Abstract
Major achievements in bone research have always relied on animal models and in vitro systems derived from patient and animal material. However, the use of animals in research has drawn intense ethical debate and the complete abolition of animal experimentation is demanded by fractions of the population. This phenomenon is enhanced by the reproducibility crisis in science and the advance of in vitro and in silico techniques. 3D culture, organ-on-a-chip, and computer models have improved enormously over the last years. Nevertheless, the overall complexity of bone tissue-cross talk and the systemic and local regulation of bone physiology can often only be addressed in entire vertebrates. Powerful genetic methods such as conditional mutagenesis, lineage tracing, and modeling of the diseases enhanced the understanding of the entire skeletal system. In this review endorsed by the European Calcified Tissue Society (ECTS), a working group of investigators from Europe and the US provides an overview of the strengths and limitations of experimental animal models, including rodents, fish, and large animals, as well the potential and short comings of in vitro and in silico technologies in skeletal research. We propose that the proper combination of the right animal model for a specific hypothesis and state-of-the-art in vitro and/or in silico technology is essential to solving remaining important questions in bone research. This is crucial for executing most efficiently the 3R principles to reduce, refine and replace animal experimentation, for enhancing our knowledge of skeletal biology, and for the treatment of bone diseases that affect a large part of society.
Introduction
After 1.4 million European citizens have signed a petition entitled “Save Cruelty-Free Cosmetics”1, the EU parliament will discuss and eventually decide whether to abandon all animal experiments in the European Union. Similarly, in the U.S., there is a growing public sentiment against animal experiments. In a Gallup poll, the moral acceptance of medical animal research dropped from 63% in 2002 to 52% in the year 20222. Reflecting this sentiment, legislation approved in 2022 eliminated the previous requirement that new drugs need to be tested in animals to receive approval from the U.S. Food and Drug Administration (FDA)3.
In this context, local ethic committees decide about animal licenses with increasing sensitivity towards the trade-off between ethics and scientific rationale. In the U.S., institutional animal care and use committees (IACUC) are responsible for the oversight of animal care and use programs. IACUCs review, at least semiannually, institutions’ experimental programs and inspect facilities to report welfare concerns, make recommendations on aspects of care, housing, or personnel training, and are authorized to suspend activities involving animals. Importantly, IACUC, as well as most grant agencies, require justification of animal numbers to be supported by statistical power analyses.
Progress in in vitro cell-based systems as well as in silico models is used as an argument to suggest that all animal experiments have become dispensable. In this light, it appears necessary for the skeletal research community to assess and defend the general reasons for animal experiments in our field, in particular when systemic diseases are investigated that still cannot be entirely modeled in vitro or in silico.
This review aims to provide a balanced view of the benefits and pitfalls of various in vitro and in vivo models and to increase awareness of their suitability to specific research questions. In addition, it should equip scientists for a discussion on the topic with laymen, stakeholders, and decision-makers with the final goal to convince them that animal experiments in the field of musculoskeletal research are still necessary, highly regulated and supervised by competent institutional bodies, and used only if in vitro and in silico models are not mimicking the systemic bone physiology.
The current landscape of animal experiments used to investigate skeletal diseases
Skeletal research focuses on understanding bone development, growth, remodeling, and aging to discover new treatments for numerous skeletal conditions including osteoporosis, arthritis, low back pain, genetic diseases, and bone tumors/metastasis that significantly impact public health systems. Their prevalence is anticipated to rise due to the increasing and aging population. Disability-adjusted life years and global deaths from low bone mineral density-related fractures have increased significantly in the last decade: 121.07% (4436789 to 9808464 cases) and 148.65% (121248 to 301482 cases) respectively, with the highest disease burden in India, China, the USA, Japan, and Germany4.
Systemic Bone diseases
Bone is crucial for systemic regulation of calcium and phosphate homeostasis, and serves as an endocrine organ. Understanding bone pathophysiology in a systemic context is therefore essential for improving patient treatment options.
Metabolic bone diseases can have genetic, hormonal, and/or environmental causes (e.g. malnutrition) and include among others osteomalacia (rickets) and hyperparathyroidism. These conditions cause weakened bone, frequent fractures, and possible growth retardation in children and are often investigated in transgenic rodents5. Using such models it was shown that the knockout of some genes that were expected to result in dramatic bone phenotypes was more subtle than expected (e.g., estrogen receptor alpha6) while other molecules involved in bone metabolism like IL-5 were only first identified by revealing their skeletal phenotype in transgenic mouse models7. Animal models have furthermore been used to study the potential effects of certain nutritional interventions, including the effects of vitamin D and calcium supplementation on bone health, influencing the development of dietary guidelines for preventing osteoporosis8–10.
Osteoporosis, another metabolic bone disease affecting millions of people worldwide, is characterized by bone loss, leading to an increased risk of fractures. For osteoporosis research, genetically modified and/or ovariectomized or glucocorticoid treated rodents – and more recently zebrafish11- are used to study underlying mechanisms of menopausal or glucocorticoid-induced bone loss respectively and to test the efficacy of new treatments, such as bisphosphonates and selective estrogen receptor modulators (SERMs)12–15. In more translational settings, large animals, mostly sheep16, and rarely non-human primate models are used.
Rare genetic bone diseases affecting the entire skeleton like osteogenesis imperfecta, skeletal dysplasias, and osteopetrosis are major concerns, as they manifest in early childhood and can be life-threatening. The research of genetic diseases can benefit from genetically modified cell lines which can be used to elucidate pathways and help to screen for potential treatment. Ultimately, treatment effects and the success of potential drugs must be investigated in vivo in vertebrate models to verify their mechanisms of action17. For historical reasons, these models have mostly been mice, but with the availability of CRISPR/Cas9, options have increased dramatically18. In this context, zebrafish offer a valuable alternative to mice due to their ease of manipulation, transparent extra-uterine development, and suitability for high-throughput drug-screening even at embryonic stages19.
Local diseases such as bone tumors (e.g. giant cell tumors) perhaps offer the best starting point for replacement methods. Here, in vitro methods have tremendous potential in shedding light on the cells and signaling involved and identifying possible treatments20. Furthermore, these diseases are suited to test the limits of in silico models or organ-on-a-chip21,22. Final validation, however, is still dependent on a vertebrate model.
Bone repair and regeneration may be studied in a clinical setting or with an interest in basic bone biology/physiology. Fin bone injury or amputation models of zebrafish have been important in determining factors involved in osteogenesis and bone regeneration23,24. Fracture models at specific anatomical locations and with different surgical interventions are routinely done in rodents. In the clinical setting, more emphasis is placed on mechanical loading thus requiring large animals25. These models are less frequently used but are essential in translational orthopedic settings.
For numerous reasons, about 90% of drug candidates, also those with promising results in vivo, never make it to the market26, but differences between animal and human physiology are a minor issue in most cases. Certainly, all in vivo and in vitro models have limitations but are in combination useful to analyze and understand particular aspects of human diseases (Figure 1). It is noteworthy, that not only in vitro but also in vivo models have been drastically refined over the last decades and years to more accurately reflect human diseases and to ensure humane animal treatment. Animal models often allow a comprehensive analysis of bone pathophysiology. Thereby, they provide the basis for functional interference with essential disease factors, which can lead to the description of druggable targets and the subsequent development of novel therapeutic strategies. The following paragraphs provide an overview of the different currently used models, their advantages, and caveats (Table1).
Figure 1: The current use of in vitro and in vivo models in pre-clinical research of bone pathologies.
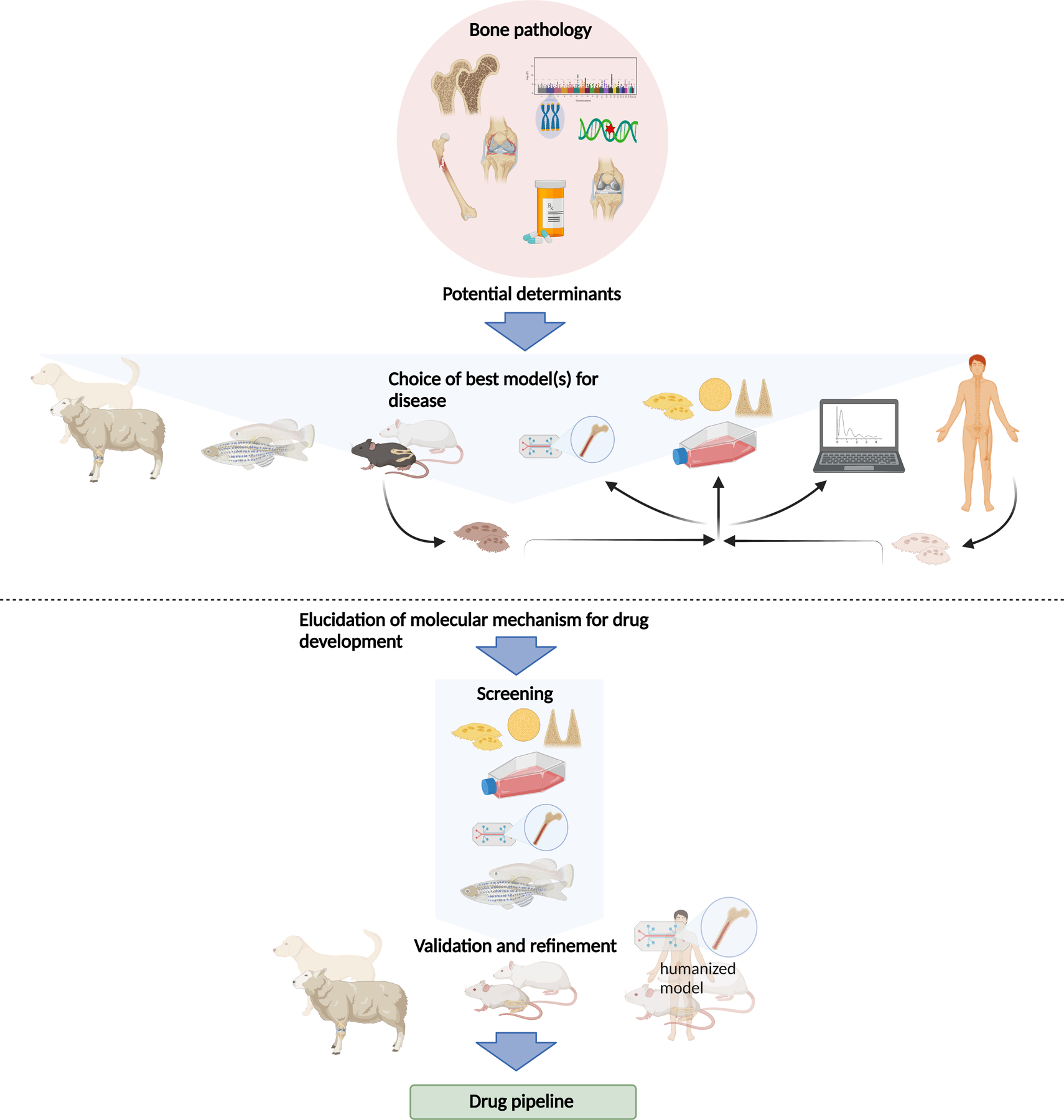
The etiology of a given bone pathology as well as the hypothesis investigated suggest one or more suitable model(s) to study the question. These may be sheep, teleost fish, rodents, or cells (from animals or human patients). Cells may be analyzed directly or grown in 2D, or 3D cultures or “on a chip”. Data gained from these experiments can be used for in-silico models. The combination of different models should allow elucidation of molecular mechanisms which can then be exploited for therapeutic strategies, where the different models can be used as readout systems. For large screens to elucidate molecular mechanisms of bone diseases, cell culture, and organ-on-the-chip models, as well as small fish are better suitable. Refinement and validation of screening results then have to be carried out in larger animal models such as rodents or larger mammals or humanized systems. This allows the application of these assays for drug testing to discover novel products to treat common and rare bone diseases.
