Abstract
Many children living with HIV in resource-limited settings remain undiagnosed and at risk for HIV-related mortality and morbidity. This article describes 5 key strategies for strengthening HIV case finding and linkage to treatment for infants, children, and adolescents. These strategies result from lessons learned during the Accelerating Children’s HIV/AIDS Treatment Initiative, a public–private partnership between the President’s Emergency Plan for AIDS Relief (PEPFAR) and the Children’s Investment Fund Foundation (CIFF). The 5 strategies include (1) implementing a targeted mix of HIV case finding approaches (eg, provider-initiated testing and counseling within health facilities, optimization of early infant diagnosis, index family testing, and integration of HIV testing within key population and orphan and vulnerable children programs); (2) addressing the unique needs of adolescents; (3) collecting and using data for program improvement; (4) fostering a supportive political and community environment; and (5) investing in health system–strengthening activities. Continued advocacy and global investments are required to eliminate AIDS-related deaths among children and adolescents.
Keywords: pediatrics, HIV case finding, HIV treatment, adolescents, resource-limited settings
INTRODUCTION
Although new HIV infections among children have declined by 47% since 2010,1–3 an estimated 160,000 children younger than 15 years acquire HIV annually.3 Without treatment, HIV infection is often rapidly progressive and fatal among infants, with an estimated 52% dying within 12 months of birth.4 Antiretroviral treatment (ART) can reduce this mortality by up to 75%.5–8 Beyond infancy, long-term survivors can remain undiagnosed throughout childhood and into adolescence.9 Although early mortality is very high among untreated infants infected prenatally and intrapartum, evidence suggests that infants infected during breastfeeding can survive much longer without treatment.10
The Accelerating Children’s HIV/AIDS Treatment (ACT) Initiative was a $200 million public–private partnership between the President’s Emergency Plan for AIDS Relief (PEPFAR) and the Children’s Investment Fund Foundation (CIFF) that aimed to dramatically scale-up pediatric HIV treatment coverage in 9 countries in Africa. Between 2014 and 2016, ACT successfully improved children’s access to HIV testing services and increased the number of children and adolescents living with HIV (C/ALHIV) on ART by 44%.11 Table 1 presents the percent increase in the number of children tested and diagnosed over the 2-year initiative in the 4 countries with complete data across all the finer age bands. The number of diagnosed children was highest during the first year of the initiative and tapered off because programs successfully identified the easiest to reach children and new pediatric cases became harder to find. ACT also improved the use of age-disaggregated data to better monitor HIV treatment coverage among infants, children, and adolescents.11
TABLE 1.
Trends in the Number of Children Tested and Diagnosed Under the ACT Initiative
| No. of Children Tested for HIV | No. of Children Diagnosed HIV-Positive | Percent Increase from October 2014 to September 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Country | Age of Child | FY15 | FY16 | FY17 | FY15 | FY16 | FY17 | Tested | Diagnosed |
|
| |||||||||
| Democratic Republic of the Congo | <01* | 2839 | 2558 | 3227 | 52 | 148 | 130 | 14% | 150% |
| 01–09 | 48,405 | 64,641 | 89,279 | 1120 | 1228 | 1448 | 84% | 29% | |
| 10–14 | 24,492 | 30,192 | 31,535 | 451 | 540 | 442 | 29% | −2% | |
| 15–19 | 106,059 | 108,631 | 98,577 | 1194 | 951 | 755 | −7% | −37% | |
| Lesotho | <01* | 9369 | 6716 | 8603 | 321 | 149 | 152 | −8% | −53% |
| 01–09 | 50,761 | 85,851 | 121,366 | 934 | 728 | 685 | 139% | −27% | |
| 10–14 | 38,919 | 51,454 | 69,165 | 752 | 521 | 490 | 78% | −35% | |
| 15–19 | 66,118 | 76,020 | 97,141 | 2131 | 1741 | 1741 | 47% | −18% | |
| Zambia | <01* | 50,008 | 56,256 | 65,190 | 2626 | 1914 | 1925 | 30% | −27% |
| 01–09 | 145,126 | 263,136 | 491,206 | 9650 | 6667 | 7696 | 238% | −20% | |
| 10–14 | 138,291 | 183,059 | 412,674 | 6162 | 4476 | 5223 | 198% | −15% | |
| 15–19 | 487,734 | 425,937 | 736,369 | 25,266 | 16,256 | 15,880 | 51% | −37% | |
| Zimbabwe | <01* | 52,148 | 37,324 | 34,306 | 2023 | 1331 | 1103 | −34% | −45% |
| 01–09 | 20,788 | 95,790 | 116,658 | 1692 | 3872 | 3915 | 461% | 131% | |
| 10–14 | 52,591 | 104,295 | 163,275 | 972 | 2410 | 2934 | 210% | 202% | |
| 15–19 | 205,290 | 288,968 | 354,440 | 8198 | 9817 | 9626 | 73% | 17% | |
<01 results from the PEPFAR monitoring, evaluation, and reporting (MER) indicator on HIV testing was replaced with results from the indicator on early infant diagnosis.
This article describes 5 key strategies for improving pediatric case finding and linkage to treatment based on lessons learned during the ACT initiative.
WHAT ARE EXISTING GAPS IN HIV CASE FINDING AMONG CHILDREN AND ADOLESCENTS?
Although diagnosis of C/ALHIV has significantly improved through ACT and other initiatives, there remain specific subpopulations with unique challenges.
HIV-Exposed Infants
Current WHO guidelines recommend virologic testing for HIV-exposed infants (HEIs) using a DNA polymerase chain reaction test at 4–6 weeks of age to optimize detection of intrauterine, intrapartum, and early postnatal HIV transmission.12 In 2016, only 50% of HEI in 21 priority countries received early infant diagnostic (EID) testing by 2 months.13 Lack of efficient systems for sample collection, transport, and processing lead to long turnaround times between laboratories and facilities, prolonging diagnosis and increasing the likelihood that infected infants will die or become lost-to-follow-up.14–16 Poor retention of HIV-infected mothers also contributes to low EID coverage.17 Even when mothers do return for clinical appointments, lack of systems for linking mothers to their infants creates missed opportunities for testing infants.
