Abstract
Background:
Alpha-gal syndrome (AGS) is an emerging immunoglobulin E (IgE)–mediated allergy to galactose-alpha-1,3-galactose (alpha-gal). The geographic distribution and burden of AGS in the United States are unknown.
Objective:
To characterize alpha-gal IgE testing patterns and describe the trends and distribution from 2010 to 2018 in the United States.
Methods:
This retrospective analysis included all persons tested for alpha-gal IgE antibodies by Viracor-IBT Laboratories (Lee’s Summit, Missouri), the primary site of testing in the United States. Data included age and sex of person tested, specimen state of origin, collection date, and result value; persons with at least 1 positive test result (≥0.1 kU/L) were compared with negatives. Proportions tested and with positive test results were calculated using the US Census population estimates.
Results:
Overall, 122,068 specimens from 105,674 persons were tested for alpha-gal IgE during July 1, 2010, to December 31, 2018. Nearly one-third (34,256, 32.4%) had at least 1 positive result. The number of persons receiving positive test results increased 6-fold from 1110 in 2011 to 7798 in 2018. Of those receiving positive test results, mean [SD] age was 46.9 (19.8) years; men were more likely to test positive than women (43.3% vs 26.0%). Arkansas, Virginia, Kentucky, Oklahoma, and Missouri had the highest number of persons who were tested and had a positive result per 100,000 population.
Conclusion:
More than 34,000 persons, most presumably symptomatic, have received positive test results for IgE antibodies to alpha-gal, suggesting AGS is an increasingly recognized public health problem. The geographic distribution of persons who tested positive is consistent with exposure to Amblyomma americanum ticks.
Introduction
Galactose-alpha-1,3-galactose (alpha-gal) syndrome (AGS) is a type 1, immunoglobulin E (IgE)–mediated allergy to the oligosaccharide alpha-gal, present in nonprimate mammals.1,2 AGS is characterized by a delayed onset allergic reaction following ingestion of mammalian meat (eg, beef, pork, lamb) or its derivatives and a positive serum IgE result to alpha-gal (≥0.1 kU/L).3 Although time from ingestion to manifestation of symptoms is typically 3 to 6 hours, route of exposure and cofactors (eg, exercise, alcohol) can influence onset. Alpha-gal is also present in many nonfood sources, including certain pharmaceuticals (eg, cetuximab, heparin), xenotransplants (eg, porcine heart valve), and vaccines containing gelatin or grown in mammalian-based culture media (eg, rabies; measles, mumps, and rubella). Delivery by intramuscular or intravenous injection allows for faster absorption, and symptoms can occur within minutes.2 The spectrum of AGS clinical presentation is broad, ranging from urticaria to angioedema and life-threatening anaphylaxis.4 A subset of patients report only gastrointestinal symptoms, which can manifest as nausea and emesis, or debilitating abdominal pain. Although alpha-gal IgE values are not directly correlated with severity of symptoms, higher levels are associated with increased likelihood of clinical manifestations.5,6 The mainstay of symptom management is the use of antihistamines together with an avoidance diet, eliminating all mammalian products, including dairy and other derrivatives.4 Typically, patients are able to reintroduce meat and mammalian products into their diet over time.
Since 2007, AGS has been reported worldwide, including in North America, Australia, Europe, and Asia.7 Various tick species have been implicated in the development of AGS, and IgE sensitization is strongly correlated with history of tick bites.8 Nonetheless, the exact causal relationship and risk factors associated with development of AGS are still unknown. Most patients tolerate mammalian products for years or decades before onset of a reaction, suggesting an inciting event.4 In the United States, most cases have been reported from the Southeast, where Amblyomma americanum (lone star tick) is most prevalent.9 A limited number of studies performed in the United States have revealed sensitization rates to alpha-gal among individuals with symptoms of esophageal dysfunction of 22% in North Carolina,10 among asymptomatic individuals of 20.8% in Tennessee,11 6.1% in regions in northern California, and less than 1% in the northeast.11 Worldwide, sensitization rates range from 8.1% in Spain and 5.5% in Denmark, to less than 1% in northern Sweden. Certain exposure groups may experience higher sensitization rates; a study revealed that 35% of people in a cohort of forest workers in Germany tested positive for alpha-gal IgE.8,12,13 Although proportion of sensitized individuals in a region does not establish burden of AGS, the correlation between elevated IgE to alpha-gal and AGS suggests that at least a portion of these individuals will be symptomatic.
The true incidence and geographic distribution of AGS in the United States and globally remain unknown. Yet, the information is critical for risk stratification and to increase provider awareness facilitating prompt and correct diagnosis. A study revealed that nearly 10% of patients with a diagnosis of idiopathic anaphylaxis were found to have AGS on further evaluation, suggesting underdiagnosis is occurring.14 AGS is neither reportable at the state level nor nationally notifiable in the United States, and so current understanding is based on limited case reports. Viracor-IBT Laboratories (Lee’s Summit, Missouri) developed the first Food and Drug Administration–approved alpha-gal IgE testing commercially in 2010 and remains the primary source of AGS testing.
