Abstract
This study aims to evaluate the clinical effects of different blood derivatives on wound healing using network meta‐analysis. PubMed, Embase, OVID, Web of Science, SCOPUS and Cochrane Central were searched to obtain studies about blood derivatives on wound healing until October 2023. R 4.2.0 and Stata 15.0 softwares were used for data analysis. Forty‐four studies comprising 5164 patients were included. The results of network meta‐analysis showed that the healing area from high to low was GF + ORCCB, ORCCB, GF, PRF, Unnas paste dressing, APG, PRP injection, PRP, PRP + thrombin gel, PPP, HPL, CT. The healing time from low to high was PRP + thrombin gel, GF, PRP, PC + K, PC, APG, PRF, CT, Silver sulfadiazine ointment. The number of patients cured from high to low was APG, PRP injection, PRP, Aurix, PRF, Leucopatch, HPL, Antimicrobial Ointment Dressing, CT, 60 μg/cm2 repifermin, 120 μg/cm2 repifermin, AFG, PPP. The order of analgesic effect from high to low was AFG, aminogam gel, PRF, PRP, Oxidised oil, APG, GF, CT. The order of the number of wound infection cases from low to high is APG, 20 μg/cm2 repifermin, 60 μg/cm2 repifermin, PRP, LeucoPatch, CT, PPP, Antiseptic ointment dressing. Healing area: GF + ORCCB had the best effect; Healing time: PRP + thrombin gel took the shortest time. The number of cured patients and the reduction of wound infection: APG has the best effect. Analgesic effect: AFG has the best effect. More studies with large sample sizes are needed to confirm the above findings.
Keywords: blood derivatives, clinical effect, network meta‐analysis, wound healing
Abbreviations
- AFG
autologous fibrin gel
- APG
autologous platelet gel
- APL
autologous platelet lysate
- Aurix
aurix gel
- CT
conventional treatment
- GF
growth factor
- HPL
autologous platelet lysate
- MCMC
Markov chain Monte Carlo
- MD
mean difference
- NOS
Newcastle Ottawa scale
- OR
odds ratio
- ORCCB
oxidised regenerated cellulose and collagen biomaterial
- Ozonated oil
ozone gas dissolved in olive oil
- PC + K
keratinocytes suspended in platelet concentrate
- PC
platelet concentrate
- PG
platelet gel
- PPP
platelet‐poor plasma
- PRF injection
injectable platelet‐rich fibrin
- PRF
platelet‐rich fibrin
- PRG
platelet rich gel
- PRP
platelet‐rich plasma
- PSRF
potential scale reduction factor
- RCT
randomised controlled trial
1. INTRODUCTION
With the acceleration of China's aging population, the number of patients with acute and chronic trauma caused by burns, surgery, diabetes, ulcers and other reasons is increasing. The increasing types and refractory coefficients of wounds have brought unprecedented challenges to clinical researchers. 1 The long‐standing wounds not only affect the quality of life of patients and increase the difficulty of nursing, but also may lead to a variety of complications (pain, electrolyte disorders, osteomyelitis, cancer, etc.), and the serious ones may face amputation or even life‐threatening risks.
Wound healing is a complex biological process that is highly regulated by a variety of growth factors, cytokines, cells and cell matrix. 2 It involves four stages (haemostasis, inflammation, proliferation and remodelling) that occur gradually and overlap with each other. 3 In addition to surgical repair, exogenous growth factors are also one of the effective ways to promote wound healing. Plasma derivatives are rich in high concentrations of platelets, which produce a large number of growth factors after activation. The main products of plasma derivatives in the clinic are platelet‐rich plasma (PRP), platelet‐gel (PG), platelet rich gel (PRG), platelet‐rich fibrin (PRF), growth factor rich plasma (GF), autologous platelet lysate (APL), platelet concentrate (PC), autologous platelet gel (APG) and autologous fibrin gel (AFG), etc. There are many kinds of blood derivatives for the treatment of wound healing, and there is a lack of efficacy comparison between them, which is not conducive to clinical promotion and the selection of the best scheme. This study intends to use a network meta‐analysis to compare the clinical efficacy of different plasma derivatives in the treatment of wound healing, to provide references and evidence for the selection of clinical drugs.
2. MATERIALS AND METHODS
2.1. Literature search
We searched PubMed, EMBASE, OVID, Web of Science, SCOPUS, and Cochrane Central, and manually included references of the retrieved literature as supplements. The retrieval time was from the establishment of each database to October 2023. The search was performed by combining subject words and free words. The retrieval strategy were as follows (‘blood product’ OR ‘plasma derivatives’ OR ‘platelet‐rich plasma’ OR ‘PRP’ OR ‘platelet‐rich in growth factors’ OR ‘GF’ OR ‘platelet rich fabric’ OR ‘PRF’ OR ‘concentrated growth factor’ OR ‘CGF’) AND (‘healing of wound’ OR ‘wound healing’ OR ‘wound’ OR ‘surgery’ OR ‘burn’).
2.2. Document selection criteria
2.2.1. Inclusion criteria
(1) The types of studies were RCT and cohort study; (2) The language is limited to English; (3) The study subjects were wounds caused by ulcers, burns, surgery, tooth extraction and other reasons, without limiting the age and gender of patients; (4) The intervention type was that the experimental group was mostly treated with blood derivatives alone or combined with other measures, without limiting the intervention time, and the control group was mostly treated with conventional/standard nursing. The experimental group and the control group should be consistent except for the inconsistent intervention measures.
2.2.2. Exclusion criteria
(1) Repeatedly published literature, reviews, meta‐analyses, treatises and conferences; (2) Literature on relevant outcomes could not be extracted; (3) Literature with errors; (4) The literature with ≤5 patients in the treatment group or the control group.
2.3. Literature quality evaluation
2.3.1. Cochrane risk of bias assessment
The Cochrane bias risk assessment tool was used for evaluation, 4 including random allocation method, allocation scheme concealment, whether the research object and scheme implementer were blinded, whether the outcome evaluator was blinded, data integrity, selective reporting and other sources of bias. Each RCT included was evaluated as ‘low‐risk’, ‘unclear’ and ‘high‐risk’ from the above seven aspects.
2.3.2. Newcastle Ottawa scale bias risk assessment
Cohort study: the Newcastle Ottawa scale (NOS) 5 was used to evaluate the quality of the five included cohort studies, including patient selection (four items, full score 4), comparability between groups (one item, full score 2) and exposure factors (three items, full score 3). A total score of ≥6 points was considered to be of high quality. Two researchers independently evaluated the literature. In case of disagreement, the third researcher ruled.
2.4. Literature screening and data extraction
Two researchers independently screened the literature according to the inclusion and exclusion criteria, extracted the data and cross‐checked them. According to the included literature, the corresponding data were extracted, mainly including the basic information of the study (research title, first author, publication time, country), the information needed for Cochrane and NOS risk of bias assessment, the characteristics of the study subjects (type of trauma, age, gender), grouping, intervention measures, follow‐up time and various outcome indicators: ① healing area; ② wound healing time; ③ number of wound healing cases; ④ pain score; ⑤ number of wound infection cases. In case of different opinions, discuss with each other to reach an agreement. If no consensus can be reached, the third investigator will be consulted.
2.5. Statistical analysis
Review Manager (version 5.3) was used to draw the risk bias chart, and the graph package of R (version 4.03) software was used to draw the network evidence chart of intervention measures. Dichotomous variables were expressed by odds ratio (OR); Mean difference (MD) was used for continuous variables. If heterogeneity was low (I 2 < 50%), the fixed‐effects model was used. Otherwise, the random‐effects model was employed. The gemtc package of R 4.1.0 software was used for Bayesian network analysis, and the probability ranking of the interventions was performed. Markov chain Monte Carlo (MCMC) random/fixed effects model was used for analysis, and the parameters were set; The initial value is set to 2.5, and the number of simulated annealing and iteration for four chains is 20 000 and 50 000. Evaluate the potential scale reduction factor (PSRF). When the PSRF is close to 1 (1.00–1.05), it indicates that the convergence of the iteration is good. Otherwise, it needs to increase the number of simulations and re‐evaluate. Finally, the comparison correction funnel plot was drawn with Stata SE (version 15.0) software to identify whether there was a small sample effect and publication bias in the results.
3. RESULTS
3.1. Results of literature search
The computer system searched PubMed (n = 120), Embase (n = 6561), OVID (n = 2232), Web of science (n = 5184), SCOPUS (n = 3011) and Cochrane CENTRAL (n = 2142) databases. After removing duplicates, 12 025 records remained. Twenty‐six references were retrieved from the references as supplements. According to the inclusion and exclusion criteria, 11 898 records were preliminarily excluded by browsing the titles and abstracts, and 153 records were obtained. After reading the full text, 42 records were finally screened, as shown in Figure 1.
FIGURE 1.
