Abstract
Background
Fibroids are common benign tumours arising in the uterus. Myomectomy is the surgical treatment of choice for women with symptomatic fibroids who prefer or want uterine conservation. Myomectomy can be performed by conventional laparotomy, by mini‐laparotomy or by minimal access techniques such as hysteroscopy and laparoscopy.
Objectives
To determine the benefits and harms of laparoscopic or hysteroscopic myomectomy compared with open myomectomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (inception to July 2014), the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of Controlled Trials (inception to July 2014), MEDLINE(R) (inception to July 2014), EMBASE (inception to July 2014), PsycINFO (inception to July 2014) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (inception to July 2014) to identify relevant randomised controlled trials (RCTs). We also searched trial registers and references from selected relevant trials and review articles. We applied no language restriction in these searches.
Selection criteria
All published and unpublished randomised controlled trials comparing myomectomy via laparotomy, mini‐laparotomy or laparoscopically assisted mini‐laparotomy versus laparoscopy or hysteroscopy in premenopausal women with uterine fibroids diagnosed by clinical and ultrasound examination were included in the meta‐analysis.
Data collection and analysis
We conducted study selection and extracted data in duplicate. Primary outcomes were postoperative pain, reported in six studies, and in‐hospital adverse events, reported in eight studies. Secondary outcomes included length of hospital stay, reported in four studies, operating time, reported in eight studies and recurrence of fibroids, reported in three studies. Each of the other secondary outcomes—improvement in menstrual symptoms, change in quality of life, repeat myomectomy and hysterectomy at a later date—was reported in a single study. Odds ratios (ORs), mean differences (MDs) and 95% confidence intervals (CIs) were calculated and data combined using the fixed‐effect model. The quality of evidence was assessed using Grades of Recommendation, Assessment, Development and Evaluation (GRADE) methods.
Main results
We found 23 potentially relevant trials, of which nine were eligible for inclusion in this review. The nine trials included in our meta‐analysis had a total of 808 women. The overall risk of bias of included studies was low, as most studies properly reported their methods.
Postoperative pain: Postoperative pain was measured on a visual analogue scale (VAS), with zero meaning 'no pain at all' and 10 signifying 'pain as bad as it could be.' Postoperative pain was significantly less, as determined by subjectively assessed pain score at six hours (MD ‐2.40, 95% CI ‐2.88 to ‐1.92, one study, 148 women, moderate‐quality evidence) and 48 hours postoperatively (MD ‐1.90, 95% CI ‐2.80 to ‐1.00, two studies, 80 women, I² = 0%, moderate‐quality evidence) in the laparoscopic myomectomy group compared with the open myomectomy group. This means that among women undergoing laparoscopic myomectomy, mean pain score at six hours and 48 hours would be likely to range from about three points lower to one point lower on a VAS zero‐to‐10 scale. No significant difference in postoperative pain score was noted between the laparoscopic and open myomectomy groups at 24 hours (MD ‐0.29, 95% CI ‐0.7 to 0.12, four studies, 232 women, I² = 43%, moderate‐quality evidence). The overall quality of these findings is moderate; therefore further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
In‐hospital adverse events: No evidence suggested a difference in unscheduled return to theatre (OR 3.04, 95% CI 0.12 to 75.86, two studies, 188 women, I² = 0%, low‐quality evidence) and laparoconversion (OR 1.11, 95% CI 0.44 to 2.83, eight studies, 756 women, I² = 53%, moderate‐quality evidence) when open myomectomy was compared with laparoscopic myomectomy. Only one study including 148 women reported injury to pelvic organs (no events were described in other studies), and no significant difference was noted between laparoscopic myomectomy and laparoscopically assisted mini‐laparotomy myomectomy (OR 3.04, 95% CI 0.12 to 75.86). Significantly lower risk of postoperative fever was observed in the laparoscopic myomectomy group compared with groups treated with all types of open myomectomy (OR 0.44, 95% CI 0.26 to 0.77, I² = 0%, six studies, 635 women). This indicates that among women undergoing laparoscopic myomectomy, the risk of postoperative fever is 50% lower than among those treated with open surgery. No studies reported immediate hysterectomy, uterine rupture, thromboembolism or mortality. Six studies including 549 women reported haemoglobin drop, but these studies were not pooled because of extreme heterogeneity (I² = 97%) and therefore could not be included in the analysis.
Authors' conclusions
Laparoscopic myomectomy is a procedure associated with less subjectively reported postoperative pain, lower postoperative fever and shorter hospital stay compared with all types of open myomectomy. No evidence suggested a difference in recurrence risk between laparoscopic and open myomectomy. More studies are needed to assess rates of uterine rupture, occurrence of thromboembolism, need for repeat myomectomy and hysterectomy at a later stage.
Keywords: Female; Humans; Fever; Fever/etiology; Hysteroscopy; Hysteroscopy/adverse effects; Hysteroscopy/methods; Laparoscopy; Laparoscopy/adverse effects; Laparoscopy/methods; Laparotomy; Laparotomy/adverse effects; Laparotomy/methods; Leiomyoma; Leiomyoma/surgery; Pain Measurement; Pain, Postoperative; Randomized Controlled Trials as Topic; Uterine Myomectomy; Uterine Myomectomy/adverse effects; Uterine Myomectomy/methods; Uterine Neoplasms; Uterine Neoplasms/surgery
Plain language summary
Minimally invasive surgical techniques versus open myomectomy for uterine fibroids
Review question: In this Cochrane review, we compared surgical results after myomectomy by open surgery versus myomectomy by laparoscopy and hysteroscopy.
Background: Fibroids are common benign tumours arising in the uterus. Sometimes they cause problems such as abnormal vaginal bleeding, pain and difficulty passing urine and bowel motions. Fibroids can be removed by an operation called myomectomy, which is performed traditionally by cutting into the abdomen (laparotomy). In this procedure, the fibroid is removed and the uterus is conserved. Myomectomy can also be performed by using keyhole surgery (laparoscopy and hysteroscopy). Laparoscopic myomectomy involves small cuts in the abdomen (three or four, about 1 cm long) followed by removal of the fibroid using a telescopic rod lens system and long laparoscopic instruments. The fibroids are then taken out by a procedure called morcellation, in which they are shaved into smaller pieces. Hysteroscopy is useful for fibroids, which are mostly inside the cavity of the uterus, and does not require any cut to the abdomen .
Study characteristics: Nine studies including 808 premenopausal women with uterine fibroids compared various methods of myomectomy. These studies were conducted in Italy (seven studies), Austria and China. The evidence is current to July 2014.
Key results: Myomectomy by laparoscopy is a less painful procedure compared with open surgery. Postoperative pain was measured on a visual analogue scale (VAS), with zero meaning 'no pain at all' and 10 signifying 'pain as bad as it could be.' Results show that among women undergoing laparoscopic myomectomy, the mean pain score at six hours (mean difference (MD) ‐2.40, 95% confidence interval (CI) ‐2.88 to ‐1.92) and at 48 hours (MD ‐1.90, 95% CI ‐2.80 to ‐1.00) would be likely to range from about three points lower to one point lower on a VAS zero‐to‐10 scale. Results of our analysis regarding pain score at 24 hours were uncertain (MD ‐0.29, 95% CI ‐0.70 to 0.12). Risk of fever after the operation was reduced by 50% in women undergoing laparoscopic myomectomy (OR 0.44, 95% CI 0.26 to 0.77). Drop in haemoglobin level (indicating reduced blood loss) could not be compared because results of the analysis were not conclusive because of differences in results with even the same surgical techniques. Risk of injury to intestines and other organs could not be determined in this meta‐analysis. No evidence was found of increased risk of recurrence of fibroids after laparoscopic surgery compared with open surgery (OR 1.12, 95% CI 0.63 to 1.99).
Quality of the evidence: The quality of the evidence ranged from very low to moderate. Some outcomes involved small numbers of participants, poor information about blinding in included studies and very wide confidence intervals.
Summary of findings
Summary of findings for the main comparison. Laparoscopic myomectomy versus open myomectomy (all types) for uterine fibroids.
| Laparoscopic myomectomy versus open myomectomy (all types) for uterine fibroids | ||||||
| Patient or population: Women with uterine fibroids Settings: Departments of Obstetrics and Gynecology of University Hospitals Intervention: Laparoscopic myomectomy versus open myomectomy (all types) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Laparoscopic myomectomy versus open myomectomy (all types) | |||||
| Postoperative pain at 6 hours VAS from 0 to 10 | Mean postoperative pain at 6 hours in the open myomectomy groups was 6.5 ± 1.5 on the VAS | Mean postoperative pain at 6 hours in the intervention groups was 2.4 lower (2.88 to 1.92 lower) | 148 (1 study) | ⊕⊕⊕⊝ moderatea | ||
| Postoperative pain at 24 hours VAS from 0 to 10 | Mean postoperative pain at 24 hours in the open myomectomy groups was 4.2 ± 2.4 on the VAS | Mean postoperative pain at 24 hours in the intervention groups was 0.29 lower (0.7 lower to 0.12 higher) | 232 (4 studies) | ⊕⊕⊕⊝ moderateb | ||
| Postoperative pain at 48 hours VAS from 0 to 10 | Mean postoperative pain at 48 hours in the open myomectomy groups was 3.8 ± 2.5 on the VAS | Mean postoperative pain at 48 hours in the intervention groups was 1.9 lower (2.8 to 1 lower) | 80 (2 studies) | ⊕⊕⊕⊝ moderateb | ||
| Perioperative in‐hospital adverse events; unscheduled return to theatre | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.04 (0.12 to 75.86) | 188 (2 studies) | ⊕⊕⊝⊝ lowb,c | Reported assumed and corresponding risks might not be representative because of the very low rate of events |
| Perioperative in‐hospital adverse events; laparoconversion | 16 per 1000 | 18 per 1000 (7 to 44) | OR 1.11 (0.44 to 2.83) | 756 (8 studies) | ⊕⊕⊕⊝ moderateb | |
| Perioperative in‐hospital adverse events; injury to pelvic organs | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.04 (0.12 to 75.86) | 148 (1 study) | ⊕⊕⊕⊝ moderated | Reported assumed and corresponding risks might not be representative because of the very low rate of events |
| In‐hospital adverse events; postoperative fever | 142 per 1000 | 68 per 1000 (41 to 113) | OR 0.44 (0.26 to 0.77) | 635 (6 studies) | ⊕⊕⊕⊝ moderateb | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aEvidence based on only 1 trial including fewer than 400 women. bEvidence to allow a judgement on blinding is lacking. cWide confidence intervals crossing the line of no effect. dEvidence was based on only 1 trial including fewer than 400 women. Wide confidence intervals crossing the line of no effect.
Background
Description of the condition
Fibroids (leiomyomas or myomas) are benign tumours that arise from individual smooth muscle cells. Fibroids can form wherever there is smooth muscle, but they are usually found in the uterus and constitute the most common benign tumours among women. They are found clinically in 25% of women (Buttram 1981). However, their true prevalence is higher, given that a large number of fibroids do not cause symptoms (Okolo 2008); Cramer 1990 found myomas in 77% of hysterectomy specimens. The prevalence of fibroids during reproductive age varies with different methods of diagnosis and is estimated to be between 5.4% and 77% (Drinville 2007; Lethaby 2002). It is known that prevalence rates vary amongst racial groups, with a lifetime risk of 70% for white women and greater than 80% for black women (Baird 2003).
The pathogenesis of fibroids is not fully understood. Factors that have been implicated include ovarian steroid hormones (oestrogen and progesterone), genetic predisposition (e.g. the genes HMGIC (Mine 2001) and MED12 (Makinen 2011)), growth factors (e.g. vascular endothelial growth factor‐A (VEGF‐A) (Gentry 2000) and transforming growth factor (Stewart 2001)) and up‐regulation of type I and type III collagen and interleukin‐8 (IL‐8) (Senturk 2001).
Although fibroids are asymptomatic in most women, they are symptomatic in others. Symptoms commonly associated with fibroids include heavy uterine bleeding, pressure‐related symptoms (e.g. on the bladder) and infertility; they can cause severe pain and complications in pregnancy (Bajekal 2000; Buttram 1981; Wallach 2004). Uterine fibroids also have a significant negative impact on quality of life and work productivity (Downes 2010). Few data are available on the non‐medical or outpatient costs associated with symptomatic fibroids; however, the estimated average inpatient cost of uterine fibroid treatment in the USA in 2004 was USD 15,405 (Viswanathan 2007).
Management of symptomatic fibroids has traditionally been surgical; however medical therapies have been tried, and, more recently, uterine artery embolisation was developed as an alternative to surgery (Levy 2008; Ravina 1995). Medical therapy with gonadotrophin‐releasing hormone analogues (GnRHa) appears useful in the short term, but side effects limit their long‐term use (Golan 1996; Lethaby 2001). Both mifepristone (a progesterone antagonist) and asoprisnil (a selective progesterone receptor modulator) are promising, but neither has shown long‐term efficacy and safety (Levy 2008).
Surgery remains the mainstay of treatment for women with large or symptomatic fibroids. In the USA, one‐third of all hysterectomies are performed as the result of uterine fibroids (Merill 2008). Myomectomy—surgical removal of fibroids with conservation of the uterus—may be the preferred option for several reasons, including retention of fertility.
The site, size and number of fibroids determine the surgical route employed. Although intracavitary fibroids can be removed hysteroscopically, intramural and subserosal fibroids are most commonly removed through the abdominal wall by myomectomy. Open myomectomy (laparotomy), once popularised by Victor Bonney (Bonney 1931), involves surgical removal of the fibroids through an incision in the abdominal wall, closure of the resulting uterine dead space and reconstitution of the remaining uterus. Decisions about choosing to proceed with myomectomy vary between gynaecologists, which may reflect the skill of the surgeon or differences among the women treated.
Open myomectomy is associated with blood loss both during the operation and afterwards. Some case series have reported transfusion rates of up to 20% (LaMorte 1993), and approximately 1% of women who undergo a myomectomy may require hysterectomy during surgery or in the first 24 hours after surgery because of uncontrollable bleeding.
Description of the intervention
Myomectomy is the traditional primary choice amongst surgical options for the removal of symptomatic fibroids that cannot be removed hysteroscopically among women who wish to remain fertile. Since 1979, various minimal access surgical techniques have been developed as alternatives to open myomectomy ( Semm 1979 ). These include hysteroscopic or laparoscopic myomectomy, laparoscopic myolysis (in situ destruction of the fibroid through diathermy or laser) and laparoscopically assisted mini‐laparotomy. For the purposes of this review, we have included both laparoscopic and hysteroscopic myomectomy; however we are including laparoscopically assisted mini‐laparotomy and mini‐laparotomy in the open myomectomy category, as these procedures involve dissection of the fibroid through a 5‐cm incision in the abdomen. Laparoscopic myomectomy is the removal of fibroids via a diathermy incision of the uterus, often assisted by morcellation, with small keyhole incisions in the abdominal wall through which instruments under telescopic control are passed (Semm 1979). Hysteroscopic resection of fibroids (or myomectomy) is the preferred method when fibroids are submucosal, or when most of an intramural fibroid protrudes into the uterine cavity. This technique involves removal of fibroids through the cervix and is generally limited to fibroids smaller than 4 cm in diameter amongst women seeking fertility (Pritts 2009). Laparoscopic myomectomy differs from open myomectomy, in which a large (approximately 12‐cm) transverse incision is made along the abdomen, the fibroid excised, large sutures tied and abdominal layers closed (usually a minimum of rectus sheath and skin layers).
