Abstract
Purpose
The intergenerational effects of ionizing radiation remain controversial. Extensive insights have been revealed for DNA mutations and cancer incidence in progeny, yet many of these results were obtained by immediate post‐radiation mating. However, conception at short times after radiation exposure is likely to be avoided. After a long period of fertility recovery, whether unexposed sperm derived from exposed spermatogonia would challenge the health of the offspring is not yet clearly demonstrated.
Methods
Ten‐week‐old C57BL/6J males underwent whole‐body acute γ irradiation at 0 and 6.4 Gy. Testes and sperm were collected at different times after radiation to examine reproductive changes. The reproductive, metabolic, and neurodevelopmental parameters were measured in the offspring of controls and the offspring derived from irradiated undifferentiated spermatogonia.
Results
Paternal fertility was lost after acute 6.4 Gy γ radiation and recovered at 10–11 weeks post irradiation in mice. The reproductive, metabolic, and neurodevelopmental health of offspring born to irradiated undifferentiated spermatogonia were comparable to those of controls.
Conclusion
The male mice could have healthy offspring after recovery from the damage caused by ionizing radiation.
Keywords: fertility recovery, intergenerational effects, ionizing radiation, offspring, undifferentiated spermatogonia
Short abstract
Male fertility remained lost after ionizing radiation (IR) exposure but was restored at 10–11 weeks pIR. The male mice could have healthy offspring after recovery from the damage caused by IR.
1. INTRODUCTION
People could be exposed to ionizing radiation (IR) by space flight, nuclear power plant accident, atomic bomb, and radiotherapy, and spermatogenesis in testes is considered as the most radiosensitive. 1 More than 6 Gy given as a single IR exposure generally produces permanent azoospermia. 2 Germ cell damage caused by IR mainly resulted from the direct ionization of cellular macromolecules and reactive oxygen species (ROS) via ionization of water. 3 Decreased testosterone and impaired sperm quality were found in male astronauts. 4 , 5 Adverse reproductive outcomes including stillbirth and congenital malformation were found in the men working in the nuclear industry who received a median annual summary dose of 1.7 mSv. 6 , 7 , 8 The similar results of testicular dysfunction and adverse effects on offspring were also observed in animal models exposed to IR. 9 , 10 , 11 , 12 Since conception at short times after radiation exposure is likely to be avoided, the fertility recovery after radiation, and the long‐time health of offspring have inevitably become important issues to be addressed.
Recently, growing evidence suggests that paternal environmental inputs can lead to adverse health outcomes in offspring. For example, paternal inflammation increases the risk of developing obesity and metabolic syndrome‐like phenotypes for the offspring. 13 Paternal exposure to stress is associated with neuropsychiatric disorders in offspring. 14 Reduced fertility of parental radiation exposure to accumulated dose of 6 Gy can also be transmitted to non‐exposed offspring via germ cells. 15 The rate of mutation in somatic cells and frequency of expanded simple‐tandem repeat (ESTR) mutation in germline remarkably increased in offspring from irradiated male mice exposed to 0.4 Gy of fission neutrons or 2 Gy of X‐rays, 16 , 17 which indicated that radiation may indirectly affect the reproductive system in offspring. Although there are increasing efforts to investigate DNA mutations and cancer incidence in offspring of paternal exposure, whether unexposed sperm derived from exposed spermatogonia would challenge the health of the offspring is not yet clearly demonstrated. Additional research on other health effects in offspring, including non‐cancer effects such as reproductive, metabolic, and neurocognitive alterations, is also required for a better understanding of intergenerational effects of ionizing radiation.
In this study, we demonstrated fertility damage and restoration in male mice after acute IR and provided evidence related to the health of reproduction, metabolism, and neurodevelopment in offspring derived from radiation‐exposed undifferentiated spermatogonia. These findings can extend our understanding of the intergenerational effects of radiation.
2. MATERIALS AND METHODS
2.1. Sires and irradiation
C57BL/6J mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. All mice were housed under a 12‐h light/12‐h dark cycle in an approved animal facility, with food and water provided ad libitum. All institutional and national guidelines for the care and use of laboratory animals were followed. This study was approved by the Institutional Animal Care and Use Committee of Zhejiang University, Hangzhou, China.
Irradiation was performed using a 137Cs gamma radiation emitting source with an activity of 20 000 Ci. Our previous gradient experiment of different doses showed that male mice treated with 9.6 Gy were successively dead within 1 month after irradiation. So we eventually chose a sublethal dose of 6.4 Gy. Ten‐week‐old C57BL/6J males (n = 49) underwent whole‐body acute gamma irradiation at 6.4 Gy with a dose rate of 0.051 Gy/min for 125 min while sham‐irradiated controls (0 Gy, n = 49) were treated in the same manner without radiation exposure. Male mice were killed at 10 days, 5, 7, and 11 weeks post irradiation (pIR).
2.2. Tissue section staining for sires
To evaluate the histological characteristics of testes, they were fixed in 4% paraformaldehyde (PFA) at room temperature for 48 h, embedded in paraffin, sectioned at 5 μm, stained with hematoxylin and eosin (H&E). These sections were imaged with Olympus BX61 (Japan).
2.3. Enzyme‐linked immunosorbent assay (ELISA) for sires
Testosterone (T, MM‐0569M1) levels in serum were measured by ELISA kits (Meimian Industry Co., Ltd, Jiangsu, China). All procedures followed the manufacturer's protocol.
2.4. Computer‐assisted semen analysis (CASA) and sperm morphology
Sperm samples of male mice were collected from the cauda epididymis. Sperm concentration and motion parameters were evaluated by CASA system (TOX IVOS, Hamilton Thorne Research, USA). Kinematic parameters contained sperm motility, average path velocity (VAP), straight‐line velocity (VSL), curvilinear velocity (VCL), lateral head displacement (ALH), beat‐cross frequency (BCF), and straightness (STR). Sperm morphology was conducted according to the manufacturer's recommendations.
2.5. Western blotting
Testes were extracted in a cell lysis buffer (RIPA buffer, phenylmethylsulfonyl fluoride and protease inhibitor cocktail). Protein concentration was determined by a BCA protein assay kit (23225, Thermo Fisher). Equal amounts of protein (15 μg) were resolved by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and blotted to the nitrocellulose filter membranes. After blocking with 5% bovine serum albumin (BSA, 4240GR250, BioFroxx), the membranes were incubated with primary antibodies overnight. After three times washes, the membranes were incubated with HRP‐conjugated secondary antibodies at room temperature, followed by washing three times. Proteins were detected using a chemiluminescence detection system (Bio‐Rad). The antibodies used are listed in Table S1.
