Abstract
Objective
This study investigated if hybrid oesophagectomy with minimally invasive gastric mobilization and thoracotomy enabled faster recovery than open surgery.
Methods
In eight UK centres, this pragmatic RCT recruited patients for oesophagectomy to treat localized cancer. Participants were randomly allocated to hybrid or open surgery, stratified by centre and receipt of neoadjuvant treatment. Large dressings aimed to mask patients to their allocation for six days post-surgery. The authors present the intention-to-treat analysis of outcome measures from the first 3 months post-randomization, including the primary outcome, the patient-reported physical function scale of the EORTC QLQ-C30, and cost-effectiveness. Current Controlled Trials registration: ISRCTN 59036820 (feasibility study), 10386621 (definitive study).
Findings
There was no evidence of a difference between hybrid (n = 267) and open (n = 266) surgery in average physical function over 3 months post-randomization: difference in means 2.1, 95% c.i. −2.0 to 6.2, P = 0.3. Complication rates were similar; for example, 88 (34%) participants in the open and 82 (32%) participants in the hybrid surgery groups experienced a pulmonary infection within 30 days. There was no evidence that hybrid surgery was more cost-effective than open surgery at 3 months.
Conclusions
Patient-reported physical function in the 3 months post-randomization provided no evidence of a difference in recovery time between hybrid and open surgery, or a difference in cost-effectiveness. Both approaches to surgery were completed safely, with a similar risk of key complications, suggesting that surgeons who have a preference for one of the two approaches need not change their practice.
This pragmatic randomized controlled trial conducted in eight UK centres allocated patients with localized oesophageal cancer to hybrid oesophagectomy with minimally invasive gastric mobilization and thoracotomy, or open surgery. The primary outcome was patient-reported recovery in the 3 months following allocation, measured using the EORTC QLQ-C30 physical function subscale. We found no evidence of a difference in recovery time between hybrid and open surgery, and a similar risk of key complications.
Introduction
Oesophageal cancer is the tenth most common cancer globally and causes one in 18 cancer deaths1. Oesophagectomy, with or without neoadjuvant therapy, is recommended for patients whose disease is confined to the oesophagus and the local lymph nodes, and who are fit to undergo surgery. The most common approach in the UK is the two-phase Ivor Lewis oesophagectomy, which involves incisions in both the abdomen and chest.
Minimally invasive approaches aim to reduce damage to healthy tissues and allow more rapid recovery, while maintaining the clinical benefits of open surgery. For oesophagectomy, two minimally invasive approaches are commonly used: hybrid or laparoscopically assisted oesophagectomy, where the chest phase uses open surgery and the abdominal phase uses laparoscopically assisted surgery; and totally minimally invasive oesophagectomy for both the abdominal and chest phases.
The use of minimally invasive techniques has been steady in England and Wales at around 50% of curative procedures since 2015. National Audit data from England and Wales, for patients diagnosed between April 2019 and March 2021, indicated that 33% of Ivor Lewis oesophagectomies were started as laparoscopically assisted, and 18% were started as totally minimally invasive2,3. National data for France between 2017 and 2019 showed greater adoption of minimally invasive approaches to Ivor Lewis oesophagectomy, with 56% of procedures being hybrid and 7% being totally minimally invasive4.
The impact of oesophagectomy on health-related quality of life is substantial and can persist for several years post-surgery5. If minimally invasive techniques can be demonstrated to lessen the impact of surgery, with significant benefit to patients, it will be important to explore their use in a greater proportion of cases. To date, two multicentre RCTs have shown that minimally invasive surgery may reduce postoperative complications, but had sample sizes too limited to confirm that minimally invasive methods achieve the same survival benefit6,7. The latter cannot be assumed when applying minimally invasive approaches to complex cancer surgery in the absence of evidence from RCTs8. The ROMIO study addresses this evidence gap, aiming to compare speed of recovery following hybrid and open oesophagectomy in a pragmatic RCT of more than 500 participants, with participants followed-up for at least 2 years for vital status, and clinical and patient-reported outcome measures. Here, outcomes up to 3 months post-randomization including the primary outcome, patient-reported postoperative recovery, are reported.
Methods
Study design and participants
The ROMIO study design has been described in detail elsewhere9. In brief, ROMIO is a pragmatic parallel group RCT comparing hybrid versus open oesophagectomy in patients with oesophageal cancer at eight National Health Service (NHS) hospitals in the UK. ROMIO began as an external pilot study at two centres (registered with Current Controlled Trials on 25 February 2013: ISRCTN 59036820) with recruitment commencing on 8 April 2013. Due to the success of the pilot, this initial phase was then adapted to an internal pilot study with recruitment continuing until funding was secured for the definitive trial10. Patients from the pilot phase were therefore included in the main trial analysis, with the exception of pilot-phase participants with high-grade dysplasia who, following changes to UK treatment recommendations during the pilot study period, did not meet the eligibility criteria for the main trial. The definitive trial was registered separately (31 May 2016, ISRCTN 10386621) and recruitment expanded to all eight centres from October 2016 until 21 August 2019, follow-up being completed on 31 August 2021.
Two ROMIO centres randomly allocated participants between three groups, the third group being part of a nested randomized IDEAL phase 2b substudy11, which will be reported separately.
Eligible adult patients had adenocarcinoma or squamous cell cancer localized in the oesophagus or oesophagogastric junction situated 5 cm below the cricopharyngeus and involving <4 cm of the stomach wall, and were referred for oesophagectomy (two- or three-phase) by the multidisciplinary cancer care team after any neoadjuvant therapy9. The decision for a two- or three-phase approach was at the discretion of the operating surgeon. Patients were invited to give written informed consent to participate in the study by surgeons who received training from an integrated QuinteT Recruitment Intervention12.
The South-West Frenchay Research Ethics Committee (main study reference 184167, pilot study reference 12/SW/0161) approved the study, with all versions of the protocol available from https://fundingawards.nihr.ac.uk/award/14/140/78. All participants provided written informed consent before taking part.
Randomization and masking
Patients were randomly allocated in a 1:1 ratio according to a blocked (varying block size of six or eight) and stratified sequence. The two centres participating in the IDEAL phase 2b substudy randomly allocated three ways in a 1:1:1 ratio, with block sizes of six and nine. Stratification was by study centre and whether the patient underwent neoadjuvant treatment prior to surgery. The allocation sequence was computer-generated by a bespoke software program during the internal pilot, and post-pilot was generated by the study statistician who was not otherwise involved with participant recruitment, using Stata statistical software version 14.1 (StataCorp 2015, College Station, Texas, USA).
Patients who gave written informed consent to participate in the ROMIO study were logged into the study and only then was the patient randomized and the surgical team informed of the allocation through the database, so ensuring concealment of allocation. Participating surgeons performed both open and hybrid surgery, according to the allocation. Participants and hospital staff (not on the surgical team) were masked to allocation until after the assessment of pain 6 days post-surgery, by covering all the wound sites for both surgical approaches with the same-sized dressings.
Procedures
The protocol for surgical quality assurance has been published and a detailed analysis of fidelity to allocated intervention will be reported separately13. Prior to participating in ROMIO, surgeons provided two anonymized unedited videos of laparoscopic cases, which had to meet the standard presented in a video assessment tool developed during the feasibility phase13.
