Abstract
Breast cancer is one of the most common types of cancer causing high mortality rates among women worldwide. This study was aimed to evaluate the effect of Pistacia terebinthus (terebinth) resin extract (TRE) on the MDA-MB-231 breast cancer cell line. In the study, the cytotoxic dose of the resin extract in MDA-MB-231 cells was evaluated by MTS analysis. The effect of TRE on apoptosis was examined by Hoechst staining. Caspase-3 and cleaved caspase-3 protein expressions were determined by western blot analysis. Based on the outcomes of our MTS analysis, the IC50 dose of TRE was calculated at 56.54 μg/mL during a 24-h application period. With Hoechst staining analysis, an increase was observed in cells that underwent apoptotic change at 10 and 100 μg/ml TRE concentrations compared to the control. At 25 and 50 μg/mL TRE concentrations, no apoptotic change was found in comparison to the control; however, a significant drop in the number of viable cells was observed because 200, 300, and 500 μg/mL TRE concentrations were above the toxic dose. The caspase-3 protein expression level was significantly higher in cells treated with 100 μg/ml TRE compared to the control group, while there was no significant change in cleaved caspase-3 protein expression. It was thought that P. terebinthus resin might cause cell death in MDA-MB-231 cells via caspase-independent apoptosis pathway or other cell death pathways, and it was concluded that it could be a supportive treatment for breast cancer treatment.
Keywords: Apoptosis, MDA-MB-231, Pistacia terebinthus, Resin
Introduction
Breast cancer is a malignant neoplasm that develops from the epithelial tissue of the mammary gland and is one of the most common types of cancer causing high mortality rates among women worldwide.1 According to the World Health Organization (WHO) report in 2020, breast cancer is the most common type of cancer with an incidence rate of 11.7% worldwide, while in Turkey it is among the most common cancers together with lung cancer with an incidence rate of 10.3%.2
Apoptosis, programmed cell death, results in the orderly and efficient elimination of damaged cells following DNA damage or during development. The mechanism of apoptosis is complex and involves many signaling pathways.3 Induction of apoptosis as a consequence of DNA damage in precancerous lesions may remove potentially harmful cells and thus block tumor growth. Deregulation of this death process is associated with uncontrolled cell proliferation, cancer development and progression, and resistance to cancer drug therapies. Therefore, deregulation of apoptosis is considered one of the indicators of cancer. Therefore, therapeutic strategies targeting molecules related to apoptotic resistance are a valid approach to restore the sensitivity of cancer cells to apoptosis and prevent treatments from becoming ineffective.4
It is known that some components naturally present in various plants are significantly effective in the prevention of cancer. It is stated that these natural components, which have cancer suppressive or preventive properties, have low toxicity in humans and may have the effect of reducing the pain of patients during treatment.5,6P. terebinthus L. is a plant belonging to the Anacardiaceae family used in alternative medicine. P. terebinthus has a rich content of substances including resin, essential oils, proteins, organic acids, sugars, flavonoids and tannins. The chemical composition of the essential oil varies according to the part of the plant from which it is obtained and the geographical origin of the plant population.7 It has been reported that different parts of the P. terebinthus tree have been widely used in phytotherapy since ancient times because they have many biological activities.8 Crude extracts, essential oils and some triterpenoid constituents of P. terebinthus are used as astringent, anti-inflammatory, anti-microbial, anti-viral and stimulants as well as for the treatment of many inflammatory diseases such as eczema, paralysis, diarrhea, throat infections, kidney stones, jaundice, asthma and stomach pains.9–12 When the literature was examined, it was observed that terebinth plant contains high antioxidants.13 It was also reported that terebinth plant extract showed anticancer effect in rats with breast cancer.14 According to the existing literature, there is no study on the cytotoxic effects of terebinth resin extract (TRE) in breast cancer cell lines. In this study, we aimed to evaluate the possible effects of TRE on cytotoxic and apoptosis signaling pathway in MDA-MB-231 human breast cancer cell line.
