Abstract
Background:
A collaborative, data-to-care strategy to identify persons with HIV (PWH) newly out-of-care, combined with an active public health intervention, significantly increases the proportion of PWH re-engaged in HIV care. We assessed this strategy’s impact on durable viral suppression (DVS).
Methods:
A multisite, prospective randomized controlled trial for out-of-care individuals using a data-to-care strategy and comparing public health field services to locate, contact, and facilitate access to care versus the standard of care. DVS was defined as the last viral load, the viral load at least 3 months before, and any viral load between the 2 were all <200 copies/mL during the 18-month postrandomization. Alternative definitions of DVS were also analyzed.
Results:
Between August 1, 2016–July 31, 2018, 1893 participants were randomized from Connecticut (n = 654), Massachusetts (n = 630), and Philadelphia (n = 609). Rates of achieving DVS were similar in the intervention and standard-of-care arms in all jurisdictions (all sites: 43.4% vs 42.4%, P = 0.67; Connecticut: 46.7% vs 45.0%, P = 0.67; Massachusetts: 40.7 vs 44.4%, P = 0.35; Philadelphia: 42.4% vs 37.3%, P = 0.20). There was no association between DVS and the intervention (RR: 1.01, CI: 0.91–1.12; P = 0.85) adjusting for site, age categories, race/ethnicity, birth sex, CD4 categories, and exposure categories.
Conclusion:
A collaborative, data-to-care strategy, and active public health intervention did not increase the proportion of PWH achieving DVS, suggesting additional support to promote retention in care and antiretroviral adherence may be needed. Initial linkage and engagement services, through data-to-care or other means, are likely necessary but insufficient for achieving DVS for all PWH.
Keywords: data to care, randomized trial, HIV, health departments, durable viral suppression
INTRODUCTION
Of the 1.2 million people in the United States with HIV persons with HIV (PWH) in 2019, an estimated 66% received some HIV care, 57% had achieved viral suppression, and 50% were retained in care.1 Retention in medical care and HIV viral suppression are beneficial to both the individual and public health.2-9 For the individual, mortality and opportunistic events decrease with a concurrent increase in life expectancy.2-7 For the community, ongoing transmission of HIV decreases dramatically.7,8
The primary goal of antiretroviral therapy is sustained or durable viral suppression (DVS), a downstream indicator of HIV control.9 Studies have highlighted the importance of measuring viral suppression longitudinally because this reflects the status of HIV care over time.7,10,11 The percentage of PWH with DVS provides a helpful indicator for monitoring national HIV prevention and treatment efforts.12 The durability of viral suppression can also help define the best timing of targeted adherence strategies and intensive viral load monitoring in individuals with multiple challenges to antiretroviral therapy adherence.12
Given the importance of DVS, the Ending the HIV Epidemic in the United States (EHE) initiative seeks to reduce the number of new HIV infections by 90% and to increase the percentage of persons virally suppressed to 95% by 2030.13 To achieve this goal, evidence-based strategies are needed to identify, engage, and retain PWH in care and to maximize the benefits of antiretroviral therapy.
Data-to-care (D2C) is a public health strategy that uses HIV surveillance and other data, such as clinical encounter data obtained from electronic health records, to support progress along the HIV care continuum and maximize viral suppression.14 Research suggests that D2C strategies involving close collaboration between clinical providers and health departments for identifying persons out of care and re-engagement activities may enhance efficiency and improve re-engagement outcomes.15-18 The influence of D2C strategies on DVS is less understood. One study suggested that combining D2C methods with intense short-term case management for up to 90 days can improve long-term viral suppression among those who achieved viral suppression.18
The Cooperative Re-Engagement Controlled Trial (CoRECT) was the first prospective randomized controlled trial to evaluate a D2C strategy among PWH newly out-of-care and implement an intervention to increase the number who engage or re-engage in HIV medical care, remain in medical care, and achieve viral suppression.15 The CoRECT study found D2C with an active public health intervention improved overall early re-engagement in care.15 This study provided evidence that this model can effectively identify, locate, and re-engage out-of-care persons, including people disproportionately affected by HIV. However, to fully understand D2C and CoRECT’s impact on the HIV care continuum, an analysis of durable viral suppression is needed. Here, we present findings from CoRECT on durable viral suppression.
METHODS
Study Design
CoRECT was designed to evaluate a D2C strategy to identify a newly out-of-care person, defined as a person who had documented HIV care at a collaborating CoRECT clinic within the last 12 months and who then subsequently disengaged from care. Participants had to be aged 18 years or older and had to be out-of-care by either or both the following criteria: (1) did not have a visit with a prescribing provider for more than 6 months or (2) no objective CD4 count or viral load test result reported to health department surveillance for more than 6 months since their last measurement. Three health departments (Connecticut Department of Public Health, Massachusetts Department of Public Health, and Philadelphia Department of Public Health) generated out-of-care lists using HIV laboratory surveillance data and 40 collaborating clinics generated newly out-of-care lists using appointment data. The combined out-of-care lists were discussed and reconciled during monthly case conferences attended by project staff from both the health department and HIV clinic. All patients deemed out-of-care at the case conference were then randomized using block randomization to either receive the clinic’s standard-of-care (SOC) linkage and engagement in care services or an active health department field services intervention. All randomizations were performed at the individual level but stratified by participating clinics in each of the 3 study sites. Each health department created its own protocol for patient enrollment, case conferencing, and active public health intervention within the context of CoRECT-specified definitions.
