Abstract
The development of musculoskeletal tissues such as tendon, enthesis, and bone relies on proliferation and differentiation of mesenchymal progenitor cells. Gli1+ cells have been described as putative stem cells in several tissues and are presumed to play critical roles in tissue formation and maintenance. For example, the enthesis, a fibrocartilage tissue that connects tendon to bone, is mineralized postnatally by a pool of Gli1+ progenitor cells. These cells are regulated by hedgehog signaling, but it is unclear if TGFβ signaling, necessary for tenogenesis, also plays a role in their behavior. To examine the role of TGFβ signaling in Gli1+ cell function, the receptor for TGFβ, TbR2, was deleted in Gli1-lineage cells in mice at P5. Decreased TGFβ signaling in these cells led to defects in tendon enthesis formation by P56, including defective bone morphometry underlying the enthesis and decreased mechanical properties. Immunohistochemical staining of these Gli1+ cells showed that loss of TGFβ signaling reduced proliferation and increased apoptosis. In vitro experiments using Gli1+ cells isolated from mouse tail tendons demonstrated that TGFβ controls cell proliferation and differentiation through canonical and non-canonical pathways and that TGFβ directly controls the tendon transcription factor scleraxis by binding to its distant enhancer. These results have implications in the development of treatments for tendon and enthesis pathologies.
Keywords: enthesis, Gli1, TGFβ, TbR2, development
Graphical Abstract

The fibrocartilaginous enthesis is mineralized postnatally by a pool of Gli1+ stem cells. These cells are regulated by hedgehog signaling, but it is unclear if TGFβ signaling, necessary for tenogenesis, also plays a role. To examine TGFβ’s role in Gli1+ cell function, the TbR2 receptor was deleted in Gli1-lineage cells in mice. TbR2 deletion in Gli1-lineage cells led to reduced enthesis mechanical properties and altered bone morphometry. Loss of TGFβ signaling reduced proliferation and increased through canonical and non-canonical pathways.
INTRODUCTION
The musculoskeletal system is formed and maintained by a wide range of mesenchymal cells. Cells positive for Gli1, an essential hedgehog signaling transcription factor, have been described as putative stem cells in several tissues; a critical role for Gli1+ cells in development and response to injury has been shown for tendon, enthesis, teeth, bone, bone marrow, skin, colon, kidney, and heart (1–11). These cells and their progenies have been localized to the tendon enthesis throughout postnatal development, eventually populating the entire fibrocartilage region between tendon and bone (11, 12). Ablation of Gli1+ cells postnatally leads to a loss of mineralized fibrocartilage, suggesting that these cells play a critical role in enthesis mineralization (12). Furthermore, these cells demonstrate clonogenicity and multipotency and therapeutic potential for enthesis regeneration (11). Similarly, during tooth development, Gli1+ cells proliferate and differentiate into various cell types required for periodontal ligament and dental pulp formation (7, 8). In bone, Gli1+ cells differentiate into various cell types at the growth plate during development and in the callus during fracture healing (10). In tissues such as muscle, skin, and colon, Gli1+ cells have been reported to serve as stem cells within tissue-specific niches and actively regenerate injured tissues (1, 2, 13). The regulation of these cells leading to their proliferation and/or differentiation is not understood, particularly for musculoskeletal tissues such as tendon and the enthesis.
Transforming growth factor beta (TGFβ) is a pleiotropic cytokine that plays important roles in several cellular functions, including proliferation, differentiation, and extracellular matrix (ECM) synthesis (14). The TGFβ family of ligands works through the TGFβ receptors 1 and 2. Specifically, for the canonical pathway, a TGFβ ligand binds to the TGFβ receptor 2 (TbR2) and initiates a signaling cascade through Smad2/3. Alternatively, the noncanonical pathway results in activation of other transcription factors such as mitogen activated protein kinase (MAPK) and Akt (14, 15). TGFβ signaling plays many critical roles in the formation of the musculoskeletal system, including tendon, bone, and intervertebral disc formation (16–20). Deletion of TbR2 in Prx1-expressing cells also severely impaired the formation of tendons and ligaments (20). Similarly, deletion of TbR2 in scleraxis (Scx) expressing tendon cells led to severe tendon disruption and eventually death by P14. Further studies revealed that TGFβ signaling was necessary to maintain tendon cell phenotype via Scx expression (21). Recently, it was shown that deletion of TbR2 in Gli1-expressing periodontal ligament progenitors reduced PDL cell numbers (22). These prior studies point to a crucial role for TGFβ in the formation of connective tissues, but its role in Gli1+ progenitor cell behavior remains unclear.
It is unknown if TGFβ signaling regulates the behavior of Gli1+ cells in tendon and the enthesis. To examine the role of TGFβ signaling in these cells, we performed a series of in vivo and in vitro studies. TbR2 was deleted in Gli1+ cells at P5 using Gli1CreERT2;TbR2fl/fl mice. To study the effect of this deletion on the development of the enthesis, the phenotype of rotator cuff entheses was examined using biomechanical, morphological, and histological assays. To study the effect of TbR2 deletion on Gli1+ tendon cells, cells were isolated from tail tendon and their in vitro behavior in response to TGFβ and hedgehog agonist stimulation were examined. Results revealed that TGFβ signaling is critical for the formation of a functional tendon enthesis and controls Gli1-lineage cells through canonical and non-canonical pathways.
MATERIALS AND METHODS
In Vivo Studies
Animal models
To examine the effect of TGFβ signaling on tendon enthesis development, TbR2 was deleted in Gli1+ cells at P5. The following mouse models were purchased from The Jackson Laboratory (Bar Harbor, ME): Gli1tms(cre/ERT2)AIj/J (Gli1CreERT2, stock number 007913), B6;129-Tgfbr2tm1Karl/J (TbR2 floxed, stock number 012603), and B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (mTmG, stock number 007676). To generate TbR2 deletion in Gli1-expressing cells, mice were crossed to produce Gli1CreERT2;TbR2fl/fl conditional knockout (CKO) mice and Gli1CreERT2; TbR2fl/wt control mice (control). In order to label Gli1-lineage cells, Gli1CreERT2;TbR2fl/fl mice were mated with mTmG reporter mice to generate Gli1CreERT2;TbR2fl/fl;mTmG mice. In these mice, Gli1-expressing cells at the time of tamoxifen (TAM, Sigma-Aldrich, catalog number 06734) injection were labeled with membrane bound GFP while all the other cells were labeled with membrane bound td-Tomato (23). To induce CreER nuclear localization and delete the TbR2 allele, mice were subjected to subcutaneous injection of TAM. TAM was dissolved in sterile corn oil (Sigma-Aldrich, catalog number: C8267) at a concentration of 10 mg/ml and subcutaneously injected at 100 mg/kg body weight at P5 and P7 (12, 23). Gli1CreERT2;TbR2fl/fl mice were used at 8 weeks of age. Due to the limitation of available mice, the control group for bone and mechanical property included both Gli1-CreERT2;TbR2fl/wt and TbR2fl/fl mice since both strains express TbR2. All the mouse experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University. The phenotypes of the mice were determined using biomechanics, bone morphometry, and histologic analyses.
Micro computed tomography (micro-CT)
Mice were euthanized at P56 (i.e., 51 days post TAM injection), humerus-supraspinatus tendon/muscle samples were isolated, and samples were stored in saline-soaked gauze at −20 °C until scanning [N=14 for control (7 males, 7 females), N=11 for CKO (7 males, 4 males)]. Specimens were thawed and micro-CT scanning was performed using a Skyscan 1271 scanner (Bruker Corporation) at an energy of 60 kV, intensity of 166 μA, 0.25 μm aluminum filter, and 5 μm resolution. Scanned images were evaluated using a custom segmentation algorithm to separate cortical and trabecular bones of the humeral head proximal to the growth plate (CTAn, Bruker Corporation). Bone mineral density (BMD) and total mineral density (TMD), bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular spacing (Tb.Sp) were measured. Tendon cross-sectional area was measured by thresholding μCT images of sagittal slices through the tendon. The minimum cross-sectional area was used for mechanical property analysis (24, 25).
Biomechanical testing
After μCT scanning, the supraspinatus muscle was carefully scraped from the tendon, the humeral bone was mounted in a custom 3D printed fixture, and a uniaxial tensile test to failure was performed using an Electroforce 3230 testing frame (TA Instruments) (N=14 for control, N=11 for CKO) (26). The testing protocol consisted of 5 cycles of preconditioning (2% strain, 0.2% /s), 180 s recovery, and extension to failure at 0.2% /s. Strain was determined from grip-to-grip displacement relative to the initial gauge length. Stress was determined using the minimum cross-sectional area of the tendon, as determined from micro-CT. Structural properties were determined from load/deformation curves, including failure load (maximum load), stiffness (slope of linear portion of load/deformation curve), and work to failure (area under load/deformation curve through yield). Material properties were determined from stress/strain curves, including strength (maximum stress), modulus (slope of linear portion of stress/strain curve), and resilience (area under the curve through yield).
Histology and immunohistochemistry
Mice were euthanized at P16 (the timepoint when the enthesis mineralizes), humeral-supraspinatus tendon/muscle samples were isolated, and samples were fixed in 4% paraformaldehyde (ThermoFisher Scientific, catalog number 50-980-487) at 4°C overnight (N=8 for control, N=3 for CKO). The specimens were then decalcified in 10% EDTA (pH 7.4) (Poly Biotech). Specimens were rinsed in PBS and dehydrated in 30% sucrose (Fisher Scientific, catalog number S5-500). The specimens in sucrose were then embedded in OCT compound (VWR, catalog number 25608–930) and sectioned into 9 μm-thick sections using a cryostat (Ag protect, CM1860, Leica). The sections were fixed in 10% neutral buffered formalin (StatLab, catalog number 28600–1) for 20 minutes, and permeabilized in 1% Triton X-100 (Sigma-Aldrich, catalog number T9284). After blocking with 5% bovine serum albumin (BSA, fraction V) (Fisher Scientific, catalog number BP1600), the sections were incubated with either 1:250 anti Ki67 (Abcam, catalog number ab15580), 1:100 anti-p-cJun (Cell Signaling Technologies, catalog number 3270), anti-SPARC (R&D Systems, catalog number: AF942-SP), or 1:100 biotinylated anti-collagen 1a (GeneTax, catalog number GTX36577) overnight at 4°C. The sections were then incubated with 1:1000 anti-rabbit IgG Alexa Fluor 555 (Invitrogen, product number A-27039), anti-mouse IgG Alexa Fluor 647 (Invitrogen, product number A-21235), or anti-goat IgG Alexa Fluor 555 (Invitrogen, catalog number A-21431) after washing 3 times with Tris Buffered Saline (Boston BioProducts, catalog number IBB-588) containing 0.5% TWEEN®−20 (Sigma-Aldrich, catalog number P1379–100ML) (TBST). The sections were then rinsed with TBST and mounted with VECTASHIELD Plus mounting media with DAPI (Vector Laboratory, reference number H-2000). Apoptotic cells were stained with Click-IT™ Plus TUNEL assay with Alexa Fluor 647 per manufacturer’s instructions, with omission of tissue deparaffinization steps (Invitrogen, catalog number C10619). The images were captured with either a Zeiss Observer 7 microscope/Axiocam 702 camera (for Alexa Fluor 555) or Nikon A1RMP Multiphoton Confocal Microscope (for Alexa Flour 647). Cells positive for antibodies and/or GFP were counted manually. The region of interest for cell counting consisted of the bony end of the enthesis through the start of tendon proper. To examine tendon and enthesis morphology, a separate group of sections were stained with Safranin O per manufacturer’s instruction (American MasterTech, reference number: KTSFO).