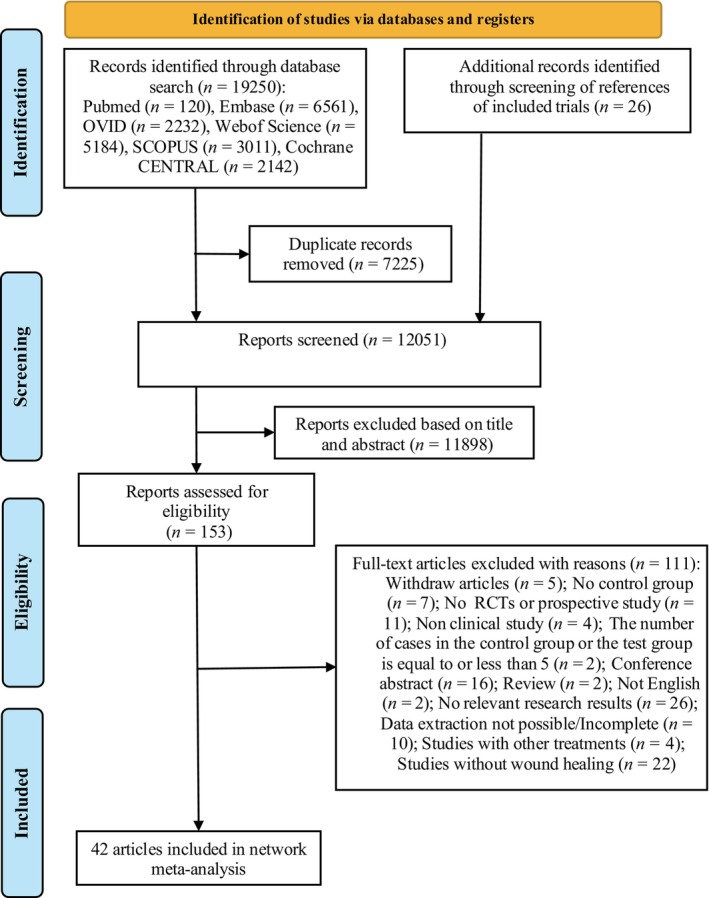
Flow chart of records search.
3.2. Basic characteristics of the included literature
Among the 42 records, 38 were RCTs and four were cohort studies, including 5164 patients. In two studies, 55 patients were randomly treated with PRF or PRP on one side and conventional treatment on the remaining side. Among them, seven were from Italy, five each from Egypt and the United States, four from China, three each from Iran, India, and Spain, two from Turkey and one each from the remaining countries (Denmark, Czech Republic, Korea, Lithuania, United Kingdom, France, Germany, Switzerland, Greece and Australia), as shown in Table 1.
TABLE 1.
Basic characteristics of literature.
| Study | Year | Study type | Country | The type of disease | Case | Median age/years | Intervention measures | Follow up (day) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (T/C) | (Male/female) | T | C | |||||||
| Amse 6 | 2021 | RCT | Egypt | Palatal wound healing | 13/13/13 | NA | 18–60 | T1: PRF, T2: ozonated oil | CT | 28 |
| Capion 7 | 2021 | RCT | Denmark | Total hip arthroplasty | 16/17 | 22/11 | T: 65.6 ± 8.5; C: 68.9 ± 7.1 | PRP | CT | 28 |
| Malekpour Alamdari 8 | 2021 | RCT | Iran | Diabetic foot ulcers | 43/47 | 56/34 | T: 56.3 ± 7.1; C: 56.7 ± 7.2 | PRP | Silver sulfadiazine ointment | 180 |
| Vaheb 9 | 2021 | RCT | Iran | Burn injury | 33/33 | 17/16 | 33.10 ± 2.60 | PRF | CT | 15 |
| Elbarbary 10 | 2020 | RCT | Egypt | Chronic venous leg ulcer | 30/30/30 | 72/18 | T1: 45.4 ± 9.35 (22–60); T2: 43.4 ± 13 (25–61); C: 41.80 ± 13.3 (23–66) | T1: PRP, T2: PRP injections | CT | 365 |
| Elsaid 11 | 2020 | RCT | Egypt | Non‐healing diabetic foot ulcers | 12/12 | 14/10 | T: 54.7 ± 6.6; C: 55.6 ± 6.5 | PRP | CT | 140 |
| Kiziltoprak 12 | 2020 | RCT | Turkey | Palatal wound healing | 12/12/12 | 9/27 | T1: 28.92 ± 9.66; T2: 33.25 ± 10.97; C: 32.08 ± 9.46 | T1: PRF, T2: AFG | CT | 90 |
| Lektemur Alpan 13 | 2020 | RCT | Turkey | Subepithelial connective tissue wound healing | 20/20 | 19/21 | T: 30.6 ± 6.45; C: 30.89 ± 6.92 | PRF | CT | 7 |
| Slaninka 14 | 2020 | RCT | Czech Republic | Trophic (vascular, diabetes) 17; Trauma 3; Burn injury 1 | 20/20 | 9/15 | Male: 60.44 (18–91), Female: 70.13 (55–84) | PRP | CT | 33 |
| Xie 15 | 2020 | RCT | China | Diabetic sinus tract wounds | 25/23 | 27/21 | T: 60.50 ± 8.27 (49–81); C: 61.10 ± 7.90 (48–80) | APG | CT | 56 |
| Yuvasri 16 | 2020 | RCT | India | Chronic venous leg ulcers | 10/10 | NA | NA | PRF | Unna's paste dressing | 28 |
| De Angelis 17 | 2019 | Prospective study | Italy | Chronic ulcers | 182/182 | NA | 20–89 | PRP | CT | 120 |
| Goda 18 | 2019 | RCT | Egypt | Diabetic foot ulcers | 25/25 | 30/20 | T: 56.88; C: 55.8 | PRP | PPP | 140 |
| Gude 19 | 2019 | RCT | US | Chronic diabetic foot ulcers | 66/63 | 100/29 | T: 64.7; C: 66.9 | Aurix | CT | 84 |
| Jeong 20 | 2019 | RCT | Korea | Ulcers | 7/7 | 12/2 | T: 72.57 ± 7.74; C: 71.57 ± 5.41 | PRP | CT | 28 |
| Rainys 21 | 2019 | RCT | Lithuania | Leg ulcers | 35/34 | 35/34 | T: 62.23 ± 14.72; C: 68.01 ± 14.89 | PRP | CT | 56 |
| Game 22 | 2018 | RCT | UK | Diabetic foot ulcers | 137/132 | 217/52 | T: 61.9 ± 11.4; C: 62.0 ± 11.9 | LeucoPatch | CT | 140 |
| Guazzo 23 | 2018 | RCT | Italy | Wound healing after mandibular third molar extraction | 62/66 | 46/82 | T: 46.9 ± 6.4; C: 48.5 ± 6.1 | Aminogam gel | CT | 14 |
| Singh 24 | 2018 | Prospective study | India | Diabetic foot ulcers | 29/26 | 34/21 | T: 53.76 ± 10.38 (30–82); C: 55.69 ± 10.35 (35–75) | PRP | CT | 28 |
| Yeung 25 | 2018 | RCT | China | Burn injury | 15/12 | 18/9 | T: 45.27 ± 15.82; C: 46.00 ± 14.03 | PRP | CT | 21 |
| Ahmed 26 | 2017 | RCT | Egypt | Diabetic foot ulcers | 28/28 | 38/28 | T: 43.2 ± 18.2; C: 49.8 ± 15.4 | PRP | Antiseptic ointment dressing | 90 |
| Escamilla Cardenosa 27 | 2017 | RCT | Spain | Venous ulcers | 55/47 | 31/71 | T: 64.09 ± 13.72; C: 64.20 ± 16.26 | GF | CT | 168 |
| Hersant 28 | 2017 | RCT | France | Necrotizing soft tissue infections | 14/13 | 13/14 | T: 54.8 ± 19.8; C: 57.7 ± 15.8 | PRP + thrombin gel | CT | 8 |
| Samani 29 | 2017 | RCT | Iran | Free gingival graft's donor site wound healing | 10/10 | NA | 20–45 | PRP | CT | 42 |
| Somani 30 | 2017 | RCT | India | Chronic venous leg ulcers | 9/6 | NA | NA | PRF | CT | 28 |
| Volpe 31 | 2017 | RCT | Italy | Diabetic foot ulcers | 10/10 | 13/7 | T: 72.8 ± 8.7; C: 72.5 ± 10.3 | APG | CT | 30 |
| Yang 32 | 2017 | RCT | China | Lower‐extremity ischemic ulcers | 38/38 | 36/40 | T: 40.1 ± 10.2; C: 43.7 ± 9.8 | APG | CT | 30 |
| Raposio 33 | 2016 | RCT | Italy | Chronic skin ulcer | 16/21 | 21/19 | T: 74.5; C: 70.75 | PRP | CT | 540 |
| Aguirre Anda 34 | 2015 | RCT | Spain | Venous ulcers | 12/11 | 9/14 | T: 68.7 ± 8.9; C: 74.3 ± 9.3 | GF | CT | 56 |
| Li 35 | 2015 | RCT | China | Cutaneous ulcers | 59/58 | 75/42 | T: 61.4 ± 13.1; C: 64.1 ± 9.4 | PRF | CT | 84 |
| Serraino 36 | 2015 | Prospective study | Italy | Sternotomy wound | 422/671 | 694/399 | NA | PRP | CT | 365 |
| Dorge 37 | 2013 | RCT | Germany | Deep sternal wound | 97/99 | 142/54 | T: 68 ± 8.6; C: 67 ± 9.5 | PRP | CT | 30 |
| Guerid 38 | 2013 | RCT | Switzerland | Trauma 13, burn 12, ulcer 6, cutaneous tumours 4, others 10 | 15/15/15 | 25/20 | T1: 46.9 ± 5.3; T2: 45.5 ± 3.9; C: 42.5 ± 3.1 | T1: PC + K; T2: PC | CT | 20 |
| Serra 39 | 2013 | Prospective study | Italy | Transmetatarsal amputation | 26/32 | 46/12 | T: 6 7.5 (43–85); C: 6 3.5 (41–79) | APG | CT | 30 |
| Anitua 40 | 2008 | RCT | Spain | Chronic cutaneous ulcers | 8/7 | 8/7 | T: 45 ± 20; C: 61 ± 16 | GF | PPP | 56 |
| Kakagia 41 | 2007 | RCT | Greece | Diabetic foot ulcers | 17/17/17 | T1: 57 ± 12; T2: 61 ± 9; C: 58 ± 10 | T1: GF + ORCCB; T2: ORCCB | GF | 56 | |
| Driver 42 | 2006 | RCT | US | Diabetic foot ulcers | 40/32 | 59/13 | T: 56.4 ± 10.2; C: 57.5 ± 9.1 | PRP | CT | 84 |
| Robson 43 | 2004 | RCT | US | Chronic venous ulcers | 123/112/117 | 216/136 | T1: 60.9 ± 13.7; T2: 61.8 ± 15.6; C: 61.0 ± 15.4 | T1: 60 μg/cm2 repifermin; T1: 120 μg/cm2 repifermin | CT | 182 |
| Saldalamacchia 44 | 2004 | RCT | Italy | Diabetic foot ulcers | 7/7 | 6/8 | T: 61.1 ± 9.4; C: 58.14 ± 7.8 | APG | CT | 35 |
| Robson 45 | 2001 | RCT | US | Venous ulcers | 31/32/31 | 61/33 | T1: 61 ± 13 (39–86); T2: 59 ± 14 (33–83); C: 59 ± 13 (35–91) | T1: 20 mg/cm2 repifermin; T2: 60 mg/cm2 repifermin | CT | 84 |
| Stacey 46 | 2000 | RCT | Australia | Venous ulcer | 44/42 | 36/50 | T: 72 (35–90); C: 70 (26–92) | HPL | CT | 140 |
| Steed 47 | 1992 | RCT | US | Chronic cutaneous ulcers | 7/6 | 9/4 | T: 58.7 ± 12.4; C: 54.2 ± 12.9 | HPL | CT | 140 |
Abbreviations: AFG, autologous fibrin gel; APG, autologous platelet gel; Aurix, aurix gel; C, control group; CT, conventional treatment; GF, growth factor; HPL, autologous platelet lysate; NA, not applicable; ORCCB, oxidised regenerated cellulose and collagen biomaterial; PC, platelet concentrate; PC + K, keratinocytes suspended in platelet concentrate; PPP, platelet‐poor plasma; PRF, platelet‐rich fibrin; PRP, platelet‐rich plasma; RCT, randomised controlled trial; T, treatment group.
