Abstract
BACKGROUND
Multidrug-resistant (MDR) bacteria that are commonly associated with health care cause a substantial health burden. Updated national estimates for this group of pathogens are needed to inform public health action.
METHODS
Using data from patients hospitalized in a cohort of 890 U.S. hospitals during the period 2012–2017, we generated national case counts for both hospital-onset and community-onset infections caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), extended-spectrum cephalosporin resistance in Enterobacteriaceae suggestive of extended-spectrum beta-lactamase (ESBL) production, carbapenem-resistant Enterobacteriaceae, carbapenem-resistant acinetobacter species, and MDR Pseudomonas aeruginosa.
RESULTS
The hospital cohort in the study accounted for 41.6 million hospitalizations (>20% of U.S. hospitalizations annually). The overall rate of clinical cultures was 292 cultures per 1000 patient-days and was stable throughout the time period. In 2017, these pathogens caused an estimated 622,390 infections (95% confidence interval [CI], 579,125 to 665,655) among hospitalized patients. Of these infections, 517,818 (83%) had their onset in the community, and 104,572 (17%) had their onset in the hospital. MRSA and ESBL infections accounted for the majority of the infections (52% and 32%, respectively). Between 2012 and 2017, the incidence decreased for MRSA infection (from 114.18 to 93.68 cases per 10,000 hospitalizations), VRE infection (from 24.15 to 15.76 per 10,000), carbapenem-resistant acinetobacter species infection (from 3.33 to 2.47 per 10,000), and MDR P. aeruginosa infection (from 13.10 to 9.43 per 10,000), with decreases ranging from −20.5% to −39.2%. The incidence of carbapenem-resistant Enterobacteriaceae infection did not change significantly (from 3.36 to 3.79 cases per 10,000 hospitalizations). The incidence of ESBL infection increased by 53.3% (from 37.55 to 57.12 cases per 10,000 hospitalizations), a change driven by an increase in community-onset cases.
CONCLUSIONS
Health care–associated antimicrobial resistance places a substantial burden on patients in the United States. Further work is needed to identify improved interventions for both the inpatient and outpatient settings. (Funded by the Centers for Disease Control and Prevention.)
Antibiotic drug resistance is a major public health problem. In 2013, the Centers for Disease Control and Prevention (CDC) published a report entitled “Antibiotic Resistance Threats in the United States, 2013,” which provided national burden estimates for selected antibiotic-resistant pathogens in the United States.1 The report was instrumental in driving national policy and investment decisions. In its wake, the U.S. National Strategy for Combating Antibiotic-Resistant Bacteria and an accompanying U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria were established. The 2013 report estimated that at least 2 million persons were infected with antibiotic-resistant pathogens each year in the United States and that at least 23,000 persons died as a result. Approximately two thirds of those deaths were associated with infections caused by multidrug-resistant (MDR) organisms that are commonly associated with health care. The methods that were used to generate the estimates for health care–associated pathogens in the 2013 report were based in part on an extrapolation of results from a multistate prevalence study that identified only hospital-onset infections.1 Because community-onset infections, which represent a substantial proportion of all health care–associated infections, could not be estimated by means of that method, the 2013 report provided only a minimum estimate of overall burden.
This article provides the CDC updates of national estimates of MDR bacterial infections associated with health care — namely, those caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), extended-spectrum cephalosporin resistance in Enterobacteriaceae suggestive of extended-spectrum beta-lactamase (ESBL) production, carbapenem-resistant Enterobacteriaceae, carbapenem-resistant acinetobacter species, and MDR Pseudomonas aeruginosa. The analysis used new methods that provide more robust national burden estimates and allow for tracking recent national incidence trends for this group of pathogens. The findings in this report serve as the basis for the updated burden estimates found in the CDC report “Antibiotic Resistance Threats in the United States, 2019.”2
METHODS
DATA SOURCES
We used three electronic health databases of deidentified data to calculate national burden estimates: the Premier Healthcare Database,3 Cerner Health Facts,4 and the BD Insights Research Database.5–8 Data from any inpatient visit in participating acute care hospitals that took place between January 1, 2012, and December 31, 2017, were included in the data sets (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org). This project underwent ethical and regulatory review in accordance with CDC institutional procedures and was determined not to be subject to review by an institutional review board or requirements for informed consent under the Common Rule. All analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
HOSPITAL COHORT
A dynamic cohort of short-term acute care hospitals was created from each of the databases for the period 2012–2017. We included hospitals at the unit of hospital-month. Any hospital-month of data for which there was at least one positive result from a microbiology culture (growth of any bacterial organism) accompanied by antimicrobial susceptibility testing data was included in the cohort. For the hospital-months that were included, we used data for all patient hospitalizations for that month, including all data regarding positive cultures. Of the positive cultures with any susceptibility result, those that had definitive susceptibility interpretations (i.e., those that were labeled as susceptible, intermediate, or resistant) were eligible to meet the case definition. Facility-level characteristics, including bed-size category, geographic region (U.S. Census division), urban or rural designation, and teaching status, were documented for every hospital-month of data. (Additional details are provided in the Methods section in the Supplementary Appendix).
CASE COHORT DEFINITION
From the hospital cohort, we identified a cohort of patients who had any clinical culture that yielded an isolate of the organisms of interest and that had accompanying susceptibility testing results sufficient for determining whether that isolate had the resistance phenotype of interest (Table S2 in the Supplementary Appendix). We categorized clinical culture specimen types as sterile, nonsterile, or surveillance on the basis of body site. Sterile specimens included those obtained from blood, bone, cerebrospinal fluid, peritoneal fluid, pleural fluid, synovial fluid, and lymph nodes. Nonsterile specimens included those obtained from urine, sputum, and wounds. We excluded specimens that were categorized as surveillance (i.e., cultures labeled as rectal, perirectal, or nasal).
