Abstract
This article summarises a selection of scientific highlights in the field of interstitial lung diseases (ILDs) presented at the International Congress of the European Respiratory Society in 2023. Translational and clinical studies focused on the whole spectrum of ILDs, from (ultra)rare ILDs to sarcoidosis, ILDs associated with connective tissue disease and idiopathic pulmonary fibrosis. The main topics of the 2023 Congress presentations were improving the diagnostic process of ILDs, better prediction of disease course and investigation of novel treatment options.
Tweetable abstract
This article summarises a selection of scientific highlights in the field of interstitial lung diseases presented at #ERSCongress 2023 https://bit.ly/3FOy10A
Introduction
During the hybrid European Respiratory Society (ERS) Congress 2023 in Milan, Italy, many sessions were dedicated to interstitial lung diseases (ILDs). Interesting new data were presented in five oral presentation sessions, one ALERT (Abstracts Leading to Evolution in Respiratory Medicine Trials) session and >15 thematic poster sessions. The 2023 symposia and hot topic sessions were focused on progressive pulmonary fibrosis (PPF), symptom burden in ILD, pulmonary hypertension and ILD and the role of novel imaging modalities in sarcoidosis.
Every year, early career members and assembly officers summarise a selection of the scientific highlights presented at the ERS Congress for the different groups of Assembly 12: group 12.01 “Idiopathic interstitial pneumonias (IIPs)”, group 12.02 “ILDs/diffuse parenchymal lung diseases (DPLDs) of known origin”, group 12.03 “Sarcoidosis and other granulomatous ILDs/DPLDs” and group 12.04 “Rare ILDs/DPLDs” [1, 2]. In this review article, we will focus on key results and take-home messages of translational and clinical research presented in oral presentation or thematic poster sessions.
Group 12.01: Idiopathic interstitial pneumonias
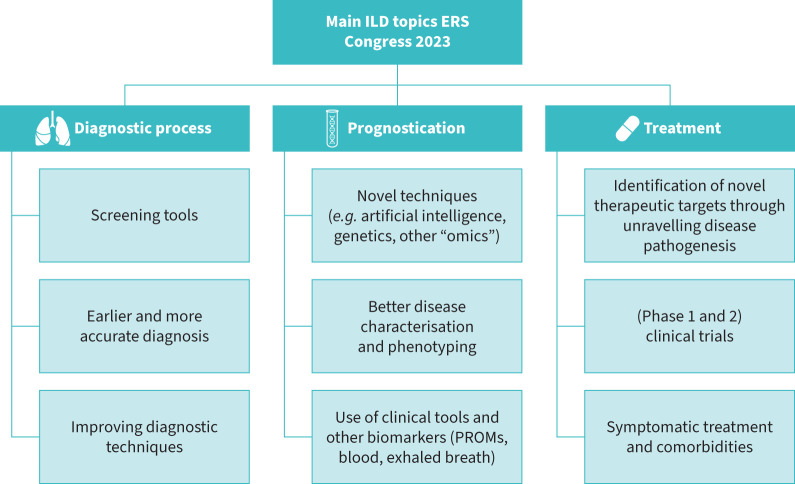
A broad range of novel research findings was presented during the ERS Congress in the field of IIPs. Translational studies focused on unravelling the pathogenesis of pulmonary fibrosis and identifying new treatment targets. Important topics for clinical studies were early and adequate diagnosis, better prediction of disease course and novel disease modifying and symptomatic pharmacological treatment options (figure 1).
FIGURE 1.
Overview of the main topics for interstitial lung disease (ILD) at the European Respiratory Society (ERS) Congress 2023. PROMs: patient reported outcome measures.
Several abstracts highlighted the potential of spatial transcriptomics to unravel disease mechanisms. Justet et al. [3] looked for similarities between patients with idiopathic pulmonary fibrosis (IPF) and long COVID. The results showed abnormal cell populations in both disease groups, compared to healthy controls. These preliminary data must be confirmed in larger cohorts but are interesting given the lung parenchymal sequelae that may follow COVID-19 infections. Bell et al. [4] also used spatial transcriptomic profiling to characterise fibroblast foci; analysis revealed a distinct gene expression profile with enrichment in complex extracellular matrix productions and bone morphogenesis. They also showed that a 3D spheroids-based culture model most closely recapitulates the transcriptome of fibroblast foci. Another promising in vitro model are the organoids used by Khan et al. [5]. The group created organoids from IPF-derived alveolar cells able to express similar functional and morphological properties to honeycomb cysts. Bozza et al. [6] developed hyaluronic acid decorated liposomes loaded with imatinib (XHALIP), which may represent a tool to deliver drugs with reduced systemic side effects. Promising data for drug development were also presented by Rosas et al. [7]. The group showed increased cGAS-STING activity contributes to senescence in IPF; thus, STING inhibitors might be a new alternative with a dual senomorphic/senolytic effect.
A few studies used animal models to investigate the potential of new treatment targets for IPF. Petersen et al. [8] studied the therapeutic effects of ALK5i in a mouse model combining bleomycin with high-fat diet. Results were promising and this model could represent a new tool to explore novel therapeutic agents for IPF. Vanegas et al. [9] investigated SMAC mimetic treatment (BI891065), targeting the inhibitor of apoptosis protein cIAP1, in a mouse model. SMAC mimetic treatment had anti-fibrotic activity by promoting myofibroblast apoptosis.
Other translational research endeavours focused on characterising various populations affected by pulmonary fibrosis. An interesting Finnish study delved into the genetic landscape of patients with IPF [10]. Through genetic mapping they found that a newly identified missense variant in KIF15 was associated with a distinctive worse clinical profile. KIF15 was identified to be enriched in the Finnish population. Patients with this genetic variant had early disease onset and a rapid progression of symptoms, shedding light on the intricate interplay between genetics and disease progression in the context of IPF.
