Abstract
In this review, early career and senior members of Assembly 5 (Airway Diseases, Asthma, COPD and Chronic Cough) present key recent findings pertinent to airway diseases that were presented during the European Respiratory Society International Congress 2023 in Milan, Italy, with a particular focus on asthma, COPD, chronic cough and bronchiectasis. During the congress, an increased number of symposia, workshops and abstract presentations were organised. In total, 739 abstracts were submitted for Assembly 5 and the majority of these were presented by early career members. These data highlight the increased interest in this group of respiratory diseases.
Tweetable abstract
Novel mechanisms of disease progression and exacerbations of airway diseases, together with superior disease models, can be used to develop disease management and novel therapies. The 2023 #ERSCongress saw several advancements in this regard. https://bit.ly/47sd2g7
Introduction
The European Respiratory Society (ERS) International Congress 2023, hosted in Milan, Italy, showcased the latest advances in respiratory medicine and basic science. Research was reported over 5 days as oral presentations, hot topics, workshops and symposia. Assembly 5, focusing on the airway diseases asthma, COPD, chronic cough (CC) and bronchiectasis, was supported by 688 members attending the congress. Of the 739 abstracts submitted for Assembly 5, an impressive 451 were presented by early career members, with an equal proportion of men and women. As done in previous years [1–3], in this review, early career members of Assembly 5 summarise highlights of the research presented and discussed during the congress.
COPD
Early diagnosis, exacerbations and “pre-COPD”
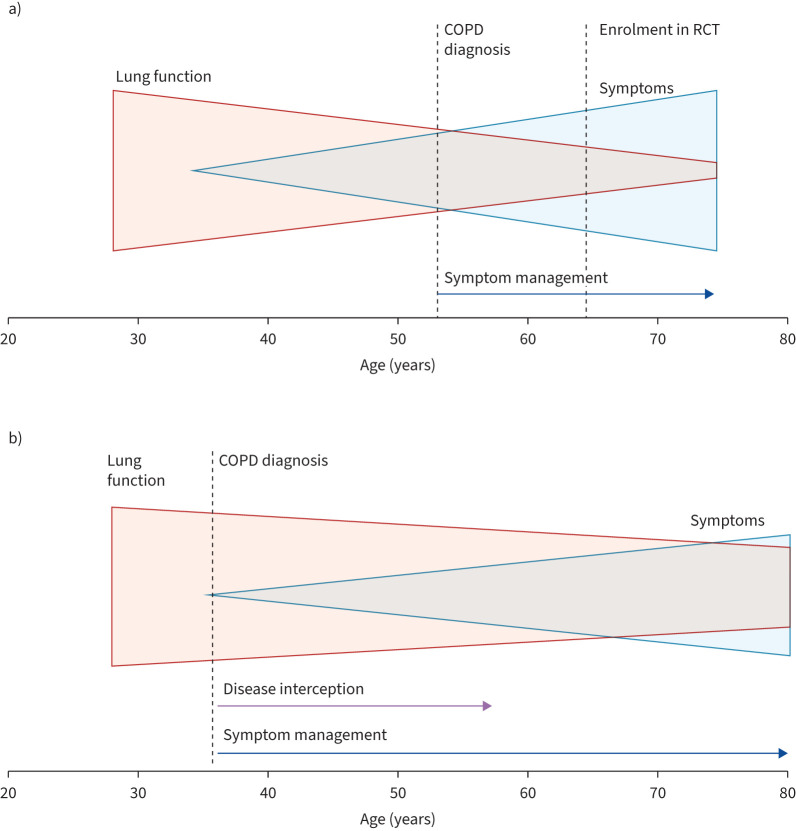
Patients with typical symptoms not fulfilling current COPD definitions are summarised under the term “pre-COPD”. The Lancet Commission 2022 statement paved the way for a more pragmatic mindset about nonlinear lung function trajectories, nonsmoking aetiologies and earlier diagnosis (figure 1) [4]. This is closely associated with the well-known insensitivity of basic spirometry [5]. A Copenhagen cohort study demonstrated that the presence of pre-COPD in young adults is an important risk for the later development of COPD [6]. Their heterogenous definition included chronic bronchitis, preserved ratio impaired spirometry (PRISm) or early airflow limitation (forced expiratory volume in 1 s (FEV1)/forced vital capacity less than the lower limit of normal (LLN)). The NOVELTY study, focusing on long-term outcomes, found comparable mortality for patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1/2 stages and PRISm, with still considerable event rates in pre-COPD. Likewise, lung function decline was similar between pre-COPD and GOLD 1/2 [7]. Quantitative chest tomography (CT) has become more accepted in this context. Swedish real-life data demonstrated that it is possible to detect typical COPD features within individuals with evidence of pre-COPD using CT [8]. An obstructive lung disease cohort study showed that cannabis use was associated with quantitative CT measures reflecting emphysema, airway inflammation and vascular pruning, independent of tobacco use [9].
FIGURE 1.
The importance of early diagnosis in COPD. a) Currently, COPD is diagnosed at a stage when pathological changes are irreversible. This late diagnosis is due to a combination of factors, including the lack of predictive biomarkers, under-recognised clinical symptoms, a long period of disease activity associated with no or minimal symptoms, and reliance on spirometry, an insensitive diagnostic tool. b) Implementation of a more inclusive diagnosis of COPD allows for the detection of early disease before irreversible pathological changes have occurred and could lead to disease interception. RCT: randomised controlled trial. Reproduced from [4] with permission.
Small airway dysfunction as assessed by oscillometry has also been increasingly investigated [10]. With “the silent zone” finally being heard [11], a whole session was dedicated to available insights into the small airways in COPD, with M. Kraft and O.S. Usmani highlighting recent research. Heated tobacco products, e-cigarettes and combustible cigarettes were reported to have significant short-term impact on vascular and small airway dysfunction [12]. The London COPD Cohort reported an association between lung function decline and biophysical components of mucus and the pulmonary microbiome [13]. An emerging mucus-microbiome signature that is characterised by increased mucin expression and presence of colonising proteobacteria, including Achromobacter, has been associated with FEV1 decline in a recent multicohort analysis [14]. The pulmonary microbiome in COPD also received attention with respect to predicting treatment response, whereby a loss of bacterial diversity and a high Gammaproteobacteria to Firmicutes ratio may be discriminative in terms of exacerbation reduction in response to astegolimab [15].
Exacerbations are known to be the main drivers for lung function decline, loss of quality of life and mortality [16–18]. Although exacerbation rates decreased during the SARS-CoV-2 pandemic, as a public health problem it is now approaching pre-pandemic levels again [19]. Single inhaler triple therapy is highly effective in reducing exacerbations [16, 20, 21]. GOLD 2023 recommends that COPD patients on inhaled corticosteroid (ICS)/long-acting β-agonist (LABA) therapy should be stepped up to ICS/LABA/long-acting muscarinic antagonist (LAMA) therapy or, for patients with a history of exacerbations or major symptoms, switched to LABA/LAMA [22]. Post hoc analyses from the KRONOS randomised clinical trial (RCT) suggest that COPD patients who remain symptomatic on ICS/LABA derive greater benefit from a step up to ICS/LAMA/LABA than from a switch to LAMA/LABA [23]. Additionally, airway smooth muscle area was shown to predict ICS response in the HISTORIC study in patients receiving triple therapy [24]. Cardiovascular events are increasingly recognised as the main driver of COPD mortality and represent an opportunity for addressing a treatable trait [25]. EXACOS-CV revealed a >10-fold increased risk for these detrimental events depending on exacerbation severity [26]. Risk remains elevated for at least 1 year after a severe COPD exacerbation. IMPACT trial data [27] demonstrated that ECG-derived parameters can identify COPD patients at risk for adverse cardiopulmonary outcomes. Increased Cardiac Infarction Injury Score (CIIS) and right heart dysfunction predict benefit from triple therapy [27].
Future directions and novel approaches in COPD
Dupilumab was demonstrated to reduce exacerbations and improve lung function and quality of life in eosinophilic COPD [28]. Dual phosphodiesterase 3/4 inhibitor ensifentrine (ENHANCE-1 RCT) showed a reduction in exacerbations in patients on non-dual-bronchodilator and non-triple therapies [29, 30]. Several compounds that inhibit interleukin (IL)-33 signalling (itepekimab, tozorakimab, astegolimab) are in clinical development, with the most pronounced effects observed in ex-smokers with evidence of intra-epithelial eosinophil infiltration [31, 32]. Interestingly, IL-33 is upregulated in the small airways within areas of epithelial remodelling [15, 33]. Innovations also included nonpharmacological interventions. In chronic bronchitis, 6-month results from the RheOx real-world registry study indicate that rheoplasty improves symptoms (assessed by the COPD Assessment Test) and quality of life, while also demonstrating that rheoplasty is a safe procedure [34].
