Abstract
Sindbis virus, the prototype alphavirus, kills cells by inducing apoptosis. To investigate potential mechanisms by which Sindbis virus induces apoptosis, we examined whether specific viral gene products were able to induce cell death. Genes encoding the three structural proteins—capsid, the precursor E1 (6K plus E1), and the precursor E2 (P62 or E3 plus E2)—were cotransfected with a β-galactosidase reporter plasmid in transient-transfection assays in rat prostate adenocarcinoma AT3 cells. Cell death, as determined by measuring the loss of blue cells, was observed in AT3 cells transfected with 6K plus E1 and with P62 but not in cells transfected with capsid. Deletion mutagenesis of P62 indicated that large regions of the cytoplasmic domain and extracellular domain were not essential for the induction of cell death. However, constructs containing the minimal E3 signal sequence fused to the E2 transmembrane domain and the minimal E3 signal sequence fused to the E1 transmembrane domain induced death as efficiently as full-length P62 and 6K plus E1, whereas no cell death was observed after transfection with a control construct containing the E3 signal sequence linked to the transmembrane domain of murine CD4. These data demonstrate that intracellular expression of the transmembrane domains of the Sindbis virus envelope glycoproteins can kill AT3 cells.
A number of viruses are known to induce apoptosis (reviewed in references 38 and 41), but the precise mechanisms by which most viruses trigger cellular apoptotic pathways are not yet understood. Mechanisms that have been postulated include the inhibition of host cell shutoff (6), deregulation of the cell cycle (7, 25), viral inhibition of cellular antiapoptotic effectors (35, 39), viral activation of CED-3/ICE-like cell death proteases (1, 2, 32), and viral envelope cross-linking of cell surface receptors involved in death signalling (3, 19, 20, 28). The evidence to support the roles of these mechanisms in cell death stems largely from studies that have focused on the importance of individual viral gene products in apoptosis induction. For example, adenovirus E1A induces apoptosis by stabilizing the p53 tumor suppressor protein and deregulating the cell cycle (7). Human immunodeficiency virus (HIV) type 1 tat protein-induced apoptosis in Jurkat T cells is associated with enhanced activation of cyclin-dependent kinases (25), further supporting the hypothesis that virus-induced cell cycle deregulation leads to apoptosis. The HIV protease induces apoptosis, which is preceded by cleavage of Bcl-2, a key negative regulator of cell death (39), and the HIV tat protein induces apoptosis which is associated with the downregulation of bcl-2 expression (35). In addition, intracellular expression of the HIV gp120-gp41 complex induces apoptosis by interacting with CD4 receptor molecules in the cell membrane (19, 20, 28), and a fusion protein comprised of the surface envelope protein of cytopathic avian leukosis virus and the Fc region of an immunoglobulin mediates apoptosis by binding to the cellular receptor, CAR1, a member of the tumor necrosis factor superfamily (3).
Sindbis virus (SIN), the prototype alphavirus, is a positive-strand RNA virus that replicates lytically in most mammalian cell lines and produces an age-dependent fatal encephalitis in mice. For several reasons, it provides a useful model for studying the mechanisms by which enveloped viruses induce apoptosis. First, apoptosis is already known to play an important role in the cytopathic effects of viral replication in vitro (21, 44) and in the disease that it causes in vivo (23, 24). Second, several gene products that can block the apoptotic pathway induced by SIN have been identified, including Bcl-2 (21, 23, 44), Bcl-xL (5), dominant inhibitory Ras (15), CrmA (32), and baculovirus and human IAP-like proteins (8). Third, the SIN genome is only 11,703 nucleotides long and has a limited number of gene products: four nonstructural proteins and three structural proteins—capsid plus two envelope glycoproteins, E2 and E1. Therefore, it is technically feasible to investigate the role of each gene product in SIN-induced apoptosis.
Although the mechanisms by which SIN induces apoptosis have not yet been defined, a previous study suggests that intracellular synthesis of viral structural proteins may be required for cell death. Using SIN replicons, Frolov et al. found that expression of the SIN nonstructural genes in BHK cells is sufficient to induce host cell shutoff but that expression of the structural genes is required for the rapid cytopathic effects (CPEs) that are similar to those observed during wild-type-virus infection (9). When BHK cells are infected with particles containing replicon RNAs that express SIN structural proteins, CPEs are observed by 12 h postinfection. In contrast, when BHK cells are infected with particles that contain RNAs that express only the nonstructural proteins, CPEs are significantly delayed. Thus, the binding of virus particles to cell surface receptors, entry, uncoating steps, and synthesis of nonstructural proteins do not appear to be related to the rapid CPEs that are characteristic of apoptosis in SIN-infected cells. The lack of a role for viral envelope attachment to cell surface receptors in SIN-induced apoptosis is further supported by the observation that the binding of UV-inactivated SIN (at a multiplicity of infection of 100) does not induce apoptosis in mouse neuroblastoma cells (45).
To investigate potential mechanisms by which SIN induces apoptosis, we evaluated whether transient expression of individual SIN structural genes could induce death in AT3 cells. AT3 cells are a rat prostate adenocarcinoma cell line which, as previously described, undergoes a characteristic apoptotic response to SIN infection that can be blocked by bcl-2 (21). Our results indicate that the transmembrane domains (TMDs) of the SIN E2 and E1 envelope glycoproteins are able to induce cell death.
MATERIALS AND METHODS
Cell culture.
Rat prostate adenocarcinoma AT3 cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum and 250 nM dexamethasone.
Expression vectors.
