Abstract
To provide guidance to medical providers, wilderness users, and travelers, the Wilderness Medical Society convened an expert panel to develop evidence-based guidelines for treating water in situations where the potability of available water is not assured, including wilderness and international travel, areas impacted by disaster, and other areas without adequate sanitation. The guidelines present the available methods for reducing or eliminating microbiological contamination of water for individuals, groups, or households; evaluation of their effectiveness; and practical considerations. The evidence base includes both laboratory and clinical publications. The panel graded the recommendations based on the quality of supporting evidence and the balance between benefits and risks/burdens according to the criteria published by the American College of Chest Physicians.
Keywords: drinking water, water disinfection, disaster water treatment, pasteurization, halogens, microfilter, ultraviolet light, coagulation–flocculation
Introduction
Safe and efficient treatment of drinking water is among the major public health advances of the last century. Without it, waterborne diseases can spread rapidly, resulting in large-scale disease and death.1,2 In high-income countries, the population is generally protected from waterborne disease by sophisticated water supply systems that disinfect water and provide continuous monitoring. In contrast, travelers to wilderness and recreational areas anywhere in the world and to low- and middle-income regions of some countries may be confronted with untreated or contaminated water that poses a risk of acquiring enteric disease. Wilderness visitors and international travelers have no reliable resources to evaluate local water system quality. Less information is available for remote surface water sources. Appearance, smell, and taste are not reliable indicators to estimate water safety from infectious organisms. In addition, disaster situations, such as earthquakes, hurricanes, and flooding events, may result in a breakdown of municipal water systems, exposing victims to nonpotable water. These situations necessitate knowledge of how to disinfect water at the point-of-use, prior to drinking.
Methods of water treatment that can be applied in the field include the use of heat, ultraviolet (UV) light, clarification, filtration, and chemical disinfection. The choices for the wilderness hiker or international traveler are increasing as new technology is applied to field applications. Different microorganisms have varying susceptibilities to these methods. The risk of waterborne illness depends on the number and type of organisms consumed, host factors, and the efficacy of the treatment system.
The Wilderness Medical Society Practice Guidelines for Water Treatment were first published in the Wilderness Environmental Medicine Journal in 2019.3 The current guideline updates this earlier one with additional information and sources. Three evidence grades were changed due to additional evidence, further evidence evaluation by the authors, or modified scope of the recommendation.
Methods
Our panel of specialists in wilderness medicine, travel medicine, public health, and microbiology was chosen in 2017 based on interest and expertise in drinking water quality, as demonstrated by research and publications to develop the initial guidelines. The same panel authored this revision. We used relevant articles from the 2019 guideline and identified recent publications through PubMed and Google Scholar databases using the following keywords or phrases: water disinfection, water purification or water treatment, waterborne illness, water sanitation and health, plus specific terms in combination with those general search terms: filtration, halogen, pasteurization, ultraviolet, SODIS, nanoparticles, household, and disaster. This was supplemented by a search of references or topics from articles in the initial results. References were not limited by publication year because much of the key research on basic methods applicable to our interest was conducted decades earlier. Websites are cited when they provide unique data, reports, or agency recommendations. Some review articles are cited to provide sources of in-depth information and to augment reference selection.
The evidence base for water treatment has 2 substantial differences from other clinical guidelines. Most of the literature concerning effectiveness of specific treatment methods against various waterborne microorganisms is laboratory-based. Evidence of the benefits of water treatment is either population-based public health research of disease outbreaks or household trials of water treatment. Therefore, the evidence grade is a combination of laboratory, population, and household or community-level studies that can be extrapolated to wilderness settings and international travel. In addition, the information is divided between medical and engineering literature.
The panel used a consensus approach to develop research questions and recommendations. Evidence grades were assigned according to the methodology stipulated by the American College of Chest Physicians guideline methodology, which was updated in 2018 (see online Supplemental Table 1).4 These recommendations are graded based on the totality of supporting evidence and balance between the benefits and risks or burdens for each modality. Water treatment techniques were primarily evaluated for removal of microbiological contaminants, not for the removal of chemicals or toxins. Laboratory research evaluating treatment impact on microorganisms was considered as high, moderate, or low quality, similar to clinical trials.
Risk and Etiology of Waterborne Infection
Even in high-income countries with low rates of diarrheal illness, regular waterborne disease outbreaks indicate that the microbiologic quality of the water, especially surface water, is not assured.5–9 The environment and activity upstream from the travelers’ surface water source define the risk. Upstream usage by humans, farm animals, or wildlife poses a major risk.10–14 Giardiasis is a zoonotic infection with numerous host species, including farm animals, deer, and other wild ungulates, beavers, and even household animals.15
Because it is very difficult to exclude animal and human activity in the watershed, the US Centers for Disease Control and Prevention (CDC) recommends treating any surface water before ingestion as a precaution to protect health.16
While substantial progress has been made in the past 20 y toward the goal of safe drinking water and sanitation worldwide, 25% of the world’s population lacks safely managed drinking water in their homes. A total of 1.7 billion people lack access to basic sanitation services, and nearly 500 million people still practice open defecation.17 Studies in low- and middle-income regions around the world show high levels of microbes in the environment and water sources.18–22 The combined roles of safe water, adequate sanitation, and hygiene in reducing diarrhea and other diseases are well documented.23–25 In any area of the world, after natural disasters such as hurricanes, tsunamis, and major earthquakes, one of the most immediate public health problems is a lack of potable water.26,27
Infectious agents with the potential for waterborne transmission include bacteria, viruses, protozoa, and nonprotozoan parasites. Most enteric organisms, including Shigella spp, Salmonella enterica serotype Typhi, hepatitis A, and Cryptosporidium spp, can retain viability for long periods in water, including when frozen in water.28–30
The risk of waterborne illness depends on the number of organisms consumed, virulence of the organism, and defenses of the host.31,32 Microorganisms with a low infectious dose (eg, Giardia, Cryptosporidium, Shigella spp, hepatitis A, enterohemorrhagic Escherichia coli, and norovirus) may cause illness even from inadvertent drinking during water-based recreational activities.33
Although the primary reason for treating and disinfecting drinking water is to destroy microorganisms from animal and human biologic wastes, water may also be contaminated with toxins and chemical pollutants from industrial sources or from the environment. Toxins can be generated by biological organisms.34 Many cyanobacteria (also known as blue-green algae) may produce toxins such as microcystins under certain conditions that can cause a wide range of symptoms, including stomach pain, vomiting, diarrhea, headache, neurological symptoms such as confusion and seizures, liver damage, and cardiovascular collapse.35
General Recommendations for Drinking Water Disinfection
We recommend treating water when traveling in low- and middle-income countries, especially in rural areas. Strong recommendation, high quality evidence.
We recommend treating water in wilderness areas with nearby agricultural use, animal grazing, or upstream human activity. Strong recommendation, high quality evidence.
We suggest treating water in wilderness settings without evidence of domestic animals and with little to no wildlife or human activity. Weak recommendation, low quality evidence.
We recommend treating water in disaster situations affecting municipal or private drinking water sources. Strong recommendation, high quality evidence.
Definitions
The term disinfection, the desired result of field water treatment, is used here to indicate the destruction or inactivation of harmful microorganisms, which reduces the risk of illness. This is sometimes used interchangeably with purification, but the latter term more accurately indicates the removal of organic or inorganic chemicals and particulate matter to improve color, taste, and odor. The term filtration is used here to refer to the process of physically removing potentially harmful microorganisms (as well as nonbiological particles) from water using a porous material, thereby reducing the risk of illness. Unless specifically designed to remove chemical contaminants, disinfection or filtration techniques may not make water safe from chemical exposures. Potable implies “drinkable” water but technically means that a water source, on average, over a period of time, contains a “minimal microbial hazard,” so that the statistical likelihood of illness is acceptably low. All enforceable standards, including water regulations in the United States, acknowledge the impracticality of trying to eliminate all microorganisms from drinking water, allowing a small risk for enteric infection.36 Elimination of all microorganisms may be especially impractical for point-of-use or household treatment methods applied to surface water.
Water Treatment Methods
Multiple techniques for improving the microbiologic quality of water are available to individuals and small groups while hiking or traveling. Bottled water may be a convenient and popular solution but has logistical challenges (eg, access, bulk, and weight) and creates ecological problems from plastic waste. Furthermore, in some low- and middle-income countries, the quality of packaged water (eg, sold in bottles or sachets) may not meet the standards of high-income countries and may contain pathogenic microbes.37,38
Clarification Techniques
Clarification refers to techniques generally used as a predisinfection step that reduce the cloudiness of water caused by natural organic and inorganic material—known as turbidity and measured in nephelometric turbidity units (NTU). Higher turbidity indicates more suspended solids in the water and creates a dirty appearance. An average person can begin to see turbidity levels starting at approximately 5 NTU, but water treatment standards generally require less than 1 NTU. Cloudy water can rapidly clog filters designed to remove microorganisms. Moreover, cloudy water requires increased levels of chemical treatment, and the combined effects of water contaminants plus chemical disinfectants result in an unpleasant taste. Clarification techniques can markedly improve the appearance and taste of water. They may reduce the number of microorganisms, but not enough to ensure potable water; however, clarifying the water facilitates disinfection by filtration or chemical treatment.
Adsorption.
Granular activated carbon is widely used in water treatment. When activated, charcoal’s regular array of carbon bonds is disrupted, making it highly reactive for adsorbing dissolved chemicals.39,40 Granular activated carbon is the best means to remove toxic organic and inorganic chemicals from water (including disinfection byproducts) and to improve odor and taste.41,42 Thus, it is widely used in municipal disinfection plants, in household undersink devices and water pitchers, and in portable water filters. Granular activated carbon does not kill microorganisms and is not designed for microbial removal.41–43
In field water treatment, granular activated carbon is often used after chemical disinfection to make water safer and more palatable by removing the taste of chemical disinfectants, disinfection byproducts, and pesticides as well as many other organic chemicals and some heavy metals. Activated charcoal will remove most but not all microcystin toxins so it may decrease the risk or severity of illness but cannot eliminate risk.44,45
Sedimentation.
Sedimentation is the separation of suspended particles, such as sand and silt, that are large enough to settle rapidly by gravity. Most microorganisms, especially protozoan cysts, also settle eventually, but this takes much longer.46 Simply allowing the water to sit undisturbed for about 1 h or until sediment has formed on the bottom of the container—then decanting or filtering the clear water from the top through a coffee filter or finely woven cloth will remove many larger particles from the water. Additional treatment is needed to obtain potable water.
Coagulation–Flocculation.
Coagulation–flocculation (C-F) is a technique in use since 2000 bc and remains a routine step in municipal water treatment.47,48 Coagulation–flocculation can also be easily used in the field to improve water quality. The process removes smaller suspended particles (colloids) and chemical complexes too small to settle by gravity. Coagulation is achieved with the addition of a chemical that causes particles to stick together by electrostatic and ionic forces. Flocculation is a physical process that promotes the formation of larger particles by gentle mixing. Alum (an aluminum salt), lime, or iron salts are commonly used coagulants. Alum is nontoxic at appropriate water treatment doses and is used in the food industry for pickling. It is readily available in most chemical supply stores and some grocery stores. Coagulation–flocculation removes 60 to 98% of microorganisms, heavy metals, and some chemicals and minerals.49,50 The tendency of microorganisms to clump with small particles or clump together to form larger aggregates enhances their removal by C-F. Coagulation-flocculation also has the benefit of reducing the amount of chemical disinfectant needed as turbidity increases demand for disinfectants such as hypochlorite.50–52
The amount of alum added in the field—approximately 1 large pinch (1 mL or 1/8 tsp) in 4 L (approximately 1 gal) of water—need not be precise. The C-F agent is stirred or shaken briskly for 1 min to mix and then agitated gently and frequently for at least 5 min to assist flocculation. If the water is still cloudy, more C-F agent may be added, followed by repeat mixing. After at least 30 min for settling, water is poured through a fine-woven cloth or paper filter. Although most microorganisms are removed with the floc, a final process of microbiologic filtration or chemical disinfection (below) should be completed to ensure disinfection. Several products combine C-F with halogen disinfection, which provide a single-step dual process for low quality water that achieves better water treatment than either alone.49,53–58
Improvisational techniques for clarification.
Many traditional plants are used by native peoples as a coagulant, as well as inorganic compounds, including lime (calcium oxide) or potash (from wood ash).59,60 In an emergency, bleaching powder, baking powder, or even the fine white ash from a campfire can be used.61
Adsorbents such as charcoal and clay and other types of organic matter have been used for water treatment for millennia.43 These substances are often used as filter media but can also act as coagulants.23 Clays can decrease turbidity and microbes in water by approximately 90 to 95%,62 but adsorption is not the main action of ceramic or clay filters.
Assessment of Supporting Evidence:
Clarification reduces cloudiness, particulate matter, and microorganisms; improves the taste and esthetics of water; and improves the effectiveness of chemical disinfectants, filtration, and UV disinfection but does not disinfect if used alone. Strong recommendation, high quality evidence.
Granular activated carbon is highly effective at removing taste and odor compounds but is not adequate for microbial removal. Strong recommendation, high quality evidence.
Sedimentation is effective for removing large particles such as sand and dirt but will not remove suspended or dissolved substances. Strong recommendation, moderate quality evidence.
Coagulation–flocculation removes most microorganisms but does not disinfect if used alone. Strong recommendation, high quality evidence.
Traditional or improvisational C-F techniques (other than alum or those used in municipal disinfection plants) have empirical evidence but do not have robust scientific evidence or practical use guidance. Weak recommendation, low quality evidence.
