Respiratory Syncytial Virus Infection in Infancy and Asthma in Childhood
Asthma is the most common chronic lung disease in children globally. From 1980 to 1995, the world’s population increased from ~4.4 to ~5.7 billion while the number of people with asthma doubled during this same period. This substantial increase in asthma prevalence over a relatively short period of time, the observation that there are marked differences in asthma prevalence among genetically homogenous populations with different environmental exposures, and the change in asthma incidence in the next generation of certain populations who geographically relocate, all support that asthma is partly driven by potentially modifiable environmental, behavioral, and lifestyle risk factors.
Early life respiratory viral infections (particularly those due to respiratory syncytial virus [RSV] and human rhinovirus [RV]) have been postulated to be a causal risk factor for asthma. RSV is a highly infectious virus and is the most common cause of lower respiratory tract infections (LRTIs) and hospitalizations in young children worldwide, although in most infants it usually causes relatively mild disease. While nearly all children are infected with RV in infancy (i.e., the first year of life), only ~50% of infants are infected with RSV and it isn’t until age 2 to 3 years that nearly all children have been infected.1 This makes RSV an ideal model to understand the causal role of early life respiratory viral infections in the development of asthma in childhood and is the focus of this commentary.
Major Limitations of Prior Research in the Field
The association between the clinical entity of bronchiolitis, an LRTI that primarily affects the small airways of children younger than age 2 years and is most commonly caused by RSV, and subsequent asthma risk was first described in 1959.2 Since then, nearly every published study in this field has focused on the association between RSV bronchiolitis or some other marker of early life severe RSV disease (such as RSV LRTI requiring hospitalization) and asthma. This has limited our understanding of the causal relationship between RSV infection in infancy —a critical period of lung and immune maturation— and asthma. First, in this causal hypothesis, bronchiolitis is not an exposure, it is a clinical phenotype that shares a cardinal symptom with asthma (i.e., wheezing). Second, there is evidence of shared heredity for both conditions (i.e., that host genetics can increase the risk of both early life severe RSV disease and asthma), but it is unknown if this explains all or just part of the association (Figure 1). Third, the control group in studies of RSV bronchiolitis typically includes young children without RSV bronchiolitis. However, this control group would include many children who may have been infected with RSV during the same period but could have had asymptomatic or milder infections, as well as those not infected with RSV. Lastly, the focus on RSV bronchiolitis has led researchers to hypothesize that the randomized clinical trials of RSV immunoprophylaxis could be used to assess asthma prevention. However, although current RSV prevention strategies are appropriately targeted to protect against early life severe RSV disease, there is no evidence that they prevent RSV infection in infancy, so equal numbers of infants in both study arms would still be expected to be infected. Thus, these study designs are all imperfect means to infer a causal relationship between RSV infection in infancy and the onset of asthma.
Figure 1.
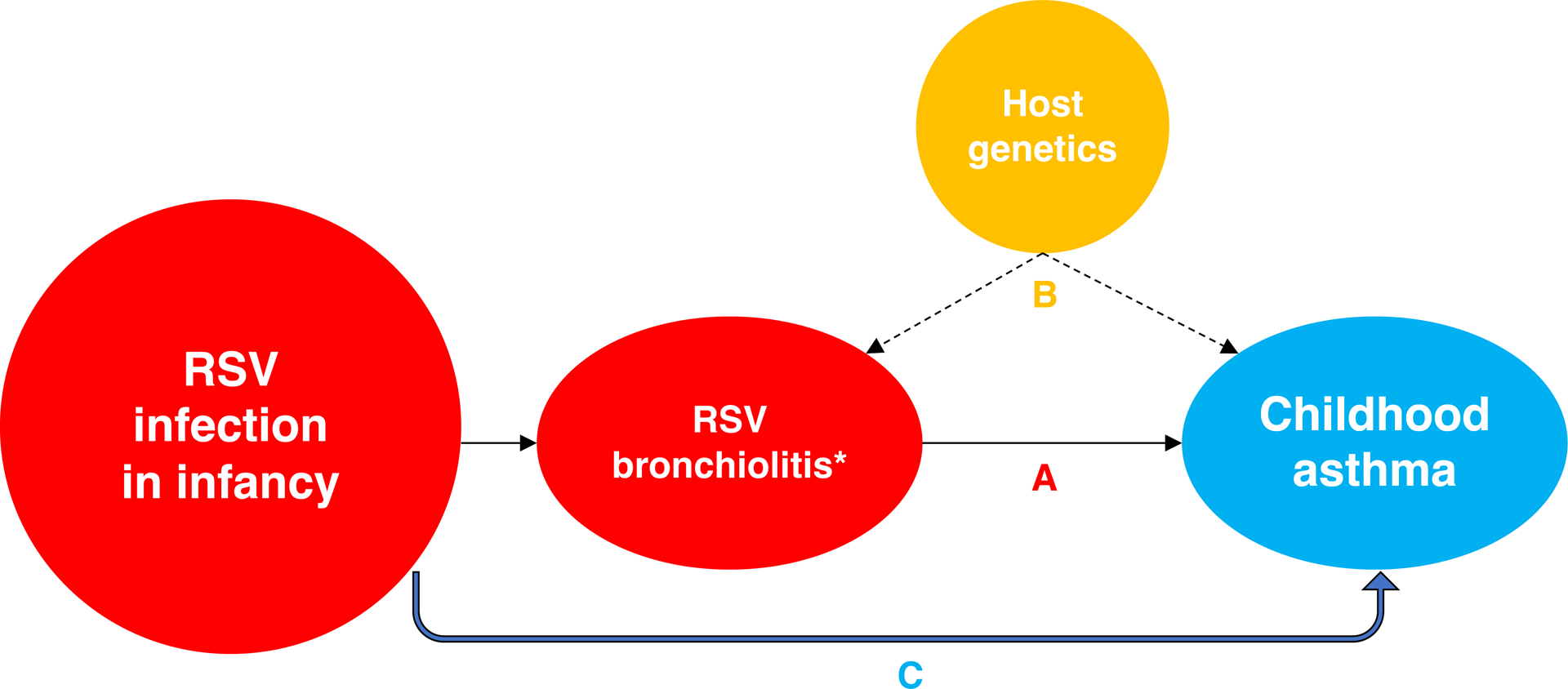