Table 1:
Advantages and limitations of various models for skeletal research
| Model | Advantages | Limitations |
|---|---|---|
| In vitro Cell culture (2D, 3D, Screens) | • Ethically preferable to animals • Allows for analysis of isolated and relatively homogeneous cell populations • Facilitates analysis of molecular pathways • Cheaper than most animal models • Amenable to high-throughput screens • Human cells allow for the modeling of patient-specific genetic variants |
• No true bony matrix formation, innervation, vascularization, or remodeling • Requires serum derived from animals (alternatives very expensive) • All in vitro models (2D, 3D, organ-on-a-chip) require the use of animals as a source of primary cells as existing cell lines do not recapitulate all the properties • 3D systems are still in a phase of development • Development and validation take years • Assays often have issues of reproducibility between laboratories • Requires specific artificial growth factors and media for each cell lineage • Multi-tissue interactions difficult or impossible to model • Sexual differences are difficult to assess accurately |
| In Silico (data-driven & knowledge-driven modeling) | • Data-driven: Genome and RNA sequencing is increasingly being employed for diagnosis and mechanistic understanding of genetic diseases. • Data-driven: high-throughput & using human samples • Knowledge-driven: allows integration of available knowledge into a coherent framework • Knowledge-driven: allows testing of hypotheses that are financially or ethically not feasible to test in vivo • All: in silico screening allows for better planning of subsequent experiments |
• Requires precise input (unknown variables as cofounders) • Rigorous credibility analysis requires high-quality physiological data • Development and validation take years |
| Fish models | • Reliably model many aspects of human skeletal development and diseases • The Zebrafish genome contains orthologues of approx. 70% of human genes (up to 82% of disease-related) • A large number of preexisting mutant and transgenic lines and efficient methods for transgenesis and gene editing • Some teleosts (e.g., killifish) have a short life span of only a few months making it attractive for the study of skeletal aging • Transparent ex utero development allows for in vivo imaging of osteogenesis to a • Small body size allows for high-resolution imaging and characterization of the entire organism including the skeleton • Mutations linked to human disease shown to alter bone quality markers similar to humans • Small size and low cost facilitate genetic and chemical screens • Microgravity can be modeled as well despite aquatic environment • Killifish have a strong sexual dimorphism |
• Differences in bone architecture (e.g. no osteonal bone remodeling present in zebrafish) • Investigation of endoskeletal fracture healing is limited in small-sized teleost models • Some (patho)physiological processes that arise in bone marrow (e.g., edema) cannot be assessed in teleost models as hematopoiesis takes place in the kidney • Limited analysis of Calcium dietary effects (uptake through gills) • water conditions (e.g. temperature, pH, salinity, mineral composition, exchange rate) play a major role during skeletal development and maintenance and need to be controlled • osteocyte-specific factors cannot be investigated in some teleosts with anosteocytic bone, such as medaka or killifish • No bone marrow so no possibility to analyze the same cell interactions between bone and BM progenitors as in mammals • Bone growth continues with age similar to mice |
| Murine models | • Genes/mechanisms discovered in mice as important for the formation, degeneration, and repair of the musculoskeletal system are generally conserved in humans • Morphophysiologically similar to humans • A large number of preexisting mutant models and transgenic lines • Efficient genetic tools for the creation of new mutant lines • Well-characterized models of bone diseases, adaptation to loading, and healing • Lower cost and larger numbers of offspring compared to large animal models • Mature quickly allows a large variety of genetic and pharmacological studies with enough power for solid interpretations. |
• Different ambulation type compared to human • No standard validation procedure • Different metabolic activity • Many Cre lines are not as specific as previously believed • Lack of osteons (Haversian system) in cortical bone • Bone acquisition and longitudinal bone growth continue in mice and rats after sexual maturity. • Lack of natural menopause |
| Ectopic models | • Can prevent having to create large bone defects in an animal • Crucial stages of bone formation/healing (inflammation, vascularization, osteoinduction) can be recapitulated in ectopic locations without confounding factors • Possible to implant up to 6 small implants on the back of a mouse or rat, thereby allowing inter-animal variability to be addressed |
• No osteoconduction from the surrounding bony environment • No physiological loading |
| Large animal models (Sheep) | • Many new therapeutic approaches, such as new implants, biomaterials, and surgical procedures can only be tested in large animals due to their bigger size • Mimic the human situation in terms of bone dimensions, structure, and turnover, as well as mechanical loading conditions • Essential for the development of new surgical techniques, orthopedic implants, the development of novel biomaterials, and tissue engineering approaches • Metaphyseal fractures. • Similar bone formation rate and fracture healing in humans |
• The difficulty of genetic engineering • Ethical considerations • High costs for animal acquisition and maintenance and limited obtainability of skeletally mature or aged animals in sufficient numbers • limited availability of transgenic organisms and specific analytical tools (e.g., antibodies) • Application of drugs in an experimental setting can be expensive due to the high body weight • Importantly, compared to humans, sheep have a much higher bone mineral density and higher mechanical strength • Seasonal changes in bone metabolism in sheep • The different gastrointestinal system of sheep as ruminant animals impedes the oral administration of drugs. • Ovariectomy (OVX), which is commonly used in rodents to mimic postmenopausal osteoporosis, seems to be less effective in sheep |
In vitro models for skeletal research
Cell culture models
Two-dimensional (2D) tissue culture models have dominated bone research for half a century. Calvarial pre-osteoblast cells, stromal mesenchymal cells from bone marrow, and outgrowths from human bone specimens can be utilized to recapitulate osteoblast differentiation27–31. Osteoclasts can be derived from peripheral blood monocytes or bone marrow of rodents, birds (mainly chickens), and rabbits to allow observation of their development32–38. Osteocytes are a challenging bone cell type in culture but particularly the development of cell lines has allowed for the investigation of some osteocyte aspects in vitro (for a review of in vitro and in vivo osteocyte models see39). Induced pluripotent stem cells (iPSCs) have recently become another prospect for bone regeneration and disease modeling40.
A wide variety of in vitro screening approaches have been employed to identify potential regulators of bone cell differentiation41–45. For example, high-throughput siRNA41,42 and compound screens have identified small molecules such as roscovitine, rapamycin, and FK506, which augment osteoblast differentiation in vitro43. These approaches allow us to reduce animal experimentation by testing potential drugs at a cellular level. As such, only successful drug targets might be exploited in animal models, limiting and specifying the use of animals. Despite successes in identifying anabolic and catabolic factors, strong limitations are obvious. The readout of early differentiation markers such as alkaline phosphatase for example is not always predictive for the entire process of bone formation.
One of the major limitations of 2D cell culture is the poor equivalence of this environment to the conditions within the bone46. There are numerous attempts now to combine multiple cell types in vitro and to increase the levels of complexity. For example, several research groups include hypertrophy and mineralization regimes in their in vitro cultures47,48 as well as altering pH and oxygen tension49,50. The highly mineralized nature of bony tissue, in addition to anatomic structure, means that plastic or other planar surfaces are a poor environmental proxy. Importantly, these cultures are unable to maintain the phenotype of osteocytes and osteoclasts for more than several weeks. As a consequence, much work has focused on the optimal culture of osteoprogenitors and osteoblasts on these surfaces. However, recruitment and control of osteoblasts in the human body also rely on the presence of other cell types, with e.g. EphA451 from osteoclasts stimulating osteoblast formation. Osteocytes are by far the most abundant cells in bone and orchestrate osteoblast and osteoclast function, yet they are missing in most 2D cultures. Another major drawback comprises the altered behavior and differentiation capacity of isolated primary cells in culture (e.g. calvarial cells differentiating into adipocytes).
To overcome these limitations, bone organ cultures are used, where harvested bone tissue is placed in a nutrient-rich medium allowing it to remain viable and functional for several days to weeks52–54. These cultures were used to study processes including bone growth, remodeling, and repair52,55,56. Here bone cell interaction can be studied in the absence of confounding factors present in vivo52. Furthermore, treatment of the organ culture with substances instead of the whole organism increases animal welfare. However, the tissue may change in response to the stress of being removed from the organism. Some limitations can be overcome by complex co-culture models which include multiple cell types to mimic, among others the remodeling of the tissue57, immune responses to the differentiation processes58, vascularization, and even the formation of a bone marrow niche59,60. Nevertheless, the use of isolated bone tissue may not capture the complex interaction between different cell types and tissues in vivo.
Further developments extend to three dimensions using scaffold structures. But even in “3D” foams61 the cells do not receive mechanical cues from all directions. To receive a continuous mechanical 3D stimulus e.g. a fibrin matrix, attached to a sparingly soluble calcium phosphate bracket has been used62. Here embedded osteoprogenitor cells differentiated and maintained an osteocyte phenotype for over a year and even produced a lacuna-canalicular network. Similar results can be achieved with fibroin63. These principles have allowed the development of tricultures that maintain the phenotype of osteoblasts, osteoclasts, and osteocytes and have successfully been used to explore the influence of unloading on the bone resorption process64. This system has also allowed new insights into osteoid deposition and mineralization and may enable researchers to focus on individual variables of bone remodeling, which is not feasible in vivo. Such models may be harnessed in screens with more variables and repetitions than would be ethically appropriate in vivo. Biological processes are also more easily controlled and monitored in these cultures than in vivo. While it is accepted that these systems still require significant development to introduce an immune and neuronal component, vasculature, and surrounding tissue to properly replicate the in vivo environment, it is clear that the combined use of modern 3D cultures with in vivo models will serve to reduce animal use and help refine both in vitro and in vivo models.
Organ-on-a-chip (multi-channel 3-D microfluidic cell culture platforms that simulate the mechanics, activities, and physiological response of an entire organ) while in their infancy in the bone field will soon very likely advance in vitro bone models making them more attractive for basic and preclinical research21,22.
While it will be several years before some of these technologies and intricate in vitro models will be sufficiently developed to replace in vivo models, some are already appropriate for more simplified experiments, such as drug toxicity screening. For characterization and discoveries of new structures in bone in vivo (e.g. innervation, blood, and lymphatic vessels) in vitro models are not suitable since they rely on that what is known from the in vivo situation. However, researchers are already performing more experiments in vitro and in silico before progressing into in vivo models. This trend will only continue to grow but may never entirely replace the intricacies of animals due to the reasons mentioned above.