Materials and methods
Preparation of terebinth resin extracts
Plant resin was collected from Kale, Malatya region in September. The plant material was macerated with 80% methanol for 24 h each and extracted four times at room temperature. The extracts were combined and concentrated under vacuum in rotary evaporator at 37 °C. All extracts were lyophilized and stored at −20 °C until analysis. For the preparation of the extract stock, 4 mg of TRE weighed on a precision balance was taken into an eppendorf tube. The extract was dissolved with 20 μL dimethyl sulfoxide (DMSO). 1980 μL of medium was added to the dissolved mixture and homogenization was achieved with a sonicator device.
Cell line and reagents
MDA-MB-231 triple negative breast cancer cell line was used in this study. These cells were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). The following reagents and chemicals were obtained from the respective suppliers: Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicilin streptomycin and L-glutamin (Sartorius, Gottingen, Germany); antibodies were purchased from Cell Signaling Technology (Cell Signaling Technology, Danvers, MA, USA). All other reagents were obtained from Sigma (Sigma Aldrich, St. Louis, Missouri, USA).
Cell culture
These cells were previously purchased from ATCC and multiplied and stored at Genome and Stem Cell Center of our university in a nitrogen tank at −196 °C. The cell line used had a passage number of 22. Cells were cultured in DMEM medium containing 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin in a 5% CO2 incubator at 37 °C. For all experiments, treatments were done after allowing cells to attach to the plate/flask overnight.
Cell viability test
The MTS cell proliferation assay was used to determine the 50% viability dose of TRE. MDA-MB-231 cells were seeded in 96-well plates (1 × 105 cells/plate) and 10, 25, 50, 100, 200, 300 and 500 μg/mL TRE were added at 72 h of culture. Cells without extract (control) were also added to one plate. After 24 h of TRE treatment, 20 μL of MTS- phenazine methosulfate (PMS) mixture (2 mL MTS-100 μL PMS mg/mL) was added to each well to measure cell viability. The cells were incubated at 37 °C. After 3–4 h, the viability of the cells was measured. The absorbance of each well was measured at 570 nm using an ELISA reader (Promega, Madison, Wisconsin, USA). Finally, the absorbance value was calculated as a percentage of viability using the following formula:
Viability (%) = (The absorbance of samples/The absorbance of controls) X 100.
The IC50 value of TRE was calculated as the dose at which 50% cell death occurred when compared to untreated cells.15 The experiments were repeated three times.
Hoechst staining analysis
To determine the effect of TRE on apoptotic cell death morphologically, cells were seeded in 6-well plates (1x105 cells/plate) and incubated in an incubator with CO2. At 72 h of incubation, cells were treated with TRE at concentrations of 10, 25, 50, 100, 200, 300 and 500 μg/mL. Some cells were left untreated to be used as a control. After 24 h of treatment, they were fixed with 4% paraformaldehyde (PFA). After the PFA was aspirated, it was washed 3 times with phosphate buffered saline (PBS) and 0.2 mL Hoechst stain was added and left to incubate for 15 min. After incubation, the stain was aspirated and images were taken under fluorescence microscope (Danaher Corporation, Washington, DC, USA).