About half of new infant infections are estimated to occur during the breastfeeding period (Fig. 1).13,18 Improving adherence and retention support to ensure that women remain virally suppressed, and strengthening routine HIV testing throughout pregnancy and breastfeeding to detect incident maternal HIV infection are key to preventing these infections. Offering sexual partner testing can also reduce HIV acquisition among pregnant and breastfeeding women involved in serodiscordant relationships.19 Finally, ensuring that infants receive an HIV test and final diagnosis once breastfeeding has ceased is critical to identify infants infected during the breastfeeding period.
FIGURE 1.
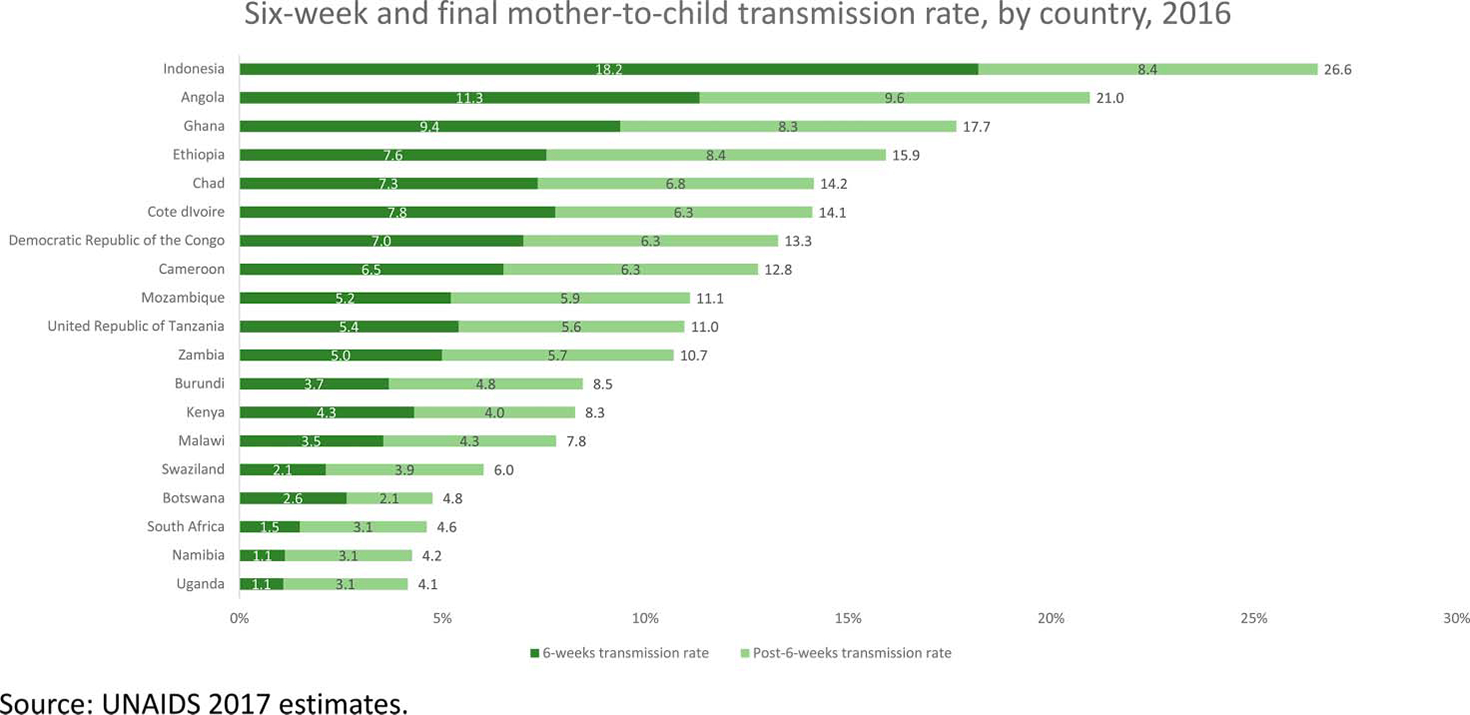
Mother-to-child HIV transmission rates at 6 weeks and after cessation of breastfeeding by country, 2016.
Perinatally Infected Children
Up to a third of HIV-infected infants are slow progressors, with a median survival of 16 years without treatment.20 These perinatally infected children may only receive HIV testing and diagnosis when they develop severe illness. Although provider-initiated testing and counseling (PITC) at health facilities is recommended to identify these children, barriers that prevent optimal implementation include lack of clarity on the definition of a legal guardian, age of consent, limited trained staff, and shortages of rapid test kits.21 Facility-based PITC may also be insufficient on its own to identify all undiagnosed C/ALHIV. In Zimbabwe, a substantial burden of undiagnosed C/ALHIV was found in the community even after 2 years of optimized PITC at nearby facilities.22
Adolescents
The number of ALHIV (aged 10–19 years) is growing because of the “aging up” of children perinatally infected in the early 2000’s and to the population boom resulting in the largest generation of adolescents in history.3 Although a third of new HIV infections occur in young people aged 15–24 years, data from recent population-based HIV impact assessment (PHIA) surveys in Lesotho, Malawi, Swaziland, Uganda, Tanzania, Zambia, and Zimbabwe support a growing body of evidence that adolescents have lower rates of HIV diagnosis, ART initiation, and viral suppression than other populations (Fig. 2).23 Barriers to adolescent uptake of HIV testing include stigma, poor health care provider attitudes, parental consent requirements, and inadequate risk perception.24
FIGURE 2.
