Abstract
As the most common non-Hodgkin lymphoma subtype, diffuse large B-cell lymphoma (DLBCL) incidence patterns generally parallel that for NHL overall. Globally, DLBCL accounts for a third of all NHLs, ranging between 20%−50% by country. Based on U.S. cancer registry data, age-standardized incidence rate for DLBCL was 7.2 per 100,000. DLBCL incidence rises with age and is generally higher in males than females; in the U.S., incidence is highest among non-Hispanic whites (9.2 per 100,000). Like NHL incidence, DLBCL incidence rose in the first half of the 20th century but has largely plateaued. However, there is some evidence that incidence rates are rising in areas of historically low rates, such as Asia; there are also estimates for rising DLBCL incidence in the near future due to the changing demographics in developed countries whose aging population is growing. Established risk factors for DLBCL include those that result in severe immune deficiency such as HIV/AIDS, inherited immunodeficiency syndromes, and organ transplant recipients. Factors that lead to chronic immune dysregulations are also established risk factors, and include a number of autoimmune conditions (e.g., Sjögren syndrome, systemic lupus erythematosus, rheumatoid arthritis), viral infections (e.g., HIV, KSHV/HHV8, HCV, EBV), and obesity. Family history of NHL/DLBCL, personal history of cancer, and multiple genetic susceptibility loci are also well-established risk factors for DLBCL. There is strong evidence for multiple environmental exposures in DLBCL etiology, including exposure to trichloroethylene, benzene, and pesticides and herbicides, with recent associations noted with glyphosate. There is also strong evidence for associations with other viruses, such as HBV. Recent estimates suggest that obesity accounts for nearly a quarter of DLBCLs that develop, but despite recent gains in the understanding of DLBCL etiology, the majority of disease remain unexplained. An understanding of the host and environmental contributions to disease etiology, and concerted efforts to expand our understanding to multiple race/ethnic groups, will be essential for constructing clinically relevant risk prediction models and develop effective strategies for disease prevention.
Patterns of Occurrence
DLBCL is the most common NHL subtype, accounting for approximately one-third of NHLs (Table 1); its incidence rates mirrors that of overall NHL and total lymphoid neoplasms (Figure 1). In the United States SEER data, for the time period 1992 through 2020, incidence rates for all lymphoid neoplasms, including NHL and DLBCL, have plateaued. In all three endpoints (all lymphoid neoplasms, NHL, and DLBCL), incidence is highest among white men, followed by Black men and Asian males. Although Asian females have the lowest rates of total lymphoid neoplasms and NHL, Black females have the lowest incidence of DLBCL in the U.S. (Figure 1 & Table 2). DLBCL risk monotonically with age (Figure 2) and its incidence is higher in men than women in all categories of race (Figure 2 and Table 3). In both men and women, Whites have the highest DLBCL incidence.
Table 1.
Incidence of hematopoietic neoplasms and DLBCL (defined by ICD-O-3 codes), 12 SEER registries, 2010–2020.
| ICD-O-3 codes# | No. | Rate* | |
|---|---|---|---|
| Lymphoid neoplasms, total | - | 165 364 | 35.7 |
| B-cell lymphoid neoplasms, total | 9591, 9597, 9670, 9671, 9673, 9675, 9678, 9679(B), 9680(B), 9684(B), 9687, 9688(B), 9687–9691, 9695, 9698, 9699, 9712(B), 9731–9735(B), 9737(B), 9738(B), 9760–9762, 9764, 9823, 9826, 9833(B), 9940 | 125 575 | 26.8 |
| Diffuse large B-cell lymphoma | 9678, 9679(B), 9680 (B), 9684(B), 9688(B), 9712(B), 9735(B), 9737(B), 9738(B), | 33 359 | 7.2 |
| T-NK-cell lymphoid neoplasms, total | 9700–9702, 9705, 9708, 9709, 9714, 9716–9719(T/NK), 9726, 9727, 9827, 9831, 9832, 9834, 9948(T/NK) | 9 866 | 2.1 |
| Hodgkin lymphoma | 9650–55, 9659, 9661–9667 | 10 726 | 2.4 |
| Unknown type lymphoid neoplasm | 9727 (unknown), 9832 (unknown) | 212 | 0.0 |
ICD-O: International classification of diseases for oncology; SEER: Surveillance, Epidemiology, and End Results
Codes followed by parentheses indicate that immunophenotyping data (B-cell, T/NK-cell, or unknown) were used to assign cases to that lymphoid neoplasm subtype
All incidence rates are age-adjusted to the 2000 US population and expressed per 100,000 person-years
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER
Research Data, 12 Registries, Nov 2022 Sub (1992–2020) - Linked To County Attributes - Time Dependent (1990–2021)
Income/Rurality, 1969–2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
Fligure 1.
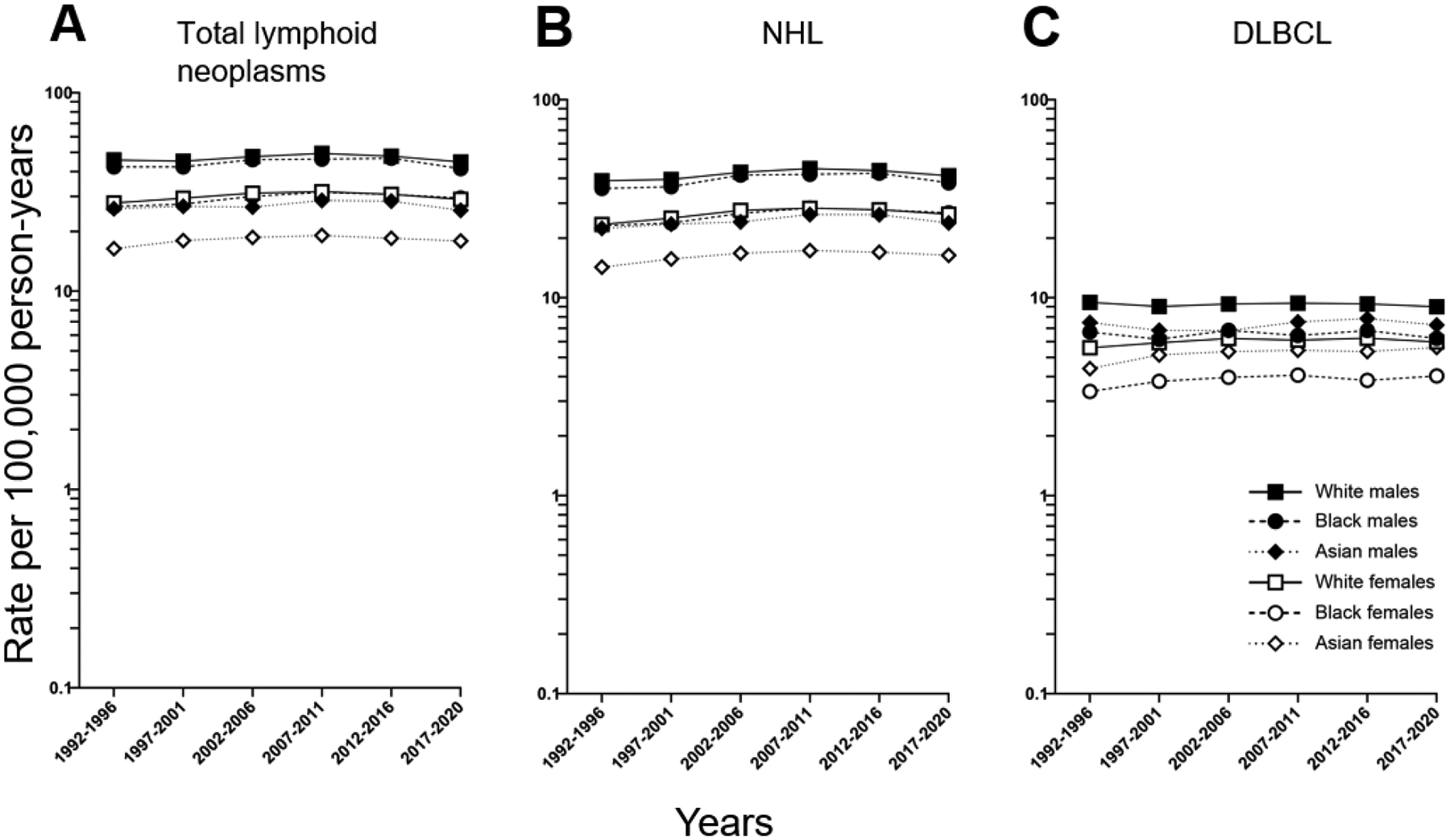
Trends in DLBCL incidence by race and sex, 12 SEER registries, 1992–2020.
Presented for 6 fixed time periods (1992–1996, 1997–2001, 2002–2006, 2007–2011, 2012–2016, 2017–2020)
Table 2.
DLBCL Incidence by Race and Sex, 12 SEER Registries, 2011–2020
| Lymphoid neoplasms, total | B-cell lymphoid neoplasms, total | DLBCL | |||||
|---|---|---|---|---|---|---|---|
| No. | Rate* | No. | Rate* | No. | Rate* | ||
| Male | White | 68 907 | 46.5 | 58 950 | 39.7 | 13 565 | 9.2 |
| Black | 7 101 | 44.2 | 5 677 | 36.3 | 1 082 | 6.5 | |
| Asian | 7 805 | 27.2 | 6 481 | 22.6 | 2 162 | 7.6 | |
| AI/AN | 696 | 23.4 | 574 | 19.9 | 128 | 4.8 | |
| Female | White | 50 894 | 30.0 | 43 704 | 25.4 | 10 450 | 6.1 |
| Black | 6 130 | 30.3 | 4 940 | 24.6 | 771 | 3.8 | |
| Asian | 6 543 | 18.3 | 5 516 | 15.3 | 1 996 | 5.5 | |
| AI/AN | 640 | 18.6 | 526 | 15.4 | 137 | 4.3 | |
AI/AN: American Indian or Alaska Native; DLBCL: diffuse large B-cell lymphoma
All incidence rates are age-adjusted to the 2000 US population and expressed per 100,000 person-years
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER
Research Data, 12 Registries, Nov 2022 Sub (1992–2020) - Linked To County Attributes - Time Dependent (1990–2021)
Income/Rurality, 1969–2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
FIGURE 2.
