Summary
Septic cardiomyopathy is associated with high mortality in septic patients, characterized by reversible systolic and diastolic dysfunction. It is essential to monitor cardiac function and hemodynamic changes in septic animals. Here, we present a protocol to monitor cardiac function and hemodynamics in septic rodents. We describe steps for performing cecal ligation and puncture on rodents to induce sepsis, acquiring two-dimensional echocardiographic and M-mode ultrasonic images, and assessing mean arterial pressure in septic animals.
For complete details on the use and execution of this protocol, please refer to Zhang et al.1
Subject areas: Immunology, Model Organisms
Graphical abstract

Highlights
-
•
Detailed steps for CLP modeling process and precautions for rats and mice
-
•
Steps to acquire long- and short-axis views of heart in CLP rats and mice using ultrasound
-
•
Assessment of cardiac contractility using indices from EchoPAC software
-
•
Evaluation of hemodynamic status of CLP rodents by measuring MBP through tail artery
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Septic cardiomyopathy is associated with high mortality in septic patients, characterized by reversible systolic and diastolic dysfunction. It is essential to monitor cardiac function and hemodynamic changes in septic animals. Here, we present a protocol to monitor cardiac function and hemodynamics in septic rodents. We describe steps for performing cecal ligation and puncture on rodents to induce sepsis, acquiring two-dimensional echocardiographic and M-mode ultrasonic images, and assessing mean arterial pressure in septic animals.
Before you begin
This protocol outlines the steps for constructing CLP model and analyzing cardiac function using echocardiography and evaluating hemodynamics in septic mice and rats. Prior to initiating the procedure, it is essential to ascertain the severity of the sepsis model (mild, moderate, severe) to be constructed and prepare the necessary materials and instruments (refer to the key resources table and Materials and Equipment).
A high-resolution ultrasound probe (such as vivid iq, GE, or an equivalent device) with a 16–18 MHz resolution and a non-invasive blood pressure monitor designed for small animals are required. To facilitate the ultrasound examination and hemodynamic monitoring, prepare the anesthesia machine and position the animals on the anesthesia system platform connected to isoflurane (ZS-MV series, Zhongshi Technology, China). The experiment utilizes an anesthesia system equipped with a waste gas recovery container to efficiently eliminate excess anesthetic gas, thereby minimizing release into the room and preventing inhalation by the operator while reducing environmental pollution. As cecal ligation and puncture (CLP) animals are susceptible to hypothermia during anesthesia, a heating pad is necessary during the ultrasound examination to maintain the mice’s body temperature and avoid hypothermia-related deaths in the CLP models. For echocardiography analysis, access the following website is required: https://www.gehealthcare.com/products/ultrasound/vivid/echopac.
Institutional permissions
6–8 weeks male C57BL/6J mice and 260–300 g weight male SD rat (Hunan SJA Laboratory Animal Co. Ltd) were housed in 12 h light and 12 h dark cycle and temperature-controlled room. Our animal research was approval by Experimental Animal Ethics Committee, Xiangya Hospital, Central South University (202110109) and strictly abided by “3R” research animal principle. It is of note that permission for conducting relevant animal experiments should be acquired from your local institutions prior to the research.
Preparation for CLP procedure
Timing: the day before the experiment
-
1.
Prepare the following equipment and materials: 8‒10-week-old C57BL/6J mice, Sprague-Dawley rats (200–300 g), alcohol swabs, surgical drapes, sterile tape, preheated 0.9% saline solution (around 20°C), isoflurane. Sterilize surgical instruments (ear punch, 21G needle, small straight scissors, small forceps, needle holder, 3.0 suture needle) one day before surgery.
-
2.
Mark mice and rats for identification: Use a permanent marker on the base of the mouse’s or rat’s tail or ear punch for marking.
-
3.
Remove abdominal and thoracic hair: Use an animal hair clipper to remove abdominal hair to prevent contamination during the CLP procedure. Similarly, removing thoracic hair facilitates cardiac ultrasound examination.
Anesthesia procedure
Timing: 8 min
-
4.
Ensure proper connection of the anesthesia machine’s tubing to avoid any leaks. Pour an appropriate amount of isoflurane into the vaporizer and tighten the lid.
-
5.Adjust the anesthesia concentration to 1% and quickly place the animals in the induction chamber.
-
a.Allow sufficient time (approximately 1–3 min) for complete anesthesia.
-
b.To verify whether the animal is fully anesthetized, gently shake the induction chamber and observe its response.