Western blot analysis
MDA-MB-231 cells (3.5 × 105 cells/flask) seeded in 25 cm2 flasks were treated with 100 μg/mL TRE for 24 h. RIPA lysis buffer was then added to the washed cells to lysed the cells and isolate the proteins. Protein concentrations of cell lysates were determined by BCA Protein Assay kit (Thermo Fisher Scientefic, Waltham, Massachusetts, USA). Aliquots containing 40 μg of total protein from each sample were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with a 4%–20% gradient for protein separation and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The PVDF membrane was blocked with 5% (w/v) non-fat milk and washed with Tris-buffered saline-Tween solution (TBS-T). The membrane was probed overnight at 4 °C with a specific primary antibody (Caspase-3, 1:1,000; rabbit polyclonal; cat. no. 9662; cleaved caspase-3, 1:1,000; rabbit polyclonal; cat. no. 9661; Cell Signaling Technology, Danvers, MA, USA). All antibodies were diluted in TBS-T containing 5% dry milk. Before testing for a new antibody, the membrane was treated with strip buffer for 10 min to remove the previous antibody from the membrane. Chemiluminescence detection was performed with Clarity Western ECL Substrate (Bio-Rad Laboratories, Hercules, California, USA), and the blots were visualized with a Chemidoc MP Imaging System (Bio-Rad Laboratories, Hercules, California, USA) and quantified with a densitometer using the imager application program (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Statistical analysis of the data was performed with GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA, USA). Independent two sample t-test was used to compare the data. P < 0.05 was considered statistically significant.
Results
Effect of terebinth resin extract on cell viability
MTS assay was performed to test whether TRE is cytotoxic for MDA-MB-231 cells and to determine the cytotoxic value, where approximately 50% of the cells die (IC value) if it has a toxic effect. For this purpose, cells were treated with TRE at concentrations of 10, 25, 50, 100, 200, 300 and 500 μg/mL for 24 h. As a result of MTS analysis, it was found that 10 and 25 μg/mL TRE did not affect cell viability and had no toxic effect at these concentrations (P > 0.05). Starting from concentration of 50 μg/mL, a statistically significant decrease in cell viability was observed at all doses compared to the control group (P < 0.0001) (Fig. 1). According to our MTS analysis results, the IC50 dose of TRE after 24 h of application was determined as 56.54 μg/mL (Fig. 2).
Fig. 1.
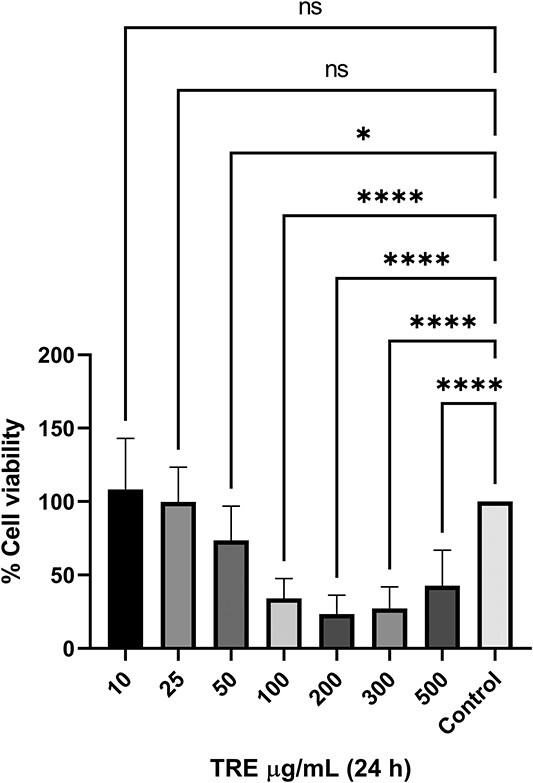
24 h cytotoxicity of TRE on MDA-MB-231 breast cancer cell line by MTS analysis method.
Fig. 2.
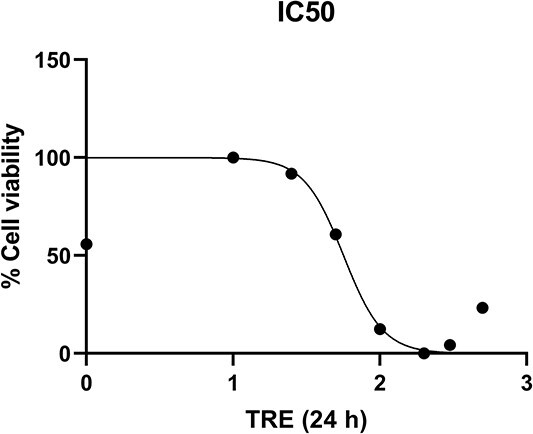
IC50 dose of TRE on MDA-MB-231 breast cancer cell line by MTS analysis method.