3.3. Quality evaluation of literature
A total of 38 RCTs were included. One literature 27 used the date of birth for numbering and grouping, and one 20 indicated nonrandom grouping, rated as ‘high risk of bias’. Nineteen records 8 , 10 , 12 , 14 , 15 , 16 , 21 , 25 , 26 , 29 , 30 , 31 , 33 , 37 , 43 , 44 , 45 , 46 , 47 only mentioned the use of random grouping, but did not specify the method of random grouping, and was rated as ‘unclear risk of bias’. Seventeen records 6 , 7 , 9 , 11 , 13 , 18 , 19 , 22 , 23 , 28 , 32 , 34 , 35 , 38 , 40 , 41 , 42 mentioned specific methods of randomisation, such as computer‐generated random table or number generators, which were rated as ‘low risk of bias’. Nine records 6 , 7 , 9 , 11 , 13 , 18 , 19 , 22 , 23 , 28 , 32 , 34 , 35 , 38 , 40 , 41 , 42 explicitly mentioned the use of envelope concealment, which was rated as ‘low risk of bias’, and 29 8 , 12 , 13 , 14 , 15 , 16 , 18 , 19 , 20 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 47 did not mention whether it was hidden, which was rated as ‘unclear risk of bias’. Ten records 6 , 9 , 18 , 23 , 38 , 42 , 43 , 45 , 46 , 47 were blinded to subjects and researchers, rated as ‘low risk of bias’, 17 records 6 , 9 , 18 , 23 , 38 , 42 , 43 , 45 , 46 , 47 did not mention whether to use blinding to subjects and researchers, rated as ‘unclear risk of bias’, and 11 records 6 , 9 , 18 , 23 , 38 , 42 , 43 , 45 , 46 , 47 were open‐label, rated as ‘high risk of bias’. Five records 7 , 9 , 13 , 22 , 23 clearly adopted the blind method for the evaluation results, and were rated as ‘low risk of bias’. Thirty‐three records 6 , 8 , 10 , 11 , 12 , 14 , 15 , 16 , 18 , 19 , 20 , 21 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 did not indicate whether the results were evaluated in a blinded manner, and were rated as ‘unclear risk of bias’. Twenty‐eight records 6 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 16 , 18 , 19 , 20 , 25 , 26 , 28 , 29 , 30 , 31 , 32 , 34 , 37 , 38 , 40 , 41 , 44 , 45 , 46 , 47 with no data loss were rated as ‘low risk of bias’, and 10 records 7 , 15 , 21 , 22 , 23 , 27 , 33 , 35 , 42 , 43 with data loss were rated as ‘high risk of bias’. None of the 38 records 7 , 15 , 21 , 22 , 23 , 27 , 33 , 35 , 42 , 43 had selective reports and were rated as ‘low risk of bias’. None of the 38 records 7 , 15 , 21 , 22 , 23 , 27 , 33 , 35 , 42 , 43 indicated whether there were other biases and were rated as ‘unclear risk of bias’, as shown in Figure 2A,B.
FIGURE 2.
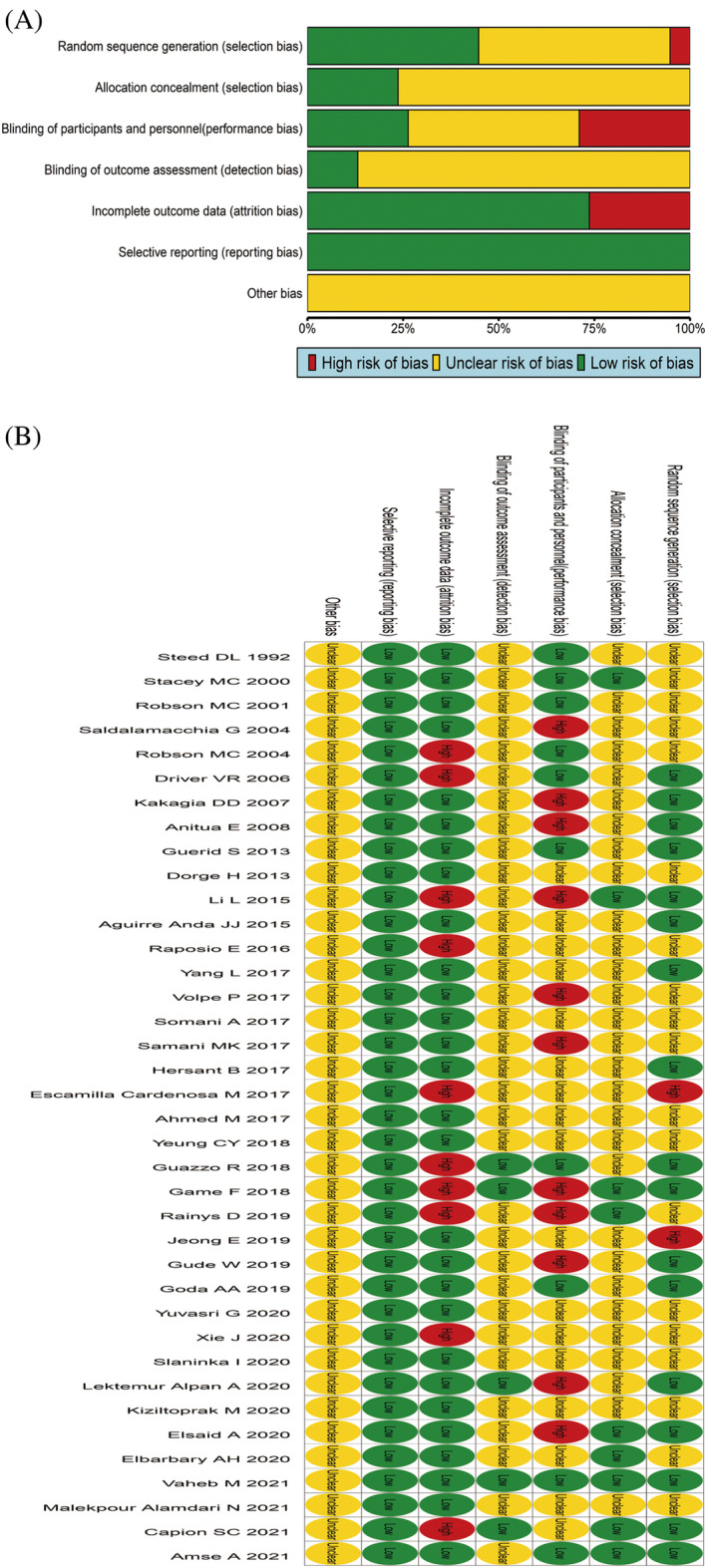
Quality assessment results of the literature included. (A) Risk of bias graph; (B) Risk of bias summary.
The quality of four cohort studies was evaluated by the NOS scoring standard, including 1 with 8 points, 17 2 with 7 points, 24 , 36 and 1 with 6 points. 39 The quality of these four records is relatively good, indicating that the risk of bias is small, as shown in Table S1.
