Abstract
Heart failure with preserved ejection fraction (HFpEF) is a multi-organ systemic syndrome that involves cardiac and extra-cardiac pathophysiological abnormalities. Its growing prevalence causes a major public concern worldwide. HFpEF is usually associated with multiple comorbidities, and non-cardiovascular death is common in patients with HFpEF. In Asia, patients with HFpEF has a younger age, higher prevalence of diabetes and chronic kidney disease than Western countries. A 2-step diagnostic algorithm is recommended in this guideline. In the first step, the diagnosis of HFpEF can be made if patients have symptoms and/or signs of heart failure, left ventricular ejection fraction ≥ 50%, increased natriuretic peptide, and objective evidence of left atrial or left ventricular abnormalities or raised left ventricular filling pressure. If diagnosis is still uncertain, invasive or noninvasive stress test can be performed in the second step. Comorbidities need to be controlled in HFpEF. Weight reduction for obesity and supervised exercise training are recommended for HFpEF. For pharmacological therapy, diuretic is used to relieve congestion and sodium-glucose cotransporter 2 inhibitor, empagliflozin or dapagliflozin, is recommended to improve prognosis of HFpEF. The research on HFpEF is advancing at a rapid pace. It is expected that newer modalities for diagnosis and management of HFpEF could appear in the near future.
Keywords: Guideline, Heart failure with preserved ejection fraction, Taiwan
Abbreviations
ACEI, Angiotensin converting enzyme inhibitor
AF, Atrial fibrillation
AHA GWTG-HF, American Heart Association Get With The Guideline-HF
ARB, Angiotensin receptor blocker
ARNI, Angiotensin receptor-neprilysin inhibitor
ATTR-CM, Transthyretin amyloid cardiomyopathy
BMI, Body mass index
BNP, B-type natriuretic peptide
CAD, Coronary artery disease
CHARM-Preserved, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity-Preserved
CKD, Chronic kidney disease
COR, Class of recommendation
CT, Computed tomography
CV, Cardiovascular
CXR, Chest X-ray
DKA, Diabetic ketoacidosis
DM, Diabetes mellitus
ECG, Electrocardiography
EF, Ejection fraction
eGFR, Estimated glomerular filtration rate
GLP1RA, Glucagon-like peptide-1 receptor agonists
HCM, Hypertrophic cardiomyopathy
HF, Heart failure
HFmrEF, HF with mildly reduced ejection fraction
HFnEF, HF with normal EF
HFpEF, Heart failure with preserved ejection fraction
HFrEF, HF with reduced ejection fraction
I-Preserve, Irbesartan in Heart Failure with Preserved Ejection Fraction Study
LA, Left atrial
LOE, Level of evidence
LV, Left ventricular
LVEF, Left ventricular ejection fraction
MI, Myocardial infarction
MRA, Mineralocorticoid receptor antagonist
MRI, Magnetic resonance imaging
NT-proBNP, N-terminal proB-type natriuretic peptide
PARAGON-HF, Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction
PCV13, 13-valent pneumococcal conjugate vaccine
PCWP, Pulmonary capillary wedge pressure
PET, Positron emission tomography
PPV23, 23-valent pneumococcal polysaccharide vaccine
RATE-AF, Rate Control Therapy Evaluation in Permanent Atrial Fibrillation
REHAB-HF, Rehabilitation Therapy in Older Acute Heart Failure Patients
RV, Right ventricular
SGLT2, Sodium-glucose cotransporter 2
STRONG-HF, Safety, Tolerability and Efficacy of Up-titration of Guideline-directed Medical Therapies for Acute Heart Failure
TOPCAT, Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
TR, Tricuspid regurgitation
TTE, Transthoracic echocardiography
US, United States
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is diagnosed as those with symptoms and/or signs of heart failure (HF), left ventricular (LV) ejection fraction (EF) of 50% or greater, elevated natriuretic peptide and cardiac structural change or diastolic dysfunction.1,2 The growing trend of HFpEF causes public health problem worldwide, including in Asia.3,4 For many years, how to make a correct diagnosis of HFpEF is still controversial. There is a consensus that the diagnosis of HFpEF needs evidence of elevated cardiac filling pressure and natriuretic peptide with the presence of structural heart disease.5 Traditionally, management of comorbidities and relief of congestion with diuretics are the main therapies for HFpEF. In recent years, a number of randomized clinical trials demonstrated that some medical therapies could change the natural course of HFpEF and effectively reduce the risk of HF hospitalization. The Taiwan Society of Cardiology published HF Guideline in 2012 and its focus update in 2019.1,6 Since there have been new scientific evidences regarding the epidemiology, diagnosis and treatment of HFpEF after 2019, the HF committee of Taiwan Society of Cardiology decided to create a new HF guideline specifically for HFpEF.
The guideline writers were invited mainly from the Taiwan Society of Cardiology HF committee which consisted of 15 members nominated by the President of Taiwan Society of Cardiology and endorsed by the Society’s board meeting in 2022. Three writer meetings were held on April 16, 2023 in Taipei, June 17, 2023 in Taichung and September 24, 2023 in Kaohsiung. During the meetings, the panel decided the main contents of the HFpEF guideline, including definition, epidemiology, pathophysiology, diagnosis, treatment and future perspective. The writers assigned to one of these sections were responsible for collecting available evidence, reviewing its scientific intensity and making proper recommendations. Consensus about guideline recommendations was achieved by all writers during the meetings. Similar to the recently published guidelines from the Taiwan Society of Cardiology,7 this guideline adopted class of recommendation (COR) to indicate whether a recommendation is useful or harmful and level of evidence (LOE) to describe the strength of scientific evidence about the recommendations. In COR, Class I recommendations indicate they are useful and beneficial for the patients. Class IIa recommendations indicate that the evidence favors the recommendations and could be used for the patients. Class IIb recommendations are those may be considered but the scientific strength is less well established. Class III recommendations are the treatment that is unnecessary or harmful. There are three levels for LOE. LOE A recommendations are supported by multiple randomized clinical trials or meta-analyses of randomized clinical trials. LOE B recommendations are from only one randomized clinical trial or non-randomized observational studies. LOE C recommendations are from case series, case reports or consensus of expert opinions.
DEFINITION
HF is a complex clinical syndrome that results from impaired cardiac function, leading to an inability of the heart to meet the body’s metabolic demand adequately.8 Conventionally, HF can be categorized into three main subtypes based on left ventricular ejection fraction (LVEF), a measure of the heart’s pumping efficiency. These subtypes are HF with reduced ejection fraction (HFrEF), HF with mildly reduced ejection fraction (HFmrEF), and HFpEF (Table 1).2,5,9 An expert consensus from the Taiwan Society of Cardiology suggests to reclassify those patients with LVEF ≥ 60% as HF with normal EF (HFnEF).10 HFpEF, previously known as diastolic HF, refers to a clinical syndrome in which patients have signs and symptoms of HF but have a relatively normal EF. The LVEF in HFpEF is typically greater than or equal to 50%.2,5,9 It is important to note that the definition of HFpEF is not solely based on the EF but includes additional clinical criteria. The EF alone is not sufficient to establish the diagnosis. Additional criteria are necessary to differentiate HFpEF from other causes of dyspnea and to ensure that the clinical syndrome is primarily due to diastolic dysfunction. These criteria include: (1) HF symptoms and/or signs. Patients with HFpEF typically present with exertional dyspnea, fatigue, exercise intolerance, and fluid retention leading to peripheral edema. Elevated jugular venous pressure, pulmonary rales, and peripheral edema often accompany these symptoms. (2) Evidence of structural heart disease. HFpEF is frequently associated with underlying structural heart abnormalities, including LV hypertrophy, increased left atrial (LA) size, and diastolic dysfunction. Echocardiography is commonly used to assess these structural abnormalities and to measure the EF. (3) Evidence of diastolic dysfunction. Diastolic dysfunction refers to abnormalities in the filling of the ventricles during diastole. In HFpEF, impaired relaxation, increased ventricular stiffness, and abnormal filling pressure are commonly observed. These abnormalities can be assessed using Doppler echocardiography which provides information on transmitral flow velocities, pulmonary venous flow, and tissue Doppler imaging. (4) Additional diagnostic tests. In some cases, additional tests may be required to establish the diagnosis of HFpEF and exclude other causes of patients’ symptoms. These tests may include cardiopulmonary exercise testing, cardiac catheterization, magnetic resonance imaging (MRI), or nuclear imaging studies.
Table 1. Definition of heart failure.
| Type of HF | HFpEF | HFmrEF | HFrEF |
| LVEF | ≥ 50% | 41-49% | ≤ 40% |
| • Symptoms/signs | • Symptoms/signs | • Symptoms/signs | |
| • Evidence of structural and/or functional cardiac abnormalities and LV diastolic dysfunction |
Natriuretic peptide is usually elevated in HF.
HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction.
* Modified from reference 9.
Recommendation
• HFpEF is defined as the presence of HF symptoms and/or signs with LVEF ≥ 50%, structural heart disease and diastolic dysfunction. (COR I, LOE A)
EPIDEMIOLOGY
Prevalence and incidence of HFpEF
In Taiwan, the prevalence of HF may be as high as 6%.11 A recent analysis using HF hospitalization as an adjudicated event showed that the HF prevalence in Taiwan is estimated to be 1.63 to 1.99% from 2020 to 2025.12 Overall, the projected HF burden in Taiwan will resemble the findings from the United States (US) with an estimated tripled HF prevalence in 2050.12 Similar to the global trend, the HF incidence is decreasing in Taiwan with an estimated incidence of 2.44 in 2001 to 2.19 per 1000 person-years in 2016.12,13 However, the age-stratified HF incidence is different with 10-20% decrease in older individuals (≥ 55 years), but approximately 4% increase among younger population (< 44 years).12,13
According to the registry data from Western countries, near half of the patients with HF have HFpEF. In patients admitted due to HF between 2005 and 2009 in the American Heart Association Get With The Guideline-HF (AHA GWTG-HF) registry, a prevalence of 46% HFpEF was found.14 The Olmsted County cohort from the US showed that 52.5% of patients with incident HF were HFpEF.15 Overall, the prevalence of HFpEF was increasing, but HFrEF prevalence seemed to be stable or declining.3,16,17 For incidence of HFpEF, the Olmsted County cohort from the US showed that the age- and sex-adjusted incidence of HF declined substantially with a 45% decrease in HFrEF compared with 28% in HFpEF.15 But more recent data from the Framingham Heart Study and Cardiovascular Health Study, a declining incidence for HFrEF and increasing incidence for HFpEF was oberved.18 Table 2 shows the prevalence and incidence of HFpEF in different registries or population-based studies.
