies: overcoming challenges and identifying opportunities. Cytotherapy. 2017;19:1140–51. doi: 10.1016/j.jcyt.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Stroncek D, Dinh A, Rai H, Zhang N, Somerville R, Panch S. The need for uniform and coordinated practices involving centrally manufactured cell therapies. J Transl Med. 2022;20:184. doi: 10.1186/s12967-022-03385-9.f2315bae65fe42d99051ff1f9bd99e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iancu EM, Kandalaft LE. Challenges and advantages of cell therapy manufacturing under good manufacturing practices within the hospital setting. Curr Opin Biotechnol. 2020;65:233–41. doi: 10.1016/j.copbio.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Locke FL, Hu ZH, Siddiqi T, Jacobson CA, Nikiforow S, Ahmed S, et al. Real-world impact of time from leukapheresis to infusion (vein-to-vein time) in patients with relapsed or refractory (r/r) large B-cell lymphoma (LBCL) treated with axicabtagene ciloleucel. Blood. 2022;140(S1):7512–5. doi: 10.1182/blood-2022-155603. [DOI] [Google Scholar]