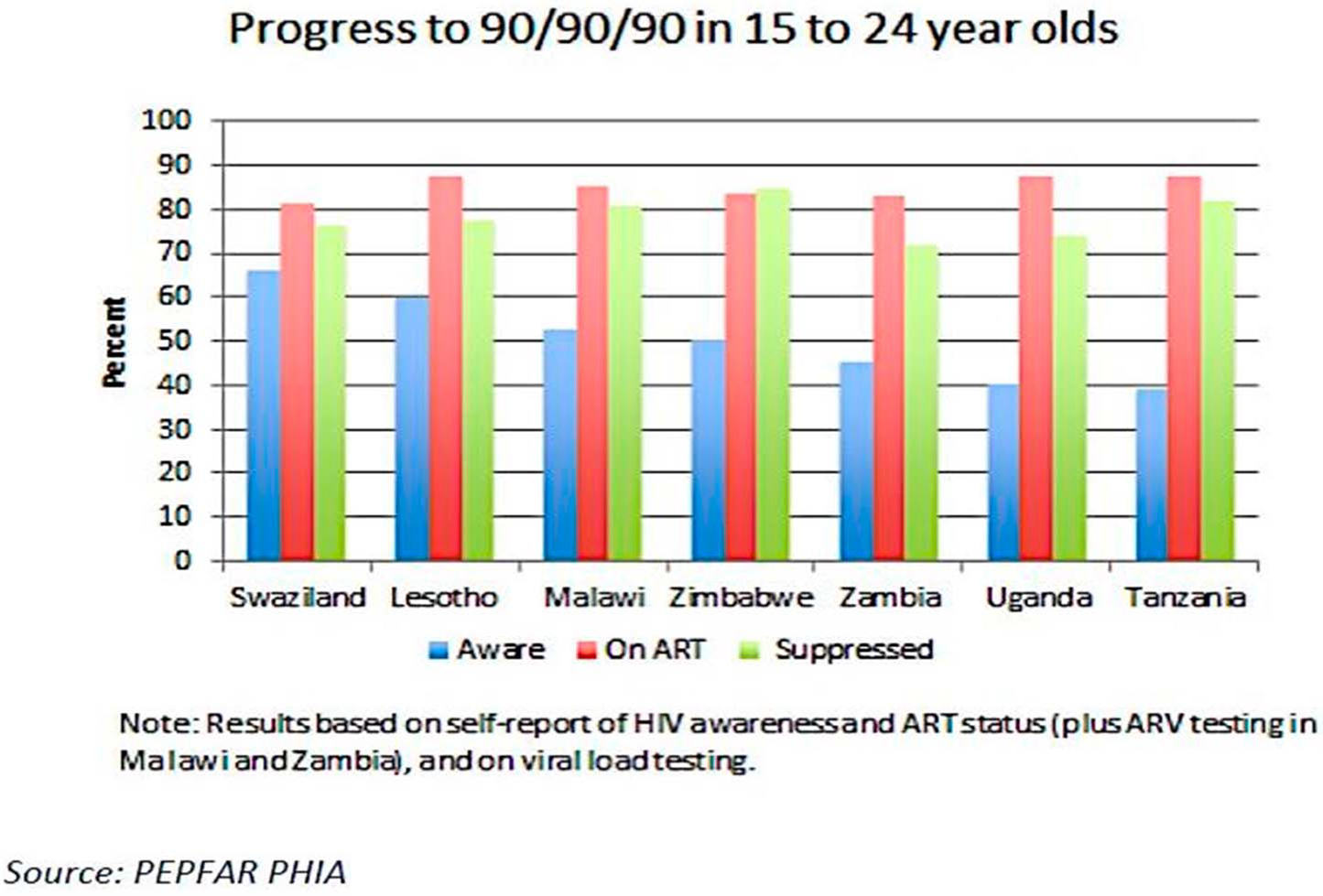
Progress to 90/90/90 among 15- to 24-year-olds in Swaziland, Lesotho, Malawi, Zimbabwe, Zambia, Uganda, and Tanzania (2016 population-based HIV impact assessments).
FIVE KEY STRATEGIES FOR ADDRESSING GAPS IN PEDIATRIC AND ADOLESCENT CASE FINDING AND LINKAGE TO TREATMENT
The ACT initiative sought to address these gaps through a variety of interventions. Below, we summarize 5 key strategies for improving pediatric case finding and linkage to treatment based on lessons learned from this initiative.
Strategy 1: Implement a Targeted Mix of Approaches to Strengthen HIV Case Finding and Linkage to Treatment
Approaches for strengthening pediatric case finding are summarized in Table 2 and in the text below.
TABLE 2.
Strategies for Improving HIV Case Finding and Linkage to Treatment Among Infants, Children, and Adolescents
| Strategies for Improving HIV Case Finding Among Infants, Children, and Adolescents |
|
|
| Scale-up PITC within health facilities in TB clinics, nutrition centers, and pediatric inpatient clinics |
| Consider HIV risk screening tools to optimize testing in facilities |
| In high-prevalence settings (>5% adult HIV prevalence), test mothers or infants attending immunization or under-5 clinics to identify HEIs |
| Use strategies that identify HIV-exposed children through connection to adults and other children living with HIV |
| Optimize PMTCT and EID for HEIs |
| Test all biologic children of adults and siblings receiving any HIV service (PMTCT and ART) through facility- or home-based family index testing |
| Test the children of key and vulnerable populations |
| Implement risk screening for all OVC |
| Offer partner notification services to adolescents |
| Consider other innovative approaches, such as HIVST, social network testing, and the use of incentives |
| Collaborate with community leaders, ALHIV networks, community members, and civil society organizations to ensure that HTS and linkage strategies meet the needs of infants, children, adolescents, and caregivers |
|
|
| Strategies for Strengthening Linkage to HIV Treatment for Infants, Children, and Adolescents |
|
|
| Provide same day ART initiation |
| Offer intensified post-test counseling on importance of starting ART |
| Provide patient escort between HIV testing and ART services |
| Offer peer navigation/case management services |
| Conduct follow-up (phone/home visit) for clients who fail to link within 14 d |
| Use ART starter packs in remotely located HIV testing sites |
| Systematically document and monitor linkage to treatment using individual-level registers and indicators |
Scale-Up PITC Within Health Facilities
Routinely testing all children and adolescents attending clinics where HIV may be an underlying cause of illness is highly effective for identifying C/ALHIV who were either missed during the early postnatal period, or who acquired HIV during breastfeeding.9 Several systematic reviews have found high prevalence rates among children tested in TB clinics, pediatric inpatient wards, malnutrition centers, and outpatient departments.9,25
In high-prevalence settings (>5% adult HIV prevalence), screening mothers or infants attending immunization or under-5 clinics may be another approach for identifying HEI.26,27 Mothers (preferably) or newborns can be screened for HIV infection or exposure, respectively, using an HIV rapid serologic test. An HIV virologic test can then be offered to all infants who test positive or whose mothers are HIV infected. Although this strategy was found to be effective in high-prevalence settings such as South Africa,28 it may not be as effective in settings with low HIV prevalence and/or high PMTCT coverage.