Effect of terebinth resin extract on nuclear morphology of cells
Hoechst staining was performed to evaluate the effect of TRE on the nucleus morphology of cells in MDA-MB-231 cell line. The effects of the applied doses on the cell line are shown in Fig. 3. The nucleus morphology of the stained cells was examined and chromatin condensation and nucleus fragmentation were investigated. When the images of the Hoechst stained cells were analyzed, it was observed that the number of cells undergoing apoptotic changes increased at TRE concentrations of 10 and 100 μg/mL compared to the control group. Since 200, 300 and 500 μg/mL TRE concentrations were above the toxic dose, a significant decrease in the number of viable cells was observed. At 25 and 50 μg/mL TRE concentrations, no apoptotic change was observed compared to the control. A few apoptotic changes were also observed in control group cells (Fig. 3).
Fig. 3.
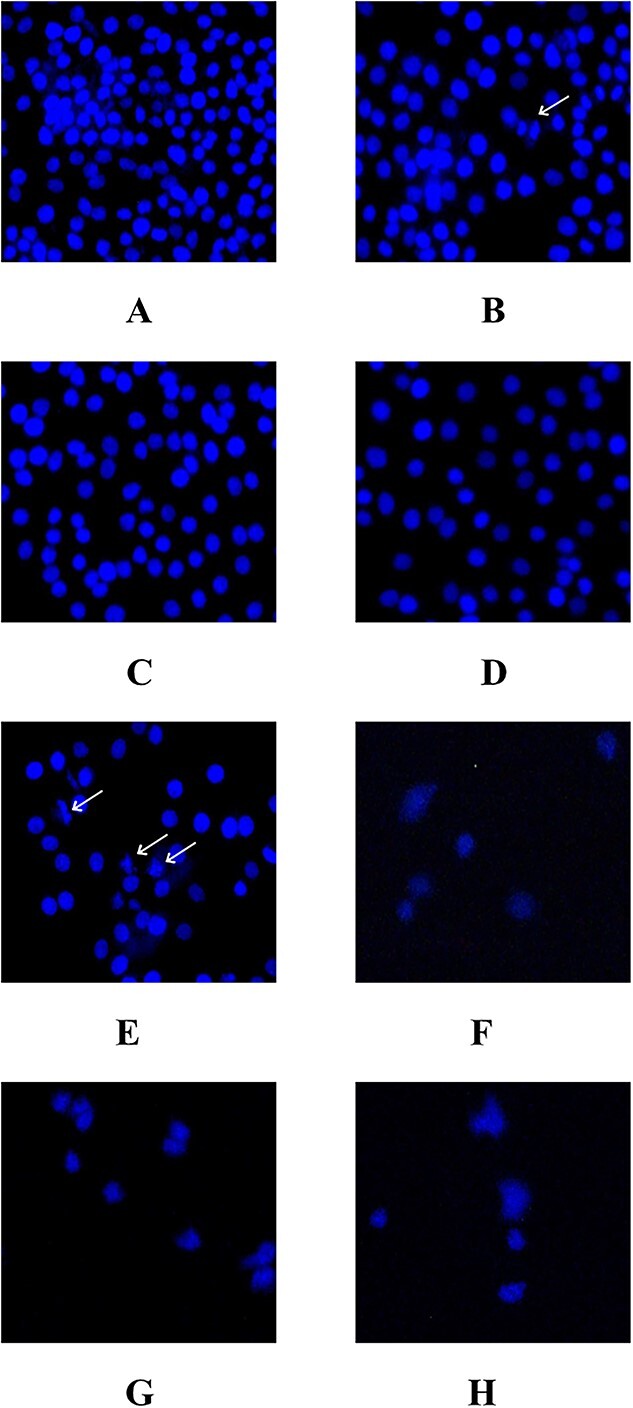
MDA-MB-231 breast cancer cells exposed to TRE and stained with Hoechst. Chromatin condensation resulting from apoptosis is seen in the cells indicated by the arrow, a: Control, B: 10 μg/mL, C: 25 μg/mL, D: 50 μg/mL, E: 100 μg/mL, F: 200 μg/mL, G: 300 μg/mL, H: 500 μg/mL.