Confounding effect from shared heredity on the association between RSV bronchiolitis and childhood asthma. “A” represents the association in observational studies examining the role of RSV bronchiolitis (*) or some other marker of early life severe RSV disease (such as RSV lower respiratory tract infection requiring hospitalization) on childhood asthma. “B” represents the shared role of host genetics on both RSV bronchiolitis and childhood asthma, an important confounder of the association that cannot be easily controlled. Thus, in observational studies of RSV bronchiolitis and childhood asthma, paths “A” and “B” cannot be differentiated. “C” represents an approach of examining the association between RSV infection in infancy (and not only RSV bronchiolitis) and childhood asthma to overcome several limitations of prior studies, including shared heredity and measuring an exposure rather than a clinical phenotype. Definition of abbreviation: RSV = respiratory syncytial virus.
Similarly, there are problems with the outcome of asthma used in studies of the association with RSV infection in infancy. The model of asthma as a single entity is now understood to be a more complex biological network of distinct and interrelated inflammatory pathways that define several diseases with distinct mechanistic pathways (endotypes) and variable clinical presentations (phenotypes). Furthermore, environmental exposures, such as RSV infection in infancy, can differentially contribute to atopic vs. non-atopic asthma phenotypes, and thus studying the impact of an exposure on a combined phenotype may dilute or even obscure an association.1, 3
Progress in Understanding if Respiratory Syncytial Virus Infection in Infancy Causes Asthma in Childhood
To address the limitations of studies aimed at assessing a causal role of RSV infection in infancy in the development of asthma, Wu et al conducted a study of birth timing in relationship to RSV circulation, using birth timing as a random event.4 The authors asked a relatively simple question: is timing of birth in relationship to the RSV season related to subsequent asthma risk? They found that the risk of asthma was associated with birth timing in relationship to RSV circulation, a finding that is difficult to explain through non-causal mechanisms. Host genetics is a potential confounder of the association between early life severe RSV disease and asthma, as noted above, but it is challenging to fully control for this confounding effect. To overcome this issue, researchers designed a study of natural exposure to precisely measure RSV infection in infancy and the outcome of asthma phenotypes.1 This study design allows for the measure of an exposure that is unlikely to be confounded by shared heredity between early life severe RSV disease and asthma (Figure 1), the ability to test for gene-by-virus interactions to understand variable susceptibility, and to test the direct effect of the presence vs. absence of RSV infection in infancy on the risk of asthma phenotypes. In the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE), a birth cohort of ~2,000 children closely followed using biweekly surveillance and PCR to identify RSV infection during the first RSV season and RSV serology at age 1 year, Rosas-Salazar et al found that being uninfected with RSV in infancy was associated with ~25% lower risk of current asthma at age 5 years.1 The authors estimated that the proportion of 5-year current asthma that could be attributed to delaying RSV infection until after infancy was ~15%. In comparison to RSV-infected infants, those not infected with RSV had a significantly lower risk of 5-year current non-atopic asthma, but not of 5-year current atopic asthma, suggesting that RSV infection in infancy contributes predominantly to non-atopic asthma phenotypes.