2.6. Fertility assay
The reproductive competency of radiation‐treated and sham‐irradiated males was determined by pairing. From 5 weeks pIR on, one male was caged with two 8‐week‐old virgin females to ensure the first filial (F1) generation derived specifically from irradiated spermatogonia, as the development of A1 differentiating spermatogonia to the point of spermiation takes 35 days. 18 To infer the time when sperm of irradiated males could successfully fertilize oocytes and irradiated males restored fertility (i.e., valid mating), plugs were assessed daily after pairing and time to first litter (numbers of days to first litter after pairing) was recorded. The time when plugs were observed, weight measure for females after plug appearance, time to first litter, and gestation period of 18–21 days in mice could help us to accurately identify the time when valid mating happened. Cumulative pregnancy rate (the percentage of pregnant females within a certain period of time) and time to pregnancy (number of days after pairing until valid mating happened) were analyzed.
2.7. The origin of offspring
After males in both groups had all produced offspring with females in the fertility assay, we re‐mated controls and the irradiated males whose fertility recovered at 10–11 weeks pIR 1:1 with 8‐week‐old virgin females at 14–17 weeks pIR to ensure that F1 offspring in both groups had similar birth dates. We also recorded the litter size and body weight of F1 offspring. When grouping the offspring, we used multiple mice (n = 1–3) from the same litter and followed the principle of “offspring in the same group were derived from at least five different females (i.e., different males).”
2.8. Tissue section staining for offspring
To evaluate the histological characteristics of testes, gastrocnemius (GAS), gonadal white adipose tissues (gWAT), and brown adipose tissues (BAT), they were fixed in 4% paraformaldehyde (PFA) at room temperature for 48 h, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). For periodic acid‐Schiff (PAS) staining, Lillie's acetic acid, alcohol, and formalin (AAF)‐fixed paraffin‐embedded sections were stained using the PAS kit (Sigma‐Aldrich) according to the manufacturer's protocol. For oil red O staining, fresh liver tissues from the same lobe were embedded in O.C.T. compound (SAKURA), sliced into 10‐μm thick sections and stained with freshly prepared Oil Red O working solution (O8010, Solarbio), and hematoxylin (BL702A, Biosharp). For Masson's trichrome staining, ovaries were embedded in paraffin and 5‐μm sections were stained with Masson's trichrome reagent to demonstrate collagen. Collagen fibers were stained blue. Cytoplasm, muscle fibers and red blood cells were stained red and the nuclei were stained black. These sections were imaged with Olympus BX61 (Japan).
For immunofluorescence (IF), the testes were fixed in 4% PFA, dehydrated in 30% sucrose solution, embedded in O.C.T. compound (SAKURA), and then cut into 6 μm sections with a freezing microtome (CryoStar NX50, Thermo Fisher). The frozen sections were permeated in 0.5% Triton X‐100, blocked in 5% BSA, incubated with primary antibodies (GFRα1, 1:100, AF560‐SP, R&D system; DDX4, 1:500, ab13840, Abcam) and secondary antibodies (1:500, ab150129, Abcam; 1:250, 111‐585‐003, Jackson ImmunoResearch, Inc.) followed by 4′,6‐diamidino‐2‐phenylindole (DAPI). Fluorescent images were captured using an Olympus FV1000 inverted confocal IX81 microscope (Japan).
For immunohistochemistry (IHC), after dewaxing and hydration, the sections were boiled in EDTA antigen retrieval solution (pH 9.0, FD7720, Fdbio) at 95°C for 15 min, blocked in 5% bovine serum albumin (BSA) for 2 h and incubated with STRA8 (1:200, ab49602, Abcam) at 4°C overnight. After washing with PBS three times, the sections were incubated with the secondary antibody (1:1, PV6001, ZSGB BIO) for 2 h at room temperature. DAB substrate kit (DA1016, Solarbio) was used according to the manufacturer's instructions. The sections were counterstained with hematoxylin and imaged with Olympus BX61.
2.9. Isolation of RNA and quantitative real‐time polymerase chain reaction (qRT‐PCR)
Testes of male offspring were homogenized in TRIzol (Takara, Japan) and total RNA was isolated according to the manufacturer's instructions. About 1 μg of total RNA (OD260/OD280 = 1.9–2.1) was reverse‐transcribed into cDNAs using the HiScript II Q RT SuperMix reagents (R223‐01, Vazyme, China) and the cDNA was mixed with ChamQ SYBR qPCR master mix reagents (Q711‐02, Vazyme, China). Primer sequences are listed in Table S2.
2.10. Computer‐assisted semen analysis (CASA) for male offspring
Sperm samples of male offspring were collected as mentioned above.
2.11. Vaginal smears and estrous cycle stage determination for female offspring
Vaginal smears stained with methylene blue had been taken daily for 31 consecutive days since F1 female offspring was 12 weeks old. The estrous cycle stage was assessed by the cytological analysis via a light microscope. Proestrus (P) exhibits round, nucleated epithelial cells. Estrus (E) consists of cornified epithelial cells. Both epithelial cells and leukocytes are present in metestrus (M). Diestrus (D) shows a few epithelial cells and a predominance of leukocytes.
2.12. Ovary serial section and follicle counting for female offspring
Ovaries from 16‐week‐old, 25‐week‐old, and 36‐week‐old diestrus F1 female offspring were fixed with 4% paraformaldehyde (PFA), embedded in paraffin wax and then serially sectioned in 5 μm thickness. Serial sections from each ovary were aligned in order on glass microscope slides and stained with H&E. The sections were examined under a light microscope and follicles were counted using the oocyte nucleus as a marker. The ovarian follicles were classified into 4 types as previously described: primordial, primary, secondary, and antral follicles. Briefly, an oocyte enclosed by a layer of squamous granulosa cells was identified as a primordial follicle and an oocyte enclosed by a layer of cuboidal granulosa cells was identified as a primary follicle. Secondary follicles had more than one layer of cuboidal granulosa cells but without antrum. Antral follicles were considered to have fluid‐filled cavity.
2.13. Enzyme‐linked immunosorbent assay (ELISA) for female offspring
Follicle‐stimulating hormone (FSH, MM‐45654M2), luteinizing hormone (LH, MM‐0582M2), progesterone (P, MM‐0568M2), and estradiol (E2, MM‐0566M2) levels in serum were measured by ELISA kits (Meimian Industry Co., Ltd, Jiangsu, China). All procedures followed the manufacturer's protocol.
2.14. Lipid measurement for offspring
High‐density lipoprotein cholesterol (HDL‐C, ADS‐W‐D011), low‐density lipoprotein cholesterol (LDL‐C, ADS‐W‐D012), total cholesterol (TC, ADS‐W‐ZF014), and triglyceride (TG, ADS‐W‐ZF013) levels were measured by kits (Aidisheng Industry Co., Ltd, Jiangsu, China) according to the manufacturer's protocol.