Mammalian expression vectors containing individual SIN structural genes under the control of a simian virus 40 (SV40) promoter were constructed. By using the double subgenomic SIN vector dsTE12Q (15) as a template, viral cDNAs encoding each of the structural genes (capsid, precursor E1 [6K plus E1], and precursor E2 [P62]) were amplified by PCR with restriction sites incorporating BglII in the upstream and downstream primers. PCR fragments were then cloned into the BglII site of the SV40 expression vector, pGH52 (provided by Marie Hardwick, Johns Hopkins University School of Medicine), and the sequence of each recombinant plasmid was confirmed by automated sequencing.
Deletion mutants of P62 were constructed by using similar methods. To construct M3, M4, and M5, downstream primers that hybridized with nucleotides 9819 to 9836, 9747 to 9764, and 9705 to 9722, respectively, were used, and each downstream primer incorporated a stop codon. To construct M1 and M2, full-length P62 fragments were digested with BspMI or HinfI, respectively, and the external restriction products (nucleotides 8439 to 8721 and 9433 to 9899 for BspMI and 8439 to 9174 and 9655 to 9899 for HinfI) were religated prior to cloning into GH52. To construct M6 and M7, the minimal E3 signal sequence (nucleotides 8439 to 8552) was ligated into a downstream E2 fragment which encoded either the four amino acids N′ terminal to the TMD, the TMD, the two amino acids C′ terminal to the TMD (M6), or the four amino acids N′ terminal to the TMD and the N′-terminal half of the E2 TMD (M7). A BamHI restriction site was added to the 3′ end of the minimal E3 signal sequence and to the 5′ end of the TMD fragment to facilitate ligation.
To construct M6/CD4 and M6/E1, the E2 TMD in M6 was replaced by either the murine CD4 receptor TMD (M6/CD4) or the SIN E1 envelope glycoprotein TMD (M6/E1). The sequences encoding the four amino acids N′ terminal and the two amino acids C′ terminal to the E2 TMD were incorporated into the upstream and downstream PCR primers used to amplify the CD4 and E1 TMDs. Thus, M6/CD4 and M6/E1 differed from M6 only in the region of the E2 TMD. Additional constructs were also made in which an eight-amino-acid (8-aa) FLAG epitope (DYKDDDDK) was added to the N′ terminus of the E3 signal.
Transfections.
Plasmid DNA was introduced into AT3 cells (5 × 105 cells/35-mm-diameter well) by cationic-liposome-mediated transfection (lipofectin, GIBCO/BRL) according to the manufacturer’s instructions. Transfection mixtures contained 4 μg of test plasmid DNA, 4 μg of pCMVβ reporter plasmid DNA (Clontech), and 6 μl of lipofectin.
β-Galactosidase assays.
For loss-of-blue-cell assays, AT3 cells were washed 60 h after transfection with phosphate-buffered saline (PBS), fixed in PBS containing 0.05% glutaraldehyde for 1 min at room temperature, and then washed twice with PBS. Fixed cells were stained overnight with PBS containing 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside per ml, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.02% Nonidet P-40, and 0.01% sodium dodecyl sulfate. Cell death was determined by counting microscopically the number of blue cells per 35-mm-diameter well. Results are expressed as 100 minus the percentage of blue cells per well after transfection with SIN structural gene expression vectors relative to the number of blue cells after transfection with empty expression vectors. For assays measuring the total number of blue cells, AT3 cells were washed and fixed at 12, 24, 48, and 60 h after transfection, β-galactosidase (β-Gal) assays were performed as above, and the total number of blue cells per 35-mm-diameter well was determined by microscopic counting.
Cell viability assays.
At serial time points after transfection, floating nonadherent and adherent AT3 cells were pelleted and resuspended in PBS, and cell viability was determined by trypan blue exclusion, with blind counting of at least 500 cells per sample.
Phase and electron microscopy.
Forty-eight hours after transfection, transfected AT3 cells were analyzed for morphologic evidence of apoptosis by phase and electron microscopy (EM). For EM analysis, floating and adherent cells were pelleted, fixed with 2.5% glutaraldehyde, postfixed in 1% OsO4 containing 1% potassium ferrocyanide, and then embedded and sectioned as described previously (11).
Immunofluorescence.
AT3 cells were fixed in 100% ethanol 48 h after transfection with FLAG epitope-tagged constructs, and immunofluorescence staining was performed with a mouse monoclonal anti-FLAG antibody (M2) (1:20 dilution) (VWR Scientific) and fluorescein isothiocyanate-conjugated horse anti-mouse antibody (1:50 dilution) (Vector Laboratories). AT3 cells were also stained with Hoechst 33258 (1 μg/ml) to detect condensed apoptotic nuclei.
RESULTS
Construction of SIN structural gene expression vectors.
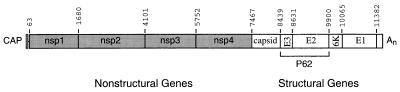
To determine whether any of the SIN structural proteins (Fig. 1) could induce apoptosis, we constructed mammalian expression vectors that expressed SIN capsid, SIN P62, and SIN 6K plus E1. P62 is the precursor of E2 and consists of the E3 signal sequence and the mature E2. The signal sequences for E2 and E1 (E3 and 6K, respectively) were included to ensure proper translocation of the viral transmembrane glycoproteins across the endoplasmic reticulum (ER) membrane. Protein expression after AT3 cell transfection of the SIN structural gene expression vectors was confirmed by immunofluorescence staining with a polyclonal anti-SIN antibody (data not shown); transfection efficiencies ranged from 10 to 20%.
FIG. 1.
Schematic diagram of the organization of the SIN genome. Numbers refer to nucleotide positions of start sites for each SIN gene.
AT3 cell death induction by SIN P62 and 6K plus E1.
