Abstract
The β-amino nitrile moiety and its derivatives frequently appear in natural product synthesis, in drug design, and as ligands in asymmetric catalysis. Herein, we describe a direct route to these complex motifs through the amino- and oxycyanation of olefins utilizing an acridinium photooxidant in conjunction with copper catalysis. The transformation can be rendered asymmetric by using a serine-derived bisoxazoline ligand. Mechanistic studies implicate olefin-first oxidation. The scope of amines for the aminocyanation reaction has been greatly expanded by undergoing a cation radical intermediate as opposed to previous N-centered radical-initiated aminocyanations. Furthermore, alkyl carboxylic acids were included as nucleophiles in this type of transformation for the first time without any decarboxylative side reactions.
INTRODUCTION
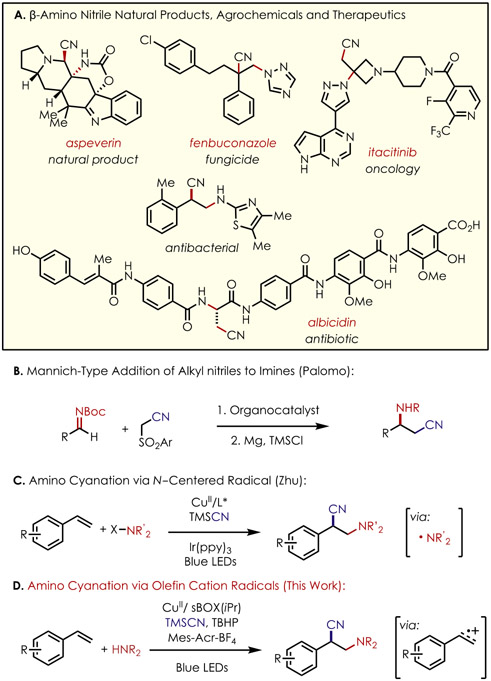
The growing interest in efficient syntheses of chiral β-amino nitriles is evident based on their prevalence in natural products, agrochemicals, and pharmaceuticals (Figure 1A).1 Furthermore, β-amino nitriles can be derivatized to chiral 1,3-diamines and β-amino acids, which are also versatile building blocks for biologically active compounds and ligands for asymmetric catalysis.2 A conventional approach to synthesize optically pure β-amino nitriles involves Mannich-type reactions between imines and alkyl nitriles (Figure 1B),2-5 While imines can be readily synthesized from abundantly available aldehydes or ketones, accessing activated alkyl nitriles can be expensive or require prefunctionalization steps.
Figure 1.
(A) Examples of β-amino nitriles. (B,C) Extant methods for aminocyanation. (D) Asymmetric aminocyantion via olefin cation radical intermediates.
To construct β-amino nitriles in a more step-economical method, the difunctionalization of alkenes was used as an alternative route. The field of amino difunctionalization has been dominated by transition metal catalysis, particularly Pd- and Ni-catalyzed systems,6-11 However, these metal-catalyzed systems are subject to deleterious side reactions such as β-hydride elimination, which forces judicious selection of starting materials, limiting the scope of reactivity or leading to low regiocontrol in the absence of directing groups.
Our laboratory has developed a number of highly regioselective anti-Markovnikov alkene hydrofunctionalization12-15 and difunctionalization16 methods. The common mechanism for all of these transformations begins with irradiation of an acridinium salt with blue LEDs, leading to a potent excited state oxidant capable of oxidizing most olefins (Ep/2 < +2.0 V vs SCE) to the corresponding cation radicals. This electrophilic intermediate readily undergoes nucleophilic addition by amines, alcohols, and carboxylic acids with complete anti-Markovnikov regioselectivity. Following addition of the nucleophilic species to the alkene cation radical, the subsequent neutral radical intermediate is captured with a hydrogen atom transfer cocatalyst to furnish the hydrofunctionalized products. We hypothesized that it might be possible to access β-amino nitriles by capture of this radical in an enantioselective cyanation event using chiral copper cocatalysts.