In silico models for skeletal research: state of the art
Over the last decades in silico computer models and simulations (referring to ‘silicon’, the main component of computer chips) have increasingly emerged in the biomedical sciences. Bone research and disease management frequently use in silico tools though they might not always be perceived as such. An example is MRI images where a physics-based model is required to transform the raw data into interpretable images. Different in silico models allow virtual testing of biological hypotheses. Data derived from these models is quantitative and unlike many biological experiments not limited to single end-points. This data can provide insight into underlying mechanisms with an accuracy not possible in biological experiments and can describe outcomes and numerical values previously unknown65.
A plethora of bioinformatics and machine learning (ML) tools are now available for processing the enormous amount of data generated by omics and sequencing initiatives (e.g. GWAS, see also Box 1). These data-driven models allow insight into basic biology as well as clinical variation. One example is the development of the skeletal cell atlas66 bringing together all available murine single-cell RNA sequencing data sets into a comprehensive framework, making use of open-source bioinformatics codes. This atlas provides a physiological blueprint of the limb development process and a platform to investigate in silico e.g. the effects of genetic mutations and knockouts. Using Boolean modeling, additive modeling or other systems biology technologies allows us to turn these blueprints into actionable gene regulatory models. These models can then be used to perform extensive in silico screening experiments to identify optimal culture conditions (medium composition) or possible (combinations of) druggable targets in skeletal diseases67. Eventually, these targets will then have to be tested in vitro and/or in vivo. Agent-based models that simulate actions and interactions of autonomous agents, such as individual molecules or bone cells have been used to investigate different drivers for limb bud outgrowth including the combination of differential cell adhesion and elasticity to determine limb bud shape68 or filopodial tension to explain the convergent extension of limb bud tissue69. These models may be utilized to predict certain outcomes of knockout animal experiments and might thereby restrict the generation of knockout experiments to those that are likely to exhibit a clear function of a gene product in an in vivo environment.
Explanatory Boxes.
Box 1. GWAS in the bone field
Genome-wide association studies (GWAS) involve scanning genetic markers across the whole genomes of individuals within large cohorts to identify genetic variants associated with disease-related traits. Over the past decade, GWAS have identified over 500 genomic loci harboring genetic variants associated with bone mineral density (BMD), as well as eBMD, a correlate of BMD measured via ultrasound94–96. The majority of BMD-associated variants are non-coding. Most likely some or even most causal variants reside in cis-regulatory elements that alter the expression of protein-coding genes in their proximity. Importantly, genes at BMD-associated loci include key members of pathways targeted by osteoporosis therapeutics, suggesting that other actionable, unidentified drug targets reside at other loci. Because the biological mechanisms underlying BMD associations may involve developmental, mechanical, autocrine, and paracrine factors that are difficult to model in vitro, gene discovery in animal models is a critical approach to identify causal genes underlying GWAS variants, and therefore for the translation of GWAS findings into clinical targets.
Tissue-level models describing bone (patho)physiology using partial differential equations are currently the most prevalent type of modeling in the bone field with applications ranging from limb development over fracture healing to bone remodeling. An example of a successful model predicted neotissue growth in 3D printed scaffolds70. This model was developed starting from the curvature-based growth principle and subsequently confirmed by in vitro experiments. It was then used to optimize the structure of a 3D printed scaffold for maxillofacial applications and tested in an in vivo rat model, confirming the superiority of the in silico-designed scaffold over the clinical golden standard71.
At the level of the whole organism, gait models are a standard tool to assess mechanical loading in muscles, joints, and bones in animal experiments as well as clinical studies72. Finally, in silico clinical trials can be used to plan, augment or replace physical trials. As an example, testing new drugs for osteoporosis using fractures as primary clinical endpoints requires thousands of patients followed up over many years. The Biomechanical Computed Tomography (BTC) provides a surrogate endpoint calculated using quantitative CT images and continuum mechanics73,74. The BTC is a good predictor of the patient’s bone biomechanical strength (and hence fracture risk) and its use, once approved by regulators, will allow for smaller cohort sizes.
In vivo models for skeletal research: state of the Art
Teleost fish models
Preclinical bone research is carried out in in vivo models. In contrast to established rodent models in skeletal research, small-sized laboratory teleost models (i.e. bony fish models) have only emerged over the last few decades. This was driven by sequencing of the zebrafish genome which allowed for a greater understanding of its similarity to that of humans and other vertebrates75. Due to evolutionarily conserved genetic, developmental, and compositional similarities between bones of teleosts and mammals, not only zebrafish19,76–79 but also medaka are used to model skeletal diseases and test drug targets11,19,76–81. The diversity of laboratory teleost species offers additional advantages in the scope of both intervention studies and studies of aging-related bone degeneration. Killifish with a lifespan of up to 6 months for example might reduce time-intensive experiments in longer-lived species82,83. Moreover, due to the transparency of teleosts up to early larval stages, they are commonly used in concert with transgenic modifications for direct visualization of the effects of genetic or chemical perturbations on osteoblast and osteoclast differentiation, skeletal morphogenesis, and mineralization84–86. Due to the small size even in adulthood, teleosts are amenable to high-resolution, whole-body imaging which enables deep phenotyping at a large number of skeletal sites87.
Despite teleost fish being suitable organisms to study many aspects of bone development, matrix mineralization, modeling and even the effect of microgravity88 there are some important differences to humans, highlighting their limitations (Table 1). The bone matrix of some species, e.g. medaka and killifish, is anosteocytic, limiting their use for modeling diseases affecting the osteocyte-lacunar network. Moreover, the mammalian bone remodeling process is not directly comparable to small-sized teleosts which have a different bone micro-architecture (e.g. lack of an osteonal cortex and extensive trabecular compartments). Given the aquatic environment of fish, further species-related differences in terms of anatomy, mineral uptake, and musculoskeletal loading apply. In general, biological mechanisms discovered in zebrafish should also be examined in mammalian models to assess their translational potential.
Genetic zebrafish models
As an experimental system, zebrafish are positioned between in vitro and in vivo models, combining the ability to perform experimental manipulations traditionally confined to cell culture, with the complex physiology of an intact organism. Due to their small size, low cost, and ease of genetic manipulation, zebrafish are well-suited for forward and reverse genetic screens. For zebrafish, there is a powerful toolbox including CRISPR-based gene editing89,90, the broad availability of mutants91, and resource centers such as the Zebrafish International Resource Center (ZIRC) and European Zebrafish Resource Center (EZRC). The pursuit of these reverse genetic screens could augment and advance ongoing systematic knockout mouse phenotyping projects such as the international mouse phenotyping consortium IMPC79,92,93 and ancillary bone phenotyping projects94–96 e.g. by prioritizing screened genes and enabling more effective use of resources79. Zebrafish screens could significantly aid follow-up functional studies to genome-wide association studies (GWAS) or sequencing studies by allowing the generation of mutant phenotype data specifically for genes at loci harboring trait-associated variants.
Models for several human genes whose mutations are linked to rare and complex skeletal genetic conditions have been established in zebrafish, e.g., Plod2 (Bruck syndrome)97, col11a2 (early-onset osteoarthritis)98, col1a1a, col1a1b, and col1a2 (osteogenesis imperfecta)99, lrp5 mutants (osteoporosis-pseudoglioma syndrome)100,101, enpp1 (ectopic mineralization)102, and kif6 mutants for modeling spinal curvature disorders103. These and numerous additional models have highlighted the value of teleost models for elucidating the pathophysiology of genetic skeletal diseases and mineralization defects19,104,105. Zebrafish mutants have evolved as particularly powerful tools for screening the effects of drugs and osteoactive compounds on the skeleton11,106,107. Molecules may be administered through the aquatic environment without the need for injections. For instance, submerging embryos of the Chihuahua zebrafish model of osteogenesis imperfecta in water containing 4-Phenylbutyric acid (4-PBA) resulted in ameliorated bone mineralization in larvae, and long-term treatment reduced skeletal deformities108. Such in vivo approaches in zebrafish allow efficient and direct assessment of systemic effects and are helpful to accelerate the search for treatment options.
The need for zebrafish to support human genetic research will grow with continuing advances in whole genome sequencing (WGS) and associated genetic studies, which discover candidate pathogenic variants at rates exceeding our ability to analyze their functions.
Rodent models
More than any other in vivo model, the use of rats and mice is a critical pillar of skeletal research and drug development. It was indeed the advent of reverse genetics in rodents that allowed researchers to decipher important factors for bone growth and remodeling in a systemic context109. One prominent example is the discovery of RANKL during osteoclastogenesis: Pivotal studies in genetically engineered mice with altered expression of the RANKL/OPG system demonstrated its influence on osteoclast formation and bone physiology110–112. Based on these fundamental discoveries the antibody Denosumab against RANKL was developed as a rational treatment for osteoporosis113,114. Another success story includes the development of Romosozumab (anti-Sclerostin)115–120. Human GWAS candidates like Wnt16121–124 and RSPO3125,126 involved in bone density have also been validated in mouse models.
For 441 known genes where mutations cause human genetic skeletal disorders, at least 260 mouse models have been phenotyped (coverage of 59%)127. Additionally, there are standard models for almost all bone diseases in rodents and other vertebrates, such as postmenopausal and age-related osteoporosis, diabetes-induced bone loss, inflammatory arthritis, and fracture healing. All of these resemble the human diseases in characteristic aspects.
In skeletal research, rats have been a preferred model for post-menopausal osteoporosis induced by ovariectomy/orchiectomy, steroid-induced bone loss, dietary interventions (e.g. low calcium), immobilization (by surgery or tail suspension)128, jaw osteonecrosis129 and fracture healing130. Molecular factors involved in human bone (re)modeling are generally well conserved in rodents so most genetic skeletal diseases can be recapitulated in mice127. Mice models however have limitations as growth plates do not necessarily close like during skeletal maturation in humans. They further do not show natural menopause and ovariectomy might not reflect this entirely, which resembles an acute loss of estrogen in contrast to the slower decline of estrogen in natural menopause. The rodent cortical bone also does not harbor Harversian channels, and their quadruped gait leads to different loading of the axial skeleton. With increasing evidence of links between whole-body metabolism and skeletal biology131, it is also necessary to keep in mind that mice have a faster metabolism and are often not raised under thermoneutrality.
Genetic Mouse models
The emergence of reverse mouse genetics and the possibility of global gene inactivation in “knockout” mice (KO) or the introduction of specific mutations in “knock-in” (KI) mice have contributed immensely to our knowledge of bone formation and treatment of monogenic skeletal diseases. Successful examples include the various forms of chondrodysplasia (for which antagonists of activated FGFR3 or downstream pathways are now clinically tested), hypophosphatemia (now successfully treated with a recombinant form of alkaline phosphatase), fibrodysplasia ossificans or osteogenesis imperfecta132–134. In the most recent IMPC data release (17.0), bone mineral density data in knockout mice are available for about one-quarter of the protein-coding genes (7,141 out of ~25,000–30,000). After characterization at the basal level, the skeletal phenotype of transgenic rodents may be combined with additional challenges such as ovariectomy (OVX) or systemic glucocorticoid (GC) treatment to mimic post-menopausal or glucocorticoid-induced osteoporosis.