Disinfection and Filtration Methods
Heat. Heat is the oldest and most reliable means of water disinfection. Heat inactivation of microorganisms is a function of time and temperature (exponential function of first-order kinetics). Thus, the thermal death point is reached in a shorter time at higher temperatures, while lower temperatures are effective if applied for a longer time. Pasteurization uses this principle to kill food pathogens and spoiling organisms at temperatures well below boiling, generally between 60°C (140°F) and 70°C (158°F) within 30 min. Flash pasteurization occurs at 70 to 72°C (158–162°F) within 30 s.63,64
All common enteric pathogens are readily inactivated by heat at pasteurization temperatures, although microorganisms vary in heat sensitivity, with protozoan cysts being the most sensitive to heat, bacteria intermediate, and viruses less sensitive (Table 1).65,66,67–77 Only bacterial spores are more resistant, but they are not generally enteric pathogens.78 After boiling, water may become contaminated again from storage containers or handling.79,80
Table I.
Heat inactivation of microorganisms.
| Organism | Lethal temperature/time | Reference |
|---|---|---|
| Protozoan cysts, including Giardia, Entamoeba histolytica | 50°C (122°F) for 10 min 55°C (131 °F) for 5 min 100°C (212°F) immediately | 67–69 |
| Cryptosporidium oocysts | 55°C (131 °F) warmed over 20 min 64°C (148°F) within 2 min | 65,70 |
| Parasitic eggs, larvae, and cercariae | 50–55°C (122–131 °F) for 30 min 60–75°C for 15–30 mina | 71,72 |
| Common bacterial enteric pathogens (E coli, Salmonella, Campylobacter, Shigella) | 55°C (131 °F) for 30 min or 65°C (149°F) for less than 1 min. (standard pasteurization temperatures) | 63,66 |
| Viruses | 56–60°C (133—140°F) within 20–40 min | 78,73,74 |
| Hepatitis A virus | 98°C (208°F) for 1 min 75C (167°F) for less than 0.5 min 85°C (185°F) for 1 min or less (in various food products) | 75–77 |
Tested in food. Expect lower temperatures and shorter times in water for inactivation.
As enteric pathogens are killed within seconds by boiling water and rapidly at temperatures >60°C (140°F), previous advice, now obsolete, was to boil water for 10 min to ensure potable water (Table 1). The time required to heat water from 55°C (131°F) to a boil works toward disinfection; therefore, any water brought to a rapid boil should be adequately disinfected.63 Boiling for 1 min is recommended by the CDC to account for user variability in identifying boiling points and to add a margin of safety. The boiling point decreases with increasing altitude, but this is not significant compared with the time required for thermal inactivation at these temperatures (Table 2).
Table 2.
Boiling temperatures at various altitudes.
| Altitude (ft) | Altitude (m) | Boiling point |
|---|---|---|
| 5000 | 1524 | 95°C (203°F) |
| 10,000 | 3048 | 90°C (194°F) |
| 14,000 | 4267 | 86°C (187°F) |
| 19,000 | 5791 | 81 °C (178°F) |
Improvisational techniques.
In wilderness or travel environments, the main limitation for using heat is availability of fuel. Although attaining boiling temperature is not necessary to kill microorganisms, boiling is the only easily recognizable endpoint without using a thermometer. If fuel is scarce, heat water until first sign of simmering (small bubbles rising from the bottom), reduce or remove heat, and leave container covered for 30 min. As a rule of thumb, water too hot to touch falls within the pasteurization range, but tolerance to touch is too variable to be reliable.81
If no reliable method of water treatment is available, tap water that has been kept hot in a tank for at least 30 min and is too hot to keep a finger immersed for 5 s (estimated 55–65°C; 131–149°F) has been suggested as a means of obtaining potable water for short periods of need based on heat disinfection and microbiological testing.82,83 In the long-term, drinking from water heaters is not advised based on relatively higher levels of metals (eg, lead) and chemical contaminants that can dissolve into water at elevated temperatures. Moreover, this improvisational measure is less useful for hotels that use on-demand water heaters without a hot water tank.
Travelers with access to electricity can boil water with either a small electric heating coil or a lightweight electric beverage warmer brought from home. In austere and desperate situations with hot, sunny climate, pasteurization temperature can be achieved with a solar oven or simple reflectors (see UV– solar UV disinfection [SODIS] below).84–86
Assessment of Supporting Evidence:
Bringing water to boil (100°C/212°F) will kill pathogenic microorganisms. Strong recommendation, high quality evidence.
Bringing water at 5000 m elevation (16,000 ft) to boil (83°C/181°F) will kill pathogenic organisms. Strong recommendation, moderate quality evidence.
Tap water that has been tanked for 30 min or longer and is too hot to touch (60°C) significantly reduces the number of pathogenic microorganisms but should be used for short periods only when other methods are unavailable. Weak recommendation, moderate quality evidence.
UV Light.
Ultraviolet radiation (UVR) and UV light disinfection systems are widely used to disinfect drinking water at the community and household levels. At sufficient doses, all waterborne enteric pathogens are inactivated by UVR.87 Ultraviolet C light in the range of 200 to 280 nm is the most effective. The germicidal effect of UV light is the result of action on the nucleic acids of microorganisms and depends on light intensity and exposure time. Bacteria and protozoan parasites generally require lower doses than do enteric viruses and bacterial spores. However, all viruses, including hepatitis A and norovirus, are susceptible and follow similar kinetics, with relatively minor differences. The vegetative cells of bacteria are significantly more susceptible to UVR than are bacterial spores or viruses. Giardia and Cryptosporidium are susceptible to practical doses of UVR and may be more sensitive because of their relatively large size.88–90 The UV waves must strike the organism, so the water must be free of particles that could act as a shield (ie, not cloudy).91 The UV waves do not alter the water, but they also do not provide any residual disinfecting power.92 Both large high-volume units and portable, lightweight battery-operated units for disinfection of small quantities of water are available. The cost of UV devices limits their use in low-income households and communities, but UV-LEDs are effective and show promise when the cost decreases.93,94
Improvisational technique: Solar Disinfection (SODIS) and solar pasteurization (SOPAR).
UV irradiation by sunlight can substantially improve the microbiologic quality of water and reduce diarrheal illness and is widely used in low-income countries and austere settings.60,85,95–103 Similar to powered UV lamps, solar UVR can effectively inactivate bacteria, viruses, and protozoan cysts. The optimal procedure for the SODIS technique is to use transparent bottles (eg, clear plastic beverage bottles), preferably lying on a dark or reflective surface to increase heat and UV, exposed to sunlight for a minimum of 4 to 6 h with intermittent agitation or 2 d under cloudy conditions.102,104 Solar UV disinfection has been studied extensively in mid latitudes, but efficacy has also been demonstrated in cool climates and cold water.105
Ultraviolet and thermal inactivation are strongly synergistic for the solar disinfection of drinking water, even if pasteurization temperatures are not reached.85,106–109 However, in warm-hot sunny climates, pasteurization temperatures of 65°C or greater can be achieved with a solar oven or simple reflectors (SOPAR).84,106,110,111 Much higher temperatures can be reached with more sophisticated solar pasteurization units.86 Very small, reusable water pasteurization indicators are available that indicate when a temperature of 65°C is reached.
Assessment of Supporting Evidence:
Ultraviolet light is an effective means of water disinfection. Strong recommendation, high quality evidence.
Full sunlight exposure to clear water in a clear plastic bottle for 4 to 6 h of exposure significantly reduces and possibly eliminates microorganism contamination. Strong recommendation, moderate quality evidence.
Pasteurization temperatures can be achieved with a solar oven. Strong recommendation, moderate quality evidence.
Filtration.
Filters are appealing to outdoor users as well as households without a reliable source of clean water because of their simplicity and suitability for commercial production.112–114 Portable water treatment products are among the most frequently purchased equipment for camping after backpacks and tents. Filtration is a standard step in municipal water treatment and widely used in the food and beverage industry, as well as many other industrial processes. Filtration can be highly effective for removing disease-causing microbes and other particulate contaminants, but unless a disinfectant chemical is incorporated into the filter media, it does not kill or inactivate microbes. Throughout the world’s history, many different types of media, from sand to vegetable products to fabric, have been used for water filtration. Filters have the advantages of being simple and requiring no wait time after passing through the filter. They do not add any unpleasant taste and may improve the taste and appearance of water. All filters eventually clog from suspended particulate matter that is present even in clear streams, requiring cleaning or replacement of the filter. As a filter clogs, it requires increasing pressure to drive the water through, which can force microorganisms through the filter or damage the filter. A crack or eroded channel in a filter will allow passage of unfiltered water. Bacteria can grow on filter media and potentially result in some bacteria in filtered water, but pathogenic bacteria have not been demonstrated.115 Silver is often incorporated into the filter media to prevent this growth, but it is not totally effective. (See additional information in the section on nanomaterials.)
The primary determinant of a microorganism’s susceptibility to filtration is its size (Table 3 and Figure 1).
Table 3.
Microorganism susceptibility to filtration.
| Organism | Approximate aize (μm) | Recommended filter rating (μm) |
|---|---|---|
| Virusesa | 0.03 | Ultrafilter, nanofilter, reverse osmosis |
| Escherichia coli Campylobacter Vibrio cholerae | 0.5 by 3–8 0.2–0.4 by 1.5–3.5 0.5 by 1.5–3.0 | 0.2–0.4 (microfilter) |
| Cryptosporidium oocyst | 2–6 | 1 (microfilter) |
| Giardia cyst Entamoeba histolytica cyst | 6–10 by 8–15 5–30 (average 10) | 3–5 (microfilter) |
| Nematode eggs | 30–40 by 50–80 | 20 (microfilter) |
| Schistosome cercariae Dracunculus larvae | 50 by 100 20 by 500 | Coffee filter or fine cloth, or double thickness closely woven cloth |
Microfilters (most filters with pore size of 0.1–0.2 μm) can filter bacteria and protozoan cysts but are not effective for virus removal unless designed to rely on electrostatic trapping of viruses. Hollow-fiber filters with 0.02 μm pores and reverse osmosis filters are capable of filtering viruses.
Figure I.
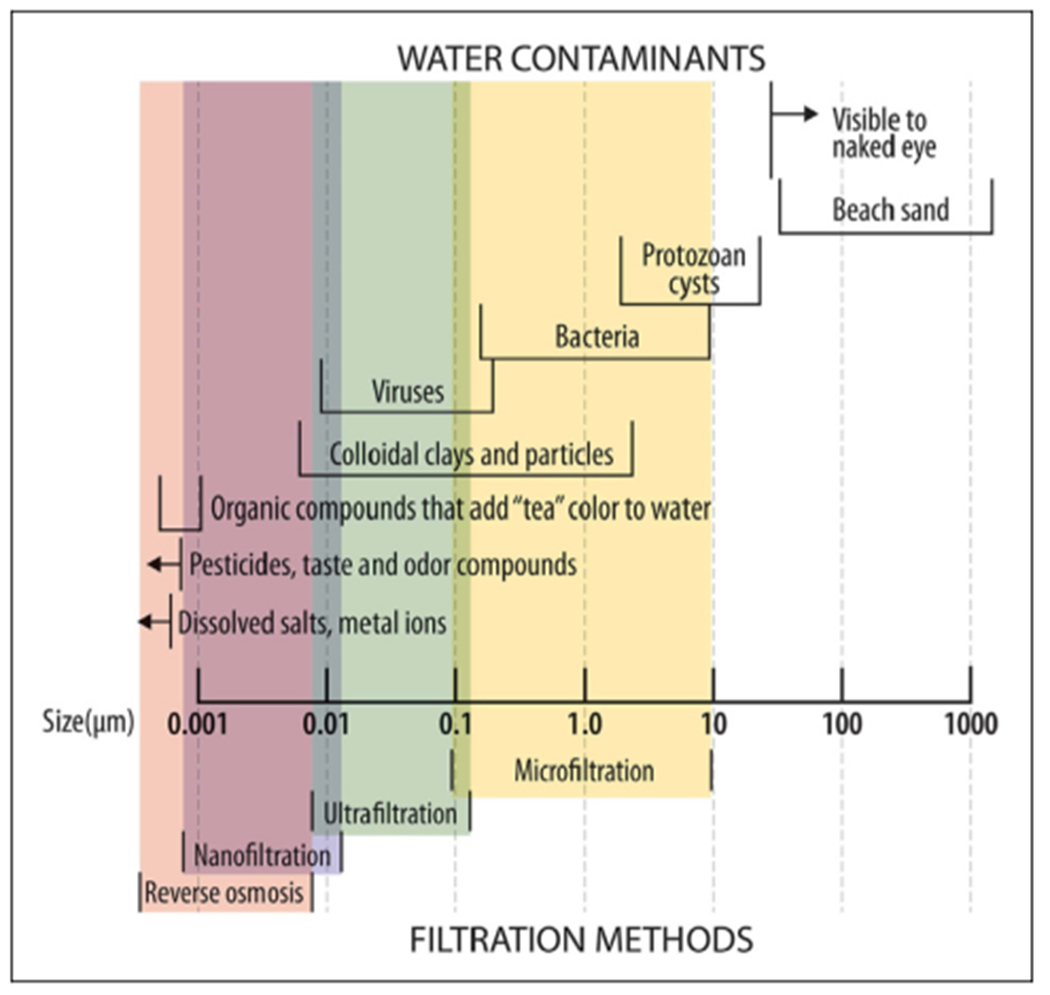
Levels of filtration and susceptibility of common microbial pathogens and other contaminants. Reproduced with permission from the Centers for Disease Control and Prevention. CDC Yellow Book 2024: Health Information for International Travel. New York: Oxford University Press, 2023. Adapted from Backer H. Field Water Disinfection. In: Auerbach PS, ed. Wilderness Medicine, 7th edition. Elsevier; 2017.
Portable filters for water treatment can be divided into microfilters with pore sizes down to 0.1 μm, ultrafilters with pore sizes of 0.002 to 0.01 μm, nanofilters with pore sizes as small as 0.001 μm or less, and reverse osmosis filters with pore sizes of 0.0001 μm or less.87 The smaller the pore size, the more pressure or greater filter surface required. Waterborne pathogens often adhere to larger particles or clump together, making them easier to remove by physical processes. Therefore, observed reductions are often greater than expected based on their individual sizes.
