ABSTRACT
Objective:
To describe the current state of the art in the therapeutic administration of botulinum toxin with indications, efficacy, and safety profile for children and adolescents with cerebral palsy.
Data source:
An integrative review was conducted. The MEDLINE/PubMed database was searched twice within the last decade using distinct terms, and only studies written in the English language were included. The study population was limited to those aged 0–18 years. Articles that were duplicates or lacked sufficient methodology information were excluded.
Data synthesis:
We found 256 articles, of which 105 were included. Among the included studies, most were conducted in developed countries. Botulinum toxin demonstrated good safety and efficacy in reducing spasticity, particularly when administered by a multidisciplinary rehabilitation team. It is primarily utilized to improve gait and upper limb function, facilitate hygiene care, reduce pain, prevent musculoskeletal deformities, and even decrease sialorrhea in patients without a functional prognosis for walking.
Conclusions:
The administration of botulinum toxin is safe and efficacious, especially when combined with a multi-professional rehabilitation team approach, which increases the probability of functional improvement. It can also be beneficial for patients with significant functional impairments to help with daily care tasks, such as hygiene, dressing, and reducing sialorrhea. Pediatricians must be familiar with this treatment and its indications to attend to and refer patients promptly when necessary, and to exploit their neuroplasticity. Further research on this topic is required in developing countries.
Keywords: Botulinum toxin, Cerebral palsy, Spasticity, Rehabilitation
RESUMO
Objetivo:
Descrever o estado da arte em aplicação terapêutica de toxina botulínica com indicações, eficácia e perfil de segurança em crianças e adolescentes com paralisia cerebral.
Fontes de dados:
Realizada revisão integrativa através de busca na base de dados MEDLINE/PubMed em dois momentos nos últimos 10 anos, e termos distintos, em inglês, numa população entre 0 e 18 anos de idade. Excluiu-se artigos duplicados ou com informações insuficientes de metodologia.
Síntese dos dados:
256 artigos foram encontrados e 105 foram incluídos, sendo a maior parte realizados em países desenvolvidos. A toxina botulínica mostrou boa segurança e efetividade na redução da espasticidade, especialmente administrada por uma equipe de reabilitação multiprofissional, usada principalmente para: melhora da marcha e da função dos membros superiores, facilitação dos cuidados de higiene, analgesia e prevenção de deformidades musculoesqueléticas, além de redução da sialorreia, inclusive em pacientes sem prognóstico funcional de marcha.
Conclusões:
A aplicação de toxina botulínica foi efetiva e segura, principalmente quando atrelada a uma abordagem por equipe de reabilitação multiprofissional, o que aumenta as chances de melhora funcional. Mostrou-se benéfica também para pacientes com grandes comprometimentos funcionais para facilitar os seus cuidados diários em relação à higiene, colocar e tirar roupas e redução da sialorreia. O pediatra deve estar familiarizado com esse tratamento e suas indicações para atender e direcionar pacientes o mais breve possível quando indicado e aproveitar o máximo de neuroplasticidade. Há necessidade de investimentos em mais pesquisas sobre este tema em países em desenvolvimento.
Palavras-chave: Toxina botulínica, Paralisia cerebral, Espasticidade, Reabilitação
INTRODUCTION
Botulinum toxin (BTX) is a biologically derived medication naturally produced by Clostridium botulinum, an anaerobic bacterium that produces eight serological types of toxins. The most potent is type A (BTX-A) and, therefore, it is used clinically in Brazil. BTX is a laboratory-produced biological agent. It is a stable crystalline substance lyophilized in human albumin and supplied in a sterile vacuum vial for dilution in saline solution. 1
Its action occurs selectively in the cholinergic peripheral nerve terminal, inhibiting the release of acetylcholine by cleaving SNAP 25 (essential protein for the coupling and release of acetylcholine from nerve terminal vesicles located within the nerve endings), which causes muscle denervation and subsequent paralysis. Typically, recovery from an intramuscular injection occurs within 12 weeks due to the growth and formation of new connections between nerve endings and terminal plates. This results in transient selective muscle relaxation for therapeutic purposes or to facilitate daily care. It is administered only to specific muscles and in controlled doses based on the child’s weight. 1 Neuromuscular blockade with BTX offers the following advantages: it allows access to specific muscles, it has a sustainable and reversible effect, and it reduces the chances of systemic adverse effects (e.g., a condition similar to botulism with generalized muscle relaxation) when compared to other oral medications (e.g., baclofen or benzodiazepines) or injectables (e.g., intrathecal baclofen pump or intravenous benzodiazepines). 1,2 Since the 1990s, BTX injections have been administered for an increasing number of indications in human therapy, and clinical research is adding new indications. 1
BTX administration for cerebral palsy (CP) has proved to be of great therapeutic value in reducing spasticity, particularly in gait improvement, upper limb function enhancement, hygiene care facilitation, pain relief, and prevention of myo-articular deformities. 3,4
Pediatricians must be familiar with this treatment and its indications to refer patients promptly when necessary and take the maximum advantage of their neuroplasticity. This study, as an integrative review, aimed to provide a comprehensive overview of the therapeutic administration of BTX in children and adolescents with CP aged 0–18 years. This review focused on the main indications, adjuvant therapies, efficacy for intended objectives (e.g., improving gait patterns, upper limb function, facilitating axillary and/or inguinal hygiene care, providing pain relief, and preventing musculoskeletal deformities), and safety profile.
METHOD
In this study, we conducted an integrative review of the therapeutic administration of BTX in children and adolescents with CP. Two authors searched the Medical Literature Analysis and Retrieval System Online (MEDLINE/PubMed) database twice using specific terms. The first search was conducted on March 2, 2022 using the search terms botulinum toxin, cerebral palsy, spasticity and child rehabilitation, and Medical Subject Headings (MeSH) terms “BOTULINUM TOXIN” AND “CEREBRAL PALSY” and “CHILD” AND “REHABILITATION” for literature published in the last ten years, in English, and limited to the age group of 0–18 years. The second search was conducted on June 2, 2022 for articles published in the last decade in English with the MeSH terms ”BOTULINUM TOXIN” AND “CEREBRAL PALSY” AND “SPASTICITY” AND “REHABILITATION” using the following search filters: “(“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“cerebral palsy”[MeSH Terms] OR (“cerebral”[All Fields] AND “palsy”[All Fields]) OR “cerebral palsy”[All Fields]) AND (“muscle spasticity”[MeSH Terms] OR (“muscle”[All Fields] AND “spasticity”[All Fields]) OR “muscle spasticity”[All Fields] OR “spastic” [All Fields] OR “spasticity”[All Fields] OR “spastics”[All Fields] OR “spasticity”[All Fields]) AND (“rehabilitant”[All Fields] OR “rehabilitants”[All Fields] OR “rehabilitate” [All Fields] OR “rehabilitated”[All Fields] OR “rehabilitates”[All Fields] OR “rehabilitating”[All Fields] OR “rehabilitation”[MeSH Terms] OR “rehabilitation”[All Fields] OR “rehabilitations”[All Fields] OR “rehabilitative”[All Fields] OR “rehabilitation”[MeSH Subheading] OR “rehabilitations”[All Fields] OR “rehabilitational”[All Fields] OR “rehabilitator”[All Fields] OR “rehabilitators”[All Fields]) AND ((y_10[Filter]) AND (allchild[Filter]))”.
Three authors extracted the data and excluded articles that were duplicated or lacked sufficient information in their methodology description. Because it is not the proposal of an integrative review, no quality analysis of the articles was performed. However, studies with more consistent outcomes were highlighted in the discussion.
All articles were analyzed by two authors. Clinical trials were analyzed separately due to their stronger evidence compared to the other studies included in this integrative review.
RESULTS
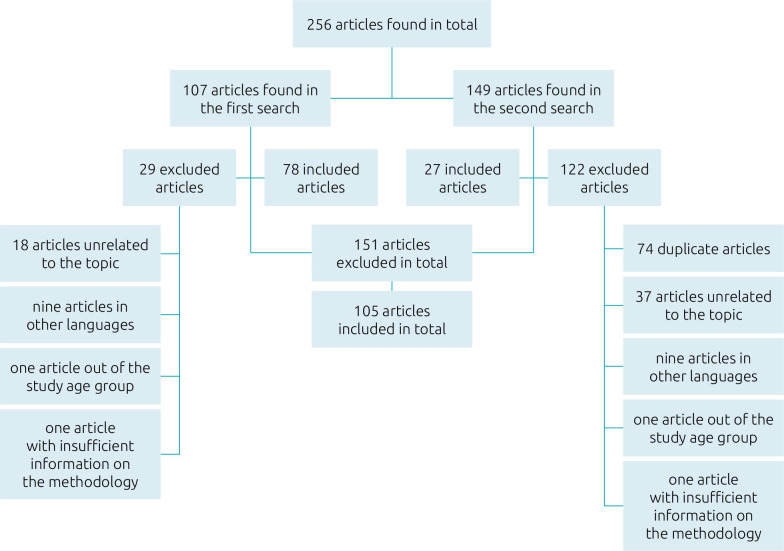
The initial search yielded 78 articles, and the subsequent search added 27, resulting in 105 articles. All disagreements between the authors during the analysis were resolved by discussion until 100% consensus. Figure 1 shows the reasons for excluding the articles from the search. The age range of the participants in the studies (n=25,910) varied from one month to 23 years old (Figure 1).
