Abstract
Introduction
5-fluorouracil (5-FU) and methotrexate (MTX) are the antineoplastic drugs most commonly used worldwide. Considered cytotoxic, these pharmaceuticals exhibit low specificity, causing damage not only to cancer cells but also to healthy cells in organisms. After being consumed and metabolized, these drugs are excreted through urine and feces, followed by wastewater treatment. However, conventional treatments do not have the capacity to completely remove these substances, risking their introduction into freshwater systems. This could pose a risk to human health even at low concentrations.
Aims
Thus, the present study aimed to investigate the genotoxicity, cytotoxicity, and mutagenicity of 5-FU and MTX at environmentally relevant concentrations after a long-term exposure, using adult male rats as an experimental model.
Methods
Male Wistar rats (70 days old) were distributed into 4 groups (n = 10/group): control, received only vehicle; MTX, received methotrexate at 10ngL−1; 5-FU received 5-fluorouracil at 10ngL−1; and MTX + 5-FU, received a combination of MTX and 5-FU at 10ngL−1 each. The period of exposure was from postnatal day (PND) 70 to PND 160, through drinking water. After that, the animals were euthanized and the samples (liver, testis, femoral bone marrow, and peripheral blood) were obtained.
Results
Increased DNA fragmentation was observed in the peripheral blood, liver, and testis, altering the parameters of the tail moment and tail intensity in the Comet assay. Besides, the change in the ratio between PCE and NCE indicates bone marrow suppression.
Conclusion
These findings warn the adverse effects for the general population worldwide chronically exposed to these drugs at trace concentration unintentionally.
Keywords: 5-fluorouracil, Methotrexate, Genetic toxicology, DNA damage, Emerging contaminants, Rats
Introduction
5-fluorouracil (5-FU) and methotrexate (MTX) are among the anticancer drugs classified as antimetabolic agents most consumed worldwide. Considered cytotoxic drugs, these substances are capable of inducing death in cells with elevated mitotic index. Therefore, these pharmaceuticals present low specificity, provoking damage not only to the cancer cells, but also to healthy cells of organisms.1
5-FU is one of the most potent antimetabolites, widely used in the treatment of advanced solid tumors.2 It enters cells by facilitated transport using the same mechanism as that of uracil, inhibiting the thymidylate synthase (TS) and incorporating its metabolites fluorodeoxyuridine diphosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP), and fluorouridine triphosphate (FUTP) into RNA and DNA, to serve as mediators of the cytotoxic effects on cancer cells in different solid tumors including colon, rectal, breast, gastric, pancreatic, ovarian, bladder, and liver.3–5 Despite the clinical advantages in cancer treatment, the administration of 5-FU in therapeutic doses can lead to bone marrow suppression and testicular toxicity, inducing DNA damage in germ cells.6,7 Also, it is known that more than 85% of 5-FU is degraded through the catabolic pathway in the liver. Therefore, an extended exposure to this substance can overload this organ, causing severe damage to hepatocytes and its functions.8
Regarding the mechanism of action of MTX, it has a similar structure to folic acid facilitating the competitive inhibition of dihydrofolate reductase (DHFR), inhibiting the de novo purine and pyrimidine synthesis.9,10 In this case, after its consumption, only 10% is converted to the metabolite 7-hydroxymethotrexate in the liver to exert their contribution to the parent compound, and subsequently excreted by the kidneys.11 This continuous process interferes with DNA synthesis, repair, and cellular replication. In high doses, i.e 500 mg/m2 or greater,12 MTX can cause hepatotoxicity leading to cirrhosis, nephrotoxicity, and bone marrow suppression in humans.13,14
In recent years, both cytotoxic drugs have been introduced into the aquatic environment, once after metabolized these drugs are excreted through urine and feces. It occurs due to the inefficiency to remove complex pharmaceuticals, considered recalcitrant, during the effluent treatment processes.15,16 Connor et al.17 recognized that the high probability of occurrence of this class of drugs in the environment may result from the use of several drugs associated during chemotherapy cycles, increasing the likelihood of unintentional human exposure to these substances. These drugs have already been detected in effluents, surface waters, and drinking water, in concentrations ranging from ngL−1 to mgL-1.18–24 The rising global rates of cancer and the insufficient information about its origins pose a risk not only to individuals directly exposed (e.g. health workers) or indirectly exposed (the general population) to antineoplastic drugs but also to the entire environment.25 Due to the presence of these substances in trace concentrations, as well as the absence of legislation that includes them as part of the investigation bases, they are considered emerging pollutants, and its presence in water bodies is a growing global concern.26
Hence, in the present study, we aimed to mimic the unintentional long-term exposure to the antineoplastic drugs 5-FU and MTX (isolated or combined) at environmentally relevant concentrations, to investigate its potential to interfere with DNA at trace concentrations, using a rat model.
