Abstract
Objective
Although large retrospective database studies have associated extranodal extension (ENE) with worse survival in several head and neck cancers, the prognostic significance of ENE in laryngeal squamous cell carcinoma (LSCC) remains unclear. Our study examines ENE and overall survival (OS) in LSCC.
Methods
The 2006–2017 National Cancer Database was queried for patients with LSCC undergoing surgical resection and neck dissection, with or without adjuvant therapy. Kaplan–Meier and multivariable Cox regression survival analyses were implemented to identify the independent impacts of pathologic nodal (pN) classification and ENE on OS.
Results
Of 4208 patients satisfying inclusion criteria, 2343 (55.7%) were pN0/ENE‐negative, 1059 (25.2%) were pN1‐2/ENE‐negative, and 806 (19.2%) were pN1‐2/ENE‐positive. The 5‐year OS of pN0/ENE‐negative, pN1‐2/ENE‐negative, and pN1‐2/ENE‐positive patients was 62.8%, 56.7%, and 32.9%, respectively (p < .001). Among pN1‐2/ENE‐positive patients undergoing no adjuvant therapy, adjuvant radiotherapy alone, and adjuvant chemoradiotherapy, 5‐year OS was 24.1%, 30.7%, and 36.7%, respectively (p < .001). After adjusting for patient demographics, clinicopathologic features, and adjuvant therapy, ENE‐positivity was associated with worse OS than ENE‐negativity (adjusted hazard ratio [aHR] 1.76, 95% confidence interval [CI] 1.53–2.02, p < .001). pN1/ENE‐positivity (aHR 1.82, 95% CI 1.31–2.54) and pN2/ENE‐positivity (aHR 1.89, 95% CI 1.49–2.40) were associated with worse OS than pN1/ENE‐negativity (p < .001). Microscopic (aHR 1.83, 95% CI 1.54–2.18) and macroscopic ENE‐positivity (aHR 1.75, 95% 1.35–2.26) were associated with worse OS than ENE‐negativity (p < .001).
Conclusion
ENE‐positivity has prognostic significance in LSCC and is associated with worse OS than ENE‐negativity. pN classification did not have prognostic significance independent of ENE. ENE should be carefully considered when determining the prognosis of LSCC and selecting adjuvant therapy.
Level of Evidence
4.
Keywords: extranodal extension, laryngeal squamous cell carcinoma, National Cancer Database, nodal metastasis, survival
Our study suggests that extranodal extension (ENE) is strongly associated with worse overall survival in laryngeal squamous cell carcinoma (LSCC) independent of pathologic nodal classification. Microscopic and macroscopic ENE were both significant, independent prognostic indicators of survival. Further studies are needed to inform adjuvant management of LSCC based on ENE status.
1. INTRODUCTION
The pathologic tumor‐node‐metastasis (pTNM) classification is one of the most widely adopted systems to stage surgically resected cancer and communicate the anatomic extent of metastasis. 1 The number, size, and location of metastatic lymph nodes determines pN classification. 1 In addition to pN classification, extranodal extension (ENE) of lymph node metastasis, defined as the expansion of tumor cells beyond the lymph node capsule into perinodal tissue, has prognostic significance in head and neck squamous cell carcinoma (HNSCC). 2 , 3 , 4 , 5
Laryngeal squamous cell carcinoma (LSCC) is one of the most common head and neck malignancies, with an incidence of approximately 13,000 patients per year in the United States. 6 , 7 , 8 Although large retrospective database studies have associated ENE with worse survival in squamous cell carcinoma of several head and neck primary sites, including the oral cavity, oropharynx, and hypopharynx, the prognostic significance of ENE in LSCC remains unclear. 9 , 10 , 11 , 12 To date, the prognostic significance of ENE in LSCC has only been investigated in institutional studies—one study of 81 patients showed a lack of statistical significance between ENE‐positivity and survival, while another study of 355 patients showed a significant association between ENE‐positivity and survival. 13 , 14 To elucidate the prognostic significance of ENE in LSCC, our study utilizes the National Cancer Database (NCDB) to comprehensively examine the independent impacts of ENE and pN classification on overall survival (OS).
2. METHODS
2.1. Data source
The NCDB is jointly sponsored by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is a hospital‐based cancer outcomes registry that collects data from >1500 CoC‐accredited hospitals within the United States and captures >70% of all newly diagnosed cancers each year. The NCDB is not responsible for the validity of the statistical analysis and conclusions derived herein. Our study was exempt from review by the Rutgers New Jersey Medical School Institutional Review Board because of the de‐identified nature of patient data.