3.4. Healing area
3.4.1. Evidence network
A total of 21 records reported the area of healing, involving 1077 patients, involving 12 dosing regimens, and the network evidence is shown in Figure 3A. The line between the two points represents the evidence of direct comparison between the two drugs, and no line indicates that there is no direct comparison, and the results can be obtained through indirect comparison. The thickness of the line indicates the number of studies using the two drugs in all included studies, and the size of the dot indicates the sample size of the included cases using the drug. The results showed that three closed loops were formed. CT had the largest sample size (n = 447), while PRP and CT had the largest number of studies (n = 8).
FIGURE 3.
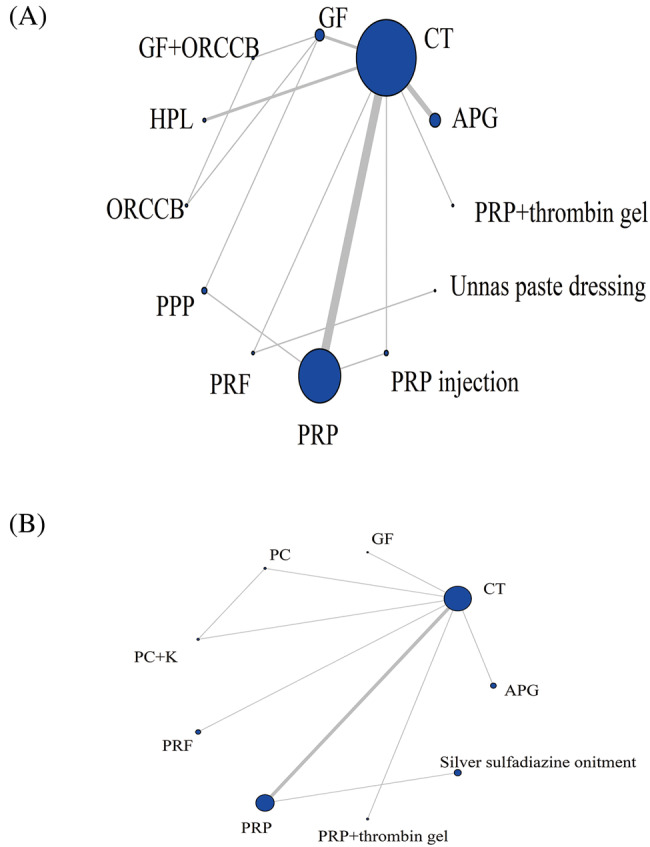
Evidence network charts of healing area (A) and healing time (B). APG, autologous platelet gel; CT, conventional treatment; GF, growth factor rich plasma; HPL, autologous platelet lysate; ORCCB, oxidised regenerated cellulose and collagen biomaterial; PPP, platelet‐poor plasma; PRF, platelet‐rich fibrin; PRP, platelet‐rich plasma.
3.4.2. Results of network meta‐analysis
A total of 21 studies reported on the healing area, involving 12 intervention measures, forming a total of 14 pairwise comparisons. The inconsistency test and node splitting method showed good consistency (I 2 < 50%), and there was no heterogeneity between studies (p > 0.05).
The results of the network meta‐analysis showed that APG, GF, GF + Oxidised regenerated cellulose and collagen biomaterial (ORCCB), ORCCB, PRF and PRP significantly increased the area of wound healing compared to the CT group. GF + ORCCB was significantly larger than Autologous Platelet Lysate (HPL), Platelet‐poor Plasma (PPP), PRP, PRP injection and Unnas paste dressing. GF was significantly higher than HPL, PPP, PRP and PRP injection. ORCCB was significantly greater than PPP, PRP and PRP injection. PRF is significantly greater than PRP. PRP + thrombin gel was significantly smaller than GF, GF + ORCCB and ORCCB. APG was significantly smaller than GF, GF + ORCCB and ORCCB. HPL was significantly smaller than ORCCB and PRF, and there was no statistically significant difference (p > 0.05) compared to other intervention measures, as shown in Table 2.
TABLE 2.
Results of network meta‐analysis of healing area.
| Intervention measure | PRP + thrombin gel | APG | GF | GF + ORCCB | HPL | ORCCB | PPP | PRF | PRP | PRP injection | Unnas paste dressing | CT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRP + thrombin gel | 0 | |||||||||||
| APG | −4.32 (−30.75, 19.85) | 0 | ||||||||||
| GF | −48.13 (−75.4, −21.56) | −43.77 (−62.26, −23.98) | 0 | |||||||||
| GF + ORCCB | −67.62 (−102.5, −33.55) | −63.33 (−91.61, −33.73) | −19.48 (−41.23, 2.25) | 0 | ||||||||
| HPL | 10.68 (−16.06, 37.29) | 14.93 (−2.54, 35.03) | 58.78 (38.18, 80.13) | 78.29 (48.54, 108.9) | 0 | |||||||
| ORCCB | −52.43 (−87.27, −18.72) | −48.14 (−76.06, −18.87) | −4.34 (−25.67, 16.9) | 15.17 (−6.82, 37.21) | −63.07 (−93.4, −33.81) | 0 | ||||||
| PPP | 2.54 (−26.89, 30.97) | 6.82 (−14.36, 29.45) | 50.61 (30.56, 70.68) | 70.11 (40.54, 99.68) | −8.16 (−32.09, 14.92) | 54.94 (25.88, 84.12) | 0 | |||||
| PRF | −29.85 (−65.83, 5.97) | −25.5 (−55.27, 6.01) | 18.25 (−13.51, 50.56) | 37.74 (−0.49, 76.58) | −40.56 (−72.4, −8.78) | 22.5 (−15.44, 61.37) | −32.37 (−65.69, 1.69) | 0 | ||||
| PRP | 0.32 (−24.05, 23.63) | 4.66 (−9.11, 19.67) | 48.43 (31.93, 64.76) | 67.97 (40.57, 95.02) | −10.29 (−27.62, 5.89) | 52.81 (25.93, 79.57) | −2.16 (−19.77, 15.2) | 30.19 (0.32, 59.33) | 0 | |||
| PRP injection | −0.58 (−29.93, 27.97) | 3.7 (−17.33, 26.44) | 47.48 (24.13, 71.07) | 66.99 (35.27, 98.95) | −11.26 (−34.99, 11.77) | 51.82 (20.48, 83.63) | −3.15 (−27.96, 22.03) | 29.27 (−4.7, 62.65) | −0.96 (−19.05, 17.59) | 0 | ||
| Unnas paste dressing | −15.76 (−62.29, 31.06) | −11.31 (−53.18, 31.97) | 32.42 (−10.92, 76.37) | 51.93 (3.6, 100.92) | −26.41 (−69.82, 16.99) | 36.68 (−11.06, 85.67) | −18.22 (−62.68, 26.99) | 14.12 (−15.3, 43.93) | −16.03 (−57.39, 26.06) | −15.11 (−59.72, 29.82) | 0 | |
| CT | 12.84 (−9.57, 35.2) | 17.13 (6.38, 30.31) | 60.93 (46.35, 76.51) | 80.44 (54.47, 107.34) | 2.14 (−12.34, 16.8) | 65.25 (39.77, 91.89) | 10.3 (−7.65, 29.22) | 42.72 (14.51, 70.96) | 12.48 (4.68, 21.41) | 13.41 (−4.57, 32.27) | 28.58 (−12.36, 69.38) | CT |
Abbreviations: APG, autologous platelet gel; CT, conventional treatment; GF, growth factor; HPL, autologous platelet lysate; ORCCB, oxidised regenerated cellulose and collagen biomaterial; PC, platelet concentrate; PPP, platelet‐poor plasma; PRF, platelet‐rich fibrin; PRF injection, injectable platelet‐rich fibrin; PRP, platelet‐rich plasma.
3.4.3. Ranking of intervention efficacy
The order of healing area from high to low is GF + ORCCB > ORCCB > GF > PRF > Unnas paste dressing > APG > PRP injection > PRP > PRP + thrombin gel > PPP > HPL > CT, see Table S2.
3.4.4. Publication bias assessment
The area of healing was assessed for publication bias. The funnel plot suggested that all studies were roughly symmetrically distributed on both sides of the vertical line with x = 0, indicating that there was less possibility of significant publication bias. Not all the dots are located inside the triangle, indicating that there may be a small sample effect, as shown in Figure S1.
3.5. Wound healing time
3.5.1. Evidence network
A total of ten records have reported the healing time of patients, a total of 481 patients, involving nine dosing regimens. The network evidence is shown in Figure 3B. The results showed that a closed loop was formed. CT had the largest sample size (n = 185), while PRP and CT had the largest number of studies (n = 4).
3.5.2. Results of network meta‐analysis
Ten records reported patient healing times involving nine interventions, forming a total of nine pairwise comparisons. The results of the inconsistency test and node splitting method showed good consistency (I 2 < 50%), with no heterogeneity among the studies (p > 0.05). The results of the network meta‐analysis showed that there was no statistical difference among all interventions (p > 0.05), as shown in Table 3.
TABLE 3.
