Abstract
Objective:
To characterize the circadian features of the trigeminal ganglion in a mouse model of headache.
Background:
Several headache disorders, such as migraine and cluster headache, are known to exhibit distinct circadian rhythms of attacks. The circadian basis for these rhythmic pain responses, however, remains poorly understood.
Methods:
We examined trigeminal ganglion ex vivo and single-cell cultures from Per2::LucSV reporter mice and performed immunohistochemistry. Circadian behavior and transcriptomics were investigated using a novel combination of trigeminovascular and circadian models: a nitroglycerin mouse headache model with mechanical thresholds measured every 6 h, and trigeminal ganglion RNA sequencing measured every 4 h for 24 h. Finally, we performed pharmacogenomic analysis of gene targets for migraine, cluster headache, and trigeminal neuralgia treatments as well as trigeminal ganglion neuropeptides; this information was cross-referenced with our cycling genes from RNA sequencing data to identify potential targets for chronotherapy.
Results:
The trigeminal ganglion demonstrates strong circadian rhythms in both ex vivo and single-cell cultures, with core circadian proteins found in both neuronal and non-neuronal cells. Using our novel behavioral model, we showed that nitroglycerin-treated mice display circadian rhythms of pain sensitivity which were abolished in arrhythmic Per1/2 double knockout mice. Furthermore, RNA-sequencing analysis of the trigeminal ganglion revealed 466 genes that displayed circadian oscillations in the control group, including core clock genes and clock-regulated pain neurotransmitters. In the nitroglycerin group, we observed a profound circadian reprogramming of gene expression, as 331 of circadian genes in the control group lost rhythm and another 584 genes gained rhythm. Finally, pharmacogenetics analysis identified 10 genes in our trigeminal ganglion circadian transcriptome that encode target proteins of current medications used to treat migraine, cluster headache, or trigeminal neuralgia.
Conclusion:
Our study unveiled robust circadian rhythms in the trigeminal ganglion at the behavioral, transcriptomic, and pharmacogenetic levels. These results support a fundamental role of the clock in pain pathophysiology.
Keywords: circadian transcriptome, clock-controlled genes, HTR2A, OPRD1, rhythmically expressed genes, von Frey
Plain Language Summary
Several headache diseases, such as migraine and cluster headache, have headaches that occur at the same time each day. We learned that the trigeminal ganglion, an important pain structure in several headache diseases, has a 24-hour cycle that might be related to this daily cycle of headaches. Our genetic analysis suggests that some medications may be more effective in treating migraine and cluster headache when taken at specific times of the day.
INTRODUCTION
Cluster headache (CH) and migraine display strong circadian rhythmicity in pain attacks and sensitivity, especially CH.1–3 Behaviorally, 70% of CH and 50% of migraine patients have a daily rhythmicity to their headaches, and circadian-related hormones (melatonin and corticosteroids) are altered in these patients.4–8 Furthermore, many medications used by both CH and migraine patients have circadian features, especially preventive treatments.2,9 These findings suggest a crucial role for the circadian clock in headache disorders.
The mammalian circadian system, at its core, consists of intracellular transcriptional-translational feedback loops with positive (Clock/Npas2, Bmal1, Rora, Rorb, and Rorc) and negative (Period1 or Per1, Period2 or Per2, Cryptochrome1 or Cry1, Cryptochrome2 or Cry2, Rev-erba, and Rev-erbb) components.10 The circadian transcriptional-translational feedback loops are present in most cell types in the body and generate cell-autonomous circadian rhythms.11 This circadian machinery drives the circadian expression of downstream genes (so-called clock-controlled genes or CCGs) and ultimately leads to daily rhythms in activities, the sleep–wake cycle, blood pressure, body temperature, metabolism, and other physiological processes.12–14 The peripheral clocks are synchronized by the central pacemaker (the suprachiasmatic nucleus) through neuronal and hormonal signaling.15 This circadian system is highly connected to primary headaches. Multiple genes involved in the circadian transcriptional-translational feedback loop have been implicated in CH and migraine,5–8 and more than half of all susceptibility genes for both disorders are CCGs.4
The canonical pain system for headaches is the trigeminovascular system,16–18 which has both peripheral and central components linked by the trigeminal ganglion (or TG, also known as the gasserian or semilunar ganglion). Located outside of the blood-brain barrier, the TG is an immensely important structure as it is the putative target of dual migraine/CH medications that do not cross the blood-brain barrier in significant amounts,19,20 such as ergotamine21 and monoclonal antibodies to calcitonin gene-related peptide (CGRP).22 The TG transcriptome has been shown to be highly conserved between mice and humans, and single-cell analysis at one time point has shown the presence of core clock gene expression in multiple cell types.23,24 Moreover, preliminary evidence suggests that the TG can cause circadian patterns of pain in humans: trigeminal neuralgia, a facial pain disorder often resulting from vascular compression of the trigeminal nerve,25 also displays a circadian periodicity of attacks.26 These observations suggest that the TG may play an important role in the circadian rhythmicity of headaches and facial pain; however, the clock network in the TG has not been characterized, and how it responds to pain remains unclear.
Here we report a robust molecular clock and a circadian transcriptome in the TG. Furthermore, using a trigeminovascular mouse model of chronic headache induced by repeated nitroglycerin (NTG) injections, we showed global reprogramming of the circadian transcriptome as well as a strong circadian time-dependent effect of NTG on the TG transcriptome. Pharmacogenetic analysis comparing our TG circadian transcriptome and time-dependent differentially expressed genes (TD-DEGs) revealed gene targets for 67 different medications used to treat migraine, CH, and/or trigeminal neuralgia. Therefore, our study illustrates the circadian circuit in the TG and its dynamic reprogramming by headache pain and medications, suggesting a nodal regulatory function of the TG clock in headache pain and therapy.
MATERIALS AND METHODS
Animal studies
C57BL/6J (Stock # 000664) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA); Per1/2 double knockout (Per1/2 dKO), Per2::LucSV, and wild-type (WT) mice were maintained in-house. All mice were housed under 12 h light/12 h dark unless otherwise noted. All animal studies, using male mice, were approved by the UTHealth Center for Laboratory Animal Medicine and Care. Cephalic allodynia studies using both male and female mice and were conducted at Washington University in St. Louis with Institutional Animal Care and Use Committee approval.
Bioluminescence measurement from ex vivo TG cultures
Our bioluminescence studies of the TG were performed similar to prior studies11,27,28 (see Supplemental Methods S1). Briefly, TGs isolated from Per2::LucSV mice were manually dissected into small pieces of equivalent size (2 pieces per TG), placed in tissue culture dishes containing bioluminescence recording media, and continuously measured by LumiCycle luminometer (Actimetrics, Wilmette, IL).
TG primary cell cultures and single cell bioluminescence recording
To measure single cell bioluminescence recordings, we used Per2::LucSV reporter mice. Primary TG cells were cultured by modifying a method from Katzenell and colleagues29,30 (see Supplemental Methods). Primary TG cells were then transduced with AAV2-Syn-GFP (Addgene #50465), which expresses GFP under the human synapsin promoter expression in neurons, for 20 h. Five days after transduction, the media was changed to bioluminescence recording media and real-time single cell bioluminescence was measured by LV-200 Olympus microscope.
NTG-induced pain models
To measure mechanical allodynia, we used the manual von Frey behavior test for hindpaw and cephalic allodynia that has been described previously31–34 (see Supplemental Methods). To measure hindpaw and cephalic mechanical allodynia during circadian time points (11 days for hindpaw and 10 days for facial after the first injection of NTG), we performed the von Frey behavior test at several times (light on at zeitgeber time or ZT2[ i.e., 2 hours after light onset in a standard 12 hour light/12 hour dark setting] and ZT8 [8 hours after light onset], light off at ZT14 [2 hours after light off] and ZT20 [8 hours after light off]) for hindpaw and two times (light on at ZT2 and light off at ZT14) for cephalic allodynia. At each timepoint, the von Frey monofilaments were applied six times and the paw or facial reaction was recorded.
Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
RNA extraction and RT-PCR analysis were performed as previously described.35 For RT-PCR analysis of the Cgrp gene in TG tissue, 10 mg/kg NTG was administered intraperitoneally every other day for 9 days, TGs were removed from mice and total RNA were extracted by using PureXtract RNAsol Reagent (GenDEPOT). The primer sets for Cgrp used F: 5′-atcaagagtcaccgcttcgc-3′, R: 5′-cctgggctgctttccaagat-3′. Gapdh was used as the control housekeeping gene.35
Immunohistochemistry of TG
For details of immunohistochemistry, see Supplemental Methods. Briefly, after anesthetization with isoflurane and systemic administration of cold phosphate-buffered saline (GenDEPOT) then paraformaldehyde in phosphate-buffered saline, the TGs were removed from the mice and prepared for staining. We used the following primary antibodies: for clock proteins BMAL1, REV-ERBα (abcam, ab174309), and CRY2; for Neuron marker NeuN (EMD Millipore Corp, MAB377); and for the satellite glial cell marker glutamine synthetase (GS; EMD Millipore Corp, MAB302). We also used the following secondary antibodies: Alexa Fluor 488 and Alexa Fluor 594, goat anti-rabbit, – mouse, –guinea pig, and –chicken, 1:500 (Fisher Scientific).
RNA sequencing
A set of control and NTG-treated mice on the same circadian schedule were sacrificed every 4 h from ZT0 to ZT20. TGs were immediately collected and frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from frozen TGs with PureXtract RNAsol (GenDEPOT). One microgram of extracted RNA was used for RNA sequencing analysis (Novogene). Poly(A-)-enriched non-stranded RNA sequencing was carried out by Novogene (US) on Illumina HiSeq 2500 with 150-bp paired-end reads (for details of RNA sequencing analysis see Supplemental Methods).
Pharmacogenetic analysis of TG circadian transcriptome
For full details of pharmacogenetic analysis, see Supplemental Methods. In brief, our analysis was performed similar to the methods of Zhang et al.36 In the Zhang et al. study, drugs of interest (from the World Health Organization and National Institutes of Health) were identified, then genes targeted by these drugs were obtained from the DrugBank database. These data were cross-referenced with cycling genes from their study. In our study, we identified drugs of interest through a systematic review (step 1), then genes targeted by these drugs were obtained from the DrugBank database (step 2), then steps 1 and 2 were cross-referenced with cycling genes from our TG data (step 3). In the first step, one author (M.J.B.) performed a systematic review of recommended medications for migraine, CH, and trigeminal neuralgia using PRISMA guidelines for systematic reviews.37 We searched a single database (PubMed) for treatment guidelines of migraine, CH, and trigeminal neuralgia between 2002 and 2022 (the search term is available in Table S1). Risk of bias within studies was assessed using the Joanna Briggs Institute critical appraisal tool for text and opinion38 (Figure S1), and risk of bias across studies was not assessed. Our outcome measures were the headache type, medication name, and medication rating. In the second step we identified gene targets for each of the medications used for migraine, CH, and trigeminal neuralgia using DrugBank39 for gene targets of each of the medications from step 1, similar to the methods of Zhang et al.36 In the third step, gene targets identified from DrugBank were cross-referenced with our mouse TG data.
Quantification and statistical analysis
Results are presented as mean ± SEM unless otherwise stated. Statistical analyses were conducted using Graph-Pad Prism (version 9, GraphPad Software, Inc.) and JTK_Cycle nonparametric algorithm.40,41 Data were analyzed using Student’s t-test, one-, or two-way analysis of variance followed by Tukey’s post hoc or Holm-Sidak multiple comparison tests. Circadian phase shifts were analyzed using the Rayleigh test and Watson’s U2 test. A value of p < 0.05 was considered statistically significant unless otherwise noted.
RESULTS
Circadian rhythms in ex vivo TG cultures and single neurons from Per2::LucSV circadian reporter mice
Using a paclitaxel model of chemotherapy-induced peripheral neuropathic pain, we previously identified circadian rhythms of pain thresholds in the dorsal root ganglia, the primary sensory neurons for nociception in the body.42 The dorsal root ganglia and TG, however, are known to differ in their gene expression.43 Here, to investigate the circadian clock in the TG, the primary sensory neurons for nociception in the face and intracranial structures, we first monitored the circadian rhythm of the TG from Per2::LucSV circadian rhythm reporter mice.28 Real-time bioluminescence monitoring of TG ex vivo cultures showed a robust circadian rhythm with a period of 24.6 h (Figure 1A,C). Dampening of PER2::LUC bioluminescence oscillation as a result of desynchronization of individual oscillators11 was recovered to a robust circadian oscillation by media change on day 9, showing that media change functions as expected as a zeitgeber and synchronizes oscillation, and that tissue death is not the cause for the dampened oscillation (Figure S2). These results suggest a persistent, self-sustained PER2::LUC rhythm in the TG. Next, we performed cell dissociation in ex vivo TG cultures and observed strong and consistent circadian reporter rhythms with a period of 24.4 h (Figure 1B,C). To further investigate the cell-autonomous clock in TG sensory neurons, we carried out single-cell bioluminescence recordings in conjunction with neuron-specific labeling with AAV2-hSyn-EGFP such that EGFP was expressed in neurons under the human synapsin promoter. We found robust circadian rhythms in individual TG neurons with an average period of 24.7 h (Figure 1D). Next, we examined the expression of the core clock proteins BMAL1, CRY2, and REV-ERBα in the TG by immunostaining. TG tissue sections collected at ZT8 were double-stained with the neuronal marker NeuN (Figure 1E) and the glial marker GS (Figure 1F). The results revealed that BMAL1, CRY2, and REV-ERBα co-localize with both NeuN-positive cells and GS-positive cells, indicating core clock proteins are expressed in both neurons and satellite glial cells (Figure 1E,F). Taken together, these findings reveal a robust cell-autonomous circadian clock in the TG.
FIGURE 1.
