Abstract
Mucus is a substance made by snails that serves a variety of purposes and is increasingly employed in the medical and cosmetic industries. It includes bioactive compounds with a range of biological characteristics that could be useful in the treatment of particular issues. This study assessed the wound-healing efficiency, antibacterial activity, chemical and mineral composition of Helix aspersa Müller slime. Inductively Coupled Argon Plasma Atomic Emission Spectrometry (ICP-OES) was used for mineral analysis, while Gas chromatography coupled to mass spectrometry (GC–MS) analysis was used for chemical characterization. The findings showed that the H. aspersa Müller slime had inhibitory activity on the test samples. Additionally, it revealed significant healing activity. These findings point to the chemical composition and various biological activities of the H. aspersa Müller slime, which may be related to the animal’s particular functions and be useful for medical applications. Our findings suggest that the H. aspersa Müller slime has biological effects related to antimicrobial activities and wound healing, and they pave the way for a more thorough investigation of its potential therapeutic effects.
Keywords: Activity antibacterial, Chemical composition, H. aspersa Müller, Slime, Wound healing
1. Introduction
Due to the serious public health issue of antibiotic resistance, researchers and medical professionals must find new, efficient antimicrobial agents [1,2]. According to the World Health Organization, Acinetobacter baumannii, Pseudomonas aeruginosa, and several species of the Enterobacteriaceae family are given worldwide priority when it comes to the discovery of novel antimicrobial medicines for these antibiotic-resistant bacteria [3].
Invertebrates only have an innate immune system to fight against invading diseases since they lack the adaptive immune system present in vertebrate species. Given this group of creatures’ exceptional evolutionary success, it is obvious that their innate immune systems are quite powerful in invertebrates [4]. This finding has led to extensive studies of several invertebrate species in recent years. Most of the antimicrobial peptides present in invertebrate hemolymph are effective against a variety of microorganisms [5–7].
For millennia, people have consumed land snails and employed them in a variety of medicinal procedures [8,9]. Snails utilize slime for various functions, serving as a defense mechanism, lubricant, emollient, adhesive, and for hydration [10,11]. This slime has found applications in the human health and cosmetics industries [12–14]. Numerous biological activities, including antibacterial, antioxidant, anti-tyrosinase, and antitumor ones, have been linked to snail slime [13,15,16]. Snail mucus has also been shown to include a wide range of substances, including allantoin, hyaluronic acid, peptides, and proteins [8,10,17].
Additionally, snails have the capacity to secrete huge amounts of mucin, which includes antibacterial proteins and confers some resistance to pathogen infection [18]. Moreover, several studies have demonstrated that bioactive substances obtained from various types of snail mucus may be employed in a broad variety of treatments, including lotions for the treatment of skin abrasions and scars, respiratory conditions, and heartburn [19].
When coupled with several conditions, such as wound length and depth, wound infections, and underlying disorders like diabetes, the wound is a complication that affects individuals, and treatment becomes more crucial [20].
Microorganisms typically reside on the skin’s surface, but when the skin is damaged, they might enter the deeper tissue. Based on factors including infection state, replication status, and tissue load of microorganisms, wound infection is classified as bacterial colonization, local infection, or invasive systemic infection [21].
Burns are one of the world’s major health problems, particularly in developing countries [22]. Microbial infections are considered a major problem for burn patients [23]. According to a recent study by Zlatko [24], certain resistant bacteria strains in burns may lessen the speed at which they recover. Major agents that induce wound infection include Staphylococcus aureus and Pseudomonas aeruginosa [25].
According to studies on the components of snail secretions, Helix aspersa slime includes a significant amount of organic compounds with advantageous and healing qualities for human skin, such as allantoin and glycolic acid [17].
The present research aimed to examine the chemical and mineral composition of the snail H. aspersa Müller slime, as well as its antibacterial activity and healing potential.
2. Material and methods
2.1. Extraction of H. aspersa Müller slime
Extraction was carried out manually by stimulating the pedal glands using a small sterile metal rod pointed at the end. It was not necessary to euthanize the snails, and their handling was carried out in compliance with the principles of animal welfare for scientific research.
2.2. GC–MS analysis of the chemical content
Gas chromatography coupled to mass spectrometry was used to evaluate extracts under the following conditions: Injector port temperature is 250 °C. The starting oven temperature is 40 °C, and the temperature is gradually increased to 8 °C.min−1 for 18 min until it reaches 260 °C. TheBR-5ns FS capillary column (30m×0.25mmID × 0.25m)was utilized. In undivided mode, the injection volume of heliumis 1.0 mLmin−1. The whole analysis took 100 min.
The mass spectrometry detector (MSD) was set to electronic impact ionization mode, with an ionizing energy of 70 eV and an m/z scan range of 50–500.
The temperature of the ion source was 230 °C, and then it quadrupled to 150 °C. With a 3-min solvent delay, the electron multiplier voltage (EM voltage) was held at 1100 V above the self-regulatory limit.
2.3. Total ash
According to ISO 936 [26], total ash from snail samples was evaluated using the gravimetric technique at 550 °C. The results were represented as a percentage (g of total ash per 100 g).
2.4. Mineral analysis
A 1g test sample was used to identify the mineral components in the calcined residue after 16 h at 480 °C. The ash was diluted in strong nitric acid (25% concentration) and then filtered. This extract was used to determine mineral composition. The trace elements studied were determined using Inductively Coupled Argon Plasma Atomic Emission Spectrometry (RF power 1500 W, plasma gas flow rate (Ar) 8 L/min, auxiliary gas flow rate (Ar) 0.2 L/min, Axial View Size, copying and Playback time of 45 min, and copying time of 15 min).