Material and methods
Materials
5-FU (purity >99% - HPLC) and MTX (Methotrexate Hydrate, purity ≥98% - HPLC) were purchased from Sigma-Aldrich (Brazil). To proceed with the experiment, a stock solution was prepared using a vehicle solution of filtered water added of 10−8% Dimethyl Sulfoxide (DMSO) – purchased from Sigma Aldrich (Brazil) (ReagentPlus® purity ≥99.5%).
Animals
Male Wistar rats, (n = 40, 63 days old), were provided by CEDEME – Center for Development of Experimental Models for Medicine and Biology, Federal University of São Paulo – UNIFESP. The animals were housed under controlled conditions of temperature (average 23 °C) and photoperiod (12 h light,12 h dark cycle and artificial light starting at 07,00 am), with free access to drinking water and rat feed (Nuvilab CR-1). All experimental procedures described were in accordance with the ethical principles from the Animal Use Ethics Committee of UNIFESP (Process 3,922,140,119) following the Directive 2010/63/EU on the protection of animals used for scientific purposes.
Experimental design
Rats were distributed into four experimental groups as detailed in Table 1. They were treated daily in an oral route from postnatal day (PND) 70–160, according to the protocols suggested by the Environmental Protection Agency of the United States (U.S. EPA) for a subchronic treatment.27–29
Table 1.
Experimental design and treatments.
| Experimental Groups Treatment | |
|---|---|
| Control (n = 10) | Received the vehicle, ad libitum, oral route. |
| 5-FU (n = 10) | Received the vehicle, ad libitum, oral route, added 5-FU at 10 ngL−1. |
| MTX (n = 10) | Received the vehicle, ad libitum, oral route, added MTX at 10 ngL−1. |
| 5-FU + MTX (n = 10) | Received the vehicle, ad libitum, oral route, added 5-FU and MTX at 10 ngL−1 each. |
The experimental concentrations used in the present study were based in previous studies which measured the concentrations of these substances in the environment,20–24 as well as, in the predicted environmental concentrations (PEC) for natural water bodies,30 wastewater31,32 and drinking water33 in several countries, revealing concentrations of these substances up 10 ng L−1. The present study is in accordance with the rationale of the European Medicine Agency,34 which reports that if predicted concentrations for the parent compound of antineoplastic drugs in the environment are higher or equal 10 ng L−1, studies in different scientific fields are necessary, aiming to understand the effects of this unintentional exposure on environmental and human health. The utilization of animal models has proven to be a valuable asset in investigating the mechanisms of DNA damage, cell, and tissue function.35 To better understand these interactions, the results presented here complement a primary study that specifically focused on the reproductive parameters of males produced in our laboratory.36
Sample collection
On postnatal day 160 (PND 160), the animals were weighed and euthanized by decapitation, with prior anesthesia by xylazine (5 mg/Kg of body weight) and ketamine (80 mg/Kg of body weight). Heparinized peripheral blood from ruptured cervical vessels after guillotine decapitation for the Comet assay, as well right testis, and liver samples, were collected. Left femoral bone marrow cells were collected for micronucleus and cytotoxicity tests.
Alkaline comet assay (single cell gel electrophoresis)
Histological slides, in duplicate for each selected organ, were previously coated with 1.5% Agarose coverage. The testis and liver samples, collected during euthanasia, were washed with PBS (Phosphate Buffer Solution pH 7.4) and trypsinized for approximately 5 min. 5 μL of the samples were diluted in 75 μL low melting agarose (LMP 0.75%), then, 40 μL of the samples were added to each precoated slide followed by the coverslip, which were immediately destined for solidification in the refrigerator. Ultimately, the coverslips were removed and the slides were immersed in vertical wells protected from light containing the lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10). After 48 h of lysis, the samples were electrophoresed (25 V and 300 mA for 20 min) following the protocol for using the pH > 13 alkaline test. Then, the slides were submerged 3 times in the neutralizing buffer (0.4 M Tris, pH 7.5), washed with distilled water and dried at room temperature for later fixation in absolute alcohol (99.6%) for 5 min.