In both study groups, two-phase (abdomen and chest) Ivor Lewis operations were expected, but with three-phases (abdomen, chest and neck) permitted. This may have been decided preoperatively or intraoperatively and this was flexible. Antibiotics and deep vein thrombosis prophylaxis were administered according to local hospital policies. Co-interventions such as perioperative analgesia were permitted according to the preferences of each centre.
For open surgery, transhiatal and thoracoabdominal approaches were prohibited. The location and length of incisions were at each surgeon’s discretion, and were recorded.
For the hybrid procedure, access to the abdominal cavity was achieved with several 12- or 5-mm incisions (as many as needed) and surgery performed laparoscopically. Laparoscopic transhiatal approaches were prohibited. Methods to create the pneumoperitoneum were at the surgeon’s discretion. If a feeding jejunostomy was placed, this could be performed laparoscopically or by creating an additional abdominal incision (maximum length of 8 cm).
For both open and hybrid surgery, complete gastric mobilization was performed based on the right gastroepiploic and right gastric arteries. Pyloroplasty, pyloromyotomy or no drainage was optional. Lymphadenectomies along the common hepatic artery, left gastric and splenic artery either en bloc or separately were performed and removal of sufficient crural fibres and a cuff of diaphragm performed if required for tumour clearance. The pericardial fat pad and strips of pleura were removed. Transection of the lesser curve could be undertaken during the abdominal or thoracic phase of the operation. Placement of a feeding jejunostomy or nasojejunal tube was at the surgeon’s discretion as was placement of intra-abdominal and intrathoracic drains. Procedures to minimize diaphragmatic herniation were permitted. The anastomotic technique and methods to close the incisions were at the surgeon’s discretion.
For both surgical approaches, the chest was opened through a right thoracotomy and the mediastinal pleura overlying the oesophagus was excised in continuity with the oesophagus. The posterior limit of the dissection was the antero-lateral wall of the aorta, so that the thoracic duct was mobilized with the oesophagus and perioesophageal tissues. The thoracic duct was tied on the aorta low in the chest cavity. The oesophagus was mobilized to the level of at least the aortic arch. Paraoesophageal nodes were removed in continuity with the oesophagus. Lymph nodes at the tracheal bifurcation, and along the right and left main bronchi to the pulmonary hilus, were removed en bloc or separately at the surgeon’s discretion. Excision of the paratracheal and recurrent laryngeal nodes was at the discretion of the surgeon and not mandated. The anastomotic technique was at the surgeon’s discretion.
Outcomes
All items in the core outcome set for oesophageal cancer managed with surgery were measured14. The primary outcome was recovery of physical function, assessed using the validated patient-reported European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30) at 3 and 6 weeks after surgery and 3 months after randomization15. The physical function scale is based on five questions, the score being transformed to a 0–100 scale with higher scores indicating better mobility and health. Longer follow-up on this and other patient-reported measures of health-related quality of life, costs and survival will be reported elsewhere.
The following secondary outcomes were recorded at days 3 and 6 after surgery for participants who were still in hospital and while the participants’ allocations were masked: forced expiratory volume in one second and forced vital capacity measured by spirometry, and patient-reported pain using a visual analogue scale16. The patient-reported EuroQoL EQ-5D-5L health-related quality of life questionnaire17 was collected at baseline, 6 days, 3 weeks, 6 weeks post-surgery and 3 months post-randomization. Survival and complications, reported using the standardized list of complications recommended by the Esophageal Complications Consensus Group18, were collected on a case report form for the 90 days following random allocation. The case report forms included the Consensus Group definitions of oesophagoenteric leak, conduit necrosis/failure, recurrent laryngeal nerve injury involvement and chyle leak severity; the Berlin definition of acute respiratory distress syndrome19; and the American College of Chest Physicians and Society of Critical Care Medicine Consensus Conference Committee definitions of generalized sepsis and multiple organ dysfunction syndrome20. In addition, pneumonia was defined as ‘New lung infiltrates plus clinical evidence that the infiltrate is of an infectious origin, which includes the new onset of fever, purulent sputum, leukocytosis or a decline in oxygenation’21. The impact of complications during the postoperative stay was assessed using the Clavien–Dindo scale22. The original plan to assess Clavien–Dindo at 30 days was amended to collect the data at discharge, to ensure all complications during the postoperative stay were included for all patients.
Statistical analysis
Two hundred and three patients recruited to each of the hybrid and open surgery groups allowed a minimum clinically important difference of 0.4 s.d.15,23 on the primary outcome to be detected with >90% power at the two-sided 5% significance level, with up to 15% of patients not following their allocated procedure and 10% failing to complete the primary outcome.
The statistical analysis plan for the ROMIO study was written prior to the completion of study follow-up by members of the study team without access to the data24. The treatment effect on the primary outcome measure was estimated in an analysis following the intention-to-treat principle (comparing the two groups of patients as randomly allocated to hybrid or open surgery) as closely as possible. The treatment effect was quantified as the difference in mean score on the EORTC QLQ-C30 physical function scale across the three time points (with 95% c.i. and two-sided P), estimated as the coefficient of a binary variable distinguishing the two treatment groups, in a two-level linear regression model. The two levels accommodated the repeated measures design by separating variation between individuals from variation between each individual’s responses at the three outcome assessment points; this is a variance components model and assumes normal distributions for both the mean responses of individuals and for the individual responses on the outcome measure. Treatment centre, assessment point, whether the patient underwent neoadjuvant treatment and the baseline value of the outcome measure were also included as covariates. The two pilot-phase centres were each included as two separate centres in this analysis, distinguishing participants recruited in the pilot and main study phases. Physical function was assumed to be zero at an assessment point for any participants who had died or who were recorded as too ill to complete patient-reported outcomes (generally if they were known to be in an intensive care or high-dependency unit), but otherwise there was no imputation of missing data in the primary analysis and participants were included if at least one of the assessments was completed. Three subgroup analyses were pre-specified: whether a patient underwent neoadjuvant chemotherapy/chemoradiotherapy prior to surgery; POSSUM physiological risk score for post-surgery mortality and morbidity25 assessed at recruitment; BMI assessed at recruitment. Sensitivity analyses investigated the potential impact of any missing primary outcome data, investigated post-surgical pain among participants whose allocated surgery was completed (as participants undergoing a different surgical procedure are likely to be informed), and further adjusted the primary analysis for time between randomization and surgery.
Secondary outcome measures are presented as summary statistics according to the groups to which participants were randomly allocated. At the request of the editors the authors have added post-hoc hypothesis tests for post-surgical outcomes (chi-square and Wilcoxon rank sum tests as appropriate) and the Clavien–Dindo classification of complications (ordered logistic regression). Stata Statistical Software version 17.0 was used for all analyses (StataCorp 2021, College Station, Texas, USA).
Economic analysis
The methods used to measure resource use in the pilot phases of ROMIO are too different from those used in the definitive trial to be combined. Therefore, the cost-effectiveness analysis includes only 328 patients recruited after the internal pilot phase. The methods were pre-specified in a health economics analysis plan26. EQ-5D-5L responses were converted to utilities using the National Institute for Health and Care Excellence (NICE)-recommended UK tariff17,27,28. These were combined with survival data to calculate quality-adjusted life years (QALYs), adjusted for differences in baseline EQ-5D-5L utility scores26,29. National unit costs were used to value resource use where available. Unit costs are reported in pounds sterling and, where applicable, were inflated to 2019/20 values. Key results were also presented in Euros using the exchange rate on 1 January 2020 (£1.00 = 1.18 Euros). A site survey identified typical theatre staff requirements for both procedures and key differences in reusable equipment and consumables. This information was combined with data (for example, duration of surgery) collected on the case report form (CRF) to micro-cost surgery. The CRF also recorded details of ICU stay, ward stay and re-interventions during the initial hospitalization and readmissions up to 3 months. Use of primary and community care after discharge was collected from patients using a resource-use questionnaire at 3 months26.