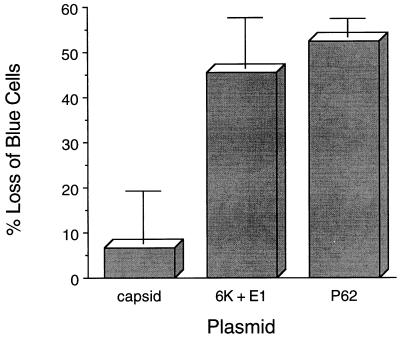
To examine whether any of the SIN structural gene plasmids could induce death, AT3 cells were cotransfected with empty vector, capsid, P62, or 6K plus E1 and with a reporter vector that expressed β-Gal. As dying cells detach from the tissue culture plate and are removed with washes that are performed prior to fixing cells for β-Gal assays, the loss of blue (β-Gal-positive) adherent cells is a commonly used parameter to quantitate cell death in transfected cells (14). We measured the loss of blue cells at 60 h after transfection (Fig. 2), as this time point is 12 h after the peak number of blue cells is observed in cells transfected with an empty control and a reporter vector (see Fig. 8 below). By measuring cell death 12 h after maximal protein expression in transfection assays, we postulated that the 60-h time point would be the most representative indicator of the cell death observed during viral infection (which occurs approximately 12 h after peak structural-protein expression).
FIG. 2.
AT3 cell death induction by SIN P62 and 6K plus E1. The x axis represents SV40 mammalian expression vectors which were cotransfected with a pCMVβ reporter plasmid. The y axis represents the loss of adherent blue cells relative to results for cells transfected with an empty control vector, GH52. The data shown represent the means ± standard errors of the mean for transfections done in triplicate from a representative experiment. Similar results were obtained in more than ten independent transfections.
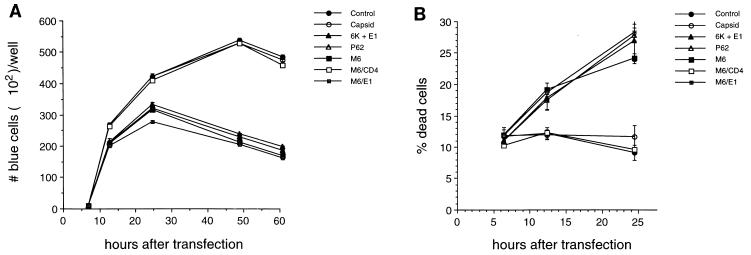
FIG. 8.
Kinetics of AT3 cell death after transfection by SIN constructs. (A) Number of blue cells at serial time points after transfection. (B) Percentage of dead cells at serial time points after transfection. Results shown represent means ± standard errors of the mean for transfections done in triplicate from a representative experiment. Similar results were obtained in three independent experiments.
Sixty hours after transfection with P62 and 6K plus E1, there were 52 and 45% losses of blue AT3 cells, compared with the numbers of blue cells after transfection with the empty vector. The number of blue cells after transfection with SIN capsid was similar to that observed after transfection with the control empty vector (Fig. 2). These data demonstrate that transient expression of the SIN envelope glycoproteins P62 and 6K plus E1, but not of the capsid protein, results in AT3 cell death.
AT3 cell apoptosis induction by SIN P62 and 6K plus E1.
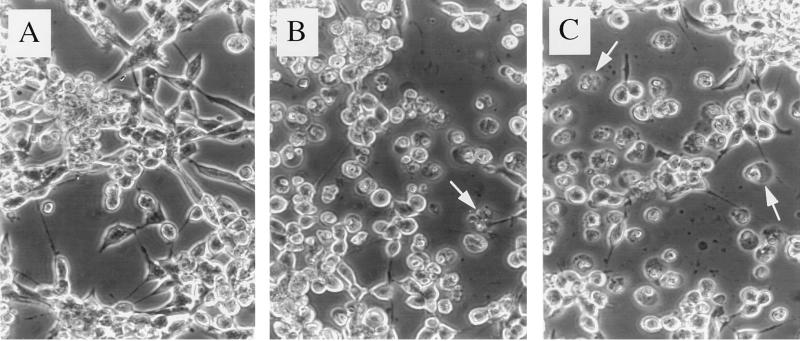
Previously, we showed that infection of AT3 cells with wild-type SIN results in morphologic changes and DNA fragmentation typical of apoptosis within 12 to 24 h (21). To determine whether apoptosis also plays a role in SIN P62- and 6K-plus-E1-induced AT3 cell death, we examined AT3 cells microscopically after transient transfection with the SIN structural gene vectors. Under phase-contrast microscopy, P62- and 6K-plus-E1-transfected cells displayed apoptotic morphology, including membrane blebbing and cytoplasmic condensation (Fig. 3B and C); these features were absent in mock-transfected cells and in those transfected with control vector (Fig. 3A) and SIN capsid (not shown). To further confirm that transfected AT3 cells were undergoing apoptosis, we performed EM on AT3 cells transfected with control vector, vector expressing 6K plus E1, or vector expressing P62. AT3 cells transfected with 6K plus E1 and with P62 displayed chromatin condensation and fragmentation into apoptotic bodies, loss of surface microvilli, and cytoplasmic vacuolization, which are all hallmarks of apoptosis (Fig. 4). EM analysis of AT3 cells transfected with the control vector failed to demonstrate these changes. Together, these observations suggest that SIN P62 and 6K plus E1 kill AT3 cells by inducing apoptosis.
FIG. 3.
Phase-contrast micrographs of AT3 cells after transfection with a control vector (A), 6K plus E1 (B), or P62 (C). Arrows denote representative apoptotic cells. Original magnification, ×300.