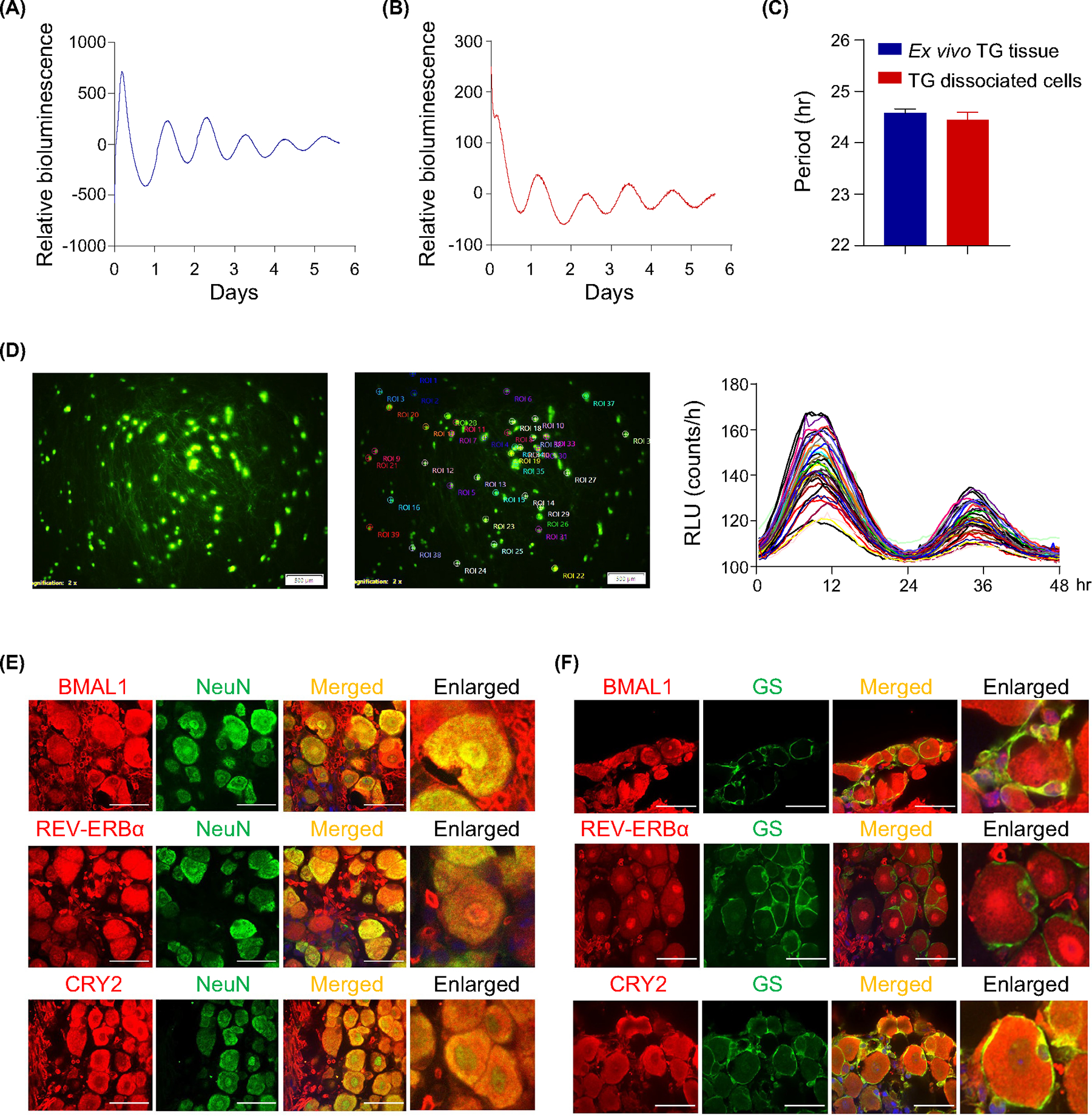
Robust circadian rhythms in trigeminal ganglion (TG). (A,B) Representative bioluminescence recordings of TG ex vivo cultures (A), and TG dissociated primary cultures (B), from Per2::LucSV mice. (C) Period calculations for TG cultures from TG ex vivo cultures (n = 10) and TG dissociated primary cultures (n = 3). (D) Dissociated TG primary culture cells from Per2::LucSV mice. Left panel: Green fluorescent protein images tracking neurons from AAV2-hSyn-EGFP virus (Addgene plasmid #50465) transduced primary cultures. Middle panel: LOI (locus of interest) indicated. Right panel: Single-cell bioluminescence recordings from marked neurons, in relative luminescence units (RLUs). Each line represents a single TG neuron. Circadian period was determined by JTK cycle. (E,F) Expression of core clock proteins BMAL1, CRY2, and REV-ERBα in TG neurons at zeitgeber time (ZT)8 and their overlap with the neuronal marker NeuN (left panels, ×60 magnification) (E), and satellite glial marker GS (right panels, ×60 magnification) (F), (scale bar: 10 μm).
Rhythmic pain responses require the circadian clock
To investigate a potential diurnal pain response, we used the established NTG headache model as an experimental paradigm for trigeminovascular-mediated pain.31,44–46 Intraperitoneal NTG injection (10 mg/kg) every other day at ZT4 increased hindpaw mechanical sensitivity as evidenced by the elevated pain sensitivity (decreased mechanical threshold) in basal and post-injection responses whereas the vehicle showed no effect (Figure 2A). In accordance, CGRP,47,48 which has been shown to increase in the TG during the NTG-induced chronic pain stage,34 was significantly increased in the TG on day 9 after the first injection of NTG (Figure 2B). Therefore, we next examined the diurnal pattern of pain sensitivity during one circadian cycle (Day 11: ZT2, ZT8, ZT14, ZT20, Day 12: ZT2 for hindpaw sensitivity and Day 10: ZT14, Day11: ZT2 for cephalic sensitivity) under normal light and red safety light (for light and dark periods respectively) on Day 11 (Figure 2C) and Day 10 (Figure 2D) after the first injection of NTG. Importantly, we found a circadian alteration of mechanical thresholds in NTG-treated mice; specifically, during the daytime mice showed significantly increased hindpaw and cephalic sensitivity (decreased mechanical thresholds) compared to the nighttime (Figure 2C,D). In contrast, vehicle-treated mice did not show significant threshold changes. These results together indicate a diurnal rhythm of headache pain hypersensitivity in the NTG mouse model.
FIGURE 2.

Nitroglycerin (NTG)-induced diurnal alteration in pain threshold was abolished from Per1/2 dKO mice. (A) Male C57BL/6J mice were treated every other day for 9 days with NTG (10 mg/kg ip) or 0.9% saline control (Cntl). Basal responses (before NTG) and post-injection responses (2 h after NTG injection) are shown (n = 9, significance was determined by t-test between Cntl and NTG on each day, **p < 0.01, ****p < 0.0001). (B) Real-time qPCR analysis of Cgrp in trigeminal ganglion (TG) tissue from Cntl (blue) and NTG (red) injected mice (n = 4/group, t-test, **p < 0.01). (C) Basal mechanical pain thresholds were measured at zeitgeber time (ZT)2, ZT8, ZT14, ZT20, and ZT2 11 days after the first NTG administration (n = 9/group); one-way ANOVA analysis, nonsignificant (NS) relative to Cntl (blue), ****p < 0.0001 relative to NTG (red) in left panel; and similarly by t-test, NS and ****p < 0.0001 for Cntl and NTG, respectively, in right panel. (D) Basal cephalic responses were measured at ZT14 10 days and ZT2 11 days after the first NTG administration (n = 14/group); 2-way repeated measures ANOVA analysis, significant effect of treatment (p < 0.001) and treatment × time interaction (p = 0.047). Holm-Sidak multiple comparisons: **p < 0.01, ***p < 0.001 relative to vehicle, #p < 0.05 relative to ZT14. (E) Male Per1/2dKO and wild-type (WT) mice (C57BL/6J) were treated every other day for 9 days with NTG (10 mg/kg ip, blue for C57BL/6J and red for Per1/2dKO) or 0.9% saline control. Basal responses (before NTG) and the post-injection responses (2 h after NTG injection) are shown (n = 7–8/group); the significance was determined by t-test between WT (NTG) and Per1/2 dKO (NTG) mice in each day. (F) Basal mechanical pain thresholds were measured during the daytime (light on; ZT2 and ZT8) and nighttime (light off; ZT14 and ZT20) 2 days after the last NTG administration (n = 5–9/group); one-way ANOVA analysis, ****p < 0.0001 relative to WT (C57BL/6J) NTG (blue), NS relative to Per1/2dKO NTG (red) in left panel; and similarly by t-test, ****p < 0.0001 and NS for WT and Per1/2dKO, respectively, in right panel. All data are presented as mean ± SEM.
Next, to examine the role of the clock in rhythmic headache pain, we used a genetic mouse model with arrhythmic locomotor behavior, namely Per1/2 dKO mice, and measured NTG-induced acute and chronic pain responses.49 During repeated NTG injections, Per1/2 dKO mice showed higher hindpaw mechanical thresholds than WT mice on Days 1 and 3, although this difference disappeared on Day 5 (Figure 2E). Importantly, the diurnal rhythm of mechanical thresholds in WT mice (increased during the day, decreased at night) was abolished in Per1/2 dKO mice (Figure 2F). This result clearly demonstrates the essential role of the clock machinery in the rhythmic pain responses in the chronic NTG headache model.