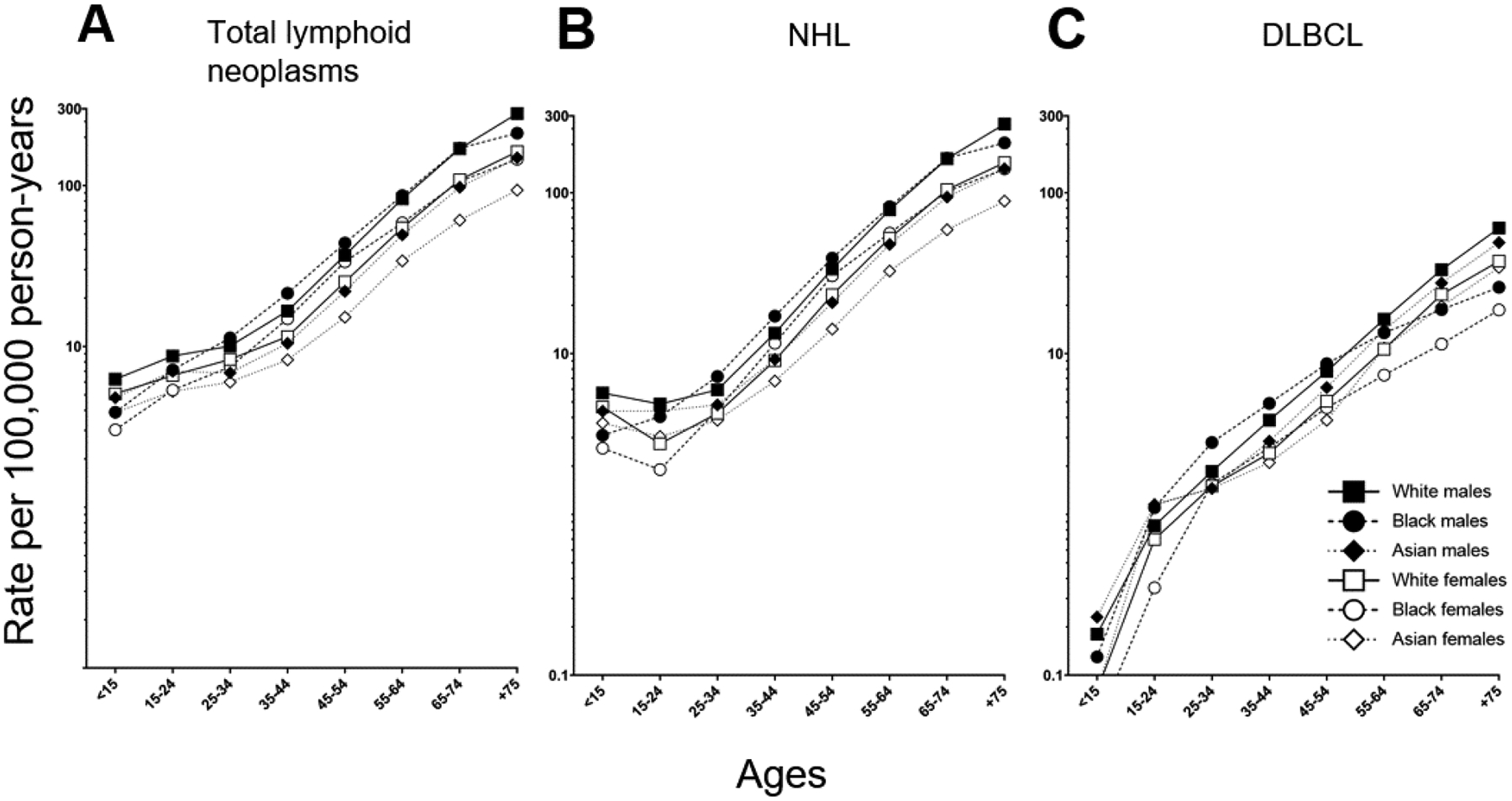
DLBCL incidence by age, race and sex, 12 SEER registries, 2011–2020. All incidence rates are age-adjusted to the 2000 United States population.
TABLE 3.
Incidence Rate Ratios (IRR) by Race and Sex, 12 SEER Registries, 2011–2020
| Lymphoid neoplasms, total | B-cell lymphoid neoplasms, total | DLBCL | ||
|---|---|---|---|---|
| IRR* | IRR* | IRR* | ||
| Male:Female IRR | White | 1.6 | 1.6 | 1.5 |
| Black | 1.5 | 1.5 | 1.7 | |
| Asian | 1.2 | 1.5 | 1.4 | |
| White:Black IRR | Males | 1.1 | 1.1 | 1.4 |
| Females | 1.0 | 1.0 | 1.6 | |
| White:Asian IRR | Males | 1.7 | 1.8 | 1.2 |
| Females | 1.6 | 1.7 | 1.1 |
DLBCL: diffuse large B-cell lymphoma
All incidence rates are age-adjusted to the 2000 US population and expressed per 100,000 person-years
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER
Research Data, 12 Registries, Nov 2022 Sub (1992–2020) - Linked To County Attributes - Time Dependent (1990–2021)
Income/Rurality, 1969–2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
Global Distribution and Incidence
Global DLBCL incidence by country and region is not available, although overall NHL rates have exhibited notable differences. According to rates estimated based on the GLOBOCAN database, NHL ranks the 5th to 9th most common cancer in most countries[1]; highest incidence was observed in Australia, U.S. (Whites), and Canada. Although global incidence rates are lacking, numerous initiatives and studies have evaluated the distribution of NHL by subtypes. The International NHL Classification Project, initiated in 1995, evaluated the distribution of NHL subtypes across 24 countries, between 1995 and 2012[2]. Overall, and across all regions – Europe, Central/South America, North Africa/Middle East/India, Africa, and Asia – and countries, DLBCL was the most common NHL subtype.
North & South America.
In the International NHL Classification Project, DLBCL accounts for ~1/3 of NHLs among Whites, based on a population derived from Omaha, Nebraska and Vancouver, Canada [2]. This is consistent with recent U.S. SEER data (Table 1) and with prior evaluation of the U.S. National Cancer Data Base which reported 32.5% of NHL as DLBCL, and DLBCL as the most common NHL subtype[3, 4]. A recent estimate of trends projects that incident DLBCL in the U.S. will rise 11% from 2020–2025 based on the changing U.S. demographics that comprises a growing aging population [5].
DLBCL is also the most common NHL subtype in South and Central America, although the distribution was higher (40%)[6]. This is consistent with an independent evaluation of NHL subtypes in a reference center in Brazil (48%)[7]. While male to female ratios are similar to that of North America, the median ages of DLBCL diagnosis was younger overall[6].
Europe.
Among 595 confirmed NHLs reviewed from Southeastern Europe as part of the International Non-Hodgkin Lymphoma Classification Project, DLBCL was the most common subtype overall (39%) in all three countries included: Croatia (37%), Macedonia (40%), and Romania (41%)[8]. Among Western European countries (United Kingdom, Germany, France, Switzerland), the International NHL Classification Project reported slightly lower proportion of DLCL among NHL subtypes of 25–30%[3]. Based on demographic characteristics, the number of incident DLBCL cases in Europe is also projected to increase from 2020 to 2025 at a rate of 7%[5].
Asia.
DLBCL is the most common NHL subtype in Asia, with reported NHL proportions of 30% in Korea[9] and ~35% across multiple populations in China[10]. Median ages also appear younger; a review of 2027 NHLs diagnosed between 2009–2014 in China yielded median age of 54 years, of which 41% were DLBCL[11]. In the International NHL Classification Project, nearly 50% DLBCL was observed in parts of China, Hong Kong, Thailand, and Jakarta based on the evaluation of 730 NHL cases[12]. Similarly in Taiwan, 48% (of 1257 NHLs) were DLBCL, based on review of 2000–2015 patients[13] and 33–38% DLBCL was observed in Japan[14, 15]. Of 194 NHL patients evaluated in India, 35.1% were DLBCL [16].
Although the incidence in Asia is lower than those reported for Europe and North America, there is some evidence of rising DLBCL incidence. Based on claims data from the National Health Insurance Service in Korea, Kim et al[17] reported DLBCL as the most common subtype (42–48% annually from 2011–2015) with standardized incidence rising for NHL overall and DLBCL (2.23 per 100,000 incidence for DLBCL). DLBCL incidence in Yogyakarta, Indonesia was also reported to rise between 2010–2014[18].
Middle East.
In the middle east, DLBCL is the most common NHL subtype, accounting for half of all NHL subtypes reported in a number of populations (e.g., 52% in Iraq [19], 51% in Saudi Arabia[19]; 54% in Lebanon[20]. Of NHL patients evaluated as part of the International NHL Classification Project, DLBCL accounted for 52.8% of NHL patients in Algeria[21], 41.5% in Egypt, 58.6% in Saudi Arabia, and 57.4% in Kuwait [16]. Studies in Kuwait have also reported similar distributions[22], and among Kuwaiti Arabs (46.5%) and non-Kuwaiti Arabs (48%)[23]. The proportion of DLBCL in Jordan was similarly high[24].
Africa.
DLBCL is the most common NHL subtype in Africa, accounting from 17% of DLBCLs in Zambia[25] to over 60% reported in the Tunisia cancer registry [26]. Data from the International NHL Classification Project [27] in Southern Africa on 487 consecutive NHL found 418 were B-cell lymphomas and DLBCL the most common subtype (38.2%). Trends in Tunisia showed rising DLBCL incidence from 2004–2007 and a projected increase, after a long period of plateau and slight decline [26].
Australia.
DLBCL incidence rose and plateaued in Australia from 1982–2014; having risen 6.2% annually prior to 1994, rates have stabilized with a 0.6% annual increase[28]. Based on Australian Cancer Registry data from 1982–2006, DLBCL accounted for 18% of all NHL. Consistent with DLBCL worldwide, DLBCL was marked by a male predominance[29].