Moving to the diagnostic fields, Furukawa et al. [11] presented results of the PROMISE study, the Japanese ILD registry, which included 2741 patients. Interestingly, after a central multidisciplinary discussion ILD diagnosis was changed in 47% of all patients and in 83% of patients that were initially diagnosed as unclassifiable ILD. This highlights the importance of collaboration between community and referral centres. Trushell-Pottinger et al. [12] presented data from a UK national pilot lung screening programme. Of 8800 participants, 651 (7.4%) had radiological interstitial lung changes on chest computed tomography (CT). Of these, 85% were successively given a diagnosis of ILD, with no differences between asymptomatic and symptomatic patients. Consequently, lung cancer screening offers an opportunity for early diagnosis and intervention. Van der Sar et al. [13] evaluated the potential of exhaled breath analysis using electronic nose (eNose) technology for diagnosis of ILD. The eNose differentiated ILD from other chronic respiratory diseases (asthma, COPD, lung cancer) with an area under the curve (AUC) of 98% (95% CI 96–100). Data should be validated in larger, external cohorts, but hold promise to shorten diagnostic delay, particularly if used as a screening tool in primary care.
Better prediction of disease progression and mortality remains an important field of study in IIPs. The CleanUP-IPF study hypothesised that a less diverse buccal microbiome would be associated with worse survival in IPF, similar to previous findings in the lung microbiome [14]. However, data showed that a greater buccal microbiome diversity was associated with worse survival. Results must be replicated in an independent cohort, but if confirmed, the buccal microbiome could be a predictive tool of survival in IPF. Guler et al. [15] analysed the predictive value of the clinical frailty scale, a very simple and widely used tool in clinical practice. Small but significant differences were found in fit versus vulnerable patients in mean annual change of forced vital capacity (FVC) and 6-min walking distance. The differences in trajectories remained similar with adjustment for age, sex, body mass index, ever-smoking, ILD diagnosis and drug treatment. If confirmed in other cohorts, the clinical frailty scale might contribute to estimating disease progression in individual patients with pulmonary fibrosis. Finally, Im and Yoo [16] developed a nomogram to predict postoperative pulmonary complications after resection of early-stage lung cancer in IPF. The nomogram is based on six clinical variables and could guide the decision-making process in this patient group. A multicentre cohort from the Netherlands evaluated the real-world use of online home spirometry in patients with different forms of pulmonary fibrosis [17]. Interestingly, lung function course in patients with IPF and other forms of pulmonary fibrosis was similar, with a rapidly progressive disease course in approximately one third of patients. This study emphasised that home spirometry is feasible and reliable as a tool for frequent monitoring in regular care.
More effective treatment for pulmonary fibrosis remains an important unmet need. Encouragingly, many research groups presented results of disease modifying and symptomatic treatment options in this field (table 1). A post hoc analysis in patients with IPF investigated the senolitic potential of a combination of dasatinib (100 mg daily) and quercetin (1250 mg daily) [18]. A significant improvement in senolitic markers (increase in alpha-klotho and a decrease in GPNMB/osteoactivin) was found, but no improvement in short-term clinical outcomes. Anti-interleukin (IL)-11 therapies showed promising anti-fibrotic effects in preclinical model of lung fibrosis, reducing collagen deposition and extracellular matrix remodelling. In a phase 1 trial, Arora et al. [19] studied the potential of LASN01, a fully monoclonal antibody targeting IL-11. In healthy volunteers, LASN01 inhibited >95% of the IL-11R signalling and was well-tolerated.
TABLE 1.
Overview of treatment trials presented at the European Respiratory Society Congress 2023
| Study | Treatment | Study population | Study phase | Outcomes |
| Kellogg et al. [18] (NCT02874989) | Dasatinib (100 mg daily) and quercetin (1250 mg daily) | 20 patients with IPF | Post hoc analysis of a first in human open-label pilot study | A significant improvement in senolitic markers was found, but no improvement in short-term clinical outcomes |
| Arora et al. [19] | LASN01 (a monoclonal antibody targeting IL-11R) | 58 healthy volunteers | Phase 1 | LASN01 inhibited >95% of the IL-11R signalling and was well-tolerated |
| Wuyts et al. [20] INTEGRIS-IPF (NCT04396756) | Bexotegrast 320 mg (dual-selective inhibitor of αvβ6 and αvβ1) | 29 patients with IPF | Phase 2a, multicentre RCT | Well-tolerated in participants with IPF up to 40 weeks of treatment; bexotegrast seemed to reduce FVC decline, and in a majority of patients QLF score improved |
| Glaspole et al. [21] (ACT001-AU-003) | ACT001 (targeting NF-κB and STAT3 signalling pathways) | 44 patients, 35 IPF, 9 fILD | Phase 2, multicentre, parallel group | ACT001 reduced FVC decline after 52 weeks |
| Borie et al. [22] ANDROTELO trial (NCT03710356) | Danazol (synthetic hormonal drug) | 25 patients with PF with a (likely) pathogenic TRG variant | Phase 2, open-label, multicentre | Danazol was poorly tolerated, only 10 patients completed 12-month treatment |
| West et al. [23] (ACTRN12618001838202) | Nebulised pirfenidone | 72 patients with IPF (41 from phase 1b, 31 newly enrolled) | Phase 2 open-label extension, multicentre | Reduction in FVC decline after 48 weeks, −165.5±242.6 mL in patients from 1b, −151.1±358.9 mL in newly enrolled patients |
| Corte et al. [24] (NCT04308681) | 30 or 60 mg of BMS-986278 (oral lysophosphatidic acid receptor 1 antagonist) | 125 patients with PPF | Phase 2 double-blind placebo-controlled RCT, multicentre | BMS-986278 reduced the rate of FVC decline after 26 weeks and was safe and well-tolerated; rates of FVC decline were 4.3% for placebo, −2.7% for 30 mg and −1.1% for 60 mg |
| Molyneaux et al. [25] PAciFy cough trial (NCT04429516) | Low-dose controlled-release morphine sulphate | 44 patients with IPF and chronic cough | Multicentre, double-blind, placebo-controlled, crossover phase 4 RCT | Morphine sulphate reduced objective cough frequency with 39.4% compared to placebo after 14 days; PROMs improved and treatment was well-tolerated |
| Myall et al. [26] | CPAP therapy in managing OSA | 44 patients with fILD and OSA, 12 started CPAP | Phase 4 study, non-randomised | Improvement in PROMs (K-BILD and PSQI) |