The cost-effectiveness analysis at 3 months was performed on an intention-to-treat basis from an NHS perspective. We used multilevel linear regression models, clustered by treatment centre, and with neoadjuvant treatment and the baseline EQ-5D-5L score as covariates to estimate the incremental cost, QALYs and Net Monetary Benefit (iNMB) of hybrid versus open oesophagectomy. The iNMB was estimated based on the lower willingness-to-pay threshold (that is, £20 000 per QALY) used by NICE to determine cost-effectiveness27.
Multiple imputation was applied for missing cost and EQ-5D-5L data. The primary economic analysis includes all patients randomized in the main phase of the trial, including those where costs and/or outcomes have been estimated by imputation. The authors present a cost-effectiveness acceptability curve to depict the probability that hybrid surgery is more cost-effective than open oesophagectomy at various willingness-to-pay thresholds.
Sensitivity analyses estimated cost-effectiveness in cases with complete cost and outcome data and extended the perspective to NHS and social care costs.
Results
Patients
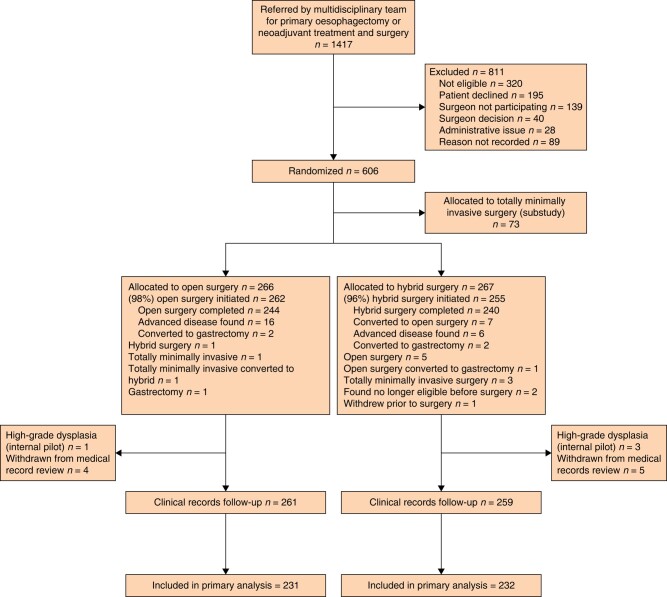
In total, 1417 patients were referred by their multidisciplinary team for oesophagectomy (Fig. 1), 811 of whom were not recruited to the study. The most common reasons recorded for this being not meeting the eligibility criteria (n = 320), the patient declining to take part (n = 195) and the patient’s surgeon not participating in ROMIO (n = 139). Details of ineligibility were available for 263 individuals from screening logs at one feasibility site plus all definitive study sites, where the most common reasons were not being suitable for all study procedures (n = 49), advanced stage (n = 42), previous procedures precluding a minimal access approach (n = 40), insufficiently fit for surgery (n = 39), extensive gastric involvement (n = 29), suitability not confirmed by the multidisciplinary team (n = 22) and co-morbidities (n = 14). Of the 1417 patients, 606 (43%) were randomly assigned, 266 to open, 267 to hybrid and 73 to totally minimally invasive oesophagectomy (to be reported separately). Table S1 presents the numbers randomized to open or hybrid surgery by recruiting centre. Two patients allocated to open surgery withdrew all their data from the study and a further four patients (one allocated to open, three to hybrid surgery) had a diagnosis of high-grade dysplasia; these six patients are not included in the results presented here. At the completion of the internal pilot 199 participants had been recruited; due to these participants being recruited and treated at just two centres, and due to some measures, notably resource use, not being collected during the pilot, the authors agreed with the funder that they would exceed the target to ensure that at least 300 participants were recruited during the definitive phase.
Fig. 1.
CONSORT flow chart
The open and hybrid groups were well balanced in terms of sociodemographic and clinical characteristics at the time of allocation (Table 1). Most participants were recruited after completing neoadjuvant treatment. The two allocated groups were also comparable on disease characteristics (Table 1).
Table 1.
Baseline patient and disease characteristics by allocated surgery
| All randomized participants | Open surgery (n = 266) | Hybrid surgery (n = 267) |
|---|---|---|
| Sex | ||
| Male | 227 | 225 |
| Female | 39 | 42 |
| Age (years), mean (s.d.) | 66 (9) | 67 (9) |
| BMI (kg/m2), mean (s.d.) | 27 (4) | 27 (4) |
| POSSUM physiological score, mean (s.d.), n* | 18 (4) n = 187 | 18 (4) n = 189 |
| Number WHO performance status score 1+, (%)* | 65/200 (32) | 64/196 (32) |
| Neoadjuvant treatment; n (%) | ||
| Chemotherapy | 176 (66) | 170 (64) |
| Chemoradiotherapy | 45 (17) | 49 (18) |
| None | 45 (17) | 48 (18) |
| Tumour histologic findings; n (%)† | ||
| Adenocarcinoma | 237 (90) | 235 (89) |
| Squamous cell carcinoma | 25 (9) | 26 (10) |
| Adenosquamous | 2 (1) | 2 (1) |
| High-grade dysplasia (internal pilot) | 1 | 3 (1) |
| Location of tumour in oesophagus; n (%)‡ | ||
| Upper third | 1 | 0 |
| Middle third | 8 (7) | 25 (10) |
| Lower third/Siewert I | 198 (75) | 201 (77) |
| Junctional tumour, Siewert II | 40 (15) | 29 (11) |
| Junctional tumour, Siewert III | 8 (3) | 7 (3) |
| Pretreatment clinical tumour category; n (%)†,‡ | ||
| cT1 | 23 (9) | 20 (8) |
| cT2 | 55 (21) | 58 (22) |
| cT3 | 179 (67) | 175 (66) |
| cT4a | 8 (3) | 11 (4) |
| Pretreatment clinical nodal category; n (%) | ||
| cN0 | 128 (48) | 109 (40) |
| cN1 | 98 (37) | 119 (45) |
| cN2/cN3 | 40 (15) | 39 (15) |
*Not collected for pilot-phase participants at Plymouth. †Not recorded for one patient in each group as patient withdrew from routine data follow-up before collection. ‡Not recorded for two patients allocated to hybrid surgery due to withdrawal from routine data follow-up before collection.
Surgery
Median time from allocation to surgery was 5 days (i.q.r. 2–12 days) in the open surgery group and 5 days (i.q.r. 1–10 days) in the hybrid surgery group. Allocated surgery was initiated for 262/266 (98%) of those in the open surgery group and 255/267 (95%) of those in the hybrid surgery group (Fig. 1). This included 16 patients allocated to open surgery and eight patients allocated to hybrid surgery who were found to have advanced disease preventing resection either before surgery was initiated (n = 2) or once surgery was underway (n = 22). For seven patients their allocated hybrid surgery was initiated but was converted to an open approach during surgery. Three patients in each of the allocated groups had a gastrectomy due to the tumour being found to extensively involve the stomach. Two patients, one allocated to each approach, were recorded as having a three-stage procedure (these data were not collected at one centre during the pilot phase). A feeding jejunostomy was inserted for 120 (45%) open surgery group and 111 (42%) hybrid surgery group participants, with this being done laparoscopically for 51 (46%) of the latter.