Although global KO mice are still an important tool in skeletal research, their usefulness may be limited by embryonic lethality, or by the inherent limitation of phenotypes caused by embryonic global gene inactivation. The latter hinders the interpretation of the phenotype at postnatal adult stages by having to tease apart the primary and secondary effects of gene modification. These drawbacks can be avoided by a conditional gene knockout approach (cKO). Based on the “Cre-LoxP” system of gene recombination, this approach allows deletion or expression of a gene in a temporospatial manner135 (Box 2). The remaining limitations stem from the irreversible nature of the gene ablation and the incomplete knowledge of which cells exactly express the Cre-recombinase under selected promoters136,137 The increasing number, availability, and lack of characterization of Cre lines have raised concern about their authentication, but solutions to this concern are being addressed138. Efficient breeding strategies can limit the number of mice. To limit animal numbers scientists commonly collect multiple tissues and power analyses are used to calculate necessary animal numbers for the most efficient outcome.
Box 2: Cre-loxP System.
The Cre recombinase was first discovered in the bacteria P1 phage. Since introducing it into transgenic modified mice in the 90ies it has become an extremely powerful tool to allow conditional loss- and gain-of-function studies, including lineage tracing. This requires a “Cre” mouse line in which the Cre-recombinase is expressed under the control of a chosen promoter active in a relatively specific population of cells or tissues. This enzyme once expressed and active, can recognize bacteriophage-derived “LoxP” sequences inserted into the genome of a second mouse line, generally in the 5’ region of a gene to be inactivated, cut the sequence in between, and join the two generated DNA ends to effectively delete the sequence between two LoxP sites. Crossing these two mouse lines leads to mice where gene inactivation occurs in all cells where the Cre-recombinase is expressed and active (and their progeny as this genomic event is not reversible). Some “Cre” lines express modified forms of the Cre-recombinase that are inactive but can be activated following injection of an inducer, thereby rendering the system inducible and allowing spatiotemporal control of gene inactivation.] In the most widely used tamoxifen-induced system (CreER(T2)) the Cre-recombinase is fused to a mutated hormone-binding domain of the estrogen receptor and can be activated by binding to tamoxifen, thus allowing translocation of the cytoplasmic Cre into the nucleus and recombination. In another approach, Doxycyclin (Dox) can be used In transgenic tTA (Tet off) mouse models to prevent Cre transcription e.g. in unborn mice by treating pregnant females. (Repeated) withdrawal then leads to a (or several) defined time window(s) of gene deletion. Less frequently a reverse tTA (rtTA) is used for a Tet-on approach that activates recombination upon Dox treatment.
This Cre-loxP strategy also allows, thanks to the use of the Rosa26 LoxP-STOP-LoxP allele, the expression of normal or mutated or fluorescent genes in a specific tissue or cell lineage and an inducible manner. This way, cell tracing can be performed, thus allowing one to follow the fate of specific cells during development, aging, or diseases, or to assess the functional consequence of mutated genes in specific cell populations. This system can also be used to ablate specific cell populations by controlling the expression of “suicide” genes in selected cell populations
Through the deletion of specific genes in restricted cell lineages, cKO mouse models can provide important information about interactions within and between bone cells and other tissues. cKO mice models combined with lineage tracing were pivotal in discovering the fate of skeletal stem cells, their diversity139,140, and their importance in bone healing141. Mouse models also enabled the discovery of a distinct type of blood vessels142, and more recently lymphatic vessels important for regeneration after bone injury143.
Ectopic Bone Models:
Human cell transplantations are often performed on matrix structures to develop ectopic sites for bone. Rodents, in particular mice, are employed to model bone formation and bone physiology with human cells while avoiding tissue culture artifacts (e.g. the absence of vasculature and innervations). These humanized structures can be used for drug testing, but also basic research questions since they recapitulate all stages of bone development or regeneration (see Box 3). Furthermore, ectopic bone formation offers an approach to investigate the osteogenic potential of molecules or biomaterials needed to assist in bone healing or augmentation.
Box 3. Ectopic Bone models.
Scientists employ ectopic models to assess several aspects of bone formation and repair processes, especially when assessing new approaches/materials/small molecules in the tissue engineering and regenerative medicine (TERM) field. There are instances where it can be desirable to use an ectopic location to test the osteoinductive properties of a drug/small molecule or a biomaterial194. Given the correct stimuli, it is possible to recapitulate almost all aspects of bone formation in an ectopic location. The most commonly used locations include the kidney capsule, intramuscular locations, and subcutaneous implantation. The benefits of the placement of a construct/drug under the fibrous outer kidney capsule where it undergoes spontaneous bone formation, are the high level of vascularization, lack of endogenous bone-forming cells (which can be useful when assessing the biology of a specific cell type or osteoinductive factor), and some level of the mechanical load caused by compression of the fibrous capsule. However, the model is technically challenging and invasive and is not commonly used195,196.
Intramuscular implantation of cells, biomaterials, and various growth factors/small molecules are often used as an ectopic model of bone formation. While this model does not involve the creation of a defect it is still relatively invasive given the requirement for some level of blunt dissection to implant a construct and/or the space required within the muscle for new bone to form. One advantage of this model compared to other ectopic models is the presence of a source of cells that have the osteogenic capacity, the satellite cells. These cells can for example undergo osteogenesis in the presence of BMPs197,198. By far the most commonly used model of ectopic bone formation is the subcutaneous implantation of various constructs to assess bone formation. In this scenario, all manner of biomaterial, in combination with various cell types and small molecules can be assessed for their ability to induce bone formation in the absence of surrounding bone-forming cells or a bony environment199–201. This can be very useful to remove any confounding effects of endogenous factors or cells on the experimental outcome. The model has further advantages of being minimally invasive and simple to perform. While these models do not contain the relevant functional mechanical loading component, it may be argued that neither do many long bone defects, due to the need for fixation/stabilization of the defect for reasons of animal welfare and to allow healing to occur. Subcutaneous bone models are also being used in the field of oncology, where researchers are actively developing new models of bone metastasis using humanized models of bone formation to create more clinically relevant models202–204
Others have shown a role in the process of endochondral ossification in atherosclerosis in mice205. What all of these models have in common is that the host animal provides a vascular supply and all of the requisite cells required for bone formation and remodeling to take place. This cannot occur in in vitro systems.
Finally, to bring the bone environment to the ectopic models, Andres Sastre et al. have developed a “semi-orthotopic” bone defect model that incorporates a viable bovine bone plug in a subcutaneous pocket of a nude mouse206. This allows for the creation of several bony defects within this implanted bone plug in one animal without the need to perform invasive surgery on the animal, thereby having several defects an order of magnitude larger in volume than could be created in a mouse femur. Whether this approach might be used to replace some of the existing experiments that would be performed in orthotopic and ectopic settings remains to be seen. In general, a lot of skeletal research is based on previous cultivation and characterization of bone cells, these may stem from cell lines or primary human cells differentiated or cultured ex vivo. The models described above can be applied in a huge range of manners that address questions surrounding bone development and healing as well as the formation of the marrow niche. They are still necessary and are now being recognized as very relevant for multiple models of cancer metastasis and leukemia and also have great utility in understanding diseases of heterotopic ossification. Subcutaneous models have the advantage of being “ectopic” and therefore are not influenced by a surrounding bony environment. This allows the testing of the true osteogenic potential of a cell, small molecule, or biomaterial without interference from the surrounding tissue. By contrast, the inclusion of a bony environment in a subcutaneous pocket brings the advantage of being able to interrogate crucial steps of bone healing such as integration and remodeling which is not possible in most subcutaneous models.
For implant testing the osteoinductive properties of materials such as calcium phosphate ceramic207 or β-tricalcium phosphate scaffolds208 can also be tested ectopically in large animals for example in sheep muscle. Also, auto-transplanted mesenchymal stem cells were able to induce bone formation in a ceramic bone substitute in sheep without the additional need for BMPs209.
Large animal models
Larger animals such as rabbits, dogs, pigs, small ruminants (sheep, goats), and non-human primates, are less frequently used in skeletal research130,144–146. The main reasons are ethical considerations. High costs, the limited obtainability of skeletally mature or aged animals, and the time-consuming breeding and experimental effort also restrict the use of large models. Furthermore, the evaluation of molecular mechanisms is restricted due to limited transgenic organisms and specific analytical tools (e.g. antibodies). Also, the application of experimental drugs in large animals can be expensive due to their body weight. Despite these disadvantages for basic molecular research, large animals are of utmost importance in translational settings because they most closely mimic the human situation in terms of bone dimensions, structure, and turnover. Therefore, they are particularly important to advance surgical techniques, orthopedic implants, fracture fixation devices, novel biomaterials (e.g. bone grafts), and tissue engineering approaches, and to investigate fracture healing16,130,144,146. A further advantage of large animal models is that the impact of mechanical loading can be considered which is not possible in mice or rats but important for bone remodeling, the osseointegration of implants, and degradation of biomaterials147.
After mostly abandoning dog models25,148 sheep have proven particularly appropriate for skeletal research due to bone dimensions similar to humans and ease of husbandry16,25,149–152. However, their bone microstructure differs in some aspects148,153,154. The cortical bone of young sheep is plexiform due to their fast body growth. With age, secondary Haversian bone becomes more prevalent148,150. Importantly, compared to humans, sheep have a higher bone mineral density and mechanical strength152,155, which must be taken into account in experimental settings, especially for implant testing or induction of osteoporosis. However, the bone formation rate of sheep (1.2– 1.5 mm/day) is similar to that of humans (1.0–1.5 mm/day)148,149 as is the healing rate of bone fractures148,156. Also, common human bone turnover markers, including alkaline phosphatase, osteocalcin, and tartrate-resistant acid phosphatase, are useful to monitor bone status in sheep157.
Sheep are often used to test anti-osteoporotic drugs, treatments for fractures, or the efficacy of implant fixation149,151,152,158. Ovariectomy (OVX) models only lead to transitional and moderate bone loss152,159–161 and were therefore combined with a calcium and vitamin D-restricted diet162,163 and with additional glucocorticoid (GC) treatment152,161,162. GC application in sheep leads to considerable bone loss, structural deterioration, and biomechanical impairment comparable to GC-induced osteoporosis in humans and has therefore been most widely used16,152,161,162. However, GC therapy can provoke major side effects depending on the treatment regime, such as susceptibility to infections and discomfort, raising ethical concerns164.
One disadvantage of sheep as an osteoporosis model is that there are seasonal changes in bone metabolism165. Another drawback is the different gastrointestinal system, which impedes the oral administration of drugs.