Due to the presence of phosphorus in organic molecules, oxidizing the organic material to release the phosphorus necessitates a digestion or calcination operation. The total phosphorus content of H. aspersa Müller flesh was then colorimetrically measured using the AOAC Method [27].
2.5. Antimicrobial assays
2.5.1. Bacterial strains
The antibacterial properties of H. aspersa Müller slime have been determined against P. aeruginosa have been isolated and identified at Plant, Animal and Agro-Industry Productions Laboratory (PAAP lab), Ibn Tofail University, and stored at −4 °C in glycol. Other reference strains are used in this study (L. monocytogenes (ATCC 7644), S. aureus (ATCC 29213), and E. coli (ATCC 35218)). In order to obtain live and cultivable bacteria, 1 mL of stored bacterial suspension was mixed with 2 mL of nutrient broth.
2.5.2. Antibiotics
The antibiotics used were ampicillin (AMP), cefuroxime sodium (CUS), cefotamine sodium (COF), erythromycin (ETM), netilmicin (NM), piperacillin (PRP) and tetracycline (TC) (Table 1).
Table 1.
Used antibiotics and their families.
| Antibiotics | Antibiotic family |
|---|---|
| Ampicilline (10 μg) | Beta - Lactame |
| Cefuroxime sodium (30 μg) | Cephalosporine |
| Cefotamine sodium (30 μg) | Beta - Lactames 3 rd Generation |
| Erythromycine (25 μg) | Macrolides |
| Netilmicin (30 μg) | Aminoside |
| Tetracycline (30 μg) | Tetracycline |
| Piperacilline (100 μg) | Beta - Lactame |
2.5.3. Disc diffusion method
In this study, the antimicrobial effect of H. aspersa Müller slime was evaluated using the Kirby Bauer disk diffusion method [28]. To assess this activity, Wathman N°3 paper disks (6 mm) were boiled for 30 min to remove any chemical that might inhibit microbial growth, sterilized, and then stored in tightly closed sterile glass vials until use. Each disk was then coated with different concentrations of slime, and antibiotic disks were placed on the surface of the MH medium, pre-inoculated by swabbing with bacterial suspensions (108 CFU). The infected Petri dishes were then incubated at 37 °C in the dark. 24 h after incubation, the diameter of the inhibitory zones, measured in millimeters, was determined. The bacteria have been grouped for the purpose of utilizing the latter [29].
The results were interpreted as follows:
Resistant: diameter ≤ 8 mm;
Moderately sensitive: diameter between 9 and 14 mm;
Sensitive: diameter between 15 and 19 mm;
Extremely sensitive: diameter > 20 mm.
2.5.4. Determination of the minimum inhibitory concentration (MIC)
Using the Wiegand et al. [30] method, the lowest inhibitory concentration of H. aspersa Müller slime against the chosen strains was calculated. 20 mL of fresh bacterial culture (1 × 104 CFU) were added and inoculated onto H. aspersa Müller slime (10, 20, 40, 60, 80, and 100 μg·mL−1). Bacterial growth was detected at 600 nm after the reaction had been incubated overnight. MIC was established as the lowest sample concentration that prevented the formation of turbidity using PBS as a negative control.
2.6. Study of the healing activity of slime of H. aspersa Müller
The aim of this study was to assess the potential for accelerating dermal tissue neoformation after application of H. aspersa Müller slime to superficial scars. The comparison was made between a group of animals receiving a reference cream (Madecassol ®) and a group treated with physiological water.
The institutional ethical committee for the care and use of the laboratory animals at the Faculty of Sciences, Ibn Tofail University, kenitra 14,000, Morocco, reviewed and approved the present study and animal rights were respected.
2.6.1. Rat preparation
Female Wistar albino rats weighing between 150 and 200 g (4–5 months old) underwent this assessment in vivo. Three groups of five animals each were used (each group was housed in its own cage). Group I functioned as the negative control (no treatment), Group II was given Madecassol ® as a positive control, and Group III received H. aspersa Müller slime as a treatment. For 28 days, wounds should be treated once per day.
2.6.2. Experimental wound
Chloral was used to anesthetize the animals (0.5 mL.100 g−1 per kilogram of body weight) intraperitoneally. Wounds were made in accordance with Akbari et al. [31] procedures.
2.6.3. Wound healing rate
Wound diameters were measured immediately after wound creation and then every 7 days after wounding. In addition, the epithelialization period was monitored for each group. In addition, the wound areas of the rats were photographed weekly over the 28 days. The length and width of the wounded areas were then measured. The area of wound contraction was then calculated according to the method of [32] using the following formula:
Where n is the day of wound surface measurement other than day 0.
2.7. Statistical analyses
The results of the tests that were run are shown as a mean ± standard deviation in triplicates. One-way analysis of variance (ANOVA) and Duncan’s test were used in the statistical analysis. P ≤ 0.05 was adopted as the significance threshold.