2.2. Inclusion criteria
Patients included in our study had (1) International Classification of Diseases for Oncology, 3rd Edition (ICD‐O‐3) histology (“8070–8074,” “8076,” “8078”), behavior (“3”), and topography (“C32.0‐C32.2”) codes consistent with conventional LSCC, (2) either pN0/ENE‐negativity, pN1‐2/ENE‐negativity, or pN1‐2/ENE‐positivity, (3) no clinically distant metastasis, and (4) definitive treatment with surgical resection and neck dissection, with or without adjuvant therapy. 12 Surgical resection was defined as local tumor destruction, local tumor excision, partial excision, total or radical laryngectomy, pharyngolargyngectomy, or unspecified surgery. Neck dissection was defined as the removal and examination of 10 regional lymph nodes. 15 , 16 Patients with unknown grade, pathologic American Joint Committee on Cancer (AJCC) group stage, pTN classification, vital status, or survival time were excluded. Patients undergoing palliative care, salvage surgery, neoadjuvant therapy, or adjuvant chemotherapy alone were also excluded. All staging and classifications were based on the AJCC Cancer Staging Manual, 7th edition.
2.3. Exposure and outcome assessment
Pathologic ENE is recorded under NCDB collaborative stage site‐specific factor 9 according to the final diagnosis in the pathology report. 11 Patients were classified as ENE‐positive if the regional lymph nodes had either microscopic or macroscopic ENE. ENE‐negativity was defined as regional lymph node involvement with no ENE on pathologic examination.
The primary outcome of our study was 5‐year OS. Survival time was calculated as the time from diagnosis to either death due to any cause or 5‐years of follow‐up.
2.4. Confounders
Potential confounders included age at diagnosis (18–55 years, 56–69 years, 70 years), sex (male, female), race (White, Black, other), Charlson‐Deyo comorbidity score (0, 1, 2), history of prior malignancy, tumor subsite (glottis, supraglottis, subglottis), tumor diameter, grade, pathologic AJCC group stage, pTN classification, lymphovascular invasion, surgical margin status, and adjuvant therapy (none, adjuvant radiotherapy alone, adjuvant chemoradiotherapy). Microscopic, macroscopic, or unspecified residual tumor were considered positive surgical margins.
2.5. Statistical analysis
The chi‐square test and independent samples t‐tests were used to compare categorical and continuous variables, respectively, across pN0/ENE‐negative, pN1‐2/ENE‐negative, and pN1‐2/ENE‐positive cohorts. Kaplan–Meier analysis and the log‐rank test were used to estimate 5‐year OS. Adjusted hazard ratios (aHRs) for the independent impacts of pN classification and ENE on OS were estimated by multivariable Cox proportional hazards regression. Multivariable Cox analyses included all variables with p < .05 on univariable Cox analysis. The proportional hazards assumption was tested with time‐dependent covariables and satisfied in all regression models. The interaction between ENE and nodal status was examined because both factors are clinically related. The significance level for all statistical testing and confidence interval (CI) calculations was set at p < 0.05. SPSS version 25 (IBM) was used for statistical analysis.
3. RESULTS
3.1. Patient demographics, clinicopathologic features, and adjuvant therapy
Of 4208 patients satisfying inclusion criteria, 2343 (55.7%) were pN0/ENE‐negative, 1059 (25.2%) were pN1‐2/ENE‐negative, and 806 (19.2%) were pN1‐2/ENE‐positive (Table 1). ENE was significantly associated with pathologic AJCC group staging, pT classification, and treatment modality. Compared with pN0/ENE‐negative patients, pN1‐2/ENE‐positive patients more frequently had pathologic AJCC group stage IV (95.0% vs. 47.2%), pT4 classification (50.4% vs. 43.4%), and treatment with adjuvant chemoradiotherapy (61.3% vs. 11.3%) (p < .001).
TABLE 1.