| Dhooria et al. [27] SARCORT trial (NCT03265405) | Prednisolone | 86 patients with pulmonary sarcoidosis | Single-centre, open-label, parallel-group RCT | Daily prednisolone dose of 40 mg is not superior to 20 mg in overall response; the frequency of relapse, treatment failure after 18 months and the mean time to these events were similar |
| Kinnersley et al. [28] (NCT03824392) | Efzofitimod (selective modulator of neuropilin-2) | 37 patients with pulmonary sarcoidosis (20 subtherapeutic dose, 17 therapeutic dose) | Phase 1b/2a randomised, double-blind, placebo-controlled, multiple ascending doses | In the therapeutic group a lower percentage of patients relapsed (7.7% versus 54.4%), FVC improved over time and health status improved in a significantly higher percentage |
| Bermudo Peloche et al. [29] FIBRO-COVID (NCT04607928) | Pirfenidone | 113 patients with post-COVID-19 pulmonary fibrosis | Phase 2, multicentre, placebo-controlled RCT | FVC improved with 12.74% in the pirfenidone group and 4.35% in the placebo group after 6 months, but differences were not statistically significant |
| Harari et al. [30] | Nintedanib | 30 patients with LAM | Phase 2, multicentre, open-label | Nintedanib stabilised lung function parameters (FVC, FEV1, DLCO) and was generally well-tolerated |
| Trapnell et al. [31] | Pulmonary macrophage transplantation | Not informed | Preclinical | Pulmonary macrophage transplantation corrected PAP and normalised disease biomarkers; approved to start a human trial |
| Bonella et al. [32] ASCEND trial (NCT02004691) | OAER therapy | 36 patients with acid sphingomyelinase deficiency | Phase 2/3, multicentre | Treatment with OAER therapy led to a persistent and progressive improvement of radiological infiltrates, FVC, DLCO and exercise capacity, and was well-tolerated |
IPF: idiopathic pulmonary fibrosis; IL-11R: interleukin 11 receptor; RCT: randomised controlled trial; FVC: forced vital capacity; QLF: Quantitative Lung Fibrosis score; fILD: fibrotic interstitial lung disease; PF: pulmonary fibrosis; TRG: telomere-related gene; PPF: progressive pulmonary fibrosis; PROMs: patient reported outcome measures; CPAP: continuous positive airway pressure; OSA: obstructive sleep apnoea; K-BILD: the King's brief interstitial lung disease questionnaire; PSQI: Pittsburgh sleep quality index; LAM: lymphangioleiomyomatosis; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; PAP: pulmonary alveolar proteinosis; OAER: olipudase alfa enzyme replacement.
Wuyts et al. [20] presented the results from the phase 2a INTEGRIS-IPF study on bexotegrast, a dual-selective inhibitor of αvβ6 and αvβ1. Bexotegrast 320 mg was well-tolerated in participants with IPF up to 40 weeks of treatment. Although not powered to assess efficacy, bexotegrast seemed to reduce FVC decline and in a majority of patients Quantitative Lung Fibrosis (QLF) score improved. The follow-up BEACON-IPF study will assess efficacy of bexotegrast. Glaspole et al. [21] evaluated ACT001, a drug derived from parthenolide that targets NF-κB and STAT3 signalling pathways, as an interesting therapeutic candidate for pulmonary fibrosis. Results showed that ACT001 reduced FVC decline after 52 weeks in a group of 44 patients. Borie et al. [22] presented the results of the ANDROTELO trial, a phase 2 study that evaluated safety and efficacy danazol, a synthetic hormonal drug, in familial pulmonary fibrosis associated with telomere-related gene mutations. While lung function remained stable in a majority of patients, unfortunately, danazol was poorly tolerated, which makes it unlikely to be an option for long-term use in telomeropathy-related pulmonary fibrosis.
West et al. [23] presented data of a phase 2 open-label extension study on nebulised pirfenidone in IPF. They found a reduction in FVC decline comparable to previous studies with oral pirfenidone, both in patients that already participated in the phase 1b study and newly treated patients. Results indicate that nebulised pirfenidone may be a well-tolerated and effective treatment for IPF in the future; a placebo-controlled trial is expected. A phase 2 study in patients with PPF evaluated efficacy and safety of BMS-986278, an oral lysophosphatidic acid receptor 1 (LPA1) antagonist in 125 patients [24]. Similar to a previous study in IPF, BMS-986278 reduced the rate of FVC decline after 26 weeks and was safe and well-tolerated. Phase 3 studies for IPF and PPF are currently being set up.
The PAciFy randomised clinical trial investigated the use of low-dose morphine sulphate for treating cough in IPF [25]. After 14 days, morphine sulphate reduced awake cough frequency by 39.4% compared to placebo and was overall well-tolerated, with acceptable side effects. The authors advocate for rapid implementation in clinical practice due to the well-established safety profile and worldwide availability. Finally, Myall et al. [26] explored the utility of continuous positive airway pressure therapy in managing obstructive sleep apnoea among patients with PPF. In this small study, quality of life and survival improved, indicating the need for larger studies to validate these findings.
Group 12.02: ILDs/DPLDs of known origin
A major challenge for ILDs of known origin lies in correct prognostication. Currently, clinical characteristics, radiographic patterns and pulmonary function tests are often used for risk stratification. However, this approach is not accurate enough to predict disease progression and prognosis in individual patients.
By using e-Lung, an artificial intelligence (AI)-powered CT tool, multiple new imaging biomarkers have been retrospectively identified. First, the weighted reticulo-vascular score (WRVS), which has previously been validated in IPF, was investigated in a cohort of 352 patients with non-IPF ILD. Baseline WRVS outperformed baseline FVC in predicting survival and was more predictive of a future decline in FVC >10% [33]. Second, the ground glass opacity (GGO) score was investigated as a prognostic marker in non-IPF ILD. Prognosis was worse for patients with a higher WRVS and less GGO, and increase in WRVS and GGO in combination with FVC decline was more predictive than FVC decline alone [33]. The potential of AI in prognosticating ILD was also presented by Guiot et al. [34]. They used LungQ to quantify the percentage of interstitial abnormalities and vascular changes in patients with systemic sclerosis (SSc)-ILD (n=68). In this exploratory study, a significant correlation between the change in interstitial lung score and the change in FVC was found. When using an experimental score, combining reticulations and GGOs, a stronger correlation was identified. These studies highlight the potential of radiology AI tools for better prediction of disease outcome in ILD.