3. Result and discussion
3.1. Chemical analysis
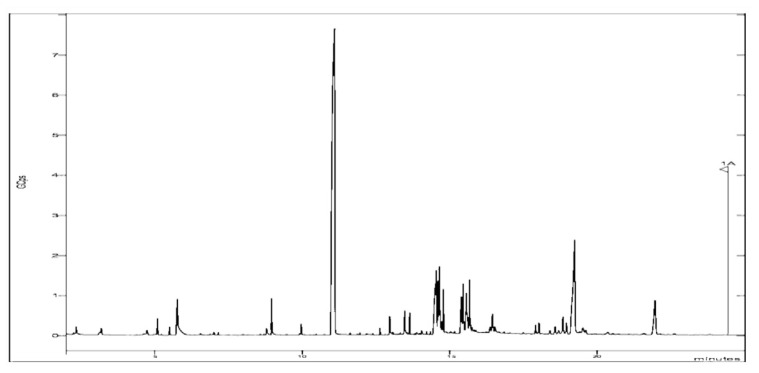
Twenty chemical substances were identified in the H. aspersa Müller slime during GC–MS analysis (Table 2, Fig. 1). The volatility of the chemicals may be inferred from the total ion current time that appeared on the TIC profile. More volatile substances were thought to elute at shorter retention periods. Esters, alcohols, aldehydes, ketones, and sulfur compounds were the primary types of volatile substances discovered in H. aspersa Müller slime.
Table 2.
Chemical composition of H. aspersa Müller slime.
| RT | Compounds | Area % |
|---|---|---|
| 02.342 | (Z)-2-heptène (C5H9N) | 00.228 |
| 03.191 | 2-éthylacridine (C15H13N) | 00.043 |
| 04.742 | 4H-Thiopyran-4-one, tétrahydro-, 1,1-dioxyde (C5H8O3S) | 00.208 |
| 05.098 | Thiophène, 3-(décyloxy)tétrahydro-, 1,1-dioxyde (C12H24O3S) | 00.667 |
| 05.507 | Méthyltris(triméthylsiloxy)silane (C10H30O3Si) | 00.295 |
| 05.771 | Methoxyphenyl-Oxime (C8H9NO2) | 02.423 |
| 07.155 | 1-Butyl-2,4,6-trimethyl benzene (C14H20OS) | 00.093 |
| 08.798 | Auramine (C17H22ClN3) | 00.414 |
| 08.963 | N-(trifluoroacétyl)-N,O,O′,O″-tetrakis(triméthylsilyl)norépinéphrine (C19H34F3NO4Si) | 01.367 |
| 09.969 | 3-isopropoxy-1,1,1,5,5,5-hexaméthyl l-3-(triméthylsiloxy)trisiloxane (C12H34O4Si4) | 00.338 |
| 11.100 | Cyclotrisiloxane, hexaméthyl (C6H18O3Si3) | 47.303 |
| 13.476 | Mercaptoéthanol (HOCH2CH2SH) | 01.098 |
| 14.498 | N-(2-acétylcyclopentylidène)cyclohexylamine (C13H21NO) | 03.057 |
| 14.645 | Octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadécaméthyl (C16H50O7Si8) | 04.325 |
| 15.560 | 6-chloro-4-phényl-2-propylquinoline (C18H16ClN) | 03.117 |
| 15.670 | 10-methylnonadecane (C20H) | 02.295 |
| 16.446 | Tetradecanal (C14H28O) | 00.755 |
| 18.025 | Furan, 2 isobutenyl-4-vinyl (C10H10O) | 00.524 |
| 19.233 | 1,2- Benzene dicarboxilic acid (C8H6O) | 11.447 |
| 21.960 | Cyclohexa-2,5-diène-1,4-dione, 2-méthyl-5-(4-morpholinyl) (C11H13NO) | 03.601 |
| Not identified | 16.402 |
Fig. 1.
GC–MS profile of H. aspersa Müller slime.
The most abundant were cyclotrisiloxane hexamethyl, 1,2-benzene dicarboxilic acid, octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl.
The compounds identified have interesting biologically relevant properties, including Methoxyphenyl-Oxime, which acts as an antimicrobial agent [33,34], 2-ethylacridine as an antioxidant and antitumor agent [35], Cyclotrisiloxane hexamethyl as an antibacterial, anti-inflammatory and anticancer agent [35], octasiloxane, 1,1,3,3,5,5,7,7,9,9, 11,11,11,15,15-hexadecamethyl has been reported for its antimicrobial properties [36].
The findings of this study thus indicate that the discovered chemical components may be the bioactive components that give H. aspersa Müller slime its effectiveness. The above-mentioned chemicals make this slime suitable for usage in a range of medicinal applications.
3.2. Mineral analysis
The total ash concentration measured after incineration was 1.09 ± 0.00%, showing that H. aspersa Müller contains more minerals. This is an indication of the rich minerals contents that are beneficial.
The Table 3 below contains the findings about the mineral composition of H. aspersa Müller slime. High calcium (Ca) concentrations (9.964 ± 0.033 mg.100 g−1) were found in H. aspersa Müller slime, which also included high levels of phosphorus (P) (6.897 ± 0.041 mg.100 g−1) and potassium (K) (4.176 ± 0.037 mg.100 g−1). Magnesium (Mg) was detected in the slime in considerable amounts (1.943 ± 0.041 mg.100 g−1), although sodium (Na), iron (Fe), and zinc (Zn) were all present in negligible amounts (0.059 ± 0.001 mg.100 g−1, 0.102 ± 0.001 mg.100 g−1, and 0.044 ± 0.002 mg.100 g−1, respectively).
Table 3.
Mineral content of H. aspersa Müller slime.
| Elements | Quantity (mg.100g−1) |
|---|---|
| Ca | 9.96 ± 0.03 |
| Fe | 0.06 ± 0.00 |
| K | 4.18 ± 0.04 |
| Mg | 1.94 ± 0.04 |
| Na | 0.04 ± 0.00 |
| Zn | 0.10 ± 0.00 |
| P | 6.90 ± 0.04 |
Although they do not work separately, the roles of these minerals are distinct [37].