Risk Factors for Diffuse Large B-cell Lymphoma
Epidemiologic studies conducted to uncover DLBCL risk factors have uniformly adopted the WHO definition for defining DLBCL and lymphoid malignancies[30–32]. This has enabled large pooled consortial efforts and meta-analyses of purported risk factors that include both genetic and non-genetic risk factors. At present, established DLBCL risk factors span hereditary, behavioral, infectious, and environmental exposures (Table 4). The strongest risk associations are those that result in severe immune deficiency such as HIV/AIDS, inherited immunodeficiency syndromes, and organ transplant recipients. Factors that lead to chronic immune dysregulations are also established risk factors, and include a number of autoimmune conditions (e.g., Sjögren syndrome, systemic lupus erythematosus, rheumatoid arthritis), viral infections (e.g., HIV, KSHV/HHV8, HCV, EBV), and obesity. Family history of NHL/DLBCL, personal history of cancer, and multiple genetic susceptibility loci are also well-established risk factors for DLBCL. There is strong evidence for multiple environmental exposures in DLBCL etiology, including trichloroethylene, benzene, and pesticides and herbicides with recent associations noted with glyphosate. There is also growing evidence supporting a role for additional viruses in DLBCL etiology, such as HBV.
Table 4.
Summary of risk associations of established risk factors for diffuse large B-cell lymphoma
| Established risk factors for DLBCL | |
|---|---|
| Risk factors | Risk association * = OR <2.0 *** = OR >2.0 |
| Family and person history | |
| Family history for any heme malignancy | * |
| Family history of DLBCL | *** |
| Personal history of cancer | * |
| Genetic susceptibility | * |
| Inherited immunodeficiency syndrome | *** |
| Organ transplants | *** |
| Autoimmune conditions | * |
| Sjogren’s syndrome | *** |
| Systemic lupus erythematosus | *** |
| Rheumatoid arthritis | * |
| Infections | |
| HIV | *** |
| KSHV/HHV8 | * |
| HCV | * |
| HBV | * |
| Anthropometric measures | |
| Adult BMI | * |
| Young adult BMI | * |
Hereditary risk factors
Family history.
Familial aggregation of NHL and elevated NHL (including DLBCL) risk among first degree relatives (parents, siblings, children) have long been established using nationwide registry data from Sweden and Denmark[33, 34]. Based on data from the Swedish Family-Cancer Database, a two-fold standard incidence ratio (SIR) in NHL risk was observed for those with family history (first-degree) of NHL; the SIR rose above two-fold (2.3) for DLBCL and when the first degree relative was a parent. When the NHL subtype of the parent was also DLBCL, the SIR increased to 11.8[35].
Population-based case control studies have also observed elevated DLBCL among those reporting a first degree relative with any hematopoietic malignancy. An international pooled study of studies across Northern America, Western Europe, and Australia conducted by the InterLymph Consortium reported a 1.4 fold increased risk of DLBCL among those reporting a family history of NHL; the risk increased to two if the relative was male[36]. In updated efforts that evaluated family history in multivariate models constructed with all DLBCL risk factors, a nearly two-fold increased DLBCL risk was observed among those reporting a family history of NHL (odds ratio, OR=1.95)[37].
Personal history.
Personal history of cancer is an established risk factor for NHL, and among the NHLs that arise as a second cancer, DLBCL is the most common NHL subtype. NHL/DLBCL risk is elevated among those who have had a lymphoid malignancy[38, 39], with a 20 year cumulative incidence rate of 3–5% for NHL after Hodgkin’s disease. Although less frequent, NHL/DLBCL has also been documented among childhood and adolescent cancer survivors[39, 40]. DLBCL is also documented to occur after primary solid tumors; in a retrospective analysis of 809 DLBCL patients in Japan, over 10% of DLBCL patients had past cancer, with stomach cancer the most frequent[41]. 836 DLBCL patients in China evaluated for personal history of solid tumors were found to have had personal histories of a number of solid tumors[42].
Genetic Susceptibility.
Several large international consortia efforts have identified genetic susceptibility loci in DLBCL risk (Table 5) [43–45]. A large international effort across populations of European ancestry based on a meta-analysis of 3 genome-wide associations studies and one prior scan comprising 3,857 cases and 7,666 controls of European ancestry and further validation in 1,359 cases and 3,557 controls yielded five genome wide significant (p<108) and independent SNPs across four loci: chromosome 6p25.4 (EXOC2), 6p21.33 (HLA-B), 2p23.3 (NCOA1), and 2 SNPs in 8q24.21 (PVT1). Defining the candidate genes require further investigation and functional analyses, but the loci at 6p21.33 strongly supports the role of HLA-B*08:01 in DLBCL risk, an association also supported by case-control studies based on HLA allelotyping [46]. In Abdou et al [46], the HLA-B*0801 and HLA-A*01-B*08-DR*03-TNF-A ancestral haplotype (AH8.1) was associated with elevated DLBCL risk. In expanded GWAS efforts that included the meta-analysis of four original GWAS discovery scans and three replication studies, additional associations between 3q13.33 and 3p24.1 were observed with DLBCL risk [44],
Table 5.
Genetic susceptibility loci associated with DLBCL risk.
| SNP | Locus | Nearest Gene | RAF* (controls) | OR | Reference |
|---|---|---|---|---|---|
| GWAS of European Ancestry | |||||
| rs79480871 | 2p23.3 | NCOA1 | 0.076 | 1.34 | [43] |
| rs6773363 | 3p24.1 | EOMES | 0.45 | 1.2 | [44] |
| rs9831894 | 3q13.33 | 0.40 | 0.83 | [44] | |
| rs2523607 | 6p21.33 | HLA-B | 0.12 0.0003 |
1.32 3.05 |
[43] [45] |
| rs116446171 | 6p25.3 | EXOC2 | 0.019 0.06 |
2.20 2.04 |
[43] [45] |
| rs13255292 | 8q24.21 | PVT1 | 0.32 0.20 |
1.22 1.34 |
[43] [45] |
| rs4733601 | 8q24.21 | PVT1 | 0.48 | 1.18 | [43] |
| rs10484561 | 6p21.32 | HLA Class II | 1.36 9, p=1.4×10–7 | [47] |
|
| GWAS of Asian Ancestry | |||||
| rs6773854 | 3q27 | BCL6/LPP | 0.22 | 1.47 | [52] |
| rs2523607 | 6p21.33 | HLA-B | 0.0003 | 3.05 | [45] |
| rs116446171 | 6p25.3 | EXOC2 | 0.06 | 2.04 | [45] |
| rs13255292 | 8q24.21 | PVT1 | 0.20 | 1.34 | [45] |
| Candidate SNPs | |||||
| rs1800629 | 6p21 | TNF −308A | 0.28 | 1.29 | [51] |
| rs3132453 | PRRC2A, BAG6/BAT3 | 0.066 | 0.68 (B cell lymphomas) | [50] | |
| rs3789068 | 2q13 | BCL1L11 | 0.46 | 1.21 (B cell lymphomas) | [50] |
| AH 8.1 | 6p21.3 | HLA A*0101-B*0801-DR*0301-TNF-A | 0.13 | 1.65 | [46] |
Risk allele frequenc
Notably, confirmed DLBCL susceptibility loci are largely distinct from susceptibility loci that have been identified for other NHL subtypes. To date, only one loci appears to be pleiotropic; a three-stage genome-wide association study of follicular lymphoma among European ancestry reported associations between rs10484561, which tags the extended haplotype HLA-DQA1*0101-HLA-DQB1*0501-HLA-DRB1*0101, with both follicular lymphoma and DLBCL (p<107), [47, 48]. An evaluation of polygenic risk scores (PRS) for each NHL subtype with GWAS data (among European ancestry) found that PRS for DLBCL was also associated with increased risk for chronic lymphocytic leukemia (CLL), follicular lymphoma, and marginal zone lymphoma[49]. Alternatively, PRS for CLL, follicular lymphoma, marginal zone lymphoma, and Waldenstrom’s macroglobulinemia were also associated with increased DLBCL risk with ORs of 1.17, 2.69, 1.28, 1.53 and 1.24, respectively, suggesting overlap in unmeasured shared heritability.
We note that early meta-analysis of candidate SNPs evaluated in populations of European ancestry yielded significant associations between rs3789068 (BCL2L11), rs3132453 (PRRC2A in HLA complex class III region) [50] and TNF-alpha [51]. Although reaching genome-wide significance in these early studies, the statistical significance of these loci in the larger pooled GWAS efforts became attenuated. It is possible that these associations were in linkage disequilibrium with the confirmed loci identified by GWAS efforts, but risk attenuation could also be due to differences in population sampling or chance.
GWAS efforts among non-European populations remain sparse. In a pooled study comprising 1124 DLBCL patients and 3596 controls from three Eastern Asian populations (Hong Kong, South Korea, and Thailand), 3 of the five GWAS SNPs from the European ancestry GWAS were replicated, despite very different minor allele frequencies for each: EXOC2, PVST1, and HLAB [45]. A GWAS among Singapore Chinese reported a new DLBCL susceptibility locus, rs6773854, located between BCL6 and LPP in chromosome 3q27 [52]. Additional efforts that build upon these data in Asian and other non-European populations are needed to construct relevant and validated PRS across race/ethnic populations. The 3q27 association reported in Singapore Chinese population [52] was not observed in the Eastern Asian or European population-based GWAS, and it is unclear whether this is a function of sample size, population, or race/ethnic differences.
The DLBCL susceptibility loci identified by GWAS in European populations are estimated to contribute to ~16% of the overall variance for DLBC [43]. The confirmed DLBCL loci are also estimated to contribute 0.09 heritability, suggesting that the most susceptibility loci have not yet been identified [43]. The familial risk estimate based on these loci is calculated to be 1.40 [49, 53], which is consistent with prior studies of family history and DLBCL.
Organ transplants and primary immunodeficiencies.