Effect of terebinth resin extract on apoptosis signaling pathway
In order to evaluate the effect of TRE on apoptosis signaling pathway in MDA-MB-231 breast cancer cell line, the expressions of caspase-3 and cleaved caspase-3 proteins in the cells treated with 100 μg/mL TRE, which was the dose found to be significant in Hoechst staining analysis, were analyzed by western blot method. β-actin protein was used as loading control. According to the results of western blot analysis, there was no statistically significant difference (P > 0.05) between the control group and cells treated with 100 μg/mL TRE for 24 h in terms of the expression of cleaved caspase-3 proteins, while caspase-3 protein expression level was higher in the 100 μg/mL TRE group (Fig. 4).
Fig. 4.

Western blot analysis results, a: Western blot images of caspase-3, cleaved caspase-3 (cl-caspase-3) and β-actin protein expression in MDA-MB-231 cells of MRE and control group, B: Results of caspase-3 protein expression, C: Results of cleaved caspase-3 protein expression.
Discussion
Plants are composed of a variety of bioactive components and the composition and concentration of these components can vary according to geographical location, harvest season, post-harvest processing and storage. Many modern drugs used in standard cancer treatment are also made from plants. In the last decade, several research studies have been conducted to identify and understand the anticancer effects of both bioactive components and extracts of plants.16 Phytochemicals from plants are more widely used in cancer therapy than other sources due to their safety, low toxicity and general availability. Phytochemicals show anti-angiogenic, anti-inflammatory, anti-oxidant, anti-proliferative and pro-apoptotic effects by affecting various signaling pathways. This makes phytochemicals effective anticancer drugs that can prevent cancer at multiple stages.17
P. terebinthus consists of a rich mixture of substances including organic acids, sugars, essential oils, resin, proteins, tannins and flavonoids.7P. terebinthus has anti-oxidant, cytotoxic, antipyretic, antiseptic and anti-inflammatory activities, especially thanks to the flavonoids and other phenolic compounds it contains and the fatty acids found in its seeds.18,19
Reactive oxygen species (ROS) are produced by various endogenous (cytokines, growth factors and metabolic processes) and exogenous substances (cigarette smoke). The ROS signaling pathway in cancer cells is bidirectional: ROS-induced tumor progression and ROS-induced tumor apoptosis. Cancer cells produce free radicals and other ROS to help stimulate growth, cell survival and inflammation, an established source of carcinogenesis. However, the presence of macrophages and neutrophils in the inflammatory process can cause the production of superoxide and other ROS to reach toxic levels, eventually leading to tumor cell death and apoptosis. Antioxidants provide an extra electron needed to stabilize or break down ROS. Therefore, it is imperative that cancer cells produce antioxidants to maintain a constant balance of ROS.20 Considering this relationship between cancer and antioxidants, it suggests that P. terebinthus, which has high antioxidant activity, may also have an effect in the prevention and treatment of cancer.
When the literature is examined, the effects of P. terebinthus seeds and coffee on various diseases have been investigated. However, the effect of P. terebinthus resin on breast cancer has not been investigated. Therefore, this study, which aims to examine the effects of P. terebinthus resin on the aggressive breast cancer cell line MDA-MB-231, is the first in the literature.