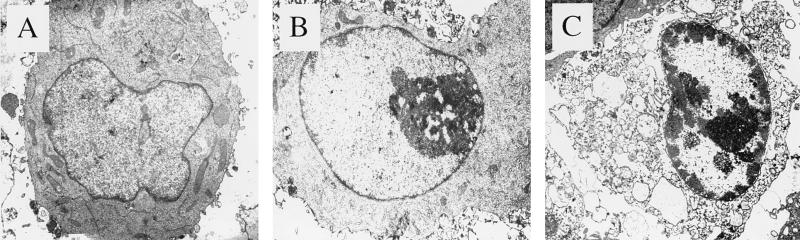
FIG. 4.
Electron micrographs of AT3 cells after transfection with a control vector (A), 6K plus E1 (B), or P62 (C). The cell shown in panel B demonstrates early apoptotic changes (chromatin condensation), and the cell shown in panel C demonstrates later apoptotic features, including cytoplasmic vacuolization and the loss of surface microvilli.
Deletion-mutational analysis of P62-induced death of AT3 cells.
After demonstrating that transient expression of SIN P62 and 6K plus E1 kills AT3 cells, we investigated which domain of P62 is responsible for the induction of AT3 cell death. As stated above, P62 is composed of the E3 signal peptide and the mature E2 envelope glycoprotein. E2 is a type 1 membrane protein with a 364-aa extracellular domain, a 28-aa TMD, and a 31-aa cytoplasmic tail. We chose to focus initially on P62 because it is an important determinant of neurovirulence. Furthermore, the previous observation that a single amino acid mutation at position 55 of E2 overcomes Bcl-2-mediated protection against SIN-induced apoptosis suggested a potential role for the extracellular domain of E2 in an apoptosis induction pathway (44).
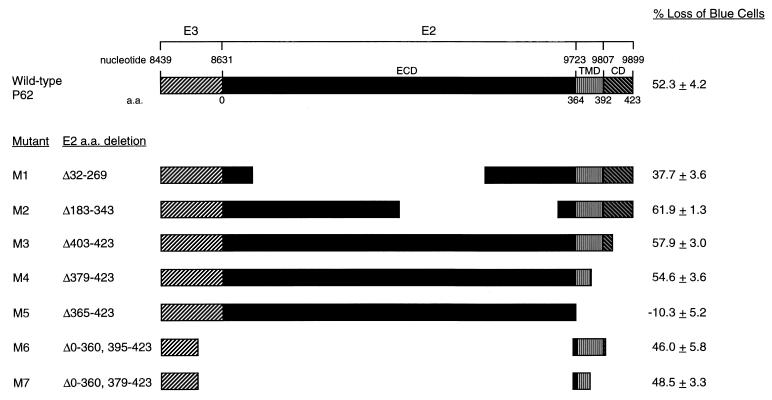
To identify the specific death domain of P62, we performed a deletion-mutational analysis. A diagram of the deletion mutants is shown in Fig. 5. All P62 deletion mutant constructs except the mutant lacking the entire TMD and cytoplasmic domain (positions 365 to 423) were able to induce death in AT3 cells as efficiently as wild-type P62. Initially, we constructed mutants 1 to 5, shown in Fig. 5. Cell death occurred with the expression of P62 mutants lacking large regions of the cytoplasmic domain (positions 403 to 423 [M3]), the cytoplasmic domain and the C-terminal half of the TMD (positions 379 to 423 [M4]), and large regions of the extracellular domain (positions 32 to 269 [M1] and 183 to 343 [M2]). However, transient expression of the P62 deletion mutant lacking the cytoplasmic domain and the entire TMD (positions 365 to 423 [M5]) was not able to induce death in AT3 cells, suggesting that either the E2 TMD itself plays a direct role in cell death induction or that it is merely required as a membrane anchor.
FIG. 5.
Deletion mutation analysis of the effects of P62 on AT3 cell death. On top, a schematic of wild-type P62 is shown. Below, schematics of P62 deletion mutants are shown. ECD, E2 extracellular domain; CD, E2 cytoplasmic domain. The percentage loss of blue cells is relative to results achieved by transfection with the control empty vector, GH52. The numbers shown represent means ± standard errors of the mean for transfections done in triplicate from a representative experiment. Similar results were obtained in more than five independent transfections.
To distinguish between these possibilities, we made two additional deletion mutant constructs, M6 and M7. Both contained the first 38 aa of E3 (which has been previously shown to constitute the minimal signal sequence required for proper trafficking of P62 [10]) and 4 charged aa residues N′ terminal to the E2 TMD. M6 contained the entire E2 TMD, and M7 contained only the first 14 aa of E2 TMD, often referred to as the stop transfer signal. Both M6 and M7 were able to induce AT3 cell death as efficiently as full-length P62, suggesting a possible direct role for the E2 TMD in AT3 cell death induction. However, these results do not exclude a role for the E3 signal sequence or the 4 aa from the extracellular domain N′ terminal to the E2 TMD, which are also present in M6 and M7.
Induction of AT3 cell death by the SIN E2 TMD.