Patient demographics, clinicopathologic features, and adjuvant therapy.
| pN0/ENE‐negative | pN1‐2/ENE‐negative | pN1‐2/ENE‐positive | p‐value | |
|---|---|---|---|---|
| No. of patients (%) | 2343 (55.7) | 1059 (25.2) | 806 (19.2) | |
| Age at diagnosis, n (%) | ||||
| Mean (years ± SD) | 61.7 ± 10.1 | 59.7 ± 9.0 | 61.2 ± 9.4 | <.001 |
| 18–55 years | 633 (27.0) | 350 (33.1) | 228 (28.3) | <.001 |
| 56–69 years | 1207 (51.5) | 562 (53.1) | 427 (53.0) | |
| 70 years | 503 (21.5) | 147 (13.9) | 151 (18.7) | |
| Sex, n (%) | ||||
| Male | 1835 (78.3) | 788 (74.4) | 587 (72.8) | .002 |
| Female | 508 (21.7) | 271 (25.6) | 219 (27.2) | |
| Race, n (%) | ||||
| White | 1910 (82.0) | 840 (80.4) | 655 (82.2) | .775 |
| Black | 364 (15.6) | 180 (17.2) | 126 (15.8) | |
| Other | 54 (2.3) | 25 (2.4) | 16 (2.0) | |
| Charlson‐Deyo comorbidity score, n (%) | ||||
| 0 | 1415 (60.4) | 677 (63.9) | 469 (58.2) | .110 |
| 1 | 631 (26.9) | 269 (25.4) | 233 (28.9) | |
| 2 | 297 (12.7) | 113 (10.7) | 104 (12.9) | |
| History of prior malignancy, n (%) | 714 (20.5) | 235 (22.2) | 183 (22.7) | <.001 |
| Subsite, n (%) | ||||
| Glottis | 1026 (43.8) | 296 (28.0) | 170 (21.1) | <.001 |
| Supraglottis | 1196 (51.0) | 731 (69.0) | 618 (76.7) | |
| Subglottis | 121 (5.2) | 32 (3.0) | 18 (2.2) | |
| Tumor diameter, cm ± SD | 3.4 ± 4.4 | 4.4 ± 7.5 | 4.5 ± 5.8 | <.001 |
| Grade, n (%) | ||||
| Well‐differentiated | 229 (9.8) | 57 (5.4) | 17 (2.1) | <.001 |
| Moderately differentiated | 1595 (68.1) | 690 (65.2) | 453 (56.2) | |
| Poorly differentiated, undifferentiated, anaplastic | 519 (22.2) | 312 (29.5) | 336 (41.7) | |
| Pathologic AJCC group stage, n (%) | ||||
| I | 261 (11.1) | 18 (1.7) | 0 (0.0) | <.001 |
| II | 330 (14.1) | 44 (4.2) | 2 (0.2) | |
| III | 646 (27.6) | 238 (22.5) | 38 (4.7) | |
| IV | 1106 (47.2) | 759 (71.7) | 766 (95.0) | |
| pT classification, n (%) | ||||
| 1 | 289 (12.3) | 67 (6.3) | 44 (5.5) | <.001 |
| 2 | 386 (16.5) | 119 (11.2) | 89 (11.0) | |
| 3 | 652 (27.8) | 354 (33.4) | 267 (33.1) | |
| 4 | 1016 (43.4) | 519 (49.0) | 406 (50.4) | |
| pN classification, n (%) | ||||
| 0 | 2343 (100.0) | 0 (0.0) | 0 (0.0) | <.001 |
| 1 | 0 (0.0) | 399 (37.7) | 197 (24.4) | |
| 2 | 0 (0.0) | 660 (62.3) | 609 (75.6) | |
| Lymphovascular invasion, n (%) | ||||
| No | 1689 (73.3) | 647 (61.9) | 267 (33.3) | <.001 |
| Yes | 416 (18.1) | 291 (27.8) | 442 (55.2) | |
| Unknown | 199 (8.6) | 108 (10.3) | 92 (11.5) | |
| Adjuvant therapy, n (%) | ||||
| None | 1321 (56.4) | 316 (29.8) | 161 (20.0) | <.001 |
| Radiotherapy alone | 758 (32.4) | 443 (41.8) | 151 (18.7) | |
| Chemoradiotherapy | 264 (11.3) | 300 (28.3) | 494 (61.3) | |
| Surgical margins, n (%) | ||||
| Negative | 2136 (92.3) | 934 (89.5) | 631 (80.1) | <.001 |
| Positive | 177 (7.7) | 110 (10.5) | 157 (19.9) |
Note: Bold values are significant at p < 0.05.