Pathology
The median number of lymph nodes retrieved was 24 for patients in the hybrid group and 26 in the open surgery group, with 19 or more lymph nodes being retrieved in 75% of patients in both groups (Table 2). A greater proportion of participants with positive margins was observed in the open surgery group (31%) compared to the hybrid surgery group (24%), with R1 defined as ‘tumours equal to or less than 1 mm from the margin’ in accordance with the UK Royal College of Pathologists criteria30. However, this difference was consistent with chance. Other pathological staging parameters of the tumour were equally distributed following the two surgical approaches.
Table 2.
Peri- and post-surgical measures
| Open surgery | Hybrid surgery | P | |
|---|---|---|---|
| Disease characteristics established post-surgery | |||
| Median lymph nodes with tumour* (Q1, Q3)†, n | 0 (0–3) | 0 (0–3) | |
| pStage, n (%) | |||
| 0 | 23 (10) | 29 (12) | |
| I or II | 106 (44) | 109 (44) | |
| III | 80 (33) | 82 (33) | |
| IV | 33 (14) | 28 (11) | |
| Measures of surgical performance | |||
| Median total lymph nodes retrieved* (Q1, Q3)†, n | 26 (19–33) | 24 (19–32) | 0.449 |
| Resection margin, n (%) | |||
| R0 | 169 (69) | 191 (76) | |
| R1 | 72 (30) | 60 (24) | |
| R2 | 3 (1) | 0 | 0.069 |
| Post-surgical outcomes | n = 261 | n = 259 | |
| Median total hospital stay in days‡ (Q1, Q3)† | 10 (8, 15) | 11 (9, 16) | 0.085 |
| Median post-surgical hospital stay in days‡ (Q1, Q3)† | 10 (8, 15) | 11 (9, 15) | 0.080 |
| Mortality within 30-days post-surgery, n (%) | 6 (2.3) | 4 (1.5) | 0.531 |
| Mortality within 90-days post-surgery, n (%) | 14 (5.4) | 8 (3.1) | 0.197 |
| Death during initial hospital stay‡, n (%)* | 4 (1.5) | 4 (1.6) | 0.987 |
*The two measures available for 244 and 252 participants allocated to open surgery and hybrid surgery, respectively. †Q1 and Q3 are the lower and upper limits of the interquartile range. ‡Not available for one participant allocated to hybrid surgery as not admitted to hospital.
Secondary outcomes
Lung function and pain were comparable between the two allocated groups during the 6 days post-surgery (Table S2), with a per-protocol sensitivity analysis of the pain scores giving near-identical findings. A greater proportion of participants allocated to open surgery received pain relief at 3 days post-surgery from an epidural infusion, compared to the hybrid surgery group. Hospital stays were very similar between the two surgical approaches (median 10 versus 11 days), with 75% of participants in both allocated groups being discharged within 15 days of surgery (Table 2). Mortality due to any cause within 90 days of surgery was low and comparable to national audit data in each group.
The impact of complications during recovery, as assessed by the Clavien–Dindo classification, was comparable between the two allocated groups (Table 3), with about one-third of participants experiencing no complications, one-third complications of moderate severity requiring pharmaceutical intervention or similar and one-third more serious complications requiring an invasive intervention (Clavien–Dindo III or above). The occurrence of five key complications was comparable between the allocated groups with pulmonary infections being the most common, observed in about a third of participants. The need for an unplanned return to the intensive treatment or high-dependency unit or for further intervention during the post-surgical hospital stay was also closely comparable between the allocated groups. Table S3 shows comparable outcomes for the full list of pre-specified complications during the 90 days following surgery. Deaths and intensive care resulted in the imputation of zero physical function scores at the 3-week (open surgery 9, hybrid surgery 8), 6-week (open surgery 8, hybrid surgery 4) and 3-month (open surgery 12, hybrid surgery 6) assessments of the primary outcome measure.
Table 3.
Overall complication severity grading and counts of key complications and further interventions
| All randomized participants continuing in follow-up | Open surgery (n = 261) | Hybrid surgery (n = 258) |
|---|---|---|
| Clavien–Dindo classification of complications | ||
| Normal recovery, no complications | 85 (33) | 84 (33) |
| Grade I (minor deviation from normal course) | 26 (10) | 25 (10) |
| Grade II (pharmaceutical intervention) | 62 (24) | 67 (26) |
| Grade IIIa/IIIb (invasive intervention) | 56 (21) | 55 (21) |
| Grade IVa/IVb (life-threatening) | 28 (11) | 23 (9) |
| Grade V (death of patient) | 4 (2) | 4 (2) |
| P for trend (ordered logistic regression) | 0.783 | |
| Key postoperative complications (any severity) within 30 days of surgery | ||
| Oesophagoenteric leak from anastomosis, staple line, or localized conduit necrosis | 21 (8) | 21 (8) |
| Conduit necrosis/failure | 7 (3) | 1 |
| Chyle leak | 10 (4) | 11 (4) |
| Pneumonia/chest infection | 91 (35) | 85 (33) |
| Bleeding requiring intervention or transfusion | 6 (2) | 2 (1) |
| Further intervention | ||
| Unexpected return to ITU/HDU during index hospitalization | 35 (13) | 30 (12) |
| Re-intervention requiring general anaesthetic during index hospitalization | 42 (16) | 49 (19) |
| Re-intervention requiring general anaesthetic within 90 days of surgery | 43 (16) | 49 (19) |
Values are n (%). Counts are of patients who may have experienced more than one complication/intervention of a given type.
Primary outcome
There was no evidence of a difference between hybrid and open surgery in recovery over the first 3 months post-random allocation, with the 95% c.i. excluding a clinically important difference in physical function (EORTC QLQ-C30 physical function scale, primary outcome) between the allocated groups (Table 4, difference in means = 2.3, 95% c.i. −1.7 to 6.4). Participants in both groups reported a negative impact on physical function following surgery, which despite some recovery of function was still apparent at 3 months.
Table 4.
Physical function (EORTC QLQ-C30 subscale*) in the 3 months post-random allocation
| Open surgery (n = 261) |
Hybrid surgery (n = 259) |
|||
|---|---|---|---|---|
| Mean (s.d.) | n | Mean (s.d.) | n | |
| Baseline | 89 (15) | 247 | 86 (17) | 241 |
| 3 weeks post-surgery† | 51 (25) | 174 | 51 (27) | 173 |
| 6 weeks post-surgery | 61 (27) | 201 | 61 (26) | 209 |
| 3 months post-randomization | 68 (27) | 205 | 69 (25) | 208 |
| Treatment effect (adjusted difference in means, n = 231 versus 232) |
2.3 (95% c.i. −1.7, 6.4) P = 0.256 | |||
*Scores are between 0 and 100, with higher scores indicating better function. A positive treatment effect indicates better mean function with hybrid surgery. †Assessment point at 3 weeks post-surgery introduced during the internal pilot.