Sufficient evidence is available in the literature to indicate the effect of mineral elements such as manganese, zinc and copper are involved in tissue, cellular, and subcellular functions, muscle contractions, membrane potential regulation, mitochondrial activity, and enzymatic reactions [38], Grela et al. [39] reported that antioxidant activity has been attributed to mineral components such as copper, manganese, and iron. Pandya [40] revealed that low doses of trace elements such as Cu and Zn inhibit the production of oxidative free radicals. A recent study showed that high antioxidant capacity was correlated with and explained by mineral richness [41], while Phan et al. [42] confirmed that Zn acts as an antibacterial agent.
3.3. Antibacterial assay
3.3.1. Disc inhibitory assay
In this study, we found that the mucus of H. aspersa Müller also has antibacterial activity. It appears that E. coli is susceptible to three antibiotics, as shown by the antibiogram. These include cefotamine sodium, netilmicin, and tetracycline. Antibiotics perform well against S. aureus include erythromycin, netilmicin, and tetracycline. P. aeruginosa, in contrast hand, has a susceptibility to the antibiotics ampicilline, piperacillin, and tetracycline. Furthermore, L. monocytogene, is inhibited by just ampicillin and tetracycline (Table 4).
Table 4.
Comparison between the effects of antibiotics on pathogenic bacteria in vitro.
| Antibiotics | P. aeruginosa | E. coli (ATCC 35218) | L. monocytogenes (ATCC 7644) | S. aureus (ATCC 29213) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| ZI (mm) | Pr | ZI (mm) | Pr | ZI (mm) | Pr | ZI (mm) | Pr | |
| AMP | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R |
| COF | 09.60 ± 0.12 b | S | 17.00 ± 0.00c | S | 10.00 ± 0.09 b | S | 00.00 ± 0.00a | R |
| CUS | 10.00 ± 1.02 b | S | 00.00 ± 0.00a | R | 28.00 ± 0.16 d | ES | 00.00 ± 0.00a | R |
| ETM | 19.00 ± 0.24 d | S | 00.00 ± 0.00a | R | 25.00 ± 0.05 cd | ES | 20.00 ± 0.00 bc | S |
| NM | 19.67 ± 0.10 de | S | 13.50 ± 0.00 b | S | 30.00 ± 0.37 de | ES | 18.00 ± 0.00 b | S |
| PRP | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R | 17.00 ± 0.20 bc | S | 00.00 ± 0.00a | R |
| TC | 00.00 ± 0.00a | R | 12.00 ± 0.00 b | S | 00.00 ± 0.00a | R | 15.00 ± 0.00 b | S |
Means in the same column with the same letter do not differ significantly from each other at the 5% level of significance according to the Duncan’s test.
ZI: Zone of Inhibition; Pr: Profil; R: Resistant; S: Sensitive; ES: Extremely Sensitive; ER: Extremely Resistant.
According to Table 5, we observe that the different concentrations of H. aspersa Müller slime used to inhibit pathogenic bacteria have a significant effect that depends on the concentration. We also note that a concentration of 100 μL is the most effective concentration against pathogens. Indeed, it showed an inhibition diameter of 30 mm against E. coli (ATCC 35218), while a zone of 23 mm appeared in the boxes containing P. aeruginosa. In contrast, similar diameters were observed against L. monocytogenes (ATCC 7644) and S. aureus (ATCC 29213) (11.1 mm and 12.3 mm, respectively).
Table 5.
Average diameter of pathogen inhibition zones generated by H. aspersa Müller slime.
| Concentration of snail mucus (μg.mL−1) | P. aeruginosa | E. coli (ATCC 35218) | L. monocytogenes (ATCC 7644) | S. aureus (ATCC 29213) |
|---|---|---|---|---|
| 25 | 10.5 ± 0.00a | 18.7 ± 0.07 ab | 03.6 ± 0.10a | 09.6 ± 0.00a |
| 50 | 12.2 ± 0.06 ab | 21.0 ± 0.20 ab | 05.0 ± 0.13a | 10.0 ± 0.01a |
| 75 | 15.0 ± 0.10 b | 28.1 ± 0.14 b | 09.4 ± 0.03 ab | 11.9 ± 0.12 ab |
| 100 | 23.1 ± 0.21c | 30.0 ± 0.32 bc | 11.1 ± 0.00 b | 12.3 ± 0.18 b |
Means in the same column with the same letter do not differ significantly from each other at the 5% level of significance according to the Duncan’s test.
These results are comparable to those of Ulagesan and Kim [15], who noted an inhibition diameter of 15.5 mm against S. aureus with a concentration of 100 μg·mL−1 of proteins extracted from snail slime (Achatina fulica), while it was 14.6 mm and 15.83 mm using proteins from C. bistrialis and P. globosa, respectively. However, none of these species inhibited the growth of P. aeruginosa. Another study showed that proteins extracted from the mantle mucus of Lissachatina fulica demonstrated the highest antibacterial activity against E. coli and S. aureus [43].
Furthermore, according to the resistance profile of the tested bacteria, we would like to note that the concentration of snail slime (H. aspersa Müller) used was better than most of the antibiotic families used.
We can say that the secreted snail slime presents a composition of biomolecules with antioxidant and antibacterial activities [10,43–45]. Various compounds in snail slime require further characterization and elucidation of their functions in the animal and their appropriate use in various applications.