How the intervention might work
The rationale of endoscopic techniques is that when a surgical incision is smaller, it causes less of an insult to the abdominal wall; therefore the woman will recover and will be pain free and mobilise more quickly after surgery. The overall aim is to reduce postoperative morbidity and mortality. Better visualisation of deep pelvic structures and increased accuracy of surgery may lessen the blood loss associated with myomectomy (Jin 2009). Other indications suggest that smaller incisions and less tissue damage may lead to reduced postoperative pain (Jin 2009). Postoperative pain is usually measured by the standardised measurement instrument called a visual analogue scale (VAS), with which patients rate their pain using numbers from zero to 10, with zero indicating complete freedom from pain and 10 representing the worst pain one can imagine. However, it should be remembered that although the incisions may be minor, this is still major surgery. In addition, minimal access techniques may be associated with complications that are not associated with open surgery, for example, visceral injury at trocar insertion (Querleu 1993).
Why it is important to do this review
Over the past 20 years, gynaecological surgery has progressed to include minimally invasive techniques such as laparoscopic myomectomy. Evidence suggests that laparoscopic myomectomy is associated with reduced morbidity compared with open myomectomy (Dubuisson 2000; Jin 2009); some studies have reported reduced blood loss, better recovery and a shorter hospital stay (Frishman 2005; Jin 2009). These studies have also reported comparable rates of pregnancy, fibroid recurrence and operative complications when the two surgical methods were compared. However, the evidence presented in these studies is limited by the rather small numbers of study participants. Another point of concern with endoscopic surgery is the potential for uterine rupture; however the level of risk is currently unclear. Several case reports have described uterine rupture (Dubuisson 2000); one review suggested that rupture is less frequent following laparoscopic myomectomy compared with the open approach (Miller 2000). A systematic review examining all relevant outcomes among a larger number of women is needed to enable women and their surgeons to make informed choices about which route of surgery is best.
Objectives
To determine the benefits and harms of laparoscopic or hysteroscopic myomectomy compared with open myomectomy.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing open myomectomy versus laparoscopic or hysteroscopic myomectomy were included in this review. We have included open studies and blinded studies. We would have included first phases of cross‐over trials in the review for completeness, but we found none.
Types of participants
Inclusion criteria
Premenopausal women with uterine fibroids diagnosed by clinical and ultrasound examination.
Types of interventions
Trials comparing open myomectomy versus myomectomy by laparoscopy or hysteroscopy. The term 'open myomectomy' encompasses laparotomy, mini‐laparotomy and laparoscopically assisted mini‐laparotomy.
Types of outcome measures
Primary outcomes
Postoperative pain, defined as the value on the subjectively assessed VAS pain scale at six, 24 and 48 hours postoperatively.
In‐hospital adverse events: perioperative and postoperative morbidity (e.g. unscheduled return to theatre in perioperative period, laparoconversion, immediate hysterectomy, injury to pelvic organs, uterine rupture, postoperative fever (defined as body temperature of 38°C or higher at two consecutive measurements at least six hours apart postoperatively excluding the first 24 hours after surgery), thromboembolism, blood transfusion, haemoglobin or hematocrit drop and mortality).
Secondary outcomes
Length of hospital stay in hours.
Operating time, defined as time from incision to closure, reported in minutes.
Improvement in menstrual symptoms such as heaviness of periods, or pressure symptoms (subjective information collected via surveys).
Change in quality of life, measured on validated quality of life scales before and at different time points after surgery.
Recurrence of fibroids, defined as clinical or ultrasound evidence of recurrence at six months or later after surgery.
Repeat myomectomy.
Hysterectomy at a later time
We have excluded fertility and pregnancy outcomes following surgical treatment of patients with fibroids, as this has already been addressed by another Cochrane review (Metwally 2012).
Search methods for identification of studies
Electronic searches
The following electronic databases, trial registers and Websites were searched using Ovid software in consultation with the Trial Search Co‐ordinator of the Cochrane Menstrual Disorders and Subfertility Group.
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1) (inception to July 2014).
Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of Controlled Trials (Appendix 2) (inception to July 2014).
MEDLINE (Appendix 3) (inception to July 2014).
EMBASE (Appendix 4) (inception to July 2014).
PsycINFO (Appendix 5) (inception to July 2014).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (Appendix 6) (inception to July 2014).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials that appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The EMBASE search was combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials: Current Controlled Trials (http://www.controlled‐trials.com/); ClinicalTrials.gov, a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home).
World Health Organization International Clinical Trials Registry Platform search portal (http://www.who.int/trialsearch/Default.aspx).
Citation indexes (http://scientific.thomson.com/products/sci/).
Conference abstracts in the Web of Knowledge (http://wokinfo.com).
Latin American and Caribbean Health Science Information Database (LILACS) database, a source of trials from the Portuguese‐ and Spanish‐speaking world (htpp://regional.bvsalud.org/php/index.php?lang=en) (choose LILACS in 'all sources' drop‐down box).
PubMed (http://www.ncbi.nlm.nih.gov/pubmed/); the random control filter for PubMed will be taken from the 'Searching' chapter of the Cochrane Handbook for Systematic Reviews of Interventions.
Open System for Information on Grey Literature in Europe (OpenSIGLE) database for grey literature from Europe (http://opensigle.inist.fr/).
Searching other resources
Reference lists of all retrieved articles and all relevant conference proceedings were handsearched. Experts in the field were personally contacted to provide additional relevant data so unpublished studies could be included.
We included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart to present results of the search and of the process of screening and selecting studies for inclusion in the review.
Data collection and analysis
Data collection and analysis were conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Study selection was undertaken by two review authors. Both review authors independently scanned titles and, when possible, abstracts of studies potentially relevant to the review (PBC, SF). Studies with clearly irrelevant titles or abstracts were removed. Full‐text articles of all potentially eligible studies were retrieved and assessed independently by both review authors. Disagreements or doubts were resolved by discussion with a third review author (CF). Further information was sought from study authors when papers contained insufficient information to permit a decision about eligibility. The selection process was documented in the PRISMA flow chart.
Data extraction and management
Data were extracted from eligible studies using a data extraction form designed and pilot‐tested by the review authors. When studies had multiple publications, the main trial report was used as the reference, and additional details were derived from secondary papers. All data were extracted independently by two review authors (PBC, SF), and differences of opinion were resolved by consensus after consultation with a third review author (CF). Additional information on trial methodology or actual original trial data were sought from the corresponding authors of trials that appeared to meet eligibility criteria, when aspects of methodology were unclear or when data were provided in a form that was unsuitable for meta‐analysis. Corresponding authors of all included trials were contacted routinely to ask whether data not reported in the published paper had been recorded.
Assessment of risk of bias in included studies
Included studies were assessed independently by two review authors to assess seven domains as having 'low risk of bias,' 'high risk of bias' or 'unclear risk of bias.' Assessments were performed independently by two review authors (PBC, SF), and disagreements were resolved by consensus or by discussion with a third review author (CF). The conclusions of the review authors are presented in the 'Risk of bias' table.
We used the Cochrane risk of bias tool, which recommends explicit reporting of the following domains.
Random sequence generation (selection bias).
Low risk of bias: use of central computer randomisation, random number table or serially numbered and sealed opaque envelopes; coin toss.
Unclear risk of bias: insufficient information about the process of sequence generation.
Allocation concealment (selection bias).
Low risk of bias: central randomisation.
High risk of bias: use of open random allocation (e.g. date of birth, medical record number, day of the week presenting).
Unclear risk of bias: insufficient information about the process of allocation concealment.
Blinding of participants, researchers and care providers (performance bias).
Low risk of bias: blinding of participants, care providers and researchers.
High risk of bias: no blinding of participants, care providers and researchers.
Unclear risk of bias: insufficient information about the process of blinding participants, researchers and care providers.
Blinding of outcome assessor (detection bias).
Low risk of bias: blinding of outcome assessors.
High risk of bias: no blinding of outcome assessors.
Unclear risk of bias: insufficient information about the process of blinding of outcome assessors.
Incomplete outcome data (attrition bias).
Low risk of bias: no missing data, or missing data with clear reasons.
High risk of bias: missing data or no reasons given for missing data.
Unclear risk of bias: insufficient information about the completeness of outcome data.
Selective outcome reporting (reporting bias).
Low risk of bias: all prespecified outcomes in the protocol reported in the published article.
High risk of bias: not all prespecified outcomes reported.
Unclear risk of bias: insufficient information about the process of outcome reporting.
Other potential sources of bias.
Low risk of bias: study free of other biases (e.g. baseline imbalance of groups, blocked randomisation in unblinded trials).
High risk of bias: other biases present (these will be specified).
Unclear risk of bias: insufficient information about the other sources of bias.
Measures of treatment effect
Dichotomous data were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) and were combined for meta‐analysis using Review Manager (RevMan) software. Increased odds of a particular outcome are displayed graphically in the meta‐analyses to the right of the centre‐line, and decreased odds of an outcome are displayed graphically to the left of the centre‐line. Continuous data were combined for meta‐analysis with RevMan software using mean differences (MDs) with 95% CIs. If only medians and ranges were given, a descriptive analysis was undertaken. We will reverse the direction of effect of individual studies, if required, to ensure consistency across trials. We will treat ordinal data (e.g. quality of life scores) as continuous data. We will present 95% CIs for all outcomes. When data needed to calculate ORs or MDs are not available, we will utilise the most detailed available numerical data, which may facilitate similar analyses of included studies (e.g. test statistics, P values). We will compare the magnitude and direction of effect reported by studies versus how they are presented in the review, while taking account of legitimate differences.
Unit of analysis issues
The analysis was per women randomly assigned. An intention‐to‐treat (ITT) analysis was performed, as far as possible.
Dealing with missing data
In the case of missing data from any of the included studies, study authors were contacted to supply relevant missing information. If missing data were not provided, an ITT analysis was applied. For dichotomous data, the denominator represented the number of women entering the trial, and we assumed that the positive event did not occur. If data for continuous outcomes were lacking, only available data were used because of the impossibility of imputation.
Assessment of heterogeneity
We assessed the characteristics of included studies to decide whether similarities among participants, interventions and outcomes were sufficient for meta‐analysis to be appropriate. An initial step was to visually inspect the forest plot for significant heterogeneity.
We used the I2 statistic to assess heterogeneity in the meta‐analysis. We interpreted results of the I2 statistic as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity (Higgins 2011).
If substantial heterogeneity was detected, possible explanations as stated in the 'Sensitivity analysis' section below were explored.
Assessment of reporting biases
We aimed to reduce reporting bias by searching for eligible studies in multiple electronic databases and in additional sources of both published and unpublished articles and remained alert for duplication of data. We searched for protocols to look for preplanned outcomes that may not have been reported in the published article. We planned to assess publication bias if 10 or more studies were included in an analysis by visually inspecting the funnel plot for asymmetry. Unfortunately, none of our comparisons included 10 or more studies.
Data synthesis
When it was not possible to combine primary studies, they were summarised in a narrative format. Statistical analysis was performed using Review Manager (RevMan) version 5.3.
Data from primary studies were combined using a fixed‐effect model. If substantial heterogeneity was detected, we used a random‐effects model.
The primary analysis compared laparoscopic myomectomy versus all types of open myomectomy.
This analysis was stratified by type of open myomectomy as follows.
Laparoscopic myomectomy versus unmodified open myomectomy.
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy.
Hysteroscopic myomectomy versus unmodified open myomectomy.
Hysteroscopic myomectomy versus myomectomy at mini‐laparotomy.
Hysteroscopic myomectomy versus laparoscopically assisted mini‐laparotomy.
Subgroup analysis and investigation of heterogeneity
When data were available, subgroup analyses were planned to gather evidence within the following subgroups.
Size of myoma(s): divided into two groups—women with myoma(s) < 4 cm and women with myoma(s) ≥ 4 cm.
Number of myoma(s): single versus multiple myomas.
Pretreatment with GnRH analogues.
If we detect substantial heterogeneity, we will explore possible explanations in sensitivity analyses by examining clinical and methodological differences between studies. We will take statistical heterogeneity into account when interpreting the results, especially if variation in the direction of effect is noted.
Sensitivity analysis
Sensitivity analyses were planned to examine the stability of study results in relation to the following factors.
Exclusion of trials with high risk of bias.
Exclusion of trials with imputation of dichotomous data.
Overall quality of the body of evidence: 'Summary of findings' table We generated a 'Summary of findings' table using GRADEPRO software. This table evaluated the overall quality of the body of evidence for our primary outcomes, using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) were justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
The search revealed 316 abstracts. After duplicates were excluded, 184 abstracts were screened. A total of 23 studies initially identified by the search as potentially eligible were retrieved in full text. When more than one report was provided on the same study, the most recent one describing the outcome of interest was included. Nine randomised controlled trials were included in the review. Fourteen trials were excluded. No studies are awaiting classification. No ongoing trials were found. See Figure 1 for details of the screening and selection process.
1.
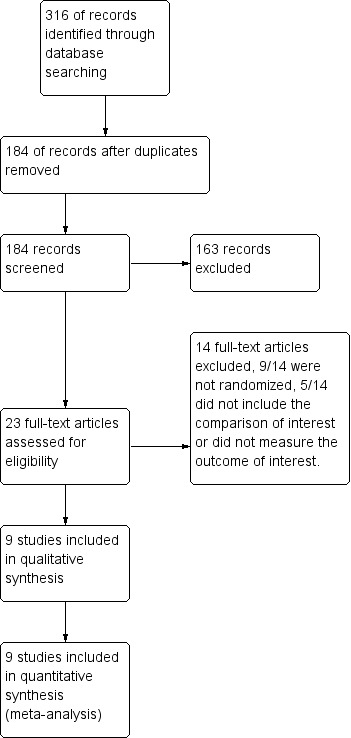
Study flow diagram.
See also Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification,
Included studies
Study design and setting: All studies were randomised controlled trials of parallel design. One was a multi‐centre study with three participating universities (Palomba 2007). Eight studies recruited women from single centres (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Rossetti 2001; Seracchioli 2000; Sesti 2008; Tan 2008). All included studies were carried out in different university departments in Italy, except Holzer 2006, which was conducted in Australia.
Participants: This review includes 808 participants with uterine fibroids diagnosed clinically and undergoing transvaginal ultrasound. All included women were premenopausal. Four studies looked at myomas smaller than 10 cm (Holzer 2006; Palomba 2007; Sesti 2008; Tan 2008), two studies included participants with myomas measuring 7 cm or less (Alessandri 2006;Cicinelli 2009) and one study included participants with myomas measuring 5 cm or larger (Seracchioli 2000). Five studies included women with four or fewer myomas (Alessandri 2006; Cicinelli 2009; Mais 1995; Palomba 2007; Tan 2008), and in one study, participants had fewer than eight myomas (Rossetti 2001).