Risk screening tools may help optimize PITC by identifying high-risk children in need of an HIV test.29 In Zimbabwe, a risk screening tool increased the percent of children testing HIV-positive from 1.4% to 4.7%. It also reduced the number of children required to be tested to identify one CLHIV from 22 to 10.30 This tool, however, was designed to diagnose perinatally infected adolescents who are symptomatic from untreated, longstanding HIV infection. It may not effectively identify asymptomatic perinatally infected children or newly infected adolescents who are likely to be asymptomatic. Adolescent risk screening tools may need additional questions on sexual health. Sensitive questions on sexual behavior or genitourinary symptoms have been challenging to operationalize in busy clinics, while still maintaining client privacy and confidentiality.29,30 Further operational research is needed to validate tools for different populations and contexts and on how best to implement this approach.
Optimize EID for HIV-Exposed Infants
Several approaches have improved EID including point of care (POC) EID testing, establishing sample transport hubs, deploying mentor mothers to increase retention of mother–infant pairs, and using mobile health strategies to return results to facilities.31,32 In Malawi, POC EID testing was shown to increase the proportion of HIV-positive infants initiating ART within 60 days of sample collection from 41.9% to 91.1%,33 suggesting the need to evaluate strategies for increasing the availability of POC EID testing. This includes use of GeneXpert platforms, which have not been used to full capacity for TB diagnosis. Adding infant testing information to the monitoring tools used to track HIV-infected mothers can help link mother–infant pairs and facilitate identification of HEI in need of an HIV test.31 The World Health Organization (WHO) recommends consideration of a virologic test at birth (known as “birth testing”) to detect in utero infections.19 Birth testing is not a replacement for the 4- to 6-week test and should only be adopted by countries who have achieved at least 80 percent coverage of the 4- to 6-week test, with timely return of results.
Test All Biologic Children of Adults and Siblings Receiving ART Through Family Index Testing
Family index testing (FIT) involves health service providers actively offering HIV testing to the biologic children of adults and siblings receiving HIV services.34,35 In a systematic review of 21 studies, FIT resulted in a 3.3% yield among CLHIV.25 In Malawi, 90% of CLHIV identified through FIT linked to treatment.36 FIT may also facilitate disclosure and communication within the family and improve adherence and retention in HIV clinical care.34
Despite its demonstrated effectiveness, FIT remains inadequately scaled-up. In Malawi, only 20% of children of adult patients with ART had received HTS,37 and only 36% of hospitalized children with HIV-infected parents received HTS in Uganda.38 Parents may be reluctant to have their children tested for fear of disclosing their own status. In Kenya, 42% of HIV-infected adults had children with unknown status. Only 14% of these adults allowed HTS for their children. Yet, high HIV prevalence rates (7.4%) were observed among the children tested.39
Reinforcing the importance of testing partner(s) and biologically exposed children at every clinic visit and offering facility- or home-based family HTS can help improve uptake of FIT. In the Malawi-based Tingathe Program, community health workers offer home-based HTS to partners and family members of ART clients, leading to a 10-fold increase in the number of CLHIV enrolled in pediatric treatment.40 A recent pooled analysis from Demographic and Health Surveys in sub-Saharan Africa found that adolescents aged 15–17 years who had lost their mother, lost both parents, or had an HIV-infected mother were 2–3 times more likely to test HIV-positive.36,41 Testing adolescents up to 15 years as part of household-based FIT can be a targeted strategy for diagnosing ALHIV.
Test the Children of Key and Vulnerable Populations
Testing the infants and children of key population (KP) living with HIV, particularly HIV-infected female sex workers and injection drug users, is another important strategy for improving pediatric case finding among hard-to-reach populations. Although this is an aspect of family index testing, many programs fail to include KP children in their index testing efforts, and many KP programs do not systematically provide counseling about the need to test infants and children as part of their routine services. In Cameroon, a program to test the children of HIV-positive female sex workers at community-based drop-in centers found a 6.1% prevalence, highlighting the potential utility of this approach (G. Fouda, personal communication, 4 May 2017). No studies on this approach seem to have been published, however.
Implement Risk Screening for All Orphan and Vulnerable Children
Orphaned children are at high risk for HIV from vertical transmission and, because of their economic vulnerability, may also be at risk from horizontal HIV transmission through sexual abuse or early sexual debut.42,43 In Zimbabwe, 18% of orphaned children attending a community-based orphan and vulnerable children (OVC) program tested HIV-positive, suggesting many undiagnosed children may be enrolled in these programs.43 HIV risk screening tools, based on behavioral and clinical factors, may help identify OVC in need of an HIV test, as described above.30
Link All HIV-Infected Infants, Children, and Adolescents to HIV Treatment Services
An HIV diagnosis without linkage to treatment confers no benefit to C/ALHIV. Strengthening linkage to treatment requires close coordination between HIV testing and treatment programs for a successful “hand-off.” After diagnosis, HTS programs can assume responsibility for linking C/ALHIV to treatment. After linkage, HIV treatment programs then assume responsibility for retaining C/ALHIV through ART initiation, clinical monitoring, and viral suppression. Designating specific linkage navigators or counselors within HTS and ART programs can facilitate communication between the 2 programs and ensure linkage takes place.11
Several promising practices to improve linkage to treatment among adults have been identified (Table 2), although further evaluation is needed to understand their utility among C/ALHIV. These include same day ART initiation,44–46 POC EID technologies,33 intensified pre- and post-test counseling,47,48 tracking mother–infant pairs,41,49 patient escorts from HTS to ART services,50,51 and family-based ART services.52,53 Follow-up phone calls or home visits, mobile text appointment reminders, and case management can also help link children who fail to enroll in ART services within 14 days of diagnosis.53–56 Use of 2-week ART starter packs within community testing programs has been proposed to improve linkage in adults but has not been widely evaluated among children.57 Patient-level registers and indicators allow for HTS programs to actively track whether C/ALHIV link to treatment and identify those who may need additional linkage support.58 These tools can also ensure that minimum benchmarks for linkage to treatment are being met and trigger quality improvement efforts in the event of underperformance.