Standard of care varied at the 40 clinical sites involved in the trial but included communication through telephone calls, letters, or e-mail. Outreach may have been performed by nurses, front desk staff, or case managers. Clinic staff were asked to work with disease intervention specialists if contacted to facilitate fast-track scheduling for participants. SOC may have changed over the course of this study in response to study participation or for other reasons external to this study. The intervention differed in composition and duration at each site (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/C30). Recruitment began in all 3 jurisdictions between August–October 2016 and was completed by July 2018. Participants were followed for a period of 18 months after randomization. Local IRB ethical approval was received for each jurisdiction.
TABLE 1.
Percentage Achieving Durable Viral Suppression by Demographic Characteristics Across all Sites and Within Each Site’s Study Arms
| Characteristics | Intervention N (%) | Standard of Care N (%) | P |
|---|---|---|---|
| Total | |||
| All sites | 415/958 (43.3) | 396/935 (42.4) | 0.67 |
| Connecticut (CT) | 155/332 (46.7) | 145/322 (45.0) | 0.67 |
| Massachusetts (MA) | 129/317 (40.7) | 139/313 (44.4) | 0.35 |
| Philadelphia (PHL) | 131/309 (42.4) | 112/300 (37.3) | 0.20 |
| Male (birth sex) | |||
| All sites | 305/672 (45.4) | 267/644 (41.5) | 0.15 |
| CT | 110/211 (52.1) | 83/197 (42.1) | 0.04 |
| MA | 95/226 (42.0) | 107/232 (46.1) | 0.38 |
| PHL | 100/235 (42.6) | 77/215 (35.8) | 0.14 |
| Race/Ethnicity | |||
| All sites | |||
| Non-Hispanic Black | 182/460 (39.6) | 184/455 (40.4) | 0.79 |
| Non-Hispanic White | 109/237 (46.0) | 117/245 (47.8) | 0.57 |
| Hispanic/Latino | 117/239 (49.0) | 81/213 (38.0) | 0.12 |
| Other | 7/22 (31.8) | 12/22 (54.5) | 0.13 |
| CT | |||
| Non-Hispanic Black | 49/123 (39.4) | 54/141 (38.3) | 0.80 |
| Non-Hispanic White | 30/63 (47.6) | 38/72 (52.8) | 0.55 |
| Hispanic/Latino | 75/138 (54.4) | 52/104 (50.0) | 0.50 |
| Other | 1/8 (12.5) | 1/5 (20.0) | 1.00 |
| MA | |||
| Non-Hispanic Black | 47/134 (35.1) | 61/119 (51.3) | 0.01 |
| Non-Hispanic White | 54/113 (47.8) | 55/118 (46.6) | 0.86 |
| Hispanic/Latino | 25/65 (38.5) | 18/69 (26.1) | 0.13 |
| Other | 3/5 (60.0) | 5/7 (71.4) | 1.00 |
| PHL | |||
| Non-Hispanic Black | 86/203 (42.4) | 69/195 (35.4) | 0.15 |
| Non-Hispanic White | 25/61 (41.0) | 26/55 (47.3) | 0.50 |
| Hispanic/Latino | 17/36 (47.2) | 11/40 (27.5) | 0.08 |
| Other | 3/9 (33.3) | 6/10 (60.0) | 0.37 |
| Age, years | |||
| All sites | |||
| 18–29 | 42/133 (31.6) | 38/126 (30.2) | 0.80 |
| 30–39 | 77/213 (36.2) | 83/221 (37.6) | 0.76 |
| 40–49 | 107/243 (44.0) | 99/234 (42.3) | 0.70 |
| 50–59 | 127/251 (50.6) | 121/258 (46.9) | 0.40 |
| 60 years or older | 62/118 (52.5) | 54/94 (57.5) | 0.48 |
| CT | |||
| 18–29 | 12/40 (30.0) | 14/51(27.5) | 0.79 |
| 30–39 | 23/59 (39.0) | 24/59 (40.7) | 0.85 |
| 40–49 | 35/90 (39.0) | 36/82 (43.9) | 0.50 |
| 50–59 | 55/94 (58.5) | 42/85 (49.4) | 0.22 |
| 60 years or older | 30/49 (61.2) | 29/45 (64.4) | 0.75 |
| MA | |||
| 18–29 | 10/32 (31.3) | 11/29 (37.9) | 0.58 |