3.3.2. Determination of the minimum inhibitory concentration (MIC)
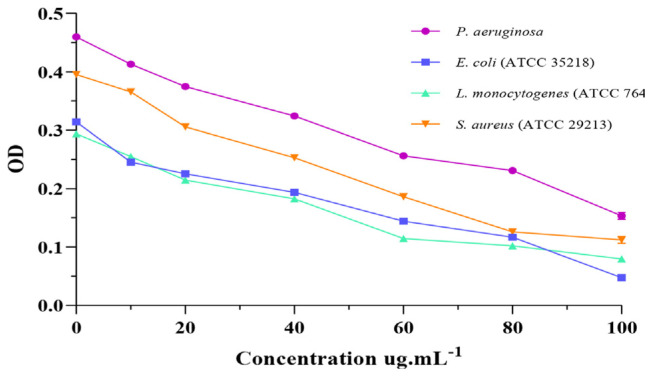
MIC is the minimum antibacterial concentration required to kill certain bacteria [46]. H. aspersa Müller slime exhibited antibacterial activity against all four bacterial strains tested with (Fig. 2). Overall, the findings clearly showed that H. aspersa Müller slime had stronger antibacterial activity.
Fig. 2.
Antibacterial activity of H. aspersa Müller slime.
These findings demonstrate that the minimum inhibitory concentration (MIC) for E. coli was 10 μg·mL−1, the MIC for L. monocytogenes was 40 μg·mL−1, P. aeruginosa and S. aureus were stopped at a minimum concentration of 20 μg·mL−1.
Our findings are in accordance with the literature on the antibacterial activity of H. aspersa Müller slime, according to a comparison of our findings with those of previous investigations.
EL-Zawawy and Mona [47] showed that slime from H. aspersa Müller and Eremina desertorum had an antibacterial effect against P. aeruginosa with MICs of 15 and 7 μg·mL−1, E. coli 20 and 5 μg·mL−1, S. aureus 15 and 5 μg·mL−1, respectively. In another study, L. fulica slime showed an antibacterial effect against S. aureus, S. epidermidis and Corynebacterium spp. with MIC values of 12.5, 25 and 25 μg·mL−1, respectively, as well as the MIC values of the in vitro antibacterial activities of Pomacea canaliculata slime against S. aureus, S. epidermidis and Corynebacterium spp. being above 50 [48].
According to several investigations, the mucus of L. fulica and H. aspersa displayed antibacterial activity against diverse species of bacteria and fungi [6,49–52]. In addition, several antimicrobial peptides from the mucus of L. fulica and H. aspersa have been studied [53–55]. Crude protein isolated from six different species of snails has recently demonstrated antibacterial efficacy against certain bacteria and fungi [15].
3.4. Effect of H. aspersa Müller slime on wound area
In the current investigation, in vivo experiments were performed to investigate the healing properties of H. aspersa Müller slime.
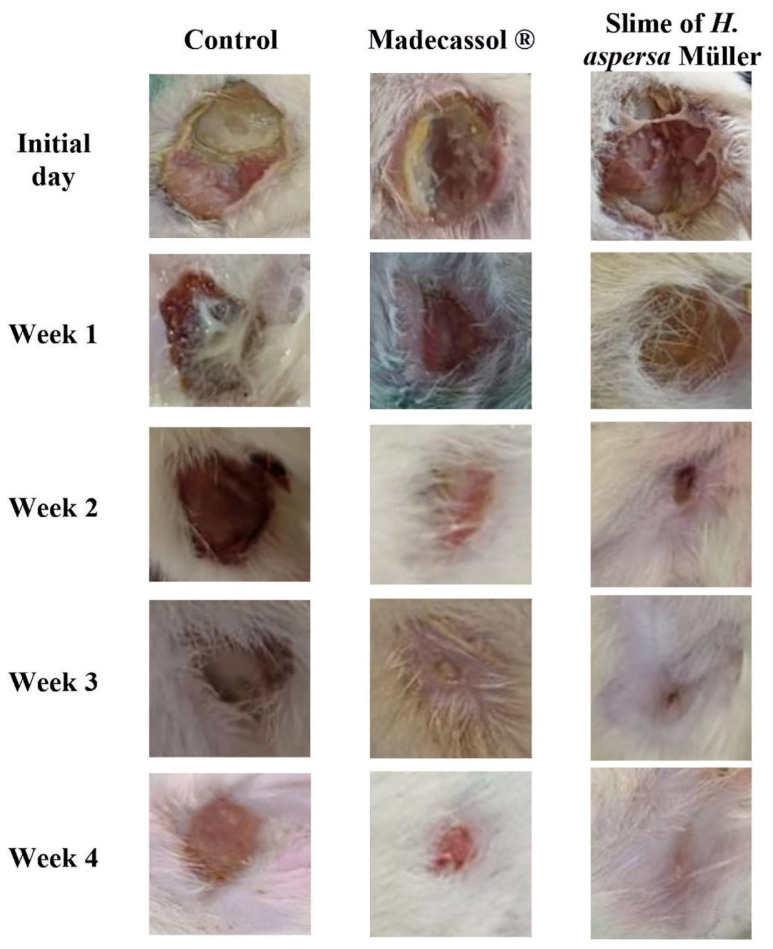
The findings of healing activity using the incision wound model are shown in the Table 6 and Fig. 3. The control, H. aspersa Müller slime therapy, and standard group healing percentages are represented at 0, 7, 14, 21, and 28 days.
Table 6.
Effect of H. aspersa Müller on percentage (%) wound closure.
| Control | Madecassol ® | Slime of H. aspersa Müller | |
|---|---|---|---|
| Initial day | 00.00 ± 0.00 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| Week 1 | 14.57 ± 3.59a | 23.16 ± 2.99 b | 39.81 ± 5.43c |
| Week 2 | 19.01 ± 4.71a | 40.01 ± 7.24 b | 64.33 ± 4.67c |
| Week 3 | 38.09 ± 6.52a | 58.44 ± 6.31 b | 76.06 ± 8.30c |
| Week 4 | 48.23 ± 4.23a | 81.14 ± 3.24 b | 91.73 ± 3.05c |
Values are expressed as mean ± SD (n = 5). All columns are significant using ANOVA.