To further address a potential causal association, researchers have examined pathways that could explain the relationship between RSV infection in infancy (and not just RSV bronchiolitis) and the development of asthma (Figure 2). Rosas-Salazar et al have shown that RSV-infected infants who develop recurrent wheeze have lower abundance of Lactobacillus species (a taxon that is known to be beneficial for human health) in the upper respiratory tract (URT).5 The authors have also shown that the URT microbiome during RSV infection in infancy is associated with the acute local immune response, disease severity, and subsequent number of wheezing episodes up to age 4 years.6 In addition, Connelly et al have demonstrated that RSV infection in infancy is associated with metabolic changes in the airway epithelium and evidence of epithelial barrier disruption, whereas Chirkova et al have shown that RSV infection in infancy can be associated with dampening of antiviral memory T cell responses (particularly type 1 and type 17 responses), changes that persist for at least several years after the initial RSV infection.3, 7 These changes in URT microbiome, airway epithelial barrier, and antiviral immune responses could predispose RSV-infected infants to allergic sensitization, to repeated acute respiratory infections, and to the detrimental effects of environmental irritants (such as air pollution), all of which further increase the risk of asthma.
Figure 2.
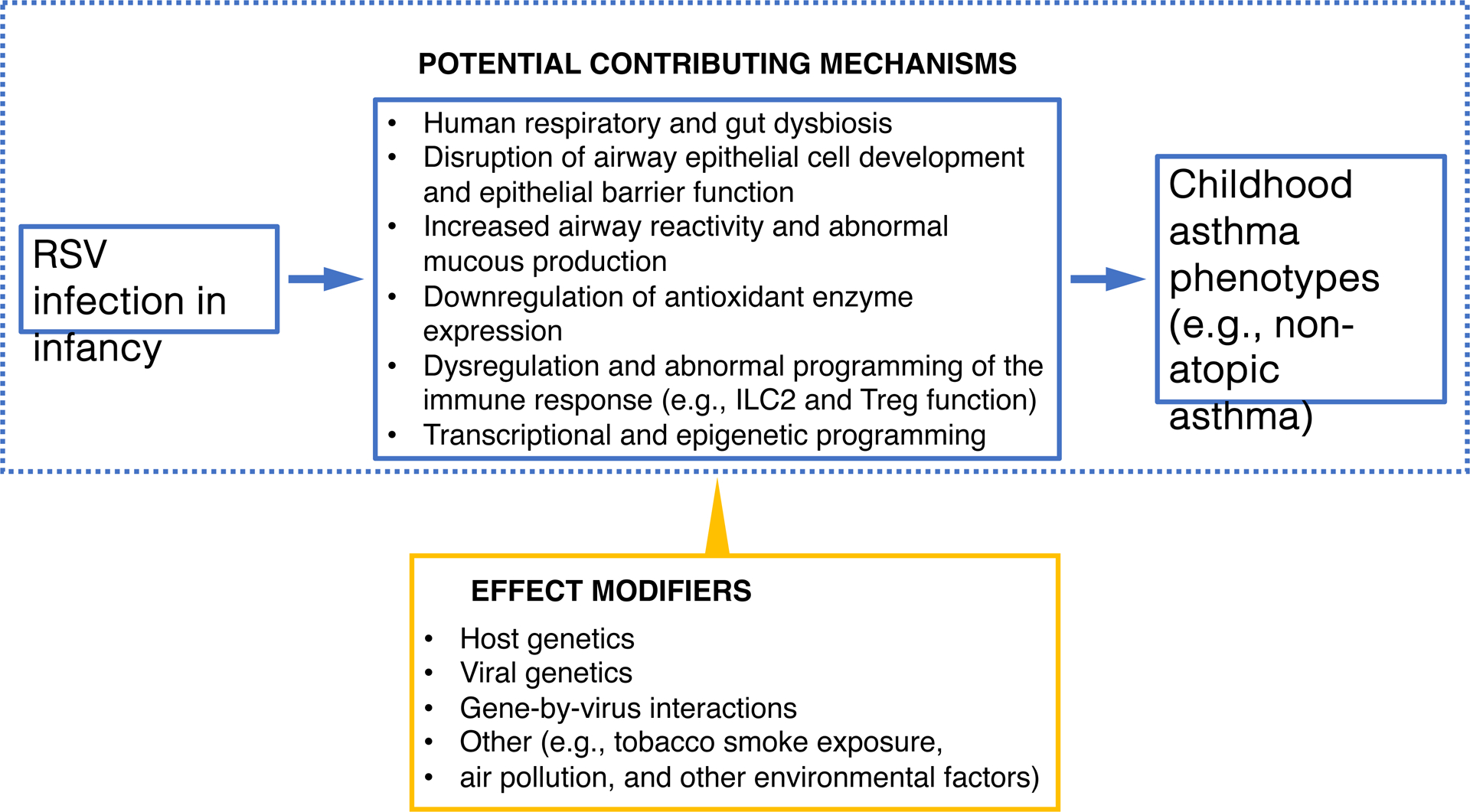
Selected potential mechanistic pathways that could underlie the association between RSV infection in infancy and childhood asthma. The results of recent studies suggest that RSV infection in infancy could lead to childhood asthma though alterations in the human microbiome, airway epithelial cell development, airway hyperresponsiveness, and immune development. Host genetics, viral genetics, and gene-by-virus interactions, among others, can also impact this association. Definition of abbreviation: RSV = respiratory syncytial virus.