2.15. Body composition for offspring
Whole body fat as well as lean mass was measured by nuclear magnetic resonance (QMR06‐090H, NIUMAG) according to the manufacturer's recommendations.
2.16. Glucose and insulin tolerance tests (GTT and ITT) for offspring
GTT and ITT were performed in F1 offspring of various ages as previously described 19 with some modifications. Briefly, mice were fasted overnight (16 h food withdrawal) for GTT and were fasted for 4 h for ITT. Either glucose (2 g/kg body weight) or insulin (0.5 U/kg body weight) was injected intraperitoneally at 0 (prior to glucose or insulin administration) and blood from the tail vein was collected at different time points (0, 30, 60, 90, 120 min) using an automatic glucometer (ACCU‐CHEK).
2.17. Behavioral tests for offspring
For the open field test (OFT), F1 offspring was placed in the center of an open field box (40 × 40 × 40 cm) and monitored for 10 min. The total distance, number of center entries and the time spent in the center square (center duration) were recorded by the video tracking system.
For the novel object recognition test (NORT), F1 offspring was initially placed in the open field box for 5 min of OFT and then trained for 5 min in the same box with the presence of two identical objects. One hour after training, the animal was reinserted into the arena where one of the objects had been replaced by a novel object in a 5‐min test session. Percentage of time exploring each object during the testing session was analyzed.
For the object location test (OLT), firstly in the habituation phase, F1 offspring was allowed to freely explore the open field box for 5 min. Then in the 5‐min training phase, the animal was placed in the arena having two identical objects on the opposite sides of the box. One hour later in the test phase, the animal was reinserted into the box where one object moved to a new location and the other remained in the same location. The place discrimination index refers to the time spent with the object moved to a new place/the total time spent in exploring both objects × 100.
2.18. Statistics
The results obtained were analyzed by GraphPad Prism version 8.0 and SPSS version 23.0. A Student's t‐test (two‐tailed) was used to evaluate the statistical significance between the two groups. To assess glucose and insulin tolerance, the area of the curve (AOC) was calculated by using the formula, the area under the curve (AUC) – the area under the baseline. 20 The values are presented as means ± SD. Data is considered statistically significant if p < 0.05.
3. RESULTS
3.1. Male fertility remained lost after irradiation but was restored at 10–11 weeks pIR
Testes underwent damage‐repair process after 6.4 Gy of acute γ irradiation. At 10 days pIR, the morphology of testes in irradiated male mice did not show visible abnormality. However, germ cells in seminiferous tubules drastically decreased at 5 weeks pIR. Various spermatogenic cells appeared in the testes once again at 7 weeks pIR. Most seminiferous tubules returned to normal morphologically at 11 weeks pIR, with some tubules including few germ cells (Figure 1A). Besides, the testes/body weight ratio, sperm concentration, and testosterone in irradiated male mice were significantly declined compared with sham‐irradiated controls at 10 days pIR and 7 weeks pIR; these values became similar between the two groups until 11 weeks pIR. Radiation‐survived sperm were proved to be viable at 10 days pIR. But sperm motility in irradiated male mice was much worse than that in the controls at 7 weeks pIR and finally recovered at 11 weeks pIR (Figure 1B). At 7 weeks pIR when various spermatogenic cells appeared, about 50% of spermatozoa in irradiated male mice were abnormal. The abnormalities in the two groups still differed until 11 weeks pIR (Figure 1C). The expression of undifferentiated spermatogonial marker promyelocytic leukemia zinc‐finger protein (PLZF), showed a trend to significantly decrease first and then increase, as well as proliferating cell nuclear antigen (PCNA), an essential protein expressed in the nuclei of all proliferating cells (Figure 1D). Significantly increased DNA damage response (DDR) proteins including poly(ADP‐ribose) polymerase (PARP) and RAD51 appeared at 10 days pIR but were expressed normally at 7 and 11 weeks pIR (Figure 1D). 93.75% of females in 0 Gy group were successfully pregnant within one week after pairing from 5 weeks pIR on whereas in 6.4 Gy group, females were not pregnant until 8–9 weeks pIR (Figure 1E). No plug was found in 6.4 Gy group from 5 weeks pIR to 7 weeks pIR, which indicated that irradiated males did not mate with females. Significantly decreased sperm concentration, sperm motility, percentage of normal spermatozoa, and level of testosterone in irradiated males at 7 weeks pIR suggest that irradiated males had low libido and fertility (Figure 1B,C). At 10–11 weeks pIR, 63.16% of females in 6.4 Gy group became pregnant and the cumulative pregnancy rate was 89.47% (Figure 1E). The average time to pregnancy (days) in 6.4 Gy group was significantly longer than that in 0 Gy group (36.47 ± 6.28 days vs. 2.69 ± 1.89 days, p < 0.0001; Figure 1F). Then we re‐mated controls and these males whose fertility recovered at 10–11 weeks pIR 1:1 with 8‐week‐old virgin females at 14–17 weeks pIR. We also recorded mating outcomes to further ensure fertility recovery. Litter size was similar between the two groups, independent of the sex of pups (Figure 1G). These data were consistent with previous results, we found related to male reproduction. Hence, 6.4 Gy of acute γ irradiation greatly impaired the fertility of male mice and fertility recovery mainly occurred at 10–11 weeks pIR.
FIGURE 1.

Male fertility underwent damage‐repair process after γ radiation. (A) Representative H&E images of testes in irradiated males and controls at different times after IR. Scale bar, 100 μm. (B) Comparison of the testes/body weight ratio, sperm concentration, sperm motility, and testosterone in irradiated males and controls at different times after IR. At each time point, n = 5 for each group. (C) Representative images of spermatozoa from irradiated males at 11 weeks pIR and the percentage of abnormal spermatozoa were shown. At least 1000 sperm were counted from five different mice in each group at each time point. (D) Testes of irradiated males and controls were obtained at different times after IR for western blotting and western blotting analysis of PLZF, PCNA, PARP, and RAD51. (E) Cumulative pregnancy rates for both groups at different times after IR. (F) Comparison of time to pregnancy in both groups. The average time to pregnancy (days) in 6.4 Gy mice was significantly longer than that in 0 Gy mice (n = 16 for 0 Gy group and n = 19 for 6.4 Gy group). (G) Litter size and number of female (male) pups per litter in fertility assays of F0 males (n = 16 for each group). Data are presented as means ± SD. A Student's t‐test (two‐tailed) was used for statistical analysis; *p value < 0.05, **p value < 0.01, ***p value < 0.001, and ****p value < 0.0001. ns, not significant.