Many portable filters are microfilters that can readily remove protozoan cysts and bacteria, but may not remove all viruses, which are much smaller than the pore size of most field filters.116–118 However, viruses often clump together or are attached to other larger particles or organisms, resulting in an aggregate large enough to be trapped by the filter; in addition, electrochemical attraction may cause viruses to adhere to the filter surface.23,62,119 Through these mechanisms, mechanical filters using ceramic elements with a pore size of 0.2 μm can reduce viral loads by 2 to 3 logs (99–99.9%) but are not adequate for complete removal of viruses.118 Ultrafiltration or nanofiltration is required for complete microbial removal, including viruses; they can also remove colloids, dissolved solids, and some large toxins.120,121 Nanofilters can also remove microcystin toxin.44
Hollow-fiber technology has been adapted for field use using bundles of tube fibers whose pore size can be engineered to achieve ultrafiltration with viral removal.122 The large surface area allows these hollow-fiber filters to have relatively high flow rates at low pressure. Hollow-fiber filters that are designed to use gravity, a squeeze bag, or a hand pump are widely available to treat water for individuals or small groups. Compact and lightweight designs have made them popular with long-distance hikers.
Some filters on the market combine filter material with other substances such as iodine or silver to add disinfection to the process. Iodine molecules can be bound in a resin engineered into field products, but the effectiveness of the resin is highly dependent on the product design and function.123–126 Most companies have abandoned iodine resin–containing portable hand-pump filters, water bottles, or drink-through straws due to reports of excess iodine or viral breakthrough in the effluent; however, iodine resins can be highly effective in carefully engineered systems, such as those developed for National Aeronautics and Space Administration space shuttles and the International Space Station.127
Several factors influence the decision of which filter to use: 1) flow volume sufficient for the number of persons relying on the filter; 2) whether the filter functional claims match the microbiologic demands that will be put on the filter; 3) the preferred means of operation (eg, hand pump or gravity); and 4) cost.
Improvisational filtration techniques.
Simple, available products, such as rice hull ash filters, crushed charcoal, sponges, and various fabrics and paper, have been used in low- and middle-income countries and in emergency situations for filtration. Typically, bacteria and viruses can be reduced by as much as 50 to 85% and larger parasites can be reduced by 99%, depending on the media. The effectiveness for decreasing turbidity may be used as an indicator that a filter material will reduce microbiologic contamination.51,128,129
Ceramic filters are a common component in portable water pump filters but are also a cost-effective means of household disinfection in low- and middle-income countries. Ceramic clay is widely available and very inexpensive to locally manufacture in the shape of a sink or flowerpot that is set into a larger container to collect the filtered water.130–132 Extensive epidemiological research has demonstrated efficacy and effectiveness of ceramic filters when properly made and maintained.125,130–134
Biosand filters employ a technology that has been used over centuries and is still used widely in municipal plants and at the household and community level.135–138 Sand filters can be highly effective at removing turbidity (in one study, from 6.2 NTU to 0.9 NTU) and improving microbiologic quality (99% efficacy), depending on their design and operation.135–139 Sand filters are constructed by forming layers of aggregate increasing in size from the top to the bottom. An emergency sand filter can be made in a 20 L (5.3 gal) bucket, composed of a 10 cm (3.9 in) layer of gravel beneath a 23 cm (9.1 in) layer of sand; a layer of cotton cloth, sandwiched between 2 layers of wire mesh, separates the sand and gravel layers.51 A sand filter also can be improvised with stacked buckets of successive filter layers with holes in the bottom to allow water passage. Many websites provide design and assembly instructions, but there are no data for comparative function.140
Assessments of Supporting Evidence:
Filtration is effective as a primary or adjunctive means of water treatment. Strong recommendation, high quality evidence.
Standard commercially available microfilters with a pore size of 0.2 μm are effective in removing protozoa and bacteria. Strong recommendation, high quality evidence.
Ultrafiltration with pore size of less than 0.01 μm or nanofiltration is needed to completely remove pathogenic viruses. Strong recommendation, high quality evidence.
Users should know how to clean their filter or consider carrying a backup method of disinfection because filters may clog. Strong recommendation, low quality evidence.
Biosand filters are an effective household and community technique that can be improvised for filtration. Strong recommendation, moderate quality evidence.
Chemical Disinfection: Halogens (Iodine and Chlorine).
Worldwide, disinfection with chemicals, chiefly halogens, is the most widely used method for improving and maintaining the microbiologic quality of drinking water.87,141 The germicidal activity of chlorine and other halogens is well-established and results from oxidation of essential cellular structures and enzymes. Disinfection effectiveness is determined by characteristics of the microorganism, the disinfectant, contact time, and environmental factors. Given adequate concentrations and contact times, both iodine and chlorine are effective disinfectants with comparable biocidal activity under most conditions (Table 4). Both are widely available in multiple formulations.
Table 4.
Disinfection data for chlorine and iodine to achieve 99.9% kill or inactivationa of select microorganisms.
| Chlorine | ||||||
|---|---|---|---|---|---|---|
| Organism | Concentration (mg·L−1) | Time (min) | pH | Temperature | Disinfection constantb | Reference |
| Escherichia coli | 0.1 | 0.16 | 6.0 | 5°C (41°F) | .016 | 141 |
| Campylobacter | 0.3 | 0.5 | 6.0–8.0 | 25°C (77°F) | 0.15 | 156 |
| 20 enteric virus | 0.5 | 60 | 7.8 | 2°C (36°F) | 30 | 142 |
| 6 enteric viruses | 0.5 | 4.5 | 6.0–8.0 | 5°C (41°F) | 2.5 | 160 |
| Norovirus | 1 | 10 | 6.0 | 5°C | 10 | 159 |
| 5 | 0.33 | 1.66 | ||||
| Hepatitis A virus | 0.36 | 6.5 | 6.0 | 5°C | 2.3 | 162 |
| Hepatitis A virus | 0.5 | 1 | 6.0 | 25°C (77°F) | 0.5 | 161 |
| Amebic cysts | 3.5 | 10 | 25°C (77°F) | 35 | 143 | |
| Giardia cysts | 2.5 | 60 | 6.0–8.0 | 5°C (41°F) | 150 | 144 |
| Giardia lamblia cysts | 0.85 | 90 | 8.0 | 2–3°C (36–37°F) | 77 | 145 |
| Giardia muris cysts | 3.05 | 50 | 7.0 | 5°C (41°F) | 153 | 165 |
| Cryptosporidium (2 strains) | 20 | 755 | 7.5 | 23°C | 15,300 | 168 |
| 20 | 501 | 7.5 | 23°C | 10,400 | ||
| Iodine | ||||||
| Escherichia coli | 1.3 | 1 | 6.0–7.0 | 2–5°C (36–41 °F) | 1.3 | 42 |
| Hepatitis Ac | 8 | 0.4 | 7.0 | 25°C | 3 | 146 |
| 8 | 1.1 | 7.0 | 5°C | 8.8 | ||
| Coxsackie virus | 0.5 | 30 | 7.0 | 5°C (41°F) | 15 | 147 |
| Amebic cysts | 3.5 | 10 | 25°C (77°F) | 35 | 143 | |
| Giardia cysts | 4 | 15 | 5.0 | 30°C (86°F) | 60d | 163 |
| Giardia cysts | 4 | 45 | 5.0 | 15° C (59°F) | 170d | 163 |
| Giardia cysts | 4 | 120 | 5.0 | 5°C (41 °F) | 480d | 163 |
99.9% is for comparison of disinfection potency and microorganism susceptibility. The standard for potable water is 99.99% inactivation for viruses and 99.999% for bacteria. This would be achieved in each example above with a higher concentration of disinfectant or a longer contact time.
Disinfection constant is the product of concentration (mg·L−1) and time (m), which are inversely related, and provides a single number for comparison for halogen susceptibility of microorganisms in specific water conditions.
Tested in purified water
More than 99.99% inactivation; viability tested only at 15, 30, 45, 60, and 120 min.
One advantage of chemical water disinfection products is flexible dosing that allows use by individual travelers, small or large groups, or communities.61,128 Unlike heat, filtration, and UV, halogens provide a disinfectant residual in water to protect against recontamination during storage.
Chlorine is still advocated by the World Health Organization (WHO) and the CDC as a mainstay of large-scale community, individual household, and emergency use.148,149 Hypochlorite, the major chlorine disinfectant, is currently the preferred means of municipal water disinfection.141 Calcium hypochlorite and sodium hypochlorite (NaOCl) readily dissociate in water to form hypochlorous acid, the active disinfectant. There are extensive data on effectiveness of chlorine-based disinfectant products in remote settings.80,87,148,150–154
Iodine is also effective in low concentrations for killing bacteria, viruses, and some protozoan cysts, and in higher concentration against fungi and even bacterial spores; however, it is a poor algaecide.124,155 Elemental iodine and hypoiodous acid are the major germicides in an aqueous solution. Of the halogens, iodine reacts least readily with organic compounds and is less affected by pH, indicating that low iodine residuals should be more stable and persistent than corresponding concentrations of chlorine. Despite these advantages, because of its physiological activity, WHO has not established a formal guideline value and recommends iodine only for short-term emergency use.124 The European Union banned the sale of iodine-based products for water disinfection in 2009.
Halogen efficacy.
Vegetative bacteria (non-spore fonning) are very sensitive to halogens (Table 4).141,156–158 Viruses, including hepatitis A, have intennediate sensitivity, requiring higher concentrations or longer contact times.125,159–162 SARS-CoV-2, the virus that causes COVID-19, can be found in water; however, it is sensitive to chlorine inactivation, and there is no evidence that COVID-19 can be transmitted through water. Protozoan cysts are more resistant than enteric bacteria and enteric viruses but some cysts (eg, Giardia) can be inactivated by field doses of halogens.163–166 Cryptosporidium oocysts, however, are much more resistant to halogens so inactivation is not practical with common doses of iodine and chlorine used in field water disinfection.167,168 Certain parasitic eggs, such as those of Ascaris, are also resistant, but these are not commonly spread by water. (All of these resistant cysts and eggs are susceptible to heat or filtration.) Bacterial spores, such as Bacillus anthracis, are relatively resistant to halogens, but not much more resistant than are Giardia cysts; furthermore, they do not normally cause waterborne enteric disease.
Halogen disinfection variables in field use.
Understanding factors that influence the disinfection reaction allows flexibility with greater reassurance of effectiveness (Table 5). The primary factors of the first-order chemical disinfection reaction are concentration and contact time.141 Lower concentrations can be used with longer contact times. In field disinfection, this can be used to minimize halogen dose and improve taste or, conversely, to minimize the required contact time with higher doses of halogen.
Table 5.
Factors affecting water disinfection with halogens.
| Factor | Impact | Common instructions | Alternative means to compensate | Reference |
|---|---|---|---|---|
| Concentration (primary factor) | Inversely correlated with the relationship quantified by their product (disinfection CT constant), which specifies inactivation of specific microorganisms | Higher concentrations are more effective | Lower concentrations can be used with longer contact times. Taste becomes distinct above 2–3 mg·L− | Table 4 and 141 |
| Time (primary factor) | Assumed priority is to minimize contact time | |||
| Temperature (secondary factor) | Cold water slows chemical disinfection reaction | Increase concentration of disinfectant | Extend contact times | |
| Organic contaminants (secondary factor) | React with halogens to form compounds with little or no disinfecting ability, decreasing the concentration of available halogen | Double the amount of chlorine or iodine in cloudy water | Clarify water to remove contaminants. (C-F, filtration, sedimentation); Due to the difficulty in estimating halogen demand, it is prudent to use 3–4 mg·L−1 as a target halogen concentration range for clear surface water. Lower concentrations (eg, 2 mg·L−) can be used for backup treatment of questionable tap water or high quality well water |
Tables 6 and 7 and 36,52,87,169–171 |
C-F, coagulation–flocculation.
Multiple products combine chlorine with a C-F powder to both clarify and disinfect in 1 step, resulting in decreased turbidity of water, high level of disinfection for bacteria and viruses, increased removal of protozoan cysts over chlorine alone, and significant removal of other water contaminants, such as arsenic.54,55,57,172
Halogen toxicity.
Chlorine has no known toxicity at the concentrations used for water disinfection. Reactions of chlorine with certain organic contaminants yield chlorinated hydrocarbons, chlorofonn, and other trihalomethanes, which are considered to have carcinogenic potential in animal models. Nevertheless, the risk of severe illness or even death from infectious diseases if disinfection is not used is far greater than any risk from byproducts of chlorine disinfection.173
Iodine has not gained general acceptance for long-term use in large populations because of concern for its physiologic activity.124 There are some older data that iodination of water with a low residual concentration ≤1 to 2 mg·L−1 appear safe, even for long periods of time, in people with normal thyroid function.174,175 However, no major agencies recommend prolonged use. The WHO did not set a guideline value for iodine in drinking water because of a paucity of data, and because it is not recommended for long-term disinfection. If the typical wilderness/intemational traveler disinfected 3 L of water each day using 2 to 4 mg·L−1 of iodine, the ingested amount of iodine per day would be 6 to 12 mg, well above US Institute of Medicine recommended dietary allowance levels. Levels produced by the recommended doses of iodine tablets are even higher and would result in ingestion of 16 to 32 mg/d. Therefore, the use of iodine for water disinfection should be limited to short periods (months). Anyone planning to use iodine for prolonged periods should have their thyroid examined and thyroid function tests done to assure that they are initially euthyroid. Certain groups should not use iodine for water treatment: pregnant women (because of concerns of neonatal goiter); those with known hypersensitivity to iodine; persons with a history of thyroid disease even if controlled on medication; persons with a strong family history of thyroid disease (thyroiditis); and persons from countries with chronic iodine deficiency.176
Improving halogen taste.