| 30–39 | 28/77 (36.4) | 33/74 (44.6) | 0.30 |
| 40–49 | 34/77 (44.2) | 31/78 (39.7) | 0.58 |
| 50–59 | 39/89 (43.8) | 47/99 (47.5) | 0.62 |
| 60 years or older | 18/42 (42.9) | 16/31 (51.6) | 0.46 |
| PHL | |||
| 18–29 | 20/61 (32.8) | 13/46 (28.3) | 0.62 |
| 30–39 | 26/77 (33.8) | 26/88 (29.6) | 0.56 |
| 40–49 | 38/76 (50.0) | 32/74 (43.2) | 0.41 |
| 50–59 | 33/68 (48.5) | 32/74 (43.2) | 0.53 |
| 60 years or older | 14/27 (51.9) | 9/18 (50.0) | 0.90 |
| Transmission category * | |||
| All sites | |||
| MSM | 167/376 (44.4) | 209/359 (58.2) | 0.18 |
| IDU | 95/198 (49.0) | 79/174 (45.4) | 0.62 |
| HET | 92/213 (43.2) | 95/223 (42.6) | 0.90 |
| Other | 61/171 (35.7) | 80/179 (44.7) | 0.09 |
| CT | |||
| MSM | 42/94 (44.7) | 38/100 (38.0) | 0.34 |
| IDU | 55/95 (57.9) | 44/83 (53.0) | 0.51 |
| HET | 37/97 (38.1) | 42/93 (45.2) | 0.33 |
| Other | 21/46 (45.7) | 21/46 (45.7) | 1.00 |
| MA | |||
| MSM | 64/133 (48.1) | 63/129 (48.8) | 0.91 |
| IDU | 9/34 (26.5) | 13/40 (32.5) | 0.57 |
| HET | 24/47 (51.1) | 17/43 (39.5) | 0.27 |
| Other | 32/103 (31.1) | 46/101 (45.5) | 0.03 |
| PHL | |||
| MSM | 61/149 (41) | 41/130 (31.5) | 0.10 |
| IDU | 31/69 (44.9) | 22/51 (43.1) | 0.85 |
| HET | 31/69 (44.9) | 36/87 (41.4) | 0.66 |
| Other | 8/22 (36.4) | 13/32 (40.6) | 0.75 |
| Viral suppression in the year before randomization | |||
| All sites | |||
| No | 33/180 (18.3) | 30/184 (16.3) | 0.61 |
| Yes | 286/538 (53.2) | 287/524 (54.8) | 0.60 |
| CT | |||
| No | 12/57 (21.1) | 11/54 (20.4) | 0.93 |
| Yes | 86/159 (54.1) | 88/163 (54.0) | 0.99 |
| MA | |||
| No | 9/54 (16.7) | 7/52 (13.5) | 0.64 |
| Yes | 100/188 (53.2) | 120/202 (59.4) | 0.22 |
| PHL | |||
| No | 12/69 (17.4) | 12/78 (15.4) | 0.74 |
| Yes | 100/191 (52.4) | 79/159 (49.7) | 0.62 |
| CD4 count in the year before randomization | |||
| All sites | |||
| ≤50 cells/μL | 82/208 (39.4) | 73/205 (35.6) | 0.42 |
| 51–199 cells/μL | 117/275 (42.6) | 125/278 (45.0) | 0.57 |
| 200–350 cells/μL | 97/205 (47.3) | 72/173 (41.6) | 0.27 |
| 351–499 cells/μL | 55/127 (43.3) | 60/140 (42.9) | 0.94 |
| ≥ 500 cells/μL | 64/143 (44.8) | 66/139 (47.5) | 0.65 |
| CT | |||
| ≤50 cells/μL | 33/78 (42.3) | 23/71 (32.4) | 0.21 |
| 51–199 cells/μL | 51/107 (47.7) | 56/106 (52.8) | 0.45 |
| 200–350 cells/μL | 19/45 (42.2) | 28/56 (50.0) | 0.44 |
| 351–499 cells/μL | 23/42 (54.8) | 17/40 (42.5) | 0.27 |
| ≥500 cells/μL | 29/60 (48.3) | 21/49 (42.9) | 0.57 |
| MA | |||
| ≤50 cells/μL | 27/69 (39.1) | 26/58 (44.8) | 0.52 |
| 51–199 cells/μL | 32/82 (39.0) | 32/83 (38.6) | 0.95 |
| 200–350 cells/μL | 36/72 (50.0) | 25/58 (43.1) | 0.43 |
| 351–499 cells/μL | 16/47 (34.0) | 28/60 (46.7) | 0.19 |
| ≥500 cells/μL | 18/47 (38.3) | 28/54 (51.9) | 0.17 |
| PHL | |||
| ≤50 cells/μL | 22/61 (36.1) | 24/76 (31.6) | 0.58 |
| 51–199 cells/μL | 34/86 (39.5) | 37/89 (41.6) | 0.78 |
| 200–350 cells/μL | 42/88 (47.7) | 19/59 (32.2) | 0.06 |
| 351–499 cells/μL | 16/38 (42.1) | 15/40 (37.5) | 0.68 |
| ≥500 cells/μL | 17/36 (47.2) | 17/36 (47.2) | 1.00 |
HET = heterosexual; IDU = injection drug use; MSM = men who have sex with men; Other = perinatal, no identified risk, or not in record. Year 2016–2019 (N= 1893).