Strategy 2: Address the Unique Needs of Adolescents to Support HIV Case Finding and Linkage to Treatment
Adolescents remain the only age group for which HIV-related deaths are increasing.3 To improve HIV case finding among adolescents, it is important to address identification of undiagnosed perinatally infected children and newly infected adolescents, who may be asymptomatic when diagnosed. Improving coverage of facility-based PITC, with the addition of entry points for sexual and reproductive health services, is critical for identifying ALHIV. Effective community-based testing is also needed because adolescents may not present to facilities unless they are very sick or become pregnant.
Partner notification is another strategy for targeted testing among adolescents.59 Active notification, whereby a health care provider assists ALHIV to notify and test their sexual partner(s), should be prioritized because passive strategies that rely solely on the adolescent to bring their partner(s) to the facility for an HIV test are less likely to be effective among adolescents who do not feel empowered to disclose to their partner(s). This is especially true if the partner is older.60
HIV self-testing (HIVST) has a high acceptance rate among older adolescents,61 and adolescents are more likely to accurately use oral self-test kits.62 Although HIVST holds potential to increase HTS coverage among adolescents, OraQuick, the most common HIVST kit, is only recommended for persons 17 years and older.63 Operational research will be needed to identify dissemination strategies and ensure that adolescents screening positive are linked to diagnostic HTS and treatment services, if confirmed positive.
Community campaigns pairing mobile HIV testing with other wellness services, such as SEARCH and POP-ART-Y, identified large numbers of previously undiagnosed adolescents.64,65 The proportion of ALHIV who knew their HIV-positive status increased 3-fold during the POP-ART-Y study in Zambia,64 and the majority (58%) of adolescents diagnosed during Project SEARCH in Uganda and Kenya were new diagnoses.65 Pairing HTS with other services may help destigmatize HIV testing.66 However, because of lower HIV prevalence among adolescents and the high cost of these campaigns, this strategy may need to be limited to “hot spots” (areas of high prevalence and low HTS coverage) and/or very remote areas.
HIV testing in schools has been widely accepted by parents.67 Yet, some countries face challenges with coordination between the Ministries of Health and Education, with HIV testing not allowed on school property.68 In studies, HIV prevalence was quite variable across schools, ranging from 1.9% to 8.3% in South Africa to only 1.4% in Botswana.69–72 Further evaluation of school-based testing models is needed to better understand the utility of this approach.
Other innovative approaches for improving adolescent HTS coverage should be explored, including demand creation through use of edutainment and social media.73 Social network testing, in which HIV-positive and high-risk, HIV-negative individuals recruit others from their social, sexual, and drug-using networks for HTS, is an effective approach among KPs.74,75 It may also be effective among adolescents. Although economic incentives to caregivers increased HIV testing uptake among children and adolescents,76,77 more research is needed on the scalability and sustainability of this approach.
Adolescents have lower rates of linkage to and retention in ART services than adults. Providing a comprehensive set of adolescent-friendly HIV testing and treatment services including adherence counseling and support, sexual and reproductive health services, and mental health support can help improve linkage and retention among adolescents.78 Support from health care workers is also critical.68 Involving adolescents in the design, delivery, and evaluation of HIV testing, treatment, and support services can ensure these programs address their unique needs.79
Strategy 3: Collect and Use Data to Strengthen Programming
Developing effective tools and systems to collect and review strategic information including epidemiologic, program, costing, and surveillance data will further optimize pediatric case finding. This information can be used to conduct real-time analyses and shift programs toward effective approaches.80,81 Information to track includes coverage and absolute number of new diagnoses to identify where there may be capacity to expand services, the yield (proportion of children who test HIV-positive), and cost per diagnosis. Disaggregation of data by age and sex allows for programs to concentrate services on populations with low testing coverage and greatest unmet need for treatment. Subnational and site-level data analysis can identify hot spots where new diagnoses of C/ALHIV are occurring and enable geographic targeting of HTS.
Strategic information can also inform quality improvement. HTS programs can set benchmarks around diagnosis and linkage to treatment for pediatric populations, use their programmatic data to monitor their progress toward achieving these benchmarks, and develop interventions to improve performance, as needed. Program managers at regional and district levels can provide supportive supervision and mentoring to health facilities and HTS programs struggling to meet performance targets.82
Strategy 4: Foster a Supportive Political and Community Environment
Supportive policy implementation is crucial to improve access to HIV testing and treatment services among C/ALHIV. In many countries, the age of consent for HTS is 18 years, which can limit access among younger adolescents.83 In some settings, the age of consent for HTS is lower than the age of consent for HIV treatment, hindering linkage to treatment for ALHIV.24,84 Continued advocacy for polices that lower the age of consent for testing and treatment is needed to improve adolescent access to these services. Clarification within national guidelines on who can consent for a minor to receive HIV testing and treatment is also needed. In many cases, the biological parent is not available to consent due to death or work-related travel. Health care providers are often reluctant to test children without the consent of one or both biologic parents, which can lead to missed opportunities for pediatric testing.83 Polices allowing for task shifting of rapid HIV testing among children over 18 months to lay counselors and community health workers can help ease human resource constraints and improve HTS access.84
Collaboration with community leaders and members, ALHIV networks, and civil society organizations is also essential to ensure that HIV testing and treatment services meet the needs of C/ALHIV and their caregivers.85 Community members can advocate for policy change, drive demand for services, and assist with linkage to and retention in ART services. Community scorecards can bridge the demand side (“service user”) and the supply side (“service provider”) to jointly address service delivery barriers.86 In Mozambique, community scorecards improved pediatric treatment services through reduced wait times and increased ARV availability.11
Strategy 5: Invest in Health Systems Strengthening
Pediatric HTS requires an adequate supply of essential commodities, including rapid test kits and EID materials. Yet, many HTS programs report frequent commodity stock-outs.87 Regular monitoring and management of the entire supply chain system—including stock levels, forecasting, procurement, storage, and delivery—is critical to address supply chain gaps and to ensure that the right commodities are in the right place at the right time.87
Avoiding misdiagnosis of HIV—both false positive and false negative results— among children and adolescents is critically important to prevent mistakenly placing an HIV-uninfected child on ART or missing a lifesaving diagnosis.88,89 Inconclusive results, while an issue at all ages, can be fatal for infants. In one study, 17% of infants with inconclusive results died due to delayed delivery of a final HIV-positive diagnosis.90 Collaboration between HTS programs and laboratory personnel to implement quality assurance measures including competency-based certification of testers, proficiency testing, and retesting for verification before ART initiation is needed to prevent incorrect or inconclusive results.91
CONCLUSIONS
In summary, despite recent success in improving pediatric case finding, many C/ALHIV in resource-limited settings remain undiagnosed and at substantial risk for HIV-related mortality and morbidity. To achieve epidemic control, national and regional programs will need to measure their progress toward achieving international benchmarks across all age and sex categories. This includes ensuring 90% of C/ALHIV have been diagnosed, 90% of those diagnosed are initiated on ART, and 90% of those on ART achieve viral suppression.92 The strategies described in this article result from lessons learned from the ACT initiative, which successfully improved pediatric case finding and treatment in 9 African countries. These strategies, if implemented at scale and with fidelity, can assist countries to achieve international benchmarks for pediatric populations. Continued advocacy and global investments are required to eliminate AIDS-related deaths among children and adolescents.