For comet evaluation, slides were stained in a dark room with SYBR® Green I DNA intercalating nucleic acid gel stain S9430-5 mL (Saint Louis – Missouri, USA), whose dilution was 1:10,000, for 4 min and examined under a fluorescence microscope (Zeiss Axio Lab F1, Jena, Thuringia, Germany) coupled to a Basler camera (Ahrensburg – Schleswig-Holsteins, Germany). For each experimental animal, 100 nucleoids were analyzed (50 per slide) in a 20x objective, using the CometAssay IV software (Perceptive Instruments Bury St Edmunds – Suffolk, England). Analyzes were conducted in a blinded trial. To calculate the average DNA damage, two parameters were considered: tail intensity (% of DNA migrated) and tail moment (the product of tail length and tail DNA fraction). The Comet Assay was performed according to recommendations of Minimum Information for Reporting on the Comet Assay (MIRCA): recommendations for describing comet assay procedures and results.37
Micronucleus assay
Left femoral bone marrow cells were collected from femur content aspiration with a syringe and homogenized with 1 mL fetal bovine serum then smeared on duplicate histological slides, fixed 24 h after collection in methanol for 10 min. The micronucleus test was performed based on the protocol proposed by the United States Environmental Protection Agency38 following adaptations by da Silva et al.39 The slides were stained with the panoptic staining kit (NewProv – Kit Instant Prov 1,319) and dried at room temperature. To evaluate the incidence of micronuclei (MNi), 2000 cells per animal were evaluated (1,000 cells from each slide prepared in duplicate), the cells were classified in polychromatic erythrocytes (PCE) to evaluation of MNi and monochromatic erythrocytes (NCE) were also quantified.
Possible cytotoxic effects were investigated by determining the PCE: NCE ratio after analysis of 2000 erythrocytes/animal. The analyzes were performed under a light microscope with a magnification of 1,000x, in a blind test, according to OECD 474.40
Statistical analysis
The Anderson-Darling, D’Agostino-Pearson, Shapiro–Wilk and Kolmogorov–Smirnov methods to validate the Gaussian distribution. Followed by the Bartlett and Brown-Forsyth to validate the homoscedasticity of the data. When necessary, to achieve such parameters, mathematical transformation was performed to fit the statistical models following the prediction: Y = sqrt(y). The statistical tests of analysis of variance - One Way or Two-Way ANOVA with Tukey’s Post-test were used when these parameters were attended.
According to the proposal, when the assumptions of homoscedasticity and Gaussian distribution were not met, the non-parametric Kruskal-Wallis test was used, with Dunn’s Post-test. GraphPad Prism 8.01 software was used for such evidence. Differences were considered statistically significant when P < 0.05.
Results
Comet assay—Peripheral blood
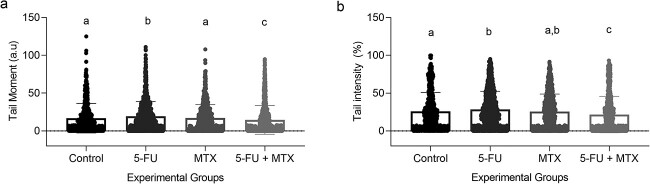
The parameters Tail Moment (A) and Tail Intensity (B) showed a significant difference in MTX (2.4 ± 4) compared to Control (1.95 ± 3.1). However, no significant differences were found among the other experimental groups in the two parameters analyzed, as shown in Fig. 1A and B respectively.
Fig. 1.
Comet assay on whole blood samples from experimental animals at PND 160. A: Tail moment (a.u). B: Tail intensity (%). Values expressed as mean ± SD, Kruskal-Wallis with Dunn’s a posteriori test. For all groups with the same letter superscript, the difference between the means is not statistically significant. Tail moment: MTX vs control, p = 0.0143. Tail intensity: MTX vs control, p = 0.0099.
Comet assay – Liver
The parameters Tail Moment (Fig. 2a) and Tail Intensity (Fig. 2b) showed significant differences among the experimental groups. 5-FU (i.e Tail Moment 16.9 ± 19.6; Tail Intensity 28.41 ± 24.05) presented an increase when compared to Control (i.e Tail Moment 16.9 ± 19.6; Tail Intensity 26 ± 25.1), MTX (i.e Tail Moment 17 ± 17.7), and 5-FU + MTX (i.e Tail Moment 14.6 ± 18.9; Tail intensity 21.75 ± 24.04). On the other hand, 5-FU + MTX showed a decrease in these parameters in comparison to Control and MTX.
Fig. 2.