Table 2. Relative distribution of HFpEF in cross-sectional and longitudinal observational studies.
| Prevalence of HFpEF | HFrEF | HFmrEF | HFpEF |
| Study name | |||
| European Society of Cardiology Long-Term Registry | 60% | 24% | 16% |
| Swedish HF Registry | 56% | 21% | 23% |
| Multicentre nationwide Italian Network (Spain) | 32% | 16% | 52% |
| UK-HEART Study | NA | NA | 31%† |
| Cardiovascular Health Study | NA | NA | 22%* |
| ADHERE Inpatient Cohort | NA | NA | 50% |
| OPTIMIZE-HF Registry (United States) | 49% | 17% | 24% |
| Framingham Heart Study (1981-2008) | NA | NA | 43%‡ |
| Get With The Guidelines-HF (GWTG-HF) | 39% | 14% | 47% |
| Strong Heart Study (American Indians) | NA | NA | 53%* |
| ADHERE Inpatient Cohort | NA | NA | 50%# |
| Global Congestive Heart Failure Registry | 54% | 21% | 24% |
| Pooled Community Cohorts (Framingham Heart Study, Cardiovascular Health Study, Prevention of Renal and Vascular End-Stage Disease, Multi-Ethnic Study of Atherosclerosis) | NA | NA | 48%‡ |
| Multicenter Inpatient Cohort (Japan, 2013 to 2014) | 36% | 21% | 43% |
| Asian-HF Registry | 81% | NA | 19% |
| China Hypertension Survey (China, 2012-2015) | 40% | 23% | 37% |
| Incidence of HFpEF | HFrEF | HFpEF |
| Study name | ||
| PREVEND Prevention of Renal and Vascular End-Stage Disease (Dutch Community) | 66% (< 40%) | 34% (≥ 50%) |
| Olmsted County, Minnesota (2008-2010) | 48% (< 50%) | 52% (≥ 50%) |
| Framingham Heart Study/Cardiovascular Health Study (≥ 60 years, 2000-2009) | 45% (< 50%) | 55% (≥ 50%) |
| Longitudinal American Community-Based Cohort | 52% | 48% |
| Among Canadian Inpatients (Ontario, 1999-2001) | 56% | 31% |
| Dutch Community Based Cohort | 66% | 34% |
Abbreviations are the same as the Table 1. NA, not applicable.
LVEF cutoffs: * LVEF ≥ 55%, † LVEF ≥ 50%, ‡ LVEF ≥ 45%, # LVEF ≥ 40%.
Recommendation
• There is an increasing prevalence of HFpEF, but the prevalence of HFrEF is stable or declining. (COR I, LOE A)
Risk factor, comorbidity and phenotype of HFpEF
Compared with HFrEF, patients with HFpEF tend to be older, predominantly female, and are associated with multiple comorbidities.16 Older age is strongly associated with HFpEF. On the contrary, the risk factors, such as male sex, previous myocardial infarction (MI), LV hypertrophy, and left bundle branch block, are more commonly associated with HFrEF rather than HFpEF.19 Lifestyle factor may also play a role as a risk of HFpEF. Previous study showed that individuals with more leisure-time physical activity had a lower risk of HFpEF than those with no leisure-time physical activity; whereas no such association was observed for the risk of HFrEF.20 Comorbidities are common in patients with HFpEF, including hypertension, diabetes mellitus (DM), chronic kidney disease (CKD), coronary artery disease (CAD), atrial fibrillation (AF), obesity, obstructive sleep apnea, and metabolically associated fatty liver disease.21-29 Because HFpEF is highly heterogeneous with multiple different comorbidities, several analyses have been performed in clustering patients with HFpEF into different phenotypes. Although there were different methods to classify the patients, several phenotypes of HFpEF are commonly identified, including diabetes and obesity, AF and CKD, young patients with milder HF symptoms and fewer comorbidities, men with AF, and elderly frail women with AF.30 The findings of diverse phenotypes in patients with HFpEF increase the potential for phenotype-specific treatment in such populations.31-33 Compared to the findings from Western countries, the comorbidities of HFpEF in Asia demonstrated a younger age, lower prevalence of CAD, AF, and obesity, but a higher prevalence of diabetes and CKD.34,35 One unique HFpEF phenotype in Asia is patients with lean diabetes.35 The clinical features of HFpEF in Taiwan were similar to those reported from the Asian studies.36,37
Recommendation
• HFpEF patients tend to be older, predominantly female, and are associated with multiple comorbidities. (COR I, LOE A)
• The Asian HFpEF patients tend to be younger and have higher prevalence of diabetes and CKD than Western countries. (COR I, LOE A)
Outcome of HFpEF
Several studies from the US showed that the secular HF hospitalization rates were increasing over time and largely driven by HFpEF events.38,39 A greater proportion of HFrEF hospitalization was attributed to the male sex, while a greater proportion of HFpEF hospitalization occurred in the female population. Hospitalization for cardiovascular (CV) causes appeared to be higher in individuals with HFrEF. However, non-CV hospitalization was comparable between HFrEF and HFpEF indicating a higher impact of non-CV related comorbidities on HFpEF hospitalization.40 Albeit the total hospitalization rate among HF patients was similar irrespective of LVEF, the short-term hospitalization rate within 1 year after discharge was still higher in patients with HFrEF than HFpEF.41,42 Overall, patients with HF have higher 30-day readmission rate after discharge compared with patients admitted with other diagnoses and HF remains the number one cause of hospitalization in the older population.43
In Taiwan, the mortality of HF was estimated to be 22.5%, 33.9%, and 42.8% at 1, 2, and 3-year follow-up, respectively. A longer duration study showed the mortality was 62.1%, 69.6%, and 75.5% at 6, 8, and 10-year follow-up.12,13 Another 16-year analysis in Taiwan revealed an in-hospital mortality rate of 4.12% in patients with newly diagnosed HF and the mortality after discharge was estimated to be 38.5%, 52.2%, 62.1%, 69.6%, and 75.5% at 2, 4, 6, 8, and 10-year follow-up, respectively.12,44 In general, patients with HFpEF tended to have better overall survival than those with HFrEF.14,16 In the Asian-HF registry, the crude annual all-cause mortality rate was higher in patients with HFrEF than in those with HFpEF.45 Importantly, most of the causes of death were CV death among patients with HFrEF.46 In contrast, non-CV death was more frequent in patients with HFpEF. In Asia, the risk of CV death was slightly higher in patients with HFrEF than in those with HFpEF, whereas non-CV death was more frequent in patients with HFpEF than HFrEF at 1-year follow-up.45
Recommendation
• Patients with HFpEF have a better survival than HFrEF and non-CV death is more frequent in patients with HFpEF. (COR I, LOE A)
Cardiomyopathy
HFpEF can be presented in most patients with obstructive hypertrophic cardiomyopathy (HCM) and approximately 10% of patients with nonobstructive HCM.47 HCM is an important genetic heart muscle disease, however, its prevalence in the general population has not yet been completely resolved. Most data indicate that the occurrence of HCM is approximately 1 in 500 individuals. However, recent studies suggest that the prevalence of HCM may be more common than previously estimated. A timely diagnosis and treatment are crucial since the development of advanced HF in HCM portends a poor prognosis.48,49 Infiltrative cardiomyopathy is a heterogeneous group of disease and may present with HFpEF. Among them, transthyretin amyloid cardiomyopathy (ATTR-CM) is an under-recognized cause of HFpEF. In Taiwan, late-onset hereditary ATTR amyloidosis with the A117S mutation and polyneuropathy is often associated with heart involvement. Recent studies have suggested that 10-15% of older adults with HF may have an unrecognized ATTR-CM. The associated carpal tunnel syndrome and lumbar spinal stenosis may raise clinical suspicion and early diagnosis.50,51 Fabry disease is a X-linked inborn error of glycosphingolipid metabolism due to alpha-galactosidase A deficiency. Newborn screening identified a high frequency of Taiwanese male with Fabry disease (approximately 1 in 1250) with 86% having the IVS4+919G>A mutation.52 HFpEF is common in infiltrative cardiomyopathy. Irrespective of the etiology, HF is a harbinger of poor outcomes of cardiomyopathy. Disease-modifying therapies are available for cardiac amyloidosis and Fabry disease and carry potential to improve outcomes.
Recommendation
• HFpEF can be presented in most patients with hypertrophic or infiltrative cardiomyopathy. (COR I, LOE A)
PATHOPHYSIOLOGY
The pathophysiology of HFpEF involves multisystem abnormalities with different mechanisms, including cardiac dysfunction (left or right heart), vascular dysfunction, peripheral tissue abnormality, or cardiomyopathy (hypertrophic or infiltrative).
Cardiac dysfunction
LV diastolic dysfunction
Although the pathophysiological mechanisms of HFpEF are multiple and cross-linked, LV diastolic dysfunction forms a major basis of the clinical syndrome of HFpEF.53-58 However, it does not fully represent the whole pathophysiological abnormalities of HFpEF.58 Diastolic dysfunction results from decreased LV chamber distensibility and causes increased diastolic pressure at any given LV volume. Patients with HFpEF display an abnormal LV diastolic pressure-volume relationship that is shifted up and to the left indicating an increase in passive chamber stiffness. The primary factors affecting myocardial stiffness include abnormalities in extracellular matrix, cardiomyocytes, matricellular proteins, calcium ion homeostasis, myocardial energy supply, and increased fat content in myocardium and pericardium.59-62 Patients with HFpEF display prolonged relaxation and inability to hasten relaxation as heart rate increases during exercise.63,64 Prolonged relaxation usually does not affect LV filling when heart rate is normal at rest, but it significantly increases LV filling pressure during exertion as cycle length shortens in tachycardia. In patients at early stage of HFpEF, LV filling pressure becomes markedly elevated only during exercise; however, at advanced stage of HFpEF, LV filling pressure is elevated even at rest.65 Increased LV filling pressure during exertion in HFpEF directly correlated with heightened inspiratory drive, symptoms of dyspnea, alterations in gas exchange and pulmonary ventilation, and reduction in aerobic capacity. Elevation in LV filling pressures alters the Starling force across the pulmonary capillaries, pushing water out of the vascular space into the interstitium. Over time, high LV filling pressure may promote vascular remodeling, particularly in pulmonary veins.
LV systolic dysfunction
HFpEF is defined as HF with normal or nearly normal LVEF, but EF is only a rough estimate of LV contractile function. Actually, LV systolic function is relatively impaired in patients with HFpEF compared with the age-matched healthy controls.66 Impairment in myocardial and chamber-level function is present at rest and becomes more severe during exercise.66-68 This limits the ability of the heart to increase stroke volume and substantially impairs the cardiac output to meet the need in exercise. In addition, LV systolic reserve deficits compromise diastolic reserve because the ability to enhance contractility plays a key role in determining the restoring force that enhances early diastolic annular motion or recoil.
Left atrial dysfunction
As the LV diastolic filling pressure elevates intermittently over time in HFpEF, secondary remodeling and dysfunction develop in the LA. Preservation of LA function may be an important adaptation in HFpEF because development of LA dysfunction is associated with worse exercise capacity, more profound pulmonary vascular disease, and increased risk of death.69 LA remodeling and dysfunction are common features in AF and HFpEF which are frequently coexisting conditions. Both conditions also share several common comorbidities such as hypertension, diabetes and obesity.17,25,70-72 HFpEF causes AF due to the structural and functional remodeling of the LA, while AF is also associated with LV myocardial fibrosis that contributes to LV diastolic dysfunction and HFpEF.