In the literature, there are studies on the antidiabetic and liver damage preventive effects of P. terebinthus plant. Bahcecioglu et al.21 examined the effect of terebinth coffee on liver damage in rats and found that fibrosis and inflammation scores were statistically significantly lower in the liver damaged group consuming terebinth coffee after 8 weeks of treatment. They suggested that this feature of P. terebinthus plant may be due to its suppression of pro-inflammatory cytokines in the NF-κB signaling pathway.21 In a study in which the antidiabetic effect of the oil extract of P. terebinthus plant was investigated in rats, it was found that blood glucose, AST, ALT, alkaline phosphatase, lactate dehydrogenase, triglyceride and LDL cholesterol levels were significantly lower in the group receiving the extract compared to the control group and acarbose group, as well as improvements in antioxidant enzyme levels.22
There are various studies examining the anticancer effect of P. terebinthus plant. In the studies, it was stated that the plant showed anticancer effect due to its antioxidant properties.14,23,24 In the study examining the effect of silver nanoparticles obtained from P. terebinthus plant on MCF-7 cells, a breast cancer cell line, it was reported that terebinth methanolic extract and silver nanoparticles at doses of 3, 6, 12.5, 25, 50, 100, 200 and 400 mg/L showed anticancer effect by showing antioxidant effect and reducing cell proliferation.24 In the study investigating the cytotoxic effects of aqueous and methanolic extracts of P. terebinthus seeds on breast cancer cell lines MCF-7 and MDA-MB-231 cell lines, MTT test was applied. The extracts were applied to both cell lines at doses of 0–1,000 μg/mL for 24 h. While no cytotoxic effect was observed at doses of 250 μg/mL and below in both cell lines, it was shown that there was a 36% and 20% decrease in the viability of MCF-7 and MDA-MB-231 cells at a dose of 500 μg/mL, respectively.23 In our study, the effects of P. terebinthus resin on MDA-MB-231 cell line were investigated. When TRE was applied for 24 h on MDA-MB-231 cells, an aggressive breast cancer cell line, the IC50 value was found to be 56.54 μg/mL, in which approximately 50% of the cells died. In our study, it was observed that TRE showed cytotoxic effect even at lower doses than aqueous and methanolic extracts of the seed form. In other words, it can be considered that the resin of P. terebinthus plant has more cytotoxic effect than the seed.
In the study examining the effect of P. terebinthus seed aqueous extract on the expression of apoptotic proteins in rats with experimental breast cancer, rats other than the control group were given 20 mg/kg terebinth seed aqueous extract by oral consumption for 16 weeks. It was observed that the expression of caspase-9 and Bax proteins was significantly higher in the extracted group compared to the control group, while Bcl-2 expression was found to be significantly lower.14 In our study, as a result of Western blotting of caspase-3 and cleaved caspase-3 protein expressions involved in the apoptosis pathway, no change was found in caspase-3 protein expression and cleaved caspase-3 expression, which is the activated protein form of caspase-3, in the TRE-treated group compared to the control group. Cleavage of caspase-3 is generally accepted as a universal marker of apoptosis because caspase-3 activity is required for most of the morphological and biochemical events associated with apoptosis. Caspase-3 cleaves at two sites resulting in three fragments; the pre-domain, the large subunit and the small subunit. The first cleavage only partially activates caspase-3 and the second autolytic cleavage results in full activation. Therefore, it is important to ensure that caspase-3 is fully activated to indicate that apoptosis has occurred.25 However, in our study, although the expression of cleaved caspase-3, the activated form of caspase-3, was higher in the TRE group, it was not statistically significant. These data suggest that TRE does not exert its cytotoxic effect through caspase-dependent apoptosis pathway. Although there was no change in caspase activity, significant changes were detected in the nuclear morphology of the cells according to our Hoechst staining results. Even a decrease in cell number was found with increasing TRE concentrations.