Comet assay in liver of experimental animals at DPN 160. A: Tail moment (a.u). B: Tail intensity (%). Values expressed as mean ± SD, Kruskal-Wallis with Dunn’s a posteriori test. For all groups with the same letter superscript, the difference between the means is not statistically significant. Tail moment: 5-FU vs control (p = 0.0052), MTX (p = 0.0043), 5-FU + MTX (<0.0001), 5-FU + MTX vs control (p = 0.0101), MTX (P = <0.0001).
Comet assay—Testis
The Tail Moment (Fig. 3a) showed a significant increase in 5-FU (2.8 ± 5.6), MTX (2.4 ± 5.1), and 5-FU + MTX (2.6 ± 5.1) when compared to Control (1.8 ± 3.9). The same pattern is shown in Tail Intensity (Fig. 3b) among the groups 5-FU (6.37 ± 10.7), MTX (5.18 ± 8.85), and 5-FU + MTX (5.7 ± 9.65) compared to Control (3.8 ± 6.5).
Fig. 3.
Comet assay in testis of experimental animals at PND 160. A: Tail moment (a.u). B: Tail intensity (%). Values expressed as mean ± SD, Kruskal-Wallis with Dunn’s a posteriori test. For all groups with the same letter superscript, the difference between the means is not statistically significant. Tail moment: 5-FU vs control (P < 0.0001), MTX vs control (p = 0.035), 5-FU + MTX vs control (p = 0.0027). Tail intensity: 5-FU vs control (P < 0.0001), MTX (p = 0.0289), and 5-FU + MTX vs control (p = 0.0053).
Micronucleus assay and cytotoxicity analysis
No significant difference among the experimental groups was observed regarding the presence of micronuclei in the bone marrow of the experimental animals at PND 160. However, there was a decrease statistically significant in the PCE:NCE ratio between MTX and the groups Control and 5-FU, as shown in Table 2.
Table 2.
Mutagenicity and cytotoxicity parameters in bone marrow samples from experimental animals at PND 160.
| Experimental groups | n |
MNi
1
(2000 cells/animal) |
Ratio PCE: NCE |
|---|---|---|---|
| Control | 9 | 0.36 ± 0.50 | 1.05 ± 0.31a |
| 5-FU | 10 | 0.58 ± 0.65 | 0.97 ± 0.64a |
| MTX | 10 | 0.49 ± 0.58 | 0.60 ± 0.23b |
| 5-FU + MTX | 9 | 0.23 ± 0.48 | 0.84 ± 0.33a,b |
Values expressed as Mean ± SD, One-Way ANOVA with Tukey’s a posteriori test.1 Values expressed as Mean ± SD, Kruskal-Wallis. For all groups with the same letter superscript, the difference between the means is not statistically significant.
Discussion
The present study addresses a current issue of extreme relevance worldwide: the potential risks of long-term exposure to chemotherapeutic agents at low concentrations on general health. This is an emerging problem since the consumption of antineoplastic drugs has increased in the last decades. Furthermore, the conventional effluent and drinking water treatment do not possess high efficiency in removing these drugs, their metabolites and many other pharmaceuticals.41,42 This is the first study to investigate the potential genetic toxicity of 5-FU and MTX, alone or combined at low concentrations, after extended exposure, using rats as an experimental model, representing an asset to pharmaceutical regulation.
The genetic toxicity exerted by chemotherapeutics under the present experimental conditions, i.e. environmental concentrations, has similarities with the findings from literature using high doses of these compounds. The decrease in the ratio of PCE in MTX is also observed by Salem et al.,43 even though the concentration used in the present study is six orders of magnitude lower than the one used by the authors (i.e. single injection of 10 mg/kg of MTX). In a precedent study conducted by Kasahara et al.,44 mice were exposed to MTX (4 or 20 mg/kg/day) in single or multiple injections. The authors reported decreased levels of DHFR in animals that received multiple injections of MTX. This imbalance can lead to unrepaired DNA damage or errors in the processes of replication of the genetic material, which would result in the inhibition of the complete cell cycle, culminating in premature cell death. This reasoning can be applied in the present study, as a reduced number of young erythrocytes (i.e. PCE) was observed, indicating bone marrow suppression, possibly due to this sensitivity of the cell cycle to external insults. Although in the present study, the number of micronucleated erythrocytes (MNE) did not change among the experimental groups, its presence would represent unrepairable DNA damage.