Organ transplants and primary immunodeficiencies are well-established risk factors for elevated NHL and DLBCL risk. Elevated B-cell lymphoma risk, including DLBCL, is well-documented among populations with primary immunodeficiencies and disorders, including common variable immune deficiency (CVID), Wiskott Aldrich, and Nijmegen Breakage syndrome[54–57]. A number of studies among recipients of solid organ transplants demonstrate elevated DLBCL risk[58–61]. Among a U.S.-based population, risk of DLBCL after solid organ transplantation was 12.6-fold (standard incidence ratio) that of a non-transplant population[62], with the highest risk was observed among those who underwent organ transplantation at younger ages and those receiving a lung or pancreas/kidney-pancreas transplant. The risk was also higher among extranodal DLBCLs at the site of transplant, and highest in the first year following the transplant. In a large cohort of solid organ transplant recipients, elevated DLBCL risk followed solid organ transplantation in a U.S. population based on linkages to 14 state and regional cancer registries[63–65]. In the U.S. Transplant Cancer Match Study, DLBCL risk among solid organ transplant recipients was >13 times higher than the general population[63, 66]. By type of organ transplanted, risk was highest for pancreas or kidney/pancreas transplant (SIR=32.6), followed by heart and/or lung transplant (SIR=18.2), liver transplant (SIR=13.1), and kidney transplant (SIR=11.0). By age of transplant, DLBCL risk was highest among those receiving a transplant 19 years and under (SIR=379).
Autoimmune Conditions
There is substantial and consistent evidence supporting positive associations between autoimmune conditions and elevated NHL and DLBCL risk. A pooled analysis of autoimmune disorders and NHL subtypes across 12 participating InterLymph Consortium case-control studies[67] reported positive associations with DLBCL with the following autoimmune disorders: hemolytic anemia (OR=3.2), systemic lupus erythematosus (OR=2.74), Sjogren’s syndrome (OR=8.92; primary Sjogren syndrome OR=6.57; Secondary Sjogren syndrome OR=12.8). Although autoimmune conditions from case control studies are inherently limited based on self-report, these associations were corroborated in data using medical billing codes from the U.S. Surveillance Epidemiology and End Results-Medicare database[68] where elevated DLBCL risk was observed for those with Sjögren syndrome and autoimmune hemolytic anemia. A modest association between rheumatoid arthritis and DLBCL (OR=1.4) and borderline association between systemic lupus erythematosus and DLBCL was also observed[68]. In a cancer registry and hospital linkage-based studies of autoimmune disorders in Sweden, elevated standardized incidence ratios (SIRs) for Sjogren syndrome (SIR=5.5), systemic lupus erythematosus (SIR=6.6), and rheumatoid arthritis (SIR=2.2) were observed[69] Associations were also observed for a number of other conditions, including: celiac disease, chronic rheumatic heart disease, Crohn’s disease, immune thrombocytopenic purpura, myasthenia gravis, primary biliary cirrhosis, sarcoidosis, systemic sclerosis, and ulcerative colitis.
Perhaps the most convincing evidence comes from studies derived of patient cohorts. A Swedish cohort reported a 16-fold NHL risk for those with Sjogren syndrome, with DLBCL the predominant NHL subtype[70]. In an international cohort of systemic lupus erythematosus (SLE) patients across 30 centers, DLBCL was the most common NHL to arise[71], and in a patient cohort of over 9500 patients with Sjogren Syndrome, DLBCL accounted for over half of the NHL subtypes that developed [72]. A Scandinavian cohort identified elevated risk among Crohn’s disease patients and inflammatory bowel disease for NHL/DLBCL[73], an association that was similarly observed in an updated evaluation of InterLymph case control studies where inflammatory bowel disease was associated with gastrointestinal DLBCL[37].
It has been posited that immunosuppressive therapies used as treatment for autoimmune conditions may at least partially explain the risk or may further increase the risk for DLBCL/NHL. Epidemiologic studies to clarify this distinction have largely comprised cohorts of specific autoimmune conditions; a number of studies have evaluated the relationship between NHL and treatment among RA patients, but results remain inconsistent and largely null [74]. Importantly, the inherent bias in treatment associated with more severe autoimmune disease severity confounds the potential to delineate treatment-related elevation in NHL risk from the underlying RA. Other autoimmune conditions and treatments have also been explored, similarly resulting in mixed though largely null associations. A prospective study of 2,105 participants with inflammatory polyarthritis reported highest lymphoma incidence among those treated with methotrexate [75, 76], but another found little evidence of elevated risk among RA patients using methotrexate, beyond that from RA alone [77]. Large epidemiologic studies have also generally not found elevated NHL risk among those on anti-TNF drugs. Mercer and colleagues [78] reported no difference in lymphoma incidence between 11,931 TNF inhibitor-treated patients from 3,367 untreated patients with RA. In a SEER-based study [77], elevated NHL risk among RA patients were modestly higher among those on anti-TNF therapies but the differences were “slight”; the study authors suggested the results were due to bias due to patients with the highest risk of lymphoma preferentially receiving anti-TNF therapy. In a meta-analysis, inflammatory bowel disease patients in 22 placebo-controlled trials were evaluated and no difference in NHL risk was observed between IBD patients on TNF-a antagonists versus placebo [79].
Infections
There are robust epidemiologic data demonstrating associations between the following infectious agents and DLBCL risk: human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), Kaposi Sarcoma herpesvirus/human herpesvirus 8 (KSHV/HHV8), hepatitis C virus (HCV). Evidence for an association between hepatitis B virus (HBV) and DLBCL continues to emerge.
Human immunodeficiency virus (HIV)
The association between HIV and NHL/DLBCL is well-established. NHL is an AIDS-defining cancer as defined by the U.S. Centers for Disease and Control[80, 81]. NHL is the second most common cancer that arises in HIV patients, but among specific AIDS subpopulations (e.g., intravenous drug users and hemophiliacs), NHL is the most frequent cancer[82]. Of NHLs, DLBCL is the most common NHL subtype that arises in HIV patients (accounting for ~50–75% of NHLs) [83, 84]. NHL DLBCL risk among HIV patients is high; compared to the general population (people without AIDS), NHL risk is 50–100-fold and DLBCL is 650-fold greater among those with HIV[80]. Standardized incidence ratios for DLBCL ranges from 100–140 per 100,000 [85]. However, this risk association has declined greatly in the era of modern antiretroviral therapy (ART) (e.g., post-ART). In the U.S., NHL risk was 11-fold greater, and DLBCL risk was 10.3-fold greater among people identified in HIV registries compared to the general population[86]. HIV is classified as a class I carcinogen by the International Agency for Research on Cancer (IARC)[87] but is believed to cause lymphoma through immunosuppression rather than oncogenic properties of the virus itself[85].
Kaposi sarcoma herpesvirus / Human Herpesvirus-8 (KSHV/HHV8)
Kaposi sarcoma herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), is also classified as a class I human carcinogen by IARC based on its role as a causative agent to Kaposi’s sarcoma (KS) and primary effusion lymphoma (PEL), a rare type of DLBCL also known as body cavity lymphoma[88–90]. KSHV-DLBCL has also been described but has overlapping clinical features with PEL and other KSHV-associated diseases[88]. KSHV preferentially occurs in immunocompromised populations such as HIV-infected or organ transplant recipienst; KSHV and EBV co-infection is also frequently seen[88].
Epstein Barr Virus (EBV)
EBV is an oncogenic virus; it is ubiquitous, infecting more than 90% of the world’s population at some point, but infection is largely asymptomatic[91]. Overall, ~10% of DLBCLs are EBV-positive, though this range varies by country and by method of EBV detection. EBV-positive DLBCL is often found among immunocompromised individuals, such as HIV/AIDS patients and organ transplant recipients[92, 93]. Over half of AIDS-related DLBCL are EBV-positive[92, 94] and standardized incidence ratios for EBV-related DLBCL is 100–140 per 100,000 [85]; among transplant recipients, EBV infection can range 70–100% [92]. EBV is posited to cause lymphoma by driving B cell hyperstimulation[92].
Hepatitis C Virus
Hepatitis C virus (HCV) is classified by IARC as a Group 1 carcinogenic agent in humans (https://gco.iarc.fr/causes/infections). HCV RNA and proteins have been identified in biopsy samples from lymphoma patients[95] and specifically in HCV-positive DLBCL [96, 97]; in addition to potential oncogenic properties, HCV is posited to contribute to NHL etiology through chronic antigenic stimulation[98, 99].
A number of case-control studies[100], cohort studies[98, 101], consortial efforts[37], and meta-analyses consistently report an association between HCV infection and elevated NHL risk[102–106], and specifically for DLBCL[37, 100]. In 2008, the InterLymph Consortium conducted a pooled analyses of 4784 NHL cases and 6260 controls across 7 studies with low HCV prevalence in North America, Europe, and Australia that measured HCV infection in serum using third-generation enzyme-linked immunosorbent assays to detect antibodies against HCV, reporting a 2.2-fold elevation in risk for DLBCL[100]. This association was robust in multivariate analysis that included other DLBCL risk factors[37]. In a meta-analysis of 8 case-control studies across populations with both high and low HCV prevalence, a 2.7-fold increased risk for DLBCL was estimated[102]. Aggregated data from 26 health care systems in the U.S compared the prevalence of DLBCL among patients with chronic hepatitis C infection between 2013–2020 to those negative for HCV, reporting a four-fold increased risk for DLBCL among those with chronic hepatitis C infection[98]. The associations are consistently supported by claims data from U.S. SEER-Medicare linkage [107] and from recent serology-based case-control studies [108]
Hepatitis B Virus
Hepatitis B virus (HBV) is classified by IARC as a Group 1 carcinogenic agent in humans (https://gco.iarc.fr/causes/infections). Although not a directly oncogenic virus, HBV infection is posited to result in chronic antigenic stimulation, the mechanism by which it would increase risk for NHL.
Multiple meta-analyses have concluded there is a positive association between HBsAg positivity and elevated NHL risk[109–112]. A 2013 meta-analysis evaluating 8 studies with NHL subtype information yielded a 2.73-fold increase in DLBCL risk among those who were HBsAg positive versus those who were HBsAg negative[110]; these studies included those with higher HBV prevalence, including Taiwan and Korea. These also included several large prospective cohort studies including the Korean Cancer Prevention Study where over 600,000 participants were followed for over a decade, yielding a 2-fold increase for DLBCL risk among HBsAg+ participants[113]. Another prospective cohort of >20,000 participants in Japan followed for other 16 years yielded a >7-fold increased risk of DLBCL[114]. A subsequent 2018 meta-analysis included 10 studies that evaluated DLBCL risk also reported a two-fold elevation in DLBCL risk among those HBsAg-positive[115]; this analysis included additional studies from non-HBV endemic regions, whose results varied. While case-control studies in Israel and Italy yielded elevated DLBCL risk[116, 117], null results were observed in a Danish cohort study with 30 years of follow-up[118]. At present, the association between HBV and DLBCL, particularly in HBV-endemic regions, appears mostly consistent.