Environmental considerations were a central theme at the 2023 ERS Congress. Lifestyle interventions can effectively reduce personal exposure to particulate matter with a 50% cut-off aerodynamic diameter of 2.5 μm, improving the quality of life and symptoms of COPD patients [35]. Individual exposure to several air pollutants can be reliably tracked using a wearable device on the wrist, as demonstrated in a study conducted locally in Milan [36]. Finally, the environmental impact of inhaled therapies is controversial. A novel propellant for extra-fine ICS/LABA/LAMA therapy was demonstrated to be bioequivalent with existing formulations. These data support therapeutic equivalence while at the same time allowing considerable reductions in the carbon footprint of pressurised metered dose inhalers [37].
Basic science research in COPD
Particulate matter derived from pollution and cigarette smoke was also a focus of basic science research at the ERS Congress 2023. Co-culture of macrophages with a BEAS-2B cell line exposed to particulate matter resulted in increased cytochrome P450 family 1 subfamily B member 1 (CYP1B1) and disrupted tumour protein P53 (TP53) and arginase 2 (ARG2) epithelial cell gene expression, which are involved in cell cycle and repair [38]. Alterations in macrophage phenotype by carbon also led to an increase in cell diameter and increased levels of CD206 [39]. Particulate matter reduces inter-α-trypsin inhibitor heavy chain 4 (ITIH4) gene expression, which is correlated with emphysema development, leading to an increase in c-Jun NH2-terminal kinase (JNK) signalling [40].
Mechanisms of senescence in COPD were discussed at the 2023 ERS Congress. Leucine-rich repeat containing G-protein coupled-receptor 6 (LGR6) was quantified in COPD and idiopathic pulmonary fibrosis lung tissue. LRG6 was upregulated in epithelial progenitors and may contribute to senescence in fibrotic areas of lung tissue in COPD patients [41]. Other polymorphisms are associated with clinically relevant outcomes, such as responsiveness to triple therapy (FKBP prolyl isomerase 5 (FKBP5)) and early onset of COPD (Hedgehog pathway) [42, 43]. Bulk RNA sequencing and proteomics analysis of bronchoalveolar lavage fluid (BALF) revealed associations between dysregulated iron metabolism and neutrophilic inflammation in COPD [44]. Polymorphisms in the iron regulatory gene iron responsive element binding protein 2 (IREB2) were also shown to be associated with decreased FEV1 % predicted and diffusing capacity of the lungs for carbon monoxide (DLCO) [45]. MicroRNA (miR)-149-3p was shown to be a key regulator of cellular senescence induced by cigarette smoke [46]. Senolytics (dasatinib and quercetin) reduced levels of senescence markers such as p16 while increasing epithelial barrier integrity in air–liquid interface cultures of COPD patients but not of healthy nonsmoker individuals [47].
Accurate cellular models of the bronchial epithelium have been generated using induced pluripotent stem cells (iPSCs), with existing models progressed to include sensory nerves [48]. The addition of both sensory neurons and Schwann cells derived from iPSCs resulted in the formation of mature neurons capable of producing axons and calcitonin gene-related peptide. The therapeutic potential of iPSCs in repairing the bronchial epithelium has been investigated [49]. Bronchial biopsies were cultured and mechanically disrupted to model injury. Both smoker and COPD bronchial epithelial cells showed incomplete repair after injury. iPSCs were generated from blood and cultured to produce lung progenitor cells, which then demonstrated complete repair of the bronchial epithelium. P63+ lung progenitor cells were used in a clinical trial to regenerate the lung epithelium [50]. Cell transplantation resulted in increased DLCO % predicted. iPSCs also have the potential to act as therapeutics themselves by aiding cellular repair in the lungs of patients [51, 52].
There is also emerging interest in extracellular vesicles and their use in the delivery of therapeutics [53]. miR-142-3p was deemed to be the most important component of small extracellular vesicles derived from airway epithelial cells in attenuating skeletal muscle dysfunction in COPD [54]. Lipofibroblast-derived vesicles were shown to suppress inflammation when lipofibroblasts were treated with a metabolic modulator, rosiglitazone [55].
Take-home messages
There is increasing evidence that the current definition of COPD does not represent the whole complexity of the disease. Improvements in refining phenotyping, identification of endotypes and awareness of risk factors other than tobacco smoking are needed. Multi-omic datasets in COPD have increased our understanding of pathophysiological mechanisms of COPD (figure 2). The mechanisms that drive exacerbations are particularly important given their effect on hospitalisations and mortality. These insights, combined with the development of better preclinical models of disease, can be used to inform the development of therapeutics.
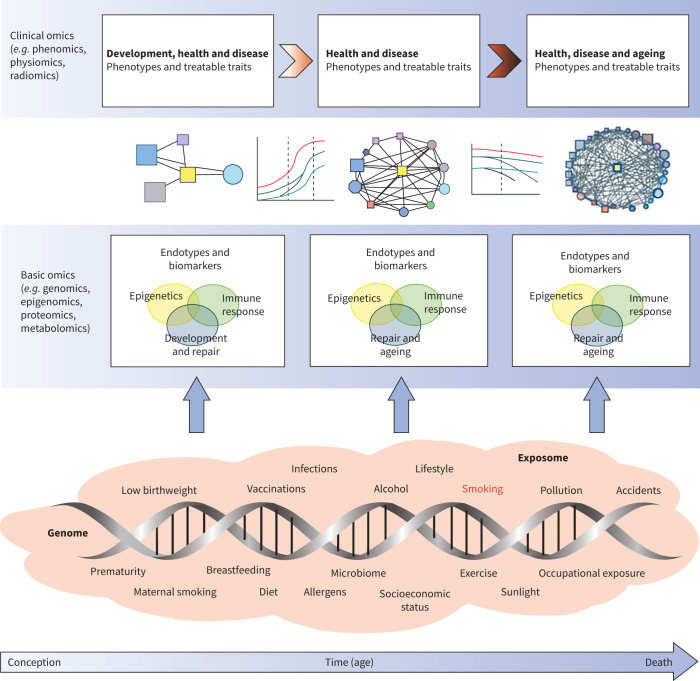
FIGURE 2.
A GETomics approach to understanding COPD and other chronic human diseases. The biological effects and clinical outcomes of different gene–environment interactions depend not only on their specific characteristics, but also on a time dimension, i.e. the age of the individual at which the interaction occurs and the cumulative history of the individual's previously encountered gene–environment interactions. We propose that future research should take a holistic approach that considers the range of interactions between genes (G) and the environment (E) that occur over an individual's lifespan (time, T) in the context of integrated omics approaches (i.e. GETomics) to better understand the pathogenesis of COPD (and probably other chronic human diseases). Examples of environmental factors (the exposome [56–59]), from conception to death, are represented by orange shading. The positions of different exposures included in the shaded area are not necessarily related to the time axis (arrow) and might occur several times during the lifespan. At different timepoints, these environmental factors interact with the genomic background of the individual through epigenetic and other mechanisms that might be identified through various basic omics approaches. These interactions induce biological responses (endotypes [60]), such as innate or acquired immune responses, that modulate organ structure (development, maintenance and repair, ageing) and function. Biomarkers for these endotypes are needed to be able to objectively characterise the pathogenic mechanisms linked to altered lung structure and function. Modulation of organ structure and function, represented here by different lung function trajectories associated with development and ageing, determines long-term phenotypes associated with health and disease, which can be explored through clinical omics approaches. Reproduced from [61] with permission.
Asthma
Novelties in the management of asthma
New insights in small airway dysfunction in asthma were presented during the ERS Congress 2023. An unbiased cluster analysis of the ATLANTIS study demonstrated four asthma clusters, with either central airway (cluster 1, neutrophilic) or both central and distal airway (cluster 4, severe eosinophilic) involvement, or normal lung function (clusters 2 and 3) [62]. A radiomics algorithm, based on CT-detected ventilation defects towards the posterior and lung base, correlated with small airway dysfunction and identified patients at risk for exacerbations [63]. From a clinical perspective, a feasibility trial demonstrated that a smart digital spacer-based education programme could identify poor inhaler adherence and technique in primary care asthma and reduce inhaler technique errors (−26%) compared to usual care (+15%) [64]. Several clinical studies of severe asthma were presented during the congress. Although lowering the dose of ICS under biological therapy is recommended [65], limited evidence supports this approach [66] and several researchers sought to address this paradigm in their presentations. The SHAMAL study (phase 4 RCT) showed ICS can be safely reduced to a low dose when asthma is optimally controlled with benralizumab, without loss of asthma control or worsening lung function [67]. In contrast, ICS as a reliever-only strategy did worsen lung function [68]. ICS dose reduction was achieved during treatment with tezepelumab in DESTINATION as compared to placebo (15% versus 10% of patients reduced from high to medium, 22% versus 15% from medium to low), without loss of lung function [69]. In another study, patients on biological therapy were found to under-report the reduction of ICS to healthcare professionals, highlighting the importance of clinicians openly discussing ICS use and dose reductions [70]. From the MESILICO study, histological evidence of improved airway remodelling was found after 6 months of mepolizumab treatment [71]. Another biopsy study showed that submucosal eosinophils at baseline predicted remission on anti-IL-5 biological therapy, better than a type 2 (T2) inflammatory score; T2 scores were derived using a combined score of exhaled nitric oxide fraction (FENO) (≥50 ppb, 25–50 ppb and <25 ppb scoring 3, 2 and 1, respectively) and blood eosinophil count (≥300, 150–300 and <150 cells·μL−1 scoring 3, 2 and 1, respectively) [72]. Real-world studies showed remission is achievable in 20–40% of patients and durable with current available biological therapies [73]. Different definitions are used, including clinical remission consisting of good asthma control, absence of exacerbations and no maintenance oral corticosteroids (OCS); and discretionary combined with lung function remission (normal or personal best FEV1). A post hoc analysis of DESTINATION showed the ability to reach remission in a broad population over the range of biomarkers with tezepelumab as compared to placebo [74]. The DESTINATION extension study showed that improvements in biomarkers, asthma symptoms and lung function gradually decrease after stopping tezepelumab [75].