This study uses Viracor testing data to describe the extent and patterns of testing and those resulting positive for alpha-gal IgE in the United States. Because individuals in this cohort were tested by healthcare providers, most likely had clinical characteristics suggestive of AGS to prompt testing. Though severity of symptoms is not directly correlated with IgE values, an IgE level greater than or equal to 0.54 is highly suggestive of AGS as revealed in an allergy clinic setting in Europe.6 We use these data to estimate the possible magnitude of AGS as a public health threat. We also reveal the geographic distribution of positive alpha-gal IgE test results in the United States.
Methods
Study Design
We performed a retrospective analysis of specimens submitted to Viracor for alpha-gal IgE testing during July 1, 2010, to December 31, 2018. The Viracor galactose-alpha-1,3-galactose IgE test is a solid-phase immunoassay using galactose-alpha-1–3-galactose as the bound antigen. It detects and quantifies IgE antibodies specific to alpha-gal in serum specimens. Results are provided in kilounits of IgE per liter, with greater than or equal to 0.1 kU/L as the limit of detection (https://www.viracor-eurofins.com). Unique identifiers were used by Viracor to distinguish multiple specimens arising from the same patient; only deidentified data were provided to the Centers for Disease Control and Prevention. Data analyzed included age and sex of person tested, specimen state of origin, collection date, and alpha-gal IgE level.
Outcomes
Persons tested more than once were considered positive (sensitized to alpha-gal) if any alpha-gal IgE test result was greater than or equal to 0.1 kU/L and negative if all specimens tested were less than 0.1 kU/L. When a person was tested more than once, the date and state of origin of the first positive result were used. The first test result was the referent for negative persons.
To account for differences in population size in state and regional analyses, the number of persons who tested positive for IgE antibodies to alpha-gal per year was divided by the respective population estimates from the US Census Bureau.15 Annual estimates were then averaged to provide the overall estimate. Similar calculations for age and sex used the US Census population estimates within each strata.16 Proportions of persons testing positive annually, seasonally, by the US Census region, age, and sex were also calculated and evaluated for trends. Percentiles were calculated based on the number of persons positive per 100,000 and mapped to find regional trends. Seasonal data used month of specimen collection and were defined as March to May (spring), June to August (summer), September to November (fall), and December to February (winter). The mean number of tests performed was determined for all persons and by result. The geometric mean and data-derived quartile ranges of the first positive value were calculated for all persons testing positive. Although greater than or equal to 0.1 kU/L is the limit of detection for Viracor alpha-gal IgE tests, a cutoff of greater than or equal to 0.35 kU/L is often reported in the literature, reflective of the limit of detection of other tests. We also calculated the proportions of tests resulting in values ranging from 0.10 to 0.34 and greater than or equal to 0.35.3
Statistical Analysis
Descriptive statistics, risk ratios (RRs), corresponding 95% confidence intervals (95% CIs), and P values were calculated for each variable to assess association with positive results. Summaries of continuous variables are expressed as means (SDs), and summaries of categorical variables are expressed as proportions. Testing began in July 2010, providing only a partial year of data; these data are reported but excluded from the analyses. Data from the first complete year (2011) was chosen as the reference group for RR. The geometric mean and corresponding 95% CI of the first positive test result were calculated by log-transforming the original values. Differences were compared using Pearson’s χ2 test for nominal variables, Cochran-Armitage tests for trend for ordinal variables, and Student’s t tests with unequal variances for continuous variables, as appropriate. Trends were analyzed using simple linear regression models; regression statistics (slopes of best fit lines, corresponding 95% CIs, and P values) are reported. Two-sided statistical tests were considered significant at a P < .05. Analyses were done using Statistical Analysis Software, version 9.4 (SAS Institute Inc, Cary, North Carolina).
Ethics
The study was reviewed by the Centers for Disease Control and Prevention Human Research Protection Office and was deemed research not involving human subjects; approval from an institutional review board was not required.
Results
Demographics
Overall, 122,068 specimens from 105,674 persons were tested for alpha-gal IgE antibodies during July 1, 2010, to December 31, 2018; 34,256 persons (32.4%) had at least 1 positive result (Table 1). The mean (SD) age of persons tested was 42.2 (19.9) years. Persons with a positive result were older on average than persons with only negative results (mean [SD], 46.9 [19.8] vs 39.3 [19.6] y; P < .001). Persons aged greater than or equal to 70 years were most likely to test positive, and 0 to 9 years was the age group least likely to receive positive test results. Women were more likely to be tested, but men were more often positive (Table 1).