Nonetheless, any osteosynthesis devices and treatment strategies were evaluated in sheep under clinically relevant conditions25,166,167. Furthermore, osteoporotic fractures in metaphyseal regions can be modeled in this species which is difficult in small animals130,168,169. Other sheep models to study centrally induced bone loss have been developed152,158,170–173. One example is the study from Bindl et al., that analyzed metaphyseal fracture healing after surgical disconnection of the hypothalamus and pituitary gland resulting in delayed bone formation, as it is often observed in osteoporotic patients174. These complex models lead to a considerable decline of bone mass158,172 and closely resemble the human situation170,171,174 but are challenging and raise similar ethical questions as the GC therapy.
Besides sheep, non-human primates and species like rhesus macaques, M. mulatta, or Papio ursinus have been used for bone research175–177. For ethical reasons, these experiments are not possible in Europe. Several successful approaches highlight the translational potential of these models, for example in demonstrating that alcohol is a risk factor for osteoporosis after HIV infection175. Non-human primates have also been used to test biologicals for osteoporosis treatment or fracture healing178,179. Particularly in translational bone research, non-human primates are the most accurate model in terms of bone metabolism and structure.
In summary, large animal models are indispensable for specific skeletal research questions, because they can facilitate the process from bench to bedside. A great drawback, the difficulty of genetic engineering, could be overcome in the next future. Indeed the first CRISPR/Cas9 transgenic sheep model for hypophosphatasia was recently generated and recapitulates dentoalveolar defects reported in humans180.
Discussion
Currently, scientists working with animal models in skeletal research are faced with fast methodological progress and rapidly changing legislative and ethical societal landscapes in which to navigate. The relatively new ease of genetic manipulation on the one hand and increased awareness of a reproducibility crisis and demands for improved animal welfare calls for an assessment of current animal experimentation and guidelines that help scientists to conduct their research as sensibly and effectively as possible and boost translation.
Standards for animal experiments in skeletal research
Around the time of the early bone cell cultures, in 1959, Russel and Burch published a milestone for humane animal research and urged the implementation of the 3 Rs (Replace, Reduce, Refine)181. It has become clear, that these 3 Rs are not enough and the data from these experiments also needs to be Robust, Registered, and Reported (6 R) to ensure scientific value182.
Since the EU Directive 2010/63/EU on the protection of animals used for scientific purposes took effect in 2013, the transition into the labs has been slow. The 3R-based directive has a wider scope and defines strict regulations on housing standards, experimentation, and care. Granted experiments require strict minimization and assessment of pain and distress and categorization of experimental severity. Furthermore, regular risk-based inspections are carried out and transparency is improved through retrospective assessments and the publication of non-technical project summaries for the lay public. Still, the legislation on animal experiments has not changed as much as the general awareness of the issue. It is therefore important to point out that granting of the planned experiment by the local authorities ensures legal safety and strict ethical regulation. Transparency protects from legal charges, and careful experimental planning with meticulous documentation ensures scientific value. Nevertheless, the limited flexibility of granted experiments and increased time demand for application and documentation result in ethical problems of their own. Mendelian ratios and gene manipulation effects may dictate a greater number of animals being born than will be used for the actual experiment. The euthanasia of (surplus) animals should only be the result of a careful breeding plan and evaluation of possible alternative uses183. Before planning animal experiments, scientists have to justify which species and strain, model, and tissues to analyze as well as the sex, age, and other treatments affecting mineral and hormonal homeostasis of the bone such as mechanical strain and nutrition. In line with ethical regulations, projects are under constant species-specific animal care supervision and subjected to scientific and ethical approvals. There is thus currently an efficient system in place to guarantee the ethical and minimal usage of animals for research.
One major step towards improving animal research and giving scientists some guidelines came with ARRIVE in 2010: a list of 20 recommendations for correct reporting of small animal experiments including, among others, study design, sample size, inclusion/exclusion criteria, and randomization. These criteria were developed in consultation with scientists, statisticians, journal editors, and several public or federal funding agencies184. Despite positive resonance, these guidelines are not comprehensively followed, although more journals now require authors to refer to them. Ten years later, ARRIVE has been revised to further facilitate its use185. Other initiatives such as the European Quality in Preclinical Data (EQIPD) aim at improving the planning stage of research186,187. Preregistration of experiments to increase liability and visibility is also increasing.
For the bone field, Manolagas and Kronenberg proposed several still valid points to overcome the reproducibility crisis in 2014188. They urged scientists and journals to endorse and use guidelines for the reporting of small animal skeletal phenotypes to ensure consistency and reproducibility (currently for animal experiments the ARRIVE (2.0) guidelines (2020)185, for bone histomorphometry the ASBMR nomenclature guidelines (2012)189, for µCT the JBMR (2010) guidelines190 as well as the ASTM standard F2721 (2014) for segmental bone defects191). The authors also advocated an increased availability of statistics and data reporting courses and encouraged the field to not shy away from vigorous scientific debate. In grant applications and manuscripts, greater emphasis was asked to be placed on scientific premises and rigor, instead of novelty and publication speed.
In addition, we believe that animal models should prioritize clinical targets for functional studies. Further important improvements towards the 6R pledge include developing well-annotated, accessible reference variant-phenotype databases, adopting phenotype description standards, data sharing, and systems to collect validated alternatives. Around 20% of all animal testing is performed for regulatory purposes192. Here, greater harmonization of the regulatory requirements between agencies of different countries can reduce required animal numbers.
Future developments for animal experiments in skeletal research
Non-animal methods (NAMs) are not a by-product of research. They require a clear vision, a dedicated development path, and a strategy for validation, standardization, and regulatory approval. Once alternatives are approved for drug testing, it should no longer be allowed to perform the corresponding in vivo experiments. This is currently the case in the toxicology community where organizations like the European Union Reference Laboratory for Alternatives to Animal Testing (EURL-ECVAM, for validation of non-animal alternatives)193 and the Organisation for Economic Co-operation and Development (OECD, for guidelines, harmonization, and good practice development) play an important role. Policy decisions and investments have allowed the development of new methodologies like serum-free cell culture, organ-on-a chip or advanced in silico modeling which will also benefit the bone field.
Nevertheless, recent discoveries using lineage tracing in genetically altered animals for the characterization of skeletal stem cells139–141, sub-types of vasculature 142,143, and lymphatic vessels demonstrate the value of the use of animal experiments for basic research that cannot be performed in in vitro models.
We believe it is obvious that the progress and success of skeletal research are highly dependent on the use of a variety of different models (Figure 1). Each model has advantages and limitations that are useful to be aware of. In vitro assays can provide answers in terms of cell signaling, gene expression, and cell behavior, but in an often non-physiological, artificial context with higher levels of oxygen and nutrients and without systemic context. In contrast, in the whole animal, cellular behavior is investigated within its physiological context and is thus more likely to represent biologically-relevant mechanisms. Furthermore, aging, a very important aspect of bone biology and health can be investigated realistically only in whole organisms. But the complexity at the structural, cellular, mechanical, and endocrine/paracrine levels makes it more challenging to correctly interpret phenotypes. In silico approaches can integrate data and/or knowledge acquired from in vitro or in vivo experiments and provide a computational framework to test biological hypotheses, run large-scale screening experiments, optimize treatment design, and perform in silico (clinical) trials. Computer models now allow the first predictions of loss- and maybe gain of functions of genes on cellular differentiation patterns in bone biology, providing an excellent tool to decide whether a genetic animal experiment is likely to reveal a phenotype and thus a functional explanation of novel factors in bone growth and physiology66. However, credibility assessment requires the availability of high-quality dedicated data that cannot be sourced sufficiently from the currently available in vitro models or human clinical studies. The goal can only be to use the best possible combination of methodology that involves scientific rigorousness and considers ethical awareness and restrictions to understand fundamental biology and develop advanced treatment options for bone diseases.
Acknowledgments
We are grateful to the members of the basic science action group of the European Calcified Tissue Society (ECTS) for critical reading of the manuscript, namely, Michaela Tencerová, Claudine Blin-Wakkach, Katherine Staines, and Sylvain Provot.
This work was supported by the 3R-Network BW to JT and by the German Research Foundation (CRC1149, project number 251293561, INST 40/492–3 to AI and JT and INST 40/682–1 to AI), and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Award Number AR074417 to RYK) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 772418 to LGe).