Anthropometric measures
Epidemiologic evidence consistently demonstrates a positive association between higher adult and young adult BMI / obesity with elevated DLBCL risk. A pooled analyses of over 10,000 NHL cases (including >3000 DLBCL) in 18 case-control studies participating in the InterLymph Consortium across North America and Europe yielded excess risk (OR=1.80) between grade 3 obesity (defined as BMI 40+ kg/m2) and DLBCL, compared to normal BMI (defined as 18.5–24.99 kg/m2) [119]. A meta-analysis of 6 case-control and 10 cohort studies conducted through 2012 also reported a positive association between overweight and obese individuals and DLBCL risk with RR of 1.14 and 1.29, respectively, with elevated DLBCL risk observed for both men and women, and for both case-control and cohort studies [120].
An updated pooled analyses conducted in the InterLymph Consortium comprising 4,667 DLBL cases and 22,639 controls across 19 case-control studies whose data were used to construct multivariate models for DLBCL risk, demonstrated positive associations between higher young adult body mass index (OR=1.58) with elevated DLBCL risk; although this measure was highly correlated with adult BMI, the group with higher young adult BMI was largely a subset of those with high adult BMI, thereby emphasizing the importance of obesity prevention early in life as avenue for DLBCL prevention [37]. The association between young adult BMI and elevated DLBCL risk has been further corroborated in a number of cohort studies, including among cohorts of U.S. physicians and nurses [121], across multiethnic populations [122], in U.S. nationwide cohorts [123], and in a cohort in the Netherlands [124]. In a recent pooled analysis of six prospective cohort studies in the United States[125], the strongest associations observed were for young adult BMI and elevated DLBCL risk, with the strong association observed among those who maintained a higher BMI from young adult into later adulthood which more than doubled their DLBCL risk (HR=2.67) compared those with a BMI of 18.5–22.9 kg/m2 [125]. Based on data from prospective cohort studies, it estimated that up to 23.5% of all DLBCLs may be preventable if young adult obesity were avoided [125].
Environmental and Occupational Exposures
Although there is relatively robust evidence linking occupations and environmental exposures to increased risk of hematopoietic malignancies, the evidence that links these exposures specifically to DLBCL remains limited due to the nature of the studies. Much of the epidemiologic data is derived from occupational cohorts that follow high-exposed populations for years but accrue relatively small numbers of NHLs, of which evaluating NHL subtypes is challenging. Nested case control studies are also used to directly measure environmental exposures in biospecimens, but due to the expense (in cost and biospecimen) of measuring exposures, these studies are also typically limited in sample size.
Trichloroethylene (TCE) is classified as a probable carcinogen (Group 2A) by IARC. A meta-analysis of 14 occupational cohort and four case-control studies linked TCE to elevated NHL risk but lacked NHL subtype data [126]. However, a pooled analysis of 3788 NHL cases and 4279 controls within four participating InterLymph Consortium studies evaluated the association between TCE based on occupation categories and NHL risk and found DLBCL risk to be elevated in the highest category of exposure intensity[127].
Benzene is classified by IARC as a human carcinogen that causes leukemia; evidence for benzene causing lymphoma has to date been considered “limited” [128]. In a 2021 meta-analysis of benzene exposure and NHL risk, data from 20 case-control studies and eight cohort studies, totaling 9587 NHL cases, yielded a 1.67-fold increased risk for DLBCL [129].
2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) is classified as a Group 1 carcinogen (Group B2) by IARC and has been linked to lymphomas [130]. However, evidence specifically linking TCDD to DLBCL risk remains lacking.
1,3-butadiene is considered a potential occupational carcinogen and teratogen by the National Institute for Occupational Safety and Health (NIOSH). Epidemiological evidence linking 1,3-butadiene to DLBCL risk is still lacking, though there is some suggestion that 1,3-butadiene may be associated with lymphoma mortality based on occupational studies of workers employed in facilities that produce styrene butadiene rubber [131, 132].
Polychlorinated Biphenyls (PCBs) are classified as a Group 1 carcinogen by IARC [133], but the evidence of association between overall PCBs and PCB congeners with NHL and DLBCL risk is inconsistent. In one of the largest studies of NHL and PCBs at the time, excess risk for NHL (and across subtypes including DLBCL) was observed for top quartiles of plasma concentrations for numerous congeners [134]. However, a comparable study in Europe of 9 PCB congeners in plasma samples yielded no overall association of PCBs with DLBCL risk [135]. Another study in Europe and Sweden measuring 6 PCB congeners in prediagnostic plasma measurements also found no overall association with NHL or DLBCL over 16 years of follow-up[136]. A 2019 case-control study of 33 PCB congeners measured in serum for NHL risk in Italy among a chemical factory similarly found no association between serum levels of total PCBs with NHL or DLBCL [137]. A 2017 meta-analysis [138] of 11 occupational cohort studies and a 2019 meta-analysis of [139] of 30 populations, including occupational cohorts, high-exposure populations, and standard populations found insufficient evidence to support an association.
The association between herbicides and insecticides and DLBCL is still developing. In a pooled analysis of nearly 8000 NHL cases from 9 case control studies across North America, Europe, and Australia in the InterLymph Consortium, long-term diazinon use (>8 years) was positively associated with DLBCL (OR=3.16). Use of insecticides, organochlorine insecticides, DDT, chlordane, organophosphate insectides and malathion were not associated with DLBCL risk [140, 141]. However, use of herbicides in a pooled analysis of 10 InterLymph case-control studies reported a positive association between 2,4-dichlorophenoxyacetic acid (2,4-D), glyphosate with DLBCL (>25.5 years, OR=1.47) [140, 141]. Overall herbicide use was also found associated with DBLCL in an evaluation of data from the United States Geological Survey (USGS), United States Census, and the Surveillance, Epidemiology, and End Results (SEER) database[142]. Calculating the association between the area density of specific agricultural pesticides and the county level annual incidence of DLBCL, DLBCL incidence was positively associated with an area density of 14 of the pesticides, of which 13 were used as herbicides, including the organophosphate Methyl Parathion and glyphosate. In a pooled analysis of three large agricultural worker cohorts including 2430 NHL cases across the United States, France, and Norway, a positive association was reported between glyphosate and DLBCL (HR=1.48)[143], which was further supported in a meta-analysis of 7 studies with a 1.3-fold DLBCL risk for the highest category of glyphosate exposure[144].
Finally, in a pooled analysis of 10 case-control studies participating in the InterLymph Consortium, 10,046 cases and 12,025 controls were evaluated for the association between occupational exposures and NHL risk[145], based on occupational coding from the 1968 International Standard Classification of Occupations (ISCO-1968). Positive associations with DLBCL were observed for the following occupations: hairdressers, textile workers, charworkers and cleaners, field crop and vegetable farm workers, metal melters and reheaters, special education teachers, and forestry workers with >10 years of employment. Further investigations are required to confirm these associations and understand the underlying biology.
Other Putative Risk Factors include atopic conditions (allergies, hay fever), smoking, alcohol consumption, sun / ultraviolet radiation exposure, hair dye use, oral contraceptives, hormone therapy, and blood transfusions. Decreased risk for atopic conditions, alcohol consumption, and sun/ultraviolet radiation exposure has been reported[37, 146–152], but discrepancy and/or inconsistent results require additional follow-up. Other risk factors such as blood transfusion and hair dye use appear to apply to specific population subsets[153, 154]. At present, there is insufficient evidence to conclude that these factors are associated with DLBCL risk.
Future Directions in Epidemiologic Research
Although there are some very strong risk factors for DLBCL, most patient’s DLBCL will likely develop through multi-factorial etiology. Continued efforts to confirm and identify novel DLBCL risk factors in prospective cohort studies where survival bias is reduced and temporality can be established are thus needed; efforts to understand how genetic and environmental risk factors contribute to risk (e.g., synergistically, independently, etc.) and whether they interact are needed. Constructing risk prediction models that combine genetic and environmental risk factors may be beneficial for those at elevated risk due to personal histories, such as family history or among those with autoimmune conditions. Pooled and consortial efforts in diverse populations will be particularly important to reach sufficient power to identify modest associations and to clarify important differences by race/ethnicity. Prospective studies further offer the ability to identify pre-diagnostic biomarkers of exposure and/or risk, a critical step for understanding the underlying biology and for constructing risk prediction models. Understanding whether DLBCL risk factors differ further by molecular subtypes, of which there are now seven[155], may also aid in understanding the multifactorial etiology of DLBCL. Preliminary data linking high body mass index and germinal center B-cell-like GCB DLBCL, have been reported [156], and new and purported risk factors may be uncovered or clarified with these molecular delineations. Data from the InterLymph Subtypes Project noted specific associations by tumor site, including positive association between heavy smoking and CNS DLBCL, and between autoimmune conditions and gastrointestinal DLBCL. Larger sample sizes will be required to confirm these results. Other DLBCL characteristics that warrant future investigations include FL-transformed DLBCL as a distinct outcome.
As genetic susceptibility loci are uncovered, it will be particularly important to interrogate gene-environment interactions to understand their joint contribution to disease risk[157–160]; further understanding whether purported environmental exposures exert their effects in the presence of susceptibility loci, or vice versa, will add to our understanding of this complex disease. To date, large population sample sizes are available for Caucasian populations, but effort is required to understand whether susceptibility loci identified to date are applicable to non-Caucasian race groups and whether risk prediction models that are constructed can be applied to non-Caucasian race groups. Finally, new and continued efforts for identifying environmental risk factors or novel infectious agents responsible for DLBCL etiology are needed. A study on HLA zygosity that demonstrated positive association with DLBCL risk suggested a role for infectious etiologies[161]. Pinpointing environmental contributions to DLBCL risk should also expand to a broader definition as defined by social and built environment, and to multi-mixture models as environmental exposures do not occur in isolation.