-
a.
Note: If the animal remains on its side without attempting to reposition, it indicates successful anesthesia.
-
6.
After induction anesthesia, transfer the animals from the induction chamber to the nose cone adapter, and set the maintenance anesthesia concentration to 0.6% (Figure 1).
CRITICAL: Monitor the animals’ breathing closely during anesthesia. If the animals show rapid and deep respiration, adjust the anesthesia machine's concentration, or move the animals away from the anesthesia source to prevent potential death due to excessive anesthesia.
Figure 1.
Anesthesia system connection and preoperative preparation for CLP
The anesthesia system mainly includes isoflurane, induction chamber, air pump, anesthesia machine, anesthesia maintenance platform, waste recycling bin, and heating pad. Preoperative preparation for CLP includes disinfectants and surgical instruments: The items in the green box are surgical instruments, and the items in the yellow box are disinfection supplies.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 0.9% saline | Kelun | N23011205 |
| Isoflurane | EZVET | 709014 |
| Ultrasound transmission gel | HYNAUT | 100051716630 |
| Buprenorphine | Shafei | H12020276 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J (male, 8–10 weeks old) | Hunan SJA Laboratory Animal | RRID: MGI:3028467 |
| Rat: Sprague-Dawley (male, 8 weeks old) | Hunan SJA Laboratory Animal | RRID: RGD:737903 |
| Other | ||
| Sterile pad | Zhonggan | N/A |
| Ear punch | Tigergene | N/A |
| 21G needle | SKT | DZ21X70-1 |
| Small tweezers | Jinzhong | JD1060 |
| Needle holder | Jinzhong | J32110 |
| Small straight scissors | Jinzhong | J21010 |
| ECG pads | Lepu | N/A |
| Temperature-controlled heating pad | Petbee | PBL-706 |
| Multi-channel anesthesia machine | Zhongshi, China | ZS-MV |
| Electric shaver | PETKIT | OKDTJ |
| Ultrasound probe L8-18i | GE | L8-18i |
| Ultrasound probe 12S | GE | 12S |
| Ultrasound machine | GE | Vivi iq |
| Blood pressure measurement system | Zhongshi, China | ZS-Z |
Step-by-step method details
Constructing the mild CLP model
Timing: 15 min
This part of the protocol includes all the necessary steps to construct the mild CLP model.2
-
1.
Induction of anesthesia: Prepare the anesthesia system as mentioned before. Turn on the induction box switch and place the animal in the induction box for anesthesia.
-
2.Gently shake the induction box and transfer the animals to the operating panel with a temperature-regulated heating pad.
-
a.Open the heating pad and adjust the temperature to 38.6°C.
-
b.Place the animals' nose gently on the operating platform with a nose cone adapter to maintain anesthesia.
-
a.
-
3.
Disinfection: Fix the limbs of the animal with tape. Use alcohol swabs to disinfect the surgical area, from the mouse’s chest to the base of the hind limbs (Figure 2A).
-
4.CLP procedure.
-
a.Make small incisions in the midline of the abdominal skin to expose the peritoneal white line, being mindful of appropriate lengths for mice (1–1.5 cm) and rats (3–4 cm) (Figure 2B). Create small incisions along the peritoneal white line to minimize bleeding and secondary damage.
-
b.Gently dissect the muscle layer from the incision to expand the surgical field and expose the cecum, typically located in the lower left abdomen.
-
i.Use small forceps to extract the cecum without damaging mesenteric blood vessels.Note: In rats, there may be a membrane extension at the mesenteric location of the cecum, which should be dissected prior to cecal ligation (Figure 2C).
-
i.
-
c.Determine the length of the cecum to be ligated by using the second branch of the cecal blood supply artery as the endpoint and the distal end as the starting point.Note: As shown in Figure 2D (green line), among all the cecal blood supply artery extending from the connection point between cecum and ileum, the second vessel branch is set as the endpoint.
-
d.Mild ligation involves constriction of 10%, moderate ligation involves 50%, and severe ligation involves 70% (Figures 2D and 2E).Note: Ligature usually makes the cecum shrink one third of the intact one. Before ligating, gently push feces in the distal part of the cecum towards the proximal end to prevent uncontrolled fecal leakage due to excessive pressure.
-
e.Use a sterile needle (21G for mice, 18G for rats) to puncture the center of the ligated distal end of the cecum twice, ensuring no blood vessels are pierced.
-
i.Squeeze out approximately 1 mm3 (the size of a grain of millet) of feces.