Coronary microvascular dysfunction
Coronary microvascular dysfunction is a common phenomenon in patients with HFpEF and causes demand-supply mismatch. In the functional aspect, low coronary flow reserve in HFpEF causes impaired myocardial oxygen delivery so that myocardial ischemia and injury occur especially during exercise.73-75 Myocardial systolic and diastolic reserves both decrease when the severity of coronary microvascular dysfunction increases. In the anatomic aspect, coronary microvascular rarefaction and myocardial fibrosis are noted in an autopsy study of patients with HFpEF.76 Based on these evidence, coronary microvascular dysfunction seems to be another mechanism that contribute to HFpEF and may be a therapeutic target.
Chronotropic incompetence
Exercise intolerance is partly related to lower peak heart rate achieved in HFpEF patients. Because increasing heart rate is the major contributor of cardiac output during exercise, impairment in heart rate reserve can lead to exertional intolerance. The mechanism of inability to increase heart rate in HFpEF patients is not fully understood. Premature cessation of exercise due to elevated LV filling pressure and beta-blocker use may account for lower maximal heart rate in some patients with HFpEF. Autonomic dysfunction and reduced cardiac beta-receptor response are also observed in some HFpEF patients. However, all these theories only partially explain the chronotropic incompetence in HFpEF.77,78
Right ventricular dysfunction
Right ventricular (RV) dysfunction leads to augmented right heart distention and elevated LV filling pressure.79 Longstanding pulmonary hypertension in HFpEF eventually causes RV dysfunction which is seen in 20%-35% of patients with HFpEF.80 However, this is not mediated purely by afterload mismatch. RV dysfunction was also independently correlated with male sex and AF which may influence RV function in a load-independent manner. Other potential contributing comorbidities include coronary disease and lung disease.81,82 The presence of RV dysfunction is associated with increased morbidity and mortality of HFpEF.83,84
Vascular dysfunction
Abnormal systemic vascular function
Peripheral vascular function is impaired in HFpEF patients. During exercise, macrovascular stiffness increases and can be reversed by inorganic nitrite.85,86 It has been reported that the increase of adipocyte free fatty acid binding protein contributes to central arterial stiffness.87 In the microvascular level, endothelial dysfunction and inflammation lead to impaired flow-mediated and NO-mediated vasodilation.65,88 Because of systemic vascular dysfunction, the arterial elastance, an estimate of afterload, elevates more than the LV end-systolic elastance, an estimate of LV chamber performance, during exertion and causes ventricular-arterial uncoupling. Abnormal ventricular-arterial coupling develops even at low-level workload.65,89 Ventricular-arterial uncoupling can be reflected by decreased carotid arterial strain and may serve as a prognostic indicator of HFpEF.90
Abnormal pulmonary vascular function
Pulmonary hypertension is defined as mean pulmonary artery pressure > 20 mmHg.91 Pulmonary hypertension and pulmonary vascular remodeling are present in about 70%-80% of patients with HFpEF.92 Impaired pulmonary vascular function displays a unique pathophysiology similar to RV dysfunction.79 In addition, even patients with HFpEF have normal pulmonary vascular resistance at rest, some of them may have inadequate pulmonary vasodilation in response to exercise.93 Cardiopulmonary exercise testing may provide some diagnostic clues of abnormal pulmonary hemodynamic response in HFpEF. Using stress echocardiography to non-invasively evaluate mean pulmonary artery pressure – cardiac output relationship, it was found that patients with HFpEF and exercise-induced pulmonary hypertension have worse exercise capacity, lower peak oxygen consumption and depressed RV systolic function.94 These patients also had higher rates of composite outcomes of all-cause mortality or HF events.94
Peripheral tissue abnormality
In addition to CV system, abnormalities in peripheral tissue also contribute to the pathophysiology of HFpEF. The oxygen diffusion and extraction in skeletal muscle are impaired in patients with HFpEF.69,95,96 Mitochondrial dysfunction, including content and structure abnormalities, in skeletal muscle leads to exercise intolerance as well.97 The coexistence of obesity and sarcopenia, so called "sarcopenic obesity", is quite often in patients with HFpEF and is associated with adverse clinical outcomes.98
Hypertrophic/infiltrative cardiomyopathy
HCM and infiltrative cardiomyopathy are HFpEF mimics which have distinct pathophysiology and clinical outcome. Both of them can present with classic symptoms of HFpEF. Careful history taking, physical examination, and echocardiography are helpful to identify cardiac amyloidosis, cardiac sarcoidosis, hemochromatosis, and Fabry disease. In addition, some gene mutations have been reported to cause cardiomyopathy, such as MYH7 and MYBPC3 gene mutations for HCM, transthyretin gene mutation for cardiac amyloidosis, and GLA gene mutation for Fabry disease.99 Diagnostic tests for these HFpEF mimics are crucial because there are specific treatments once the diagnosis is made.100
In summary, HFpEF is a common form of HF in the elderly, especially in women, and those with multiple comorbidities.101,102 Table 3 summarizes the pathophysiology of HFpEF that maybe involved in aging, female, and different comorbidities.
Table 3. Pathophysiology of HFpEF involved in aging, female, and different comorbidities.
| Common comorbidities in HFpEF | Involved pathophysiology |
| General | |
| Aging | LV diastolic dysfunction |
| LV systolic dysfunction | |
| Chronotropic incompetence | |
| Systemic vascular dysfunction | |
| Peripheral tissue abnormality | |
| Female | LV diastolic dysfunction |
| Coronary microvascular dysfunction | |
| Systemic vascular dysfunction | |
| Pulmonary vascular dysfunction | |
| Cardiovascular | |
| Atrial fibrillation | LV diastolic dysfunction |
| LA dysfunction | |
| Hypertension | LV diastolic dysfunction |
| LV systolic dysfunction | |
| LA dysfunction | |
| Coronary microvascular dysfunction | |
| Systemic vascular dysfunction | |
| Coronary artery disease | LV diastolic dysfunction |
| LV systolic dysfunction | |
| Coronary microvascular dysfunction | |
| Respiratory | |
| Chronic obstructive pulmonary disease | LV diastolic dysfunction |
| RV dysfunction | |
| Sleep apnea syndrome | Pulmonary vascular dysfunction |
| Metabolic | |
| Diabetes mellitus | LV diastolic dysfunction |
| Coronary microvascular dysfunction | |
| Chronotropic incompetence | |
| Systemic vascular dysfunction | |
| Peripheral tissue abnormality | |
| Obesity | LV diastolic dysfunction |
| LV systolic dysfunction | |
| Coronary microvascular dysfunction | |
| Systemic vascular dysfunction | |
| Peripheral tissue abnormality | |
| Nephrogenic | |
| Chronic kidney disease | LV diastolic dysfunction |
| Coronary microvascular dysfunction | |
| Systemic vascular dysfunction |
HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; RV, right ventricular.
Recommendation
• Pathophysiology of HFpEF involves cardiac dysfunction, vascular dysfunction, peripheral tissue abnormality, and cardiomyopathy. (COR I, LOE A)
• Correct diagnosis of hypertrophic or infiltrative cardiomyopathy in patients presenting with HFpEF is important. (COR I, LOE A)
DIAGNOSIS
HF symptoms and signs
To identify symptoms and signs of HF is the beginning step to diagnose HFpEF. The Framingham Diagnostic Criteria describe the major symptoms or signs indicating the presence of HF (Table 4). The criteria are derived from the information gathered during the Framingham Heart Study.103 A HF diagnosis requires the presence of two major criteria or one major and two minor criteria. The major criteria include orthopnea or paroxysmal nocturnal dyspnea, jugular venous distension, hepatojugular reflux, rales, S3 gallop rhythm, pulmonary edema, and cardiomegaly. The minor criteria include exertional dyspnea, nighttime cough, ankle edema, tachycardia with a heart rate surpassing 120 beats per minute, hepatomegaly, and pleural effusion. Dyspnea or ankle edema are common first presentations of HFpEF. However, it is necessary to make a differential diagnosis and consider other causes before making the diagnosis of HF. For dyspnea, the differential diagnosis includes cardiac diseases other than HF, pulmonary diseases and other causes, such as anemia, neuromuscular disease and anxiety. For edema, renal failure, lymphedema, liver cirrhosis, and drug-related ankle edema (for example, dihydropyridine calcium-channel blockers) should be ruled out.
Table 4. The Framingham diagnostic criteria of heart failure.
| Major criteria | Minor criteria |
| • Acute pulmonary edema | • Ankle edema |
| • Cardiomegaly | • Dyspnea on exertion |
| • Hepatojugular reflux | • Hepatomegaly |
| • Neck vein distention | • Nocturnal cough |
| • Paroxysmal nocturnal dyspnea or orthopnea | • Pleural effusion |
| • Pulmonary rales | • Tachycardia (heart rate greater than 120 beats per minute) |
| • Third heart sound (S3 gallop) |
* Modified from reference 103. Diagnosis of heart failure requires the presence of two major criteria or one major and two minor criteria.
History and physical examination
Initial assessment of patient with suspected HFpEF includes history and physical examination. An important part of the history and physical evaluation is the scrutiny of clinical congestion which is the manifestation arising from heightened cardiac filling pressures. Addressing congestion is pivotal for adjusting medications and is intertwined with the quality of life and prognosis of HF patients. Various methods exist to evaluate clinical congestion, encompassing the presence of jugular venous distention, orthopnea, bendopnea, a square-wave response to the Valsalva maneuver, and leg edema.104-106 History and physical examination also help to identify the potential causes of clinical deterioration in stable HF, such as myocardial ischemia, pulmonary emboli, or systemic infection. History is important in identification of inherited cardiomyopathies through familial history or other specific conditions such as amyloid heart disease. Body weight should be evaluated because the severity of obesity is another important issue in HFpEF.
Laboratory test
Electrocardiography (ECG) is a routine examination for suspected HFpEF because the data of rhythm, heart rate, QRS morphology/duration provide vital information of the potential underlying causes and prognosis of HFpEF. Chest X-ray (CXR) is another valuable initial diagnostic tool. It enables the evaluation of cardiac size, pulmonary congestion, as well as pleural effusion. Additionally, it has the potential to unveil alternative pulmonary causes of the patient’s symptoms.107 Initial blood examination include a complete blood count, electrolytes, urea nitrogen, creatinine, glucose, fasting lipid profile, and liver function test. Other studies, including iron profile (serum iron, ferritin, transferrin saturation) and thyroid-stimulating hormone level, may be necessary to find coexisting conditions that are contributors or confounders of patients’ symptoms.