Comorbidities have been considered with respect to treatment response, specifically OCS use in asthma. In REALITI-A, patients with depression or anxiety had higher exacerbation rates and maintenance OCS use and worse Asthma Control Questionnaire scores compared to those without after 2 years of mepolizumab [76]. Furthermore, a post hoc analysis of the REDES RCT demonstrated that the reduction in OCS daily dose while on mepolizumab was lower in asthma patients with concomitant bronchiectasis than in those without [77]. Elsewhere, OCS-dependent patients on anti-IL-5/IL-5 receptor subunit α (IL-5Rα) biologicals were shown to lose weight in a real-world setting [78].
Promising targets for pharmacological treatment of asthma
Receptor for advanced glycation end-products (RAGE) is required for allergen-induced release of IL-33, accumulation of type 2 innate lymphoid cells (ILC2s) and upregulation of IL-5 and IL-13. An ongoing phase 1/2a trial, ARO-RAGE, showed reductions in soluble RAGE in BALF and serum of asthma patients [79]. Adermastat, a matrix metalloproteinase-12 inhibitor, attenuated late allergen-induced asthmatic response in mild allergic asthma [80]. Another phase 1 trial showed good tolerability and safety of LQ036, an inhaled IL-4Rα antagonist [81]. A single dose of a novel anti-thymic stromal lymphopoietin/anti-IL-13 biological molecule improved small airway dysfunction in asthma [82]. A novel inhaled phosphodiesterase 4A inhibitor, tanimilast, was demonstrated to modulate inflammation both alone and in combination with ICS in both T2 and non-T2 asthma phenotypes. In fact, the use of tanimilast resulted in a significant decrease of T2, type-1 (T1) and type-17 (T17) cytokines [83].
The evaluation of anti-IL-6 as a potential new drug for patients with non-eosinophilic IL-6-high asthma was also reported: higher local airway IL-6/soluble IL-6Rα signalling in asthma patients with low sputum eosinophils is the rationale for these targets [84].
Novel immunological mechanisms in asthma pathogenesis
Single-cell transcriptomics has been used to demonstrate novel epithelial–immune cell crosstalk in asthma, e.g. between goblet cells and CD4+ cells, which may be mediated via major histocompatibility complex II, providing a plausible mechanism for potentiating inflammatory responses in asthma [85].
Obese and overweight asthma patients are challenging to treat optimally, and many experience poor asthma control [86]. It was reported that alterations in the CD8+ terminally differentiated effector memory re-expressing CD45 (TEMRA) cell and ILC2 compartments in overweight and obese individuals with asthma serve to immunologically distinguish these groups [87]. ILC2s are an important source of T2 cytokine production in asthma and may therefore be a promising therapeutic target. Zinc has been shown to promote T2 cytokine production by ILC2s, thereby acting as a potential target for modulation of ILC2 activity [88]. A significant effect of mepolizumab on the function of eosinophils was reported: the number and activation status of eosinophils in peripheral blood was decreased by mepolizumab treatment. This treatment also induced a transition of inflammatory subsets of eosinophils to resident eosinophils (with regulatory properties) [89]. For benralizumab treatment, the response of innate immunity mediated by natural killer (NK) cells seemed to be a good marker. Benralizumab, in fact, shifted NK cell phenotypes towards maturity [90].
The effect of in vitro azithromycin treatment on epithelial antiviral immunity was also reported, augmenting bronchial epithelial cells' antiviral response [91].
Non-T2 asthma
The T2 inflammatory phenotype in asthma is characterised by an eosinophilic/T-helper 2 (Th2) inflammatory pattern. Conversely, a non-T2 phenotype of asthma is becoming increasingly recognised and can be identified by a neutrophilic/paucigranulocytic inflammatory infiltrate with associated cytokines [92].
Non-T2 asthma is defined by a combination of the effect of corticosteroid treatments and the nonspecific nature of asthma symptoms. Recently Th1-Th17 and regulatory T-cell-related cytokines were demonstrated to have similar levels in T2-high and T2-low asthma, as discussed by R. Costello.
Some thromboxane and prostaglandin metabolites are associated with persistent symptoms in patients with non-T2 asthma, independently from obesity [93]. Urine eicosanoid concentrations increase with asthma severity and they are differentially associated with clinical phenotypes of asthma. In this study, high concentrations of leukotriene E4 and prostaglandin D2 metabolites were associated with lower lung function and increased amounts of exhaled nitric oxide and eosinophil markers in blood and sputum [94]. Metabolomics was used to analyse several novel metabolites associated with blood neutrophils that may prove useful for stratifying non-T2 asthma. Of these, the ratio of dihydroceramide and sphingosines to glucocorticoids seemed to be the most significant in characterising non-T2-driven asthma [95].
Changes in serum chitinase-3-like protein 1 (YKL-40) negatively correlated with changes in annual FEV1. Serum YKL-40 can be a biomarker of neutrophilic airway inflammation [96, 97]. Neutrophilic airway inflammation seemed to be a driver of inflammatory processes in these patients. Subacute exposure to house dust mites and diesel exhaust particles was associated with neutrophil extracellular trap formation [98].
Take-home messages
Management and treatment of asthma, especially severe asthma, seem to be the crucial points in the field of asthma. Several novel monoclonal antibodies will be available in the future, and the molecular and immunological characteristics of the patients as well as the presence of comorbidities could support clinicians in making the right choice. Different studies in basic and clinical research on non-T2 asthma were reported.
Chronic cough
Cough is a protective reflex that can be triggered by various stimuli. When cough persists for more than 8 weeks, it is termed CC. The features and treatment of CC were extensively discussed during the ERS Congress 2023, underscoring the increasing significance and interest in this condition.
Pathophysiology and definitions of chronic cough
The definitions of several subtypes of CC have been discussed and confirmed. Refractory CC is characterised by a persistent cough that continues despite receiving appropriate treatment; unexplained CC refers to a persistent cough that cannot be attributed to any underlying condition, even after a comprehensive evaluation [99].
The genesis of a cough originates from the activation of a complex reflex arc. It commences with the stimulation of receptors distributed throughout various airway regions. Receptors located in the larynx and tracheobronchial branches can be triggered by mechanical and chemical stimuli [100], e.g. cation channel heat, capsaicin, citric acid and derivatives of arachidonic acid, which elicit the cough reflex by activating vanilloid receptors known as transient receptor potential cation channel subfamily V member 1 (TRPV1) [101]. The involvement of both the central and peripheral nervous systems has been extensively examined as the primary drivers of CC, essentially treating CC as a neurological disorder and a potential target for therapeutic intervention.
A novel paradigm called cough hypersensitivity syndrome is characterised by CC triggered by low-level exposures to thermal, mechanical or chemical stimuli [102, 103]. The concept of the “exposome” is noteworthy because it emphasises the pivotal role of the environment in the development and aggravation of respiratory conditions [104].
Management of chronic cough
CC significantly affects multiple facets of a patient's quality of life, including chronic pain [105], anxiety and depression [106, 107], and urinary incontinence [108, 109]. The correlation between CC and urinary incontinence has been described: this condition typically manifests as leaks occurring during coughing episodes [110]. Moreover, an analysis of emotions associated with CC using the Discrete Emotions Questionnaire revealed that anger and anxiety are emotions frequently associated with CC, particularly in women [111].
The initial diagnostic steps for CC involve taking a thorough medical history, which includes inquiries about the cough's history; cardiac, gastrointestinal and nasal symptoms; angiotensin-converting enzyme inhibitor use; and exposure to smoke or environmental irritants. Diagnostic tests such as spirometry with a bronchodilator test, methacholine challenge test, chest radiography and a complete blood count are recommended when CC is suspected [110, 112]. The initial approach focuses on identifying and addressing potential triggers and treating any underlying conditions that may be causing the cough; if it persists, further evaluations should be undertaken to determine the presence of unexplained or refractory CC. In such cases, a trial with neuromodulator therapy is suggested [110, 112].