Another potential tool to predict the development of lung function impairment in patients with SSc is the non-invasive skin transcriptome [35]. By using publicly available datasets, a weighted gene correlation analysis was performed. Multiple gene networks were associated with skin involvement and lung function evolution. Pathway analysis showed that these pathways were enriched for extracellular matrix interactions, chemokine signalling and cytokine–cytokine receptor interaction. Possibly, these signatures can identify patients at risk for lung involvement and identify targetable pathways for treatment in the future.
Hoffmann-Vold et al. [36] examined whether progression in the preceding year could predict progression in the following year among 231 patients with SSc-ILD. Progression was defined based on four different definitions (FVC >5%, FVC >10%, American Thoracic Society PPF criteria, progressive fibrosing ILD INBUILD criteria). Using multivariable logistic regression adjusted for known risk factors, they found that disease progression in the previous year, no matter the definition, did not predict future progression, even when excluding patients who changed medication due to progression (OR 0.33, p=0.013). These results can have important implications for clinical practice and trial design.
Moving to cellular biomarkers, Moraes et al. [37] examined the role of dendritic cells as a predictive tool in airway-centred interstitial fibrosis. 24 patients with a definitive diagnosis of connective tissue disease (CTD), micro-aspiration-induced pneumonitis and hypersensitivity pneumonitis were included after undergoing a surgical lung biopsy. In all groups, patients with more CLEC9a-positive dendritic cells, as well as patients with low CD1c-positive cells, had a worse survival.
Another important challenge is the differentiation of CTD-ILD from other forms of ILD. Moor et al. [38] used an electronic nose (eNose) to examine the differences between the breath prints of patients with CTD-ILD and other ILDs. 132 CTD-ILD patients were compared to 122 other ILD patients, using partial least square discriminant analysis in a training and test set. The model could differentiate well between CTD-ILD and other ILDs (test AUC 0.92). Furthermore, an even better separation was seen between interstitial pneumonia with autoimmune features and CTD-ILD (test AUC 0.99). In an exploratory follow-up study, the potential of eNose in the differentiation of SSc without ILD and SSc-ILD was investigated, highlighting the potential future role of eNose as a screening tool for ILD in SSc (test AUC 0.84).
Two studies studied the underlying genetic background of different ILDs. Solomon et al. [39] showed that patients with genes associated with the development of seropositive rheumatoid arthritis (RA), had a moderate protective effect on the development of IPF, using Mendelian randomisation. In contrast, patients predisposed to IPF had a significantly higher risk of developing seropositive RA. Upon future validation, this research could help to gain further insights into the pathogenesis of usual interstitial pneumonia (UIP) in patients with RA. Additionally, Novais Bastos et al. [40] showed that fibrotic hypersensitivity pneumonitis and IPF seem to share a genetic background as the minor MUC5B allele (rs35705950) was present in nearly 75% of IPF and fibrotic hypersensitivity pneumonitis cases, while 75% of control patients carried the wild type (GG).
Group 12.03: Sarcoidosis and other granulomatous ILDs/DPLDs
One of the main sarcoidosis highlights from the ERS Congress was the presentation of the SARCORT trial results. In a single-centre, open-label, parallel-group randomised controlled trial (n=86) over 26 weeks Dhooria et al. [27] reported that an initial daily prednisolone dose of 40 mg was not superior to 20 mg in overall response. The frequency of relapse, treatment failure after 18 months and the mean time to these events were similar. No significant differences in change in FVC at 6 and 18 months, overall response, incidence of adverse effects or changes in health-related quality of life were observed between groups. Overall side effects were frequent in both groups, iterating the need for better tolerated first-line pulmonary sarcoidosis treatments.
A phase 1b/2a randomised, double-blind, placebo-controlled, multiple ascending dose study evaluated the use efzofitimod in chronic pulmonary sarcoidosis [28]. Efzofitimod is a novel immunomodulator binding neuropilin-2, which is upregulated on immune cells in response to lung inflammation. Placebo and 1 mg·kg−1 arms were considered subtherapeutic (n=20); 3 mg·kg−1 and 5 mg·kg−1 arms were deemed therapeutic (n=17). In the therapeutic group a lower percentage of patients relapsed (7.7% versus 54.4%). Moreover, FVC improved over time in the therapeutic group and declined in the subtherapeutic group. Finally, health status improved in a significantly higher percentage of patients in the therapeutic group. A phase 3 multicentre, randomised, double-blind study (EFZO-FIT) on efficacy and safety of 3 mg·kg−1 and 5 mg·kg−1 intravenous efzofitimod is currently enrolling.
Sarcoidosis is associated with various comorbidities and treatment of sarcoidosis often leads to relevant side effects. Cameli et al. [41] analysed 252 patients with chronic sarcoidosis from a referral centre in Italy and compared the prevalence of bone fractures with 250 sex- and age-matched healthy controls. Bone mineral density (BMD) was significantly lower in patients with sarcoidosis than healthy controls and prevalence of fragility fractures was higher (30.6% versus 12.3%). Interestingly, presence of bone fractures was not associated with use of glucocorticoids. In contrast, Møller et al. [42] found that BMD increased over a 12-month interval. There was no difference in BMD between patients with and without glucocorticoid treatment.
Sarcoidosis is a complex disease with a heterogeneous disease course. A German research group evaluated informational needs and resources of patients with sarcoidosis [43]. They found that many patients (40.6%) felt not adequately informed. Patients expressed the need for better information early in their disease course, especially with regard to future perspectives and symptoms, such as fatigue and pain. In a second study, they analysed whether the internet is a reliable source of information for sarcoidosis. Their conclusion was that the quality of information was moderate (according to standardised criteria) and important aspects of the disease were frequently not addressed. These studies highlight the importance of providing accurate information on sarcoidosis, and collaboration with different medical specialties and patient organisations.
