Abstract
The Extracorporeal Life Support Organization (ELSO) maintains the world’s largest extracorporeal membrane oxygenation (ECMO) registry by volume, center participation, and international scope. This 2022 ELSO Registry Report describes the program characteristics of ECMO centers, processes of ECMO care, and reported outcomes. Neonates (0–28 days), children (29 days–17 years), and adults (≥18 years) supported with ECMO from 2009 through 2022 and reported to the ELSO Registry were included. This report describes adjunctive therapies, support modes, treatments, complications, and survival outcomes. Data are presented descriptively as counts and percent or median and interquartile range (IQR) by year, group, or level. Missing values were excluded before calculating descriptive statistics. Complications are reported per 1,000 ECMO hours. From 2009 to 2022, 154,568 ECMO runs were entered into the ELSO Registry. Seven hundred and eighty centers submitted data during this time (557 in 2022). Since 2009, the median annual number of adult ECMO runs per center per year increased from 4 to 15, whereas for pediatric and neonatal runs, the rate decreased from 12 to 7. Over 50% of patients were transferred to the reporting ECMO center; 20% of these patients were transported with ECMO. The use of prone positioning before respiratory ECMO increased from 15% (2019) to 44% (2021) for adults during the coronavirus disease-2019 (COVID-19) pandemic. Survival to hospital discharge was greatest at 68.5% for neonatal respiratory support and lowest at 29.5% for ECPR delivered to adults. By 2022, the Registry had enrolled its 200,000th ECMO patient and 100,000th patient discharged alive. Since its inception, the ELSO Registry has helped centers measure and compare outcomes across its member centers and strategies of care. Continued growth and development of the Registry will aim to bolster its utility to patients and centers.
Keywords: extracorporeal life support, extracorporeal membrane oxygenation, survival, cardiogenic shock, respiratory failure, extracorporeal cardiopulmonary resuscitation
In 1989, the Extracorporeal Life Support Organization (ELSO) and its Registry were launched by pioneers of extracorporeal membrane oxygenation (ECMO). Since its creation, more than 200,000 patients have received ECMO for refractory respiratory failure, cardiac failure, or both, and more than 100,000 survivors have been discharged alive from ELSO centers.1 Those patients’ data have contributed to more than 300 peer-reviewed publications from the ELSO Registry.2,3
Over the years, scientific research has progressed in ECMO for respiratory failure,4-10 cardiogenic shock,11-14 and refractory cardiac arrest,15-18 and the registry has evolved in data collection and quality assurance. The 2022 ELSO Registry Report characterizes adjunctive therapy use, support mode, complications, and mortality for ECMO-supported patients submitted from 2009 through December 31, 2022. The previous Registry report reported data from inception through year-end of 2015. This report utilizes data from the start of 2009, which is a significant year both because it is when the 2009 Influenza A (H1N1) pandemic drove a dramatic increase in adult venovenous (VV) ECMO use,19 and also when new ECMO technology using oxygenators made with hollow fiber polymethylpentene (PMP) and centrifugal pumps came to market, which increased safety and efficacy of the ECMO circuit.
Methods
Data are de-identified upon release, are not considered human subjects research, and are exempt from Institutional Review Board review. The data analyzed were obtained from the ELSO Registry, including the Cardiac Addendum and the ECPR Addendum. We included all ECMO runs that began on or after January 1, 2009, were completed by December 31, 2022 and were submitted to the ELSO registry by April 18, 2023. The ELSO Registry records unique ECMO runs and patients; patient-level outcomes are reported at the patient level, and ECMO run-level outcomes are reported at the ECMO run level. Patients are categorized both by age and by support type. The age categories are neonatal: birth–28 days, pediatric: 29 days–17 years, adult: ≥18 years. Support types are respiratory, cardiac, and extracorporeal cardiopulmonary resuscitation (ECPR).20 Support modes include venoarterial (VA), VV, and less commonly utilized modes, including veno-venoarterial (VVA) and venopulmonary (VP). These terms are defined in detail previously.20,21
Data Quality Assurance
ELSO site managers receive detailed instructions and definitions on data entry. Data definitions and entry instructions are publicly available.22 Since 2019, data managers must pass data entry examinations to submit data to the registry. Accuracy is checked through an assessment at point-of-entry into the database, including data value hard limits. Once data entry is completed, full record validation is performed to ensure mandatory data fields are complete.23
Original ELSO Registry data elements have been previously described1 and include patient demographics; patient hemodynamics on ECMO, ventilator settings, and blood gas values before ECMO and at 24 hours after ECMO initiation; pre-ECMO support including hemodynamic support devices and select medications; International Classification of Disease (ICD) coded diagnoses; Common Procedural Terminology (CPT) coded procedures; complications; hospital admission and discharge; intubation and extubation; vital status at hospital discharge; and disposition location.
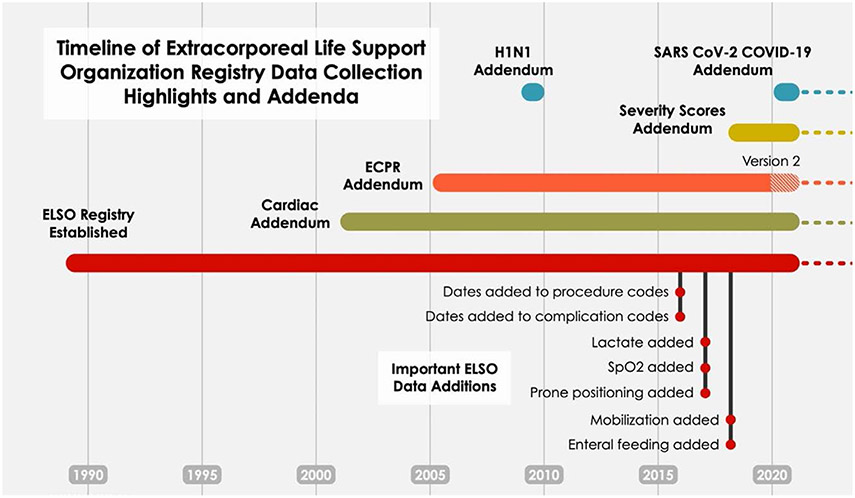
New data elements have been added to the main Registry with Addenda (Figure 1). These newer data elements include dates and times of procedures and complications, additional support measures, cannula sizes, postcannulation management, outcomes, and condition-specific addenda. Addenda were created or updated for patients with COVID-19, trauma, cardiac ECMO, and ECPR.30-32 Complications definitions can be found in the eMethods http://links.lww.com/ASAIO/B185.
Figure 1.
Timeline of the Extracorporeal Life Support Organization registry data collection highlights and addenda. New data elements have been added to the main registry and with addenda. These newer data elements include dates and times of procedures and complications, level of mobilization during ECMO,24 the use of prone positioning before ECMO,25 and ICU type.26 Mobilization on ECMO was defined as score of >0 on the ICU mobility scale (IMS),24 which indicates active or active assisted exercise, such as rolling, bridging or cycle ergometry, or greater. A score of 0 is passive activity/movement. The cardiac addendum contains additional diagnostic, procedural, and echocardiographic data, as well as indications for support, postoperative and cardiac function.27 Version 1 was designed for neonates and children, a revised cardiac addendum (Version 2) appropriate for adults, will be released in 2023. The ECPR addendum (Version 1) began in 200527 and was revised in January 2020 (Version 2) and now contains precipitating and antecedent events, comorbid conditions, arrest and cannulation location, subsequent intra-arrest management, initial arrest rhythms, details on post arrest clinical management28,29 and neurologic outcomes. ELSO began collecting data specific to the ECMO care of patients with SARS-CoV-2 in early 2020.30-32 Dates and times of procedures and complications were added September 15, 2016. Lactate (worst value) was added January 1, 2017, and then transitioned to pre-ECMO value (as defined for other pre-ECMO variables) January 15, 2018. SpO2 was added January 15, 2017. Prone positioning was added December 1, 2017. Mobilization and enteral feeding data were added January 15, 2018.
Statistical Analysis
Data are presented descriptively as counts and percent, median and interquartile range (IQR) stratified by year, group, or level. Missing values were excluded before calculating descriptive statistics (unless otherwise noted), and counts of missing values are provided, where applicable.
We report in-hospital mortality at the ELSO center and mortality within 24 hours of ECMO decannulation (eMethods, http://links.lww.com/ASAIO/B185). Complication rates were defined as the sum of all reported instances of each complication code across all runs divided by the sum of ECMO durations across all runs. These rates were calculated based only on runs completed on or after 2017, corresponding to the first full year in which multiple instances of a complication code per run could be reported in the ELSO registry. Some complication codes were combined to calculate a single rate, as detailed in the Supplement. Mobility on ECMO was defined as an ICU Mobility Score >0,24 and was only collected for patients ≥8 years of age, beginning in 2018. Functional outcomes after ECPR were recorded for pediatric patients using the Pediatric Cerebral Performance Category (PCPC) score (range: 1—normal; 6—brain death),33 and for adults using the Cerebral Performance Category (CPC) score (range: 1—normal; 5—brain death).34 Prone positioning data are reported from 2019 to 2022 to evaluate positioning before and after COVID.