Table 1.
Characteristics of Persons Tested for Alpha-Gal Immunoglobulin E by ResultdUnited States, 2010 to 2018
| Characteristics | Total (n = 105,674) | Positivea (n = | 34,256) | Negativeb (n = 71,418) | RR (95% CI) | P valuec | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age (y), mean (SD) | 42.2 (19.9) | 46.9 | (19.8) | 39.9 | (19.6) | 1.1 (1.1–1.1) | <.001 |
| Age category (y), n (%) | |||||||
| 0–9 | 5357 | 1127 | (21.0) | 4230 | (79.0) | Reference | <.001 |
| 10–19 | 12,411 | 3372 | (27.2) | 9039 | (72.8) | 1.3 (1.2–1.4) | |
| 20–29 | 12,680 | 2932 | (23.1) | 9748 | (76.9) | 1.1 (1.0–1.2) | |
| 30–39 | 16,169 | 4037 | (25.0) | 12,132 | (75.0) | 1.2 (1.1–1.3) | |
| 40–49 | 17,986 | 5527 | (30.7) | 12,459 | (69.3) | 1.5 (1.4–1.5) | |
| 50–59 | 17,672 | 6839 | (38.7) | 10,833 | (61.3) | 1.8 (1.7–1.9) | |
| 60–69 | 14,427 | 6322 | (43.8) | 8105 | (56.2) | 2.1 (2.0–2.2) | |
| >70 | 8969 | 4098 | (45.7) | 4871 | (54.3) | 2.2 (2.1–2.3) | |
| Sex, n (%) | |||||||
| Male | 38,983 | 16,881 | (43.3) | 22,102 | (56.7) | 1.7 (1.6–1.7) | <.001 |
| Female | 65,904 | 17,118 | (26.0) | 48,786 | (74.0) | Reference | |
| Year, n (%) | |||||||
| 2010d | 372 | 209 | (56.2) | 163 | (43.8) | – | <.001 |
| 2011 | 2330 | 1110 | (47.6) | 1220 | (52.4) | Reference | |
| 2012 | 4412 | 1952 | (44.2) | 2460 | (55.8) | 0.9 (0.9–1.0) | |
| 2013 | 7200 | 2846 | (39.5) | 4354 | (60.5) | 0.8 (0.8–0.9) | |
| 2014 | 11,571 | 4295 | (37.1) | 7276 | (62.9) | 0.8 (0.7–0.8) | |
| 2015 | 14,766 | 4722 | (32.0) | 10,044 | (68.0) | 0.7 (0.6–0.7) | |
| 2016 | 17,795 | 5077 | (28.5) | 12,718 | (71.5) | 0.6 (0.6–0.6) | |
| 2017 | 21,080 | 6247 | (29.6) | 14,833 | (70.4) | 0.6 (0.6–0.7) | |
| 2018 | 26,148 | 7798 | (29.8) | 18,350 | (70.2) | 0.6 (0.6–0.7) | |
| Seasone, n (%) | |||||||
| March to May | 22,771 | 6477 | (28.4) | 16,294 | (71.6) | Reference | .022 |
| June to August | 28,987 | 10,120 | (34.9) | 18,867 | (65.1) | 1.2 (1.2–1.3) | |
| September to November | 30,850 | 10,766 | (34.9) | 20,074 | (65.1) | 1.2 (1.2–1.3) | |
| December to February | 23,066 | 6883 | (29.8) | 16,183 | (70.2) | 1.0 (1.0–1.1) | |
| US census region, n (%) | |||||||
| West | 831 | 54 | (6.5) | 777 | (93.5) | Reference | |
| Northeast | 5328 | 1658 | (31.1) | 3670 | (68.9) | 4.8 (3.7–6.2) | <.001 |
| Midwest | 8717 | 2613 | (30.0) | 6104 | (70.0) | 4.6 (3.6–6.0) | <.001 |
| South | 30,955 | 10,766 | (34.8) | 20,189 | (65.2) | 5.4 (4.1–6.9) | <.001 |
| Unknown | 59,840 | 19,165 | (32.0) | 40,678 | (68.0) | 4.9 (3.8–6.4) | <.001 |
| Number of specimens per personf, n (%) | |||||||
| Mean (SD; range) | 1.2 (0.6; 1–33) | 1.4 (1.0; 1–33) | 1.0 (0.2; 1–5) | – | <.001 | ||
| 1 | 95,603 | 26,177 | (27.4) | 69,426 | (72.6) | Reference | <.001 |
| 2–3 | 8545 | 6571 | (76.9) | 1974 | (23.1) | 2.8 (2.8–2.9) | |
| 4–5 | 1204 | 1186 | (98.5) | 18 | (1.5) | 3.6 (3.5–3.6) | |
| >6 | 322 | 322 | (100) | 0 | (0) | 3.7 (3.6–3.7) | |
Abbreviations: Alpha-gal, galactose-alpha-1,3-galactose; CI, confidence interval; IgE, immunoglobulin E; RR, risk ratio.