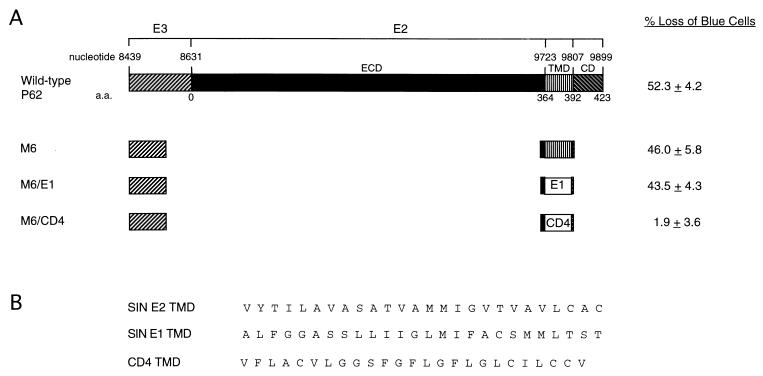
To exclude the possibility that death induced by M6 and M7 was caused by the E3 signal or the 4 aa extracellular to the E2 TMD, as well as to evaluate whether death induction was specific to the SIN E2 TMD (as opposed to a nonspecific effect of overexpressing a transmembrane protein in AT3 cells), we constructed a control plasmid (M6/CD4) in which the E2 TMD in M6 was replaced by the TMD of the mouse CD4 receptor (27). The CD4 TMD has previously been used successfully as a membrane anchor in the construction of chimeric proteins (37, 46), and its overexpression has not been associated with the induction of cell death in HeLa and mouse MOP8 cells. Similarly, in AT3 cells, we found that cotransfection of M6/CD4 with pCMVβ did not lead to a loss of blue cells, compared to cotransfection with empty vector and pCMVβ. This is in contrast to the 46% loss of blue cells after cotransfection with M6 (containing the E2 TMD) and pCMVβ (Fig. 6). FLAG-epitope-tagged M6 and M6/CD4 displayed identical patterns of immunoreactivity after AT3 cell transfection: both proteins demonstrated a punctate pattern in the perinuclear region and cytoplasm, consistent with the expected association with intracellular membranes (data not shown). These findings suggest that the SIN E2 TMD has a direct and specific role in the induction of cell death in AT3 cells.
FIG. 6.
(A) Effects of E2, E1, and CD4 TMDs on AT3 cell death. A schematic of wild-type P62 is shown. The construct M6 contains amino acid deletions shown in Fig. 5. In M6/CD4, the E2 TMD of M6 is replaced by the TMD of mouse CD4. In M6/E1, the E2 TMD of M6 is replaced by the TMD of SIN E1. The percentage loss of blue cells is relative to results achieved by transfection with the control empty vector, GH52. The numbers shown represent means ± standard errors of the mean for transfections done in triplicate from a representative experiment. Similar results were obtained in more than five independent transfections. (B) Amino acid sequences of the TMDs of the constructs depicted schematically in panel A. SIN E2 TMD is from M6, SIN E1 TMD is from M6/E1, and CD4 TMD is from M6/CD4.
Induction of AT3 cell apoptosis by the SIN E2 TMD.
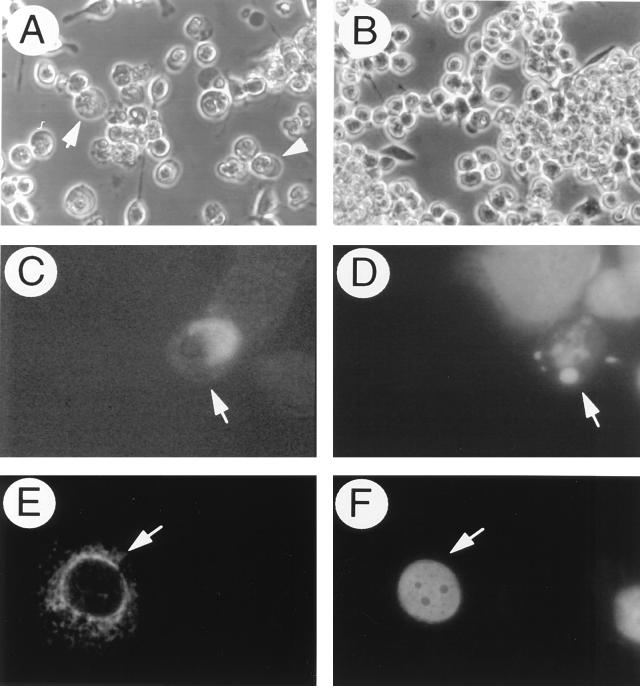
While the assessment of the loss of blue cells is an accurate means of quantitating cell death in AT3 cells cotransfected with pCMVβ and SIN structural gene plasmids, we wished to confirm the presence of apoptotic morphology in AT3 cells expressing the SIN E2 TMD. Therefore, we performed immunofluorescent staining to detect FLAG-M6 expression in transfected AT3 cells with an anti-FLAG antibody and simultaneously labeled the cells with Hoechst 33258 to detect apoptotic condensed nuclei. First, we confirmed that the loss of blue cells with AT3 cells transfected with FLAG epitope-tagged M6 and M6/CD4 was similar to that with non-epitope-tagged constructs (data not shown); we also confirmed that cell morphology, as visualized by light microscopy, was similar for FLAG epitope-tagged (Fig. 7A and B) and non-epitope-tagged constructs. For immunofluorescence staining, adherent cells were fixed earlier (48 h after transfection) than for experiments quantitating the loss of blue cells (60 h after transfection) to facilitate the detection of apoptotic cells prior to detachment. AT3 cells that expressed FLAG-M6 typically displayed apoptotic nuclei, whereas nonimmunoreactive cells displayed normal nuclear morphology (Fig. 7C and D). Similar apoptotic changes were not seen in the nuclei of cells that expressed FLAG-M6/CD4 (Fig. 7E and F). These observations confirmed that apoptosis occurred in cells expressing M6.
FIG. 7.
AT3 cell apoptosis induction by FLAG-M6. Light micrographs of AT3 cells transfected with FLAG-M6 (A) or FLAG-M6/CD4 (B). Arrowheads in panel A denote representative apoptotic cells. Simultaneous immunofluorescent staining (C and E) to detect FLAG epitope expression and Hoechst staining (D and F) to detect apoptotic nuclei in FLAG-M6- (C and D) and FLAG-M6/CD4-transfected (E and F) AT3 cells was done. Arrows in panels C and D denote the same FLAG-M6-transfected cell, and the arrows in panels E and F denote the same FLAG-M6/CD4-transfected cell. Magnifications are ×300 for panels A and B and ×1,000 for panels C and F.
