Abstract
Objectives/Background:
To estimate prevalence and severity of excessive daytime sleepiness among patients with obstructive sleep apnea (OSA) who were prescribed treatment; assess perception and satisfaction of OSA-related care; describe relationships between excessive daytime sleepiness, treatment adherence, and patient satisfaction.
Patients/Methods:
A national population-based cross-sectional sample of US adults with clinician-diagnosed OSA was surveyed in January 2021 via Evidation Health’s Achievement App. Patients completed the Epworth Sleepiness Scale, rated satisfaction with healthcare provider and overall OSA care, and reported treatment adherence. Covariates affecting excessive daytime sleepiness (average weekly sleep duration, treatment adherence, sleepiness-inducing medications, age, sex, body mass index, nasal congestion, smoking status, and comorbidities) were adjusted in multivariate regression models.
Results:
In 2289 participants (50.3% women; 44.8±11.1 years), EDS was highly prevalent (42%), and was experienced by 36% of patients with high positive airway pressure (PAP) therapy adherence. Each additional hour of nightly PAP use was associated with improved sleepiness (a 0.28-point lower Epworth score; p<0.001). Excessive daytime sleepiness was associated with lower patient satisfaction with healthcare providers and overall care (OR [95% CI] 0.62 [0.48–0.80] and 0.50 [0.39–0.64], respectively; p<0.0001), whereas PAP adherence was associated with higher patient satisfaction (OR [95% CI] 2.37 [1.64–3.43] and 2.91 [2.03–4.17]; p<0.0001), after adjusting for confounders.
Conclusions:
In a real-world population-based study of patients with OSA, excessive daytime sleepiness was highly prevalent and associated with poor patient satisfaction ratings. Better patient-centered care among patients with OSA may require interventions aimed at addressing excessive daytime sleepiness and treatment adherence.
Keywords: patient satisfaction, apnea, excessive daytime sleepiness, treatment adherence
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder that is estimated to affect as many as 33% of US adults [1–3]. Positive airway pressure (PAP) is the primary therapy recommended for OSA [4], but PAP adherence varies widely. Insufficient PAP use can reduce the potential benefit of treatment and may lead to increased risk of cardiovascular events and death [5].
Excessive daytime sleepiness (EDS) is a key symptom associated with OSA that negatively impacts health-related quality of life [6]. EDS is common among individuals with OSA and is an important reason for patients to seek treatment [7–9]. As reported in previous European studies, persistent EDS has been estimated to be 10% to 28% among continuous positive airway pressure (CPAP)-treated patients [10–12]. Importantly, no such studies have been conducted in the United States and, to our knowledge, no study has assessed the relationship between EDS and patient satisfaction with healthcare.
Patient satisfaction is an important, subjective metric used in healthcare practices to assess the quality of care a patient receives [13]. Both theory and published evidence suggest that patient satisfaction measures are accurate and distinctive indicators of healthcare quality [14–16]. Domains contributing to patient satisfaction with care may include the provision of essential care, patient-requested treatments, and healthcare provider behaviors encompassing patient-centered care [17]. Positive healthcare experiences are generally favorably associated with patient adherence to treatment and, subsequently, broadly associated with better clinical outcomes [13, 15, 18]; however, this has not been specifically studied in the context of OSA. Specifically, the relationship between persistent EDS and patient satisfaction with healthcare in patients with OSA has not been studied adequately.
This real-world study had the following objectives: 1) to estimate the prevalence and severity of EDS among patients with OSA who were prescribed treatment, 2) to assess the perception and satisfaction of OSA-related medical care, and 3) to describe the relationships between EDS, PAP adherence, and patient satisfaction with care in a large, real-world population of patients with OSA.
2. MATERIALS AND METHODS
2.1. Study design
This was a decentralized, cross-sectional, real-world survey study. Participants were recruited through Evidation Health’s Achievement App (Evidation Health, San Mateo, CA) from 10 December 2020 to 30 January 2021. Users who previously self-reported a physician diagnosis of OSA were invited to participate in the survey. The study received institutional review board approval prior to participant recruitment.
A total of 24,441 individuals were sent an invitation to take the OSA Care Satisfaction survey. Invitations were sent in a series of batches; the first batch targeted a small sample of individuals to gather initial engagement and funnel metrics. Evaluation of the initial batch responses indicated that female participants engaged with and/or responded to the survey approximately 4 times the rate of males. To account for this difference, approximately 80% of invited participants were male.
Following screening, eligible participants were asked to complete an electronic informed consent form. Consenting participants were compensated $30 for completing the study survey. Participants were excluded if they did not complete the survey within 7 days. As part of the screening and survey process, participants input data relating to their demographics and background, medical history (participants were asked to select comorbidities from a list), current medications (questions relating to use of medications categorized by clinical application [eg, prescription medications to stay awake, medications that cause daytime sleepiness]), symptom and treatment experience, treatment journey, satisfaction with care, and general health and wellbeing.