Conclusion
According to these results, TRE showed a cytotoxic effect on aggressive breast cancer cells and inhibited cell proliferation/viability but did not affect caspase-dependent apoptosis signaling pathway. All these results suggest that TRE suppresses cell viability via caspase-independent apoptosis pathway or other cell death pathways. Further studies are required to elucidate which death signaling pathway TRE affects to exert its cytotoxic effect, i.e. the molecular mechanism of TRE in aggressive breast cancer cells.
Acknowledgments
We would like to thank Erciyes University Scientific Research Projects Unit and its staff and Erciyes University Betül Ziya Eren Genom and Stem Cell Center Management and its staff.
Contributor Information
Kerim Fırat, Department of Anatomy, Medical Faculty, Erciyes University, Kayseri 38039, Turkey.
Mehtap Nisari, Department of Anatomy, Medical Faculty, Erciyes University, Kayseri 38039, Turkey.
İrem Metin, Genome and Stem Cell Center (GENKOK), Erciyes University, Kayseri 38039, Turkey.
Yağmur Yaşar Fırat, Department of Nutrition and Dietetic, Faculty of Health Sciences, Erciyes University, Kayseri 38039, Turkey.
Gökçe Şeker Karatoprak, Department of Pharmacognosy, Faculty of Pharmacy, Erciyes University, Kayseri 38039, Turkey.
Zühal Hamurcu, Genome and Stem Cell Center (GENKOK), Erciyes University, Kayseri 38039, Turkey; Department of Medical Biology, Medical Faculty, Erciyes University, Kayseri 38039, Turkey.
Author contribution
The authors confirm contribution to the paper as follows: study conception and design: K.F, M.N, Z.H; data collection: K.F, İ.M., G.Ş.K., Z.H.; analysis and interpretation of results: K.F, İ.M., Y.Y.F., G.Ş.K.; draft manuscript preparation: K.F, M.N., Y.Y.F. All authors reviewed the results and approved the final version of the manuscript.
Funding
This research was supported by Erciyes University Scientific Research Projects Unit with the project numbered TYL-2021-11155.
Conflict of interest statement. No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
References
- 1. Li H, Giger ML. Breast cancer. In: Li R, Xing L, Napel S, Rubin D, editors. Radiomics and Radiogenomics. Milton Park: Taylor and Francis; 2019:229–249. [Google Scholar]
- 2. World Health Organisation . The global cancer observatory: Turkey. https://gco.iarc.fr/today/data/factsheets/populations/792-turkey-fact-sheets.pdf(accessed 2022 November 9).
- 3. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007:35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016:8(4):603–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang Q, Yang M, Qu Z, Zhou J, Zhang Q. Acupuncture for primary Sjögren syndrome (pSS) on symptomatic improvements: study protocol for a randomized controlled trial. BMC Complement Altern Med. 2017:17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang C-Z, Calway T, Yuan C-S. Herbal medicines as adjuvants for cancer therapeutics. Am J Chin Med. 2012:40(04):657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pulaj B, Mustafa B, Nelson K, Quave CL, Hajdari A. Chemical composition and in vitro antibacterial activity of Pistacia terebinthus essential oils derived from wild populations in Kosovo. BMC Complement Altern Med. 2016:16(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kallis M, Sideris K, Kopsahelis N, Bosnea L, Kourkoutas Y, Terpou A, Kanellaki M. Pistacia terebinthus resin as yeast immobilization support for alcoholic fermentation. Food Secur. 2019:8(4):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, Izumi S, Hirata T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 2003:74(1–2):170–176. [DOI] [PubMed] [Google Scholar]
