Abstract
There is a growing body of evidence that inadequate maternal nutrition during gestation can have immediate and life-long effects on offspring. However, little is known about the effects of maternal nutrition during gestation on male offspring reproduction. Here, using a sheep model of maternal restricted- and over-nutrition (60% or 140% of NRC requirements) during gestation, we found that maternal gestational restricted- and over-nutrition does not affect semen characteristics (i.e., volume, sperm concentration, pH, sperm motility, sperm morphology) or scrotal circumference in male F1 offspring. However, using small RNA sequencing analysis, we demonstrated that both restricted- and over-nutrition during gestation induced marked changes in composition and expression of sperm small non-coding RNAs (sncRNAs) subpopulations including tsRNA, rsRNAs, and miRNAs in male F1 offspring. Whole genome bisulfite sequencing analysis further identified specific genomic loci where poor maternal nutrition resulted in alterations in DNA methylation. These findings indicate that maternal restricted- and over-nutrition during gestation induces epigenetic modifications in sperm of F1 offspring sperm in sheep, which may contribute to environmentally influenced phenotypes in ruminants.
Keywords: Maternal gestational nutrition, sncRNAs, DNA methylation, sperm, sheep
In brief
Inadequate maternal nutrition during gestation can have immediate and life-long effects on offspring. This study shows that maternal restricted- and over-nutrition during gestation does not affect semen characteristics in F1 male offspring, but alters offspring sperm sncRNA profiles and DNA methylome in sheep.
Introduction
In ruminants, poor maternal nutrition during gestation is common in production settings due to established management practices (Wu et al., 2006, Reynolds et al., 2010). In portions of the U.S., pregnant animals graze on rangeland and are not supplemented with additional feedstuffs. This becomes problematic when forage is of poor quality or in limited quantity due to weather or season, especially in species like sheep, which gestate through the fall and winter months. Alternatively, in other regions where animals are managed much more intensely (e.g., university settings or in the Northeast and Mid-Atlantic), maternal obesity during gestation may occur. In the ruminant artificial insemination (AI) industry, virtually all males used for semen collection and AI are the result of embryo transfer from elite females (Ștefan Gregore, 2021). A common problem within the embryo transfer industry is over-conditioning of embryo donors followed by under-conditioning of the recipients after transfer (Jones and Lamb, 2008, Santos et al., 2008). Therefore, maternal nutritional imbalance can affect the development and future function of the reproductive systems in both female and male offspring into adulthood (Mossa et al., 2015).
In livestock, inadequate maternal nutrition during gestation can have immediate and life-long negative effects on offspring growth across multiple generations. For example, in sheep, the diet of pregnant ewes affected the weight of their granddaughters (Nijland et al., 2008). Additionally, poor maternal nutrition (restricted- or over-feeding) has negative consequences for the growth and metabolism of the offspring (Shasa et al., 2015, Hoffman et al., 2017, Jones et al., 2018, Pillai et al., 2017a, Reed et al., 2014, Ford et al., 2007, Ford and Long, 2011, Ford et al., 2009, Huang et al., 2010, Huang et al., 2012, Long et al., 2010, Pankey et al., 2017). Over the past decade, a burgeoning collection of literature from rodents has linked parental diet and other environmental stresses to offspring phenotypes and demonstrated that the sperm epigenetics act as an essential factor in mediating offspring phenotypes. For instance, poor parental diets can induce changes to DNA methylation (Radford et al., 2014, Ly et al., 2017, Watkins et al., 2018), small non-coding RNAs (sncRNAs; (Gapp et al., 2014, Rodgers et al., 2015, Chen et al., 2016, Sharma et al., 2016, Zhang et al., 2018b, Sarker et al., 2019), and histone modifications (Ben Maamar et al., 2020, Lismer et al., 2021) in mouse sperm and transmit parental environmental exposures to the next generation. These studies reveal a thus far neglected epigenetic programming that underpins the development of animal health.
The knowledge about whether or how maternal gestational nutrition impacts male reproductive function in domestic species is limited, and the underlying epigenetic mechanisms are still unclear. In addition, male infertility and subfertility represent one of the greatest costs associated with reproductive problems in the U.S. animal industry (Butler et al., 2020, Maquivar et al., 2021), though it remains understudied. In the present study, using our established model of poor maternal nutrition in sheep (Tillquist et al., 2023), we sought to test our hypothesis that maternal gestational nutrition affects F1 semen characteristics and sperm epigenetics.
Materials and Methods
Ethical approval
All animal procedures were completed in accordance with guidelines established and approved by the University of Connecticut Animal Care and Use Committee (A22–017).
Animals
A detailed description of experimental design, animals, and diets used was previously reported (Tillquist et al., 2023). Briefly, multiparous Dorset ewes (F0; n = 46) from the University of Connecticut flock were estrus synchronized (Reed et al., 2014, Hoffman et al., 2016, Pillai et al., 2017b) and bred with one of three genetically related Dorset rams. At day 26 of gestation, ewes were confirmed pregnant with twins via transabdominal ultrasound (Jones et al., 2016) and assigned to one of three dietary treatments (balanced for ram exposure), which were 60% (Restricted nutrition, RES, n = 17), 100% (Control, CON, n = 13) or 140% (Over nutrition, OVER, n = 16) of NRC requirements for ewes pregnant with twins (Figure 1). Diets were gradually introduced over a 4-day period such that full dietary treatment were achieved by day 30 of gestation and ewes remained on these diets until parturition. Day 30 was selected for onset of diets to be consistent with our previous reports and allow for confirmation of fetal number. Before the start of dietary treatments, all ewes were of similar body weight (BW) and body condition score (BCS) (P ≥ 0.69; Tillquist et al., 2023). Throughout gestation, restricted-nutrition F0 ewes were 11.8% lighter than control F0 ewes and 20.3% lighter than over-nutrition F0 ewes (Tillquist et al., 2023). The BCS of F0 ewes throughout gestation was 15.5% and 14.3% less in restricted-nutrition F0 ewes compared with control and over-nutrition ewes, respectively, as previously reported (Tillquist et al., 2023). These reports of altered BW and BCS throughout gestation confirm our model of poor maternal nutrition. After lambing, all ewes were fed to 100% NRC requirements for lactating ewes so that observed effects on offspring can be attributed to maternal gestational diet. For F1 lamb management, to mimic production settings after birth, F1 ram lambs were maintained with their dam and allowed ad libitum access to creep feed (Lamb BT, Blue Seal Feeds, Litchfield, CT) and second cutting hay until weaning at 60 d of age. After weaning, lambs were housed as a group and fed grower feed (Home Fresh Shepherd 16, Blue Seal) to 100% of NRC requirements and second cutting hay. Between 6 and 8 months of age, lambs were maintained on an ad libitum complete feed diet as part of a residual feed intake trial. By using the restricted- and over-feeding models with defined diet formulation (Table S1), we were able to represent the production settings when forage may be limited and where the animals are intensively managed (e.g., universities and small-scale farm), respectively.