It is known that the Comet assay is able to detect recent lesions to the genetic material that can be repaired, such as single-strand breaks and alkaline site lesions.45 The increase in the damage rate to the genetic material observed in the group that received MTX at environmentally relevant concentrations (10 ng/L) is similarly observed in the literature that investigated its effects at therapeutic doses. According to a study performed by Rjiba-Touati et al.,46 the treatment of male Wistar rats with MTX (20 mg/kg) caused a significant increase in DNA fragmentation in the liver and kidney in the Comet assay. Similarly, Mughal et al.47 who used the same concentration to expose Sprague–Dawley rats for up to 96 h, reported alterations in several parameters in the Comet assay using peripheral blood samples. As observed in the present study, the experimental groups that received environmental concentrations of these anticancer drugs showed increased DNA fragmentation in the liver, testis, and peripheral blood in the two observed parameters. It is important to consider that the testis is the organ programmed to undergo constant cell division (mitosis and meiosis) during spermatogenesis. Therefore, this organ can be a target tissue for antineoplastic drugs. The observed increase in DNA fragmentation can compromise several fertility parameters, even at low concentrations, as observed in a previous study conducted in our laboratory.35
Tian and Cronstein11 described the mechanism of action of MTX through the inhibition of thymidylate synthase pathways and the by-products of this reaction, blocking purine and pyrimidine synthesis. This can lead to several toxicities, such as bone marrow suppression and liver toxicity, even at low concentrations as used in the present study. In addition, another pathway of toxicity can be attributed to oxidative damage to genetic material, as evidenced by Zeng et al.48 Their experiment using 5-FU (150 mg/kg) in mice was able to induce a decrease in the enzymatic activities of superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT). This indicates that 5-FU can damage the liver by limiting its antioxidant capacity, resulting in an acute inflammatory response and apoptosis. In a complementary study conducted in our laboratory,35 we observed intense immunolabeling of 8-OHdG (indicative of oxidative damage to genetic material) in germinative and somatic cells of the testis after exposure to MTX or 5-FU, either isolated or combined. This further supports the relevance of this oxidative stress scenario.
Considering that the combination between compounds is, in most situations, less than additive due to the sequential sites of action of the different drugs,49 it is not surprising that the association between MTX and 5-FU led to antagonist effects under our conditions. This reveals a more active function of MTX and its mechanisms than 5-FU at the concentration used in this experiment, probably due to the fact that low concentrations in mixtures can present the same toxicity as a higher single dose, which was observed previously in different studies.25
According to Vanneste et al.,50 unintentional human exposure occupationally demands attention to the link between concentration and the extent of exposure. The present subchronic experiment with 5-FU and MTX also demonstrates the relevance of time as a factor for toxicity at constant exposure to observe subtle but genotoxic and cytotoxic effects even at environmentally relevant concentrations. Nevertheless, the possibility of chromosomal alterations resulting in mutagenicity cannot be dismissed. Once DNA damage occurs, it has the potential to lead to MNE formation. The critical factor influencing this process is the time of exposure. The type of damage observed both in the comet assay and the MNE assay differs according to the length of exposure, in which MNE depends on medium/long, i.e. chronic exposure, and the stage of the cell cycle.51
Conclusion
This study highlights the emerging contaminants, 5-FU and MTX, as significant risk factors for the integrity of genetic material, leading to DNA fragmentation in the peripheral blood, liver, and testis, along with bone marrow suppression. These findings raise concerns about the adverse effects on the general population worldwide who are unintentionally chronically exposed to these drugs at trace concentrations.
Author contributions
Paloma da Cunha de Medeiros: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Project administration, Writing – original draft. E A Nunes: Methodology, Investigation. G R M Barcelos: Methodology, Investigation. Juliana E Perobelli: Conceptualization, Supervision, Resources, Project administration, Writing – review & editing, Funding acquisition.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) [grant number # n° 2019/04108–7] and the Brazilian National Council for Scientific and Technological Development (CNPq) [grant number 307022/2022–6].
Conflict of interest statement. There are no conflicts of interest to declare.
Contributor Information
P da Cunha de Medeiros, Laboratory of Experimental Toxicology – LATOEX, Universidade Federal de São Paulo, Instituto do Mar, Carvalho de Mendonça, 144, Santos 11070-100, SP, Brazil.
E A Nunes, Department of Biosciences, Laboratory of Gene-Environmental Interactions in Toxicology – GENINTOX, Universidade Federal de São Paulo, XV de novembro 195, sala 614, Santos 11.010-151, SP, Brazil.
G R M Barcelos, Department of Biosciences, Laboratory of Gene-Environmental Interactions in Toxicology – GENINTOX, Universidade Federal de São Paulo, XV de novembro 195, sala 614, Santos 11.010-151, SP, Brazil.