The indication for myomectomy was symptomatic myoma (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Palomba 2007; Rossetti 2001; Sesti 2008; Tan 2008). Two trials included only infertile women with myomas (Palomba 2007; Seracchioli 2000). All trials excluded women with submucous myomas.
Interventions
Four studies compared laparoscopic myomectomy versus unmodified open myomectomy (Holzer 2006;Mais 1995;Rossetti 2001;Seracchioli 2000).
Four studies were available for the comparison of laparoscopic myomectomy versus mini‐laparotomy myomectomy (Alessandri 2006;Cicinelli 2009;Palomba 2007;Sesti 2008).
One study compared laparoscopy versus laparoscopically assisted mini‐laparotomy (Tan 2008).
Only one trial compared laparoscopic myomectomy versus the open technique of laparoscopy (Alessandri 2006); all other trials used the conventional technique of entry by the Verres needle.
Gasless laparoscopy with an abdominal wall‐lifting device was used in the study by Sesti 2008 and Tan 2008.
No eligible studies compared hysteroscopic myomectomy versus open myomectomy.
No study reported preoperative GnRH analogue administration.
Outcomes
6/9 studies reported postoperative pain (within the first seven days) (Alessandri 2006;Holzer 2006;Mais 1995;Palomba 2007;Sesti 2008;Tan 2008). Two studies reported postoperative pain graphically, and no values were described (Mais 1995;Palomba 2007). Values were calculated from the bar graph in Mais 1995 and were used for meta‐analysis.
8/9 studies reported in‐hospital adverse events (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Palomba 2007; Seracchioli 2000; Sesti 2008; Tan 2008).
4/9 studies reported length of hospital stay (Alessandri 2006;Cicinelli 2009;Seracchioli 2000;Tan 2008).
8/9 studies reported operating time (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Palomba 2007; Seracchioli 2000; Sesti 2008; Tan 2008). However, Sesti 2008 described operating time as time from induction of anaesthesia to closure and was not included in the analysis.
1/9 studies reported improvement in menstrual symptoms (Alessandri 2006).
1/9 studies reported changes in quality of life (Palomba 2007).
3/9 studies reported the recurrence of fibroids (Alessandri 2006;Rossetti 2001;Seracchioli 2000). Sesti 2008 reported the recurrence rate only in a subgroup of women who had had a follow‐up contact and excluded the others from analysis.
1/9 studies reported repeat myomectomy (Seracchioli 2000).
1/9 studies reported hysterectomy at a later date (Seracchioli 2000).
Excluded studies
Fourteen studies were excluded from the review.
9/14 studies were excluded because they were non‐randomised.
5/14 did not include the comparison of interest or did not measure any outcomes of interest to this review.
Risk of bias in included studies
2.
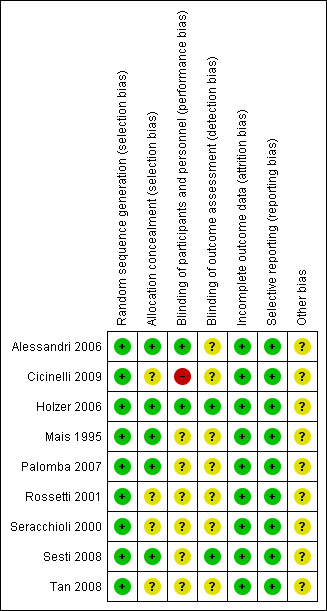
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
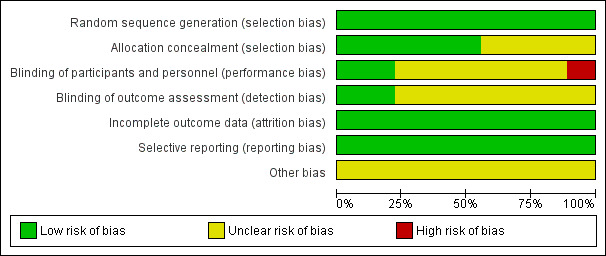
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All nine studies were at low risk of selection bias related to sequence generation, as they used computer randomisation or a random numbers table.
Five studies were at low risk of selection bias related to allocation concealment (Alessandri 2006; Holzer 2006; Mais 1995; Palomba 2007; Sesti 2008). Allocation concealment was not described in four studies, and all were at unclear risk of this bias (Cicinelli 2009; Rossetti 2001; Seracchioli 2000; Tan 2008).
Blinding
Surgeons cannot be blinded to the type of surgery performed. The risk of performance bias due to blinding of participants was judged to be low in two trials (Alessandri 2006; Holzer 2006) because participants were blind to the type of surgery performed. In one trial, the surgeons were unaware of the women undergoing surgery who were included in the trial (Cicinelli 2009). This risk was unclear in the other included trials. In most studies, it was not possible to differentiate between blinding of participants, personnel or outcome assessors, as this was not clearly reported.
Outcome assessors were blind in two studies, and this was unclear in seven trials.
Incomplete outcome data
All studies included in our analyses reported no dropouts; therefore we judged them to be at low risk of bias.
Selective reporting
All studies included in our analyses reported all outcomes stated in the methods section, or in the protocol, if available, in the results section. Therefore, we rated them at low risk of bias.
Other potential sources of bias
We found no potential sources of other within‐study bias in all of the nine studies included in the analysis.
Effects of interventions
See: Table 1
Laparoscopic myomectomy compared with all types of open myomectomy
Primary outcomes
Postoperative pain
Postoperative pain at six hours
Only one study made this comparison. It compared laparoscopic myomectomy versus myomectomy at mini‐laparotomy (Alessandri 2006).
Postoperative pain on the VAS was significantly less in women undergoing laparoscopic myomectomy (MD ‐2.40, 95% CI ‐2.88 to ‐1.92, one study, 148 women, moderate‐quality evidence). This means that among women undergoing laparoscopic myomectomy, the mean pain score at six hours would be likely to range from about three points lower to one point lower on a zero‐to‐10 VAS (Analysis 1.1),
1.1. Analysis.
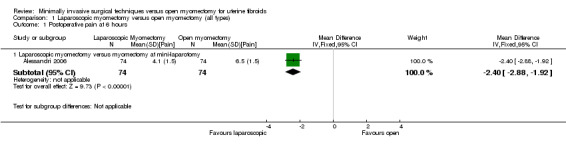
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 1 Postoperative pain at 6 hours.
Postoperative pain at 24 hours
Four studies made this comparison (Holzer 2006; Mais 1995; Sesti 2008; Tan 2008).
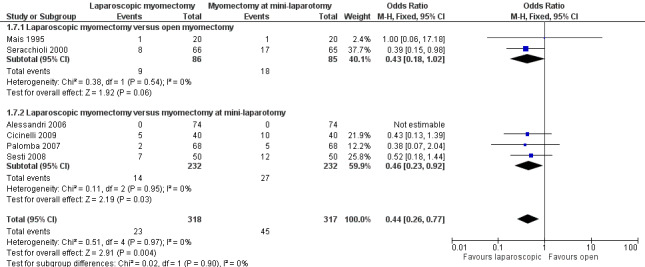
No significant difference in postoperative pain was noted on the VAS between laparoscopic and open myomectomy groups (MD ‐0.29, 95% CI ‐0.7 to 0.12, four studies, 232 women, I² = 43%, moderate‐quality evidence) (Analysis 1.2; Figure 4).
1.2. Analysis.
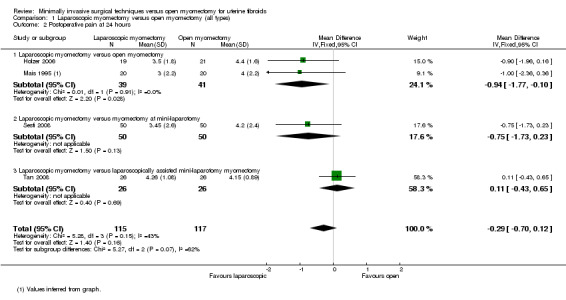
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 2 Postoperative pain at 24 hours.
4.
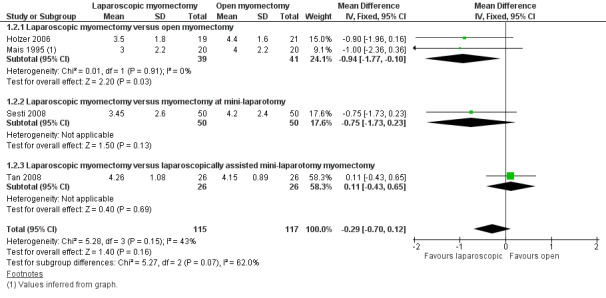
Forest plot of comparison: 1 Laparoscopic myomectomy versus open myomectomy (all types), outcome: 1.2 Postoperative pain at 24 hours.
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy.
Two studies made this comparison (Holzer 2006; Mais 1995). Postoperative pain on VAS score was significantly less in the laparoscopic group (MD ‐0.94, 95% CI ‐1.77 to ‐0.10, two studies, 80 women, I2 = 0%). This means that among women undergoing laparoscopic myomectomy, the mean pain score at 24 hours would be likely to range from about two points lower to zero points lower on a zero‐to‐10 VAS.
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
One study made this comparison (Sesti 2008). No significant difference in postoperative pain was seen in VAS score between laparoscopic and open myomectomy groups (MD ‐0.75, 95% CI 1.73 to 0.23, 100 women).
Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy myomectomy.
One study made this comparison (Tan 2008). No significant difference in postoperative pain was seen in VAS score between laparoscopic and open myomectomy groups (MD 0.11, 95% CI ‐0.43 to 0.65, 52 women).
Postoperative pain at 48 hours
Two studies made this comparison (Holzer 2006; Mais 1995). Both compared laparoscopic myomectomy versus unmodified open myomectomy.
Pain reported on the VAS at 48 hours was significantly less in the laparoscopic group than in the open myomectomy group (MD ‐1.90, 95% CI ‐2.80 to ‐1.00, two studies, 80 women, I² = 0%, moderate‐quality evidence). This means that among women undergoing laparoscopic myomectomy, the mean pain score at 48 hours would be likely to range from about three points lower to one point lower on a zero‐to‐10 VAS (Analysis 1.3; Figure 5).
1.3. Analysis.
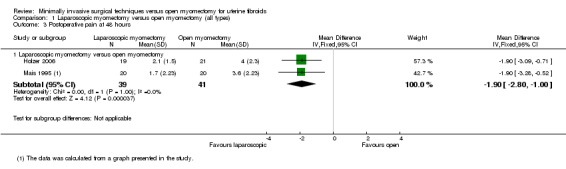
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 3 Postoperative pain at 48 hours.
5.
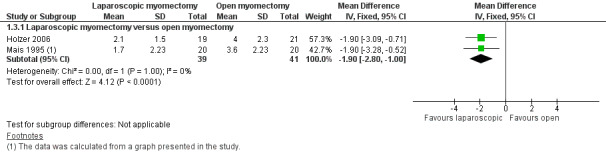
Forest plot of comparison: 1 Laparoscopic myomectomy versus open myomectomy (all types), outcome: 1.3 Postoperative pain at 48 hours.
In‐hospital adverse events: perioperative and postoperative
Perioperative in‐hospital adverse events
Eight studies reported perioperative in‐hospital adverse events (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Palomba 2007; Rossetti 2001; Seracchioli 2000; Sesti 2008). Events were reported as follows.
Unscheduled return to theatre.
Two studies reported this outcome.
No evidence of a significant difference between treatment groups was found (OR 3.04, 95% CI 0.12 to 75.86, two studies, 188 women, I2 = 0%) (Analysis 1.4).
1.4. Analysis.
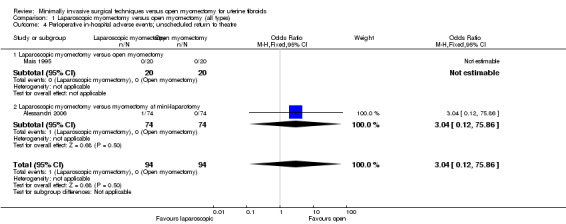
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 4 Perioperative in‐hospital adverse events; unscheduled return to theatre.
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy.
One study made this comparison. No events were reported in either group (Mais 1995).
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
One study made this comparison. A single case of bowel injury required return to theatre in the laparoscopic group, and no significant difference was noted between groups (OR 3.04, 95% CI 0.12 to 75.86, 148 women).
Laparoconversion
Eight studies reported this outcome (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Palomba 2007; Rossetti 2001; Seracchioli 2000; Sesti 2008) (Analysis 1.5).
1.5. Analysis.
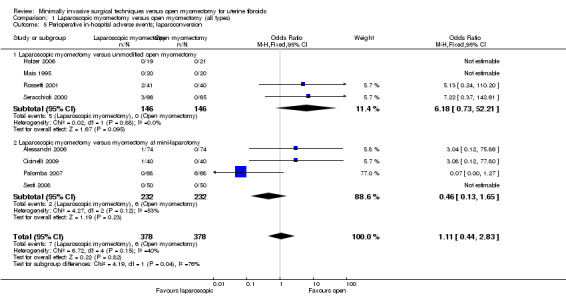
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 5 Perioperative in‐hospital adverse events; laparoconversion.
No significant difference was noted between groups in risk of laparoconversion (OR 1.11, 95% CI 0.44 to 2.83, eight studies, 756 women, I2 = 40%) (Figure 6).
6.
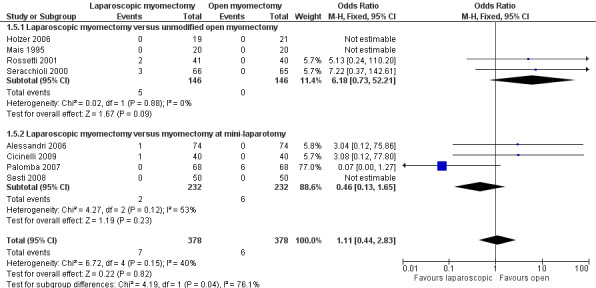
Forest plot of comparison: 1 Laparoscopic myomectomy versus open myomectomy (all types), outcome: 1.5 Perioperative in‐hospital adverse events; laparoconversion.
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy.
Four studies made this comparison (Holzer 2006; Mais 1995; Rossetti 2001; Seracchioli 2000).
No significant difference in risk of laparoconversion was noted between groups (OR 6.18, 95% CI 0.73 to 52.21, four studies, 292 women, I² = 0%) (Analysis 1.5).
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
Four studies made this comparison (Alessandri 2006; Cicinelli 2009; Palomba 2007; Sesti 2008).
No significant difference in risk of laparoconversion was noted between groups (OR 0.46, 95% CI 0.13 to 1.65, four studies, 464 women, I2 = 53%), and heterogeneity was substantial (Analysis 1.5).
Injury to pelvic organs
One study reported this outcome (Alessandri 2006). It compared laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
A single case of bowel injury was reported in the laparoscopic group, and no significant difference between groups was noted (OR 3.04, 95% CI 0.12 to 75.86, 148 women) (Analysis 1.6).