3.2. Paternal irradiation did not influence F1 spermatogenesis and spermiogenesis
The growth curves from 3 to 12 weeks of age in F1 males were comparable between the two groups (Figure 2A). And fertility assay suggested that F1 males in 6.4 Gy group had normal sexual function and could produce their offspring (Figure 2B). To explore whether germ cells in offspring could be influenced by paternal exposure, we first collected the testes of F1 male offspring at 7 and 12 days post‐partum (P7 and P12) when the first wave of spermatogenesis is initiated. 21 , 22 Generally, spermatogonia in seminiferous tubules are undifferentiated at P7 and spermatocytes enter meiotic prophase around P12. 22 Notable difference in testes by H&E staining was absent between the two groups both at P7 and P12 (Figure S1A). IF staining of the undifferentiated spermatogonia marker GFRα1 showed the F1 male offspring of irradiated males had seminiferous tubules filled with spermatogonia at P7 (Figure S1B). Meiosis initiation marker STRA8 also showed similar expression levels in the two groups at P12 (Figure S1C). In addition, no significance was observed in the expression of PLZF (undifferentiated spermatogonial marker), PCNA (proliferation marker), PARP (DDR proteins), and SOX9 (a marker for Sertoli cells) in the two groups at P7 and P12 (Figure S1D). To further investigate spermatogenesis and spermiogenesis in 12‐week‐old F1 male mice, we examined sperm functions by CASA, the germ cell marker DDX4 expression by IF staining, and specific genes of various cells in testes by qPCR. The testes/body weight ratio, sperm concentration, sperm motility, testosterone levels, and other different parameters of CASA including VAP, VSL, VCL, ALH, BCF, and STR were similar (Figure 2C,D). F1 male mice in both groups had seminiferous tubules filled with germ cells (Figure 2E). We also used Sohlh1, Smc3, Acrv1, Izumo3, Hsd3β1, and Rhox5 to reflect the alterations of spermatogonia, spermatocytes, round spermatids, elongated spermatids, Leydig cells, and Sertoli cells, respectively. There were no significant differences between the two groups (Figure 2F). PLZF, PCNA, PARP, and SOX9 were also similar between the two groups in 12‐week‐old and 36‐week‐old F1 males (Figure 2G). Collectively, these data signify normal spermatogenesis and spermiogenesis in F1 male offspring derived from exposed undifferentiated spermatogonia.
FIGURE 2.

F1 male offspring of irradiated males had normal spermiogenesis. (A) Growth curve of F1 males (n = 9 for each group). (B) Fertility assay in F1 males (n = 8 for each group). (C) Comparison of the testes/body weight ratio, sperm concentration, sperm motility, and testosterone in 12‐week‐old F1 males (n = 12 for each group). (D) Comparison of CASA parameters in 12‐week‐old F1 males. (E) IF staining of the germ cell marker DDX4 (red) in F1 male offspring at P12. Scale bar, 50 μm. (F) The expression levels of the genes associated with different testicular cells were determined in 12‐week‐old F1 males (n = 9 for each group). (G) Testes of F1 males at 12 and 36 weeks old were obtained for western blotting and western blotting analysis of PLZF, PCNA, PARP, and SOX9. Data are presented as means ± SD. A Student's t‐test (two‐tailed) was used for statistical analysis.
3.3. Paternal irradiation did not influence F1 ovarian functions
To investigate whether paternal radiation affects ovarian function, we conducted experiments on F1 females. The growth curves from 3 to 12 weeks of age in F1 females were comparable between the two groups (Figure 3A). And fertility assay also suggested that F1 females in 6.4 Gy group had the ability to conceive and produce their offspring (Figure 3B). Their serum levels of FSH, LH, P, and E2 at 16 weeks of age, as well as at 25 and 36 weeks of age were also analogous (Figure 3C). We then collected the ovaries of F1 female offspring of different ages in diestrus. Follicle counting indicated that the number of primordial, primary, secondary, and antral follicles were similar in both groups at 16 weeks of age, as well as at 25 and 36 weeks of age (Figure 3D). Additionally, vaginal smears for at least six estrous cycles exhibited regular estrous cycles (Figure 3E) and a similar percentage of time on cycle phase in F1 female offspring of irradiated male mice and the controls (Figure 3F). Morphology and collagen volume fraction of ovaries from F1 female offspring of irradiated male mice was comparable with that of sham‐irradiated controls by H&E and Masson's trichrome staining (Figure 3G). These data indicate that F1 female offspring of irradiated males had normal ovarian functions.
FIGURE 3.

Reproductive system in F1 female offspring of irradiated males functioned without distinct defects. (A) Growth curve of F1 females (n = 9 for each group). (B) Fertility assay in F1 males (n = 8 for each group). (C) The levels of reproductive hormones in F1 female offspring (n = 5 for each group) at different ages. (D) Representative H&E images of ovaries in F1 female offspring at different ages. Scale bar, 200 μm. And comparison of the numbers of primordial follicles (PrFs), primary follicles (PFs), secondary follicles (SFs), and antral follicles (AFs) of mice (n = 5 for each group) at different ages. (E) Representative estrus cycles in 12‐week‐old F1 female offspring. (F) The percent time in cycle phases of 12‐week‐old F1 female offspring (n = 10 for each group). (G) Masson's trichrome staining and statistics of collagen volume fraction (%) in ovaries of 36‐week‐old F1 female offspring (n = 5 for each group). Left panel, scale bar, 200 μm. Right panel, scale bar, 100 μm. Data are presented as means ± SD. A Student's t‐test (two‐tailed) was used for statistical analysis.
3.4. Paternal irradiation did not influence F1 glucose and lipid metabolic health
Next, we traced the metabolic change during F1 offspring's growth after birth. Body composition, GTT, and ITT were performed in adolescence (3–5 weeks) and adulthood (10–12 weeks). Neither F1 male offspring or F1 female offspring showed any difference in lean mass or body fat percentage between the two groups (Figure 4A). F1 offspring of irradiated male mice also had normal glucose tolerance and insulin sensitivity in adolescence and adulthood, regardless of sex (Figure 4B,C). Since lipid is more prone to accumulate with increasing age, 23 we explored lipid metabolism in 36‐week‐old F1 offspring. No significant change was detected in lipid levels (Figure 5A), liver glycogen, and lipid droplets (Figure 5B,C) in F1 offspring at 36 weeks of age. Besides, F1 offspring of irradiated male mice had normal GAS and gWAT in CSA, as well as BAT orderly arranged with similar size (Figure 5D,E). These results showed that F1 offspring of irradiated males had normal glucose and lipid metabolic health.
FIGURE 4.

F1 offspring of irradiated males had normal glucose metabolic health. (A) Comparison of body composition in F1 offspring (n = 9 for each group) at different ages. (B) GTT and ITT for F1 males (n = 9 for each group) at different ages. (C) GTT and ITT for F1 females (n = 9 for each group) at different ages. Data are presented as means ± SD. A Student's t‐test (two‐tailed) was used for statistical analysis.
FIGURE 5.