Figure 1. Organization chart on the data source to compose the integrative review.
Regarding the continent where the studies were conducted, the distribution was as follows: Europe (n=31; 29.5%), Asia (n=27; 25.7%), North America (n=17; 16.1%), Euro-Asia (Turkey) (n=15; 14.3%), Oceania (n=10; 9.5%), South America (n=3; 2.8%), and Africa (n=2; 1.9%).
As of the absence of relevant data in the included articles, three studies (2.9%) did not specify the age range, 36 (34.3%) did not indicate whether the patient was undergoing rehabilitation treatment, and 53 (50.5%) did not provide information on adverse effects (Tables 1–6). 4-105
Table 1. Results: Gait improvement (Part 1).
| SRN | Year (country) | Type | Sample (age)* | RP | Adverse events |
|---|---|---|---|---|---|
| 5 | 2020 (Belgium) | CT | 24 (7–9) | NI | NI |
| 6 | 2020 (Turkey) | CT | 241 (2–17) | POT | A |
| 7 | 2020 (Spain) | CS | 69 (2–18) | NI | NI |
| 8 | 2020 (South Korea) | CT | 29 (3–14) | NI | NI |
| 9 | 2020 (Australia) | CT | 40 (8–16) | NI | A |
| 10 | 2019 (South Korea) | CT | 591 (2–13) | P | NI |
| 11 | 2019 (Turkey) | CT | 118 (3–10) | POT | pain at the application site and bruising |
| 12 | 2019 (Norway) | CT | 1414 (5–8) | POT | NI |
| 13 | 2019 (Turkey) | CT | 30 (3–13) | P | redness at the application site |
| 14 | 2019 (Egypt) | CT | 60 (4–7) | P | A |
| 15 | 2019 (USA) | CT | 52 (1mo–17yrs) | NI | redness at the application site, erythema at the application site (face), mild dysphagia, dry mouth, and thick saliva |
| 16 | 2019 (Turkey) | SR | 153 (0–15) | P | pain and redness at the application site |
| 17 | 2018 (Poland) | CT | 60 (2–16) | P | NI |
| 18 | 2018 (Brazil) | SR | 111 (0–18) | P | temporary muscle weakness, dysesthesia, and pain at the injection site |
| 19 | 2018 (USA) | CT | 10 (2–12) | NI | NI |
| 20 | 2018 (Japan) | CT | 9 (4–8) | NI | NI |
| 21 | 2017 (India) | CT | 29 (2–7) | POT | fever associated with respiratory tract infection, and muscle weakness for a few days at the application site |
| 22 | 2017 (Australia) | RC | 17 (2–6) | P | NI |
| 23 | 2017 (South Korea) | CT | 144 (2–10) | NI | NI |
| 24 | 2017 (South Korea) | CT | 144 (2–10) | NI | urticaria and dysphonia in the Botox® group |
| 25 | 2017 (Sweden) | CT | 40 (4–12) | P | A |
| 26 | 2017 (Turkey) | CT | 51 (3–17) | P | A |
| 27 | 2016 (Turkey) | SR | 893 (1–19) | NI | NI |
| 28 | 2016 (Australia) | CT | 42 (2–5) | P | flu-like symptoms, localized muscle pain, vomiting, and occasional gait instability |
*Years. SRN: study reference number; RP: rehabilitation program; CT: clinical trial; NI: not informed; POT: physiotherapy and occupational therapy; A: absent; CS: case series; P: physiotherapy; SR: systematic review; RC: retrospective cohort.
Table 6. Muscle structure effects, cost-effectiveness assessment, and recommended procedure techniques.
| Muscle structure effects | |||||
|---|---|---|---|---|---|
| SRN | Year (country) | Type | Sample (age)* | RP | Adverse events |
| 90 | 2021 (South Korea) | CT | 15 (2–12) | NI | NI |
| 91 | 2019 (South Korea) | CT | 14 (2–10) | P | discomfort, decline in general condition, headache, dry mouth, pain at the injection site, viral infection, dizziness, back pain, and fatigue |
| 92 | 2019 (Belgium) | CT | 67 (7–11) | P | NI |
| 93 | 2018 (Australia) | PC | 11 (5–13) | NI | A |
| 94 | 2017 (Turkey) | PC | 12 (6–14) | NI | A |
| 95 | 2014 (Brazil) | SR | 480 (1.5–16) | POT | NI |
| 96 | 2014 (South Korea) | PC | 13 (4–8) | P | A |
| 97 | 2022 (Belgium) | PC | 26 (2–9) | P | A |
| Cost-effectiveness assessment | |||||
| 4 | 2020 (Netherlands) | CES | 60 (NI) | P | NI |
| Recommended procedural techniques | |||||
| 98 | 2018 (USA) | SC | 307 (NI) | NI | NI |
| 99 | 2018 (Turkey) | CT | 25 (3–16) | P | A |
| 100 | 2018 (USA) | CT | 14 (4–13) | NI | A |
| 101 | 2018 (USA) | RC | 249 (5–9) | NI | NI |
| 102 | 2017 (Thailand) | CS | 116 (30–46) | NI | NI |
| 103 | 2016 (Netherlands) | PC | 75 (4–18) | NI | NI |
| 104 | 2013 (Netherlands) | CT | NI | NI | NI |
| 105 | 2020 (France) | CT | 59 (5–11) | NI | NI |
*Years. SRN: study reference number; RP: rehabilitation program; CT: clinical trial; NI: not informed; P: physiotherapy; PC: prospective cohort; A: absent; SR: systematic review; POT: physiotherapy and occupational therapy; CES: cost-effectiveness study; SC: specialists’ consensus; RC: retrospective cohort; CS: case series.
Table 2. Results: Gait improvement (Part 2).
| SRN | Year (country) | Type | Sample (age)* | RP | Adverse events |
|---|---|---|---|---|---|
| 29 | 2016 (Belgium) | RC | 53 (4–18) | P | NI |
| 30 | 2016 (South Korea) | CT | 25 (3–15) | P | NI |
| 31 | 2016 (Australia) | PC | 10 (6–16) | NI | NI |
| 32 | 2016 (USA) | CT | 241 (2–17) | P | temporary muscle weakness |
| 33 | 2014 (Australia) | CT | 6 (8–9) | P | A |
| 34 | 2014 (Belgium) | CT | 31 (3–18) | P | NI |
| 35 | 2014 (China) | CT | 37 (3–15) | P | NI |
| 36 | 2014 (Turkey) | CT | 33 (2–8) | P | A |
| 37 | 2014 (Belgium) | CT | 19 (3–18) | P | NI |
| 38 | 2013 (Australia) | CT | 15 (5–12) | P | NI |
| 39 | 2012 (South Korea) | CT | 40 (2–14) | NI | NI |
| 40 | 2012 (South Korea) | CT | 17 (2–9) | P | A |
| 41 | 2022 (Czech Republic) | CT | 370 (2–17) | POT | seizure in patient with epilepsy, constipation and muscle weakness |
| 42 | 2022 (USA) | CT | 381 (2–17) | P | upper respiratory tract infections, followed by fever and cough |
| 43 | 2021 (USA) | CSR | 1508 (0–19) | P | NI |
| 44 | 2021 (Poland) | NSR | 945 (NI) | POT | respiratory tract infection, bronchitis, pharyngitis, asthma, muscle weakness, urinary incontinence, falls, seizures, temporary low-grade fever, and pain at the application site |
| 45 | 2019 (Turkey) | SR | 153 (11mo–15yrs) | P | pain, redness, and dysesthesia at the application site, and muscle weakness |
| 46 | 2018 (Canada) | Q | 15 (5–17) | NI | NI |
| 47 | 2016 (China) | CT | 4 (5–8) | POT | NI |
| 48 | 2014 (Poland) | CT | 41 (2–15) | P | A |
| 49 | 2014 (South Korea) | CT | 25 (2–6) | NI | NI |
| 50 | 2013 (Brazil) | CT | 14 (2–18) | P | A |
| 51 | 2013 (China) | CT | 244 (1–23) | P | NI |
*Years. SRN: study reference number; RP: rehabilitation program; RC: retrospective cohort; P: physiotherapy; NI: not informed; CT: clinical trial; PC: prospective cohort; A: absent; POT: physiotherapy and occupational therapy; CSR: Cochrane systematic review; NSR: non-systematic review; SR: systematic review; Q: qualitative.