Among clinical isolates with sufficient susceptibility testing results, those with the resistance phenotype of interest were eligible to be considered as incident cases. Only isolates that were obtained from patients having no culture yielding the same resistance phenotype of interest in the previous 14 days were counted as incident cases. For patients who had isolates with the resistance phenotype of interest from both a sterile and nonsterile positive culture obtained within 14 days of each other, only the sterile culture was counted as an incident case. For the reporting of carbapenem-resistant Enterobacteriaceae and ESBL-producing organisms, denominator definitions account for potential antimicrobial susceptibility cascade reporting by hospitals (Table S2).
Cases were defined as community-onset infections when the culture was obtained immediately before admission or within the first 3 days of hospitalization. Cases were defined as hospital-onset infections when the culture was obtained on day 4 or later of hospitalization.
NATIONAL ESTIMATE OF CASES
For each year, we extrapolated national estimates from our cohort data. We used a raking procedure (iterative proportional fitting) to generate a weighted adjustment to the data so that hospitals with characteristics that were over- or under-represented in the sample could be accurately represented in the final extrapolated results, which would ensure that our estimates would be representative of all U.S. hospitals.9–13 The procedure generated hospital-specific weights for the extrapolation that resulted in a distribution of hospitalizations in the final data set that matched the distribution of hospitalizations for all hospitals in the United States according to the American Hospital Association survey for that respective year.14 Weights for the extrapolating procedure were based on the following hospital characteristics: bed size, U.S. Census division, urban or rural designation, and teaching status. We applied a weighted-means survey procedure to calculate pathogen-specific national case estimates for each year (see the Methods section in the Supplementary Appendix).
STATISTICAL ANALYSIS
We calculated pooled rates using the weighted number of cases and hospitalizations in each month. We examined temporal trends using a multivariable logistic model that incorporated a survey design with the corresponding weights and hospital designation as the specific cluster.15,16 Using monthly hospital-level data from the period 2012–2017, we modeled cases per hospitalization, controlling for hospital characteristics, month of hospitalization, proportion of patients in specific age ranges, and database. The variable of year, representing the trend, was modeled in two ways: as a continuous trend and as a series of five dummy variables representing each year. Because results were similar, only linear trends with 95% confidence intervals are reported throughout. Confidence intervals were not adjusted for multiple comparisons.
To assess the effect of changes in participating hospitals over time, we performed a subanalysis for each of our microbiologic outcomes that was restricted to hospitals with consistent reporting over the course of the study period. In addition, to address the possibility that observed trends may have been influenced by changes in individual hospital practices related to pathogen or resistance detection, we examined whether there were temporal changes in the rate of obtaining clinical cultures, and we estimated annual incidence trends for each of the data sources independently, comparing them with each other to assess the consistency of the findings in different hospital populations.
RESULTS
CHARACTERISTICS OF THE HOSPITAL COHORT
In the period 2012–2017, the hospital cohort comprised 890 hospitals overall, with numbers ranging from 532 to 722 depending on year; this cohort accounted for 41.6 million hospitalizations (>20% of U.S. hospitalizations annually). The characteristics of the cohort hospitals were stable over time and similar in distribution to all U.S. acute care hospitals (Table 1). Between 88% and 94% of all possible hospital-months of data were included, depending on year. The overall rate of clinical cultures was 292 cultures per 1000 patient-days and was stable during the study period.