The TG circadian transcriptome is dynamically remodeled by NTG administration
To further explore the role of the TG molecular clock in regulating rhythmic pain responses, we examined the circadian transcriptome in the collected TG tissues from control and NTG-treated chronic pain mice every 4 h over one circadian cycle and performed RNA-seq (n = 3–4 per time point in each group). All RNA-seq samples were sequenced to a depth of approximately 30 million reads and aligned to the mouse genome. To identify the circadian transcriptome, we used the MetaCycle package in R language.50 In the control TG we found 466 rhythmically expressed genes (REGs),51 which constitute 2.9% (466/16,285) of the total expressed genes in the TG; remarkably, 71.0% (331/466) of the REGs in the control TG lost circadian rhythms of expression in the NTG group, indicating a profound impact on the circadian transcriptome by NTG-induced headache pain (Figure 3A,B and Tables S2 and S3). In comparison, we identified 584 (81.2%, 584/719) de novo REGs in the NTG group that were not cyclic in the control group (and 4.4% or 719/16,285 of the total expressed genes in the TG52), suggesting a surprisingly large-scale NTG-induced reprogramming of REGs (Figure 3A,C, and Tables S2 and S3).
FIGURE 3.
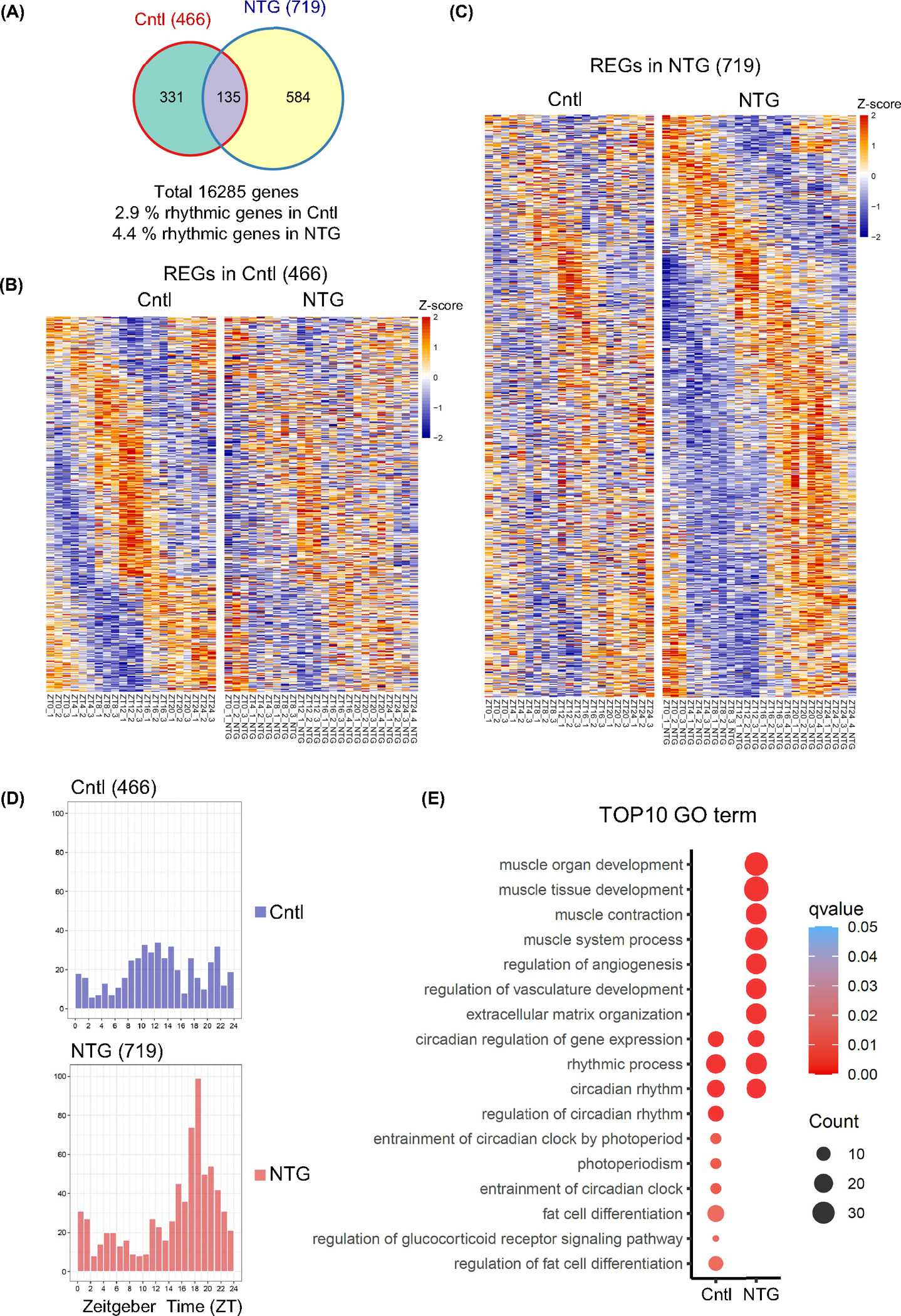
Robust circadian transcriptome in trigeminal ganglion (TG) and reprogramming of transcriptomic landscape by nitroglycerin (NTG)-induced chronic pain model. (A) Venn diagrams of rhythmically expressed genes (REGs) in the control and NTG groups. (B) Heatmap view of control REGs (466) in control (Cntl) and NTG groups. (C) Heatmap view of NTG REGs (719) in Cntl and NTG groups. Each gene is represented as a horizontal line, ordered vertically by phase determined by MetaCycle. (D) Histograms showing peak phase distributions of REGs in Cntl and NTG-treated groups. The control group showed a unimodal distribution of REG at ZT14, while the NTG group shifted to ZT18 (Rayleigh test, p < 0.05 and Watson’s U2 test, p < 0.05). (E) Top10 enriched gene ontology (GO) terms for REGs in control and NTG groups. Color gradient ranging from red to blue corresponds to the order of increasing q-value, and the size of the bubbles indicates the gene count of each GO term.
We analyzed phase distribution of REGs in the control and NTG groups and observed robust effects of NTG on the circadian phase. While the control group showed a strong peak of REG expression at ZT12, the NTG group shifted to ZT18 (Figure 3D). Next, we performed gene ontology term analysis53,54 to analyze the biological processes enriched for control and/or NTG REGs. Both groups shared the enrichment of genes involved in circadian gene expression and rhythmic processes (Figure 3E). Glucocorticoid signaling was enriched in control REGs; glucocorticoids such as prednisone and dexamethasone are not only strong circadian modulators at the molecular level, but also guideline-recommended treatments for CH and migraine.55–57 In the NTG group we found strong enrichment related to vasculature development and muscle contraction, consistent with the NTG’s role as a vasodilator.58 Circadian core clock genes, including Bmal1, Cry1, and Rev-erbb, were found to oscillate in both control and NTG groups with only moderate changes in amplitude and phase (Figure 4A). We also found that TG circadian rhythms from both control and NTG-treated chronic pain mice showed similar period lengths and amplitudes (Figure S3). In contrast to the core clock genes, other REGs shared between the control and NTG groups (135) displayed significant phase alterations, including both effect size and direction, in response to NTG treatment (Figure S4). Interestingly several migraine and CH susceptibility genes4 showed a circadian rhythm of expression including Ccm2l and Jag1, and NTG altered their circadian expression (Figure 4B and Table S4). Furthermore, in an examination of 66 TG neuropeptides and receptors reported previously,59,60 we observed 3 REGs (GABRA2 and OPRD1 in the control TG group and OPRM1 in the NTG group). These results indicate a marked circadian remodeling by NTG and support the notion that circadian oscillation of core clock genes is resilient to perturbations whereas clock output genes are more susceptible.61,62
FIGURE 4.