Participants were US residents ≥18 years who could speak, read, and understand English with a self-reported clinician diagnosis of OSA between 1/1/2015 and 3/31/2020. Participants with a history of bariatric surgery since OSA diagnosis or weight loss >50 pounds in a 1-year period after OSA diagnosis were excluded from the study.
Participants’ self-reported present PAP use was used to categorize participants as: nonuse (no PAP therapy; participants may have used other forms of OSA therapy [eg, oral devices, hypoglossal nerve stimulator devices]), nonadherent (<4 hours/night or <5 days/week), intermediate adherent (≥4 and <6 hours/night, ≥5 days/week), or highly adherent (≥6 hours/night, ≥5 days/week). Participants were also categorized by the presence or absence of EDS, defined by an Epworth Sleepiness Scale (ESS) score >10 [19]; where applicable, EDS was defined as mild (ESS 11–12), moderate (ESS 13–15), or severe (ESS ≥16).
2.2. Outcome measures
Self-reported outcomes from the screener and cross-sectional surveys comprised responses to questions relating to PAP use, daytime sleepiness (measured with the validated ESS) [20], and patient satisfaction across several specific aspects of care: overall healthcare provider (HCP) and OSA care; PAP and OSA treatment effectiveness; OSA symptom management; coordination of care; and education on impact of OSA on cardiovascular health, importance of PAP use, and availability of prescription medications to treat OSA symptoms. Consistent with previous studies that assessed patient satisfaction with healthcare [21–24], satisfaction was rated on a 5-point Likert scale as ‘very satisfied,’ ‘satisfied,’ ‘neither satisfied nor unsatisfied,’ ‘unsatisfied,’ or ‘very unsatisfied.’ The 5-point Likert scale has been used in more than 700 publications across various sleep disorders [21–27], as well as other medical conditions and clinical settings [28–34]. The 5-point Likert scale as used for patient satisfaction assessments has excellent discriminant and predictive validity that have been demonstrated through regression analyses [32–34]. Questions were designed to inquire about patients’ perceptions on satisfaction with various aspects of care (eg, ‘Overall satisfaction with OSA care,’ ‘Overall satisfaction with management of OSA symptoms,’ ‘Overall satisfaction with overall effectiveness of OSA treatments’). Patients who rated their care as ‘very satisfied’ or ‘satisfied’ were considered to be satisfied with their care, whereas those who rated their care as ‘neither satisfied nor unsatisfied,’ ‘unsatisfied,’ or ‘very unsatisfied’ were considered to not be satisfied with their care.
2.3. Statistical analysis
No formal sample size calculations were conducted due to the exploratory nature of the study. P values were not adjusted for multiple comparisons, and p<0.05 was considered to be statistically significant. The relationship between PAP use and ESS score was assessed with a linear model and the impacts of EDS and PAP adherence on measures of patient satisfaction with care were assessed with multivariate logistic regression models. The linear and logistic regression models controlled for age, sex, BMI, nasal congestion, smoking, presence of comorbidities, use of medications that cause sleepiness, and average weekly sleep. All other data were characterized descriptively. Because PAP adherence was a key factor in the linear and logistic regression models related to patient satisfaction with care, summary data for satisfaction with care (ie, percentage of participants satisfied/not satisfied with care) are reported for PAP users only, unless otherwise noted. Statistical analyses were conducted with SAS Version 9.2 and Python.
3. Results
3.1. Participant demographics
Of 6225 participants screened, 3207 met the eligibility criteria, and 2352 completed a consent form. Of these, 2289 completed the survey and were included in the analysis (see Figure S1 in the supplemental material). Overall, the majority of participants were White and using PAP treatment, sex was evenly distributed, and the mean (SD) age was 44.8 (11.1) years (Table 1).
Table 1.
Participant characteristics.