Structure, Process, and Outcomes
We present our results within the framework of Registry Structure, Process Measures, and Clinical Outcomes.
Results
Registry Structure
Overall trends.
From 2009 to 2022, 154,568 ECMO runs were entered into the ELSO Registry (108,265 adult, 25,739 pediatric, and 20,564 neonatal). These patients were distributed across centers that cared for neonatal, pediatric, and adult patients, or a combination. Since its inception in 1989, 780 unique centers have entered data at some point. The growth in ECMO utilization is predominantly adult, increasing from 851 (2009) to 17,975 (2021) (Figure 2). Pediatric ECMO runs have doubled from 1,154 (2009) to 2,394 (2021), and neonatal runs were relatively unchanged between 2009 and 2021 (1,258 and 1,527, respectively).
Figure 2.
Runs over time by age group. Y2-axis shows the number of runs over time by age group (colors), using data from 2009 to 2022 (inclusive). Run counts are indicated in the figure for color (age group) for the first and last years of the chart for reference. Y1-axis shows the number of centers (orange line with box counts in 2009 and 2022). Run counts in 2009 for adult age group: 850. Run counts in 2021: neonatal: 1,527; pediatric: 2,394; adult: 17,975.
Center growth.
The ELSO Registry is international, with 57 countries reporting data in 2022. The ELSO regions North America, Asia-Pacific, Latin American, and South and West Asian regions have grown in numbers of centers and runs (Figure 3). From 2009 to 2022, the median annual ELSO center adult patient volume has increased from 4 [IQR, 1–12] to 15 [6–36] patients (Figure 4). For neonates and pediatric patients, the median annual center volume has decreased from 12 [6–23] in 2009 and 7 [2–17] in 2022 over the same period. From 2009 to 2022, neonatal and pediatric ECMO cases have become more centralized, with the top 50% of centers (ranked by their annual volume) accounting for 90% of the cases in 2022 (Figure S1, http://links.lww.com/ASAIO/B185).
Figure 3.
Run count by region over time. Number of runs over time by ELSO chapter (colors), using data from 2009 to 2022 (inclusive). Boxed numbers indicate runs counts for years 2009 and 2022 for visual reference according to color (ELSO Chapter). Run counts in 2009: North American: 2,300; European: 529; Asia-Pacific: 381; Latin American: 45; South and West Asian: 7. Run counts in 2021: North American: 15,430; European: 3,390; Asia-Pacific: 1,542; Latin American: 707; South and West Asian: 827. Run counts in 2022: North American: 12,587; European: 2,248; Asia-Pacific: 959; Latin American: 470; South and West Asian: 539. Center counts in 2009: North American: 124; European: 24; Asia-Pacific: 13; Latin American: 4; South and West Asian: 1. Center counts in 2021: North American: 350; European: 108; Asia-Pacific: 57; Latin American: 45; South and West Asian: 31. Center counts in 2022: North American: 352; European: 107; Asia-Pacific: 43; Latin American: 32; South and West Asian: 31.
Figure 4.
Center-level volume over time, log scale. Distribution of annual center-level volume (log2-scale) over time by age group (colors). Boxes cover the middle 50% and whiskers cover the middle 80%. Center volume for neonatal/pediatric centers: 2009: median 12, interquartile range (IQR) 6, 23; 2021: 8 (2, 18.5); 2022: 7 (2, 17); Center volume for adult centers: 2009: 4 (1, 12); 2021: 20 (7, 48); 2022: 15 (6, 36).
Intensive care unit (ICU) patient distribution.
ECMO in neonatal intensive care units is dominated by neonatal respiratory ECMO (79%, 2,205/2,797) (Table S1, http://links.lww.com/ASAIO/B185). Pediatric ICUs care for a diversity of ECMO support and age groups, including 80% (3,040/3,790) of pediatric respiratory ECMO, 38% (1,445/3,850) of neonatal respiratory ECMO, 86% (4,875/5,659) of pediatric cardiac ECMO, 79% (2,295/2,901) of neonatal cardiac ECMO, and 86% (2,310/2,694) of pediatric ECPR runs. Adult cardiovascular ICUs (including cardiac) care for a diversity of ECMO patients as well: 63% (18,936/30,021) of adult cardiac, 51% (4,727/9,327) of adult ECPR, and 42% (13,665/32,803) of adult respiratory ECMO.
Transfers.
Since 2018, approximately 50% of patients reported to the ELSO Registry were transported to the ECMO center (Table S2, http://links.lww.com/ASAIO/B185). In 2022, 49% (6,413/12,963) of adult patients, 52% (1,283/2,457) of pediatric patients, and 70% (912/1,383) of neonatal patients were transported to an ECMO center. In the same year, the percentage of patients who were transported on ECMO was fifteen percent (15%) (1,906/12,963) for adult patients, 9% (216/2,457) for pediatric patients, and 5% (73/1,383) for neonatal patients.
The percentage of patients who were transported did vary by ELSO Chapter (Table S3, http://links.lww.com/ASAIO/B185). In Europe in 2022 there was roughly equal distribution among the groups (“Transported on ECMO”/“Transported not on ECMO”/“Not Transported”) with (23% [359], 38% [598]/32% [505)]). In contrast, in the same year, more patients were not transported in North America and Latin America (44%–50%); this was more pronounced in the Asia-Pacific (AP) and the South West Asia and Africa Chapter (SWAAC), with approximately 65% of patients not transported.
COVID-19.
During the COVID-19 pandemic, adult respiratory ECMO support was dominated by COVID-19. Patients with confirmed or suspected COVID-19 accounted for 58% (5,123/8,786) in 2020 and 74% (7,521/10,219) in 2021. By 2022, COVID-19 had diminished considerably, and only 22% (961/4,348) of patients had confirmed or suspected COVID-19 (Figure 5). During the COVID-19 pandemic (2020 and 2021), adult cardiac support, adult ECPR, and pediatric respiratory support declined relative to 2019 numbers for the first time in the last 5 years (Figure S2, http://links.lww.com/ASAIO/B185). Further, beginning in 2020, fewer adults receiving ECPR were age 65 or older (2019: 29%; 2020: 25.8%; Figure S3, http://links.lww.com/ASAIO/B185). In contrast, in 2021, more adults without COVID-19 who were offered and received respiratory ECMO were age 65 or older (2019: 15%; 2020: 14%; 2021: 18%).
Figure 5.
Distribution of COVID-19 status in adult receiving respiratory support ECMO in 2020 through 2022. COVID-19 test status is indicated by colors for each of three years. Bar height represents proportion from 0 to 1, shown on the Y-axes.
Process Measures
ECMO support type and mode.
The distribution of ECMO support types (respiratory, cardiac, ECPR) according to age group (neonatal, pediatric, adult) over time is depicted in Figure 6. In 2022, respiratory support accounted for the plurality of neonatal ECMO cases, comprising 49% of all cases. The use of the carotid artery for respiratory support remains ~50% each year (Figure S4, http://links.lww.com/ASAIO/B185). Conversely, cardiac was the primary pediatric ECMO support, representing 46% of all cases in the same year. Before 2020, adults primarily received ECMO for cardiac support (61% in 2019). However, during the COVID-19 pandemic, a transient shift to respiratory support was observed (51% in 2020, 57% in 2021), before, again, being superseded by cardiac support (50% in 2022). The newly defined VP ECMO support21 represents only a small fraction of ECMO support (1.4% [181/12,963] of adult ECMO in 2022).
Figure 6.
Runs over time by age group and support type. Number of runs over time by support type (colors) and age group (panels), from 2009 to 2022 (inclusive). Boxed numbers indicate runs counts for years 2009 and 2022 for visual reference according to color (support type). Run counts: neonatal ECPR (2009): 112; neonatal ECPR (2021): 175; neonatal Cardiac (2021): 589; neonatal Respiratory (2021): 763: neonatal ECPR (2022): 168; pediatric ECPR (2009): 219; pediatric ECPR (2021): 501; pediatric Cardiac (2021): 1,162; pediatric Respiratory; adult ECPR (2009): 86; adult Cardiac (2009): 269; adult Respiratory (2009): 495; adult ECPR (2022): 2,069.
Mobility.
Mobilization on ECMO21 was greatest among those receiving respiratory support. Among adults, 22% (5,426/24,684) were mobilized, and 20% (206/1,056) of pediatric patients 8 or older were mobilized. Mobilization was less in other forms of ECMO support: adult cardiac 14% (2,934/20,957), adult ECPR 8% (451/5,604), pediatric cardiac patients 8 years or older 11% (128/1,150), pediatric ECPR 8 years or older 5% (21/451). Overall, the majority of ECMO patients remain immobile and bed-bound during their ECMO run.