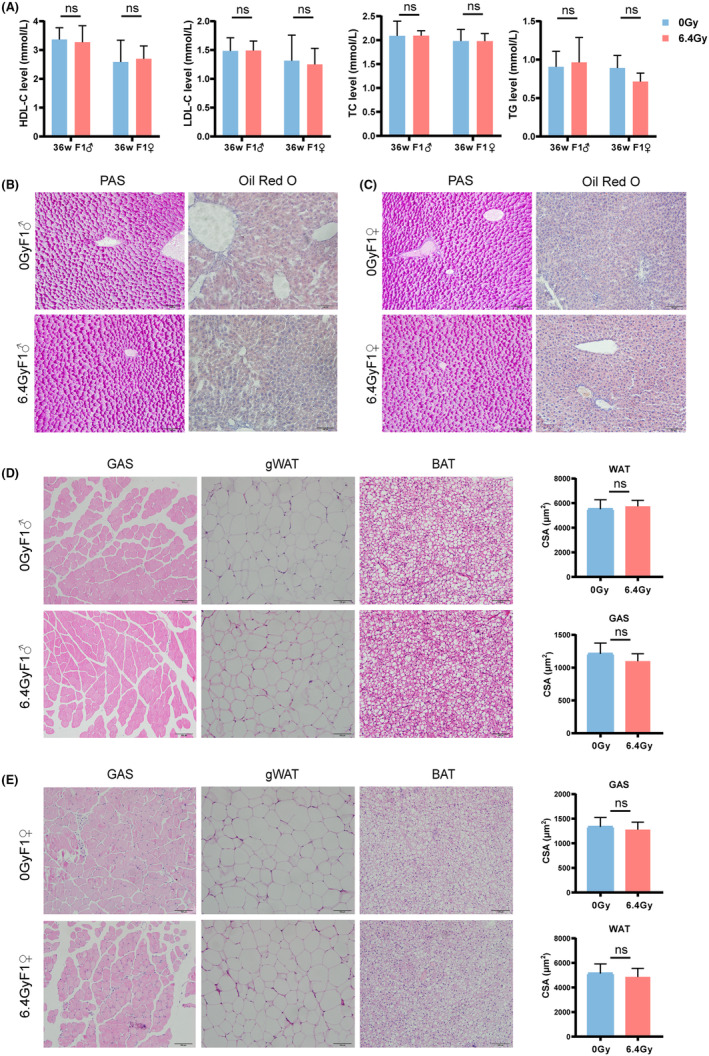
F1 offspring of irradiated males had normal lipid metabolic health. (A) Lipid levels in 36‐week‐old F1 offspring (n = 5 for each group). (B) Representative PAS and oil red O staining of liver sections in F1 males. Scale bar, 100 μm. (C) Representative PAS and oil red O staining of liver sections in F1 females. Scale bar, 100 μm. (D) Representative H&E images of GAS, gWAT, and BAT in 36‐week‐old F1 males. Statistics of CSA in GAS and gWAT (n = 9 for each group). Scale bar, 100 μm. (E) Representative H&E images of GAS, gWAT, and BAT in 36‐week‐old F1 females. Statistics of CSA in GAS and gWAT (n = 9 for each group). Scale bar, 100 μm. Data are presented as means ± SD. A Student's t‐test (two‐tailed) was used for statistical analysis.
3.5. Paternal irradiation did not influence F1 neurodevelopment
To explore whether the offspring of irradiated male mice has any phenotypes of neurodevelopmental disability, we conducted OFT for 3‐week‐old offspring. The trace plots suggested that neither F1 males nor F1 females of irradiated male mice displayed anxiety‐like behaviors. According to distance in open field, center duration, and number of center entries, we found that locomotor activity and spatial exploration were similar in both groups, regardless of sex (Figure 6A,B). Then we performed OLT and NORT in offspring of different ages. There were no significant differences in the place discrimination index of OLT (Figure 6C). Besides in NORT, 8‐week‐old F1 males and females showed higher preference to the novel object, with no significance between the radiation group and control group (Figure 6D). Twelve‐week‐old offspring did not perform as well as they did at 8 weeks of age in NORT. F1 males in both groups still had more than 50% preference to the novel object while F1 females in both groups showed less interest in the novel object (Figure 6D). The two groups still did not differ in preference to the novel object, irrespective of sex.
FIGURE 6.

No apparent anomalies existed in neurodevelopment of F1 offspring. (A) OFT for F1 males (n = 9 for each group) at 3 weeks old. Left, representative movement trace images of the two groups. Right, comparison of distance, center duration, and the number of center entries in the OFT. (B) OFT for F1 females (n = 9 for each group) at 3 weeks old. Left, representative movement trace images of the two groups. Right, comparison of distance, center duration, and the number of center entries in the OFT. (C) Place discrimination index of F1 offspring (n = 9 for each group) at 8 and 12 weeks old in the OLT. (D) Representative movement trace images of NORT in F1 offspring at 8 and 12 weeks old. And the percentage of exploration time in the old and novel objects for NORT in F1 offspring (n = 9 for each group) at 8 and 12 weeks old. All data are shown as the mean ± SD. A Student's t‐test (two‐tailed) was used for statistical analysis.
4. DISCUSSION
Here, we first traced the damage‐repair process in testes after acute IR. Male fertility remained lost after IR exposure but was restored at 10–11 weeks pIR. Then, we found that spermatogenesis, spermiogenesis, and oogenesis were normal in F1 offspring of irradiated males. Besides, F1 offspring of irradiated males showed similar glucose and lipid metabolism in adolescence and adulthood to those of the controls. Neurocognitive development was also comparable between the two groups, without apparent abnormality.
IR does cause great damage to paternal fertility. In our study, we did not find any pregnant female caged with irradiated males prior to 8 weeks pIR. It was contributed to the fact that mating did not occur, as no plug was found in 6.4 Gy group. Consequently, radiation‐induced fertility loss is not only contributed to the lack of available spermatozoa but also associated with sexual dysfunction. Testosterone is a well‐recognized crucial factor in regulating male sexual function. 24 Low testosterone is often associated with reduced libido, erectile dysfunction, reduced spontaneous erection, and delayed ejaculation. 25 Our results showed that testosterone levels in irradiated males were significantly decreased at 10 days pIR and 7 weeks pIR, which may explain the absence of mating prior to 8 weeks pIR. Similar results have been reported in rat experiments. Male rats receiving 5 Gy dose of X‐rays had a fertility rate of 0% at 8 weeks pIR. 26 To the best of our knowledge, no one has previously reported on the timing of fertility recovery after IR either. Male mice regained fertility at 10–11 weeks pIR, but abnormal spermatozoa were still markedly increased at that time. It has been reported that the progeny of irradiated males exhibited developmental delay in preimplantation embryo stage, 27 a high incidence of post‐natal death, 28 an elevated frequency of chromosomal aberrations, 29 and mutations at protein‐coding genes. 30 However, the data observed in progeny were almost from irradiated sperm. As revealed by our results, radiation‐survived sperm were proved to be still viable at 10 days pIR. Since cells of various stages in seminiferous tubules are characterized by significant differences in the chromosome conformation and the ability to repair DNA damage, 31 the heritable effects of paternal IR exposure are attributed to whether the spermatozoa at the time of fertilization are directly exposed to IR or derived from directly exposed spermatogenic cells at certain stage. Spermatogonia are more resistant to radiation. 3 Sustained spermatogenesis in adult males and fertility recovery following germ cell depletion are dependent on undifferentiated spermatogonia. 32 Therefore, unlike these studies, we were more interested in the impacts on F1 offspring born to exposed spermatogonia, knowledge of which remains very limited.