Table 1.
Characteristics of All Included Hospitals and Patients, According to Year, as Compared with the Distribution of U.S. Hospitals as Provided by the AHA.*
| Characteristic | Hospitals Included in Study | All Hospitals, 2017 (N=4847)† | ||||||
|---|---|---|---|---|---|---|---|---|
| All Years (N = 890) | 2012 (N = 532) | 2013 (N = 587) | 2014 (N = 614) | 2015 (N = 653) | 2016 (N = 707) | 2017 (N = 722) | ||
| Hospitals | ||||||||
| Electronic database— no. (%) | ||||||||
| BD Insights Research | 394 (44.3) | 188 (35.3) | 209 (35.6) | 236 (38.4) | 283 (43.3) | 325 (46.0) | 355 (49.2) | NA |
| Cerner Health Facts | 240 (27.0) | 180 (33.8) | 187 (31.9) | 200 (32.6) | 212 (32.5) | 217 (30.7) | 178 (24.7) | NA |
| Premier Healthcare | 256 (28.8) | 164 (30.8) | 191 (32.5) | 178 (29.0) | 158 (24.2) | 165 (23.3) | 189 (26.2) | NA |
| Location — no. (%) | ||||||||
| Rural | 218 (24.5) | 112 (21.1) | 134 (22.8) | 143 (23.3) | 155 (23.7) | 182 (25.7) | 194 (26.9) | 1882 (38.8) |
| Urban | 672 (75.5) | 420 (78.9) | 453 (77.2) | 471 (76.7) | 498 (76.3) | 525 (74.3) | 528 (73.1) | 2965 (61.2) |
| Teaching status — no. (%)‡ | ||||||||
| Nonteaching | 589 (66.2) | 334 (62.8) | 377 (64.2) | 396 (64.5) | 421 (64.5) | 458 (64.8) | 489 (67.7) | 3079 (63.5) |
| Teaching | 301 (33.8) | 198 (37.2) | 210 (35.8) | 218 (35.5) | 232 (35.5) | 249 (35.2) | 233 (32.3) | 1768 (36.5) |
| No. of beds — no. (%) | ||||||||
| <300 | 609 (68.4) | 349 (65.6) | 395 (67.3) | 412 (67.1) | 440 (67.4) | 478 (67.6) | 498 (69.0) | 4025 (83.0) |
| ≥300 | 281 (31.6) | 183 (34.4) | 192 (32.7) | 202 (32.9) | 213 (32.6) | 229 (32.4) | 224 (31.0) | 822 (17.0) |
| U.S. Census division — no. (%) | ||||||||
| New England | 28 (3.1) | 18 (3.4) | 18 (3.1) | 18 (2.9) | 18 (2.8) | 21 (3.0) | 23 (3.2) | 181 (3.7) |
| Middle Atlantic | 113 (12.7) | 75 (14.1) | 76 (12.9) | 74 (12.1) | 74 (11.3) | 81 (11.5) | 77 (10.7) | 412 (8.5) |
| South Atlantic | 156 (17.5) | 88 (16.5) | 98 (16.7) | 103 (16.8) | 105 (16.1) | 114 (16.1) | 133 (18.4) | 714 (14.7) |
| East North Central | 176 (19.8) | 78 (14.7) | 106 (18.1) | 116 (18.9) | 135 (20.7) | 152 (21.5) | 154 (21.3) | 735 (15.2) |
| East South Central | 80 (9.0) | 67 (12.6) | 66 (11.2) | 67 (10.9) | 69 (10.6) | 71 (10.0) | 69 (9.6) | 392 (8.1) |
| West North Central | 48 (5.4) | 23 (4.3) | 28 (4.8) | 26 (4.2) | 27 (4.1) | 32 (4.5) | 37 (5.1) | 700 (14.4) |
| West South Central | 129 (14.5) | 80 (15.0) | 90 (15.3) | 94 (15.3) | 99 (15.2) | 106 (15.0) | 101 (14.0) | 738 (15.2) |
| Mountain | 52 (5.8) | 33 (6.2) | 36 (6.1) | 40 (6.5) | 42 (6.4) | 43 (6.1) | 44 (6.1) | 418 (8.6) |
| Pacific | 108 (12.1) | 70 (13.2) | 69 (11.8) | 76 (12.4) | 84 (12.9) | 87 (12.3) | 84 (11.6) | 557 (11.5) |
| Patients | ||||||||
| Age — no. (% of all discharges) | ||||||||
| <1 yr | 3,994,181 (9.6) | 598,709 (9.7) | 648,094 (9.9) | 679,866 (9.8) | 684,078 (9.6) | 699,463 (9.3) | 683,971 (9.3) | NA |
| 1–17 yr | 1,439,633 (3.5) | 248,885 (4.0) | 253,871 (3.9) | 268,776 (3.9) | 235,762 (3.3) | 225,160 (3.0) | 207,179 (2.8) | NA |
| 18–54 yr | 14,790,525 (35.5) | 2,233,783 (36.2) | 2,329,837 (35.6) | 2,493,632 (36.1) | 2,534,993 (35.7) | 2,658,272 (35.3) | 2,540,008 (34.4) | NA |
| 55–64 yr | 6,264,510 (15.0) | 890,807 (14.4) | 952,496 (14.6) | 1,023,557 (14.8) | 1,078,884 (15.2) | 1,170,155 (15.5) | 1,148,611 (15.5) | NA |
| 65–74 yr | 6,439,533 (15.5) | 896,057 (14.5) | 978,642 (15.0) | 1,034,080 (15.0) | 1,100,578 (15.5) | 1,208,782 (16.1) | 1,221,394 (16.5) | NA |
| >75 yr | 8,554,506 (20.5) | 1,285,696 (20.8) | 1,349,268 (20.6) | 1,375,925 (19.9) | 1,444,712 (20.3) | 1,541,100 (20.5) | 1,557,805 (21.1) | NA |
| Unknown | 154,402 (0.4) | 22,450 (0.4) | 25,836 (0.4) | 27,968 (0.4) | 24,296 (0.3) | 23,798 (0.3) | 30,054 (0.4) | NA |
| Annual no. of hospitalizations (96)§ | 41,637,309 (20.2) | 6,176,400 (17.6) | 6,538,050 (19.0) | 6,903,804 (20.4) | 7,103,303 (20.8) | 7,526,730 (21.9) | 7,389,022 (21.4) | 34,554,279 (100) |
| Annual no. of patient-days (%)§ | 183,510,066 (16.5) | 28,262,293 (15.1) | 29,263,095 (15.9) | 30,461,326 (16.7) | 31,237,196 (16.9) | 32,769,964 (17.7) | 31,516,192 (17.0) | 185,636,360 (100) |
Percentages may not total 100 because of rounding. AHA denotes American Hospital Association, and NA not available.