Figure 1.
Experimental design. CON: control-fed (100% National Research Council (NRC) requirements), RES: restricted (60% NRC), OVER: over-fed (140% NRC).
Semen collection and sperm isolation for DNA/RNA extraction
The F1 rams used in this study were from both same-sex and opposite-sex twins, and within each treatment group, the number of rams was balanced in terms of the sex of the siblings. Semen was collected from F1 rams at day 256 ± 1.85 of age (n = 12 to 20 per maternal diet) by electro-ejaculation (EEJ), which does not affect the morphometric characteristics in ram sperm (Tapaloaga and Tapaloaga, 2016, Yaniz et al., 2012). The same semen collection was used for semen analysis and sperm RNA/DNA isolation for epigenome analysis (Figure 1). For RNA/DNA isolation, sperm cells (20 to 30 × 106) were isolated following the Sperm Separation Protocol (Irvine Scientific, Santa Ana, CA). The sperm pellet was re-suspended in somatic cell lysis reagent (0.1% SDS,0.5% Triton X-100) and washed with RNase-free PBS to separate the sperm cells from the diluent and any contaminating somatic cells. The sperm pellet was then lysed in Trizol Reagent (Invitrogen) to extract RNA and DNA.
Semen analysis
Semen was collected in a graduated 15 mL Falcon tube and volume was recorded. Semen quality (sperm motility and concentration) was immediately analyzed following standard procedures (Moraes et al., 2019) using a mini/mobile computer assisted sperm analyzer (mCASA; iSperm, Aidmics Biotechnology Co., LTD). Semen pH was then determined via plastic pH indicator strip (5.5–8.0; Fisher Scientific, Hampton, NH) and a 10 uL aliquot was fixed in 1 mL buffered formalin saline for histological analysis to determine percent morphologically abnormal sperm visually with phase contrast microscopy (Soler et al., 2016). Abnormal sperm were characterized following the Society for Theriogenology guidelines (Hopkins and Spitzer, 1997). Briefly, fixed samples were mixed well and 100 random individual sperm per animal were assessed for sperm head abnormalities (% Head), midpiece abnormalities (% MP), and tail abnormalities (% Tail). The percent normal sperm (% Normal) was calculated as the difference between the total number of sperm assessed (100) and the sum of the abnormalities (Barth and Oko, 1989). Additionally, the scrotal circumference of each F1 ram was measured before semen collection using a scrotal tape. Data were analyzed using R Studio (version 4.4.2) using the packages car and emmeans. Semen variables were analyzed using a one-way analysis of variance (ANOVA) to evaluate the effect of maternal diet on each variable. Post hoc pairwise comparisons were made using emmeans if an effect of maternal diet was significant. Statistical significance was considered at P ≤ 0.05 and a tendency at P > 0.05 and < 0.10.
Small RNA-seq analysis
Sperm RNA samples of three F1 rams (n = 3) from each maternal diet group were used for small RNA sequencing analysis. For small RNA profiling, the sperm were treated with somatic cell lysis buffer (0.1 % SDS, 0.5 % Triton X in DEPC H2O) for 40 min on ice to eliminate somatic cell contamination, followed by centrifugation at 600 g for 5 minutes. The sperm pellet was washed and used for RNA extraction using TRIzol reagent. Small RNA libraries were constructed according to the NEBNext Small RNA Library Prep Kit protocol, employing 15 to 50 nt small RNA pre-size selection, followed by the standard cDNA library construction using NEBNext Small RNA Library Prep Kit and sequencing with Illumina NovaSeq 6000 platform. Approximately 10 million paired end reads per library were generated. The raw reads pre-process, mapping and annotation to the sheep genome were performed using the software SPORTS1.0 (Shi et al., 2018a). Specific small RNA composition from treatment groups were established and differential expression of small RNA were determined using the package edgeR with cutoff set at FDR <0.05 and fold change >2 after normalization of reads per million (RPM).
Whole genome bisulfite sequencing (WGBS) analysis
Sperm samples (CON rams, n = 4; OVER rams, n = 5; RES rams, n = 5) were prepared for WGBS analytical procedures. These samples included the 3 offspring used for RNA-seq. All male offspring used for RNA-seq and WGBS analysis were from twin pregnancies. The procedures for WGBS were conducted as previously described (Jiang et al., 2018, Duan et al., 2019). Briefly, DNA extraction, MspI digestion, end-repair, dA-tailing, adaptor ligation, and bisulfite conversion were conducted in a single tube to minimize DNA loss. Amplified DNA fragments of 200 to 500 bp were size selected using a 2% agarose gel. WGBS libraries were multiplexed and sequenced in Illumina NovaSeq 6000 platform. The raw sequencing reads were pre-processed as previously described (Jiang et al., 2018, Duan et al., 2019), and the clean reads were then aligned to the bisulfite-converted reference sheep genome (Oar_v4.0/oviAri4). Following alignment, the 300-bp-tile-based DNA methylation calculation algorithm was performed (Smith et al., 2012). DNA methylation of each sample was calculated as the average of DNA methylation in each 300-bp tile. The CpG sites with less than 3x coverage were excluded from analysis. Additionally, only CpG sites and tiles that commonly show up in at least one control and at least one treatment group were used for downstream analysis. Sperm DNA methylation levels between CON, RES and OVER rams were compared. The differentially methylated regions were determined in each comparison if FDR-corrected Fisher’s exact test of P-value < 0.01 and >25% differential methylation level. The DMRs were intersected to the genome to determine associated genes. Gene ontology analysis was performed using the DAVID tool (https://david.ncifcrf.gov).
Data availability
The raw FASTQ files and normalized read accounts for small RNA-seq and WGBS data are available at Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE225988.
Results
Effect of maternal gestational nutrition on semen variables in F1 rams
At the time of semen collection, male offspring from restricted-nutrition ewes (78.7 kg ± 1.86) were lighter (P = 0.02) and offspring from over-nutrition ewes (85.6 kg ± 2.31) tended to be lighter (P = 0.07) compared with male offspring from control ewes (88.2 kg ± 2.63). Despite the effects of poor maternal nutrition on offspring growth, there was a minimal impact of maternal diet observed on ram semen characteristics, including volume (P = 0.5317), pH (P = 0.4666), and sperm concentration (P = 0.9010) and motility (P = 0.8261; Table 1). Maternal diet tended to impact the sperm head and tail morphology (P = 0.0504). Specifically, rams from restricted- and over-nutrition dams had decreased instance of sperm head abnormality compared with rams from control dams (P = 0.0504). Rams from over-nutrition dams tended to have greater instance of tail abnormality compared with rams from restricted-nutrition and control dams (P = 0.0826). Overall, the lack of significant differences on semen characteristics suggests that maternal restricted- and over-nutrition during gestation in the current settings did not largely affect F1 offspring semen quality.
Table 1.