- 10. Giner-Larza EM, Máñez S, Recio MC, Giner RM, Prieto JM, Cerdá-Nicolás M, Ríos JL. Oleanonic acid, a 3-oxotriterpene from Pistacia, inhibits leukotriene synthesis and has anti-inflammatory activity. Eur J Pharmacol. 2001:428(1):137–143. [DOI] [PubMed] [Google Scholar]
- 11. Lamiri A, Lhaloui S, Benjilali B, Berrada M. Insecticidal effects of essential oils against hessian fly, Mayetiola destructor (say). F Crop Res. 2001:71(1):9–15. [Google Scholar]
- 12. Mouhajir F, Hudson JB, Rejdali M, Towers GHN. Multiple antiviral activities of endemic medicinal plants used by Berber peoples of Morocco. Pharm Biol. 2001:39(5):364–374. [Google Scholar]
- 13. Özcan MM, Al Juhaimi F, Uslu N, Ahmed IAM, Babiker EE, Osman MA, Gassem MA, Alqah HAS, Ghafoor K. Effect of sonication process of terebinth (Pistacia terebinthus L.) fruits on antioxidant activity, phenolic compounds, fatty acids and tocopherol contents. J Food Sci Technol. 2020:57(6):2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erdem Erişir F, Zengin H. in Conference: 4th International Eurasian Conference on Biological and Chemical Sciences (EurasianBioChem 2021), Ankara, Turkey, 2021. [Google Scholar]
- 15. Shandiz SAS, Khosravani M, Mohammadi S, Noorbazargan H, Mirzaie A, Inanlou DN, Jalali MD, Jouzaghkar H, Baghbani-Arani F, Keshavarz-Pakseresht B. Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR. Asian Pac J Trop Biomed. 2016:6(2):159–163. [Google Scholar]
- 16. Subramani R, Lakshmanaswamy R. Complementary and alternative medicine and breast cancer. Prog Mol Biol Transl Sci. 2017:151:231–274. [DOI] [PubMed] [Google Scholar]
- 17. Shu L, Cheung K-L, Khor TO, Chen C, Kong A-N. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010:29(3):483–502. [DOI] [PubMed] [Google Scholar]
- 18. Dhifi W, Mnif W, Ouerhani B, Ghrissi K. Chemical composition and antibacterial activity of essential oil from the seeds of Pistacia terebinthus grown in Tunisia. J Essent Oil Bear Plants. 2012:15(4):582–588. [Google Scholar]
- 19. Topçu G, Ay M, Bilici A, Sarıkürkcü C, Öztürk M, Ulubelen A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem. 2007:103(3):816–822. [Google Scholar]
- 20. Athreya K, Xavier MF. Antioxidants in the treatment of cancer. Nutr Cancer. 2017:69(8):1099–1104. [DOI] [PubMed] [Google Scholar]
- 21. Bahcecioglu IH, Ispiroglu M, Tuzcu M, Orhan C, Ulas M, Demirel U, Yalniz M, Özercan IH, Ilhan N, Sahin K. Pistacia Terebinthus coffee protects against Thioacetamide-induced liver injury in rats. Acta Medica Cordoba. 2015:58(2):56–61. [DOI] [PubMed] [Google Scholar]
- 22. Uyar A, Abdulrahman NT. A histopathological, immunohistochemical and biochemical investigation of the antidiabetic effects of thePistacia terebinthusin diabetic rats. Biotech Histochem. 2020:95(2):92–104. [DOI] [PubMed] [Google Scholar]
- 23. İçen MS, Karakuş F, Tosun E, Yılmaz K, in Conference: Gazi Pharma Symposium Series, 2015.
- 24. Naghmachi M, Raissi A, Baziyar P, Homayoonfar F, Amirmahani F, Danaei M. Green synthesis of silver nanoparticles (AgNPs) by Pistacia terebinthus extract: comprehensive evaluation of antimicrobial, antioxidant and anticancer effects. Biochem Biophys Res Commun. 2022:608:163–169. [DOI] [PubMed] [Google Scholar]
- 25. Crowley LC, Waterhouse NJ. Cold Spring Harb Protoc. 2016:pdbprot087312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