Data on all U.S. hospitals (short-term, acute care) are from the AHA.14
A total of 20 hospitals in the Cerner Health Facts Database were missing values for teaching status. In these cases, we imputed teaching status by comparing the number of beds with distributions of hospital sizes as reported among the other two hospital data systems, data from the AHA, and data from the Centers for Medicare and Medicaid Services Healthcare Cost Report Information System.17
Percentages were calculated on the basis of all hospitalizations or patient-days reported by the AHA each year.14
NATIONAL ESTIMATES
The national estimate of the number of incident cases for all six pathogens combined in 2017 was 622,390 (95% confidence interval [CI], 579,125 to 665,655); a total of 517,818 infections (83%) had their onset in the community, and 104,572 infections (17%) had their onset in the hospital. Pathogen-specific national estimates, according to year, are shown in Figure 1 and Table 2. MRSA and ESBL infections were the most common and together accounted for 84% of all the cases in 2017 (52% for MRSA and 32% for ESBL infection).
Figure 1. Estimated Number of Cases of Multidrug-Resistant Bacterial Infection in the United States, According to Year and Location Onset, 2012–2017.
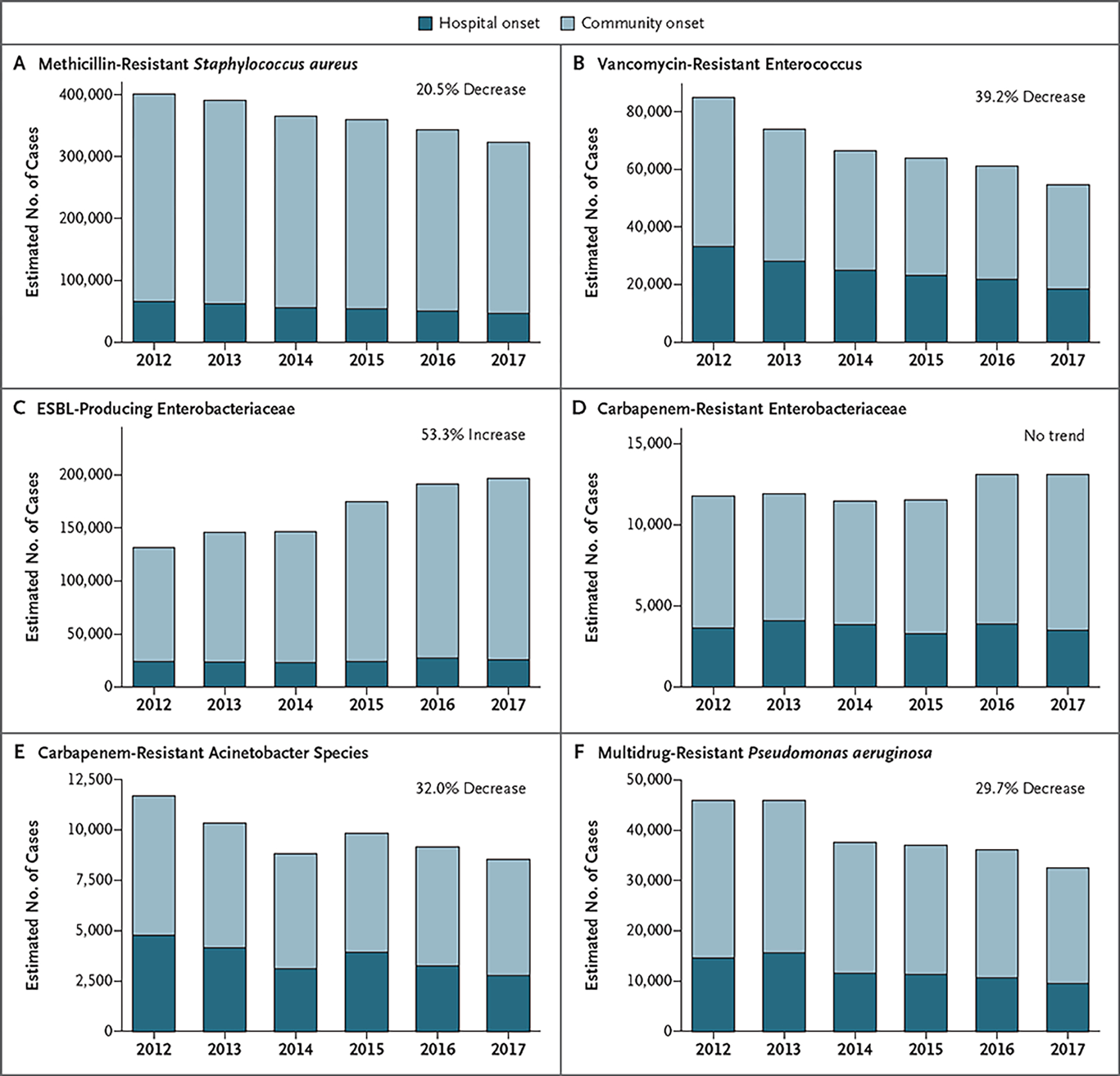
Shown are cases of infection with onset in the hospital and in the community over time. Trend estimates were based on modeled overall (hospital-onset and community-onset) 5-year incidence trends relative to the 2012 estimate. ESBL denotes extended-spectrum beta-lactamase.
Table 2.