Effect of nitroglycerin (NTG) on circadian rhythm of core clock and headache susceptibility genes. (A) Core clock gene expression in trigeminal ganglion (TG) control (blue) and NTG-induced chronic pain (red) groups from RNA-Seq (two-way ANOVA analysis, ****p < 0.0001 for Cry2, ***p < 0.001 for Clock and Rev-erbb, **p < 0.01 for Cry1, *p < 0.05 for Dbp). (B) Headache susceptibility gene expression in TG control (blue) and NTG-induced chronic pain (red) groups from RNA-Seq. Data shown are mean ± SEM (n = 3–4). For full list of headache susceptibility genes see Table S4 (two-way ANOVA analysis, ***p < 0.001 for Ccm2l, *p < 0.05 for Itgb5, jag1 and Plce1). (C) Enriched pathways (Metascape) in control (Cntl; left) and NTG (right) rhythmically expressed genes (REGs) based on their expression phases. Top pathway in de novo NTG-induced REGs are indicated in orange. The separate phases of the REGs shown in Figure 3D are represented in rose plots.
Extending the above analysis in order to understand time-specific events, we further divided REGs of the control and NTG groups according to their peak time and performed Metascape enrichment analysis.63 Interestingly, the control and NTG groups showed distinct phase-specific enriched pathways (Figure 4C). In the control group, enrichment was observed in response to redox state (strongest at ZT0–4), negative regulation of inflammation (at ZT4–8), circadian rhythm (at ZT8–12), carboxylic acid transport (at ZT12–16), negative regulation of fatty acid transport (at ZT16–20), and microtubule cytoskeleton organization involved in mitosis (at ZT20–24). In contrast, enrichment in the NTG group was seen in dilated cardiomyopathy (strongest at ZT0–4), adenylate cyclase inhibiting GPCR signaling (at ZT4–8), circadian rhythm (at ZT8–12), microtubule depolymerization (at ZT12–16), muscle system process (at ZT16–20), and ER localized multiprotein process (at ZT20–24). Interestingly, of de novo NTG-induced REGs, sensory perception of pain was the top pathway for both ZT0–4 and ZT4–8 (Figure 4C), suggesting a molecular mechanism for the increased pain sensitivity during the day (Figure 2). These results together revealed a predominant effect of circadian timing on NTG-induced transcriptome remodeling.
Circadian TD-DEGs
We next sought to identify circadian TD-DEGs, namely genes that are differentially expressed between the two groups at each of 6 circadian time points (Figure S5). Interestingly, we observed a remarkable circadian time-dependent gene enrichment where 90.6% of all DEGs were found at the consecutive time points ZT0 and ZT20 (Figure 5A). Functional enrichment analysis of gene clusters in TD-DEGs provided additional insights (Figure 5B). Specifically, ZT20 showed strong enrichment in extracellular matrix organization that may be a functional prerequisite for neurotransmitter transport/secretion and synapse signaling at ZT0.64,65 Our analysis therefore identified a pivotal circadian time window (ZT20) where NTG elicits a functional cascade of differential gene expression.
FIGURE 5.
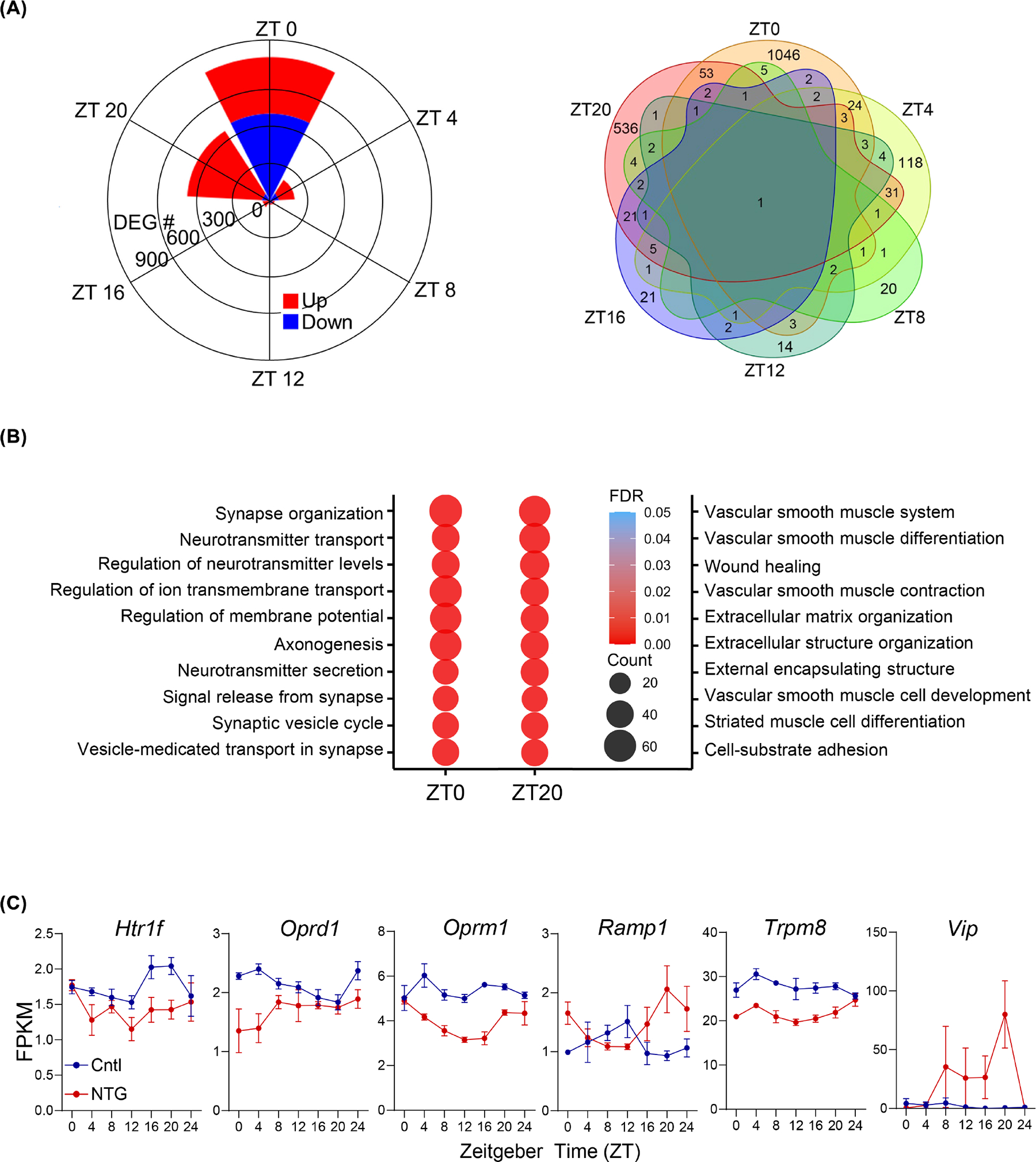
Time-dependent differentially-expressed genes (DEGs) in the trigeminal ganglion (TG) between control (Cntl) and nitroglycerin (NTG)-induced chronic pain groups. (A) Circular plot (left panel) and Venn diagram (right panel) showing the number of DEGs between the Cntl and NTG at each time point and their overlap. Left panel: Up- and down-regulated genes in the NTG group are represented in red and blue, respectively. A total of 1935 DEGs between the Cntl and NTG groups were found. (B) Top10 enriched gene ontology (GO) terms in DEGs between Cntl and NTG groups at ZT0 and ZT20. (C) Expression of neuropeptides and their receptors in TG Cntl (blue) and NTG-induced chronic pain (red) groups from RNA-Seq (two-way ANOVA analysis, *p < 0.05 for Oprm1). All data are presented as mean ± SEM. Additional neuropeptides and receptors can be found in Figure S6.