1.6. Analysis.
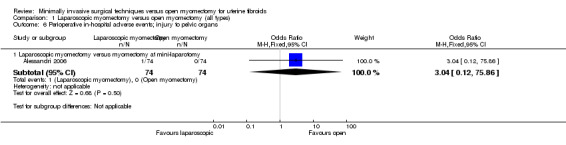
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 6 Perioperative in‐hospital adverse events; injury to pelvic organs.
Postoperative in‐hospital adverse events
Eight studies reported this outcome (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Palomba 2007; Seracchioli 2000; Sesti 2008; Tan 2008). Events were reported as follows.
Postoperative fever
Six studies reported this outcome (Alessandri 2006; Cicinelli 2009; Mais 1995; Palomba 2007; Seracchioli 2000; Sesti 2008) (Analysis 1.7).
1.7. Analysis.
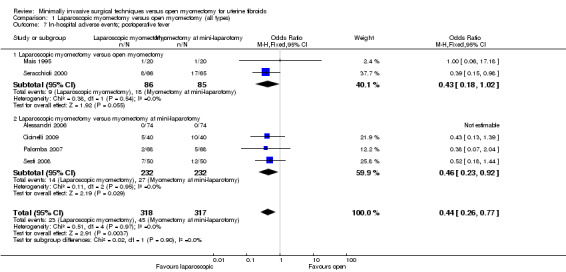
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 7 In‐hospital adverse events; postoperative fever.
A significantly lower risk of postoperative fever was noted with laparoscopic myomectomy compared with all types of open myomectomy (OR 0.44, 95% CI 0.26 to 0.77, six studies, 635 women, I² = 0%, moderate‐quality evidence). This indicates that among women undergoing laparoscopic myomectomy, the risk of postoperative fever is 50% less (Figure 7Analysis 1.7).
7.
Forest plot of comparison: 1 Laparoscopic myomectomy versus open myomectomy (all types), outcome: 1.7 In‐hospital adverse events; postoperative fever.
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy
Two studies made this comparison (Mais 1995; Seracchioli 2000). No significant difference between groups was noted (OR 0.43, 95% CI 0.18 to 1.02, two studies, 171 women, I² = 0%) (Figure 7Analysis 1.7).
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
Four studies made this comparison (Alessandri 2006; Cicinelli 2009; Palomba 2007; Sesti 2008). Evidence of lower postoperative fever was seen with laparoscopic myomectomy when compared with myomectomy at mini‐laparotomy (OR 0.46, 95% CI 0.23 to 0.92, four studies, 464 women, I² = 0%). This indicates that among women undergoing laparoscopic myomectomy, the risk of postoperative fever is 50% less (Figure 7; Analysis 1.7).
Haemoglobin drop (measured in grams per decilitre)
Six studies (of 549 women) reported this outcome (Alessandri 2006; Cicinelli 2009; Holzer 2006; Seracchioli 2000; Sesti 2008; Tan 2008).
Studies were not pooled because of extreme heterogeneity (I2 = 97%). Four of the six studies reported a significantly reduced drop in the laparoscopy group, and one reported a significantly reduced drop in the open myomectomy group. The sixth study found no significant differences between groups. Findings for each study are detailed below by type of open myomectomy (Analysis 1.8).
1.8. Analysis.
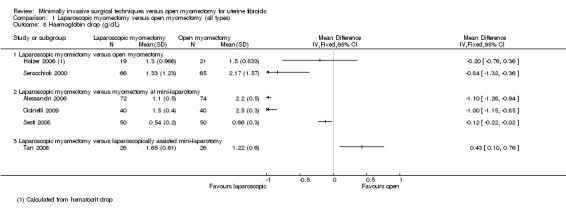
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 8 Haemoglobin drop (g/dL).
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy.
Two studies made this comparison (Holzer 2006; Seracchioli 2000). These findings were not pooled because of heterogeneity. One study (Holzer 2006) found no significant differences between groups (MD ‐0.20, 95% CI ‐0.76 to 0.36, 40 women), and the other reported a significantly reduced drop in the laparoscopy group (MD ‐0.84, 95% CI ‐1.32 to ‐0.36, 131 women).
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
Three studies made this comparison (Alessandri 2006; Cicinelli 2009; Sesti 2008). These findings were not pooled because of heterogeneity.
Two studies reported a significantly reduced drop in the laparoscopy group (Alessandri 2006: MD ‐1.10, 95% CI ‐1.26 to ‐0.94, 146 women; Cicinelli 2009: MD ‐1.00, 95% CI ‐1.15, ‐0.85, 80 women). One reported no differences between groups (Sesti 2008: ‐0.12, 95% CI ‐0.22, ‐0.02, 100 women).
Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy.
One study made this comparison (Tan 2008).
No significant differences were noted between the two groups (MD 0.43, 95% CI 0.10 to 0.76, 52 women).
Secondary outcomes
Length of hospital stay
Four studies (of 411 women) reported this outcome (Alessandri 2006; Cicinelli 2009; Seracchioli 2000; Tan 2008). Studies were not pooled because of extreme heterogeneity (I2 = 97%) (Analysis 1.9).
1.9. Analysis.
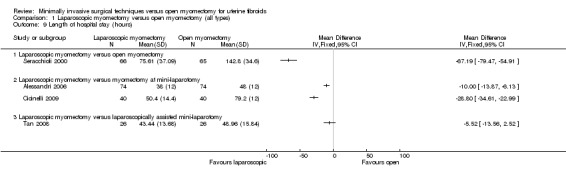
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 9 Length of hospital stay (hours).
Three of the studies reported a significantly shorter in‐hospital stay in hours for the laparoscopy group, and the fourth reported a significantly shorter stay in the open myomectomy group. Findings for each study are detailed below, by type of open myomectomy.
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy.
One study (Seracchioli 2000) reported this comparison.
Length of stay in hours was significantly shorter in the laparoscopy group (MD ‐67.19, 95% CI ‐79.47 to ‐54.91, 131 women).
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
Two studies reported this comparison (Alessandri 2006; Cicinelli 2009). Their findings were not pooled because of heterogeneity.
Both reported a significantly shorter length of stay in hours in the laparoscopy group (Alessandri 2006: MD ‐10.00, 95% CI ‐13.87 to ‐6.13, 148 women; Cicinelli 2009: MD ‐28.8, 95% CI ‐34.61 to ‐22.99, 80 women).
Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy.
One study made this comparison (Tan 2008). No evidence showed a difference in length of stay in hours between the two study groups (MD 5.52, 95% CI ‐13.56 to 2.52, 52 women).
Operating time
Six studies made this comparison (Alessandri 2006; Cicinelli 2009; Holzer 2006; Mais 1995; Seracchioli 2000; Tan 2008).
Operating time in minutes was significantly longer in the laparoscopy group (MD 13.08, 95% CI 9.61 to 16.56, six studies, 487 women, I² = 13%).
Analysis was stratified by type of open myomectomy.
Laparoscopic myomectomy versus unmodified open myomectomy
Three studies made this comparison (Holzer 2006; Mais 1995; Seracchioli 2000).
Operating time in minutes was significantly longer in the laparoscopy group (MD 14.33, 95% CI 5.76 to 22.91, 211 women, I2 = 48%).
Laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
Two studies made this comparison (Alessandri 2006; Cicinelli 2009).
Operating time in minutes was significantly longer in the laparoscopy group (MD 12.24, 95% CI 8.30 to 16.18, 226 women, I2 = 0%).
Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy.
One study made this comparison (Tan 2008).
Operating time in minutes was significantly longer in the laparoscopy group (MD 20.50, 95% CI 6.39 to 34.61, 52 women).
Improvement in menstrual symptoms
One study including 148 participants reported on improvement in menstrual symptoms (Alessandri 2006). This study compared laparoscopic myomectomy versus myomectomy at mini‐laparotomy.
All participants in both groups showed improvement in menstrual symptoms.
Change in quality of life
No studies reported this outcome.
Recurrence of fibroids
Four studies reported this outcome (Alessandri 2006; Rossetti 2001; Seracchioli 2000; Sesti 2008).
No evidence showed a significant difference in recurrence rate between the two groups (OR 1.12, 95% CI 0.63 to 1.99, four studies, 460 women, I² = 0%) (Analysis 1.12).
1.12. Analysis.
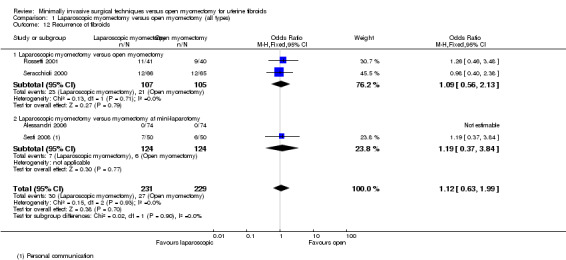
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 12 Recurrence of fibroids.
Repeat myomectomy
One study reported this outcome (Seracchioli 2000). It compared laparoscopic myomectomy versus open myomectomy.
No evidence showed a significant difference between groups (OR 0.13, 95% CI 0.01 to 2.65, 313 women).
Hysterectomy at a later time
One study reported this outcome (Seracchioli 2000). It compared laparoscopic myomectomy versus open myomectomy.
No evidence showed a significant difference between groups (OR 0.32, 95% CI 0.01 to 8.08, 313 women).
Subgroup and sensitivity analyses
We did not conduct the subgroup analyses planned in our protocol, as we had insufficient data for subgroup analyses on size of fibroids, number of fibroids or pretreatment with GnRH analogues.
We conducted a sensitivity analysis while excluding one study with high risk of performance bias (Cicinelli 2009), using a random‐effects model rather than a fixed‐effect model, and using the summary effect measure risk ratio rather than the odds ratio. These sensitivity analyses did not yield different results.
Discussion
Summary of main results
Laparoscopic myomectomy versus open myomectomy
Laparoscopic myomectomy is a less painful procedure compared with all types of open myomectomy, as indicated by lower pain VAS scores at six hours and at 48 hours. However, no evidence of a difference in pain scores was noted on the VAS at 24 hours after surgery between laparoscopic myomectomy and all types of open myomectomy. Moderate heterogeneity (43%) for this comparison could be explained by the study by Tan 2008, which included laparoscopically assisted mini‐laparotomy myomectomy, in which laparoscopy is used for fibroid enucleation and dissection, and specimen removal and suturing are done through a small abdominal incision. This might reduce tissue damage and operating time compared with open myomectomy and may skew the results of pain scores. The overall level of evidence for postoperative pain is moderate, which means that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate (see Table 1). The sensitivity analysis that we performed showed that results for postoperative pain are stable when a random‐effects model was used, or when the summary effect was measured by risk ratio rather than odds ratio.
Risk of visceral injury and unscheduled return to theatre could not be estimated because of their low incidence in the included studies. No evidence suggested a difference in the need for laparoconversion between laparoscopic myomectomy and all types of open myomectomy. However, the incidence of laparoconversion is low, and the number of women included in the trials was not sufficient to result in a stable conclusion. Furthermore, heterogeneity for this outcome is mild, which can be explained by the different operating techniques used for open myomectomy. Additionally, Palomba 2007 showed an unusually high incidence of laparoconversion in the open myomectomy group, and the study had a high impact on the overall results of this outcome. Therefore, larger studies are needed before a definitive answer can be provided regarding perioperative in‐hospital adverse events.
Results of our analysis have shown that postoperative febrile morbidity is less common in laparoscopic myomectomy than in all types of open myomectomy. The power of the evidence for these results seems to be moderate, indicating that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate (Table 1). No heterogeneity was noted for this outcome (I² = 0%).
When laparoscopic myomectomy was compared with open myomectomy, a reduced haemoglobin drop was noted postoperatively, indicating decreased intraoperative blood loss in laparoscopic myomectomy. However, heterogeneity was substantial (I² = 97%) in our analysis, which can be explained by different surgical techniques, different surgery protocols, different operating room settings or different skilled surgeons.
Another Cochrane review has shown that many women choose minimally invasive surgery because of benefits such as shorter postoperative recovery time and reduced risk of infection for laparoscopic hysterectomy or myomectomy compared with abdominal hysterectomy or myomectomy (Nieboer 2009). However, an important aspect of safety associated with laparoscopic hysterectomy or myomectomy is discussed in the recently published Food and Drug Administration (FDA) safety communication about laparoscopic uterine power morcellation in hysterectomy and myomectomy. Authors of this report state that the prevalence of unsuspected uterine sarcoma among patients undergoing hysterectomy or myomectomy for presumed benign leiomyoma is one in 352, and the prevalence of unsuspected uterine leiomyosarcoma is one in 498. Therefore the FDA concludes that when "using power morcellation in women with unsuspected uterine sarcoma, there would be a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient's likelihood of long‐term survival. For this reason, and because there is no reliable method for predicting whether a woman with fibroids may have a uterine sarcoma, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids" (FDA 2014). Nevertheless, more specific studies and a detailed analysis are needed before an evidence‐based decision can be made about whether morcellation should be used and which patient characteristics might increase or lower the risk of spread of a malignant type of cancer.
Overall completeness and applicability of evidence
All randomised controlled clinical trials comparing laparoscopic versus open myomectomy for uterine fibroids are included in this review. Our primary outcomes—postoperative pain and perioperative and postoperative morbidity—were reported in included trials. Because of insufficient data, we could not conduct the subgroup analyses planned in our protocol. Another limitation of our analysis is that unfortunately no trials reported on uterine rupture; therefore our analysis includes no results on this outcome.
All included trials treated participants with myomas measuring no larger than 10 cm. For this reason, trials do not provide outcome data for women with fibroids of diameter larger then 10 cm.
None of the studies used GnRH pretreatment, so we cannot provide conclusions for women using GnRH pretreatment.
Repeat myomectomy and hysterectomy at a later time were reported in only one study. The study population and the numbers of events for these outcomes were too small to show evidence of a difference between the two study groups.
Unfortunately, we were not able to perform subgroup analyses because reporting of group characteristics was insufficient.
No studies compared hysteroscopic myomectomy versus open myomectomy.
Quality of the evidence
We included nine studies with a total of 808 women. The quality of evidence varied for different analyses. We stated in our protocol that we would perform different subgroup analyses on size of myomas, number of myomas and GnRH pretreatment. Because data were insufficient, we could not perform these analyses. We included a small number of studies, so we did not make a funnel plot. Our conclusions for different outcomes are unstable, and additional studies are most likely to have an impact on our results. This is so because a high number of studies described unclear allocation concealment and only small subgroups of the included studies reported different outcomes. Therefore, our different analyses included rather small study populations. Additionally, only one of our studies blinded both participants and outcome assessors, and the other included studies were not double blind, or it was unclear whether any blinding was provided. The consistency of pooled estimates for our different outcomes when we have pooled data was good, as an overall low percentage of heterogeneity was reported. Our analyses of the outcomes haemoglobin drop and operating time showed substantial heterogeneity; therefore we have chosen to not pool the data for these outcomes. In conclusion, additional studies are very likely to have an impact on our results and might change the estimate.