Abbreviations: AJCC, American Joint Committee on Cancer; ENE, extranodal extension; pTN, pathologic tumor‐nodal; SD, standard deviation.
3.2. Kaplan–Meier survival analysis
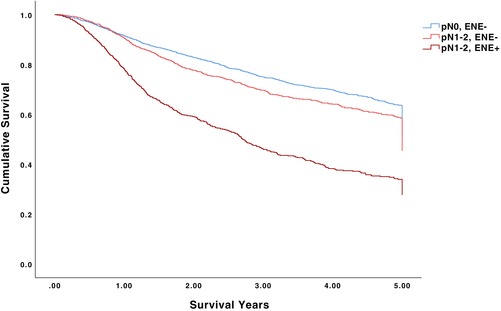
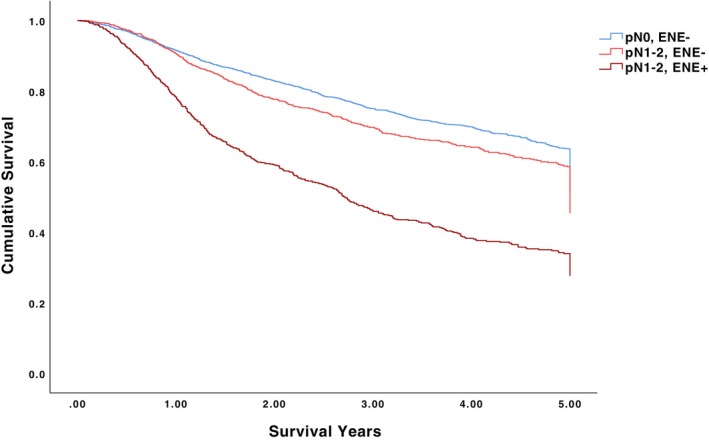
The 5‐year OS for pN0/ENE‐negative, pN1‐2/ENE‐negative, and pN1‐2/ENE‐positive patients was 62.8%, 56.7%, and 32.9%, respectively (p < .001) (Table 2, Figure 1). Among pN0/ENE‐negative patients undergoing no adjuvant therapy, adjuvant radiotherapy alone, and adjuvant chemoradiotherapy, 5‐year OS was 63.7%, 61.9%, and 60.7%, respectively (p = .496). Among pN1‐2/ENE‐positive patients undergoing no adjuvant therapy, adjuvant radiotherapy alone, and adjuvant chemoradiotherapy, 5‐year OS was 24.1%, 30.7%, and 36.7%, respectively (p < .001). The 5‐year OS for pN1/ENE‐negative, pN1/ENE‐positive, pN2/ENE‐negative, and pN2/ENE‐positive patients was 60.5%, 31.9%, 54.5%, and 31.4%, respectively (p < .001) (Figure 2). The 5‐year OS for ENE‐negative, microscopic ENE‐positive, and macroscopic ENE‐positive patients was 60.9%, 31.6%, and 30.4%, respectively (p < .001) (Figure 3).
TABLE 2.
5‐year overall survival (%) within strata.