Several neuropeptides and their receptors also displayed pronounced time-dependent differential expression in the NTG group compared to the control, including vasoactive intestinal peptide or VIP, a neuropeptide important in CH,66,67 displaying a 50-fold increase in gene expression at ZT20 (Figure 5C). Several receptors known to play a role in migraine also showed time-dependent differential expression, including OPRD1 (the delta opioid receptor),68 the RAMP1 component of the CGRP receptor,48 and the TRPM8 receptor69 (Figure 5C). Other neuropeptides and receptors implicated in headache disorders, including pituitary adenylate cyclase-activating peptide-38 (PACAP) and its receptors70 and serotonin 1 receptors, also exhibited differential gene expression in the TG (Figure S6).
Pharmacogenetic analysis of the TG circadian transcriptome
To perform pharmacogenetic analysis for the crosstalk between the genetic network and medication effects, we identified shared genes between the TG circadian transcriptome and target genes of medications used to treat migraine, CH, and trigeminal neuralgia. We chose to add trigeminal neuralgia because of its direct relationship to trigeminal nerve-mediated pain and because of previously reported daily cycles in trigeminal neuralgia.26 First, we carried out a systematic review of guideline-recommended medications for migraine, CH, and trigeminal neuralgia. Ultimately 28 studies were included in the qualitative synthesis: 19 migraine, 2 CH, 6 trigeminal neuralgia, and 1 for both migraine and CH (for flow diagram see Figure S7). Next, using a methodology similar to a prior circadian gene expression atlas of the best-selling medications,36 we searched DrugBank39 for gene targets of each of the medications used for migraine, CH, and trigeminal neuralgia. We found 103 total medications (90 migraine, 22 CH, and 8 trigeminal neuralgia) that target 200 genes (Table S5).
We identified 10 REGs from our TG transcriptome targeted by migraine, CH, and trigeminal neuralgia medications which are targets for 28 medications: 25 medications treat migraine, 6 treat CH, and 3 treat trigeminal neuralgia (Figure 6, Table S6, and Supplemental Results). The most targeted REG was Htr2a: HTR2A was modulated by 9 different medications (both acute and preventive) which include treatments for all three diseases.
FIGURE 6.

Pharmacogenetics analysis of rhythmically expressed genes (REGs). (A) REGs in the control (Cntl) trigeminal ganglion (TG; top) or nitroglycerin (NTG)-treated (bottom) TG that are targeted by medications used to treat migraine (purple), cluster headache (yellow), and/or trigeminal neuralgia (green). (B) Examples of genes from panel A that either gained (Htr2a, Oprm1, Calr) or lost (Oprd1) rhythmic expression in the NTG-treated TG compared to the Cntl TG. Data for Oprm1 and Oprd1 are the same as the data in Figure 5B (two-way ANOVA analysis, *p < 0.05 for Oprm1). All data are presented as mean ± SEM.
We also identified 29 TD-DEGs targeted by migraine, CH, and trigeminal neuralgia medications (Figure S8, Table S7, and Supplemental Results). These genes are targets for 54 medications that treat migraine, CH, and/or trigeminal neuralgia. Almost all (93%, or 27/29) of TD-DEGs targeted by medications were differentially expressed at ZT0 and/or ZT20.
Our findings demonstrate that several preventive medications for migraine, CH, and trigeminal neuralgia target REGs, and several acute medications target genes that are downregulated at either ZT0 or ZT20 in the headache (NTG) model.
DISCUSSION
Here we demonstrated and characterized a robust circadian clock in the TG using multiple methodologies. Employing Per2::LucSV reporter bioluminescence, we revealed a robust circadian rhythm in ex vivo explants consistent with a previous study.71 Importantly, we found robust circadian rhythms in individual TG neurons and demonstrated a cell-autonomous, self-sustained molecular clock in the TG. We built on these findings by examining dissociated cultures and single cells to show the presence of cell-autonomous clocks in the TG. Immunostaining results revealed core clock proteins (BMAL1, Cry2, and REV-ERBα) in NeuN-positive cells and GS-positive cells, indicating that both neuronal and non-neuronal cells in the TG have a circadian clock. We also unveiled a unique circadian transcriptome in the TG, where 2.9% and 4.4% of the transcriptome are REGs for the control and NTG groups respectively. Remarkably, 331 of the control TG REGs lost circadian rhythmicity in the NTG group, indicating a profound reprogramming of the TG circadian transcriptome by NTG-induced headache pain.4 This level of reprogramming is comparable to that of high fat diet and time-restricted feeding, two experimental conditions known to have profound circadian changes.72,73 In similar RNA sequencing studies with samples collected every 2–4 h for 24 h, high fat diet treatment leads to 72.5%–87.8% differential expression of REGs in the liver and meibomian glands compared to a control diet61,74,75; in time-restricted feeding experiments, 63%–85.1% of all REGs are differentially expressed in a variety of tissues compared to an ad libitum diet.76,77 In our study, a similar 81.2% of all REGs were differentially expressed between the control and NTG groups. Finally, our comprehensive pharmacogenetic analysis showed that many preventive medications for migraine, CH, and/or trigeminal neuralgia target genes that are rhythmically expressed in the TG, while several acute medications for migraine and/or CH target genes that are differentially expressed at specific times points in the TG of NTG-treated mice. Together, our study represents the first to provide an in-depth analysis of the TG clock network and reveals important circadian principles for headache and facial pain disorders.
The circadian behaviors of migraine and CH have long been suspected to be linked to the hypothalamus given its prominent role in primary headache disorders. The finding of a strong circadian clock in the TG, a peripheral component of the headache system, suggests a more broadly distributed circadian headache network than previously thought. This distributed system may explain how a CH attack initially activates the posterior hypothalamus78 but not the anterior hypothalamus (where the suprachiasmatic nucleus is located). Our finding of a peripheral circadian clock in the trigeminovascular system suggests that headache medications with circadian effects may still be effective even if they cannot cross the blood-brain barrier. Furthermore, it could explain why diseases such as trigeminal neuralgia, which can be caused entirely by damage to the trigeminal nerve portion of the TG, can have circadian patterns of pain. While cluster headache is more common in men,79 both migraine and trigeminal neuralgia are more common in women.80,81 Sex differences have been found in circadian behavior (i.e., greater activity precision in males in normal light and greater activity duration in females in 24 h darkness) and in circadian gene expression in tissues (i.e., phase-delays in the pituitary and liver).82 Our cephalic allodynia study used equal numbers of male and female mice and showed phenotypic similarities; however, for hindpaw and ex vivo experiments, our study was limited to male mice. Additional studies are needed in female mice to determine the circadian sex differences in the TG and the NTG model.
In the current study, we demonstrated for the first time the impact of an NTG-induced chronic headache paradigm on the circadian clock. Injection of NTG (10 mg/kg) every other day for 9 days generated a diurnal change in mechanical pain thresholds that was not present in vehicle-injected control mice. This diurnal pattern required an intact circadian transcriptional-translational feedback loop, as arrhythmic Per1/2 dKO mice exposed to the NTG treatment paradigm showed no diurnal rhythm. Interestingly the Per1/2 dKO mice initially showed higher pain thresholds, suggesting an acute antinociceptive role for Per1 and/or Per2.
Our circadian pain responses and TG transcriptome analysis revealed that the time of day is an important variable in behavior and molecular research of the trigeminovascular system. These findings suggest that the NTG chronic headache paradigm is a useful model for examining the circadian rhythm of migraine and CH in both behavior and molecular mechanisms.