National Estimates of Resistant Infections, According to Pathogen, 2012–2017.*
| Pathogen and Year | Estimated Cases of Resistant Infection (95% CI) | Cases with Hospital Onset | Cases in Sterile Site | Cases in Patients ≥65 Yr of Age |
|---|---|---|---|---|
| number | percent | |||
| MRSA | ||||
| 2012 | 400,961 (345,267–456,655) | 16.4 | 17.1 | 41.5 |
| 2013 | 391,042 (341,715–440,368) | 15.8 | 17.1 | 41.5 |
| 2014 | 365,421 (318,714–412,128) | 15.2 | 16.9 | 40.7 |
| 2015 | 359,481 (316,807–402,155) | 15.0 | 17.8 | 40.9 |
| 2016 | 343,138 (305,252–381,024) | 14.5 | 17.7 | 40.5 |
| 2017 | 323,718 (287,967–359,469) | 14.3 | 18.1 | 41.0 |
| VRE | ||||
| 2012 | 84,812 (70,151–99,472) | 39.0 | 13.8 | 63.2 |
| 2013 | 73,837 (63,475–84,199) | 37.9 | 14.3 | 63.5 |
| 2014 | 66,364 (57,323–75,406) | 37.5 | 14.3 | 63.5 |
| 2015 | 63,734 (55,396–72,072) | 36.3 | 14.1 | 61.0 |
| 2016 | 61,064 (52,433–69,694) | 35.7 | 15.3 | 59.0 |
| 2017 | 54,457 (47,356–61,559) | 33.6 | 14.3 | 60.1 |
| ESBL-producing Enterobacteriaceae | ||||
| 2012 | 131,869 (111,732–152,007) | 18.4 | 11.2 | 65.4 |
| 2013 | 146,067 (125,903–166,231) | 16.2 | 11.3 | 66.0 |
| 2014 | 146,694 (127,684–165,705) | 15.5 | 12.1 | 65.0 |
| 2015 | 175,096 (152,621–197,572) | 13.9 | 12.8 | 64.3 |
| 2016 | 191,756 (168,997–214,514) | 14.3 | 13.4 | 63.9 |
| 2017 | 197,378 (173,913–220,842) | 13.1 | 13.4 | 63.4 |
| Carbapenem-resistant Enterobacteriaceae | ||||
| 2012 | 11,786 (8918–14,655) | 30.8 | 11.4 | 61.7 |
| 2013 | 11,901 (9883–13,918) | 34.4 | 12.4 | 59.8 |
| 2014 | 11,438 (9240–13,636) | 33.6 | 13.1 | 55.8 |
| 2015 | 11,530 (9484–13,576) | 28.6 | 11.6 | 51.3 |
| 2016 | 13,091 (10,828–15,354) | 29.5 | 14.1 | 57.0 |
| 2017 | 13,106 (11,136–15,075) | 26.7 | 10.7 | 56.2 |
| Carbapenem-resistant acinetobacter species | ||||
| 2012 | 11,684 (8869–14,499) | 40.9 | 10.9 | 48.9 |
| 2013 | 10,333 (8245–12,421) | 40.3 | 10.6 | 41.3 |
| 2014 | 8807 (6992–10,622) | 35.4 | 12.2 | 46.5 |
| 2015 | 9834 (7697–11,970) | 40.1 | 8.9 | 47.2 |
| 2016 | 9146 (7385–10,906) | 35.6 | 9.3 | 45.8 |
| 2017 | 8531 (6747–10,315) | 32.5 | 8.7 | 42.1 |
| MDR Pseudomonas aeruginosa | ||||
| 2012 | 45,989 (38,213–53,765) | 31.9 | 5.5 | 54.2 |
| 2013 | 45,978 (38,933–53,022) | 33.9 | 5.1 | 52.9 |
| 2014 | 37,596 (31,868–43,324) | 30.8 | 4.6 | 51.3 |
| 2015 | 36,994 (31,640–42,348) | 30.7 | 5.0 | 51.3 |
| 2016 | 36,186 (30,428–41,944) | 29.4 | 4.6 | 50.2 |
| 2017 | 32,577 (28,046–37,109) | 29.3 | 4.2 | 49.3 |
ESBL denotes extended-spectrum beta-lactamase, MDR multidrug-resistant, MRSA methicillin-resistant Staphylococcus aureus, and VRE vancomycin-resistant enterococcus.
RATES AND TRENDS
National incidence trends, according to pathogen, are shown in Figure 1 and Table 3 and in Table S3B. Between 2012 and 2017, decreases in incidence were seen for MRSA (from 114.18 to 93.68 cases per 10,000 hospitalizations), for VRE (from 24.15 to 15.76 cases per 10,000 hospitalizations), for carbapenem-resistant acinetobacter species (from 3.33 to 2.47 cases per 10,000 hospitalizations), and for MDR P. aeruginosa (from 13.10 to 9.43 cases per 10,000 hospitalizations) (Table S3B). The overall decreases in the incidence of these pathogens, as estimated from multivariable models over the 5-year study period, ranged from −20.5% to −39.2%. For MRSA and VRE, the incidence of hospital-onset infections decreased faster than the incidence of community-onset infections (Table 3). There was no significant change in the incidence of carbapenem-resistant Enterobacteriaceae (from 3.36 to 3.79 cases per 10,000 hospitalizations). The only pathogen to increase in rate was ESBL-producing Enterobacteriaceae, which increased 53.3% between 2012 and 2017 (from 37.55 to 57.12 cases per 10,000 hospitalizations) — a change driven by increases in community-onset cases (Table 3). Increases in the incidence of ESBL-producing Escherichia coli infection accounted for 86% of the overall increase in the incidence of ESBL infection.
Table 3.