Flow cytometry
To analyze the number of Gli1-lineage cells in the supraspinatus, infraspinatus, and Achilles tendon entheses, tissues were dissected from Gli1-CreERT2;TbR2fl/wt;mTmG heterozygous control and Gli1-CreERT2;TbR2fl/fl;mTmG CKO mice. The pooled tissues were chopped into small pieces with a razor blade and digested in 5 ml of complete medium MEMα (ThermoFisher Scientific, catalog number 1256107) containing 10% fetal bovine serum (Gemini Bio-products, catalog number 900–108), 100 U/ml penicillin-streptomycin (ThermoFisher Scientific, catalog number 15140122) and 4–5 mg/ml type II collagenase (Worthington Biochemical Corp, catalog number 004117) at 37 °C with 225 rpm shaking. After 1.5 hours, 5 ml of complete medium was added to the digestion. The digested tendon fragments were then passed through a 20 G needle to break any clumps and then filtered through a 70 μm cell strainer (Fisher Scientific, catalog number 08-771-2). The cells were then spun down at 350 xG for 5 min and washed once with 5 ml fresh complete medium. The cell suspensions were stained with CD45-Brilliant violet 711™ (1:100, Biolegend, catalog number: 103147) and DAPI (1:5,000, Invitrogen, catalog number: D1306). The stained cells were passed through a LSRII cell analyzer (BD Biosciences) and analyzed using FCS Express software. The percentage of positive cells for each population was presented after CD45+ hematopoietic cells and DAPI+ dead cells were excluded.
In vitro studies
Cell culture
To examine the role of TGFβ signaling in Gli1-lineage cell function, Gli1-lineage cells labeled with GFP were isolated from supraspinatus/infraspinatus tendon entheses or tail tendons from 6–8-week old mice and cultured, as previously described (27). Briefly, tissues were chopped into small pieces with a razor blade, and digested in 5 ml of complete medium MEMα (ThermoFisher Scientific, catalog number 1256107) containing 10% fetal bovine serum (Gemini Bio-products, catalog number 900–108), 100 U/ml penicillin-streptomycin (ThermoFisher Scientific, catalog number 15140122) and 4–5 mg/ml type II collagenase (Worthington Biochemical Corp, catalog number 004117) at 37 °C with 225 rpm shaking. After 1.5 hours, 5 ml of complete medium was added to the digestion. The digested tendon fragments were then passed through a 20 G needle to break any clumps and then filtered through a 70 μm cell strainer (Fisher Scientific, catalog number 08-771-2). The cells were then spun down at 350 xG for 5 min and washed once with 5 ml fresh complete medium. The cells were then re-suspended in 3 ml of complete medium and plated into a 35 mm cell culture plate until they reached 80% confluence. The cells were then lifted by trypsin/EDTA (ThermoFisher Scientific, catalog number 25200056) and re-plated at a 1:2 ratio.
The cultured cells were separated by flow cytometry (Influx, BD Bioscience) at passage 3 based on their expression of either GFP or td-Tomato and analyzed using FCS Express software. The sorted cells were then further expanded. The cells were then replated at 3,000 /cm2 and starved in MEMα with 1% FBS overnight. For Western blot analysis, the starved cells were then treated with 5 ng/ml of recombinant murine TGFβ1 (PEPROTECH, catalog number AF-100–21C). Cell responses were evaluated at 0, 15, 30, 60, 120, and 1440 minutes. Cells were lysed with RIPA Lysis and Extraction Buffer (ThermoFisher Scientific, catalog number: 89900) supplemented with Halt™ Protease and Phosphatase Inhibitor Cocktail (ThermoFisher Scientific, catalog number 78440) according to manufacturer’s instructions. For gene expression analysis, the starved cells were treated with recombinant murine TGFβ1 (5 ng/ml) or hedgehog agonist (Hh-Ag1.5, Xcess Bioscicences Inc., CAS# 612542-14-0, 0.1μM) for 1, 4, 24, 48, and 72 hours. Total RNA was extracted using RNeasy Mini Kit (Qiagen, catalog number:74104). For immunofluorescence, cells were grown on a cover slip and treated with recombinant TGFβ1 (5 ng/ml) for 60 minutes.
Western blot analysis
Western blot analyses were performed on FACS sorted GFP+ Gli1+ cells to determine signaling pathways induced by TGFβ (N=5 cell isolations for control, N=3 cell isolations for CKO). Whole cell lysates were quantified with the Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific, catalog number: 20227). 20 μg of total protein was separated on an 8% SDS-PAGE, transferred to a 0.2 μm nitrocellulose membrane (BioRad, catalog number 1620112), and probed with 1:1000 of anti-phospho-Smad2 (Cell Signaling Technology, catalog number 18338S), anti-Smad2 (Cell Signaling Technology, catalog number 5339S), anti phospho-cJun (Cell Signaling Techology, catalog number 3270S), anti cJun (Cell Signaling Technology, catalog number 9165S), anti-phospho-SAPK/JNK (Cell Signaling Technology, catalog number 4668S), anti JNK (Cell Signaling Technology, catalog number 9252S), anti-phospho-p38 MAPK (Cell Signaling Technology, catalog number 4511S), anti-p38 MAPK (Cell Signaling Technology, catalog number 8690S), anti-phospho-p44/p42 MAPK (pErk, Cell Signaling Technology, catalog number 8690S), anti-p44/p42 MAPK (Erk, Cell Signaling Technology, catalog number 4695S), and anti-GAPDH (Cell Signaling Technology, catalog number 5174S). The primary antibody was detected with an anti-rabbit IgG, HRP-linked secondary antibody (Cell Signaling Technology, catalog number 7074S; 1:4,000) and visualized with either SuperSignalTM West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific, catalog number 34094) or SuperSignal™ West Pico PLUS Chemiluminescent Substrate (ThermoFisher Scientific, catalog number 34577) for phospho-JNK and GAPDH. Images were captured with an Azure 600C Imager (Azure Biosystems) and quantified using Azurespot software. Between application of different antibodies, the nitrocellulose membrane was stripped with Restore™ Plus Western Blot Stripping Buffer (ThermoFisher Scientific, catalog number 21059) according to manufacturer’s instructions.
Quantitative polymerase chain reaction (qPCR)
To determine expression patterns of cells induced with TGFβ or Hh Agonist, qPCR was performed on cultured Gli1+ cells (N=8 cell isolations for control, N=3 cell isolations for CKO). One hundred nanograms of total RNA were reversely transcribed into complementary DNA (cDNA) using Maxima First Strand cDNA Synthesis Kit for RT-qPCR (ThermoFisher Scientific, catalog number K1641). The cDNA was diluted 10-fold with water and 2 μL of diluted cDNA was quantified for each specific gene using Power SYBR™ Green PCR Master Mix (ThermoFIsher Scientific, catalog number 4368706) in a QuantStudio™ 6 Pro Real-Time PCR System (ThermoFisher Scientific, catalog number: A44288) according to manufacturer’s instructions. Gene expression was determined as fold change relative to the vehicle treated sample (defined as 1) for the corresponding genotype. The primer pairs for each gene were designed using an online primer design tool (Primer3, http://primer3.ut.ee/). The primer sequences are listed in Table 1.
Table 1.
Primer sequences for qPCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Scx | 5’ ccaaacagatctgcaccttc | 5’ tgtcacggtctttgctcaac |
| Sox9 | 5’ tgcagcacaagaaagaccac | 5’ cgttcttcaccgacttcctc |
| Gli1 | 5’ tgtgtgagcaagaaggttgc | 5’ atggcttctcattggagtgg |
| GAPDH | 5’ gcacagtcaaggccgagaat | 5’ gccttctccatggtggtgaa |
Chromatin immunoprecipitation (ChIP)
ChIP analysis was performed to explore how TGFβ controls expression of Scx, a key tendon enthesis transcription factor (28) (N=2 cell isolations for control and N=2 cell isolations for CKO). Tail tendon fibroblasts from control and CKO mice were grown to 90% confluence in 15 cm cell culture plates. The cells were treated with TGFβ1 (5ng/ml) for 1 hour up to 24 hours in MEMα−1. The chromatins were harvested using Cell Signaling Technology’s SimpleChIPR Enzymatic Chromatin IP Kit (Magnetic Beads) and precipitated with anti-mouse Smad4 antibody (Cell Signaling, catalog number 46535, lot 2) according to manufacturer’s protocols (Cell Signaling, #9003). Briefly, tendon fibroblasts were seeded at 2×106 per 15 cm plate in complete media. When the cells reached to 90–95% confluence, the media was switched to MEMα −1 overnight. The cells were treated with TGFβ1 (5ng/ml) for 1 hour to 24 hours on the following day in 20 mL MEMα −1 or fresh 20 mL MEMα −1 for un-treated controls. At the end the stimulation, 540 μL of 37% formaldehyde (Fisher Scientific, catalog number BP531) were added to cross-link the proteins with DNA. The cross-linking was stopped by adding glycine. The cells were then washed with cold PBS and pelleted in 2 ml cold PBS at 2,000 × G for 5 min. The cell pellets were then digested with 0.3 μL of Micrococcal Nuclease for 20 min at 37 °C. The digestion was stopped by adding 10 μL of 0.5 M EDTA. The digested chromatins were then fractioned by sonication via 3 × 30 s pulses with 20% Duty Cycle, Output Control setting at 3 in a Branson SONIFIER 250 sonicator (Branson Ultrasonics Corp). The chromatins were then split into two parts: 1 part was saved as an input control and 1 part was incubated with anti Smad4 rabbit monoclonal antibody at 1:100 overnight at 4 °C. The following day, 30 μL of Protein G magnetic beads were added to the antibody-chromatin mix and incubated for 2 hours at 4 °C. The magnetic beads-Smad4-chromatin complexes were then purified in a DynaMag2 magnetic particle concentrator (Invitrogen). The purified complexes were eluted in a high salt solution and reverse cross-linked by proteinase K. The immunoprecipitated chromatins and input control DNA were further purified with a DNA purification kit (Cell Signaling, catalog number 14209). The precipitated DNA was quantified using qPCR with specific primers for each potential binding site (Table 2). The binding capacity was calculated as the percentage of input using the formula: percentage of input = 2×2(Ct of input-Ct of IP). To pool data from different experiments, the fold change between TGFβ treated samples was compared to untreated samples. Those samples were then examined for potential Smad4 consensus binding sites using ALGGEN-PROMO from 10 kb upstream and 10 kb downstream of mouse Scx gene transcription start site (TSS) (29) and compared with the conserved regulatory regions reported by Pryce et al (30).