The effect of maternal gestational nutrition on semen variables in F1 rams. The data represent means ± SEM.
| Treatment1 | ||||
|---|---|---|---|---|
|
| ||||
| Item | CON (n=10) | RES (n=20) | OVER (n=13) | P-value |
| Volume, mL 2 | 0.980 ± 0.171 | 0.980 ± 0.121 | 0.777 ± 0.150 | 0.5317 |
| Concentration, million/mL 3 | 1240 ± 254 | 1237 ± 180 | 1360 ± 233 | 0.9010 |
| pH 4 | 6.93 ± 0.275 | 6.88 ± 0.151 | 6.62 ± 0.169 | 0.4666 |
| % Motility 5 | 67.5 ± 5.02 | 64.0 ± 3.55 | 66.5 ± 4.41 | 0.8261 |
| % Normal 6 | 86.1 ± 2.93 | 85.5 ± 2.13 | 82.8 ± 2.57 | 0.6444 |
| % Head 7 | 5.4 ± 1.106a | 2.0 ± 0.782b | 2.7 ± 0.970b | 0.0504 |
| % MP 8 | 7.7 ± 2.61 | 11 ± 1.85 | 10.8 ± 2.29 | 0.5583 |
| % Tail 9 | 0.80 ± 1.136x | 0.90 ± 0.803x | 3.62 ± 0.997y | 0.0826 |
| Scrotal Circumference, cm 10 | 35.05 ± 0.366 | 34.00 ± 0.366 | 34.17 ± 0.366 | 0.5200 |
Means with different superscripts within row represent differences among treatment (P ≤ 0.05).
Means with different superscripts within row represent trend among treatment (P < 0.10).
Multiparous Dorset ewes pregnant with twins were fed 100%, 60%, or 140% of NRC requirements from d 30 of gestation until parturition. Offspring are referred to as CON, RES, and OVER, respectively.
Semen was collected from rams via electro-ejaculation into a 15mL graduated conical and semen volume was recorded.
Concentration was determined via iSperm.
A plastic pH indicator strip (pH 5.5–8.0) was used.
Percent motility was determined via iSperm.
Percent nomal (% Normal) refers to [Sum of total abnormalities identified per sample - 100]
Guidelines established by Society for Theriogenology for percent head abnormalities (Acrosome defect, detached head, underdeveloped head, narrow head etc.) per 100 evaluated sperm.
Guidelines established by Society for Theriogenology for percent midpiece abnormalities (Proximal droplet, distal midpiece reflex, swollen midpiece, etc.) per 100 evaluated sperm.
Guidelines established by Society for Theriogenology for percent tail abnormalities (Terminally coiled tail) per 100 evaluated sperm.
Scrotal tape was used prior to semen collection.
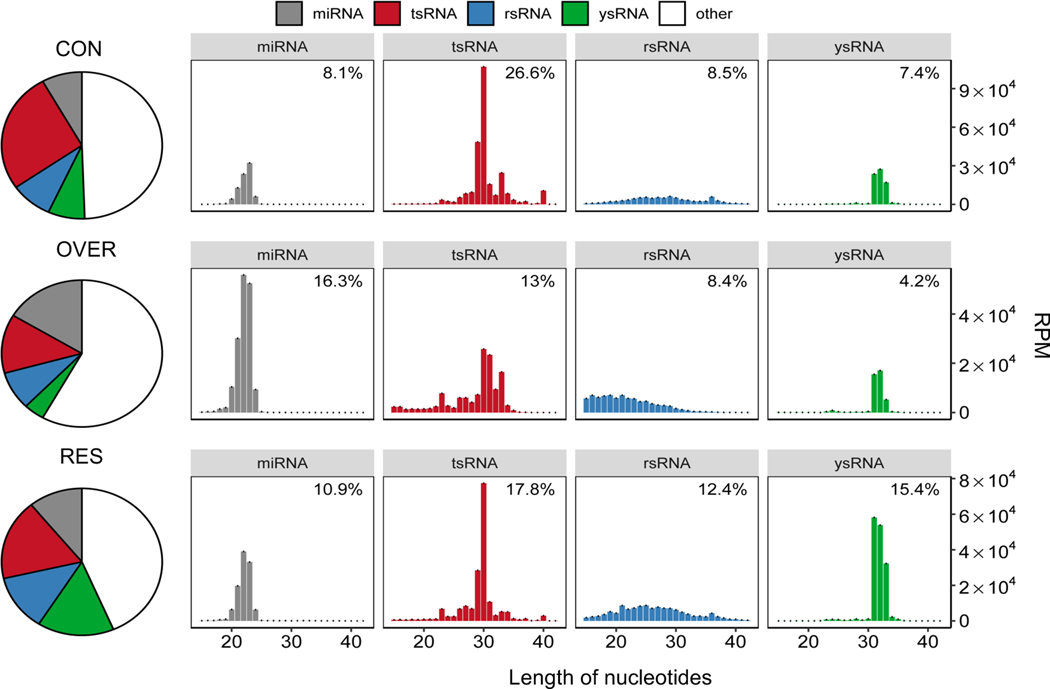
Maternal restricted- and over-nutrition during gestation induced marked changes in sperm sncRNA composition in F1 offspring
Principal Component Analysis (PCA) and Pearson correlation analysis of sncRNA profiles indicated consistent measurements across biological replicates in each treatment (Figure S1). Overall, among all annotated sncRNAs, the majority of sncRNAs in sheep sperm were identified as microRNA (miRNA), tRNA-derived small RNA (tsRNA), rRNA-derived small RNA (rsRNA), and yRNA-derived small RNA (ysRNA; Figure 2). Surprisingly, both restricted- and over-nutrition during gestation induced marked changes in sperm sncRNA composition in F1 offspring (Figure 2). Specifically, we identified an increased percentage of miRNA (16.3% vs 8.1%; P = 0.0002) and decreased percentage of tsRNA (13% vs 26.6%; P < 0.0001) in OVER compared with CON rams (Figure 2). In contrast, a decreased percentage of ysRNA (4.2% vs 7.4%; P = 0.0286) were observed in OVER rams while an increased percentage of rsRNA (12.4% vs 8.5%; P = 0.3875) and ysRNA (15.4% vs 7.4%; P < 0.0001) were observed in RES rams (Figure 2). rsRNA profiles were not changed in RES (P = 0.3875) or OVER (P = 0.9224) rams (Figure 2). Interestingly, length distribution of rsRNAs shifted to the left in OVER rams (Figure 2).
Figure 2.
Sperm sncRNAs (miRNAs, tsRNAs, rsRNAs and rsRNAs) are dynamically regulated in response to maternal restricted- and over-nutrition (RES and OVER) during gestation in F1 males. The results show length distribution and pattern changes (right panel) of different sncRNAs in sperm across different diet groups (CON, OVER, and RES). The data represent means ± s.e.m. The histogram details the percentage of each sncRNA as shown in the bar chart. The abundance of sncRNAs is measured as reads per million (RPM) as shown in the y-axis corresponding to the length of each category of sncRNA as shown in the x-axis.