In this vein, the Stahl and Liu groups established the first enantioselective cyanation method of benzylic C─H bonds employing chiral copper complexes.17 Upon oxidative addition of copper(I) into the nitrogen─fluorine bond of N-fluorosulfonamide (NFSI), fragmentation to form an N-centered radical can occur. This persistent radical can act as an H atom abstraction agent to form a stable benzylic radical. Upon capture of the benzylic radical by a copper(II) cyanide species, reductive elimination can occur to deliver the benzylic nitrile in good yields and in excellent levels of enantiocontrol using chiral bisoxazoline ligands.17
Since then, several groups have reported cyanofunctionalizations of alkenes via this radical relay strategy.18-22 More specifically, several examples of enantioselective aminocyanations have been disclosed using a similar copper system23-25 (Figure 1C). N-centered radicals that are derived from NFSI, trimethylsilyl azide, and N-hydroxypthalamide (NHP) esters add to alkenes, resulting in an alkyl radical primed for a copper-mediated cyanation event, delivering the β-amino nitrile products. However, the scope of amines is severely limited by the lack of N-centered radical precursors.
We envisioned that by combining our alkene anti-Markovnikov hydrofunctionalization reaction conditions with the enantioselective cyanation conditions reported by Stahl and Liu, we would effect the asymmetric formation of β-amino nitriles (Figure 1D). With this reaction mechanism, we hoped that we might be able to greatly expand the range of amines that could be utilized in this alkene difunctionalization without the need for preoxidation of the amine component. Herein, we report the enantioselective aminocyanation of alkenes using a dual organic photoredox and chiral copper catalytic system.
RESULTS AND DISCUSSION
Reaction optimization was initiated by combining our laboratory’s previously reported hydroamination conditions with the Stahl and Liu benzylic cyanation conditions.12,17 With an oxidation potential of +1.6 V vs SCE, 4-tert-butylstyrene (1a)26 was selected as a suitable model substrate with N-Boc amine (4.0 equiv) as the nucleophilic partner. Although previous cyanation methods have employed copper(I) salts to form in situ copper(II)-cyanide intermediates to capture benzylic radicals, we observed our best yields with copper(II) triflate. Furthermore, an external oxidant in the form of tert-butyl hydrogen peroxide (TBHP) was presumably necessary for reoxidation of Mes-Acr-BF4 and the copper(I) intermediate formed upon reductive elimination of the product. After extensive screening, we identified that irradiating (456 nm LEDs) a mixture of trimethylsilylcyanide (TMSCN; 4.0 equiv), TBHP (2.0 equiv), Mes-Acr-BF4 (5.0 mol %), Cu(OTf)2 (5.0 mol %), and an isopropyl cyclopropane serinate-derived bisoxazoline ligand (sBOX (iPr); 7.5 mol %) in acetonitrile (MeCN; 0.2 M) at 5 °C delivered product 1 in 35% yield and 80% enantiomeric excess (ee) (Table 1, entry 1). A systematic evaluation of other bisoxazoline ligands (L1–L3) that have been used in other Cu-catalyzed cyanation reactions did not give rise to higher yields or enantioselectivities compared with the sBOX(iPr) ligand (entries 2–4). Evaluation of solvents identified that MeCN gave the best enantioselectivity; however, ethyl acetate (EtOAc) increased yields of 1 to 49% (entry 6). Varying reaction concentration also proved ineffective in improving selectivity. Unsurprisingly, reduced elevated temperatures resulted in decreased enantioselectivities (entry 9). Finally, control reactions conducted in the absence of a photocatalyst and copper catalyst and in the dark revealed no conversion to product (entry 10).
Table 1.
Optimization for Enantioselective Aminocynation

| |||
|---|---|---|---|
| entry | deviations from above conditions | yield (%)a |
ee (%)b |
| 1 | none | 35 | 80 |
| 2 | L1 instead of sBOX(iPr) | 23 | 72 |
| 3 | L2 instead of sBOX(iPr) | 10 | 33 |
| 4 | L3 instead of sBOX(iPr) | 31 | 52 |
| 5 | DCE instead of MeCN | 24 | 71 |
| 6 | EtOAc instead of MeCN | 49 | 69 |
| 7 | Cu(MeCN)4BF4 instead of Cu(OTf)2 | 20 | 74 |
| 8 | 0.05 M | 30 | 75 |
| 9 | 45 °C | 16 | 75 |
| 10 | No Cu(OTf)2 or no Mes-Acr-BF4 or no light | <5 | – |
Reactions were run at 0.2 mmol scale with 2 mL of solvent.
ee was determined by HPLC with chiral stationary phase.