Objectionable taste and smell limit the acceptance of halogens, with taste becoming distinctive above 3 mg·L−1.177 Taste preference between chlorine and iodine is individual and can be improved by several means. One method is to use the minimum necessary dose with a longer contact time (30–60 m) (Table 6). The CDC and Pan American Health Organization developed the Safe Water System for household disinfection, which provides a low dosage of 1.875 or 3.75 mg·L−1 of NaOCl with a contact time of 30 min, sufficient to inactivate most bacteria, viruses, and some protozoa that cause water-borne diseases.148,154
Table 6.
Recommendations for contact time using halogen disinfection in the field.
| Concentration of halogen | Contact time (min) at various water temperatures |
||
|---|---|---|---|
| 5°C (41 °F) | 15°C (59°F) | 30°C (86°F) | |
| 2 ppm | 240 | 180 | 60 |
| 4 ppm | 180 | 60 | 45 |
| 8 ppm | 60 | 30 | 15 |
Concentration and contact time are based on the most resistant target organism, which is the Giardia cyst. These are well beyond the time needed to kill bacteria and viruses. These contact times have been extended from the usual recommendations in cold water to account for the extended inactivation time required in very cold water and for the uncertainty of residual concentration.
Another method is to remove the taste after the disinfection time prior to drinking through chemical reduction of chlorine to chloride or iodine to iodide, which have no color or taste and no disinfectant activity. The best and most readily available agent is ascorbic acid (vitamin C), available in crystalline or powder form. A small pinch in a liter, mixed after the required contact time, will usually suffice. Ascorbic acid is a common ingredient of flavored drink mixes, accounting for their effectiveness in removing the taste of halogens. Granular activated carbon (see above) adsorbs organic and inorganic chemicals, including iodine and chlorine byproducts, thereby improving odor and taste—the reason for its common inclusion in field and household filters.141 Techniques that reduce residual disinfectant levels should only be used at the time of consumption, not if water will be stored.
Sources of Halogen Disinfectants.
There is no comparable substitute for proven chemical disinfectants, but there are many common substances that contain halogens (Table 7). Unscented household bleach containing NaOCl is available in most parts of the world. Products that contain calcium hypochlorite provide much higher concentrations (70%) than household bleach. Iodine is also available in liquid, tablets, or resins. A common household source is tincture of iodine or similar topical disinfectant with an iodine concentration of 2 to 8%. These products also contain iodide, which has no disinfecting power, but does contribute to iodine toxicity. Colorless iodine solution contains only iodide, so it should not be used for water disinfection. Povidone–iodine, a topical disinfectant commonly used in medical settings, contains active iodine bound to a neutral polymer of high molecular weight that gives the iodine greater solubility and stability. In dilute aqueous solution, povidone-iodine provides a sustained-release reservoir, releasing free iodine in a concentration of 2 to 10 mg·L−1 that increases with dilution in a bell-curve manner.178,179
Table 7.
Halogen disinfection products and recommended doses for point-of-use disinfection.
| Iodination techniquesa | Add to 1 L or qt of water |
|
|---|---|---|
| Amount to achieve 4 mg·L−1 | Amount to achieve 8 mg·L−1 | |
| Iodine tabsb | 0.5 tab (or 1 tab in 2 L) | 1 tab |
| Tetraglycine hydroperiodide | ||
| Emergency drinking water germicidal tablet | ||
| Potable aqua | ||
| Globaline | ||
| 2% iodine solution (tincture) | 0.25 mL | 0.5 mL |
| 5 dropsc | 10 drops | |
| 10% povidone–iodine solutiond | 0.4 mL | 0.8 mL |
| 8 drops | 16 drops | |
| Saturated solution: iodine crystals in watere | 13 mL | 26 mL |
| Add to 1 L or qt of water |
||
| Chlorination techniquesf | Amount to achieve 2 mg·L−1 | Amount to achieve 5 mg·L−1 |
|
| ||
| Sodium hypochlorite (household bleach 5%) | 1 drop | 0.1 mL 2 drops |
| Sodium hypochlorite (household bleach 8.25%) | 1 drop (in 2 L) | 1 drop |
| 1% bleach (CDC-WHO Safe Water System)g | 4–5 drops | 8–10 drops |
| Calcium hypochloriteh (Redi Chlor [0.1-g tab]) | Cannot use in small quantities of water for low concentrations | 0.25 tab |
| NaDCCi (Aquatab, Kintab) | ¼ tab of 8.5 mg NaDCC (may be impractical) | ½ tab (8.5 mg NaDCC) |
| Chlorine plus flocculating agent (Chlor-Floc tablets or powder sachets, PUR) | Not practical for small volumes and low concentrations | ½ tablet/yields 5 mg·L−1j |
CDC, Centers for Disease Control and Prevention; WHO, World Health Organization; NaDCC, sodium dichloroisocyanurate.
World Health Organization recommends only for short-term emergency use.
Iodine tablets were developed by the military with the criteria that they will disinfect water, including Giardia, with a short contact (holding) time of 10 min since troops in the field may not wait longer. This high concentration is not necessary for field disinfection of clear water; it is preferable to target 4 mg·L−1 and wait longer. Additionally, the recommendation to use 8 mg·L−1 for cloudy water will result in poor taste, so it is recommended to clarify the water first.
Measure of a drop varies from 16 to 24 gtt·mL−1, standard 20 gtt·mL−1 is used here.
Povidone-iodine solutions release free iodine in levels adequate for disinfection, but scant data are available. (See text above)
A small amount of elemental iodine goes into solution (no significant iodide is present); the saturated solution is used to disinfect drinking water. Water can be added to the crystals hundreds of times before they are completely dissolved.
Can easily be adapted to large or small quantities of water. Simple field test kits or swimming pool test kits with color strips are widely available to ensure adequate residual chlorine. In usual situations, EPA recommends a target residual of 4 mg·L−1. For household use, CDC recommends less than 2 mg·L−1. Many of the recommended emergency doses exceed this threshold.128 For treatment of large volumes, see formula to calculate in Lantagne 2008.177
Safe Water System for long-term routine household point-of-use water disinfection recommends a hypochlorite dose of about 2 mg·L−1 in clear water and 4 mg·L−1 in slightly turbid water. This results in a low yet effective target residual concentration but requires testing in a particular water source to ensure sufficient residual.
Stable, concentrated (70%), dry source of hypochlorite that is used for chlorination of swimming pools. Multiple products are available in various size tablets or granular form. Best formulation for large quantities of water.
Available in different strengths to treat different volumes of water. Check packaging to determine proper dose.
Use full tablet or sachet in highly turbid water.
Mixed Species Disinfectant (Electrolysis).
Passing a current through a simple brine salt solution generates free available chlorine as well as other “mixed species” disinfectants, giving the resulting solution greater disinfectant ability than a simple solution of NaOCl.150,151 The process has been engineered for use on both large and small scales.180
Assessments of Supporting Evidence:
Chlorine and iodine (halogens) are effective means of disinfecting water contaminated with bacteria, viruses, and Giardia in the field or household using appropriate contact time and halogen concentration. Strong recommendation, high quality evidence.
Usual field concentrations of iodine and chlorine are not effective for other protozoa, including Cryptosporidium and Cyclospora. Strong recommendation, high quality evidence.
Simple techniques for improving the taste of halogenated water are available for field use. Strong recommendation, moderate quality evidence.
Mixed species electrolytic disinfection is effective for water disinfection of microbes that are susceptible to halogens. Strong recommendation, moderate quality evidence.
Miscellaneous Disinfectants
Chlorine dioxide and ozone.
Both chlorine dioxide (ClO2) and ozone (O3) are potent biocides due to their strong oxidizing potential that have been used for many years to disinfect municipal water and numerous other industrial applications. Until recently, their benefits have been limited to large-scale applications because standard formulations must be made on-site and are associated with a risk for producing volatile or toxic gas. Newer methods may enable cost-effective and portable generation for use in an array of small-scale applications.
Chlorine dioxide has no taste or odor in water at concentrations used for treating drinking water. It is capable of inactivating most waterborne pathogens, including Cryptosporidium parvum oocysts.181,182 It is at least as effective a bactericide as chlorine and far superior for virus and parasite inactivation. There are several commercial point-of-use applications using ClO2 in liquid or tablet form but relatively few data are available on testing of these products.183 Chlorine dioxide–production tablets contain 6.4% sodium chlorite as the active ingredient. After a tablet is added to water, a series of complex chemical reactions occur, generating ClO2. Some of the intermediary chemical compounds may also have antimicrobial activity. A major disadvantage for field use of tablets is the long reaction or contact time required, upwards of 2 to 4 h needed to achieve dependable disinfection. Chlorine dioxide does not produce a lasting residual and water undergoing ClO2 disinfection must be protected from sunlight.
Ozone is an unstable form of pure oxygen that is colorless and tasteless in water. It is one of the strongest oxidants and disinfectants used in treating water and wastewater, rapidly killing organisms by oxidizing organic material in the membranes of bacteria, viruses, and parasites.125,141,181,183–186 It also oxidizes metals, facilitating their removal and improving taste, smell, and color.141 Generally, O3 is generated from air using electrical current or UV light. Small portable products using a low battery current to generate O3 from oxygen in water are now available; however, data on their effectiveness are limited. Ozone breaks down rapidly, so adequate concentration and contact time must be assured.
Assessment of Supporting Evidence:
Chlorine dioxide and O3 are widely used and potent water disinfectants, including efficacy against the protozoan parasite Cryptosporidium. Strong recommendation, high quality evidence.
Portable, point-of-use products generating ClO2 or O3 have limited data demonstrating effective concentration and contact time. Weak recommendation, moderate quality evidence.
Silver.
Silver ion has bactericidal effects in low doses and some attractive features, including absence of color, taste, and odor. Limited data for disinfection of viruses and protozoan cysts indicate incomplete effect, even at high doses. Moreover, the concentrations are strongly affected by adsorption onto the surface of any container. Silver is physiologically active but not likely to cause a problem in concentrations found in drinking water. Long-term effects are discoloration of the skin (argyria), considered cosmetic and not toxic. The WHO does not recommend silver for primary water disinfection and the US Environmental Protection Agency has not approved it for this use in the United States, but silver is approved as a water preservative to prevent bacterial growth in previously treated and stored water.187,188 In Europe, silver tablets are sold for field water disinfection. One product combines silver with hypochlorite for both effective disinfection and preservation. There is some promise in steady release products and incorporation into nanoparticles, which seem to be more effective than silver ions.141,189,190 Silver is commonly incorporated into filter material to aid in disinfection and to prevent bacterial growth on filter media; however, these effects are limited.191
Assessment of Supporting Evidence:
We recommend that use of silver should be limited to water preservation and not as a primary disinfectant. Strong recommendation, moderate quality evidence.
Hydrogen Peroxide.
Hydrogen peroxide is a strong oxidizing agent that is widely used as a preservative in food, as a sterilant for medical and food equipment, and many other applications. Although hydrogen peroxide can sterilize water, it is not widely used as a field water disinfectant, perhaps because high concentrations that are known to be effective are very caustic, and there is a lack of data for protozoal cysts and quantitative data for dilute solutions. It can be used to remove the taste of hypochlorite and in combination with other processes.192,193
Assessment of Supporting Evidence:
We recommend that hydrogen peroxide in typical concentration of 3% should not be used as a primary drinking water disinfectant; whereas effective concentrations are not practical for field use. Strong recommendation, moderate quality evidence.
Citrus and Potassium Permanganate.
Both citrus juice and potassium permanganate have some demonstrated antibacterial effects in an aqueous solution.194 However, data are limited and not available for effect on cysts. In municipal water disinfection, potassium permanganate is used primarily for reducing contaminants to improve taste and odor.188 Either substance could be used in an emergency to reduce bacterial and viral contamination or as an adjunct in combination with another technique but cannot be recommended as a primary means of water disinfection. Citrus can also enhance SODIS.60
Assessment of Supporting Evidence:
We suggest not using citrus juice and potassium permanganate as the primary point-of-use drinking water disinfectant. Weak recommendation, low quality evidence.
Nanoparticles: Solar Photocatalytic Disinfection.
Nanomaterials have structures measuring 100 nm or less that may be in the form of particles, tubes, rods, or fibers and comprised of organic, inorganic, carbon, or composite materials.195 Several nanomaterials have been shown to have strong anti-microbial properties and are being evaluated for use in water disinfection and purification.190,196–198 Inorganic-based nanomaterials, including different metal and metal oxides, such as titanium, zinc, iron, and silver, are of particular interest for water disinfection applications because they can be activated by UV to produce potent oxidizers that are excellent disinfectants for microorganisms. In addition, they can break down complex organic contaminants and even most heavy metals into nontoxic forms. Titanium dioxide (TiO2) is the most effective photocatalytic substance identified to date. Recent work demonstrated inactivation of Cryptosporidium by TiO2.197,199 These methods are widely used in industry, but few products have incorporated the technology into individual or small group point-of-use products.200,201 Commercial point-of-use products have incorporated silver or TiO2 into filter media or membranes.190
Assessment of Supporting Evidence:
New technology using nanoparticles and photocatalytic disinfection is highly promising for translation into point-of-use water disinfection. Strong recommendation, high quality evidence.
Preferred Technique
The optimal water treatment technique for an individual or group will depend on the number of persons to be served, space and weight accommodations, quality of source water, personal taste preferences, duration of use, and resources (eg, fuel availability) or devices available. Because halogens are not effective for killing Cryptosporidium at drinking water concentrations and common microfilters are not reliable for virus removal, optimal protection for all situations may require a 2-step process of filtration or C-F followed by chemical disinfection. Heat (boiling) is effective as a one-step process but will not improve the esthetics of the water or provide protective residual disinfection during storage. UVR is an effective 1-step process for clear water. Table 8 summarizes effects of major water disinfection methods on categories of microorganisms. Several authors have reviewed efficacy data for point-of-use methods for household disinfection in low- and middle-income countries24,25,56,113,202 (Table 9).203,204 In practice, there is a difference between laboratory and field application of any water treatment method due to differences in water quality and use of the product or method.
Table 8.