Recommendations
• A thorough history and physical examination should be performed to search for HF symptoms/signs. (COR I, LOE C)
• Initial evaluation should include 12-lead ECG and CXR. (COR I, LOE C)
• Initial blood examination includes complete blood count, renal function, electrolytes, lipid profile and liver function test. (COR I, LOE C)
HF biomarker
Natriuretic peptide assay, including B-type natriuretic peptide (BNP) or N-terminal proB-type natriuretic peptide (NT-proBNP), is commonly used to confirm the presence and severity of HFpEF. Generally, the levels of BNP and NT-proBNP are comparable, rendering either one is suitable for clinical use.108,109 When evaluating dyspnea of potentially cardiac origin, measuring BNP and NT-proBNP levels offers important diagnostic insight, particularly when the cause of dyspnea remains uncertain and physical examination yields inconclusive results. Normal BNP and NT-proBNP levels aid in excluding the diagnosis of HF, however, the natriuretic peptide levels are usually not increased in HF patients with obesity and diminish their diagnostic accuracy.110,111 Elevated levels of natriuretic peptide possess a strong positive predictive value for HFpEF diagnosis. But it is noteworthy that both BNP and NT-proBNP levels can rise in individuals with a range of noncardiac conditions.112,113 The normal levels of natriuretic peptide can be considered as a first-line tool to exclude the diagnosis of HF. To avoid overdiagnosis, we suggest NT-proBNP > 300 pg/mL or BNP > 100 pg/mL as the cut off values to diagnose HF in sinus rhythm and NT-proBNP > 600 pg/mL or BNP > 150 pg/mL for AF. In Taiwan, other biomarkers including galectin-3 and matrix metalloproteinase-2 were reported to be significantly associated with global cardiac fibrosis in HFpEF patients.114 Connective tissue growth factor was documented to be associated with the presence of HFpEF.115 Serum CA-125 was also mentioned to serve as a novel biomarker for HFpEF in women.116
Recommendations
• NT-proBNP or BNP should be measured in patients with suspected HFpEF. (COR I, LOE A)
• HF should be considered when NT-proBNP > 300 pg/mL or BNP > 100 pg/mL in sinus rhythm and NT-proBNP > 600 pg/mL or BNP > 150 pg/mL for AF. (COR I, LOE A)
Cardiac imaging
Cardiac imaging plays a pivotal role in the assessment of individuals with suspected HFpEF. Transthoracic echocardiography (TTE) stands as the most valuable initial diagnostic imaging tool. It determines LVEF which is the main criterion to differentiate HFrEF and HFpEF. TTE provides in-depth insights into cardiac structure and function. It identifies abnormalities within the myocardium, heart valves, and pericardium and the information could offer diagnostic value for HFpEF.117-119 TTE evaluation encompasses RV dimension and function, atrial size, and comprehensive valve analysis, addressing both anatomical and flow-related abnormalities. Diastolic function with estimated LV filling and LA pressures are also covered. Furthermore, indices reflecting myocardial deformation, including global longitudinal strain, have the potential to uncover subclinical LV systolic dysfunction.120-122
In addition to TTE, supplementary noninvasive imaging tools can be helpful in evaluating cardiac structure and function. Cardiac MRI offers a precise and consistently replicable evaluation of cardiac volumes, mass, and EF for both LV and RV.123 Cardiac MRI boasts exceptional anatomical resolution across all aspects of the heart and surrounding structures, without involving ionizing radiation. It is helpful in the diagnosis of HCM, cardiac amyloidosis, cardiac sarcoidosis, and Fabry disease.51,124-126 ECG-gated cardiac computed tomography (CT) similarly delivers accurate assessments of ventricular size, EF, and abnormalities of wall motion.127 Radionuclide ventriculography offers highly reproducible LVEF measurements, but exposes patients to ionizing radiation.128 Positron emission tomography (PET) is a non-invasive imaging technique that provides information on myocardial metabolism, perfusion, inflammation, and fibrosis.129
Recommendation
• Transthoracic echocardiography should be performed for cardiac structural and functional evaluation in patients with suspected HFpEF. (COR I, LOE A)
• Other imaging tools, such as CT, MRI, radionuclide study or PET could be considered in patients with suspected HFpEF. (COR I, LOE C)
Diagnostic algorithm of HFpEF
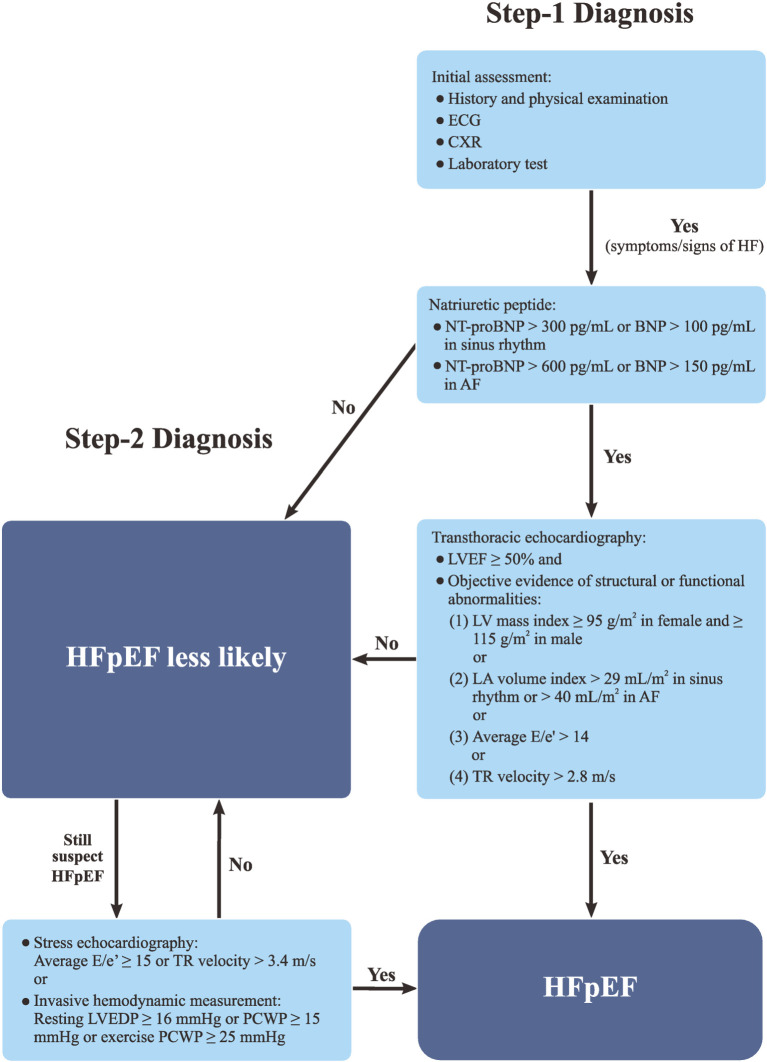
In this guideline, we proposed a 2-step diagnostic algorithm for HFpEF. When patients have HF symptoms and signs fulfilling the criteria of Framingham Diagnostic Criteria and HFpEF is suspected, step-1 work up should be started (Figure 1). NT-proBNP/BNP should be checked. The presence of cardiac structural or functional abnormalities should be evaluated with TTE. The structural abnormalities encompass an enlargement in LA size and/or volume or an increase in LV mass. The functional abnormalities indicate the presence of increased LV filling pressure. In step-1 diagnosis, the patients should fulfill all the following four criteria to make a diagnosis of HFpEF: (1) symptoms and/or signs of HF, (2) LVEF ≥ 50%, (3) increased natriuretic peptide (NT-proBNP > 300 pg/mL or BNP > 100 pg/mL in sinus rhythm and NT-proBNP > 600 pg/mL or BNP > 150 pg/mL in AF), (4) objective evidence of LA or LV abnormalities or raised LV filling pressure. The objective evidence of LA or LV abnormalities or raised LV filling pressure include: (1) LV mass index ≥ 95 g/m2 in female and ≥ 115 g/m2 in male, or (2) LA volume index > 29 mL/m2 in sinus rhythm or > 40 mL/m2 in AF, or (3) average E/e′ > 14, or (4) tricuspid regurgitation (TR) velocity > 2.8 m/s.1,100,130
Figure 1.
A 2-step diagnostic algorithm of HFpEF. AF, atrial fibrillation; BNP, B-type natriuretic peptide; CXR, chest X-ray; ECG, electrocardiography; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LVEDP, left ventricular end diastolic pressure; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal proB-type natriuretic peptide; PCWP, pulmonary capillary wedge pressure; TR, tricuspid regurgitation.
If patients do not fulfill the four criteria of step-1 diagnosis but HFpEF is still highly suspected or in any situation that uncertainty persists after step-1 diagnosis, step-2 diagnosis with stress test could be considered (Figure 1). An exercise stress test combined with echocardiographic evaluation of diastolic parameters should be performed. A diagnosis of HFpEF could be made if an average E/e′ ≥ 15 or a TR velocity > 3.4 m/s are observed after exercise.131 If the results of stress echocardiography is still questionable, an invasive hemodynamic measurement during rest and/or exercise could be considered. The invasive hemodynamic measurement serves as the golden criteria for the diagnosis of HEpEF. If a patient has a resting LV end diastolic pressure ≥ 16 mmHg or pulmonary capillary wedge pressure (PCWP) ≥ 15 mmHg or an exercise PCWP ≥ 25 mmHg, a definite diagnosis of HEpEF could be made.131
Recommendation
• In step-1 diagnosis, HFpEF can be diagnosed if patients have symptoms and/or signs of HF, LVEF ≥ 50%, increased NT-proBNP or BNP, and objective evidence of LA or LV abnormalities or raised LV filling pressure. (COR I, LOE A)
• Step-2 diagnosis using invasive or noninvasive stress test can be performed if HFpEF diagnosis is questionable. (COR I, LOE C)
TREATMENT
The major goals of treatment for patients with HFpEF are to reduce symptoms, improve functional status, and lower the risk of hospitalization for HF. Specific treatment of cardiomyopathy is beyond the scope of this guideline.
Non-pharmacological treatment
Weight reduction
Obesity, especially the central obesity, is associated with a higher incidence of HF and all-cause mortality.132 Obesity is recognized as an important phenotype of HFpEF and up to 80% of individuals with HFpEF are either overweight or have obesity.100,133 Physical inactivity and obesity are also linked with poor prognosis of HFpEF. Low cardiorespiratory fitness has been associated with a higher risk of HF across all categories of body mass index (BMI) and may explain about 50% of HF risk associated with BMI.134 Implementation of lifestyle modification to increase physical activity and weight reduction are important strategies to lower the risk of HF.