NOTE. Referent dates for analysis are the date of first positive test (for persons with at least 1 positive test result) and that of first test (for persons with all negative test results).
Persons with at least 1 alpha-gal IgE test result greater than or equal to 0.1 kU/L were considered positive.
Persons with all alpha-gal IgE test results less than 0.1 kU/L were considered negative.
P values were calculated to identify significant differences in trends (season, year, age category, number of specimens per person) or interactions (US Census region, sex) between those testing positive and negative; P < .05 indicates statistical significance.
Risk ratio and 95% CI not calculated for 2010 because data represent persons tested during July 2010 to December 2010.
Seasons are defined as Spring (March to May), Summer (June to August), Fall (September to November), and Winter (December to February).
Data are based on 122,068 specimens collected and tested for alpha-gal IgE from 105,674 persons from 2010 to 2018, of which 47,706 specimens (39.1%) were positive.
The number of testing positive per 100,000 population rose for both males and females as age increased; at 70 years, there was a decline (Fig 1). There was a steep rise from ages 0 to 9 to 10 to 19 years, particularly for males (Fig 1). Both males and females aged 60 to 69 years had the highest number positive compared with all other age groups (2.1 and 1.9 per 100,000, respectively); the lowest number positive for both sexes was among 0 to 9 years (0.4 for males and 0.2 for females, per 100,000).
Figure 1.
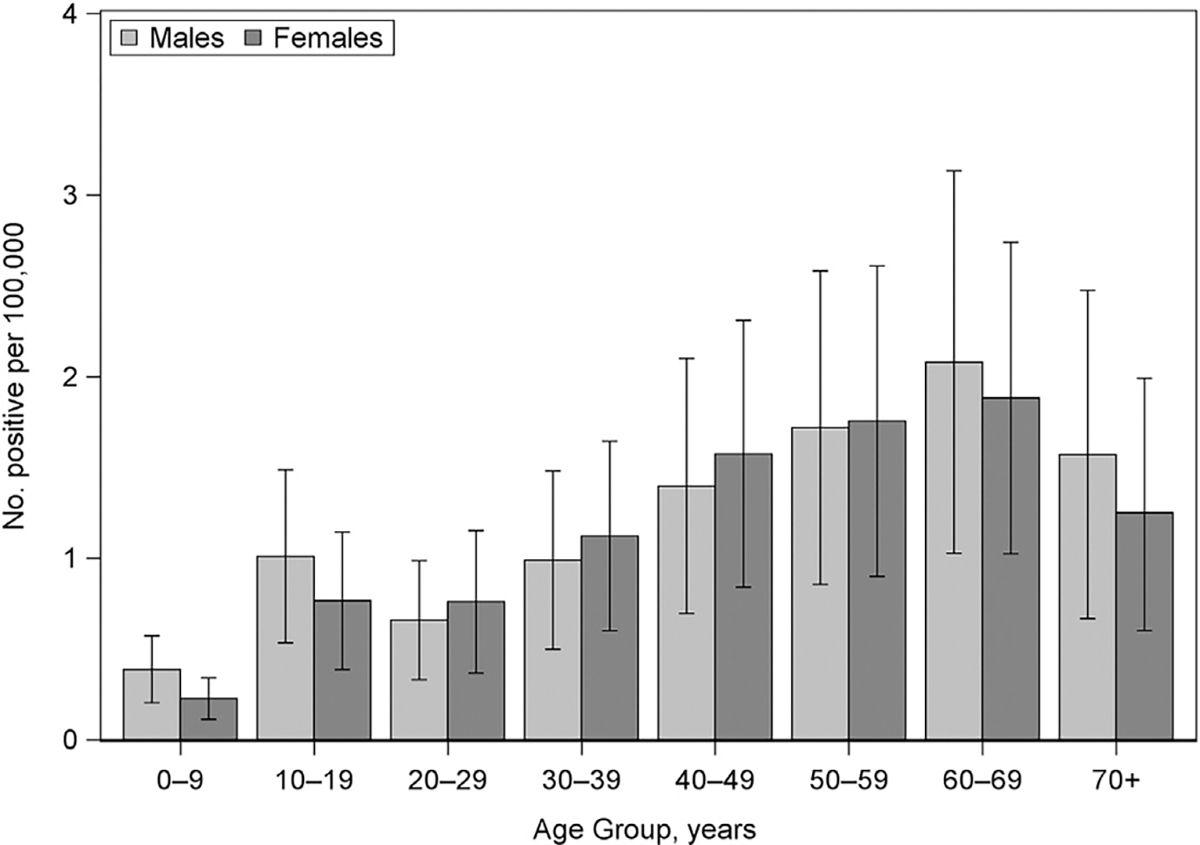
Number of persons who received positive test results per 100,000 population by age group and sex—United States, 2010 to 2018 (N = 33,999). Number of persons receiving positive test results per 100,000 was calculated using the average US Census population by age and sex during the study period. Mean estimates for each age group among males and females are represented by the clustered bars with 95% confidence intervals included for each mean estimate. No., number.