Sperm tsRNAs, rsRNAs, and miRNAs are altered in response to maternal restricted- and over-nutrition during gestation
In addition to analyzing changes of sncRNA composition, we also examined changes of each category of F1 offspring sperm sncRNAs in response to maternal gestational restricted- and over-nutrition. Mitochondrial tRNAs showed an overall different tsRNA production pattern (tsRNA populations) compared with that of genomic tsRNAs in response to maternal restricted- and over-nutrition (Figure 3A). Expression of genomic tsRNAs were down-regulated in OVER and RES rams compared with CON rams (P < 0.0001), while mitochondrial tsRNAs were up-regulated in RES rams compared with CON rams (P = 0.0001, Figure 3A). We identified 24 genomic tsRNAs and 20 mitochondrial tsRNAs that were significantly differentially regulated as a result of maternal nutrition during gestation (Figure 3B). Specifically, the top regulated tsRNAs in OVER rams included genomic tsRNA-Pro (log2FC = −3.31; P < 0.0001) and tsRNA-Glu (log2FC = −3.33; P < 0.0001) with more than 9-fold down-regulation, and mitochondrial tsRNA-Asp (log2FC = 2.64; P < 0.0001), tsRNA-Ala (log2FC = 2.43; P < 0.0001) and tsRNA-Val (log2FC = 1.97; P < 0.0001) with more than 4-fold up-regulation (Figure 3B, Table S2). In addition, genomic tsRNA-Pro (log2FC = −1.04; P < 0.0001) and tsRNA-Glu (log2FC = −1.10; P < 0.0001) and mitochondrial tsRNA-Val (log2FC = 2.03; P < 0.0001) were also identified to be top regulated tsRNAs in RES rams (Figure 3B, Table S3).
Figure 3.
Maternal restricted- and over-nutrition during gestation altered sperm sncRNA populations and expression in F1 ram. A. Overall length mapping showing the distribution of relative tsRNA reads from mature genomic and mitochondrial tRNA in F1 sperm from CON, OVER, and RES rams. The abundance of the tsRNAs is measured as reads per million (RPM) as shown in the y-axis corresponding to the length of each category of sncRNA as shown in the x-axis. B. Heatmaps showing the differential expression of tsRNAs of genomic and mitochondrial origin under different maternal nutrition conditions during gestation. Relative expression was normalized to total miRNA and based on a log2-transformed scale in the row direction. C. Comparison of rsRNA-generating loci by rsRNA mapping data on 5S rRNA, 5.8S rRNA, 18S rRNA and 28S rRNA, detected from F1 sperm from CON, OVER, and RES rams. The data represent means ± s.e.m.The peaks in the chart represent the abundance of rsRNA mapped to the corresponding loci across the whole length of each rRNA. D. Heatmaps showing differential expression of annotated miRNAs under different maternal nutrition conditions during gestation.
Detailed mapping of rsRNAs on the specific loci of different rRNAs revealed differing expression levels and a shift of rsRNA populations as a result of maternal nutrition (Figure 3C). In general, rsRNAs derived from 5s rRNA have decreased abundance in OVER rams (P < 0.0001, Figure 3C). To note, both loci of origin and abundance of rsRNA derived from 28S rRNA were altered in OVER and RES rams (P < 0.0001, Figure 3C). We identified a total of 32 differentially expressed miRNAs in sperm as a result of maternal nutrition (Figure 3D). Notably, mir-133 had the most significant change where OVER rams had a 500-fold up-regulation (log2FC = 9.04; P < 0.0001) and RES rams had a 6-fold up-regulation (log2FC = 2.60; P < 0.0001) compared with CON rams, respectively (Figure 3D, Table S2, Table S3).
Taken together, our analyses indicate that F1 offspring sperm sncRNA subpopulations are dynamically regulated as a result of poor maternal nutrition during gestation in sheep.
Maternal restricted- and over-nutrition during gestation induced changes in sperm DNA methylome in F1 offspring
It is well documented in mice that sperm DNA methylation and histone modifications contribute to transgenerational epigenetic inheritance (Radford et al., 2014). We next aimed to understand if maternal gestational restricted- and over-nutrition induced alterations in F1 sperm DNA methylation. We generated high-quality sperm WGBS libraries for each experimental group (Table S4). In the present experiment, the most highly methylated regions in sheep sperm were found in repeat elements including LINE, LTR, and SINE (Figure 4A). Hypermethylated regions also included exon, intron, DNA replication terminus (TER), simple sequence repeats (SSR), and intergenic regions, while the hypomethylated regions were in CGI and promoters (Figure 4A). The overall DNA methylation levels ranged from 67.9% to 80.8% among F1 rams, with no apparent effects of maternal diet (Figure 4B, Figure S2, P = 0.3809 for OVER group and P = 0.4457 for RES group). However, differences were identified in the specific genome regions. Specifically, LTR is hypermethylated in OVER rams compared with CON rams (P = 0.0463), while LINE is hypermethylated in both OVER (P = 0.0132) and RES (P = 0.0412) rams compared with CON rams, indicating these two genomic elements are sensitive to DNA methylation change in response to maternal diet (Figure 4B).
Figure 4.
Maternal gestational restricted- and over-nutrition altered sperm DNA methylation in F1 offspring. A, and B. Average CpG methylation level of different genomic regions (promoter, TER, exon, intron, intergenic, CGI, SINE, LINE, LTR, and SSR) and genome-wide in F1 sperm from CON, OVER, and RES rams. Statistical significance was determined by two-sided one-way ANOVA with Dunnett’s multiple comparisons test. All data are plotted as means ± s.e.m. *P ≤ 0.05, **P ≤ 0.01. C. The number of differentially methylated cytosine (DMC)/region (DMR) and annotated genes following OVER and RES diet comparing with CON. D. GO analysis of differentially methylated genes in OVER and RES diet. The size of circles represent the number of genes in the enriched pathways. The color of circle represents –log10 (false discovery rate) as a statistical evaluation. The X-axis show the fold enrichment of those top pathways. CGI: CpG island; LTR: long terminal repeats; SSR: short simple repeat; TER: DNA replication terminus.
Although overall DNA methylation levels did not differ as a result of maternal nutrition, a number of differentially methylated cytosines (DMCs) were identified in F1 rams as a result of maternal nutrition. We identified a total number of 1,064,188 and 1,227,721 CpG in OVER and RES rams (3x genome coverage), respectively. Of these, 13,230 and 22,440 DMCs were identified in OVER and RES rams compared with CON rams, respectively (Figure 4C). These DMCs were associated with 27,037 and 33,055 differentially methylated regions (DMRs), and annotated to 6,633, and 7,470 genes (Figure 4C), respectively. Interestingly, GO analysis revealed that the hypomethylated genes were enriched in nervous system development including neuron development, differentiation, and neurogenesis in both OVER and RES rams (Figure 4D), while hypermethylated genes are mostly associated with cell adhesion, nervous system development and cell organization (Figure 4D). Specifically, in OVER rams, Eukaryotic Translation Elongation Factor 2 (EEF2) and Proline Rich Transmembrane Protein 2 (PRRT2) had a significant reduction (over 80%) of its promoter DNA methylation compared with CON rams (Table S5). On the contrary, 60% DNA methylation was identified in the promoter region of genes including Tumor Suppressor Candidate (TUSC3), RELA Proto-Oncogene, NF-KB Subunit (RELA), Myosin Light Chain 11 (MYL11) and Synaptotagmin 15 (SYT15) in RES rams (Table S5).