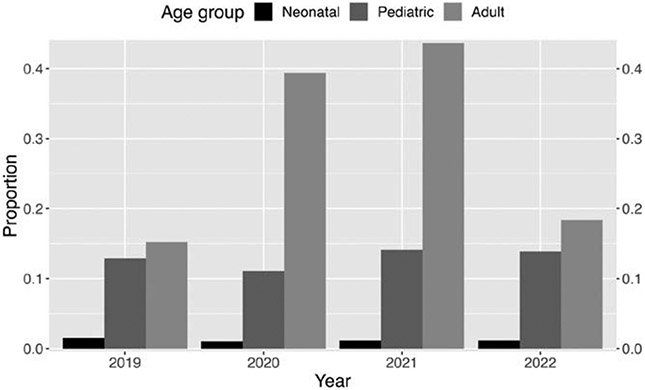
Prone positioning in respiratory ECMO.
Figure 7 shows the use of prone positioning before initiation of respiratory ECMO by age. The use of prone positioning has increased among adult patients and was found primarily among patients with COVID-19 (Table S4, http://links.lww.com/ASAIO/B185). Among patients without COVID-19, prone positioning use before ECMO remained relatively unchanged over the years 2019–2022 (2019 rate: 15.2% (784/5,156), 2020 rate: 13.7% (480/3,506), 2021 rate: 10.0% (270/2,698), 2022 (10.6% (358/3,387). Among patients with COVID-19, prone positioning increased compared to the baseline rate of 15% seen in adult respiratory ECMO in 2019 (COVID prone rate 2019: 0% (0/0); 2020 rate: 57.7% (2,956/5,123); 2021 rate: 55.7% (4,190/7,521); 2022 rate: 45.9% (441/961). Prone positioning in children remained unchanged over the years 2019–2022. In 2022, it was 14% (113/815) for pediatric and 1% (8/675) for neonatal respiratory support.
Figure 7.
Proportion of prone positioning use before respiratory support ECMO by age group from 2019 through 2022. Age groups are indicated by colors for each of three years. Bar height represents proportion from 0 to 1, shown on the Y-axes.
Cardiac support.
Since 2009, 60% (4,128/6,911) of neonatal cardiac runs, 50% (5,797/11,504) of pediatric cardiac runs, and 16% (7,133/45,830) of adult cardiac runs had a cardiac addendum reported. Low cardiac output was the most common indication for ECMO (Table S5, http://links.lww.com/ASAIO/B185). The median cardiopulmonary bypass (CPB) duration is listed in Table S6, http://links.lww.com/ASAIO/B185, and the cross-clamp duration in Table S7, http://links.lww.com/ASAIO/B185.
ECPR.
There are ECPR Addendum data available for 8,050 adults, 4,176 pediatric patients, and 1,639 neonates. The distribution of arrest locations is shown in Table S8, http://links.lww.com/ASAIO/B185. Adult arrests were more evenly spread across hospital units: 23% (1,857/8,050) occurring in the ICU,15% (1,190/8,050) occurring in the operating room or a procedural suite, 12% (995/8,050) occurring in the Emergency Department, and 12% (944/8,050) occurring prehospital. In contrast, 65% (2,727/4,176) of pediatric and 79% (1,302/1,639) of neonatal arrests occurred in the ICU. Cannulation location was most commonly ICU for pediatric (74% [3,098/4,176]) and neonatal groups (82% [1,342/1,484]), but adult cannulations were more evenly distributed across the ICU (25% [1,981/8,050]), operating room (24.0% [1,934/8,050]) and emergency department (23% [1,808/8,050]) (Table S9, http://links.lww.com/ASAIO/B185). The most common initial cardiac arrest rhythm was pulseless electrical activity (PEA) (20% (821/4,176]) for pediatrics, other for neonatal patients (25% [410/1,639]), and ventricular fibrillation (VF (31% [2,486/8,050]) for adults (Table S10, http://links.lww.com/ASAIO/B185).
Clinical Outcomes
Survival.
Survival to hospital discharge was 57% (27,701/48,338) for adult respiratory, 44% (20,264/45,830) for adult cardiac, 30% (4,162/14,097) for adult ECPR; 64% (5,423/8,495) for pediatric respiratory, 58% (6,636/11,504) pediatric cardiac, 41% (2,355/5,740) pediatric ECPR, 69% (7,888/11,511) neonatal respiratory, 48% (3,337/6,911) neonatal cardiac, and 44% (949/2,142) neonatal ECPR (Table 1). For respiratory support, absolute survival decreased by approximately 10% from 24 hours after ECMO decannulation until hospital discharge. In contrast, for cardiac and ECPR support, absolute survival decreased by approximately 20% over the same interval (Table 1).
Table 1.
ECMO Case Counts, Center Counts, and Survival, 2009–2022
| Age Group | Support Type | Runs | Centers | Survival to 24 Hours After ECMO Decannulation* |
Survival to Hospital Discharge† |
|---|---|---|---|---|---|
| Adult | Respiratory | 48,338 | 658 | 67.2% (32,008/47,663) | 57.3% (27,701) |
| Cardiac | 45,830 | 634 | 62.6% (28,138/44,927) | 44.2% (20,264) | |
| ECPR | 14,097 | 504 | 44% (6,124/13,910) | 29.5% (4,162) | |
| Pediatric | Respiratory | 8,495 | 421 | 74.5% (6,214/8,342) | 63.8% (5,423) |
| Cardiac | 11,504 | 393 | 76.9% (8,621/11,213) | 57.7% (6,636) | |
| ECPR | 5,740 | 309 | 58.9% (3,311/5,617) | 41.0% (2,355) | |
| Neonatal | Respiratory | 11,511 | 242 | 82.7% (9,327/11,277) | 68.5% (7,888) |
| Cardiac | 6,911 | 264 | 71.5% (4,827/6,750) | 48.3% (3,337) | |
| ECPR | 2,142 | 181 | 67.5% (1,411/2,091) | 44.3% (949) |
This excludes run records in which the patient was not reported as being discharged alive to home and for which the patient’s time of death/discharge was not recorded.
This is the percentage of patients discharged alive and off ECMO.
Discharge location.
There were differences in the discharge locations by age group in 2022 among patients discharged alive (Table 2). Forty-two percent of adults (2,750/6,623), 63% (938/1,481) of pediatric, and 71% (591/838) of neonates were discharged directly to home. For adults, 36% (2,373/6,623) of patients were discharged to a long-term acute care (LTAC) or rehabilitation facility, compared to 13% (191/1,481) of pediatric patients and 5% (41/838) of neonatal patients.
Table 2.
Discharge Location for Decannulated ECMO Patients Discharged Alive From the Hospital, 2022
| Discharge Location |
Neonatal | Pediatric | Adult |
|---|---|---|---|
| Other, unknown | 1.7% (14) | 3.6% (53) | 1.8% (120) |
| Home | 70.5% (591) | 63.3% (938) | 41.5% (2,750) |
| Transferred to another hospital | 20.5% (172) | 14.4% (214) | 15.3% (1,012) |
| LTAC or rehab | 4.9% (41) | 12.9% (191) | 35.8% (2,373) |
| Transfer to hospice | 0.4% (3) | 0.3% (5) | 1.5% (98) |
| (missing) | 2.0% (17) | 5.4% (80) | 4.1% (270) |
LTAC, long-term acute care.
Duration of ECMO run.
The median duration of an ECMO run (2009–2022) was longest for respiratory and shortest for ECPR, across all age groups (Table 3). Among adults, the median run duration for respiratory support was 233 hours [IQR 110, 484]) and shortest for ECPR (68 hours [22, 140]) (Table 3). The median duration of respiratory support declined with age for pediatrics, the median duration of respiratory support was (188 hours [97, 357]) and for neonates, the median duration of respiratory support was (159 hours [100, 276]).
Table 3.
Duration of ECMO Run, 2009–2022
| Age Group | Support Type | Duration (Hours)* | Missing |
|---|---|---|---|
| Adult | Respiratory | 233 (110,484) | 45 |
| Cardiac | 113 (53,196) | 43 | |
| ECPR | 68 (22,140) | 12 | |
| Pediatric | Respiratory | 188 (97,357) | 20 |
| Cardiac | 114 (65,190) | 7 | |
| ECPR | 84 (38,151) | 3 | |
| Neonatal | Respiratory | 159 (100,276) | 7 |
| Cardiac | 111 (65,189) | 5 | |
| ECPR | 95 (53,167) | 1 |
Median (interquartile range).
ECPR, extracorporeal cardiopulmonary resuscitation.
Complications.
The rates of complications and renal replacement therapy use on ECMO, per 1,000 ECMO hours are described in Tables 4-6, as well as Table S11, http://links.lww.com/ASAIO/B185, S12 http://links.lww.com/ASAIO/B185, and S13 http://links.lww.com/ASAIO/B185). The use of renal replacement therapy (RRT) was high across all age groups. Complication rates varied by age group and support type. The incidence of limb ischemia, fasciotomy, and amputation were highest among adult patients. Cannulation complications were most prevalent for pediatric and neonatal age groups. Circuit thromboses and hemolysis was highest in neonatal support, decreasing in pediatric patients and further in adult patients.