-
ii.Gently push the cecum back into the abdominal cavity (Figures 2F and 2G).
-
i.
-
f.Close the peritoneum, muscle layer, and skin (Figures 2H and 2I).
-
i.Provide postoperative care by disinfecting the abdominal incision with an alcohol swab.
-
ii.Administer preheated saline solution subcutaneously for fluid resuscitation (1 mL for mouse, 5 mL/100 g for rat).
-
i.
-
g.Place the animals on a heating pad to regain consciousness and return them to a clean cage.Note: The animals were group-housed with 3–4 mice per cage and 2–3 rats per cage.
-
h.Observe the wound tearing and bleeding of the mice and rats every hour for 6 h after surgery.
-
i.Administer buprenorphine (0.05 mg/kg body weight subcutaneously) for postoperative pain relief and repeat administration every 6 h for a minimum of 2 days.
-
j.In terms of diet, to reduce the tearing of the postoperative wounds, replace fresh food daily and place it inside the cages for easy access of food.
-
k.Ensure a normal light-dark cycle, access to food and water, and maintain a temperature-controlled environment (22°C). Monitor the animal closely every 6 h after surgery.
 CRITICAL: Previous studies have shown that mice induced with infection at night develop sepsis faster than that induced at daytime.3 Therefore, to ensure the homogeneity of the model, CLP and sham operation should be performed during the day or at night.
CRITICAL: Previous studies have shown that mice induced with infection at night develop sepsis faster than that induced at daytime.3 Therefore, to ensure the homogeneity of the model, CLP and sham operation should be performed during the day or at night.
-
a.
Figure 2.
Steps in the CLP procedure in mice
(A) Disinfection of the abdominal area after shaving.
(B) Incise the skin to expose the muscle layer.
(C) Cut the white line of the peritoneum to expose the caecum.
(D) Confirm the ligation position. The green line indicates the starting point as the second branch of the cecal artery and the end point as the cecal end. The red line indicates the first branch of the cecal artery and the yellow line indicates a point 10% from the end.
(E) Ligature at the yellow point.
(F) Use a 21G needle to pass through the cecal twice.
(G) Extract 1 mm3 of feces and return it to the abdominal cavity.
(H and I) Suture the muscle layer and skin with 4‒0 thread and disinfect.
Acquisition of long and short axis of the heart
Timing: 15–20 min
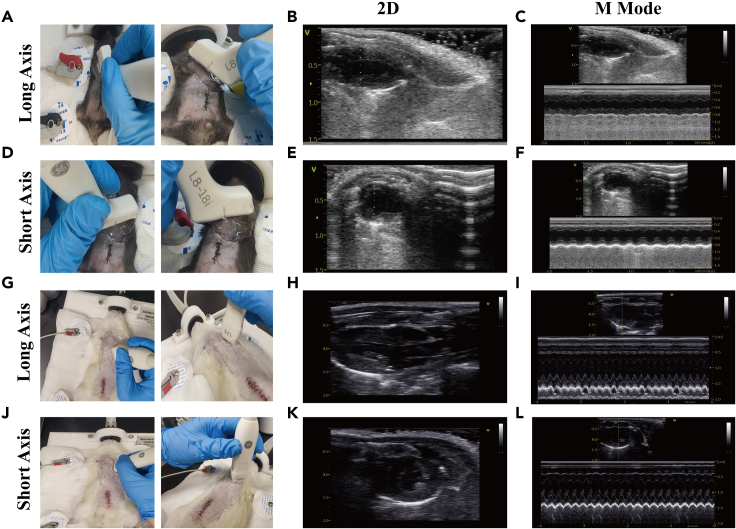
The following steps mainly describe how to obtain the long and short axis sections of the hearts of rats and mice, including the use of anesthesia systems, positioning of the animals, methods for using probes in different sections, and obtaining the long and short axis of the heart.4,5
-
5.Preparation of the ultrasound station.
-
a.Turn on the power switch located at the upper right corner and enter the login page by inputting the login password.
-
b.Connect the small animal ultrasound probe.
-
c.Input the animal grouping information and identification number.
-
a.
-
6.
Animal anesthesia: The operational steps are the same as the first step of constructing the CLP mouse model. During the ultrasound process, closely monitor the animals’ vital signs and adjust the anesthetic concentration in a timely manner.
-
7.Place the animals’ supine on the operating platform,
-
a.Adjust the animals’ posture, use electrode pads to fix the four limbs of the mouse.