| Demographics | Total Population (N = 2289) |
|---|---|
| Age, years, mean (SD) | 44.8 (11.1) |
| BMI, kg/m2, mean (SD) | 35.4 (8.7) |
| Sex, female, n (%) | 1152 (50.3) |
| Race/ethnicity*, n (%) | |
| American Indian or Alaskan Native | 68 (3.0) |
| Asian | 102 (4.5) |
| Black or African American | 216 (9.4) |
| Hispanic, Latin, or Spanish | 147 (6.4) |
| Middle Eastern or North African | 11 (0.5) |
| Native Hawaiian or Other Pacific Islander | 16 (0.7) |
| White | 1889 (82.5) |
| Other | 16 (0.7) |
| Prefer not to answer | 4 (0.2) |
| Current OSA treatment*, n (%) | |
| PAP | 1589 (69.4) |
| Oral device | 137 (6.0) |
| Hypoglossal nerve stimulator | 7 (0.3) |
| PAP use, n (%) | |
| Nonuse | 700 (30.6) |
| Nonadherent | 153 (6.7) |
| Adherent (intermediate + highly adherent) | 1436 (62.7) |
| Intermediate | 225 (9.8) |
| Highly | 1211 (52.9) |
| Smoker, n (%) | 712 (31.1) |
| Nasal congestion, n (%) | 1657 (72.4) |
| Any comorbidity, n (%) | 2007 (87.7) |
| Selected comorbidities*,†, n (%) | |
| Anxiety | 1017 (44.4) |
| Depression | 962 (42.0) |
| Hypertension | 885 (38.7) |
| Dyslipidemia | 603 (26.3) |
| Asthma | 488 (21.3) |
| Chronic pain | 366 (16.0) |
| Type 2 diabetes | 320 (14.0) |
| Hypothyroidism | 298 (13.0) |
| Prediabetes | 217 (9.5) |
| Cancer | 113 (4.9) |
| Takes medication that can cause sleepiness, n (%) | 360 (15.7) |
| ESS score, mean (95% CI) | |
| Participants with EDS | 14.1 (13.9, 14.3) |
| Participants without EDS | 6.7 (6.5, 6.8) |
Participants could select >1 response.
Comorbidities reported by ≥5% of the total population.
BMI; body mass index; CI, confidence interval; EDS; excessive daytime sleepiness; ESS; Epworth Sleepiness Scale; OSA; obstructive sleep apnea; PAP; positive airway pressure; SD, standard deviation.
Psychiatric and cardiovascular/cardiometabolic comorbidities were common (Table 1). Other comorbidities assessed by this survey and frequently reported by participants included asthma (21.3%), chronic pain (16.0%), and hypothyroidism (13.0%). Comorbid sleep disorders are detailed in Table S1.
The majority (69%) of participants reported PAP use. Among PAP users, most (76%) were highly adherent. Demographics were similar across PAP use subgroups (see Table S2 in the supplemental material).
Overall, the majority of participants in this study were satisfied with their care, with 72% of the total population satisfied with their HCP and 65% satisfied with their overall OSA care. Among PAP users, 79% and 77% were satisfied with their HCPs and overall OSA care, respectively. Among PAP nonusers, 55% and 38% were satisfied with their HCPs and overall OSA care, respectively.
3.2. Prevalence of EDS and relationship to patient satisfaction
Overall, a very high proportion of participants had EDS (42%). The mean (95% CI) ESS score among those with EDS was 14.1 (13.9, 14.3) compared with 6.7 (6.5, 6.8) among those without EDS.
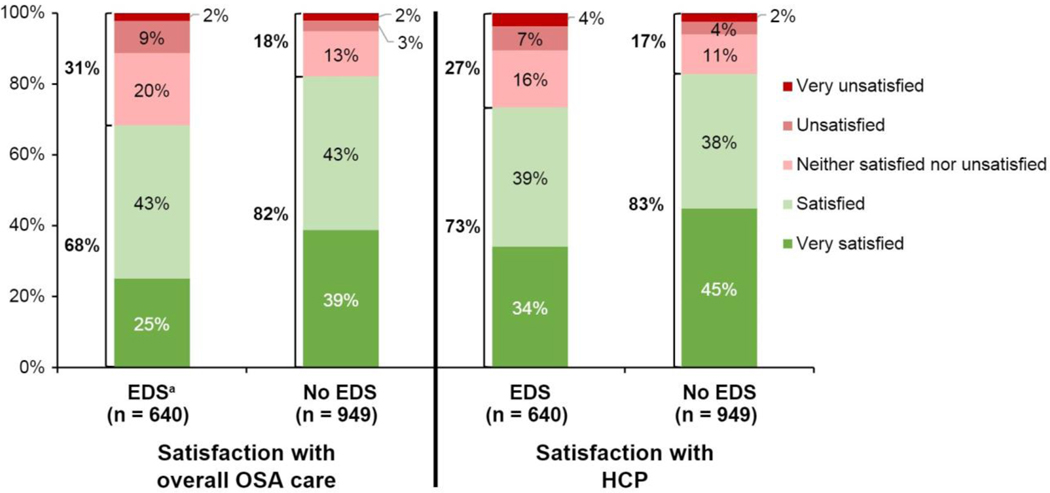
Among PAP users, participants with EDS were less likely to be satisfied with their HCP and overall care compared with participants without EDS (Figure 1). The percentage of participants who indicated they were not satisfied with their HCP and overall OSA care was 27% and 32%, respectively, among those with EDS and 17% and 18%, respectively, among those without EDS. In a multivariate logistic regression, EDS was negatively associated (odds ratio [95% CI]) with satisfaction with HCP (0.62 [0.48, 0.80], p<0.0001) and OSA care (0.50 [0.39, 0.64], p<0.0001).
Fig. 1.
PAP users with EDS were less satisfied with their overall OSA care and HCPs compared with PAP users without EDS.