Summary of field water disinfection techniques.
| Bacteria | Viruses | Giardia/ameba | Cryptosporidium | Nematodes/cercarea | |
|---|---|---|---|---|---|
| Heat | + | + | + | + | + |
| Filtration | + | +/−a | + | + | + |
| Halogens | + | + | + | − | +/−b |
| UV | + | + | + | + | DNA |
| Chlorine dioxide, ozone, and photocatalytic | + | + | + | + | DNAc |
DNA, data not available.
Most filters make no claims for viruses. Ultrafiltration with hollow-fiber technology and reverse osmosis is effective.
Eggs are not very susceptible to halogens but very low risk of waterborne transmission.
No data available for photocatalytic disinfection.
Table 9.
Efficacy and effectiveness of point-of-use technologies for low-income world households.
| Treatment process | Pathogen | Optimal log reductiona | Expected log reductionb | Diarrheal disease reduction (%)c |
|---|---|---|---|---|
| Ceramic filters | Bacteria | 6 | 2 | 63 (51–72) for candle filters 46 (29–59) for bowl filters |
| Viruses | 4 | 0.5 | ||
| Protozoa | 6 | 4 | ||
| Free chlorine | Bacteria | 6 | 3 | 37 (25–48) |
| Viruses | 6 | 3 | ||
| Protozoa | 5 | 3 | ||
| Coagulation/Chlorination | Bacteria | 9 | 7 | 31 (18–42) |
| Viruses | 6 | 2–4.5 | ||
| Protozoa | 5 | 3 | ||
| Biosand filtration | Bacteria | 3 | 1 | 47 (21–64) |
| Viruses | 3 | 0.5 | ||
| Protozoa | 4 | 2 | ||
| SODIS | Bacteria | 5.5 | 3 | 31 (26–37) |
| Viruses | 4 | 2 | ||
| Protozoa | 3 | 1 |
SODIS, Solar UV disinfection.
Data from multiple studies analyzed and summarized by Sobsey et al,113 Bielefeldt et al,203 WHO,36 Clasen et al,202 and data from additional references.23,123,204
Skilled operators using optimal conditions and practices (efficacy); log reduction: pretreatment minus post-treatment concentration of organisms (eg, 6 log = 99.999% removal).
Actual field practice by unskilled persons (effectiveness) depends on water quality, quality and age of filter or materials, following proper procedure, and other factors.
Summary estimates from published data vary with consistency and correct use of technique, integrity of techniques (eg, cracked filter), and other household sanitation measures; thus, these estimates represent effectiveness not efficacy, and real world not ideal conditions.
In disaster situations, such as floods, hurricanes, and earthquakes, sanitation and water treatment facilities are frequently damaged or inundated, so household or point-of-use water disinfection is advised, as in regions where there is no sanitation or improved water sources. Surface water quality is likely to be poor with turbidity, bacteriological, and chemical contamination. Optimally, cloudy water should first be clarified, followed by application of heat, filtration, or chemical disinfection. Granular activated carbon can remove many chemical contaminants. Chlorination may be the simplest method and sufficient alone for disinfecting contaminated water for most classes of microorganisms (Table 8).26,27,128,177 other point-of-use methods described above can also improve water quality after a disaster. In sunny climates, if time allows, SODIS requires no special resources other than the container.
On long-distance ocean-going boats where water must be desalinated as well as disinfected during the voyage, only reverse osmosis membrane filters are adequate. Water storage also requires consideration. Iodine will work for short periods only (ie, weeks) because it is a poor algaecide. For prolonged storage, water should be chlorinated and kept in a tightly sealed container to reduce the risk of contamination and to maintain chlorine levels. For daily use, narrow-mouthed jars or containers with water spigots prevent contamination from repeated contact with hands or utensils.92,153,205
Few studies compare multiple techniques or devices.114,118,123,206–210 For additional reviews of water treatment methods, effectiveness, and efficacy data, see the following additional references.56,87,123,202,211
Sanitation
Sanitation and water treatment are inextricably linked.17,132 Wilderness and remote travelers typically lose access to accustomed sanitation with toilets and running water, and their hygiene practices diminish, similar to conditions for many residents in low- and middle-income countries. While there are little to no data on these practices and interventional impacts on the former group, there are extensive studies on the latter. Studies in low- and middle-income countries have demonstrated a clear benefit in the reduction of diarrheal illness and other infections from safe drinking water, hygiene, and adequate sanitation.24,25,212–216 The benefit is greater when all are applied together, especially with appropriate education.17,217,218
Personal hygiene, particularly hand washing, prevents the spread of infection from food contamination during preparation of meals.219–221 Disinfection of dishes and utensils is accomplished by rinsing in water containing enough household bleach to achieve a distinct chlorine odor. Travelers to remote low- and middle-income settings and wilderness areas should practice proper fecal waste disposal to prevent additional contamination of water supplies. Human waste should be buried 8 to 12 in deep, at least 100 ft from any water, and at a location where water run-off is not likely to wash organisms into nearby water sources. Groups of 3 persons or more should dig a common latrine to avoid numerous individual potholes and inadequate disposal. Victims in disaster situations without functional toilets can use latrines or plastic bags for fecal waste.
Assessment of Supporting Evidence:
We recommend that after a disaster with lack of safe tap water, the same water treatment methods described above should be used. Strong recommendation, high quality evidence.
We recommend that sanitation and hygiene practices be paired with water treatment to reduce further source contamination, prevent contamination of food and utensils, and reduce recontamination of treated water. Strong recommendation, high quality evidence.
Conclusions
Wilderness and international travelers should be aware of water and sanitation conditions and plan in advance to use an effective means of disinfecting water. It is important for disaster and medical relief workers to understand the common methods of water treatment as well as improvisational methods. It is not possible for travelers to judge the microbiologic quality of water by sight, smell, or taste alone, and it is prudent to assume that even tap water is nonpotable in many low- and middle-income locations and in emergency situations. Simple and effective field techniques to improve microbiologic water quality are available.
Supplementary Material
Footnotes
Disclosures
None.
Disclaimer
Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the US Department of Health and Human Services, the Wilderness Medical Society, or the Wilderness and Environmental Medicine Journal. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention, US Department of Health and Human Services, or the University of California.
Supplemental Materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.wem.2023.08.021.
References
- 1.World Health Organization. Combating waterborne disease at the household level. Accessed November 16, 2022. https://www.who.int/publications/i/item/9789241595223
- 2.Schoenen D. Role of disinfection in suppressing the spread of pathogens with drinking water: possibilities and limitations. Water Res. 2002;36(15):3874–3888. [DOI] [PubMed] [Google Scholar]
- 3.Backer HD, Derlet RW, Hill VR. Wilderness Medical Society clinical practice guidelines for water disinfection for wilderness, international travel, and austere situations. Wilderness Environ Med. 2019;30(4 s):S100–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diekemper RL, Patel S, Mette SA, Ornelas J, Ouellette DR, Casey KR. Making the GRADE: CHEST updates its methodology. Chest. 2018;153(3):756–759. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds KA, Mena KD, Gerba CP. Risk of waterborne illness via drinking water in the United States. Rev Environ Contam Toxicol. 2008;192:117–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colford JM, Hilton JF, Wright CC, Arnold BF, Saha S, Wade TJ, et al. The Sonoma water evaluation trial: a randomized drinking water intervention trial to reduce gastrointestinal illness in older adults. Am J Public Health. 2009;99(11):1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict KM, Reses H, Vigar M, Roth DM, Roberts VA, Mattioli M, et al. Surveillance for waterborne disease outbreaks associated with drinking water - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017; 66(44):1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira NA, Bondelind M. Safe drinking water and waterborne outbreaks. J Water Health. 2017;15(1):83–96. [DOI] [PubMed] [Google Scholar]
- 9.McClung RP, Roth DM, Vigar M, Roberts VA, Kahler AM, Cooley LA, et al. Waterborne disease outbreaks associated with environmental and undetermined exposures to water - United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66(44):1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derlet RW, Richards JR, Tanaka LL, Hayden C, Ger KA, Goldman CR. Impact of summer cattle grazing on the Sierra Nevada watershed: aquatic algae and bacteria. J Environ Public Health. 2012;2012:760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derlet RW, Ger KA, Richards JR, Carlson JR. Risk factors for coliform bacteria in backcountry lakes and streams in the Sierra Nevada mountains: a 5-year study. Wilderness Environ Med. 2008;19(2):82–90. [DOI] [PubMed] [Google Scholar]
- 12.Myers L, Whited B. The impact of cattle grazing in high elevation Sierra Nevada mountain meadows over widely variable annual climatic conditions. J Environ Prot (Irvine, Calif). 2012;3:823–837. [Google Scholar]
- 13.Pendergraph DP, Ranieri J, Ermatinger L, Baumann A, Metcalf AL, DeLuca TH, et al. Differentiating sources of fecal contamination to wilderness waters using droplet digital PCR and fecal indicator bacteria methods. Wilderness Environ Med. 2021;32(3):332–339. [DOI] [PubMed] [Google Scholar]
- 14.Derlet RW, Carlson JR, Noponen MN. Coliform and pathologic bacteria in Sierra Nevada national forest wilderness area lakes and streams. Wilderness Environ Med. 2004;15(4):245–249. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24(1):110–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Water treatment options when hiking, camping or traveling. Updated October 14, 2022. Accessed June 7, 2023. https://www.cdc.gov/healthywater/drinking/travel/index.html
- 17.WHO/UNICEF Joint Monitoring Programme for Water Supply Sanitation and Hygiene. Progress on household drinking water, sanitation and hygiene 2000-2020: five years into the SDGs. Water, Sanitation and Hygiene (WASH); 2021. Licence: CC BY-NC-SA 3.0 IGO. Accessed September 11, 2022. https://data.unicef.org/resources/progress-on-household-drinking-water-sanitation-and-hygiene-2000-2020/ [Google Scholar]
- 18.Clasen TF, Bastable A. Faecal contamination of drinking water during collection and household storage: the need to extend protection to the point of use. J Water Health. 2003;1(3):109–115. [PubMed] [Google Scholar]
- 19.Gil AI, Lanata CF, Hartinger SM, Mäusezahl D, Padilla B, Ochoa TJ, et al. Fecal contamination of food, water, hands, and kitchen utensils at the household level in rural areas of Peru. J Environ Health. 2014;76(6): 102–106. [PubMed] [Google Scholar]
- 20.Kravitz JD, Nyaphisi M, Mandel R, Petersen E. Quantitative bacterial examination of domestic water supplies in the Lesotho Flighlands: water quality, sanitation, and village health. Bull World Health Organ. 1999;77(10):829–836. [PMC free article] [PubMed] [Google Scholar]
- 21.Rai SK, Ono K, Yanagida JI, Ishiyama-Imura S, Kurokawa M, Rai CK. A large-scale study of bacterial contamination of drinking water and its public health impact in Nepal. Nepal Med Coll J. 2012;14(3):234–340. [PubMed] [Google Scholar]
- 22.Haramoto E. Detection of waterborne protozoa, viruses, and bacteria in groundwater and other water samples in the Kathmandu Valley, Nepal. IOP Conference Series: Earth Environ Sci. 2018;120:1–7. [Google Scholar]
- 23.Sobsey M. Managing Water in the Home: Accelerated Health Gains from Improved Water Supply. Water, Sanitation and Hygiene (WASH): 2002:69. WHO/SDE/WSH/02.07. Accessed November 20, 2022. https://www.who.int/publications/i/item/WHO-SDE-WSH-02.07
- 24.Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ. 2007;334(7597):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clasen T Household water treatment and safe storage to prevent diarrheal disease in developing countries. Curr Environ Health Rep. 2015;2(1):69–74. [DOI] [PubMed] [Google Scholar]
- 26.Lantagne DS, Clasen TF. Use of household water treatment and safe storage methods in acute emergency response: case study results from Nepal, Indonesia, Kenya, and Haiti. Environ Sci Technol. 2012;46(20):11352–11360. [DOI] [PubMed] [Google Scholar]
- 27.Lantagne D, Clasen T. Effective use of household water treatment and safe storage in response to the 2010 Haiti earthquake. Am J Trop Med Hyg. 2013;89(3):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickens DL, DuPont HL, Johnson PC. Survival of bacterial enteropathogens in the ice of popular drinks. JAMA. 1985;253(21):3141–3143. [PubMed] [Google Scholar]
- 29.Robertson LJ, Campbell AT, Smith HV. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl Environ Microbiol. 1992;58(11):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Doyle M. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J Food Prot. 1998;61(6):662–667. [DOI] [PubMed] [Google Scholar]