Taken together, our analyses demonstrate that the sperm DNA methylome is sensitive to maternal gestational restricted- and over-nutrition in sheep, and that regions with increased or decreased 5mC methylation occur at distinct genomic loci.
Discussion
Sheep are an important agricultural species. Poor maternal nutrition in sheep during gestation often occurs during routine management practices, which is problematic due to implications on offspring growth and health. The effects of poor maternal nutrition during gestation on reproductive development in female offspring are characterized in ruminant species (Mossa et al., 2015). However, there are limited data evaluating the effects of poor maternal diet on the reproductive function in male offspring. In sheep, nutrient restriction during gestation can impact testicular development in offspring at birth, as assessed by a reduction in the number of Sertoli cells (Alejandro et al., 2002, Rae et al., 2002, Kotsampasi et al., 2009). Alternately, over-nutrition of the dam during gestation increases testicular volume of offspring in cattle (Sullivan et al., 2010). However, the impact of poor maternal nutrition on offspring semen characteristics and sperm function are not well-defined. Understanding the nutritional cues that program male reproduction and the mechanisms of how males can pass along the effects of parental diet to future generations will result in new strategies for improving animal health, reproduction, and production.
Our study demonstrates that both maternal restricted- and over-nutrition during gestation do not affect semen characteristics including volume, pH, and sperm concentration and motility. It should be recognized that our diets are from day 30 of gestation until birth, where nutrition during the first 30 days of gestation are similar across all ewes, when primordial germ cells (PGCs) originate and give rise to the male gonads in the sheep. Day 30 was selected for the onset of diets to be consistent with previous studies and to ensure that only twin pregnancies were used for the experiment (Table S6). There is a possibility that PGCs are sensitive to maternal nutrition which may persist to spermatogonia stem cell development and mature sperm. It should also be recognized that the sperm motility and concentration were determined via an mCASA (iSperm), which is a cost-effective technology commonly used on-farm and has previously been validated for use in several livestock species (Medica et al., 2023, Matsuura et al., 2019, Bulkeley et al., 2021). We recognize that a more detailed functional characterization of fertilizing capability of semen is warranted to conclude the effects of maternal gestational nutrition on fertility of animals.
Although maternal nutrition during gestation does not affect offspring sperm phenotypes in the present study; in sheep, poor maternal nutrition (restricted- or over-feeding) of dams has consequences for the growth and metabolism of the offspring (Reed et al., 2014, Raja et al., 2016, Pillai et al., 2016, Martin et al., 2019). This is also observed in the present study, where male offspring from restricted- and over-nutrition dams were smaller than male offspring from control dams at the time of semen collection (Tillquist et al., 2023). These persistent negative effects are likely due to fetal programming as a result of maternal diet, which can then be passed on to subsequent generations. However, the mechanisms through which these changes occur remain unknown. Our study was able to capture marked changes in F1 sperm sncRNAs induced by maternal restricted- and over-nutrition during gestation. This is consistent with the emerging evidence established in a mouse model, that sperm sncRNAs mediate the environmental-induced phenotypes to future generations (Chen, 2022, Zhang et al., 2019). It is logical to infer that the altered sperm small RNAs may lead to poor health and performance in future generations in sheep.
In this study, we identified specific sperm sncRNAs categories that are altered as a result of maternal nutrition. First, we found that tsRNAs of mitochondrial and genomic origin respond in opposite directions due to maternal diet (Figure 2, 3A and B). Notably, mitochondrial tsRNA-Val is found to be highly up-regulated in both OVER and RES rams, while genomic tsRNA-Pro and tsRNA-Glu are commonly down-regulated in both groups. Given that tsRNA-Val is efficient in regulating gene expression (Chen et al., 2016), our findings indicate tsRNA-Val may mediate metabolic and developmental changes. However, the underlying mechanism remains unknown. Second, following the discovery of tsRNAs, sperm small RNAs derived from the other housekeeping RNA, namely rRNA, have also been identified to be altered in offspring in response to maternal nutrition. Our results have shown that sperm rsRNA-28S are more sensitive to maternal nutrition compared with those derived from 5S, 5.8S and 18S RNA (Figure 2 and 3C). Thus far, rsRNAs have been shown to be involved in inflammation (Chu et al., 2017). Also, rsRNA-28S are enriched in leukocytospermia semen samples, which can lead to impaired sperm function and subfertility (Chu et al., 2017). Our findings implicate that maternal restricted- or over-nutrition during gestation may affect F1 male fertility through sperm-carried rsRNA-28S. Finally, we found many sperm microRNAs were altered in OVER and RES rams compared with CON. For example, mir-133, which is essential in muscle development (Ivey et al., 2008) was identified as the most differentially regulated microRNAs. It may provide an epigenetic mechanism through which poor maternal nutrition affects muscle development in ovine offspring (Reed et al., 2014). sncRNAs have been identified to have a regulatory function in the processes of spermatogenesis and sperm maturation. It has been shown that sperm sncRNAs consist of remnants from spermatogenesis as well as post-testicular sncRNAs obtained through interactions with extracellular vesicles along the epididymis (Sellem et al., 2021). These findings have helped generate the hypothesis that sncRNAs could potentially serve as valuable biomarkers for assessing semen fertility.
DNA methylation is another major epigenetic regulator mediating epigenetic inheritance. In our sheep model, although overall genome-wide DNA methylation level did not differ among CON, RES, and OVER rams, we did identify DNA methylation changes in specific genomic regions including repetitive elements like LTR and LINE as a result of maternal nutrition. Repetitive elements can be a source of genome instability due to their intrinsic nature. Therefore, they are usually methylated in a repressed state (Pappalardo and Barra, 2021). Given that repetitive elements can also regulate gene expression as promoters, enhancers, and polyadenylation sequences (Liu and Eiden, 2011), it is of particular interest to further investigate the association between maternal nutrient status during gestation on sperm repetitive elements, methylation, and downstream regulated genes.
We identified a significant number of genes that were differentially methylated in sperm from OVER and RES rams compared with CON rams, which are mostly involved in neuronal development and cell adhesion. Promoter DNA methylation regulates gene expression. In OVER rams, we found the promoter of EEF2, a gene that promotes protein synthesis and is closely related to tumorigenesis (Shi et al., 2018b, Zhang et al., 2018a) and TUSC3, a candidate tumor suppressor gene and inhibits cell proliferation (Sun et al., 2022) were hypermethylated. We also identified several differentially hypomethylated genes in RES rams including MYL11, though their specific roles in development and spermiogenesis remain unexplored and warrant further investigation. The effect of sperm DNA methylation epimutations has been found to corelate with semen characteristics and fertility in both humans and cattle (Uysal et al., 2019, Stiavnicka et al., 2022). Further research to investigate if poor maternal nutrition affects F1 ram fertility through sperm DNA methylation and sncRNAs epimutation is warranted in livestock models such as sheep.