P ≤ 0.05 when compared to control.
Fig. 3.
Photos depicting the effect of H. aspersa Müller slime on the wound-healing model at different stages of the study.
In order to restore the morphological and functional integrity of the epithelial tissues and ensure barrier function, wound healing is a complicated physiological process that involves tissue remodeling and repair [56]. Re-epithelialization and tissue granulation are crucial throughout the healing process [57]. Similar to the re-epithelialization procedure, the time frame in which the barrier function is recovered is crucial [25]. As a result, crucial metrics for measuring the effectiveness of healing include wound contraction, epithelialization, and granulation [58].
In this study, wounds treated with H. aspersa Müller slime had a significantly greater capacity for contraction than the standard ointment-treated group, the latter being superior to the control group (p ≤ 0.05) (Table 6).
Both groups hastened wound healing and displayed greater results than the control group by day 14, when wound evaluation revealed that 64% of the wounds in the group treated with H. aspersa Müller slime had healed, as opposed to group II, where this proportion was only discovered as early as 21 days. H. aspersa Müller slime had a healing effect that was equivalent to that of common, over-the-counter ointment.
In the present study, we demonstrated that H. aspersa Müller snail slime also displayed antibacterial action and healing activity, which is consistent with its chemical makeup.
4. Conclusion
This study examined the antibacterial, wound-healing properties, chemical and mineral composition of H. aspersa Müller slime. According to the study’s findings, this slime exhibits a variety chemical composition of bioactive compounds, a respectable level of minerals, and a sizable amount of antibacterial and wound-healing activity. Due to the presence of bioactive chemicals, it can be used for future clinical and commercial studies, and can be considered as an adjuvant therapy for skin wounds. The biological activities of H. aspersa Müller slime suggest that it has positive effects for both medicinal and aesthetic uses. In conclusion, the data reported here might be a useful resource for future study into the use of novel natural compounds to fight microbial diseases.
Footnotes
Author contributions: All authors read and approved the final manuscript.
Conflict of interest: The authors declare no conflict of interest.
Funding: This research received no external funding.
Availability of data and materials
No new data was generated for this review.
References
- 1. Totsika M. Benefits and challenges of antivirulence antimicrobials at the dawn of the post-antibiotic era. Curr Med Chem. 2016;6:30–7. [Google Scholar]
- 2.World Health Organization. Critically important antimicrobials for human medicine: ranking of antimicrobial agents for risk management of antimicrobial resistance due to nonhuman use; 5th revision. Geneva, Switzerland: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO; Available online: https://apps.who.int/iris/bitstream/handle/10665/255027/9789241512220-eng.pdf;jsessionid=DD014C6E76979E5E3ECE1BA519E14CA1?sequence=1. [Google Scholar]
- 3.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. [Accessed 17 April 2019]. Available online: http://www.who.int/medicines/publications/global-priority-list-antibioticresistant-bacteria.
- 4. Smith VJ, Desbois AP, Dyrynda EA. Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar Drugs. 2010;8:1213–62. doi: 10.3390/md8041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhuang J, Coates CJ, Zhu H, Zhu P, Zujian W, Xie L. Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev Comp Immunol. 2015;49:96–102. doi: 10.1016/j.dci.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 6. Pitt S, Graham MA, Ded CG, Taylor-Harris PM, Gunn A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br J Biomed Sci. 2015;72:174–81. doi: 10.1080/09674845.2015.11665749. [DOI] [PubMed] [Google Scholar]
- 7. Li H, Parisi MG, Parrinello N, Cammarata M, Roch P. Molluscan antimicrobial peptides, a review from activity-based evidences to computer-assisted sequences. Invertebr Surviv J. 2011;8:85–97. [Google Scholar]
- 8. Cilia G, Fratini F. Antimicrobial properties of terrestrial snail and slug mucus. J Compl Integr Med. 2018;15(3):20170168. doi: 10.1515/jcim-2017-0168. [DOI] [PubMed] [Google Scholar]
- 9. Meyer-Rochow VB. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: a comparative survey and review. J Ethnobiol Ethnomed. 2017;13:9. doi: 10.1186/s13002-017-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trapella C, Rizzo R, Gallo S, Alogna A, Bortolotti D, Casciano F, et al. HelixComplex snail mucus exhibits prosurvival, proliferative and pro-migration effects on mammalian fibroblasts. Sci Rep. 2018;8:17665–74. doi: 10.1038/s41598-018-35816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greistorfer S, Klepal W, Cyran N, Gugumuck A, Rudoll L, Suppan J, et al. Snail mucus glandular origin and composition in Helix pomatia. Zoology. 2017;122:126–38. doi: 10.1016/j.zool.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 12. Truchuelo MT, Vitale M. A cosmetic treatment based on the secretion of Cryptomphalus aspersa 40% improves the clinical results after the use of nonablative fractional laser in skin aging. J Cosmet Dermatol. 2019;19:622–8. doi: 10.1111/jocd.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabi SG, Cohen JL, Peterson JD, Kiripolsky MG, Goldman MP. The effects of filtrate of the secretion of the Cryptomphalus aspersa on photoaged skin. J Drugs Dermatol JDD. 2013;12(4):453–7. [PubMed] [Google Scholar]