| pN0/ENE‐negative | pN1‐2/ENE‐negative | pN1‐2/ENE‐positive | p‐value | |
|---|---|---|---|---|
| Overall | 62.8 | 56.7 | 32.9 | <.001 |
| Age at diagnosis, years | ||||
| 18–55 | 74.1 | 60.1 | 35.0 | <.001 |
| 56–69 | 63.8 | 57.7 | 35.1 | <.001 |
| 70 | 47.0 | 45.5 | 23.5 | <.001 |
| Sex | ||||
| Male | 61.0 | 55.3 | 31.8 | <.001 |
| Female | 69.2 | 61.1 | 36.0 | <.001 |
| Race | ||||
| White | 62.5 | 57.1 | 33.3 | <.001 |
| Black | 62.3 | 55.2 | 32.7 | <.001 |
| Other | 68.3 | 40.0 | 25.9 | .001 |
| Subsite | ||||
| Glottis | 63.2 | 56.9 | 24.3 | <.001 |
| Supraglottis | 62.8 | 56.9 | 35.4 | <.001 |
| Subglottis | 60.2 | 51.0 | 29.0 | .025 |
| Grade | ||||
| Well‐differentiated | 69.3 | 63.4 | 46.2 | .229 |
| Moderately differentiated | 64.3 | 55.3 | 29.7 | <.001 |
| Poorly differentiated, undifferentiated, anaplastic | 55.6 | 58.8 | 36.7 | <.001 |
| Pathologic AJCC group stage | ||||
| I | 78.4 | 93.5 | ‐ | .317 |
| II | 72.5 | 80.8 | 50.0 | .514 |
| III | 59.3 | 66.4 | 43.3 | .040 |
| IV | 58.4 | 51.5 | 32.3 | <.001 |
| pT classification | ||||
| 1 | 77.6 | 75.4 | 38.5 | <.001 |
| 2 | 68.9 | 67.0 | 46.7 | <.001 |
| 3 | 56.8 | 60.0 | 41.1 | <.001 |
| 4 | 60.2 | 49.5 | 24.1 | <.001 |
| pN classification | ||||
| 0 | 62.8 | ‐ | ‐ | ‐ |
| 1 | ‐ | 60.5 | 40.1 | <.001 |
| 2 | ‐ | 54.5 | 30.6 | <.001 |
| Adjuvant therapy | ||||
| None | 63.7 | 61.0 | 24.1 | <.001 |
| Radiotherapy alone | 61.9 | 55.8 | 30.7 | <.001 |
| Chemoradiotherapy | 60.7 | 53.7 | 36.7 | <.001 |
Note: Bold values are significant at p < 0.05.
Abbreviations: AJCC, American Joint Committee on Cancer; ENE, extranodal extension; pTN, pathologic tumor‐nodal.
FIGURE 1.
5‐year overall survival for pN0/ENE‐negative, pN1‐2/ENE‐negative, and pN1‐2/ENE‐positive patients.
FIGURE 2.
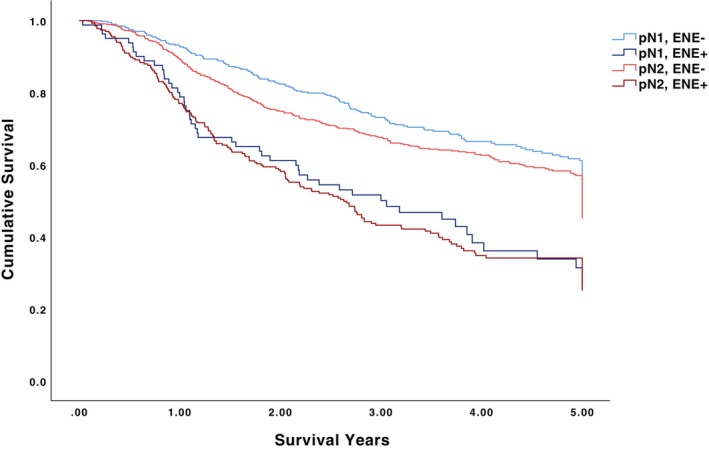
5‐year overall survival for pN1/ENE‐negative, pN1/ENE‐positive, pN2/ENE‐negative, and pN2/ENE‐positive patients.
FIGURE 3.
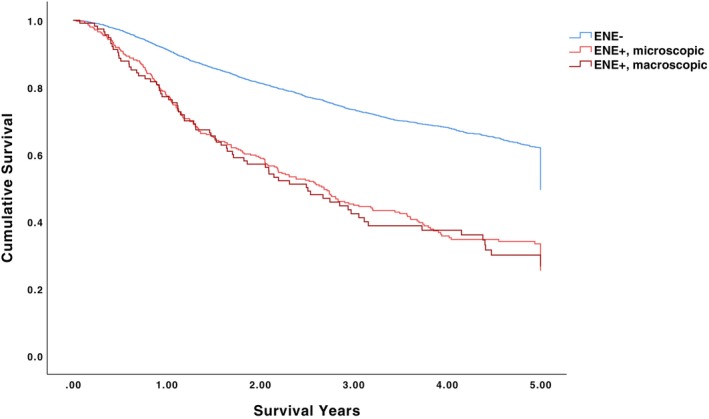
5‐year overall survival for ENE‐negative, microscopic ENE‐positive, and macroscopic ENE‐positive patients.