Table 2.
Primer sequences for ChIP
| Binding site | Forward primer | Reverse primer |
|---|---|---|
| −2.5 kb | 5’ gacctgcaggagaagaagagtg | 5’ cgtaagtgtggagtcagaggtg |
| −1.2 kb | 5’ caacctgctcatcagttagcc | 5’ gcactgtgcaagacaagatgac |
| +4.9 kb | 5’ ctgtctgcgagagaggaagc | 5’ ggcagaacgaggagagagtg |
Immunocytochemistry
To examine the effectiveness of TGFβ deletion in the mouse models, sorted cells from reporter mice were cultured and examined using immunofluorescence. After TGFβ treatment, tendon fibroblasts were washed 2 times with cold PBS and fixed in 0.4% formaldehyde (Fisher Scientific, catalog number BP531–500) for 20 min. The cells were then permeabilized in 1% Triton-X100 for 2 min. After three 5 min washes with TBST, the cells were blocked with 5% bovine serum albumin (Sigma-Aldrich, catalog number A3803–100G) for 30 minutes and 1:100 anti-Smad2 (Cell Signaling Technology) for 2 hours. After 3 washes with TBST, the cells were incubated with 1:1000 Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (ThermoFisher Scientific, catalog number A21206) for td-Tomato positive cells or 1:1000 of Donkey anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (ThermoFisher Scientific, catalog number: A-31572) for 1 hr. The cover slips of cells were then mounted onto slides with VECTASHIELD® Antifade Mounting Media with DAPI (VECTOR Laboratories, catalog number: H-1200) after 5 TBST washes. Images were taken on a Nikon A1RMP Multiphoton Confocal Microscope.
Statistics
All data are presented as mean plus/minus standard deviation. For biomechanics, microCT, FACS, and histomorphometry data, CKO was compared to control using student t-tests. qPCR and Western blot data was compared using a two-factor ANOVA design for the factors genotype (CKO, control) and time (1–72hr for qPCR, 0–1440 minutes for Western blots). All statistical tests were performed using Prism software (GraphPad).
RESULTS
TGFβ signaling was inhibited in Gli1-lineage cells
To isolate Gli1-expressing cells whose TGFβ receptor 2 (TbR2) was deleted, Gli1CreERT2;TbR2fl/fl mice were crossed to Rosa26R/mTmG reporter mice to generate Gli1CreERT2;TbR2fl/fl;mTmG mice. Tamoxifen (TAM) injection at P5 and P7 led to deletion of TbR2 and enhanced expression of GFP in Gli1-lineage cells (Figure 1A). Thus, TbR2-deleted cells expressed GFP and all other cells (that did not express Gli1) expressed td-Tomato (Figure 1A). Since the numbers of Gli1-lineage cells isolated from the supraspinatus tendon enthesis were insufficient for in vitro studies, Gli1-lineage cells were isolated from tail tendons to validate the model. Cells derived from supraspinatus tendon enthesis and tail tendon showed similar responses to TGFβ in pilot studies (Figure S1). Use of tendon-derived Gli-lineage cells was further motivated by recent work demonstrating this cell population in the periodontal ligament (22). Tail-derived tendon fibroblasts (TFs) were expanded and separated based on their GFP (green fluorescence) and td-Tomato (red fluorescence) expression in a flow cytometer and cultured. To test if TGFβ signaling was blocked in Gli1CreERT2;TbR2fl/fl cells, the sorted td-Tomato+ and GFP+ cells were treated with TGFβ1 (5ng/ml) for 60 minutes and then stained for Smad2. In td-Tomato positive (i.e., wild type) cells, Smad2 protein was primarily localized to the nuclei (97.3±4% nuclear Smad2, Figure 1B, bottom row, right-most panel). In contrast, Smad2 remained in the cytoplasm of TbR2-deleted GFP+ cells (3.0±7.8% nuclear Smad2, p<0.001 compared to td-Tomato+ cells, Figure 1B, top row, right-most panel). These results showed that GFP+ cells were Gli1-lineage cells whose TGFβ signaling was blocked. Consistent with this result, Western blot analysis demonstrated marked decreases in pSmad2 and TbR2 in TbR2-deleted GFP+ cells compared to wild type control cells (Figure 1C, S2). During the peak induction time at 60 minutes, Smad2 phosphorylation was reduced by an average of 45% in CKO cells (13.1 mean fold induction in control cells vs. 7.2 mean fold induction in CKO cells). These data demonstrate that TGFβ signaling was substantially (but not completely) inhibited in Gli1CreERT2;TbR2fl/fl tendon cells.
Figure 1. Generation and validation of Gli1CreERT2;TbR2fl/fl;mTmG mice.
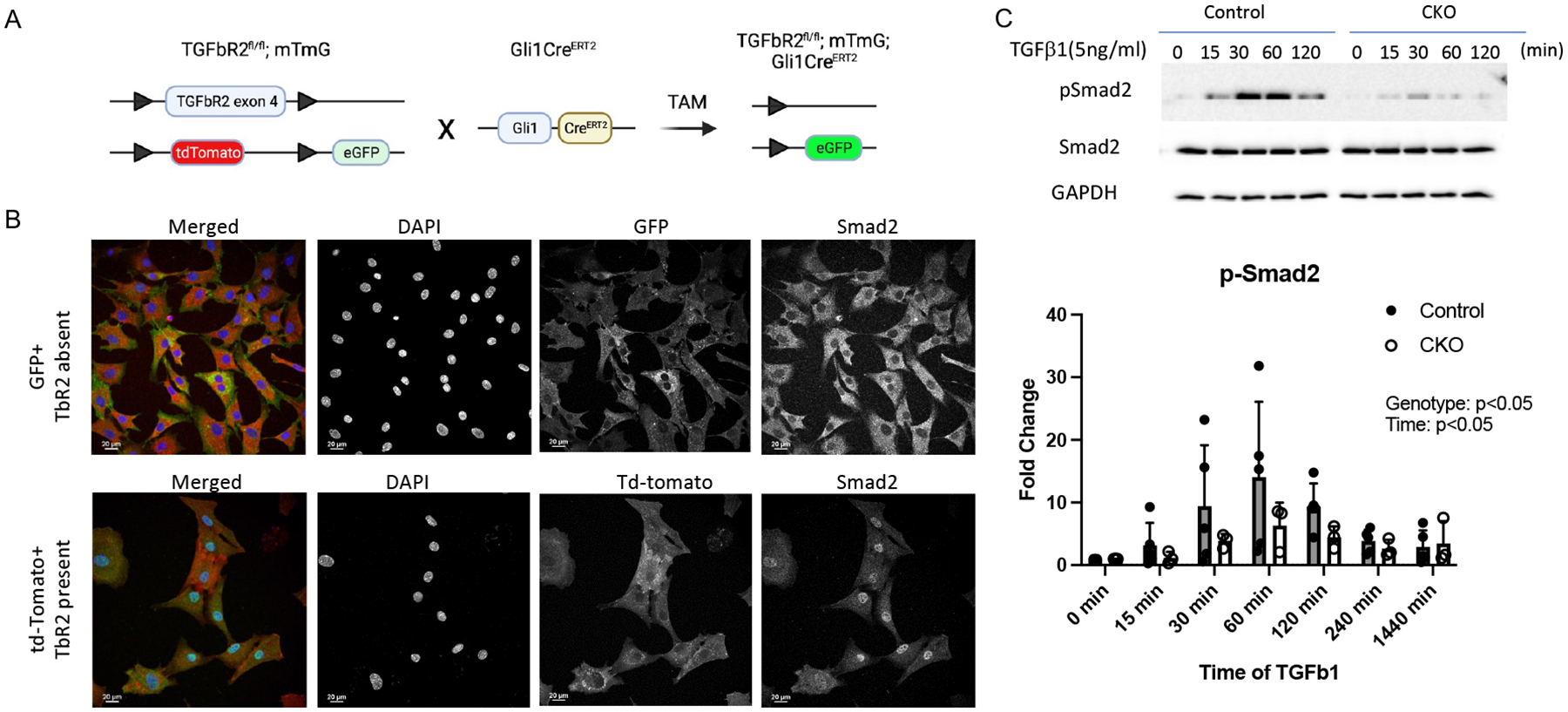
(A) Strategy for generating reporter mice whose Gli1-lineage TbR2-deleted cells were tagged with eGFP. (B) Immunocytochemistry of cultured cells showed that TGFβ-induced Smad2 nuclear localization was blocked in Gli1-lineage (green) cells with TbR2 deletion (top row), in contrast to non-Gli1-lineage cells (red) (bottom row). (C) Western blot analysis of cultured cells demonstrated substantially reduced Smad2 phosphorylation in Gli1CreERT2;TbR2fl/fl cells (CKO) cells compared to control cells.