Finally, many studies presented during the congress focused on disease mechanisms and pathogenesis. A few posters from the same group presented results of flow-cytometric analysis of peripheral blood, bronchoalveolar lavage (BAL) and lymph nodes collected from patients with pulmonary sarcoidosis before start of treatment. Fanetti et al. [44] demonstrated a relationship between an imbalance of follicular helper CD4+ T-cells (Tfh) in these three anatomical compartments. They found higher percentages of proinflammatory Tfh17 and Tfh2 cells and lower percentages of Tfh1 cells in peripheral blood than in BAL and lymph nodes. These results suggest a link between the imbalance of Tfh subsets and pathogenesis of sarcoidosis. Ventura et al. [45] found a higher percentage of natural killer cell concentration in peripheral blood compared to BAL and lymph nodes. Messina et al. [46] showed that the highest percentage of T-helper type 1 (Th1) cells was identified in BAL, whereas the highest percentages of Th2 and cytotoxic T-cells were observed in peripheral blood. According to the authors, their findings were similar to what occurs in autoimmune diseases.
The RESPISAM study investigated the role of immune dysregulation in different sarcoidosis phenotypes [47]. They found significantly higher levels of type 1 inflammation biomarkers from bronchial mucosal lining fluid (IFN-γ, IP-10, MCP-4, IL-15, IL12-p40 and IL12-p70) in pulmonary sarcoidosis than in isolated mediastinal lymph node sarcoidosis.
Group 12.04: Rare ILDs/DPLDs
A large variety of studies about rare ILDs were presented at the 2023 ERS Congress. The main focus was on novel treatment options and better characterisation and phenotyping of rare ILDs.
A phase 2 randomised controlled trial from Spain (FIBRO-COVID) evaluated efficacy of pirfenidone compared to placebo for 6 months in 113 patients who had evidence of pulmonary fibrosis (>5% fibrotic abnormalities on chest CT) at least 1 month after hospital discharge for severe COVID-19 and receiving oral glucocorticosteroids [29]. FVC improved with 12.74% in the pirfenidone group and 4.35% in the placebo group, although differences were not statistically significant.
A multicentre, open-label, single-arm study from Italy (MILES) investigated the use of nintedanib in 30 patients with lymphangioleiomyomatosis (LAM) and progressive pulmonary function decline over the last 12 months despite the use of sirolimus, or patients who did not tolerate the treatment [30]. Patients were treated for 12 months and then followed for an additional 12 months. Nintedanib stabilised lung function (FVC, forced expiratory volume in 1 s (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO)) and was generally well-tolerated.
Results from preclinical studies were presented for hereditary pulmonary alveolar proteinosis (hPAP) [31]. After discovering that lentiviral-mediated CSF2RA gene transfer restores granulocyte–macrophage colony-stimulating factor (GM-CSF) signalling in hPAP patients, the investigators developed and validated Csf2raKO mice as a model of hPAP. The authors found that pulmonary macrophage transplantation corrected hPAP and normalised disease biomarkers. Based on these results, the study was approved to start a human clinical trial.
Bonella et al. [32] investigated the use of the olipudase alfa enzyme replacement (OAER) therapy in adult patients with acid sphingomyelinase deficiency (ASCEND trial). They evaluated whether there was a difference in change in pulmonary function, radiology and exercise capacity between the first-year primary analysis of the randomised controlled trial and after 2 years in the open-label extension follow-up. Notably, treatment with OAER therapy led to a significant and sustained improvement in these outcome parameters and was well-tolerated.
A retrospective study proposed a novel radiological method for risk stratification in patients with LAM [48]. Cyst volume index (CVI) was assessed using YACTA (Yet Another Computed Tomography Analyzer) software and correlated with lung function parameters. A significant negative correlation between CVI and FEV1 and between CVI and DLCO were found. The authors concluded that YACTA software might be useful as a tool for risk stratification and prognosis estimation in patients with LAM.
Krymskaya et al. [49] assessed alveolar fibroblast activation and epithelial injury/repair in LAM in a mouse model. Their results suggest that in the late stage of the LAM disease, there is an overall decrease in pulmonary healing that might indicate the exhaustion of alveolar epithelial cell renewal capacity. The results also showed that abnormal epithelial–mesenchymal crosstalk during injury/repair facilitates the fibroblast activation in lung cells of patients with LAM.
A French multicentre retrospective study evaluated 36 participants with mutations in surfactant genes SFTPC and ABCA3 and investigated their clinical, radiological and lung function characteristics [50]. This study shows that patients with these mutations have a heterogeneous phenotype, often classified as unclassifiable ILD on high-resolution CT. Histological pattern was most frequently nonspecific interstitial pneumonia in the ABCA3 group and UIP in the SFTPC group. Multicentre collaborations are needed to gain more insights in these rare causes of ILD.
Ishii et al. [51] evaluated 63 patients with myelodysplastic syndrome complicated with secondary pulmonary alveolar proteinosis in the largest case series of this patient group yet studied. The authors found a poor prognosis with a mean survival time of 17 months. There was a high prevalence of trisomy 18 and U2A1F mutations, which may be associated with disease pathogenesis.
Finally, a Greek cohort retrospectively analysed 36 patients with autoimmune pulmonary alveolar proteinosis from 2002 to 2022 [52]. Whole lung lavage was performed in 30.5% of patients, but less frequently in the last 10 years. 58.3% of patients were treated with inhaled GM-CSF which led to an overall improvement in lung function parameters and oxygen saturation regardless of disease severity.
Concluding remarks
In conclusion, the 2023 International Congress of the ERS offered exciting and much needed research endeavours throughout the entire spectrum of ILDs. From innovative therapeutic breakthroughs to comprehensive population studies dissecting the genetic and clinical nuances of this complex and heterogeneous group of diseases, the congress reaffirmed the commitment of the medical community to unravel the mysteries of ILD and its aim to discover new therapeutic targets.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: L. Fabbri, J. Guiot and E. Bargagli have nothing to disclose.
Conflict of interest: M. Vermant reports travel fees from Sanofi, outside the submitted work.
Conflict of interest: E. Miądlikowska reports travel fees from Boehringer Ingelheim, outside the submitted work.
Conflict of interest: D. Estrella reports travel fees from Sanofi and Roche/Genentech outside the submitted work.