With the optimized reaction conditions in hand, we investigated the scope for the enantioselective aminocyanation reaction (Figure 2). We first investigated simple carbamate nucleophiles (1 and 2) which furnished the expected products in moderate yields (45–49%) and ee’s (69–70%). We were pleased to see that nucleophilic azole compounds afforded higher yields along with excellent levels of stereocontrol, likely due to more efficient nucleophilic addition to cation radical intermediates. Azoles with a variety of substituents (3–9) were able to undergo this transformation effectively giving the β-amino nitriles in moderate to good yields (43–69%) with good to excellent enantioselectivities (79–90% ee). Further olefin examination revealed that both terminal and internal styrene derivatives are well tolerated under standard reaction conditions. Common styrene derivatives (10, 13–21) were successfully coupled with different nucleophiles, forming desired products in moderate to good yields (33–70%) with good to excellent enantioselectivities (81–99% ee). Both electron rich and electron deficient aromatic systems bearing ortho- and meta-substitutions were compatible. In the cases where lower yields of the three-component coupling were observed, the remaining mass balance can be attributed to the formation of four possible side products (see Supporting Information).
Figure 2.
Nucleophile and alkene scope for enantioselective aminocyanation and carboxylic acid scope for oxycyanation. aReactions were run in EtOAc instead of MeCN. bReactions were run with 1,10-phenanthroline instead of the sBOX(iPr) ligand.
Unfortunately, for alkenes with two prochiral carbon centers, little to no diastereoselectivity was observed. Although the chiral ligand controls facial selectivity for the cyanation event, there appears to be no influence from the chiral copper species on the nucleophilic addition to the alkene cation radical, resulting in meager to nonexistent diastereoselectivity. Despite this, diastereomeric mixtures were easily separated by using column chromatography with the exception of 23. The low enantioselectivity of the major diastereomer of 23 could be attributed to the high steric demand in a five-membered ring system, as the formation of the benzylic stereogenic center is also partially substrate controlled. Olefins bearing functional groups such as esters (25) were tolerated in moderate yields with good enantioselectivities. Application of this catalytic transformation to drug derivatization was demonstrated with ibuprofen and estrone derivatives, giving the expected adducts (11 and 12). Finally, the absolute configurations of both diastereomers were unambiguously confirmed by the X-ray crystal structures of (S)-18.
Once the scope for aminocyanation was established, we set out to expand this general catalytic strategy to include carboxylic acid nucleophiles. The challenge with implementing carboxylic acid nucleophiles is preventing premature oxidative decarboxylation before anionic addition to the alkene cation radical. A recent asymmetric olefin oxycyanation report by Bao and co-workers24 circumvents this issue as it relies on the generation of aryl carboxy radicals (derived from the requisite dibenzoyl peroxides), which are slower than their aliphatic counterparts to undergo decarboxylation. Additionally, recent reports by Guan et al. achieve oxycyanation reactions enabling the use of aliphatic carboxylic acids via cross coupling with a copper(I) cocatalyst, avoiding an oxidative decarboxylation mechanism.27,28 We hoped that, proceeding via an olefin cation radical intermediate, the formation of a carboxy radical and subsequent decarboxylation is avoided, and thus we expected to achieve the asymmetric oxycyanation of simple alkyl carboxylic acids.
We were pleased to find that using an alkyl carboxylic acid, 26 was formed in 95% yield with 87% ee and 92% ee for the major and minor diastereomers, respectively, and importantly, with no decarboxylation product observed. Similarly, cyanolactonization of substituted alkenoic acids (29–32) was successful, providing products in moderate to good yields (50–89%) and good to excellent enantioselectivities (67–95% ee). More sterically congested naphthyl groups were well tolerated (27 and 28) as well as a spirocyclic lactone motif (33), which are privileged scaffolds in drug design. This method can also be applied for functionalization of unactivated olefins (34, 35), though in this case, enantioselectivity was not possible as the absolute stereochemistry of the addition step is not controlled by the chiral copper cocatalyst.