In summary, our study represents the first insights into sperm epigenome dynamics with respects to maternal gestational restricted- and over-nutrition in sheep. We demonstrated that there are marked changes in offspring sperm sncRNAs and DNA methylation induced by maternal gestational restricted- and over-nutrition. There is growing interest in studying correlations between reproductive epigenome and environmental conditions (e.g., heat stress and nutrition) in livestock production and human reproductive health. In this regard, we have established a highly informative sheep model for studying sperm-mediated epigenetic inheritance and understanding how developmental programming may impact animal health.
Supplementary Material
Supplementary Figure 1. Principal component analysis (PCA) (A) and heat map showing the clustering analysis (B) of sncRNA profiles in F1 sperm across different diet groups (CON, OVER, and RES).
Supplementary Figure 2. The average DNA methylation levels in genomic features (promoter, exon, intron, intergenic region, CGI, SSR, SINE and ter) in F1 sperm across different diet groups (CON, OVER, and RES).
Supplementary Table 1. Diet formulations with chemical analyses used in the study.
Supplementary Table 2. Differential expression of sncRNAs in F1 sperm between OVER and CON diet group.
Supplementary Table 3. Differential expression of sncRNAs in F1 sperm between RES and CON group.
Supplementary Table 4. Summary of the sequencing qualities, read mapping, and the covered CpG sites.
Supplementary Table 5. The promoter DNA methylation level in all detected genes in F1 sperm across different diet groups (CON, OVER, and RES).
Supplementary Table 6. Sibling sex of the F1 rams used in the study.
Funding
This work was supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD102533) and USDA National Institute of Food and Agriculture (2019-67016-29863, W4171).
Footnotes
Competing Interest Statement
The authors declare no competing interests.
References:
- ALEJANDRO B, PEREZ R, PEDRANA G, MILTON JT, LOPEZ A, BLACKBERRY MA, DUNCOMBE G, RODRIGUEZ-MARTINEZ H. & MARTIN GB 2002. Low maternal nutrition during pregnancy reduces the number of Sertoli cells in the newborn lamb. Reprod Fertil Dev, 14, 333–7. [DOI] [PubMed] [Google Scholar]
- BARTH AD & OKO RJ 1989. Abnormal morphology of bovine spermatozoa, Ames, Iowa State University Press. [Google Scholar]
- BEN MAAMAR M, BECK D, NILSSON E, MCCARREY JR & SKINNER MK 2020. Developmental origins of transgenerational sperm histone retention following ancestral exposures. Dev Biol, 465, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULKELEY E, COLLINS C, FOUTOUHI A, GONZALES K, POWER H. & MEYERS S. 2021. Assessment of an iPad-based sperm motility analyzer for determination of canine sperm motility. Transl Anim Sci, 5, txab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER ML, BORMANN JM, WEABER RL, GRIEGER DM & ROLF MM 2020. Selection for bull fertility: a review. Transl Anim Sci, 4, 423–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Q. 2022. Sperm RNA-mediated epigenetic inheritance in mammals: challenges and opportunities. Reprod Fertil Dev, 35, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Q, YAN M, CAO Z, LI X, ZHANG Y, SHI J, FENG GH, PENG H, ZHANG X, ZHANG Y, QIAN J, DUAN E, ZHAI Q. & ZHOU Q. 2016. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science, 351, 397–400. [DOI] [PubMed] [Google Scholar]
- CHU C, YU L, WU B, MA L, GOU LT, HE M, GUO Y, LI ZT, GAO W, SHI H, LIU MF, WANG H, CHEN CD, DREVET JR, ZHOU Y. & ZHANG Y. 2017. A sequence of 28S rRNA-derived small RNAs is enriched in mature sperm and various somatic tissues and possibly associates with inflammation. J Mol Cell Biol, 9, 256–259. [DOI] [PubMed] [Google Scholar]
- DUAN JE, JIANG ZC, ALQAHTANI F, MANDOIU I, DONG H, ZHENG X, MARJANI SL, CHEN J. & TIAN XC 2019. Methylome Dynamics of Bovine Gametes and in vivo Early Embryos. Front Genet, 10, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD SP, HESS BW, SCHWOPE MM, NIJLAND MJ, GILBERT JS, VONNAHME KA, MEANS WJ, HAN H. & NATHANIELSZ PW 2007. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. Journal of animal science, 85, 1285–1294. [DOI] [PubMed] [Google Scholar]
- FORD SP & LONG NM 2011. Evidence for similar changes in offspring phenotype following either maternal undernutrition or overnutrition: potential impact on fetal epigenetic mechanisms. Reprod Fertil Dev, 24, 105–11. [DOI] [PubMed] [Google Scholar]
- FORD SP, ZHANG L, ZHU M, MILLER MM, SMITH DT, HESS BW, MOSS GE, NATHANIELSZ PW & NIJLAND MJ 2009. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol, 297, R835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAPP K, JAWAID A, SARKIES P, BOHACEK J, PELCZAR P, PRADOS J, FARINELLI L, MISKA E. & MANSUY IM 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci, 17, 667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN ML, PECK KN, FORELLA ME, FOX AR, GOVONI KE & ZINN SA 2016. The effects of poor maternal nutrition during gestation on postnatal growth and development of lambs. J Anim Sci, 94, 789–99. [DOI] [PubMed] [Google Scholar]
- HOFFMAN ML, REED SA, PILLAI SM, JONES AK, MCFADDEN KK, ZINN SA & GOVONI KE 2017. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM:The effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J Anim Sci, 95, 2222–2232. [DOI] [PubMed] [Google Scholar]
- HOPKINS FM & SPITZER JC 1997. The new Society for Theriogenology breeding soundness evaluation system. Vet Clin North Am Food Anim Pract, 13, 283–93. [DOI] [PubMed] [Google Scholar]
- HUANG Y, YAN X, ZHU MJ, MCCORMICK RJ, FORD SP, NATHANIELSZ PW & DU M. 2010. Enhanced transforming growth factor-beta signaling and fibrogenesis in ovine fetal skeletal muscle of obese dams at late gestation. Am J Physiol Endocrinol Metab, 298, E1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG Y, ZHAO JX, YAN X, ZHU MJ, LONG NM, MCCORMICK RJ, FORD SP, NATHANIELSZ PW & DU M. 2012. Maternal obesity enhances collagen accumulation and cross-linking in skeletal muscle of ovine offspring. PloS one, 7, e31691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVEY KN, MUTH A, ARNOLD J, KING FW, YEH RF, FISH JE, HSIAO EC, SCHWARTZ RJ, CONKLIN BR, BERNSTEIN HS & SRIVASTAVA D. 2008. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell, 2, 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Z, LIN J, DONG H, ZHENG X, MARJANI SL, DUAN J, OUYANG Z, CHEN J. & TIAN XC 2018. DNA methylomes of bovine gametes and in vivo produced preimplantation embryos. Biol Reprod, 99, 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES AK, GATELY RE, MCFADDEN KK, ZINN SA, GOVONI KE & REED SA 2016. Transabdominal ultrasound for detection of pregnancy, fetal and placental landmarks, and fetal age before Day 45 of gestation in the sheep. Theriogenology, 85, 939–945 e1. [DOI] [PubMed] [Google Scholar]