Results of network meta‐analysis of healing time.
| Intervention measures | APG | GF | PC | PC + K | PRF | PRP | PRP + thrombin gel | Silver sulfadiazine onitment | CT |
|---|---|---|---|---|---|---|---|---|---|
| APG | 0 | ||||||||
| GF | 9.16 (−41.29, 59.18) | 0 | |||||||
| PC | 1.78 (−48.31, 52.08) | −7.41 (−57.18, 42.96) | 0 | ||||||
| PC + K | 3.35 (−46.63, 53.33) | −5.84 (−55.81, 44.45) | 1.51 (−34.03, 37.09) | 0 | |||||
| PRF | −0.43 (−50.31, 49.9) | −9.54 (−59.46, 40.9) | −2.15 (−52.66, 47.96) | −3.63 (−53.99, 46.4) | 0 | ||||
| PRP | 4.44 (−35.6, 43.56) | −4.74 (−44.72, 34.95) | 2.67 (−37.57, 42.33) | 1.16 (−39.09, 40.51) | 4.8 (−34.98, 44.23) | 0 | |||
| PRP + thrombin gel | 30.42 (−25.66, 87.2) | 21.23 (−34.74, 77.93) | 28.62 (−27.86, 85.38) | 27.06 (−29.47, 83.9) | 30.8 (−25.75, 87.13) | 26.09 (−21.4, 73.99) | 0 | ||
| Silver sulfadiazine onitment | −20.56 (−74.2, 32.66) | −29.67 (−83.46, 23.59) | −22.29 (−76.14, 30.83) | −23.78 (−77.54, 28.94) | −20.18 (−73.78, 32.98) | −24.98 (−60.47, 10.64) | −50.96 (−110.1, 7.91) | 0 | |
| CT | −4.9 (−40.38, 30.47) | −14.06 (−49.41, 21.38) | −6.64 (−42.27, 28.79) | −8.19 (−43.74, 27.22) | −4.53 (−39.89, 30.86) | −9.34 (−26.93, 8.94) | −35.32 (−79.81, 8.89) | 15.63 (−23.88, 55.66) | 0 |
Abbreviations: APG, autologous platelet gel; CT, conventional treatment; GF, growth factor; PC, platelet concentrate; PC + K, keratinocytes suspended in platelet concentrate; PRF, platelet‐rich fibrin; PRP, platelet‐rich plasma.
3.5.3. Ranking of intervention efficacy
The order of healing time from low to high is PRP + thrombin gel <GF < PRP < Keratinocytes Suspended in Platelet Concentrate (PC + K) < PC < APG < PRF < CT < Silver sulfadiazine ointment, as shown in Table S3.
3.5.4. Publication bias assessment
Evaluate publication bias on healing time and use Stata SE15.0 for comparison correction funnel plot. The funnel plot shows that all records are roughly symmetrically distributed on both sides of the x = 0 vertical line, indicating a small possibility of significant publication bias. Some dots are scattered outside the triangle, indicating a possible small sample effect, as shown in Figure S1.
3.6. Number of wound healing cases
3.6.1. Evidence network
A total of 15 records have reported the number of patients who have fully recovered, with a total of 1553 patients involved in 13 medication regimens. The network evidence is shown in Figure 4A. The results showed the formation of three closed loops. CT had the largest sample size (n = 650), while PRP and CT had the largest number of studies (n = 5).
FIGURE 4.
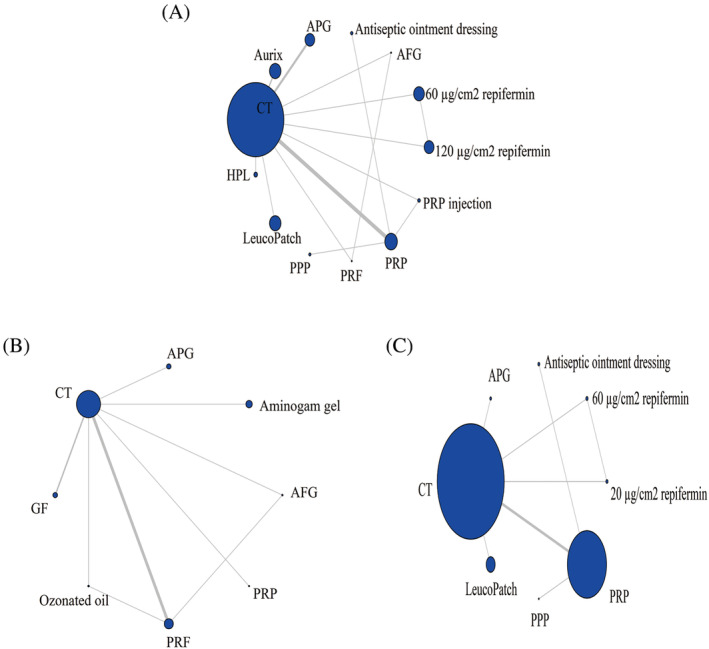
Evidence network charts of wound healing cases (A), pain score cases (B) and wound infection cases (C). AFG, autologous fibrin gel; APG, autologous platelet gel; CT, conventional treatment; GF, growth factor rich plasma; HPL, autologous platelet lysate; PPP, platelet‐poor plasma; PRF, platelet‐rich fibrin; PRP, platelet‐rich plasma.
3.6.2. Results of network meta‐analysis
Fifteen records have reported the number of patients who have recovered, involving 13 intervention measures, forming a total of 15 pairwise comparisons. The inconsistency test and node splitting method showed good consistency (I 2 < 50%), and there was no heterogeneity between studies (p > 0.05). The results of the network meta‐analysis showed that the therapeutic effect of APG was significantly greater than that of 120 μg/cm2 replifermin and CT, and the PRP was significantly greater than that of CT (p < 0.05). There was no statistically significant difference between other intervention measures (p > 0.05), as shown in Table 4.
TABLE 4.
Results of network meta‐analysis of wound healing cases.
| Intervention measures | 120 μg/cm2 repifermin | 60 μg/cm2 repifermin | AFG | Antiseptic ointment dressing | APG | Aurix | HPL | LeucoPatch | PPP | PRF | PRP | PRP injection | CT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 120 μg/cm2 repifermin | 0 | ||||||||||||
| 60 μg/cm2 repifermin | −0.29 (−2.13, 1.52) | 0 | |||||||||||
| AFG | 16.22 (−40.08, 73.89) | 16.52 (−39.73, 74.2) | 0 | ||||||||||
| Antiseptic ointment dressing | −0.34 (−3.41, 2.53) | −0.05 (−3.12, 2.86) | −16.57 (−74.3, 39.73) | 0 | |||||||||
| APG | −2.08 (−4.85, −0.19) | −1.78 (−4.55, 0.1) | −18.42 (−76.18, 37.89) | −1.76 (−4.79, 0.73) | 0 | ||||||||
| Aurix | −1.16 (−3.39, 1.1) | −0.86 (−3.1, 1.39) | −17.31 (−75, 38.83) | −0.81 (−3.46, 1.97) | 0.93 (−0.63, 3.31) | 0 | |||||||
| HPL | −0.46 (−3.14, 2.24) | −0.17 (−2.82, 2.53) | −16.65 (−74.32, 39.63) | −0.11 (−3.13, 3.08) | 1.64 (−0.43, 4.5) | 0.7 (−1.66, 3.08) | 0 | ||||||
| LeucoPatch | −1.04 (−3.61, 1.55) | −0.75 (−3.29, 1.83) | −17.22 (−74.9, 39) | −0.68 (−3.58, 2.38) | 1.05 (−0.83, 3.8) | 0.13 (−2.12, 2.37) | −0.57 (−3.26, 2.09) | 0 | |||||
| PPP | 0.23 (−2.85, 3.11) | 0.52 (−2.54, 3.42) | −16.04 (−73.78, 40.37) | 0.57 (−2.47, 3.61) | 2.34 (−0.16, 5.39) | 1.39 (−1.43, 4.03) | 0.69 (−2.51, 3.69) | 1.27 (−1.8, 4.16) | 0 | ||||
| PRF | −6.44 (−70.84, 49.94) | −6.14 (−70.53, 50.2) | −21.91 (−116.97, 72.28) | −6.07 (−70.63, 50.22) | −4.18 (−68.55, 52.1) | −5.29 (−69.71, 50.9) | −6.03 (−70.44, 50.22) | −5.42 (−69.81, 50.89) | −6.68 (−71.18, 49.71) | 0 | |||
| PRP | −1.44 (−3.66, 0.5) | −1.15 (−3.36, 0.82) | −17.69 (−75.41, 38.5) | −1.11 (−3.29, 1) | 0.66 (−0.86, 2.67) | −0.29 (−2.07, 1.24) | −0.98 (−3.33, 1.11) | −0.4 (−2.63, 1.52) | −1.67 (−3.88, 0.44) | 4.98 (−51.24, 69.38) | 0 | ||
| PRP injection | −2.07 (−4.74, 0.47) | −1.76 (−4.42, 0.76) | −18.28 (−75.93, 38) | −1.71 (−4.55, 1.13) | 0.05 (−2.07, 2.7) | −0.9 (−3.25, 1.3) | −1.59 (−4.41, 1.03) | −1.02 (−3.69, 1.49) | −2.28 (−5.16, 0.58) | 4.33 (−51.83, 68.64) | −0.6 (−2.45, 1.3) | 0 | |
| CT | −0.37 (−2.19, 1.45) | −0.08 (−1.89, 1.77) | −16.56 (−74.2, 39.64) | −0.02 (−2.34, 2.42) | 1.73 (0.61, 3.6) | 0.79 (−0.53, 2.12) | 0.09 (−1.87, 2.05) | 0.66 (−1.14, 2.48) | −0.6 (−2.92, 1.87) | 6.11 (−50.12, 70.46) | 1.08 (0.17, 2.21) | 1.69 (−0.09, 3.64) | 0 |
Abbreviations: AFG, autologous fibrin gel; APG, autologous platelet gel; Aurix, aurix gel; CT, conventional treatment; GF, growth factor; HPL, autologous platelet lysate; PPP, platelet‐poor plasma; PRF, platelet‐rich fibrin; PRF injection, injectable platelet‐rich fibrin; PRP, platelet‐rich plasma.