- 14. Tribó-Boixareu MJ, Parrado-Romero C, Rais B, Reyes E, Vitale-Villarejo MA, Gonzalez S. Clinical and histological efficacy of a secretion of the mollusk Cryptomphalus aspersa in the treatment of cutaneous photoaging. J Cosmet Dermatol. 2009;22:247–52. [Google Scholar]
- 15. Ulagesan S, Kim HJ. Antibacterial and antifungal activities of proteins extracted from seven different snails. Appl Sci. 2018;8:1362. [Google Scholar]
- 16. Ellijimi C, Hammouda MB, Othman H, Moslah W, Jebali J, Mabrouk HB, et al. Helix aspersa maxima mucus exhibits antimelanogenic and antitumoral effects against melanoma cells. Biomed Pharmacother. 2018;101:871–80. doi: 10.1016/j.biopha.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 17. El Mubarak MA, Lamari FN, Kontoyannis C. Simultaneous determination of allantoin and glycolic acid in snail mucus and cosmetic creams with high performance liquid chromatography and ultraviolet detection. J Chromatogr A. 2013;1322:49–53. doi: 10.1016/j.chroma.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 18. Adikwu M, Alozie BU. Application of snail mucin dispersed in detarium gum gel in wound healing. Sci Res Essays. 2007;2:195–8. [Google Scholar]
- 19. Dolashka-Angelova P, Stefanova T, Livaniou E, Velkova L, Klimentzou P, Stevanovic S, et al. Potentiel immunologique de Helix vulgaris et Rapana venosa Hemocyanins. Immunol Invest. 2008;37(8):822–40. doi: 10.1080/08820130802403366. [DOI] [PubMed] [Google Scholar]
- 20. Valizadeh N, Holasou HA, Mohammadi SA, Khawar KM. A comparison of genomic DNA extraction protocols in Artemisia annua L. for large scale genetic analyses studies. Iran J Sci Technol Trans A-Science. 2021;45(5):1587–95. [Google Scholar]
- 21. Nwankwo IU, Edward KC, Nwoba CN, Okwudiri CV. Evaluation of bacterial species in patients with skin infection and their antibiogram. South Asian J Res Microbiology. 2021;9(4):10–6. [Google Scholar]
- 22. Ali SS, Morsy R, El-Zawawy NA, Fareed M, Bedaiwy MY. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): a novel antimicrobial, anti-elastase, antikeratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int J Nanomed. 2017;12(12):6059–607. doi: 10.2147/IJN.S141201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. At Ar, Hafez SF, Abdelhakam SM, Ali-Eldin ZA, Esmat IM, Elsayed MS, et al. Antimicrobial resistant bacteria among health care workers in intensive care units at Ain Shams University Hospitals. J Egypt Soc Parasitol. 2010;40(1):71–83. [PubMed] [Google Scholar]
- 24. Zlatko K. Commentary Development of next-generation antimicrobial hydrogel dressing to combat burn wound infection. Biosci Rep. 2021;41:202–9. doi: 10.1042/BSR20203404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daemi A, Lotfi M, Farahpour MR, Oryan A, Ghayour SJ, Sonboli A. Topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing reepithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharmaceut Biol. 2019;57(1):799–806. doi: 10.1080/13880209.2019.1687525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ISO. Meat and meat products-Determination of total ash. Pub. L n°. Vol. 936. ISO; 1998. p. 1998. [Google Scholar]
- 27.Horwitz W, Latimer G. Association of Officiating Analytical Chemists; Washington, DC, USA: 2005. [Google Scholar]
- 28. Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. American Soc Microbiology. 2009;15:55–63. [Google Scholar]
- 29. El Moussaoui A, Jawhari FZ, Almehdi AM, Elmsellem H, Fikri Benbrahim K, Bousta D, et al. Antibacterial, antifungal and antioxidant activity of total polyphenols of withania frutescens L. Bioorg Chem. 2019;93:103337. doi: 10.1016/j.bioorg.2019.103337. [DOI] [PubMed] [Google Scholar]
- 30. Wiegand I, Hilpert K, Hancock R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substance. Nat Protoc. 2008;3:163–75. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 31. Akbari F, Azadbakht M, Bagheri A, Vahedi L. In vitro and in vivo wound healing activity of Astragalus Floccosus Boiss. (Fabaceae) Advances in Pharmacological and Pharmaceutical Sci. 2022;2022:1. doi: 10.1155/2022/7865015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farmoudeh A, Akbari J, Saeedi M, Ghasemi M, Asemi N, Nokhodchi A. Methylene blue-loaded niosome: preparation, physicochemical characterization, and in vivo wound healing assessment. Drug Delivery and Translational Res. 2020;10(5):1428–41. doi: 10.1007/s13346-020-00715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barghouthi SA, Ayyad I, Ayesh M, Abu-Lafi S. Isolation, identification, and characterization of the novel antibacterial agent methoxyphenyl-oxime from streptomyces pratensis QUBC97 isolate. J Antibio Res. 2017;1(1):105. doi: 10.15744/2574-5980.1.105. [DOI] [Google Scholar]
- 34. Ismail GA, Gheda SF, Abo-Shady AM, Abdel-Karim OH. In vitro potential activity of some seaweeds as antioxidants and inhibitors of diabetic enzymes. Food Sci Technol, Campinas. 2020;40(3):681–91. [Google Scholar]
- 35. Muthukrishnan S, Prakathi P, Sivakumar T, Thiruvengadam M, Jayaprakash B, Baskar V, et al. Composants bioactifs etpotentiel de santé de la diversité microfongique endophytique dans les plantes médicinales. Antibiotiques. 2022;11:1533. doi: 10.3390/antibiotics11111533. [DOI] [Google Scholar]