EDS, excessive daytime sleepiness; HCP, healthcare provider; OSA, obstructive sleep apnea; PAP, positive airway pressure.
aTotal % of subgroups does not add to 100 due to rounding.
3.3. EDS and relationship to PAP use
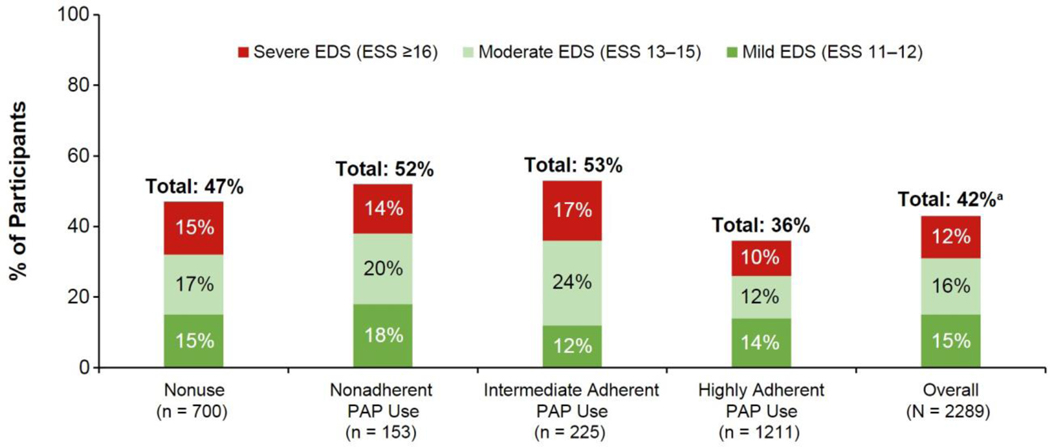
EDS was common even among those who were highly adherent to PAP (36% [95% CI: 33.7, 39.1]), with higher rates among participants who were not using PAP (47% [43.7, 51.1]), nonadherent to PAP (52% [44.4, 60.2]), and intermediately adherent to PAP (53% [46.4, 59.4]) (Figure 2).
Fig. 2.
Frequency of EDS across PAP use subgroups.
EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; PAP, positive airway pressure.
aIndividual % values may not add up to total % due to rounding.
Among the 36% of participants with EDS in the highly adherent PAP subgroup, EDS severity was mild in 14.1% (95% CI: 12.2, 16.1), moderate in 12.5% (10.6, 14.3), and severe in 9.8% (8.2, 11.5) of participants. Among the 47% of participants with EDS in the PAP nonuse subgroup, 15.3% (12.6, 18.0), 17.3% (14.5, 20.1), and 14.9% (12.2, 17.5) had mild, moderate, and severe EDS, respectively. Among the 52% of participants with EDS in the nonadherent PAP subgroup, 17.7% (11.6, 23.7), 20.3% (13.9, 26.6), and 14.4% (8.8, 19.9) had mild, moderate, and severe EDS, respectively. Among the 53% of participants with EDS in the intermediate adherent PAP subgroup, 12.4% (8.1, 16.8), 23.6% (18.0, 29.1), and 16.9% (12.0, 21.8) had mild, moderate, and severe EDS, respectively.
Lower ESS scores were associated with higher self-reported nightly PAP use (see Table S3 in the supplemental material). Each additional hour of nightly PAP use was associated with a 0.28-point lower ESS score (p<0.001). It is important to note that this model does not account for all variability in the data, and PAP use did not account for most variability in the model; the residual plots did not show obvious patterns. Other factors associated with lower ESS scores (p<0.05) included sex (male) and weekly sleep (calculated based on self-reported hours of sleep during weekdays and weekends). The presence of nasal congestion, comorbidities, and the use of medications that can cause sleepiness were associated with higher ESS scores.