Treatment of chronic cough
Managing CC can be a complex task, necessitating a personalised treatment approach. The latest ERS guidelines emphasise the importance of identifying a likely cause for CC and implementing tailored therapy targeted at either resolving the underlying pathology or addressing the pathological mechanisms driving it [110].
CC remains a prevalent issue with limited treatment options, and discussions surrounding its management have been extensive. There are currently no approved therapies for refractory CC, except in Japan and Switzerland, where gefapixant is approved [113]. Table 1 summarises current and future therapeutic opportunities. The safety and efficacy of gefapixant, a selective inhibitor of purinoceptor 3 (P2X3), was tested in patients with either short (<1 year) or long (>1 year) duration of refractory/unexplained CC [114]. The analysis revealed that regardless of the duration of CC, there was a similar and favourable response to gefapixant treatment.
TABLE 1.
Current and future therapeutic options in chronic cough
| Target receptor | Type of receptor | Drug name | Mechanism of action |
| P2X3 | Peripheral receptor | Gefapixant#, eliapixant, sivopixant, camlipixant | Antagonist |
| NaV1.7 | Peripheral receptor | NTX-1175 | Blockers |
| TRPM8 | Peripheral receptor | AX-8 | Antagonist |
| NK-1 | Central receptor | Aprepitant, orvepitant | Antagonist |
P2X3: purinoceptor 3; TRPM8: transient receptor potential cation channel subfamily M (melastatin) member 8; NK-1: neurokinin-1 receptor. #: phase 3 trial completed.
Camlipixant is a selective inhibitor of P2X3 receptors implicated in the neuronal hyperreflexia of the airways [115]. The SOOTHE study indicates that camlipixant is effective in reducing cough frequency over a 24-h period and in enhancing the quality of life of CC patients. The most common adverse events reported are related to taste disturbances [116].
Neurokinin-1 and N-methyl-d-aspartic acid, as well as peripheral receptors like P2X3, NaV and transient receptor potential cation channel subfamily M (melastatin) member 8, play a role in the hyperreflexia observed in CC. Research into modulating these receptors could provide new avenues for the treatment of CC (table 1).
There is some debate over the actual impact of the placebo effect in RCTs for CC. It is noteworthy that RCT arms often demonstrate significant improvement in CC symptoms, prompting the suggestion to include a “no treatment group” in future studies to better understand this phenomenon.
Take-home messages
CC is a complex condition associated with a spectrum of conditions that significantly reduce quality of life. Promising novel treatments, such as P2X3 receptor antagonists, could be a valid therapeutic option to reduce 24-h cough frequency and to improve quality of life.
Bronchiectasis
Non-cystic fibrosis bronchiectasis
At the ERS Congress 2023, four sessions were centred around bronchiectasis, with 32 presentations, 109 abstracts and 97 posters. Bronchiectasis is defined as a clinical syndrome characterised by abnormal bronchial dilatation that can be identified on high-resolution CT [117]. From the clinical perspective, the patient presents with productive cough and recurrent lower airway infections [118]. Most of the research is focused on registries and cohorts to learn more about the aetiology, diagnostics, treatments and outcomes of this not-uncommon disease.
Novel findings from EMBARC
Some of the posters presented at ERS were based on data from EMBARC, the European Bronchiectasis Registry, and the cohorts derived from this registry. A study of the metabolomic profile of patients with bronchiectasis showed that peripheral blood eosinophils have differences in multiple pathways, including metabolism of purine, nicotinate and nicotinamide [119].
There is also interesting information about the genetic diversity of Pseudomonas aeruginosa virulence factors in bronchiectasis, using samples from patients in the EMBARC registry. The bacterial growth and biofilm formation are influenced in vivo by different regulatory genes. The effect of these variants on virulence remains to be tested in vivo [120].
Pathophysiology of bronchiectasis
Regarding the pathogenesis of bronchiectasis, Cole's vicious cycle is an interesting theory. This hypothesis proposes that the host-mediated inflammatory response to any foreign material and the bacterium in the airway causes tissue damage resulting in bronchiectasis. It also demonstrates that airway damage contributes to abnormal mucus clearance and further bacterial colonisation [121].
Key pathogens were identified and compared in trials like EMBRACE, BAT, BLESS and the NCFB US Bronchiectasis Research Registry [122–125]. In Europe, the main pathogen detected was Haemophilus influenzae, while in the USA it was nontuberculous mycobacteria like Mycobacterium avium. Therefore, the pathogens isolated from patients with bronchiectasis may depend on the geographic area of residence and should be considered with respect to personalised treatment.
Diagnostics and outcomes in bronchiectasis
An interesting symposium regarding the controversial issues of bronchiectasis from the perspective of infection and inflammation caught much attention at the congress. It highlighted that most research to date is focused on bronchial infection as the main risk factor for bronchiectasis. However, approximately 38% of patients with bronchiectasis do not have a bronchial infection as a risk factor of the disease [117]. Research presented at ERS Congress 2023 highlighted the importance of understanding the inflammatory endotypes present within the disease, because chronic airway inflammation is a pertinent feature [126].
The aetiology of bronchiectasis has been explored in the EMBARC study, with the roles of inflammation, infection and impaired mucociliary clearance characterised in the early stages of the disease [127]. It has been suggested that most bronchiectasis patients or those in a pre-bronchiectasis state have clear risk factors for developing the airway disease, e.g. genetic predisposition or systemic inflammatory conditions like asthma, allergic bronchopulmonary aspergillosis and rheumatoid arthritis [127, 128].
Treatment of bronchiectasis
ICS are an option for anti-inflammatory therapy for patients with airway diseases like asthma and COPD. ERS guidelines do not suggest the use of this treatment for bronchiectasis patients [129]. Some research suggests that ICS could reduce exacerbation frequency in patients with “pure bronchiectasis” and eosinophilia (blood eosinophil count ≥400 cells·µL−1) [130]. There is also biological plausibility in using macrolides in respiratory disease, such as azithromycin, owing to their anti-inflammatory effects [131]. It was demonstrated that dipeptidyl peptidase 1 inhibitors reduce neutrophil serine protease activity and this was associated with improvements in clinical outcomes such as exacerbation frequency [132].
Take-home messages
As eloquently highlighted by T. Welte, bronchiectasis has become one of the hot topics of respiratory medicine this decade. Most research to date has focused on registries and cohorts, allowing promising advances in the understanding of the disease, including the importance of genetic diversity and bronchial infections in bronchiectasis aetiology.
Concluding remarks
An increased number of presentations in the field of airway diseases were communicated at the ERS International Congress 2023. Advances in several novel treatments were reported and increased understanding of the pathogenetic mechanisms of airways diseases were described by several groups from around the world. Further important research in airways disease will be presented at the 2024 ERS International Congress in Vienna (7–11 September).
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: A. Bossios reports grants from the Swedish Heart–Lung Foundation and lecture honoraria to his institution, not related to this manuscript.
Conflict of interest: G-J. Braunstahl reports honoraria for lectures and consultancy from GlaxoSmithKline, AstraZeneca, Novartis and Sanofi Genzyme, as well as research grants from Sanofi Genzyme, GlaxoSmithKline and AstraZeneca, not related to this manuscript.
Conflict of interest: L.H. Conemans reports honoraria from GlaxoSmithKline, Sanofi, AstraZeneca and Vertex, as well as travel support from TEVA and Novartis.
Conflict of interest: A.G. Mathioudakis reports lecture fees from GlaxoSmithKline.
Conflict of interest: P. Pobeha reports consulting fees from Pfizer; consulting fees and honoraria from Chiesi, Angeliny and Boehringer Ingelheim; and honoraria from Berlin-Chemie.
Conflict of interest: F.L.M. Ricciardolo reports grants from Chiesi, Sanofi and GlaxoSmithKline; consulting fees and honoraria from Sanofi Novartis and GlaxoSmithKline; and personal fees from AstraZeneca, Sanofi, GlaxoSmithKline and Novartis.
Conflict of interest: F. Schleich reports grants, consulting fees and honoraria from Chiesi, AstraZeneca, GlaxoSmithKline and Novartis, as well as grants and consulting fees from TEVA.
Conflict of interest: R.J. Snelgrove reports grants from The Wellcome Trust, Rosetrees Trust and The Stoneygate Trust.
Conflict of interest: F. Trinkmann reports grants from AstraZeneca, Bayer Boehringer Ingelheim, Chiesi, Novartis, Roche, BMBF, DZL, Markedsmodningsfonden and E+H Knorr Stiftung; consulting fees and honoraria from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Fisher and Paykel, GlaxoSmithKline, Janssen-Cilag, Merck Healthcare, Novartis, Omron, OM-Pharma, Roche, Sanofi, Aventis and Thorasys; and travel support from AstraZeneca, Actelion, Bayer, Berlin-Chemie, Boehringer Ingelheim, Chiesi, Mundipharma, Novartis, Pfizer and TEVA.