Statistical Analysis
For this analysis, DVS was the primary outcome. DVS was defined by the study protocol as having at least 2 viral loads (VL) and fulfilling 3 criteria: (1) the last VL recorded in the 18-month follow-up period after randomization is less than 200 copies/mL, (2) the VL immediately before, but at least 3 months apart from, the last recorded VL is less than 200 copies/mL, and (3) all VL results between times (1) and (2) are less than 200 copies/mL. Additional analyses were conducted for varying definitions of DVS: (1) the above criteria with the last VL occurring at least 6 months postrandomization, (2) the above criteria with VLs restricted to 6–18 months, and (3) all VLs postrandomization being less than 200 copies/mL, including if only one VL was recorded. An intent-to-treat approach was used meaning all participants randomized in the study were included in the analysis regardless of disposition or acceptance of the intervention. Missing VLs were presumed as failure. The number and interval of VL tests performed were variable.
The proportion of individuals who achieved DVS was calculated and then compared by study arm overall and for each of the 3 sites. DVS was also compared by demographic and clinical variables by study arm and overall. Demographic variables included sex at birth, current sex (including transgender), race/ethnicity, age (median, interquartile range; IQR) at the time of randomization, and HIV transmission category designated at the time of diagnosis. Clinical variables included time since HIV diagnosis, number of VL results (median, IQR), a categorical variable for the last VL before randomization (<200, 200–1,000, 1001–10,000, >10,000 copies/mL, and a categorical variable of the last CD4 count before randomization (<50, 51–199, 200–349, 350–499, ≥500 cells/mL). χ2, Fisher exact, and rank-sum tests (for continuous variables) were used to compare demographic and clinical characteristics between study arms. A log-binomial model was used to assess the relationship between DVS and the intervention as well as to identify possible factors associated with DVS. Risk ratios (RRs), or relative benefits, were estimated by pooling data from across the 3 sites while controlling for the aforementioned variables. All statistical analyses were conducted using SAS version 9.4. This trial is registered with ClinicalTrials.gov, Number NCT02693145.
Role of the Funding Source
This study was funded by the Division of HIV Prevention, Centers for Disease Control and Prevention. The funders participated in the study design, data collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
RESULTS
Baseline Characteristics
The number of newly out-of-care PWH randomized by the site was Connecticut (CT): 654 (332 intervention and 322 SOC); Massachusetts (MA): 630 (317 intervention and 313 SOC); Philadelphia (PHL): 609 (309 intervention and 300 SOC) for a total of 1893 (958 intervention and 935 SOC). Demographic variables did not differ by study arm at any of the 3 sites, although there were differences in study populations between jurisdictions. Full CoRECT study characteristics and demographics can be found in previous study publications.15 Notably, most participants were men: CT 62.4%, MA 72.7%, PHL 73.9%. The largest race/ethnicity group was non-Hispanic Black: CT 40.4%, MA 40.2%, and PHL 65.3%. Of those participants with data available, the last VL before randomization was <200 copies/mL in a large percentage: CT 74.2%, MA 78.9%, and PHL 70.4%. Demographic and clinical characteristics of all participants and percentage who achieved DVS by study arm are summarized in Table 1.
Durable Viral Suppression
The median number (IQR) of VL tests performed per participant comparing intervention with SOC across all sites was 2 (1–4) vs 2 (1–4), P = 0.17; CT: 3 (1–4) vs 2 (1–4), P = 0.44; MA: 2 (1–4) vs 2 (1–3), P = 0.61; PHL: 3 (2–4) vs 2 (1–4), P = 0.02. Of the 1893 participants during the observation period, 68.8%, 14.4%, and 16.8% had at least 2, one, and zero VLs recorded, respectively; those with zero or one VLs were defined as failures.
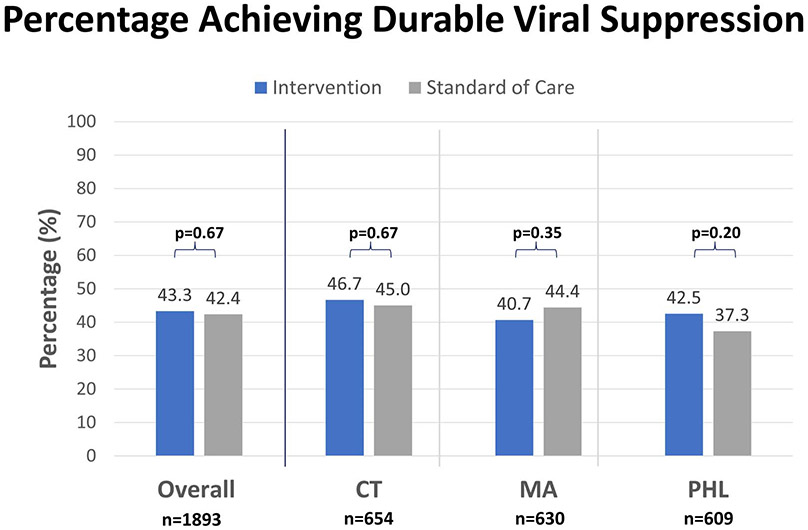
Overall, 811 (42.8%) of participants had 2 or more VLs and met criteria for DVS, 25.9% had 2 or more VL tests but did not meet criteria for DVS, and 31.2% did not have more than one test in the relevant period. The percentage of participants achieving DVS comparing intervention with SOC across all sites were 43.3% vs 42.4%, P = 0.67; CT: 46.7% vs 45%, P = 0.67; MA: 40.7% vs 44.4%, P = 0.35; PHL: 42.5% vs 37.3%, P = 0.20 (Table 1, Fig. 1). Proportions of persons achieving DVS using 3 alternative definitions of DVS were not significantly different between the intervention and SOC arms at each of the sites (Table 2). Among the participants who did not achieve DVS (n = 1082), overall, 501 (46.3%) had at least one suppressed VL measure of <200 copies/mL during the follow-up. When excluding PWH who never achieved viral suppression during the observation period, the overall rates for achieving DVS comparing intervention to SOC slightly increased to 61.9% vs 61.8%, P = 0.98.