- 31.Ford TE. Microbiological safety of drinking water: United States and global perspectives. Environ Health Perspect. 1999;107(suppl 1):191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst C, Clark R, Regli S. Estimating the risk of acquiring infectious disease from ingestion of water. In: Hurst C, ed. Modeling Disease Transmission and its Prevention by Disinfection. Cambridge University Press; 1996:99–139. [Google Scholar]
- 33.Yoder JS, Hlavsa MC, Craun GF, Hill V, Roberts V, Yu PA, et al. Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events–United States, 2005-2006. MMWR Surveill Summ. 2008;57(9):1–29. [PubMed] [Google Scholar]
- 34.Parsaeimehr A, Lutzu GA, Rahman Shah M, Parra Saldivar R. Detection to treatment and global impacts of algal toxins. Front Biosci (Schol Ed). 2019;11(2):214–235. [DOI] [PubMed] [Google Scholar]
- 35.Lad A, Breidenbach JD, Su RC, Murray J, Kuang R, Mascarenhas A, et al. As we drink and breathe: adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life (Basel). 2022;12(3):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda. 4th ed. World Health Organization; 2022. Accessed 10/20/2023. https://www.who.int/publications/i/item/9789240045064 [PubMed] [Google Scholar]
- 37.Evans DP. Non-pharmacotherapeutic interventions in travellers diarrhoea (TD). J Travel Med. 2018;25(suppl 1):S38–S45. [DOI] [PubMed] [Google Scholar]
- 38.Muiphy JL, Kahler AM, Nansubuga I, Nanyunja EM, Kaplan B, Jothikumar N, et al. Environmental survey of drinking water sources in Kampala, Uganda, during a typhoid fever outbreak. Appl Environ Microbiol. 2017;83(23):e01706–01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geldreich EE, Reasoner DJ. Home treatment devices and water quality. In: McFeters G, ed. Drinking Water Microbiology. Springer-Verlag; 1990:147–168. [Google Scholar]
- 40.Rosen AA, Booth RL. Taste and odor control. In: American Water Works Association, ed. Water Quality and Treatment: A handbook of Public Water Supplies. 2nd ed. McGraw Hill; 1971. [Google Scholar]
- 41.Calabrese EJ. Health Effects of Drinking Water Treatment Technologies. 1st ed. CRC Press; 1989. [Google Scholar]
- 42.National Research Council (US) Safe Drinking Water Committee. The Disinfection of drinking water. Vol 2. Drinking Water and Health. National Academies Press; 1980:5–139. [Google Scholar]
- 43.Le Chevallier MW, McFeters GA. Microbiology of activated carbon. In: McFeters GA, ed. Drinking Water Microbiology. Springer-Verlag; 1990:104–120. [Google Scholar]
- 44.Hall T, Hart J, Croll B, Gregory R. Laboratory-scale investigations of algal toxin removal by water treatment. Water Environ J. 2000;14(2):143–149. [Google Scholar]
- 45.Lambert TW, Holmes CFB, Hrudey SE. Adsorption of microcystin-LR by activated carbon and removal in full scale water treatment. Water Res. 1996;30(6):1411–1422. [Google Scholar]
- 46.Medema GJ, Schets FM, Teunis PF, Havelaar AH. Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Appl Environ Microbiol. 1998;64(11):4460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coagulation Committee AWWA. Committee report: coagulation as an integrated water treatment process. J Am Water Works Assoc. 1989;81(10):74–78. [Google Scholar]
- 48.Cohen JM, Hannah SA. Water Quality and Treatment: A Handbook of Public Water Supplies. McGraw-Hill: 1971. [Google Scholar]
- 49.Crump JA, Otieno PO, Slutsker L. Keswick BH, Rosen DH, Hoekstra RM, et al. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhoea in areas with turbid source water in rural western Kenya: cluster randomised controlled trial. BMJ. 2005;331(7515):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston K, Lantagne D, Kotlarz N, Jellison K. Turbidity and chlorine demand reduction using alum and Moringa flocculation before household chlorination in developing countries. J Water Health. 2010;8(1):60–70. [DOI] [PubMed] [Google Scholar]
- 51.Kotlarz N, Lantagne D, Preston K, Jellison K. Turbidity and chlorine demand reduction using locally available physical water clarification mechanisms before household chlorination in developing countries. J Water Health. 2009;7(3):497–506. [DOI] [PubMed] [Google Scholar]
- 52.LeChevallier MW, Evans TM, Seidler RJ. Effect of turbidity on chlorination efficiency and bacterial persistence in drink,ing water. Appl Environ Microbiol. 1981;42(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reller ME, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Olson CA, et al. A randomized controlled trial of household-based flocculant-disinfectant drinking water treatment for diarrhea prevention in rural Guatemala. Am J Trop Med Hyg. 2003;69(4):411–419. [PubMed] [Google Scholar]
- 54.Powers EM, Hernandez C, Boutros SN, Harper BG. Biocidal efficacy of a flocculating emergency water purification tablet. Appl Environ Microbiol. 1994;60(7 ):2316–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powers E Efficacy of flocculating and other emergency water purification tablets. 1993. Report Natick/TR-93/033. Accessed 10/20/2023. https://apps.dtic.mil/sti/pdfs/ADA266695.pdf
- 56.Bielefeldt AR. Appropriate and sustainable water disinfection methods for developing communities In: Buchanan KM, ed. Water Disinfection. Nova Science Publishers; 2011:45–75. [Google Scholar]
- 57.Marois-Fiset JT, Shaheed A, Brown J, Dorea CC. Laboratory evaluation of a new coagulant/disinfectant point-of-use water treatment product for emergencies. J Appl Microbiol. 2016;121(3):892 –902. [DOI] [PubMed] [Google Scholar]
- 58.Crump JA, Okoth GO, Slutsker L, Ogaja DO, Keswick BH, Luby SP. Effect of point-of-use disinfection, flocculation and combined flocculation-disinfection on drinking water quality in western Kenya. J Appl Microbiol. 2004;97(1):225–231. [DOI] [PubMed] [Google Scholar]
- 59.Islam MS, Ansaruzzaman M, Mahmud ZH, Matin MA, Islam MS, Mallik AK, et al. A novel and simple mixture as point-of-use water treatment agent to produce safe drinking water. Trans R Soc Trop Med Hyg. 2014;108(5):290–296. [DOI] [PubMed] [Google Scholar]
- 60.Pandit AB, Kumar JK. Clean water for developing countries. Annu Rev Chem Biomol Eng. 2015;6:217–246. [DOI] [PubMed] [Google Scholar]
- 61.Army US. Sanitary control and surveillance of field water supplies. 2005. Department of Army Technical Bulletin (TB Med 577). Dec 15. Accessed 10/20/2023. https://armypubs.army.mil/epubs/DR_pubs/DR__a/pdf/web/tbmed577.pdf [Google Scholar]
- 62.Clasen T, Brown J, Suntura O, Collin S. Safe household water treatment and storage using ceramic drip filters: a randomised controlled trial in Bolivia. Water Sci Technol. 2004; 50(1): 111–115. [PubMed] [Google Scholar]
- 63.Frazier WC, Westhoff DC. Preservation by use of high temperatures. In: Food microbiology. McGraw-Hill; 1978. [Google Scholar]
- 64.Islam MF, Johnston RB. Household pasteurization of drinking-water: the chulli water-treatment system. J Health Popul Nutr. 2006;24(3):356–362. [PMC free article] [PubMed] [Google Scholar]
- 65.Fayer R Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl Environ Microbiol. 1994;60(8):2732–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bandres JC, Mathewson JJ, DuPont HL. Heat susceptibility of bacterial enteropathogens. Implications for the prevention of travelers’ diarrhea. Arch Intern Med. 1988;148(10):2261 –2263. [PubMed] [Google Scholar]
- 67.Aukerman R, Monzingo D. Water treatment to inactivate Giardia. J For. 1989;87(11):18–21. [Google Scholar]
- 68.Ongerth JE, Johnson RL, MacDonald SC, Frost F, Stibbs HH. Back-country water treatment to prevent giardiasis. Am J Public Health. 1989;79(12):1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bingham AK, Jr Jarroll EL, Mayer EA, Radulescu S. Giardia sp.: physical factors of exeystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol. 1979;47(2):284 –291. [DOI] [PubMed] [Google Scholar]
- 70.Anderson BC. Moist heat inactivation of Cryptosporidium sp. Am J Public Health. 1985;75(12):1433–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shephart M Helminthological aspects of sewage treatment. In: Feachem R, McGarry M, Mara D, eds. Water, Wastes and Health in Hot Climates. John Wiley and Sons; 1977:299–310. [Google Scholar]
- 72.Franssen F, Gerard C, Cozma-Petrut A, Vieira-Pinto M, Jambrak AR, Rowan N, et al. Inactivation of parasite transmission stages: efficacy of treatments on food of animal origin. Trends Food Sci Technol. 2019;83:114–128. [Google Scholar]
- 73.Perkins J Thermal destruction of microorganisms: heat inactivation of viruses. In: Thomas C, ed. Principles and Methods of Sterilization in Health Sciences. Springfield, IL: John Wiley and Sons; 1969:63–94. [Google Scholar]
- 74.Tuladhar E, Bouwknegt M, Zwietering MH, Koopmans M, Duizer E. Thermal stability of structurally different viruses with proven or potential relevance to food safety. J Appl Microbiol. 2012;112(5):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baert L, Debevere J, Uyttendaele M. The efficacy of preservation methods to inactivate foodborne viruses. Int J Food Microbiol. 2009;131(2–3):83–94. [DOI] [PubMed] [Google Scholar]
- 76.Bidawid S, Farber JM, Sattar SA, Hayward S. Heat inactivation of hepatitis A virus in dairy foods. J Food Prot. 2000;63(4):522–528. [DOI] [PubMed] [Google Scholar]
- 77.Krugman S, Giles JP, Hammond J. Hepatitis virus: effect of heat on the infectivity and antigenicity of the MS-1 and MS-2 strains. J Infect Dis. 1970;122(5):432–436. [DOI] [PubMed] [Google Scholar]
- 78.Alder V, Simpson R. Sterilization and disinfection by heat methods. In: Russel A, Hugo W, Ayliffe G, eds. Principles and Practice of Disinfection, Preservation, and Sterilization. 2nd ed. Blackwell Scientific; 1992:483. [Google Scholar]
- 79.Brown J, Sobsey MD. Boiling as household water treatment in Cambodia: a longitudinal study of boiling practice and microbiological effectiveness. Am J Trop Med Hyg. 2012;87(3):394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fagerli K, Trivedi KK, Sodha SV, Blanton E, Ati A, Nguyen T, et al. Comparison of boiling and chlorination on the quality of stored drinking water and childhood diarrhoea in Indonesian households. Epidemiol Infect. 2017;145(15):3294–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groh CD, MacPherson DW, Groves DJ. Effect of heat on the sterilization of artificially contaminated water. J Travel Med. 1996;3(1):11–13. [DOI] [PubMed] [Google Scholar]
- 82.Neumann HH. Alternatives to water chlorination. Rev Infect Dis. 1981;3(6):1255–1257. [DOI] [PubMed] [Google Scholar]
- 83.Neumann HH. Bacteriological safety of hot tapwater in developing countries. Public Health Rep (1896). 1969;84(9):812–814. [PMC free article] [PubMed] [Google Scholar]
- 84.Ciochetti DA, Metcalf RH. Pasteurization of naturally contaminated water with solar energy. Appl Environ Microbiol. 1984;47(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.García-Gil A, García-Muñoz RA, McGuigan KG, Marugán J. Solar water disinfection to produce safe drinking water: a review of parameters, enhancements, and modelling approaches to make SODIS faster and safer. Molecules. 2021;26(11):3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaúque BJM, Brandão FG, Rott MB. Development of solar water disinfection systems for large-scale public supply, state of the art, improvements and paths to the future—a systematic review. J Environ Chem Eng. 2022;10(3):107887. [Google Scholar]
- 87.LeChevallier MW, Au KK; World Health Organization. Water treatment and pathogen control: process efficiency in achieving safe drinking water. Published 2004. WHO Drinking Water Quality Series. Accessed 10/20/2023 http://apps.who.int/iris/bitstream/10665/42796/1/9241562552.pdf [Google Scholar]
- 88.Hijnen WA, Beerendonk EF, Medema GJ. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 2006;40(1):3–22. [DOI] [PubMed] [Google Scholar]
- 89.Linden KG, Shin GA, Faubert G, Cairns W, Sobsey MD. UV disinfection of Giardia lamblia cysts in water. Environ Sci Technol. Jun 1 2002;36(11):2519–2522. [DOI] [PubMed] [Google Scholar]
- 90.Zimmer JL, Slawson RM, Huck PM. Inactivation and potential repair of Cryptosporidium parvum following low- and medium-pressure ultraviolet irradiation. Water Res. 2003;37(14):3517–3523. [DOI] [PubMed] [Google Scholar]
- 91.Abd-Elmaksoud S, Naranjo JE, Gerba CP. Assessment of a portable handheld UV light device for the disinfection of viruses and bacteria in water. Food Environ Virol. 2013;5(2):87–90. [DOI] [PubMed] [Google Scholar]
- 92.Reygadas F, Gruber JS, Ray I, Nelson KL. Field efficacy evaluation and post-treatment contamination risk assessment of an ultraviolet disinfection and safe storage system. Water Res. 2015;85:74–84. [DOI] [PubMed] [Google Scholar]
- 93.Chatterley C, Linden K. Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. J Water Health. 2010;8(3):479–486. [DOI] [PubMed] [Google Scholar]
- 94.Mariita RM, Blumenstein SA, Beckert CM, Gombas T, Randive RV. Disinfection Performance of a drinking water bottle system with a UV subtype C LED cap against water-borne pathogens and heterotrophic contaminants. Front Microbiol. 2021:12:719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King BJ, Hoefel D, Daminato DP, Fanok S, Monis PT. Solar UV reduces Cryptosporidium parvum oocyst infectivity in environmental waters. J Appl Microbiol. 2008;104(5):1311–1323. [DOI] [PubMed] [Google Scholar]
- 96.Tedeschi CM, Barsi C, Peterson SE, Carey KM. A pilot study of solar water disinfection in the wilderness setting. Wilderness Environ Med. 2014;25(3):340–345. [DOI] [PubMed] [Google Scholar]