This same pattern of results was apparent when the analysis of the primary outcome measure was stratified by the internal pilot and main trial phases (Table S4). Greater resources were available during the definitive trial phase for monitoring the return of primary outcome questionnaires and sending reminders as necessary, these measures resulting in only 11 participants in the definitive trial phase not having primary outcome data compared to 46 during the internal pilot. Four of the 11 participants stopped completing questionnaire measures after they were found to be unsuitable for oesophagectomy, either before or during surgery, and an additional participant stopped after experiencing significant postoperative complications. Repeating the analysis of the primary outcome with the addition of time between randomization and surgery (median 5 days for participants in both allocated groups) as a further covariate resulted in the same estimated treatment effect.
Subgroup analyses
The results of three pre-specified subgroup analyses are presented in Table S5. There was no evidence that the treatment effect on recovery during the first 3 months post-surgery was affected by whether the participant underwent neoadjuvant treatment pre-surgery, or by whether the participants physical condition was relatively poor (lower POSSUM Physiology scores). There was strong evidence that the relative benefits of open and hybrid surgery for recovery in the first 3 months post-surgery differed according to BMI (P = 0.004). Participants with a lower BMI had a faster recovery following hybrid compared to open surgery. This benefit of hybrid surgery was not seen in participants with higher BMI, where the observed difference, while consistent with chance, was in the direction favouring open surgery.
Economic analysis
The cost of the initial procedure was marginally higher in the hybrid surgery arm due to greater equipment and consumable costs and a longer mean procedure time (Table 5). Higher ward and re-intervention costs in the hybrid surgery arm were largely offset by lower ICU and readmissions costs. Primary and other ambulatory care costs after discharge were very similar between study groups. Total health service costs at 3 months were somewhat higher in the hybrid surgery arm (£16 712 versus £16 304, or 19 740 versus 19 258 Euros), but confidence intervals were wide and included zero, indicating no strong evidence of cost differences (adjusted mean difference from the multilevel regression £206; 95% c.i. −£3381 to £3794). Mean (s.d.) EQ-5D-5L scores across both study groups improved from 0.389 (0.275) at 6 days to 0.660 (0.246) at 3 months (Fig. S1). However, differences in QALYs between study groups were small (adjusted mean difference from the multilevel regression −0.005; 95% c.i. −0.016 to 0.006). The incremental net monetary benefit at a willingness-to-pay threshold of £20 000 of hybrid surgery was small and negative −£367 (95% c.i. −£4010 to £3276; Table 5), providing no evidence that it is more cost-effective than open surgery at 3 months across a range of willingness-to-pay thresholds (Fig. S2). The cost-effectiveness of hybrid surgery was worse in sensitivity analyses limiting the analysis to cases with complete cost and outcome data (Table S6). Other sensitivity analyses included social care costs, and assumed quality-of-life scores were zero when participants were in ICU supported the findings of the primary economic analysis.
Table 5.
Incremental costs, QALYs and net benefit of hybrid versus open surgery
| Open surgery (n = 162) | Hybrid surgery (n = 162) | |||
|---|---|---|---|---|
| Units* | Mean (s.d.), n | Units* | Mean (s.d.), n | |
| Procedure equipment/consumables | £514 (£424), 162 | £626 (£232), 162 | ||
| Procedure staff time cost† | 353 min | £2972 (£717), 162 | 364 min | £3067 (£662), 162 |
| ICU | 5.04 days | £7148 (£15 342), 162 | 4.72 days | £6446 (£12 545), 162 |
| Ward | 9.91 days | £4103 (£3413), 162 | 11.93 days | £4938 (£4757), 162 |
| Re-interventions | 0.18 procedures | £286 (£1293), 162 | 0.42 procedures | £708 (£2046), 162 |
| Readmissions‡ | 2.40 days | £1166 (£3184), 162 | 1.89 days | £929 (£2918), 162 |
| Total inpatient costs | £16 189 (£17 252), 162 | £16 715 (£16 376), 162 | ||
| Primary and other ambulatory§ care | £212 (£306), 141 | £253 (£419), 139 | ||
| Total health service cost | £16 304 (£16 094), 141 | £16 712 (£15 007), 139 | ||
| EQ-5D-5L score | ||||
| Baseline | 0.822 (0.156), 162 | 0.812 (0.169), 160 | ||
| 6 days | 0.398 (0.276), 143 | 0.381 (0.275), 143 | ||
| 21 days | 0.578 (0.206), 127 | 0.546 (0.221), 133 | ||
| 42 days | 0.629 (0.227), 134 | 0.638 (0.203), 137 | ||
| 90 days | 0.655 (0.250), 143 | 0.665 (0.243), 145 | ||
| QALYs | 0.157 (0.042), 100 | 0.150 (0.045), 109 | ||
| iNMB# | −£367 (95% c.i.: −£4010 to £3276) P = 0.84 | |||
*Where applicable. †Time includes procedure time only; cost includes monitoring and recovery suite costs, if applicable. ‡Including costs of readmission for outpatient procedures. §Including emergency department. #Multilevel model after multiple imputation (n = 324) of missing costs and EQ-5D-5L scores.
Discussion
In this pragmatic RCT comparing minimally invasive versus open surgery to treat localized oesophageal cancer, there was no evidence of differences in perioperative measures such as positive resection margins, the occurrence and severity of complications including chest infection, and no difference in post-surgical recovery as measured by patient-reported physical function during 3 months post-randomization (primary outcome). The 95% c.i. for the treatment effect on patient-reported physical function excluded the minimum clinically important difference of 10.4 points23. There was evidence that the relative benefits of open and hybrid surgery for post-surgical recovery differed according to the patient’s BMI, with hybrid surgery associated with faster recovery in those patients with a lower BMI. The marginally higher procedure costs of hybrid surgery were not offset by lower subsequent inpatient or ambulatory care costs. Differences in QALYs between study groups were small and there was no evidence that hybrid surgery was more cost-effective than open surgery at 3 months.
ROMIO has not replicated the findings of minimally invasive oesophagectomy reducing the occurrence of major complications within 30 days of surgery as reported by the MIRO study7, or reducing the occurrence of pulmonary infections during the hospital stay following surgery as reported in the TIME study6. Comparing participant characteristics between the three studies, the ROMIO participants are older (average age: ROMIO 67 years, MIRO 61 years, TIME 62 years), slightly heavier (average BMI: ROMIO 27 kg/m2, MIRO 25 kg/m2, TIME 25 kg/m2) and with a higher prevalence of adenocarcinoma (ROMIO 89%, MIRO 59%, TIME 62%). In the MIRO study, a greater proportion of participants allocated to open surgery were disabled (WHO performance status score 1+: 49%) compared to those allocated to the hybrid surgery group (35%), which may have influenced the occurrence of and response to complications. In the ROMIO study, 32% of participants allocated to each of the allocated groups were classified as disabled by this measure. The risk of pulmonary complications in the open surgery groups of the TIME, MIRO and ROMIO studies were comparable, affecting approximately one in three patients. The reduction of pulmonary infection risk in the TIME study’s totally minimally invasive group to 12%, which was not seen in ROMIO’s hybrid surgery group, cannot entirely be explained by the chest phase also being conducted with minimally invasive methods in the TIME trial, as a smaller but still substantial risk reduction to 18% was seen in the MIRO study’s hybrid surgery group. The risk of major complications (Clavien–Dindo category II or above, primary outcome measure in the MIRO study) was slightly less in the ROMIO open surgery group (57%) compared to the same group in the MIRO study (64%), but again the reduction in risk of major complications in the MIRO study’s hybrid surgery group to 35% was not seen in the ROMIO study hybrid surgery group. Much of the reduction in major complications seen in the MIRO study was due to a lower risk of Clavien–Dindo category II complications with hybrid surgery (14%) compared to open surgery (36%); category II complications are those requiring a pharmacological intervention such as antibiotics. Notably, despite the substantial reduction in complications following hybrid surgery observed by the MIRO study, this was not reflected by patient-reported physical function at 30 days post-surgery (EORTC QLQ-C30 Physical Function, mean scores hybrid surgery 66 versus open surgery 64)31. Complications were assessed by the operating surgeons themselves in the MIRO study. Finally, it is acknowledged that the R1 rates are higher in both groups in the ROMIO study compared to the MIRO study, which reported an R1/2 rate of only 3.4%. This may be related to the different pathological definitions of R1, different frequencies of complete tumour regression between the studies, or that surgery was less radical in the ROMIO study compared to the MIRO study. If one or both of the latter two possibilities are the case, then survival differences between participants in the two trials would be expected, and this will be examined during analysis of the long-term data from the ROMIO study.