Potential biases in the review process
All data were extracted by two independent review authors (PBC and SF), who also compared extracted data and entered them into RevMan. Disagreements or doubts were discussed by CF and PBC, and SF wrote the review with input from the other review authors. We followed all good practice guidelines of The Cochrane Collaboration regarding inclusion and data extraction and analysis.
Agreements and disagreements with other studies or reviews
The conclusions of this review are in agreement with those of the other review on this subject conducted by Jin 2009, who found less haemoglobin drop, reduced operative blood loss, more women fully recuperated at day 15, diminished postoperative pain and fewer overall complications but longer operation time for laparoscopic myomectomy compared with open myomectomy.
Authors' conclusions
Implications for practice.
Laparoscopic myomectomy is associated with less postoperative pain, lower incidence of postoperative febrile morbidity and a shorter hospital stay. Results on drop in haemoglobin level were not conclusive. The incidence of visceral injuries could not be estimated because of their low prevalence in this meta‐analysis.
Implications for research.
Only one trial addressed changes in quality of life and menstrual symptoms after myomectomy. Further research is needed to address changes in quality of life after open or laparoscopic myomectomy performed with well‐validated instruments. Future trials should include longer follow‐up to assess the need for repeat surgery (myomectomy or hysterectomy) among women undergoing myomectomy laparoscopically or by laparotomy.
The prevalence of visceral injury was low in studies included in the meta‐analysis. However, studies with larger sample sizes are needed to detect differences between laparoscopic and open myomectomy.
With technical advances, better electrical morcellators have shortened the time required for removal of specimens and thus have reduced total operating time for laparoscopic myomectomy. Our review suggests that laparoscopic myomectomy requires significantly longer operative time, but more recent trials might report contrary findings as the result of better experience with laparoscopy and use of better instruments.
More studies are needed to evaluate laparoscopically assisted mini‐laparotomy myomectomy compared with open and laparoscopic myomectomy. This procedure is less challenging technically, and it avoids endo suturing and morcellation.
No studies have compared hysteroscopic myomectomy versus open myomectomy. No studies have looked at uterine rupture, which is an important concern associated with myomectomy. More studies are needed to compare improvement in menstrual symptoms and changes in quality of life after myomectomy by laparoscopy versus open myomectomy.
History
Protocol first published: Issue 1, 2004 Review first published: Issue 10, 2014
| Date | Event | Description |
|---|---|---|
| 2 February 2012 | Amended | A protocol entitled 'Laparoscopic versus open myomectomy for uterine fibroids' was published in 2004. This current protocol has a wider scope, incorporating the earlier work |
Notes
A protocol entitled 'Laparoscopic versus open myomectomy for uterine fibroids' was published in 2004. This current review includes that topic and incorporates text from the previous protocol. The title has been changed to reflect the new wider scope of the review.
Acknowledgements
We would like to thank the authors of the previous version of this protocol (Alex Taylor, Malini Sharma, Andrew Prentice and Adam Magos) and the Cochrane Menstrual Disorders and Subfertility Group, in particular, Marian Showell (Trial Search Co‐ordinator) for writing and running the search, and Vanessa Jordan (New Zealand Cochrane Fellow), Helen Nagels (Managing Editor of MDSG) and Julie Brown (systematic reviewer) for answering our questions.
We acknowledge Antonia Steed's contribution to the published protocol.
Appendices
Appendix 1. CENTRAL search strategy
EBM Reviews—Cochrane Central Register of Controlled Trials 1 exp leiomyoma/ or exp myoma/ (384) 2 leiomyoma$.tw. (209) 3 myoma$.tw. (230) 4 fibroid$.tw. (261) 5 fibroma$.tw. (22) 6 fibromyoma$.tw. (11) 7 hysteromyoma$.tw. (14) 8 myomectom$.tw. (221) 9 or/1‐8 (758) 10 exp Laparotomy/ (558) 11 laparotom$.tw. (1203) 12 minilaparotom$.tw. (94) 13 open surg$.tw. (656) 14 abdomin$ incision.tw. (94) 15 myomectom$.tw. (221) 16 exp Surgical Procedures, Minimally Invasive/ (18163) 17 Minimally Invasive Surg$.tw. (238) 18 laparoscop$.tw. (5936) 19 hysteroscop$.tw. (482) 20 or/10‐19 (22294) 21 9 and 20 (342) 22 limit 21 to yr="2013 ‐Current" (15)
Appendix 2. MDSG search strategy
Keywords CONTAINS "uterine fibroids"or"uterine leiomyomas"or"uterine myoma"or"uterine myomas"or "myoma"or"myomas"or"Leiomyoma"or"leiomyomata"or"fibroids" or Title CONTAINS "uterine fibroids"or"uterine leiomyomas"or"uterine myoma"or"uterine myomas"or "myoma"or"myomas"or"Leiomyoma"or"leiomyomata"or"fibroids"
AND
Keywords CONTAINS "myomectomy"or "Laparoscopic‐Assisted Minilaparotomy" or"abdominal myomectomy"or "laparoscopic myomectomy"or "laparoscopic surgery" or"laparotomy" or"laparotomy"or "*Surgical‐Procedures,‐Laparoscopic" or"laparoscopic surgical treatment" or"laparoscopy" or Title CONTAINS "myomectomy"or "Laparoscopic‐Assisted Minilaparotomy" or"abdominal myomectomy"or "laparoscopic myomectomy"or "laparoscopic surgery" or"laparotomy" or"laparotomy"or "*Surgical‐Procedures,‐Laparoscopic" or"laparoscopic surgical treatment" or"laparoscopy" or Title CONTAINS "myomectomy"or "Laparoscopic‐Assisted Minilaparotomy" or"abdominal myomectomy"or "laparoscopic myomectomy"or "laparoscopic surgery" or"laparotomy" or"laparotomy"or "*Surgical‐Procedures,‐Laparoscopic" or"laparoscopic surgical treatment" or"laparoscopy" or Title CONTAINS "myomectomy"or "Laparoscopic‐Assisted Minilaparotomy" or"abdominal myomectomy"or "laparoscopic myomectomy"or "laparoscopic surgery" or"laparotomy" or"laparotomy"or "*Surgical‐Procedures,‐Laparoscopic" or"laparoscopic surgical treatment" or"laparoscopy"
Appendix 3. MEDLINE search strategy
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1 exp leiomyoma/ or exp myoma/ (18853) 2 leiomyoma$.tw. (10599) 3 myoma$.tw. (4524) 4 fibroid$.tw. (4183) 5 fibroma$.tw. (9573) 6 fibromyoma$.tw. (629) 7 hysteromyoma$.tw. (49) 8 myomectom$.tw. (2242) 9 or/1‐8 (33922) 10 exp Laparotomy/ (15581) 11 laparotom$.tw. (38441) 12 minilaparotom$.tw. (916) 13 open surg$.tw. (14995) 14 abdomin$ incision.tw. (964) 15 myomectom$.tw. (2242) 16 exp Surgical Procedures, Minimally Invasive/ (370527) 17 Minimally Invasive Surg$.tw. (6802) 18 laparoscop$.tw. (83229) 19 hysteroscop$.tw. (4755) 20 or/10‐19 (436988) 21 9 and 20 (5696) 22 randomized controlled trial.pt. (378779) 23 controlled clinical trial.pt. (88839) 24 randomized.ab. (299270) 25 placebo.tw. (160357) 26 clinical trials as topic.sh. (171019) 27 randomly.ab. (216251) 28 trial.ti. (128790) 29 (crossover or cross‐over or cross over).tw. (61378) 30 or/22‐29 (936207) 31 exp animals/ not humans.sh. (3967409) 32 30 not 31 (862643) 33 21 and 32 (373) 34 (201311$ or 201312$).ed. (180892) 35 2014$.ed. or 2014$.dp. (917002) 36 34 or 35 (1097614) 37 33 and 36 (21)
Appendix 4. EMBASE search strategy
EMBASE.com 1 exp leiomyoma/ or exp uterus tumor/ (159503) 2 leiomyoma$.tw. (12965) 3 myoma$.tw. (6074) 4 fibroid$.tw. (6242) 5 fibroma$.tw. (10670) 6 fibromyoma$.tw. (600) 7 hysteromyoma$.tw. (106) 8 exp myomectomy/ (3526) 9 myomectom$.tw. (3544) 10 or/1‐9 (176079) 11 laparotom$.tw. (48741) 12 minilaparotom$.tw. (1097) 13 open surg$.tw. (21124) 14 abdomin$ incision.tw. (1252) 15 abdomin$ surg$.tw. (15365) 16 exp laparotomy/ (53479) 17 exp myomectomy/ (3526) 18 myomectom$.tw. (3544) 19 or/11‐18 (113410) 20 10 and 19 (9077) 21 Clinical Trial/ (836488) 22 Randomized Controlled Trial/ (347343) 23 exp randomization/ (62555) 24 Single Blind Procedure/ (18468) 25 Double Blind Procedure/ (116578) 26 Crossover Procedure/ (39375) 27 Placebo/ (254546) 28 Randomi?ed controlled trial$.tw. (100115) 29 Rct.tw. (14184) 30 random allocation.tw. (1356) 31 randomly allocated.tw. (20577) 32 allocated randomly.tw. (1951) 33 (allocated adj2 random).tw. (794) 34 Single blind$.tw. (14602) 35 Double blind$.tw. (147725) 36 ((treble or triple) adj blind$).tw. (398) 37 placebo$.tw. (204002) 38 prospective study/ (254842) 39 or/21‐38 (1385042) 40 case study/ (26713) 41 case report.tw. (269007) 42 abstract report/ or letter/ (913318) 43 or/40‐42 (1203374) 44 39 not 43 (1346864) 45 20 and 44 (986) 46 (201311$ or 201312$).em. (45467) 47 2014$.em. or 2014$.dp. (903286) 48 46 or 47 (948753) 49 45 and 48 (79)
Appendix 5. PsycINFO search strategy
1 leiomyoma$.tw. (12) 2 myoma$.tw. (23) 3 fibroid$.tw. (39) 4 fibroma$.tw. (19) 5 fibromyoma$.tw. (1) 6 hysteromyoma$.tw. (2) 7 or/1‐6 (92) 8 exp Surgery/ (41791) 9 laparotom$.tw. (117) 10 minilaparotom$.tw. (0) 11 open surg$.tw. (57) 12 abdomin$ incision.tw. (11) 13 or/8‐12 (41892) 14 7 and 13 (21) 15 limit 14 to yr="2013 ‐Current" (2)
Appendix 6. CINAHL search strategy
| Search ID# | Search terms |
| S16 | S7 and S15 |
| S15 | S8 or S9 or S10 or S11 or S12 or S13 or S14 |
| S14 | (MH "Laparoscopy") |
| S13 | (MH "Surgery, Laparoscopic") |
| S12 | "abdominal incision" |
| S11 | "open surgery" |
| S10 | "minilaparotomy" |
| S9 | (MH "Laparotomy") |
| S8 | "myomectomy" |
| S7 | S1 or S2 or S3 or S4 or S5 or S6 |
| S6 | "hysteromyoma" |
| S5 | "fibromyoma" |
| S4 | "fibroma" |
| S3 | "fibroid" |
| S2 | (MH "Myoma") |
| S1 | (MH "Leiomyoma") |
Appendix 7. Clinicaltrials.gov search strategy
We have searched clinicaltrials.gov very broadly using the following search terms: (Fibromyoma OR fibroid OR hysteromyoma OR fibroma OR myoma OR leiomyoma) AND (myomectomy OR laparotomy OR minilaparotomy OR open surgery OR abdominal incision OR laparoscopic OR laparoscopy), resulting in 105 hits (July 2014).
Data and analyses
Comparison 1. Laparoscopic myomectomy versus open myomectomy (all types).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain at 6 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐2.88, ‐1.92] |
| 2 Postoperative pain at 24 hours | 4 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.70, 0.12] |
| 2.1 Laparoscopic myomectomy versus open myomectomy | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.77, ‐0.10] |
| 2.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.73, 0.23] |
| 2.3 Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy myomectomy | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.43, 0.65] |
| 3 Postoperative pain at 48 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Laparoscopic myomectomy versus open myomectomy | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.80, 1.00] |
| 4 Perioperative in‐hospital adverse events; unscheduled return to theatre | 2 | 188 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 75.86] |
| 4.1 Laparoscopic myomectomy versus open myomectomy | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 75.86] |
| 5 Perioperative in‐hospital adverse events; laparoconversion | 8 | 756 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.44, 2.83] |
| 5.1 Laparoscopic myomectomy versus unmodified open myomectomy | 4 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.18 [0.73, 52.21] |
| 5.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 4 | 464 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.13, 1.65] |
| 6 Perioperative in‐hospital adverse events; injury to pelvic organs | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 75.86] |
| 7 In‐hospital adverse events; postoperative fever | 6 | 635 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.26, 0.77] |
| 7.1 Laparoscopic myomectomy versus open myomectomy | 2 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.02] |
| 7.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 4 | 464 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.23, 0.92] |
| 8 Haemoglobin drop (g/dL) | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Laparoscopic myomectomy versus open myomectomy | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Length of hospital stay (hours) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Laparoscopic myomectomy versus open myomectomy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Operating time (minutes) | 6 | 489 | Mean Difference (IV, Fixed, 95% CI) | 13.08 [9.61, 16.56] |
| 10.1 Laparoscopic myomectomy versus unmodified open myomectomy | 3 | 211 | Mean Difference (IV, Fixed, 95% CI) | 14.33 [5.76, 22.91] |
| 10.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 12.24 [8.30, 16.18] |
| 10.3 Laparoscopic myomectomy versus laparoscopically assisted mini‐laparotomy | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 20.5 [6.39, 34.61] |
| 11 Improvement in menstrual symptoms such as heaviness of periods, or pressure symptoms | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Recurrence of fibroids | 4 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.63, 1.99] |
| 12.1 Laparoscopic myomectomy versus open myomectomy | 2 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.13] |
| 12.2 Laparoscopic myomectomy versus myomectomy at mini‐laparotomy | 2 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.37, 3.84] |
| 13 Repeat myomectomy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Laparoscopic myomectomy versus open myomectomy | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.65] |
| 14 Hysterectomy at a later time | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Laparoscopic myomectomy versus open myomectomy | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.08] |
1.10. Analysis.
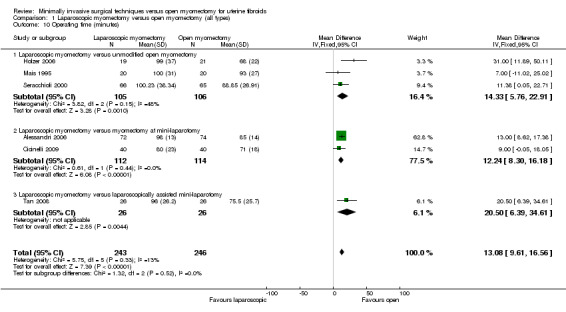
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 10 Operating time (minutes).
1.11. Analysis.
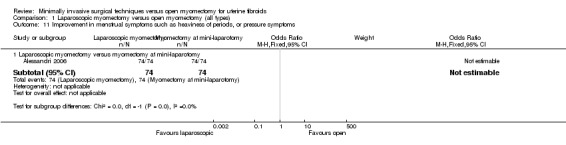
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 11 Improvement in menstrual symptoms such as heaviness of periods, or pressure symptoms.