Postnatal deletion of TbR2 in Gli1-lineage cells led to mechanical and morphological defects in the enthesis
Deletion of TbR2 in Gli1-lineage cells in the early post-natal period led to functional defects in the tendon enthesis (Figure 2A–E). Uniaxial tensile testing of supraspinatus tendon entheses revealed that the Gli1CreERT2;TbR2fl/fl (CKO) mice had lower maximum force, work to failure, and resilience compared to TbR2fl/wt control (CTRL) mice (Figure 2B). Maximum stress (p=0.072), stiffness, and modulus were not significantly different between CKO and CTRL mice. Microcomputed tomography (microCT) analysis of humeral head bone near the supraspinatus tendon enthesis showed that, compared to CTRL mice, CKO mice had lower cortical bone thickness, smaller total cross-sectional area (TtAr), less bone volume to total volume ratio (BV/TV), lower bone mineral density of articular bone (trabecular BMD), and lower trabecular bone thickness (TbTh) (Figure 2C,E). Trabecular thickness and spacing were not significantly different between CKO and CTRL mice. Histologic appearance of the supraspinatus tendon enthesis at P16 (i.e., immediately after enthesis mineralization) was similar in the CKO mice compared to control mice, with some decreased staining for fibrocartilage (Figure 2D).
Figure 2. TbR2 deletion in Gli1-lineage cells led to reduced enthesis mechanical properties and altered bone morphometry.
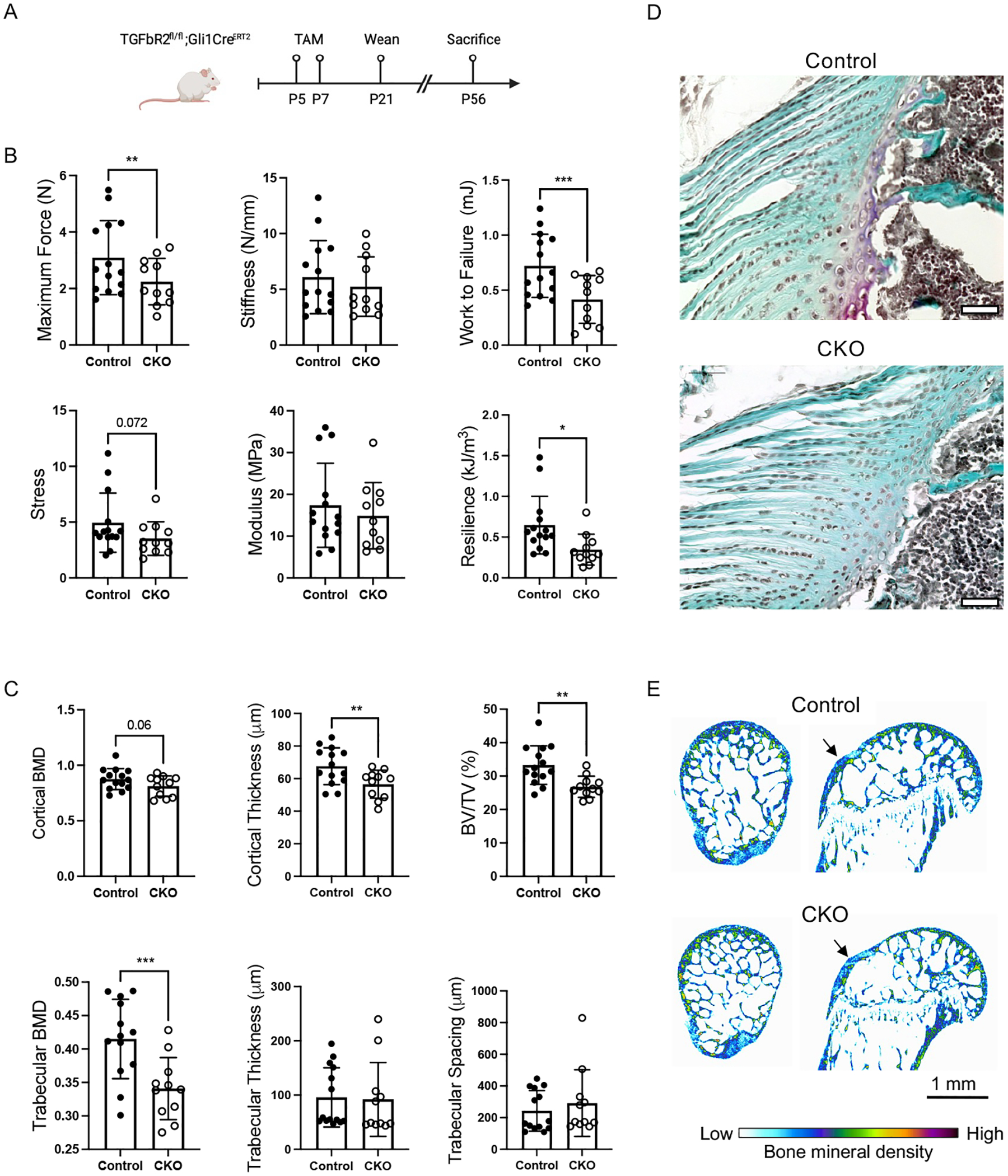
(A) Tamoxifen (TAM) was administrated at P5 and P7 to induce deletion of TbR2 in Gli1-expressing cells (CKO). (B) The mechanical properties of the supraspinatus tendon enthesis were reduced in CKO mice compared to CTRL mice. * p<0.05, ** p<0.01, *** p<0.001. (C) Deletion of TbR2 in Gli1-expressing cells led to altered bone morphometry of the humeral head cortical and trabecular bone. (D) Representative histologic sections for control and CKO supraspinatus tendon entheses (Safranan O stain, tendon is on the left and bone is on the right of the sections; scale bars = 50 μm). (E) Representative microCT images show reduced trabecular bone and lower bone mineral density adjacent to the supraspinatus tendon enthesis (arrows). ** p<0.01, *** p<0.001.
ECM changes in Gli1CreERT2;TbR2fl/fl CKO mice were limited to Gli1-lineage cells
Collagen 1a (COL1a) production is an established consequence of TGFβ signaling in musculoskeletal tissues, as seen during tissue fibrosis and scar formation, including for tendon and enthesis (31–34). Histomorphometric analysis revealed a trend towards increased numbers of COL1a+ cells in CKO entheses compared to Control entheses, however the percentage of COL1a+ cells was not different between the two groups (Figures 3A, Supplemental Figure S3). When considering only Gli1-lineage (i.e., GFP+) cells, there were trends for decreased numbers and percentage of COL1a+ cells in CKO compared to Control enthesis (Figure 3A). Secreted protein acidic and rich in cysteine (SPARC, a.k.a osteonectin) is a major non-collagen ECM protein found in tendon and mineralized tissues (35, 36). The numbers and percentage of SPARC+ cells were similar between CKO and Control entheses (Figures 3B and Supplemental Figure S3). When considering only Gli1-lineage (i.e., GFP+) cells, there was a significant reduction in the percentage of SPARC+ cells in CKO compared to Control enthesis (Figure 3B).
Figure 3. ECM changes in Gli1CreERT2;TbR2fl/fl CKO mice were limited to Gli1-lineage cells.
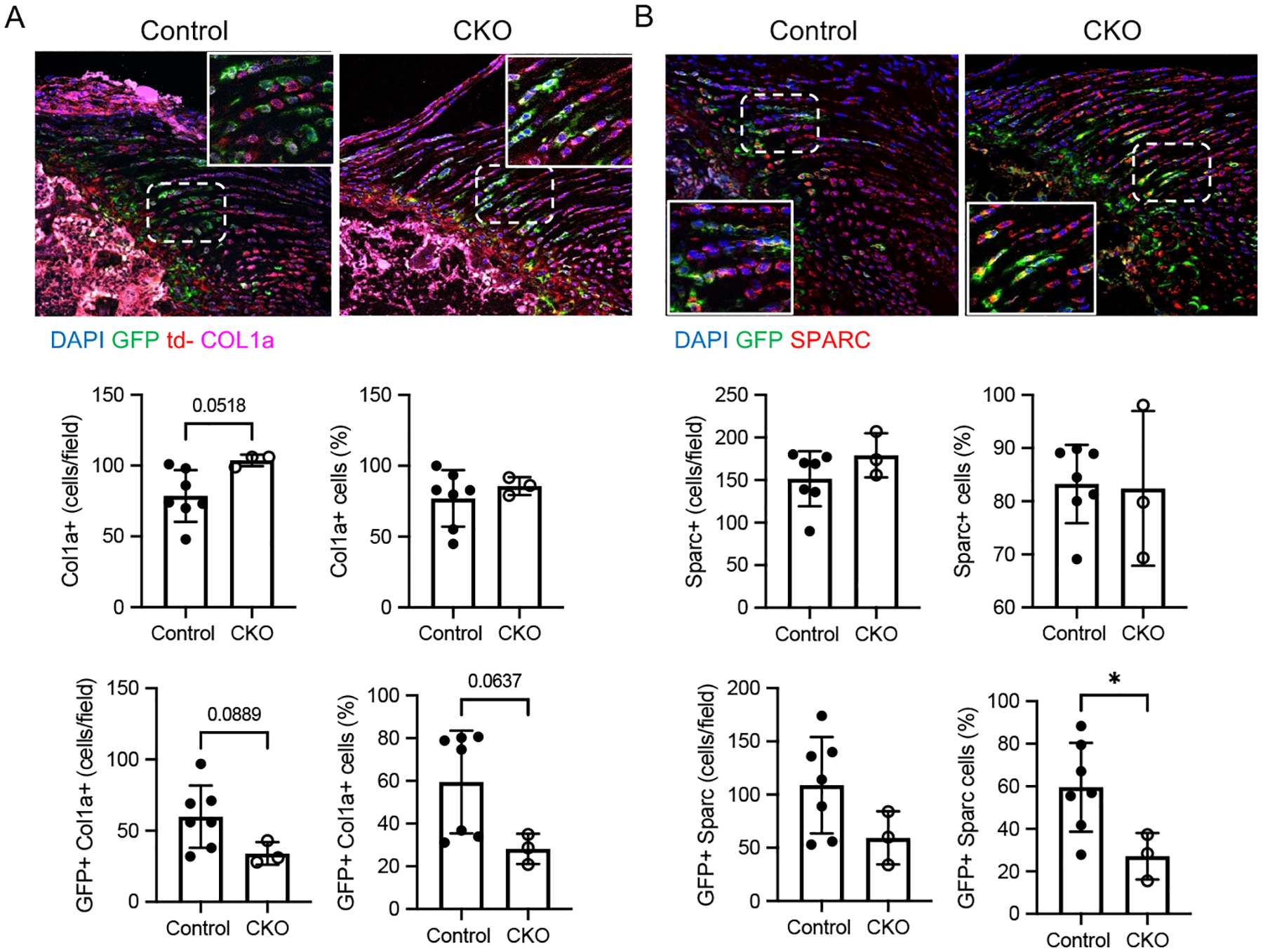
Immunohistochemistry for (A) COL1a (magenta) and (B) SPARC (red) in Control and CKO supraspinatus tendon entheses. Gli1-lineage cells are GFP+ (green) (bone is on the left and tendon is on the right of the sections; scale bars = 50 μm). The insert is an enlarged view of the white boxed area of the enthesis (positive cells indicated by arrowheads). Histomophometric analyses demonstrated numbers and percentage of cells positive for (A) COL1a and (B) SPARC for the enthesis and for Gli1-lineage cells (i.e., colocalization of GFP with either COL1a or SPARC). * p<0.05.