3.4. Relationship between PAP use and satisfaction with care
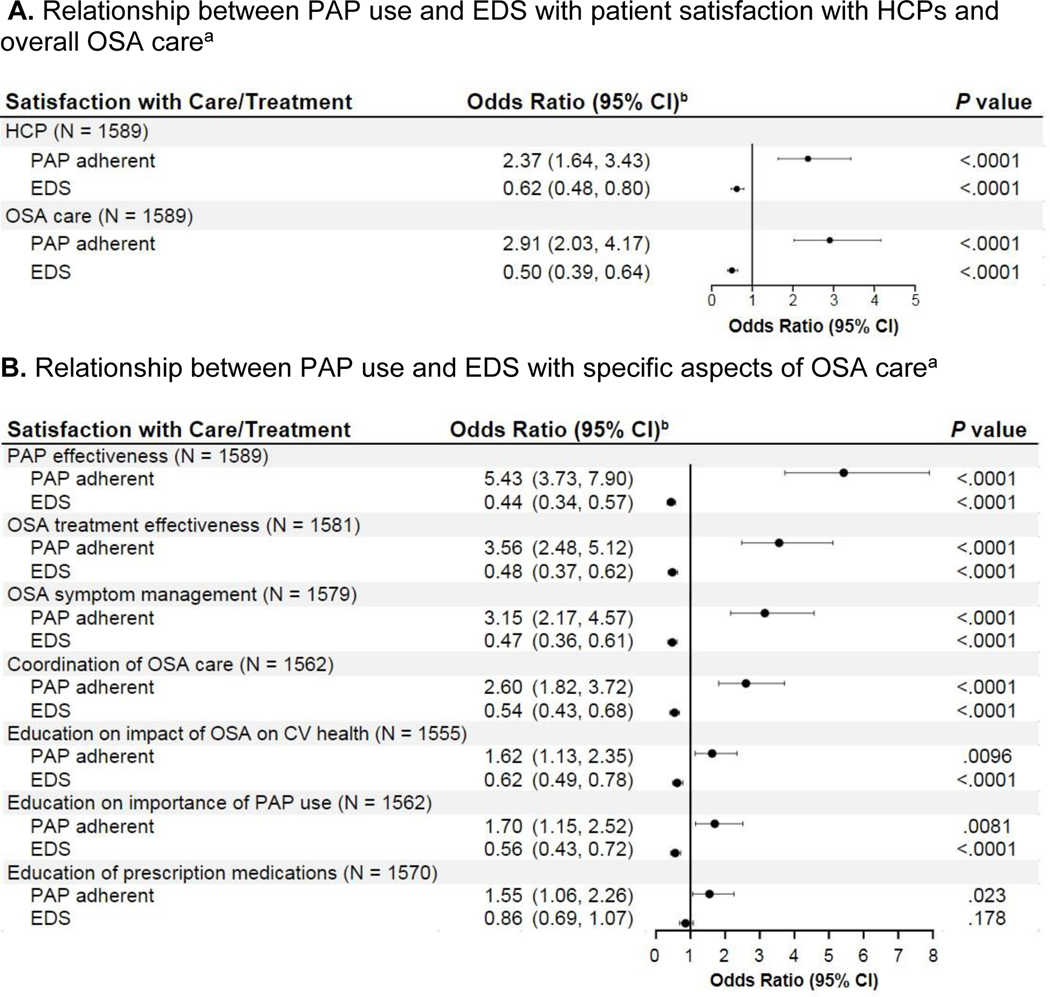
Participants who reported being adherent to PAP were 2.4 and 2.9 times as likely to be satisfied with their HCP and OSA care, respectively, compared with those who were nonadherent (Figure 3A).
Fig. 3.
Relationship between PAP use and EDS with patient satisfaction with HCPs, overall OSA care, and specific aspects of OSA care.
CI, confidence interval; EDS, excessive daytime sleepiness; HCP, healthcare provider; OSA, obstructive sleep apnea; PAP, positive airway pressure.
aPAP nonuse subgroup excluded.
bControlled for age, sex, BMI, presence of congestion, smoking status, and presence of medical comorbidity in addition to the factors listed in the table. The satisfaction dependent variable was binary: “satisfied” (very satisfied, satisfied) and “not satisfied” (neither satisfied nor unsatisfied, unsatisfied, very unsatisfied). PAP adherence level was a binary variable: “adherent” (intermediate and highly adherent groups) and “nonadherent.”
3.5. Relationships between EDS, PAP use, and satisfaction with specific aspects of care
Among PAP users, at least 1 out of 5 participants were not satisfied with specific aspects of care included in the survey, including PAP effectiveness (25%), OSA treatment effectiveness (21%), OSA symptom management (19%), coordination of OSA care (28%), and education from their HCP on impact of OSA on cardiovascular health (26%) and importance of using a PAP device (20%). Further, nearly half of participants (47%) were not satisfied with education from their HCP on availability of prescription medications to treat OSA symptoms. The percentage of participants who were not satisfied tended to be higher among PAP users with EDS than PAP users without EDS (Table S4).
In logistic regression models, participants with EDS were 14% to 56% less likely than those without EDS to be satisfied with individual aspects of care, except education on the availability of prescription medications for OSA symptoms. Participants who were adherent to PAP were 55% to 443% more likely to be satisfied across all surveyed aspects of care (Figure 3B).
4. Discussion
The findings of this study were based on robust, established measures of EDS (ESS score) and patient satisfaction [20–24]. In this real-world survey of participants with OSA, EDS was very common. Stratifying by self-reported PAP adherence demonstrated that increased PAP use was associated with lower ESS scores, but EDS remained frequent even among participants who were highly adherent to PAP. Participant satisfaction with care was negatively associated with the presence of EDS and positively associated with PAP adherence. Comorbidities (particularly depression and anxiety) were also commonly reported.