Table 4.
ECMO Rates of Select Complications (Per 1,000 ECMO Hours) by Support Type, Adults, 2017–2022
| Complication | Respiratory | Cardiac | ECPR |
|---|---|---|---|
| Cannula problems | 0.155 | 0.206 | 0.377 |
| Air in circuit | 0.025 | 0.051 | 0.083 |
| Circuit change | 0.258 | 0.177 | 0.180 |
| Clots and air emboli | 0.006 | 0.008 | 0.016 |
| Thrombosis/clots in circuit component | 0.103 | 0.225 | 0.282 |
| Renal replacement therapy | 0.574 | 1.655 | 1.924 |
| Cannula site bleeding | 0.123 | 0.751 | 1.059 |
| Surgical site bleeding | 0.156 | 0.871 | 0.643 |
| GI hemorrhage | 0.136 | 0.272 | 0.392 |
| Tamponade (blood) | 0.020 | 0.212 | 0.171 |
| CNS hemorrhage by US/CT/MRI | 0.107 | 0.154 | 0.272 |
| CNS infarction by US/CT/MRI | 0.031 | 0.208 | 0.365 |
| Seizures (clinical or on EEG) | 0.019 | 0.068 | 0.241 |
| Brain death | 0.024 | 0.072 | 0.433 |
| Hemolysis (all types) | 0.160 | 0.282 | 0.351 |
| Limb Ischemia | 0.021 | 0.241 | 0.410 |
| Compartment syndrome | 0.005 | 0.053 | 0.092 |
| Fasciotomy | 0.010 | 0.156 | 0.232 |
| Amputation | 0.004 | 0.042 | 0.052 |
CNS, central nervous system; CT, computed tomography; dL, deciliter; ECPR, extracorporeal cardiopulmonary resuscitation; EEG, electroencephalogram; GI, gastrointestinal; mg, milligram; MRI, magnetic resonance imaging; US, ultrasound; WBC, white blood cell.
Table 6.
ECMO Rates of Select Complications (Per 1,000 ECMO Hours) by Support Type, Neonatal, 2017–2022
| Complication | Respiratory | Cardiac | ECPR |
|---|---|---|---|
| Cannula problems | 0.666 | 0.589 | 0.718 |
| Air in circuit | 0.146 | 0.173 | 0.230 |
| Circuit change | 0.862 | 0.767 | 0.761 |
| Clots and air emboli | 0.074 | 0.031 | 0.029 |
| Thrombosis/clots in circuit component | 1.242 | 0.977 | 1.069 |
| Renal Replacement Therapy | 0.907 | 1.772 | 2.175 |
| Cannula site bleeding | 0.370 | 1.019 | 1.522 |
| Surgical site bleeding | 0.294 | 0.902 | 0.811 |
| GI hemorrhage | 0.073 | 0.074 | 0.122 |
| Tamponade (blood) | 0.051 | 0.166 | 0.194 |
| Seizures (clinical or on EEG) | 0.524 | 0.749 | 1.084 |
| Brain death | 0.108 | 0.191 | 0.301 |
| Hemolysis (all types) | 0.352 | 0.556 | 1.342 |
| Limb Ischemia | 0.005 | 0.037 | 0.057 |
| Compartment syndrome | 1.222 | 1.200 | 1.629 |
| Fasciotomy | 0.013 | 0.050 | 0.022 |
| Amputation | 0.001 | 0.006 | 0.014 |
CNS, central nervous system; CT, computed tomography; dL, deciliter; ECPR, extracorporeal cardiopulmonary resuscitation; EEG, electroencephalogram; GI, gastrointestinal; mg, milligram; MRI, magnetic resonance imaging; US, ultrasound; WBC, white blood cell.
Neurologic outcomes after ECPR.
Seventeen percent of all neonatal or pediatric patients with an ECPR addendum, and 32% of neonatal or pediatric patients reported a PCPC score of 1–2 (normal/mild disability) (Table S14, http://links.lww.com/ASAIO/B185). Thirty-one percent of all neonatal or pediatric patients, and 60% of patients with a reported PCPC value, had a score of 6 (brain death). 17% of adult patients reported a CPC value, had a score of 1–2 (normal/mild disability) (Table S15, http://links.lww.com/ASAIO/B185). Sixty-seven percent of all adult patients, and 74% of patients with a reported CPC value, had a score of 5 (brain death) or death.
Discussion
The 2022 ELSO Registry Report details ECMO support between 2009 and 2022, when the Registry’s 200,000th ECMO patient was reported and 100,000th patient was discharged alive. Through this period, there were shifts in ECMO structure, process, and outcomes; many were associated with the COVID-19 pandemic, while others predated the pandemic and continue beyond it.
During 2020 and 2021, the COVID-19 pandemic was associated with growth in the number of ECMO-supported patients and changes in the proportion of ECMO support types delivered. In 2020 and 2021, adult respiratory ECMO predominated, while adult cardiac, adult ECPR, and pediatric respiratory ECMO support saw absolute declines for the first time in years. Among adult patients, cardiac ECMO case volume declines coincided with temporary suspension of nonurgent cardiac operations,35 which may have also led to reduced ECMO support. ECPR was somewhat restricted to younger (presumably healthier) adult patients. By 2021, ECMO use had liberalized with proportionally more older adults receiving non-COVID ECMO. Pediatric respiratory support may have decreased due to masking and isolation leading to decreased transmission of influenza and respiratory syncytial virus (RSV).36,37 Additionally, ELSO and other professional organizations issued caution around ECPR at the start of the pandemic, and many resources were constrained, all of which may have contributed to stymied ECMO growth.38
However, the impact of COVID-19 on volume appears to have been temporary. In 2022, the ELSO Registry demonstrated a reduction in ECMO support for COVID-19 and return to pre-COVID-19 patterns of ECMO use. We also observed center-level changes that predated COVID-19 and persisted thereafter. The number of centers in the 2016 report was 310, with 303 adult centers providing ECMO, compared to over 650 in the 2022 report. ELSO center volumes of median adult annual ECMO center volume continued to rise from 4 to 18.5/center, while pediatric median annual volumes began to decline.
There have been multiple process changes and resultant additions to data collection since the last Registry report. During COVID-19 pandemic years of 2020 and 2021, 39%–43% of respiratory ECMO patients received prone positioning (for at least 16/24 hours before ECMO initiation); this was a nearly a threefold increase compared to the 15%–18% of adults who had received prone positioning prior, in 2019, and after, in 2022. As prone positioning has been demonstrated to reduce ARDS mortality,25 and is aligned with best practice in severe ARDS,39 the addition of this variable to the Registry in 2017 highlights how the Registry continues to incorporate evidenced-based and best practices metrics for research and quality reporting.23 Another process that gained attention during the COVID-19 pandemic was VP ECMO, which enables right ventricular hemodynamic support in addition to gas exchange.20 Though rarely used, select centers reported improved outcomes with VP ECMO support,40,41 prompting ELSO to begin reporting VP ECMO cannulation separately.
The increasing awareness of the potential benefits of mobilization during critical illness42 was reflected in Registry data collection, which began collecting mobilization data in 2018. For respiratory ECMO support, one in five children and adults are reported to achieve some mobility during ECMO.43 While this remains modest, the practice is poised to grow with the field, given the associated benefits.44-47
Despite the increasing use of ECMO, hospital survival was broadly similar between the 2016 and 2022 ELSO Registry Reports.27 In respiratory ECMO support, the 2016 report observed mortality at hospital discharge for respiratory ECMO support to be 74% for neonates, 58% for pediatrics, and 58% for adults compared to 69%, 64%, and 57%, respectively, in 2022. It is unclear whether these changes in mortality reflect different management strategies, that sicker patients are more recently being placed on ECMO48 or whether other factors are involved.49 Since 2016, ELSO has also added additional outcome measures. These outcomes include details on extubation, transition to transplant or ventricular assist device, ICU discharge, and neurologic outcome for ECPR patients using the PCPC33 and CPC.34 The 2022 report reports complications per 1,000 hours of ECMO, similar to the COVID-19 Registry Reports.30,31 This has the advantage of accounting for the exposure to ECMO when reporting complications.30 This was not feasible historically because the Registry only collected the presence or absence of complications, but since 2017 dates were added to complications, enabling collection of repeated events.
Finally, the ELSO Registry has increasingly supported academic research, with exponentially increasing publications utilizing Registry data;2,50 data are released under license but free of charge to ELSO member centers, pending scientific review and approval of research proposals.