TbR2 deletion led to fewer Gli1-lineage cells
To see if TbR2 deletion in Gli1-lineage cells affected their proliferation, cells from tail tendons and rotator cuff entheses were analyzed. Control and CKO cells from tail tendons were cultured to passage 3 and analyzed using FACS. The number of GFP+ Gli1-lineage cells was significantly reduced in CKO mice compared to CTRL mice (Figure 4A). Similar results were observed for enthesis cells isolated from supraspinatus and infraspinatus tendon entheses (Figure S4). Immunohistochemistry of mouse supraspinatus entheses for the proliferation marker Ki67 qualitatively supported the in vitro results, however histomorphometric analysis showed high variability and no significant difference in the number of Ki67+ cells in CKO entheses compared to control entheses (Figure 4B). Considering a possible alternative cause of decreased cell numbers, apoptotic Gli1 lineage cells were compared between CTRL and CKO mice using the TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay. The number of apoptotic was higher in CKO entheses compared to CTRL entheses, suggesting that TGFβ signalling not only maintains Gli1 lineage cell proliferation, but also prevents them from undergoing apoptosis (Figure 4C). To further investigate if anti-apoptotic Bcl-2 was involved in this process, we performed immunostaining for Bcl-2 in control and CKO enthesis. Bcl-2 was detected in a subset of bone marrow cells, but was not detected in Gli1-lineage cells, suggesting that another pathway was involved in Gli1+ cells apoptosis (Figure S5). Overall, these data suggest that Gli1-lineage cell proliferation is in part dictated by TGFβ signaling.
Figure 4. TbR2 deletion led to fewer Gli1-lineage cells.
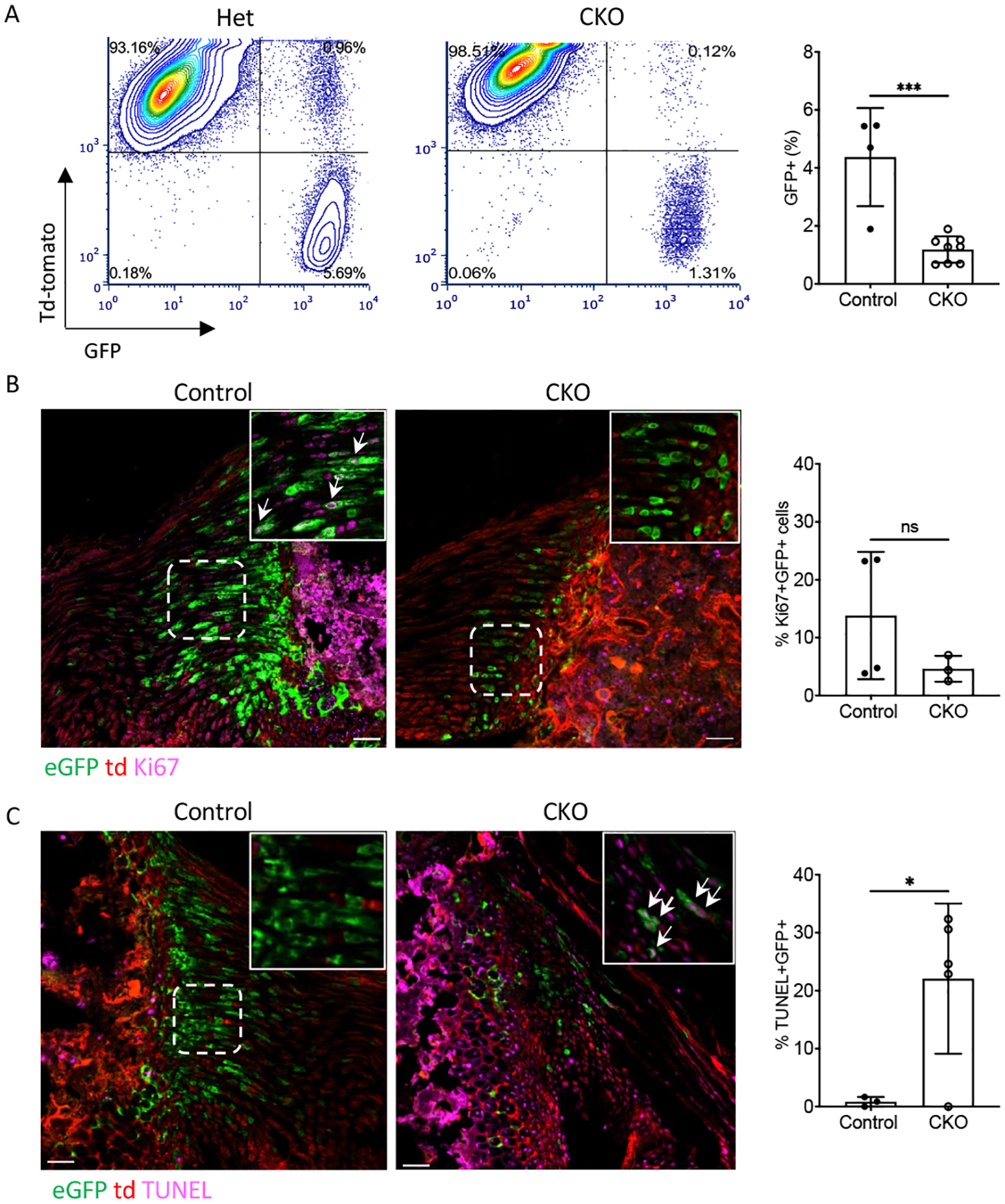
(A) FACS plots and quantification showed reduced numbers of Gli1-lineage cells in CKO mice during cell culture (passage 3). (B) Ki67 immunostaining revealed Ki67+ in Gli1-lineage cells in control and CKO supraspinatus entheses. Arrows indicate Ki67+ Gli1-lineage cells (tendon is on the left and bone is on the right of the sections). (C) TUNEL staining revealed apoptotic cells in Gli1-lineage cells in control and CKO supraspinatus enthesis. Arrows indicate TUNEL-positive Gli1-lineage cells (tendon is on the right and bone is on the left of the sections) (scale bars=50μm). ns: not significant, * p<0.05, *** p<0.001.
Smad-dependent canonical and Smad-independent non-canonical pathways were affected in TbR2-deficient mice
Due to limited numbers of Gli1-lineage cells in the enthesis, mechanistic experiments exploring the pathways by which TbR2 control Gli1-lineage cell behavior focused only on cells derived from tendon. FACS-sorted Gli1-lineage cells from tail tendons from Gli1CreERT2;TbR2fl/wt;mTmG control and Gli1CreERT2;TbR2 fl/fl;mTmG CKO mice were stimulated with TGFβ1 or hedgehog agonist (HhAg). TGFβ treatment increased Scx gene expression in a time-dependent manner in control cells, and this induction was significantly reduced in CKO cells (Figure 5). Similarly, HhAg induced Scx expression in a time dependent manner, but there was no difference between responses in the control and CKO cells (Figure 5). Sox9 was not significantly induced by either TGFβ or HhAg (Figure 5). As expected, TGFβ induced expression of TGFβ1 (Figure S6) and HhAg induced expression of Gli1 (Figure S7). Despite substantial variation in absolute responses between cell isolations, statistically significant effects were seen at the ANOVA factor level for genotype and time.
Figure 5. Responses of Gl1-lineage cells to TGFβ and HhAg in vitro.
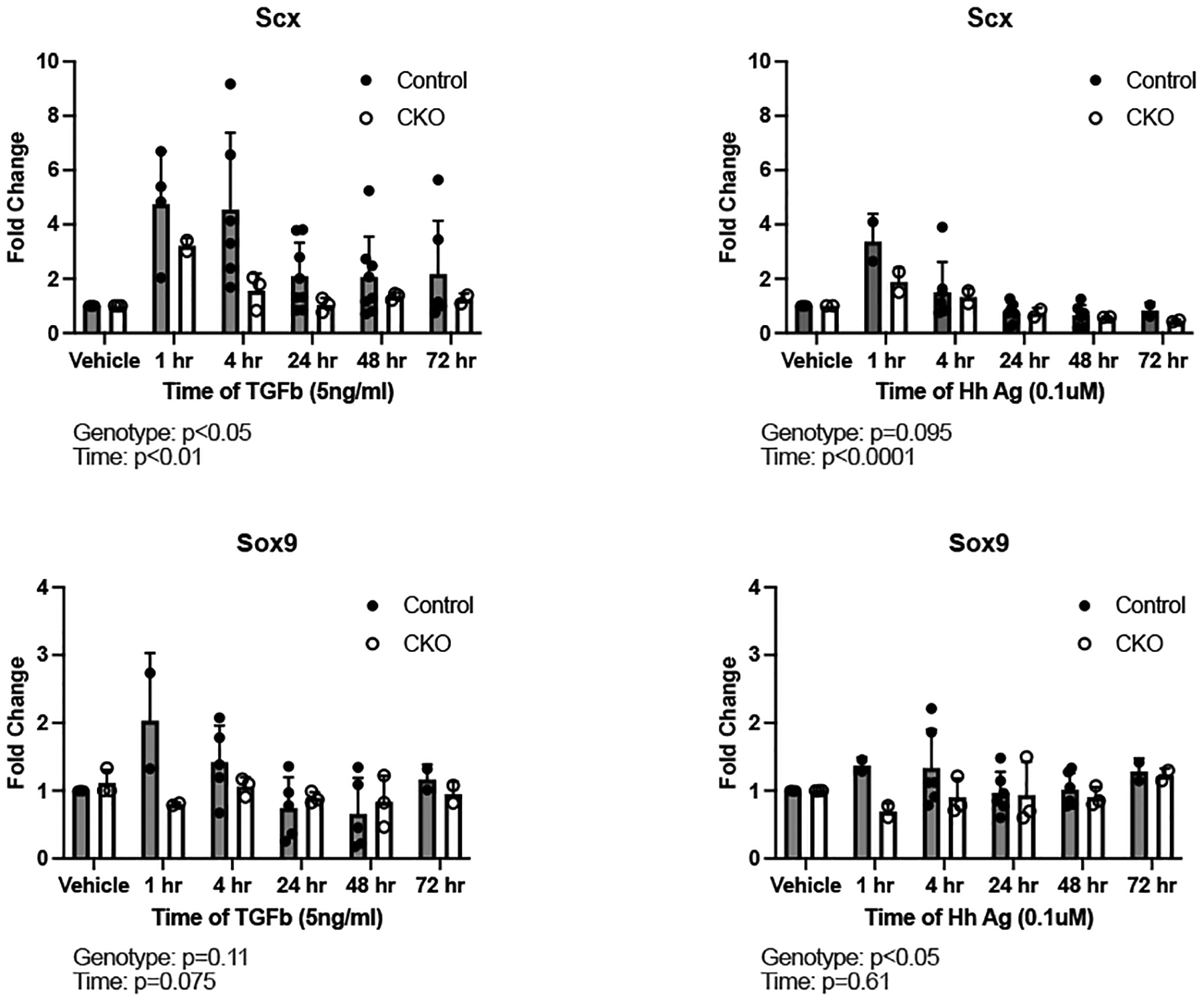
Quantification of Scx and Sox9 expression by qPCR in cultured Gli1-lineage cells between control and CKO mice. Left panel: cells treated with TGFβ, right panel: cells treated with hedgehog agonist (HhAg). TbR2 deletion impaired TGFβ-induced Scx expression and HhAg-induced Sox9 expression in Gli1-lineage cultured cells. Statistically significant effects of genotype and time were determined by two factor ANOVA.