Although much progress on uncovering DLBCL risk factors has been made over the last two decades, early detection and screening does not yet exist. Continued efforts to identify risk factors that can explain the causes of DLBCL are thus needed to construct risk prediction models, tailored to identify those at highest risk for disease. Critically, continued efforts to identify precursor conditions for early detection efforts are needed. Potentially promising precursors include clonal hematopoiesis; while studies have largely reported CH in myeloid, follicular, and CLL/SLL as endpoints, continued discovery efforts to uncover clonal hematopoiesis in DLBCL could hold promise for early detection [162]. In the meantime, ascertaining personal and medical histories will remain important for disease prevention and early detection; prevention of and monitoring individuals with many of these risk factors, such as young adult obesity and infections, will be pertinent for multiple health outcomes, including DLBCL.
Acknowledgements
We thank Emily Caubler for her expertise and contributions on descriptive statistics and graphical presentation. We are also deeply grateful to all patients and study participants who tirelessly contribute their information to help uncover the etiology of DLBCL.
The authors confirm no conflict of interest; there are no affiliations or involvement in any organization or entity with financial interests.
Funding:
This work was supported by the National Institutes of Health (R01CA202712).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Miranda-Filho A, et al. , Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control, 2019. 30(5): p. 489–499. [DOI] [PubMed] [Google Scholar]
- 2.Perry AM, et al. , Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica, 2016. 101(10): p. 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JR, Armitage JO, and Weisenburger DD, Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol, 1998. 9(7): p. 717–20. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hamadani M, et al. , Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol, 2015. 90(9): p. 790–5. [DOI] [PubMed] [Google Scholar]
- 5.Kanas G, et al. , Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020–2025. Leuk Lymphoma, 2022. 63(1): p. 54–63. [DOI] [PubMed] [Google Scholar]
- 6.Laurini JA, et al. , Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood, 2012. 120(24): p. 4795–801. [DOI] [PubMed] [Google Scholar]
- 7.Caminha B VG, Pimenta M, et al. , Epidemiological Analysis of Lymphoma Subtypes in a Reference Center in João Pessoa, Paraiba, Brazil. International Journal of Physical Medicine & Rehabilitation, 2018. 6(3). [Google Scholar]
- 8.Dotlic S, et al. , Classification of non-Hodgkin lymphoma in South-eastern Europe: review of 632 cases from the international non-Hodgkin lymphoma classification project. Br J Haematol, 2015. 171(3): p. 366–72. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SO, et al. , Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. Am J Hematol, 2010. 85(10): p. 760–4. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, et al. , Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol, 2012. 138(3): p. 429–34. [DOI] [PubMed] [Google Scholar]
- 11.Meng J, et al. , Epidemiologic characteristics of malignant lymphoma in Hubei, China: A single-center 5-year retrospective study. Medicine (Baltimore), 2018. 97(35): p. e12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry AM, et al. , Non-Hodgkin lymphoma in the Far East: review of 730 cases from the international non-Hodgkin lymphoma classification project. Ann Hematol, 2016. 95(2): p. 245–51. [DOI] [PubMed] [Google Scholar]
- 13.Chuang SS, et al. , Lymphoma in Taiwan: Review of 1347 neoplasms from a single institution according to the 2016 Revision of the World Health Organization Classification. J Formos Med Assoc, 2017. 116(8): p. 620–625. [DOI] [PubMed] [Google Scholar]
- 14.Miura Y, et al. , Clinicopathological features of malignant lymphoma in Japan: the Miyagi Study. Tohoku J Exp Med, 2011. 224(2): p. 151–60. [DOI] [PubMed] [Google Scholar]
- 15.The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int, 2000. 50(9): p. 696–702. [DOI] [PubMed] [Google Scholar]
- 16.Perry AM, et al. , Relative frequency of non-Hodgkin lymphoma subtypes in selected centres in North Africa, the middle east and India: a review of 971 cases. Br J Haematol, 2016. 172(5): p. 699–708. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, et al. , Increasing Incidence of B-Cell Non-Hodgkin Lymphoma and Occurrence of Second Primary Malignancies in South Korea: 10-Year Follow-up Using the Korean National Health Information Database. Cancer Res Treat, 2020. 52(4): p. 1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dwianingsih EK, et al. , Histopathological Features of Lymphoma in Yogyakarta, Indonesia. Asian Pac J Cancer Prev, 2016. 17(9): p. 4213–4216. [PubMed] [Google Scholar]
- 19.Yaqo RT, et al. , Non-Hodgkin Lymphoma in the Middle East Is Characterized by Low Incidence Rates With Advancing Age. J Glob Oncol, 2019. 5: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touma E, et al. , Non Hodgkin lymphoma in Lebanon: a retrospective epidemiological study between 1984 and 2019. BMC Public Health, 2021. 21(1): p. 1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudjerra N, et al. , Classification of non-Hodgkin lymphoma in Algeria according to the World Health Organization classification. Leuk Lymphoma, 2015. 56(4): p. 965–70. [DOI] [PubMed] [Google Scholar]
- 22.Ameen R, et al. , Frequencies of non-Hodgkin’s lymphoma subtypes in Kuwait: comparisons between different ethnic groups. Ann Hematol, 2010. 89(2): p. 179–84. [DOI] [PubMed] [Google Scholar]
- 23.Temmim L, et al. , Clinical characteristics and pathological classification of non-Hodgkin’s lymphoma in Kuwait. Results of a collaborative study with the International Lymphoma Study Group (ILSG). Leuk Lymphoma, 2004. 45(9): p. 1865–71. [DOI] [PubMed] [Google Scholar]
- 24.Almasri NM, Habashneh MA, and Khalidi HS, Non-Hodgkin lymphoma in Jordan. Types and patterns of 111 cases classified according to the WHO classification of hematological malignancies. Saudi Med J, 2004. 25(5): p. 609–14. [PubMed] [Google Scholar]
- 25.Polepole P, et al. , Spectrum of common Hodgkin lymphoma and non-Hodgkin lymphomas subtypes in Zambia: a 3-year records review. J Health Popul Nutr, 2021. 40(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benhassine A, et al. , Incidence Trend for Non-Hodgkin Lymphoma in the North Tunisian Population, 1998–2009. Asian Pac J Cancer Prev, 2016. 17(5): p. 2513–8. [PubMed] [Google Scholar]
- 27.Perry AM, et al. , Non-Hodgkin lymphoma in Southern Africa: review of 487 cases from The International Non-Hodgkin Lymphoma Classification Project. Br J Haematol, 2016. 172(5): p. 716–23. [DOI] [PubMed] [Google Scholar]
- 28.Farrall AL and Smith JR, Changing Incidence and Survival of Primary Central Nervous System Lymphoma in Australia: A 33-Year National Population-Based Study. Cancers (Basel), 2021. 13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Leeuwen MT, et al. , Lymphoid neoplasm incidence by WHO subtype in Australia 1982–2006. Int J Cancer, 2014. 135(9): p. 2146–56. [DOI] [PubMed] [Google Scholar]
- 30.Morton LM, et al. , Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood, 2007. 110(2): p. 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner JJ, et al. , InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood, 2010. 116(20): p. e90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffe ES, The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program, 2009: p. 523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldin LR, et al. , Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev, 2005. 14(10): p. 2402–6. [DOI] [PubMed] [Google Scholar]
- 34.Altieri A, Bermejo JL, and Hemminki K, Familial risk for non-Hodgkin lymphoma and other lymphoproliferative malignancies by histopathologic subtype: the Swedish Family-Cancer Database. Blood, 2005. 106(2): p. 668–72. [DOI] [PubMed] [Google Scholar]
- 35.Goldin LR, et al. , Highly increased familial risks for specific lymphoma subtypes. Br J Haematol, 2009. 146(1): p. 91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SS, et al. , Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood, 2007. 109(8): p. 3479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerhan JR, et al. , Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr, 2014. 2014(48): p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cappelaere P, [Secondary non-Hodgkin’s lymphomas]. Bull Cancer, 1998. 85(3): p. 217–31. [PubMed] [Google Scholar]
- 39.Eguiguren JM, et al. , Secondary non-Hodgkin’s lymphoma after treatment for childhood cancer. Leukemia, 1991. 5(10): p. 908–11. [PubMed] [Google Scholar]
- 40.Zahnreich S and Schmidberger H, Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies. Cancers (Basel), 2021. 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanba K, et al. , Prognostic impact of a past or synchronous second cancer in diffuse large B cell lymphoma. Blood Cancer J, 2018. 8(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y, et al. , Second malignant neoplasms in lymphomas, secondary lymphomas and lymphomas in metabolic disorders/diseases. Cell Biosci, 2022. 12(1): p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerhan JR, et al. , Genome-wide association study identifies multiple susceptibility loci for diffuse large B cell lymphoma. Nat Genet, 2014. 46(11): p. 1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleinstern G, et al. , Inherited variants at 3q13.33 and 3p24.1 are associated with risk of diffuse large B-cell lymphoma and implicate immune pathways. Hum Mol Genet, 2020. 29(1): p. 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassig BA, et al. , Genetic susceptibility to diffuse large B-cell lymphoma in a pooled study of three Eastern Asian populations. Eur J Haematol, 2015. 95(5): p. 442–8. [DOI] [PubMed] [Google Scholar]
- 46.Abdou AM, et al. , Human leukocyte antigen (HLA) A1-B8-DR3 (8.1) haplotype, tumor necrosis factor (TNF) G-308A, and risk of non-Hodgkin lymphoma. Leukemia, 2010. 24(5): p. 1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smedby KE, et al. , GWAS of follicular lymphoma reveals allelic heterogeneity at 6p21.32 and suggests shared genetic susceptibility with diffuse large B-cell lymphoma. PLoS Genet, 2011. 7(4): p. e1001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong C, et al. , The role of HLA variation in lymphoma aetiology and survival. J Intern Med, 2019. 286(2): p. 154–180. [DOI] [PubMed] [Google Scholar]