Strengths of the ELSO Registry include its size and breadth. Including over 200,000 patients since 1989, across 557 centers in 57 countries, the ELSO Registry is the world’s largest ECMO Registry and offers an unprecedented ability to query local, regional, and global clinical characteristics and outcomes over time. Responsive to changing world events, the ELSO Registry allows for dynamic additions of data collection through focused and temporally discrete addenda, such as H1N1 or COVID-19, or for clinical conditions, such as trauma, or ECPR. The scope of center participation facilitates prospective studies.51 Since 2016, the US Food and Drug Administration encourages the use of registry data, such as ELSO, for regulatory approval and monitoring, which ELSO has facilitated.52 The ELSO Registry admittedly has limitations. As a voluntary Registry, data entry falls below 100, and lacks external validation at this time. The goal of increasing global participation and reporting may be impaired by calls for expanded data collection, especially with manual data entry. Further, the twin goals of research and quality mean that the Registry is inherently broad in scope with limits to granularity. Long-term outcomes and many process measures are not yet collected, though remain future targets. The changing needs of our global ECMO community, including patients, hospitals, industry and regulators encourage a continuous cycle of iterative Registry changes, ensuring continued relevance to all our patients, members and stakeholders.
Conclusions
The ELSO Registry has expanded tremendously in its 34 year history in terms of patients, centers, and depth and breadth of data included. Since its inception, it has helped centers to measure and compare outcomes across its member centers. During the COVID-19 pandemic, it helped the ECMO community observe and disseminate observations to inform care as care was being provided. In the coming years, it will continue to build on its legacy of providing data to inform improved ECMO care delivered as measured by the ELSO Quality Reporting Platform and in the creation of new knowledge through its own resource and embedded studies.
Supplementary Material
Table 5.
ECMO Rates of Select Complications (Per 1,000 ECMO Hours) by Support Type, Pediatric, 2017–2022
| Complication | Respiratory | Cardiac | ECPR |
|---|---|---|---|
| Cannula problems | 0.534 | 0.424 | 0.748 |
| Air in circuit | 0.152 | 0.171 | 0.202 |
| Circuit change | 0.636 | 0.621 | 0.554 |
| Clots and air emboli | 0.025 | 0.025 | 0.016 |
| Thrombosis in circuit component | 0.545 | 0.816 | 0.775 |
| Renal Replacement Therapy | 0.875 | 1.515 | 2.074 |
| Cannula site bleeding | 0.343 | 1.006 | 1.097 |
| Surgical site bleeding | 0.227 | 0.850 | 0.631 |
| GI hemorrhage | 0.123 | 0.137 | 0.264 |
| Tamponade (blood) | 0.064 | 0.175 | 0.136 |
| CNS hemorrhage by US/CT/MRI | 0.179 | 0.331 | 0.695 |
| CNS infarction by US/CT/MRI | 0.097 | 0.256 | 0.636 |
| Seizures (clinical or on EEG) | 0.134 | 0.311 | 1.283 |
| Brain death | 0.054 | 0.094 | 0.492 |
| Hemolysis (all types) | 1.039 | 0.983 | 1.017 |
| Limb Ischemia | 0.021 | 0.102 | 0.130 |
| Compartment Syndrome | 0.009 | 0.031 | 0.064 |
| Fasciotomy | 0.021 | 0.066 | 0.104 |
| Amputation | 0.010 | 0.023 | 0.013 |
CNS, central nervous system; CT, computed tomography; dL, deciliter; ECPR, extracorporeal cardiopulmonary resuscitation; EEG, electroencephalogram; GI, gastrointestinal; mg, milligram; MRI, magnetic resonance imaging; US, ultrasound; WBC, white blood cell.
Acknowledgments
We would first and foremost like to acknowledge ELSO Centers worldwide; without the clinical care and effort of these providers and staff, and the time and effort it takes to enter these data, this report would not be possible. Furthermore, we would like to acknowledge the patients included in this report, both those who have survived, and those who have died. Finally, we would like to acknowledge the contributions of Jonathon P. Fanning MBBS, PhD to this report. Collaborating authors to this report from the ELSO Member Centers are named with their center (Supplement 2, http://links.lww.com/ASAIO/B186).
Disclosure
J.E.T. is the Chair of the Registry Committee of the Extracorporeal Life Support Organization (ELSO). P.S.B. receives salary support from ELSO. G.M. is the President of ELSO. M.P. is the Immediate past President of ELSO. D.B. receives research support from and consults for LivaNova. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira, and Cellenkos. He is the President-elect of ELSO and the Chair of the Executive Committee of the International ECMO Network (ECMONet), and he writes for UpToDate. M.A., A.H., and K.R. are the Immediate Past Co-Chairs of the Scientific Oversight Committee of ELSO. P.R. is the Executive Director of ELSO. C.S. is the Chief Executive Officer (CEO) of ELSO. N.A.B. is the President of European Chapter of ELSO. N.A.B. has been on the medical advisory boards for Xenios and Baxter. T.M. is on the Board of Directors of ELSO. R.D.G. is the President of the Latin-American Chapter of ELSO. P.M.K. is the President of the South West Asia and Africa Chapter of ELSO. J.F.F. is the President of Asia-Pacific Chapter of ELSO. P.M.A.A. is Treasurer of ELSO Board of Directors. P.M.A.A. is funded by U.S. DoD PRMRP Clinical Trial Award #W81XWH2210301, NIH (R13HD104432) and FDA UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation (U01FD004979/U01FD005978). None of the funding sources were involved in the design or conduct of the study, collection, management, analysis, or interpretation of the data, or preparation, review, or approval of the manuscript. No other conflicts of interest reported. R.P.B. is a member of the Board of Directors for ELSO and receives funding from the National Heart, Lung, And Blood Institute (R01 HL153519).
J.E.T. is supported by a Career Development Award from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23 HL141596). This study was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002538 (formerly 5UL1TR001067-05, 8UL1TR000105, and UL1RR025764). P.M.A.A. is funded by U.S. DoD PRMRP Clinical Trial Award #W81XWH2210301, NIH (R13HD104432), and FDA UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation (U01FD004979/U01FD005978). None of the funding sources were involved in the design or conduct of the study, collection, management, analysis, or interpretation of the data, or preparation, review, or approval of the manuscript.
The Extracorporeal Life Support Organization (ELSO) Member Centers investigators are as follows:
Adeel Abbasi, Ahmad Said Abdalmohsen, Akram M. Abdelbary, Francisco Abecasis, Peter Abel, Yasir Abu-Omar, Douglas R Adams, Juan Manuel Africano, Devon Aganga, Salvatore Agati, Cara Agerstrand, Mario V. Aguillon, Crystal S. Akers, Julia Akhtarekhavari, Mohammad Izzat Salah Alazzam, Martin Albert, Angela Alberti, Peta M.A. Alexander, Abdulrahman A. Al-Fares, Huda Alfoudri, Silvie Allaert, Keesha N. Allbert, Christopher T. Allen, Miguel Ángel Lescano Alva, Cory M. Alwardt, Angela Amigoni, Balaram Anandamurthy, Kyriakos Anastasiadis, Nicholas R. Anders, Scott A. Anderson, Patricia L. Anderson, Ana Andrijević, Alice Annoni, Michael Anselmi, James R. Anstey, Marta V. Antonini, Polychronis Antonitsis, Tays Stein Araujo, Rhodney Arcalas, Igor Areinamo, Anibal Martin Arias, Veronica Armijo-Garcia, Vladimir Aronsky, Lovkesh Arora, Madhur Arora, Marit Leigh Aspenleiter, Fernando A. Atik, Erin Colleen AugustGeorg Auzinger, Ismail Azzam, Matthew Bacchetta, Erica I. Bak, Joan Balcells, Jesús Sánchez Ballesteros, Igor S. Banjac, Jacqueline M. BarbariaNicholas A. Barrett, Cleide L. Barrigoto, Stephanie D. Bass, Uroš Batranović, Matthew H. Bauer, Diego Fernando Bautista, Robert M. Beck, Estefania Giraldo Bejarano, Jan Belohlavek, Melania M. Bembea, Jan Benes, Peyman Benharash, Lynne A. Benish, Suzanne Bennett, Luís F.N. Bento, Christian A. Bermudez, Pietro Bertini, Derek Best, Ankit Bharat, Omar