Conflict of interest: M.S. Wijsenbeek reports grants from Boehringer Ingelheim and Hoffmann la Roche, and fees from Bristol Myers Squibb, Boehringer Ingelheim, Galapagos, Galecto, Hoffman la Roche, Horizon Therapeutics, Kinevant Sciences, Molecure, Nerre Therapeutics, Novartis, PureTech Health, Respivant and CSL Behring, outside the submitted work, paid to her institution.
Conflict of interest: W. Wuyts reports grants and fees from Boehringer Ingelheim, Hoffman la Roche, Galapagos and Sanofi, outside the submitted work, paid to his institution.
Conflict of interest: A. Froidure reports grants and fees from Boehringer Ingelheim.
Conflict of interest: P. Spagnolo reports grants from PPM Services, Roche and Boehringer Ingelheim, outside the submitted work, paid to his institution; and fees from PPM Services, Novartis, Pieris, Glycocore Pharma, Galapagos, Boehringer Ingelheim and Menarini outside the submitted work.
Conflict of interest: M. Veltkamp reports fees from Boehringer Ingelheim and Chiesi outside the submitted work.
Conflict of interest: M. Molina-Molina reports grants and fees from Boehringer Ingelheim, Roche, Chiesi and Ferrer, outside the submitted work.
Conflict of interest: C. McCarthy reports grants and fees from Boehringer Ingelheim, Savara Inc., AI Therapeutics and Theravance Inc., outside the submitted work.
Conflict of interest: K. Antoniou reports grants and fees from Boehringer Ingelheim, Hoffmann la Roche, GlaxoSmithKline, AstraZeneca, Chiesi and Menarini outside the submitted work.
Conflict of interest: M. Kreuter reports grants and fees from Boehringer Ingelheim, Hoffman la Roche and Nichtraucherhelden/Sanero outside the submitted work.
Conflict of interest: C.C. Moor reports grants and fees from Boehringer Ingelheim, Hoffmann la Roche, AstraZeneca and Daiichi Sankyo, outside the submitted work, paid to her institution.
References
- 1.Guler SA, Cuevas-Ocaña S, Nasser M, et al. ERS International Congress 2021: highlights from the Interstitial Lung Diseases Assembly. ERJ Open Res 2022; 8: 00640-2021. doi: 10.1183/23120541.00640-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karampitsakos T, Diep PP, Loth DW, et al. ERS International Congress 2022: highlights from the Interstitial Lung Diseases Assembly. ERJ Open Res 2023; 9: 00584-2022. doi: 10.1183/23120541.00584-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justet A, Ravaglia C, Zhao A, et al. Spatial transcriptomic analysis reveals similar gene expression patterns in the long COVID and IPF lungs. Eur Respir J 2023; 62: Suppl. 67, OA4198. doi: 10.1183/13993003.congress-2023.OA4198 [DOI] [Google Scholar]
- 4.Bell J, Eyres M, Davies E, et al. Defining and modelling the fibrotic niche in lung fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA4197. doi: 10.1183/13993003.congress-2023.OA4197 [DOI] [Google Scholar]
- 5.Khan P, Blumer S, Savic S, et al. A 3D organoid model mimicking honeycomb cysts in idiopathic pulmonary fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA4204. doi: 10.1183/13993003.congress-2023.OA4204 [DOI] [Google Scholar]
- 6.Bozza E, Bozzini S, Bagnera C, et al. Hyaluronic acid decorated-liposome loaded with imatinib: an in vitro study as targeted drug delivery system for lung fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA4201. doi: 10.1183/13993003.congress-2023.OA4201 [DOI] [Google Scholar]
- 7.Rosas L, Vanegas N-D, Riley M, et al. STING inhibitor ameliorates senescence via inhibiting cGAS-STING pathway in idiopathic pulmonary fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA4199. doi: 10.1183/13993003.congress-2023.OA4199 [DOI] [Google Scholar]
- 8.Petersen AG, Bousamaki J, Andreasen LJ, et al. Therapeutic effects of ALK5i on pulmonary function and fibrosis in a high-fat diet + bleomycin-induced and spirometry-confirmed mouse model of IPF. Eur Respir J 2023; 62: Suppl. 67, OA4202. doi: 10.1183/13993003.congress-2023.OA4202 [DOI] [Google Scholar]
- 9.Vanegas N, Rosas L, Garcia PA, et al. SMAC mimetic as potential anti-fibrotic therapy in an in vivo model of bleomycin-induced pulmonary fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA4203. doi: 10.1183/13993003.congress-2023.OA4203 [DOI] [Google Scholar]
- 10.Ainola M, Hollmén M, Laaka A, et al. KIF15 missense variant is associated with a distinct prognostic clinical phenotype in idiopathic pulmonary fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA2579. doi: 10.1183/13993003.congress-2023.OA2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa T, Kondoh Y, Oyama S, et al. Nationwide all ILD registry with central MDD in Japan; providing multidisciplinary ILD diagnoses (PROMISE) study. Eur Respir J 2023; 62: Suppl. 67, OA1424. doi: 10.1183/13993003.congress-2023.OA1424 [DOI] [Google Scholar]
- 12.Trushell-Pottinger D, Holbourn A, Nandakumar K, et al. Experience of making early interstitial lung disease diagnosis from patients taking part in UK national pilot lung screening programme. Eur Respir J 2023; 62: Suppl. 67, OA1425. doi: 10.1183/13993003.congress-2023.OA1425 [DOI] [Google Scholar]