- 36. More K, Tayade S, Gawande P, Manik S, Shelke D. Antioxidant and antimicrobial potential of Canavalia gladiata (Jacq.) DC. leaves and seeds: GC-MS based metabolic profiling. Indian J Natural Products and Resources. 2022;13(2):163–9. [Google Scholar]
- 37. Engmann FN, Afoakwah NA, Darko PO, Sefah W. Proximate and mineral composition of snail (Achatina achatina) meat; any nutritional justification for acclaimed health benefits? J Basic Appl Sci Res. 2013;3(4):8–15. [Google Scholar]
- 38. Siddiqui K, Bawazeer N, Joy SS. Variation in macro and trace elements in progression of type 2 diabetes. Sci World J. 2014;2014:461591. doi: 10.1155/2014/461591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grela ER, Samolinska W, Kiczorowska B, Klebaniuk R, Kiczorowski P. Content of minerals and fatty acids and their correlation with phytochemical compounds and antioxidant activity of leguminous seeds. Biol Trace Elem Res. 2017;180(2):338–48. doi: 10.1007/s12011-017-1005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pandya DP. Oxidant injury and antioxidant prevention: role of dietary antioxidants, minerals, and drugs in the management of coronary heart disease. Compr Ther. 2002;28(1):62–73. doi: 10.1007/s12019-002-0043-7. [DOI] [PubMed] [Google Scholar]
- 41. Lachkar N, Lamchouri F, Bouabid K, Boulfia M, Senhaji S, Stitou M, et al. Mineral composition, phenolic content, and in vitro antidiabetic and antioxidant properties of aqueous and organic extracts of Haloxylon scoparium aerial parts. Evid base Compl Alternative Med. 2021;2021:20. doi: 10.1155/2021/9011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–8. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 43. Noothuan N, Apitanyasai K, Panha S, Tassanakajon A. Snail mucus from the mantle and foot of two land snails, Lissachatina fulica and Hemiplecta distincta, exhibits different protein profile and biological activity. BMC Res Notes. 2021;14:138. doi: 10.1186/s13104-021-05557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gugliandolo E, Cordaro M, Fusco R, Peritore AF, Syracuse R, Genovese T, et al. Protective effect of snail secretion filtrate against ethanol-induced gastric ulcer in mice. Sci Rep. 2021;11:3638. doi: 10.1038/s41598-021-83170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atta SA, Ibrahim AM, Megahed FAK. In-vitro anticancer and antioxidant activities of eremina desertorum (forsskal, 1775) snail mucin. Asian Pac J Cancer Prev APJCP. 2021;22(11):3467–74. doi: 10.31557/APJCP.2021.22.11.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goins M.Minimum inhibitory (MIC) and minimum bactericidal concentration (MBC) evaluations as R&D tools. Q Laboratories. 2017. [Accessed 20 April 2023]. Available online: https://www.qlaboratories.com/minimum-inhibitory-mic-and-minimum-bactericidal-concentration-mbc-evaluations-as-rd-tools/
- 47. El-Zawawy NA, Mona MM. Antimicrobial efficacy of Egyptian Eremina desertorum and Helix aspersa snail mucus with a novel approach to their anti-inflammatory and wound healing potencies. Sci Rep. 2021;11:24317. doi: 10.1038/s41598-021-03664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nantarat N, Tragoolpua Y, Gunama P. Antibacterial activity of the mucus extract from the giant african snail (lissachatina fulica) and golden Apple snail (Pomacea canaliculata) against pathogenic bacteria causing skin diseases. Tropical Natural History. 2019;19(2):103–12. [Google Scholar]
- 49. Bortolotti D, Trapella C, Bernardi T, Rizzo R. Letter to the Editor: antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br J Biomed Sci. 2016;73:49–50. doi: 10.1080/09674845.2016.1155377. [DOI] [PubMed] [Google Scholar]
- 50. Santana WA, Melo CM, Cardoso JC, Pereira-Filho RN, Rabelo AS, Reis FP, et al. Assessment of antimicrobial activity and healing potential of mucous secretion of Achatina fulica. Int J Morphol. 2012;30:365–73. [Google Scholar]
- 51. Etim L, Aleruchi C, Obande G. Antibacterial properties of snail mucus on bacteria isolated from patients with wound infection. Br Microbiol Res J. 2016;11:1–9. [Google Scholar]
- 52. Zodape GV. A study on presence of bioactive compounds in snail Achantina fulica. J Nat Appl Sci. 2010;2:266–8. [Google Scholar]
- 53. Dolashka P, Dolashki A, Beeumen JV, Floetenmeyerd M, Velkova L, Stevanovic S, et al. Antimicrobial activity of molluscan hemocyanins from Helix and Rapana snails. Curr Pharmaceut Biotechnol. 2016;17:263–70. doi: 10.2174/1389201016666150907113435. [DOI] [PubMed] [Google Scholar]
- 54. Mukherjee S, Barman S, Mandal NC, Bhattacharya S. Antibacterial activity of Achatina CRP and its mechanism of action. Indian J Exp Biol. 2014;52:692–704. [PubMed] [Google Scholar]
- 55. Zhong J, Wang W, Yang X, Yan X, Liu R. A novel cysteinerich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides. 2013;39:1–5. doi: 10.1016/j.peptides.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 56. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Galiano RD, Michaels VJ, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12(4):485–92. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 58. Farahpour MR, Pirkhezr E, Ashrafian A, Sonboli A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed Pharmacother. 2020;128:110120. doi: 10.1016/j.biopha.2020.110120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data was generated for this review.