3.3. Multivariable Cox regression survival analysis
After adjusting for age at diagnosis, sex, race, CCI, history of prior malignancy, pathologic AJCC group stage, pT classification, and surgical margin status, ENE‐positivity was associated worse OS than ENE‐negativity (aHR 1.76, 95% CI 1.53–2.02, p < .001) while also adjusting for pN classification (Table 3). Adjusting for pN classification and ENE together, pN1/ENE‐positivity (aHR 1.82, 95% CI 1.31–2.54) and pN2/ENE‐positivity (aHR 1.89, 95% CI 1.49–2.40) were both associated with worse OS than pN1/ENE‐negativity (p < .001) (Table 4). Microscopic (aHR 1.83, 95% CI 1.54–2.18) and macroscopic (aHR 1.75, 95% CI 1.35–2.26) ENE were both associated with worse OS than ENE‐negativity while also adjusting for pN classification (p < 0.001) (Table 5).
TABLE 3.
Adjusted hazard ratios for ENE and pN classification among all pN1‐2 patients.
| n (%) | aHR a (95% CI) | p‐value | |
|---|---|---|---|
| ENE | |||
| Negative | 1030 (56.9) | Ref | |
| Positive | 779 (43.1) | 1.76 (1.53–2.02) | <.001 |
| pN classification | |||
| 1 | 576 (31.8) | Ref | |
| 2 | 1233 (68.2) | 1.15 (0.99–1.34) | .065 |
Note: Bold values are significant at p < 0.05.
Abbreviations: aHR, adjusted hazard ratio; AJCC, American Joint Committee on Cancer; CI, confidence interval; ENE, extranodal extension; pTN, pathologic tumor‐nodal; Ref, reference.
Adjusted for age at diagnosis, sex, race, Charlson‐Deyo comorbidity score, history of prior malignancy, pathologic AJCC group stage, pT classification, and surgical margin status.
TABLE 4.
Adjusted hazard ratios for combined ENE and pN classification among all pN1‐2 patients.
| n (%) | aHR a (95% CI) | p‐value | |
|---|---|---|---|
| pN classification/ENE | |||
| pN1/ENE‐negative | 386 (28.2) | Ref | |
| pN1/ENE‐positive | 79 (5.8) | 1.82 (1.31–2.54) | <.001 |
| pN2/ENE‐negative | 644 (47.1) | 1.03 (0.84–1.26) | .779 |
| pN2/ENE‐positive | 258 (18.9) | 1.89 (1.49–2.40) | <.001 |
Note: Bold values are significant at p < 0.05.
Abbreviations: aHR, adjusted hazard ratio; AJCC, American Joint Committee on Cancer; CI, confidence interval; ENE, extranodal extension; pTN, pathologic tumor‐nodal; Ref, reference.
Adjusted for age at diagnosis, sex, race, Charlson‐Deyo comorbidity score, history of prior malignancy, pathologic AJCC group stage, pT classification, and surgical margin status.
TABLE 5.
Adjusted hazard ratios for negative, microscopic, and macroscopic ENE and pN classification among all pN1‐2 patients.
| n (%) | aHR a (95% CI) | p‐value | |
|---|---|---|---|
| ENE | |||
| Negative | 1030 (69.6) | Ref | |
| Microscopic | 337 (22.8) | 1.83 (1.54–2.18) | <.001 |
| Macroscopic | 112 (7.6) | 1.75 (1.35–2.26) | <.001 |
| pN classification | |||
| 1 | 490 (33.1) | Ref | |
| 2 | 989 (66.9) | 1.06 (0.89–1.25) | .519 |
Note: Bold values are significant at p < 0.05.
Abbreviations: aHR, adjusted hazard ratio; AJCC, American Joint Committee on Cancer; CI, confidence interval; ENE, extranodal extension; pTN, pathologic tumor‐nodal; Ref, reference.
Adjusted for age at diagnosis, sex, race, Charlson‐Deyo comorbidity score, history of prior malignancy, pathologic AJCC group stage, pT classification, and surgical margin status.
4. DISCUSSION
The prognostic significance of ENE in patients with LSCC undergoing definitive treatment with surgical resection and neck dissection is not well understood. Our study utilizing the NCDB to investigate the prognostic significance of ENE in LSCC found that ENE‐positive patients had worse OS than ENE‐negative patients, even after stratification of survival analysis by patient demographics, clinicopathologic features, and adjuvant therapy. The survival detriment of ENE‐positivity was more pronounced among patients with age 70 years, female sex, and glottic tumors. Among pN0/ENE‐negative patients, adjuvant therapy, adjuvant radiotherapy alone, and adjuvant chemoradiotherapy were all associated with similar OS. Among pN1‐2/ENE‐positive patients, adjuvant chemoradiotherapy was associated with higher OS than both adjuvant radiotherapy alone or no adjuvant therapy. On multivariable analysis, ENE‐positivity remained associated with worse OS, regardless of pN classification and other confounders. The survival detriment of ENE‐positivity persisted with both microscopic and macroscopic ENE. pN classification was not associated with OS independent of ENE.