Strengths of the ROMIO study include its relatively large sample size, the pragmatic design and good participation (at least 55% of eligible patients agreed to participate), blinding of participants and care staff to allocation in the 6 days post-surgery and high retention rates. An examination of the impact of missing data was considered unnecessary, as 95% of participants recruited in the main phase of the trial were included in the primary outcome analysis, giving an estimated treatment effect very similar to the cohort as a whole (87% of all randomized participants completed the primary outcome). Allocated surgery was initiated for 97% of participants, completed for 91%, and high surgeon fidelity to the allocated approach—ensured by quality assurance of the surgery—provide a clear and unbiased comparison of the two surgical approaches. Consultation with patients identified the importance of a patient-reported measure of post-surgical recovery as the primary outcome, but also of conducting a study big enough to highlight any unexpected differences in survival. The economic analysis provides evidence for surgeons and policy makers on the relative costs and benefits of hybrid surgery.
Limitations include the participants being aware of the surgery they had undergone when completing the patient-reported primary outcome measure, meaning a placebo-type effect is possible. There is currently interest in new robot-assisted approaches to oesophagectomy32. However, these new approaches should only be utilized as part of an RCT until sufficient evidence of their effectiveness and safety has accumulated; until that point, the ROMIO results remain relevant to clinical practice.
The ROMIO pragmatic RCT did not confirm previous findings of a reduction in complications with minimally invasive approaches to oesophagectomy, and furthermore found no evidence of differences in short-term clinical outcomes or patient-reported recovery of physical function over 3 months between the hybrid and open approaches. The evidence of an advantage of hybrid surgery in patients with lower BMI requires further examination and replication before influencing clinical guidelines. It is not clear that the higher equipment and consumable costs of hybrid surgery are justified by better patient outcomes. These findings do not require surgeons who have a preference for one of the two approaches to change their practice.
Collaborators
Khurshid Akhtar, Salford Royal NHS Foundation Trust. Bilal Alkhaffaf, Salford Royal NHS Foundation Trust. Arun Ariyarathenam, University Hospitals Plymouth NHS Trust. Kerry Avery, University of Bristol. Paul Barham, University Hospitals Bristol and Weston NHS Foundation Trust. Adrian Bateman, University Hospital Southampton NHS Foundation Trust. Chloe Beard, University of Bristol. Richard Berrisford, University Hospitals Plymouth NHS Trust. Jane M. Blazeby, University of Bristol. Natalie Blencowe, University of Bristol. Alex Boddy, University Hospitals of Leicester NHS Trust. David Bowrey, University Hospitals of Leicester NHS Trust. Tim Bracey, University Hospitals Plymouth NHS Trust. Rachel C. Brierley, University of Bristol. Kate Briton, Royal Infirmary of Edinburgh. James Byrne, University Hospital Southampton NHS Foundation Trust. James Catton, Nottingham University Hospitals NHS Trust. Ram Chaparala, Salford Royal NHS Foundation Trust. Sarah K. Clark, Royal Infirmary of Edinburgh. Tonia Clarke, Royal United Hospital Bath NHS Trust. Jill Cooke, University Hospitals of Leicester NHS Trust. Graeme Couper, Royal Infirmary of Edinburgh. Lucy Culliford, University of Bristol. Heidi Dawson, Royal Infirmary of Edinburgh. Chris Deans, Royal Infirmary of Edinburgh. Jenny L. Donovan, University of Bristol. Charlotte Ekblad, Royal United Hospital Bath NHS Trust. Jackie Elliott, Independent advisor, patient & Carer Perspective. David Exon, University Hospitals of Leicester NHS Trust. Stephen Falk, University Hospitals Bristol and Weston NHS Foundation Trust. Naheed Farooq, Salford Royal NHS Foundation Trust. Kirsty Garfield, University of Bristol. Daisy M. Gaunt, University of Bristol. Fran Gill, University Hospitals Bristol and Weston NHS Foundation Trust. Robert Goldin, Imperial College, London. Athanasia Gravani, University of Bristol. George Hanna, Imperial College, London. Stephen Hayes, Salford Royal NHS Foundation Trust. Rachael Heys, University of Bristol. Carolyn Hindmarsh, Salford Royal NHS Foundation Trust. Sandra Hollinghurst, University of Bristol. William Hollingworth, University of Bristol. Andrew Hollowood, University Hospitals Bristol and Weston NHS Foundation Trust. Rebecca Houlihan, University Hospitals Bristol and Weston NHS Foundation Trust. Benjamin Howes, University Hospitals Bristol and Weston NHS Foundation Trust. Lucy Howie, Royal United Hospital Bath NHS Trust. Lee Humphreys, University Hospitals Plymouth NHS Trust. David Hutton, University of Bristol. Rosina Jarvis, University Hospitals Bristol and Weston NHS Foundation Trust. Marcus Jepson, University of Bristol. Rebecca Kandiyali, University of Warwick. Surinder Kaur, University of Bristol. Philip Kaye, Nottingham University Hospitals NHS Trust. Jamie Kelly, University Hospital Southampton NHS Foundation Trust. Anni King, University of Bristol. Jana Kirwin, University of Bristol. Richard Krysztopik, Royal United Hospital Bath NHS Trust. Peter Lamb, Royal Infirmary of Edinburgh. Alistair Lang, Royal Infirmary of Edinburgh. Vivienne Lee, University Hospitals Bristol and Weston NHS Foundation Trust. Sally Maitland, Nottingham University Hospitals NHS Trust. Nicholas Mapstone, Salford Royal NHS Foundation Trust. Georgia Melia, Nottingham University Hospitals NHS Trust. Chris Metcalfe, University of Bristol. Rachel Melhado, Salford Royal NHS Foundation Trust. Aida Moure-Fernandez, University of Bristol. Beena Nair, Lancashire Teaching Hospitals NHS Foundation Trust. Joanna Nicklin, University Hospitals Bristol and Weston NHS Foundation Trust. Fergus Noble, University Hospital Southampton NHS Foundation Trust. Sian M Noble, University of Bristol. Abby O’Connell, University of Bristol. Stephen Palmer, University of Bristol. Simon Parsons, Nottingham University Hospitals NHS Trust. Kish Pursnani, Lancashire Teaching Hospitals NHS Foundation Trust. Nicola Rea, Royal Infirmary of Edinburgh. Fiona Reed, University Hospitals Plymouth NHS Trust. Caoimhe Rice, University of Bristol. Cathy Richards, University Hospitals of Leicester NHS Trust. Chris Rogers, University of Bristol. Grant Sanders, University Hospitals Plymouth NHS Trust. Vicki Save, Royal Infirmary of Edinburgh. Chas Shaw, University of Bristol. Michael Schiller, University Hospitals Bristol and Weston NHS Foundation Trust. Rachel Schranz, University Hospital Southampton NHS Foundation Trust. Vinutha Shetty, Lancashire Teaching Hospitals NHS Foundation Trust. Beverly Shirkey, University of Bristol. Jo Singleton, Royal Infirmary of Edinburgh. Richard Skipworth, Royal Infirmary of Edinburgh. Joanne Smith, University Hospitals Plymouth NHS Trust. Christopher Streets, University Hospitals Bristol and Weston NHS Foundation Trust. Dan Titcomb, University Hospitals Bristol and Weston NHS Foundation Trust. Paul Turner, Lancashire Teaching Hospitals NHS Foundation Trust. Sukhbir Ubhi, University Hospitals of Leicester NHS Trust. Tim Underwood, University Hospital Southampton NHS Foundation Trust. Cellins Vinod, Salford Royal NHS Foundation Trust. Ravinder Vohra, Nottingham University Hospitals NHS Trust. Elizabeth M. Ward, University of Bristol. Rhian Warman, Nottingham University Hospitals NHS Trust. Neil Welch, Nottingham University Hospitals NHS Trust. Tim Wheatley, University Hospitals Plymouth NHS Trust. Katie White, Royal United Hospital Bath NHS Trust. Robin A. Wickens, University of Bristol. Paul Wilkerson, University Hospitals Bristol and Weston NHS Foundation Trust. Alexandra Williams, Lancashire Teaching Hospitals NHS Foundation Trust. Rob Williams, University Hospitals of Leicester NHS Trust. Natasha Wilmshurst, University Hospitals Plymouth NHS Trust. Newton A.C.S. Wong, North Bristol NHS Trust.