Table 3. Results: Upper limbs function improvement.
| SRN | Year (country) | Type | Sample (age)* | RP | Adverse events |
|---|---|---|---|---|---|
| 52 | 2021 (USA) | CT | 372 (2–17) | POT | pain at the application site |
| 53 | 2020 (Turkey) | CT | 210 (2–17) | OT | Nausea |
| 7 | 2020 (Spain) | CS | 69 (2–18) | NI | NI |
| 54 | 2020 (Saudi Arabia) | CT | 64 (6–10) | P | NI |
| 12 | 2019 (Norway) | CT | 1414 (5–8) | POT | NI |
| 15 | 2019 (USA) | CT | 52 (1mo–17yrs) | NI | redness at the application site, erythema at the application site (face), mild dysphagia, dry mouth, and thick saliva |
| 27 | 2016 (Turkey) | SR | 893 (1–19) | NI | NI |
| 31 | 2016 (Australia) | PC | 10 (6–16) | NI | NI |
| 55 | 2015 (China) | CT | 12 (3–12) | OT | A |
| 56 | 2015 (Sweden) | CT | 20 (1.5–10) | OT | A |
| 57 | 2015 (Netherlands) | CT | 35 (4–9) | POT | A |
| 58 | 2014 (Italy) | CT | 27 (3–9) | P | A |
| 41 | 2022 (Czech Republic) | CT | 370 (2–17) | POT | seizure in patient with epilepsy, constipation, and muscle weakness |
| 44 | 2021 (Poland) | NSR | 945 (NI) | POT | respiratory tract infection, bronchitis, pharyngitis, asthma, muscle weakness, urinary incontinence, falls, seizures, temporary low-grade fever, and pain at the application site |
| 59 | 2020 (Sweden) | CS | 25 (1–23) | POT | NI |
| 60 | 2019 (Sweden) | CT | 20 (1–4) | OT | NI |
| 46 | 2018 (Canada) | Q | 15 (5–17) | NI | NI |
| 50 | 2013 (Brazil) | CT | 14 (2–18) | P | A |
*Years. SRN: study reference number; RP: rehabilitation program; CT: clinical trial; POT: physiotherapy and occupational therapy; OT: occupational therapy; CS: case series; NI: not informed; P: physiotherapy; SR: systematic review; PC: prospective cohort; A: absent; NSR: non-systematic review; Q: qualitative.
Table 4. Results: deformities prevention, hygiene improvement, and pain relief.
| Deformities prevention | |||||
|---|---|---|---|---|---|
| SRN | Year (country) | Type | Sample (age)* | RP | Adverse events |
| 61 | 2018 (Turkey) | CT | 17 (4–8) | P | NI |
| 17 | 2018 (Poland) | CT | 60 (2–16) | P | NI |
| 19 | 2018 (USA) | CT | 10 (2–12) | NI | NI |
| 62 | 2017 (South Korea) | CT | 144 (2–10) | NI | urticaria and dysphonia in the Botox® group |
| 26 | 2017 (Turkey) | CT | 51 (3–17) | P | A |
| 41 | 2022 (Czech Republic) | CT | 370 (2–17) | POT | seizure in patient with epilepsy, constipation and muscle weakness |
| 63 | 2021 (South Korea) | CT | 20 (2–10) | NI | NI |
| 14 | 2019 (Egypt) | CT | 60 (4–7) | P | NI |
| 46 | 2018 (Canada) | Q | 15 (5–17) | NI | NI |
| 48 | 2014 (Poland) | CT | 41 (2–15) | P | A |
| 49 | 2014 (South Korea) | CT | 25 (2–6) | NI | NI |
| 50 | 2013 (Brazil) | CT | 14 (2–18) | P | A |
| 64 | 2014 (Australia) | CT | 41 (2–16) | POT | pain and bruising at the application site |
| Hygiene improvement | |||||
| 7 | 2020 (Spain) | CS | 69 (2–18) | NI | NI |
| 12 | 2019 (Norway) | CT | 1414 (5–8) | POT | NI |
| 15 | 2019 (USA) | CT | 52 (1mo–17yrs) | NI | redness at the application site, erythema at the application site (face), mild dysphagia, dry mouth, and thick saliva |
| 64 | 2014 (Australia) | CT | 41 (2–16) | POT | pain and bruising at the application site |
| 46 | 2018 (Canada) | Q | 15 (5–17) | NI | NI |
| 50 | 2013 (Brazil) | CT | 14 (2–18) | P | A |
| Pain relief | |||||
| 65 | 2021 (Lithuania) | SR | 644 (0–18) | POT | A |
| 31 | 2016 (Australia) | PC | 10 (6–16) | NI | NI |
| 64 | 2014 (Australia) | CT | 41 (2–16) | POT | pain and bruising at the application site |
| 15 | 2019 (USA) | RC | 52 (1mo–18yrs) | NI | redness at the application site, erythema at the application site (face), mild dysphagia, dry mouth, and thick saliva |
| 46 | 2018 (Canada) | Q | 15 (5–17) | NI | NI |
*Years. SRN: study reference number; RP: rehabilitation program; CT: clinical trial; P: physiotherapy; NI: not informed; A: absent; POT: physiotherapy and occupational therapy; Q: qualitative; CS: case series; SR: systematic review; PC: prospective cohort; RC: retrospective cohort.
Table 5. Safety and effects assessments of Botulinum toxin injections and adjunct therapies effects and Botulinum toxin injections effects.
| Safety and effects assessments of BTX injections | |||||
|---|---|---|---|---|---|
| SRN | Year (country) | Type | Sample (age)* | RP | Adverse events |
| 66 | 2021 (Thailand) | RC | 1405 (0–18) | NI | A |
| 67 | 2021 (Turkey) | SC | 60 (0–18) | POT | A |
| 68 | 2020 (Turkey) | CT | 33 (3–7) | NI | NI |
| 69 | 2020 (Japan) | CT | 24 (2–11) | P | NI |
| 70 | 2019 (USA) | CT | 256 (1–21) | NI | NI |
| 71 | 2018 (Netherlands) | O | 65 (4–12) | P | A |
| 72 | 2017 (USA) | CR | 2 (6–11) | A | A |
| 73 | 2016 (Turkey) | CS | 24 (3–12) | NI | NI |
| 74 | 2016 (Turkey) | CT | 25 (4–22) | POT | excessive weakness at the application site |
| 75 | 2015 (Australia) | CT | 41 (2–16) | NI | pain at the application site and temporary muscle weakness |
| 76 | 2015 (USA) | NSR | 98 (3–11) | NI | temporary muscle weakness, transient pain, irritability, nausea, vomiting, fatigue, increased seizure episodes, increased salivation, slower speech, transient low-grade fever, and blister-like lesion at the application site |
| 77 | 2021 (Sweden) | CT | 8817 (4–19) | NI | NI |
| 78 | 2021 (USA) | SR | 761 (0–2) | POT | local or generalized weakness, pain at the application site, bruising, and vomiting |
| 79 | 2019 (France) | NSR | NI | NI | pseudo influenza, rash, pain, cramps, and bruising at the application site |
| 17 | 2018 (Poland) | CT | 60 (2–16) | NI | NI |
| 80 | 2016 (China) | CR | 1 (17) | POT | NI |
| 81 | 2022 (China) | CT | 91 (2–10) | NI | NI |
| Adjunct therapies effects assessment and BTX injections effects | |||||
| 82 | 2019 (France) | SR | 662 (3–13) | POT | pain, skin irritation, and muscle atrophy associated with plaster immobilization |
| 83 | 2018 (Netherlands) | CT | 65 (4–12) | P | A |
| 84 | 2018 (USA) | CT | 65 (2–17) | POT | NI |
| 85 | 2017 (Italy) | CT | 10 (3–14) | P | NI |
| 86 | 2014 (South Korea) | CT | 38 (2–14) | P | NI |
| 87 | 2014 (Australia) | CT | 30 (4–14) | P | NI |
| 88 | 2013 (India) | CT | 36 (2–8) | P | NI |
| 89 | 2019 (South Korea) | SR | 1264 (1–9) | POT | NI |
*Years. BTX: Botulinum toxin; SRN: study reference number; RP: rehabilitation program; RC: retrospective cohort; NI: not informed; A: absent; SC: specialists’ consensus; POT: physiotherapy and occupational therapy; CT: clinical trial; O: others; P: physiotherapy; CR: case report; CS: case series; NSR: non-systematic review; SR: systematic review.
Concerning clinical trials, 22 (31.4%) were conducted in Asia, 20 (28.6%) in Europe, nine (12.9%) in North America, nine (12.9%) in Euro-Asia (specifically Turkey), seven (10.0%) in Oceania, two (2.9%) in Africa, and one (1.4%) in South America.
Referring to systematic reviews, three (30%) originated from Euro-Asia (Turkey), two (20%) from Europe, two (20%) from North America, two (20%) from South America, and one (10%) was from Asia (Tables 1–6) 4-105
The description of the objectives of the BTX administration included in the studies was categorized into different domains: improvement of gait (n=71; 67.6%), enhancement of upper limb motor function (n=28; 26.7%), facilitation of axillary and inguinal hygiene care (n=15; 14.3%), reduction of sialorrhea (n=14; 13.3%), prevention of musculoskeletal deformities (n=9; 8.6%), and improvement of pain (n=5; 4.8%). Furthermore, a significant portion of the studies aimed to cover more than one domain (n=30; 28.6%), and three studies (2.9%) lacked data on their objectives. Notably, 92.4% (n=97) of the studies achieved the proposed objective.
The most commonly used BTX in the studies was Botox® (n=73; 69.5%), followed by Dysport® (n=27; 25.7%), Xeomin® (n=8; 7.6%), Myobloc® (n=4; 3.8%), Medytox®/Botulift® (n=3; 2.9%), Prosigne® (n=1; 0.9%), and Botulim®/Botulax® (n=1; 0.9%); and 4 (3.8%) did not specify which BTX was administered. It is important to note that many studies used more than one type of BTX.