The treatment approach for HFpEF with obesity involves a combination of lifestyle modification, pharmacotherapy, and bariatric surgery. People who achieved ≥ 10% weight loss had a significant 24% risk reduction of nonfatal MI, stroke, hospitalization for HF, or CV death.135 The goal of lifestyle modification, including dietary change and regular physical activity, is to achieve weight loss while managing the symptoms and underlying causes of HF. Aerobic physical activity with 30-60 minutes of moderate to vigorous intensity in most days of the week is recommended for obese adults who want to achieve body weight loss. Lifestyle intervention with liraglutide have been reported to decrease more visceral adipose tissue compared with lifestyle intervention only.136 Pharmacotherapy for weight loss (liraglutide or orlistat) can be used for persons with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with adiposity-related complications, such as type 2 diabetes, nonalcoholic fatty liver disease or gout.137 In the Semaglutide Treatment Effect in People with obesity and HFpEF (STEP-HFpEF) randomized trial, administration of semaglutide to patients with HFpEF and obesity resulted in significant weight loss, enhancement in exercise capacity, greater decrease in NT-proBNP levels, and reduced occurrence of adjudicated HF events compared to the placebo group.138 The ongoing SUMMIT (A Study of Tirzepatide in Participants With Heart Failure With Preserved Ejection Fraction and Obesity) trial may provide important insight into the potential benefit and safety of pharmacological weight loss in HFpEF. Bariatric surgery can be considered for people with BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with at least 1 adiposity-related complication to reduce long-term overall mortality. Those with BMI ≥ 35 kg/m2 should be considered to refer to a multidisciplinary team of medical, surgical, and nutritional experts for obesity treatment.100
Recommendation
• Weight reduction is recommended for obese patients with HFpEF. (COR I, LOE B)
• Pharmacotherapy for weight reduction should be considered for obese patients with HFpEF to improve HF symptoms, exercise capacity, and quality of life. (COR I, LOE B)
Exercise training
A significant number of patients with HFpEF have multiple comorbidities which contribute to pathophysiology of HFpEF and hinder exercise capacity.31,139 Engaging in appropriate physical activity is widely recognized for its numerous beneficial effects on CV system and exercise-based therapy is emerging as a non-pharmacological intervention for HFpEF.140 Exercise training improves peak oxygen uptake and quality of life but causes no significant changes in LV systolic or diastolic function in patients with HFpEF.141 The 2022 AHA/ACC/HFSA heart failure guideline suggested a Class I recommendation for exercise training in patients with HF regardless of the LVEF.2 A meta-analysis of supervised exercise training in stable HFpEF patients indicated that regular aerobic exercise could significantly increase peak oxygen uptake by 14% and increase total exercise time by 21% in the exercise group compared with 1% decrease in the control group.142 Aerobic exercise also improved the six-minute walk distance by 9% compared with only 3% increase in control subjects.142 Regarding acute decompensated HF and hospitalized patients, the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial showed that early, transitional, tailored rehabilitation improved physical function and quality of life but had no beneficial effect on rehospitalization or death than usual care.143 Among the individuals in REHAB-HF study, patients with HFpEF might derive greater benefits from early physical rehabilitation for the outcomes of all-cause death, HF rehospitalization, and physical performance than those with HFrEF.144
However, there are some controversial issues regarding the exercise-based therapy in HFpEF. First, the evidence is still limited for exercise training to improve clinical outcomes and LV diastolic function of HFpEF. In addition, the setting and modalities of exercise training varied significantly among clinical trials and there is no standard protocol for the exercise-based therapy for HF. Despite the uncertainties, this guideline still recommends exercise training as a non-pharmacological therapy for HFpEF because it is safe and offers substantial improvement in exercise capacity and quality of life for patients with HFpEF. It is crucial to note that these exercise prescriptions should be supervised, individualized, and carefully monitored. A multidisciplinary approach involving collaboration among cardiologists, exercise physiologists, and physical therapists should be considered to optimize the implementation of exercise training and ensure patient safety.
Recommendation
• Supervised exercise training is recommended for patients with HFpEF. (COR I, LOE B)
Vaccination
Pneumonia and respiratory tract infection are important triggers of HF decompensation and cause hospitalization. The risk of pneumonia is high in HF patients especially for HFpEF.145 The infection events not only increase the in-hospital mortality of HF, but also results in poor long-term prognosis.146 Influenza and pneumococcal vaccines help to prevent respiratory infections that may probably reduce the risk of exacerbation of HF.
Numerous studies have provided evidence that the influenza vaccine has the potential to decrease CV morbidity and mortality in patients undergoing secondary prevention for CAD, particularly among the elderly population.147 In Taiwan, elderly MI patients who received influenza vaccination had a lower risk of all-cause mortality and hospitalization for HF.148 So far, there has been no large-scale randomized clinical trial to evaluate the efficacy of influenza vaccination in HF patients. Retrospective analysis of the Prospective Comparison of ARNI with angiotensin converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial demonstrated that HF patients received influenza vaccination had a significant lower risk of all-cause mortality, but the CV death and HF hospitalization did not reach statistical significance.149 The current European guidelines recommend annual influenza vaccinations for HF patients, especially the elderly.9
Previous observational study found that patients diagnosed with pneumococcal pneumonia faced a significant risk of acute CV events, including MI, arrhythmia, and development or worsening of HF.150 Currently, there are two types of vaccines for prevention of S. pneumoniae infection: the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PCV13). The effectiveness of vaccines in preventing invasive pneumococcal infections varies, ranging from approximately 56% to 75%.7,151 The efficacy for pneumococcal vaccination specifically for HF patients is not well established due to lack of large-scale randomized controlled trials. Based on expert consensus, in adults ≥ 65 years of age who have not previously received a pneumococcal vaccine, the administration of PCV13 followed by PPV23 1 year later is recommended for HF patients.
Recommendation
• Influenza and pneumococcal vaccinations are recommended for patients with HFpEF, especially for the elderly. (COR I, LOE B)
Management of comorbidities
HFpEF is usually associated with multiple comorbidities, such as hypertension, DM, CKD, CAD, or AF. Delivering therapy for underlying comorbidities and treating modifiable HF risk factors are mandatory for HFpEF treatment.
Hypertension and diabetes
Hypertension is the leading cause of HFpEF with a prevalence ranging from 60% to 89% in HFpEF.17 In line with the Hypertension Guideline of Taiwan Society of Cardiology, the systolic blood pressure target for HFpEF should be less than 130 mmHg.152 The patient’s other comorbidities, such as diabetes, CKD, or CAD, should guide the personalized choice of antihypertensive agents. The preferred agents for hypertension control in HFpEF include diuretics, angiotensin receptor blocker (ARB), and mineralocorticoid receptor antagonist (MRA) because these agents also have some beneficial effects for HFpEF in addition to blood pressure reduction. Since chronotropic incompetence is a potential mechanism contributing to exercise functional limitation in HFpEF, use of beta-blockers in HFpEF may be avoided if there are no other specific indications for beta-blockers.153 Given the recently demonstrated benefits of sodium-glucose cotransporter 2 (SGLT2) inhibitors in improving outcomes in patients with HFpEF,154-156 SGLT2 inhibitors should be prescribed as first-line therapy for diabetic patients with HFpEF. However, the use of SGLT2 inhibitors is not recommended if estimated glomerular filtration rate (eGFR) is < 20 mL/min/1.73 m2. SGLT2 inhibitors should also be avoided in all patients with type 1 diabetes, or in type 2 diabetes with prior diabetic ketoacidosis (DKA) or a condition predisposing to DKA, including pancreatic insufficiency, drug or alcohol addiction, and prolonged fasting. Metformin is also recommended as first-line therapy for glycemic control in diabetic patients with HFpEF. Given the substantial weight loss effect of the glucagon-like peptide-1 receptor agonists (GLP1RA), these agents should be considered for HFpEF patients with DM and obesity. Due to an increased risk of fluid retention, weight gain, and HF events, thiazolidinediones are relatively contraindicated in diabetic patients with HFpEF.
Recommendation
• Diuretics, ARB, and MRA are recommended for hypertension in patients with HFpEF. (COR IIa, LOE C)
• SGLT2 inhibitors are recommended for diabetes in patients with HFpEF. (COR I, LOE A)
• GLP1RA should be considered for diabetes and obesity in patients with HFpEF. (COR I, LOE B)
CKD, CAD and AF
Patients with HFpEF and CKD should be treated with evidence-based therapies that reduce the progression of CKD. SGLT2 inhibitors have been shown to improve renal outcome in patients with CKD. Although there is an expected initial decline in eGFR of approximately 4 mL/min/1.73 m2 when initiation of SGLT2 inhibitors, the rate of eGFR decline is slower compared to patients not on SGLT2 inhibitors after long term follow up.154-156 Clinical trials also demonstrated significant slow-down of eGFR decline in patients treated with ARB or angiotensin receptor-neprilysin inhibitor (ARNI).157 CAD is prevalent in HFpEF.158 To determine the severity of CAD and assess the need for revascularization, CT coronary angiography should be considered in patients with a low to intermediate pretest probability of CAD or those with equivocal non-invasive stress tests. Invasive coronary angiography may be considered in patients with an intermediate to high pretest probability of CAD. Medical therapies of CAD should be given according to the recommendations from the Guidelines of the Taiwan Society of Cardiology on the diagnosis and management of chronic coronary syndrome.7 Currently, there are no prospective randomized trials to evaluate the effect of revascularization on patients with HFpEF and CAD. For AF, anticoagulant remains the cornerstone therapy for AF and HFpEF to prevent stroke and the indication for anticoagulation is determined by CHA2DS2-VASc score. Randomized control trials did not demonstrate an advantage of rhythm control with antiarrhythmic medications over rate control.159 Ablation of AF may be a better strategy than antiarrhythmic medications for rhythm con-trol.160 This guideline recommends that patients with HFpEF and AF should have adequate rate control. Beta-blockers and non-dihydropyridine calcium-channel blockers are the usual first-line agents. A recent clinical trial demonstrated that digoxin may improve functional capacity and reduce more NT-proBNP over bisoprolol in AF patients with HF symptoms.161
Recommendation
• SGLT2 inhibitors, ARNI or ARB are recommended for CKD in patients with HFpEF. (COR I, LOE A)
• Beta-blockers, non-dihydropyridine calcium-channel blockers or digoxin are recommended for rate control in patients with HFpEF and AF (COR IIa, LOE B)
Pharmacological treatment
Diuretics
HFpEF patients with volume overload should be treated with diuretics.48 Loop diuretics should be initiated as the first-line therapy with the type and dose depending on the severity of congestion. For those patients with loop diuretic resistance, sequential nephron blockade can be achieved using thiazide diuretics and/or MRA.
Recommendation
• Diuretics are recommended for HFpEF patients with congestion to relief symptoms. (COR I, LOE C)
SGLT2 inhibitor
Both empagliflozin and dapagliflozin have shown their clear benefits in the management of HFpEF. The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) trial first demonstrated the advantages of empagliflozin over placebo on the reduction of HF hospitalization or CV death among 5988 patients with HF and LVEF > 40% (hazard ratio: 0.79, 95% confidence interval: 0.69-0.90).154 In addition, empagliflozin also reduced total HF hospitalization by 27% and delayed the decline of eGFR.154 The pre-specified analysis clearly showed empagliflozin reduced the risk of HF hospitalization or CV death by 17% in patients with LVEF ≥ 50%. The Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial investigated the effect of dapagliflozin in 6263 patients with HF and LVEF > 40% and showed similar results.156 Patients can be enrolled as outpatients or during hospitalization after stabilization for HF. The trial demonstrated a significant 18% reduction in the risk of the primary composite endpoint (CV death or worsening heart failure) with dapagliflozin, primarily due to the reduced HF events, but not CV mortality. The benefit of dapagliflozin were found to be consistent in the subgroup analyzes comparing patients with LVEF ≥ 60% and LVEF < 60% suggesting no attenuation of benefit in patients with higher LVEF.156 The therapeutic benefit with dapagliflozin was observed consistently, independent of age, BMI, frailty class, presence of AF, or New York Heart Association functional class. The time to first statistically significant reduction of the primary endpoint was only 13 days in DELIVER trial and 18 days in EMPEROR-Preserved study.154-156 SGLT2 inhibitor, empagliflozin or dapagliflozin, is recommended as a foundation therapy for HFpEF and should be initiated as early as possible.