- JONES AK, HOFFMAN ML, PILLAI SM, MCFADDEN KK, GOVONI KE, ZINN SA & REED SA 2018. Gestational restricted- and over-feeding promote maternal and offspring inflammatory responses that are distinct and dependent on diet in sheep. Biol Reprod, 98, 184–196. [DOI] [PubMed] [Google Scholar]
- JONES AL & LAMB GC 2008. Nutrition, synchronization, and management of beef embryo transfer recipients. Theriogenology, 69, 107–15. [DOI] [PubMed] [Google Scholar]
- KOTSAMPASI B, CHADIO S, PAPADOMICHELAKIS G, DELIGEORGIS S, KALOGIANNIS D, MENEGATOS I. & ZERVAS G. 2009. Effects of maternal undernutrition on the hypothalamic-pituitary-gonadal axis function in female sheep offspring. Reprod Domest Anim, 44, 677–84. [DOI] [PubMed] [Google Scholar]
- LISMER A, DUMEAUX V, LAFLEUR C, LAMBROT R, BRIND’AMOUR J, LORINCZ MC & KIMMINS S. 2021. Histone H3 lysine 4 trimethylation in sperm is transmitted to the embryo and associated with diet-induced phenotypes in the offspring. Dev Cell, 56, 671–686 e6. [DOI] [PubMed] [Google Scholar]
- LIU M. & EIDEN MV 2011. Role of human endogenous retroviral long terminal repeats (LTRs) in maintaining the integrity of the human germ line. Viruses, 3, 901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG NM, GEORGE LA, UTHLAUT AB, SMITH DT, NIJLAND MJ, NATHANIELSZ PW & FORD SP 2010. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. Journal of animal science, 88, 3546–3553. [DOI] [PubMed] [Google Scholar]
- LY L, CHAN D, AARABI M, LANDRY M, BEHAN NA, MACFARLANE AJ & TRASLER J. 2017. Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation. Mol Hum Reprod, 23, 461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAQUIVAR MG, SMITH SM & BUSBOOM JR 2021. Reproductive Management of Rams and Ram Lambs during the Pre-Breeding Season in US Sheep Farms. Animals (Basel), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN DE, JONES AK, PILLAI SM, HOFFMAN ML, MCFADDEN KK, ZINN SA, GOVONI KE & REED SA 2019. Maternal Restricted- and Over-Feeding During Gestation Result in Distinct Lipid and Amino Acid Metabolite Profiles in the Longissimus Muscle of the Offspring. Front Physiol, 10, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUURA K, WANG WH, CHING A, CHEN Y. & CHENG CM 2019. Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm. Micromachines (Basel), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDICA AJ, LAMBOURNE S. & AITKEN RJ 2023. Predicting the Outcome of Equine Artificial Inseminations Using Chilled Semen. Animals (Basel), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORAES CR, RUNCAN EE, BLAWUT B. & COUTINHO DA SILVA MA 2019. Technical Note: The use of iSperm technology for on-farm measurement of equine sperm motility and concentration. Transl Anim Sci, 3, 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSSA F, WALSH SW, IRELAND JJ & EVANS ACO 2015. Early nutritional programming and progeny performance: Is reproductive success already set at birth? Animal Frontiers, 5, 18–24. [Google Scholar]
- NIJLAND MJ, FORD SP & NATHANIELSZ PW 2008. Prenatal origins of adult disease. Curr Opin Obstet Gynecol, 20, 132–8. [DOI] [PubMed] [Google Scholar]
- PANKEY CL, WALTON MW, ODHIAMBO JF, SMITH AM, GHNENIS AB, NATHANIELSZ PW & FORD SP 2017. Intergenerational impact of maternal overnutrition and obesity throughout pregnancy in sheep on metabolic syndrome in grandsons and granddaughters. Domest Anim Endocrinol, 60, 67–74. [DOI] [PubMed] [Google Scholar]
- PAPPALARDO XG & BARRA V. 2021. Losing DNA methylation at repetitive elements and breaking bad. Epigenetics Chromatin, 14, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLAI SM, JONES AK, HOFFMAN ML, MCFADDEN KK, REED SA, ZINN SA & GOVONI KE 2017a. Fetal and organ development at gestational days 45, 90, 135 and at birth of lambs exposed to under- or over-nutrition during gestation. Translational Animal Science, 1, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLAI SM, JONES AK, HOFFMAN ML, MCFADDEN KK, REED SA, ZINN SA & GOVONI KE 2017b. Fetal and organ development at gestational days 45, 90, 135 and at birth of lambs exposed to under- or over-nutrition during gestation(,)(). Transl Anim Sci, 1, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLAI SM, SEREDA NH, HOFFMAN ML, VALLEY EV, CRENSHAW TD, PARK YK, LEE JY, ZINN SA & GOVONI KE 2016. Effects of Poor Maternal Nutrition during Gestation on Bone Development and Mesenchymal Stem Cell Activity in Offspring. PLoS One, 11, e0168382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADFORD EJ, ITO M, SHI H, CORISH JA, YAMAZAWA K, ISGANAITIS E, SEISENBERGER S, HORE TA, REIK W, ERKEK S, PETERS A, PATTI ME & FERGUSON-SMITH AC 2014. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science, 345, 1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAE MT, RHIND SM, FOWLER PA, MILLER DW, KYLE CE & BROOKS AN 2002. Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses. Reproduction, 124, 33–9. [PubMed] [Google Scholar]
- RAJA JS, HOFFMAN ML, GOVONI KE, ZINN SA & REED SA 2016. Restricted maternal nutrition alters myogenic regulatory factor expression in satellite cells of ovine offspring. Animal, 10, 1200–3. [DOI] [PubMed] [Google Scholar]
- REED SA, RAJA JS, HOFFMAN ML, ZINN SA & GOVONI KE 2014. Poor maternal nutrition inhibits muscle development in ovine offspring. J Anim Sci Biotechnol, 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS LP, BOROWICZ PP, CATON JS, VONNAHME KA, LUTHER JS, HAMMER CJ, MADDOCK CARLIN KR, GRAZUL-BILSKA AT & REDMER DA 2010. Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J Anim Sci, 88, E61–72. [DOI] [PubMed] [Google Scholar]