Key institutions
The University of Bristol (Methodology expertise and coordination, including from the Bristol Trials Centre). University Hospitals Bristol and Weston NHS Foundation Trust (Methodology expertise, coordination, sponsorship, participant facing activities). University Hospitals Plymouth NHS Trust (Methodology expertise, coordination, participant facing activities). Lancashire Teaching Hospitals NHS Foundation Trust, Nottingham University Hospitals NHS Trust, NHS Lothian, Royal United Hospital Bath NHS Trust, Salford Royal NHS Foundation Trust, University Hospitals of Leicester NHS Trust and University Hospital Southampton NHS Foundation Trust (Participant-facing activities).
Supplementary Material
Acknowledgements
The authors thank the large teams at each hospital who have worked on the ROMIO study. They also thank staff at the University of Bristol who supported the study. This study was sponsored by University Hospitals Bristol and Weston NHS Foundation Trust, Research and Innovation, Level 3, UH Bristol Education and Research Centre, Upper Maudlin Street, Bristol BS2 8AE, UK. The funder and sponsors approved any amendments to the study but had no direct involvement in study design; collection, management, analysis and interpretation of data; writing of the report and the decision to submit this report for publication. The study is overseen by an independent steering committee and an independent data monitoring committee. Steering Committee membership: Craig Ramsay (Chair, Professor in Health Services Research, University of Aberdeen, Scotland); Tony Ingold (Trustee, Oesophageal Patients Association, England); Heike Grabsch (Professor of Gastrointestinal Pathology, Maastricht University, Netherlands); William Allum (Consultant Upper Gastrointestinal Surgeon, Royal Marsden NHS Foundation Trust, London, UK); Richard Hardwick (Consultant Upper Gastrointestinal Surgeon & Lead Clinician for Upper GI Cancer, Cambridge University Hospitals NHS FT, Cambridge, UK); Colin Green (Professor of Health Economics, University of Exeter, Exeter, UK); Neil Corrigan (Senior Medical Statistician, University of Leeds). Previous members were: Sally Stenning (Professor of Medical Statistics, MRC Clinical Trials Unit at University College London); Helen Marshall (Principal Statistician, University of Leeds, Leeds, UK). Data Monitoring Committee membership: Judith Bliss (Chair, Professor of Clinical Trials, Institute of Cancer Research, London, UK); William Robb (Consultant Upper Gastrointestinal Surgeon, Beaumont Hospital & Royal College of Surgeons in Ireland, Dublin, Ireland); Derek Alderson (President of the Royal College of Surgeons and emeritus Professor of Surgery, University of Birmingham, Birmingham, UK). The authors are grateful to patients and members of the public who were involved in the study’s planning, design, conduct and dissemination, through regular consultation with their public co-applicant and patient/public advisory group including members of the Gastro/Oesophageal Support and Help Cancer Group (Bristol): www.goshbristol.com. This study was designed and delivered in collaboration with the Bristol Trials Centre (BTC), a UKCRC-registered clinical trials unit in receipt of National Institute for Health Research Clinical Trials Unit support funding. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. This work uses data provided by patients and collected by the NHS as part of their care and support.
Contributor Information
The ROMIO Study Group:
Khurshid Akhtar, Bilal Alkhaffaf, Arun Ariyarathenam, Kerry Avery, Paul Barham, Adrian Bateman, Chloe Beard, Richard Berrisford, Jane M Blazeby, Natalie Blencowe, Alex Boddy, David Bowrey, Tim Bracey, Rachel C Brierley, Kate Briton, James Byrne, James Catton, Ram Chaparala, Sarah K Clark, Tonia Clarke, Jill Cooke, Graeme Couper, Lucy Culliford, Heidi Dawson, Chris Deans, Jenny L Donovan, Charlotte Ekblad, Jackie Elliott, David Exon, Stephen Falk, Naheed Farooq, Kirsty Garfield, Daisy M Gaunt, Fran Gill, Robert Goldin, Athanasia Gravani, George Hanna, Stephen Hayes, Rachael Heys, Carolyn Hindmarsh, Sandra Hollinghurst, William Hollingworth, Andrew Hollowood, Rebecca Houlihan, Benjamin Howes, Lucy Howie, Lee Humphreys, David Hutton, Rosina Jarvis, Marcus Jepson, Rebecca Kandiyali, Surinder Kaur, Philip Kaye, Jamie Kelly, Anni King, Jana Kirwin, Richard Krysztopik, Peter Lamb, Alistair Lang, Vivienne Lee, Sally Maitland, Nicholas Mapstone, Georgia Melia, Chris Metcalfe, Rachel Melhado, Aida Moure-Fernandez, Beena Nair, Joanna Nicklin, Fergus Noble, Sian M Noble, Abby O’Connell, Stephen Palmer, Simon Parsons, Kish Pursnani, Nicola Rea, Fiona Reed, Caoimhe Rice, Cathy Richards, Chris Rogers, Grant Sanders, Vicki Save, Chas Shaw, Michael Schiller, Rachel Schranz, Vinutha Shetty, Beverly Shirkey, Jo Singleton, Richard Skipworth, Joanne Smith, Christopher Streets, Dan Titcomb, Paul Turner, Sukhbir Ubhi, Tim Underwood, Cellins Vinod, Ravinder Vohra, Elizabeth M Ward, Rhian Warman, Neil Welch, Tim Wheatley, Katie White, Robin A Wickens, Paul Wilkerson, Alexandra Williams, Rob Williams, Natasha Wilmshurst, and Newton A C S Wong
Funding
This study is funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme (project references: feasibility study HTA10/50/65; definitive study HTA14/140/78). The authors also received support from the Bristol Biomedical Research Centre, the Medical Research Council (MRC) ConDuCT-II (Collaboration and innovation for Difficult and Complex randomized controlled Trials In Invasive procedures) Hub for Trials Methodology Research (MR/K025643/1) and the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Following publication of the main results, anonymized individual patient data will be made available for secondary research through https://data.bris.ac.uk/data/, along with supporting documentation (the ROMIO study protocol and statistical analysis plan have been published elsewhere9,24, a data dictionary will be available). Access will be conditional on assurance from the secondary researcher that the proposed use of the data is compliant with the MRC Policy on Data Preservation and Sharing regarding scientific quality, ethical requirements and value for money.