Continuous Trend Analysis of Adjusted National Incidence Trends, According to Pathogen and Location Onset, 2012–2017.*
| Variable | MRSA | VRE | ESBL-Producing Enterobacteriaceae | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Hospital | Community | Overall | Hospital | Community | Overall | Hospital | Community | |
| Linear estimate | −0.046 | −0.079 | −0.040 | −0.100 | −0.134 | −0.080 | 0.086 | 0.008† | 0.099 |
| 95% CI | −0.057 to −0.035 | −0.094 to −0.064 | −0.051 to −0.029 | −0.121 to −0.078 | −0.157 to −0.110 | −0.102 to −0.057 | 0.070 to 0.102 | −0.017 to 0.032† | 0.083 to 0.115 |
| Annual percent chang‡ | −4.5 | −7.6 | −3.9 | −9.5 | −12.5 | −7.6 | 8.9 | 0.8† | 10.4 |
| 95% CI | −5.5 to −3.5 | −9.0 to −6.2 | −5.0 to −2.9 | −11.4 to −7.5 | −14.6 to −10.4 | −9.7 to −5.5 | 7.2 to 10.7 | −1.7 to 3.3† | 8.7 to 12.2 |
| 5-Yr percent chang§ | −20.5 | −32.6 | −18.1 | −39.2 | −48.8 | −32.8 | 53.3 | 4.1† | 64.1 |
| 95% CI | −24.6 to −16.2 | −37.4 to −27.3 | −22.4 to −13.6 | −45.3 to −32.4 | −54.5 to −42.4 | −40.0 to −24.7 | 41.6 to 66.1 | −8.1 to 17.4† | 51.4 to 78.0 |
| Variable | Carbapenem-Resistant Enterobacteriaceae | Carbapenem-Resistant Acinetobacter Species | MDR P. aeruginosa | ||||||
| Overall | Hospital | Community | Overall | Hospital | Community | Overall | Hospital | Community | |
| Linear estimate | 0.015† | −0.031† | 0.035† | −0.077 | −0.119 | −0.052 | −0.070 | −0.105 | −0.055 |
| 95% CI | −0.018 to 0.049† | −0.068 to 0.007† | −0.007 to 0.077 | −0.124 to −0.030 | −0.180 to −0.059 | −0.097 to −0.006 | −0.094 to −0.047 | −0.144 to −0.065 | −0.076 to −0.034 |
| Annual percent chang‡ | 1.6† | −3.0† | 3.6† | −7.4 | −11.2 | −5.0 | −6.8 | −9.9 | −5.4 |
| 95% CI | −1.8 to 5.0† | −6.6 to 0.7† | −0.7 to 8.0† | −11.7 to −2.9 | −16.4 to −5.7 | −9.3 to −0.6 | −8.9 to −4.6 | −13.4 to −6.3 | −7.3 to −3.4 |
| 5-Yr percent chang§ | 7.8† | −14.4† | 19.l† | −32.0 | −44.9 | −22.8 | −29.7 | −40.7 | −24.1 |
| 95% CI | −8.6 to 27.8† | −28.8 to 3.6† | −3.4 to 47.0† | −46.2 to −13.9 | −59.3 to −25.4 | −38.5 to −3.1 | −37.3 to −21.1 | −51.4 to −27.7 | −31.6 to −15.7 |
Analyses were adjusted for hospital characteristics, including bed size, U.S. Census division, urban or rural designation, teaching status, month of hospitalization, patient age distributions, and database. Hospital-onset infection was defined as a positive culture obtained 4 or more days after the time of admission. Community-onset infection was defined as a positive culture obtained less than 4 days after the time of admission.
The 95% confidence interval crosses the null value.
The annual percent change was calculated as (ep − 1) × 100%, where p is the modeled parameter estimate for the variable year.
The 5-year percent change was calculated as (e5p − 1) × 100%, where p is the modeled parameter estimate for the variable year.
The consistency of findings among the databases and the results of the subanalyses of hospitals with consistent reporting are shown in Figures S2 and S3, respectively. The results of these subanalyses do not differ substantially from the findings or conclusions of the combined analysis.
DISCUSSION
The burden of health care–associated MDR organisms in the United States remains substantial, with an estimated 622,390 cases among hospitalized patients annually. The burden decreased between 2012 and 2017 for four of the pathogens examined in this analysis, but the incidence did not decrease for all pathogens. The incidence of carbapenem-resistant Enterobacteriaceae remained stable, and the incidence of ESBL increased. Efforts to improve the implementation of existing prevention efforts and to identify more effective prevention strategies are needed.
This study did not determine reasons for the observed trends, but there is evidence suggesting that prevention efforts in health care settings contributed to the declining rates for some pathogens. First, the organisms that decreased all have well-documented associations with health care. For example, VRE, carbapenem-resistant acinetobacter species, and MDR P. aeruginosa are identified almost exclusively among patients with substantial health care exposure and appear to be rarely acquired in the community. Decreases in the incidence of these organisms are very likely to be attributable to a change in transmission in health care settings rather than in the community.