Recommendation
• SGLT2 inhibitor, empagliflozin or dapagliflozin, is recommended for patients with HFpEF to reduce the risk of worsening HF event or CV death. (COR I, LOE A)
Renin-angiotensin system inhibitor and MRA
Randomized clinical trials of ACEI and ARB, including perindopril in the Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) trial,162 candesartan in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity-Preserved (CHARM-Preserved) trial163 and irbesartan in the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve) trial,164 failed to achieve a significant reduction in the primary outcome compared with placebo for patients with HFpEF. In the Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction (PARAGON-HF) trial, ARNI did not significantly reduce the risk of HF hospitalization or CV death compared with valsartan (p = 0.059), but the benefit of ARNI over ARB was greater in selected groups of HFpEF patients, such as recently hospitalized patients, women and those with an LVEF at or below the median value of 57%.165,166 For MRA, although the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial did not demonstrate mortality benefit or reduction in HF hospitalization in the whole study with LVEF 45% or greater, the benefits of spironolactone was shown at the lower end (up to 55%) of HFpEF.167 Its subgroup geographic analyses also demonstrated that there was a significant improved prognosis for the 1767 enrolled patients from north and south America.168 Several upcoming studies (FINEARTS-HF, SPIRRIT, and SPIRIT-HF) are still undergoing to clarify the definite role of MRAs, including finerenone or spironolactone, in HFpEF. An individual patient-level meta-analysis demonstrated the benefits of candesartan, spironolactone, and ARNI extending to the lower end of the LVEF range of HFpEF.169 Therefore, in selected patients with HFpEF, MRA and ARNI may be considered to decrease HF hospitalizations. An ARB may be considered for patients with HFpEF who are eligible for ARNI but cannot take it due to cost or intolerance. Combination therapy of ARNI, MRA and SGLT2 inhibitor is reasonable and was estimated to reduce CV death and HF hospitalization among HF patients with LVEF between 45% to 65%.170 An expert consensus from the Taiwan Society of Cardiology suggests a sequencing strategy starting with SGLT2 inhibitor first and combining it with ARNI if systolic blood pressure is ≥ 100 mmHg or MRA if systolic BP is < 100 mmHg for HFpEF treatment.10
Recommendation
• ARNI and MRA are recommended for selected groups of patients with HFpEF. (COR IIa, LOE B)
• ARB is recommended as an alternative for HFpEF who cannot tolerate or afford ARNI. (COR IIa, LOE B)
• Combination therapy of SGLT2 inhibitor, ARNI and/or MRA is reasonable in selected group of patients with HFpEF. (COR IIb, LOE C)
Beta-blocker
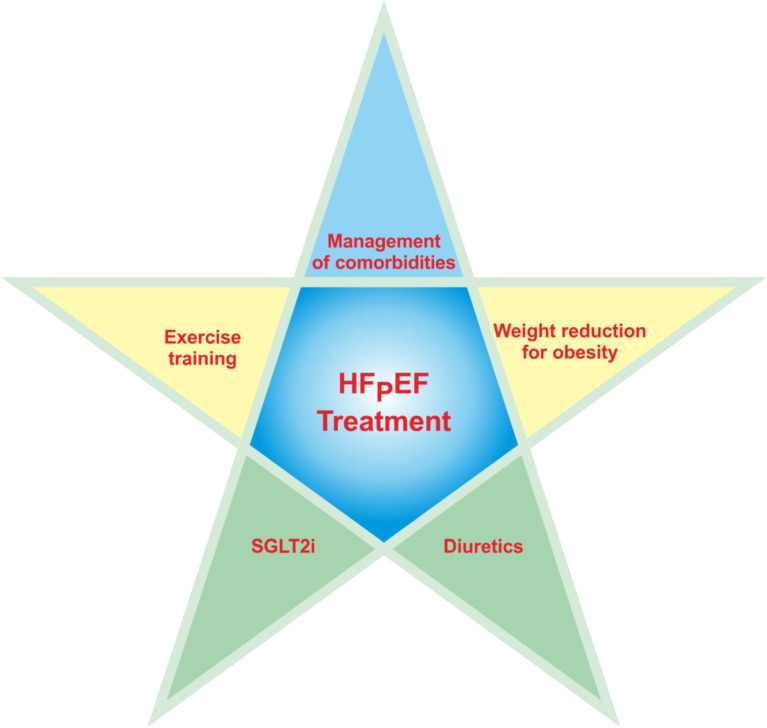
In the Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) study that compare digoxin with bisoprolol in AF patients with HF symptoms, the primary outcome of quality of life was comparable between patients treated with bisoprolol and digoxin at 6 months.161 But beta-blocker caused more adverse effects, such as dizziness, drowsiness, and hypotension than digoxin.161 Additionally, the improved functional capacity and lower NT-proBNP levels favored digoxin group at 12 months. An individual patient-level meta-analysis failed to demonstrate the benefit of beta-blockers over placebo among HFpEF patients.171 A beta-blocker is not recommended in patients with HFpEF without compelling indications, such as CAD, prior MI or rate control for AF, especially for those with chronotropic incompetence. Figure 2 summarizes the current major non-pharmacological and pharmacological therapies for HFpEF.
Figure 2.
Current major non-pharmacological and pharmacological therapies for HFpEF. HFpEF, heart failure with preserved ejection fraction; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Recommendation
• Beta-blocker is not recommended in patients with HFpEF without compelling indications (COR III, LOE C)
Early initiation strategy
Early initiation strategy means starting recommended HF pharmacological therapies as soon as possible, preferably at the clinic visit as the HF is definitely diagnosed or before discharge following HF hospitalization. The Safety, Tolerability and Efficacy of Up-titration of Guideline-directed Medical Therapies for Acute Heart Failure (STRONG-HF) trial, enrolled patients with HFrEF or HFpEF and introduced an intensive care strategy involving early initiation and up-titration of foundation medications for HF. The strategy has been shown to significantly reduce the rates of all-cause death and HF readmission within 180 days after acute decompensated HF hospitalization.172 In the trial, 15% patients were HFpEF and the clinical benefit of early initiation and up-titration strategy was observed regardless of the baseline LVEF is ≤ 40% vs. > 40%.173 It is important to note that STRONG-HF focused on triple therapy with renin-angiotensin system inhibitor, beta-blocker and MRA. For patients diagnosed with HFpEF in outpatient clinic or admitted due to acute HFpEF, an early initiation strategy should include the initiation of SGLT2 inhibitor, empagliflozin or dapagliflozin, and/or diuretics to mitigate the risk of HF readmission or mortality.
Recommendation
• An early initiation and titration strategy of HF foundation therapy before discharge and in the first few weeks following a HF hospitalization is recommended to reduce the risk of HF rehospitalization or death. (COR IIa, LOE B)
FUTURE PERSPECTIVE
HFpEF is an escalating public health concern accounting for more than half of all HF cases and marked by elevated morbidity and mortality.174 HFpEF often goes unnoticed and leads to extensive utilization of health resource. Treating HFpEF poses formidable challenges involving management of comorbidities, non-pharmacological treatment, and guideline-based medical therapies. In addition to current understanding, gaining more insight into the pathophysiology, including inflammation, vascular dysfunction, fibrosis, and tissue remodeling, is essential for developing novel diagnostic method and treatment of HFpEF.175 Research is now concentrating on comprehensive phenotyping of HFpEF patients and evaluating targeted treatment in various subtype of HFpEF.176 Employing machine learning with the help of artificial intelligence system will become a more rapid method to facilitate HFpEF diagnosis and aids in identifying subtypes with more precise intervention.177,178 Clinical trials with precision medicine approach in various phenotypes of HFpEF have been conducted.179 Multidisciplinary collaboration is pivotal for administering quality care for HFpEF. Capitalizing on evolving therapies and resolving diagnostic complexities will be a pivotal prospect to redefine HFpEF care. The recommendations in this guideline are proposed based on recent study results. Since the landscape is rapidly evolving, establishing new pathways for diagnosis and management of HFpEF driven by emerging clinical trial data is important in the near future.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 4.Rajadurai J, Tse HF, Wang CH, et al. Understanding the epidemiology of heart failure to improve management practices: an Asia-Pacific perspective. J Card Fail. 2017;23:327–339. doi: 10.1016/j.cardfail.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Chen JH, Yu WC, et al. 2012 guidelines of the Taiwan Society of Cardiology (TSOC) for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2012;28:161–195. [Google Scholar]
- 7.Ueng KC, Chiang CE, Chao TH, et al. 2023 guidelines of the Taiwan Society of Cardiology on the diagnosis and management of chronic coronary syndrome. Acta Cardiol Sin. 2023;39:4–96. doi: 10.6515/ACS.202301_39(1).20221103A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis GS, Cohn JN. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J. 1990;4:3068–3075. doi: 10.1096/fasebj.4.13.2210153. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 10.Chiang CE, Hung CL, Wu YW, et al. 2023 consensus of Taiwan Society of Cardiology on the pharmacological treatment of chronic heart failure. Acta Cardiol Sin. 2023;39:361–390. doi: 10.6515/ACS.202305_39(3).20230301A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes EB, Ha JW, Firdaus I, et al. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol. 2016;223:163–167. doi: 10.1016/j.ijcard.2016.07.256. [DOI] [PubMed] [Google Scholar]
- 12.Hung CL, Chao TF, Tsai CT, et al. Prevalence, incidence, lifetime risks, and outcomes of heart failure in Asia: a nationwide report. JACC Heart Fail. 2023;11:1454–1456. doi: 10.1016/j.jchf.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang TD, Huang ST, Wang CY, et al. Nationwide trends in incidence, healthcare utilization, and mortality in hospitalized heart failure patients in Taiwan. ESC Heart Fail. 2020;7:3653–3666. doi: 10.1002/ehf2.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 15.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 17.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 18.Tsao CW, Lyass A, Enserro D, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho JE, Enserro D, Brouwers FP, et al. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9:e003116. doi: 10.1161/CIRCHEARTFAILURE.115.003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142. doi: 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teo LY, Chan LL, Lam CS. Heart failure with preserved ejection fraction in hypertension. Curr Opin Cardiol. 2016;31:410–416. doi: 10.1097/HCO.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 22.McHugh K, DeVore AD, Wu J, et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:602–611. doi: 10.1016/j.jacc.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Ter Maaten JM, Damman K, Verhaar MC, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–598. doi: 10.1002/ejhf.497. [DOI] [PubMed] [Google Scholar]
- 24.John JE, Claggett B, Skali H, et al. Coronary artery disease and heart failure with preserved ejection fraction: The ARIC Study. J Am Heart Assoc. 2022;11:e021660. doi: 10.1161/JAHA.121.021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotecha D, Lam CS, Van Veldhuisen DJ, et al. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 26.Harrington J, Felker GM, Lingvay I, et al. Managing obesity in heart failure: a chance to tip the scales? JACC Heart Fail. 2024;12:28–34. doi: 10.1016/j.jchf.2023.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Piccirillo F, Crispino SP, Buzzelli L, et al. A state-of-the-art review on sleep apnea syndrome and heart failure. Am J Cardiol. 2023;195:57–69. doi: 10.1016/j.amjcard.2023.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Lee PL, Wu YW, Cheng HM, et al. Recommended assessment and management of sleep disordered breathing in patients with atrial fibrillation, hypertension and heart failure: Taiwan Society of Cardiology/Taiwan Society of Sleep Medicine/Taiwan Society of Pulmonary and Critical Care Medicine joint consensus statement. J Formos Med Assoc. 2024;123:159–178. doi: 10.1016/j.jfma.2023.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Kara Wegermann K, Fudim M, Henao R, et al. Serum metabolites are associated with HFpEF in biopsy-proven nonalcoholic fatty liver disease. J Am Heart Assoc. 2023;12:e029873. doi: 10.1161/JAHA.123.029873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teramoto K, Teng TK, Chandramouli C, et al. Epidemiology and clinical features of heart failure with preserved ejection fraction. Card Fail Rev. 2022;8:e27. doi: 10.15420/cfr.2022.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CY, Sung HY, Chen YJ, et al. Personalized management for heart failure with preserved ejection fraction. J Pers Med. 2023;13:746. doi: 10.3390/jpm13050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli E, Bourg C, Kosmala W, et al. Phenomapping heart failure with preserved ejection fraction using machine learning cluster analysis: prognostic and therapeutic implications. Heart Fail Clin. 2021;17:499–518. doi: 10.1016/j.hfc.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Tromp J, Teng TH, Tay WT, et al. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail. 2019;21:23–36. doi: 10.1002/ejhf.1227. [DOI] [PubMed] [Google Scholar]