J E Perobelli, Laboratory of Experimental Toxicology – LATOEX, Universidade Federal de São Paulo, Instituto do Mar, Carvalho de Mendonça, 144, Santos 11070-100, SP, Brazil.
References
- 1. Yadav A, Rene ER, Mandal MK, Dubey KK. Threat and sustainable technological solution for antineoplastic drugs pollution: review on a persisting global issue. Chemosphere. 2021:263:128285. [DOI] [PubMed] [Google Scholar]
- 2. Shiga S, Machida T, Yanada T, Machida M, Hirafuji M, Iizuka K. The role of nitric oxide in small intestine differs between a single and a consecutive administration of methotrexate to rats. J Pharmacol Sci. 2020:143(1):30–38. [DOI] [PubMed] [Google Scholar]
- 3. Ciaffaglione V, Modica MN, Pittalà V, Romeo G, Salerno L, Intagliata S. Mutual prodrugs of 5-fluorouracil: from a classic chemotherapeutic agent to novel potential anticancer drugs. ChemMedChem. 2021:16(23):3496–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sethy C, Kundu CN. 5-fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed Pharmacother. 2021:137:111285. [DOI] [PubMed] [Google Scholar]
- 5. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003:3(5):330–338. [DOI] [PubMed] [Google Scholar]
- 6. Naren G, Guo J, Bai Q, Fan N, Nashun B. Reproductive and developmental toxicities of 5-fluorouracil in model organisms and humans. Expert Rev Mol Med. 2022:24:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian ZY, Du GJ, Xie SQ, Zhao J, Gao WY, Wang CJ. Synthesis and bioevaluation of 5-fluorouracil derivatives. Molecules (Basel, Switzerland). 2007:12(11):2450–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Innocenti F, Danesi R, Bocci G, Natale G, Del Tacca M. 5-fluorouracil catabolism to 5-fluoro-5, 6-dihydrouracil is reduced by acute liver impairment in mice. Toxicol Appl Pharmacol. 2005:203(2):106–113. [DOI] [PubMed] [Google Scholar]
- 9. Maksimovic V, Pavlovic-Popovic Z, Vukmirovic S, Cvejic J, Mooranian A, Al-Salami H, et al. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol Biol Rep. 2020:47(6):4699–4708. [DOI] [PubMed] [Google Scholar]
- 10. Chan ES, Cronstein BN. Methotrexate—how does it really work? Nat Rev Rheumatol. 2010:6(3):175–178. [DOI] [PubMed] [Google Scholar]
- 11. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007:65(3):168–173. [PubMed] [Google Scholar]
- 12. Hamed KM, Dighriri IM, Baomar AF, Alharthy BT, Alenazi FE, Alali GH, Alenazy RH, Alhumaidi NT, Alhulayfi DH, Alotaibi YB, et al. Overview of methotrexate toxicity: a comprehensive literature review. Cureus. 2022:14(9):e29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams M, Urban C. Pharmacology connections to nursing practice. 2nd ed. Boston, MA: Pearson; 2013. p. 752.1031-1032. [Google Scholar]
- 14. Iqbal S, Armaghani A, Aiyer R, Kazory A. Methotrexate nephrotoxicity: novel treatment, new approach. J Oncol Pharm Pract. 2013:19(4):373–376. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Ye C, Li J, Yu X, Feng M. Treatment-driven removal efficiency, product formation, and toxicity evolution of antineoplastic agents: current status and implications for water safety assessment. Water Res. 2021:206:117729. [DOI] [PubMed] [Google Scholar]
- 16. Goirand F, Lemaitre F, Launay M, Tron C, Chatelut E, Boyer JC, Bardou M, Schmitt A. How can we best monitor 5-FU administration to maximize benefit to risk ratio? Expert Opin Drug Metab Toxicol. 2018:14(12):1303–1313. [DOI] [PubMed] [Google Scholar]
- 17. Connor TH, DeBord DG, Pretty JR, Oliver MS, Roth TS, Lees PSJ, McDiarmid MA. Evaluation of antineoplastic drug exposure of health Care Workers at Three University-Based US cancer Centers. J Occup Environ Med. 2010:52(10):1019–1027. [DOI] [PubMed] [Google Scholar]
- 18. Škvára P, Santana-Viera S, Montesdeoca-Esponda S, Mordačíková E, Santana-Rodríguez JJ, Vojs Staňová A. Determination of 5-fluorocytosine, 5-fluorouracil, and 5-fluorouridine in hospital wastewater by liquid chromatography-mass spectrometry. J Sep Sci. 2020:43(15):3074–3082. [DOI] [PubMed] [Google Scholar]
- 19. Kračun-Kolarević M, Kolarević S, Atanacković A, Marković V, Gačić Z, Paunović M, Vuković-Gačić B. Effects of 5-fluorouracil, etoposide and CdCl2 in aquatic Oligochaeta Limnodrilus udekemianus Claparede (Tubificidae) measured by comet assay. Water Air Soil Pollut. 2015:226(8):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosjek T, Perko S, Žigon D, Heath E. Fluorouracil in the environment: analysis, occurrence, degradation and transformation. J Chromatogr A. 2013:1290:62–72. [DOI] [PubMed] [Google Scholar]