Regarding the type of BTX adopted in clinical trials (n=70), the majority exclusively used Botox® (n=36; 51,4%), while Dysport® was the second most commonly used (n=10; 14.3%).
Regarding the number of administrations, most studies (n=75; 71.4%) performed up to two administrations, followed by five or more (n=7; 6.7%), up to three administrations (n=5; 4.8%), and up to four (n=2; 1.9%); and 16 studies (15.2%) did not report the number of administrations performed.
Regarding orthoses, 39 studies (37.1%) utilized this device; however, most did not report this information (n=65; 31.9%).
Considering the effective improvement in gait outcomes, this result was achieved in a clinical trial conducted by Dursun et al. 6 The trial evaluated the efficacy of Dysport® injections in 241 patients aged 2–17 years. Kim et al. carried out a clinical trial with 29 patients between the ages of 3–14 years, demonstrating an improvement in gait performance compared to knee flexion without altering the muscular structure. 8 In another clinical trial conducted by Wesseling M involving 24 patients aged 7–9 years, there was no deterioration in gait stability after the administration of BTX. 5 Valentine J carried out a clinical trial involving 40 children and adolescents (8–16 years old) with CP classified in Gross Motor Function Classification System (GMFCS) level II, which demonstrated an increase in gait functionality after the administration of BTX. 9 Hastings-Ison et al. performed a clinical trial with 42 children aged 2–5 years to compare the efficacy of one annual Botox® administration versus three annual administrations for the treatment of spastic dynamic equinus. The annual administration demonstrated the same level of efficacy as the three annual administrations. This study found that 36.3% of the participants experienced moderate adverse effects, including flu-like symptoms, localized muscle pain, vomiting, and occasional gait instability. 28
Regarding the functional improvement outcome of the upper limbs, a favorable result was achieved with a good safety profile in a clinical trial conducted by Delgado et al. with 210 patients between 2–17 years of age with the administration of Dysport®. 53 There were good functional results for BTX administration in upper limbs with the association of functional electrical stimulation in a clinical trial conducted by Elnaggar et al. 54
In the clinical trial by Lee et al., the administration of Meditoxin® in the hips of CP children to prevent dislocation yielded positive results. 63 Regarding the prevention of equinus deformity, Chang et al. conducted a clinical trial involving 144 children aged 2–10 years who received two types of BTX toxin: Botulax® (letibotulinumtoxinA) and Botox® (onabotulinumtoxinA), with improvement of gait and prevention of equinus deformity in both types of BTX. There were records of two patients (2.86%) who experienced adverse effects due to the administration of Botox®, including urticaria and short-term dysphonia. 24
In a systematic review by Almina et al., the administration of BTX for spastic hips reduced pain. It facilitated daily care for non-ambulatory children with GMFCS levels IV and V, who could not walk. 65 Botox® and Dysport® were used in the study.
The BTX administration program, provided by the public health system of Vale do Jequitinhonha, Brazil, effectively improved gait and functional independence for daily activities. This conclusion was drawn from a clinical trial conducted by Silva et at. involving 14 patients aged 2–18 years. 50
The study conducted by Bussmann et al., which evaluated the cost-effectiveness of BTX in 60 children with walking capacity, found that BTX was efficacious when administered by specialist doctors in physical medicine and rehabilitation and/or trained neurologists. 4
Systematic outpatient treatment with BTX injections, combined with physiotherapy, occupational therapy, plaster, and/or orthoses associated with rehabilitation team treatment, has been recommended in several studies to achieve the set objectives successfully. 3,4,45,59,82,60 Hareb et al. conducted a non-systematic review. They reported that the administration of BTX is well-supported in the literature, especially for spasticity in children over 2 years old, requiring a multidisciplinary approach. The following BTXs were included in the studies in this review: Botox®, Dysport®, and Xeomin®. There were reports of adverse effects such as pseudo-influenza, skin rashes, pain, cramps, and bruises at the administration site. 79
Yi et al. conducted a clinical trial with 14 children aged 2–10 years with hemiplegia who had equinus gait on the affected side to evaluate whether the repeated administrations of Botox® could impact the growth and strength of the gastrocnemius muscle fibers. The study reported no significant difference in muscle structure due to successive administration. There were reports of possible adverse effects during administration, including discomfort, general weakness, headache, dry mouth, pain at the injection site, viral infection, dizziness, back pain, and fatigue. 91
Shoval et al. conducted a clinical trial with 52 patients aged one month to 17 years to evaluate the administration of Botox® in salivary glands and multiple muscle segments to treat sialorrhea and axillary and inguinal hygiene. The majority of patients had severe functional CP sequelae. Consequently, there was a decrease in drooling and an improvement in upper limb function, gait, and axillary and inguinal hygiene. Adverse effects were observed in 4% of patients who received Botox®, in the salivary glands, and 7% of those who received BTX, in the glands and muscles. The most severe adverse effects included redness at the administration site lasting for one day, dry mouth for two weeks, and mild difficulty swallowing thick saliva for two months. 15
Juneja et al. conducted a clinical trial involving 29 children aged 2–7 years who received Botox® injections and an intensive rehabilitation program, which improved the gait pattern. Adverse effects were fever caused by mild upper respiratory tract infection (10.3%) and temporary muscular weakness (6.9%). 21 Yana et al. conducted a systematic review on the efficacy of Botox® and/or Dysport® injections in the lower limbs of spastic children with CP. The study reported that the administration improved spasticity and range of motion when combined with physiotherapy. However, the results of this systematic review were inconclusive regarding whether the combination of BTX and physiotherapy is more effective than isolated physiotherapy at improving motor function. The adverse effects described were pain at the administration site, muscle weakness, and localized dysesthesia. 45
Regarding the adverse effects reported in clinical trials, 13 participants (12.4%) experienced systemic adverse effects. Out of these, seven (53.8%) were treated with Botox®, two (15.4%) with both Botox® and Dysport®, one (7.7%) with Botulax® and Botox®, one (7.7%) was treated with Botox®, Dysport®, and Xeomin®, and one (7.7%) did not specify which BTX was used (Tables 1-6). 4-105
Regarding safety and efficacy, Ploypetch et al. conducted a medical chart review on the adverse effects of combined multilevel chemical blocks of Botox® and 5% Phenol for spasticity at a university medical center in the United States. The study included 98 children aged 3–11 years who received at least one multilevel chemical block, resulting in a total of 146 administrations. The most frequent adverse effect was temporary muscle weakness, which occurred in 11.0% of cases and was accompanied by a higher incidence of falls, at 3.0%. The incidence of muscle weakness was significantly higher in the group that administered only Botox® (20.0%) compared to the group that combined the administration of Botox® and 5% Phenol (7.6%). Pain at the administration site (4.8%) and local blister (0.7%) only occurred in the group that performed combined administration. Other less frequent adverse effects were observed, including irritability (4.1%), nausea (4.1%), vomiting (4.1%), fatigue (4.1%), increased frequency of seizures (4.1%), sialorrhea (4.1%), slower speech (0.7%), and temporary low-grade fever (0.7%). 76 In a clinical trial conducted by Kaňovský et al. involving 370 patients aged 2–17 years, the administration of Xeomin® was safe and efficacious for spastic upper and lower limbs. There were reports of adverse effects in 4.3% of cases, including muscle weakness (0.3%), seizure in a patient with epilepsy (0.3%), and constipation (0.3%). 41 In a clinical trial conducted by Dimitrova et al. involving 381 patients aged 2–17 years, physiotherapy was associated with significantly reduced spasticity and improved lower limb movement. Adverse effects were observed in 2.8% of cases, including mild to moderate exacerbation of seizures, which were more frequent in the group that received a dose of 8 U/K, but similar to the placebo group. 42 Ayala et al. conducted a systematic review of interventions for infants with a confirmed diagnosis of CP suffering from spasticity. In cases of moderate to severe spasticity, they recommended the administration of BTX for this age group, as it positively impacts functionality and social participation despite the paucity of evidence. In this review, the authors observed several adverse effects, including muscle weakness, injection site pain, bruising, and vomiting. 78 Bonikowski and Sławek conducted a non-systematic review to compare the safety and efficacy of Botox®, Xeomin®, and Dysport®, taking into consideration their pre-administration preparation. These treatments were safe and efficacious against both upper and lower limb hypertonia. However, some adverse effects have been reported, including respiratory tract infections, bronchitis, pharyngitis, asthma, muscle weakness, urinary incontinence, falls, seizures, temporary low-grade fever, and pain at the injection site. It is important to note that these adverse effects were temporary and had a low incidence rate. 44
DISCUSSION
The integrative review method enables the synthesis of knowledge and the incorporation of relevant study results into practice. It is the most thorough methodological approach among reviews, as it permits the inclusion of both experimental and non-experimental studies, thereby providing a comprehensive understanding of the phenomenon under consideration. This method combines theoretical and empirical literature data for a variety of purposes, which includes defining concepts, reviewing theories and evidence, and analyzing methodological problems related to a particular topic. The extensive sample and variety of proposals should generate a coherent and comprehensible overview of intricate concepts, theories, or health issues. Since 1980, the integrative review has been reported in the literature as a research method. 106,107
It is extremely relevant the fact that it is a broad review on the subject, being the first integrative review on this topic worldwide on the therapeutic administration of BTX in children and adolescents with CP, according to PubMed. Furthermore, there were no significant study limitations and there was no conflict of interests by the authors, particularly with the pharmaceutical industry.