Induction of AT3 cell death by the SIN E1 TMD.
Since 6K plus E1 induces AT3 cell death as efficiently as P62, we investigated whether the TMD of E1 is like the E2 TMD in being able to induce death directly in AT3 cells. We replaced the E2 TMD in M6 with the E1 TMD and compared the abilities of M6 and M6/E1 to induce death in AT3 transient-transfection assays. Cotransfection of AT3 cells with M6/E1 and pCMVβ resulted in a percentage loss of blue cells comparable to that obtained by cotransfection of AT3 cells with M6 (Fig. 6). Thus, the TMDs of both the SIN E2 and E1 envelope glycoproteins, but not the control murine CD4 TMD, are able to induce death in AT3 cells.
Kinetics of SIN protein-induced AT3 cell death.
To confirm that the death induced by SIN P62, SIN 6K plus E1, SIN E2 TMD, and SIN E1 TMD, as measured by the loss of blue cells, was not the result of decreased protein expression of these constructs at the assayed time point (60 h), we examined the numbers of blue cells and the percentages of trypan blue-positive dead cells at serial time points after transfection. In the assays measuring blue cells, detached dying cells in the nonadherent layer were removed, and only remaining viable cells were counted, whereas in the assay measuring the percentage of dead cells, both the nonadherent and adherent layers were collected together.
At 6 h after transfection, no blue cells were detected with AT3 cells cotransfected with reporter plasmid and SIN structural gene expression plasmids, and the viabilities of AT3 cells transfected with all of the SIN structural gene plasmids were comparable to those of AT3 cells transfected with the control vector. At 12 h after transfection, there was already a small, but significant, decrease in the number of blue cells in wells containing cells transfected with death-inducing constructs (P62, 6K plus E1, M6, and M6/E1), compared to wells with cells transfected with the control vector, capsid, or M6/CD4 (P < 0.001 by Student’s t test). The corresponding increase in cell death 12 h after transfection with P62, 6K plus E1, M6, and M6/E1 suggested that this decreased number of blue cells was a reflection of cell death occurring rather than of decreased transfection efficiency or protein expression. At 24 h after transfection, the differences between P62, 6K plus E1, M6, and M6/E1, on the one hand, and control vector, capsid, and M6/CD4, on the other hand, were similar, but of greater magnitude. At 48 h after transfection, the peak number of blue cells was observed with AT3 cells transfected with control vector, capsid, or M6/CD4, whereas at this time point, the number of blue cells with AT3 cells transfected with P62, 6K plus E1, M6, or M6/E1 had already declined. (The percentage of dead cells was not measured after 24 h after transfection because of the confounding influence of the proliferation of nontransfected viable cells.) Taken together, the kinetic data in Fig. 8A and B suggest that the loss of blue cells at later time points after AT3 cell transfection with P62, 6K plus E1, M6, and M6/E1 is a reflection of cell death induced by expression of these proteins.
DISCUSSION
We investigated the roles of the SIN structural proteins in the induction of apoptosis in rat prostate adenocarcinoma AT3 cells. Our findings demonstrate that transient expression of the E2 and E1 envelope glycoproteins, but not of the capsid protein, results in apoptosis. Furthermore, deletion of large regions of the extracellular domain (encompassing previously defined determinants of neurovirulence) and of the cytoplasmic domain of E2 does not diminish the death-inducing ability of the precursor E2 protein. However, a mutant precursor E2 lacking the TMD is unable to induce cell death, and expression of the TMDs of E1 and E2, but not of the control protein murine CD4, results in the induction of AT3 cell death. Taken together, these findings suggest that the TMDs of the SIN E2 and E1 envelope glycoproteins are capable of inducing apoptosis in AT3 cells.
It is well established that SIN induces apoptosis in vitro in several different cell types (e.g., AT3, BHK, N18, and PC12 cells [5, 15, 21, 44, 45]) as well as in vivo (23, 24) in mouse brains. In addition, several cellular factors that play roles in regulating SIN-induced apoptosis have been identified, including antiapoptotic genes of the bcl-2 family (5, 21, 23, 44), caspases (32), ras (15), nitric oxide (43), and NF-κB (26). Yet little is known about the viral factors that trigger the cell death program in infected cells. Our findings are the first to define viral gene products (e.g., SIN E2 and E1) that may play roles in SIN-induced apoptosis as well as to define a potential mechanism by which such gene products induce apoptosis (e.g., via effects on cellular membranes).
At present, it is not known whether the abilities of the SIN E2 and E1 TMDs to induce apoptosis in transfected cells are indicative of actual roles for these protein domains in apoptosis in SIN-infected cells. The biologic activities of viral proteins, when expressed individually in transfected cells, may or may not mimic the activities of the proteins when expressed in infected cells. However, since SIN usually induces apoptosis in infected cells, it is unlikely that other SIN viral proteins expressed in infected cells have antiapoptotic effects that would antagonize the actions of the SIN E2 and E1 TMDs. The identification of loss-of-function point mutations in the death domains of E2 and E1, with subsequent introduction of such mutations into recombinant SINs, will be important in evaluating the roles of the E2 and E1 TMDs in virus-induced apoptosis.