Our pharmacogenetic analysis of REGs may guide future chronotherapy for headaches. One aspect of chronotherapy is time-dependent medication delivery: This has been adopted in the treatment of hypertension,83 asthma,84 and other disorders,36 but it has not been extensively investigated for primary headaches. For neurodegenerative disorders, chronotherapy aims to improve proteostasis from CCGs85; along similar lines for headache, it would be sensible to time agonists at the trough of protein expression and antagonists at the peak. For example, NSAIDs are prostaglandin G/H synthase 1 (Ptgs1) inhibitors, and Ptgs1 expression is upregulated in the TG of NTG-induced mice at ZT20. Thus, NSAIDs might be most effective when taken at night. Meanwhile the REG HTR2A has a peak at ZT5, so dihydroergotamine might be most effective when administered in the morning. Thus, some patients might be best treated with two acute medications timed to their gene expression: one when the attacks start during the daytime, another when they start at night.
Another aspect of chronotherapy is the targeting of clock genes and REGs to alter their circadian expression. We found that many preventive headache medications target one of 10 REGs targeted by migraine, CH, and trigeminal neuralgia medications, suggesting the use of these REGs as an interesting screen for a new line of preventive treatments. For example, OPRD1 agonists alleviate pain in animal models of chronic migraine and medication overuse headache,68,86,87 while HTR2A is the canonical target for hallucinogens such as psilocybin that have recently been studied in early-phase trials of migraine and CH.88,89 Our data would predict that chronotherapy with OPRD1 agonists and the HTR2A agonist psilocybin would be useful preventives for migraine or CH.
CONCLUSION
Our study demonstrated robust circadian rhythms in a mouse model of headache at the behavioral, transcriptomic, and pharmacogenetic levels. Future studies should explore the contributions of specific genes, brain areas, and medications on the circadian pattern of pain in this model.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Hyun-Kyoung Lee and Dr. Min-Hee Yi for expert advice on immunostaining.
FUNDING INFORMATION
This work is in part supported by NIH/NIA (RF1AG061901, R01AG065984) to Z.C., The Welch Foundation (AU-2127–20220331) and NIH/NIGMS (R01GM114424, R35GM145232–01) to S.-H.Y., and the Will Erwin Headache Research Foundation to M.J.B.
Abbreviations:
- CCG
clock-controlled genes
- CGRP
calcitonin gene related peptide
- CH
cluster headache
- GS
glutamine synthetase
- NTG
nitroglycerin
- Per1/2dKO
Per1/2 double knockout
- REG
rhythmically expressed gene
- RT-PCR
reverse transcription polymerase chain reaction
- TD-DEG
time-dependent differentially-expressed gene
- TG
trigeminal ganglion
- WT
wild-type
- ZT
zeitgeber time
Footnotes
CONFLICT OF INTEREST STATEMENT
Amynah A. Pradhan has a research contract with Lundbeck for work unrelated to this study. Mark J. Burish was an unpaid medical advisor for Praxis Precision Medicines (in lieu of compensation a fee was paid to the UTHealth Houston), was an unpaid consultant for Beckley Psytech Limited (in lieu of compensation a donation was made to the Will Erwin Headache Research Foundation), and was a site investigator for a cluster headache clinical trial funded by Lundbeck. Chorong Han declares no disclosures, Ji Ye Lim declares no disclosures, Nobuya Koike declares no disclosures, Sun Young Kim declares no disclosures, Kaori Ono declares no disclosures, Celia K. Tran declares no disclosures, Elizaveta Mangutov declares no disclosures, Eunju Kim declares no disclosures, Yanping Zhang declares no disclosures, Lingyong Li declares no disclosures, Kazuhiro Yagita declares no disclosures, Zheng Chen declares no disclosures, and Seung-Hee Yoo declares no disclosures.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Burish MJ, Chen Z, Yoo SH. Cluster headache is in part a disorder of the circadian system. JAMA Neurol. 2018;75:783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burish MJ, Chen Z, Yoo SH. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol (Oxf). 2019;225:e13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naber WC, Fronczek R, Haan J, et al. The biological clock in cluster headache: a review and hypothesis. Cephalalgia. 2019;39:1855–1866. [DOI] [PubMed] [Google Scholar]
- 4.Benkli B, Kim SY, Koike N, et al. Circadian features of cluster headache and migraine: a systematic review, meta-analysis, and genetic analysis. Neurology. 2023;100:e2224–e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fourier C, Ran C, Zinnegger M, et al. A genetic CLOCK variant associated with cluster headache causing increased mRNA levels. Cephalalgia. 2018;38:496–502. [DOI] [PubMed] [Google Scholar]
- 6.Fourier C, Ran C, Sjostrand C, Waldenlind E, Steinberg A, Belin AC. The molecular clock gene cryptochrome 1 (CRY1) and its role in cluster headache. Cephalalgia. 2021;41:1374–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farahani S, Solgi L, Bayat S, et al. RAR-related orphan receptor a: one gene with multiple functions related to migraine. CNS Neurosci Ther. 2020;26:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan KC, Bates EA, Shapiro RE, et al. Casein kinase Iδ mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5:183ra–56, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burish MJ, Han C, Mawatari K, et al. The first-line cluster headache medication verapamil alters the circadian period and elicits sex-specific sleep changes in mice. Chronobiol Int. 2021;38:839–850. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allada R, Bass J. Circadian mechanisms in medicine. N Engl J Med. 2021;384:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duhart JM, Inami S, Koh K. Many faces of sleep regulation: beyond the time of day and prior wake time. FEBS J. 2023;290:931–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz WJ, Klerman EB. Circadian neurobiology and the physiologic regulation of sleep and wakefulness. Neurol Clin. 2019;37:475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buijs RM, Scheer FA, Kreier F, et al. Organization of circadian functions: interaction with the body. Prog Brain Res. 2006;153:341–360. [DOI] [PubMed] [Google Scholar]
- 16.May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115–127. [DOI] [PubMed] [Google Scholar]
- 17.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May A, Schwedt TJ, Magis D, Pozo-Rosich P, Evers S, Wang SJ. Cluster headache. Nat Rev Dis Primers. 2018;4:18006. [DOI] [PubMed] [Google Scholar]
- 19.Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109. [DOI] [PubMed] [Google Scholar]
- 20.Wiggers A, Ashina H, Hadjikhani N, et al. Brain barriers and their potential role in migraine pathophysiology. J Headache Pain. 2022;23(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhoeff NP, Visser WH, Ferrari MD, Saxena PR, van Royen EA. Dopamine D2-receptor imaging with 123I-iodobenzamide SPECT in migraine patients abusing ergotamine: does ergotamine cross the blood brain barrier? Cephalalgia. 1993;13:325–329. [DOI] [PubMed] [Google Scholar]
- 22.Edvinsson L, Warfvinge K. Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia. 2019;39:366–373. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Xu M, Bhuiyan SA, et al. Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron. 2022;110:1806–1821.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Wu Y, Jia S, et al. Single-nucleus transcriptome analysis reveals transcriptional profiles of circadian clock and pain related genes in human and mouse trigeminal ganglion. Front Neurosci. 2023;17:1176654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 26.Knezevic NN, Nader A, Pirvulescu I, Pynadath A, Rahavard BB, Candido KD. Circadian pain patterns in human pain conditions—a systematic review. Pain Pract. 2022;23:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo SH, Kojima S, Shimomura K, et al. Period2 3’-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci U S A. 2017;114:E8855–E8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzenell S, Cabrera JR, North BJ, Leib DA. Isolation, purification, and culture of primary murine sensory neurons. Methods Mol Biol. 2017;1656:229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzenell S, Cabrera JR, North BJ, Leib DA. Correction to: isolation, purification, and culture of primary murine sensory neurons. Methods Mol Biol. 2017;1656:E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgos-Vega CC, Ahn DD, Bischoff C, et al. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia. 2016;36:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelmayer RM, Ossipov MH, Porreca F. An experimental model of headache-related pain. Methods Mol Biol. 2012;851:109–120. [DOI] [PubMed] [Google Scholar]