- 35.Tromp J, Tay WT, Ouwerkerk W, et al. Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN-HF Registry. PLoS Med. 2018;15:e1002541. doi: 10.1371/journal.pmed.1002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CK, Lee JK, Chiang FT, et al. Prognostic factors of heart failure with preserved ejection fraction: a 12-year prospective cohort follow-up study. Int J Cardiol. 2014;171:331–337. doi: 10.1016/j.ijcard.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Chien SC, Lo CI, Lin CF, et al. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Fail. 2019;6:953–964. doi: 10.1002/ehf2.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC Study Community Surveillance. Circulation. 2018;138:12–24. doi: 10.1161/CIRCULATIONAHA.117.027551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark KAA, Reinhardt SW, Chouairi F, et al. Trends in heart failure hospitalizations in the US from 2008 to 2018. J Card Fail. 2022;28:171–180. doi: 10.1016/j.cardfail.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Desai AS, Claggett B, Pfeffer MA, et al. Influence of hospitalization for cardiovascular versus noncardiovascular reasons on subsequent mortality in patients with chronic heart failure across the spectrum of ejection fraction. Circ Heart Fail. 2014;7:895–902. doi: 10.1161/CIRCHEARTFAILURE.114.001567. [DOI] [PubMed] [Google Scholar]
- 41.Crespo-Leiro MG, Anker SD, Maggioni AP, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–625. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 42.Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1574–1585. doi: 10.1002/ejhf.813. [DOI] [PubMed] [Google Scholar]
- 43.Azad N, Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol. 2014;11:329–337. doi: 10.11909/j.issn.1671-5411.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung CL, Chao TF, Su CH, et al. Income level and outcomes in patients with heart failure with universal health coverage. Heart. 2021;107:208–216. doi: 10.1136/heartjnl-2020-316793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDonald MR, Tay WT, Teng TK, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF Registry. J Am Heart Assoc. 2020;9:e012199. doi: 10.1161/JAHA.119.012199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahim B, Kapelios CJ, Savarese G, Lund LH. Global public health burden of heart failure: an updated review. Card Fail Rev. 2023;9:e11. doi: 10.15420/cfr.2023.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgiopoulos G, Figliozzi S, Pateras K, et al. Comparison of demographic, clinical, biochemical, and imaging findings in hypertrophic cardiomyopathy prognosis: a network meta-analysis. JACC Heart Fail. 2023;11:30–41. doi: 10.1016/j.jchf.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 48.McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 49.Bayonas-Ruiz A, Muñoz-Franco FM, Sabater-Molina M, et al. Current therapies for hypertrophic cardiomyopathy: a systematic review and meta-analysis of the literature. ESC Heart Fail. 2023;10:8–23. doi: 10.1002/ehf2.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang YH, Lin YH, Yen RF, et al. 2021 advocacy statements for the role of 99mTc-pyrophosphate scintigraphy in the diagnosis of transthyretin cardiac amyloidosis: a report of the Taiwan Society of Cardiology and the Society of Nuclear Medicine of the Republic of China. Acta Cardiol Sin. 2021;37:221–231. doi: 10.6515/ACS.202105_37(3).20210420A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CC, Chang WT, Lin YH, et al. 2023 expert consensus of the Taiwan Society of Cardiology on the diagnosis and treatment of cardiac amyloidosis. Acta Cardiol Sin. 2023;39:511–543. doi: 10.6515/ACS.202307_39(4).20230610A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung CL, Wu YW, Lin CC, et al. 2021 TSOC expert consensus on the clinical features, diagnosis, and clinical management of cardiac manifestations of Fabry disease. Acta Cardiol Sin. 2021;37:337–354. doi: 10.6515/ACS.202107_37(4).20210601A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zile MR. Heart failure with preserved ejection fraction: is this diastolic heart failure? J Am Coll Cardiol. 2003;41:1519–1522. doi: 10.1016/s0735-1097(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 54.van Heerebeek L, Borbély A, Niessen HW, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 55.Borbély A, van der Velden J, Papp Z, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 56.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351:1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 57.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 58.Zile MR, Baicu CF, Bonnema DD. Diastolic heart failure: definitions and terminology. Prog Cardiovasc Dis. 2005;47:307–313. doi: 10.1016/j.pcad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 59.van Heerebeek L, Franssen CP, Hamdani N, et al. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293–302. doi: 10.1007/s11897-012-0109-5. [DOI] [PubMed] [Google Scholar]
- 60.Czuriga D, Paulus WJ, Czuriga I, et al. Cellular mechanisms for diastolic dysfunction in the human heart. Curr Pharm Biotechnol. 2012;13:2532–2538. [PubMed] [Google Scholar]
- 61.Wu CK, Lee JK, Hsu JC, et al. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:445–454. doi: 10.1002/ejhf.1617. [DOI] [PubMed] [Google Scholar]
- 62.Jin X, Hung CL, Tay WT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail. 2022;24:1346–1356. doi: 10.1002/ejhf.2513. [DOI] [PubMed] [Google Scholar]
- 63.Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 64.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borlaug BA, Lam CS, Roger VL, et al. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 68.Shah AM, Claggett B, Sweitzer NK, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation. 2018;137:148–161. doi: 10.1161/CIRCULATIONAHA.117.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cikes M, Claggett B, Shah AM, et al. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail. 2018;6:689–697. doi: 10.1016/j.jchf.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Kuo JY, Jin X, Sun JY, et al. Insights on distinct left atrial remodeling between atrial fibrillation and heart failure with preserved ejection fraction. Front Cardiovasc Med. 2022;9:857360. doi: 10.3389/fcvm.2022.857360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. doi: 10.1093/eurheartj/ehy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. doi: 10.1161/JAHA.114.001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Obokata M, Reddy YNV, Melenovsky V, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018;72:29–40. doi: 10.1016/j.jacc.2018.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohammed SF, Hussain S, Mirzoyev SA, et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phan TT, Shivu GN, Abozguia K, et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 78.Sarma S, Stoller D, Hendrix J, et al. Mechanisms of chronotropic incompetence in heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13:e006331. doi: 10.1161/CIRCHEARTFAILURE.119.006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorter TM, Obokata M, Reddy YNV, et al. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. doi: 10.1093/eurheartj/ehy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorter TM, Hoendermis ES, van Veldhuisen DJ, et al. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2016;18:1472–1487. doi: 10.1002/ejhf.630. [DOI] [PubMed] [Google Scholar]
- 81.Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammed SF, Hussain I, AbouEzzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorter TM, van Veldhuisen DJ, Bauersachs J, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:16–37. doi: 10.1002/ejhf.1029. [DOI] [PubMed] [Google Scholar]
- 85.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70:136–148. doi: 10.1016/j.jacc.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tartière-Kesri L, Tartière JM, Logeart D, et al. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 87.Yen CH, Lin JL, Sung KT, et al. Association of free fatty acid binding protein with central aortic stiffness, myocardial dysfunction and preserved ejection fraction heart failure. Sci Rep. 2021;11:16501. doi: 10.1038/s41598-021-95534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D'Amario D, Migliaro S, Borovac JA, et al. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol. 2019;10:1347. doi: 10.3389/fphys.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 90.Zhou KN, Sung KT, Yen CH, et al. Carotid arterial mechanics as useful biomarker of extracellular matrix turnover and preserved ejection fraction heart failure. ESC Heart Fail. 2020;7:1615–1625. doi: 10.1002/ehf2.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 92.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang W, Oliveira RKF, Lei H, et al. Pulmonary vascular resistance during exercise predicts long-term outcomes in heart failure with preserved ejection fraction. J Card Fail. 2018;24:169–176. doi: 10.1016/j.cardfail.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Saito Y, Obokata M, Harada T, et al. Disproportionate exercise-induced pulmonary hypertension in relation to cardiac output in heart failure with preserved ejection fraction: a non-invasive echocardiographic study. Eur J Heart Fail. 2023;25:792–802. doi: 10.1002/ejhf.2821. [DOI] [PubMed] [Google Scholar]
- 95.Haykowsky MJ, Brubaker PH, John JM, et al. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Molina AJ, Bharadwaj MS, Van Horn C, et al. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail. 2016;4:636–645. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139:1435–1450. doi: 10.1161/CIRCULATIONAHA.118.036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirkman DL, Bohmke N, Billingsley HE, Carbone S. Sarcopenic obesity in heart failure with preserved ejection fraction. Front Endocrinol (Lausanne) 2020;11:558271. doi: 10.3389/fendo.2020.558271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olivotto I, Udelson JE, Pieroni M, Rapezzi C. Genetic causes of heart failure with preserved ejection fraction: emerging pharmacological treatments. Eur Heart J. 2023;44:656–667. doi: 10.1093/eurheartj/ehac764. [DOI] [PubMed] [Google Scholar]
- 100.Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81:1835–1878. doi: 10.1016/j.jacc.2023.03.393. [DOI] [PubMed] [Google Scholar]
- 101.Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction in older adults. Heart Fail Clin. 2017;13:485–502. doi: 10.1016/j.hfc.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lam CSP, Arnott C, Beale AL, et al. Sex differences in heart failure. Eur Heart J. 2019;40:3859–368c. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 103.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 104.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 105.Thibodeau JT, Turer AT, Gualano SK, et al. Characterization of a novel symptom of advanced heart failure: bendopnea. JACC Heart Fail. 2014;2:24–31. doi: 10.1016/j.jchf.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 106.Felker GM, Cuculich PS, Gheorghiade M. The Valsalva maneuver: a bedside "biomarker" for heart failure. Am J Med. 2006;119:117–122. doi: 10.1016/j.amjmed.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 107.Badgett RG, Mulrow CD, Otto PM, Ramirez G. How well can the chest radiograph diagnose left ventricular dysfunction? J Gen Intern Med. 1996;11:625–634. doi: 10.1007/BF02599031. [DOI] [PubMed] [Google Scholar]