Acknowledgments
Supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention, the United States Agency for International Development, or the U.S. Government.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kim MH, Ahmed S, Abrams EJ. Paediatric HIV: progress on prevention, treatment, and cure. Curr Pediatr Rep. 2015;3:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chibwesha CJ, Chi BH. Expanding coverage of paediatric HIV testing. Lancet. 2016;3:e451–e452. [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV and AIDS (UNAIDS). Fact Sheet for World AIDS Day 2017. Geneva, Switzerland; 2018. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed March 5, 2018. [Google Scholar]
- 4.Newell ML, Coovadia H, Cortina-Borja M, et al. Ghent international AIDS society (IAS) working group on HIV infection in women and children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV infected infants. N Engl J Med. 2008;359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luzuriaga K, McManus M, Mofenson L, et al. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–2480. [DOI] [PubMed] [Google Scholar]
- 7.Goetghebuer T, Haelterman E, Le Chenadec J, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23:597–604. [DOI] [PubMed] [Google Scholar]
- 8.Joint United Nations Programme on HIV and AIDS (UNAIDS). 2015 Progress Report on the Global Plan. Geneva, Switzerland; 2015. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf. Accessed November 20, 2017. [Google Scholar]
- 9.Cohn J, Whitehouse K, Tuttle J, et al. Paediatric HIV testing beyond the context of prevention of mother-to-child transmission: a systematic review and meta-analysis. Lancet HIV. 2016;3:e473–e481. [DOI] [PubMed] [Google Scholar]
- 10.Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.President’s Emergency Plan for AIDS Relief (PEPFAR). Promising Practices and Lessons Learned from Implementation of the ACT Initiative. Washington, DC; 2017. Available at: https://www.pepfar.gov/documents/organization/270700.pdf. Accessed May 30, 2017. [Google Scholar]
- 12.World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Prevention HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland; 2016. Available at: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1. Accessed November 1, 2018. [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV and AIDS (UNAIDS). Get on the Fast Track: The Life-cycle Approach to HIV. Geneva, Switzerland; 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/Get-on-the-Fast-Track_en.pdf. Accessed March 6, 2018. [Google Scholar]
- 14.Vojnov L, Markby J, Boeke C, et al. Impact of SMS/GPRS printers in reducing time to early infant diagnosis compared with routine result reporting: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2017;76:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diallo K, Kim AA, Lecher S, et al. Early diagnosis of HIV infection in infants—one Caribbean and six sub-Saharan African countries. Morb Mortal Wkly Rep. 2011–2015;65:1285–1290. [DOI] [PubMed] [Google Scholar]
- 16.Wagner A, Slyker J, Langat A, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr. 2015;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumede-Moyo S, Filteau S, Munthali T, et al. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: a systematic literature review. Medicine. 2017;96:e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Njuguna IN, Wagner AD, Cranmer LM, et al. Hospitalized children reveal health systems gaps in the mother-child HIV care cascade in Kenya. AIDS Patient Care STDS. 2016;30:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). Consolidated Guidelines on HIV Testing Services. Geneva, Switzerland; 2015. Available at: http://apps.who.int/iris/bitstream/handle/10665/179870/9789241508926_eng.pdf?sequence=1. Accessed September 18, 2017. [PubMed] [Google Scholar]
- 20.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simms V, Dauya E, Dakshina, et al. Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: a cross-sectional survey. PLoS Med. 2017;14:e1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown K, Williams DB, Kinchen S, et al. Status of HIV epidemic control among adolescent girls and young women aged 15–24 years—seven African countries, 2015–2017. Morb Mortal Wkly Rep. 2018;67:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sam-Agudu NA, Folayan MO, Echezona EE. Seeking wider access to HIV testing for adolescents in sub-Saharan Africa. Pediatr Res. 2016;79: 838–845. [DOI] [PubMed] [Google Scholar]
- 25.Govindasamy D, Ferrand RA, Wilmore S, et al. Uptake and yield of HIV testing and counseling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2015;18:20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamla D, Mbori-Ngacha D, Newman M, et al. Evidence from the field: missed opportunities for identifying and linking HIV-infected children for early initiation of ART. AIDS. 2013;27(suppl 2):S139–S146. [DOI] [PubMed] [Google Scholar]
- 27.De Schacht C, Mabunda N, Ferreira OC, et al. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in Southern Mozambique. J Int AIDS Soc. 2014;17:18808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. [DOI] [PubMed] [Google Scholar]
- 29.Ferrand RA, Weiss HA, Nathoo K, et al. A primary care level algorithm for identifying HIV-infected adolescents in populations at high risk through mother-to-child transmission. Trop Med Int Health. 2011;16: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandason T, McHugh G, Dauya E, et al. Validation of a screening tool to identify older children living with HIV in primary care facilities in high HIV prevalence settings. AIDS. 2016;30:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The World Health Organization (WHO). Pediatric Advocacy Toolkit: For Improved Pediatric HIV Diagnosis, Care and Treatment in High HIV Prevalence Countries and Regions. Geneva, Switzerland; 2011. Available at: http://whqlibdoc.who.int/hq/2011/WHO_HIV_11.04_eng.pdf?ua=1. Accessed November 1, 2018. [Google Scholar]