Second, for at least two of the pathogens, MRSA and VRE, our analysis shows that the incidence of hospital-onset infections decreased approximately twice as fast as the incidence of community-onset infections. In addition, two recent studies provide evidence that decreases in MRSA rates in the United States are explained by a decrease in the incidence of health care–associated MRSA — specifically, to a decrease in the incidence of USA100, a strain that has been strongly associated with transmission in the health care setting.18,19 During the past decade, health care decision makers have placed increased emphasis on infection control in health care, including efforts to improve implementation of a wide array of infection-control strategies.20–25 These practices appear to have had a substantial effect on overall health care–associated infections26 and may have played a role in the decrease in the incidence in resistant pathogens that we observed.27
Why the incidence of carbapenem-resistant Enterobacteriaceae, another pathogen almost exclusively associated with health care, did not decrease between 2012 and 2017 is unclear, given that this was a time when health care interventions may have been contributing to the decrease in the incidence of other MDR organisms. Carbapenem-resistant Enterobacteriaceae has shown a capacity for rapid spread in the United States and other countries.27 Nevertheless, data from the National Healthcare Safety Network suggest that the proportion of health care–associated Enterobacteriaceae resistant to carbapenems decreased sharply in the United States between 2007 and 2012, but those reductions subsequently plateaued27,28; this is consistent with our observation that the burden of carbapenem-resistant Enterobacteriaceae remained at low, stable levels after 2012. Holding the incidence of carbapenem-resistant Enterobacteriaceae at a constant low level since 2012 may represent an important success, especially given that modeling studies have predicted that the prevalence would increase rapidly in the absence of intervention.27,29,30 Further progress may require both the identification of strategies more effective at preventing spread in high-risk populations, particularly those in highest-risk post–acute care settings, and better regional coordination of surveillance and prevention activities.29,30
The only MDR organisms for which we observed an increase in incidence among hospitalized patients were ESBL-producing Enterobacteriaceae. We hypothesize that this increase may be attributable to epidemiologic characteristics that are distinct from those of the other pathogens we studied — namely, that a greater proportion of cases might result from community-based transmission, such that health care–based interventions would have less effect. Evidence supporting this hypothesis includes the observation that the increase in the burden of ESBL-related infection was driven largely by increases in the incidence of community-onset cases and by published literature showing increases in the incidence of community-associated ESBL infections.31,32 These trends might be related to the emergence of the E. coli clonal group ST131, a strain that has enhanced virulence characteristics, can colonize for longer periods of time, and has been strongly associated with the ESBL phenotype.33,34 A better understanding of the epidemiology of ESBL-producing Enterobacteriaceae is essential in order to inform more effective containment measures.
Our analysis has several limitations. First, identification and categorization of our cases were based on clinical microbiology test results combined with test and admission dates. We could not make a clinical determination of whether an isolate from a nonsterile site represented a true infection. Although a subset of such isolates may not represent true infection, these isolates represent an important epidemiologic burden in that they serve as potential reservoirs for transmission, potentially put carriers at risk for having progression to infection in the future, and may affect decisions regarding antibiotic treatment for the patient as well as others in the facility. Second, we were only able to categorize community-onset and hospital-onset cases according to the timing of culture relative to admission and were therefore not able to determine whether community-onset cases were attributable to previous health care exposures.
Third, data were derived from a large but not randomly selected sample of hospitals that may not have been nationally representative. We sought to overcome this limitation by combining three different data sources to increase the sample size and by taking methodologic steps to ensure that our estimates were representative of all U.S. hospitals. We also tested the validity of our data sources by performing the analysis independently for all three data sources; each analysis yielded similar results, which shows internal consistency. Similarly, in analyses that were restricted to hospitals that had consistent reporting, we found no meaningful differences from the full analysis. In addition, comparison of our findings with national estimates of burden and trends that are based on independent CDC surveillance systems and peer-reviewed publications provides strong corroborating evidence for the validity of our estimates.18,28,31,35,36 Finally, it is possible that changes in culturing practices, interpretive criteria for susceptibility tests, or microbiologic diagnostic systems may have affected observed trends, but this seems unlikely given divergent trends across pathogens and epidemiologic categories within pathogens.
Antimicrobial resistance remains an important threat to health in the United States. For some resistant pathogens, encouraging reductions in their incidence have been observed in recent years, which suggests that current prevention efforts, such as in health care settings, are yielding important benefits, although the burden remains high. Not all antibiotic resistance threats are decreasing, however. Further work is needed to sustain progress, including the continued development of new and more effective antibiotics, better antibiotic stewardship, and the identification of innovative interventions and strategies tailored to the spectrum of health care and community settings.
Supplementary Material
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention (CDC) or the Department of Veterans Affairs.
Supported by the CDC.
We thank Vikas Gupta, Adam Zerda, Kalvin Yu, and Gang Ye of BD for their assistance in making data from the BD Insights Research Database available for this analysis.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
John A. Jernigan, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Kelly M. Hatfield, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Hannah Wolford, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Richard E. Nelson, IDEAS Center, Veterans Affairs Salt Lake City Health Care System, and the Department of Internal Medicine, University of Utah School of Medicine — both in Salt Lake City
Babatunde Olubajo, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Sujan C. Reddy, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Natalie McCarthy, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Prabasaj Paul, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
L. Clifford McDonald, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Alex Kallen, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Anthony Fiore, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
Michael Craig, Division of Healthcare Quality Promotion and the Office of Antimicrobial Resistance, Centers for Disease Control and Prevention, Atlanta
James Baggs, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. (https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf).
- 2.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. (https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf).