-
b.Record the animals’ electrocardiogram, and wait for the electrocardiogram to stabilize.
-
a.
-
8.Cover the shaved chest area of the animals with a large amount of warm ultrasound gel (ensure that no bubbles form in the gel, as they may cause imaging artifacts).
-
a.Keep the probe long axis parallel to the long axis of the animals' body.
-
b.Gently place the probe vertically on the chest.
-
c.Slowly move it up, down, left, and right until the beating heart is found.
-
a.
Note: Use different probes for mice (L8-18i probe) and rats (12S probe), respectively.
-
9.Acquisition of long axis of the heart.
- a.
-
b.Keep the probe still, adjust the imaging mode to M-mode ultrasound, and place the sampling line parallel to the two ventricular walls on the long axis of the heart.
-
c.Wait for the interface to show a clear endocardial line, and then freeze the interface (Figures 3C and 3I).
-
d.Use the measurement key of the machine to anchor and measure the left ventricular end-diastolic and end-systolic diameters (LVIDd and LVIDs).
-
e.Automatically calculate the fractional shortening (FS) and ejection fraction (EF) through EchoPAC software.
-
10.Acquisition of short axis of the heart.
-
a.When the probe is in the long axis position, clockwise rotation of 90° will reveal the short axis section of the heart. Similarly, adjust the angle by left and right tilt to obtain a clear image (Figures 3D and 3J).
-
b.The heart image should be centered on the screen, and the right ventricular cavity is almost invisible on this section.
 CRITICAL: The interventricular septum is arched towards the right ventricular side, and two strong echogenic papillary muscles are located on the left ventricular short axis section (Figures 3E and 3K).
CRITICAL: The interventricular septum is arched towards the right ventricular side, and two strong echogenic papillary muscles are located on the left ventricular short axis section (Figures 3E and 3K). -
c.Keep the probe still, adjust the imaging mode of the ultrasound machine to M-mode ultrasound, and.
-
i.Place the sampling line in the middle of the short axis section of the heart.
-
ii.Wait for the interface to show a clear endocardial line (Figures 3F and 3L).
-
i.
-
d.Use the measurement key of the machine to anchor and measure the LVIDd and LVIDs.
-
e.Automatically calculate the FS and EF through EchoPAC software.
-
f.Save the image data, wipe off the ultrasound gel on the mouse or rat, and put it on a warming pad to recover and revive.
 CRITICAL: To obtain reliable cardiac ultrasound results, the heart rates of rats and mice should be maintained at a minimum of 350 and 450 beats per minute, respectively. Isoflurane is commonly used as an anesthetic agent in most animal cardiac ultrasounds. To minimize its impact on cardiac function, we recommend to use a lower dosage.
CRITICAL: To obtain reliable cardiac ultrasound results, the heart rates of rats and mice should be maintained at a minimum of 350 and 450 beats per minute, respectively. Isoflurane is commonly used as an anesthetic agent in most animal cardiac ultrasounds. To minimize its impact on cardiac function, we recommend to use a lower dosage.
-
a.
Figure 3.
Two-dimensional (2D) ultrasound images and M-mode ultrasound of the long and short axis of the heart in CLP mice and rats
(A and D) Representative pictures show the placement of the heart probe when acquiring the long and short axis views of the mouse heart.
(B and C) The 2D and M-mode ultrasound images of the long axis of the mouse heart.
(E and F) The 2D and M-mode ultrasound images of the short axis of the mouse heart.
(G and J) Representative pictures showing the placement of the heart probe when acquiring the long and short axis views of the rat heart.
(H and I) The 2D and M-mode ultrasound images of the long axis of the rat heart.
(K and L) The 2D and M-mode ultrasound images of the short axis of the rat heart.
Measurement of MBP (mean blood pressure)
Timing: 15–20 min
After assessing the cardiac function of the rats and mice, we perform the non-invasive blood pressure measurements on the animals after they fully recovered to evaluate the hemodynamic status. The following steps will provide a detailed description of the process for measuring MBP in CLP rats using a non-invasive tail artery blood pressure measurement system. The operation is the same for both mice and rats, with the only difference being the size of coils to measure tail artery pressure. This section will focus on the procedure using CLP rats.
-
11.Device installation.
-
a.Follow the instructions provided by the reagent supplier to install the blood pressure monitor and connect it to the multi-channel physiological recorder, oscilloscope, and media biological function experiment system.