1.13. Analysis.
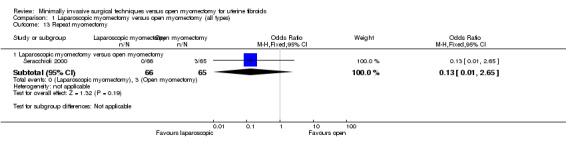
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 13 Repeat myomectomy.
1.14. Analysis.
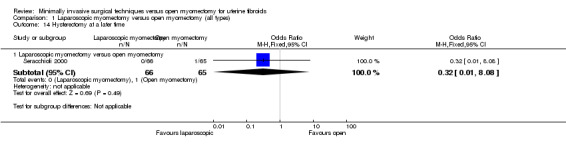
Comparison 1 Laparoscopic myomectomy versus open myomectomy (all types), Outcome 14 Hysterectomy at a later time.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alessandri 2006.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: "symptomatic subserous or intramural myomas, number of myomas ranging from 1 to 4 and size of the largest myoma ranging from 3 to 7 cm" Exclusion criteria: "submucosal myomas, association with ovarian or uterine lesions, age > 45 years, body mass index 29, contraindications for general anaesthesia, systemic infections and psychiatric disorders precluding consent. No participant included in the study underwent medical treatment for ovarian suppression before surgery" Setting: Department of Obstetrics and Gynecology, San Martino Hospital, University of Genoa, Italy Duration of the trial: October 1, 2002, to October 31, 2004 Duration of follow‐up: six months postoperative |
|
| Interventions | Mini‐laparotomy myomectomy (n = 74) versus laparoscopic myomectomy (n = 74) Minilaparotomy myomectomy: A suprapubic transverse incision 4 to 6 cm in length was given, the fascia was opened crosswise and the abdominal muscle was opened longitudinally at the midline with a longitudinal peritoneal incision. A linear uterine incision was made at the most prominent portion of the myoma. After the myoma pseudocapsule was identified, enucleation was possible following the cleavage plane. Uterine defects were closed with interrupted sutures Laparoscopic myomectomy: An open laparoscopy technique with a 10‐mm umbilical port and one 5‐mm and another 10‐mm ancillary port on each side was used. A uterine manipulator was used, and an incision was made through the uterine wall and the pseudocapsule of the myoma. The myoma was fixed with a Manhes grasping forceps, the cleavage plane identified and the myoma enucleated. All uterine defects were closed with interrupted intracorporeal sutures, and the myoma was removed with an automatic morcellator Both groups: "The surgical procedures were performed with the patients under general anaesthesia that was induced by use of propofol 2.5 mg/kg, atracurium 0.5 mg/kg, fentanyl 0.05 mg. During surgery, anaesthesia was maintained with atracurium 0.01 mg/kg/30 min, remifentanil 0.2‐0.5 g/kg/min, sevoflurane 1 minimum alveolar concentration (MAC; 60% air, 40% O2)." When all surgical procedures had been performed, all participants received tramadol (100 mg) and ketorolac trimethamine (30 mg) intravenously. No participant included in the study systematically received analgesics during the 8 hours after surgery. In both groups, postoperative analgesics (intramuscular ketorolac 30 mg) were given when requested by the participant |
|
| Outcomes |
(primary and secondary outcomes not specified in the trial report) |
|
| Notes |
Source of funding: not mentioned Ethics approval: "The trial was approved by the local ethics committee" Informed consent: "Each woman was informed of the experimental design of the study and gave informed consent to participate" Clinical trial registration: not provided Sample size calculation: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by using a computer generated randomisation list drawn up by a statistician" |
| Allocation concealment (selection bias) | Low risk | "Immediately before surgery, each woman was randomly assigned to either minilaparotomy or laparoscopy by an operating room nurse not otherwise engaged in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Wounds and site ports were dressed with identical dressings regardless of surgical procedure; therefore, during the hospital stay, the patients were unaware of the procedure performed" Participants were blinded during the hospital stay. Surgeons cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the trial report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all participants were described, including two participants in the laparoscopy group who had complications |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | None stated |
Cicinelli 2009.
| Methods | Prospective randomised controlled trial | |
| Participants |
Inclusion criteria: presence of no more than 3 symptomatic subserous or intramural myomas no larger than 7 cm Exclusion criteria: presence of more than 3 myomas, at least 1 myoma larger than 7 cm, cardiopulmonary disease contraindicating the LPS approach and preoperative haemoglobin level less than 9 g/dL Setting: Department of Obstetrics and Gynecology, University of Bari Medical School, Italy Duration of the trial: January 2007 to December 2007 Duration of follow‐up: six months postoperative |
|
| Interventions | Laparoscopic myomectomy (n = 40) versus mini‐laparotomy myomectomy (n = 40) Laparoscopic myomectomy: Laparoscopy was done using Verres needle and 3 ports 5 mm at 3 cm below the umbilicus and 2 5‐mm ancillary ports. A uterine manipulator was used, and a serosal incision was made without vasoconstriction using a monopolar needle. Manhes grasping forceps was used to obtain adequate traction on the myoma; the myoma was enucleated and the uterine wall sutured in two layers with extracorporeal sutures. The myoma was morcellated using an electromechanical morcellator Mini‐laparotomy myomectomy: A 5‐cm transverse suprapubic incision was made about 1 to 3 cm above the pubic symphysis. The fascia was opened longitudinally and the peritoneum vertically. The most prominent part of the uterine serosa overlying each myoma was cut with a monopolar knife. Enucleation was performed following the cleavage plane between the myoma and the pseudocapsule. Myoma beds were sutured with interrupted polyglactin 910 sutures Both groups: Bowel preparation and antithrombotic prophylaxis were performed, and short‐term intraoperative prophylactic antibiotic therapy with a second‐generation cephalosporin was administered to all participants |
|
| Outcomes |
No mention was made in the report about primary and secondary outcomes |
|
| Notes |
Sources of funding: not mentioned in the trial report Ethics approval: "The study was approved by the institutional review board, and all women gave informed consent" Informed consent: "The study was approved by the institutional review board, and all women gave informed consent" Clinical trial registration: not mentioned in the study report Sample size calculation: not mentioned in the trial report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At admission, women were randomly assigned to either the MLPT or the LPS group. Randomization order was obtained by using a computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | "Surgeons were informed of the type of intervention planned (LPS or MLPT) just before performing the operation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. Surgeons cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | More information was sought from study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | None was stated |
Holzer 2006.
| Methods | Randomised controlled trial | |
| Participants | 19– to 45‐year‐old patients who were referred for myomectomy of symptomatic myoma Inclusion criteria: intramural myomas, 3‐ to 10‐cm diameter size for the largest myoma, American Society of Anesthesiologists (ASA) physical status of I to III Exclusion criteria: contraindications to general anaesthesia, ASA physical status of IV or V, all pregnant patients and all women unable to understand the study or give informed consent Setting: Department of Obstetrics and Gynecology, Medical University of Vienna, Austria Duration of the trial: not mentioned Duration of follow‐up : Women were followed for at least 72 hours in hospital, and evaluated in a non‐hospital setting after 4 weeks. |
|
| Interventions | Laparoscopic myomectomy (n = 19) versus laparotomy myomectomy (n = 21) Laparoscopic myomectomy: done as described by Mais et al Laparotomy myomectomy: done as described by Mais et al Both groups: Quote: "All patients were premedicated with midazolam 7.5 mg orally 1 h before the operation. For the induction of anaesthesia, we administered 0.003 mg/kg body weight (BW) of fentanyl, a maximum dose of 3 mg/kg BW of propofol, and 0.1 mg/kg BW of vecuronium to facilitate endotracheal intubation. Anesthesia was maintained with a continuous infusion of propofol (5 mg · kg BW1 · h1) and fentanyl as determined by the anaesthesia provider. All patients’ lungs were ventilated conventionally. Nitrous oxide was avoided" After surgery, 10 mL of bupivacaine 0.5% was instilled in the incision of all participants (laparoscopy and laparotomy) after surgery. Identical wound dressings were applied in both groups, with neither the participant nor the observer who recorded the postoperative pain aware of the surgical approach. All participants had drains inserted for 24 hours after surgery in the lateral port or at the possible site of a lateral port in open surgery. For analgesia, we offered a patient‐controlled system (PCA) for 24 hours after the operation. Participants were allowed a maximum of 4 doses of piritramide (0.05 mg · kg BW1 · h1). The numbers of doses of piritramide were recorded for each participants. After 24 h after surgery, subcutaneous injections of 7.5 mg of piritramide were given if requested by the participant. No additional analgesics were allowed during the study period |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Baseline imbalances: Randomisation produced two groups, which were similar in baseline characteristics such as age, weight, body mass index, indication for surgery (rapidly growing myoma, pain, infertility, habitual abortion, anaemia), number of myomas, diameter of largest myoma and additive diameter of myomas Sources of funding: not mentioned in the trial report; on contact, study authors mentioned that no funding was obtained Ethics approval: "The local IRB approved the protocol" Informed consent: "After written and verbal informed consent, 40 patients entered the study" Clinical trial registration: not mentioned Sample size calculation: Quote: "A sample size of 40 patients provides a power of 90% to detect a difference of 1.7 (on a scale of 0–10) in the mean overall VAS scores between the 2 groups" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The patients were assigned by computer‐generated randomisation to either laparotomy (n 21) or laparoscopy (n 19) when the patients were already in the operating room (central telephone). Before randomisation, patients were stratified according to the size of the largest myoma, the additive diameter of all myomas,and the individual surgeon who would perform the operation" Comment: Random sequence generation is mentioned |
| Allocation concealment (selection bias) | Low risk | Participants were assigned by central telephone by computer‐generated randomisation to laparotomy or laparoscopy when they were already in the operating room |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "After surgery, identical wound dressings were applied in both groups, with neither the patient nor the observer who recorded the postoperative pain aware of the surgical approach" Participants were blinded, surgeons cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After surgery, identical wound dressings were applied in both groups, with neither the patient nor the observer who recorded the postoperative pain aware of the surgical approach" Outcome assessors who recorded pain were blinded as per study report. On further enquiry as to whether assessors who recorded data other than pain were blind or not, study authors responded: "Blinding was done for 48 hours by wound coverings. Assessors were blinded for the access and recorded all date blinded until day 2 after the operation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | None were stated |
Mais 1995.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: absence of associated ovarian lesions, symptomatic subserous or intramural myomas, number of myomas ranging from 1 to 4, and size of the largest myoma ranging from 3 to 6 cm Exclusion criteria: Submucosal fibroids were looked for by hysteroscopy and were excluded from the study. Participants unfit for general anaesthesia or who had systemic infections or psychiatric disorders precluding consent were excluded Setting: Department of Obstetrics and Gynecology of the University of Cagliari, Italy Duration of the trial: January 1993 to July 1994 Duration of follow‐up: Six months postoperative |
|
| Interventions | Laparoscopic myomectomy versus laparotomy myomectomy Laparoscopic myomectomy (n = 20): Using Verres needle for insufflation, a 10‐mm primary port, 2 ancillary side ports on each side (the first 5 mm, the other 10 mm), an incision was made over the myoma pseudocapsule. The myoma was fixed with a myoma drill and enucleated. The myoma was removed by morcellation using a serrated edge macromorcellator. Uterine defects were closed with interrupted extracorporeal sutures, and the serosa was left open Laparotomy myomectomy (n = 20): The mini‐Pfannenstiel incision was used for laparotomy. No pharmacological vasoconstriction or mechanical vascular occlusion techniques were used before the uterine incision was made, usually anteriorly. Uterine defects were closed with interrupted sutures of Vicryl 1‐0 polyglactin 910 (Ethicon SpA, Pratica di Mare, Rome). The serosa was approximated with Vicryl 4‐0 Both groups: Postoperatively, analgesics were given when requested by the participant. Analgesics consisted of intramuscular ketoprofen (100 mg) or buprenorphine hydrochloride (0.3 mg). The latter was used for participants with glueose‐6‐phosphate dehydrogenase deficiency. No oral medication was given postoperatively and after discharge |
|
| Outcomes |
Primary outcome: Intensity of postoperative pain at 1, 2 and 3 days postoperative Secondary outcomes:
|
|
| Notes |
Sources of funding: not mentioned Ethics approval: Quote: "The trial was approved by the university ethical committee, and written informed consent was obtained from each patient" Informed consent: "The trial was approved by the university ethical committee, and written informed consent was obtained from each patient" Clinical trial registration: not mentioned in the trial report Sample size calculation: "The preliminary feasibility study indicated that after laparoscopic myomectomy at least 80% of patients were analgesic free on day 2, discharged from the hospital by day 3, and feeling fully recuperated on day 15. By contrast, no more than 20% of patients were usually analgesic‐free on day 2, discharged from the hospital by day 3, and feeling fully recuperated on day 15 after abdominal myomectomy. This sample size provides a power of 90% to detect such differences in comparing the two proportions at the 5% level. Moreover, the same sample size provides a power higher than 90% to detect a difference between group means as small as 1.7 cm of the visual analogue scale by use of two‐factor analysis of variance for repeated measures at the 5% level" Comment: Sample size was estimated on the basis of a preliminary feasibility study performed on 26 patients from January to December 1992 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assignment to laparotomy or laparoscopy was done by use of table of random digits" |
| Allocation concealment (selection bias) | Low risk | "Numbered sealed envelopes were used for allocating patients, seal was broken in anaesthesia room before surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The report does not mention whether participants were blinded. When a surgical intervention is provided, surgeons cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned; information was sought from study authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | None were stated |
Palomba 2007.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: ambulatory premenopausal women with uterine leiomyomas with leiomyoma‐related symptoms or unexplained infertility Exclusion criteria: major medical conditions, endocrine disease (including basal FSH > 10 UI/L), psychiatric disorders, current or past history of acute or chronic physical illness, premenstrual syndrome, current or past (washout period of at least 6 months was considered appropriate before enrolment), use of hormonal drugs or drugs influencing cognition, vigilance, or mood, inability to complete the daily diary and a history of alcohol abuse were considered as exclusion criteria. No desire to conceive, presence of more than 3 uterine leiomyomas and of leiomyomas with a main diameter less than 3 cm or greater than 10 cm, hypoechoic or calcified leiomyomas diagnosed at ultrasound (23), presence of submucosal leiomyomas or alterations of the uterine cavity screened by hysteroscopy and of other uterine or adnexal abnormalities at ultrasound (e.g. adenomyosis, abnormal endometrial thickness), pattern of hyperplasia with cytological atypia in the endometrial biopsy performed for abnormal uterine bleedings under hysteroscopy on suspected areas, an abnormal Papanicolau smear and a positive urine pregnancy test Setting: Three university Departments of Obstetrics and Gynecology (Catanzaro, Rome and Florence), Italy Duration of the trial: January 2002 to March 2003 Duration of follow‐up: 3 months postoperative Premenopausal women with symptomatic uterine fibroids or unexplained infertility |
|