Our findings that E2 or E1 alone can kill AT3 cells contrast somewhat with previous studies which found that expression of both E2 and E1 was required for rapid CPEs in BHK cells (9). However, we have also found that BHK cell transfection with SIN structural gene plasmids containing either P62 or 6K plus E1 does not induce BHK cell death (16). Thus, the ability of E2 or E1 to induce cell death independently is likely to be a cell type-specific phenomenon. It is possible that the mechanisms by which the E2 and E1 glycoproteins induce death in AT3 cells are fundamentally different from the mechanisms operating in BHK cells. Alternatively, it is possible that coexpression of E2 and E1 is required in BHK cells for protein stability, protein trafficking, protein-protein interactions, or the protein effects on membranes that are necessary for death induction, but that similar phenomena can occur in AT3 cells without E2-E1 heterodimer formation.
Our finding that aa 32 to 269 of the extracellular domain of E2 were not required for P62-induced apoptosis in AT3 cells was somewhat unexpected, in view of the observation that a Gln→His mutation at position 55 of E2 overcomes the ability of Bcl-2 to protect AT3 cells against virus-induced apoptosis (44). The ability of a mutation at position 55 of E2 to both confer neurovirulence (22, 42, 44) and overcome AT3 cellular blocks to apoptosis (44) raised the possibility that this region of E2 was somehow directly involved in apoptosis induction. While we cannot exclude this possibility on the basis of the findings of the present study, our observation that P62 containing a 238-aa deletion encompassing position 55 of E2 still induces apoptosis suggests that other explanations for the effects of the E2 Gln→His mutation on cellular apoptosis may be more likely. For example, this mutation has been shown to increase viral replication in mouse brain, neuroblastoma cells, and BHK cells (42), as well as to block an antiviral effect of Bcl-2 in AT3 cells (44). Thus, the ability of the E2 position 55 Gln→His change to block Bcl-2-mediated protection may reflect effects on the regulation of viral replication in Bcl-2-overexpressing cells rather than direct participation of this region of E2 in apoptosis induction. This hypothesis is consistent with our observation that transfection of AT3 cells with a P62 construct containing a Gln→His mutation at E2 position 55 results in a level of cell death comparable to, but not greater than, that achieved by transfection with P62 containing wild-type glutamine at E2 position 55 (16).
The mechanism(s) by which the SIN E1 and SIN E2 TMDs trigger apoptosis in AT3 cells is unknown. Interestingly, there is accumulating evidence to suggest that cellular regulators of apoptosis of the Bcl-2 family may function, at least in part, by virtue of their effects on intracellular membranes with which they associate, namely the outer mitochondrial membrane, the ER, and the nuclear envelope. Earlier cell transfection studies indicated that the cell death inhibitor Bcl-2 might have a membrane transport function, with reported effects on Ca2+ flux (18) and protein translocation (including the apoptogenic protease activators cytochrome c [17, 47] and apoptosis-inducing factor [40]) across intracellular membranes. Recently, it was shown that the structure of Bcl-xL, another death repressor member of the Bcl-2 family, is similar to that of the pore-forming domain of bacterial toxins (30). This finding led to several subsequent studies demonstrating that both Bcl-2 and Bcl-xL, as well as the proapoptotic Bcl-2 family member Bax, have ion channel activity (29, 34, 36). Thus, it is tempting to speculate that viral envelope protein TMDs may be part of an evolutionarily conserved functional family including bacterial toxins and cellular apoptotic regulators and that the SIN E1 and SIN E2 TMDs may have a membrane-associated death-inducer function analogous to that of the cellular death inducer Bax.
Alternatively, the SIN E1 and SIN E2 TMDs might heterodimerize with other membrane-associated molecules involved in apoptotic pathways or participate in ER nuclear death signalling pathways. The signal transduction pathway involving NF-κB activation represents one such candidate pathway. Previously, Lin et al. demonstrated NF-κB activation in AT3 cells undergoing apoptosis, as well as inhibition of NF-κB activity and apoptosis following AT3 cell treatment with NF-κB transcription factor decoys (26). These findings suggest that NF-κB-dependent signalling pathways may be important in SIN-induced apoptosis of AT3 cells. Although we have not yet determined whether E2 and E1 TMD expression are like SIN infection in leading to NF-κB activation in AT3 cells, Pahl and Baeuerle have demonstrated that expression of the influenza virus transmembrane protein, the virion surface hemagglutinin (HA), strongly activates NF-κB DNA binding and transactivation (33). However, it is not yet known whether the NF-κB activation that occurs after influenza HA transfection plays a role in the apoptosis that is induced by influenza virus infection (12). In addition, several studies have shown that in tumor necrosis factor signalling pathways, NF-κB activation is part of a divergent pathway that is distinct from apoptosis induction (4, 13, 31). Thus, it remains to be determined whether NF-κB activation by viral envelope proteins in the ER is important in apoptosis induction.
Cellular proteases of the caspase family are activated by death stimuli and appear to be a nearly universal, if not universal, part of cellular apoptotic pathways. Thus, it is likely that the expression of SIN E1 and E2 TMDs leads to activation of one or more caspases. However, at present it is unknown which caspase(s) is important for mediating SIN E1 and E2 TMD-induced death in AT3 cells. Nava et al. have shown that SIN activates caspases and that SIN-induced apoptosis in BHK and N18 cells can be blocked by the caspase inhibitors CrmA (a serpin from cowpox virus) and zVAD-FMK (a peptide-fluoromethyl ketone) (32). However, we have found that CrmA does not block SIN-induced apoptosis in AT3 cells and that zVAD-FMK does not block SIN E1 and E2 TMD-induced AT3 cell death (16), suggesting that proteases with different substrate specificities are important in BHK and N18 cells compared to AT3 cells. Elucidation of the specific cell death protease(s) activated by SIN E1 and SIN E2 in AT3 cells will be important in unravelling the signalling events linking viral transmembrane protein expression with cell death.