Annual and Seasonal Trends
The number of persons tested annually rose from 2330 in 2011 to 26,148 in 2018, and those receiving positive test results per year also rose from 1110 in 2011 to a high of 7798 in 2018. The proportion of persons receiving positive test results decreased from 47.6% (1110) in 2011 to 29.8% (7798) in 2018, with a low of 28.5% (5077) in 2016 (line of best fit: slope = −2.6% per year; 95% CI, −3.8 to −1.3; P = .003) (Fig 2A). The number of newly positive persons averaged 1.2 per 100,000 population per year and substantially increased during the study period from 0.4 in 2011 to 2.4 in 2018 (line of best fit: slope = 0.3; 95% CI, 0.2–0.3; P < .001) (Fig 2B).
Figure 2.
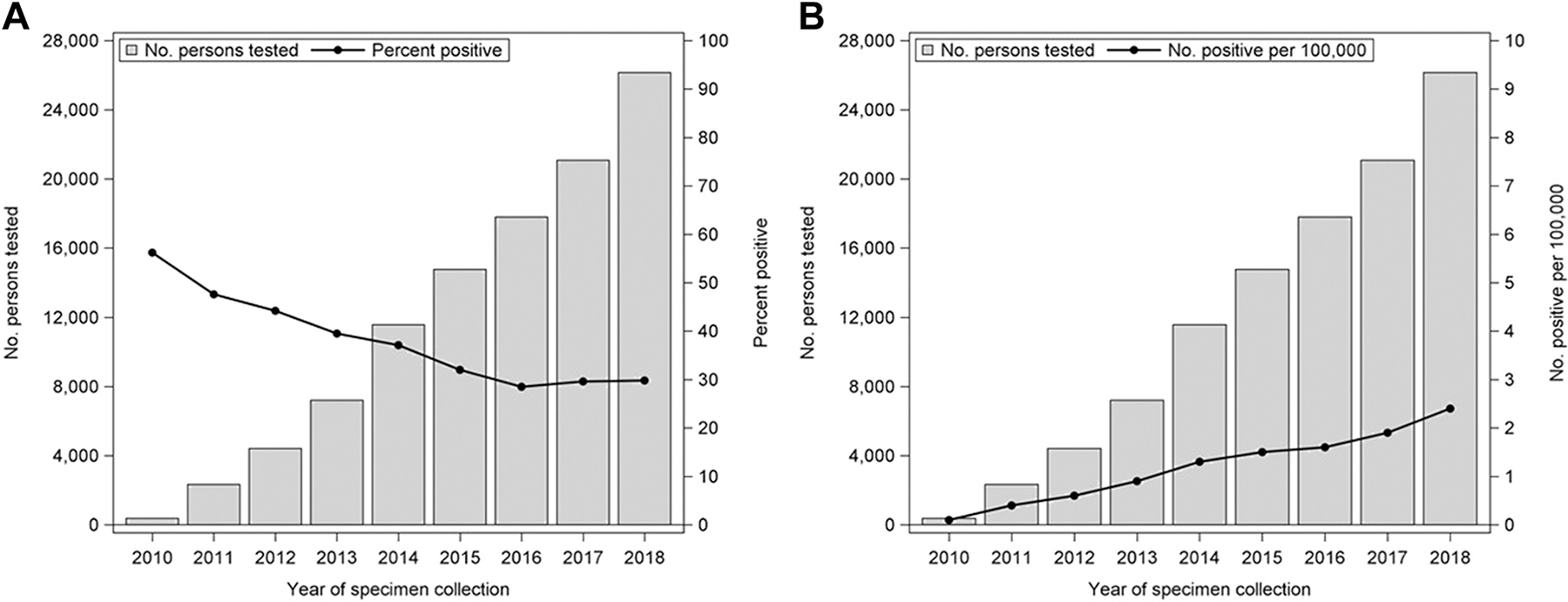
Number of persons tested for alpha-gal IgE and (A) percent positive annually. (B) Number of positive per 100,000 population annually—United States, 2010 to 2018. The line of best fit for (A): slope = −2.6; 95% CI, −3.8 to −1.3; P = .003. The line of best fit for (B): slope = 0.3; 95% CI, 0.2–0.3; P <.001. Alpha-gal, galactose-alpha-1,3-galactose; CI, confidence interval; IgE, immunoglobulin E.