3.6.3. Ranking of intervention efficacy
The order of number of wound healing cases from high to low is APG > PRP injection > PRP > Aurix > PRF > LeucoPatch > HPL > Antiseptic Ointment Dressing > CT > 60 μg/cm2 replifermin > 120 μg/cm2 replifermin > AFG > PPP, as shown in Table S4.
3.6.4. Publication bias assessment
Evaluate publication bias and small sample effects on the number of recovered cases, and use Stata SE 15.0 for comparison correction funnel plots. The funnel plot indicates that all studies are roughly symmetrically distributed on both sides of the x = 0 vertical line, indicating a small possibility of significant publication bias. All the dots are inside the triangle, indicating that there cannot be a small sample effect, as shown in Figure S1.
3.7. Pain score
3.7.1. Evidence network
There are a total of nine articles reporting VAS pain scores, with a total of 457 patients involved in eight medication regimens. The network evidence is shown in Figure 4B. The results show the formation of two closed loops. CT has the largest sample size (n = 211), while PRF and CT have the highest number of studies (n = 4).
3.7.2. Results of network meta‐analysis
Nine records reported pain scores involving eight intervention measures, resulting in a total of nine pairwise comparisons. The inconsistency test and node splitting method showed good consistency (I 2 < 50%), and there was no heterogeneity between studies (p > 0.05). The results of the network meta‐analysis showed that the AFG score was significantly lower than that of CT (p < 0.05), and there was no statistically significant difference (p > 0.05) compared to other intervention measures, as shown in Table 5.
TABLE 5.
Results of network meta‐analysis of pain score.
| Intervention measures | AFG | Aminogam_gel | APG | GF | Ozonated oil | PRF | PRP | CT |
|---|---|---|---|---|---|---|---|---|
| AFG | 0 | |||||||
| Aminogam gel | −2.35 (−21.73, 15.57) | 0 | ||||||
| APG | −11.42 (−30.89, 6.49) | −9.08 (−27.94, 9.72) | 0 | |||||
| GF | −11.68 (−28.47, 3.76) | −9.3 (−25.78, 7.07) | −0.2 (−16.43, 16.01) | 0 | ||||
| Ozonated oil | −9.28 (−26.52, 7.1) | −6.97 (−24.58, 11.34) | 2.1 (−15.37, 20.16) | 2.33 (−12.54, 17.85) | 0 | |||
| PRF | −6.83 (−19.83, 5.82) | −4.5 (−19.03, 11.16) | 4.55 (−9.76, 20.25) | 4.78 (−6.28, 17.17) | 2.44 (−9.16, 14.72) | 0 | ||
| PRP | −9.34 (−28.52, 8.5) | −6.99 (−25.64, 11.79) | 2.09 (−16.49, 20.83) | 2.3 (−13.85, 18.57) | −0.02 (−18.21, 17.39) | −2.46 (−18.02, 11.77) | 0 | |
| CT | −12.56 (−26.42, −0.14) | −10.17 (−23.61, 3.14) | −1.11 (−14.27, 12.13) | −0.88 (−10.36, 8.55) | −3.22 (−15.51, 8.37) | −5.69 (−13.26, 0.61) | −3.18 (−16.45, 9.94) | 0 |
Abbreviations: AFG, autologous fibrin gel; APG, autologous platelet gel; CT, conventional treatment; GF, growth factor; ozonated oil, ozone gas dissolved in olive oil; PG, platelet gel; PRF, platelet‐rich fibrin; PRG, platelet‐rich gel; PRP, platelet‐rich plasma.
3.7.3. Ranking of intervention efficacy
The order of pain scores from low to high is AFG < Aminogem gel < PRF < PRP < Ozoned oil < APG < GF < CT (Table S5).
3.7.4. Publication bias assessment
The pain score was evaluated using Stata SE 15.0 for publication bias and small sample effects. The funnel plot indicates that all studies are roughly symmetrically distributed on both sides of the x = 0 vertical line, indicating a small possibility of significant publication bias. The two dots are scattered outside the triangle, indicating a possible small sample effect, as shown in Figure S1.
3.8. Number of wound infection cases
3.8.1. Evidence network
There are a total of eight records reports on the number of wound infection cases during treatment, with a total of 1818 patients involved in eight medication regimens. The network evidence is shown in Figure 4C. The results show the formation of a closed loop. CT has the largest sample size (n = 983), while PRP and CT have the largest number of studies (n = 4).
3.8.2. Results of network meta‐analysis
Eight records have reported the number of wound infection cases, involving eight intervention measures, forming a total of eight pairwise comparisons. The inconsistency test and node splitting method showed good consistency (I 2 < 50%), and there was no heterogeneity between studies (p > 0.05). The results of the network meta‐analysis showed that APG had the fewest number of infection cases and was significantly lower than other intervention measures (p < 0.05). There was no statistically significant difference (p > 0.05) between other intervention measures, as shown in Table 6.
TABLE 6.
Results of network meta‐analysis of wound infection cases.
| Intervention measures | 20 μg/cm2 repifermin | 60 μg/cm2 repifermin | Antiseptic ointment dressing | APG | LeucoPatch | PPP | PRP | CT |
|---|---|---|---|---|---|---|---|---|
| 20 μg/cm2 repifermin | 0 | |||||||
| 60 μg/cm2 repifermin | −0.85 (−5.5, 3.71) | 0 | ||||||
| Antiseptic ointment dressing | −2.71 (−9.7, 4.04) | −1.86 (−8.78, 4.84) | 0 | |||||
| APG | 32.59 (2.42, 77.68) | 33.5 (3.31, 78.54) | 35.44 (5.03, 80.43) | 0 | ||||
| LeucoPatch | −1.5 (−7.71, 4.71) | −0.63 (−6.77, 5.4) | 1.21 (−5.4, 8.02) | −34.18 (−79.14, −3.98) | 0 | |||
| PPP | −2.34 (−9.81, 4.7) | −1.48 (−8.86, 5.51) | 0.38 (−6.52, 7.12) | −35.09 (−80.19, −4.5) | −0.82 (−8.13, 6.08) | 0 | ||
| PRP | −1.27 (−6.59, 3.82) | −0.41 (−5.61, 4.55) | 1.43 (−3.04, 5.99) | −33.98 (−78.85, −4.04) | 0.23 (−4.8, 5.1) | 1.03 (−3.91, 6.34) | 0 | |
| CT | −1.8 (−6.4, 2.66) | −0.95 (−5.38, 3.42) | 0.91 (−4.19, 6.19) | −34.53 (−79.24, −4.68) | −0.3 (−4.55, 3.9) | 0.52 (−4.98, 6.43) | −0.53 (−3.02, 2.08) | 0 |
Abbreviations: APG, autologous platelet gel; CT, conventional treatment; PPP, platelet‐poor plasma; PRP, platelet‐rich plasma.
3.8.3. Ranking of intervention efficacy
The order of the number of wound infection cases from low to high is APG < 20 μg/cm2 replifermin < 60 μg/cm2 replifermin < PR < LeucoPatc < CT < PPP < Antiseptic Ointment Dressing, as shown in Table S6.
3.8.4. Publication bias assessment
Evaluate publication bias and small sample effects on wound infections. The funnel plot indicates that all studies are roughly symmetrically distributed on both sides of the x = 0 vertical line, indicating a small possibility of significant publication bias. All the dots are inside the triangle, indicating that there cannot be a small sample effect, as shown in Figure S1.
4. DISCUSSION
Plasma derivatives are substances prepared from healthy blood using separation and purification techniques such as chromatography and centrifugation. The mechanisms by which plasma derivatives promote wound healing are as follows: ① Platelets in plasma derivatives are activated to release powerful cell/growth factors (TGF‐β, PDGF, IGF‐1 and EGF, etc.), which act on a variety of target cells to promote cell proliferation, matrix synthesis, collagen deposition, and ultimately achieving tissue repair. 48 ② The anti‐inflammatory cytokines in plasma derivatives can regulate the process of inflammatory response. 49 ③ Adhesion factors (fibrin, fibronectin and hyalonin) in plasma derivatives construct a three‐dimensional structure locally (required for tissue repair), repairing cell movement and facilitating wound repair. 50 ④ Plasma derivatives induce the release of local growth factors, cytokines, or microRNAs through autocrine and paracrine pathways, promoting wound healing. 51 ⑤ Platelet activation in plasma derivatives releases bioactive substances (chemokines, histamines and adenosine) that directly induce platelet aggregation, alleviate pain and indirectly chemotactic leukocytes to exert bactericidal effects. 52 In recent years, with the rapid development of regenerative medicine, the use of plasma derivatives for wound treatment has achieved remarkable results, 53 but the lack of direct or indirect comparison of efficacy among derivatives is not conducive to clinical promotion and the selection of the best program. In this study, network meta‐analysis was used to analyse the healing area, healing time, healing number, late infection number and analgesia score, in order to provide evidence for the selection of clinical drugs.