The frequency of EDS was high regardless of PAP adherence in this real-world population. The presence of EDS among more than one-third of highly adherent participants suggests that high levels of PAP use in some patients may not resolve EDS. Indeed, each additional hour of nightly PAP use was associated with only a 0.28-point lower ESS score. PAP use did not account for most variability in the linear model of ESS scores; male sex and higher amounts of sleep were also associated with lower ESS scores while nasal congestion, having a comorbidity, and taking medications that cause sleepiness were associated with higher ESS scores.
Participant satisfaction with their healthcare provider and overall OSA care were negatively associated with EDS and positively associated with PAP adherence. Further, participants with EDS were approximately 38% and 50% less likely to be satisfied with their healthcare provider and overall OSA care, respectively, demonstrating that those who have unresolved EDS are substantially more likely to be dissatisfied with their care. These relationships were maintained across more specific aspects of patient satisfaction with care, including treatment effectiveness, symptom management, coordination of care, and education by healthcare providers on OSA-related topics. Reciprocally, positive patient care experiences are broadly associated with treatment adherence [18], and education on medication and health management improves patient satisfaction in various clinical settings and chronic conditions [35]. Collectively, these relationships illustrate the importance of resolving EDS and its impact on patient satisfaction and PAP adherence. A recent systematic review concluded that future studies are needed to understand how treatment of EDS with wake-promoting agents may impact PAP adherence as no studies to date have examined this association [36].
Comorbidities, including those frequently associated with OSA, were common among this real-world cohort, particularly anxiety and depression. The frequency of most comorbidities was similar across PAP adherence groups. These findings align with numerous studies describing an increased risk for the development of psychiatric disorders, such as post-traumatic stress disorder [37], depression [37–39], bipolar disorder [37, 39], anxiety [37, 38], and psychosis [37] in patients with OSA. Although not examined in the current study, the severity of OSA has previously been shown to impact the level of increased risk for the onset of depression, even after controlling for other potential confounders [40].
4.1. Limitations
This study had several limitations. First, PAP adherence was based on self-report. Typically, patients tend to overestimate CPAP adherence compared with objective data from devices [41, 42]. This is important considering PAP adherence has been shown to improve quality of life in patients with severe OSA [43]; however, findings on the severity of OSA and quality of life were not reported here. Since all adherence data in this study were self-reported, any overestimation would be systematic. Second, conclusions regarding causality cannot be drawn regarding the associations observed here (eg, that participants who adhere to PAP experience improvements in EDS and are thus more satisfied with care received, or that participants who are satisfied with care received are more inclined to adhere to PAP). Additionally, the nonrandomized, observational nature of the trial and different sample sizes within PAP use subgroups complicate the interpretation of the odds ratios observed. Further, the recruitment method (via a digital app) may have some inherent limitations, such as a likelihood to recruit younger participants or to incentivize reporting better self-care behaviors; therefore, these findings may not be fully generalizable to all patients with OSA.
5. Conclusions
In this real-world study of participants with OSA, EDS was common and was associated with lower patient satisfaction with multiple aspects of OSA care. Those with EDS were 38% and 50% less likely than those without EDS to be satisfied with their healthcare provider and OSA care, respectively.
PAP adherence was associated with higher patient satisfaction ratings across high level and specific aspects of care, and each hour of additional nightly PAP use was associated with a 0.28-point lower ESS score. The results of this study, based on robust measures of EDS and patient satisfaction with care, provide an update for providers to understand the frequency of EDS in OSA and its relationship with care satisfaction and PAP use in a real-world population, which may help providers improve patient care.
Supplementary Material
HIGHLIGHTS.
Excessive daytime sleepiness (EDS) is common in obstructive sleep apnea (OSA)
EDS can persist despite airway therapy, leading to greater public health burden
Residual EDS may adversely impact patient satisfaction with healthcare quality
Acknowledgments
Under the direction of the authors, Sean Anderson, PhD, and Hannah Ritchie, PhD, of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support for this manuscript, which was funded by Axsome Therapeutics, Inc. and Jazz Pharmaceuticals.
Funding/Support
This study was supported by Jazz Pharmaceuticals. The development of this manuscript was supported by Axsome Therapeutics, Inc. and Jazz Pharmaceuticals. At the time the study was conducted, Jazz Pharmaceuticals had worldwide development, manufacturing, and commercialization rights to solriamfetol, excluding certain jurisdictions in Asia. Axsome Therapeutics, Inc. and its affiliates completed acquisition of the rights to Sunosi® (solriamfetol) from Jazz Pharmaceuticals in the US on May 9, 2022 and ex-US on November 14, 2022. SK Biopharmaceuticals, the discoverer of the compound (also known as SKL-N05), maintains rights in 12 Asian markets, including Korea, China, and Japan.