Since ENE was first defined by Bennett et al., many studies have highlighted the association between ENE‐positivity and poor survival. 17 Large retrospective database studies of SCC of the oral cavity, oropharynx, and other head and neck primary sites document the survival detriment associated with ENE‐positivity. 18 , 19 The 8th edition of the AJCC Cancer Staging Manual has therefore incorporated evidence of ENE in the N classification of HNSCC. 20 However, literature describing the role of ENE in outcomes of LSCC is limited. Several smaller institutional studies describe ENE‐positivity as portending poorer survival. 13 , 14 , 21 To our knowledge, our study is the first to implement a large‐scale, multi‐institutional design to evaluate the impact of ENE in LSCC survival and distinguish between the microscopic and macroscopic ENE.
Until recently, there was little consensus among pathologists regarding the histologic definition of ENE across head and neck primary sites. 17 , 18 , 22 However, the 8th edition of the AJCC Cancer Staging Manual and the College of American Pathologist have created standardized criteria allowing for comparable studies between microscopic (≤2 mm from the capsule) and macroscopic ENE (>2 mm from the capsule). 18 , 19 A prior study describing ENE of the larynx and hypopharynx defined macroscopic ENE as intraoperative transcapsular spread and microscopic ENE as pathologic examination revealing microscopic invasion. 23 With this definition, microscopic ENE was shown to not have an association with poorer survival. 23 The lack of standardization of numerical parameters may explain the discrepancy between the results of this study and the results of our study.
Our study has several limitations. Some data in retrospective databases such as the NCDB may be missing or inaccurate. Our retrospective study design precludes controlling for treatment selection bias. The NCDB does not encode medical comorbidities, tobacco use, disease‐specific survival, and locoregional recurrence, which all affect LSCC management and survival. Defining neck dissection as the removal and examination of 10 lymph nodes may have underestimated the delivery of neck dissection and included patients undergoing less selective nodal resections. The 8th edition of the AJCC Cancer Staging Manual is the first to provide an updated, unified definition of ENE, but reporting in the NCDB was only mandated starting in 2018. The number of involved lymph nodes was not adjusted for in multivariable analysis. Despite these limitations, our study presents a large analysis of the NCDB and suggests that both microscopic and macroscopic ENE have prognostic significance independent of patient demographics, pN classification, adjuvant therapy, and other clinicopathologic confounders.
5. CONCLUSION
Our study demonstrates that microscopic and macroscopic ENE are both strongly associated with worse OS in LSCC independent of pN classification. pN classification was not associated with OS independent of ENE. Further studies are necessary to inform management of LSCC based on ENE status.
AUTHOR CONTRIBUTIONS
Aman M. Patel: Design, analysis, interpretation, manuscript writing. Sudeepti Vedula: Design, analysis, interpretation, manuscript writing. Ariana L. Shaari: Design, analysis, interpretation, manuscript writing. Hannaan S. Choudhry: Design, analysis, interpretation, manuscript writing. Andrey Filimonov: Design, analysis, interpretation, manuscript writing, final approval.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Patel AM, Vedula S, Shaari AL, Choudhry HS, Filimonov A. Extranodal extension in laryngeal squamous cell carcinoma. Laryngoscope Investigative Otolaryngology. 2024;9(2):e1232. doi: 10.1002/lio2.1232
REFERENCES
- 1. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93‐99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 2. Huang SH, Chernock R, O'Sullivan B, Fakhry C. Assessment criteria and clinical implications of Extranodal extension in head and neck cancer. Am Soc Clin Oncol Educ Book. 2021;41:265‐278. doi: 10.1200/EDBK_320939 [DOI] [PubMed] [Google Scholar]