FIGURE 1.
Percentage of adults with HIV participating in CoRECT achieving durable viral suppression* by study arm and location, year 2016–2019 (N = 1893). * Durable viral suppression was defined as fulfilling 3 criteria: (1) the last viral load recorded in the 18-month follow-up period after randomization is less than 200 copies/mL; (2) and the viral load immediately before, but at least 3 months apart from, the last recorded viral load is less than 200 copies/mL;and (3) all viral load results between times 1 and 2 are less than 200 copies/mL.
TABLE 2.
Proportions Achieving Durable Viral Suppression Among Adults With HIV Participating in CoRECT by Alternative Definitions and Study Arm, Year 2016–2019 (N = 1893)*
| Original Definition | Alternative Definition 1 | Alternative Definition 2 | Alternative Definition 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criteria | (1) The most recent viral load (VL) in the 18-month follow-up period after randomization is <200 copies/mL; and (2) the VL immediately before, but at least three months apart from, the most recent VL is <200 copies/mL; and (3) all VLs between time (1) and time (2) are <200 copies/mL |
Original definition plus the last VL occurring at least 6 months postrandomization |
Original definition but VLs restricted to 6–18 months† |
All VLs postrandomization are <200 copies/mL including if only 1 VL was recorded |
||||||||
| Site | Standard of Care N (%) |
Intervention N (%) |
P | Standard of Care N (%) |
Intervention N (%) |
P | Standard of Care N (%) |
Intervention N (%) |
P | Standard of Care N (%) |
Intervention N (%) |
P |
| All sites | 396/935 (42.3%) |
415/958 (43.3%) |
0.67 | 391/935 (41.8%) |
411/958 (42.9%) |
0.63 | 303/935 (32.4%) |
301/958 (31.42%) |
0.65 | 407/935 (43.5%) |
415/958 (43.3%) |
0.93 |
| CT | 145/322 (45.0%) |
155/332 (46.7%) |
0.67 | 144/322 (44.7%) |
154/332 (46.4%) |
0.67 | 113/322 (35.1%) |
117/332 (35.2%) |
0.97 | 148/322 (46.0%) |
159/332 (47.9%) |
0.62 |
| MA | 139/313 (44.4%) |
129/317 (40.7%) |
0.35 | 136/313 (44.4%) |
129/317 (40.7%) |
0.48 | 101/313 (32.3%) |
88/317 (27.8%) |
0.22 | 147/313 (47.0%) |
137/317 (43.2%) |
0.35 |
| PHL | 112/300 (37.3%) |
131/309 (42.4%) |
0.20 | 111/300 (37.0%) |
128/309 (41.4%) |
0.26 | 89/300 (29.7%) |
96/309 (31.1%) |
0.71 | 112/300 (37.3%) |
119/309 (38.5%) |
0.76 |
An intent-to-treat approach was used, meaning all participants randomized in this study were included in the analysis regardless of disposition or acceptance of the intervention and missing VLs were presumed as failure.
The number of individuals achieving durable viral suppression in Alternative Definition 2 are lower for each cell compared with the original definition due primarily to having <2 VLs available during the time frame of 6–18 months.
In the log-binomial model analysis (Table 3), the intervention was not associated with DVS when compared with the SOC and, overall, the relative benefit for achieving DVS varied by site, but we could not exclude the null of no difference between study arms (unadjusted RR CT 1.04, 95% CI: 0.88–1.22; P = 0.67; unadjusted RR MA 0.92, 95% CI: 0.76–1.10; P = 0.35; unadjusted RR PHL 1.14, 95% CI: 0.93–1.38; P = 0.20; unadjusted RR combined by site RR 1.02, 95% CI: 0.92–1.14; P = 0.67). This was also true after adjusting for site, age categories, race/ethnicity, birth sex, CD4 categories, and exposure categories (RR 0.98, 95% CI: 0.88–1.09; P = 0.71). Controlling for all variables in the model, the following were associated with DVS: being in the older age groups when compared with being in the 18–29 years age group (40–49 years old: RR 1.34, CI: 1.07–1.68; 50–59 years old: RR 1.43, CI: 1.14–1.79; 60 years or older: RR 1.58, CI: 1.24–2.02), being virally suppressed before randomization when compared with those who were not virally suppressed before randomization (RR 2.86, CI: 2.26–3.62; P > 0.0001), and having a higher CD4 count when compared with individuals with CD4 counts less than 50 cells/μL (CD4 count ≥500: RR 2.26, CI: 1.04–1.53; CD4 count 200–349: RR 1.30, CI: 1.09–1.54).