Role of the Funder/Sponsor
The authors, including the Axsome Therapeutics, Inc. and Jazz Pharmaceuticals authors, were involved with: designing the study; collecting, analyzing, and interpreting the data; and writing the manuscript. Although Axsome Therapeutics, Inc. and Jazz Pharmaceuticals were involved in the review of the manuscript, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in Sleep Medicine were made by the authors independently.
Declarations for Each Author:
S Parthasarathy is a consultant for Jazz Pharmaceuticals, Inc., Abbvie, Inc., and Apria Healthcare. He reports receiving research grants to institution from Verily Lifesciences, Inc., Philips, Inc., Sommetrics, Inc., WHOOP, Inc., Regeneron, Inc., and USBiotest, Inc. Additionally, he reports receiving personal fees from UpToDate, Inc. Dr. Parthasarathy reports grants from NIH (R25-HL126140, R33-HL151254; OT2-HL161847; R21-HD109777; C06-OD028307; HL140144; HL138377; 1OT2HL156812; OT2-HL156912 and OT2HL158287), PCORI (DI-2018C2–13161, CER-2018C2–13262), Department of Defense (W81XWH20C0051 and W81XWH2110025), Pima County Health Department (CPIMP211275), Arizona Commerce Authority (LTR DTD 021822), and Sergey Brin Foundation. In addition, Dr. Parthasarathy has a patent US20160213879A1 licensed to SaiOx, Inc. that is unrelated to this manuscript.
D Hyman, R Saad, S Morris, J Zhang, and G Parks are former employees of Jazz Pharmaceuticals who, in the course of this employment, received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc.
G Parks is currently a full-time employee of Axsome Therapeutics, Inc who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Axsome Therapeutics, Inc.
J Doherty is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc.
L Eldemir, B Fox, MKY Vang, and J Schroeder, are former employees of Evidation, a consulting firm that received research funding from Jazz Pharmaceuticals to conduct this study.
NJ Marshall is an employee of Evidation, a consulting firm that received research funding from Jazz Pharmaceuticals to conduct this study.
ABBREVIATIONS USED IN MANUSCRIPT
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- HCP
healthcare provider
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
Footnotes
Clinical Trial Registration: n/a
Data Sharing Statement
All relevant data are provided within the manuscript and supporting files.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
D Hyman, R Saad, S Morris, and GS Parks are former employees of Jazz Pharmaceuticals.
L Eldemir, B Fox, MKY Vang, and J Schroeder are former employees of Evidation Health.
REFERENCES
- [1].Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009;108(5):246–9. [PMC free article] [PubMed] [Google Scholar]
- [3].Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7(8):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Epstein LJ, Kristo D, Strollo PJ Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- [5].Jacobsen AR, Eriksen F, Hansen RW, et al. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One 2017;12(12):e0189614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stepnowsky C, Sarmiento KF, Bujanover S, Villa KF, Li VW, Flores NM. Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey. J Clin Sleep Med 2019;15(2):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].American Academy of Sleep Medicine. International Classification of Sleep Disorders – Third Edition. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- [8].Pagel JF. Excessive daytime sleepiness. Am Fam Physician 2009;79(5):391–6. [PubMed] [Google Scholar]
- [9].Dongol EM, Williams AJ. Residual excessive sleepiness in patients with obstructive sleep apnea on treatment with continuous positive airway pressure. Curr Opin Pulm Med 2016;22(6):589–94. [DOI] [PubMed] [Google Scholar]
- [10].Gasa M, Tamisier R, Launois SH, et al. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res 2013;22(4):389–97. [DOI] [PubMed] [Google Scholar]
- [11].Pepin JL, Viot-Blanc V, Escourrou P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J 2009;33(5):1062–7. [DOI] [PubMed] [Google Scholar]
- [12].Bonsignore MR, Pepin JL, Cibella F, et al. Excessive daytime sleepiness in obstructive sleep apnea patients treated with continuous positive airway pressure: data from the European Sleep Apnea Database. Front Neurol 2021;12:690008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glickman SW, Boulding W, Manary M, et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2010;3(2):188–95. [DOI] [PubMed] [Google Scholar]
- [14].Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med 2013;368(3):201–3. [DOI] [PubMed] [Google Scholar]
- [15].Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med 2008;359(18):1921–31. [DOI] [PubMed] [Google Scholar]
- [16].Hickson GB, Federspiel CF, Pichert JW, Miller CS, Gauld-Jaeger J, Bost P. Patient complaints and malpractice risk. JAMA 2002;287(22):2951–7. [DOI] [PubMed] [Google Scholar]
- [17].Patient satisfaction surveys. NEJM Catalyst, 2018. Available at: 10.1056/CAT.18.0288. Accessed: November 30, 2021. [DOI] [Google Scholar]
- [18].Anhang Price R, Elliott MN, Zaslavsky AM, et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev 2014;71(5):522–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep 1997;20(10):844–9. [DOI] [PubMed] [Google Scholar]