J. Bhutta, Samantha J. Bizzell, Stephanie A. Blakeman, Pablo Blanco-Schweizer, Jessica K. Blanton, Peggy S. Blood, Allison S. Bohlmann, John Kyle Bohman, Michela Bombino, Desiree Kathleen Bonadonna, Ashley Bond, Kristina M. Borgmann, Pierre Bourgoin, Brian M. Boville, Raquel Boza, Heather L. Brady, Alison Brady, Jessica M. Braunlich, Brian C. Bridges, Karen K. Brinkley, Robert S. Brookshire, Nicolas A. BrozziNicole Brueggemann, Dwight P. Buckley Jr., Klayton Buckley, Irfan B. Budhani, Nazar Bukamal, Lucrecia M Burgos, Filip Burša, Landon K. Busby, Hergen Buscher, Menoly Butler, Warwick W. Butt, Jonathan W. Byrnes, Christos Calaritis, Lisa R. Caldwell, Gregory L. Calligaro, Patrick T. Campbell, Luigi Camporota, Luiz Fernando Caneo, Vladimir Jovo Carapic, Cristina Carrasco-Carrasco, Nestor Ivan Carrizo, Heidi Carrow, Edmund G. Carton, Christian Casabella, Vanesa Gomez Casal, Francis L. Casey III, Andres Castillo, Anthony W. Castleberry, Yiorgos Alexandros Cavayas, Karey Cerqua, Kai Man ChanWai Ming Chan, Jason Brian Chapman, Hari Brahma Chari, Omair ChaudharyIra M. Cheifetz, Robin H.S Chen, Weiting Chen, Eva W. Cheung, Anson Cheung, Juan I. Chico, Roberto Chiletti, Hwa Jin Cho, Jill M. Cholette, Steffen Christensen, Betty S. Chui, Alessandro Circelli, Katherine C. Clement, Julie Cleuziou, Brian Clouse, Gwendolen Cole, Garrett M. Coles, Monika F. Collins, Monika F. Collins, James Connelly, Steven A. Conrad, Marlene Cook, Hannah Copeland, Scott C. Copus, Charles S. Cox Jr, Lynne K. Craig, Natasha Crain, Ricardo V. Cremonese, Emily A. Criswell, Lisa M. Cross, Moira A. Crowley, Jerome C. Crowley, Leonora Cruz, Marcelo Cypel, Tomasz Czarnik, Miroslaw E. Czuczwa, Taís Sica da Rocha, Samuel Daddow, Dante C. Dali, Heidi J. Dalton, Kathleen J.R. Daly, Emily Damuth, Dennis A. Daniel, John M. Daniel IV, Josiane M. Daniel, Max D. Danis, Melissa E. Danko, Joao Alberto Rodrigues Dantas, Isabelle Daoust, Dieter F. Dauwe, Mark Davidson, Joel C. Davis, Mitchell Davis, Jonathan D’Cunha, Bruno de Arruda Bravim, Willem P. de BoodeKim T. De La Cruz, Kathryn Gray DeAngelis, Gerdy Debeuckelaere, Matthew A. Deitemyer, Jeffrey DellaVolpe, Jamie L. Deneau, Walter F. DeNino, Christopher G. Denmark, Derek Denney, Patrick A. DeValeria, Petra Dewulf, Matteo Di Nardo, Daniel J. DiBardino, Joseph DiMartino, Stavros Dimopoulos, Michele B. Domico, Meaghan E. Dominy, Dirk W. Donker, Till Dresbach, Joep M. Droogh, Tiffany W. Dunlap, Allsion Dupon, Lucian A. Durham III, Andrew Durward, Anna Dvorak, John F. Dyett, Carol L. Dziedzina, Carmen L. Eaken, Jonathan S. Eaton, Christopher J. Eberle, Linda Edwards, Christakis Efseviou, Juliann M. Eigner, Hazem Ahmed Elhamrawi, Alyaa M. Elhazmi, Tammy Elizondo, Beverly L. Ellersick, Jonathan A. Emling, Andreas Ernst, Juan Pablo Escalante, Otoniel Espinoza, Lee W. Evey, Eddy Fan, Gary Fang, Jeffrey J. Fanning, Gail M. Faulkner, Karen R Fauman, Niall Ferguson, Benigno Ferreira, Arnt E. Fiane, , Dario Andrade Fierro, , María Martha Filippi, Michael C. Findeisen, Katie Finlay, Gordon Finlayson, Gwenyth A. Fischer, Courtney D. Fischer, William J. Fischer III, Caleb M. Fisher, Reni Fitriasari, Jillian Fitzgerald, Melissa K. Fix, Sarah B. Fleming, Brigid C. Flynn, Beth A. Forst, Philip P. Fortuna, Giuseppe Foti, Matthew P. Fox, Thais O. Franco, C. David Freeland, Justin A. Fried, Matthew L. Friedman, Beatriz Furlanetto, Thomas Fux, Sérgio Gaião, Michael J. Gale, Joann Kathleen G. Garcia, Romel Garcia-Montilla, Eric R. Gardner, Meena Garg, Lawrence L. Garrison, Srdjan M. Gavrilovic, Ryszard Gawda, Laura W. Geer, Elton A. Gelandt, Michael G. Gelvin, Bradley M. Genovese, Jeffrey A. George, Timothy J George, Sangley George, Anup Ghimire, Marco Giani, Baljit S. Gill, Erin Glikes, Michael Golecki, Rene D. Gomez-Gutierrez, Enrique Gongora, Sara Govener, Amanda Graf, Giacomo Grasselli, Brian W. Gray, Joseph A. Greenlee III, Igor D. Gregoric, Melinda Gregory, Edgars Grins, Heinrich Volker Groesdonk, Kimberly F. Group, Fabio Guarracino, Alexandra Joy Guidi-Solloway, Tyler M. Gunn, Pramod K Guru, John C. Haddle, Jonathan W. Haft, Emma Haisz, Julie L. Hall, Cameron Hall, Jun Hamaguchi, Terese C. Hammond, Peggy K. Han, Daphne C. Hardison, Dickwelle T. Harischandra, Shaun M. Hart, Matthew T. Harting, Louise Hartley, Chris J. Harvey, Zubair Hasan, Ibrahim Fawzy Hassan, Jennifer R. Hastings, Renee’ Hatcher, Kevin W. Hatton, Christopher K. Haught, Jeremiah Awori Hayanga, Timothy Peter Haydon, Aaron H. Healy, Micheal L. Heard, Beth M. Heather, Rik H.J. Hendrix, Felix Hennig, Greet HermansJeannine A.J. Hermens, Deborah A. Hernandez, Jaime Hernandez-Montfort, Guillermo Herrera, Keri Hickman, Ashley Hittel, Crystal Hobbs, Jordan R.H. Hoffman, Laura E. Hollinger, Michael Homishak, Nelson K. Horigoshi, Kota Hoshino, Shu-Chien Huang, Katharina Huenges, Alexander D. Hussey, Robert W. Hyslop, Rayan E. Ihle, Ola Ingemansson, Daniel Ivulich, Amanda L. Jackson, Rogelio Garcia Jacques, Harsh Jain, Sharon M. Jakobs, Robert Jan, Lisa M. Janowiak, Claire B. Jara, Angela M. Jarden, Jamie L. Jarzembowski, Andrew Jaudon, Venkata Krishna Kishore Jayanthi, Joseph A. Jennings, Inseok Jeong, Rafael Meza Jiménez, Gian M. Jimenez-Rodriguez, Sabrina Joachim, Daniel Joelsons, Caroline A. Johnson, Andrea L. Johnson, Jeffry H. Jones, Mark Joseph, Sunimol Joseph, Raja Joshi, Christopher J. Joyce, Jae Seung Jung, José Carone Junior, Harry J. Kallas, Asavari KamerkarPilje Kang, Biswajit Kar, , Georgios T. Karapanagiotidis, Javier Kattan, David A. Kaufman, Akira Kawauchi, Sarah D. Keene, Norma M. Keller, Roberta Keller, Emily W. Kelley, Kellie Kelley, Janet F. Kelly-Geyer, Peter Kenderessy, Laura E. Kenny, Shaf Keshavjee, D. Kessel, Heather Kessler, Suzanne Keuler, Sanjay Khicha, Do Wan Kim, Richard Y. Kim, Aaron Maxwell Kime, Robert C. Kincade, Florian Kipfmueller, Douglas A. Kirk, Liviu Klein, Randall S. Knapp, Randall S. Knapp, Martin C.J. Kneyber, Andrea L. Knowles, Jillian M. Koch, Stephanie Koepke, Klaus M. Kogelmann, Carlos Elzo Kraemer, Amanda Krauklis, Samantha L. Krumroy, Madhan Kumar, Arun Kumar, Matthias E Kumpf, Kimberly Kyle, Anna Laffin, Wim Kees Lagrand, Parshawn A. Lahiji, Peter Chi Keung Lai, Cally Ho Ka Lai, Amanda Danielle Laird, Michelle LaMarreDavid M. Landsberg, Pia Lanmueller, Annemieke Oude Lansink-Hartgring, Sharon Beth Larson, De’Ann M. Laufenberg, Jayshree Lavana, Tracie L. Layne, Michael John Lazar, Matthew R. Ledoux, Raymond C. Lee, Thomas M. Leek, Laurance Lequier, Timur Lesbekov, Robert Leslie, Kit Hung Anne Leung, Jon Lillie, Yeong Phang Lim, Sang-Hyun Lim, Ling Lin, Thomas Lindsey, Steven Kin Ho Ling, Kaitlyn J. Lingle, Jed Lipes, Songqiao Liu, Judit Llevadias, Erin A. Lomas, Robert D. Longenecker, Roberto Lorusso, Tracy Ann Low, Anthony Steven Lubinsky, Matthias LubnowMark T. Lucas, , Alberto Lucchini, Lisa E. Luze, William R. Lynch, Manoj M.C., Jacinta J. Maas, Graeme MacLaren, Vanessa MacNamara, Jesse L. Madden, Justin Maimone, Rajiv Malhotra, Matthew P. Malone, Chirantan Mangukia, Daniel Manzur-Sandoval, Veronika Maráczi, Jonathan L. Marinaro, Christina R. Marinucci, Tammy Marshall, Mark Martin, Eva M. Marwali, Anna Maslach-Hubbard, Jovan Matijašević, Adrian Mattke, Joseph Mattucci, Timothy M. Maul, Marc O. Maybauer, Michael Mayette, Joni R. Mayville, Catherine McAllister, Martha W. McBride, David Scott McCaul, Samantha L.S. McClelland, Colin Gregory McCloskey, Randy McGregor, Wesley A. McKamie, Andrew D. McKee, Chelsea M. McMahon, Kaye McMullin, Jane McNicol, John P. McNulty, Thomas McRae, Maureen E. Meade, Philippe Meersseman, Michael Mekeirele, Elisa Ito Mendes, Anuradha P. Menon, Jason P. Meyer, Jourdan E. Meyers, Bart Meyns, John L. Mignone, Brittany D. Miller, Malcolm G.A. Miller, Deborah Miller, Renee Mintak, Sarah M. Minter, Dinis Reis Miranda, Farrukh Mirza, Joseph D. Mishkin, Paul Modelewski, Rajeev C. Mohan, Yee Hui Mok, Dustin Money, Julie Monteagudo, Russell R. Moores Jr., Patrick Moran, Shawn Morelock, Marsha R. Moreno, Juan Blanco Morillo, Tracy Morrison, John