The healing area shows that GF + ORCCB has the largest healing area, and the combination of wound environment regulator (ORCCB) and GF can significantly accelerate the healing area of the wound. This may be due to: ① ORCCB can change the external environment of the wound by binding and inactivating proteases and gelatinases that seep out of the wound, creating a protective environment for growth factor healing. 54 ② ORCCB can physically bind proteases. When the proteases are fully controlled, this material has the ability to bind and protect growth factors, and can send 70% of the growth factors back to the wound surface, which is beneficial for wound healing. 55 Zhang et al. 56 confirmed through meta‐analysis that ORCCB is beneficial in improving the wound healing rate and relative percentage reduction compared to other traditional nursing materials (non‐MMP, inhibitory biomaterials and modern excipients).
PRP + thrombobin gel has the shortest healing time. PRP increases the rate of wound healing by increasing haemostasis, releasing growth factors, re‐epithelialization, inducing fibroblast proliferation of extracellular matrix and promoting angiogenesis. Thrombin is another important component of wound healing. 57 The combination of the two can convert fibrin in PRP into a network structure, embed platelets, white blood cells, and growth factors, and produce a certain adhesive effect, retaining the sample at the delivery site and stimulating the release of growth factors, accelerating wound healing time. 58
APG has the best effect on healing and reducing post‐infection. APG can accelerate wound healing and promote tissue regeneration by increasing the production of extracellular matrix and granulation tissue, 59 which is consistent with the results of this analysis. The activated platelets in APG contain a series of microbicidal proteins with antibacterial activity, which exert strong antibacterial effects mainly by altering the permeability of cell membranes and inhibiting the synthesis of large molecules. 60 This may be a key factor in reducing the number of infections in the later stages of surgery.
Postoperative pain assessment is an important component of wound healing and plays a decisive role in wound healing. Segal et al. 61 found through a randomised trial that there was no significant difference in the analgesic effect of AFG compared to not using AFG (p = 0.988). Contrary to Segal N's research, this article found that AFG has a significantly higher postoperative analgesic effect than CT, which may be due to the release of mediators such as histamine, 5‐hydroxytryptamine and dopamine from concentrated platelets, thereby reducing the occurrence of pain. 12 This indicates that there are relatively few included literature on some blood derivatives with low quality, and more high‐quality studies with larger sample sizes are needed to confirm them in the later stage.
Limitations of this study: ① Most of the 38 RCT studies included were of low quality, and the vast majority did not indicate specific randomised, hidden, or blind methods, which poses a certain risk of bias and affects the authenticity and reliability of the results.② Some studies include a small sample size, which may reduce the credibility of the results.③ The inclusion of research implemented in multiple countries around the world has a certain impact on the results due to differences in medical standards and standard protocols. ④ Inconsistent inclusion of causes of trauma (surgical wounds, ulcers, burns, etc.) may affect the accuracy of the results. ⑤ The lack of direct comparison between different blood derivatives affects the credibility of the results. ⑥ This study included four retrospective cohort studies, which may have a certain impact on the results. Given the above limitations, clinical practice should maintain a rigorous and cautious attitude, conduct more multi‐centre, large‐sample clinical studies and provide more sources of evidence for further verifying its efficacy.
5. CONCLUSIONS
GF + ORCCB is the best in reducing wounds, and PRP + thrombin gel has the shortest treatment time. The number of people who have recovered from APG and the reduction of wound infections are the most prominent, and the analgesic effect of APG is the best. Clinical medical staff can choose appropriate treatment plans based on the needs of patients.
FUNDING INFORMATION
This research was supported by the Yucai Foundation of General Hospital of Southern Theater Command (Grant No. 2022NZB002).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Supporting information
Figure S1. Corrected funnel plots of healing area comparison (A), healing time comparison (B), wound healing cases comparison (C), pain score comparison (D) and wound infection cases comparison (E).
Table S1. NOS quality evaluation results of cohort study.
Table S2. Efficacy ranking of healing area interventions.
Table S3. Efficacy ranking of healing time intervention.
Table S4. Efficacy ranking of intervention measures on the number of wound healing cases.
Table S5. Efficacy ranking of pain score interventions.
Table S6. Efficacy ranking of intervention measures on the number of wound infection cases.
ACKNOWLEDGEMENTS
The authors thank all the participants of this work.
Wu Y, Peng G, Wang Y, et al. Clinical efficacy of blood derivatives on wound healing: A systematic review and network meta‐analysis. Int Wound J. 2024;21(4):e14622. doi: 10.1111/iwj.14622
DATA AVAILABILITY STATEMENT
The data used in this network meta‐analysis are available to the authors upon reasonable request.
REFERENCES
- 1. Huang Y, Fu X. The establishment and development of wound repair discipline in China. Front Surg. 2022;9:1046494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chester D, Brown AC. The role of biophysical properties of provisional matrix proteins in wound repair. Matrix Biol. 2017;60‐61:124‐140. [DOI] [PubMed] [Google Scholar]
- 3. El Mohtadi M, Whitehead K, Dempsey‐Hibbert N, Belboul A, Ashworth J. Estrogen deficiency‐a central paradigm in age‐related impaired healing? EXCLI J. 2021;20:99‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amse A, Nfh B. Clinical and cytological assessment of platelet‐rich fibrin versus topical ozonated oil in palatal wound healing after free gingival graft harvesting: randomized controlled trial. J Oral Maxillofac Surg Med Pathol. 2021;34:343‐351. [Google Scholar]
- 7. Capion SC, Jorgensen HBL, Agren MS, et al. The wound healing effect of local leukocyte platelet‐rich plasma after total hip arthroplasty: a randomized controlled trial. Wound Repair Regen. 2021;29(6):988‐995. [DOI] [PubMed] [Google Scholar]
- 8. Malekpour Alamdari N, Shafiee A, Mirmohseni A, Besharat S. Evaluation of the efficacy of platelet‐rich plasma on healing of clean diabetic foot ulcers: a randomized clinical trial in Tehran, Iran. Diabetes Metab Syndr. 2021;15(2):621‐626. [DOI] [PubMed] [Google Scholar]
- 9. Vaheb M, Karrabi M, Khajeh M, Asadi A, Shahrestanaki E, Sahebkar M. Evaluation of the effect of platelet‐rich fibrin on wound healing at Split‐thickness skin graft donor sites: a randomized, placebo‐controlled, triple‐blind study. Int J Low Extrem Wounds. 2021;20(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 10. Elbarbary AH, Hassan HA, Elbendak EA. Autologous platelet‐rich plasma injection enhances healing of chronic venous leg ulcer: a prospective randomised study. Int Wound J. 2020;17(4):992‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elsaid A, El‐Said M, Emile S, Youssef M, Khafagy W, Elshobaky A. Randomized controlled trial on autologous platelet‐rich plasma versus saline dressing in treatment of non‐healing diabetic foot ulcers. World J Surg. 2020;44(4):1294‐1301. [DOI] [PubMed] [Google Scholar]
- 12. Kiziltoprak M, Uslu MO. Comparison of the effects of injectable platelet‐rich fibrin and autologous fibrin glue applications on palatal wound healing: a randomized controlled clinical trial. Clin Oral Investig. 2020;24(12):4549‐4561. [DOI] [PubMed] [Google Scholar]
- 13. Lektemur Alpan A, Torumtay Cin G. PRF improves wound healing and postoperative discomfort after harvesting subepithelial connective tissue graft from palate: a randomized controlled trial. Clin Oral Investig. 2020;24(1):425‐436. [DOI] [PubMed] [Google Scholar]
- 14. Slaninka I, Fibir A, Kaska M, Paral J. Use of autologous platelet‐rich plasma in healing skin graft donor sites. J Wound Care. 2020;29(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 15. Xie J, Fang Y, Zhao Y, Cao D, Lv Y. Autologous platelet‐rich gel for the treatment of diabetic sinus tract wounds: a clinical study. J Surg Res. 2020;247:271‐279. [DOI] [PubMed] [Google Scholar]
- 16. Yuvasri G, Rai R. Comparison of efficacy of autologous platelet‐rich fibrin versus Unna's paste dressing in chronic venous leg ulcers: a comparative study. Indian Dermatol Online J. 2020;11(1):58‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Angelis B, D'Autilio M, Orlandi F, et al. Gentile P:wound healing: in vitro and in vivo evaluation of a bio‐functionalized scaffold based on hyaluronic acid and platelet‐rich plasma in chronic ulcers. J Clin Med. 2019;8(9):1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goda AA, Ewada A, Ewees H, Hamza M. Platelet‐rich plasma for the treatment of diabetic foot ulcer: a randomized, double‐blind study. Egypt J Surg. 2019;37:178. [Google Scholar]