Conflict of interest: L. Uller reports lecture fees from AstraZeneca.
Conflict of interest: L. Bergantini, J. Baker, F. Lombardi, L.P. Prada Romero and A. Beech have no conflicts of interest.
Support statement: J. Baker, A.G. Mathioudakis and A. Beech were supported by the National Institute for Health and Care Research (NIHR) Manchester Biomedical Research Centre (NIHR203308). A.G. Mathioudakis was supported by an NIHR Clinical Lectureship in Respiratory Medicine. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. R.J. Snelgrove is a Wellcome Trust Senior Research Fellow in Basic Biomedical Sciences (209458/Z/17/Z). A. Bossios is supported by the Swedish Heart–Lung Foundation (20220478 and 20210434).
References
- 1.Schleich F, Bikov A, Mathioudakis AG, et al. . Research highlights from the 2018 European Respiratory Society International Congress: airway disease. ERJ Open Res 2019; 5: 00225-2018. doi: 10.1183/23120541.00225-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahousse L, Bahmer T, Cuevas-Ocaña S, et al. ERS International Congress, Madrid, 2019: highlights from the Airway Diseases, Asthma and COPD Assembly. ERJ Open Res 2020; 6: 00341-2019. doi: 10.1183/23120541.00341-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beech A, Portacci A, Herrero-Cortina B, et al. . ERS International Congress 2022: highlights from the Airway Diseases Assembly. ERJ Open Res 2023; 9: 00034-2023. doi: 10.1183/23120541.00034-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolz D, Mkorombindo T, Schumann DM, et al. . Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 2022; 400: 921–972. doi: 10.1016/S0140-6736(22)01273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinkmann F, Watz H, Herth FJF. Why do we still cling to spirometry for assessing small airway function? Eur Respir J 2020; 56: 2001071. doi: 10.1183/13993003.01071-2020 [DOI] [PubMed] [Google Scholar]
- 6.Çolak Y, Lange P, Vestbo J, et al. . Pre-COPD in young adults and COPD later in life: a population-based cross-cohort comparison study. Eur Respir J 2023; 62: Suppl. 67, PA1013. doi: 10.1183/13993003.congress-2023.PA1013 [DOI] [Google Scholar]
- 7.Rapsomaniki E, Hughes R, Make B, et al. . Long-term outcomes in patients with pre-COPD or PRISm in NOVELTY. Eur Respir J 2023; 62: Suppl. 67, OA4936. doi: 10.1183/13993003.congress-2023.OA4936 [DOI] [Google Scholar]
- 8.Ercan S, Alpaydin AO, Canturk A, et al. . The comparison of quantitative CT features of COPD and pre-COPD: results from a real-life study. Eur Respir J 2023; 62: Suppl. 67, PA2282. doi: 10.1183/13993003.congress-2023.PA2282 [DOI] [Google Scholar]
- 9.Virdee S, Hogg J, Boudreau J, et al. . Quantitative computed tomography assessment of pulmonary structure in cannabis smokers. Eur Respir J 2023; 62: Suppl. 67, PA2285. doi: 10.1183/13993003.congress-2023.PA2285 [DOI] [Google Scholar]
- 10.Kostorz-Nosal S, Jastrzębski D, Błach A, et al. . Window of opportunity for respiratory oscillometry: a review of recent research. Respir Physiol Neurobiol 2023; 316: 104135. doi: 10.1016/j.resp.2023.104135 [DOI] [PubMed] [Google Scholar]
- 11.Valach C, Veneroni C, Wouters E, et al. . Oscillometry in the assessment of early smoking-induced airway changes. Eur Respir J 2023; 62: Suppl. 67, OA4236. doi: 10.1183/13993003.congress-2023.OA4236 [DOI] [Google Scholar]
- 12.Franzen KF, Buchwald I, Hauck A, et al. . Impact of heated tobacco products, e-cigarettes, and combustible cigarettes on small airways and arterial stiffness. Eur Respir J 2023; 62: Suppl. 67, PA5316. doi: 10.1183/13993003.congress-2023.PA5316 [DOI] [Google Scholar]
- 13.Meldrum OW, Donaldson GC, Narayana JK, et al. . Mucus, microbiomes, and lung function decline in COPD. Eur Respir J 2023; 62: Suppl. 67, PA2193. doi: 10.1183/13993003.congress-2023.PA2193 [DOI] [Google Scholar]
- 14.Fang H, Liu Y, Yang Q, et al. . Prognostic biomarkers based on proteomic technology in COPD: a recent review. COPD 2023; 18: 1353–1365. doi: 10.2147/COPD.S410387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldar K, Choy DF, Yosuf A, et al. . Heterogeneity of the lung microbiome influences response to astegolimab, an anti ST2, in COPD. Eur Respir J 2023; 62: Suppl. 67, PA1291. doi: 10.1183/13993003.congress-2023.PA1291 [DOI] [Google Scholar]
- 16.Ferguson GT, Rabe KF, Martinez FJ, et al. . Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med 2018; 6: 747–758. doi: 10.1016/S2213-2600(18)30327-8 [DOI] [PubMed] [Google Scholar]
- 17.Rothnie KJ, Müllerová H, Smeeth L, et al. . Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 464–471. doi: 10.1164/rccm.201710-2029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev 2010; 19: 113–118. doi: 10.1183/09059180.00002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter V, Skinner D, Chan JSK, et al. . COPD exacerbations during and beyond the COVID-19 pandemic in the UK. Eur Respir J 2023; 62: Suppl. 67, PA1014. doi: 10.1183/13993003.congress-2023.PA1014 [DOI] [Google Scholar]
- 20.Lipson DA, Barnhart F, Brealey N, et al. . Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018; 378: 1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 21.Rabe KF, Martinez FJ, Ferguson GT, et al. . Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med 2020; 383: 35–48. doi: 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 22.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med 2023; 11: 18. doi: 10.1016/S2213-2600(22)00494-5 [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Bafadhel M, Bowen K, et al. . Step up to ICS/LAMA/LABA versus switch to LAMA/LABA in patients with COPD on ICS/LABA: post hoc analysis of KRONOS. Eur Respir J 2023; 62: Suppl. 67, PA4687. doi: 10.1183/13993003.congress-2023.PA4687 [DOI] [Google Scholar]
- 24.Stolz D, Papakonstantinou E, Pascarella M, et al. . Airway smooth muscle area to predict steroid responsiveness in COPD patients receiving triple therapy (HISTORIC): a randomised, placebo-controlled, double-blind, investigator-initiated trial. Eur Respir J 2023; 62: 2300218. doi: 10.1183/13993003.00218-2023 [DOI] [PubMed] [Google Scholar]
- 25.Cardoso J, Ferreira AJ, Guimarães M, et al. . Treatable traits in COPD – a proposed approach. Int J Chron Obstruct Pulmon Dis 2021; 16: 3167–3182. doi: 10.2147/COPD.S330817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelmeier C, Simons S, Garbe E, et al. . Increased risk of severe cardiovascular events following exacerbations of COPD: a multi-database cohort study. Eur Respir J 2023; 62: Suppl. 67, PA3013. doi: 10.1183/13993003.congress-2023.PA3013 [DOI] [Google Scholar]
- 27.Wade R, Martinez FJ, Criner GJ, et al. . ECG-derived risk factors for adverse cardiopulmonary outcomes in COPD patients: IMPACT post hoc analysis. Eur Respir J 2023; 62: Suppl. 67, OA4929. doi: 10.1183/13993003.congress-2023.OA4929 [DOI] [Google Scholar]
- 28.Bhatt SP, Rabe KF, Hanania NA, et al. . Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med 2023; 389: 205–214. doi: 10.1056/NEJMoa2303951 [DOI] [PubMed] [Google Scholar]
- 29.Anzueto A, Barjaktarevic IZ, Siler TM, et al. . Ensifentrine, a novel phosphodiesterase 3 and 4 inhibitor for the treatment of chronic obstructive pulmonary disease: randomized, double-blind, placebo-controlled, multicenter phase III trials (the ENHANCE trials). Am J Respir Crit Care Med 2023; 208: 406–416. doi: 10.1164/rccm.202306-0944OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watz H, Rheault T, Bengtsson T, et al. . Inhaled ensifentrine, decreased healthcare research utilization and reduced moderate exacerbation rate and risk in COPD over 24 weeks. Eur Respir J 2023; 62: Suppl. 67, OA2602. doi: 10.1183/13993003.congress-2023.OA2602 [DOI] [Google Scholar]
- 31.Rabe KF, Celli BR, Wechsler ME, et al. . Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 32.Kim RY, Oliver BG, Wark PAB, et al. . COPD exacerbations: targeting IL-33 as a new therapy. Lancet Respir Med 2021; 9: 1213–1214. doi: 10.1016/S2213-2600(21)00182-X [DOI] [PubMed] [Google Scholar]