- 3. Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92(12):3030‐3036. doi: [DOI] [PubMed] [Google Scholar]
- 4. Ferlito A, Rinaldo A, Devaney KO, et al. Prognostic significance of microscopic and macroscopic extracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncol. 2002;38(8):747‐751. doi: 10.1016/s1368-8375(02)00052-0 [DOI] [PubMed] [Google Scholar]
- 5. Suh S, Pak K, Seok JW, Kim IJ. Prognostic value of Extranodal extension in thyroid cancer: a meta‐analysis. Yonsei Med J. 2016;57(6):1324‐1328. doi: 10.3349/ymj.2016.57.6.1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 7. Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(9):951‐962. doi: 10.1001/archotol.124.9.951 [DOI] [PubMed] [Google Scholar]
- 8. Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(9):855‐860. doi: 10.1001/jamaoto.2014.1671 [DOI] [PubMed] [Google Scholar]
- 9. Ho AS, Kim S, Tighiouart M, et al. Association of quantitative metastatic lymph node burden with survival in hypopharyngeal and laryngeal cancer. JAMA Oncol. 2018;4(7):985‐989. doi: 10.1001/jamaoncol.2017.3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: a systematic review and meta‐analysis. Oral Oncol. 2016;62:60‐71. doi: 10.1016/j.oraloncology.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 11. Bauer E, Mazul A, Chernock R, et al. Extranodal extension is a strong prognosticator in HPV‐positive oropharyngeal squamous cell carcinoma. Laryngoscope. 2020;130(4):939‐945. doi: 10.1002/lary.28059 [DOI] [PubMed] [Google Scholar]
- 12. Cheraghlou S, Kuo P, Judson BL. Treatment delay and facility case volume are associated with survival in early‐stage glottic cancer. Laryngoscope. 2017;127(3):616‐622. doi: 10.1002/lary.26259 [DOI] [PubMed] [Google Scholar]
- 13. Bulgurcu S, Idil M, Kucuk U, Cukurova I. The effect of Extranodal extension on survival in laryngeal carcinoma. Ear Nose Throat J. 2020;99(5):305‐308. doi: 10.1177/0145561319862211 [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Zeng Q, Li Y, Lu T, Liu C, Hu G. Extranodal extension as an independent prognostic factor in laryngeal squamous cell carcinoma patients. J Cancer. 2020;11(24):7196‐7201. doi: 10.7150/jca.47700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crawford KL, Jafari A, Qualliotine JR, et al. Elective neck dissection for T3/T4 cN0 sinonasal squamous cell carcinoma. Head Neck. 2020;42(12):3655‐3662. doi: 10.1002/hed.26418 [DOI] [PubMed] [Google Scholar]
- 16. Patel AM, Vedula S, Haleem A, Choudhry HS, Tseng CC, Park RC. Elective neck dissection for cT1‐4 N0M0 head and neck verrucous Carcinoma. Otolaryngol Head Neck Surg. 2023;169:1187‐1199. [DOI] [PubMed] [Google Scholar]
- 17. Bennett SH, Futrell JW, Roth JA, Hoye RC, Ketcham AS. Prognostic significance of histologic host response in cancer of the larynx or hypopharynx. Cancer. 1971;28(5):1255‐1265. doi: [DOI] [PubMed] [Google Scholar]
- 18. Quinton BA, Cabrera CI, Tamaki A, et al. The impact of microscopic versus macroscopic extranodal extension in oral cavity squamous cell carcinoma: national cancer database analysis and review of the literature. Am J Otolaryngol. 2022;43(4):103511. doi: 10.1016/j.amjoto.2022.103511 [DOI] [PubMed] [Google Scholar]
- 19. Lewis JS Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24(11):1413‐1420. doi: 10.1038/modpathol.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanoni DK, Patel SG. New AJCC: how does it impact oral cancers? Oral Oncol. 2020;104:104607. doi: 10.1016/j.oraloncology.2020.104607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirabayashi H, Koshii K, Uno K, et al. Extracapsular spread of squamous cell carcinoma in neck lymph nodes: prognostic factor of laryngeal cancer. Laryngoscope. 1991;101(5):502‐506. doi: 10.1288/00005537-199105000-00010 [DOI] [PubMed] [Google Scholar]
- 22. Abdel‐Halim CN, Rosenberg T, Larsen SR, et al. Histopathological definitions of Extranodal extension: a systematic review. Head Neck Pathol. 2021;15(2):599‐607. doi: 10.1007/s12105-020-01221-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brasilino de Carvalho M. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck. 1998;20(1):16‐21. doi: [DOI] [PubMed] [Google Scholar]