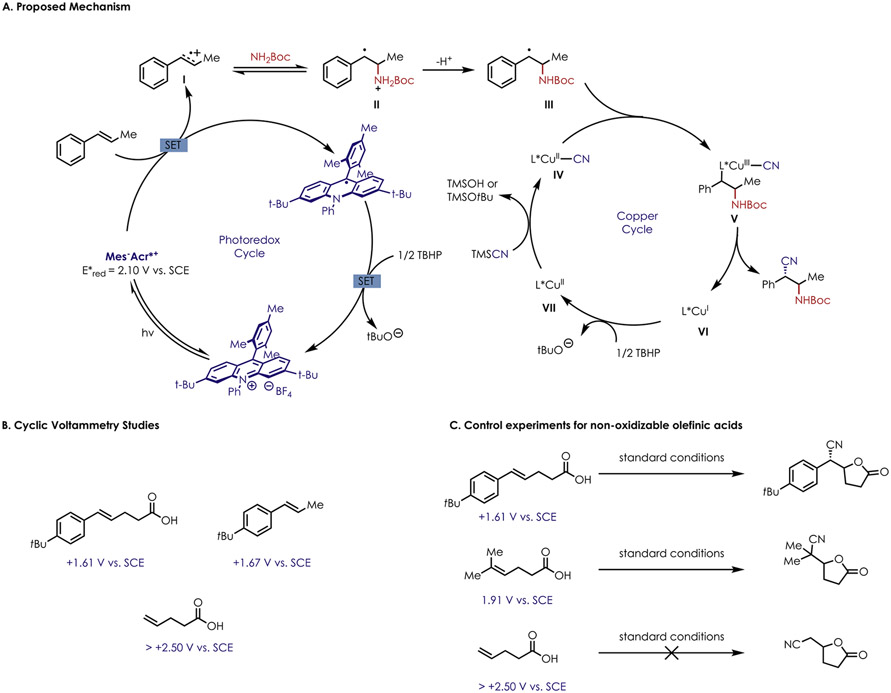
The general mechanism of these transformations is presumed to be rooted in the prior anti-Markovnikov olefin functionalization methods that have been extensively studied in our group.29 First, excitation of the Mes-Acr-BF4 catalyst by 456 nm LEDs gives rise to the potent excited state oxidant, Mes-Acr-BF4*. Single electron oxidation of the substrate yields cation radical intermediate I that can be intercepted via nucleophilic addition by an amine or carboxylic acid nucleophile to give II.
To support formation of a cation radical as opposed to a carboxy radical, cyclic voltammetry studies were performed, demonstrating that the oxidation potential of alkenoic acid is more aligned with the oxidation potential of the corresponding β-methylstyrene than that of the unsubstituted olefinic acid (Scheme 1B). Furthermore, nonoxidizable olefinic acid substrates did not participate in the oxycyanation reaction, presumably because the alkene is outside of the oxidation range of the acridinium excited state. In an analogy to the mechanism presented by the Stahl and Liu groups,17 the alkyl radical likely adds to an in situ generated L*Cu(II)-CN (IV) species. The L*Cu(III) intermediate V is now primed to undergo reductive elimination, releasing the desired product. Previous DFT calculations reveal that the reductive elimination step is enantiodetermining,17 and we believe that the stereocontrol arises in this step as well. The Mes-Acr-BF4 radical and L*Cu(I) VI can both be oxidized by TBHP to close both the photoredox and copper catalytic cycles.
Scheme 1.
(A) Proposed Mechanism for Enantioselective Difunctionalization of Olefins, (B) Cyclic Voltammetry Study of Fragments of p-tBu Acid Substrate, (C) Control Experiments for Nonoxidizable Unsaturated Carboxylic Acid
Previous studies have demonstrated post-transformations to make chiral β-amino acids and 1,3-diamines with excellent stereocontrol and high yields.25 Such reactions can be applied to the products showcased in this work, as well.
CONCLUSION
In conclusion, we have developed an enantioselective difunctionalization of olefins via a cation radical intermediate. Circumventing formation of an N-centered radical, the scope of amines has been widened extensively to include protected amines and azole. Furthermore, alkyl carboxylic acids are amenable in this reaction as decarboxylation pathways are avoided. The wide array of nucleophiles in this three-component coupling allows for diversification of drug molecules as showcased by the functionalization of estrone and ibuprofen derivates. Therefore, we foresee ample application of such a methodology in the development of drugs and natural products.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided in part by the National Institutes of Health (NIGMS) Award R35 GM136330. The authors would like to thank the University of North Carolina’s Department of Chemistry NMR Core Laboratory for the use of their NMR spectrometers. This material is based upon work supported by the National Science Foundation under Grant No. CHE-2117287.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c06936.