TABLE 3.
Unadjusted and Multivariate Logistic Regression Models to Assess the Relationship Between Achieving Durable Viral Suppression and the Intervention Among Adults With HIV Participating in CoRECT, Year 2016–2019, Pooled (N = 1893)
| Effect | Risk Ratio | 95% CI | |
|---|---|---|---|
| Unadjusted Risk Ratio by Site | |||
| Combined by site | 1.02 | 0.92 | 1.14 |
| Connecticut | 1.04 | 0.88 | 1.22 |
| Massachusetts | 0.92 | 0.76 | 1.10 |
| Philadelphia | 1.14 | 0.93 | 1.38 |
| Adjusted risk ratio | |||
| Intervention vs. Standard of Care | 0.98 | 0.88 | 1.09 |
| Connecticut vs. Philadelphia | 1.03 | 0.95 | 1.12 |
| Massachusetts vs. Philadelphia | 1.00 | 0.92 | 1.08 |
| Age (vs. 18–29 years) | |||
| 30–39 | 1.16 | 0.92 | 1.46 |
| 40–49 | 1.34 | 1.07 | 1.68 |
| 50–59 | 1.43 | 1.14 | 1.79 |
| 60 years or older | 1.58 | 1.24 | 2.02 |
| Race (vs. Non-Hispanic Black) | |||
| Hispanic/Latino | 1.00 | 0.86 | 1.16 |
| Non-Hispanic White | 1.10 | 0.95 | 1.27 |
| Other | 1.10 | 0.78 | 1.56 |
| Birth sex (vs. Male) | |||
| Female | 0.95 | 0.82 | 1.11 |
| Viral load suppression before randomization | |||
| Yes | 2.87 | 2.26 | 3.62 |
| CD4 count (vs. <50 cells/uL) | |||
| 51–199 | 1.08 | 0.91 | 1.28 |
| 200–349 | 1.30 | 1.09 | 1.54 |
| 350–499 | 1.13 | 0.92 | 1.39 |
| 500+ | 1.26 | 1.04 | 1.53 |
| Exposure category (vs. MSM, MSM/HET) * | |||
| IDU (IDU or HET/IDU) | 0.99 | 0.83 | 1.18 |
| MSM/IDU or MSM/HET/IDU | 1.08 | 0.90 | 1.29 |
| HET | 0.93 | 0.72 | 1.21 |
| Other | 1.07 | 0.878 | 1.30 |
HET = heterosexual; IDU = injection drug use; MSM = men who have sex with men; Other = perinatal, no identified risk, or not in record.
In a separate but similar model adding re-engagement at day 90 to examine whether it was a contributing factor, we found that higher CD4 (≥50 cells/μL) was again associated with DVS (RR 1.23, CI: 1.06–1.42) compared with <50 cells/μL; differences by study arm were not observed (RR 0.92, CI: 0.83–1.15) (results not displayed in the table).
DISCUSSION
The CoRECT, which was the first multisite randomized controlled trial to evaluate D2C linked with an active public health intervention and its effect on the HIV care continuum, showed that a collaborative, D2C strategy, and active public health intervention led by health departments increases the proportion of PWH re-engaged in HIV care and may improve retention in care and decrease time to viral suppression.15 In this extended analysis, we observed no increase in the proportion of PWH achieving durable viral suppression in the intervention arm.
Although the CoRECT demonstrated the effectiveness of the intervention to improve re-engagement, that did not translate to DVS in any of the 3 sites. One possible explanation is that the intervention focused primarily on re-engagement and did not continue to work with patients beyond re-engagement, which without sustained intervention, may have resulted in disengagement from care or in failure to translate into medication adherence.19 During intervention periods, services to assist with barriers to care such as obtaining health insurance and linking patients to community resources were rendered. Patients who are out-of-care and determined to be eligible for public health intervention may be at risk of recurrent disengagement from care because of the reasons that may have initially led them to become out-of-care. This issue, however, is beyond the scope of this analysis but deserves additional analysis. Other studies have shown insurance status, income, housing, transportation, mental health diagnoses, and substance use disorders may affect achieving DVS.7,20-24 These factors suggest essential social support services (and unmet needs) may affect DVS, consistent with barriers to linkage to HIV care identified in qualitative studies of US clinics.20,21 Strategies that further address underlying and persistent population-, system-, clinic-, and individual-level barriers to care beyond the initial linkage and re-engagement of care are needed.15
Addressing the barriers to care and structural inequalities that may contribute to lower levels of DVS such as social determinants of health, stigma, and lack of essential support services may improve clinical outcomes. The overall 43% of individuals in this study who achieved DVS and the higher relative benefit of achieving DVS for those who re-engaged at 90 days suggest that clinics were able to address issues for many, but other barriers remain for those who did not achieve DVS. Clinics should consider that, without intensified interventions, patients who re-engage in care do not achieve DVS in more than half the cases, owing possibly to disengaging from care or nonadherence; therefore, clinics should consider prioritizing efforts in attending to these patients’ ongoing needs and identified barriers to care.19 Our regression analysis suggests that previous viral suppression is associated with achieving DVS; clinics could therefore consider prioritizing those who do not have prior viral suppression with additional support services. Another strategy could be employing disease intervention specialists, case managers, or other patient navigators to more frequently interact with patients who are at high risk for disengagement, through telephone, telemedicine, or other means which may not require face-to-face visits. Many evidence-based interventions and best practices exist for HIV care providers and clinics to help effectively assist patients in staying in HIV care.25 However, systems-level and structural barriers such as lack of transportation, housing, time away from work, childcare, or insurance coverage should not be overlooked.