- 33.Booth S, Cunoosamy D, Vestbo J, et al. . Relationship between IL-33 expression and epithelial remodelling in COPD small airways. Eur Respir J 2023; 62: Suppl. 67, OA4298. doi: 10.1183/13993003.congress-2023.OA4298 [DOI] [Google Scholar]
- 34.Brock J, Herth F, Darwiche K, et al. . Bronchial rheoplasty for chronic bronchitis: 6-month results from the European Registry Study. Eur Respir J 2023; 62: Suppl. 67, OA2594. doi: 10.1183/13993003.congress-2023.OA2594 [DOI] [Google Scholar]
- 35.Kang J, Jung JY, Ji HW, et al. . Lifestyle interventions to reduce particulate matter exposure in patients with COPD. Eur Respir J 2023; 62: Suppl. 67, OA2606. doi: 10.1183/13993003.congress-2023.OA2606 [DOI] [Google Scholar]
- 36.Bernasconi S, Angelucci A, Rossi A, et al. . A wearable system for personal air pollution exposure: a walk-about in Milan. Eur Respir J 2023; 62: Suppl. 67, PA2908. doi: 10.1183/13993003.congress-2023.PA2908 [DOI] [Google Scholar]
- 37.Rony F, Klein J, Valent A, et al. . Preserving treatment for asthma and COPD patients while minimizing carbon footprint: relative lung bioavailability and total systemic exposure of a medium dose formulation of BDP/FF/GB MDI with novel propellant. Eur Respir J 2023; 62: Suppl. 67, PA2388. doi: 10.1183/13993003.congress-2023.PA2388 [DOI] [Google Scholar]
- 38.Frias DP, Vieira GL, Smelan J, et al. . Anthracosis particulate matter causes changes in macrophages inflammatory response and in expression of genes related to xenobiotics metabolism in co-culture of macrophages with BEAS-2B airway cells. Eur Respir J 2023; 62: Suppl. 67, PA4047. doi: 10.1183/13993003.congress-2023.PA4047 [DOI] [Google Scholar]
- 39.Baker J, Booth B, Dungwa J, et al. . Alveolar macrophage carbon is associated with COPD severity. Eur Respir J 2023; 62: Suppl. 67, PA4065. doi: 10.1183/13993003.congress-2023.PA4065 [DOI] [Google Scholar]
- 40.Lee K-Y, Chen K-Y, Wu S-M, et al. . The clinical and pathogenetic roles of ITIH4 in particulate matter-related emphysema. Eur Respir J 2023; 62: Suppl. 67, PA1264. doi: 10.1183/13993003.congress-2023.OPA1264 [DOI] [Google Scholar]
- 41.Cortesi EE, Antoranz A, Geudens V, et al. . Single-cell spatial proteomics depicts novel dynamics involving senescent progenitors in fibrotic lesions in COPD and IPF. Eur Respir J 2023; 62: Suppl. 67, OA1772. doi: 10.1183/13993003.congress-2023.OA1772 [DOI] [Google Scholar]
- 42.Lahmar MZ, Ahmed E, Vachier I, et al. . Early COPD and Hedgehog interacting protein (HHIP) polymorphisms. Eur Respir J 2023; 62: Suppl. 67, PA5206. doi: 10.1183/13993003.congress-2023.PA5206 [DOI] [Google Scholar]
- 43.Ntenti C, Goulas A, Papakonstantinou E, et al. . FKBP5 rs4713916: a potential genetic predictor of differential response to triple therapy among COPD patients. Eur Respir J 2023; 62: Suppl. 67, PA5200. doi: 10.1183/13993003.congress-2023.PA5200 [DOI] [Google Scholar]
- 44.Baker J, McCrae C, Higham A, et al. . Neutrophilic inflammation associates with dysregulated iron metabolism in COPD. Eur Respir J 2023; 62: Suppl. 67, PA3051. doi: 10.1183/13993003.congress-2023.PA3051 [DOI] [Google Scholar]
- 45.Ntenti C, Goulas A, Grize L, et al. . Genetic variants in the iron responsive element binding protein 2 (IREB2) are associated with spirometry parameters and cough prevalence. Eur Respir J 2023; 62: Suppl. 67, PA5198. doi: 10.1183/13993003.congress-2023.PA5198 [DOI] [Google Scholar]
- 46.Park JW, Son ES, Jeong SH. MicroRNA-149-3p regulates cigarette smoke extract-induced autophagy and cellular senescence in human small airway epithelial cells (NHBE) cultured under the air–liquid interface (ALI). Eur Respir J 2023; 62: Suppl. 67, PA5199. doi: 10.1183/13993003.congress-2023.PA5199 [DOI] [Google Scholar]
- 47.Baker J, Kimura G, Nishimoto Y,et al. . The senolytic effect of dasatinib and quercetin on cellular senescence in COPD in vitro and in vivo models. Eur Respir J 2023; 62: Suppl. 67, PA4046. doi: 10.1183/13993003.congress-2023.PA4046 [DOI] [Google Scholar]
- 48.Foisset F, Lehalle C, Nasri A, et al. Development of a bronchial epithelium with a sensory innervation both derived from induced pluripotent stem cells. Eur Respir J 2023; 62: Suppl. 67, OA892. doi: 10.1183/13993003.congress-2023.OA892 [DOI] [Google Scholar]
- 49.Nadaud M, Bourdais C, Fort-Petit A, et al. . Benefits of bronchial epithelial repair by iPSC in COPD. Eur Respir J 2023; 62: Suppl. 67, PA5240. doi: 10.1183/13993003.congress-2023.PA5240 [DOI] [Google Scholar]
- 50.Wang Y, Meng Z, Ming L, et al. , Autologous transplantation of P63+ lung progenitor cells for chronic obstructive pulmonary disease therapy. Eur Respir J 2023; 62: Suppl. 67, OA4297. doi: 10.1183/13993003.congress-2023.OA4297 [DOI] [PubMed] [Google Scholar]
- 51.Calzetta L, Aiello M, Frizzelli A, et al. . Stem cell-based regenerative therapy and derived products in COPD: a systematic review and meta-analysis. Cells 2022; 11: 1797. doi: 10.3390/cells11111797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guarnier LP, Moro LG, Lívero FAR, et al. Regenerative and translational medicine in COPD: hype and hope. Eur Respir Rev 2023; 32: 220223. doi: 10.1183/16000617.0223-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez N, James V, Onion D, et al. . Extracellular vesicles and chronic obstructive pulmonary disease (COPD): a systematic review. Respir Res 2022; 23: 82. doi: 10.1186/s12931-022-01984-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng M, Hou G. Small extracellular vesicles mediated pathological communications between dysfunctional airway epithelium cells and skeletal muscle cells as a novel mechanism induced skeletal muscle dysfunction in COPD. Eur Respir J 2023; 62: Suppl. 67, PA4040. doi: 10.1183/13993003.congress-2023.PA4040 [DOI] [Google Scholar]
- 55.Fujimoto S, Fujita Y, Watanabe N, et al. . Lipofibroblast derived-extracellular vesicles attenuate cigarette smoke-induced lung injury. Eur Respir J 2023; 62: Suppl. 67, PA5238. doi: 10.1183/13993003.congress-2023.PA5238 [DOI] [Google Scholar]
- 56.Rappaport SM. Biomarkers intersect with the exposome. Biomarkers 2012; 17: 483–489. 10.3109/1354750X.2012.691553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 2005; 14: 1847–1850. 10.1158/1055-9965.EPI-05-0456 [DOI] [PubMed] [Google Scholar]
- 58.Wild CP. The exposome: from concept to utility. Int J Epidemiol 2012; 41: 24–32. 10.1093/ije/dyr236 [DOI] [PubMed] [Google Scholar]
- 59.Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax 2014; 69: 876–878. [DOI] [PubMed] [Google Scholar]
- 60.Woodruff PG, Agusti A, Roche N, et al. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet 2015; 385: 1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agustí A, Melén E, DeMeo DL, et al. . Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene–environment interactions across the lifespan. Lancet Respir Med 2022; 10: 512–524. doi: 10.1016/S2213-2600(21)00555-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuks P, Karp T, Hartman J, et al. . Cluster analysis to identify distinct clinical asthma phenotypes within ATLANTIS. Eur Respir J 2023; 62: Suppl. 67, OA1525. doi: 10.1183/13993003.congress-2023.OA1525 [DOI] [Google Scholar]
- 63.Clemeno FA, Bell A, Richardson M, et al. . Associations of imaging biomarkers with physiological markers of small airways dysfunction in asthma in the ATLANTIS study. Eur Respir J 2023; 62: Suppl. 67, OA1529. doi: 10.1183/13993003.congress-2023.OA1529 [DOI] [Google Scholar]
- 64.Dierick B, Achterbosch M, Eikholt A, et al. . Electronic monitoring with a digital smart spacer to support personalized inhaler use education in patients with asthma: the randomized controlled OUTERSPACE feasibility trial. Eur Respir J 2023; 62: Suppl. 67, OA3181. doi: 10.1183/13993003.congress-2023.OA3181 [DOI] [PubMed] [Google Scholar]