- 34.Moye LS, Siegersma K, Dripps I, et al. Delta opioid receptor regulation of calcitonin gene-related peptide dynamics in the trigeminal complex. Pain. 2021;162:2297–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han C, Wirianto M, Kim E, Burish MJ, Yoo SH, Chen Z. Clock-modulating activities of the anti-arrhythmic drug moricizine. Clocks Sleep. 2021;3:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 38.McArthur A, Klugarova J, Yan H, Florescu S. Systematic reviews of text and opinion. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 39.Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes ME, Abruzzi KC, Allada R, et al. Guidelines for genome-scale analysis of biological rhythms. J Biol Rhythms. 2017;32:380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HK, Lee SY, Koike N, et al. Circadian regulation of chemotherapy-induced peripheral neuropathic pain and the underlying transcriptomic landscape. Sci Rep. 2020;10:13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Megat S, Ray PR, Tavares-Ferreira D, et al. Differences between dorsal root and trigeminal ganglion nociceptors in mice revealed by translational profiling. J Neurosci. 2019;39:6829–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei DY, Goadsby PJ. Comprehensive clinical phenotyping of nitroglycerin infusion induced cluster headache attacks. Cephalalgia. 2021;41:913–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moye LS, Pradhan AAA. Animal model of chronic migraine-associated pain. Curr Protoc Neurosci. 2017;80:9.60.1–9.60.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harriott AM, Strother LC, Vila-Pueyo M, Holland PR. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J Headache Pain. 2019;20:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei DY, Goadsby PJ. Cluster headache pathophysiology—insights from current and emerging treatments. Nat Rev Neurol. 2021;17:308–324. [DOI] [PubMed] [Google Scholar]
- 48.Russo AF, Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev. 2023;103: 1565–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng B, Albrecht U, Kaasik K, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. [DOI] [PubMed] [Google Scholar]
- 50.Wu G, Anafi RC, Hughes ME, Kornacker K, Hogenesch JB. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics. 2016;32:3351–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolff CA, Gutierrez-Monreal MA, Meng L, et al. Defining the age-dependent and tissue-specific circadian transcriptome in male mice. Cell Rep. 2023;42:111982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nassini R, Materazzi S, Vriens J, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. [DOI] [PubMed] [Google Scholar]
- 53.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gene OC. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obermann M, Nagel S, Ose C, et al. Safety and efficacy of prednisone versus placebo in short-term prevention of episodic cluster headache: a multicentre, double-blind, randomised controlled trial. Lancet Neurol. 2021;20:29–37. [DOI] [PubMed] [Google Scholar]
- 56.Ailani J, Burch RC, Robbins MS. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021–1039. [DOI] [PubMed] [Google Scholar]
- 57.Evers S, Áfra J, Frese A, et al. Cluster headache and other trigemino-autonomic cephalgias. In: Gilhus NE, Barnes MP, Brainin M, eds. European Handbook of Neurological Management. Blackwell Publishing Ltd; 2011:179–190. [Google Scholar]
- 58.Divakaran S, Loscalzo J. The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J Am Coll Cardiol. 2017;70:2393–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. [DOI] [PubMed] [Google Scholar]
- 60.Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 2019;39:1661–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim E, Yoo SH, Chen Z. Circadian stabilization loop: the regulatory hub and therapeutic target promoting circadian resilience and physiological health. F1000Res. 2022;11:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dankovich TM, Rizzoli SO. The synaptic extracellular matrix: long-lived, stable, and still remarkably dynamic. Front Synaptic Neurosci. 2022;14:854956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117(Pt 3):427–434. [DOI] [PubMed] [Google Scholar]
- 67.Vollesen ALH, Snoer A, Chaudhry B, et al. The effect of pituitary adenylate cyclase-activating peptide-38 and vasoactive intestinal peptide in cluster headache. Cephalalgia. 2020;40:1474–1488. [DOI] [PubMed] [Google Scholar]
- 68.Charles A, Pradhan AA. Delta-opioid receptors as targets for migraine therapy. Curr Opin Neurol. 2016;29:314–319. [DOI] [PubMed] [Google Scholar]
- 69.Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem Nerosci. 2014;5:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vollesen ALH, Ashina M. PACAP38: emerging drug target in migraine and cluster headache. Headache. 2017;57(Suppl 2):56–63. [DOI] [PubMed] [Google Scholar]
- 71.Shirakawa Y, Ohno SN, Yamagata KA, et al. Circadian rhythm of PERIOD2::LUCIFERASE expression in the trigeminal ganglion of mice. Front Neurosci. 2023;17:1142785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Potter GD, Cade JE, Grant PJ, Hardie LJ. Nutrition and the circadian system. Br J Nutr. 2016;116:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato T, Sassone-Corsi P. Nutrition, metabolism, and epigenetics: pathways of circadian reprogramming. EMBO Rep. 2022;23:e52412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan L, Sundaram S, Rust BM, Palmer DG, Johnson LK, Zeng H. Consumption of a high-fat diet alters transcriptional rhythmicity in liver from pubertal mice. Front Nutr. 2022;9:1068350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou S, Liu J, Si H, et al. High-fat intake reshapes the circadian transcriptome profile and metabolism in murine meibomian glands. Front Nutr. 2023;10:1146916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenwell BJ, Trott AJ, Beytebiere JR, et al. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep. 2019;27:649–657.e645. [DOI] [PubMed] [Google Scholar]
- 77.Deota S, Lin T, Chaix A, et al. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab. 2023;35:150–165.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.May A, Goadsby PJ. Hypothalamic involvement and activation in cluster headache. Curr Pain Headache Rep. 2001;5:60–66. [DOI] [PubMed] [Google Scholar]
- 79.Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28:614–618. [DOI] [PubMed] [Google Scholar]
- 80.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. [DOI] [PubMed] [Google Scholar]
- 81.De Toledo IP, Conti Réus J, Fernandes M, et al. Prevalence of trigeminal neuralgia: a systematic review. J Am Dent Assoc. 2016;147:570–576. e572. [DOI] [PubMed] [Google Scholar]
- 82.Kuljis DA, Loh DH, Truong D, et al. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gumz ML, Shimbo D, Abdalla M, et al. Toward precision medicine: circadian rhythm of blood pressure and chronotherapy for hypertension—2021 NHLBI workshop report. Hypertension. 2023;80:503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paudel KR, Jha SK, Allam V, et al. Recent advances in chronotherapy targeting respiratory diseases. Pharmaceutics. 2021;13:2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colwell CS. Defining circadian disruption in neurodegenerative disorders. J Clin Invest. 2021;131(19):e148288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moye LS, Tipton AF, Dripps I, et al. Delta opioid receptor agonists are effective for multiple types of headache disorders. Neuropharmacology. 2019;148:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pradhan AA, Smith ML, Zyuzin J, Charles A. Delta-opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br J Pharmacol. 2014;171:2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schindler EAD, Sewell RA, Gottschalk CH, et al. Exploratory controlled study of the migraine-suppressing effects of psilocybin. Neurotherapeutics. 2021;18:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schindler EAD, Sewell RA, Gottschalk CH, et al. Exploratory investigation of a patient-informed low-dose psilocybin pulse regimen in the suppression of cluster headache: results from a randomized, double-blind, placebo-controlled trial. Headache. 2022;62:1383–1394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