More recently, researchers have demonstrated that bronchiolitis is not a single entity but that, like asthma, is also comprised of unique endotypes and phenotypes. For example, using unsupervised clustering technique, Dumas et al found 4 distinct phenotypic profiles in children hospitalized with bronchiolitis. In this study, the RSV-dominated profile had a younger age, more severe disease, and a lower proportion of prior wheeze than the most common RV-dominated profile.8 Likewise, the use of novel molecular and systems biology approaches has allowed the linking of pathobiology to phenotypes with the goal of identifying endotypes of early life severe RSV disease and their association with asthma. In a multicenter cohort study of infants hospitalized for RSV bronchiolitis, Raita et al identified 4 clinically and biologically distinct bronchiolitis endotypes through an integrated omics approach to the URT transcriptome, metatranscriptome, and metabolome.9 The endotype characterized by a high proportion of parental asthma, IgE sensitization, RV coinfection, dominance by Streptococcus pneumoniae and Moraxella catarrhalis, and upregulated IFN-α and -γ responses was most strongly associated with subsequent asthma risk. These analyses highlight not only the complex interplay between respiratory viruses, the URT microbiome, and the host immune response, but also the heterogeneity in molecular mechanisms of early life severe RSV disease, identifying another challenge for causal inference studies of RSV infection in infancy and asthma.
Lastly, an interesting and challenging aspect of studying viruses as an environmental risk factor is that, similar to the host, these pathogens also have genetic variability that may influence clinical outcomes. Human et al previously identified a mutation in the RSV G gene associated with childhood wheezing illnesses, which in an animal model is better able to infect in the presence of preexisting immunity.10 The idea that there are RSV genetic variants that evade immune recognition and can either reinfect or result in prolonged infection led Lawless et al to study a small group of term healthy infants with repeated RSV identification in whom they discovered an RSV genetic variant that was associated with viral persistence.11 The authors speculate that these RSV genetic variants may have a capacity for chronicity that could influence the normal development of infant airway epithelium.