Author contributions
Chris Metcalfe (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing), Jane Blazeby (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing), Paul Barham (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing), Kerry Avery (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing—review & editing), Richard Berrisford (Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing—review & editing), Natalie Blencowe (Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—review & editing), Rachel Brierley (Data curation, Investigation, Project administration, Resources, Supervision, Validation, Writing—original draft, Writing—review & editing), Lucy Culliford (Investigation, Project administration, Supervision, Writing—review & editing), Jenny Donovan (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing—review & editing), Jackie Elliott (Conceptualization, Funding acquisition, Resources, Writing—review & editing), Kirsty Garfield (Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing), Daisy Gaunt (Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—review & editing), William Hollingworth (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing), Rebecca Houlihan (Data curation, Project administration, Supervision, Validation, Writing—review & editing), Ben Howes (Conceptualization, Funding acquisition, Investigation, Methodology, Writing—review & editing), Marcus Jepson (Formal analysis, Investigation, Methodology, Writing—review & editing), Rebecca Kandiyali (Data curation, Formal analysis, Investigation, Methodology, Writing—review & editing), Anni King (Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—review & editing), Chris A. Rogers (Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing—review & editing), Elizabeth Ward (Data curation, Investigation, Project administration, Resources, Supervision, Writing—review & editing), Paul Wilkerson (Data curation, Investigation, Project administration, Validation, Writing—review & editing), and Newton A. C. S. Wong (Conceptualization, Data curation, Funding acquisition, Investigation, Validation, Writing—review & editing).
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249 [DOI] [PubMed] [Google Scholar]
- 2. Cromwell D, Wahedally H, Park MH, Maynard N, Crosby T, Trudgill N et al. National Oesophago-Gastric Cancer Audit 2019. London: Royal College of Surgeons of England, 2019 [Google Scholar]
- 3. Park MH, Wahedally MAH, Maynard N, Crosby T, Thomas B, Trudgill N et al. National Oesophago-Gastric Cancer Audit. 2022 Annual Report. London: The Royal College of Surgeons of England, 2023 [Google Scholar]
- 4. Nuytens F, Lenne X, Clement G, Bruandet A, Eveno C, Piessen G. Effect of phased implementation of totally minimally invasive Ivor Lewis esophagectomy for esophageal cancer after previous adoption of the hybrid minimally invasive technique: results from a French nationwide population-based cohort study. Ann Surg Oncol 2022;29:2791–2801 [DOI] [PubMed] [Google Scholar]
- 5. Lagergren P, Avery KN, Hughes R, Barham CP, Alderson D, Falk SJ et al. Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer 2007;110:686–693 [DOI] [PubMed] [Google Scholar]
- 6. Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892 [DOI] [PubMed] [Google Scholar]
- 7. Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med 2019;380:152–162 [DOI] [PubMed] [Google Scholar]
- 8. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895–1904 [DOI] [PubMed] [Google Scholar]
- 9. Brierley RC, Gaunt D, Metcalfe C, Blazeby JM, Blencowe NS, Jepson M et al. Laparoscopically assisted versus open oesophagectomy for patients with oesophageal cancer-the Randomised Oesophagectomy: Minimally Invasive or Open (ROMIO) study: protocol for a randomised controlled trial (RCT). BMJ Open 2019;9:e030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avery KN, Metcalfe C, Berrisford R, Barham CP, Donovan JL, Elliott J et al. The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer–the ROMIO (Randomized Oesophagectomy: Minimally Invasive or Open) study: protocol for a randomized controlled trial. Trials 2014;15:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG, Group I. IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ 2013;346:f3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blencowe NS, Skilton A, Gaunt D, Brierley R, Hollowood A, Dwerryhouse S et al. Protocol for developing quality assurance measures to use in surgical trials: an example from the ROMIO study. BMJ Open 2019;9:e026209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avery KNL, Chalmers KA, Brookes ST, Blencowe NS, Coulman K, Whale K et al. Development of a core outcome set for clinical effectiveness trials in esophageal cancer resection surgery. Ann Surg 2018;267:700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376 [DOI] [PubMed] [Google Scholar]
- 16. Hauser K, Walsh D. Visual analogue scales and assessment of quality of life in cancer. J Support Oncol 2008;6:277–282 [PubMed] [Google Scholar]
- 17. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286–294 [DOI] [PubMed] [Google Scholar]
- 19. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 20. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 21. Cunha AB. Pneumonia Essentials. 3rd edn. Royal Oak, MI: Physicians Press, 2010 [Google Scholar]
- 22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011;29:89–96 [DOI] [PubMed] [Google Scholar]
- 24. Metcalfe C, Barham CP. The ROMIO Study: Statistical Analysis Plan. https://research-information.bris.ac.uk/files/209886797/ROMIO_SAP_September_2019_v1.pdf (2019).
- 25. Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 2005;78:355–360 [DOI] [PubMed] [Google Scholar]
- 26. Kandiyali R, Hollingworth W, Barham CP. The ROMIO Study: Health Economic Analysis Plan. https://research-information.bris.ac.uk/en/publications/the-romio-study-health-economic-analysis-plan. University of Bristol; 2020 (accessed 11 March 2020).
- 27. NICE . NICE Health Technology Evaluations: The Manual. www.nice.org.uk/process/pmg36 (2022).
- 28. Hernández Alava M, Pudney S Wailoo A. Estimating the relationship between EQ-5D-5L and EQ-5D-3L: results from a UK population study. PharmacoEconomics 2023;41:199–207. doi: 10.1007/s40273-022-01218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487–496 [DOI] [PubMed] [Google Scholar]
- 30. Grabsch HI, Mapstone NP Novelli M. Standards and Datasets for Reporting Cancers. Dataset for Histopathological Reporting of Oesophageal and Gastric Carcinoma. London: The Royal College of Pathologists, 2019 [Google Scholar]
- 31. Mariette C, Markar S, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D et al. Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open-label, randomized phase III controlled trial: the MIRO trial. Ann Surg 2019;6:1023–1029 [DOI] [PubMed] [Google Scholar]
- 32. Tagkalos E, van der Sluis PC, Berlth F, Poplawski A, Hadzijusufovic E, Lang H et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer 2021;21:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Following publication of the main results, anonymized individual patient data will be made available for secondary research through https://data.bris.ac.uk/data/, along with supporting documentation (the ROMIO study protocol and statistical analysis plan have been published elsewhere9,24, a data dictionary will be available). Access will be conditional on assurance from the secondary researcher that the proposed use of the data is compliant with the MRC Policy on Data Preservation and Sharing regarding scientific quality, ethical requirements and value for money.