As IR severely impairs paternal fertility, we first focused on the reproductive system in F1. The fertility assays indicated that both F1 males and F1 females had normal time to pregnancy and litter size. Burruel et al. found that spermatozoa obtained 6 weeks after 1 Gy paternal F0 irradiation can transmit a decrease in fertilization rate to F1 males. 33 In Daphnia magna, the fertility of F0 and F1 gradually declined with the dose of parental exposure and significantly decreased at dose of 0.1 Gy and at higher doses of acute γ‐rays. 34 These contradictory results may be related to differences in model organisms, radiation doses, and mating timing after IR.
No significant difference in the reproductive system was observed in F1 offspring, we then chose to test their metabolic function. Metabolic syndrome (MS) has become a worldwide epidemic. And cancer survivors who underwent radiotherapy exhibited a wide range of long‐term complications, in particular circulatory diseases and their major risk factors, metabolic diseases. 35 But the evidence associated with the metabolic phenotypes of F1 offspring from irradiated survivors was scarce. We found that paternal irradiation did not influence F1 glucose and lipid metabolic health. IR results in pro‐inflammatory, pro‐thrombotic phenotype, and elevated levels of oxidative stress, which were also features of MS. 36 MS in mice with total body irradiation was related to long‐term damage to the liver, long‐lasting perturbations in skeletal muscles metabolism, adipocytes, and pancreatic functions. 37 From our results of F1 offspring, it seems that this effect does not affect gametes and could not be passed on to the next generation.
Additionally, IR might shape offspring neurodevelopment via genomic and epigenetic changes. 38 , 39 Some phenotypes might be related to autism spectrum disorder (ASD) or other neurodevelopmental disorders. 40 Zhang et al. demonstrated that utero exposure to 9.417 GHz microwave throughout gestation (Days 3.5–18) caused increased anxiety‐related behavior in F1 and decreased learning and memory in F1 males. 41 Prenatal exposure to electromagnetic field (EMF) affected cognitive processes and may cause damage to both the fetus and adult brain tissue. 42 However, a meta‐analysis demonstrated that EMF exposure during pregnancy in offspring was not associated with detrimental effects on learning and memory functions. 43 Behavioral evidence of F1 from parents exposed to IR was limited. In our study, both hippocampus‐dependent memory (measured through an OLT) and hippocampus‐independent or partially hippocampus‐dependent memory function (measured through an NORT) in offspring were not apparently affected by parental exposure. This may be due to the fact that our study did not involve intrauterine exposure.
Consistent with our results, both F1 offspring of 6 Sv of acute X‐ray‐irradiated male mice and controls lived similar lifespan, and no significant differences were observed in the frequency, severity, or age distribution of neoplasms and other diseases between experimentals and controls by autopsy. 44 Furthermore, previous studies on human populations are primarily from the offspring of atomic bomb, nuclear accident, and childhood cancer survivors. The studies were related to chromosome abnormalities, birth defects, cancer mortality, and cancer incidence. 45 , 46 , 47 , 48 The rate, class distribution, and single‐nucleotide variants (SNVs) type distribution of de novo mutations (DNMs) in adult children born to sires exposed to IR (mean cumulative preconception gonadal paternal exposure = 0.365 Gy, range = 0–4.08 Gy) from the Chernobyl accident, are comparable to those reported in the general population. 46 Increased risk of chromosome aberrations, Mendelian diseases, malformations, and cancers in relation to parental radiation dose was absent. 48 These data indicate that IR‐exposed spermatogonia seem not to cause distinct diseases in progeny.
Nevertheless, our study lacked mechanistic elucidation. DNA damage in IR‐exposed mature sperm was repaired by maternally provided error‐prone polymerase theta‐mediated end joining (TMEJ), which contributed to genome instability in F1 and embryonic lethality in F2. 33 On the other hand, spermatogonia inherently has the capacity of DNA repair. DNA repair proteins involved in homologous recombination (MRE11, RAD51) and in non‐homologous end joining (KU70) are expressed in spermatogonia. 49 But DDR is not sufficiently accurate to eliminate all DNA damage. 50 Persistent DNA damage in spermatogonia was still detected even several spermatogenic cycles after fractionated low‐dose radiation with 5 Gy. 51 , 52 Additionally, advanced age is a major risk factor for metabolic disorders 53 and diseases of nervous system 54 but we did not examine glucose metabolism and neurodevelopment in F1 at older ages.
In conclusion, our results suggest that the male mice could have healthy offspring after recovery from the damage caused by IR. At least the reproduction, metabolism, and neurodevelopment in progeny of irradiated males, were not significantly affected, as revealed by our results and the evidence mentioned above. We did not detect F2 offspring's health and future studies are certainly needed to explore the transgenerational effects of IR exposure as well as related mechanism to address the health status of the children born to exposed population at long times after IR.
FUNDING INFORMATION
This work was supported by grants from the National Key Research and Development Program of China (2022YFC2703500, D.Z.), the National Natural Science Foundation of China (No. 81974224, D.Z.), the Key Research and Development Program of Zhejiang Province (No. 2021C03098, D.Z.), and Zhejiang University Global Partnership Fund (188170 + 194452205/001, D.Z.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Data S1.
ACKNOWLEDGMENTS
We also appreciate the valuable comments from other members of our laboratory. We acknowledge the Core Facilities of Zhejiang University School of Medicine.
Li J, Liu J, Zhang Y, Qiu H, Zheng J, Xue J, et al. Effects of paternal ionizing radiation exposure on fertility and offspring's health. Reprod Med Biol. 2024;23:e12567. 10.1002/rmb2.12567
Jiaqun Li, Juan Liu, Yanye Zhang contributed equally.