- 32.Chi B, Bolton-Moore C, Holmes C. Prevention of mother-to-child HIV transmission within the continuum of maternal, newborn, and child health services. Curr Opin HIV AIDS. 2013;8:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mwenda R, Fong Y, Magombo T, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Infect Dis. 2018. doi: 10.1093/cid/ciy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed S, Kim MH, Sugandhi N, et al. Beyond early infant diagnosis: case finding strategies for identification of HIV-infected infants and children. AIDS. 2013;27:S235–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellerman S, Essajee S. HIV testing for children in resource-limited settings: what are we waiting for? PLoS Med. 2010;7:e1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed S, Sabeli RA, Simon K. Index case finding facilitates identification and linkage to care of children and young persons living with HIV/AIDS in Malawi. Trop Med Int Health. 2017;22:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen D, Lungu M, van Oosterhout JJ. HIV testing coverage of family members of adult antiretroviral therapy patients in Malawi. AIDS Care. 2010;22:1346–1349. [DOI] [PubMed] [Google Scholar]
- 38.Wanyenze RK, Nawavvu C, Ouma J, et al. Provider-initiated HIV testing for paediatric inpatients and their caretakers is feasible and acceptable. Trop Med Int Health. 2010;15:113–119. [DOI] [PubMed] [Google Scholar]
- 39.Wagner AD, Mugo C, Njuguna IN, et al. Implementation and operational research: active referral of children of HIV-positive adults reveals high prevalence of undiagnosed HIV. J Acquir Immune Defic Syndr. 2016;73:e83–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MH, Ahmed S, Buck WC, et al. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15(suppl 2):17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidman R, Anglewicz P. Are adolescent orphans more likely to be HIV-positive? A pooled data analyses across 19 countries in sub-Saharan Africa. J Epidemiol Community Health. 2016;70:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.PEPFAR. Guidance for Orphans and Vulnerable Children Programming. Washington, DC; 2012. Available at: http://www.pepfar.gov/documents/organization/195702.pdf. Accessed June 3, 2014. [Google Scholar]
- 43.Patel D, Matyanga P, Nyamundaya T, et al. Facilitating HIV testing, care, and treatment for orphans and vulnerable children aged 5 years and younger through community-based early childhood development play-centres in rural Zimbabwe. J Int AIDS Soc. 2012;15(suppl 2):17404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koenig SP, Dorvil N, Dévieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. 2017;14:e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med. 2016;13:e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhamadi L, Tumwesigye NM, Kadobera D, et al. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre-antiretroviral care in Eastern Uganda. Trials. 2011;12: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braun M, Kabue MM, McCollum ED, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mavegam BO, Pharr JR, Cruz P, et al. Effective interventions to improve young adults’ linkage to HIV care in Sub-Saharan Africa: a systematic review. AIDS Care. 2017;29:1198–1204. [DOI] [PubMed] [Google Scholar]
- 51.Hewett PC, Nalubamba M, Bozzani F, et al. Randomized evaluation and cost-effectiveness of HIV and sexual and reproductive health service referral and linkage models in Zambia. BMC Public Health. 2016;16:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Betancourt TS, Abrams EJ, McBain R, et al. Family centered approaches to the prevention of mother to child transmission of HIV. J Int AIDS Soc. 2010;13(suppl 2):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elul B, Lamb MR, Lahuerta M, et al. A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: a cluster-randomized study. PLoS Med. 2017; 14:e1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox MP, Rosen S, Geldsetzer P, et al. Interventions to improve the rate or timing of initiation of antiretroviral therapy for HIV in sub-Saharan Africa: meta-analyses of effectiveness. J Int AIDS Soc. 2016;19:20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrazo AC, Firth J, Amzel A, et al. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health. 2018;23: 136–148. [DOI] [PubMed] [Google Scholar]
- 56.Barnabas RV, van Rooyen H, Tumwesigye E, et al. Uptake of antiretroviral therapy and male circumcision after community-based HIV testing and strategies for linkage to care versus standard clinic referral: a multisite, open-label, randomised controlled trial in South Africa and Uganda. Lancet HIV. 2016;3:e212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.PEPFAR. PEPFAR 2018 Country Operational Plan Guidance for Standard Process Countries. Washington, DC; 2018. Available at: https://www.pepfar.gov/documents/organization/276459.pdf. Accessed March 6, 2018. [Google Scholar]
- 58.Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3: e539–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization (WHO). Guidelines on HIV Self-Testing and Partner Notification. Geneva, Switzerland; 2016. Available at: http://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en/. Accessed March 3, 2017. [PubMed] [Google Scholar]
- 60.Toska E, Pantelic M, Meinck F, et al. Sex in the shadow of HIV: a systematic review of prevalence, risk factors, and interventions to reduce sexual risk-taking among HIV-positive adolescents and youth in sub-Saharan Africa. PLoS One. 2017;12:e0178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choko AT, MacPherson P, Webb E, et al. Uptake, accuracy, safety, and linkage into care over two years of promoting annual self-testing for HIV in Blantyre, Malawi: a community-based prospective study. PLoS Med. 2015;12:e1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotsche CI, Simwinga M, Muzumara A et al. HIV Self Testing in Zambia: User Ability to Follow the Manufacturer’s Instructions for Use [MOPED1167]. Presented at: 9th International AIDS Society Conference on HIV Science; July 23–36, 2017; Paris, France. [Google Scholar]
- 63.Orasure Technologies, Inc. Frequently Asked Questions about OraQuick. Bethlehem, PA; 2016. Available at: www.oraquick.com/faqs. Accessed March 9, 2018. [Google Scholar]