The methodology employed in this type of review enables the inclusion of more articles than in a systematic review. All 105 articles included demonstrated that BTX is safe and efficacious in treating spasticity, particularly when administered in a multi-professional rehabilitation team approach. This approach increases the likelihood of functional improvement in both gait and voluntary activities involving the upper limbs. However, it can be beneficial for patients who do not have a prognosis for walking or active movement of their upper limbs to facilitate their daily care in terms of hygiene, dressing and undressing, and reducing sialorrhea.
Most studies were conducted in Europe, Asia, and North America. Only 1.9% of the participants were from Africa, which may be attributed to socioeconomic challenges and limited access to medication. This highlights the urgent need for BTX administration research funding in developing countries.
The primary outcome was an improvement in gait, followed by an improvement in upper limb motor skills and in daily care, including axillary and inguinal hygiene. This demonstrates the vast therapeutic potential of this medication in children and adolescents with CP, even in patients without a functional prognosis for gait.
Most clinical trials (51.4%) were exclusively conducted with Botox®, suggesting that this BTX is the most commonly administered.
The articles highlighted the improvement in gait 5,6,8 and upper limb 53 functions due to BTX administration. These findings demonstrate the efficacy of BTX in these areas and its safety in terms of adverse effects. Ideally, the administration should be performed by medical specialists in physical medicine and rehabilitation (physiatry) and/or neurologists 106 affiliated with a multi-professional rehabilitation program. 50
The fact that 50.5% of articles lack information on adverse effects is extremely relevant, as such data is crucial for the clinical administration of BTX in terms of safety and effectiveness. There were reports of systemic adverse effects in 12.4% of clinical trials; however, no deaths were directly related to the administration of BTX. This reaffirms the safety of BTX administration in children with CP. The fact that Botox® is the most commonly administered BTX in clinical trials justifies that 53.8% of clinical trials with systemic adverse effects involved Botox®. In 34.3% of the articles, no data was available on rehabilitation therapies administered concurrently with BTX. The effectiveness of BTX administration depends on multidisciplinary rehabilitation treatment. 106
Our study has some limitations: the search for only the last ten years, the fact that only articles in English were analyzed, and no database other than MEDLINE/PubMed was used in the search for articles.
The administration of BTX for spasticity in children with CP is efficacious and safe. This is particularly true when combined with a multidisciplinary rehabilitation team approach, which increases the likelihood of functional improvement in both gait and voluntary upper limb movement. However, patients who do not have a prognosis of walking or active movement of their upper limbs can still benefit from using applications that facilitate their daily care, such as hygiene, dressing and undressing, and reducing sialorrhea, all these indications with safety and effectivity. Experienced and well-trained doctors, typically found in rehabilitation centers, physiatrists, and neuro-pediatricians, safely and effectively perform chemical blockade using BTX.
Footnotes
Funding This study did not receive any funding.
REFERENCES
- 1.Sposito MM. Botulinum toxin type A: pharmacological properties and clinical use. Acta Fisiátr. 2004;11(Supl.1):S7–44. [Google Scholar]
- 2.Simon O, Yelnik AP. Managing spasticity with drugs. Eur J Phys Rehabil Med. 2010;46:401–10. [PubMed] [Google Scholar]
- 3.Jost W. Pictorial atlas of botulinum toxin injection: dosage, localization, application. 2nd. Berlin: Quintessence Pub Co; 2013. [Google Scholar]
- 4.Bussmann JB, Pangalila RF, Stam HJ, Schasfoort F. The role of botulinum toxin in multimodal treatment of spasticity in ambulatory children with spastic Cerebral Palsy: extensive evaluation of a cost-effectiveness trial. J Rehabil Med. 2020;52:jrm00059. doi: 10.2340/16501977-2680. [DOI] [PubMed] [Google Scholar]
- 5.Wesseling M, Kainz H, Hoekstra T, Van Rossom S, Desloovere K, Groote F, et al. Botulinum toxin injections minimally affect modelled muscle forces during gait in children with cerebral palsy. Gait Posture. 2020;82:54–60. doi: 10.1016/j.gaitpost.2020.08.122. [DOI] [PubMed] [Google Scholar]
- 6.Dursun N, Bonikowski M, Dabrowski E, Matthews D, Gormley M, Tilton A, et al. Efficacy of repeat abobotulinumtoxin A (Dysport®) injections in improving gait in children with spastic cerebral palsy. Dev Neurorehabil. 2020;23:368–74. doi: 10.1080/17518423.2019.1687602. [DOI] [PubMed] [Google Scholar]
- 7.León-Valenzuela A, Palacios JS, Del Pinho Algarrada R. IncobotulinumtoxinA for the treatment of spasticity in children with cerebral palsy – a retrospective case series focusing on dosing and tolerability. BMC Neurol. 2020;20:126. doi: 10.1186/s12883-020-01702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SK, Rha DW, Park ES. Botulinum toxin type A injections impact hamstring muscles and gait parameters in children with flexed knee gait. Toxins (Basel) 2020;12:145. doi: 10.3390/toxins12030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentine J, Davidson SA, Bear N, Blair E, Paterson L, Ward R, et al. A prospective study investigating gross motor function of children with cerebral palsy and GMFCS level II after long-term Botulinum toxin type A use. BMC Pediatr. 2020;20:7. doi: 10.1186/s12887-019-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JY, Kim SK, Park ES. The effect of botulinum toxin injections on gross motor function for lower limb spasticity in children with cerebral palsy. Toxins (Basel) 2019;11:651. doi: 10.3390/toxins11110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dursun N, Akarsu M, Gokbel T, Akyuz M, Karacan C, Dursun E. Switching from onabotulinumtoxin-A to abobotulinumtoxin-A in children with cerebral palsy treated for spasticity: A retrospective safety and efficacy evaluation. J Rehabil Med. 2019;51:390–4. doi: 10.2340/16501977-2550. [DOI] [PubMed] [Google Scholar]
- 12.Raftemo AE, Mahendran A, Hollung SJ, Jahnsen RB, Lydersen S, Vik T, et al. Use of botulinum toxin A in children with cerebral palsy. Tidsskr Nor Laegeforen. 2019:139. doi: 10.4045/tidsskr.18.0554. [DOI] [PubMed] [Google Scholar]
- 13.Okur SÇ, Uğur M, Şenel K. Effects of botulinum toxin A injection on ambulation capacity in patients with cerebral palsy. Dev Neurorehabil. 2019;22:288–91. doi: 10.1080/17518423.2018.1502832. [DOI] [PubMed] [Google Scholar]
- 14.Elnaggar RK, Elbanna MF. Evaluation of independent versus integrated effects of reciprocal electrical stimulation and botulinum toxin-A on dynamic limits of postural stability and ankle kinematics in spastic diplegia: a single-blinded randomized trial. Eur J Phys Rehabil Med. 2019;55:241–9. doi: 10.23736/S1973-9087.18.05196-1. [DOI] [PubMed] [Google Scholar]
- 15.Shoval H, Levin J, Friel K, Kim H. Safety of combined salivary gland and multilevel intramuscular onabotulinumtoxinA injections with and without ethanol in pediatric patients with cerebral palsy: a retrospective study. J Pediatr Rehabil Med. 2019;12:189–96. doi: 10.3233/PRM-180552. [DOI] [PubMed] [Google Scholar]
- 16.Yana M, Tutuola F, Westwater-Wood S, Kavlak E. The efficacy of botulinum toxin A lower limb injections in addition to physiotherapy approaches in children with cerebral palsy: a systematic review. NeuroRehabilitation. 2019;44:175–89. doi: 10.3233/NRE-182581. [DOI] [PubMed] [Google Scholar]
- 17.Mirska A, Kułak W, Okurowska-Zawada B, Dmitruk E. Effectiveness of multiple botulinum toxin sessions and the duration of effects in spasticity therapy in children with cerebral palsy. Childs Nerv Syst. 2019;35:141–7. doi: 10.1007/s00381-018-3923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca PR, JR, Moura RC, Galli M, Oliveira CS. Effect of physiotherapeutic intervention on the gait after the application of botulinum toxin in children with cerebral palsy: systematic review. Eur J Phys Rehabil Med. 2018;54:757–65. doi: 10.23736/S1973-9087.17.04940-1. [DOI] [PubMed] [Google Scholar]