- 108.Zaphiriou A, Robb S, Murray-Thomas T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail. 2005;7:537–541. doi: 10.1016/j.ejheart.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 109.Booth RA, Hill SA, Don-Wauchope A, et al. Performance of BNP and NT-proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev. 2014;19:439–451. doi: 10.1007/s10741-014-9445-8. [DOI] [PubMed] [Google Scholar]
- 110.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 111.Mehra MR, Uber PA, Park MH, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 112.Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol. 2006;47:91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 113.Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 114.Wu CK, Su MM, Wu YF, et al. Combination of plasma biomarkers and clinical data for the detection of myocardial fibrosis or aggravation of heart failure symptoms in heart failure with preserved ejection fraction patients. J Clin Med. 2018;7:427. doi: 10.3390/jcm7110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu CK, Wang YC, Lee JK, et al. Connective tissue growth factor and cardiac diastolic dysfunction: human data from the Taiwan diastolic heart failure registry and molecular basis by cellular and animal models. Eur J Heart Fail. 2014;16:163–172. doi: 10.1002/ejhf.33. [DOI] [PubMed] [Google Scholar]
- 116.Hung CL, Hung TC, Liu CC, et al. Relation of carbohydrate antigen-125 to left atrial remodeling and its prognostic usefulness in patients with heart failure and preserved left ventricular ejection fraction in women. Am J Cardiol. 2012;110:993–1000. doi: 10.1016/j.amjcard.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 117.Nauta JF, Hummel YM, van der Meer P, et al. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20:1303–1311. doi: 10.1002/ejhf.1220. [DOI] [PubMed] [Google Scholar]
- 118.Al Saikhan L, Park C, Hardy R, Hughes A. Prognostic implications of left ventricular strain by speckle-tracking echocardiography in the general population: a meta-analysis. Vasc Health Risk Manag. 2019;15:229–251. doi: 10.2147/VHRM.S206747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen ZW, Huang CY, Cheng JF, et al. Stress echocardiography-derived E/e′ predicts abnormal exercise hemodynamics in heart failure with preserved ejection fraction. Front Physiol. 2019;10:1470. doi: 10.3389/fphys.2019.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Biering-Sorensen T, Biering-Sorensen SR, Olsen FJ, et al. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging. 2017;10:e005521. doi: 10.1161/CIRCIMAGING.116.005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 122.Huang CY, Lee JK, Chen ZW, et al. Inhaled prostacyclin on exercise echocardiographic cardiac function in preserved ejection fraction heart failure. Med Sci Sports Exerc. 2020;52:269–277. doi: 10.1249/MSS.0000000000002145. [DOI] [PubMed] [Google Scholar]
- 123.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 124.Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142:e7–e22. doi: 10.1161/CIR.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 125.Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021;107:1591–1599. doi: 10.1136/heartjnl-2019-316442. [DOI] [PubMed] [Google Scholar]
- 126.Pieroni M, Moon JC, Arbustini E, et al. Cardiac involvement in Fabry disease: JACC review topic of the week. J Am Coll Cardiol. 2021;77:922–936. doi: 10.1016/j.jacc.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 127.Kaniewska M, Schuetz GM, Willun S, et al. Noninvasive evaluation of global and regional left ventricular function using computed tomography and magnetic resonance imaging: a meta-analysis. Eur Radiol. 2017;27:1640–1659. doi: 10.1007/s00330-016-4513-1. [DOI] [PubMed] [Google Scholar]
- 128.van Royen N, Jaffe CC, Krumholz HM, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–850. doi: 10.1016/s0002-9149(97)89179-5. [DOI] [PubMed] [Google Scholar]
- 129.Saraste A, Knuuti J. PET imaging in heart failure: the role of new tracers. Heart Fail Rev. 2017;22:501–511. doi: 10.1007/s10741-017-9620-9. [DOI] [PubMed] [Google Scholar]
- 130.Chang SN, Sung KT, Huang WH, et al. Sex, racial differences and healthy aging in normative reference ranges on diastolic function in ethnic Asians: 2016 ASE guideline revisited. J Formos Med Assoc. 2021;120:2160–2175. doi: 10.1016/j.jfma.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 131.Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 132.Lin PL. Obesity and cardiovascular disease. Str Circ J. 2021;3:1–3. [Google Scholar]
- 133.Clerico A, Zaninotto M, Passino C, Plebani M. Obese phenotype and natriuretic peptides in patients with heart failure with preserved ejection fraction. Clin Chem Lab Med. 2018;56:1015–1025. doi: 10.1515/cclm-2017-0840. [DOI] [PubMed] [Google Scholar]
- 134.Pandey A, Cornwell WK, 3rd, Willis B, et al. Body mass index and cardiorespiratory fitness in mid-life and risk of heart failure hospitalization in older age:findings from the Cooper Center Longitudinal Study. JACC Heart Fail. 2017;5:367–374. doi: 10.1016/j.jchf.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 135.Wilding JPH, Jacob S. Cardiovascular outcome trials in obesity: a review. Obes Rev. 2021;22:e13112. doi: 10.1111/obr.13112. [DOI] [PubMed] [Google Scholar]
- 136.Santilli F, Simeone PG, Guagnano MT, et al. Effects of liraglutide on weight loss, fat distribution, and beta-cell function in bbese subjects with prediabetes or early type 2 diabetes. Diabetes Care. 2017;40:1556–1564. doi: 10.2337/dc17-0589. [DOI] [PubMed] [Google Scholar]
- 137.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 139.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135. doi: 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sachdev V, Sharma K, Keteyian SJ, et al. Supervised exercise training for chronic heart failure with preserved ejection fraction: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2023;147:e699–e715. doi: 10.1161/CIR.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 143.Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mentz RJ, Whellan DJ, Reeves GR, et al. Rehabilitation intervention in older patients with acute heart failure with preserved versus reduced ejection fraction. JACC Heart Fail. 2021;9:747–757. doi: 10.1016/j.jchf.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shen L, Jhund PS, Anand IS, et al. Incidence and outcomes of pneumonia in patients with heart failure. J Am Coll Cardiol. 2021;77:1961–1973. doi: 10.1016/j.jacc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 146.Chen CY, Lee CH, Lin HW, et al. Impact of infection-related admission in patients with heart failure: a 10 years national cohort study. Sci Rep. 2023;13:6941. doi: 10.1038/s41598-023-34028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fountoulaki K, Tsiodras S, Polyzogopoulou E, et al. Beneficial effects of vaccination on cardiovascular events: myocardial infarction, stroke, heart failure. Cardiology. 2018;141:98–106. doi: 10.1159/000493572. [DOI] [PubMed] [Google Scholar]
- 148.Wu HH, Chang YY, Kuo SC, Chen YT. Influenza vaccination and secondary prevention of cardiovascular disease among Taiwanese elders-a propensity score-matched follow-up study. PLoS One. 2019;14:e0219172. doi: 10.1371/journal.pone.0219172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vardeny O, Claggett B, Udell JA, et al. Influenza vaccination in patients with chronic heart failure: The PARADIGM-HF Trial. JACC Heart Fail. 2016;4:152–158. doi: 10.1016/j.jchf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis. 2007;45:158–165. doi: 10.1086/518849. [DOI] [PubMed] [Google Scholar]
- 151.Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Vaccine. 2015;33(Suppl 4):D60–D65. doi: 10.1016/j.vaccine.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 152.Wang TD, Chiang CE, Chao TH, et al. 2022 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. Acta Cardiol Sin. 2022;38:225–325. doi: 10.6515/ACS.202205_38(3).20220321A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palau P, Seller J, Domínguez E, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78:2042–2056. doi: 10.1016/j.jacc.2021.08.073. [DOI] [PubMed] [Google Scholar]
- 154.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 155.Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 157.Mc Causland FR, Lefkowitz MP, Claggett B, et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020;142:1236–1245. doi: 10.1161/CIRCULATIONAHA.120.047643. [DOI] [PubMed] [Google Scholar]
- 158.Rush CJ, Berry C, Oldroyd KG, et al. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6:1130–1143. doi: 10.1001/jamacardio.2021.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 160.Packer DL, Piccini JP, Monahan KH, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA. 2020;324:2497–2508. doi: 10.1001/jama.2020.23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 163.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 164.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 165.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 166.Vaduganathan M, Claggett BL, Desai AS, et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol. 2020;75:245–254. doi: 10.1016/j.jacc.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–462. doi: 10.1093/eurheartj/ehv464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 169.Dewan P, Jackson A, Lam CSP, et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail. 2020;22:898–901. doi: 10.1002/ejhf.1776. [DOI] [PubMed] [Google Scholar]
- 170.Vaduganathan M, Claggett BL, Inciardi RM, et al. Estimating the benefits of combination medical therapy in heart failure with mildly reduced and preserved ejection fraction. Circulation. 2022;145:1741–1743. doi: 10.1161/CIRCULATIONAHA.121.058929. [DOI] [PubMed] [Google Scholar]
- 171.Cleland JGF, Bunting KV, Flather MD, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26–35. doi: 10.1093/eurheartj/ehx564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400:1938–1952. doi: 10.1016/S0140-6736(22)02076-1. [DOI] [PubMed] [Google Scholar]
- 173.Pagnesi M, Metra M, Cohen-Solal A, et al. Uptitrating treatment after heart failure hospitalization across the spectrum of left ventricular ejection fraction. J Am Coll Cardiol. 2023;81:2131–2144. doi: 10.1016/j.jacc.2023.03.426. [DOI] [PubMed] [Google Scholar]
- 174.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329:827–838. doi: 10.1001/jama.2023.2020. [DOI] [PubMed] [Google Scholar]
- 175.Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9:242. doi: 10.3390/cells9010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shah SJ, Borlaug BA, Kitzman DW, et al. Research priorities for heart failure with preserved ejection fraction. Circulation. 2020;141:1001–1026. doi: 10.1161/CIRCULATIONAHA.119.041886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tromp J, Seekings PJ, Hung CL, et al. Automated interpretation of systolic and diastolic function on the echocardiogram: a multicohort study. Lancet Digit Health. 2022;4:e46–e54. doi: 10.1016/S2589-7500(21)00235-1. [DOI] [PubMed] [Google Scholar]
- 178.Chiou YA, Hung CL, Lin SF. AI-assisted echocardiographic prescreening of heart failure with preserved ejection fraction on the basis of intrabeat dynamics. JACC Cardiovasc Imaging. 2021;14:2091–2104. doi: 10.1016/j.jcmg.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 179.Heinzel FR, Shah SJ. The future of heart failure with preserved ejection fraction: deep phenotyping for targeted therapeutics. Herz. 2022;47:308–323. doi: 10.1007/s00059-022-05124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]