-
b.Connect the wires from the pressure transducer, pulse transducer, and temperature-controlled rodent cage to their respective sockets and connect them to the data acquisition system.
-
a.
-
12.Animal fixation and tail heating.
-
a.Place the rat into the fixation device, turn on the power switch, and start warming the rodent cage.Note: Generally, after 15–20 minutes, the rat will settle down in the cage. Continuously monitor the vital signs of the rat.
- b.
-
a.
-
13.Measurement of systolic pressure.
-
a.Position the pressure transducer of the blood pressure monitor near the proximal end of the rat’s tail.Note: Place the high-sensitivity pulse transducer in the upper 1/3 of the tail, with the transducer surface facing the ventral side of the tail.
-
b.Once the rat is calm, turn on the recording system. Pulse waves will be visible.
-
c.Wait for the waveform to stabilize, then use an amplifier to enhance the pulse wave, typically by 2–3 cm.
-
d.Inflate the cuff to increase the pressure within the cuff until the pulse wave disappears completely.
-
e.Increase the pressure by an additional 30 mmHg and slowly deflate the cuff while observing the time it takes for the first pulse wave to reappear, as indicated by the black arrow in Figure 4B.
-
f.The corresponding pressure at the pressure channel is the systolic pressure. The interface on the right side of the channel displays the systolic blood pressure value.
-
a.
-
14.Measurement of diastolic pressure.
-
a.Use the multi-channel recorder to simultaneously record the pulse wave and the pressure changes within the cuff. The measurement process is the same as described above.
-
b.When the pulse wave is occluded, begin to decrease the pressure.
 CRITICAL: When the first pulse wave reappears, continue to lower the pressure until the first maximum pulse wave peak appears (as indicated by the red arrow in Figure 4B).
CRITICAL: When the first pulse wave reappears, continue to lower the pressure until the first maximum pulse wave peak appears (as indicated by the red arrow in Figure 4B). -
c.The corresponding diastolic pressure data at the pressure channel is the diastolic pressure. Similarly, on the right side of the channel, the interface can display the size of the diastolic pressure value.
-
a.
-
15.
Calculation of MAP: MAP = diastolic pressure + 1/3 (systolic pressure - diastolic pressure). The software can automatically calculate this value.
Figure 4.
Measurement of mean arterial pressure (MAP) in rats
(A) The non-invasive tail artery blood pressure measurement system for rats mainly includes blood pressure analysis system, biological signal acquisition and processing system, pressure gauges, inflation device, blood pressure and pulse cuff, pulse transducer, fixing device and warmer.
(B) Representative recording of the blood pressure and pulse channels for rats. The red box indicates the blood pressure channel, and the green box indicates the pulse channel. The black arrow indicates the first pulse appearing after releasing pressure, corresponding to the systolic pressure in the blood pressure channel. The red arrow indicates the first maximum pulse wave appearing after releasing pressure, corresponding to the diastolic pressure in the blood pressure channel. On the right side of the blood pressure channel, the recorded values include systolic pressure, diastolic pressure, MAP, and pulse pressure.
Expected outcomes
Successfully constructing CLP rat and mouse models and obtaining the following parameters from CLP rat/mouse M-mode echocardiography analysis: EF, FS. In addition to the EF and FS, the MBP can also be obtained from rats through a non-invasive tail artery blood pressure measurement system. MBP is used to evaluate volume status. Due to the heterogeneity of rodents from different batches and the variability of operators, the reference values for EF and FS are mainly compared to the normal values of the control group measured in each experiment. The normal EF of mice ranges as 65%–90%. In the CLP model, three types of EF were observed: EF > 90%, EF < 65%, and EF between 65% and 90%. MBP ranged from 80‒90 mmHg in the control group, while MBP ranged from 70‒80 mmHg at 24 h after CLP induction.1
Quantification and statistical analysis
The statistical methods will depend on the specific experimental design. Usually, we use t-tests to compare the differences in heart function between two given groups. For comparing the differences in heart function among three or more groups of mice, we use analysis of variance (ANOVA).
Limitations
One common reason for the variation in modeling sepsis between individuals is the difference in the volume of feces squeezed out and the difference in cecum contents. And echocardiography is highly dependent on the operator’s familiarity with ultrasound techniques and the interpretation of the results by the researchers, which can affect the authenticity and objectivity of the data. Ideally, before performing echocardiograms on mouse hearts, you should learn how to obtain echocardiograms of hearts from human or bigger animals and master the basics of ultrasound image acquisition, as ultrasound is a highly experiential technique.