- RODGERS AB, MORGAN CP, LEU NA & BALE TL 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A, 112, 13699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS JE, CERRI RL & SARTORI R. 2008. Nutritional management of the donor cow. Theriogenology, 69, 88–97. [DOI] [PubMed] [Google Scholar]
- SARKER G, SUN W, ROSENKRANZ D, PELCZAR P, OPITZ L, EFTHYMIOU V, WOLFRUM C. & PELEG-RAIBSTEIN D. 2019. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc Natl Acad Sci U S A, 116, 10547–10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLEM E, JAMMES H. & SCHIBLER L. 2021. Sperm-borne sncRNAs: potential biomarkers for semen fertility? Reprod Fertil Dev, 34, 160–173. [DOI] [PubMed] [Google Scholar]
- SHARMA U, CONINE CC, SHEA JM, BOSKOVIC A, DERR AG, BING XY, BELLEANNEE C, KUCUKURAL A, SERRA RW, SUN F, SONG L, CARONE BR, RICCI EP, LI XZ, FAUQUIER L, MOORE MJ, SULLIVAN R, MELLO CC, GARBER M. & RANDO OJ 2016. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science, 351, 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHASA DR, ODHIAMBO JF, LONG NM, TUERSUNJIANG N, NATHANIELSZ PW & FORD SP 2015. Multigenerational impact of maternal overnutrition/obesity in the sheep on the neonatal leptin surge in granddaughters. Int J Obes (Lond), 39, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI J, KO EA, SANDERS KM, CHEN Q. & ZHOU T. 2018a. SPORTS1.0: A Tool for Annotating and Profiling Non-coding RNAs Optimized for rRNA- and tRNA-derived Small RNAs. Genomics Proteomics Bioinformatics, 16, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI N, CHEN X, LIU R, WANG D, SU M, WANG Q, HE A. & GU H. 2018b. Eukaryotic elongation factors 2 promotes tumor cell proliferation and correlates with poor prognosis in ovarian cancer. Tissue Cell, 53, 53–60. [DOI] [PubMed] [Google Scholar]
- SMITH ZD, CHAN MM, MIKKELSEN TS, GU H, GNIRKE A, REGEV A. & MEISSNER A. 2012. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature, 484, 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLER C, GARCIA-MOLINA A, SANCHO M, CONTELL J, NUNEZ M. & COOPER TG 2016. A new technique for analysis of human sperm morphology in unstained cells from raw semen. Reprod Fertil Dev, 28, 428–33. [DOI] [PubMed] [Google Scholar]
- ȘTEFAN GREGORE C. 2021. Embryo Transfer. In: YUSUF B. & MUSTAFA NUMAN B. (eds.) Animal Reproduction. Rijeka: IntechOpen. [Google Scholar]
- STIAVNICKA M, CHAULOT-TALMON A, PERRIER JP, HOSEK P, KENNY DA, LONERGAN P, KIEFER H. & FAIR S. 2022. Sperm DNA methylation patterns at discrete CpGs and genes involved in embryonic development are related to bull fertility. BMC Genomics, 23, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN TM, MICKE GC, GREER RM & PERRY VE 2010. Dietary manipulation of Bos indicusxheifers during gestation affects the prepubertal reproductive development of their bull calves. Anim Reprod Sci, 118, 131–9. [DOI] [PubMed] [Google Scholar]
- SUN F, JIE Q, LI Q, WEI Y, LI H, YUE X. & MA Y. 2022. TUSC3 inhibits cell proliferation and invasion in cervical squamous cell carcinoma via suppression of the AKT signalling pathway. J Cell Mol Med, 26, 1629–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPALOAGA D. & TAPALOAGA P-R 2016. Assessment of Some Morphometric Parameters in Ram Sperm Correlated with the Collection Method. Agriculture and Agricultural Science Procedia, 10, 340–345. [Google Scholar]
- TILLQUIST NM, REED SA, KAWAIDA MY, REITER AS, SMITH BI, JANG H, LEE JY, LEE EC, ZINN SA & GOVONI KE 2023. Restricted- and over-feeding during gestation decreases growth of offspring throughout maturity. Transl Anim Sci, 7, txad061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UYSAL F, AKKOYUNLU G. & OZTURK S. 2019. Decreased expression of DNA methyltransferases in the testes of patients with non-obstructive azoospermia leads to changes in global DNA methylation levels. Reprod Fertil Dev, 31, 1386–1394. [DOI] [PubMed] [Google Scholar]
- WATKINS AJ, DIAS I, TSURO H, ALLEN D, EMES RD, MORETON J, WILSON R, INGRAM RJM & SINCLAIR KD 2018. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc Natl Acad Sci U S A, 115, 10064–10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU G, BAZER FW, WALLACE JM & SPENCER TE 2006. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci, 84, 2316–37. [DOI] [PubMed] [Google Scholar]
- YANIZ JL, VICENTE-FIEL S, CAPISTROS S, PALACIN I. & SANTOLARIA P. 2012. Automatic evaluation of ram sperm morphometry. Theriogenology, 77, 1343–50. [DOI] [PubMed] [Google Scholar]
- ZHANG X, HU L, DU M, WEI X, ZHANG J, HUI Y, CHEN C, LI G. & HOU J. 2018a. Eukaryotic Elongation Factor 2 (eEF2) is a Potential Biomarker of Prostate Cancer. Pathol Oncol Res, 24, 885–890. [DOI] [PubMed] [Google Scholar]
- ZHANG Y, SHI J, RASSOULZADEGAN M, TUORTO F. & CHEN Q. 2019. Sperm RNA code programmes the metabolic health of offspring. Nat Rev Endocrinol, 15, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y, ZHANG X, SHI J, TUORTO F, LI X, LIU Y, LIEBERS R, ZHANG L, QU Y, QIAN J, PAHIMA M, LIU Y, YAN M, CAO Z, LEI X, CAO Y, PENG H, LIU S, WANG Y, ZHENG H, WOOLSEY R, QUILICI D, ZHAI Q, LI L, ZHOU T, YAN W, LYKO F, ZHANG Y, ZHOU Q, DUAN E. & CHEN Q. 2018b. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol, 20, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Principal component analysis (PCA) (A) and heat map showing the clustering analysis (B) of sncRNA profiles in F1 sperm across different diet groups (CON, OVER, and RES).
Supplementary Figure 2. The average DNA methylation levels in genomic features (promoter, exon, intron, intergenic region, CGI, SSR, SINE and ter) in F1 sperm across different diet groups (CON, OVER, and RES).
Supplementary Table 1. Diet formulations with chemical analyses used in the study.
Supplementary Table 2. Differential expression of sncRNAs in F1 sperm between OVER and CON diet group.
Supplementary Table 3. Differential expression of sncRNAs in F1 sperm between RES and CON group.
Supplementary Table 4. Summary of the sequencing qualities, read mapping, and the covered CpG sites.
Supplementary Table 5. The promoter DNA methylation level in all detected genes in F1 sperm across different diet groups (CON, OVER, and RES).
Supplementary Table 6. Sibling sex of the F1 rams used in the study.
Data Availability Statement
The raw FASTQ files and normalized read accounts for small RNA-seq and WGBS data are available at Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE225988.