- 97.MÄusezahl D, Christen A, Pacheco GD, Tellez FA, Iriarte M, Zapata ME, et al. Solar drinking water disinfection (SODIS) to reduce childhood diarrhoea in rural Bolivia: a cluster-randomized, controlled trial. PLoS Med. 2009;6(8):e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.du Preez M, Conroy RM, Ligondo S, Hennessy J, Elmore-Meegan M, Soita A, et al. Randomized intervention study of solar disinfection of drinking water in the prevention of dysentery in Kenyan children aged under 5 years. Environ Sci Technol. 2011;45(21):9315–9323. [DOI] [PubMed] [Google Scholar]
- 99.Conroy RM, Meegan ME, Joyce T, McGuigan K, Barnes J. Solar disinfection of drinking water protects against cholera in children under 6 years of age. Arch Dis Child. 2001;85(4):293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGuigan KG, Conroy RM, Mosler HJ, du Preez M, Ubomba-Jaswa E, Fernandez-Ibañez P . Solar water disinfection (SODIS): a review from bench-top to roof-top. J Hazard Mater. 2012;235-236:29–16. [DOI] [PubMed] [Google Scholar]
- 101.Berney M, Weilenmann HU, Simonetti A, Egli T. Efficacy of solar disinfection of Escherichia coli, Shigella flexneri, Salmonella Typhimurium and Vibrio cholerae. J Appl Microbiol. 2006;101(4):828–836. [DOI] [PubMed] [Google Scholar]
- 102.Luzi S, Tobler M, Suter F, Meierhofer R SODIS Manual. Swiss federal institute of envimomental science and technology (EAWAG); 2016. Accessed 10/20/2023. https://www.sodis.ch/methode/anwendung/ausbildungsmaterial/dokumente_material/sodismanual_2016_lr.pdf [Google Scholar]
- 103.McGuigan KG, Méndez-Hermida F, Castro-Hermida JA, Ares-Mazás E, Kehoe SC, Boyle M, et al. Batch solar disinfection inactivates oocysts of Cryptosporidium parvum and cysts of Giardia muris in drinking water. J Appl Microbiol. 2006;101(2):453–463. [DOI] [PubMed] [Google Scholar]
- 104.Centers for Disease Control and Prevention. Solar disinfection. Updated January 10, 2022. Accessed September 15, 2022. https://www.cdc.gov/healthywater/global/household-water-treatment/solardisinfection.html
- 105.Juvakoski A, Singhal G, Manzano MA, Moriñigo M, Vahala R, Levchuk I. Solar disinfection - an appropriate water treatment method to inactivate faecal bacteria in cold climates. Sci Total Environ. June 25: 827:154086. [DOI] [PubMed] [Google Scholar]
- 106.McGuigan KG, Joyce TM, Conroy RM. Solar disinfection: use of sunlight to decontaminate drinking water in developing countries. J Med Microbiol. 1999;48(9):785–787. [DOI] [PubMed] [Google Scholar]
- 107.McGuigan KG, Joyce TM, Conroy RM, Gillespie JB, Elmore-Meegan M. Solar disinfection of drinking water contained in transparent plastic bottles: characterizing the bacterial inactivation process. J Appl Microbiol. 1998; 84(6):1138–1148. [DOI] [PubMed] [Google Scholar]
- 108.Rijal GK, Fujioka RS. Synergistic effect of solar radiation and solar heating to disinfect drinking water sources. Water Sci Technol. 2001;43(12):155–162. [PubMed] [Google Scholar]
- 109.Joyce TM, McGuigan KG, Elmore-Meegan M, Conroy RM. Inactivation of fecal bacteria in drinking water by solar heating. Appl Environ Microbiol. 1996;62(2):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Safapour N, Metcalf RH. Enhancement of solar water pasteurization with reflectors. Appl Environ Microbiol. 1999;65(2):859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strauss A, Dobrowsky PH, Ndlovu T, Reyneke B, Khan W. Comparative analysis of solar pasteurization versus solar disinfection for the treatment of harvested rainwater. BMC Microbiol. 2016;16(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larson KL, Hansen C, Ritz M, Carreño D. Acceptance and impact of point-of-use water filtration systems in rural Guatemala. J Nurs Scholarsh. 2017;49(1):96–102. [DOI] [PubMed] [Google Scholar]
- 113.Sobsey MD, Stauber CE, Casanova LM, Brown JM, Elliott MA. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ Sci Technol. 2008;42(12):4261–4267. [DOI] [PubMed] [Google Scholar]
- 114.UNICEF. Household water treatment filters product guide. Accessed October 30, 2022. https://www.unicef.org/innovation/media/14316/file/Household_Water_Treatment_Filters_Product_Guide.pdf
- 115.Zhang Y, Wang Q, Lou W, Wang Y, Zhu X. Microbiological safety of household membrane water filter. J Environ Biol. 2013;34(2 Spec No):481–487. [PubMed] [Google Scholar]
- 116.Environmental Health Directorate Health Protection Branch. Assessing the effectiveness of small filtration systems for point-of-use disinfection of drinking water supplies. Department of National Health and Welfare, Ottawa; 1980. 80-EHD-54. [Google Scholar]
- 117.Schlosser O, Robert C, Bourderioux C, Rey M, de Roubin MR. Bacterial removal from inexpensive portable water treatment systems for travelers. J Travel Med. 2001;8(1):12–18. [DOI] [PubMed] [Google Scholar]
- 118.Hörman A, Rimhanen-Finne R, Maunula L, von Bonsdorff CH, Rapala J, Lahti K, et al. Evaluation of the purification capacity of nine portable, small-scale water purification devices. Water Sci Technol. 2004;50(1):179–183. [PubMed] [Google Scholar]
- 119.Rao VC, Symons JM, Ling A, Wang P, Metcalf TG, Hoff JC, et al. Removal of hepatitis A virus and rotavirus by drinking water treatment. J Am Water Works Assoc. 1988;80(2):59–67. [Google Scholar]
- 120.Mull B, Hill VR. Recovery of diverse microbes in high turbidity surface water samples using dead-end ultrafiltration. J Microbiol Methods. 2012;91(3):429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raphael BH, Lautenschlager M, Kahler A, Pai S, Parks BA, Kalb SR, et al. Ultrafiltration improves ELISA and Endopep MS analysis of botulinum neurotoxin type A in drinking water. J Microbiol Methods. 2012;90(3):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lindquist ED, George CM, Perin J, Nciswender de Calani KJ, Nonnan WR, Davis TP, et al. A cluster randoized controlled trial to reduce childhood diarrhea using hollow fiber water filter and/or hygiene-sanitation educational interventions. Am J Trop Med Hyg. 2014;91(1):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clasen T, Menon S. Microbiological performance of common water treatment devices for household use in India. Int J Environ Health Res. 2007;17(2):83–93. [DOI] [PubMed] [Google Scholar]
- 124.Bevan R, Nocher A; World Health Organization. Iodine as a drinking-water disinfectant. Alternative drinking-water disinfectants: bromine, iodine and silver. Published 2018. Accessed September 213, 2022. https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/AQ52iodine-0203201.pdf?sfvrsn=4d414c11_5 [Google Scholar]
- 125.Vasudevan P, Tandon M. Antimicrobial properties of iodine based products. J Sci Ind Res. 2010;69:376–383. [Google Scholar]
- 126.Naranjo JE, Chaidez C, Quiñonez M, Gerba CP, Olson J, Dekko J. Evaluation of a portable water purification system for the removal of enteric pathogens. Water Sci Techol. 1997;35(11–12):55–58. [Google Scholar]
- 127.NASA. An innovation for global clean water. Accessed September 21, 2022. https://spinoff.nasa.gov/node/9563
- 128.Lantagne D, Person B, Smith N, Mayer A, Preston K, Blanton E, et al. Emergency water treatment with bleach in the United States: the need to revise EPA recommendations. Environ Sci Technol. 2014;48(9):5093–5100. [DOI] [PubMed] [Google Scholar]
- 129.Kozlicic A, Hadzic A, Bevanda H. Improvised purification methods for obtaining individual drinking water supply under war and extreme shortage conditions. Prehosp Disaster Med. 1994;9(2)suppl 1):S25–S28. [DOI] [PubMed] [Google Scholar]
- 130.Clasen TF, Brown J, Collin S, Suntura O, Caimcross S. Reducing diarrhea through the use of household-based ceramic water filters: a randomized, controlled trial in rural Bolivia. Am J Trop Med Hyg. 2004;70(6):651–657. [PubMed] [Google Scholar]
- 131.Brown J, Sobsey MD, Loomis D. Local drinking water filters reduce diarrheal disease in Cambodia: a randomized, controlled trial of the ceramic water purifier. Am J Trop Med Hyg. 2008;79(3):394–400. [PubMed] [Google Scholar]
- 132.Morris JF, Murphy J, Fagerli K, Schneeberger C, Jaron P, Moke F, et al. A randomized controlled trial to assess the impact of ceramic water filters on prevention of diarrhea and cryptosporidiosis in infants and young children-Westem Kenya, 2013. Am J Trop Med Hyg. 2018;98(5):1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moropeng RC, Budeli P. Mpenyana-Monyatsi L, Momba MNB. Dramatic reduction in diarrhoeal diseases through implementation of cost-effective household drinking water treatment systems in Makwane Village, Limpopo Province, South Africa. Int J Environ Res Public Health. 2018;15(3):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.du Preez M, Conroy RM, Wright JA, Moyo S, Potgieter N, Gundry SW. Use of ceramic water filtration in the prevention of diarrheal disease: a randomized controlled trial in rural South Africa and Zimbabwe. Am J Trop Med Hyg. Nov 2008;79(5):696–701. [PubMed] [Google Scholar]
- 135.Tiwari SS, Schmidt WP, Darby J, Kariuki ZG, Jenkins MW. Intermittent slow sand filtration for preventing diarrhea among children in Kenyan households using unimproved water sources: randomized controlled trial. Trop Med Int Health. Nov 2009;14(11):1374–1382. [DOI] [PubMed] [Google Scholar]
- 136.Stauber CE, Kominek B, Liang KR, Osman MK, Sobsey MD. Evaluation of the impact of the plastic BioSand filter on health and drinking water quality in rural Tamale, Ghana. Int J Environ Res Public Health. 24 2012;9(11):3806–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stauber CE, Ortiz GM, Loomis DP, Sobsey MD. A randomized controlled trial of the concrete biosand filter and its impact on diarrheal disease in Bonao, Dominican Republic. Am J Trop Med Hyg. 2009;80(2):286–293. [PubMed] [Google Scholar]
- 138.Fabiszewski de Aceituno AM, Stauber CE, Walters AR, Meza Sanchez RE, Sobsey MD. A randomized controlled trial of the plastic-housing BioSand filter and its impact on diarrheal disease in Copan, Honduras. Am J Trop Med Hyg. 2012;86(6):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Duke WF, Nordin RN, Baker D, Mazumder A. The use and performance of BioSand filters in the Artibonite Valley of Haiti: a field study of 107 households. Rural Remote Health. 2006;6(3):570. [PubMed] [Google Scholar]
- 140.Center for Affordable Water and Sanitation Technology. Biosand filter construction manual. Published; August 2012. Accessed 10/20/2023 https://washresources.cawst.org/en/resources/b6be2637/biosand-filter-construction-manual
- 141.White GC. White’s Handbook of Chlorination and Alternative Disinfectants. 5th ed. Wiley; 2010. [Google Scholar]
- 142.Briton G. Introduction to environmental virology. Wiley; 1980. [Google Scholar]
- 143.Chang S Modern concepts of disinfection: water treatment in the seventies. Proceedings of the National Specialty Conference on Disinfection. American Society of Civil Engineers; 1970:635–679. [Google Scholar]
- 144.Rice EW, Hoff JC, Schaefer FW. Inactivation of Giardia cysts by chlorine. Appl Environ Microbiol. 1982;43(1):250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wallis P, Davies JS, Nutbrown R. Buchanan-Mappin JM, Roach PD, van Roodselaar A. Removal and inactivation of Giardia cysts in a mobile water treatment plant under field condition: preliminary results. In: Wallis P, Hammond B, eds. Advances in Giardia Research. University of Calgary Press; 1988:137–144. [Google Scholar]
- 146.Sobsey MD, Oldham CE, McCall DE. Comparative inactivation of hepatitis A virus and other enteroviruses in water by iodine. Water Sci Technol. 1991;24(2):331–337. [Google Scholar]
- 147.Berg G, Chang S, Harris E. Devitalization of microorganisms by iodine. I. Dynamics of the devitalization of enteroviruses by elemental iodine. Virology. Apr : 22:469–481. [DOI] [PubMed] [Google Scholar]
- 148.Centers for Disease Control and Prevention. Safe water systems for the developing world: a handbook for implementing household-based water treatment and safe storage projects. Published 2001. Accessed 10/20/2023. http://www.cdc.gov/safewater/manual/sws_manual.pdf
- 149.Lantagne DS. Viability of commercially available bleach for water treatment in developing countries. Am J Public Health. 2009;99(11):1975–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arnold BF, Colford JM Jr. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg. 2007;76(2):354–364. [PubMed] [Google Scholar]
- 151.Pickard B, Clarke S, William B. Chlorine disinfection in the use of individual water purification devices. US Army Center for Health Promotion and Preventive Medicine; 2006. Technical Report. TIP 31-002-0306. Accessed 10/20/2023. https://apps.dtic.mil/sti/pdfs/ADA454058.pdf [Google Scholar]
- 152.Mengistie B, Berhane Y, Worku A. Household water chlorination reduces incidence of diarrhea among under-five children in mral Ethiopia: a cluster randomized controlled trial. PloS One. 2013;8(10):e77887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mintz ED, Reiff FM, Tauxe RV. Safe water treatment and storage in the home. A practical new strategy to prevent waterborne disease. JAMA. 1995;273(12):948–953. [PubMed] [Google Scholar]
- 154.Shrestha RK, Marseille E, Kahn JG, Lule JR, Pitter C, Blandford JM, et al. Cost-effectiveness of home-based chlo,rination and safe water storage in reducing diarrhea among HIV-affected households in mral Uganda. Am J Trop Med Hyg. 2006;74(5):884–890. [PubMed] [Google Scholar]
- 155.US Army Public Health Command. Iodine disinfection in the use of individual water purification devices. Published 2011. Accessed 10/20/2023. https://phc.amedd.army.mil/PRC%20Resource%20Library/Iodine%20Disinfection%20in%20the%20Use%20of%20Individual%20Water%20Purification%20Devices.pdf