Seasonal patterns revealed that testing was performed most frequently in the fall (29.2%) and least often in the spring (21.5%) (Table 1). Persons were more likely to test positive in the summer and fall compared with the spring (RR,1.2; 95% CI,1.2–1.3; P = .002).
Geographic Distribution
Data on state of specimen origin were provided for 45,834 (43.4%) persons; regionally, most specimens (30,955; 67.5%) originated from the South US Census region, where the percent testing positive was highest (10,766; 34.8%), and the fewest (831; 1.8%) originated from the West, where the percent positive was lowest (6.5%) (RR, 5.4; 95% CI, 4.1–6.9; P <.001). Although fewer specimens originated from the Northeast (5328; 11.6%) and Midwest (8717; 19.0%), the percent positive in these regions (31.1% and 30.0%) was similar to the South.
In total, 15,091 persons with known state of origin received positive test results from 2010 to 2018 (Fig 3). Arkansas, Virginia, Kentucky, Oklahoma, and Missouri had the highest number of positive persons per 100,000 population (≥2.35). States with the lowest number were Idaho, New Mexico, and Arizona (0-<0.01 per 100,000). Hawaii was the only state without any persons tested during the study period (eTable 1).
Figure 3.
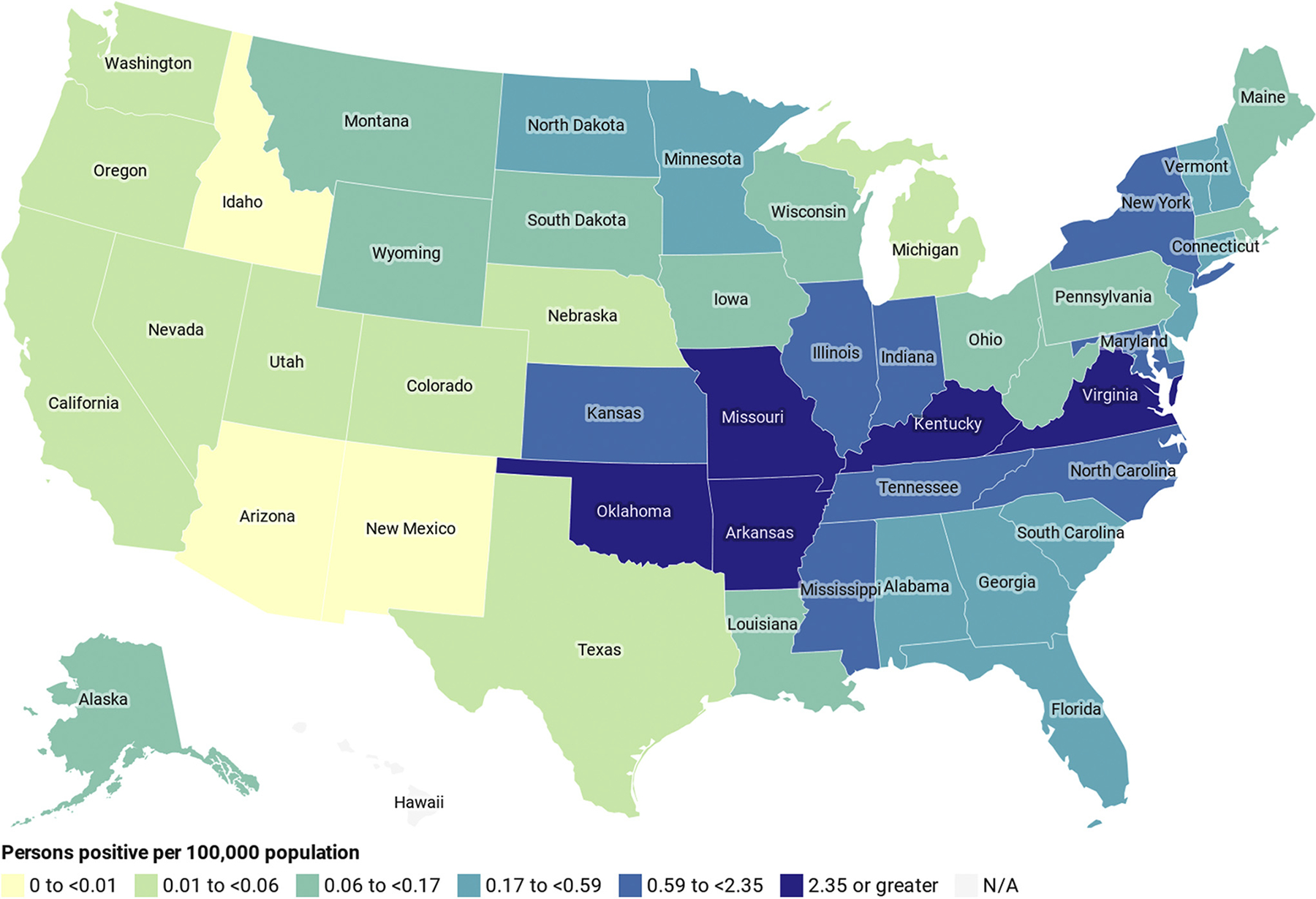
Geographic distribution of alpha-gal IgE-positive persons per 100,000 population by state—United States, 2010 to 2018 (N = 15,091). Data are based on the state of origin of the first specimen testing positive for 15,091 persons. Number of persons tested and number of positive per 100,000 were calculated using the average US Census population by state during the study period. Legend categories were created using percentile distribution of data. Alpha-gal, galactose-alpha-1,3-galactose; IgE, immunoglobulin E; N/A, not applicable; No., number.