- [20].Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- [21].Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna S. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med 2013;9(6):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Parthasarathy S, Subramanian S, Quan SF. A multicenter prospective comparative effectiveness study of the effect of physician certification and center accreditation on patient-centered outcomes in obstructive sleep apnea. J Clin Sleep Med 2014;10(3):243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Parthasarathy S, Haynes PL, Budhiraja R, Habib MP, Quan SF. A national survey of the effect of sleep medicine specialists and American Academy of Sleep Medicine Accreditation on management of obstructive sleep apnea. J Clin Sleep Med 2006;2(2):133–42. [PubMed] [Google Scholar]
- [24].Cimas M, Ayala A, García-Pérez S, Sarria-Santamera A, Forjaz MJ. The patient satisfaction questionnaire of EUprimecare project: measurement properties. Int J Qual Health Care 2016;28(3):275–80. [DOI] [PubMed] [Google Scholar]
- [25].Braun M, Wollny M, Schoebel C, Sommer JU, Heiser C. Patient-reported experience with hypoglossal nerve stimulation in the treatment of obstructive sleep apnea. Sleep Breath 2023;Aug 5 Online Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Waibel KH, Cain SM, Hall TE, Keen RS. Multispecialty Synchronous Telehealth Utilization and Patient Satisfaction Within Regional Health Command Europe: A Readiness and Recapture System for Health. Mil Med 2017;182(7):e1693–e7. [DOI] [PubMed] [Google Scholar]
- [27].Ng ET, Perez-Garcia A, Lagravère-Vich MO. Development and initial validation of a questionnaire to measure patient experience with oral appliance therapy. J Clin Sleep Med 2023;19(8):1437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miller MJ, Pak SS, Keller DR, Barnes DE. Evaluation of Pragmatic Telehealth Physical Therapy Implementation During the COVID-19 Pandemic. Phys Ther 2021;101(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Derby R, Rohal P, Jackson C, Beutler A, Olsen C. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med 2011;24(1):69–74. [DOI] [PubMed] [Google Scholar]
- [30].Santoro AJ, Ford EA, Pontes M, Busconi BD, McMillan S. Patient-Specific E-mailed Discharge Instructions Improve Patient Satisfaction and Patient Understanding After Surgical Arthroscopy. Arthroscopy, sports medicine, and rehabilitation 2022;4(4):e1315–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Satpathy S, Wundaville LT, Satapathy S, et al. A Systematic Review of Patient Satisfaction Scales and Their Applicability to Covid-19 Hospitalized Patients: Gaps and Emerging Needs. Journal of patient experience 2022;9:23743735221079132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Anderson RT, Weisman CS, Camacho F, Scholle SH, Henderson JT, Farmer DF. Women’s satisfaction with their on-going primary health care services: a consideration of visit-specific and period assessments. Health Serv Res 2007;42(2):663–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Scholle SH, Weisman CS, Anderson R, Weitz T, Freund KM, Binko J. Women’s satisfaction with primary care: a new measurement effort from the PHS National Centers of Excellence in Women’s Health. Womens Health Issues 2000;10(1):1–9. [DOI] [PubMed] [Google Scholar]
- [34].Scholle SH, Weisman CS, Anderson RT, Camacho F. The development and validation of the primary care satisfaction survey for women. Womens Health Issues 2004;14(2):35–50. [DOI] [PubMed] [Google Scholar]
- [35].Yen PH, Leasure AR. Use and Effectiveness of the Teach-Back Method in Patient Education and Health Outcomes. Fed Pract 2019;36(6):284–9. [PMC free article] [PubMed] [Google Scholar]
- [36].Saad R, Somers VK, Parks GS, Pushkarna D, Fazeli MS, Black J. Determinants of adherence/persistence to positive airway pressure therapy in patients with obstructive sleep apnoea [abstract]. Sleep Med 2022;100(suppl 1):S245–S6. [Google Scholar]
- [37].Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005;28(11):1405–11. [DOI] [PubMed] [Google Scholar]
- [38].Kim JY, Ko I, Kim DK. Association of obstructive sleep apnea with the risk of affective disorders. JAMA Otolaryngol Head Neck Surg 2019;145(11):1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lu MK, Tan HP, Tsai IN, Huang LC, Liao XM, Lin SH. Sleep apnea is associated with an increased risk of mood disorders: a population-based cohort study. Sleep Breath 2017;21(2):243–53. [DOI] [PubMed] [Google Scholar]
- [40].Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006;166(16):1709–15. [DOI] [PubMed] [Google Scholar]
- [41].Choi J-A, Yoon I-Y, Han E-G, Lee S. Subjective and objective CPAP compliance in patients with obstructive sleep apnea syndrome. Sleep Med Res 2011;2(2):63–8. [Google Scholar]
- [42].Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care 2013;58(9):1467–73. [DOI] [PubMed] [Google Scholar]
- [43].Batool-Anwar S, Goodwin JL, Kushida CA, et al. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). J Sleep Res 2016;25(6):731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.