- 156.Blaser MJ, Smith PF, Wang WL, Hoff JC. Inactivation of Campylobacter jejuni by chlorine and monochloramine. Appl Environ Microbiol. 1986;51(2):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang XW, Li JS, Jin M, Zhen B, Kong QX, Song N, et al. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126(1–2):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Craun G, Swedlow D, Tauxe R, Clark R, Fox K, Geldreich E, et al. Prevention of waterborne cholera in the United States. J Am Water Works Assoc. 1991;83(11):40–45. [Google Scholar]
- 159.Shin GA, Sobsey MD. Inactivation of norovirus by chlorine disinfection of water. Water Res. 2008;42(17)4562–4568. [DOI] [PubMed] [Google Scholar]
- 160.Engelbrecht RS, Weber MJ, Salter BL, Schmidt CA. Comparative inactivation of viruses by chlorine. Appl Environ Microbiol. 1980;40(2):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grabow WO, Gauss-Müller V, Prozesky OW, Deinhardt F. Inactivation of hepatitis A virus and indicator organisms in water by free chlorine residuals. Appl Environ Microbiol. 1983. ;46(3):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sobsey MD, Fuji T, Hall RM. Inactivation of cell-associated and dispersed hepatitis A virus in water. J Am Water Works Assoc. 1991;83(11):64–67. [Google Scholar]
- 163.Fraker LD, Gentile DA, Krivoy D, Condon M, Backer HD. Giardia cyst inactivation by iodine. J Wilderness Med. 1992;3(4):351–357. [Google Scholar]
- 164.Hibler C, Hancock C, Perger L, Wegrzyn J, Swabby K. Inactivation of Giardia cysts with chlorine at 0.5C to 5.0C. AWWA Research Report. AWWA Research Foundation; 1987. [Google Scholar]
- 165.Rubin AJ, Evers DP, Eyman CM, Jarroll EL. Inactivation of Gerbil-cultured Giardia lamblia cysts by free chlorine. Appl Environ Microbiol. 1989;55(10):2592–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clark RM, Read EJ, Hoff JC. Analysis of inactivation of Giardia lamblia by chlorine. J Environ Eng. 1989;115(1):80–90. [Google Scholar]
- 167.Carpenter C, Fayer R, Trout J, Beach MJ. Chlorine disinfection of recreational water for Cryptosporidium parvum. Emerg Infect Dis. 1999;5(4):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shields JM, Hill VR, Arrowood MJ, Beach MJ. Inactivation of Cryptosporidium parvum under chlorinated recreational water conditions. J Water Health. 2008;6(4):513–520. [DOI] [PubMed] [Google Scholar]
- 169.Wilhelm N, Kaufmann A, Blanton E, Lantagne D. Sodium hypochlorite dosage for household and emergency water treatment: updated recommendations. J Water Health. 2018;16(1):112–125. [DOI] [PubMed] [Google Scholar]
- 170.Gerba PC, Johnson DC, Hasan MN. Efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med. 1997;8(2):96–100. [DOI] [PubMed] [Google Scholar]
- 171.Mohamed H, Brown J, Njee RM, Clasen T, Malebo HM, Mbuligwe S. Point-of-use chlorination of turbid water: results from a field study in Tanzania. J Water Health. 2015;13(2):544–552. [DOI] [PubMed] [Google Scholar]
- 172.Souter PF, Cruickshank GD, Tankerville MZ, Keswick BH, Ellis BD, Langworthy DE, et al. Evaluation of a new water treatment for point-of-use household applications to remove microorganisms and arsenic from drinking water. J Water Health. 2003; 1(2):73–84. [PubMed] [Google Scholar]
- 173.Lantagne DS, Cardinali F, Blount BC. Disinfection by-product formation and mitigation strategies in point-of-use chlorination with sodium dichloroisocyanurate in Tanzania. Am J Trop Med Hyg. 2010;83(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Black AP, Kinman RN, Thomas WC, Freund G, Bird ED. Use of iodine for disinfection. J Am Water Works Assoc. 1965;57(11):1401–1421. [Google Scholar]
- 175.Thomas WC, Malagodi MH, Oates TW, McCourt JP. Effects of an iodinated water supply. Trans Am Clin Climatol Assoc. 1979;90:153–162. [PMC free article] [PubMed] [Google Scholar]
- 176.Backer H, Hollowell J. Use of iodine for water disinfection: iodine toxicity and maximum recommended dose. Environ Health Perspect. 2000;108(8):679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lantagne DS. Sodium hypochlorite dosage for household and emergency water treatment. J Am Water Works Assoc. 2008;100(8):106–119. [DOI] [PubMed] [Google Scholar]
- 178.Gottardi W. Iodine and iodine compounds. In: Block S, ed. Disinfection, Sterilization, and Preservation. 4th. ed. Lea & Febiger; 1991:152–167. [Google Scholar]
- 179.Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986;151(3):400–406. [DOI] [PubMed] [Google Scholar]
- 180.Reiff FM, Roses M, Venczel L, Quick R, Witt VM. Low-cost safe water for the world: a practical interim solution. J Public Health Policy. 1996;17(4):389–408. [PubMed] [Google Scholar]
- 181.Clark RM, Sivaganesan M, Rice EW, Chen J. Development of a Ct equation for the inactivation of Cryptosporidium oocysts with chlorine dioxide. Water Res. 2003;37(11):2773–2783. [DOI] [PubMed] [Google Scholar]
- 182.Murphy JL, Haas CN, Arrowood MJ, Hlavsa MC, Beach MJ, Hill VR. Efficacy of chlorine dioxide tablets on inactivation of Cryptosporidium oocysts. Environ Sci Technol. 2014;48(10):5849–5856. [DOI] [PubMed] [Google Scholar]
- 183.Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol 1990;56(5):1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.US Environmental Protection Agency. Wastewater technology fact sheet: ozone disinfection. Accessed November 2, 2022. https://www3.epa.gov/npdes/pubs/ozon.pdf
- 185.Finch GR, Black EK, Labatiuk CW, Gyürék L, Bclosevic M. Comparison of giardia lamblia and giardia muris cyst inactivation by ozone. Appl Environ Microbiol. 1993;59(11):3674–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Peeters JE, Mazás EA, Masschelein WJ, Villacorta Martiez dc Maturana I, DeBacker E. Effect of disinfection of drinking water with ozone or chlorine dioxide on survival of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1989;55(6):1519–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bevan R, Nocher A; World Health Organization. Silver as a drinking-water disinfectant. Alternative drinking-water disinfectants: bromine, iodine and silver. Published 2018. Accessed September 12, 2022. https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/silver-020320188b233a75-5b6f-489d-bff5-99d7f2e77d98.pdf?sfvrsn=fed055313 [Google Scholar]
- 188.US Environmental Protection Agency, Office of Water. Alternative disinfectants and oxidants guidance manual. Published 1999. Accessed 10/20/2023. https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=2000229L.txt
- 189.Marchin GL, Fina LR, Hoff JC. Contact and demand-release disinfectants. Crit Rev Environ Control. 1989;19(4):227–290. [Google Scholar]
- 190.Khan ST, Malik A. Engineered nanomaterials for water decontamination and purification: from lab to products. J Hazard Mater. 2019; Feb 5 : 363:295–308. [DOI] [PubMed] [Google Scholar]
- 191.Brown J, Sobsey MD. Microbiological effectiveness of locally produced ceramic filters for drinking water treatment in Cambodia. J Water Health. 2010;8(1):1–10. [DOI] [PubMed] [Google Scholar]
- 192.Fisher MB, Keenan CR, Nelson KL, Voelker BM. Speeding up solar disinfection (SODIS): effects of hydrogen peroxide, temperature, pH, and copper plus ascorbate on the photoinactivation of E. coli. J Water Health. 2008;6(1):35–51. [DOI] [PubMed] [Google Scholar]
- 193.Li W, Bonakdarpour A, Gyenge E, Wilkinson DP. Drinking water purification by electrosynthesis of hydrogen peroxide in a power-producing PEM fuel cell. ChemSusChem. 2013;6(11):2137–2143. [DOI] [PubMed] [Google Scholar]
- 194.D’Aquino M, Teves SA. Lemon juice as a natural biocide for disinfecting drinking water. Bull Pan Am Health Organ. 1994;28(4):324–330. [PubMed] [Google Scholar]
- 195.National Institute of Environmental Health Sciences. Nanomaterials. Updated September 8, 2021. Accessed September 20, 2022. https://www.niehs.nih.gov/health/topics/agents/sya-nano/index.cfm
- 196.Chong MN, Jin B, Chow CW, Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. May 2010;44(10):2997–3027. [DOI] [PubMed] [Google Scholar]
- 197.Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–4602. [DOI] [PubMed] [Google Scholar]
- 198.Kumar S, Ahlawat W, Bhanjana G, Heydarifard S, Nazhad MM, Dilbaghi N. Nanotechnology-based water treatment strategies. J Nanosci Nanotechnol. 2014;14(2):1838–1858. [DOI] [PubMed] [Google Scholar]
- 199.Sunnotel O, Verdoold R, Dunlop PS, Snelling WJ, Lowery CJ, Dooley JSG, et al. Photocatalytic inactivation of Cryptosporidium parvum on nanostructured titanium dioxide films. J Water Health. 2010;8(1):83–91. [DOI] [PubMed] [Google Scholar]
- 200.Blanco-Galvez J, Fernández-Ibáñez P, Malato-Rodríguez S. Solar photocatalytic detoxification and disinfection of water: recent overview. J Sol Energy Eng. 2007;129(1):4–15. [Google Scholar]
- 201.Alrousan DM, Dunlop PS, McMurray TA, Byrne JA. Photocatalytic inactivation of E. coli in surface water using immobilised nanoparticle TiO2 films. Water Res. 2009;43(1):47–54. [DOI] [PubMed] [Google Scholar]
- 202.Clasen TF, Alexander KT, Sinclair D, Boisson S, Pcletz R, Chang HH, et al. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev. 2015;2015(10):CD004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bielefeldt AR, Kowalski K, Summers RS. Bacterial treatment effectiveness of point-of-use ceramic water filters. Water Res. 2009;43(14):3559–3565. [DOI] [PubMed] [Google Scholar]
- 204.Mwabi JK, Mamba BB, Momba MN. Removal of Escherichia coli and faecal coliforms from surface water and groundwater by household water treatment devices/systems: a sustainable solution for improving water quality in rural communities of the Southern African Development Community region. Int J Environ Res Public Health. 2012;9(1):139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sobel J, Mahon B, Mendoza CE, Passaro D, Cano F, Baier K, et al. Reduction of fecal contamination of street-vended beverages in Guatemala by a simple system for water purification and storage, handwashing, and beverage storage. Am J Trop Med Hyg. 1998;59(3):380–387. [DOI] [PubMed] [Google Scholar]
- 206.Logsdon GS, Symons JM, Hoye RL, Arozarena MM. Alternative filtration methods for removal of Giardia cysts and cyst models. J Am Water Works Assoc. 1981;73(2):111–118. [Google Scholar]
- 207.Jarroll EL, Bingham AK, Meyer EA. Giardia cyst destruction: effectiveness of six small-quantity water disinfection methods. Am J Trop Med Hyg. 1980;29(1):8–11. [DOI] [PubMed] [Google Scholar]
- 208.Tobin RS. Water treatment for the home or cottage. Can J Public Health. 1984;75(1):79–82. [PubMed] [Google Scholar]
- 209.Ibeto CN, Oparaku NF, Okpara CG. Comparative study of renewable energy based water disinfection methods for developing countries. J Environ Sci Technol. 2010;3(4):226–231. [Google Scholar]
- 210.Naranjo JE , Gerba CP. Evaluation of portable water treatment devices by a condensed version of the Guide of standard protocol for microbiological purifiers (USEPA, 1987). 1995. [Google Scholar]
- 211.McDonnell GE. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance. John Wiley & Sons; 2020. [Google Scholar]
- 212.Opryszko MC, Majeed SW, Hansen PM, Myers JA, Baba D, Thompson RE, et al. Water and hygiene interventions to reduce diarrhoea in mral Afghanistan: a randomized controlled study. J Water Health. 2010;8(4):687–702. [DOI] [PubMed] [Google Scholar]
- 213.Jung S, Doh YA, Bizuneh DB, Beyene H, Seong J, Kwon H, et al. The effects of improved sanitation on diarrheal prevalence, incidence, and duration in children under five in the SNNPR state, Ethiopia: study protocol for a randomized controlled trial. Trials. 2016;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Garrett V, Ogutu P, Mabonga P, Ombeki S, Mwaki A, Aluoch G, et al. Diarrhoea prevention in a high-risk rural Kenyan population through point-of-use chlorination, safe water storage, sanitation, and rainwater harvesting. Epidemiol Infect. 2008;136(11):1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dreibelbis R, Freeman MC, Greene LE, Saboori S, Rlieingans R. The impact of school water, sanitation, and hygiene interventions on the health of younger siblings of pupils: a cluster-randomized trial in Kenya. Am J Public Health. 2014;104(1):e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cha S, Lee J, Seo D, Park BM, Mansiangi PM, Bernard K, et al. Effects of improved sanitation on diarrheal reduction for children under five in Idiofa, DR Congo: a cluster randomized trial. Infect Dis Poverty. 2017;6(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Quick RE, Kimura A, Thevos A, Tembo M, Shamputa I, Hutwagner L, et al. Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am J Trop Med Hyg. 2002;66(5):584–589. [DOI] [PubMed] [Google Scholar]
- 218.Sobsey MD, Handzel T, Venczel L. Chlorination and safe storage of household drinking water in developing countries to reduce waterborne disease. Water Sci Technol. 2003;47(3):221–228. [PubMed] [Google Scholar]
- 219.Luby SP, Agboatwalla M, Painter J, Altai A, Billhimer W, Keswick B, et al. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health. 2006;11(4):479–489. [DOI] [PubMed] [Google Scholar]
- 220.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer WL, Hoekstra RM. Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA. 2004;291(21):2547–2554. [DOI] [PubMed] [Google Scholar]
- 221.Hashi A, Kumie A, Gasana J. Hand washing with soap and WASH educational intervention reduces under-five childhood diarrhoea incidence in Jigjiga District, Eastern Ethiopia: a community-based cluster randomized controlled trial. Prev Med Rep. 2017; Apr 27: 6:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