Summary and Future Opportunities
Despite more than 60 years of research examining the relationship between RSV infection in infancy and asthma, misclassification of both the exposure and outcome have impeded progress in establishing a causal association and advancing asthma prevention efforts. RSV bronchiolitis is a clinical phenotype that has a similar clinical presentation to asthma and there is evidence of shared heredity between these two conditions. The outcome of asthma also continues to be a poorly defined syndrome. These limitations in the ascertainment of the exposure and outcome must inform future causal inference studies. Nonetheless, although multiple recent lines of evidence support a causal role of RSV infection in infancy on the development of asthma in childhood, observational studies can never definitively establish causality. Through capitalizing on large consortiums of carefully harmonized birth cohorts, using novel study designs, applying modern technologies and improved statistical methods to better endotype and phenotype children, and studying novel RSV preventive strategies, researchers have the opportunity to make major progress in understanding if preventing RSV infection in infancy or delaying the first infection decreases asthma and long-term respiratory morbidity. This will ultimately allow us to design effective primary prevention strategies for asthma.
Funding:
This work was supported in whole or in part with funds from the National Institutes of Health (under award numbers U19AI095227, UG3OD023282, K24AI77930, R01AI137091, R01AI134940, R01AI148338, and K23HL148638). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflicts of Interests:
CRS serves as a consultant for Amgen and AstraZeneca. TVH has served on vaccine Data Safety Monitoring Boards for Pfizer and as a consultant for Sanofi-Pasteur. KH does not have a commercial or other association that might pose a conflict of interest.
References:
- 1.Rosas-Salazar C, Chirkova T, Gebretsadik T, Chappell JD, Peebles RS Jr., Dupont WD, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet 2023;401:1669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittig HJ, Glaser J. The relationship between bronchiolitis and childhood asthma; a follow-up study of 100 cases of bronchiolitis. J Allergy 1959;30:19–23. [DOI] [PubMed] [Google Scholar]
- 3.Chirkova T, Rosas-Salazar C, Gebretsadik T, Jadhao SJ, Chappell JD, Peebles RS Jr., et al. Effect of Infant RSV Infection on Memory T Cell Responses at Age 2–3 Years. Front Immunol 2022;13:826666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008;178:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Nasopharyngeal Lactobacillus is Associated with Childhood Wheezing Illnesses Following Respiratory Syncytial Virus Infection in Infancy. J Allergy Clin Immunol 2018;142:1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosas-Salazar C, Tang ZZ, Shilts MH, Turi KN, Hong Q, Wiggins DA, et al. Upper Respiratory Tract Bacterial-Immune Interactions during Respiratory Syncytial Virus Infection in Infancy. J Allergy Clin Immunol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connelly AR, Jeong BM, Coden ME, Cao JY, Chirkova T, Rosas-Salazar C, et al. Metabolic Reprogramming of Nasal Airway Epithelial Cells Following Infant Respiratory Syncytial Virus Infection. Viruses 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas O, Erkkola R, Bergroth E, Hasegawa K, Mansbach JM, Piedra PA, et al. Severe bronchiolitis profiles and risk of asthma development in Finnish children. J Allergy Clin Immunol 2022;149:1281–5 e1. [DOI] [PubMed] [Google Scholar]
- 9.Raita Y, Perez-Losada M, Freishtat RJ, Harmon B, Mansbach JM, Piedra PA, et al. Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma. Nat Commun 2021;12:3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Human S, Hotard AL, Rostad CA, Lee S, McCormick L, Larkin EK, et al. A Respiratory Syncytial Virus Attachment Gene Variant Associated with More Severe Disease in Infants Decreases Fusion Protein Expression, Which May Facilitate Immune Evasion. J Virol 2020;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawless D, McKennan CG, Das SR, Junier T, Xu ZM, Anderson LJ, et al. Viral genetic determinants of prolonged respiratory syncytial virus infection among infants in a healthy term birth cohort. J Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]