| Interventions | Laparoscopic myomectomy (n = 68) versus mini‐laparotomic myomectomy (n = 68) Laparoscopic myomectomy: In the laparoscopic group, procedures were performed using a 10‐mm scope with 2 or 3 ancillary ports. A longitudinal incision, using a unipolar hook scissor, was performed close to the midline. After cleavage plane identification, the leiomyoma was enucleated by means of adequate traction with a myoma drill or a strong grasper and countertraction maneuvers with Manhes forceps or scissors, or a hydrodissection technique. Myometrium was sutured in 1 or 2 layers, according to the depth of the uterine defect, with interrupted figure‐8 sutures, using intracorporeal knots. Morcellation was done with a 12‐mm Steiner automatic morcellator Mini‐laparotomic myomectomy (n = 68): A 4‐ to 7‐cm transverse incision was made for mini‐laparotomy access. To avoid extension of the mini‐laparotomies, 2 double sutures were made at the ends of the skin incisions. The anterior rectus fascia was incised vertically, and the muscular fibers were dissected. The parietal peritoneum was exposed and incised vertically. The hysterotomy was performed with a monopolar knife on the most prominent part of each tumour, and the myoma was enucleated; interrupted figure‐8 sutures in 1 or 2 layers were used to repair the uterus. Progressive morcellement of the leiomyomas was performed with a cold knife scalpel if the tumour was larger than the skin incision Both groups: Immediately before surgery, each participant received 2 g of IV cephalosporin as antibiotic prophylaxis. In both groups (laparoscopic and minilaparotomy), haemostasis was attained by bipolar coagulation of the tissue and vessels around the fibroids. A manipulator probe was used in both groups. Infiltration of the serosa or myometrium overlying the leiomyoma was performed before the uterine incision was made, with a solution of 50 mL of bupivacaine cloridrate 0.25% and 0.5 mL of epinephrine (0.5 vial of 1 mg/mL) in all cases. The uterine incision was performed using the same electrosurgical device with a cutting current of 70 W. At the end of each intervention, the pelvis was washed with saline solution. After the intervention, all participants received an IV bolus of tramadol (100 mg), followed by patient‐controlled analgesia (tramadol 200 mg [2 vials] in 500 mL of saline solution). Postoperative analgesia was administered during the first 12 hours after surgery |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Sources of funding: not stated in the trial report Ethics approval: "The study was approved by the Institutional Review Board (IRB) of the University ‘‘Magna Graecia’’ of Catanzaro, Italy" Informed consent: "Before entering the study, the protocol was explained to the patients, including the possibility of conversion to traditional laparotomic surgery, and a written consent was obtained from each patient." Clinical trial registration: not mentioned in the trial report Sample size calculation: not mentioned in the trial report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out using online software of randomisation (www.randomization.it) to generate a random allocation sequence in single blocks as method of restriction" |
| Allocation concealment (selection bias) | Low risk | "The random allocation sequence was concealed in closed and dark‐coloured envelope until surgeries were assigned (before entering in the operating room)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The report does not mention whether participants were blind. In a surgical trial, surgeons cannot be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors is not mentioned; information from study authors was sought but not obtained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were missing |
| Selective reporting (reporting bias) | Low risk | All outcomes were described |
| Other bias | Unclear risk | None were stated |
Rossetti 2001.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: women younger than 42 years of age with at least 1 symptomatic myoma > 3 cm and 7 or fewer myomas Exclusion criteria: more than 7 myomas Setting: One Department of obstetrics and Gynaecology at a University Hospital, Italy Duration of the trial: 7 years, from January 1991 to June 1998 Duration of follow‐up: 40 months |
|
| Interventions | Abdominal myomectomy (n = 40) versus laparoscopic myomectomy (n = 41) "Myomectomies were performed with a standard technique using three suprapubic ports." Operating techniques were not further specified |
|
| Outcomes |
|
|
| Notes |
Sources of funding: not stated Ethics approval: not stated Informed consent: not stated whether written informed consent was obtained Clinical trial registration: not stated Sample size calculation: not stated The authors of the study were contacted via email to provide additional information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised using computer assisted random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was provided about blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | None were stated |
Seracchioli 2000.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: women of reproductive age (range 21‐42 years) with an anamnesis of primary or secondary infertility and the presence of at least 1 large uterine myoma with a diameter of at least 5 cm Exclusion criteria: women with pedunculated myomata, uterine size above the transverse umbilical line, more than 3 myomata > 5 cm, presence of concomitant alterations in tubal patency or other infertility factors including male factors or alterations of uterine cavity such as septum or subseptum Setting: a reproductive medicine unit in Italy Duration of the trial: 5 years, from January 1993 to January 1998 Duration of follow‐up: at least 12 months |
|
| Interventions | Laparoscopic myomectomy (n = 66) versus abdominal myomectomy (n = 65) Abdominal laparotomy (n = 65): A low transverse abdominal incision was made, followed by a linear uterine incision as small as possible on the most prominent part of the leiomyoma. Different incisions were made, depending on the position of the myomata. Enucleation was done following the cleavage plain, the uterus was sutured in 1 or 2 planes according to depth and size of myomata. Afterwards, the endometrium was closed and sutured as well; this was followed by washing of the pelvis with a saline solution Laparoscopy (n = 66): A pneumoperitoneum was obtained with carbon dioxide insufflation, followed by a standard umbilical incision and introduction of the laparoscope. Two suprapubic access routes were inserted lateral, and a third was inserted in the midline. Uterine cannulation was used for optimal exposure of the myoma, and a methylene blue test was carried out. To incise the serosa of the myoma, a monopolar pointed knife was used. Dissection was done via traction on the myoma and countertraction on the uterus. Coagulation was performed with bipolar current before cutting. Afterwards, the uterine wall was sutured. The myomata were removed from the abdominal cavity with a manual laparoscopic morcellator, followed by washing of the pelvis with saline solution |
|
| Outcomes |
Surgical outcomes: mean operative time, perioperative and postoperative morbidity (average drop in haemoglobin, transfusions, fever > 38°C), length of hospitalisation, myoma recurrence, repeat myomectomy, hysterectomy Fertility outcomes: pregnancy rate, miscarriage rate, ongoing pregnancies, ectopic pregnancies, deliveries, preterm deliveries, vaginal deliveries, caesarean sections, uterine ruptures |
|
| Notes |
Sources of funding: not stated Ethics approval: not stated Informed consent: yes: "All women signed an informed consent form and a surgical consent form to participate in this study" Clinical trial registration: not stated Sample size calculation: not stated Study authors were contacted via email to provide more information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out the day before surgery using total random digits" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated whether women were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Unclear risk | None were stated |
Sesti 2008.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: 1 to 5 intramural or subserous symptomatic uterine myomas with infertility or fast growth measuring 4 to 10 cm Exclusion criteria: body mass index (BMI) of 30 kg/m² or more, previous uterine surgery and suspicion of malignant gynaecological disease Setting: Section of Gynaecology, Department of Surgery, Tor Vergata Hospital University, Rome, Italy Duration of the trial: 3 years, from January 2003 to March 2006 Duration of follow‐up: not stated |
|
| Interventions | Gasless laparoscopy versus mini‐laparotomy | |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes |
Sources of funding: “Research funds were financed by the Italian Ministry of University. There was no financial interest or any arrangement with the companies producing the instruments used in the study or with competitor companies. There also was no direct payment to the authors from any source for the purpose of financing the writing of the manuscript, nor was there any other financial connections, direct or indirect, or other situations that might raise the question of bias in the work” Ethics approval: "The study was previously approved by the local ethics committee and research funds were financed by the Italian Ministry of University” Informed consent: yes: “A written informed consent was taken before randomisation” Clinical trial registration: not stated Sample size calculation: "A power calculation verified that more than 26 patients in each group would be necessary to detect a difference of more than 24h in discharge time with an alpha error level of 5% and a beta error of 80%" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned the day before surgery using a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque, sealed envelopes were used. The sequence was kept concealed by a physician until interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Each participant was blindly allocated by a physician. Surgeons did not know which participants were included in the study. Does not mention whether participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blind to group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | None were stated |
Tan 2008.
| Methods | Randomised controlled trial | |
| Participants |
Inclusion criteria: "presence of 1 to 3 symptomatic intramural or subserosal myomas (without pedicle) associated with either infertility or fast growth and having a diameter of 5 to 10 cm" Exclusion criteria: major medical conditions, psychiatric disorders, current or past history of acute or chronic physical illness, premenstrual syndrome, current or past use (6 months) of hormonal drugs or drugs that influence cognition, vigilance, or mood, inability to complete the daily diary and a history of alcohol abuse. Additionally, specific exclusion criteria included the following: no wish to conceive, hypoechoic or calcified leiomyomas diagnosed at ultrasound scanning, presence of submucosal leiomyomas or alterations of the uterine cavity screened by hysteroscopy and of other uterine or adnexal abnormalities at ultrasound scanning (e.g. adenomyosis, abnormal endometrial thickness), pattern of hyperplasia with cytological atypia in the endometrial biopsy specimen obtained, abnormal uterine bleeding observed with hysteroscopy of suspected areas, an abnormal Pap smear result and a positive urine pregnancy test result, as well as no previous conception resulting in a live baby or with tubal/male factor subfertility Setting: Department of Gynaecology, Jiangyin Hospital, affiliated with Southeast of China University, China Duration of the trial: 2 years, from January 2005 to March 2007 Duration of follow‐up: not stated |
|
| Interventions | Laparoscopic myomectomy (n = 26) versus laparoscopically assisted mini‐laparotomy myomectomy (n = 26) Laparoscopic myomectomy (LA): "Incise the umbilical opening, place a 10‐mm trocar, insert the laparoscope, pass a Kirschner's needle (diameter: 2 mm) vertically through the subcutaneous tissue of the lower abdomen at an interval of 10 cm, suspend both ends of the steel needle at the cross arm of the lifting/pulling device via a saddle chain, connect the cross arm with a vertical arm, and fix at 1 side of the operating table. incise a 1.5‐ to 2.0‐cm aperture to the peritoneum at the left lower abdomen, put an incision protective sleeve via this opening, and start apparatus operation. Exise the myomas one by one according to their growing positions" Laparoscopically assisted mini‐laparotomy (LA‐MLT): A 10‐mm port was inserted through the umbilicus to introduce the laparoscope, a 5‐mm trocar was inserted left of the umbilicus. MlT was performed with a 4‐ to 7‐cm suprapubic incision. Size of the incision was related to number, position and size of myomas. Subcutaneous fat and abdominal fascia were transversely opened in the abdominal muscle and parietal peritoneum longitudinally on the midline. The dominant myoma was grasped with a tenaculum clamp and dissected. Enucleation was executed by traction on the myoma and by countertraction on the uterus In both groups, a 70 W electrosurgical device was used for uterine incision, and the pelvis was washed with saline solution |
|
| Outcomes | Total operative time (min), intraoperative blood loss (mL), decrease in Hb, postoperative ileus (h), hospitalisation (days), conversion to laparotomy (n), postoperative abdominal pain (VAS scores) (12 and 24 hours) | |
| Notes |
Sources of funding: “There was no financial interest or any arrangement with the companies producing the instruments used in the study or with competitor companies. There also was no direct payment to the authors from any source for the purpose of financing the writing of the manuscript, nor were there any other financial connections, direct or indirect, or other situations that might raise the question of bias in the work" Ethics approval: yes: "The study was previously approved by the ethics committee" Informed consent: yes: Each woman gave informed consent to be included in the study Clinical trial registration: not stated Sample size calculation: "A power calculation verified that 26 patients in each group would be necessary to detect a difference of 24 hours in discharge time, with an alpha error level of 5% and a beta error of 80%" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated list of randomisation was used |
| Allocation concealment (selection bias) | Unclear risk | "After enrolment by a physician, each woman was randomised by a nurse to the LA (n = 26), or LA‐MLT (n = 26) group, in sealed and dark‐colored envelopes until surgeries were assigned (before entering the operating room)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | None were stated |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angioni 2005 | Conference abstract comparing pregnancy outcomes in infertile women undergoing laparoscopic and open myomectomy |
| Benassi 2005 | Randomised controlled trial comparing myomectomy by mini‐laparotomy versus laparotomy myomectomy; no arm of laparoscopic myomectomy |
| Birsan 2003 | Prospective pilot study, not randomised. Participants with single large posterior wall fibroid (> 5 cm) allocated to vaginal or laparoscopic myomectomy, no arm that underwent laparotomy or mini‐laparotomy |
| Cagnacci 2003 | Randomised controlled trial comparing early outcomes in myomectomies performed by 3 laparotomic approaches (laparotomy, mini‐laparotomy, laparoscopically assisted mini‐laparotomy). In no arm was laparoscopic myomectomy performed |
| Campo 2003 | Not an RCT |
| Falcone 2002 | Not an RCT, review article analysing data for different techniques of myomectomy |
| Fanfani 2005 | Prospective study comparing myomectomy by laparoscopy versus mini‐laparotomy myomectomy, not randomised (decision about type of surgery was made per surgeon preference) |
| Holub 2006 | Prospective non‐randomised study comparing degree of inflammatory response in laparoscopic versus open myomectomy |
| Kalogiannidis I. | Prospective non‐randomised study comparing laparoscopically assisted myomectomy versus abdominal myomectomy. Participants were allocated to 2 groups as per their preference, and no laparoscopic myomectomy arm was included |
| Malzoni 2010 | Retrospective study comparing feasibility, safety, morbidity and pregnancy outcomes of laparoscopic versus mini‐laparotomy myomectomy |
| Palomba 2005 | Conference abstract of "semi‐randomised" study comparing mini‐laparotomic and laparoscopic myomectomy |
| Signorile 2000 | Not an RCT |
| Wang 2011 | Randomised controlled trial comparing myomectomy by gasless laparoscopy versus myomectomy by conventional laparoscopy. No arm of laparotomy, mini‐laparotomy or laparoscopically assisted mini‐laparotomy myomectomy |
| Zhang 2005 | No relevant outcomes measured |
Differences between protocol and review
Based on the outcomes of postoperative pain described by different study authors, we have chosen to report postoperative pain at six, 24 and 48 hours after surgery, rather than within the first seven days, to present a more detailed analysis of reported results.
Postoperative ileus was removed from the list of adverse events, as it is reflected in the length of hospital stay.
The perioperative mortality outcome was pooled with the outcome of in‐hospital adverse events.
We also conducted sensitivity analyses using a random‐effects rather than a fixed‐effect model, and using the summary effect measure of risk ratio rather than odds ratio. We conducted these additional sensitivity analyses in accordance with current guidelines of the Cochrane Menstrual Disorders and Subfertility Group.
Contributions of authors
AS and AWP drafted the protocol.
PBC and SF independently selected studies, extracted data and performed the meta‐analysis for the full review. PBC and SF wrote the review with input from other review authors. CF helped to draft the review, acted as a clinical expert and commented on the review.
Sources of support
Internal sources
University College, London, UK.
University of Cambridge, UK.
University of Auckland, New Zealand.