To investigate whether Smad-dependent canonical or Smad-independent non-canonical pathways were affected by TbR2 deletion, Gli1-lineage cells were FACS sorted and stimulated with TGFβ. Whole cell lysates were probed for phosphorylated Smad2, the key downstream signaling molecule for the canonical TGFβ pathway, as well as phosphorylated cJun, JNK, p38, and Erk, involved in non-canonical pathways. As expected, Smad2 phosphorylation was significantly decreased in CKO cells (Figure 1C), suggesting that the canonical pathway was affected by TbR2 deletion. Both cJun and Erk were induced by TGFβ in a time dependent manner, but only cJun and JNK activation were TbR2-dependent, showing reduced phosphorylation in CKO cells (Figure 6). cJun activation was verified on tissue section using immunohistochemistry for the phosphorylated form of the cJun protein. The numbers and percentage of cJun+ cells were similar in CKO and Control entheses (Figure 6C). Of note, trends in Erk phosphorylation were opposite to those for other transcription factors, with increased levels in CKO cells. This may represent a compensatory mechanism by cells in response to reduction of other transcription pathways, activation of a TbR2-independent pathway, and/or incomplete deletion of TbR2 in the cultured cells. When considering only Gli1-lineage (i.e., GFP+) cells, there was a dramatic reduction in the percentage of cJun+ cells in CKO compared to ccontrol enthesis (Figure 6C). Notably, non-canonical TGFβ signaling through MAPK/JNK/cJun pathways may controls cell proliferation (37–39), consistent with the result that TbR2 deletion led to fewer Gli1-lineage cells and reduced proliferation (Figure 4). Akt activation was not induced by TGFβ (Figure 6A and data not shown). These results suggest that both Smad2 canonical and cJun non-canonical pathways were dependent on TbR2 in Gli1-lineage cells.
Figure 6. TGFβ induced non-canonical pathways in Gli1-lineage cells.
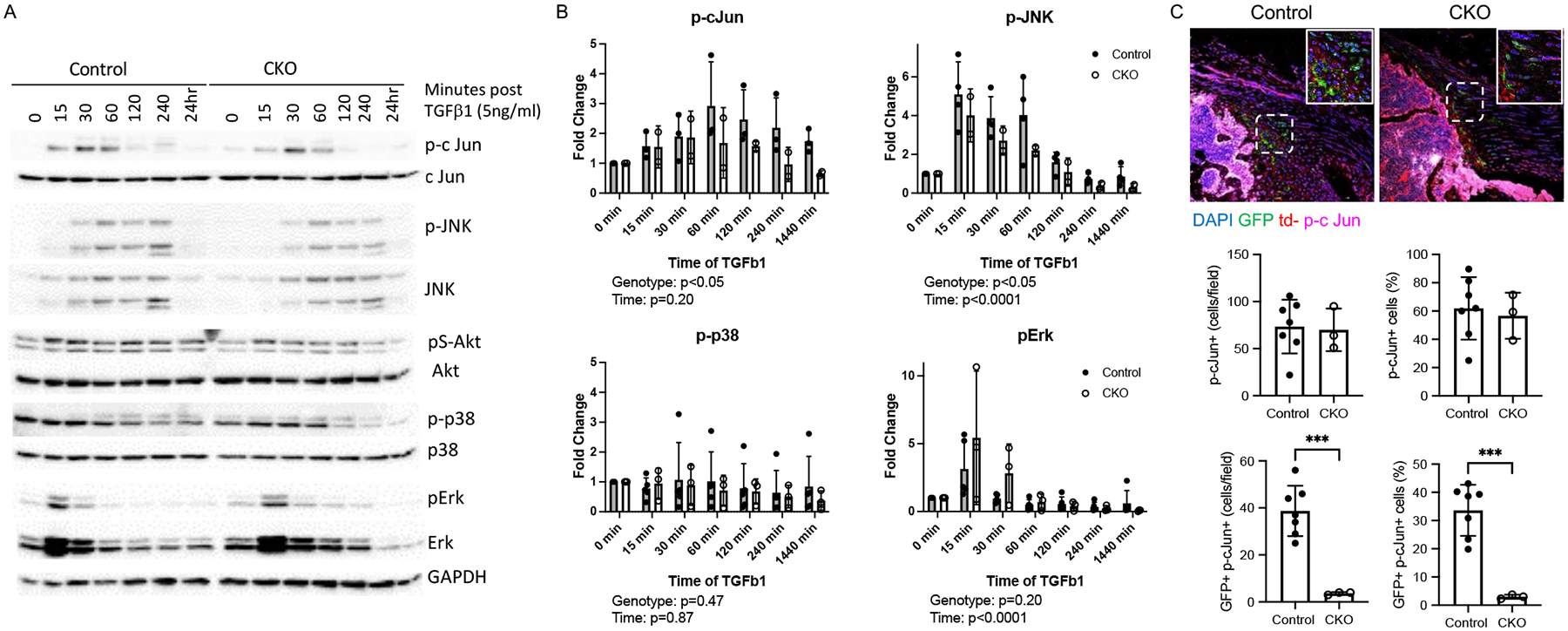
(A) Representative western blots for control and CKO Gli1-lineage cells. (B) Quantification of phosphorylated cJun, JNK, p38, and Erk in control and CKO Gli1-lineage cells. Statistical significance for the effect of genotype and time was determined by two factor ANOVA. (C) Immunofluorescent staining of p-cJun (magenta) in Control and CKO supraspinatus tendon entheses. Gli1-lineage cells are GFP+ (green) (bone is on the lower left and tendon is on the upper left of the sections; positive cells indicated by arrowheads; scale bars = 50 μm.
TGFβ signaling drove Scx expression through its distant enhancer
Our data and previous studies have shown that TGFβ drives Scx gene expression in tendon cells (Figure 4) (20). However, this has not been explored in Gli1-lineage cells and the molecular mechanism by which TGFβ regulates Scx remains elusive. PROMO software was used to search 10 kilobases (kb) up- and down-stream of the mouse Scx gene transcription start site (TSS), revealing three potential Smad4 binding sites when the dissimilarity parameter was set at 4.0 (Figure 7A). Using chromatin immuno-precipitation (ChIP) with an anti-Smad4 antibody, the strongest binding site was located ~4.9 kb downstream of the Scx TSS (Figure 7B). Binding of Smad4 to this site depended on TbR2, as TGFβ-induced binding was diminished in TbR2 deficient cells (Figure 7C). Notably, the binding of Smad4 to the Scx enhancer was transient, likely indicating tight control of Scx expression. However, stability of Scx mRNA may result in prolonged Scx expression for longer periods, as seen in Figure 5. These data provide direct evidence that TGFβ controls Scx through Smad4 binding to the Scx enhancer 4.9 kb downstream from its transcription starting site in those cells.
Figure 7. Identification of Smad4 binding site on Scx enhancer.