Troubleshooting
Problem 1
Heterogeneity in CLP models lead to various results (step 4).
Potential solution
-
•
Ensure complete consistency in the materials used during the modeling process for the same batch of animals. Be gentle during CLP operation to avoid damaging the cecal and mesenteric vessels and causing additional injuries.
-
•
Due to the influence of circadian rhythms on the inflammatory state of animals, all animals used for CLP modeling should be in the same period.7
-
•
Pay attention to maintaining consistency in the size of the needle used for perforation and the pressure applied while expressing feces.
Problem 2
Severity of modeling affects the acquisition of cardiac ultrasound images. It is difficult to obtain cardiac ultrasound images when the modeling is too severe (steps 9–10).
Potential solution
-
•
Performing mild or moderate CLP modeling or using a higher frequency ultrasound probe.
Problem 3
High dependence on operators for ultrasound data, prone to subjective influences (steps 9–10).
Potential solution
-
•
When performing cardiac ultrasound examinations, two personnel who are blind to the experimental groups but familiar with cardiac operations are required.
-
•
It is necessary to acquire 3–5 data points per section and determine the final value by taking the average.
-
•
In addition to evaluating cardiac function using cardiac ultrasound, the changes in cardiac function can be corroborated by combining myocardial injury biomarkers and cardiac morphology.
Problem 4
The type, concentration, and duration of anesthetics may affect cardiac function (step 6).
Potential solution
-
•
Using the same type of anesthetic and recording anesthesia parameters in real-time, including the concentration and time used.
-
•
Minimize the duration of the ultrasound procedure to reduce the stress time for the mice. Isoflurane, which is used in this study, is a commonly used anesthetic for most small animal ultrasound research.
Problem 5
The stress caused by immobilizing rats in an unanesthetized state may affect the accuracy of MBP measurement (step 12).
Potential solution
Two days before the modeling, place the animal in the fixation device to acclimate to the environment. Conduct the experimental procedure as soon as possible once the animal is stable in the device.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Lina Zhang: zln7095@csu.edu.cn.
Technical contact
Further technical problems should be directed to the technical contacts: xymmbb@csu.edu.cn, wangxr1893@csu.edu.cn.
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate new datasets or code.
Acknowledgments
This work was supported by the Key Research and Development Programs from Hunan Province (# 2023SK2020 and 2022SK2039).
Author contributions
B.M., X.W., L.L., H.Z., and W.L. performed the experiments and data analysis. Z.H., L.Z., and Z.Q. designed the protocol, B.M. wrote the manuscript, and X.W. prepared the figures. Z.H., L.Z., and Z.Q. designed and supervised the study.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Zhonghua Hu, Email: huzhonghua@csu.edu.cn.
Lina Zhang, Email: zln7095@csu.edu.cn.
Zhaoxin Qian, Email: xyqzx@csu.edu.cn.
References
- 1.Zhang L., Qi D., Peng M., Meng B., Wang X., Zhang X., Zuo Z., Li L., Wang Z., Zou W., et al. Decoding molecular signature on heart of septic mice with distinct left ventricular ejection fraction. iScience. 2023;26 doi: 10.1016/j.isci.2023.107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rittirsch D., Huber-Lang M.S., Flierl M.A., Ward P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver A.C., Arjona A., Walker W.E., Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zacchigna S., Paldino A., Falcão-Pires I., Daskalopoulos E.P., Dal Ferro M., Vodret S., Lesizza P., Cannatà A., Miranda-Silva D., Lourenço A.P., et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: a position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021;117:43–59. doi: 10.1093/cvr/cvaa110. [DOI] [PubMed] [Google Scholar]
- 5.Pistner A., Belmonte S., Coulthard T., Blaxall B. Murine echocardiography and ultrasound imaging. J. Vis. Exp. 2010:42:2100. doi: 10.3791/2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigiarelli K.J. Rodent Thermoregulation: Considerations for Tail-Cuff Blood Pressure Measurements. J. Am. Assoc. Lab. Anim. Sci. 2022;61:406–411. doi: 10.30802/AALAS-JAALAS-22-000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heipertz E.L., Harper J., Lopez C.A., Fikrig E., Hughes M.E., Walker W.E. Circadian Rhythms Influence the Severity of Sepsis in Mice via a TLR2-Dependent, Leukocyte-Intrinsic Mechanism. J. Immunol. 2018;201:193–201. doi: 10.4049/jimmunol.1701677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate new datasets or code.