- 49.Berndt SI, et al. , Distinct germline genetic susceptibility profiles identified for common non-Hodgkin lymphoma subtypes. Leukemia, 2022. 36(12): p. 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nieters A, et al. , PRRC2A and BCL2L11 gene variants influence risk of non-Hodgkin lymphoma: results from the InterLymph consortium. Blood, 2012. 120(23): p. 4645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothman N, et al. , Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: a report from the InterLymph Consortium. Lancet Oncol, 2006. 7(1): p. 27–38. [DOI] [PubMed] [Google Scholar]
- 52.Tan DE, et al. , Genome-wide association study of B cell non-Hodgkin lymphoma identifies 3q27 as a susceptibility locus in the Chinese population. Nat Genet, 2013. 45(7): p. 804–7. [DOI] [PubMed] [Google Scholar]
- 53.Sampson JN, et al. , Analysis of Heritability and Shared Heritability Based on Genome-Wide Association Studies for Thirteen Cancer Types. J Natl Cancer Inst, 2015. 107(12): p. djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Resnick ES, et al. , Morbidity and mortality in common variable immune deficiency over 4 decades. Blood, 2012. 119(7): p. 1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salavoura K, et al. , Development of cancer in patients with primary immunodeficiencies. Anticancer Res, 2008. 28(2B): p. 1263–9. [PubMed] [Google Scholar]
- 56.Schuetz C, et al. , Autoimmunity, autoinflammation and lymphoma in combined immunodeficiency (CID). Autoimmun Rev, 2010. 9(7): p. 477–82. [DOI] [PubMed] [Google Scholar]
- 57.Tran H, et al. , Immunodeficiency-associated lymphomas. Blood Rev, 2008. 22(5): p. 261–81. [DOI] [PubMed] [Google Scholar]
- 58.Vajdic CM, et al. , Second Cancer Risk and Late Mortality in Adult Australians Receiving Allogeneic Hematopoietic Stem Cell Transplantation: A Population-Based Cohort Study. Biol Blood Marrow Transplant, 2016. 22(5): p. 949–56. [DOI] [PubMed] [Google Scholar]
- 59.Vajdic CM, McCaughan GW, and Grulich AE, Cancer risk after organ transplantation. JAMA, 2012. 307(7): p. 663; author reply 663–4. [DOI] [PubMed] [Google Scholar]
- 60.Vajdic CM and van Leeuwen MT, Cancer incidence and risk factors after solid organ transplantation. Int J Cancer, 2009. 125(8): p. 1747–54. [DOI] [PubMed] [Google Scholar]
- 61.Quinlan SC, et al. , Increased risk for lymphoid and myeloid neoplasms in elderly solid-organ transplant recipients. Cancer Epidemiol Biomarkers Prev, 2010. 19(5): p. 1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson TM, et al. , Risk of diffuse large B-cell lymphoma after solid organ transplantation in the United States. Am J Hematol, 2014. 89(7): p. 714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clarke CA, et al. , Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer, 2013. 109(1): p. 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Leeuwen MT, et al. , Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood, 2009. 114(3): p. 630–7. [DOI] [PubMed] [Google Scholar]
- 65.Quinlan SC, et al. , Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol, 2011. 86(2): p. 206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engels EA, et al. , Spectrum of cancer risk among US solid organ transplant recipients. JAMA, 2011. 306(17): p. 1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekstrom Smedby K, et al. , Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood, 2008. 111(8): p. 4029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson LA, et al. , Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer, 2009. 125(2): p. 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hemminki K, et al. , Origin of B-Cell Neoplasms in Autoimmune Disease. PLoS One, 2016. 11(6): p. e0158360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theander E, et al. , Lymphoma and other malignancies in primary Sjogren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis, 2006. 65(6): p. 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernatsky S, et al. , Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun, 2013. 42: p. 130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voulgarelis M, et al. , Prognosis and outcome of non-Hodgkin lymphoma in primary Sjogren syndrome. Medicine (Baltimore), 2012. 91(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 73.Olen O, et al. , Increasing Risk of Lymphoma Over Time in Crohn’s Disease but Not in Ulcerative Colitis: A Scandinavian Cohort Study. Clin Gastroenterol Hepatol, 2023. [DOI] [PubMed] [Google Scholar]
- 74.Yadlapati S and Efthimiou P, Autoimmune/Inflammatory Arthritis Associated Lymphomas: Who Is at Risk? Biomed Res Int, 2016. 2016: p. 8631061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller EB, Autoimmunity and Lymphoma: A Brief Review. J Rheum Dis Treat 2018. 4: p. 062. [Google Scholar]
- 76.Franklin J, Lunt M, Bunn D, Symmons D, Silman A, Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis. Ann Rheum Dis, 2006. 65: p. 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolfe F and Michaud K, Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum, 2004. 50(6): p. 1740–51. [DOI] [PubMed] [Google Scholar]
- 78.Mercer LK, et al. , Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis, 2017. 76(3): p. 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams CJ, Peyrin-Biroulet L, and Ford AC, Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-alpha therapy in inflammatory bowel disease. Aliment Pharmacol Ther, 2014. 39(5): p. 447–58. [DOI] [PubMed] [Google Scholar]
- 80.Cote TR, et al. , Non-Hodgkin’s lymphoma among people with AIDS: incidence, presentation and public health burden. AIDS/Cancer Study Group. Int J Cancer, 1997. 73(5): p. 645–50. [DOI] [PubMed] [Google Scholar]
- 81.Caceres W, Cruz-Amy M, and Diaz-Melendez V, AIDS-related malignancies: revisited. P R Health Sci J, 2010. 29(1): p. 70–5. [PubMed] [Google Scholar]
- 82.Huguet M, et al. , Diffuse Large B-Cell Lymphoma in the HIV Setting. Cancers (Basel), 2023. 15(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berhan A, Bayleyegn B, and Getaneh Z, HIV/AIDS Associated Lymphoma: Review. Blood Lymphat Cancer, 2022. 12: p. 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LD K HIV-related lymphomas: Epidemiology, risk factors, and pathobiology. 2023.
- 85.Engels EA, Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev, 2007. 16(3): p. 401–4. [DOI] [PubMed] [Google Scholar]
- 86.Hernandez-Ramirez RU, et al. , Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV, 2017. 4(11): p. e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.in Report on Carcinogens Monograph on Human Immunodeficiency Virus Type 1: RoC Monograph 08. 2016: Research Triangle Park (NC). [Google Scholar]
- 88.Cesarman E, et al. , Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med, 1995. 332(18): p. 1186–91. [DOI] [PubMed] [Google Scholar]
- 89.Mesri EA, Cesarman E, and Boshoff C, Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer, 2010. 10(10): p. 707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.in Report on Carcinogens Monograph on Kaposi Sarcoma-Associated Herpesvirus: RoC Monograph 10. 2016: Research Triangle Park (NC). [PubMed] [Google Scholar]
- 91.in Report on Carcinogens Monograph on Epstein-Barr Virus: RoC Monograph 07. 2016: Research Triangle Park (NC). [PubMed] [Google Scholar]
- 92.Healy JA and Dave SS, The Role of EBV in the Pathogenesis of Diffuse Large B Cell Lymphoma. Curr Top Microbiol Immunol, 2015. 390(Pt 1): p. 315–37. [DOI] [PubMed] [Google Scholar]
- 93.Andreone P, et al. , Posttransplantation lymphoproliferative disorders. Arch Intern Med, 2003. 163(17): p. 1997–2004. [DOI] [PubMed] [Google Scholar]
- 94.Mbulaiteye SM, Parkin DM, and Rabkin CS, Epidemiology of AIDS-related malignancies an international perspective. Hematol Oncol Clin North Am, 2003. 17(3): p. 673–96, v. [DOI] [PubMed] [Google Scholar]
- 95.Peveling-Oberhag J, et al. , Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol, 2013. 59(1): p. 169–77. [DOI] [PubMed] [Google Scholar]
- 96.Canioni D, et al. , In Situ Hepatitis C NS3 Protein Detection Is Associated with High Grade Features in Hepatitis C-Associated B-Cell Non-Hodgkin Lymphomas. PLoS One, 2016. 11(6): p. e0156384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Couronne L, et al. , From hepatitis C virus infection to B-cell lymphoma. Ann Oncol, 2018. 29(1): p. 92–100. [DOI] [PubMed] [Google Scholar]
- 98.Alkrekshi A, et al. , Risk of Non-Hodgkin’s Lymphoma in HCV Patients in the United States Between 2013 and 2020: A Population-Based Study. Clin Lymphoma Myeloma Leuk, 2021. 21(11): p. e832–e838. [DOI] [PubMed] [Google Scholar]
- 99.Tasleem S and Sood GK, Hepatitis C Associated B-cell Non-Hodgkin Lymphoma: Clinical Features and the Role of Antiviral Therapy. J Clin Transl Hepatol, 2015. 3(2): p. 134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Sanjose S, et al. , Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol, 2008. 6(4): p. 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giordano TP, et al. , Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA, 2007. 297(18): p. 2010–7. [DOI] [PubMed] [Google Scholar]
- 102.Dal Maso L and Franceschi S, Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev, 2006. 15(11): p. 2078–85. [DOI] [PubMed] [Google Scholar]
- 103.Gisbert JP, et al. , Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology, 2003. 125(6): p. 1723–32. [DOI] [PubMed] [Google Scholar]
- 104.Matsuo K, et al. , Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci, 2004. 95(9): p. 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pozzato G, et al. , Hepatitis C virus and non-Hodgkin’s lymphomas: Meta-analysis of epidemiology data and therapy options. World J Hepatol, 2016. 8(2): p. 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu X, Jing L, and Li X, Hepatitis C virus infection is a risk factor for non-Hodgkin lymphoma: A MOOSE-compliant meta-analysis. Medicine (Baltimore), 2019. 98(11): p. e14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahale P, et al. , Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer, 2017. 123(7): p. 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iqbal T, et al. , Prevalence and association of hepatitis C virus infection with different types of lymphoma. Int J Cancer, 2016. 138(4): p. 1035–7. [DOI] [PubMed] [Google Scholar]
- 109.Nath A, et al. , Prevalence of hepatitis B virus infection in non-Hodgkin lymphoma: a systematic review and meta-analysis. Intern Med J, 2010. 40(9): p. 633–41. [DOI] [PubMed] [Google Scholar]