Experimental procedures and supporting 1H and 13C NMR spectra (PDF)
Accession Codes
CCDC 2278367–2278368 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
REFERENCES
- (1).López R; Palomo C Cyanoalkylation: Alkylnitriles in Catalytic C─C Bond-Forming Reactions. Angew. Chem., Int. Ed 2015, 54 (45), 13170–13184. [DOI] [PubMed] [Google Scholar]
- (2).Zhao J; Liu X; Luo W; Xie M; Lin L; Feng X Asymmetric Synthesis of β-Amino Nitriles through a ScIII-Catalyzed Three-Component Mannich Reaction of Silyl Ketene Imines. Angew. Chem., Int. Ed 2013, 52 (12), 3473–3477. [DOI] [PubMed] [Google Scholar]
- (3).Anderson JC; Campbell IB; Campos S; Rundell CD; Shannon J; Tizzard GJ Base-Controlled Diastereoselective Synthesis of Either Anti- or Syn-β-Aminonitriles. Org. Lett 2017, 19 (7), 1918–1921. [DOI] [PubMed] [Google Scholar]
- (4).Diosdado S; López R; Palomo C Ureidopeptide-Based Brønsted Bases: Design, Synthesis and Application to the Catalytic Enantioselective Synthesis of β-Amino Nitriles from (Arylsulfonyl)-Acetonitriles. Chem.—Eur. J 2014, 20 (21), 6526–6531. [DOI] [PubMed] [Google Scholar]
- (5).Poisson T; Gembus V; Oudeyer S; Marsais F; Levacher V Product-Catalyzed Addition of Alkyl Nitriles to Unactivated Imines Promoted by Sodium Aryloxide/Ethyl(Trimethylsilyl)Acetate (ETSA) Combination. J. Org. Chem 2009, 74 (9), 3516–3519. [DOI] [PubMed] [Google Scholar]
- (6).He J; Xue Y; Han B; Zhang C; Wang Y; Zhu S Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem., Int. Ed 2020, 59 (6), 2328–2332. [DOI] [PubMed] [Google Scholar]
- (7).Kang T; González JM; Li Z-Q; Foo K; Cheng PTW; Engle KM Alkene Difunctionalization Directed by Free Amines: Diamine Synthesis via Nickel-Catalyzed 1,2-Carboamination. ACS Catal. 2022, 12 (7), 3890–3896. [Google Scholar]
- (8).Tambe SD; Iqbal N; Cho EJ Nickel-Catalyzed Trans-Carboamination across Internal Alkynes to Access Multifunctionalized Indoles. Org. Lett 2020, 22 (21), 8550–8554. [DOI] [PubMed] [Google Scholar]
- (9).Manna MK; Hossian A; Jana R Merging C-H Activation and Alkene Difunctionalization at Room Temperature: A Palladium-Catalyzed Divergent Synthesis of Indoles and Indolines. Org. Lett 2015, 17 (3), 672–675. [DOI] [PubMed] [Google Scholar]
- (10).Wu Y; Wu L; Zhang Z-M; Xu B; Liu Y; Zhang J Enantioselective Difunctionalization of Alkenes by a Palladium-Catalyzed Heck/Borylation Sequence. Chem. Sci 2022, 13 (7), 2021–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Liu Z; Ni H-Q; Zeng T; Engle KM Catalytic Carbo- and Aminoboration of Alkenyl Carbonyl Compounds via Five- and Six-Membered Palladacycles. J. Am. Chem. Soc 2018, 140 (9), 3223–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nguyen TM; Nicewicz DA Anti-Markovnikov Hydroamination of Alkenes Catalyzed by an Organic Photoredox System. J. Am. Chem. Soc 2013, 135 (26), 9588–9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Perkowski AJ; Nicewicz DA Direct Catalytic Anti-Markovnikov Addition of Carboxylic Acids to Alkenes. J. Am. Chem. Soc 2013, 135 (28), 10334–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nguyen TM; Manohar N; Nicewicz DA Anti-Markovnikov Hydroamination of Alkenes Catalyzed by a Two-Component Organic Photoredox System: Direct Access to Phenethylamine Derivatives. Angew. Chem., Int. Ed 