REFERENCES
- 1. De Felice F, Marchetti C, Marampon F, Cascialli G, Muzii L, Tombolini V. Radiation effects on male fertility. Andrology. 2019;7(1):2–7. 10.1111/andr.12562 [DOI] [PubMed] [Google Scholar]
- 2. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100(5):1180–1186. 10.1016/j.fertnstert.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mishra B, Luderer U. Reproductive hazards of space travel in women and men. Nat Rev Endocrinol. 2019;15(12):713–730. 10.1038/s41574-019-0267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strollo F, Riondino G, Harris B, Strollo G, Casarosa E, Mangrossa N, et al. The effect of microgravity on testicular androgen secretion. Aviat Space Environ Med. 1998;69(2):133–136. [PubMed] [Google Scholar]
- 5. Smith SM, Heer M, Wang Z, Huntoon CL, Zwart SR. Long‐duration space flight and bed rest effects on testosterone and other steroids. J Clin Endocrinol Metab. 2012;97(1):270–278. 10.1210/jc.2011-2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker L, Pearce MS, Dickinson HO, Aitkin M, Craft AW. Stillbirths among offspring of male radiation workers at Sellafield nuclear reprocessing plant. Lancet. 1999;354(9188):1407–1414. 10.1016/S0140-6736(99)04138-0 [DOI] [PubMed] [Google Scholar]
- 7. Draper GJ, Little MP, Sorahan T, Kinlen LJ, Bunch KJ, Conquest AJ, et al. Cancer in the offspring of radiation workers: a record linkage study. BMJ. 1997;315(7117):1181–1188. [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar D, Salian SR, Kalthur G, Uppangala S, Kumari S, Challapalli S, et al. Semen abnormalities, sperm DNA damage and global hypermethylation in health workers occupationally exposed to ionizing radiation. PloS One. 2013;8(7):e69927. 10.1371/journal.pone.0069927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin F, Liu N, Nie J, Shen T, Xu Y, Pan S, et al. Circadian effects of ionizing radiation on reproductive function and clock genes expression in male mouse. Environ Health Prev Med. 2021;26(1):103. 10.1186/s12199-021-01021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurem S, Martin LM, Lindeman L, Brede DA, Salbu B, Lyche JL, et al. Parental exposure to gamma radiation causes progressively altered transcriptomes linked to adverse effects in zebrafish offspring. Environ Pollut. 2018;234:855–863. 10.1016/j.envpol.2017.12.023 [DOI] [PubMed] [Google Scholar]
- 11. Dobrzynska MM, Gajowik A, Jankowska‐Steifer EA, Radzikowska J, Tyrkiel EJ. Reproductive and developmental F1 toxicity following exposure of pubescent F0 male mice to bisphenol a alone and in a combination with X‐rays irradiation. Toxicology. 2018;410:142–151. 10.1016/j.tox.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 12. Baulch JE, Li MW, Raabe OG. Effect of ATM heterozygosity on heritable DNA damage in mice following paternal F0 germline irradiation. Mutat Res. 2007;616(1–2):34–45. 10.1016/j.mrfmmm.2006.11.020 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Ren L, Sun X, Zhang Z, Liu J, Xin Y, et al. Angiogenin mediates paternal inflammation‐induced metabolic disorders in offspring through sperm tsRNAs. Nat Commun. 2021;12(1):6673. 10.1038/s41467-021-26909-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33(21):9003–9012. 10.1523/JNEUROSCI.0914-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buisset‐Goussen A, Goussen B, Della‐Vedova C, Galas S, Adam‐Guillermin C, Lecomte‐Pradines C. Effects of chronic gamma irradiation: a multigenerational study using Caenorhabditis elegans . J Environ Radioact. 2014;137:190–197. 10.1016/j.jenvrad.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 16. Barber R, Plumb MA, Boulton E, Roux I, Dubrova YE. Elevated mutation rates in the germ line of first‐ and second‐generation offspring of irradiated male mice. Proc Natl Acad Sci USA. 2002;99(10):6877–6882. 10.1073/pnas.102015399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dubrova YE, Plumb M, Gutierrez B, Boulton E, Jeffreys AJ. Transgenerational mutation by radiation. Nature. 2000;405(6782):37. 10.1038/35011135 [DOI] [PubMed] [Google Scholar]
- 18. Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17. 10.1152/physrev.00013.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vinue A, Gonzalez‐Navarro H. Glucose and insulin tolerance tests in the mouse. Methods Mol Biol. 2015;1339:247–254. 10.1007/978-1-4939-2929-0_17 [DOI] [PubMed] [Google Scholar]
- 20. Virtue S, Vidal‐Puig A. GTTs and ITTs in mice: simple tests, complex answers. Nat Metab. 2021;3(7):883–886. 10.1038/s42255-021-00414-7 [DOI] [PubMed] [Google Scholar]
- 21. Huang XY, Guo XJ, Shen J, Wang YF, Chen L, Xie J, et al. Construction of a proteome profile and functional analysis of the proteins involved in the initiation of mouse spermatogenesis. J Proteome Res. 2008;7(8):3435–3446. 10.1021/pr800179h [DOI] [PubMed] [Google Scholar]
- 22. Xu K, Yang Y, Feng GH, Sun BF, Chen JQ, Li YF, et al. Mettl3‐mediated m(6)a regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27(9):1100–1114. 10.1038/cr.2017.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toth MJ, Tchernof A. Lipid metabolism in the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S121–S125. 10.1038/sj.ejcn.1601033 [DOI] [PubMed] [Google Scholar]
- 24. Corona G, Maggi M. The role of testosterone in male sexual function. Rev Endocr Metab Disord. 2022;23(6):1159–1172. 10.1007/s11154-022-09748-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late‐onset hypogonadism in middle‐aged and elderly men. N Engl J Med. 2010;363(2):123–135. 10.1056/NEJMoa0911101 [DOI] [PubMed] [Google Scholar]
- 26. Cavalim Vale AP, Dos Santos G, da Silva TP, Mansano NDS, Chies AB, Chagas EFB, et al. Influence of the AT1 receptor antagonists telmisartan and losartan on reproduction and offspring after paternal exposure to ionizing radiation. Reprod Sci. 2019;26(5):639–648. 10.1177/1933719118783251 [DOI] [PubMed] [Google Scholar]
- 27. Adiga SK, Toyoshima M, Shiraishi K, Shimura T, Takeda J, Taga M, et al. p21 provides stage specific DNA damage control to preimplantation embryos. Oncogene. 2007;26(42):6141–6149. 10.1038/sj.onc.1210444 [DOI] [PubMed] [Google Scholar]
- 28. Kumar D, Upadhya D, Salian SR, Rao SB, Kalthur G, Kumar P, et al. The extent of paternal sperm DNA damage influences early post‐natal survival of first generation mouse offspring. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):164–167. 10.1016/j.ejogrb.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 29. Kropacova K, Slovinska L, Misurova E. Cytogenetic changes in the liver of progeny of irradiated male rats. J Radiat Res. 2002;43(2):125–133. 10.1269/jrr.43.125 [DOI] [PubMed] [Google Scholar]