- 65.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2023. Available from: http://ginasthma.org/ [Google Scholar]
- 66.Louis R, Harrison TW, Chanez P, et al. . Severe asthma standard-of-care background medication reduction with benralizumab: ANDHI in practice substudy. J Allergy Clin Immunol Pract 2023; 11: 1759–1770. doi: 10.1016/j.jaip.2023.03.009 [DOI] [PubMed] [Google Scholar]
- 67.Jackson DJ, Heaney LG, Humbert M, et al. SHAMAL: reduction of maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab: a randomised phase 4 study. Eur Respir J 2023; 62: Suppl. 67, RCT798. 10.1183/13993003.congress-2023.RCT798 [DOI] [PubMed] [Google Scholar]
- 68.Jackson DJ, Pavord ID, Christian Virchow J, et al. . The proportion of patients achieving low biomarker levels with tezepelumab treatment in the phase 3 NAVIGATOR study. Eur Respir J 2023; 62: Suppl. 67, OA1418. doi: 10.1183/13993003.congress-2023.OA1418 [DOI] [Google Scholar]
- 69.Stolz D, Lugogo N, Lawson K, et al. . Inhaled corticosteroid dose reduction with tezepelumab in patients with severe, uncontrolled asthma over 2 years. Eur Respir J 2023; 62: Suppl. 67, PA4721. doi: 10.1183/13993003.congress-2023.PA4721 [DOI] [Google Scholar]
- 70.Smidt-Hansen T, Andersen HH, Pedersen LM, et al. . Patients with severe asthma reducing ICS treatment during treatment with biologicals do not have more frequent exacerbations. Eur Respir J 2023; 62: Suppl. 67, PA636. doi: 10.1183/13993003.congress-2023.PA636 [DOI] [Google Scholar]
- 71.Domvri K, Tsiouprou I, Bakakos P, et al. Effect of mepolizumab on airways remodeling in patients with late-onset severe eosinophilic asthma and fixed obstruction (preliminary data of the MESILICO study). Eur Respir J 2023; 62: Suppl. 67, OA3152. 10.1183/13993003.congress-2023.OA3152 [DOI] [Google Scholar]
- 72.Shafiek H, Iglesias A, Mosteiro M, et al. . Bronchial submucosal eosinophils predict clinical remission better than blood eosinophils and FENO in severe uncontrolled asthma treated with biological therapy. Eur Respir J 2023; 62: Suppl. 67, OA1413. doi: 10.1183/13993003.congress-2023.OA1413 [DOI] [Google Scholar]
- 73.Tran T, Le TT, Scelo G, et al. . Real world biologic treatment response in severe asthma: an analysis of the International Severe Asthma Registry (ISAR). Eur Respir J 2023; 62: Suppl. 67, PA1894. doi: 10.1183/13993003.congress-2023.PA1894 [DOI] [Google Scholar]
- 74.Wechsler ME, Brusselle G, Virchow JC, et al. On-treatment clinical remission with tezepelumab in patients with severe, uncontrolled asthma in the phase 3 DESTINATION study. Eur Respir J 2023; 62: Suppl. 67, PA4722. 10.1183/13993003.congress-2023.PA4722 [DOI] [Google Scholar]
- 75.Brightling CE, Jackson D, Kotalik A, et al. . Biomarkers and clinical outcomes after cessation of tezepelumab after 2 years of treatment (DESTINATION). Eur Respir J 2023; 62: Suppl. 67, OA1415. doi: 10.1183/13993003.congress-2023.OA1415 [DOI] [Google Scholar]
- 76.Chaudhuri R, Liu MC, Bagnasco D, et al. . Real-world outcomes with mepolizumab in patients with severe asthma and comorbid anxiety/depression: REALITI-A at 2 years. Eur Respir J 2023; 62: Suppl. 67, PA4757. doi: 10.1183/13993003.congress-2023.PA4757 [DOI] [Google Scholar]
- 77.García Rivero JL, Bañas Conejero D. Multicentric real life experience of mepolizumab in bronchiectasis concomitant to severe asthma. Eur Respir J 2023; 62: Suppl. 67, PA1905. doi: 10.1183/13993003.congress-2023.PA1905 [DOI] [Google Scholar]
- 78.ten Have L, Visser E, Sont JK, et al. . Long-term weight changes after starting anti-interleukin-5/5Ra biologics in severe asthma. Eur Respir J 2023; 62: Suppl. 67, PA1896. doi: 10.1183/13993003.congress-2023.PA1896 [DOI] [Google Scholar]
- 79.O'Carroll M, Huetsch J, Hamilton J, et al. . A first-in-human study of ARO-RAGE, an RNAi therapy designed to silence pulmonary RAGE expression. Eur Respir J 2023; 62: Suppl. 67, OA2601. doi: 10.1183/13993003.congress-2023.OA2601 [DOI] [Google Scholar]
- 80.Diamant Z, Lee Y, Yang W, et al. . Effect of MMP-12 inhibitor, adermastat-(FP-025), on allergen-induced late response in asthmatic subjects. Eur Respir J 2023; 62: Suppl. 67, OA2494. doi: 10.1183/13993003.congress-2023.OA2494 [DOI] [Google Scholar]
- 81.Wan Y, Gai J, Zhu M, et al. . Phase I safety and tolerance study of the inhalable anti IL-4Rα single domain antibody, LQ036, demonstrates promising clinical profile to treat asthma. Eur Respir J 2023; 62: Suppl. 67, OA1412. doi: 10.1183/13993003.congress-2023.OA1412 [DOI] [Google Scholar]
- 82.Deiteren A, Krupka E, Imberdis K, et al. . Early improvement in asthma small airway dysfunction after one dose of SAR443765, a novel bispecific anti-thymic stromal lymphopoietin/anti-IL-13 nanobody molecule. Eur Respir J 2023; 62: Suppl. 67, OA4296. doi: 10.1183/13993003.congress-2023.OA4296 [DOI] [Google Scholar]
- 83.Pisano AR, Fragni D, Allen A, et al. . The inhaled PDE4 inhibitor tanimilast shows efficacy in both Th2 and non-Th2 murine models of asthma. Eur Respir J 2023; 62: Suppl. 67, OA4305. doi: 10.1183/13993003.congress-2023.OA4305 [DOI] [Google Scholar]
- 84.El-Husseini Z, Khalenkow D, Lan A, et al. . SIL-6Rα amplified IL-6 signaling in bronchial epithelial cells defines a subgroup of asthma patients low in sputum eosinophils. Eur Respir J 2023; 62: Suppl. 67, OA4300. doi: 10.1183/13993003.congress-2023.OA4300 [DOI] [Google Scholar]
- 85.Oliver A, Madissoon E, Sungnak W, et al. . Decoding the epithelial-T helper cell signalling axis in human asthmatic airways one cell at a time. Eur Respir J 2023; 62: Suppl. 67, OA774. doi: 10.1183/13993003.congress-2023.OA774 [DOI] [Google Scholar]
- 86.Peters U, Dixon A, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018; 141: 1169–1179. doi: 10.1016/j.jaci.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tibbitt C, Björkander S, Maier P, et al. . Innate lymphoid cells type 2 and CD8+ T cells are perturbed in overweight and obese individuals with asthma. Eur Respir J 2023; 62: Suppl. 67, OA2502. doi: 10.1183/13993003.congress-2023.OA2502 [DOI] [PubMed] [Google Scholar]
- 88.Kabata H, Irie M, Baba R, et al. . Zinc plays an essential role in type 2 cytokine production from ILC2s in asthma. Eur Respir J 2023; 62: Suppl. 67, OA2501. doi: 10.1183/13993003.congress-2023.OA2501 [DOI] [Google Scholar]
- 89.Miguéns-Suárez P, Vázquez-Mera S, Martelo-Vidal L, et al. . Mepolizumab alters the phenotype and activation status of peripheral blood eosinophils in patients with severe eosinophilic asthma. Eur Respir J 2023; 62: Suppl. 67, OA4301. doi: 10.1183/13993003.congress-2023.OA4301 [DOI] [Google Scholar]
- 90.Bergantini L, d'Alessandro M, Pianigiani T, et al. . Benralizumab affects NK cell maturation and proliferation in severe asthmatic patients. Clin Immunol 2023; 253: 109680. doi: 10.1016/j.clim.2023.109680 [DOI] [PubMed] [Google Scholar]
- 91.Ghanizada M, Millgren SM, Said NM, et al. . Effect of in vitro azithromycin treatment on epithelial antiviral immunity in eosinophilic and non-eosinophilic asthma phenotypes. Eur Respir J 2023; 62: Suppl. 67, OA4303. doi: 10.1183/13993003.congress-2023.OA4303 [DOI] [Google Scholar]
- 92.Ricciardolo FLM, Sprio AE, Baroso A, et al. . Characterization of T2-low and T2-high asthma phenotypes in real-life. Biomedicines 2021; 9: 1684. doi: 10.3390/biomedicines9111684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eastwood MC, Busby J, Kolmert J, et al. . Urinary eicosanoids – novel biomarkers in T2-low severe asthma. Eur Respir J 2023; 62: Suppl. 67, OA1531. doi: 10.1183/13993003.congress-2023.OA1531 [DOI] [Google Scholar]
- 94.Kolmert J, Gómez C, Balgoma D, et al. . Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterized by type 2 inflammation. A clinical observational study. Am J Respir Crit Care Med 2021; 203: 37–53. doi: 10.1164/rccm.201909-1869OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wheelock C, Chen Y, Checa A, et al. . Identification of neutrophilic asthma via the ratio of sphingolipids to glucocorticoids. Eur Respir J 2023; 62: Suppl. 67, OA1532. doi: 10.1183/13993003.congress-2023.OA1532 [DOI] [Google Scholar]