- 19.Brandenburg JE, Eby SF, Song P, Bamlet WR, Sieck GC, An KN. Quantifying effect of onabotulinum toxin A on passive muscle stiffness in children with cerebral palsy using ultrasound shear wave elastography. Am J Phys Med Rehabil. 2018;97:500–6. doi: 10.1097/PHM.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda M, Tomita K, Yozu A, Nakayama T, Nakayama J, Ohguro H, et al. Effect of botulinum toxin type A treatment in children with cerebral palsy: sequential physical changes for 3 months after the injection. Brain Dev. 2018;40:452–7. doi: 10.1016/j.braindev.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Juneja M, Jain R, Gautam A, Khanna R, Narang K. Effect of multilevel lower-limb botulinum injections & intensive physical therapy on children with cerebral palsy. Indian J Med Res. 2017;146:S8–14. doi: 10.4103/ijmr.IJMR_1223_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Read FA, Boyd RN, Barber LA. Longitudinal assessment of gait quality in children with bilateral cerebral palsy following repeated lower limb intramuscular Botulinum toxin-A injections. Res Dev Disabil. 2017;68:35–41. doi: 10.1016/j.ridd.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Hong BY, Chang HJ, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy of repeated botulinum toxin type A injections for spastic equinus in children with cerebral palsy-A secondary analysis of the randomized clinical trial. Toxins (Basel) 2017;9:253. doi: 10.3390/toxins9080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang HJ, Hong BY, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy and safety of letibotulinum toxin A for the treatment of dynamic equinus foot deformity in children with cerebral palsy: a randomized controlled trial. Toxins (Basel) 2017;9:252. doi: 10.3390/toxins9080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löwing K, Thews K, Haglund-Åkerlind Y, Gutierrez-Farewik EM. Effects of botulinum toxin-A and goal-directed physiotherapy in children with cerebral palsy GMFCS levels I & II. Phys Occup Ther Pediatr. 2017;37:268–82. doi: 10.3109/01942638.2016.1150384. [DOI] [PubMed] [Google Scholar]
- 26.Dursun N, Gokbel T, Akarsu M, Dursun E. Randomized controlled trial on effectiveness of intermittent serial casting on spastic equinus foot in children with cerebral palsy after botulinum toxin-A treatment. Am J Phys Med Rehabil. 2017;96:221–5. doi: 10.1097/PHM.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 27.Kahraman A, Seyhan K, Değer Ü, Kutlutürk S, Mutlu A. Should botulinum toxin A injections be repeated in children with cerebral palsy? A systematic review. Dev Med Child Neurol. 2016;58:910–7. doi: 10.1111/dmcn.13135. [DOI] [PubMed] [Google Scholar]
- 28.Hastings-Ison T, Blackburn C, Rawicki B, Fahey M, Simpson P, Baker R, et al. Injection frequency of botulinum toxin A for spastic equinus: a randomized clinical trial. Dev Med Child Neurol. 2016;58:750–7. doi: 10.1111/dmcn.12962. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwenhuys A, Papageorgiou E, Pataky T, Laet T, Molenaers G, Desloovere K. Literature review and comparison of two statistical methods to evaluate the effect of botulinum toxin treatment on gait in children with cerebral palsy. PLoS One. 2016;11:e0152697. doi: 10.1371/journal.pone.0152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi JY, Jung S, Rha DW, Park ES. Botulinum toxin Type A injection for spastic equinovarus foot in children with spastic cerebral palsy: effects on gait and foot pressure distribution. Yonsei Med J. 2016;57:496–504. doi: 10.3349/ymj.2016.57.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentine J, Stannage K, Fabian V, Ellis K, Reid S, Pitcher C, et al. Muscle histopathology in children with spastic cerebral palsy receiving botulinum toxin type A. Muscle Nerve. 2016;53:407–14. doi: 10.1002/mus.24763. [DOI] [PubMed] [Google Scholar]
- 32.Delgado MR, Tilton A, Russman B, Benavides O, Bonikowski M, Carranza J, et al. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: a randomized controlled trial. Pediatrics. 2016;137:e20152830. doi: 10.1542/peds.2015-2830. [DOI] [PubMed] [Google Scholar]
- 33.Mudge A, Harvey LA, Lancaster A, Lowe K. Electrical stimulation following botulinum toxin A in children with spastic diplegia: a within-participant randomized pilot study. Phys Occup Ther Pediatr. 2015;35:342–53. doi: 10.3109/01942638.2014.990548. [DOI] [PubMed] [Google Scholar]
- 34.Bar-On L, Van Campenhout A, Desloovere K, Aertbeliën E, Huenaerts C, Vandendoorent B, et al. Is an instrumented spasticity assessment an improvement over clinical spasticity scales in assessing and predicting the response to integrated botulinum toxin type a treatment in children with cerebral palsy? Arch Phys Med Rehabil. 2014;95:515–23. doi: 10.1016/j.apmr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Liu JJ, Ji SR, Wu WH, Zhang Y, Zeng FY, Li NL. The relief effect of botulinum toxin-A for spastic iliopsoas of cerebral palsy on children. Eur Rev Med Pharmacol Sci. 2014;18:3223–8. [PubMed] [Google Scholar]
- 36.Boyaci A, Tutoglu A, Boyaci N, Koca I, Calik M, Sakalar A, et al. Changes in spastic muscle stiffness after botulinum toxin A injections as part of rehabilitation therapy in patients with spastic cerebral palsy. NeuroRehabilitation. 2014;35:123–9. doi: 10.3233/NRE-141107. [DOI] [PubMed] [Google Scholar]
- 37.Bar-On L, Aertbeliën E, Molenaers G, Van Campenhout A, Vandendoorent B, Nieuwenhuys A, et al. Instrumented assessment of the effect of Botulinum Toxin-A in the medial hamstrings in children with cerebral palsy. Gait Posture. 2014;39:17–22. doi: 10.1016/j.gaitpost.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Williams SA, Elliott C, Valentine J, Gubbay A, Shipman P, Reid S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: the impact on muscle morphology and strength. Disabil Rehabil. 2013;35:596–605. doi: 10.3109/09638288.2012.711898. [DOI] [PubMed] [Google Scholar]
- 39.Jang DH, Sung IY, Kang YJ. Usefulness of the tendon reflex for assessing spasticity after botulinum toxin-a injection in children with cerebral palsy. J Child Neurol. 2013;28:21–6. doi: 10.1177/0883073812450615. [DOI] [PubMed] [Google Scholar]
- 40.Park GY, Kwon DR. Sonoelastographic evaluation of medial gastrocnemius muscles intrinsic stiffness after rehabilitation therapy with botulinum toxin a injection in spastic cerebral palsy. Arch Phys Med Rehabil. 2012;93:2085–9. doi: 10.1016/j.apmr.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Kaňovský P, Heinen F, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. Safety and efficacy of repeat long-term incobotulinumtoxinA treatment for lower limb or combined upper/lower limb spasticity in children with cerebral palsy. J Pediatr Rehabil Med. 2022;15:113–27. doi: 10.3233/PRM-210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitrova R, Kim H, Meilahn J, Chambers HG, Racette BA, Bonikowski M, et al. Efficacy and safety of onabotulinumtoxinA with standardized physiotherapy for the treatment of pediatric lower limb spasticity: a randomized, placebo-controlled, phase III clinical trial. NeuroRehabilitation. 2022;50:33–46. doi: 10.3233/NRE-210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Kolaski K. Is botulinum toxin type A more effective and safer than other treatments for the management of lower limb spasticity in children with cerebral palsy? A Cochrane Review summary with commentary. NeuroRehabilitation. 2021;49:161–4. doi: 10.3233/NRE-218003. [DOI] [PubMed] [Google Scholar]
- 44.Bonikowski M, Sławek J. Safety and efficacy of Botulinum toxin type A preparations in cerebral palsy – an evidence-based review. Neurol Neurochir Pol. 2021;55:158–64. doi: 10.5603/PJNNS.a2021.0032. [DOI] [PubMed] [Google Scholar]
- 45.Yana M, Tutuola F, Westwater-Wood S, Kavlak E. The efficacy of botulinum toxin A lower limb injections in addition to physiotherapy approaches in children with cerebral palsy: a systematic review. NeuroRehabilitation. 2019;44:175–89. doi: 10.3233/NRE-182581. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen L, Di Rezze B, Mesterman R, Rosenbaum P, Gorter JW. Effects of Botulinum toxin treatment in nonambulatory children and adolescents with cerebral palsy: understanding parents’ perspectives. J Child Neurol. 2018;33:724–33. doi: 10.1177/0883073818786567. [DOI] [PubMed] [Google Scholar]
- 47.Lin YC, Lin IL, Chou TF, Lee HM. Quantitative evaluation for spasticity of calf muscle after botulinum toxin injection in patients with cerebral palsy: a pilot study. J Neuroeng Rehabil. 2016;13:25. doi: 10.1186/s12984-016-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirska A, Cybula K, Okurowska-Zawada B, Kułak W, Dmitruk E, Okulczyk K, et al. Use of botulinum toxin in the treatment of ankle plantar flexor spasticity in children with cerebral palsy. J Pediatr Orthop B. 2014;23:517–22. doi: 10.1097/BPB.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 49.Rha DW, Park ES, Jung S, Lee SC, Suh M, Choi HS. Comparison of ultrasound-guided anterior and posterior approaches for needle insertion into the tibialis posterior in hemiplegic children with spastic cerebral palsy. Am J Phys Med Rehabil. 2014;93:841–8. doi: 10.1097/PHM.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 50.Silva GF, Teles MC, Santos SA, Ferreira FO, Almeida KM, Camargos AC. Evaluation of a program of application of the botulinum toxin type A in children with cerebral palsy in Vale do Jequitinhonha. Cien Saude Colet. 2013;18:2075–84. doi: 10.1590/s1413-81232013000700023. [DOI] [PubMed] [Google Scholar]