- 19. Gude W, Hagan D, Abood F, Clausen P. Aurix gel is an effective intervention for chronic diabetic foot ulcers: a pragmatic randomized controlled trial. Adv Skin Wound Care. 2019;32(9):416‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong E, Yoo IK, Cakir OO, et al. Effectiveness of autologous platelet‐rich plasma for the healing of ulcers after endoscopic submucosal dissection. Clin Endosc. 2019;52(5):472‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rainys D, Cepas A, Dambrauskaite K, Nedzelskiene I, Rimdeika R. Effectiveness of autologous platelet‐rich plasma gel in the treatment of hard‐to‐heal leg ulcers: a randomised control trial. J Wound Care. 2019;28(10):658‐667. [DOI] [PubMed] [Google Scholar]
- 22. Game F, Jeffcoate W, Tarnow L, et al. LeucoPatch IItt: LeucoPatch system for the management of hard‐to‐heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer‐masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):870‐878. [DOI] [PubMed] [Google Scholar]
- 23. Guazzo R, Perissinotto E, Mazzoleni S, Ricci S, Penarrocha‐Oltra D, Sivolella S. Effect on wound healing of a topical gel containing amino acid and sodium hyaluronate applied to the alveolar socket after mandibular third molar extraction: a double‐blind randomized controlled trial. Quintessence Int. 2018;49(10):831‐840. [DOI] [PubMed] [Google Scholar]
- 24. Singh SP, Kumar V, Pandey A, Pandey P, Gupta V, Verma R. Role of platelet‐rich plasma in healing diabetic foot ulcers: a prospective study. J Wound Care. 2018;27(9):550‐556. [DOI] [PubMed] [Google Scholar]
- 25. Yeung CY, Hsieh PS, Wei LG, et al. Efficacy of lyophilised platelet‐rich plasma powder on healing rate in patients with deep second degree burn injury: a prospective double‐blind randomized clinical trial. Ann Plast Surg. 2018;80(2S Suppl 1):S66‐S69. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet‐rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. 2017;38:206‐211. [DOI] [PubMed] [Google Scholar]
- 27. Escamilla Cardenosa M, Dominguez‐Maldonado G, Cordoba‐Fernandez A. Efficacy and safety of the use of platelet‐rich plasma to manage venous ulcers. J Tissue Viability. 2017;26(2):138‐143. [DOI] [PubMed] [Google Scholar]
- 28. Hersant B, SidAhmed‐Mezi M, Bosc R, Meningaud JP. Autologous platelet‐rich plasma/thrombin gel combined with split‐thickness skin graft to manage postinfectious skin defects: a randomized controlled study. Adv Skin Wound Care. 2017;30(11):502‐508. [DOI] [PubMed] [Google Scholar]
- 29. Samani MK, Saberi BV, Ali Tabatabaei SM, Moghadam MG. The clinical evaluation of platelet‐rich plasma on free gingival graft's donor site wound healing. Eur J Dent. 2017;11(4):447‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Somani A, Rai R. Comparison of efficacy of autologous platelet‐rich fibrin versus saline dressing in chronic venous leg ulcers: a randomised controlled trial. J Cutan Aesthet Surg. 2017;10(1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Volpe P, Marcuccio D, Stilo G, et al. Efficacy of cord blood platelet gel application for enhancing diabetic foot ulcer healing after lower limb revascularization. Semin Vasc Surg. 2017;30(4):106‐112. [DOI] [PubMed] [Google Scholar]
- 32. Yang L, Gao L, Lv Y, JJIJoC W. Medicine E: autologous platelet‐rich gel for lower‐extremity ischemic ulcers in patients with type 2 diabetes. Int J Clin Exp Med. 2017;10(9):13796‐13801. [Google Scholar]
- 33. Raposio E, Bertozzi N, Bonomini S, et al. Adipose‐derived stem cells added to platelet‐rich plasma for chronic skin ulcer therapy. Wounds. 2016;28(4):126‐131. [PubMed] [Google Scholar]
- 34. Aguirre Anda JJ, Anitua E, Francisco S, Cabezas A, Orive G, Algorta J. Efficacy and safety of plasma rich in growth factors in the treatment of venous ulcers: a randomized clinical trial controlled with conventional treatment. Clin Dermatol. 2015;3:13‐20. [Google Scholar]
- 35. Li L, Chen D, Wang C, et al. Autologous platelet‐rich gel for treatment of diabetic chronic refractory cutaneous ulcers: a prospective, randomized clinical trial. Wound Repair Regen. 2015;23(4):495‐505. [DOI] [PubMed] [Google Scholar]
- 36. Serraino GF, Dominijanni A, Jiritano F, et al. Platelet‐rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int Wound J. 2015;12(3):260‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dorge H, Sellin C, Bury MC, et al. Incidence of deep sternal wound infection is not reduced with autologous platelet rich plasma in high‐risk cardiac surgery patients. Thorac Cardiovasc Surg. 2013;61(3):180‐184. [DOI] [PubMed] [Google Scholar]
- 38. Guerid S, Darwiche SE, Berger MM, Applegate LA, Benathan M, Raffoul W. Autologous keratinocyte suspension in platelet concentrate accelerates and enhances wound healing—a prospective randomized clinical trial on skin graft donor sites: platelet concentrate and keratinocytes on donor sites. Fibrogenesis Tissue Repair. 2013;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J. 2013;10(5):612‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anitua E, Aguirre JJ, Algorta J, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84(2):415‐421. [DOI] [PubMed] [Google Scholar]
- 41. Kakagia DD, Kazakos KJ, Xarchas KC, et al. Synergistic action of protease‐modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Complications. 2007;21(6):387‐391. [DOI] [PubMed] [Google Scholar]
- 42. Driver VR, Hanft J, Fylling CP, Beriou JM. Autologel Diabetic Foot Ulcer Study G: a prospective, randomized, controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68‐70. 72, 74 passim. [PubMed] [Google Scholar]
- 43. Robson MC, Hanfnt J, Garner W, Jenson J. Cooper DMJTJoAR: healing of chronic venous ulcers is not enhanced by the addition of topical repifermin (KGF2) to standardized care. J Appl Res. 2004;4(2):302‐311. [Google Scholar]
- 44. Saldalamacchia G, Lapice E, Cuomo V, et al. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovasc Dis. 2004;14(6):395‐396. [DOI] [PubMed] [Google Scholar]
- 45. Robson MC, Phillips TJ, Falanga V, et al. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor‐2) to accelerate wound healing in venous ulcers. Wound Repair Regen. 2001;9(5):347‐352. [DOI] [PubMed] [Google Scholar]
- 46. Stacey MC, Mata SD, Trengove NJ, Mather CA. Randomised double‐blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur J Vasc Endovasc Surg. 2000;20(3):296‐301. [DOI] [PubMed] [Google Scholar]
- 47. Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. CT‐102 activated platelet supernatant, topical versus placebo. Diabet Care. 1992;15(11):1598‐1604. [DOI] [PubMed] [Google Scholar]
- 48. Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet‐rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen. 2015;23(5):638‐643. [DOI] [PubMed] [Google Scholar]
- 49. Papait A, Cancedda R, Mastrogiacomo M, Poggi A. Allogeneic platelet‐rich plasma affects monocyte differentiation to dendritic cells causing an anti‐inflammatory microenvironment, putatively fostering wound healing. J Tissue Eng Regen Med. 2018;12(1):30‐43. [DOI] [PubMed] [Google Scholar]
- 50. Miron RJ, Fujioka‐Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet‐rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng Part B Rev. 2017;23(1):83‐99. [DOI] [PubMed] [Google Scholar]
- 51. De Pascale MR, Sommese L, Casamassimi A, Napoli C. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev. 2015;29(1):52‐61. [DOI] [PubMed] [Google Scholar]
- 52. Edelblute CM, Donate AL, Hargrave BY, Heller LC. Human platelet gel supernatant inactivates opportunistic wound pathogens on skin. Platelets. 2015;26(1):13‐16. [DOI] [PubMed] [Google Scholar]
- 53. Fu X. Wound care in China: from repair to regeneration. Int J Low Extrem Wounds. 2012;11(3):143‐145. [DOI] [PubMed] [Google Scholar]
- 54. Smeets R, Ulrich D, Unglaub F, Woltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5(2):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cullen B, Watt PW, Lundqvist C, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34(12):1544‐1556. [DOI] [PubMed] [Google Scholar]
- 56. Zhang L, Wang S, Tan M, Zhou H, Tang Y, Zou Y. Efficacy of oxidized regenerated cellulose/collagen dressing for management of skin wounds: a systematic review and meta‐analysis. Evid Based Complement Alternat Med. 2021;2021:1058671. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57. Pradnyandari NKPD. Natasha RRJISM: the role of platelet‐rich plasma (PRP) in burn wound healing: a literature‐review. Intisari Sains Medis. 2022;13(2):507‐510. [Google Scholar]
- 58. Matuska AM, Klimovich ML, Anz AW, Podesta L, Chapman JR. Autologous thrombin preparations: biocompatibility and growth factor release. Wound Repair Regen. 2021;29(1):144‐152. [DOI] [PubMed] [Google Scholar]
- 59. Whitlow J, Shackelford A, Sievert A, Sistino J. Barriers to the acceptance and use of autologous platelet gel. Perfusion. 2008;23(5):283‐289. [DOI] [PubMed] [Google Scholar]
- 60. Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70(12):6524‐6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Segal N, Puterman M, Rotem E, et al. A prospective randomized double‐blind trial of fibrin glue for reducing pain and bleeding after tonsillectomy. Int J Pediatr Otorhinolaryngol. 2008;72(4):469‐473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Corrected funnel plots of healing area comparison (A), healing time comparison (B), wound healing cases comparison (C), pain score comparison (D) and wound infection cases comparison (E).
Table S1. NOS quality evaluation results of cohort study.
Table S2. Efficacy ranking of healing area interventions.
Table S3. Efficacy ranking of healing time intervention.
Table S4. Efficacy ranking of intervention measures on the number of wound healing cases.
Table S5. Efficacy ranking of pain score interventions.
Table S6. Efficacy ranking of intervention measures on the number of wound infection cases.
Data Availability Statement
The data used in this network meta‐analysis are available to the authors upon reasonable request.