The analysis of DVS fills an important gap in understanding D2C’s distal impacts on the HIV care continuum. Since the initiation of this study, D2C has become a required core strategy and activity for federally funded health departments to implement as part of an integrated HIV surveillance and prevention program.26 The key aspects of the CoRECT are (1) the collaborative D2C model whereby the health department and clinical providers hold joint case conferences for successful identification and determination of care status using surveillance data and contributions from clinical care providers and (2) implementation of an active public health intervention using disease intervention specialists or field epidemiologists to locate, contact, and provide assistance with re-engagement in care. Given CoRECT’s previous findings of improvement in re-engagement, it is worthwhile to consider prioritizing re-engagement into public health field services of persons out of HIV care and potentially adjusting workflow to allow for focusing on multilevel barriers to retention in care and antiretroviral therapy adherence to achieve DVS.
Our regression analysis also suggests younger age and lower CD4 counts are associated with not achieving DVS. Older age groups have previously been found to have higher levels of retention and DVS. One national study reported a lower proportion of those aged 25–34 years vs. older than 55 years (52% and 72%, respectively) achieving a 2-year DVS.27 A cross-sectional analysis, among 14 cohorts, in the United States and Canada, reported the older the individual, the greater the probability of viral suppression.28 In addition, in our study, higher CD4 counts are associated with achieving DVS compared with those who had CD4 counts less than 50 cells/μL. It is possible that PWH with lower CD4 counts may have late HIV diagnosis, or longstanding or advanced HIV, which may affect the likelihood of achieving DVS.
The EHE in the US initiative aims to end the HIV epidemic by 2030. The health of PWH and the success of HIV prevention efforts are determined primarily by the ability to achieve and maintain viral suppression. Mathematical modeling indicates that disengagement from HIV care contributes the most (61%) of new annual infections.3 Our findings that nearly half (43%) of re-engaged PWH achieved DVS suggests that to optimally reduce new HIV infections in the United States, our D2C strategy should likely be linked to other strategies to engage a larger proportion of PWH for longer periods, perhaps through better engagement with medical case management, treatment for mental health and substance use disorders, and addressing systems-level and structural barriers. To improve the health of PWH and reduce onward transmission of HIV, multifaceted sustained interventions and evidence-based strategies are needed to identify, engage, and retain PWH in care, maximize the benefits of antiretroviral therapy, and enhance efforts to address social determinants of health that influence HIV clinical outcomes.2
Our study has several limitations. First, all sites were in the Northeast United States. Factors such as health insurance coverage, availability of HIV treatment providers, and availability of supplemental support for HIV treatment through state drug assistance programs among other factors affect access to and continuity of HIV care. Because there are local, state, and regional variations in these factors, the findings have unknown generalizability for other areas. In addition, the definition of newly out-of-care used here may only comprise a specific group that were recently out-of-care, for example, in care for at least once during a 12-period followed by out-of-care for at least 6 months. These factors may limit generalizability to other population cohorts, including people out-of-care for longer periods of time, and jurisdictions. Second, the standard of care may have changed over time, potentially improving re-engagement procedures and thereby decreasing the impact of the intervention over time. In addition, it is possible that individuals who re-engaged on their own or through the SOC may be more likely to continue care and reach DVS, potentially decreasing the impact of the intervention on DVS. Third, health departments and their respective design and implementation of the active public health intervention were structurally and operationally different which allowed for some variation in design and implementation of that intervention, the effects of which could affect generalizability and account for site-level differences in outcomes. Finally, participants had differential opportunities for achieving DVS as the number and interval of VL tests performed were variable.
In conclusion, the CoRECT recruited more than 1800 PWH at multiple clinical sites in 3 geographic areas who were identified as newly out-of-care. Although the collaborative, data-to-care strategy, and active public health intervention improved re-engagement and retention in care, it did not translate to durable viral suppression. These findings underscore the possible need for additional system-level and individual-level support to keep all PWH engaged and retained in HIV care.