- 21. Yin J, Shao B, Zhang J, Li K. A preliminary study on the occurrence of cytostatic drugs in hospital effluents in Beijing, China. Bull Environ Contam Toxicol. 2010:84(1):39–45. [DOI] [PubMed] [Google Scholar]
- 22. Kovalova L, McArdell CS, Hollender J. Challenge of high polarity and low concentrations in analysis of cytostatics and metabolites in wastewater by hydrophilic interaction chromatography/tandem mass spectrometry. J Chromatogr A. 2009:1216(7):1100–1108. [DOI] [PubMed] [Google Scholar]
- 23. Catastini C, Mullot JU, Boukari S, Mazellier P, Levi Y, Cervantes P, Ormsby JN. Identification de molécules anticancéreuses dans les effluents hospitaliers. European journal of water quality. 2008:39(2):171–180. [Google Scholar]
- 24. Mahnik SN, Lenz K, Weissenbacher N, Mader RM, Fuerhacker M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere. 2007:66(1):30–37. [DOI] [PubMed] [Google Scholar]
- 25. Viegas S, Ladeira C, Costa-Veiga A, Perelman J, Gajski G. Forgotten public health impacts of cancer–an overview. Arch Ind Hyg Toxicol. 2017:68(4):287–297. [DOI] [PubMed] [Google Scholar]
- 26. Khan HK, Rehman MYA, Malik RN. Fate and toxicity of pharmaceuticals in water environment: an insight on their occurrence in South Asia. J Environ Manag. 2020:271:111030–111049. 10.1016/j.jenvman.2020.111030. [DOI] [PubMed] [Google Scholar]
- 27. U.S. Environmental Protection Agency . Reproductive and fertility effects. In: Pesticide assessment guidelines, subdivision F. Hazard evaluation: human and domestic animals. Washington, D.C.: Office of Pesticides and Toxic Substances (OPPTS); 1982. EPA540/9-82-025. [Google Scholar]
- 28. U.S. Environmental Protection Agency . Toxic substances control act test guidelines: final rules. Fed Regist. 1985b:50(188):39426–39436. [PubMed] [Google Scholar]
- 29. U.S. Environmental Protection Agency . Guidelines for reproductive toxicity risk assessment. Fed Regist. 1996:61(212):56274–56322. [Google Scholar]
- 30. Besse J-P, Latour J-F, Garric J. Anticancer drugs in surface waters. Environ Int. 2012:39(1):73–86. [DOI] [PubMed] [Google Scholar]
- 31. Brooker V, Halsall C, Llewellyn N, Johnson A, Williams R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci Total Environ. 2014:473:159–170. [DOI] [PubMed] [Google Scholar]
- 32. Johnson AC, Jurgens MD, Williams RJ, Kümmerer K, Kortenkamp A, Sumpter JP. Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study. J Hydrol. 2008:348(1–2):167–175. [Google Scholar]
- 33. Garcia-Ac A, Segura PA, Viglino L, Fürtös A, Gagnon C, Prévost M, Sauvé S. On-line solid-phase extraction of large-volume injections coupled to liquid chromatography-tandem mass spectrometry for the quantitation and confirmation of 14 selected trace organic contaminants in drinking and surface water. J Chromatogr A. 2009:1216(48):8518–8527. [DOI] [PubMed] [Google Scholar]
- 34. European Medicines Agency (EMEA) . Guideline on the environmental risk assessment of medicinal products for human use Doc. Ref.EMEA/CHMP/SWP/4447/00.London: EMEA; 2006. [Google Scholar]
- 35. King-Herbert A, Vasbinder MA. Toxicology. The Laboratory Rat. 2020:849–862. 10.1016/b978-0-12-814338-4.00022-2. [DOI] [Google Scholar]
- 36. Cunha de Medeiros P, Nascimento CC, Perobelli JE. Antineoplastic drugs in environmentally relevant concentrations cause endocrine disruption and testicular dysfunction in experimental conditions. Environ Toxicol Pharmacol. 2023:100:104122. [DOI] [PubMed] [Google Scholar]
- 37. Møller P, Azqueta A, Boutet-Robinet E, Koppen G, Bonassi S, Milić M, Gajski G, Costa S, Teixeira JP, Costa Pereira C, et al. Minimum information for reporting on the comet assay (MIRCA): recommendations for describing comet assay procedures and results. Nat Protoc. 2020:15(12):3817–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. U.S. Environmental Protection Agency . Health effects test guidelines. In: OPPTS 870.5395 Mammalian erythrocyte micronucleus test. Washington, D.C.: Office of Prevention, Pesticides and Toxic Substance (OPPTS); 1998. EPA712-C-98-226.