The authors would like to acknowledge that the figure was drawn using BioRender.com
References
- 1.Munro Chelsea. History in the Making! European Citizens’ Initiative Closes With 1,413,383 Signatures. History in the Making! European Citizens’ Initiative Closes With 1,413,383 Signatures https://www.peta.org.uk/blog/eci-closes/ (2022). [Google Scholar]
- 2.Gallup Ratings of U.S. moral values. Moral Issues https://news.gallup.com/poll/1681/Moral-Issues.aspx (2023). [Google Scholar]
- 3.Meredith W FDA no longer needs to require animal tests before human drug trials. https://www.science.org/content/article/fda-no-longer-needs-require-animal-tests-human-drug-trials (2023). [Google Scholar]
- 4.Shen Y et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 13, 882241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda DK et al. Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. 98, 7498–7503 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCaulao LE, Tozum TF & Rosol TJ Estrogen Receptors in Skeletal Metabolism: Lessons from Genetically Modified Models of Receptor Function. Crit. Rev. Eukaryot. Gene Expr. 12, 89–100 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Macias MP et al. Expression of IL-5 alters bone metabolism and induces ossification of the spleen in transgenic mice. J. Clin. Invest. 107, 949–959 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshizawa T et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 16, 391–396 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Bouillon R et al. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 29, 726–776 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray EJB, Murray SS, Grisanti M, Duarte MEL & Urist MR Effect of low dietary calcium on bone metabolism in the SENCAR mouse. J. Orthop. Res. 15, 585–592 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Bergen DJM, Kague E & Hammond CL Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds. Front. Endocrinol. 10, 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kangas L, Härkönen P, Väänänen K & Peng Z Effects of the Selective Estrogen Receptor Modulator Ospemifene on Bone in Rats. Horm. Metab. Res. 46, 27–35 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Black LJ et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J. Clin. Invest. 93, 63–69 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke HZ, Simmons HA, Pirie CM, Crawford DT & Thompson DD Droloxifene, a new estrogen antagonist/agonist, prevents bone loss in ovariectomized rats. Endocrinology 136, 2435–2441 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Kippo K et al. The effects of clodronate on increased bone turnover and bone loss due to ovariectomy in rats. Bone 17, 533–542 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Oheim R, Amling M, Ignatius A & Pogoda P Large animal model for osteoporosis in humans: the ewe. Eur. Cell. Mater. 24, 372–385 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Lv F, Cai X & Ji L An Update on Animal Models of Osteogenesis Imperfecta. Calcif. Tissue Int. 111, 345–366 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Mohamed FF et al. Dentoalveolar Defects of Hypophosphatasia are Recapitulated in a Sheep Knock-In Model. J. Bone Miner. Res. jbmr.4666 (2022) doi: 10.1002/jbmr.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonelli F et al. Zebrafish: A Resourceful Vertebrate Model to Investigate Skeletal Disorders. Front. Endocrinol. 11, 489 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y, Nizami S, Goto H & Lee FY Modern Interpretation of Giant Cell Tumor of Bone: Predominantly Osteoclastogenic Stromal Tumor. Clin. Orthop. Surg. 4, 107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadida M & Marchat D Strategy for achieving standardized bone models. Biotechnol. Bioeng. 117, 251–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelan IT, Moeendarbary E, Hoey DA & Kelly DJ Biofabrication of vasculature in microphysiological models of bone. Biofabrication 13, 032004 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Knopf F et al. Bone Regenerates via Dedifferentiation of Osteoblasts in the Zebrafish Fin. Dev. Cell 20, 713–724 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Geurtzen K et al. Mature osteoblasts dedifferentiate in response to traumatic bone injury in the zebrafish fin and skull. Development 141, 2225–2234 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Martini L, Fini M, Giavaresi G & Giardino R Sheep model in orthopedic research: a literature review. Comp. Med. 51, 292–299 (2001). [PubMed] [Google Scholar]
- 26.Sun D, Gao W, Hu H & Zhou S Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 12, 3049–3062 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger EH, Boonekamp PM & Nijweide PJ Osteoblast and osteoclast precursors in primary cultures of calvarial bone cells. Anat. Rec. 214, 32–40 (1986). [DOI] [PubMed] [Google Scholar]
- 28.Bjurholm A, Kreicbergs A, Schultzberg M & Lerner UH Neuroendocrine regulation of cyclic AMP formation in osteoblastic cell lines (UMR-106–01, ROS 17/2.8, MC3T3-E1, and Saos-2) and primary bone cells. J. Bone Miner. Res. 7, 1011–1019 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Soleimani M & Nadri S A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 4, 102–106 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Baustian C, Hanley S & Ceredig R Isolation, selection and culture methods to enhance clonogenicity of mouse bone marrow derived mesenchymal stromal cell precursors. Stem Cell Res. Ther. 6, 151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson KB, Frost A, Nilsson O, Ljunghall S & Ljunggren Ö Three isolation techniques for primary culture of human osteoblast-like cells: A comparison. Acta Orthop. Scand. 70, 365–373 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Ash P, Loutit JF & Townsend KMS Osteoclasts derived from haematopoietic stem cells. Nature 283, 669–670 (1980). [DOI] [PubMed] [Google Scholar]
- 33.Jee WSS & Nolan PD Origin of Osteoclasts from the Fusion of Phagocytes. Nature 200, 225–226 (1963). [DOI] [PubMed] [Google Scholar]
- 34.Manley MT, Kotzar G, Stern LS & Wilde A Effects of repetitive loading on the integrity of porous coatings. Clin. Orthop. 293–302 (1987). [PubMed] [Google Scholar]
- 35.Walker DG Enzymatic and electron microscopic analysis of isolated osteoclasts. Calcif. Tissue Res. 9, 296–309 (1972). [DOI] [PubMed] [Google Scholar]
- 36.Martini MC, Osdoby P & Caplan AI Adhesion of osteoclasts and monocytes to developing bone. J. Exp. Zool. 224, 345–354 (1982). [DOI] [PubMed] [Google Scholar]
- 37.Collin-Osdoby P & Osdoby P Isolation and Culture of Primary Chicken Osteoclasts. in Bone Research Protocols (eds. Helfrich MH & Ralston SH) vol. 816 119–143 (Humana Press, 2012). [DOI] [PubMed] [Google Scholar]
- 38.Kanehisa J & Heersche JNM Osteoclastic bone resorption: In vitro analysis of the rate of resorption and migration of individual osteoclasts. Bone 9, 73–79 (1988). [DOI] [PubMed] [Google Scholar]
- 39.Kalajzic I et al. In vitro and in vivo approaches to study osteocyte biology. Bone 54, 296–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csobonyeiova M, Polak S, Zamborsky R & Danisovic L iPS cell technologies and their prospect for bone regeneration and disease modeling: A mini review. J. Adv. Res. 8, 321–327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad M et al. Inhibition of Cdk5 increases osteoblast differentiation and bone mass and improves fracture healing. Bone Res. 10, 33 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad M et al. Cell-based RNAi screening and high-content analysis in primary calvarian osteoblasts applied to identification of osteoblast differentiation regulators. Sci. Rep. 8, 14045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darcy A et al. A novel library screen identifies immunosuppressors that promote osteoblast differentiation. Bone 50, 1294–1303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.AlMuraikhi N et al. Stem cell library screen identified ruxolitinib as regulator of osteoblastic differentiation of human skeletal stem cells. Stem Cell Res. Ther. 9, 319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuyasu S et al. Cell-Based Double-Screening Method to Identify a Reliable Candidate for Osteogenesis-Targeting Compounds. Biomedicines 10, 426 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beresford JN, Graves SE & Smoothy CA Formation of mineralized nodules by bone derived cells in vitro: A model of bone formation? Am. J. Med. Genet. 45, 163–178 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Freeman FE, Haugh MG & McNamara LM An In Vitro Bone Tissue Regeneration Strategy Combining Chondrogenic and Vascular Priming Enhances the Mineralization Potential of Mesenchymal Stem Cells In Vitro While Also Allowing for Vessel Formation. Tissue Eng. Part A 21, 1320–1332 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Freeman FE, Haugh MG & McNamara LM Investigation of the optimal timing for chondrogenic priming of MSCs to enhance osteogenic differentiation in vitro as a bone tissue engineering strategy: Optimal timing for chondrogenic priming of MSCs. J. Tissue Eng. Regen. Med. 10, E250–E262 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Díaz-Payno PJ et al. Gremlin-1 Suppresses Hypertrophy of Engineered Cartilage In Vitro but Not Bone Formation In Vivo. Tissue Eng. Part A 28, 724–736 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Lee PS et al. Recapitulating bone development events in a customised bioreactor through interplay of oxygen tension, medium pH, and systematic differentiation approaches. J. Tissue Eng. Regen. Med. 13, 1672–1684 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Chen X et al. Osteoblast–osteoclast interactions. Connect. Tissue Res. 59, 99–107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellido T & Delgado‐Calle J Ex Vivo Organ Cultures as Models to Study Bone Biology. JBMR Plus 4, jbm4.10345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cramer EEA, Ito K & Hofmann S Ex vivo Bone Models and Their Potential in Preclinical Evaluation. Curr. Osteoporos. Rep. 19, 75–87 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staines KA, Brown G & Farquharson C The Ex Vivo Organ Culture of Bone. in Bone Research Protocols (ed. Idris AI) vol. 1914 199–215 (Springer New York, 2019). [DOI] [PubMed] [Google Scholar]
- 55.Park Y et al. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 7, eabd6495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vainieri ML, Wahl D, Alini M, van Osch GJVM & Grad S Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies. Acta Biomater. 81, 256–266 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Simfia I, Schiavi J & McNamara LM Alterations in osteocyte mediated osteoclastogenesis during estrogen deficiency and under ROCK-II inhibition: An in vitro study using a novel postmenopausal multicellular niche model. Exp. Cell Res. 392, 112005 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Kiernan CH et al. Allogeneic Chondrogenic Mesenchymal Stromal Cells Alter Helper T Cell Subsets in CD4+ Memory T Cells. Tissue Eng. Part A 26, 490–502 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Bessy T, Itkin T & Passaro D Bioengineering the Bone Marrow Vascular Niche. Front. Cell Dev. Biol. 9, 645496 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souquet B, Opitz M, Vianay B, Brunet S & Théry M Manufacturing a Bone Marrow-On-A-Chip Using Maskless Photolithography. in Bone Marrow Environment (eds. Espéli M & Balabanian K) vol. 2308 263–278 (Springer US, 2021). [DOI] [PubMed] [Google Scholar]
- 61.Pavek A et al. Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines 9, 551 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iordachescu A et al. An In Vitro Model for the Development of Mature Bone Containing an Osteocyte Network. Adv. Biosyst. 2, 1700156 (2018). [Google Scholar]
- 63.Akiva A et al. An Organoid for Woven Bone. Adv. Funct. Mater. 31, 2010524 (2021). [Google Scholar]
- 64.Iordachescu A et al. Trabecular bone organoids: a micron-scale ‘humanised’ prototype designed to study the effects of microgravity and degeneration. Npj Microgravity 7, 17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattazio RR, Noritomi PY & Silveira ZC An In Silico Model for the Prediction of Changes in Mineral Density in Cortical Bone Remodeling. J. Biomech. Eng. 142, 011008 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Herpelinck T et al. An integrated single-cell atlas of the skeleton from development through adulthood. 10.1101/2022.03.14.484345 (2022) doi: 10.1101/2022.03.14.484345. [DOI] [Google Scholar]
- 67.Lesage R et al. An integrated in silico - in vitro approach for identification of therapeutic drug targets for osteoarthritis. 10.1101/2021.09.27.461207 (2021) doi: 10.1101/2021.09.27.461207. [DOI] [Google Scholar]