- 13.van der Sar IG, Wijsenbeek MS, Braunstahl GJ, et al. Electronic nose technology differentiates interstitial lung diseases from other chronic respiratory diseases. Eur Respir J 2023; 62: Suppl. 67, OA1427. doi: 10.1183/13993003.congress-2023.oa1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Das P, Ma S-F, et al. Buccal microbiome and survival in idiopathic pulmonary fibrosis: CleanUP-IPF. Eur Respir J 2023; 62: Suppl. 67, OA1432. doi: 10.1183/13993003.congress-2023.OA1432 [DOI] [Google Scholar]
- 15.Guler SA M, Cox D-C, Durand G, et al. The clinical frailty scale is associated with progression of fibrotic interstitial lung disease – a multicentre cohort study. Eur Respir J 2023; 62: Suppl. 67, OA1430. doi: 10.1183/13993003.congress-2023.OA1430 [DOI] [Google Scholar]
- 16.Im Y, Yoo H. Development and validation of a nomogram for predicting pulmonary complications after curative resection in idiopathic pulmonary fibrosis patients with early stage lung cancer. Eur Respir J 2023; 62: Suppl. 67, OA1428.doi: 10.1183/13993003.congress-2023.OA1428 [DOI] [Google Scholar]
- 17.Nakshbandi G, Moor CC, Veltkamp M, et al. Home spirometry in regular care for patients with pulmonary fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA3283. doi: 10.1183/13993003.congress-2023.OA3283 [DOI] [Google Scholar]
- 18.Kellogg D, Nambiar A, Justice J, et al. Post hoc pooled analyses of open-label and randomized controlled trials of senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA2583. doi: 10.1183/13993003.congress-2023.OA2583 [DOI] [Google Scholar]
- 19.Arora P, Lickliter J, Richardson B, et al. A novel interleukin-11 receptor antibody, LASN01 is well tolerated and demonstrates target engagement in phase 1. Eur Respir J 2023; 62: Suppl. 67, OA2581. doi: 10.1183/13993003.congress-2023.OA2581 [DOI] [Google Scholar]
- 20.Wuyts WA, Valenzuela C, Jenkins G, et al. Safety, tolerability and antifibrotic activity of bexotegrast: phase 2a INTEGRIS-IPF study (NCT04396756). Eur Respir J 2023; 62: Suppl. 67, OA1423. doi: 10.1183/13993003.congress-2023.OA1423 [DOI] [Google Scholar]
- 21.Glaspole I, Cai D, Chambers D. Safety and efficacy study of ACT001 in IPF and fILD patients. Eur Respir J 2023; 62: Suppl. 67, OA2585. doi: 10.1183/13993003.congress-2023.OA2585 [DOI] [Google Scholar]
- 22.Borie R, De Fontbrune FS, Jouneau S, et al. Efficacy and safety of DANAZOL for pulmonary fibrosis associated with telomere related gene mutation. Eur Respir J 2023; 62: Suppl. 67, OA2580. doi: 10.1183/13993003.congress-2023.OA2580 [DOI] [Google Scholar]
- 23.West A, Lawrence A, Bao H, et al. Nebulised pirfenidone in idiopathic pulmonary fibrosis (IPF): first look at FVC data. Eur Respir J 2023; 62: Suppl. 67, OA2582. doi: 10.1183/13993003.congress-2023.OA2582 [DOI] [Google Scholar]
- 24.Corte T, Behr J, Cottin V, et al. BMS-986278 for progressive pulmonary fibrosis (PPF): results from a phase 2 randomised controlled trial. Eur Respir J 2023; 62: Suppl. 67, RCT800. doi: 10.1183/13993003.congress-2023.RCT800 [DOI] [Google Scholar]
- 25.Molyneaux P, Wu Z, Spencer LG, et al. Morphine sulfate for the treatment of cough in idiopathic pulmonary fibrosis: the PAciFy cough randomised clinical trial. Eur Respir J 2023; 62: Suppl. 67, OA4195. doi: 10.1183/13993003.congress-2023.OA4195 [DOI] [Google Scholar]
- 26.Myall K, West A, Martinovic J, et al. Treatment of obstructive sleep apnoea in patients with interstitial lung disease improves quality of life and survival. Eur Respir J 2023; 62: Suppl. 67, OA2586. doi: 10.1183/13993003.congress-2023.OA2586 [DOI] [Google Scholar]
- 27.Dhooria S, Sehgal IS, Agarwal R, et al. High-dose (40 mg) versus low-dose (20 mg) prednisolone for treating sarcoidosis: a randomised trial (SARCORT trial). Eur Respir J 2023; 62: 2300198. doi: 10.1183/13993003.00198-2023 [DOI] [PubMed] [Google Scholar]
- 28.Kinnersley N, Chandrasekaran A, Ramesh P, et al. Therapeutic doses of efzofitimod significantly improve multiple pulmonary sarcoidosis efficacy measures. Eur Respir J 2023; 62: Suppl. 67, PA1744. doi: 10.1183/13993003.congress-2023.PA1744 [DOI] [Google Scholar]
- 29.Bermudo Peloche G, Del Río B, Vicens-Zygmunt V, et al. Pirfenidone in post-covid19 pulmonary fibrosis (FIBRO-COVID): phase-II randomised clinical trial (NCT04607928). Eur Respir J 2023; 62: Suppl. 67, OA3282. doi: 10.1183/13993003.congress-2023.OA3282 [DOI] [Google Scholar]
- 30.Harari SA, Elia D, Caminati A, et al. Nintedanib for lymphangioleiomyomatosis: an open-label phase II study. Eur Respir J 2023; 62: Suppl. 67, OA3274. doi: 10.1183/13993003.congress-2023.OA3274 [DOI] [Google Scholar]
- 31.Trapnell B, Arumugam P, Carey B, et al. Preclinical studies supporting a first-in-human trial of pulmonary macrophage transplantation therapy of CSF2RA-associated PAP. Eur Respir J 2023; 62: Suppl. 67, OA3276. doi: 10.1183/13993003.congress-2023.OA3276 [DOI] [Google Scholar]
- 32.Bonella F, Wasserstein M, Giugliani R, et al. Reversal of interstitial lung disease after olipudase alfa enzyme replacement therapy in adults with acid sphingomyelinase deficiency. Eur Respir J 2023; 62: Suppl. 67, OA3281. doi: 10.1183/13993003.congress-2023.OA3281 [DOI] [Google Scholar]