- 39. Silva J, Freitas TR, Heuser V, Marinho JR, Erdtmann B. Genotoxicity biomonitoring in coal regions using wild rodent Ctenomys torquatus by comet assay and micronucleus test. Environ Mol Mutagen. 2000:35(4):270–278. [PubMed] [Google Scholar]
- 40. Organisation for Economic Co-operation and Development (OECD) . Test No. 474: Mammalian Erythrocyte Micronucleus Test. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris; 2016. 10.1787/9789264264762-en. [DOI]
- 41. Patel M, Kumar R, Kishor K, Mlsna T, Pittman CU Jr, Mohan D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem Rev. 2019:119(6):3510–3673. [DOI] [PubMed] [Google Scholar]
- 42. Verlicchi P, Al Aukidy M, Zambello E. Occurrence of pharmaceutical compounds inurban wastewater: removal, mass load and environmental risk after a secondary treatment—a review. Sci Total Environ. 2012:429:123–155. [DOI] [PubMed] [Google Scholar]
- 43. Salem NIS, Noshy MM, Said AA. Modulatory effect of curcumin against genotoxicity and oxidative stress induced by cisplatin and methotrexate in male mice. Food Chem Toxicol. 2017:105:370–376. [DOI] [PubMed] [Google Scholar]
- 44. Kasahara Y, Nakai Y, Miura D, Yagi K, Hirabayashi K, Makita T. Mechanism of induction of micronuclei and chromosome aberrations in mouse bone marrow by multiple treatments of methotrexate. Mutat Res. 1992:280(2):117–128. [DOI] [PubMed] [Google Scholar]
- 45. De Oliveira NC, Sarmento MS, Nunes EA, Porto CM, Rosa DP, Bona SR, et al. Rosmarinic acid as a protective agent against genotoxicity of ethanol in mice. Food Chem Toxicol. 2012:50(5):1208–1214. [DOI] [PubMed] [Google Scholar]
- 46. Rjiba-Touati K, Amara I, Bousabbeh M, Salem IB, Azzebi A, Guedri Y, Abid S. Recombinant human erythropoietin prevents etoposide- and methotrexate-induced toxicity in kidney and liver tissues via the regulation of oxidative damage and genotoxicity in Wistar rats. Human & Experimental Toxicology. 2017:37(8):848–858. [DOI] [PubMed] [Google Scholar]
- 47. Mughal A, Vikram A, Ramarao P, Jena GB. Micronucleus and comet assay in the peripheral blood of juvenile rat: establishment of assay feasibility, time of sampling and the induction of DNA damage. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010:700(1–2):86–94. [DOI] [PubMed] [Google Scholar]
- 48. Zeng D, Wang Y, Chen Y, Li D, Li G, Xiao H, et al. Angelica polysaccharide antagonizes 5-FU-induced oxidative stress injury to reduce apoptosis in the liver through Nrf2 pathway. Front Oncol. 2021:11:720620. 10.3389/fonc.2021.720620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gottlieb A, Stein GY, Oron Y, Ruppin E, Sharan R. INDI: a computational framework for inferring drug interactions and their associated recommendations. Mol Syst Biol. 2012:8(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vanneste D, Verscheure E, Srinivasan AN, Godderis L, Ghosh M. Systematic review of genotoxicity induced by occupational exposure to antineoplastic drugs. Arch Toxicol. 2023:97(6):1453–1517. [DOI] [PubMed] [Google Scholar]
- 51. Maluf SW. Monitoring DNA damage following radiation exposure using cytokinesis–block micronucleus method and alkaline single-cell gel electrophoresis. Clin Chim Acta. 2004:347(1–2):15–24. [DOI] [PubMed] [Google Scholar]