M. Morton, Brenda Morton, Andrea Moscatelli, Jarrod M. Mosier, Ralf M. Muellenbach, Andreas Mueller, Dale Mueller, Steven C. Musca, Dave Nagpal, Tasnim Najaf, Mangala Narasimhan, Melissa Nater, Zynthia Natividad, Djordje Nedeljkov, Bryan D. Nelson, Sally F. Newman, Debra E. Newton, Jonathan L. Neyman, Wing Yiu George Ng, Meghan C. Nicholson, Christine Nicolaas, Charlie Nix, Raymond Nkwantabisa, Shirley Nolan, Mariano Norese, Bridget M. Norton, Bridget M. Norton, Serena G. O’Brien, Maura O’Callaghan, Peter Oishi, Tony D. O’Leary, Salim E. Olia, Carlisle O’Meara, Emily E. Oppel, Julian Arias Ortiz, Pranay L. Oza, Caroline P. Ozment, Marjorie Pacific, Matthew L. Paden, Fernando Pálizas, David Palmer, Luca Paoletti, Diego H. Pardo, Pablo Paredes, Thomas PasgaardMrunal G. Patel, Sandeep M. Patel, Vijay S. Patel, Brijesh V. Patel, Sameer PatelDrisya Paul, Amit A. Pawale, Nicole M. Pearson, Crystal Renee Pearson, Giles J. Peek, Crescens M. Pellecchia, , Vincent Pellegrino, Harlinde Peperstraete, Rebecca L. Perkins, Brandon Perkins, Steven Peterec, Claire Peterman, Cooper W. Phillips, Richard R. Piekutowski, Maráa L. Pilan, Maria Luisa Pilan, Jason Mark Pincus, Melissa Pino, Robert W. Plambeck, Michael S. Plisco, Donald A. Plumley, Mark D. Plunkett, Robinson Poffo, Pei-Fen Poh, Angelo Polito, Travis L Pollema, Matteo Pozzi, Matteo Pozzi, Thomas Pranikoff, Matthew E. Prekker, Erik F. Prossen, Pramod S. Puligandla, Mateusz Puslecki, Muhammad Raheel Qureshi, Lily Emilia Rabanal, Ahmed Abdulhamid Rabie, Craig R. Rackley, Rajko Radovancevic, Matthias Raes, Lauren Desiree Allen Raff, Youssef Rahban, Patricia L. Raimer, Bijoy G. Rajbanshi, Raj Ramanan, Jerome Rambaud, Jorge A. Ramírez-Arce, Ana Carolina Simões Ramos, Suresh G. Rao, Raymond Rector, Bengt Redfors, Ashim Regmi, Jose Alejandro Rey, Joao Miguel Ribeiro, Chelsea E Richards, C. Joan Richardson, Christy C. Riddle, Jordi Riera, Marina Ripardo, Fernando M. Rivas, Ronald M. Roan, Elizabeth Robertson, Megan Robinson, Daniel Röder, Inez E.R. Rodrigus, Peter Paul Roeleveld, Jennifer C. Romano, Roberto Rona, Carol Ann Rosenberg, Felix Rosenow, Robert J. Rowe, Katy E. Rower, Kristina L. Rudolph, Luis Fernando Rueda, Bettina Ruf, Hyde M. Russell, Nichole Russell, Kathleen Ryan, Asif A. Saberi, Ahmed S. Said, Caitlin Sailor, Angela Sakal, Gisela Lujan Salas, Leonardo Salazar, Kashif Saleem, Gordan Samoukovic, Pablo G. Sanchez, Lian Marie Santiago, Murat Sargin, Assad Miguel Sassine, Nancy L. Satou, Paul C. Saunders, Scott Schachinger, Thomas Schaible, Peter Schellongowski, Gerald W. Schlager, Christof Schmid, Joachim Schmitt, LeeAndra Schnell, Janos Schnur, Lukas Schroeder, Scott Schubach, Michael T. Schuetz, Gary S. Schwartz, Patricia Schwarz, Nicole M. Scriven, Ruth B. Seabrook, Cassandra Seefeldt, Troy G. Seelhammer, Susana Segura-Matute, Ayan Sen, Leonardo Adrian Seoane, Jamie Shaffer, Bilal M. Shafi, Shannon Shambley, Shyam Shankar, Amanda Shapland, Yih Sharng, David Shavelle, Jayne Sheldrake, Rajesh Mohan Shetty, Joseph R. Shiber, Naoki Shimzu, Billie Lou Short, Kay A. Sichting, Keith E. Sidehamer, Teka Siebenaler, Scott C. Silvestry, Jennifer T Sinclair, Andrew Sinclair, Aalok R. Singh, Gurmeet Singh, Sean C. Skinner, Alexandra Smart, Reanna M. Smith, Adam Smith, Karen Smith, Sherri Sommer-Candelario, Seunghwan Song, Gro Sorensen, Eduardo Sousa, Christopher T. Sower, Nicholas V Spadea, April Spangle, David G. Speicher, Peter M. Spieth, Ankur Srivastava, Neeraj Srivastava, Mark Stahl, Eric D. Stallkamp Jr, Vanessa J. Stanley, Joanne P. Starr, Thomas Staudinger, Berkeley E. Stevens, Kimberly Stevens, Christian Stocker, Richard Strickland, Erik E. Suarez, Rakesh Kumar Subramanian, Serhii Sudakevych, Charlene Summerall, Santosh Sundararajan, Attapoom Susupaus, Hiroyuki Suzuki, Todd Sweberg, Troy Sydzyik, Tuan Anh Ta, Luciana Tagliari, Hiroyuki Tanaka, Christopher T. Tanski, Mark Tasset, Donna M. Taylor, Nicholas R. Teman, Paul Ramesh Thangaraj, Ravi R. Thiagarajan, Timothy Thiruchelvam, James A. Thomas, Owain D. Thomas, Shaun L. Thompson, David A. Thomson, Roopa Thukaram, Mark L. Todd, Hadi Toeg, Joseph Tonna, Silvio F. Torres, Simon Trautner, Terry Trombino, Divina M. Tuazon, Julie Tuel, Monika Tukacs, April N. Turner, Melissa M. Tyree, Makoto Uchiyama, Prashant Vaijyanath, Judith M.D. van den Brule, Marlice A. van Dyck, Mascha van Gijlswijk, Krisa P. Van Meurs, Tyler J. VanDyck, Amir Vardi, Alejandra Vega, Corey E. Ventetuolo, Magdalena Vera, Leen Vercaemst, Philippe Vets, Heather Viamonte, Mårten Vidlund, Sally H. Vitali, Alexander P.J. Vlaa, Alain Vuylsteke, Kah Loon Wan, Reuben Watkins, Pia Watson, Travis A. Weast, Karen E. Weaver, Norbert Welkovics, Heidi L. Wellner, Jason C. Wells, Karen Welter, Amber G. Westpheling, Lesta D.S. Whalen, Stephen Whebell, Ubbo Wiersema, Matthew E. Wiisanen, Bradley Eugene Wilcox, Keith Wille, Ellyne Jan Will, Brock J. Wilson, April M. Win, James R. Winearls, Linda J. Wise, Tobias Witter, Hoi Mei Ruby Wong, Berhane Worku, Tina M Wright, James K. Wu, Larissa A. Yalon, Garrett Yantosh, Dmitry M. Yaranov, Pat Yee, Cassia Yi, Christian C. Yost, John Young, Katrina Younger, Steven Zaborowski, Brenda Zachmann, Asma Zainab, Rosanna Zanai, Ju Zhao, Chengbin Zhou, and Marcia Zinger.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
References
- 1.Toomasian JM, Snedecor SM, Cornell RG, Cilley RE, Bartlett RH: National experience with extracorporeal membrane oxygenation for newborn respiratory failure Data from 715 cases. ASAIO Trans 34: 140–147, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Tonna JE, Barbaro RP, Rycus PT, et al. : On the academic value of 30 years of the extracorporeal life support organization registry. ASAIO J 67: 1–3, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolar CJ, Delosh T, Bartlett RH: Extracorporeal life support organization 1993. ASAIO J 39: 976–979, 1993. [PubMed] [Google Scholar]
- 4.UK Collaborative Randomised Trial of Neonatal Extracorporeal Membrane Oxygenation: UK Collaborative ECMO Trail Group. Lancet 348: 75–82, 1996. [PubMed] [Google Scholar]
- 5.Mugford M, Elbourne D, Field D: Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev (3): CD001340, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 374: 1351–1363, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet: Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378: 1965–1975, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Shaefi S, Brenner SK, Gupta S, et al. ; STOP-COVID Investigators: Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med 47: 208–221, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combes A, Peek GJ, Hajage D, et al. : ECMO for severe ARDS: Systematic review and individual patient data meta-analysis. Intensive Care Med 46: 2048–2057, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamee JJ, Gillies MA, Barrett NA, et al. ; REST Investigators: Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: The rest randomized clinical trial. JAMA 326: 1013–1023, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser CD Jr, Jaquiss RD, Rosenthal DN, et al. ; Berlin Heart Study Investigators: Prospective trial of a pediatric ventricular assist device. N Engl J Med 367: 532–541, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Brechot N, Hajage D, Kimmoun A, et al. ; International ECMO Network: Venoarterial extracorporeal membrane oxygenation to rescue sepsis-induced cardiogenic shock: a retrospective, multicentre, international cohort study. Lancet 396: 545–552, 2020. [DOI] [PubMed] [Google Scholar]
- 13.Ostadal P, Rokyta R, Karasek J, et al. ; ECMO-CS Investigators: Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: Results of the ECMO-CS randomized clinical trial. Circulation 147: 454–464, 2023. [DOI] [PubMed] [Google Scholar]