- 68.Popławski NJ, Swat M, Scott Gens J & Glazier JA Adhesion between cells, diffusion of growth factors, and elasticity of the AER produce the paddle shape of the chick limb. Phys. Stat. Mech. Its Appl. 373, 521–532 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belmonte JM, Swat MH & Glazier JA Filopodial-Tension Model of Convergent-Extension of Tissues. PLOS Comput. Biol. 12, e1004952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guyot Y et al. A computational model for cell/ECM growth on 3D surfaces using the level set method: a bone tissue engineering case study. Biomech. Model. Mechanobiol. 13, 1361–1371 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Van Hede D et al. 3D‐Printed Synthetic Hydroxyapatite Scaffold With In Silico Optimized Macrostructure Enhances Bone Formation In Vivo. Adv. Funct. Mater. 32, 2105002 (2022). [Google Scholar]
- 72.Kuo AD & Donelan JM Dynamic Principles of Gait and Their Clinical Implications. Phys. Ther. 90, 157–174 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benemerito I et al. Delivering computationally-intensive digital patient applications to the clinic: An exemplar solution to predict femoral bone strength from CT data. Comput. Methods Programs Biomed. 208, 106200 (2021). [DOI] [PubMed] [Google Scholar]
- 74.La Mattina AA, Baruffaldi F, Taylor M & Viceconti M Statistical Properties of a Virtual Cohort for In Silico Trials Generated with a Statistical Anatomy Atlas. Ann. Biomed. Eng. (2022) doi: 10.1007/s10439-022-03050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howe K et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busse B, Galloway JL, Gray RS, Harris MP & Kwon RY Zebrafish: An Emerging Model for Orthopedic Research. J. Orthop. Res. 38, 925–936 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dietrich K et al. Skeletal Biology and Disease Modeling in Zebrafish. J. Bone Miner. Res. 36, 436–458 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Kague E & Karasik D Functional Validation of Osteoporosis Genetic Findings Using Small Fish Models. Genes 13, 279 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwon RY, Watson CJ & Karasik D Using zebrafish to study skeletal genomics. Bone 126, 37–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witten PE, Huysseune A, Maisey JG, Winkler C & Gong Z A boost for fish skeletal research. J. Fish Biol. 98, 903–905 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Lleras-Forero L, Winkler C & Schulte-Merker S Zebrafish and medaka as models for biomedical research of bone diseases. Dev. Biol. 457, 191–205 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Cui R, Willemsen D & Valenzano DR Nothobranchius furzeri (African Turquoise Killifish). Trends Genet. 36, 540–541 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Hu C-K & Brunet A The African turquoise killifish: A research organism to study vertebrate aging and diapause. Aging Cell 17, e12757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarasco M, Laizé V, Cardeira J, Cancela ML & Gavaia PJ The zebrafish operculum: A powerful system to assess osteogenic bioactivities of molecules with pharmacological and toxicological relevance. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 197, 45–52 (2017). [DOI] [PubMed] [Google Scholar]
- 85.DeLaurier A et al. Zebrafish sp7:EGFP: A transgenic for studying otic vesicle formation, skeletogenesis, and bone regeneration. genesis 48, 505–511 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caetano-Lopes J et al. Unique and non-redundant function of csf1r paralogues in regulation and evolution of post-embryonic development of the zebrafish. Development dev.181834 (2020) doi: 10.1242/dev.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hur M et al. MicroCT-based phenomics in the zebrafish skeleton reveals virtues of deep phenotyping in a distributed organ system. eLife 6, e26014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chatani M et al. Microgravity promotes osteoclast activity in medaka fish reared at the international space station. Sci. Rep. 5, 14172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah AN, Davey CF, Whitebirch AC, Miller AC & Moens CB Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods 12, 535–540 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watson CJ et al. Phenomics-Based Quantification of CRISPR-Induced Mosaicism in Zebrafish. Cell Syst. 10, 275–286.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kettleborough RNW et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496, 494–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown SDM & Moore MW The International Mouse Phenotyping Consortium: past and future perspectives on mouse phenotyping. Mamm. Genome 23, 632–640 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koscielny G et al. The International Mouse Phenotyping Consortium Web Portal, a unified point of access for knockout mice and related phenotyping data. Nucleic Acids Res. 42, D802–D809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Estrada K et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 44, 491–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kemp JP et al. Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat. Genet. 49, 1468–1475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris JA et al. An Atlas of Human and Murine Genetic Influences on Osteoporosis. 10.1101/338863 (2018) doi: 10.1101/338863. [DOI] [Google Scholar]
- 97.Gistelinck C et al. Loss of Type I Collagen Telopeptide Lysyl Hydroxylation Causes Musculoskeletal Abnormalities in a Zebrafish Model of Bruck Syndrome: LOSS OF TYPE I COLLAGEN TELOPEPTIDE HYDROXYLATION IN ZEBRAFISH BS MODEL. J. Bone Miner. Res. 31, 1930–1942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawrence EA et al. The mechanical impact of col11a2 loss on joints; col11a2 mutant zebrafish show changes to joint development and function, which leads to early-onset osteoarthritis. Philos. Trans. R. Soc. B Biol. Sci. 373, 20170335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gistelinck C et al. Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies. Proc. Natl. Acad. Sci. 115, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bek JW, De Clercq A, Coucke PJ & Willaert A The ZE-Tunnel: An Affordable, Easy-to-Assemble, and User-Friendly Benchtop Zebrafish Swim Tunnel. Zebrafish 18, 29–41 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Khrystoforova I et al. Zebrafish mutants reveal unexpected role of Lrp5 in osteoclast regulation. Front. Endocrinol. 13, 985304 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Apschner A, Huitema LFA, Ponsioen B, Peterson-Maduro J & Schulte-Merker S Pathological mineralization in a zebrafish enpp1 mutant exhibits features of Generalized Arterial Calcification of Infancy (GACI) and Pseudoxanthoma Elasticum (PXE). Dis. Model. Mech. dmm.015693 (2014) doi: 10.1242/dmm.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gray RS et al. Postembryonic screen for mutations affecting spine development in zebrafish. Dev. Biol. 471, 18–33 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marí-Beffa M, Mesa-Román AB & Duran I Zebrafish Models for Human Skeletal Disorders. Front. Genet. 12, 675331 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fiedler IAK et al. Severely Impaired Bone Material Quality in Chihuahua Zebrafish Resembles Classical Dominant Human Osteogenesis Imperfecta. J. Bone Miner. Res. 33, 1489–1499 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Patton EE, Zon LI & Langenau DM Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 20, 611–628 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fleming A, Sato M & Goldsmith P High-Throughput In Vivo Screening for Bone Anabolic Compounds with Zebrafish. SLAS Discov. 10, 823–831 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Gioia R et al. The chaperone activity of 4PBA ameliorates the skeletal phenotype of Chihuahua, a zebrafish model for dominant osteogenesis imperfecta. Hum. Mol. Genet. 26, 2897–2911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brunetti G, D’Amelio P, Wasniewska M, Mori G & Faienza MF Editorial: Bone: Endocrine Target and Organ. Front. Endocrinol. 8, 354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong Y-Y et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999). [DOI] [PubMed] [Google Scholar]
- 111.Dougall WC et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 13, 2412–2424 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. 97, 1566–1571 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cummings SR et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. Obstet. Gynecol. Surv. 64, 805–807 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Lacey DL et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am. J. Pathol. 157, 435–448 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka S & Matsumoto T Sclerostin: from bench to bedside. J. Bone Miner. Metab. 39, 332–340 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Cosman F et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Lin C et al. Sclerostin Mediates Bone Response to Mechanical Unloading Through Antagonizing Wnt/β-Catenin Signaling. J. Bone Miner. Res. 24, 1651–1661 (2009). [DOI] [PubMed] [Google Scholar]
- 118.Li X et al. Sclerostin Antibody Treatment Increases Bone Formation, Bone Mass, and Bone Strength in a Rat Model of Postmenopausal Osteoporosis*. J. Bone Miner. Res. 24, 578–588 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Li X et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J. Bone Miner. Res. 23, 860–869 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Leanza G et al. Gain‐of‐Function Lrp5 Mutation Improves Bone Mass and Strength and Delays Hyperglycemia in a Mouse Model of Insulin‐Deficient Diabetes. J. Bone Miner. Res. 36, 1403–1415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohlsson C et al. Inducible Wnt16 inactivation: WNT16 regulates cortical bone thickness in adult mice. J. Endocrinol. 237, 113–122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wergedal JE, Kesavan C, Brommage R, Das S & Mohan S Role of WNT16 in the Regulation of Periosteal Bone Formation in Female Mice. Endocrinology 156, 1023–1032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng H-F et al. WNT16 Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk. PLoS Genet. 8, e1002745 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gori F, Lerner U, Ohlsson C & Baron R A new WNT on the bone: WNT16, cortical bone thickness, porosity and fractures. BoneKEy Rep. 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duncan EL et al. Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk. PLoS Genet. 7, e1001372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nilsson KH et al. RSPO3 is important for trabecular bone and fracture risk in mice and humans. Nat. Commun. 12, 4923 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brommage R & Ohlsson C High Fidelity of Mouse Models Mimicking Human Genetic Skeletal Disorders. Front. Endocrinol. 10, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP & Dontas IA The Laboratory Rat as an Animal Model for Osteoporosis Research. Comp. Med. 8. [PMC free article] [PubMed] [Google Scholar]
- 129.Aguirre JI, Castillo EJ & Kimmel DB Preclinical models of medication-related osteonecrosis of the jaw (MRONJ). Bone 153, 116184 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haffner-Luntzer M & Ignatius A Animal models for studying metaphyseal bone fracture healing. Eur. Cell. Mater. 40, 172–188 (2020). [DOI] [PubMed] [Google Scholar]
- 131.Chevalier C et al. Warmth Prevents Bone Loss Through the Gut Microbiota. Cell Metab. 32, 575–590.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee Y-C, Song I-W, Pai Y-J, Chen S-D & Chen Y-T Knock-in human FGFR3 achondroplasia mutation as a mouse model for human skeletal dysplasia. Sci. Rep. 7, 43220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakkalakal SA et al. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J. Bone Miner. Res. 27, 1746–1756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rossi V, Lee B & Marom R Osteogenesis imperfecta: advancements in genetics and treatment. Curr. Opin. Pediatr. 31, 708–715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elefteriou F & Couasnay G Advantages and Limitations of Cre Mouse Lines Used in Skeletal Research. in Skeletal Development and Repair (ed. Hilton MJ) vol. 2230 39–59 (Springer US, 2021). [DOI] [PubMed] [Google Scholar]
- 136.Couasnay G, Madel M, Lim J, Lee B & Elefteriou F Sites of Cre‐recombinase activity in mouse lines targeting skeletal cells. J. Bone Miner. Res. 36, 1661–1679 (2021). [DOI] [PubMed] [Google Scholar]
- 137.Dallas SL, Xie Y, Shiflett LA & Ueki Y Mouse Cre Models for the Study of Bone Diseases. Curr. Osteoporos. Rep. 16, 466–477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Couasnay G, Frey C & Elefteriou F Promoter Cre-Specific Genotyping Assays for Authentication of Cre-Driver Mouse Lines: BONE PROMOTER CRE-SPECIFIC GENOTYPING ASSAYS. JBMR Plus 3, e10128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Debnath S et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562, 133–139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mizuhashi K et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 563, 254–258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tournaire G et al. Nestin-GFP transgene labels skeletal progenitors in the periosteum. Bone 133, 115259 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Kusumbe AP, Ramasamy SK & Adams RH Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Biswas L et al. Lymphatic vessels in bone support regeneration after injury. Cell 186, 382–397.e24 (2023). [DOI] [PubMed] [Google Scholar]