- 51.Jianjun L, Shurong J, Weihong W, Yan Z, Fanyong Z, Nanling L. Botulinum toxin-A with and without rehabilitation for the treatment of spastic cerebral palsy. J Int Med Res. 2013;41:636–41. doi: 10.1177/0300060513488515. [DOI] [PubMed] [Google Scholar]
- 52.Dabrowski E, Chambers HG, Gaebler-Spira D, Banach M, Kaňovský P, Dersch H, et al. IncobotulinumtoxinA efficacy/safety in upper-limb spasticity in pediatric cerebral palsy: randomized controlled trial. Pediatr Neurol. 2021;123:10–20. doi: 10.1016/j.pediatrneurol.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Delgado MR, Tilton A, Carranza-Del Río J, Dursun N, Bonikowski M, Aydin R, et al. Efficacy and safety of abobotulinumtoxinA for upper limb spasticity in children with cerebral palsy: a randomized repeat-treatment study. Dev Med Child Neurol. 2021;63:592–600. doi: 10.1111/dmcn.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elnaggar RK, Alqahtani BA, Elbanna MF. Functional outcomes of botulinum neurotoxin-A injection followed by reciprocal electrical stimulation in children with cerebral palsy: a randomized controlled trial. Restor Neurol Neurosci. 2020;38:431–41. doi: 10.3233/RNN-201088. [DOI] [PubMed] [Google Scholar]
- 55.Lin YC, Huang CY, Lin IL, Shieh JY, Chung YT, Chen KL. Evaluating functional outcomes of botulinum toxin type A injection combined with occupational therapy in the upper limbs of children with cerebral palsy: a 9-month follow-up from the perspectives of both child and caregiver. PLoS One. 2015;10:e0142769. doi: 10.1371/journal.pone.0142769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lidman G, Nachemson A, Peny-Dahlstrand M, Himmelmann K. Botulinum toxin A injections and occupational therapy in children with unilateral spastic cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 2015;57:754–61. doi: 10.1111/dmcn.12739. [DOI] [PubMed] [Google Scholar]
- 57.Speth L, Janssen-Potten Y, Leffers P, Rameckers E, Defesche A, Winkens B, et al. Effects of botulinum toxin A and/or bimanual task-oriented therapy on upper extremity impairments in unilateral cerebral palsy: an explorative study. Eur J Paediatr Neurol. 2015;19:337–48. doi: 10.1016/j.ejpn.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Ferrari A, Maoret AR, Muzzini S, Alboresi S, Lombardi F, Sgandurra G, et al. A randomized trial of upper limb botulimun toxin versus placebo injection, combined with physiotherapy, in children with hemiplegia. Res Dev Disabil. 2014;35:2505–13. doi: 10.1016/j.ridd.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Andersson G, Renström B, Blaszczyk I, Domellöf E. Upper-extremity spasticity-reducing treatment in adjunct to movement training and orthoses in children with cerebral palsy at gross motor function- and manual ability classification system levels IV-V: a descriptive study. Dev Neurorehabil. 2020;23:349–58. doi: 10.1080/17518423.2019.1655677. [DOI] [PubMed] [Google Scholar]
- 60.Lidman GR, Nachemson AK, Peny-Dahlstrand MB, Himmelmann KM. Long-term effects of repeated botulinum neurotoxin A, bimanual training, and splinting in young children with cerebral palsy. Dev Med Child Neurol. 2020;62:252–8. doi: 10.1111/dmcn.14298. [DOI] [PubMed] [Google Scholar]
- 61.Aydil S, Akpinar FM, Akpinar E, Beng K, Yagmurlu MF. Effectiveness of multilevel botulinum toxin A injection with integrated treatment program on spasticity reduction in non-ambulatory young children with cerebral palsy. Med Princ Pract. 2019;28:309–14. doi: 10.1159/000499369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang HJ, Hong BY, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy and safety of letibotulinum toxin A for the treatment of dynamic equinus foot deformity in children with cerebral palsy: a randomized controlled trial. Toxins (Basel) 2017;9:252. doi: 10.3390/toxins9080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y, Lee S, Jang J, Lim J, Ryu JS. Effect of botulinum toxin injection on the progression of hip dislocation in patients with spastic cerebral palsy: a pilot study. Toxins (Basel) 2021;13:872. doi: 10.3390/toxins13120872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne-Pees L, Kentish M, et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. J Pediatr. 2014;165:140–46.e4. doi: 10.1016/j.jpeds.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 65.Almina S, Karile Y, Audrone P, Indre B. Analgesic effect of botulinum toxin in children with cerebral palsy: a systematic review. Toxicon. 2021;199:60–7. doi: 10.1016/j.toxicon.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Lin CY, Chung CH, Matthews DJ, Chu HY, Chen LC, Yang SS, et al. Long-term effect of botulinum toxin A on the hip and spine in cerebral palsy: a national retrospective cohort study in Taiwan. PLoS One. 2021;16:e0255143. doi: 10.1371/journal.pone.0255143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yalçınkaya EY, Saygı EK, Taşkıran ÖO, Çapan N, Kutlay Ş, Tur BS, et al. Consensus recommendations for botulinum toxin injections in the spasticity management of children with cerebral palsy during COVID-19 outbreak. Turk J Med Sci. 2021;51:385–92. doi: 10.3906/sag-2009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertan H, Oncu J, Vanli E, Alptekin K, Sahillioglu A, Kuran B, et al. Use of shear wave elastography for quantitative assessment of muscle stiffness after botulinum toxin injection in children with cerebral palsy. J Ultrasound Med. 2020;39:2327–37. doi: 10.1002/jum.15342. [DOI] [PubMed] [Google Scholar]
- 69.Dağ N, Cerit MN, Şendur HN, Zinnuroğlu M, Muşmal BN, Cindil E, et al. The utility of shear wave elastography in the evaluation of muscle stiffness in patients with cerebral palsy after botulinum toxin A injection. J Med Ultrason (2001) 2020;47:609–15. doi: 10.1007/s10396-020-01042-6. [DOI] [PubMed] [Google Scholar]
- 70.McLaughlin MJ, Fisher MT, Vadivelu S, Ramsey J, Ratnasingam D, McGhee E, et al. A risk-stratified approach toward safely resuming OnabotulinumtoxinA injections based on dosing and ambulatory status in pediatric patients with cerebral palsy during the Coronavirus pandemic of 2019 (COVID-19) J Pediatr Rehabil Med. 2020;13:273–9. doi: 10.3233/PRM-200756. [DOI] [PubMed] [Google Scholar]
- 71.Schasfoort F, Dallmeijer A, Pangalila R, Catsman C, Stam H, Becher J, et al. Value of botulinum toxin injections preceding a comprehensive rehabilitation period for children with spastic cerebral palsy: a cost-effectiveness study. J Rehabil Med. 2018;50:22–9. doi: 10.2340/16501977-2267. [DOI] [PubMed] [Google Scholar]
- 72.Workinger MS, Kent RD, Meilahn JR. The effect of botulinum toxin A (Botox) injections used to treat limb spasticity on speech patterns in children with dysarthria and cerebral palsy: a report of two cases. J Pediatr Rehabil Med. 2017;10:137–43. doi: 10.3233/PRM-170433. [DOI] [PubMed] [Google Scholar]
- 73.Safer VB, Demir SO, Ozkan E, Guneri FD. Effects of botulinum toxin serotype A on sleep problems in children with cerebral palsy and on mothers sleep quality and depression. Neurosciences (Riyadh) 2016;21:331–7. doi: 10.17712/nsj.2016.4.20160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karaca B, Ünlü E, Köse G, Gönen E, Çakcı A. Outcomes of botulinum toxin type A injection followed by rehabilitation in cases of cerebral palsy with upper extremity involvement. J Child Neurol. 2016;31:357–63. doi: 10.1177/0883073815596609. [DOI] [PubMed] [Google Scholar]
- 75.Edwards P, Sakzewski L, Copeland L, Gascoigne-Pees L, McLennan K, Thorley M, et al. Safety of botulinum toxin type a for children with nonambulatory cerebral palsy. Pediatrics. 2015;136:895–904. doi: 10.1542/peds.2015-0749. [DOI] [PubMed] [Google Scholar]