- 14.Levy B, Girerd N, Amour J, et al. ; HYPO-ECMO Trial Group and the International ECMO Network (ECMONet): Effect of moderate hypothermia vs normothermia on 30-day mortality in patients with cardiogenic shock receiving venoarterial extracorporeal membrane oxygenation: A randomized clinical trial. JAMA 327: 442–453, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YS, Lin JW, Yu HY, et al. : Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372: 554–561, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Yannopoulos D, Bartos J, Raveendran G, et al. : Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 396: 1807–1816, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belohlavek J, Smalcova J, Rob D, et al. ; Prague OHCA Study Group: Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: A randomized clinical trial. JAMA 327: 737–747, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad SA, Rycus PT, Dalton H: Extracorporeal life support registry report 2004. ASAIO J 51: 4–10, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Davies A, Jones D, Bailey M, et al. ; Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators: Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA 302: 1888–1895, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Conrad SA, Broman LM, Taccone FS, et al. : The extracorporeal life support organization Maastricht Treaty for nomenclature in extracorporeal life support A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med 198: 447–451, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broman LM, Taccone FS, Lorusso R, et al. : The ELSO Maastricht Treaty for ECLS Nomenclature: Abbreviations for cannulation configuration in extracorporeal life support - a position paper of the extracorporeal life support organization. Crit Care 23: 36, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Organization ELS: resources for submission of patient data to the ELSO registry. Available at: https://elso.org/registry/datadefinitions,forms,instructions.aspx. Accessed August 3, 2023.
- 23.Lorusso R, Alexander P, Rycus P, Barbaro R: The extracorporeal life support organization registry: Update and perspectives. Ann Cardiothorac Surg 8: 93–98, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgson C, Needham D, Haines K, et al. : Feasibility and interrater reliability of the ICU mobility scale. Heart Lung 43: 19–24, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Guerin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368: 2159–2168, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Owyang CG, Donnat C, Brodie D, et al. : Similarities in extracorporeal membrane oxygenation management across intensive care unit types in the United States: An analysis of the Extracorporeal Life Support Organization Registry. Artif Organs 46: 1369–1381, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiagarajan RR, Barbaro RP, Rycus PT, et al. ; ELSO member centers: Extracorporeal life support organization registry international report 2016. ASAIO J 63: 60–67, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Tonna JE, Selzman CH, Bartos JA, et al. : The association of modifiable postresuscitation management and annual case volume with survival after extracorporeal cardiopulmonary resuscitation. Crit Care Explor 4: e0733, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonna JE, Selzman CH, Bartos JA, et al. : The association of modifiable mechanical ventilation settings, blood gas changes and survival on extracorporeal membrane oxygenation for cardiac arrest. Resuscitation 174: 53–61, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization: Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the extracorporeal life support organization registry. Lancet 396: 1071–1078, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization: Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international extracorporeal life support organization registry. Lancet 398: 1230–1238, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbaro RP, Paden ML, Guner YS, et al. ; ELSO member centers: Pediatric extracorporeal life support organization registry international report 2016. ASAIO J 63: 456–463, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiser DH: Assessing the outcome of pediatric intensive care. J Pediatr 121: 68–74, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Jennett B, Bond M: Assessment of outcome after severe brain damage. Lancet 1: 480–484, 1975. [DOI] [PubMed] [Google Scholar]
- 35.Grasselli G, Pesenti A, Cecconi M: Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 323: 1545–1546, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Chow EJ, Uyeki TM, Chu HY: The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol 21: 195–210, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groves HE, Piche-Renaud PP, Peci A, et al. : The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg Health Am 1: 100015, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartlett RH, Ogino MT, Brodie D, et al. : Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J 66: 472–474, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 195: 1253–1263, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Mustafa AK, Alexander PJ, Joshi DJ, et al. : Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg 155: 990–992, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Banayosy AM, El Banayosy A, Brewer JM, et al. : The ProtekDuo for percutaneous V-P and V-VP ECMO in patients with COVID-19 ARDS. Int J Artif Organs 45: 1006–1012, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonna JE: Is active mobility the most underdelivered care component for patients on extracorporeal membrane oxygenation? Ann Am Thorac Soc 19: 9–11, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tonna JE, Bailey M, Abrams D, Brodie D, Hodgson CL: Predictors of early mobilization in patients requiring VV ECMO for greater than 7 days: An international cohort study. Heart Lung 62: 57–63, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrams D, Madahar P, Eckhardt CM, et al. ; MORE-PT Investigators: Early mobilization during extracorporeal membrane oxygenation for cardiopulmonary failure in adults: Factors associated with intensity of treatment. Ann Am Thorac Soc 19: 90–98, 2022. [DOI] [PubMed] [Google Scholar]
- 45.Abrams D, Garan AR, Brodie D: Awake and fully mobile patients on cardiac extracorporeal life support. Ann Cardiothorac Surg 8: 44–53, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eggmann S, Luder G, Verra ML, Irincheeva I, Bastiaenen CHG, Jakob SM: Functional ability and quality of life in critical illness survivors with intensive care unit acquired weakness: A secondary analysis of a randomised controlled trial. PLoS One 15: e0229725, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodgson CL, et al. ; Investigators TS, the ACTG: Early active mobilization during mechanical ventilation in the ICU. N Engl J Med 387: 1747–1758, 2022. [DOI] [PubMed] [Google Scholar]
- 48.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL: Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med 39: 364–370, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Salluh JIF, Chiche JD, Reis CE, Soares M: New perspectives to improve critical care benchmarking. Ann Intensive Care 8: 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasr VG, Raman L, Barbaro RP, et al. ; ELSO Registry Scientific Oversight Committee: Highlights from the extracorporeal life support organization registry: 2006-2017. ASAIO J 65: 537–544, 2019. [DOI] [PubMed] [Google Scholar]
- 51.ARDS in Children and ECMO Initiation Strategies Impact on Neurodevelopment (ASCEND) (ASCEND) NCT05388708. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT05388708. Accessed September 15, 2023.
- 52.USFDA: Use of real-world evidence to support regulatory decision-making for medical devices. Guidance for industry and food and drug administration staff. Available at: https://www.fda.gov/files/medical%20devices/published/Use-of-Real-World-Evidence-to-Support-Regulatory-Decision-Making-for-Medical-Devices---Guidance-for-Industry-and-Food-and-Drug-Administration-Staff.pdf. Accessed September 15, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.