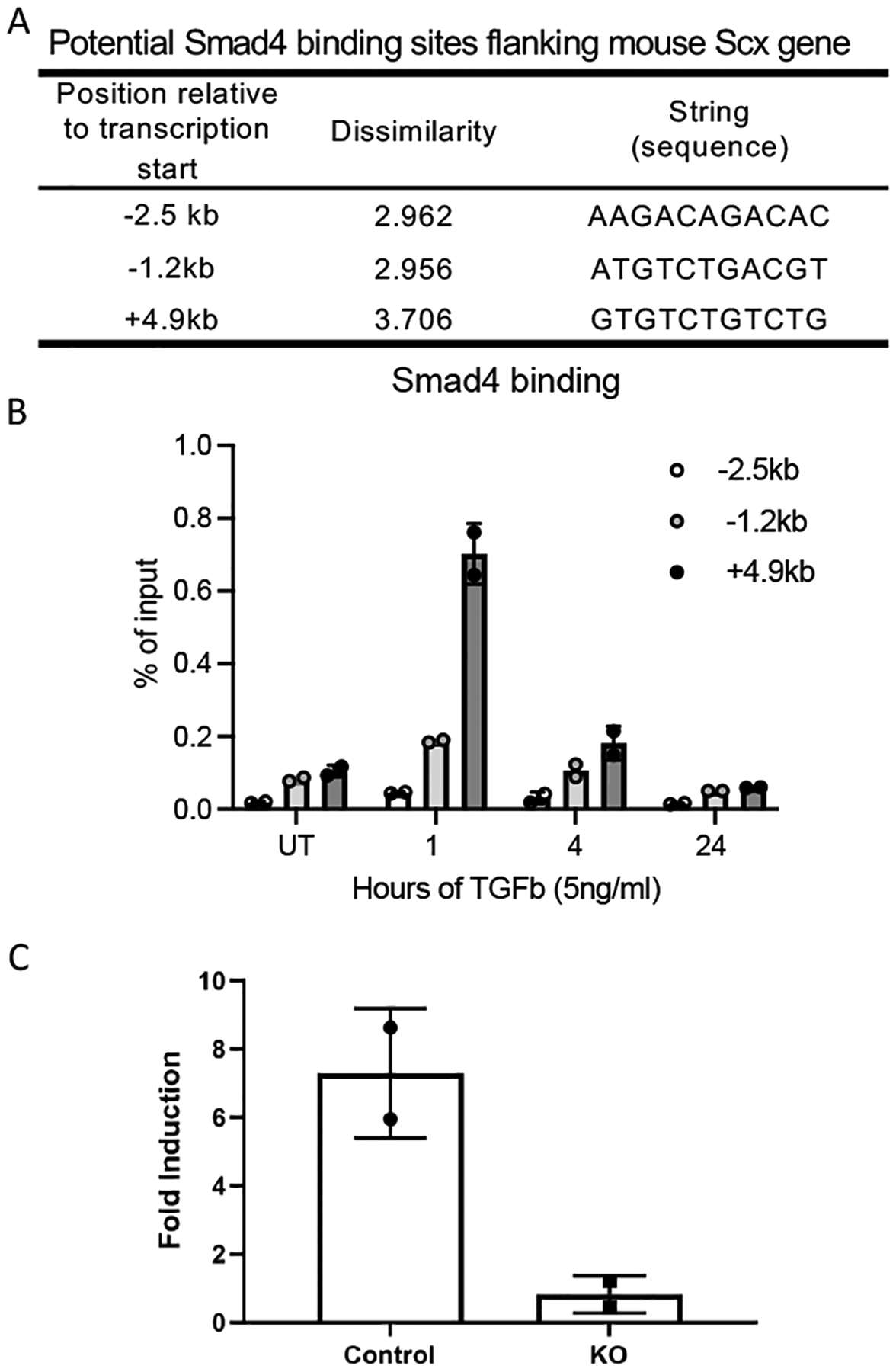
(A) Three potential Smad4 binding sites in the mouse Scx locus, as predicted by Promo software. (B) Time-dependent Smad4 binding to 3 potential sites. (C) Comparison of TGFβ induced Smad4 binding to 4.9 kb Scx enhancer between control and CKO cells.
DISCUSSION
The enthesis is a unique mineralized fibrocartilage tissue that bridges tendon and bone (40, 41). This functionally graded transitional tissue is formed by a pool of Gli1+ (i.e., hedgehog responsive) cells that eventually populate the entire enthesis (12, 23). As a transcription factor crucial for Hh signaling, Gli1 lineage cells have been reported in many stem/progenitor cells, including entheses and periodontal ligament cells (11, 22, 23, 40, 42, 43). The TGFβ family of growth factors have been shown to play important roles in the development, maintenance, and healing of musculoskeletal tissues, including tendon, bone, cartilage, and muscle (20, 21, 44, 45). TGFβ signaling has been shown to drive the formation of tendon and the enthesis; loss of TGFβ signaling results in defective tendon and ligament formation (20, 21) and abnormal enthesis mineralization (46). Furthermore, recent reports have suggested that TGFβ controls fibrocartilage and ligament cell proliferation (22, 47). Notably, there is an abundance of TGFβ ligands embedded in the bone and tendon ECM. However, the molecular mechanisms by which TGFβ controls tendon and enthesis formation remain unclear. Therefore, the current study used an inducible conditional knockout model to delete TbR2 in Gli1-lineage cells postnatally. We hypothesized that deletion of TbR2 in Gli1-lineage tendon enthesis cells would negatively affect development and mineralization of the enthesis. Since enthesis mineralization occurs at approximately P14 (48, 49), we induced TbR2 deletion at P5-P7 (23). Loss of TGFβ signaling at the postnatal enthesis led to defects in humeral head bone morphology and enthesis mechanical properties by P56 and reduction of ECM proteins COL1a and SPARC in Gli1-lineage cells. This is consistent with prior studies showing the importance of the TGFβ superfamily for tendon and enthesis development (20, 50).
To explore mechanisms by which TGFβ regulates tendon enthesis cell differentiation and proliferation, and apoptosis, a mouse reporter model was developed. The model was based on the important role that Gli1-lineage cells play in the development of a variety of tissues, including the tendon enthesis (11, 23). The enthesis is composed of a range of cell phenotypes, including tendon fibroblasts, fibrocartilage cells, mineralized fibrocartilage cells, and bone cells, all derived from a Gli1+ pool of progenitors (11, 49, 51). In the current study, we used tail tendon-derived Gli1-lineage cells for the mechanistic in vitro studies. These experiments provided valuable insights on how TGFβ regulates Hh-activated (i.e., Gli1+) cells. However, further studies using cells derived from the enthesis are needed to link regulation of these cells directly to the enthesis. Nevertheless, enthesis- and tendon-derived Gli1-lineage cells showed similar responses to TGFβ in vitro. Isolating these cells to certain purity is a crucial step to investigate their function and molecular mechanisms.
Using Gli1CreERT2;mTmG reporter mice combined with flow cytometry, we successfully isolated these cells to a high level of purity from mouse rotator cuff as well as tail tendons. In these mice, a membrane bound td-Tomato (mT) flanked a pair of loxP sequences, which was followed by a membrane bound enhanced GFP (mG) cloned in the R26 locus. In cells that did not contain Cre recombinase, only td-Tomato was expressed. In cells that expressed Cre recombinase, the td-Tomato was excised and eGFP was expressed. Therefore, Gli1CreERT2;TbR2fl/fl;mTmG mice allowed for the isolation of GFP-expressing Gli1-lineage cells that had TbR2 deleted and td-Tomato expressing cells that did not express Gli1 and had TbR2 intact. This tool allowed us to explore TGFβ regulation of Gli1+ cells both in vitro and in vivo. By immunofluorescence staining, the nuclear localization of Smad2 was blocked only in Gli1 lineage cells, suggesting that the deletion was specific to that lineage. By western blot, the phosphorylation of Smad2 was reduced, but not completed blocked, suggesting deletion efficiency on the order of 50%. The reduction in TGFβ signaling led to a reduction in total and proliferating Gli1-lineage cells in CKO mice, implying that JNK/cJun-mediated non-conical TGFβ signaling is necessary for proliferation of Gli1-lineage; for instance, TGFβ promotes regulatory T cell (Treg) survival by inhibiting Treg apoptosis while increasing short lived effector cytotoxic T cell apoptosis (52, 53). Here, we showed that removal of TGFβ signaling led to increased apoptosis in Gli1-lineage cells. Overall, the data support the premise that reduced numbers of Gli1 lineage cells in CKO mice was due to decreased proliferation and increase apoptosis.
At the cellular level, TGFβ signaling is initiated by binding of ligands such as TGFβ1, β2, and β3, leading to dimerization of TbR2 with TbR1 and downstream phosphorylation of Smad2 and 3. Phosphorylated Smad2/3 then further phosphorylate and associate with Smad4. Phosphorylated Smad4 brings other Smad proteins to the nucleus and binds to DNA to promote gene transcription leading to cell proliferation, differentiation, and/or ECM deposition (54, 55). Other than TGFβ/Smad canonical signaling pathway, TGFβ also activates non-canonical pathways through TAK/MAPK and mTOR, which controls cell proliferation and differentiation (44, 56, 57). These pathways are activated in normal cells as well as malignant cells (34, 58). In vitro, the JNK/cJun non-canonical pathway showed TbR2 dependency in Gli1-lineage cells, while p38 MARK pathway was not induced in these cells. Erk induction appeared to be independent of TbR2 and could be due to an unrealized signaling complex (although, we cannot rule out the possibilities that this was due to incomplete deletion of TbR2 in the cultured cells leading to overcompensation and/or an ineffective anti-pErk antibody whose signal was saturated in the control cells). In vivo, reduced c-Jun activation may be restricted to Gli1-expressing cells. These results coincided well a reduction in total and proliferating Gli1-lineage cells in CKO mice and imply that JNK/cJun-mediated non-conical TGFβ signaling is necessary for proliferation of Gli1-lineage cells.
At the molecular level, TGFβ has been shown to upregulate key transcription factors for tendon and enthesis development (e.g., Scx, Mohawk) (20, 59, 60). Furthermore, it was reported that Smad3 could physically associate with Scx and Mohawk and regulate their expression (61). By comparing gene sequences between human and mouse, Pryce et al proposed 9 potential regulatory regions in mouse Scx gene (30). To explore how TGFβ controls Scx expression at the molecular level, we carried out a ChIP assay using an anti-Smad4 antibody, as Smad4 mediates all Smad protein DNA binding activities (62, 63). Among three potential binding sites identified by PROMO software, a conservative Smad4 binding sequence 4.9kb downstream from transcription start site (TSS, +4.9kb) was the highest induced by TGFβ treatment. TGFβ induced a 7-fold Smad4 binding to this site, and the induction was absent in TbR2 deficient cells (figure 7C). A recent study reported a similar binding motif for Smad4 in CD8+ T cells (64). Notably, this distal enhancer is conserved among mammals, and is thus highly likely to function similarly in other animals, including human (30). Further mutagenesis studies are needed to prove this is the bona fide Smad4 binding site regulating Scx expression. Smad proteins may also associate with other transcription factors, such as Scx, to bind more proximal region of Scx promoter/enhancer.
A limitation of the study was that only ~50% of the TGFb signal was blocked in GFP+ cells. This may be simply due to 50% efficiency, i.e., only 50% of GFP+ cells had TbR2 deleted. Alternatively, it is possible that some cells had td-Tomato deleted in the Rosa locus but TbR2 was not deleted. This is a possibility because the two loci may be modified differently at the epigenetic level. If this was the case, i.e., that the two loci may be accessed differently by Cre recombinase, the reporter activity would not truly reflect the deletion efficiency.
Using tools ranging from knockout animals to cellular and molecular biology, the results of the current study add to the growing body of literature defining the regulation of the Gli1-lineage cells that build and mineralize the tendon enthesis. TGFβ signaling is necessary for the formation of a functional enthesis, affecting both differentiation and proliferation of Gli1-lineage enthesis cells as well as its mineralization process, likely via canonical and non-canonical pathways. This work can be applied in future translational studies to drive the regeneration of the enthesis during tendon-to-bone healing via growth factor and/or stem cell therapies.
Supplementary Material
ACKNOWLEDGEMENTS
The study was supported by the NIH/NIAMS through R01 AR057836. Flow cytometry analysis was supported by NIH grant S10 BR027050 and cell sorting was supported by NIH grant S10 OD020056. Microscopic core was supported by NIH grant P30 CA013696.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Declarations of interest: none.