Laboratory Characteristics
Most persons (95,603; 90.5%) had only 1 test performed, and 26,177 (27.4%) of those were positive (Table 1). Persons with at least 1 positive result were tested more often than those with negative results (mean [SD], 1.4 [1.0] vs 1.0 [0.2] tests; P < .001). Among persons with a positive result, the geometric mean of the first positive value was 2.6 kU/L (95% CI, 2.5–2.6); data-derived quartile ranges were Q1 [0.1-<0.5], Q2 [0.5-<2.4], Q3 [2.4-<13], and Q4 [13]. Most persons (27,209; 79.4%) testing positive had an alpha-gal IgE value of greater than or equal to 0.35 on the first positive test.
Discussion
Our data reveal both a remarkable utilization of testing and a dramatic increase in capture of persons sensitized to alpha-gal. From 2010 to 2018, more than 105,000 persons were tested for alpha-gal IgE, the number of tests performed annually increased more than 10-fold, and the annual number of persons testing positive increased 6-fold. The increase in testing volume was accompanied initially by a steep decline in the proportion of persons testing positive, which stabilized approximately 30% from 2015 to 2018. A likely explanation for this level of increased testing is provider awareness and increased access to diagnostics. However, the high proportion of positive test results throughout the study period suggests that physicians continue to have a high degree of clinical suspicion and diagnostic acumen when ordering diagnostic testing for alpha-gal IgE.
We identified 34,256 persons whose samples were positive for IgE antibodies to alpha-gal. Presumably, persons tested had clinical characteristics suggestive of AGS to prompt testing and nearly three-quarters (72.5%) of persons who received positive test results had IgE levels greater than or equal to 0.54, a value found to be highly predictive of AGS in an allergy clinic setting in Europe.6 These results suggest that the number of persons with AGS is an order of magnitude larger than the hundreds to thousands of cases previously reported in the literature in the United States.9 Increased clinical awareness and access to diagnostics is likely responsible for much of this increased detection. However, this increase may also stem from increasing sensitization of the population presumably through tick exposure, or by an increase in the likelihood that sensitized persons develop AGS.
Exposure to lone star ticks has been strongly implicated in the development of AGS, but a causal relationship has not been confirmed.9 Our understanding of the geographic distribution of AGS in the United States is based on small case series,9 and to date, nearly all cases have been reported from the known geographic distribution of the lone star tick.17 Similarly, our study revealed that most tests were submitted from the South US Census region and the highest numbers of persons testing positive relative to state population were in states highly endemic for Ehrlichia chaffeensis and Ehrlichia ewingii ehrlichiosis, also transmitted by the lone star tick. Oklahoma, Arkansas, Missouri, Kentucky, and Virginia had the highest number of persons testing positive for alpha-gal IgE per 100,000 population. Arkansas, Missouri, Virginia, and Oklahoma also have the highest incidence rates for E chaffeensis ehrlichiosis.18,19
Although most samples originated from the South US Census region, a high proportion of persons whose samples originated from the Midwest and Northeast tested positive (30%-31%). In states such as Minnesota and New Hampshire, the lone star tick has only rarely been observed, but these states have large established populations of the black-legged tick, Ixodes scapularis.20 Recent studies have implicated I scapularis in the development of AGS, and our data reveal individuals sensitized to alpha-gal in areas within the geographic distribution of I scapularis.21 American dog ticks (Dermacentor variabilis) are also widely distributed in the United States and are found in states such as North Dakota and in northern Minnesota.17 This may reflect travel to areas where A americanum is present, or a role of tick species other than A americanum in the development of AGS in the United States. Other Ixodes species have been linked to the development of AGS in other parts of the world, with Ixodes ricinus the primary species implicated in Europe, and Ixodes holocyclus recently joined by Ixodes australiensis associated with the condition in Australia.22,23 However, the most prominent geographic patterns in our study further solidify the primary role of A americanum in the development of AGS.