- 3.Data that informs and performs: Premier Healthcare Database white paper. November 4, 2019. (https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper). [Google Scholar]
- 4.DeShazo JP, Hoffman MA. A comparison of a multistate inpatient EHR database to the HCUP Nationwide Inpatient Sample. BMC Health Serv Res 2015;15:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 2013;34:588–96. [DOI] [PubMed] [Google Scholar]
- 6.Ridgway JP, Sun X, Tabak YP, Johannes RS, Robicsek A. Performance characteristics and associated outcomes for an automated surveillance tool for bloodstream infection. Am J Infect Control 2016;44:567–71. [DOI] [PubMed] [Google Scholar]
- 7.McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis 2018;5(10):ofy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brossette SE, Hacek DM, Gavin PJ, et al. A laboratory-based, hospital-wide, electronic marker for nosocomial infection: the future of infection control surveillance? Am J Clin Pathol 2006;125:34–9. [PubMed] [Google Scholar]
- 9.Izrael D, Battaglia MP, Frankel MR. Extreme survey weight adjustment as a component of sample balancing (a.k.a. raking). Paper 247–2009. In: Proceedings of SAS Global Forum 2009. (http://support.sas.com/resources/papers/proceedings09/247-2009.pdf). [Google Scholar]
- 10.Pierannunzi C, Xu F, Wallace RC, et al. A methodological approach to small area estimation for the Behavioral Risk Factor Surveillance System. Prev Chronic Dis 2016;13:E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dal Grande E, Chittleborough CR, Campostrini S, Tucker G, Taylor AW. Health estimates using survey raked-weighting techniques in an Australian Population Health Surveillance System. Am J Epidemiol 2015;182:544–56. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Mawokomatanda T, Flegel D, et al. Surveillance for certain health behaviors among states and selected local areas — Behavioral Risk Factor Surveillance System, United States, 2011. MMWR Surveill Summ 2014;63:1–149. [PubMed] [Google Scholar]
- 13.Pretz CR, Cuthbert JP, Whiteneck GG. A validation study for using iterative proportional fitting to weight the Traumatic Brain Injury Model Systems National Database: an NIDRR-sponsored study. Arch Phys Med Rehabil 2015;96:746–9. [DOI] [PubMed] [Google Scholar]
- 14.AHA annual survey database. Chicago: American Hospital Association, 2017. (http://www.ahadata.com/).
- 15.Robust inference with multi-way clustering. Cambridge, MA: National Bureau of Economic Research, September 2006. (https://www.nber.org/papers/t0327.pdf). [Google Scholar]
- 16.Thompson SB. Simple formulas for standard errors that cluster by both firm and time. J Financ Econ 2011;99:1–10. [Google Scholar]
- 17.Healthcare Cost Report Information System. Baltimore: Centers for Medicare and Medicaid Services, 2012–2017. (https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Cost-Reports/Cost-Reports-by-Fiscal-Year). [Google Scholar]
- 18.Kourtis AP, Hatfield K, Baggs J, et al. Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections — United States. MMWR Morb Mortal Wkly Rep 2019;68:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.See I, Mu Y, Albrecht V, et al. Trends in incidence of methicillin-resistant Staphylococcus aureus bloodstream infections differ by strain type and healthcare exposure, United States, 2005–2013. Clin Infect Dis 2020;70:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725–32. [DOI] [PubMed] [Google Scholar]
- 21.Reduction in central line-associated bloodstream infections among patients in intensive care units — Pennsylvania, April 2001–March 2005. MMWR Morb Mortal Wkly Rep 2005;54:1013–6. [PubMed] [Google Scholar]
- 22.Cardo D, Dennehy PH, Halverson P, et al. Moving toward elimination of healthcare-associated infections: a call to action. Infect Control Hosp Epidemiol 2010;31: 1101–5. [DOI] [PubMed] [Google Scholar]
- 23.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30. [DOI] [PubMed] [Google Scholar]
- 24.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol 2003;24:362–86. [DOI] [PubMed] [Google Scholar]
- 25.Weiner LM, Webb AK, Walters MS, Dudeck MA, Kallen AJ. Policies for controlling multidrug-resistant organisms in US healthcare facilities reporting to the National Healthcare Safety Network, 2014. Infect Control Hosp Epidemiol 2016;37:1105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Healthcare-associated infections: data summary of HAIs in the US: assessing progress 2006–2016 (https://www.cdc.gov/hai/data/archive/data-summary-assessing-progress.html).
- 27.Woodworth KR, Walters MS, Weiner LM, et al. Containment of novel multi-drug-resistant organisms and resistance mechanisms — United States, 2006–2017. MMWR Morb Mortal Wkly Rep 2018;67:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Antibiotic resistance & patient safety portal (https://arpsp.cdc.gov/). [Google Scholar]
- 29.Paul P, Slayton RB, Kallen AJ, Walters MS, Jernigan JA. Modeling regional transmission and containment of a healthcare-associated multidrug-resistant organism. Clin Infect Dis 2020;70:388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toth DJA, Khader K, Slayton RB, et al. The potential for interventions in a long-term acute care hospital to reduce transmission of carbapenem-resistant enterobacteriaceae in affiliated healthcare facilities. Clin Infect Dis 2017;65:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaden JT, Fowler VG, Sexton DJ, Anderson DJ. Increasing incidence of extended-spectrum β-lactamase-producing Escherichia coli in community hospitals throughout the southeastern United States. Infect Control Hosp Epidemiol 2016;37:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi Y, Park YS, Rivera JI, et al. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 2013;56:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen LF, Freeman JT, Nicholson B, et al. Widespread dissemination of CTX-M-15 genotype extended-spectrum-β-lactamase-producing enterobacteriaceae among patients presenting to community hospitals in the southeastern United States. Antimicrob Agents Chemother 2014;58:1200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 2010;51:286–94. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. ABCs report: methicillin-resistant Staphylococcus aureus, 2012. (https://www.cdc.gov/abcs/reports-findings/survreports/mrsa12.html).
- 36.Goto M, McDanel JS, Jones MM, et al. Antimicrobial nonsusceptibility of gram-negative bloodstream isolates, Veterans Health Administration System, United States, 2003–2013. Emerg Infect Dis 2017;23:1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