DATA AVAILABILITY
The data that support the findings of this study are available in the methods and/or supplementary material of this article.
REFERENCES
- 1.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, and Basler KJN (2018) GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. 558, 449–453 [DOI] [PubMed] [Google Scholar]
- 2.Brownell I, Guevara E, Bai CB, Loomis CA, and Joyner A. L. J. C. s. c. (2011) Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. 8, 552–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, and Humphreys B. D. J. C. s. c. (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. 16, 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, and Gratwohl S. J. C. s. c. (2016) Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. 19, 628–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen B, Stenberg W, Chai Y, Ge W-P, and Feng JQJDC (2020) Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. 54, 639–654. e636 [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, and Chai Y. J. C. s. c. (2014) Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. 14, 160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, and Schober MJD (2010) Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. 137, 3753–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Feng J, Li J, Zhao H, Ho T-V, and Chai YJD (2015) An Nfic-hedgehog signaling cascade regulates tooth root development. 142, 3374–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PM, Bindels EM, Heckl D, Büsche G, and Fleck D. J. C. s. c. (2017) Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. 20, 785–800. e788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, He G, Lee W-C, McKenzie JA, Silva MJ, and Long FJNC (2017) Gli1 identifies osteogenic progenitors for bone formation and fracture repair. 8, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang F, Xiao Y, Zelzer E, Leong KW, and Thomopoulos S (2022) A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential. Cell Stem Cell 29, 1669–1684 e1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz AG, Long F, and Thomopoulos S (2015) Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao L, Tichy ED, Zhong L, Mohanty S, Wang L, Ai E, Yang S, Mourkioti F, Qin L. J. J. o. B., and Research, M. (2021) Gli1 Defines a Subset of Fibro‐adipogenic Progenitors that Promote Skeletal Muscle Regeneration With Less Fat Accumulation. [DOI] [PMC free article] [PubMed]
- 14.Massague J (2012) TGFbeta signalling in context. Nat Rev Mol Cell Biol 13, 616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bensimon-Brito A, Ramkumar S, Boezio GLM, Guenther S, Kuenne C, Helker CSM, Sanchez-Iranzo H, Iloska D, Piesker J, Pullamsetti S, Mercader N, Beis D, and Stainier DYR (2020) TGF-beta Signaling Promotes Tissue Formation during Cardiac Valve Regeneration in Adult Zebrafish. Dev Cell 52, 9–20 e27 [DOI] [PubMed] [Google Scholar]
- 16.Baffi MO, Moran MA, and Serra R (2006) Tgfbr2 regulates the maintenance of boundaries in the axial skeleton. Dev Biol 296, 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Shen J, Wang B, Wang M, Shu B, and Chen D (2011) TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett 585, 1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sueyoshi T, Yamamoto K, and Akiyama H (2012) Conditional deletion of Tgfbr2 in hypertrophic chondrocytes delays terminal chondrocyte differentiation. Matrix Biol 31, 352–359 [DOI] [PubMed] [Google Scholar]
- 19.Qiu T, Wu X, Zhang F, Clemens TL, Wan M, and Cao X (2010) TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol 12, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, and Schweitzer R (2009) Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan GK, Pryce BA, Stabio A, Brigande JV, Wang C, Xia Z, Tufa SF, Keene DR, and Schweitzer R (2020) Tgfbeta signaling is critical for maintenance of the tendon cell fate. Elife 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu C, Xie X, Zhao H, Wu Y, Wang J, and Feng JQ (2021) TGF-Beta Receptor II Is Critical for Osteogenic Progenitor Cell Proliferation and Differentiation During Postnatal Alveolar Bone Formation. Front Physiol 12, 721775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz AG, Galatz LM, and Thomopoulos S (2017) Enthesis regeneration: a role for Gli1+ progenitor cells. Development 144, 1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, and Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AG, Lipner JH, Pasteris JD, Genin GM, and Thomopoulos S (2013) Muscle loading is necessary for the formation of a functional tendon enthesis. Bone 55, 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtaliaj I, Golman M, Abraham AC, and Thomopoulos S (2019) Biomechanical Testing of Murine Tendons. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Pongkitwitoon S, Lu H, Lee C, Gelberman R, and Thomopoulos S (2019) CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J Orthop Res 37, 574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killian ML, and Thomopoulos S (2016) Scleraxis is required for the development of a functional tendon enthesis. FASEB J 30, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, and Alba MM (2002) PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 18, 333–334 [DOI] [PubMed] [Google Scholar]
- 30.Pryce BA, Brent AE, Murchison ND, Tabin CJ, and Schweitzer R (2007) Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 236, 1677–1682 [DOI] [PubMed] [Google Scholar]
- 31.Tsukada S, Westwick JK, Ikejima K, Sato N, and Rippe RA (2005) SMAD and p38 MAPK signaling pathways independently regulate alpha1(I) collagen gene expression in unstimulated and transforming growth factor-beta-stimulated hepatic stellate cells. J Biol Chem 280, 10055–10064 [DOI] [PubMed] [Google Scholar]
- 32.Verrecchia F, and Mauviel A (2002) Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118, 211–215 [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Fang L, Tang X, Lu S, Tamm M, Stolz D, and Roth M (2019) TGF-beta Upregulated Mitochondria Mass through the SMAD2/3-->C/EBPbeta-->PRMT1 Signal Pathway in Primary Human Lung Fibroblasts. J Immunol 202, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumucio JP, Sugg KB, and Mendias CL (2015) TGF-beta superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev 43, 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gehwolf R, Wagner A, Lehner C, Bradshaw AD, Scharler C, Niestrawska JA, Holzapfel GA, Bauer HC, Tempfer H, and Traweger A (2016) Pleiotropic roles of the matricellular protein Sparc in tendon maturation and ageing. Sci Rep 6, 32635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosset EM, and Bradshaw AD (2016) SPARC/osteonectin in mineralized tissue. Matrix Biol 52–54, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao HF, Wang J, and Tony To SS (2015) The phosphatidylinositol 3-kinase/Akt and c-Jun N-terminal kinase signaling in cancer: Alliance or contradiction? (Review). Int J Oncol 47, 429–436 [DOI] [PubMed] [Google Scholar]
- 38.Sun K, Luo J, Guo J, Yao X, Jing X, and Guo F (2020) The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage 28, 400–409 [DOI] [PubMed] [Google Scholar]
- 39.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, and Sandri M (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280, 4294–4314 [DOI] [PubMed] [Google Scholar]
- 40.Fang F, Sup M, Luzzi A, Ferrer X, and Thomopoulos S (2022) Hedgehog signaling underlying tendon and enthesis development and pathology. Matrix Biol 105, 87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu HH, and Thomopoulos S (2013) Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng 15, 201–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui CC, and Angers S (2011) Gli proteins in development and disease. Annu Rev Cell Dev Biol 27, 513–537 [DOI] [PubMed] [Google Scholar]
- 43.Jing D, Chen Z, Men Y, Yi Y, Wang Y, Wang J, Yi J, Wan L, Shen B, Feng JQ, Zhao Z, Zhao H, and Li C (2022) Response of Gli1(+) Suture Stem Cells to Mechanical Force Upon Suture Expansion. J Bone Miner Res 37, 1307–1320 [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Chen G, and Li YP (2016) TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman MS, Akhtar N, Jamil HM, Banik RS, and Asaduzzaman SM (2015) TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res 3, 15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Xie L, Crane J, Zhen G, Li F, Yang P, Gao M, Deng R, Wang Y, Jia X, Fan C, Wan M, and Cao X (2018) Aberrant TGF-beta activation in bone tendon insertion induces enthesopathy-like disease. J Clin Invest 128, 846–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia C, Ge Q, Fang L, Yu H, Zou Z, Zhang P, Lv S, Tong P, Xiao L, Chen D, Wang PE, and Jin H (2020) TGF-beta/Smad2 signalling regulates enchondral bone formation of Gli1(+) periosteal cells during fracture healing. Cell Prolif 53, e12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelzer E, Blitz E, Killian ML, and Thomopoulos S (2014) Tendon-to-bone attachment: from development to maturity. Birth Defects Res C Embryo Today 102, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz AG, Pasteris JD, Genin GM, Daulton TL, and Thomopoulos S (2012) Mineral distributions at the developing tendon enthesis. PLoS One 7, e48630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, Johnson RL, Tabin CJ, Schweitzer R, and Zelzer E (2009) Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell 17, 861–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomopoulos S, Genin GM, and Galatz LM (2010) The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J Musculoskelet Neuronal Interact 10, 35–45 [PMC free article] [PubMed] [Google Scholar]
- 52.Massague J, and Sheppard D (2023) TGF-beta signaling in health and disease. Cell 186, 4007–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouyang W, Beckett O, Ma Q, and Li MO (2010) Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massague J (2008) TGFbeta in Cancer. Cell 134, 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng XH, and Derynck R (2005) Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 56.Moustakas A, and Heldin CH (2009) The regulation of TGFbeta signal transduction. Development 136, 3699–3714 [DOI] [PubMed] [Google Scholar]
- 57.Choi ME, Ding Y, and Kim SI (2012) TGF-beta signaling via TAK1 pathway: role in kidney fibrosis. Semin Nephrol 32, 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derynck R, and Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 59.Farhat YM, Al-Maliki AA, Chen T, Juneja SC, Schwarz EM, O’Keefe RJ, and Awad HA (2012) Gene expression analysis of the pleiotropic effects of TGF-beta1 in an in vitro model of flexor tendon healing. PLoS One 7, e51411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakabe T, Sakai K, Maeda T, Sunaga A, Furuta N, Schweitzer R, Sasaki T, and Sakai T (2018) Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J Biol Chem 293, 5766–5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berthet E, Chen C, Butcher K, Schneider RA, Alliston T, and Amirtharajah M (2013) Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization. J Orthop Res 31, 1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kretzschmar M, and Massague J (1998) SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev 8, 103–111 [DOI] [PubMed] [Google Scholar]
- 63.Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67, 753–791 [DOI] [PubMed] [Google Scholar]
- 64.Igalouzene R, Hernandez-Vargas H, Benech N, Guyennon A, Bauche D, Barrachina C, Dubois E, Marie JC, and Soudja SM (2022) SMAD4 TGF-beta-independent function preconditions naive CD8+ T cells to prevent severe chronic intestinal inflammation. J Clin Invest 132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the methods and/or supplementary material of this article.