- 33.George P, Benvenuti G, Rennison-Jones C, et al. Novel e-Lung CT biomarkers combine to provide higher prognostic discrimination than FVC in patients with non-IPF fibrotic interstitial lung disease. Eur Respir J 2023; 62: Suppl. 67, OA855. Doi: 10.1183/13993003.congress-2023.OA855 [DOI] [Google Scholar]
- 34.Guiot J, Gester F, Henket M, et al. AI-based quantification of ILD and pulmonary vasculature in a retrospective SSc-ILD cohort correlates with pulmonary function test: a perspective from the PROFILE.net ERS Clinical Research Collaboration. Eur Respir J 2023; 62: Suppl. 67, OA853. Doi: 10.1183/13993003.00577-2023.OA853 [DOI] [Google Scholar]
- 35.Zielonka J, Li N, Wang Z, et al. Skin transcriptomes link severity of skin involvement and lung function impairment in systemic sclerosis. Eur Respir J 2023; 62: Suppl. 67, OA856. Doi: 10.1183/13993003.congress-2023.OA856 [DOI] [Google Scholar]
- 36.Hoffmann-Vold AM, Petelytska L, Fretheim H, et al. Progression of interstitial lung disease in systemic sclerosis does not predict further progression. Eur Respir J 2023; 62: Suppl. 67, OA857. Doi: 10.1183/13993003.congress-2023.OA857 [DOI] [Google Scholar]
- 37.Moraes MML, Telini WM, Batah SS, et al. Dendritic cells as a potential new tool in predicting patient's survival in airway-centered interstitial fibrosis. Eur Respir J 2023; 62: Suppl. 67, OA851. Doi: 10.1183/13993003.congress-2023.OA851 [DOI] [Google Scholar]
- 38.Moor K, Van Der Sar IG, Wijsenbeek MS. Electronic nose technology detects connective tissue disease-associated interstitial lung disease. Eur Respir J 2023; 62: Suppl. 67, OA852. Doi: 10.1183/13993003.congress-2023.OA852 [DOI] [Google Scholar]
- 39.Solomon J, Leavy OC, Kawano-Dourado L, et al. Rheumatoid arthritis and idiopathic pulmonary fibrosis: a bidirectional Mendelian randomisation study. Eur Respir J 2023; 62: Suppl. 67, OA858. Doi: 10.1183/13993003.congress-2023.OA858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novais Bastos H, Santos R, Cardoso CG, et al. Unveiling common molecular pathways linked to ILDs with progressive fibrosing phenotype: the role of MUC5B promoter variants. Eur Respir J 2023; 62: Suppl. 67,OA854. Doi: 10.1183/13993003.congress-2023.OA854 [DOI] [Google Scholar]
- 41.Cameli P, Caffarelli C, Al Refaie A, et al. Evaluation of bone mineral density and fracture risk in sarcoidosis population. Eur Respir J 2023; 62: Suppl. 67, PA1747. Doi: 10.1183/13993003.congress-2023.PA1747 [DOI] [Google Scholar]
- 42.Møller J, Rejnmark L, Bendstrup E. Change in bone mineral density in Danish patients with sarcoidosis. Eur Respir J 2023; 62: Suppl. 67, PA2228. Doi: 10.1183/13993003.congress-2023.PA2228 [DOI] [Google Scholar]
- 43.Kreuter M, Buschulte K, Höger P, et al. How informed are patients with pulmonary sarcoidosis about their disease? Eur Respir J 2023; 62: Suppl. 67, PA2218. Doi: 10.1183/13993003.congress-2023.PA2218 [DOI] [PubMed] [Google Scholar]
- 44.Fanetti M, D'Alessandro M, Gangi S, et al. T follicular helper cell subsets in sarcoidosis patients: a comparison between three anatomical compartment. Eur Respir J 2023; 62: Suppl. 67, PA2219. Doi: 10.1183/13993003.congress-2023.PA2219 [DOI] [Google Scholar]
- 45.Ventura V, Cassai L, Pordon E, et al. CD56dimCD16bright in peripheral blood, lung lymph node and bronchoalveolar lavage from sarcoidosis patients. Eur Respir J 2023; 62: Suppl. 67, PA2225. Doi: 10.1183/13993003.congress-2023.PA2225 [DOI] [Google Scholar]
- 46.Messina M, D'Alessandro M, Gangi S, et al. T helper and T cytotoxic cells subsets in peripheral, alveolar and lymph nodes of sarcoidosis patients. Eur Respir J 2023; 62: Suppl. 67, PA2221. Doi: 10.1183/13993003.congress-2023.PA2221 [DOI] [Google Scholar]
- 47.Hatem O, Jarvis H, Thwaites R, et al. RESPISAM: type 1 inflammation biomarkers from bronchial mucosal lining fluid in parenchymal versus nodal sarcoidosis. Eur Respir J 2023; 62: Suppl. 67, PA2223. Doi: 10.1183/13993003.congress-2023.PA2223 [DOI] [Google Scholar]
- 48.Lederer C, Guth K, Bohács A, et al. New diagnostic approaches for lymphangioleiomyomatosis (LAM). Eur Respir J 2023; 62: Suppl. 67, OA3278. Doi: 10.1183/13993003.congress-2023.OA3278 [DOI] [Google Scholar]
- 49.Krymskaya V, Obraztsova K, Mukhitov A, et al. Alveolar fibroblast activation and epithelial injury/repair in pulmonary lymphangioleiomyomatosis (LAM). Eur Respir J 2023; 62: Suppl. 67, OA3279. Doi: 10.1183/13993003.congress-2023.OA3279 [DOI] [Google Scholar]
- 50.Diesler R, Legendre M, Si-Mohamed S, et al. Phenotypic characterisation of interstitial lung disease associated with mutations in SFTPC and ABCA3 in adults. Eur Respir J 2023; 62: Suppl. 67, OA3280. Doi: 10.1183/13993003.congress-2023.OA3280 [DOI] [Google Scholar]
- 51.Ishii H, Tazawa R, Handa T, et al. Exploring the prognosis and pathogenesis of secondary pulmonary alveolar proteinosis. Eur Respir J 2023; 62: Suppl. 67, OA3277. Doi: 10.1183/13993003.congress-2023.OA3277 [DOI] [Google Scholar]
- 52.Manali ED, Papiris SA, Kallieri M, et al. Autoimmune pulmonary alveolar proteinosis (aPAP) in Greece: 20 years in progress. Eur Respir J 2023; 62: Suppl. 67, OA3275. Doi: 10.1183/13993003.congress-2023.OA3275 [DOI] [Google Scholar]