- 64.Shanaube K, Schaap A, Chaila MJ, et al. Community interventionimproves knowledge of HIV status of adolescents in Zambia: findings from HPTN 071-PopART for youth study. AIDS. 2017;31(suppl 3): S221–S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadede K, Ruel T, Kabami J, et al. Increased adolescent HIV testing with a hybrid mobile strategy in Uganda and Kenya. AIDS. 2016;30:2121–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coates TJ, Kulich M, Celentano DD, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2:e267–e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madiba S, Mokgatle M. Parents support implementation of HIV testing and counseling in school: cross-sectional study with parents of adolescent attending high school in Gauteng and North West Provinces, South Africa. AIDS Res Treat. 2016;2016:4842814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Health Organization (WHO). HIV and Adolescents: Guidance for HIV Testing and Counseling and Care for Adolescents Living With HIV. Geneva, Switzerland; 2013. Available at: http://apps.who.int/iris/bitstream/10665/94334/1/9789241506168_eng.pdf?ua=1. Accessed February 20, 2015. [Google Scholar]
- 69.Bandason T, Langhaug LF, Makamba M, et al. Burden of HIV amongprimary school children and feasibility of primary school-linked HIV testing in Harare, Zimbabwe: a mixed methods study. AIDS Care. 2013; 25:1520–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kharsany AB, Mlotshwa M, Frohlich JA, et al. HIV prevalence among high school learners—opportunities for schools-based HIV testing programmes and sexual reproductive health services. BMC Public Health. 2012;12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdool Karim Q, Kharsany AB, Leask K, et al. Prevalence of HIV, HSV-2 and pregnancy amongst high school students in rural KwaZulu-Natal: a bio-behavioral cross-sectional survey. Sex Transm Infect. 2014; 90:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ministry of Education, Republic of Botswana. Second Botswana Youth Risk Behavioural and Biological Surveillance Survey Report. Gaborone, Botswana: Botswana Ministry of Basic Education; 2016. [Google Scholar]
- 73.Daniels J, Komarek A, Forgreive B, et al. Shout-It-Now: a mobile HCT model employing technology and edutainment in South Africa. J Int Assoc Providers AIDS Care. 2017;16:506–511. [DOI] [PubMed] [Google Scholar]
- 74.Lightfoot M, Lippman S, Campbell C, et al. Increasing Testing and Diagnosis for Men Who Have Sex with Men Using Social Networks for HIV Self-test Distribution [MOPEB0260] . Presented at: 9th International AIDS Society Conference on HIV Science; July 23–26, 2017; Paris, France. [Google Scholar]
- 75.Smyrnov P, Williams LD, Korobchuk A, et al. Risk network approaches to locating undiagnosed HIV cases in Odessa, Ukraine. J Int AIDS Soc. 2018;21:e25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kranzer K, Simms V, Bandason T, et al. Economic incentives for HIV testing by adolescents in Zimbabwe: a randomised controlled trial. Lancet HIV. 2017;5:e79–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Njuguna IN, Wagner AD, Omondi VO, et al. Financial incentives for pediatric HIV testing in Kenya. Pediatr Infect Dis. 2018. doi: 10.1097/INF.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murray KR, Dulli LS, Ridgeway K, et al. Improving retention in HIV care among adolescents and adults in low- and middle income countries:a systematic review of the literature. PLoS One. 2017;12:e0184879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson KS, Beima-Sofie KM, Moraa H, et al. “At our age, we would like to do things the way we want:’’ a qualitative study of adolescent HIV testing services in Kenya. AIDS. 2017;31(suppl 3):S213–S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rice B, Sanchez T, Baral S, et al. Know your epidemic, strengthen your response: developing a new HIV surveillance architecture to guide HIV resource allocation and target decisions. JMIR Public Health Surveill. 2018;4:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rehle T, Lazzari S, Dallabetta G, et al. Second-generation HIV surveillance: better data for decision-making. Bull World Health Organ. 2004;82:121–127. [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner AD, Mugo C, Bluemer-Miroite S, et al. Continuous quality improvement intervention for adolescent and young adult HIV testing services in Kenya improves HIV knowledge. AIDS. 2017;31(suppl 3):S243–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eba PM, Lim H. Reviewing independent access to HIV testing, counselling, and treatment for adolescents in HIV-specific laws in sub-Saharan Africa: implications for the HIV response. J Int AIDS Soc. 2017; 20:21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penazzato M, Davies MA, Apollo T, et al. Task shifting delivery of pediatric antiretroviral treatment: a systematic review. J Acquir Immune Defic Syndr. 2014;65:414–422. [DOI] [PubMed] [Google Scholar]
- 85.Kismodi E, Kiragu K, Sawicki O, et al. Where public health meets human rights: integrating human rights into the validation of the elimination of mother-to-child transmission of HIV and syphilis. Health Hum Rights. 2017;19:237–247. [PMC free article] [PubMed] [Google Scholar]
- 86.Matheson R, Brion S, Sharma A, et al. Realizing the promise of the global plan: engaging communities and promoting the health and human rights of women living with HIV. J Acquir Immune Defic Syndr. 2017;75 (suppl 1):S86–S93. [DOI] [PubMed] [Google Scholar]
- 87.Modi S, Callahan T, Rodrigues J, et al. Overcoming health system challenges for women and children living with HIV through the global plan. J Acquir Immune Defic Syndr. 2017;75(suppl 1):S76–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Health Organization (WHO). Improving the Quality of HIV-Related Point-of-Care Testing: Ensuring the Reliability and Accuracy of Results. Geneva, Switzerland; 2015. Available at: http://apps.who.int/iris/bitstream/10665/199799/1/9789241508179_eng.pdf?ua=1. Accessed May 5, 2016. [Google Scholar]
- 89.Sacks E, Cohn J. Penazzato. HIV misdiagnosis in paediatrics: unpacking the complexity. J Int AIDS Soc. 2017;20(suppl 6):21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Technau KM, Kuhn AH, Hands L, et al. Prevalence and outcomes ofHIV-1 diagnostic challenges during universal birth testing—an urban South African observational cohort. J Int AIDS Soc. 2017;20(suppl 6): 21761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson CC, Dalal S, Baggaley R, et al. A public health approach to addressing and preventing misdiagnosis in the scale-up of HIV rapid testing programmes. J Int AIDS Soc. 2017;20(suppl 6):22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.UNAIDS. Ambitious Treatment Targets: Writing the Final Chapter of the AIDS Epidemic. Geneva, Switzerland; 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2670_UNAIDS_Treatment_Targets_en.pdf. Accessed October 21, 2017. [Google Scholar]