The number of persons receiving positive test results increased with age, which may reflect cumulative exposure to ticks, or an increased likelihood of development of AGS with age among previously sensitized persons. Although positive tests were equally distributed by sex, more females were tested with a lower proportion of positive test results. The reason for this testing pattern is unknown. A higher proportion of persons whose samples were obtained in the summer and fall tested positive, which would be consistent with a condition induced by tick bite as ticks are more prevalent during these months. Nevertheless, seasonal variation in the proportion positive was not substantial, likely owing to variability in the timing between symptom onset and testing.
This study is limited by the lack of clinical information. Furthermore, the geographic data were only available for less than half of the specimens and reflected the specimen state of origin, not necessarily where the exposure occurred or where the condition was acquired. Differential awareness among healthcare providers may have led to geographic variation in testing practices and thus identification of symptomatic persons sensitized to alpha-gal. Finally, it is possible that some persons could have been counted twice because of missing data elements needed to determine if an individual was unique. We think that this represents an infrequent occurrence and does not significantly affect findings. It is also possible that additional testing is performed in laboratories using their own assays, and so, these data may be an underestimate.
Our data reveal that the number of people tested for alpha-gal IgE in the United States has been rapidly increasing since the test became available. The number of people with positive alpha-gal IgE test results at levels consistent with clinical AGS suggests that AGS is an increasingly recognized public health problem. Although trends in seasonality and geographic distribution provide further evidence of an association with A americanum exposure, persons receiving positive test results outside of areas endemic for this tick raise the possibility that other tick species contribute to the development of AGS in the United States. Awareness among healthcare providers of the possibility of AGS in their patients, even in regions not endemic for the lone star tick, is critical for accurate and timely diagnosis.
Supplementary Material
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/.janai.2020.12.019.
References
- 1.Galili U The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83(6):674e686. [DOI] [PubMed] [Google Scholar]
- 2.Hilger C, Fischer J, Wölbing F, Biedermann T. Role and mechanism of galactose-alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr Allergy Asthma Rep. 2019;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin M, Apostolovic D, Biedermann T, et al. Galactose α-1,3-galactose phenotypes: lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer J, Huynh H-N, Hebsaker J, Forchhammer S, Yazdi AS. Prevalence and impact of type I sensitization to alpha-gal in patients consulting an allergy unit. Int Arch Allergy Immunol. 2020;181(2):119–127. [DOI] [PubMed] [Google Scholar]
- 7.van Nunen S Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac Allergy. 2015;5(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Quintela A, Dam Laursen AS, Vidal C, Skaaby T, Gude F, Linneberg A. IgE antibodies to alpha-gal in the general adult population: relationship with tick bites, atopy, and cat ownership. Clin Exp Allergy. 2014;44(8):1061–1068. [DOI] [PubMed] [Google Scholar]
- 9.Commins SP, James HR, KellyLA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–1293.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burk CM, Beitia R, Lund PK, Dellon ES. High rate of galactose-alpha-1,3-galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis Esophagus. 2016;29(6):558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11): 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy. 2017;72(10):1540–1547. [DOI] [PubMed] [Google Scholar]
- 13.Commins SP, Kelly LA, Rönmark E, et al. Galactose-α-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med. 2012;185(7):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter MC, Ruiz-Esteves KN, Workman L, et al. Identification of alpha-gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy. 2018;73(5):1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Population Division, US Census Bureau. Table 1. Annual estimates of the resident population for the United States, Regions, States, and Puerto Rico: April 1, 2010 to July 1, 2018. (NST-EST2018–01; ). Available at: https://www.census.gov/newsroom/press-kits/2018/pop-estimates-national-state.html. Accessed 24 January 2018. [Google Scholar]
- 16.Population Division, US Census Bureau. Annual estimates of the resident population for selected age groups by sex for the United States, States, Counties and Puerto Rico Commonwealth and municipals: April 1, 2010 to July 1, 2018. Available at: https://www.census.gov/data/tables/time-series/demo/popest/2010s-state-detail.html. Accessed 24 January 2018.
- 17.Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans. Available at: https://www.cdc.gov/ticks/geographic_distribution.Html. Accessed November 26, 2019. [Google Scholar]
- 18.Division of Health Informatics and Surveillance, Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention. Notifiable infectious diseases and conditions data tables. Atlanta, GA. Available at: https://www.cdc.gov/nndss/infectious-tables.Html. Accessed November 22, 2018. [Google Scholar]
- 19.Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51(2):342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crispell G, Commins SP, Archer-Hartman SA, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak M, Somerville C, van Nunen S. A novel Australian tick Ixodes (Endopalpiger) australiensis inducing mammalian meat allergy after tick bite. Asia Pac Allergy. 2018;8(3):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apostolovic D, Mihailovic J, Commins SP, et al. Allergenomics of the tick Ixodes ricinus reveals important α-Gal–carrying IgE-binding proteins in red meat allergy. Allergy. 2020;75(1):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