External sources
No sources of support supplied
Declarations of interest
No declarations of interest were made.
New
References
References to studies included in this review
Alessandri 2006 {published data only}
- Alessandri F, Lijoi D, Mistrangelo E, Ferrero S, Ragni N. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. Journal of Minimally Invasive Gynecology 2006;13(2):92‐7. [CENTRAL: CN‐00563190; DOI: 10.1016/j.jmig.2005.11.008] [DOI] [PubMed] [Google Scholar]
Cicinelli 2009 {published data only}
- Cicinelli E, Tinelli R, Colafiglio G, Saliani N. Laparoscopy vs minilaparotomy in women with symptomatic uterine myomas: a prospective randomized study. Journal of Minimally Invasive Gynecology 2009;16(4):422‐6. [CENTRAL: CN‐00721670 ; DOI: 10.1016/j.jmig.2009.03.011] [DOI] [PubMed] [Google Scholar]
Holzer 2006 {published data only}
- Holzer A, Jirecek ST, Illievich UM, Huber J, Wenzl RJ. Laparoscopic versus open myomectomy: a double‐blind study to evaluate postoperative pain. Anesthesia and Analgesia 2006;102(5):1480‐4. [CENTRAL: CN‐00564509 ] [DOI] [PubMed] [Google Scholar]
Mais 1995 {published data only}
- Mais V, Ajossa S, Guerriero S, Mascia M, Solla E, Melis GB. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome.. American Journal of Obstetrics and Gynecology 1996;174(2):654‐8. [CENTRAL: CN‐00123957] [DOI] [PubMed] [Google Scholar]
Palomba 2007 {published data only}
- Palomba S, Marconi D, Falbo A, Russo T, Mattei A, Zupi E, et al. A randomized controlled trial comparing the laparoscopic and minilaparotomic approach for uterine leiomyomas: surgical and fertility outcomes. Journal of Minimally Invasive Gynecology 2006;13(Suppl 5):S15‐16. [Google Scholar]
- Palomba S, Zupi E, Falbo A, Russo T, Marconi D, Tolino A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: reproductive outcomes.. Fertility and Sterility 2007;88(4):933‐41. [CENTRAL: 00609541] [DOI] [PubMed] [Google Scholar]
- Palomba S, Zupi E, Russo T, Falbo A, Marconi D, Tolino A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: short‐term outcomes. Fertility and Sterility 2007;88(4):942‐51. [CENTRAL: CN‐00609333] [DOI] [PubMed] [Google Scholar]
Rossetti 2001 {published data only}
- Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long‐term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Human Reproduction 2001;16(4):770‐4. [DOI] [PubMed] [Google Scholar]
Seracchioli 2000 {published data only}
- Seracchioli R, Rossi S, Govoni F, Rossi E, Venturoli S. Efficacy of laparoscopic myomectomy in cases of large myoma(s): randomized comparison with laparotomic myomectomy. Human Reproduction 1999;14:274. [DOI] [PubMed] [Google Scholar]
- Seracchioli R, Rossi S, Govoni F, Rossi E, Venturoli S, Bulletti C, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Human Reproduction 2000;15(12):2663‐8. [CENTRAL: CN‐00330415] [DOI] [PubMed] [Google Scholar]
Sesti 2008 {published data only}
- Sesti F, Capobianco F, Capozzolo T, Pietropolli A, Piccione E. Isobaric gasless laparoscopy versus minilaparotomy in uterine myomectomy: a randomized trial. Surgical Endoscopy 2008;22(4):917‐23. [CENTRAL: CN‐00636939] [DOI] [PubMed] [Google Scholar]
Tan 2008 {published data only}
- Tan J, Sun Y, Dai H, Zhong B, Wang D. A randomized trial of laparoscopic versus laparoscopic‐assisted minilaparotomy myomectomy for removal of large uterine myoma: short‐term outcomes. Journal of Minimally Invasive Gynecology 2008;15(4):402‐9. [CENTRAL: CN‐00649521 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angioni 2005 {published data only}
- Angioni 2005. Laparoscopic vs abdominal myomectomy: role on fertility and pregnancy outcome. Gynecological Surgery 2005;2(Suppl 1):S13. [Google Scholar]
Benassi 2005 {published data only}
- Benassi L, Marconi L, Benassi G, Accorsi F, Angeloni M, Besagni F. Minilaparotomy vs laparotomyfor uterine myomectomies: a randomized controlled trial. Minerva Gynecology 2005;57:169‐73. [PubMed] [Google Scholar]
Birsan 2003 {published data only}
- Birsan A, Brunao D, Detchev R, Ponclet C, Darai E. Vaginal and laparoscopic myomectomy for large posterior myomas: results of a pilot study. European Journal of Obstetrics and Gynecology and Reproductive Biology 2003;110:89‐93. [DOI] [PubMed] [Google Scholar]
Cagnacci 2003 {published data only}
- Cagnacci A, Pirillo D, Malmusi S, Arangino S, Alessandrini C, Volpe A. Early outcome of myomectomy by laparotomy, minilaparotomy and laparoscopically assisted minilaparotomy. A randomized prospective study. Human Reproduction 2003;18(12):2590‐4. [DOI] [PubMed] [Google Scholar]
Campo 2003 {published data only}
- Campo S, Campo V, Gambadauro P. Reproductive outcome before and after laparoscopic or abdominal myomectomy for subserous or intramural myomas. European Journal of Obstetrics, Gynecology and Reproductive Biology 2003;110(2):215‐9. [DOI] [PubMed] [Google Scholar]
Falcone 2002 {published data only}
- Falcone T, Bedaiwy MA. Minimally invasive management of uterine fibroids. Current Opinion in Obstetrics & Gynaecology 2002;14(4):401‐7. [DOI] [PubMed] [Google Scholar]
Fanfani 2005 {published data only}
- Fanfani F, Fagotti A, Bifulco G, Ercoli A, Malzoni M, Scambia G. A prospective study of laparoscopy versus minilaparotomy in the treatment of uterine myomas. Journal of Minimally Invasive Gynecology 2005;12(6):470‐4. [CENTRAL: CN‐00600174] [DOI] [PubMed] [Google Scholar]
Holub 2006 {published data only}
- Holub Z. Surgical results of myomectomy using laparoscopic and minilaparotomic access. Women's Health 2007;3(5):537‐9. [DOI] [PubMed] [Google Scholar]
Kalogiannidis I. {published data only}
- Kalogiannidis I, Prapas N, Xiromeritis P, Prapas Y. Laparoscopically assisted myomectomy versus abdominal myomectomy in short‐term outcomes: a prospective study. Archives of Gynecology and Obstetrics 2010;281(5):865‐70. [DOI] [PubMed] [Google Scholar]
Malzoni 2010 {published data only}
- Malzoni M, Tinelli R, Cosentino F, Iuzzolino D, Surico D, Reich H. Laparoscopy versus minilaparotomy in women with symptomatic uterine myomas: short‐term and fertility results. Fertility Sterility 2010;93(7):2368‐73. [DOI] [PubMed] [Google Scholar]
Palomba 2005 {published data only}
- Palomba S, Zupi E. Minilaparotomic and laparoscopic myomectomy: a comparative study. Journal of Minimally Invasive Gynecology 2005;12:S122‐S124. [Google Scholar]
Signorile 2000 {published data only}
- Signorile P, Gargiulo F, Latavo C. Laparoscopic and ultraminilaparotomic (3cm) myomectomy (LUM): preliminary report of a new surgical technique. XVI FIGO World Congress of O & G. 2000; Vol. Abstract book 5:25‐6.
Wang 2011 {published data only}
- Wang J, Yang F, Gao T, Li L, Xia H, Li H. Gasless laparoscopy versus conventional laparoscopy in uterine myomectomy: a single centre randomized trial. The Journal of International Medical Research 2011;39:172‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2005 {published data only}
- Zhang GL, Wen WQ, Xing FQ. Effect of enucleation of hysteromyoma by laparoscopic surgery on protein oxidation and lipid hyperoxidation. Zhonghua Yi Xue Za Zhi 2005;85(3):177‐80. [PubMed] [Google Scholar]
Additional references
Baird 2003
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American Journal of Obstetrics and Gynecology 2003;188(1):100‐7. [DOI] [PubMed] [Google Scholar]
Bajekal 2000
- Bajekal N, Li TC. Fibroids, infertility and pregnancy wastage. Human Reproduction Update 2000;6(6):614‐20. [DOI] [PubMed] [Google Scholar]
Bonney 1931
- Bonney V. The technique and results of myomectomy. Lancet 1931;220:171‐7. [Google Scholar]
Buttram 1981
- Buttram VC, Reiter RC. Uterine leiomyomata. Fertility and Sterility 1981;36:433‐45. [DOI] [PubMed] [Google Scholar]
Cramer 1990
- Cramer SF, Patel A. The frequency of uterine leiomyomas. American Journal of Clinical Pathology 1990;94(4):435‐8. [DOI] [PubMed] [Google Scholar]
Downes 2010
- Downes E, Sikirica V, Gilabert‐Estelles J, Bolge SC, Dodd SL, Maroulis C, et al. The burden of uterine fibroids in five European countries. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2010;152(1):96‐102. [DOI] [PubMed] [Google Scholar]
Drinville 2007
- Drinville SJ, Memarzadeh S. Benign disorders of the uterine corpus. Current Diagnosis & Treatment Obstetrics & Gynecology 2007;10:639‐53. [Google Scholar]
Dubuisson 2000
- Dubuisson JB, Fauconnier A, Babaki‐Fard K, Chapron C. Laparoscopic myomectomy: a current view. Human Reproduction Update 2000;6(6):588‐94. [DOI] [PubMed] [Google Scholar]
FDA 2014
- Food, Drug Administration. Quantitative Assessment of the Prevalence of Unsuspected Uterine Sarcoma in Women Undergoing Treatment of Uterine Fibroids. Summary and Key Findings. FDA Safety Communications April 17, 2014.
Frishman 2005
- Frishman GN, Jurema MW. Myomas and myomectomy. Journal of Minimally Invasive Gynecology 2005;12(5):443‐56. [DOI] [PubMed] [Google Scholar]
Gentry 2000
- Gentry C, Okolo S, Fong L, Crow J, Maclean A, Perret C. Quantification of vascular endothelial growth factor‐A in leiomyomas and adjacent myometrium. Clinical Science 2001;101:691‐5. [PubMed] [Google Scholar]
Golan 1996
- Golan A. GnRH analogues in the treatment of uterine fibroids. Human Reproduction 1996;11(Suppl 3):33‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of interventions Version 5.1.0 [updated March 2011]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Jin 2009
- Jin C, Hu Y, Chen XC, Zheng FY, Lin F, Zhou K, et al. Laparoscopic versus open myomectomy—a meta‐analysis of randomized controlled trials. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;145(1):14‐21. [DOI] [PubMed] [Google Scholar]
LaMorte 1993
- LaMorte A, Lalwani S, Diamond M. Morbidity associated with abdominal myomectomy. Obstetrics and Gynecology 1993;82(6):897‐900. [PubMed] [Google Scholar]
Lethaby 2001
- Lethaby A, Vollenhoven B, Sowter MC. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy foruterine fibroids. Cochrane Database of Systematic Reviews 2001, (2). [DOI: 10.1002/14651858.CD000547] [DOI] [PubMed] [Google Scholar]
Lethaby 2002
- Lethaby A, Vollenhoven B. Fibroids. Clinical Evidence 2002;14:1666‐78. [PubMed] [Google Scholar]
Levy 2008
- Levy BS. Modern management of uterine fibroids. Acta Obstetricia et Gynecologica Scandinavica 2008;87(8):812‐23. [DOI] [PubMed] [Google Scholar]
Makinen 2011
- Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011;334(6053):252‐5. [DOI] [PubMed] [Google Scholar]
Merill 2008
- Merill RM, Layman AB, Oderda D, Asche C. Risk estimates of hysterectomy and selected conditions commonly treated with hysterectomy. Annals of Epidemiology 2008;18(3):253‐60. [DOI] [PubMed] [Google Scholar]
Metwally 2012
- Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database of Systematic Reviews 2012, Issue Issue 11. [Art. No.: CD003857] [DOI] [PubMed] [Google Scholar]
Miller 2000
- Miller CE. Myomectomy. Comparison of open and laparoscopic techniques. Obstetrics and Gynecology Clinics of North America 2000;27(2):407‐20. [DOI] [PubMed] [Google Scholar]
Mine 2001
- Mine N, Kurose K, Nagai H, Doi D, Ota Y, Yoneyama K, et al. Gene fusion involving HMGIC is a frequent aberration in uterine leiomyomas. Journal of Human Genetics 2001;46:408‐12. [DOI] [PubMed] [Google Scholar]
Nieboer 2009
- Nieboer TE, Johnson N, Lethaby A, Tavender E, Curr E, Garry R, et al. Surgical approach to hysterectomy for benign gynecological disease. Cochrane Database of Systematic Reviews 2009;3. [DOI: 10.1002/14651858.CD003677.pub4] [DOI] [Google Scholar]
Okolo 2008
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Practice & Research. Clinical Obstetrics & Gynaecology 2008;22(4):571‐88. [DOI] [PubMed] [Google Scholar]
Pritts 2009
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertility and Sterility 2009;91(4):1215‐23. [DOI] [PubMed] [Google Scholar]
Querleu 1993
- Querleu D, Chevallier L, Chapron C, Bruhat M. Complications of gynaecological surgery. A French multicenter collaborative study. Gynaecological Endoscopy 1993;2:3‐6. [DOI] [PubMed] [Google Scholar]
Ravina 1995
- Ravina JH, Herbreteau D, Ciraru‐Vigneron N, Bouret J, Houdart E, Aymard A, et al. Arterial embolisation to treat uterine myomata. Lancet 1995;346:671‐2. [DOI] [PubMed] [Google Scholar]
Semm 1979
- Semm K. New methods of pelviscopy (gynaecologic laparoscopy) for myomectomy, ovariectomy, tubectomy and adnectomy. Endoscopy 1979;11:85‐93. [DOI] [PubMed] [Google Scholar]
Senturk 2001
- Senturk LM, Sozen I, Gutierrez L, Atrici A. Interleukin 8 production and interleukin 8 receptor expression in human myometrium and leiomyoma. American Journal of Obstetrics and Gynecology 2001;184:559‐66. [DOI] [PubMed] [Google Scholar]
Stewart 2001
- Stewart EA. Uterine fibroids. Lancet 2001;357:293‐8. [DOI] [PubMed] [Google Scholar]
Viswanathan 2007
- Viswanathan M, Hartmann K, McKoy N, Stuart G, Rankins N, Thieda P, et al. Management of Uterine Fibroids: An Update of the Evidence. AHRQ Publication No. 07‐E011. Evidence Report/Technology Assessment No. 154 Prepared by RTI International–University of North Carolina Evidence‐based Practice Center under Contract No. 290‐02‐0016. Rockville, MD, USA: Agency for Healthcare Research and Quality, July 2007. [Google Scholar]
Wallach 2004
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstetrics and Gynecology 2004;104(2):393‐406. [DOI] [PubMed] [Google Scholar]