Interestingly, the N′-terminal half of the SIN E2 TMD (which is sufficient for death induction) shares 9 of 14 aa with the TMD of the Japan/305/57 strain of influenza A virus HA protein (VYTILAVASATVAM in SIN E2; VYQILAIYATVAG in influenza A virus HA Japan/305/57). While we have not yet tested whether the Japan/305/57 HA peptide also induces death, it seems probable that overexpression of other viral TMDs may also lead to cell death. The activation of an ER-dependent death signalling pathway may be one of many general mechanisms by which enveloped viruses can destroy their cellular targets. As many transcription factors are dually involved in apoptosis and antiviral cytokine gene expression (e.g., NF-κB, IRF1, c-Jun), it is possible that such ER-dependent signalling pathways are turned on as a cellular defense against the presence of viral proteins.
ACKNOWLEDGMENTS
We thank J. Marie Hardwick for providing the plasmid GH52, Gerald Siu for providing a murine CD4 plasmid, Wanda Setlik for assistance with electron microscopy, and Hui Hui Jiang for excellent technical assistance.
This work was supported by a James S. McDonnell Foundation Scholar Award (B.L.) and NIH grants KO8 AIO1217 (B.L.) and R29 AI40246 (B.L.). A.K.J. was supported by a Hatch Foundation Fellowship and a Markey Scholar Fellowship. B.L. was supported by an American Cancer Society Junior Faculty Research Award and an Irma T. Hirschl Trust Career Scientist Award.
REFERENCES
- 1.Bertin J, Mendrysa S M, LaCount D J, Gaur S, Krebs J F, Armstrong R C, Tomaselli K J, Friesen P D. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulakia C, Chen G, Ng F W, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Bcl-2 and adenovirus E1B 19 kDA protein prevent EIA-induced processing of CPP32 and cleavage of poly (ADP-ribose) polymerase. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- 3.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 4.Cai Z, Korner M, Tarantino N, Chouiab S. IκB α overexpression in human breast carcinoma MCF7 cells inhibits nuclear factor-κB activation but not tumor necrosis factor-α-induced apoptosis. J Biol Chem. 1997;272:96–101. doi: 10.1074/jbc.272.1.96. [DOI] [PubMed] [Google Scholar]
- 5.Cheng E H Y, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 6.Clem R J, Miller L K. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 8.Duckett C S, Nava V E, Gedrich R W, Clem R J, VanDongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 9.Frolov I, Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994;68:1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garoff H, Huylebroeck D, Robinson A, Tillman U, Liljestrom P. The signal sequence of the P62 protein of Semliki Forest virus is involved in initiation but not in completing chain translocation. J Cell Biol. 1987;111:867–876. doi: 10.1083/jcb.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 14.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 15.Joe A K, Ferrari G, Jiang H H, Liang X H, Levine B. Dominant inhibitory Ras delays Sindbis virus-induced apoptosis in neuronal cells. J Virol. 1996;70:7744–7751. doi: 10.1128/jvi.70.11.7744-7751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joe, A. K., and B. Levine. Unpublished data.
- 17.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 18.Lam M, Dubyak G, Chen L, Nunez G, Miesfeld R L, Distelhorst C W. Evidence that Bcl-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1995;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 20.Laurent-Crawford A G, Coccia E, Krust B, Hovanessian A G. Membrane-expressed HIV envelope glycoprotein heterodimer is a powerful inducer of cell death in uninfected CD4+ target cells. Res Virol. 1995;146:5–17. doi: 10.1016/0923-2516(96)80585-1. [DOI] [PubMed] [Google Scholar]
- 21.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 22.Levine B, Griffin D E. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993;67:6872–6875. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis J, Wesslingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 26.Lin K-I, Lee S-H, Narayanan R, Baraban J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littman D R, Gettner S N. Unusual intron in the immunoglobulin domain of the newly isolated murine CD4 (L3T4) gene. Nature. 1987;325:453–455. doi: 10.1038/325453a0. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y Y, Koga Y, Tanaka K, Sasaki M, Kimura G, Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994;68:390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Bcl-xL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 30.Muchmore S W, Sattler M, Liang H, Meadows R P, Harian J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S L, Ng S L, Fesik S W. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 31.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 32.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahl H L, Baeuerle P A. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J Virol. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 35.Sastry K J, Marin M C, Nehete P N, McConnell K, el-Naggar A K, McDonnell T J. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene. 1996;13:487–493. [PubMed] [Google Scholar]
- 36.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Channel formation by anti-apoptotic protein, Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selinka H-C, Zibert A, Wimmer E. A chimeric poliovirus-CD4 receptor confers susceptibility to poliovirus on mouse cells. J Virol. 1992;66:2523–2526. doi: 10.1128/jvi.66.4.2523-2526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 39.Strack P R, Frey M W, Rizzo C J, Cordova B, George H J, Meade R, Ho S P, Corman J, Tritch R, Korant B D. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci USA. 1996;93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tucker P C, Strauss E G, Kuhn R J, Strauss J H, Griffin D E. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993;67:4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker P C, Griffin D E, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ubol S, Park S, Budihardjo I, Desnoyers S, Montrose M H, Poirier G G, Kaufman S H, Griffin D E. Temporal changes in chromatin, intracellular calcium, and poly(ADP-ribose) polymerase during Sindbis virus-induced apoptosis of neuroblastoma cells. J Virol. 1996;70:2215–2220. doi: 10.1128/jvi.70.4.2215-2220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willey R L, Buckler-White A, Strebel K. Sequences present in the cytoplasmic domain of CD4 are necessary and sufficient to confer sensitivity to the human immunodeficiency virus type 1 Vpu protein. J Virol. 1994;68:1207–1212. doi: 10.1128/jvi.68.2.1207-1212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]