- 30. Barber RC, Hickenbotham P, Hatch T, Kelly D, Topchiy N, Almeida GM, et al. Radiation‐induced transgenerational alterations in genome stability and DNA damage. Oncogene. 2006;25(56):7336–7342. 10.1038/sj.onc.1209723 [DOI] [PubMed] [Google Scholar]
- 31. Satoh Y, Asakawa JI, Nishimura M, Kuo T, Shinkai N, Cullings HM, et al. Characteristics of induced mutations in offspring derived from irradiated mouse spermatogonia and mature oocytes. Sci Rep. 2020;10(1):37. 10.1038/s41598-019-56881-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan AL, La HM, Legrand JMD, Makela JA, Eichenlaub M, De Seram M, et al. Germline stem cell activity is sustained by SALL4‐dependent silencing of distinct tumor suppressor genes. Stem Cell Rep. 2017;9(3):956–971. 10.1016/j.stemcr.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burruel VR, Raabe OG, Wiley LM. In vitro fertilization rate of mouse oocytes with spermatozoa from the F1 offspring of males irradiated with 1.0 Gy 137Cs gamma‐rays. Mutat Res. 1997;381(1):59–66. 10.1016/s0027-5107(97)00148-6 [DOI] [PubMed] [Google Scholar]
- 34. Sarapultseva EI, Dubrova YE. The long‐term effects of acute exposure to ionising radiation on survival and fertility in Daphnia magna. Environ Res. 2016;150:138–143. 10.1016/j.envres.2016.05.046 [DOI] [PubMed] [Google Scholar]
- 35. Tapio S, Little MP, Kaiser JC, Impens N, Hamada N, Georgakilas AG, et al. Ionizing radiation‐induced circulatory and metabolic diseases. Environ Int. 2021;146:106235. 10.1016/j.envint.2020.106235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal. 2017;26(9):445–461. 10.1089/ars.2016.6756 [DOI] [PubMed] [Google Scholar]
- 37. Amorim NML, Kee A, Coster ACF, Lucas C, Bould S, Daniel S, et al. Irradiation impairs mitochondrial function and skeletal muscle oxidative capacity: significance for metabolic complications in cancer survivors. Metabolism. 2020;103:154025. 10.1016/j.metabol.2019.154025 [DOI] [PubMed] [Google Scholar]
- 38. Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA. 2015;112(44):13699–13704. 10.1073/pnas.1508347112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Meyer DH, Schumacher B. Inheritance of paternal DNA damage by histone‐mediated repair restriction. Nature. 2023;613(7943):365–374. 10.1038/s41586-022-05544-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rein B, Yan Z. 16p11.2 copy number variations and neurodevelopmental disorders. Trends Neurosci. 2020;43(11):886–901. 10.1016/j.tins.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Li Z, Gao Y, Zhang C. Effects of fetal microwave radiation exposure on offspring behavior in mice. J Radiat Res. 2015;56(2):261–268. 10.1093/jrr/rru097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan B, Canturk Tan F, Yalcin B, Dasdag S, Yegin K, Yay AH. Changes in the histopathology and in the proteins related to the MAPK pathway in the brains of rats exposed to pre and postnatal radiofrequency radiation over four generations. J Chem Neuroanat. 2022;126:102187. 10.1016/j.jchemneu.2022.102187 [DOI] [PubMed] [Google Scholar]
- 43. Cordelli E, Ardoino L, Benassi B, Consales C, Eleuteri P, Marino C, et al. Effects of radiofrequency electromagnetic field (RF‐EMF) exposure on pregnancy and birth outcomes: a systematic review of experimental studies on non‐human mammals. Environ Int. 2023;180:108178. 10.1016/j.envint.2023.108178 [DOI] [PubMed] [Google Scholar]
- 44. Cosgrove GE, Selby PB, Upton AC, Mitchell TJ, Steele MH, Russell WL. Lifespan and autopsy findings in the first‐generation offspring of X‐irradiated male mice. Mutat Res. 1993;319(1):71–79. 10.1016/0165-1218(93)90032-9 [DOI] [PubMed] [Google Scholar]
- 45. Ogura K, Ayabe Y, Harada C, Tanaka IB 3rd, Tanaka S, Komura JI. Increased frequency of copy number variations revealed by Array comparative genomic hybridization in the offspring of male mice exposed to low dose‐rate ionizing radiation. Int J Mol Sci. 2021;22(22):12437. 10.3390/ijms222212437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeager M, Machiela MJ, Kothiyal P, Dean M, Bodelon C, Suman S, et al. Lack of transgenerational effects of ionizing radiation exposure from the Chernobyl accident. Science. 2021;372(6543):725–729. 10.1126/science.abg2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakamura N. Why genetic effects of radiation are observed in mice but not in humans. Radiat Res. 2018;189(2):117–127. 10.1667/RR14947.1 [DOI] [PubMed] [Google Scholar]
- 48. Nakamura N, Suyama A, Noda A, Kodama Y. Radiation effects on human heredity. Annu Rev Genet. 2013;47:33–50. 10.1146/annurev-genet-111212-133501 [DOI] [PubMed] [Google Scholar]
- 49. Marjault HB, Allemand I. Consequences of irradiation on adult spermatogenesis: between infertility and hereditary risk. Mutat Res Rev Mutat Res. 2016;770(Pt B):340–348. 10.1016/j.mrrev.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 50. Ahmed EA, van der Vaart A, Barten A, Kal HB, Chen J, Lou Z, et al. Differences in DNA double strand breaks repair in male germ cell types: lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair (Amst). 2007;6(9):1243–1254. 10.1016/j.dnarep.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 51. Haines GA, Hendry JH, Daniel CP, Morris ID. Germ cell and dose‐dependent DNA damage measured by the comet assay in murine spermatozoaa after testicular X‐irradiation. Biol Reprod. 2002;67(3):854–861. 10.1095/biolreprod.102.004382 [DOI] [PubMed] [Google Scholar]
- 52. Grewenig A, Schuler N, Rube CE. Persistent DNA damage in spermatogonial stem cells after fractionated low‐dose irradiation of testicular tissue. Int J Radiat Oncol Biol Phys. 2015;92(5):1123–1131. 10.1016/j.ijrobp.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 53. Farkhondeh T, Abedi F, Samarghandian S. Chrysin attenuates inflammatory and metabolic disorder indices in aged male rat. Biomed Pharmacother. 2019;109:1120–1125. 10.1016/j.biopha.2018.10.059 [DOI] [PubMed] [Google Scholar]
- 54. Taams NE, Drenthen J, Hanewinckel R, Ikram MA, van Doorn PA. Age‐related changes in neurologic examination and sensory nerve amplitude in the general population: aging of the peripheral nervous system. Neurology. 2023;101(13):e1351–e1358. 10.1212/WNL.0000000000207665 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