2014, 53 (24), 6198–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hamilton DS; Nicewicz DA Direct Catalytic Anti-Markovnikov Hydroetherification of Alkenols. J. Am. Chem. Soc 2012, 134 (45), 18577–18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Griffin JD; Cavanaugh CL; Nicewicz DA Reversing the Regioselectivity of Halofunctionalization Reactions through Cooperative Photoredox and Copper Catalysis. Angew. Chem., Int. Ed. Engl 2017, 56 (8), 2097–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zhang W; Wang F; McCann SD; Wang D; Chen P; Stahl SS; Liu G Enantioselective Cyanation of Benzylic C-H Bonds via Copper-Catalyzed Radical Relay. Science 2016, 353 (6303), 1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Fu N; Song L; Liu J; Shen Y; Siu JC; Lin S New Bisoxazoline Ligands Enable Enantioselective Electrocatalytic Cyanofunctionalization of Vinylarenes. J. Am. Chem. Soc 2019, 141 (37), 14480–14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wang J-Y; Zhang S; Tang Y; Yan S; Li G Copper-Catalyzed Annulation-Trifluoromethyl Functionalization of Enynones. Org. Lett 2023, 25 (14), 2509–2514. [DOI] [PubMed] [Google Scholar]
- (20).Bao X; Wang Q; Zhu J Dual Photoredox/Copper Catalysis for the Remote C(Sp3)-H Functionalization of Alcohols and Alkyl Halides by N-Alkoxypyridinium Salts. Angew. Chem., Int. Ed 2019, 58 (7), 2139–2143. [DOI] [PubMed] [Google Scholar]
- (21).Drennhaus T; Leifert D; Lammert J; Drennhaus JP; Bergander K; Daniliuc CG; Studer A Enantioselective Copper-Catalyzed Fukuyama Indole Synthesis from 2-Vinylphenyl Isocyanides. J. Am. Chem. Soc 2023, 145 (15), 8665–8676. [DOI] [PubMed] [Google Scholar]
- (22).Zhang G; Zhou S; Fu L; Chen P; Li Y; Zou J; Liu G Asymmetric Coupling of Carbon-Centered Radicals Adjacent to Nitrogen: Copper-Catalyzed Cyanation and Etherification of Enamides. Angew. Chem 2020, 132 (46), 20619–20624. [DOI] [PubMed] [Google Scholar]
- (23).Zheng M; Gao K; Qin H; Li G; Lu H Metal-to-Ligand Ratio-Dependent Chemodivergent Asymmetric Synthesis. Angew. Chem., Int. Ed 2021, 60 (42), 22892–22899. [DOI] [PubMed] [Google Scholar]
- (24).Zeng Y; Li Y; Lv D; Bao H Copper-Catalyzed Three-Component Oxycyanation of Alkenes. Organic Chemistry Frontiers 2021, 8 (5), 908–914. [Google Scholar]
- (25).Forster D; Guo W; Wang Q; Zhu J Dual Photoredox and Copper Catalysis: Enantioselective 1,2-Amidocyanation of 1,3-Dienes. ACS Catal. 2023, 13 (11), 7523–7528. [Google Scholar]
- (26).Roth H; Romero N; Nicewicz D Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27 (05), 714–723. [Google Scholar]
- (27).Chen J; Liang Y-J; Wang P-Z; Li G-Q; Zhang B; Qian H; Huan X-D; Guan W; Xiao W-J; Chen J-R Photoinduced Copper-Catalyzed Asymmetric C-O Cross-Coupling. J. Am. Chem. Soc 2021, 143 (33), 13382–13392. [DOI] [PubMed] [Google Scholar]
- (28).Wang P-Z; Liang Y-J; Wu X; Guan W; Xiao W-J; Chen J-R Copper-Catalyzed Three-Component Photo-ATRA-Type Reaction for Asymmetric Intermolecular C-O Coupling. ACS Catal. 2022, 12 (17), 10925–10937. [Google Scholar]
- (29).Romero NA; Nicewicz DA Mechanistic Insight into the Photoredox Catalysis of Anti-Markovnikov Alkene Hydrofunctionalization Reactions. J. Am. Chem. Soc 2014, 136 (49), 17024–17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.