- 76.Ploypetch T, Kwon JY, Armstrong HF, Kim H. A retrospective review of unintended effects after single-event multi-level chemoneurolysis with botulinum toxin-a and phenol in children with cerebral palsy. PM R. 2015;7:1073–80. doi: 10.1016/j.pmrj.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 77.Hägglund G, Hollung SJ, Ahonen M, Andersen GL, Eggertsdóttir G, Gaston MS, et al. Treatment of spasticity in children and adolescents with cerebral palsy in Northern Europe: a CP-North registry study. BMC Neurol. 2021;21:276. doi: 10.1186/s12883-021-02289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ayala L, Winter S, Byrne R, Fehlings D, Gehred A, Letzkus L, et al. Assessments and interventions for spasticity in infants with or at high risk for cerebral palsy: a systematic review. Pediatr Neurol. 2021;118:72–90. doi: 10.1016/j.pediatrneurol.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 79.Hareb F, Bertoncelli CM, Rosello O, Rampal V, Solla F. Botulinum toxin in children with cerebral palsy: an update. Neuropediatrics. 2020;51:1–5. doi: 10.1055/s-0039-1694988. [DOI] [PubMed] [Google Scholar]
- 80.Zhu YL, Zhang B, Li F. A potential new indication for botulinum toxin injection: a case study of spasticity with mirror movements. Chin Med J (Engl) 2016;129:2514–5. doi: 10.4103/0366-6999.191833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang H, Peng T, Yang X, Liu L, Xu Y, Zhao Y, et al. Plasma metabolomic changes in children with cerebral palsy exposed to botulinum neurotoxin. J Proteome Res. 2022;21:671–82. doi: 10.1021/acs.jproteome.1c00711. [DOI] [PubMed] [Google Scholar]
- 82.Mathevon L, Bonan I, Barnais JL, Boyer F, Dinomais M. Adjunct therapies to improve outcomes after botulinum toxin injection in children: a systematic review. Ann Phys Rehabil Med. 2019;62:283–90. doi: 10.1016/j.rehab.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Schasfoort F, Pangalila R, Sneekes EM, Catsman C, Becher J, Horemans H, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. J Rehabil Med. 2018;50:732–42. doi: 10.2340/16501977-2369. [DOI] [PubMed] [Google Scholar]
- 84.Shierk A, Jiménez-Moreno AC, Roberts H, Ackerman-Laufer S, Backer G, Bard-Pondarre R, et al. Development of a pediatric goal-centered upper limb spasticity home exercise therapy program for use in a phase-III trial of Abobotulinumtoxina (Dysport®) Phys Occup Ther Pediatr. 2019;39:124–35. doi: 10.1080/01942638.2018.1486346. [DOI] [PubMed] [Google Scholar]
- 85.Picelli A, La Marchina E, Gajofatto F, Pontillo A, Vangelista A, Filippini R, et al. Sonographic and clinical effects of botulinum toxin type A combined with extracorporeal shock wave therapy on spastic muscles of children with cerebral palsy. Dev Neurorehabil. 2017;20:160–4. doi: 10.3109/17518423.2015.1105320. [DOI] [PubMed] [Google Scholar]
- 86.Jang DH, Sung IY. The influence of physical therapy and anti-botulinum toxin antibody on the efficacy of botulinum toxin-A injections in children with spastic cerebral palsy. Dev Neurorehabil. 2014;17:414–9. doi: 10.3109/17518423.2014.938834. [DOI] [PubMed] [Google Scholar]
- 87.Thomas RE, Johnston LM, Boyd RN, Sakzewski L, Kentish MJ. GRIN: “GRoup versus INdividual physiotherapy following lower limb intra-muscular Botulinum toxin-A injections for ambulant children with cerebral palsy: an assessor-masked randomised comparison trial”: study protocol. BMC Pediatr. 2014;14:35. doi: 10.1186/1471-2431-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaturvedi SK, Rai Y, Chourasia A, Goel P, Paliwal VK, Garg RK, et al. Comparative assessment of therapeutic response to physiotherapy with or without botulinum toxin injection using diffusion tensor tractography and clinical scores in term diplegic cerebral palsy children. Brain Dev. 2013;35:647–53. doi: 10.1016/j.braindev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Kim SW, Jeon HR, Youk T, Kim J. The nature of rehabilitation services provided to children with cerebral palsy: a population-based nationwide study. BMC Health Serv Res. 2019;19:277. doi: 10.1186/s12913-019-4111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee D, Kim J, Oh JY, Han MH, Kim DY, Kang JH, et al. Changes in muscle mass after botulinum toxin injection in children with spastic hemiplegic cerebral palsy. Toxins (Basel) 2021;13:278. doi: 10.3390/toxins13040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yi YG, Jang DH, Lee D, Oh JY, Han MH. Botulinum toxin injection in children with hemiplegic cerebral palsy: correction of growth through comparison of treated and unaffected limbs. Toxins (Basel) 2019;11:688. doi: 10.3390/toxins11120688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schless SH, Cenni F, Bar-On L, Hanssen B, Kalkman B, O’brien T, et al. Medial gastrocnemius volume and echo-intensity after botulinum neurotoxin A interventions in children with spastic cerebral palsy. Dev Med Child Neurol. 2019;61:783–90. doi: 10.1111/dmcn.14056. [DOI] [PubMed] [Google Scholar]
- 93.Alexander C, Elliott C, Valentine J, Stannage K, Bear N, Donnelly CJ, et al. Muscle volume alterations after first botulinum neurotoxin A treatment in children with cerebral palsy: a 6-month prospective cohort study. Dev Med Child Neurol. 2018;60:1165–71. doi: 10.1111/dmcn.13988. [DOI] [PubMed] [Google Scholar]
- 94.Bilgici MC, Bekci T, Ulus Y, Bilgici A, Tomak L, Selcuk MB. Quantitative assessment of muscle stiffness with acoustic radiation force impulse elastography after botulinum toxin A injection in children with cerebral palsy. J Med Ultrason (2001) 2018;45:137–41. doi: 10.1007/s10396-017-0780-y. [DOI] [PubMed] [Google Scholar]
- 95.Salazar LF, Santos GL, Pavão SL, Rocha NA, Russo TL. Intrinsic properties and functional changes in spastic muscle after application of BTX-A in children with cerebral palsy: systematic review. Dev Neurorehabil. 2015;18:1–14. doi: 10.3109/17518423.2014.948640. [DOI] [PubMed] [Google Scholar]
- 96.Park ES, Sim E, Rha DW, Jung S. Architectural changes of the gastrocnemius muscle after botulinum toxin type A injection in children with cerebral palsy. Yonsei Med J. 2014;55:1406–12. doi: 10.3349/ymj.2014.55.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beukelaer N, Weide G, Huyghe E, Vandekerckhove I, Hanssen B, Peeters N, et al. Reduced cross-sectional muscle growth six months after botulinum toxin type-A injection in children with spastic cerebral palsy. Toxins (Basel) 2022;14:139. doi: 10.3390/toxins14020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paulson A, Zigler CK, Houtrow A, Pruitt D. Botulinum toxin: techniques within pediatric physiatry. PM R. 2019;11:38–44. doi: 10.1016/j.pmrj.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 99.Büyükavcı R, Büyükavcı MA. Effects of ultrasound-guided botulinum toxin type-A injections with a specific approach in spastic cerebral palsy. Acta Neurol Belg. 2018;118:429–33. doi: 10.1007/s13760-018-0929-5. [DOI] [PubMed] [Google Scholar]
- 100.Chau B, Chi B, Wilson T. Decreasing pediatric pain and agitation during botulinum toxin injections for spasticity with virtual reality: lessons learned from clinical use. J Pediatr Rehabil Med. 2018;11:199–204. doi: 10.3233/PRM-180534. [DOI] [PubMed] [Google Scholar]
- 101.Fisher MT, Zigler CK, Houtrow AJ. Factors affecting procedural pain in children during and immediately after intramuscular botulinum toxin injections for spasticity. J Pediatr Rehabil Med. 2018;11:193–7. doi: 10.3233/PRM-170516. [DOI] [PubMed] [Google Scholar]
- 102.Buntragulpoontawee M, O’Brien TE, Kovindha A. Influence of rehabilitation medicine residency training in performing chemodenervation in children with cerebral palsy in Thailand. J Med Assoc Thai. 2017;100:347–52. [PubMed] [Google Scholar]
- 103.Warnink-Kavelaars J, Vermeulen RJ, Buizer AI, Becher JG. Botulinum neurotoxin treatment in children with cerebral palsy: validation of a needle placement protocol using passive muscle stretching and relaxing. Dev Med Child Neurol. 2016;58:1281–7. doi: 10.1111/dmcn.13176. [DOI] [PubMed] [Google Scholar]
- 104.Warnink-Kavelaars J, Vermeulen RJ, Becher JG. Study protocol: precision of a protocol for manual intramuscular needle placement checked by passive stretching and relaxing of the target muscle in the lower extremity during BTX-A treatment in children with spastic cerebral palsy, as verified by means of electrical stimulation. BMC Pediatr. 2013;13:129. doi: 10.1186/1471-2431-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Houx L, Dubois A, Brochard S, Pons C. Do clowns attenuate pain and anxiety undergoing botulinum toxin injections in children? Ann Phys Rehabil Med. 2020;63:393–9. doi: 10.1016/j.rehab.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 106.Souza MT, Silva MD, Carvalho R. Integrative review: what is it? How to do it? Einstein (Sao Paulo) 2010;8:102–6. doi: 10.1590/S1679-45082010RW1134. [DOI] [PubMed] [Google Scholar]
- 107.Mendes KD, Silveira RC, Galvão CM. Integrative literature review: a research method to incorporate evidence in health care and nursing. Texto & Contexto Enferm. 2008;17:758–64. doi: 10.1590/S0104-07072008000400018. [DOI] [Google Scholar]