- 110.Dalia S, et al. , Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res, 2013. 37(9): p. 1107–15. [DOI] [PubMed] [Google Scholar]
- 111.Yi HZ, et al. , Association between infection of hepatitis B virus and onset risk of B-cell non-Hodgkin’s lymphoma: a systematic review and a meta-analysis. Med Oncol, 2014. 31(8): p. 84. [DOI] [PubMed] [Google Scholar]
- 112.Qi Z, Wang H, and Gao G, Association of risk of non-Hodgkin’s lymphoma with hepatitis B virus infection: a meta-analysis. Int J Clin Exp Med, 2015. 8(12): p. 22167–74. [PMC free article] [PubMed] [Google Scholar]
- 113.Engels EA, Cho ER, and Jee SH, Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol, 2010. 11(9): p. 827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abe SK, et al. , Hepatitis B and C virus infection and risk of lymphoid malignancies: A population-based cohort study (JPHC Study). Cancer Epidemiol, 2015. 39(4): p. 562–6. [DOI] [PubMed] [Google Scholar]
- 115.Li M, et al. , Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J Viral Hepat, 2018. 25(8): p. 894–903. [DOI] [PubMed] [Google Scholar]
- 116.Kleinstern G, et al. , Associations between B-cell non-Hodgkin lymphoma and exposure, persistence and immune response to hepatitis B. Haematologica, 2016. 101(7): p. e303–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taborelli M, et al. , Hepatitis B and C viruses and risk of non-Hodgkin lymphoma: a case-control study in Italy. Infect Agent Cancer, 2016. 11: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andersen ES, et al. , Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat, 2015. 22(10): p. 828–34. [DOI] [PubMed] [Google Scholar]
- 119.Willett EV, et al. , Non-Hodgkin lymphoma and obesity: a pooled analysis from the InterLymph Consortium. Int J Cancer, 2008. 122(9): p. 2062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castillo JJ, et al. , Obesity is associated with increased relative risk of diffuse large B-cell lymphoma: a meta-analysis of observational studies. Clin Lymphoma Myeloma Leuk, 2014. 14(2): p. 122–30. [DOI] [PubMed] [Google Scholar]
- 121.Bertrand KA, et al. , A prospective analysis of body size during childhood, adolescence, and adulthood and risk of non-Hodgkin lymphoma. Cancer Prev Res (Phila), 2013. 6(8): p. 864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maskarinec G, et al. , Overweight and obesity at different times in life as risk factors for non-Hodgkin’s lymphoma: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev, 2008. 17(1): p. 196–203. [DOI] [PubMed] [Google Scholar]
- 123.Troy JD, et al. , Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Epidemiol, 2010. 171(12): p. 1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pylypchuk RD, et al. , Body mass index, height, and risk of lymphatic malignancies: a prospective cohort study. Am J Epidemiol, 2009. 170(3): p. 297–307. [DOI] [PubMed] [Google Scholar]
- 125.Teras LR, et al. , Body size and risk of non-Hodgkin lymphoma by subtype: A pooled analysis from six prospective cohorts in the United States. Br J Haematol, 2022. 197(6): p. 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mandel JH, et al. , Occupational trichloroethylene exposure and non-Hodgkin’s lymphoma: a meta-analysis and review. Occup Environ Med, 2006. 63(9): p. 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cocco P, et al. , Occupational exposure to trichloroethylene and risk of non-Hodgkin lymphoma and its major subtypes: a pooled InterLymph [correction of IinterLlymph] analysis. Occup Environ Med, 2013. 70(11): p. 795–802. [DOI] [PubMed] [Google Scholar]
- 128.in Benzene. 2018: Lyon (FR). [Google Scholar]
- 129.Rana I, et al. , Benzene exposure and non-Hodgkin lymphoma: a systematic review and meta-analysis of human studies. Lancet Planet Health, 2021. 5(9): p. e633–e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Apostoli P, Bergonzi R, and Catalani S, [Classification as carcinogenic for 2,3,7,8-tetrachlorodibenzo-para-dioxin: an eventful journey]. G Ital Med Lav Ergon, 2011. 33(1): p. 84–99. [PubMed] [Google Scholar]
- 131.Humans, I.W.G.o.t.E.o.C.R.t., IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr Eval Carcinog Risks Hum, 2008. 97: p. 3–471. [PMC free article] [PubMed] [Google Scholar]
- 132.Rice JM and Boffetta P, 1,3-Butadiene, isoprene and chloroprene: reviews by the IARC monographs programme, outstanding issues, and research priorities in epidemiology. Chem Biol Interact, 2001. 135–136: p. 11–26. [DOI] [PubMed] [Google Scholar]
- 133.Polychlorinated Biphenyls and Polybrominated Biphenyls. IARC Monogr Eval Carcinog Risks Hum, 2016. 107: p. 9–500. [PMC free article] [PubMed] [Google Scholar]
- 134.Spinelli JJ, et al. , Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer, 2007. 121(12): p. 2767–75. [DOI] [PubMed] [Google Scholar]
- 135.Cocco P, et al. , Plasma polychlorobiphenyl and organochlorine pesticide level and risk of major lymphoma subtypes. Occup Environ Med, 2008. 65(2): p. 132–40. [DOI] [PubMed] [Google Scholar]
- 136.Kelly RS, et al. , Prediagnostic plasma concentrations of organochlorines and risk of B-cell non-Hodgkin lymphoma in envirogenomarkers: a nested case-control study. Environ Health, 2017. 16(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Magoni M, et al. , Serum levels of polychlorinated biphenyls and risk of non-Hodgkin lymphoma: A hospital-based case-control study. Chemosphere, 2019. 235: p. 969–975. [DOI] [PubMed] [Google Scholar]
- 138.Zani C, et al. , Do polychlorinated biphenyls cause cancer? A systematic review and meta-analysis of epidemiological studies on risk of cutaneous melanoma and non-Hodgkin lymphoma. Chemosphere, 2017. 183: p. 97–106. [DOI] [PubMed] [Google Scholar]
- 139.Catalani S, et al. , Occupational and environmental exposure to polychlorinated biphenyls and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis of epidemiology studies. Eur J Cancer Prev, 2019. 28(5): p. 441–450. [DOI] [PubMed] [Google Scholar]
- 140.De Roos AJ, et al. , Herbicide use in farming and other jobs in relation to non-Hodgkin’s lymphoma (NHL) risk. Occup Environ Med, 2022. 79(12): p. 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Roos AJ, et al. , Occupational insecticide exposure and risk of non-Hodgkin lymphoma: A pooled case-control study from the InterLymph Consortium. Int J Cancer, 2021. 149(10): p. 1768–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Altahan A, et al. , Association Between Pesticide Use and Incidence of Diffuse Large B-Cell Lymphoma. Anticancer Res, 2020. 40(10): p. 5423–5426. [DOI] [PubMed] [Google Scholar]
- 143.Leon ME, et al. , Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int J Epidemiol, 2019. 48(5): p. 1519–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Donato F, et al. , Exposure to glyphosate and risk of non-Hodgkin lymphoma and multiple myeloma: an updated meta-analysis. Med Lav, 2020. 111(1): p. 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.t Mannetje A, et al. , Occupation and Risk of Non-Hodgkin Lymphoma and Its Subtypes: A Pooled Analysis from the InterLymph Consortium. Environ Health Perspect, 2016. 124(4): p. 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vajdic CM, et al. , Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res, 2009. 69(16): p. 6482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang ET, et al. , Adulthood residential ultraviolet radiation, sun sensitivity, dietary vitamin D, and risk of lymphoid malignancies in the California Teachers Study. Blood, 2011. 118(6): p. 1591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kricker A, et al. , Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer, 2008. 122(1): p. 144–54. [DOI] [PubMed] [Google Scholar]
- 149.Besson H, et al. , Tobacco smoking, alcohol drinking and non-Hodgkin’s lymphoma: A European multicenter case-control study (Epilymph). Int J Cancer, 2006. 119(4): p. 901–8. [DOI] [PubMed] [Google Scholar]
- 150.Chang ET, et al. , Alcohol consumption over time and risk of lymphoid malignancies in the California Teachers Study cohort. Am J Epidemiol, 2010. 172(12): p. 1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morton LM, et al. , Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr, 2014. 2014(48): p. 130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morton LM, et al. , Alcohol consumption and risk of non-Hodgkin lymphoma: a pooled analysis. Lancet Oncol, 2005. 6(7): p. 469–76. [DOI] [PubMed] [Google Scholar]
- 153.Cerhan JR, et al. , Blood transfusion history and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Causes Control, 2019. 30(8): p. 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y, et al. , Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. Am J Epidemiol, 2008. 167(11): p. 1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wright GW, et al. , A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell, 2020. 37(4): p. 551–568 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maignan K, Cell of Origin (COO) Association with High Body Mass Index (BMI) and Overall Survival (OS) in Patients with Diffuse Large B-Cell Lymphoma (DLBCL). Blood, 2019. 134. [Google Scholar]
- 157.Wang SS, et al. , Associations of non-Hodgkin Lymphoma (NHL) risk with autoimmune conditions according to putative NHL loci. Am J Epidemiol, 2015. 181(6): p. 406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang SS, et al. , B-Cell NHL Subtype Risk Associated with Autoimmune Conditions and PRS. Cancer Epidemiol Biomarkers Prev, 2022. 31(5): p. 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang SS, et al. , Variation in effects of non-Hodgkin lymphoma risk factors according to the human leukocyte antigen (HLA)-DRB1*01:01 allele and ancestral haplotype 8.1. PLoS One, 2011. 6(11): p. e26949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang SS, et al. , Immune mechanisms in non-Hodgkin lymphoma: joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors. Cancer Res, 2007. 67(10): p. 5042–54. [DOI] [PubMed] [Google Scholar]
- 161.Wang SS, et al. , HLA Class I and II Diversity Contributes to the Etiologic Heterogeneity of Non-Hodgkin Lymphoma Subtypes. Cancer Res, 2018. 78(14): p. 4086–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Y, et al. , Clonal hematopoiesis in diffuse large B-cell lymphoma: clinical impact and genetic relatedness to lymphoma and therapy-related myeloid neoplasm. Haematologica, 2023. 108(3): p. 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]