- 144.McGovern JA, Griffin M & Hutmacher DW Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 11, dmm033084 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oheim R et al. Sheep model for osteoporosis: The effects of peripheral hormone therapy on centrally induced systemic bone loss in an osteoporotic sheep model. Injury 48, 841–848 (2017). [DOI] [PubMed] [Google Scholar]
- 146.van Griensven M Preclinical testing of drug delivery systems to bone. Adv. Drug Deliv. Rev. 94, 151–164 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Meakin LB, Price JS & Lanyon LE The Contribution of Experimental in vivo Models to Understanding the Mechanisms of Adaptation to Mechanical Loading in Bone. Front. Endocrinol. 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pearce A, Richards R, Milz S, Schneider E & Pearce S Animal models for implant biomaterial research in bone: A review. Eur. Cell. Mater. 13, 1–10 (2007). [DOI] [PubMed] [Google Scholar]
- 149.Turner AS The Sheep as a Model for Osteoporosis in Humans. Vet. J. 163, 232–239 (2002). [DOI] [PubMed] [Google Scholar]
- 150.Newman E The Potential of Sheep for the Study of Osteopenia: Current Status and Comparison with Other Animal Models. Bone 16, 277S–284S (1995). [DOI] [PubMed] [Google Scholar]
- 151.Dias IR et al. Preclinical and Translational Studies in Small Ruminants (Sheep and Goat) as Models for Osteoporosis Research. Curr. Osteoporos. Rep. 16, 182–197 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Oheim R, Schinke T, Amling M & Pogoda P Can we induce osteoporosis in animals comparable to the human situation? Injury 47, S3–S9 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Hillier ML & Bell LS Differentiating Human Bone from Animal Bone: A Review of Histological Methods. J. Forensic Sci. 52, 249–263 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Mori R et al. Preliminary Study of Histological Comparison on the Growth Patterns of Long-Bone Cortex in Young Calf, Pig, and Sheep. J. Vet. Med. Sci. 67, 1223–1229 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Aerssens J, Boonen S, Lowet G & Dequeker J Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research*. Endocrinology 139, 663–670 (1998). [DOI] [PubMed] [Google Scholar]
- 156.Bigham-Sadegh A & Oryan A Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect. Tissue Res. 56, 175–194 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Camassa JA et al. Bone turnover markers in sheep and goat: A review of the scientific literature. An. Acad. Bras. Ciênc. 89, 231–245 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Oheim R et al. Targeting the lateral but not the third ventricle induces bone loss in ewe: An experimental approach to generate an improved large animal model of osteoporosis. J. Trauma Acute Care Surg. 72, 720–726 (2012). [DOI] [PubMed] [Google Scholar]
- 159.Turner AS, Alvis M, Myers W, Stevens ML & Lundy MW Changes in bone mineral density and bone-specific alkaline phosphatase in ovariectomized ewes. Bone 17, S395–S402 (1995). [DOI] [PubMed] [Google Scholar]
- 160.Augat P et al. Glucocorticoid-treated sheep as a model for osteopenic trabecular bone in biomaterials research. J. Biomed. Mater. Res. 66A, 457–462 (2003). [DOI] [PubMed] [Google Scholar]
- 161.Schorlemmer S et al. Glucocorticoid Treatment of Ovariectomized Sheep Affects Mineral Density, Structure, and Mechanical Properties of Cancellous Bone. J. Bone Miner. Res. 18, 2010–2015 (2003). [DOI] [PubMed] [Google Scholar]
- 162.Lill CA, Fluegel AK & Schneider E Effect of Ovariectomy, Malnutrition and Glucocorticoid Application on Bone Properties in Sheep: A Pilot Study. Osteoporos. Int. 13, 480–486 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Lill CA, Fluegel AK & Schneider E Sheep Model for Fracture Treatment in Osteoporotic Bone: A Pilot Study About Different Induction Regimens: J. Orthop. Trauma 14, 559–565 (2000). [DOI] [PubMed] [Google Scholar]
- 164.Egermann M, Goldhahn J, Holz R, Schneider E & Lill CA A sheep model for fracture treatment in osteoporosis: Benefits of the model versus animal welfare. Lab. Anim. 42, 453–464 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Arens D et al. Seasonal changes in bone metabolism in sheep. Vet. J. 174, 585–591 (2007). [DOI] [PubMed] [Google Scholar]
- 166.Claes L Improvement of clinical fracture healing – What can be learned from mechano-biological research? J. Biomech. 115, 110148 (2021). [DOI] [PubMed] [Google Scholar]
- 167.Mills LA & Simpson AHRW In vivo models of bone repair. J. Bone Joint Surg. Br. 94-B, 865–874 (2012). [DOI] [PubMed] [Google Scholar]
- 168.Claes L et al. Metaphyseal fracture healing follows similar biomechanical rules as diaphyseal healing. J. Orthop. Res. 29, 425–432 (2011). [DOI] [PubMed] [Google Scholar]
- 169.Alagboso F et al. Establishment of a clinically relevant large animal model to assess the healing of metaphyseal bone. Eur. Cell. Mater. 37, 444–466 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Beil FT et al. Low turnover osteoporosis in sheep induced by hypothalamic-pituitary disconnection. J. Orthop. Res. 30, 1254–1262 (2012). [DOI] [PubMed] [Google Scholar]
- 171.Oheim R et al. Sheep model for osteoporosis: Sustainability and biomechanical relevance of low turnover osteoporosis induced by hypothalamic-pituitary disconnection. J. Orthop. Res. 31, 1067–1074 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Pogoda P et al. Leptin Inhibits Bone Formation Not Only in Rodents, but Also in Sheep. J. Bone Miner. Res. 21, 1591–1599 (2006). [DOI] [PubMed] [Google Scholar]
- 173.Egermann M et al. Pinealectomy affects bone mineral density and structure - an experimental study in sheep. BMC Musculoskelet. Disord. 12, 271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bindl R et al. Metaphyseal fracture healing in a sheep model of low turnover osteoporosis induced by hypothalamic-pituitary disconnection (HPD). J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 31, 1851–1857 (2013). [DOI] [PubMed] [Google Scholar]
- 175.Denys A et al. Impact of Alcohol on Bone Health in People Living With HIV: Integrating Clinical Data From Serum Bone Markers With Morphometric Analysis in a Non-Human Primate Model. JBMR Plus 7, e10703 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pandruvada SN et al. Bone biology-related gingival transcriptome in ageing and periodontitis in non-human primates. J. Clin. Periodontol. 43, 408–417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ripamonti U, Ramoshebi LN, Teare J, Renton L & Ferretti C The induction of endochondral bone formation by transforming growth factor-beta(3): experimental studies in the non-human primate Papio ursinus. J. Cell. Mol. Med. 12, 1029–1048 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kostenuik PJ et al. Effects of Denosumab, Alendronate, or Denosumab Following Alendronate on Bone Turnover, Calcium Homeostasis, Bone Mass and Bone Strength in Ovariectomized Cynomolgus Monkeys: TRANSITION FROM ALENDRONATE TO DENOSUMAB. J. Bone Miner. Res. 30, 657–669 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Ominsky MS et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J. Bone Miner. Res. 26, 1012–1021 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Mohamed FF et al. Dentoalveolar Defects of Hypophosphatasia are Recapitulated in a Sheep Knock‐In Model. J. Bone Miner. Res. jbmr.4666 (2022) doi: 10.1002/jbmr.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Russell WMS & Burch RL The Principles of Humane Experimental Technique. (1959). [Google Scholar]
- 182.Strech D & Dirnagl U 3Rs missing: Animal research without scientific value is unethical. BMJ Open Sci. 3, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wewetzer H, Wagenknecht T, Bert B & Schönfelder G The fate of surplus laboratory animals: Minimizing the production of surplus animals has greatest potential to reduce the number of laboratory animals. EMBO Rep. 24, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kilkenny C, Browne WJ, Cuthill IC, Emerson M & Altman DG Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 8, 6–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.du Sert NP et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biology vol. 18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vollert J et al. Protocol for a systematic review of guidelines for rigour in the design, conduct and analysis of biomedical experiments involving laboratory animals. BMJ Open Sci. 2, 2–5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bespalov A et al. Introduction to the EQIPD quality system. eLife 10, e63294 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Manolagas SC & Kronenberg HM Reproducibility of results in preclinical studies: A perspective from the bone field. J. Bone Miner. Res. 29, 2131–2140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dempster DW et al. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry : A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. 28, 1–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bouxsein ML et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 (2010). [DOI] [PubMed] [Google Scholar]
- 191.F04 Committee. Guide for Pre-clinical in vivo Evaluation in Critical Size Segmental Bone Defects. http://www.astm.org/cgi-bin/resolver.cgi?F2721-09R14 doi:10.1520/F2721-09R14. [Google Scholar]
- 192.Summary Report on the statistics on the use of animals for scientific purposes in the Member States of the European Union and Norway in 2019. https://ec.europa.eu/environment/chemicals/lab_animals/pdf/SWD2019_Part_A_and_B.pdf (2022). [Google Scholar]
- 193.European Commission. Joint Research Centre. Non-animal methods in science and regulation: EURL ECVAM status report 2021. (Publications Office, 2022). [Google Scholar]
- 194.Fahmy-Garcia S et al. Novel In Situ Gelling Hydrogels Loaded with Recombinant Collagen Peptide Microspheres as a Slow-Release System Induce Ectopic Bone Formation. Adv. Healthc. Mater. 7, 1800507 (2018). [DOI] [PubMed] [Google Scholar]
- 195.O’Neill HC et al. Transplanted spleen stromal cells with osteogenic potential support ectopic myelopoiesis. PLOS ONE 14, e0223416 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Scott MA et al. Brief Review of Models of Ectopic Bone Formation. Stem Cells Dev. 21, 655–667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Urist MR Bone: Formation by Autoinduction. Science 150, 893–899 (1965). [DOI] [PubMed] [Google Scholar]
- 198.Urist MR & Strates BS Bone Morphogenetic Protein. J. Dent. Res. 50, 1392–1406 (1971). [DOI] [PubMed] [Google Scholar]
- 199.Kenswil KJG et al. Endothelium-derived stromal cells contribute to hematopoietic bone marrow niche formation. Cell Stem Cell 28, 653–670.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 200.Knuth C, Witte-Bouma J, Ridwan Y, Wolvius E & Farrell E Mesenchymal stem cell-mediated endochondral ossification utilising micropellets and brief chondrogenic priming. Eur. Cell. Mater. 34, 142–161 (2017). [DOI] [PubMed] [Google Scholar]
- 201.Stojanović S et al. Tissue response to biphasic calcium phosphate covalently modified with either heparin or hyaluronic acid in a mouse subcutaneous implantation model. J. Biomed. Mater. Res. A 109, 1353–1365 (2021). [DOI] [PubMed] [Google Scholar]
- 202.Quent V et al. A humanised tissue‐engineered bone model allows species‐specific breast cancer‐related bone metastasis in vivo. J. Tissue Eng. Regen. Med. 12, 494–504 (2018). [DOI] [PubMed] [Google Scholar]
- 203.Wagner F et al. A Validated Preclinical Animal Model for Primary Bone Tumor Research. J. Bone Jt. Surg. 98, 916–925 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Grigoryan A et al. Engineering human mini-bones for the standardized modeling of healthy hematopoiesis, leukemia, and solid tumor metastasis. Sci. Transl. Med. 14, eabm6391 (2022). [DOI] [PubMed] [Google Scholar]
- 205.Leszczynska A et al. Differentiation of Vascular Stem Cells Contributes to Ectopic Calcification of Atherosclerotic Plaque. Stem Cells 34, 913–923 (2016). [DOI] [PubMed] [Google Scholar]
- 206.Andrés Sastre E et al. A new semi-orthotopic bone defect model for cell and biomaterial testing in regenerative medicine. Biomaterials 279, 121187 (2021). [DOI] [PubMed] [Google Scholar]
- 207.Lenihouannen D et al. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 36, 1086–1093 (2005). [DOI] [PubMed] [Google Scholar]
- 208.Spalthoff S et al. Heterotopic bone formation in the musculus latissimus dorsi of sheep using β-tricalcium phosphate scaffolds: evaluation of an extended prefabrication time on bone formation and matrix degeneration. Int. J. Oral Maxillofac. Surg. 44, 791–797 (2015). [DOI] [PubMed] [Google Scholar]
- 209.Boos AM et al. Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J. Cell. Mol. Med. 15, 1364–1378 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