Supplementary Material
Acknowledgments
This work was supported by the Division of HIV Prevention, Centers for Disease Control and Prevention Research gGrant FOA PS14-001(Clinical-Trials.gov, NCT02693145). The 3 grantees were Philadelphia Department of Public Health, Massachusetts Department of Public Health, and Connecticut Department of Public Health. The funders participated in the study design, data collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
The authors have declared that no competing interests exist.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 Dependent Areas, 2019. HIV Surveillance Supplemental Report; 2021;26(No.2). Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.No.2 [Google Scholar]
- 2.The White House. National HIV/AIDS Strategy for the United States 2022–2025. Washington, DC; 2021. Available at: https://www.whitehouse.gov/wp-content/uploads/2021/11/National-HIV-AIDS-Strategy.pdf [Google Scholar]
- 3.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175:588–596. [DOI] [PubMed] [Google Scholar]
- 4.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001; 135:17–26. [DOI] [PubMed] [Google Scholar]
- 5.Lesko CR, Cole SR, Hall HI, et al. The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009–11. Int J Epidemiol. 2016;45:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi AeA, Hall HI, Hu X, et al. Population-based estimates of life expectancy after HIV diagnosis: United States 2008–2011. JAIDS 2016; 72:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammed DY, Koumoulos LM, Martin E, et al. Annual and durable HIV retention in care and viral suppression among patients of Peter Ho Clinic, 2013-2017. PLoS One 2020;15:e0244376. Published 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Understanding the HIV care continuum 2019. Available at: https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf
- 10.Enns EA, Reilly CS, Horvath KJ, et al. HIV care trajectories as a novel longitudinal assessment of retention in care. AIDS Behav. 2019;23:2532–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications JAIDS. 2016;73:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maina EK, Mureithi H, Adan AA, et al. Incidences and factors associated with viral suppression or rebound among HIV patients on combination antiretroviral therapy from three counties in Kenya Int J Infect Dis. 2020; 97:151–158. [DOI] [PubMed] [Google Scholar]
- 13.Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019;321:844–845. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Data to care; 2020. Available at: https://www.cdc.gov/hiv/effective-interventions/respond/data-to-care/index.html
- 15.Fanfair RN, Khalil G, Williams T, et al. The Cooperative Re-Engagement Controlled trial (CoRECT): a randomised trial to assess a collaborative data to care model to improve HIV care continuum outcomes. Lancet Reg Health Americas 2021;3:100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arey AL, Cassidy-Stewart H, Kurowski PL, et al. Evaluating HIV surveillance completeness along the continuum of care: supplementing surveillance with health center data to increase HIV data to care efficiency. JAIDS 2019;82:S26–S32. [DOI] [PubMed] [Google Scholar]
- 17.Hart-Malloy R, Brown S, Bogucki K, et al. Implementing data-to-care initiatives for HIV in New York state: assessing the value of community health centers identifying persons out of care for health department follow-up. AIDS Care 2018;303:391–396. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev DD, Mara E, Hughes AJ, et al. "Is a bird in the hand worth 5 in the bush?": a comparison of 3 data-to-care referral strategies on HIV care continuum outcomes in san francisco. Open Forum Infect Dis. 2020;7: ofaa369. Published 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udeagu CCN, Shah S, Misra K, et al. Where are they now? Assessing if persons returned to HIV care following loss to follow-up by public health case workers were engaged in care in follow-up years. AIDS Patient Care and STDs 2018;32:181–190. [DOI] [PubMed] [Google Scholar]
- 20.Philbin MM, Tanner AE, Duval A, et al. Linking HIV-positive adolescents to care in 15 different clinics across the United States: creating solutions to address structural barriers for linkage to care. AIDS Care 2014;26:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan DM, Dawit R, Gbadamosi SO, et al. Sustained HIV viral suppression among men who have sex with men in the Miami-Dade County Ryan White Program: the effect of demographic, psychosocial, provider and neighborhood factors. BMC Public Health 2020; 20:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen S. HRSA’s ryan white HIV/AIDS program: a critical component to ending the HIV epidemic. In: American Public Health Association Annual Meeting; 2020. Oct 24–28; Virtual. [cited 2020 Nov 1] Available at: https://apha.confex.com/apha/2020/meetingapp.cgi/Session/61686 [Google Scholar]
- 23.Zhong Y, Beattie CM, Rojas J, et al. Enrollment length, service category, and HIV health outcomes among low-income HIV-positive persons newly enrolled in a housing program, New York City, 2014–2017. Am J Public Health 2020;110:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley H, Viall AH, Wortley PM, et al. Ryan White HIV/AIDS program assistance and HIV treatment outcomes. Clin Infect Dis. 2016; 62:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Linkage to, Retention in, and Re-engagement in HIV Care (LRC) Chapter. Atlanta, GA: CDC; 2022. Available at: https://www.cdc.gov/hiv/research/interventionresearch/compendium/lrc/index.html [Google Scholar]
- 26.Centers for Disease Control and Prevention. Funding Opportunity Announcement (FOA) PS18-1802: Integrated Human Immunodeficiency Virus (HIV) Surveillance and Prevention Programs for Health Departments; 2017. Available at: https://www.cdc.gov/hiv/funding/announcements/ps18-1802/index.html. [Google Scholar]
- 27.Crepaz N, Tang T, Marks G, et al. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clin Infect Dis. 2016;63:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yehia BR, Rebeiro P, Althoff KN, et al. Impact of age on retention in care and viral suppression. JAIDS 2015;68:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.