- 96.Liu L, Zhang X, Liu Y, et al. . Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir Res 2019; 20: 95. doi: 10.1186/s12931-019-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki Y, Saito J, Rikimaru M, et al. . Serum YKL-40 as a biomarker for predicting loss of lung function and neutrophilic airway inflammation in asthma, COPD and asthma–COPD overlap. Eur Respir J 2023; 62: Suppl. 67, OA3148. doi: 10.1183/13993003.congress-2023.OA3148 [DOI] [Google Scholar]
- 98.De Volder J, Bontinck A, De Grove K, et al. . Neutrophilic responses in a subacute and chronic mouse model of pollutant-aggravated allergic asthma. Eur Respir J 2023; 62: Suppl. 67, OA4211. doi: 10.1183/13993003.congress-2023.OA4211 [DOI] [PubMed] [Google Scholar]
- 99.McGarvey L, Gibson PG. What is chronic cough? Terminology . J Allergy Clin Immunol Pract 2019; 7: 1711–1714. doi: 10.1016/j.jaip.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 100.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008; 371: 1364–1374. doi: 10.1016/S0140-6736(08)60595-4 [DOI] [PubMed] [Google Scholar]
- 101.Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther 2009; 22: 65–70. doi: 10.1016/j.pupt.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 102.Song WJ, Morice AH. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res 2017; 9: 394–402. doi: 10.4168/aair.2017.9.5.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung KF, McGarvey L, Song W-J, et al. . Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022; 8: 45. doi: 10.1038/s41572-022-00370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.López-Cervantes JP, Lønnebotn M, Jogi NO, et al. . The exposome approach in allergies and lung diseases: is it time to define a preconception exposome? Int J Environ Res Public Health 2021; 18: 12684. doi: 10.3390/ijerph182312684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arinze JT, Verhamme KMC, Luik AI, et al. . The interrelatedness of chronic cough and chronic pain. Eur Respir J 2021; 57: 2002651. doi: 10.1183/13993003.02651-2020 [DOI] [PubMed] [Google Scholar]
- 106.Song W-J, Chang Y-S, Faruqi S, et al. . The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 107.Satia I, Mayhew AJ, Sohel N, et al. . Prevalence, incidence and characteristics of chronic cough among adults from the Canadian Longitudinal Study on Aging. ERJ Open Res 2021; 7: 00160-2021. doi: 10.1183/23120541.00160-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang C, Feng Z, Chen Z, et al. . The risk factors for urinary incontinence in female adults with chronic cough. BMC Pulm Med 2022; 22: 276. doi: 10.1186/s12890-022-02069-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.French CL, Irwin RS, Curley FJ, et al. . Impact of chronic cough on quality of life. Arch Intern Med 1998; 158: 1657–1661. doi: 10.1001/archinte.158.15.1657 [DOI] [PubMed] [Google Scholar]
- 110.Morice AH, Millqvist E, Bieksiene K, et al. . ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arismendi E, Puente-Maestu L,Dávila I, et al. . Emotions associated with bouts of cough. A study in patients with refractory/unexplained chronic cough. Eur Respir J 2023; 62: Suppl. 67, PA3822. doi: 10.1183/13993003.congress-2023PA3822 [DOI] [Google Scholar]
- 112.Satia I, Wahab M, Kum E, et al. . Chronic cough: investigations, management, current and future treatments. Can J Respir Crit Care Sleep Med 2021; 5: 404–416. doi: 10.1080/24745332.2021.1979904 [DOI] [Google Scholar]
- 113.Dicpinigaitis PV, Morice AH, Smith JA, et al. . Efficacy and safety of eliapixant in refractory chronic cough: the randomized, placebo-controlled phase 2b PAGANINI study. Lung 2023; 201: 255–266. doi: 10.1007/s00408-023-00621-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McGarvey LP, Birring SS, Morice AH, et al. . Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022; 399: 909–923. doi: 10.1016/S0140-6736(21)02348-5 [DOI] [PubMed] [Google Scholar]
- 115.Birring SS, Passant C, Patel RB, et al. . Chronic tonsillar enlargement and cough: preliminary evidence of a novel and treatable cause of chronic cough. Eur Respir J 2004; 23: 199–201. doi: 10.1183/09031936.03.00066403 [DOI] [PubMed] [Google Scholar]
- 116.McGarvey L, Smith J, Birring SS, et al. . Response in patient-reported cough severity in SOOTHE, a phase 2b trial of camlipixant in refractory chronic cough. Am J Respir Crit Care Med 2023; 207: A2533. doi: 10.1164/ajrccm-conference.2023.207.1_MeetingAbstracts.A2533 [DOI] [Google Scholar]
- 117.King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis 2009; 4: 411–419. doi: 10.2147/COPD.S6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mocelin HT, Fischer GB, Piccini JD, et al. . Surgical treatment of non-cystic fibrosis bronchiectasis in children and adolescents: a review. Paediatr Respir Rev 2023; 46: 57–62. [DOI] [PubMed] [Google Scholar]
- 119.Shoemark A, Giam YH, Gilmour A, et al. . Neutrophil metabolomics in bronchiectasis: data from the EMBARC BRIDGE study. Eur Respir J 2023; 62: Suppl. 67, OA1456. doi: 10.1183/13993003.congress-2023.OA1456 [DOI] [Google Scholar]
- 120.Hull RC, Gilmour A, Mcintosh E, et al. . Genetic diversity of Pseudomonas aeruginosa virulence factors in bronchiectasis. Eur Respir J 2023; 62: Suppl. 67, OA1463. doi: 10.1183/13993003.congress-2023.OA1463 [DOI] [Google Scholar]
- 121.McShane PJ, Naureckas ET, Tino G, et al. . Non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013; 188: 647–656. doi: 10.1164/rccm.201303-0411CI [DOI] [PubMed] [Google Scholar]
- 122.Wong C, Jayaram L, Karalus N, et al. . Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380: 660–667. doi: 10.1016/S0140-6736(12)60953-2 [DOI] [PubMed] [Google Scholar]
- 123.Altenburg J, de Graaff CS, Stienstra Y, et al. . Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis. JAMA 2013; 309: 1251–1259. doi: 10.1001/jama.2013.1937 [DOI] [PubMed] [Google Scholar]
- 124.Serisier DJ, Martin ML, McGuckin MA, et al. . Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis. JAMA 2013; 309: 1260–1267. doi: 10.1001/jama.2013.2290 [DOI] [PubMed] [Google Scholar]
- 125.Aksamit TR, O'Donnell AE, Barker A, et al. . Adult patients with bronchiectasis. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chalmers JD, Polverino E, Crichton ML, et al. . Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med 2023; 11: 637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 127.Chalmers JD, Aliberti S, Altenburg J, et al. . Transforming clinical research and science in bronchiectasis: EMBARC3, a European Respiratory Society Clinical Research Collaboration. Eur Respir J 2023; 61: 2300769. doi: 10.1183/13993003.00769-2023 [DOI] [PubMed] [Google Scholar]
- 128.Chang AB, Grimwood K, Gibson PG, et al. . PBB: definition, mechanisms, and treatment. Lancet Respir Med 2015; 3: 743–744. doi: 10.1016/S2213-2600(15)00243-X [DOI] [PubMed] [Google Scholar]
- 129.Polverino E, Goeminne PC, McDonnell MJ, et al. . European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 130.Pollock J, Polverino E, Crichton ML, et al. . Blood eosinophils, inhaled corticosteroids and exacerbations in bronchiectasis: data from the EMBARC registry. Eur Respir J 2023; 62: Suppl. 67, OA1457. doi: 10.1183/13993003.congress-2023.OA1457 [DOI] [Google Scholar]
- 131.Chalmers JD, Boersma W, Lonergan M, et al. . Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med 2019; 7: 845–854. doi: 10.1016/S2213-2600(19)30191-2 [DOI] [PubMed] [Google Scholar]
- 132.Chalmers JD, Haworth CS, Metersky ML, et al. . Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 2020; 383: 2127–2137. doi: 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]